Abstract
Janus kinase inhibitors [JAKi] are a new class of small molecule drugs that modulate inflammatory pathways by blocking one or more JAK receptors, and are increasingly being used in the treatment of immune-mediated diseases. Tofacitinib, a non-selective JAKi, is now approved for moderate-to-severe ulcerative colitis [UC] that is refractory or intolerant to tumour necrosis factor inhibitors [TNFi]. Whereas tofacitinib is associated with the advantages of oral administration, rapid onset of action, and lack of immunogenicity over TNFi, there are many safety considerations to take into account such as the risk of thromboembolism, infections, and hyperlipidaemia: each with specific nuances pertaining to prevention and monitoring strategies. Considerations such as pregnancy, breastfeeding, and history of malignancy also are to be navigated with utmost caution, given that very few data are available for guidance. With the use of JAKi in the real world progressively over time, safety implications will become more lucid, including caveats pertaining to JAK selectivity and gut-selective JAKi, as well as mechanistic data pertaining to adverse effects. This Viewpoint serves as a practical guide for clinicians managing inflammatory bowel disease [IBD] patients to navigate safety concerns around JAKi, including preventive and monitoring strategies.
Keywords: Inflammatory bowel diseases, ulcerative colitis, JAK inhibitors, tofacitinib, drug safety, adverse effects, monitoring, preventive, clinical care
1. Introduction
Janus kinase inhibitors [JAKi] are being increasingly used for the treatment of inflammatory bowel diseases [IBD] and other immune-mediated diseases. These are small molecule drugs with the advantages of oral administration, rapid onset of action, short half-life, and lack of immunogenicity, over biologics. JAKs represent a group of four membrane-bound receptors, which via the signal transducer and activator of transcription proteins [STAT] pathway, mediate regulation of genes that code for diverse inflammatory proteins.1 These receptors are JAK1, JAK2, JAK3, and TYK2, each with mainly discrete and some overlapping functions.1,2 Therefore, JAK selectivity as well as dose impact on the efficacy and safety of various JAKi.1 Tofacitinib, a pan-JAK inhibitor with higher inhibitory activity for JAK1 and JAK3, is approved for the treatment of moderate-to-severe ulcerative colitis [UC], non-responsive to conventional therapy, after its efficacy over placebo was demonstrated for induction and maintenance of remission in the OCTAVE 1 and 2 and SUSTAIN trials.3 Upadacitinib and filgotinib are JAK1 selective and are in phase 3 clinical trials for the therapy of Crohn’s disease [CD] and UC [NCT03345836, NCT02819635, NCT02914561],4–6 and TD-1473 [non-selective inhibitor of JAK1, 2, and 3 and TYK2 with exclusive enteral distribution] is in phase 2–3 trials for CD and UC [NCT03635112, NCT03758443].7,8 Other JAKi have been studied for rheumatoid arthritis [RA] treatment, and additional ones are in development.
In this brief viewpoint, we focus on the main adverse effects associated with tofacitinib for the treatment of UC, which are relevant to the practising clinician. We then propose practical recommendations on how to manage JAKi therapy in UC patients, including preventive care and monitoring. For a comprehensive review of JAKi safety, we refer readers to the several excellent and detailed works on the topic referenced in this Viewpoint.
