Abstract
Given the ubiquity of the α-helix in the proteome, there has been much research in developing mimics of α-helices, and most of this research has been toward developing protein-protein interaction inhibitors. A common strategy for mimicking α-helices has been through the use of constrained, helical peptides. The addition of a constraint typically provides for conformational and proteolytic stability and, in some cases, cell permeability. Some of the most well-known strategies included lactam formation and hydrocarbon “stapling.” Beyond those strategies, there have been many recent advances in developing constrained peptides. The purpose of this review is to highlight recent advances in the development of new helix-stabilizing technologies, constraint diversification strategies, tether diversification strategies, and combination strategies that create new bicyclic helical peptides.
INTRODUCTION
There are a multitude of protein-protein interactions in the cell that are mediated by α-helices, and in many disease states it would be advantageous to mimic features of an α-helix with a small molecule or peptide. A common strategy for mimicking α-helices has been through the use of constrained, helical peptides. (1)(2) (3) (4) (5)(6)(7)(8)(9) Doing so provides for conformational stability by reducing the number of degrees of freedom of the peptide and/or by facilitating α-helical hydrogen bonding. This strategy also often provides for proteolytic stability: many proteases recognize an extended peptide conformation, but because of the conformational rigidity imparted by the constraint, helical peptides are not readily recognized by proteases that otherwise might recognize an unconstrained peptide of similar sequence.(10) Interestingly, peptide macrocyclization sometimes also provides for cell permeability, although this is not universally true. While there is much interest in understanding the determinants of cell permeability of constrained peptides, (11) predicting cell permeability a priori with complete accuracy is not possible currently.
Macrocyclization strategies usually fall into two broad categories: those that link sidechains together (“sidechain-to-sidechain”) or those that link one of the termini to a sidechain (“sidechain-to-terminus”). Most of the sidechain-to-sidechain strategies have attempted to place the constraint on the same face of an α-helix. Because the α-helix contains 3.6 residues per turn, many of these strategies have relied upon linking sidechains at residues that are near a multiple of 3.6 (i.e., at the i,i+4; i,i+7; and i,i+11 positions). This approach is often implemented by designing the constraint so that it replaces non-interacting residues of protein-protein interfaces. There are, however, several examples in the literature where the tether has been observed to interact directly with the surface of the target protein, (12) (13) (14) so that, in some cases, the constraint can be designed to replace interacting residues. The other major approach has been to link a sidechain to one of the termini of the peptide in a sidechain-to-terminus fashion. This method is perhaps best exemplified by hydrogen-bond surrogate (HBS) peptides. A notable advantage of this approach is its ability to nucleate the formation of an α-helix. (15)(16) Sidechain-to-terminus peptides have the added advantage that they do not replace two sidechains, which can serve to simplify the design and to minimize the overall size and molecular weight of a constrained peptide.
A successful and widely employed sidechain-to-sidechain technology has been “hydrocarbon stapling,” in which two olefin-terminated sidechains are linked together using ruthenium-catalyzed ring-closing metathesis. First described by Blackwell and Grubbs(17) and championed by Verdine and coworkers(18)(19)(20) (21) hydrocarbon stapling has gained attention for its ease of implementation and for its use in a number of different high-profile systems. (12) (13) (14) (22) (23) (24) Stapled peptides are currently in the clinic for treating orphan endocrine diseases (NCT01775358A). Although the term “staple” was originally coined to refer to the resemblance of an olefin to a literal staple, it has since been appropriated to refer to cyclized peptides in general—even those that are not helical.(25) As a result, technologies that were discovered and optimized before hydrocarbon-stapled peptides, such as helical disulfide- and lactam-constrained peptides,(26)(27) (28) (29) (30) are now sometimes referred to as “stapled” peptides. To avoid confusion, in this review we have reserved the term “staple” for hydrocarbon-stapled peptides formed by ring-closing metathesis.
While much of the earliest helical peptide literature focused on lactams and disuflides, and later on hydrocarbon staples, there has been much recent work on new constraints. More recently, researchers have begun to focus on using the constraint itself as a diversity-generating element. Likewise, functionalization of the tether that links the constraint to the peptide has seen accelerating interest, especially in those cases where the constraint interacts with the surface of the target. Lastly, researchers have begun to examine bicyclic peptides that contain at least two different helix-stabilizing technologies.
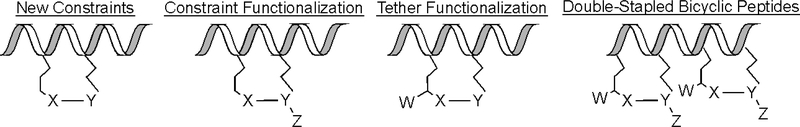
Others have published excellent recent reviews of the literature surrounding constrained helical peptides that focus on the application and future of constrained peptides (Walensky & Bird, Hydrocarbon-Stapled Peptides: Principles, Practice, and Progress, 2014) (Robertson & Spring, 2018) (Fairlie & Dantas de Araujo, 2016) (4) on new macrocyclic constraints (31) (2) on biological activity (7)(32) (5) (6), and on cysteine-crosslinking, (3) among others. The purpose of this review is to highlight recent structural advances in the development of new helix-stabilizing technologies, constraint diversification, tether diversification, and combination strategies to create new bicyclic peptides (Figure 1).
Figure 1.
The focus of this review is to highlight advancements in the development of (left to right) new constraints, functionalization of constraints and tethers, and combination strategies to develop bicyclic peptides.
NEW CONSTRAINTS
Thiol-Ene
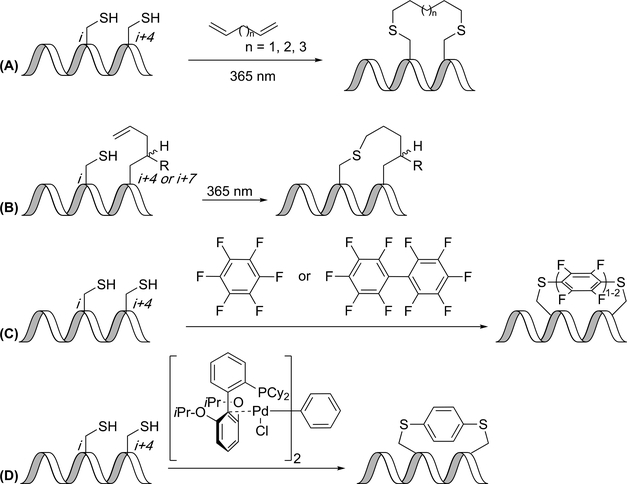
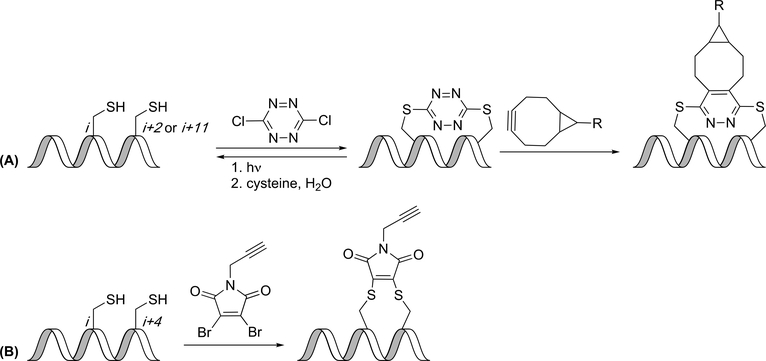
Chou and coworkers described the use of thiol-ene chemistry to create helical peptides, where two thioethers are installed by linking cysteines with an α,ω-diene in the presence of UV light (Figure 2A). Advantages of the thiol-ene reaction are its specificity for olefins, its ease of synthesis (no unnatural amino acids), and its lack of potentially contaminating metal catalysts. When cysteines in the i,i+4 positions were joined with an eight-carbon linker, α-helicity was similar to that of hydrocarbon-stapled peptides. Moving the cysteines to the i,i+7 positions also showed an increase in α-helicity with the eight-carbon or nine-carbon linker. The efficiency of blocking the p53-MDM2 interaction was measured using an ELISA assay, and a thiole-ene constrained peptide was as potent as a hydrocarbon stapled peptide.(33)
Figure 2.
Thioether linkages. (A) Intermolecular thiol-ene reaction gives thioethers. (B) Intramolecular thiol-ene reaction gives thioether-linked peptides. (C) SNAr reaction at cysteines gives fluorophenyl thioethers. (D) Aryl-palladium complex converts cysteines to phenyl thioethers.
Intermolecular thiol-ene reactions can be problematic in that they require multiple equivalents of the linker due to the radical-mediated mechanism. To address this, Li and coworkers developed an intramolecular thiol-ene reaction that could be performed on-resin (Figure 2B). The intramolecular thiol-ene reaction occurred between a thiol from a cysteine residue and a terminal double bond of a non-natural amino acid in i,i+4 positions, respectively. As with the intermolecular variant, addition of a photo-initiator and irradiation by UV light catalyzed the intramolecular thiol-ene reaction. The thiol-ene reaction appeared to be tolerated by a variety of amino acids including Arg, Asp, Lys and Glu. To determine the usefulness of this new linkage method, Li and coworkers synthesized an 11-mer peptide to mimic a steroid receptor coactivator sequence to bind to estrogen receptor α (ERα). This new method of carrying out an on-resin thiol-ene reaction has provided a simplified, efficient, and selective method for cyclization.(34)
A typical trend observed in α-helical peptides has been that constrained peptides are more proteolytically stable than unconstrained peptides. Perhaps surprisingly, Wang et al. showed that some thiol-ene constrained peptides were more rapidly degraded than unconstrained peptides. Molecular dynamics simulations suggested that the unconstrained peptides exhibited an extended helical conformation, while the single- and double-thiol-ene constrained peptides adopted a “collapsed” conformation. This atypical conformation was hypothesized to be the cause of more rapid degradation.(35)
A modification to the thiol-ene technique has been the development of thiol-yne constrained peptides. A cysteine residue was placed at the i position, and an alkyne stapling amino acid was placed at the i+4 position. After cleavage from the resin, the two residues form a vinyl thioether after exposure to UV light and a photoinitiator. The constrained peptide was observed to be helical as compared to the linear peptide. The constrained peptide was also stable in acidic and basic conditions and had 5-fold greater cellular uptake as compared to the linear peptide while blocking the estrogen receptor-coactivator interaction at nanomolar concentrations.(36)
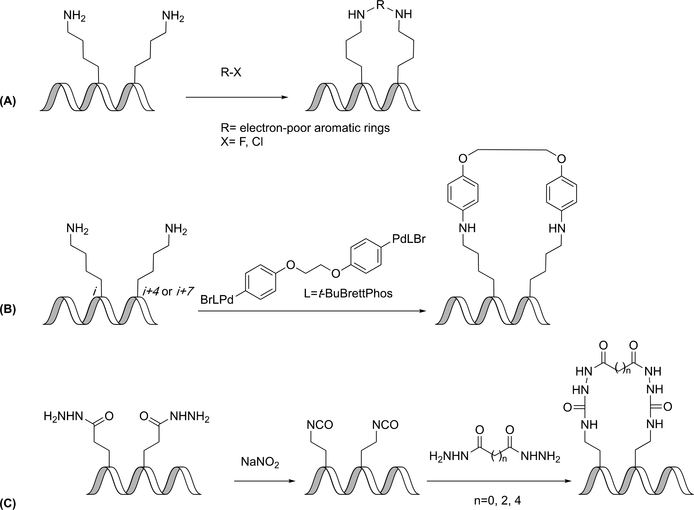
Cysteine Arylation
Often, cysteines are selected for the development of new linkers due to the ease with which they are able to form thioether linkages. An arylation approach, different from the alkylations previously described, was developed by Pentelute and coworkers, who used SNAr reactions to link two cysteines with a perfluoroaromatic molecule (Figure 2C). The reaction was highly regioselective in that the only observed product was the 1,4-disubstituted benzene. The reaction also showed good chemoselectivity, as no reaction occurred between perfluorobenzene and carboxylate-, amine-, and imidazole-containing amino acids. This new method allowed for the synthesis of peptides with increased protease stability, binding affinity for the C-terminal domain of HIV-1 capsid assembly polyprotein, and cell permeability, compared to unconstrained peptides; however, because of interference in the circular dichroism spectrum due to the perfluoroaryl linker, only a semi-quantitative estimate of the helicity of the peptide could be determined. In addition to peptide stapling, this method was also used in conjunction with native chemical ligation to create modified proteins. Advantages of this method include that it does not require metal catalysts or unnatural amino acids, and that it can provide additional lipophilicity in cases where that is beneficial.(37) Pentelute and coworkers have also used this strategy to prepare blood/brain-barrier-penetrant BIM BH3 analogue constrained peptides.(38)
In the examples of cysteine arylation given above, the aryl linker is activated for nucleophilic attack by the presence of electronegative fluorine atoms. Buchwald, Pentelute, and coworkers have developed a method that does not require fluoroarenes but instead relies upon palladium-catalyzed cysteine arylation, beginning from aryl halides or trifluoromethanesulfonates (Figure 2D). The aryl-palladium complexes were stable when exposed to acids, bases, oxidants and thiol nucleophiles, and they could be stored for up to 20 weeks at 4 °C. Upon addition of the palladium complex, RuPhos ligand, and trifluoromethanesulfonate, peptides were converted to the desired product in as little as five minutes. The reaction appeared to be highly selective for cysteine and could be carried out at a variety of pH’s and in the presence of various co-solvents and buffers. This provides a method of constraining peptides using a variety of aryl linkers inaccessible by other methods. This newly developed palladium complex provides an alternative method of bioconjugation of aryl moieties to cysteine residues and can be used to functionalize unprotected peptides.(39)
Since their initial reports, Rojas et al. have developed a water-soluble palladium arylation complex. This new palladium arylation complex allowed for a wide range of conjugation reactions that could take place in aqueous solutions with only minimal amounts of organic solvent—an important extension for biomolecules that would otherwise denature in organic solvent.(40)
A comprehensive investigation of the effect of linker length, chemical composition and rigidity on cysteine arylation has been completed by Rojas et al. This comprehensive study provided information necessary to select and screen for optimal linkers for use with deprotected peptides.(41)
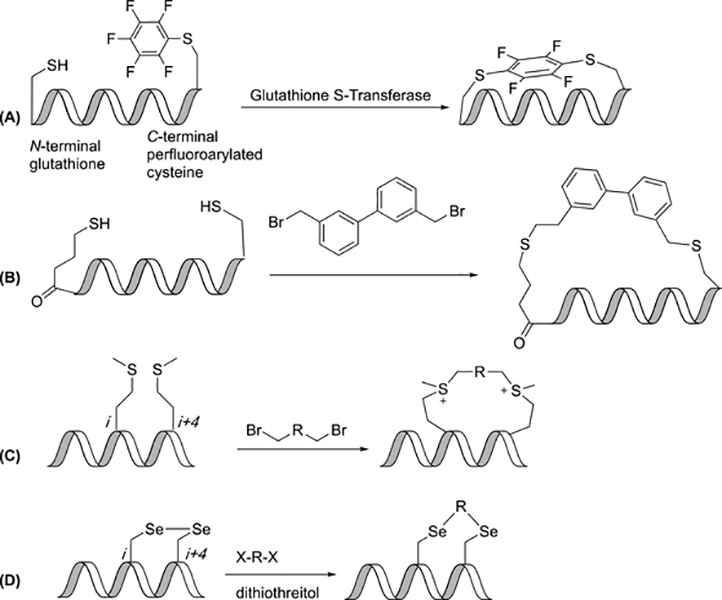
Macrocyclization of peptides has mostly been achieved through the use of synthetic methodology; however, an untapped source of efficient synthesis is through enzyme catalysis. Zhang et al. developed a method in which an SNAr reaction between cysteine and perfluoroaryl groups was catalyzed by glutathione S-transferase (GST) enzyme to yield constrained peptides with a large number of residues. A cysteine in a glutathione tag near the N-terminus reacted with a perfluoroarylated cysteine, which was positioned near the C-terminus (Figure 3A). The cysteine residues were positioned in i,i+1 to i,i+14 positions. Even the longer peptides were observed to be linked at a much greater rate with GST. For example, an i,i+7 peptide with a decafluorobiphenyl linker was observed to be cyclized at a rate 900-fold faster with GST than without enzyme. Although yields continued to decrease as ring size increased, the yields for the GST-catalyzed reactions were ~20 times greater than that for the reaction without the enzyme. This technique allowed for the development of constrained peptides which could not have been developed due to the limitations associated with synthetic methods.(42)
Figure 3.