2. Infections
2.1. Herpes zoster
JAKi, across studies in RA, psoriasis, and UC, are associated with an increased incidence of herpes zoster [HZ] infection or shingles.9–11 In a pooled analysis of phase 2, 3, maintenance, and open label extension [OLE] global tofacitinib data on 1157 UC patients (1612.8 patient-years [PY] of exposure), 65 [5.6%] patients developed 69 events of HZ infection with incidence rate of 4.1 per 100 PY (95% confidence interval [CI] 3.1–5.2).11 In this analysis, the risk of HZ with tofacitinib 10 mg twice daily was higher [6.6 per 100 PY; 95% CI 3.2–12.2], compared with 5 mg twice daily [2.1 per 100 PY; 95% CI 0.4–6.0] and placebo [1.0 per 100 PY; 95% CI 0–5.4]: suggesting a dose-response relationship. The majority of HZ events [51/69, 74%] had limited dermatomal distribution11 which is consistent with tofacitinib data from clinical trial in RA and psoriasis.9,10 Twelve events were multidermatomal HZ, and six were disseminated HZ, of which one was complicated by encephalitis; 16 patients withheld tofacitinib temporarily and five ultimately discontinued the study drug.11 Risk factors for HZ include older age (hazard ratio [HR] 1.58; 95% CI 1.34–1.87; p <0.0001 for every 10-year increase in age) and previous tumour necrosis factor inhibitor [TNFi] failure [HR 1.92; 95% CI 1.15–3.21; p = 0.0122]. Whereas there was a trend toward higher risk in Asian patients [HR 1.76; 95% CI 0.97–3.19; p = 0.0612], this was not significant.11 In a meta-analysis of 21 RA clinical trials, the risk of HZ was higher for baricitinib than placebo (incidence rate ratio [IRR] 2.86; 95% CI 1.26–6.50), but not for tofacitinib or upadacitinib [IRR 1.38; 95% CI 0.66–2.88, and 0.78 95% CI 0.19–3.22, respectively].12
Other data suggest a higher risk of HZ with JAKi use in Asia, particularly in Japan and Korea, compared with Western countries. In a post-hoc analysis of pooled data on tofacitinib in RA patients in the Asia Pacific, the incidence rate per 100 PY of HZ was higher [0.7; 95% CI 0.5–1.0], compared with that in the global population [0.3; 95% CI 0.2–0.3].13 Genome-wide meta-analysis data suggest that these differences may be mediated by single nucleotide polymorphisms [SNPs] near the CD83 or IL17RB genes; such SNPs are implicated in immune pathways that mediate response to HZ, and are more frequent in East Asians than Caucasians.14
In phase 2 clinical trials for the treatment of moderate-severe CD, one case of HZ in the filgotinib trial [1/152, up to 20 weeks of follow-up] and one case in the upadacitinib trial [1/183, up to 16 weeks of follow-up] were observed. Although no significant risk was found compared with placebo, these results should be interpreted with caution as the trials were of short duration and tested a range of doses, some of which were ineffective.15,16
Current recommendations are to treat HZ with anti-virals in all IBD patients regardless of immunosuppression, in consultation with an infectious diseases specialist, for at least 7 days if immunosuppressed, or for 2 additional days after all skin lesions have crusted over.17 The choice of anti-viral can be oral in uncomplicated cases, and intravenous in complicated, ophthalmic, multidermatomal, or disseminated HZ. Urgent ophthalmological consultation is recommended in ophthalmic HZ.17 Most patients in the UC, RA, and psoriasis trials were able to continue tofacitinib through the course of HZ infection, whereas others temporarily discontinued and restarted after resolution of infection; even fewer patients discontinued it permanently.10,11,18
HZ risk associated with tofacitinib is preventable with vaccination. The adjuvanted recombinant HZ subunit vaccine [Shingrix] became available in 2017, is administered intramuscularly as two doses 2 months apart, and is effective in preventing HZ in 97.2% [95% CI 93.7–99.0] of persons older than 50 years, including those older than 70 years of age.19 Although the attenuated subunit zoster vaccine is not studied specifically in UC patients, we recommend that the first dose be administered before starting tofacitinib therapy, followed by the second dose in 2 months. Zostavax, a live attenuated vaccine, is contraindicated in those on immunosuppressive medications,20 a recommendation that is applicable to JAKi.
2.2. Other infections
In 4.4 years of follow-up data from global UC clinical trials, non-HZ opportunistic infections [OI] were very few and included one case each of cytomegalovirus colitis, cytomegalovirus hepatitis, pulmonary cryptococcosis, and histoplasmosis. Serious non-HZ infections included appendicitis [n = 4], anal abscess [n = 2], and Clostridium difficile infection [n = 2]. In the overall cohort, the IR per 100 PY of both non-HZ OI and serious non-HZ infections was 0.2 [95% CI 0.1–0.6]. Higher body weight [90 kg] was a risk factor for serious infections [HR 2.3; 95% CI 1.1–4.8; p = 0.0318].21 In a meta-analysis of 21 RA trials, serious infections with JAKi were rare and comparable to the baseline risk in the population, [IRR for tofacitinib 1.22; 95% CI 0.60–2.45, for upadacitinib 1.14; 95% CI 0.24–5.43, and for baricitinib 0.80; 95% CI 0.46–1.38].12
The risk of tuberculosis [TB] with tofacitinib in pooled RA trials data varied with background risk in the population; IR [per 100 PY] was 0.02 [95% CI 0.003–0.15] in low-, 0.08 [95% CI 0.03–0.21] in medium-, and 0.75 [95% CI 0.49–1.15] in high-incidence countries.22 In phase 3 studies, no case of TB was reported in the 263 patients with latent TB infection who were given isoniazid prophylaxis concurrently with tofacitinib.22 The risk of hepatitis B among those on tofacitinib is reported in a small real-world retrospective Taiwanese study, in which 75/116 persons with RA, who were positive for hepatitis B core antibody, did not develop hepatitis B reactivation regardless of surface antigen status. Of those with chronic hepatitis B [hepatitis B surface antigen positive, n = 6], four persons did not receive prophylactic nucleotide analogues, of whom two had reactivation of hepatitis B; both were recaptured with therapy, and the other two who received prophylactic therapy did well.23
Based on these data, we recommend testing for TB and hepatitis B and institution of prophylactic therapy as needed, before tofacitinib therapy, similarly to guidance pertaining to TNFi therapy.24
3. Venous thromboembolism
In February 2019, the FDA issued a black-box warning after venous thromboembolism [VTE] was reported with the 10 mg twice daily dose [but not lower doses] of tofacitinib for RA among patients >50 years of age and with at least one other cardiovascular risk factor, in an ongoing safety trial, with VTE risk five times that associated with TNFi.25 This trial is expected to be completed imminently, and will be highly informative. Robust long-term safety data for tofacitinib, especially in the context of the higher dose, are lacking. Tofacitinib has been approved for use in RA and psoriatic arthritis since November 2012 and December 2017, respectively, the approved doses being 5 mg daily or twice daily.26 The higher dose of 10 mg twice daily has been approved only since May 2018 for the treatment of moderate to severe UC.26 Therefore, long-term safety data pertaining to this dose of tofacitinib are even more sparse.
In a pooled analysis of phase 2, phase 3, and OLE studies of tofacitinib for moderate-to-severe UC, with 1613 patients-years’ exposure, 4.4 years of follow-up, and with most patients on the 10 mg twice daily dose [83.9%], one death due pulmonary embolism was reported in a patient with pre-existing metastatic cholangiocarcinoma. Major adverse cardiovascular events [MACEs] occurred in four persons, of whom three had underlying cardiovascular risk factors [IR 0.2; 95% CI 0.1–0.6].21 In a meta-analysis of 26 randomised controlled trials [RCTs], including 11 799 RA patients on various JAKi, there was no significant increase in the risk of overall cardiovascular events [CVEs] (odds ratio [OR] 1.04; 95% CI 0.6–1.76), MACEs [OR 0.80; 95% CI 0.36–1.75], or VTEs [OR 1.16; 95% CI 0.48–2.81]. There was no difference in the risk of CVEs, MACEs, or VTEs between 5 mg or 10 mg a day of tofacitinib, or between 15 mg or 30 mg a day of upadacitinib.27 Similarly, in a pooled analysis of over 50 000 persons with RA, using claims data from the Truven Marketscan database [2012–2016] and Medicare [2012–2015], the risk of VTE, although numerically higher for tofacitinib (pooled IR per 100 PY for tofacitinib 0.77 [95% CI 0.43–1.27]; for TNFi 0.74 [95% CI 0.65–0.83]), was statistically comparable between the two groups, with a propensity-score adjusted hazard ratio of 1.33 [95% CI 0.78–2.24].28 Last, using the FDA’s Adverse Event Reporting System [FAERS] pertaining to JAKi use between approval and March 31, 2017, there was a trend towards increase in composite thromboembolic adverse events as a class effect, but no increase in deep vein thrombosis and pulmonary embolism.29