Modifications to thioether linkages. (A) Glutathione S-Transferase catalyzes SNAr reactions to give fluorophenyl thioethers. (B) Addition of biphenyl linker to thiols gives biphenyl thioether. (C) Bis-alkylation of methionine gives sulfonium-linked peptides. (D) Alkylation of diselenocysteine peptides gives diselenoether constrained peptides.
Wu et al have developed a method in which a “capped-strapped” peptide was produced. This method of constraining peptides incorporates an aromatic linker between the N-terminus and C-terminus of a peptide. A cysteine is placed at the C-terminus, a 4-thiobutyric acid is placed at the N-terminus, and a biphenyl or bipyridyl linker is used off-resin to constrain the peptide (Figure 3B). According to circular dichroism results, the capped-strapped peptides were helical, while the acyclic peptides exhibited a random-coil conformation. Interestingly, this peptide exhibited a quaternary hexamer structure that could be useful in the design of foldamers.(43)
Methionine-linked peptides
Methionine-linked peptides have not been as thoroughly investigated as cysteine-linked peptides. Work completed by Li and coworkers highlighted a reversible stapling technique of methionine residues using a bis-alkylation/dealkylation sequence for unprotected peptides. This two-component stapling technique used two methionines placed in i,i+3; i,i+4; or i,i+7 positions (Figure 3C). A di-halide is added with acetonitrile, water and formic acid. The linear peptide could be regenerated by adding 2-mercaptopyridine. The cellular uptake of these peptides was found to be greater than that observed for non-constrained peptides. These results were attributed to the presence of positive charges on the tether itself.(44)
Selenocysteine-crosslinks
The increased acidity of selenocysteine relative to cysteine allows for incorporation of a greater variety of electrophilic linkers under mild aqueous conditions. Two selenocysteine residues were placed at the i,i+4; i,i+7; or i,i+11 positions with a dihalide linker added to form the constraint (Figure 3D). Circular dichroism results indicated that the diselenoether peptides were more α-helical than the initial linear sequence. Long alkane linkers were best at inducing helicity in the i,i+7 peptide, aliphatics were best for i,i+4 peptide, and the ten-atom aliphatic linker was best for the i,i+11 peptide. In general, cross-linked peptides displayed enhanced binding affinity to MDM2 relative to the linear form. A peptide with an i,i+7 12-atom aliphatic crosslink had the greatest potential as an inhibitor of the MDM2/p53 complex.(45)
1,3-Diynyl Linker
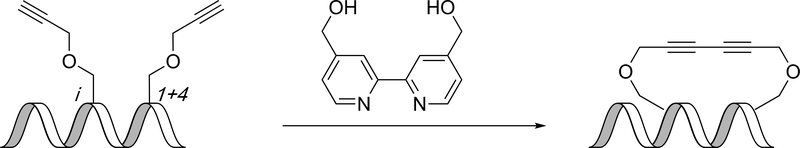
An on-resin intramolecular Glaser reaction between two propargyl serine residues at the i,i+4 positions in the presence of copper chloride, 4,4’-bis(hydroxymethyl)-2,2’-bipyridine (bpy-diol) ligand, and diisopropylethylamine at room temperature produced a 1,3-diynyl peptide macrocycle (Figure 4). The i,i+5, i,i+6, and i,i+7 positions were also investigated; however, the i,i+4 and i,i+7 positions induced the greatest helicity at 56% and 40%, respectively, as compared to the linear peptides. The i,i+5 and i,i+6 peptides were observed to be mostly disordered. These peptides were prepared as analogues of a segment of B-cell CLL/lymphoma 9 protein in an effort to target the Wnt signaling pathway.(46)
Figure 4.
Glaser reaction gives 1,3-diynyl peptide.
Nitrogen arylation
As mentioned above, cysteine arylation is a powerful method used for cyclizing peptides due to the ease with which the reaction takes place; however, under basic conditions the aryl thioether can eliminate a thiophenol to produce dehydroalanine. Disulfide bond formation and oxidation are also competing side reactions during the formation of the thioether. In an effort to eliminate these limitations, Pentelute and coworkers investigated arylation of nitrogen-containing amino acids. Arylation of nitrogen species has not been well-developed for peptide synthesis using conventional methods like Ullman-type N-arylation or Buchwald-Hartwig coupling, which may require high temperatures and oxygen-sensitive catalysts. The authors developed a new approach in which lysine N-arylation can be performed in high yields, under mild conditions, and with a variety of macrocycle sizes for unprotected peptides (Figure 5A). Perfluoroaryl compounds were investigated due to their ability to complete SNAr cysteine arylation. Certain perfluoroaryls gave stable Meisenheimer complexes but resulted in low yields and long reaction times; therefore, a perfluorosulfone was investigated in order to eliminate the stabilization of the Meisenheimer complex. A model peptide containing nucleophilic residues, except cysteine, was synthesized and the arylation was observed to be specific for lysine. To determine optimized macrocyclic ring size, a scan was completed at i, i+1 to i, i+14 sites. Only i, i+9 and i, i+10 had low conversions, which were attributed to ring strain and preference for double-arylation product. To determine the optimal length of the amine tether, ornithine, diaminobutyric acid, and diaminopropionic acid were incorporated at i, i+7 positions and crosslinked with two separate aryl halides. Conversions of 72–97% indicated that peptides could be designed with highly diverse scaffolds. The lysine-arylated peptide proved stable to oxidative and basic conditions. The same conditions resulted in degradation of the cysteine-arylated peptide. The macrocyclic peptides had improved proteolytic stability compared to the linear peptide, and flow cytometry and confocal microscopy confirmed enhanced cellular uptake for constrained peptides that targeted MDM2. This N-arylation method could resolve some stability issues associated with the arylation of cysteines and has provided a new method of macrocyclization.(47)
Figure 5.
Amine and Amide linkages. (A) SNAr reaction at lysines gives secondary anilines. (B) Bis-palladium complex converts lysines to secondary anilines. (C) Addition of bifunctional nucleophile to isocyanates gives semicarbazide constraint.
Similarly to cysteine arylation, a palladium-catalyzed nitrogen arylation method has been developed by Lee et al. Upon addition of the bis-palladium complex, sodium phenoxide,and t-BuBrettPhos or BrettPhos, peptides can be converted to the desired product within 6 hours (Figure 5B). The reaction appeared to be selective for lysine as compared to other nucleophilic amino acids. This provided a method of stapling peptides using a variety of aryl linkers inaccessible through other methods by eliminating the requirement of a pre-activated nucleophile.(48)
Isocyanates
Pentelute and coworkers have developed a two-component macrocyclization using isocyanates in which two glutamic acid γ-hydrazide residues were oxidized with sodium nitrite, yielding acyl azide side chains in unprotected peptides. Curtius rearrangement afforded isocyanates, which could be trapped with bifunctional nucleophiles to give constrained peptides (Figure 5C). Initial work was completed with dihydrazides yielding two diaminobutyric acid side chains linked by an N’-substituted semicarbazide bridge. A variety of linkers and amino acid positions were investigated, with cyclization most efficient for the i, i+4 substitution pattern. As is commonly observed, the constrained peptides were more resistant to proteolytic degradation than the unconstrained peptides, although, in general, the longer linkers provided more protease resistance than the shorter linkers. The constrained peptides were stable in acidic, basic, reducing, and oxidizing conditions. Likewise, the α-helicity of the constrained peptides with longer linkers was increased compared to the unconstrained peptide. Peptides with shorter linkers had limited observable differences in α-helicity indicating that both the length and rigidity of the linker may have an effect on the α-helicity of the peptide. The α-helicity of the constrained peptides was found to be an indicator of the binding affinity of the peptides to C-CA (C-terminal domain of HIV-1 capsid assembly polyprotein), with highly α-helical peptides having the highest affinity. This approach has contributed to current methods in that it is a two-component macrocyclization technique that does not depend on the presence of either a cysteine or unnatural amino acid and is simple enough that it can be used to synthesize a diverse library of peptides using easily accessible bifunctional nucleophiles.(49)
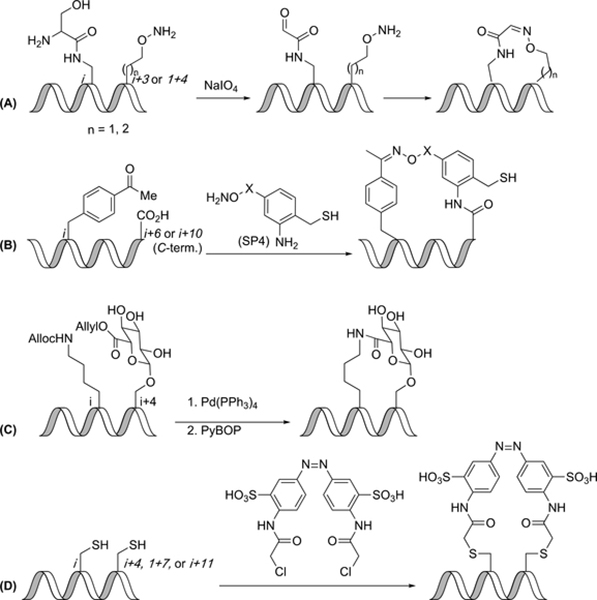
Oximes
Horne and coworkers have described the use of oximes as constraints in α-helical peptides. The aminooxy component of the reaction was an isostere of either ornithine or lysine, and the aldehyde component came from a serine-acylated amine side-chain that was oxidatively cleaved to give a glyoxyl aldehyde (Figure 6A). The aminooxy residue was placed at the i+3 or i+4 position, and the glyoxyl functionalized amine was placed at the i position. The spacing of the constraint affected helicity in that the i, i+4 oxime constrained peptide led to greater helicity than both the i, i+3 peptide and the unconstrained peptide. Under buffered conditions, a mixture of E and Z oximes formed quickly upon the addition of periodate.(50)
Figure 6.