Although reassuring, the applicability of these data is limited in the context of VTE in UC patients, as they do not reflect risk associated with 10 mg twice daily, which is the dose implicated in VTE risk and used most frequently in UC. They also do not account for the hypercoagulable state associated with active UC. Long-term safety data on high-dose tofacitinib in UC are needed. While we await these, it is prudent to avoid tofacitinib among persons with risk factors for VTE which include age >50 years [based on data from the ongoing safety trial25], history of VTE, hypercoagulable state, smoking, immobilisation or reduced mobility, recent trauma or surgery, cardiovascular disease, cancer, obesity, lower limb paralysis, frequent long flights, and hormonal therapy.30 We also recommend a trial of de-escalation to the maintenance dose of 5 mg twice daily from 10 mg twice daily, once the patient is in endoscopic remission.3,31
4. Hyperlipidaemia
In a pooled analysis of 1157 UC patients enrolled in tofacitinib clinical trials, of whom 83.9% of patients received the higher dose of 10 mg twice daily, there was a dose-dependent and reversible increase in total cholesterol, low- and high-density lipoprotein cholesterol [LDL-c, HDL-c] but no increase in MACEs. There were no clinically significant changes in lipid ratios.32 The Reynolds Risk Score [RRS], which is a predictor of 10-year cardiovascular events taking into account C-reactive protein in addition to traditional risk factors, remained unchanged with tofacitinib therapy. As reported above, four cases of MACEs occurred in these cohorts, of which three had pre-existing risk factors.32 Similar data indicating increase in lipids, but no increase in MACE risk, are reported in clinical trials of tofacitinib in RA and psoriatic arthritis.33,34
We recommend checking the lipid profile at baseline before initiation of tofacitinib, and at Week 8 of treatment. If significantly abnormal, or if additional CV risk factors are noted, cardiac work-up and consideration of lipid-lowering agents are indicated.
5. Pregnancy and breastfeeding considerations
5.1. Pregnancy
There are very few human data on the safety of tofacitinib or other JAKi during pregnancy. Most of our knowledge in this regard is extrapolated from experimental studies. Although there are no data on placental transfer of tofacitinib, it is reasonable to assume that tofacitinib, a small-sized molecule, crosses the placenta.35 Animal data demonstrate that in rats, at a dose 73 times, but not at 29 times, the therapeutic dose of 10 mg twice daily, tofacitinib was teratogenic and foeticidal.36 Similarly in rabbits, at a dose 6.3 times, but not 1.5 times, the therapeutic dose of 10 mg twice daily, teratogenic and foeticidal effects and post-implantation loss were noted.36 Tofacitinib had no impact on male fertility or sperm quality or motility in animal studies.36 JAK1 maintains primordial ovarian follicle reserve in mice, and JAK1 blockage with ruxolitinib is associated with increased apoptosis, accelerated follicular activation, and loss of follicular reserve.37 However, clinical data pertaining to these pathways are lacking.
In a pooled analysis of five UC studies, outcomes of maternal [n = 11] and paternal [n = 14] exposure to tofacitinib in the periconception period or during pregnancy were reported.35 There were 15 healthy newborns, two spontaneous abortions, two medical terminations, and no foetal or neonatal death or congenital malformation. Data from RA and psoriasis studies are similar reassuring, with healthy newborns being the most common outcome.38 However, these are very few data over a short period of follow-up, and long-term exposure across multiple pregnancies in the real world will be needed to inform the safety of JAKi in pregnancy.
In the absence of conclusive safety data, JAKi should be avoided in women planning pregnancy; tofacitinib should be avoided for at least i week before conception, given its short half-life.39 When unavoidable, we recommend discussing the limited safety data around pregnancy with the patient, and advising contraception [non-hormonal] for the duration of JAKi therapy.
5.2. Breastfeeding
Similar to the limited pregnancy data, no human studies have reported outcomes of breastfeeding with JAKi. Tofacitinib is present in rat milk at twice the concentration of that in the serum of lactating rats. The presence of drug in animal milk makes it likely that it would be present in human milk as well.
Until more data become available, the recommendation is to not breastfeed for 18 h after taking tofacitinib.36,39
6. Malignancy
In the five tofacitinib trials in UC patients, 11 persons were diagnosed with a malignancy excluding non-melanoma skin cancer [NMSC], all of whom had previous treatment with thiopurines and eight had previously been treated with TNFi.21 All reported malignancies were discrete and included cervical cancer, hepatic angiosarcoma, cholangiocarcinoma, cutaneous leiomyosarcoma, Epstein-Barr virus-associated lymphoma, renal cell carcinoma, essential thrombocythaemia, acute myeloid leukaemia, adenocarcinoma of colon, lung cancer, and breast cancer. Eleven patients with NMSC were reported, of whom 6, 10, and 10 had previous history of NMSC, previous therapy with thiopurines, and previous therapy with TNFi, respectively The incidence rates of malignancy [excluding NMSC] and NMSC were each 0.7 [95% CI, 0.3–1.2] per 100 PY of exposure.21 In a network meta-analysis comparing various biologic therapies and JAKi for RA in clinical trials and long-term extension studies, malignancy risk was insignificant across all drugs [OR 1.68; 95% CI 0.48–5.92 for infliximab, 1.15; 95% CI 0.24–5.47 for tofacitinib].40
At this time, there are not enough data to determine cancer risk due to tofacitinib and other JAKi. In the absence of clear evidence, it is prudent to avoid JAKi, or use them with caution among those with history of cancer. Similarly, for patients who develop cancer while on JAKi therapy, we would advise switching to a medication with a more acceptable safety profile, if feasible.
7. Gastrointestinal perforation
In the overall cohort of UC patients on tofacitinib, the three cases of perforation were: one patient on tofacitinib 10 mg twice daily who had active colitis, concomitant steroid use, and recent endoscopy; another in OLE, on non-steroidal anti-inflammatory drugs, who was noted to have perforated appendicitis; and a third in OLE with sigmoid colon perforation at the site of Epstein-Barr virus lymphoma.21 Two patients with CD, who received the higher dose of upadacitinib [each received 24 mg twice daily and 24 mg daily], experienced small bowel perforations.16 Similarly, in pooled data from tofacitinib trials in RA, 22 perforations [IR 0.11; 95% CI 0.07–0.17] were reported. These rates were similar between the two doses of 5 mg and 10 mg twice daily. Interestingly, all patients were on concomitant therapy with corticosteroids [n = 3], non-steroidal anti-inflammatory drugs [n = 9], or both [n = 10].41
No signal pertaining to perforation risk has been reported with JAKi in the real world, but we advise vigilance, especially in the context of concerning symptoms.
8. Others
Given that JAK2 signalling is involved in the haematopoiesis,42 JAK inhibition may lead to altered blood cell counts. Tofacitinib, a pan-JAK inhibitor, has been associated with an initial decrease in haemoglobin and neutrophil, lymphocyte, and platelet counts, although these changes were mild and reversible.2,43 In contrast, no significant change in lymphocyte or neutrophil counts was reported with filgotinib, a JAK1 selective inhibitor. It is reasonable to monitor complete blood count [CBC] after initiating tofacitinib and to be specially cautious in initiating tofacitinib in persons with significant anaemia or leucopenia. JAK inhibitors were related to the minimally elevated level of serum liver transaminases and creatine kinase, but they did not result in clinically noticeable changes.44
9. Conclusion
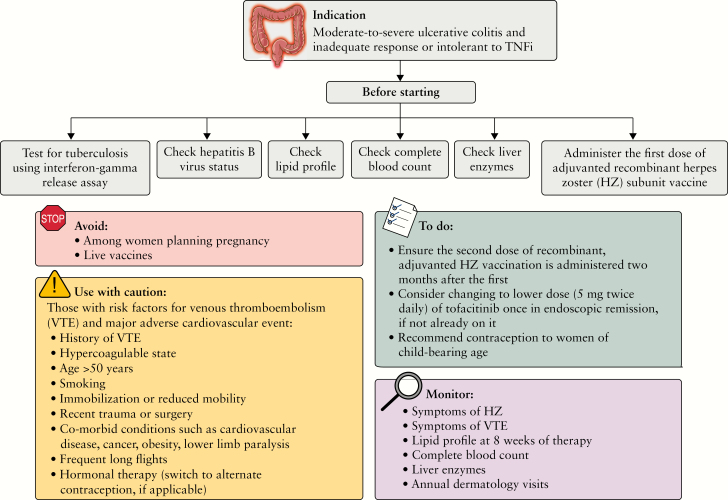