Diversification of linkages. (A) Reaction of aminooxy residue and aldehyde gives glyoxyl aldehyde. (B) Oximes are formed by addition of p-acetylphenylalanine to constraint through oxime formation. (C) Amide formation gives glucuronic acid-constrained peptide. (D) Addition of 3,3’-bis(sulfonate)-4,4’-bis (chloroacetamido)azobenzene (BSBCA) to cysteines yield a photo-isomerizable constraint.
Fasan and coworkers have described a two-component, sidechain-to-terminus cyclization strategy that depended on oxime formation with SP4 (Figure 6B), followed by amide formation facilitated by a thioesterification. p-Acetylphenylalanine was placed at the i position, and alanine at the C-terminus, either i+6 or i+10 residues away. The constrained peptides were found to be two-fold better inhibitors of the p53-HDM2 interaction than unconstrained peptides. Interestingly, the most potent inhibitor was observed to have decreased α-helicity as compared to the control linear peptide; however, this lack of direct correlation between α-helicity and inhibitory activity has been observed in other p53-HDM2 protein-protein interaction inhibitors.(51)
Glucuronic acids
Glucuronic acid was investigated as a constraint due to the high frequency of glycosylation of proteins that occurs naturally. Computational studies carried out by Fairlie and coworkers suggested the addition of a glucuronic acid constraint could induce both α-helical and 310-helical formation. A lysine residue was placed at the i position, and a glucuronyl-functionalized serine was placed at the i+4 position (Figure 6C). The allyl group on the glucuronic acid residue and alloc group of lysine were removed using Pd(Ph3)4, and PyBOP was used to cyclize the peptide. Circular dichroism results and Ramachandran plots indicated that both α-helical and 310-helical conformations were possible.(52)
Diazo-linkers
Nevola et al. have developed a photoisomerizable linker, 3,3’-bis(sulfonate)-4,4’-bis(chloroacetamido)azobenzene (BSBCA), to link two cysteine residues (Figure 6D). This method was developed due to the unique characteristic of BSBCA to isomerize between cis and trans configurations when exposed to 380 nm or 500 nm light, respectively. Binding affinity was dependent on cis/trans configuration of BSBCA. An i,i+11 peptide was helical when BSBCA was in the trans configuration, and an i,i+7 peptide was helical when BSBCA was in the cis configuration. Flow cytometry and confocal microscopy imaging indicated that the two active peptides were taken up by cells, and total internal reflection microscopy was used to confirm that the constraint could function as a photo-controllable switch to regulate the activity of the peptide in living cells. This strategy provides a method in which the structure of a constrained peptide could be manipulated by exposure to specific wavelengths of light.(53)
CONSTRAINT FUNCTIONALIZATION/DIVERSIFICATION
Dihydroxylation
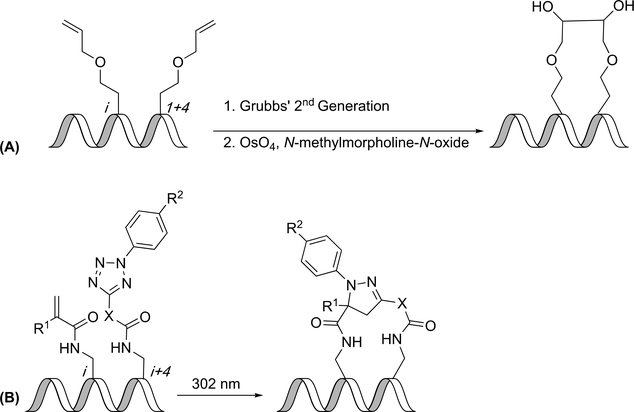
Most of the derivatization of stapling techniques in the literature has focused on development of new constraining reactions; however, recent work has also included modification of constraints post-installation. Similar to the typical ring-closing metathesis strategy, two allyl-homoserine derivatives were placed at i,i+4 positions and were exposed to 2nd generation Grubbs catalyst to form an alkene. Upjohn dihydroxylation was used to form a dihydroxylated linker (Figure 7A). This technique was used to develop an inhibitor of vitamin D receptor/coactivator interactions. Most constrained peptides developed were shown to have minimal activity against the vitamin D receptor/coactivator interaction; however, the dihydroxylated constrained peptide was found to have 70-fold greater activity. The circular dichroism spectrum of the dihydroxylated peptide as well as the non-hydroxylated peptide indicated helical structures.(54)
Figure 7.
Constraint diversification. (A)Upjohn Dihydroxylation of alkene gives dihydroxylated constraint. (B) Formal 1,3-dipolar cycloaddition reaction yields pyrazoline-constrained peptides.
Pyrazolines
Madden et al. reported a pyrazoline-constrained peptide using a photoinduced formal 1,3-dipolar cycloaddition reaction. This reaction is used to connect an alkene-containing amino acid and a tetrazole-containing amino acid to form a pyrazoline cross-linker, after extrusion of N2 (Figure 7B). These amino acids were developed by adding either an α,β-unsaturated carbonyl or a functionalized tetrazole moiety to a lysine or ornithine residue. The alkene residue was placed at the i position, and the tetrazole residue was placed at the i+4 position. Lysine-derived amino acids were observed to induce higher yields than amino acids derived from ornithine—a finding which was ascribed to a larger macrocycle causing less strain. N-4-methoxyphenyltetrazole and N-4-(dimethylamino)phenyltetrazole substituents induced higher yields than N-phenyltetrazole, which was attributed to the reactivity of the respective azomethine-imide-type intermediate formed. Circular dichroism data indicated that the constrained peptides had 28–64% helicity compared to 53% helicity for a linear peptide. Of those constrained peptides with greater helicity, all contained a methyl group at the 3-position of the pyrazoline ring, indicating that it may aid in inducing helical structure. The peptides were also observed to be cell-permeable. The pyrazoline constraint was intrinsically fluorescent; therefore, it has the potential to be used for fluorescent microscopy, as Madden et al. illustrate.(55)
1,2,3-Triazoles
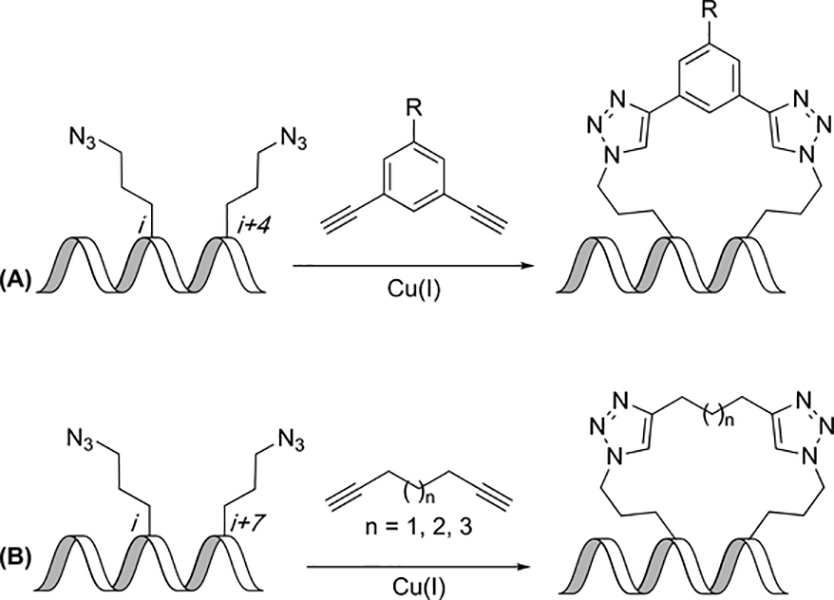
Although others have described one-component approaches to create 1,2,3-traizole-constrained peptides, (56)Spring and coworkers have described the use of a two-component Huisgen 1,3-dipolar cycloaddition to give 1,2,3-triazole-constrained peptides (Figure 8A). Given the ease of synthesis of azide-containing amino acids from their corresponding amines, this method provides a straightforward route to constrained peptides. A linker formed from 3,5-dialkynylbenzene and azido-terminated amino acids gave the highest helicity. An additional advantage of this method was the ability to use the constraint itself as a diversity-generating element. For instance, the authors functionalized a linker with varying numbers of arginine residues to increase cell permeability without changing the peptide sequence; this addition did not lead to significant alteration of helicity.(57)
Figure 8.