Overall, tofacitinib can be used safely to treat refractory UC so long as all relevant risks are considered before treatment, informed decisions are made jointly with the patient, and preventive and monitoring strategies are in place [Figure 1]. Of course, while considering the risk/benefit profile of the drug, we must also take into account the risks associated with untreated disease, and those associated with other available therapeutic options. It is also relevant to reiterate here that rare, serious, and clinically meaningful adverse effects can take several years of real-word data before becoming apparent, and that post-marketing surveillance strategies, such as the Sentinal Network and spontaneous adverse event reporting, are critical to recognising a drug’s safety profile over time.45,46
Figure 1.
Strategies to navigate safety concerns pertaining to tofacitinib for the treatment of moderate-to-severe ulcerative colitis. TNFi, tumour necrosis factor inhibitors.
Future research pertaining to the impact of JAK selectivity on the adverse effect profile of JAKi will be highly informative. Phase 1b data on TD-1473, a pan-JAK inhibitor distributed exclusively in the intestinal tract, suggest minimal systemic distribution in UC patients,15 and further safety and efficacy data are awaited. Last, mechanistic data on the risk of VTE, hyperlipidaemia, and other adverse effects associated with JAKi will help identify strategies to minimise and prevent these.
Acknowledgement
We thank Jill Gregory, Certified Medical Illustrator, Icahn School of Medicine at Mount Sinai, for assistance with the illustration.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The corresponding author confirms on behalf of all authors that there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated. MA receives intramural research support from the Dickler Family Fund. JFC reports receiving research grants from AbbVie, Janssen Pharmaceuticals, and Takeda; receiving payment for lectures from AbbVie, Amgen, Allergan, Ferring Pharmaceuticals, Shire, and Takeda; receiving consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene Corporation, Celltrion, Eli Lilly, Enterome, Ferring Pharmaceuticals, Genentech, Janssen Pharmaceuticals, Landos, Ipsen, Medimmune, Merck, Novartis, Pfizer, Shire, Takeda, Tigenix, Viela bio; and holds stock options in Intestinal Biotech Development and Genfit.
Author Contributions
MA and ESK: concept, literature search, drafting of manuscript and critical revision of the manuscript for important intellectual content. JFC: concept and critical revision of the manuscript for important intellectual content. All authors approved the final version of the manuscript.
References
- 1. Danese S, Argollo M, Le Berre C, Peyrin-Biroulet L. JAK selectivity for inflammatory bowel disease treatment: does it clinically matter? Gut 2019;68:1893–9. [DOI] [PubMed] [Google Scholar]
- 2. Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol 2017;13:234–43. [DOI] [PubMed] [Google Scholar]
- 3. Sandborn WJ, Su C, Sands BE, et al. ; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. [DOI] [PubMed] [Google Scholar]
- 4.U.S National Library of Medicine. A Study of the Efficacy and Safety of Upadacitinib [ABT-494] in Subjects With Moderately to Severely Active Crohn’s Disease Who Have Inadequately Responded to or Are Intolerant to Biologic Therapy.2019. https://clinicaltrials.gov/ct2/show/NCT03345836?term=upadacitinib&cond=Crohn+Disease&draw=2&rank=1. Accessed November 23, 2019.
- 5.U.S National Library of Medicine. A Study to Evaluate the Safety and Efficacy of Upadacitinib [ABT-494] for Induction and Maintenance Therapy in Subjects With Moderately to Severely Active Ulcerative Colitis [UC] 2019. https://clinicaltrials.gov/ct2/show/NCT02819635?term=upadacitinib&cond=ulcerative+colitis&draw=2&rank=3. Accessed November 23, 2019.
- 6. Filgotinib in the Induction and Maintenance of Remission in Adults With Moderately to Severely Active Crohn’s Disease [Diversity1]. 2019. https://clinicaltrials.gov/ct2/show/NCT02914561?term=filgotinib&cond=crohn%27s&draw=2&rank=3. Accesses November 23, 2019. [Google Scholar]
- 7.U.S National Library of Medicine. Efficacy and Safety of TD-1473 in Crohn’s Disease [DIONE]. 2019. [https://clinicaltrials.gov/ct2/show/NCT03635112?term=td+1473&cond=Crohn+Disease&draw=2&rank=1. Accessed September 23, 2019.
- 8.U.S National Library of Medicine. Efficacy ans Safety of TD-1473 in Ulcerative Colitis [RHEA]. 2019. https://clinicaltrials.gov/ct2/show/NCT03758443?term=td1473&cond=Ulcerative+Colitis&draw=2&rank=3. Accessed November 26, 2019. [Google Scholar]
- 9. Winthrop KL, Yamanaka H, Valdez H, et al. . Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol 2014;66:2675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winthrop KL, Lebwohl M, Cohen AD, et al. . Herpes zoster in psoriasis patients treated with tofacitinib. J Am Acad Dermatol 2017;77:302–9. [DOI] [PubMed] [Google Scholar]
- 11. Winthrop KL, Melmed GY, Vermeire S, et al. . Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis 2018;24:2258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bechman K, Subesinghe S, Norton S, et al. . A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology 2019;58:1755–66. [DOI] [PubMed] [Google Scholar]
- 13. Lee EB, Yamanaka H, Liu Y, et al. . Efficacy and safety of tofacitinib for the treatment of rheumatoid arthritis in patients from the Asia-Pacific region: Post-hoc analyses of pooled clinical study data. Int J Rheum Dis 2019;22:1094–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bing NZ, Zhou H, Zhang B. Genome‐wide trans‐ancestry metaanalysis of herpes zoster in RA and Pso patients treated with tofacitinib. Arthritis Rheumatol. 2015;67:799–800 [abstract 566]. [Google Scholar]
- 15. Vermeire S, Schreiber S, Petryka R, et al. . Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib [the FITZROY study]: results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017;389:266–75. [DOI] [PubMed] [Google Scholar]
- 16. Sandborn WJ, Feagan BG, Panes J, et al. . Safety and efficacy of ABT-494 [Upadacitinib], an oral JAK1 inhibitor, as induction therapy in patients with Crohn’s disease: results from Celest. Gastroenterology. 2017;152:S1308–9.. [Google Scholar]
- 17. Colombel JF. Herpes zoster in patients receiving JAK inhibitors for ulcerative colitis: mechanism, epidemiology, management, and prevention. Inflamm Bowel Dis 2018;24:2173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Winthrop KL, Curtis JR, Lindsey S, et al. . Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol 2017;69:1960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lal H, Cunningham AL, Godeaux O, et al. ; ZOE-50 Study Group Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015;372:2087–96. [DOI] [PubMed] [Google Scholar]
- 20. Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol 2017;112:241–58. [DOI] [PubMed] [Google Scholar]
- 21. Sandborn WJ, Panés J, D’Haens GR, et al. . Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol 2019;17:1541–50. [DOI] [PubMed] [Google Scholar]
- 22. Winthrop KL, Park SH, Gul A, et al. . Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis 2016;75:1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen YM, Huang WN, Wu YD, et al. . Reactivation of hepatitis B virus infection in patients with rheumatoid arthritis receiving tofacitinib: a real-world study. Ann Rheum Dis 2018;77:780–2. [DOI] [PubMed] [Google Scholar]
- 24. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 25. Pfizer. XELJANZ [tofacitinib]: Increased risk of pulmonary embolism and mortality in rheumatoid arthritis patients receiving 10mg twice daily in a clinical trial. 2019. https://www.fda.gov/media/120485/download Accessed November 15, 2019. [Google Scholar]