Formation of triazole constraints. (A) Addition of dialkynylbenzene to azido-terminated residues yields 1,2,3-triazole-constrained peptides. (B) Addition of aliphatic dialkynes to azido-terminated residues yields 1,2,3-triazole-constrained peptides.
Lau et al. carried out a systematic analysis of the impact of the azide-containing sidechain length and constraint position on helicity and biological activity. Three sidechain lengths were compared that had two, three, or four methylene units in the sidechain: azidohomoalanine (Aha(N3)), azidoornithine (Orn(N3)), and azidolysine (Lys(N3)). These amino acids were placed at the i,i+7 positions. The highest helicity peptides resulted from placing the Aha(N3) amino acids at the i,i+7 positions. Although the peptide with Aha(N3) residues had the highest affinity, Lau et al. found that the highest affinity for MDM2 resulted from a peptide which had Orn(N3) amino acids. A second linker, containing three pendant arginines, was used to determine whether affinity for MDM2 would remain stable with the additional tag; however, binding affinity was found to decrease for peptides with the second linker.(58)
Two-component, copper-assisted azide/alkyne cycloaddition (CuAAC), mentioned above, was adapted to provide both a stapling reagent and a photo-crosslinking motif using a multifunctional photoactive benzophenone linker that included an additional alkyne handle for pull-down assays. Two azide stapling amino acid residues were placed at the i,i+7 positions, and the benzophenone linker was added using two-component CuAAC. Isothermal titration calorimetry experiments indicated that the constraint did not negatively impact binding affinity with the benzophenone constrained peptide exhibiting a binding affinity of 18 nM for MDM2.(59)
The biophysical characteristics of the linkers used in constrained peptides are an important factor that can influence the activity of the peptide in cells. Spring and coworkers have also focused on the effects of using linear aliphatic dialkynes instead of aromatic linkers with azidoornithine residues installed in peptides. Azidoornithine residues were in the i,i+7 positions, and the addition of the aliphatic linker yielded the constrained peptide(60) (Figure 8B). Circular dichroism indicated that the aliphatic-linked peptides were helical with hepta-1,6-diyne linker producing the most highly helical peptides at 78%. All of the constrained peptides had higher binding affinity to MDM2 than the unconstrained peptide, and the peptide with the hepta-1,6-diyne linker had similar affinity as a peptide containing an aromatic linker (2.6 nM vs. 3.2 nM, respectively).(61)
Spring and coworkers also used the azido alkynyl stapling method to develop an α-helical peptide with the ability to bind to Ctf4. Interestingly, the authors constrained the peptide at the i, i+6 positions, and this arrangement gave good helicity and target binding affinity. (62)
Two-component stapling strategies have not been easily adapted to high throughput screening due to the need for a secondary purification that results from the stapling reaction being completed in solution-phase in the presence of copper catalyst and additives. Spring and coworkers developed an azido alkynyl stapling procedure in which a secondary purification is unnecessary. This technique is a strain-promoted azide-alkyne cycloaddition reaction that is catalyst-free and can be completed in cell culture (for a review of strain-promoted cycloaddition, see (63)). In parallel to the in situ reactions, peptides were also synthesized and constrained using standard procedures including a secondary purification to confirm and compare bioactivity observed in the 96-well assay. Cellular activity related to MDM2 targeting was found to be greater for pure peptides, which could be attributed to gradual product formation. An advantage to using this method was that, while the compounds were not as pure as preparing them serially, high-throughput synthesis gave a greater diversity of compounds that could be prepared and assayed quickly.(64)
Tetrazines
The introduction of an s-tetrazine linker has provided an additional handle for diversification by the use of inverse demand Diels-Alder reactions. An S,S-tetrazine bridge was formed between two cysteine residues at the i,i+2 positions linked by a reaction with dichlorotetrazine. As an example of diversification from inverse demand Diels-Alder reactions, Brown and Smith incorporated a fluorescein label appended to a cyclooctyne (Figure 9A). The dual applicability of the linker, ease of removability and addition of probes, means that this linker has potential to be very useful for synthesizing peptides targeting a variety of protein-protein interactions. The disulfhydryl peptide can be re-formed by exposing an unfunctionalized tetrazine-bridged peptide to 365 nm light and cysteine. An alternative method for larger macrocycles includes exposure to 312 nm light and oxygen.(65)
Figure 9.
Diversification of thioether linkages. (A) Addition of dichlorotetrazine to cysteines gives S,S-tetrazine constraint, which can be further modified using inverse demand Diels-Alder reactions. (B) Addition of dibromomaleimides to cysteines gives S,S’-maleimide constrained peptides.
Dibromomaleimides can be efficiently added to cysteine or homocysteine residues in the i,i+4 positions to form a S,S’-maleimide crosslink (Figure 9B). This constraint can subsequently be removed by addition of excess thiol. The constrained peptides were more helical (42–43%) than the unconstrained peptides (24–29%) and were more proteolytically stable than the unconstrained peptides. The binding affinity of these peptides in comparison to the native sequence was found to be comparable for BID/Mcl-1 and improved for BAK/Bcl-xL. The addition of an alkyne onto the linker was used to provide a handle for further functionalization including to biotin, fluorescein, and PEG azide. (66)
TETHER FUNCTIONALIZATION
Tether-functionalized hydrocarbon-stapled peptides
Speltz et al. developed new stapling amino acids with γ-methylation in an effort to mimic native leucine and isoleucine residues. Evans’ N-acyloxazolidinone chemistry was used to synthesize enantiomerically enriched, branched alkyl side chains which were reacted with Schöllkopf’s bis-lactim ethers to produce the desired R- and S-γ-methylated stapling amino acids (Figure 11B). The amino acids were positioned to replace the i and i+4 residues of a steroid receptor coactivator ILXXLL motif to create estrogen receptor antagonists. The authors found that placing the S-configured methyl on the i side resulted in high helicity and binding affinity, whereas placing R-configured methyl on the i side maintained binding affinity but resulted in reduced helicity. Placing the R-configured methyl on the i+4 side resulted in poor affinity and helicity. Placing the S-configured methyl stapling amino acid at the i+4 position led to unsuccessful olefin ring-closing metathesis, indicating that this stapling amino acid in this position was not conducive for ring closure. Minimization of syn-pentane strain between α- and γ-methyl groups was responsible for the conformations taken up by the sidechains.(67)
Figure 11.
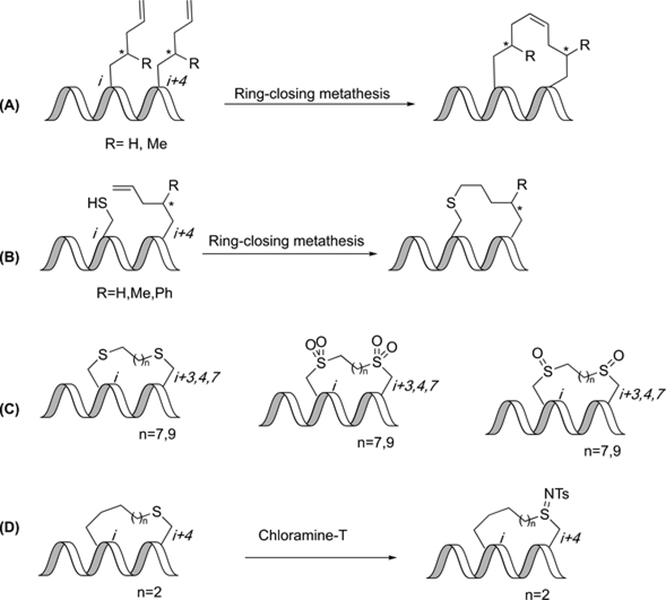
Tether functionalized constrained peptides. (A) Ring-closing metathesis of γ-methylated olefin tethers gives chiral all-hydrocarbon constraints. (B) Ring-closing metathesis of cysteine and γ-substituted olefin tethers gives chiral thioether constraint. (C) Thioether, sulfone, and sulfoxide peptides. (D) Addition of chloramine-T to a thioether-constrained peptide gives a sulfilimine.
α-Carbon-functionalized hydrocarbon stapled peptides
Wu et al. developed an approach to synthesize hydrocarbon stapling amino acids with retained sidechains. Leucine, methionine, serine, tyrosine, lysine, arginine and phenylalanine derivatives were synthesized using this universal method. These modified stapling amino acids were placed in the i position and either S5 or R5 was placed at the i,i+3 and i,i+4 positions (Figure 10). All of the peptides with the retained sidechain stapling amino acids were observed to have higher helicity (22–71%) than that observed for the control peptide, Axin, which had helicity of 20%. These peptides were also stable to protease degradation after 1 hour as compared to Axin, which had a half-life of 9 minutes. This method was used to develop BID BH3 peptide mimics. (68)
Figure 10.
Ring-closing metathesis of α-disubstituted olefin tethers gives all-hydrocarbon constraint while retaining natural amino acid sidechains.
Tether-functionalized thioether peptides
Li and coworkers used γ-methyl and γ-phenyl stapling amino acids to develop thioether-linked peptides with substitution on the tether. The l-amino acids containing an additional chiral center in the sidechain were synthesized as a diastereomeric mixture and separated using chromatographic methods. Pentapeptides were used as the model with a cysteine at the i position and the substituted S5 residues in the i+4 position (Figure 11B). Thiol-ene chemistry was used to close the macrocycle. The optimal side chain length for maintaining helicity was 5 carbon atoms long. Li and coworkers used this stapling strategy to develop peptides that target the estrogen receptor and MDM2. The R-γ-substituted peptides displayed better helicity, binding affinity and protease stability.(69)
Sulfur-functionalized constrained thioethers
The thioether oxidation state is one of several that a sulfur atom may exhibit. Others include the sulfoxide, sulfone, and sulfonium oxidation states. Perell et al. have compared the effects of sulfur oxidation states on the secondary structure of constrained helical peptides. Cysteine residues were placed at the i and i+3, i+4, or i+7 positions. The residues were then alkylated using either eight-carbon or ten-carbon dibromoalkanes to afford the constrained peptides. The i, i+4 constrained peptide was observed to have a slight decrease in helicity; the i, i+3 and i, i+7 constrained peptides were observed to have slight increases in helicity. The peptides were oxidized to sulfoxides and sulfones, which led to a decrease in helicity (Figure 11C). These oxidized peptides were observed to have much greater trypsin stability as compared to the control peptide. Up to 79% intact oxidized peptide was observed at 200 minutes for an oxidized peptide, vs. a t1/2 of 5 min for the unconstrained peptide. These peptides were used to target the MLL-KIX protein-protein interaction. Although the addition of the oxidized sulfur atoms did not lead to increases in helicity, this method has potential to be used in an effort to modify the polarity of constrained peptides.(70)
Lin et al. expanded upon their work with thioether-constrained peptides containing a chiral center by developing an in-tether sulfilimine chiral center. A cysteine was positioned near the N-terminus and a hydrocarbon stapling amino acid (S3, S4, S5 or S6) were placed at the C-terminus. The unconstrained peptides were converted using chloramine-T oxidation into constrained sulfilimine peptides (Figure 11D). The R-peptides were observed to be helical and the S-peptides were observed to have a random coil conformation. The helical constrained peptides were also observed to be thermally stable.(71)
Dual In-Tether-functionalized peptides
A combination strategy was used by Li and coworkers to develop dual chiral centers in the tether of a constrained peptide. A cysteine and a γ-methyl or γ-phenyl hydrocarbon stapling amino acid were placed in i,i+4 positions so that each peptide contained one of each of these residues (Figure 12 A). The linkage reaction between the olefin and thiol occurred under UV light. The oxidation of the sulfur to the sulfoxide occurred in solution. Helicity of the peptides was determined using circular dichroism, and it was observed that the helicity of the peptide with one chiral center was only dependent on the stereochemistry of the γ-position of the residue closest to the C-terminus. Interestingly, the stereochemistry of the chiral centers of the stapling amino acids at the i and i+4 positions became important for the helicity of the peptide, with i-γ-S and i+4-γ-R configurations optimal for inducing a helical conformation. The sulfoxide chiral center placed on the i residue appeared to have a positive effect on the helicity of the overall peptide.(72)
Figure 12.
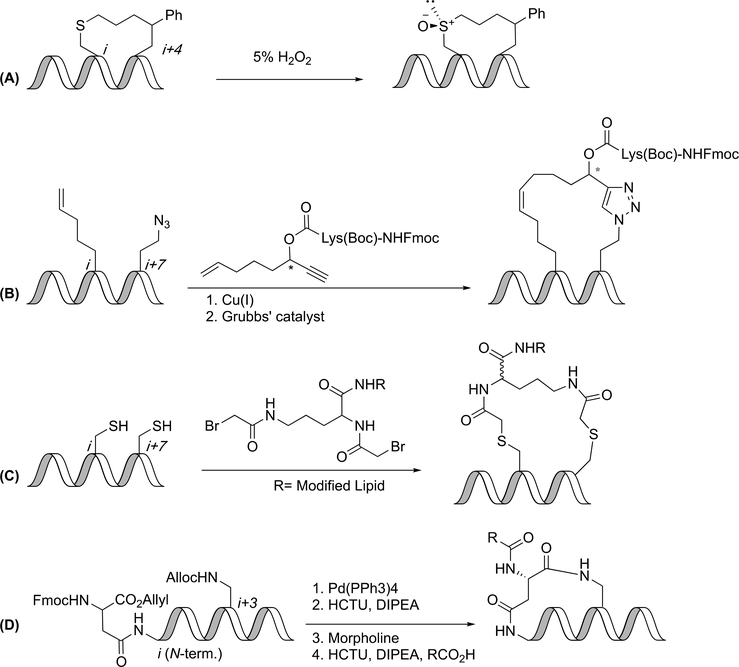
Functionalization of tethers. (A) Oxidation of thioether gives a dual-chiral center linker. (B) Orthogonal linkage strategies (CuAAC and ring-closing metathesis) give alkene/tetrazole constraints which can be functionalized on the tether. (C) Addition of modified lipid linker gives glucagon-like peptide 2 analog. (D) Sidechain-to-terminus macrocyclization of succinic acid and diaminopimelic acid.
Tether-functionalized 1,2,3-triazole peptides
A two-component strategy was used by Ley and coworkers to create unsymmetrically substituted constrained peptides targeting MDM2. The authors combined two orthogonal reaction strategies: CuAAC and ring-closing metathesis. Incorporation of an acylated alcohol along the tether allowed for tether functionalization and provided for a new chiral center. A hydrocarbon stapling amino acid was placed at the i position, and an azide-containing amino acid was placed at the i+7 position. CuAAC was completed first, followed by ruthenium-catalyzed ring closing metathesis (Figure 12B). The peptides constrained with the S-chiral center linker were observed to have comparable α-helicity to the unconstrained peptide. The peptides constrained with the R-chiral center linker were observed to have enhanced α-helicity. As with other examples above, this trend indicated that the stereochemistry of the chiral center on the linker could have a great effect on the overall structure of the peptide.(73)
Lipid modified thiol-constrained peptides
Yang et al. developed a glucagon-like peptide 2 analog with an extended half-life. The linear peptide was constrained by a thiol-selective modified lipid linker and cysteines in i,i+7 positions to incorporate a serum protein binding motif (Figure 12C). The constrained peptide was observed to have increased helicity, improved in vitro activity, 10-fold longer in vivo half-life and improved efficacy in mouse models as compared to the native peptide and teduglutide, the current treatment for short bowel syndrome. (74)
Sidechain-to-N-terminus lactams
Li and coworkers have developed a new sidechain-to-terminus macrocyclization in which an N-terminal acid is linked to the side chain of a diaminopimelic acid. In the first iteration, succinic acid was placed at the N-terminus, and diaminopimelic acid was placed in the i+3 position (Figure 12D). Circular dichroism indicated that the constrained peptide was more α-helical than the unconstrained peptide; however, the increase was minimal, and the helicity of the constrained peptide was only modest.(75) Using molecular dynamics simulations as a guide, the group developed a peptide with an iso-d-aspartic acid linker, which showed enhanced helicity and targeted estrogen receptor. An advantage of the isoaspartic acid technique is the possibility of using the N-terminal amine as a modification site.(76) This approach is conceptually similar to that of hydrogen bond surrogate peptides created by Arora and coworkers,(77) who developed a technique in which an olefin stapling amino acid is placed at the terminal and another olefin sidechain is added to the amine of the backbone at the i+4 position. The olefins undergo a ring-closing metathesis reaction.