- 26. Xeljanz approval History. 2019. https://www.drugs.com/history/xeljanz.html. Accessed November 18, 2019. [Google Scholar]
- 27. Xie W, Huang Y, Xiao S, Sun X, Fan Y, Zhang Z.Response to: Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis 2019, July 4. doi: 10.1136/annrheumdis-2019-215841. [Epub ahead of print,] [DOI] [PubMed] [Google Scholar]
- 28. Desai RJ, Pawar A, Weinblatt ME, Kim SC. Comparative risk of venous thromboembolism in rheumatoid arthritis patients receiving tofacitinib versus those receiving tumor necrosis factor inhibitors: an observational cohort study. Arthritis Rheumatol 2019;71:892–900. [DOI] [PubMed] [Google Scholar]
- 29. Verden A, Dimbil M, Kyle R, Overstreet B, Hoffman KB. Analysis of spontaneous postmarket case reports submitted to the FDA regarding thromboembolic adverse events and JAK Inhibitors. Drug Saf 2018;41:357–61. [DOI] [PubMed] [Google Scholar]
- 30. Schünemann HJ, Cushman M, Burnett AE, et al. . American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv 2018;2:3198–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sands BE, Armuzzi A, Marshall JK, et al. . Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: results from OCTAVE Open. Aliment Pharmacol Ther 2020;51:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sands BE, Taub PR, Armuzzi A, et al. . Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2020;18:123–32.e3. [DOI] [PubMed] [Google Scholar]
- 33. Kremer JM, Bloom BJ, Breedveld FC, et al. . The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum 2009;60:1895–905. [DOI] [PubMed] [Google Scholar]
- 34. Gladman DD, Charles-Schoeman C, McInnes IB, et al. . Changes in lipid levels and incidence of cardiovascular events following tofacitinib treatment in patients with psoriatic arthritis: a pooled analysis across phase III and long-term extension studies. Arthritis Care Res 2019;71:1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mahadevan U, Dubinsky MC, Su C, et al. . Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis 2018;24:2494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pfizer Inc. Xeljanz Full Prescribing Information.2019. https://labeling.pfizer.com/ShowLabeling.aspx?id=959#data. Accessed August 15, 2019.
- 37. Sutherland JM, Frost ER, Ford EA, et al. . Janus kinase JAK1 maintains the ovarian reserve of primordial follicles in the mouse ovary. Mol Hum Reprod 2018;24:533–42. [DOI] [PubMed] [Google Scholar]
- 38. Clowse ME, Feldman SR, Isaacs JD, et al. . Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf 2016;39:755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mahadevan U, Robinson C, Bernasko N, et al. . Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology 2019;156:1508–24. [DOI] [PubMed] [Google Scholar]
- 40. Maneiro JR, Souto A, Gomez-Reino JJ. Risks of malignancies related to tofacitinib and biological drugs in rheumatoid arthritis: systematic review, meta-analysis, and network meta-analysis. Semin Arthritis Rheum 2017;47:149–56. [DOI] [PubMed] [Google Scholar]
- 41. Cohen SB, Tanaka Y, Mariette X, et al. . Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 2012;36:542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Strober B, Buonanno M, Clark JD, et al. . Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol 2013;169:992–9. [DOI] [PubMed] [Google Scholar]
- 44. Wollenhaupt J, Silverfield J, Lee EB, et al. . Safety and efficacy of tofacitinib, an oral janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol 2014;41:837–52. [DOI] [PubMed] [Google Scholar]
- 45. Coloma PM, Trifirò G, Patadia V, Sturkenboom M. Postmarketing safety surveillance: where does signal detection using electronic healthcare records fit into the big picture? Drug Saf 2013;36:183–97. [DOI] [PubMed] [Google Scholar]
- 46. Platt R, Wilson M, Chan KA, Benner JS, Marchibroda J, McClellan M. The new Sentinel Network – improving the evidence of medical-product safety. N Engl J Med 2009;361:645–7. [DOI] [PubMed] [Google Scholar]