DOUBLE-STAPLED BICYCLIC PEPTIDES
“Stitched” Peptides
Verdine and coworkers developed bicyclic peptides in which two ring-closing metathesis reactions could be carried out to form “stitched” peptides (Figure 13A). The R5, R8, S5 or S8 stapling amino acid was placed in the i position, the newly developed B5 stapling amino acid in the i+4 or i+7 position and the second R5, R8, S5 or S8 stapling amino acid was placed in the i+4+4, i+4+7, i+7+4 or i+7+7 position. These “stitched” peptides were observed to have 60% or greater helicity and improved cell permeability as compared to singular stapled peptides. These bicyclic peptides were developed due to the rigidifying nature of the B5 stapling amino acid, allowing for increased helicity.(78)
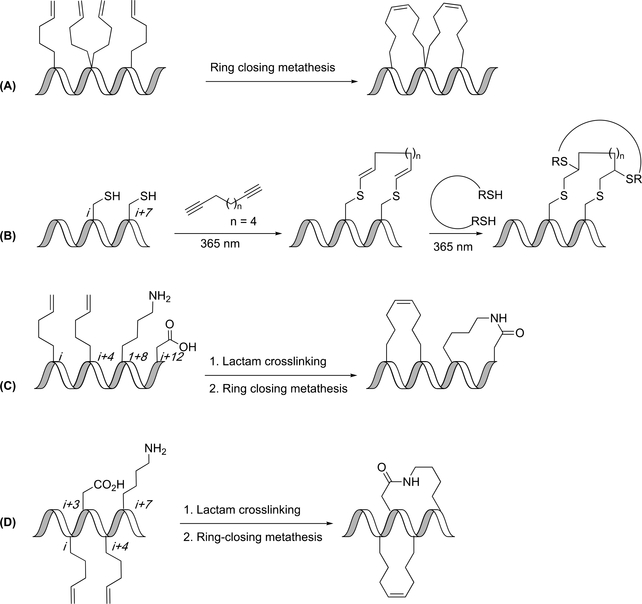
Figure 13.
Modifications of stitched peptides. (A) Ring-closing metathesis yields bicyclic “stitched” peptide. (B) Reaction of cysteines with diyne gives di-vinyl sulfide linker that can undergo a thiol-ene reaction. (C) Sequential lactam crosslinking and ring-closing metathesis yields a bicyclic peptide. (D) Sequential lactam crosslinking and ring closing metathesis yields a cross-stitched peptide.
Thiol-yne/Thiol-ene
Wang et al. combined thiol-yne and thiol-ene chemistry to create bicyclic peptides. Reacting an α,ω-diyne with two cysteines formed a di-vinyl sulfide linker which was reacted in a thiol-ene reaction with a second peptide containing two cysteines to form a bicyclic constrained peptide (Figure 13B). This linker also allowed for other types of functionalization, including groups that enhance water solubility and cell permeability, as illustrated by Wang et al. with the addition of a CRRRRC motif to the constrained peptide.(35)
Ring-closing metathesis/lactam formation
Fairlie and coworkers combined ring-closing metathesis with lactam closure to develop a dual inhibitor of Mcl-1 and Bcl2A1 proteins. Multiple variations of the doubly-constrained peptide were developed with all bicyclic peptides having increased helicity as compared to the monocyclic peptides. The authors studied hydrocarbon stapling and lactam formation, alone or in combination. The optimal positioning of the constraints for helicity was to place two lactam linkages in the i (Lys),i+4 (Glu) and i+8 (Lys),i+12 (Glu) positions (Figure 13C). This positioning of the constraints allowed for 96% helicity. Fairlie and coworkers used this double-stapling technique in order to develop an inhibitor of Mcl-1 and Bcl2A1. The optimal positioning of the constraints for affinity was to place a hydrocarbon staple at the i,i+4 positions and a lactam at the i+8 (Lys),i+12 (Glu) positions.(79)
Speltz et al developed a “cross-stitched” peptide containing a hydrocarbon staple at i, i+4 positions and a lactam linker at i+3 (Asp), i+7 (Lys) positions (Figure 13D). This cross-stitched peptide was observed to have highest helicity (53%) as compared to singly stapled hydrocarbon (33%) or lactam-constrained (23%) peptides. When treated with proteinase K, the cross-stitched peptide was observed to have a proteolytic half-life of ~2000 minutes as compared to the 0.27 minutes of the unconstrained peptide. The affinity of the cross-stitched peptide for the estrogen receptor was greater than the unconstrained peptide, but lower than with the lactam alone.(80)
CONCLUSION
The methods used to constrain peptides have developed greatly since hydrocarbon stapling was first introduced. Variations of lactam, cysteine, triazole, oxime, semicarbazide, amine, pyrazoline, glucuronic acid, and selenocysteine constraining techniques have been developed with the resulting constrained peptides often having increased helicity, protease stability and cellular uptake, relative to their unconstrained analogs. Tether variations have also been studied and appear to be an important method in which constrained peptide characteristics can be altered. Lastly, combining one or more constraining techniques has led to bicyclic helical peptides.
Although there are a variety of constraints being developed, a few trends have been observed to be consistent. de Araujo et al. compared the helicity of pentapeptides constrained using a variety of constraints. The results indicated that the lactam constraint induced the greatest helicity (100%), followed by hydrocarbon and triazole constraints (62%), and finally by thioether-constrained peptides, which, in their work, appeared to be minimally helical and mostly unstructured.(81) Tian et al. also confirmed these helicity results and observed a general trend for cellular permeability. Peptides with highly polar linkages were observed to have minimal cellular uptake as compared to peptides with more non polar linkages, which were observed to be three- to fivefold greater.(82)
Selecting a constraining technique for a particular application requires balancing a number of different factors. To select a constraining technique that will allow for optimal binding affinity, the structure of the protein target has to be taken into consideration, but beyond binding affinity, other considerations should also be taken into account. When increased hydrophobicity is needed, an all-hydrocarbon stapling technique may be optimal. For high peptide helicity, a lactam constraint may be desirable.
The solubility of constrained peptides has been described as one of the limitations of using these methods for developing these molecules as drugs. Some peptides are soluble in aqueous solution, while highly hydrophobic peptides must be dissolved in DMSO and serially diluted into aqueous buffer to the desired concentrations. (21) (83) Another consideration with respect to hydrophobicity is that Shoichet and coworkers have shown that steep dose-response curves may be indicative of aggregation. In cases where steep dose-response curves are obtained in vitro, it may be prudent to consider whether aggregation may give rise to false positive results.(85) A convenient method of determining peptide aggregation is through the use of dynamic light scattering.(86)
There is no set of “rules” for designing cell-permeable peptides; however, several groups have published reports about the hydrophobicity and charges observed in cell-permeable peptides. Chu et al. used an epifluorescence microscopy assay to analyze the cell permeability of more than 200 peptides. They reported that staple type and formal charge were directly related to the cell permeability of the stapled peptide with −1 to +1 charge inducing moderate cell permeability and +1 to +7 charge inducing high cell permeability.(87) Bird et al. used a statistical approach to analyze biophysical properties that have an effect on the uptake of constrained peptides. (11) Peptide uptake was driven by optimal hydrophobicity and helicity, as well as the placement of the staple at the amphipathic boundary. Tian et al. determined that polar linkages lead to decreased cell permeability and that non-polar linkages aid in inducing cell permeability.(82)
The continuous development of new linkers and modifications to previously developed linkers adds to a growing collection of peptide constraining techniques, which have different properties to choose for a particular application. Constrained peptides have provided a straightforward strategy for developing α-helical mimetics that have the potential to be further developed into effective drugs against a variety of diseases including cancer and neurodegenerative diseases.
Acknowledgments
This work was funded by the University of Illinois Cancer Center (to T.W.M.). T.E.S. was funded by training grant T32AT007533, Office of the Director, National Institutes of Health (OD) and National Center for Complementary and Integrative Health (NCCIH).
Biosketches
Miss. Kornelia Joanna Skowron is currently a graduate student in medicinal chemistry at the University of Illinois Chicago in the Terry W. Moore lab. She received her bachelor’s degree from Roosevelt University in Biochemistry in 2017. Her interests are in medicinal 52 chemistry. Her current work includes the development of stapled peptide inhibitors of the estrogen receptor-coactivator interaction.
Dr. Thomas Speltz is a postdoctoral fellow at the University of Chicago under the guidance of Prof. Raymond Moellering. He earned a Ph.D. in medicinal chemistry under the guidance of Prof. Terry Moore at the University of Illinois at Chicago in 2018. He holds bachelor’s and master’s degrees from DePaul University, where he worked with Prof. Justin Maresh. His research interests are in medicinal chemistry, biochemistry, and chemical biology.
Prof. Terry Moore is an assistant professor of medicinal chemistry at the University of Illinois at Chicago. He received his Ph.D. in chemistry in 2008 from the University of Illinois at Urbana-Champaign, where he worked with Prof. John Katzenellenbogen. He did postdoctoral studies in drug discovery from 2009–2013 at Emory University with Prof. Dennis Liotta. He started as an assistant professor of medicinal chemistry at the University of Illinois at Chicago in 2013. His research interests include the medicinal chemistry, biochemistry, and chemical biology of transcription factor/protein interactions.
Bibliography
- 1.Robertson S, Spring DR. Using Peptidomimetics and Constrained Peptides as Valuable Tools for Inhibiting Protein-Protein Interactions. Molecules. 2018;23:959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein M Stabilized helical peptides: overview of the technologies and its impact on drug discovery. Expert Opin Drug Disc. 2017;12:1117–1125. [DOI] [PubMed] [Google Scholar]
- 3.Fairlie DP, Dantas de Araujo A. Stapling Peptides Using Cysteine Crosslinking. Biopolymers (Peptide Science). 2016;106:843–852. [DOI] [PubMed] [Google Scholar]
- 4.Tan YS, Lane DP, Verma CS. Stapled peptide design: principles and roles of computation. Drug Discov Today. 2016; 21:1642–1653. [DOI] [PubMed] [Google Scholar]
- 5.Wojcik P, Berlicki L. Peptide-based inhibitors of protein–protein interactions. Bioorganic Med Chem. Lett. 2016;26:707–713. [DOI] [PubMed] [Google Scholar]
- 6.Iyer VV. A Review of Stapled Peptides and Small Molecules to Inhibit Protein–Protein Interactions in Cancer. Curr Med Chem. 2016;23:3025–3043. [DOI] [PubMed] [Google Scholar]
- 7.Cromm PM, Spiegel J, Grossmann TN. Hydrocarbon Stapled Peptides as Modulators of Biological Function. ACS Chem Biol. 2015;10:1362–1375. [DOI] [PubMed] [Google Scholar]
- 8.Walensky LD, Bird GH. Hydrocarbon-Stapled Peptides: Principles, Practice, and Progress. J Med Chem. 2014;57:6275–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henchey LK, Jochim AL, Arora PS. Contemporary Strategies for the Stabilization of Peptides in the α-Helical Conformation. Curr Opin Chem Biol. 2008:12;692–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyndall JDA, Nall T, Fairlie DP. Proteases Universally Recognize Beta Strands In Their Active Sites. Chem Rev. 2005;105:973–1000. [DOI] [PubMed] [Google Scholar]
- 11.Bird GH, Mazzola E, Opoku-Nsiah K, et al. Biophysical determinants for cellular uptake of hydrocarbon stapled peptide helices. Nat Chem Biol. 2016;10:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douse CH, Maas SJ, Thomas JC, et al. Crystal Structures of Stapled and Hydrogen Bond Surrogate Peptides Targeting a Fully Buried Protein–Helix Interaction. ACS Chem Biol. 2014;9:2204–2209. [DOI] [PubMed] [Google Scholar]
- 13.Chang YS, Graves B, Guerlavais V, et al. Stapled α−helical peptide drug development: A potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. PNAS. 2013;110: E3445–E3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Philips C, Roberts LR, Schade M, et al. Design and Structure of Stapled Peptides Binding to Estrogen Receptors. J Am Chem Soc. 2011;133: 696–9699. [DOI] [PubMed] [Google Scholar]
- 15.Chapman RN, Dimartino G, Arora PS. A Highly Stable Short a-Helix Constrained by a Main-Chain Hydrogen-Bond Surrogate. J Am Chem Soc. 2004;126:12252–12253. [DOI] [PubMed] [Google Scholar]
- 16.Patgiri A, Jochim AL, Arora PS. A Hydrogen Bond Surrogate Approach for Stabilization of Short Peptide Sequences in α-Helical Conformation. Acc Chem Res. 2008;41:1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blackwell HE, Grubbs RH. Highly Efficient Synthesis of Covalently Cross-Linked Peptide Helices by Ring-Closing Metathesis. Angew Chem Int Ed. 1998;37:3281–3284. [DOI] [PubMed] [Google Scholar]
- 18.Schafmeister CE, Po J, Verdine GL. An All-Hydrocarbon Cross-Linking System for Enhancing the Helicity and Metabolic Stability of Peptides. J Am Chem Soc. 2000;24:5891–5892. [Google Scholar]
- 19.Walensky LD, Kung AL, Escher I, et al. Activation of Apoptosis in Vivo by a Hydrocarbon-Stapled BH3 Helix. Science. 2004;305:1466–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernal F, Tyler AF, Korsmeyer SJ, Walenksy LD, Verdine GL. Reactivation of the p53 Tumor Suppressor Pathway by a Stapled p53 Peptide. J Am Chem Soc. 2007;129:2456–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walensky LD, Pitter K, Morash J, et al. A Stapled BID BH3 Helix Directly Binds and Activates BAX. Mol Cell. 2006; 24:199–210. [DOI] [PubMed] [Google Scholar]
- 22.Moellering RE, Cornejo M, Davis TN, et al. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009;462:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bird GH, Madani N, Perry AF, et al. Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic. PNAS. 2010;107:14093–14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dietrich L, Rathmer B, Ewan K, et al. Cell Permeable Stapled Peptide Inhibitor of Wnt Signaling that Targets β-Catenin Protein-Protein Interactions. Cell Chem Biol. 2017;24:958–968. [DOI] [PubMed] [Google Scholar]
- 25.Whelan J Stapled peptide induces cancer cell death. Drug Discov Today. 204;9:907. [DOI] [PubMed] [Google Scholar]
- 26.Bracken C, Gulyas J, Taylor JW, Baum J. Synthesis and Nuclear Magnetic Resonance Structure Determination of an alpha-Helical, Bicyclic, Lactam-Bridged Hexapeptide. J Am Chem Soc. 1994;116:6431–6432. [Google Scholar]
- 27.Felix AM, Heimer EP, Wang C-T, et al. Synthesis, biological activity and conformational analysis of cyclic GRF analogs. Int J Pept Prot Res. 1988;32:441–454. [DOI] [PubMed] [Google Scholar]
- 28.Judice JK, Tom JY, Huang W, et al. Inhibition of HIV type 1 infectivity by constrained alpha-helical peptides: implications for the viral fusion mechanism. Proc Natl Acad Sci USA. 197;94:13426–13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geistlinger TR, Guy, R. Novel Selective Inhibitors of the Interaction of Individual Nuclear Hormone Receptors with a Mutually Shared Steroid Receptor Coactivator 2. J Am Chem Soc. 2003;125:6852–6853. [DOI] [PubMed] [Google Scholar]
- 30.Leduc A-M, Trent JO, Wittliff JL, et al. F. Helix-stabilized cyclic peptides as selective inhibitors of steroid receptor–coactivator interactions. PNAS. 2003;100:11273–11278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau YH, de Andrade P, Wu Y, Spring DR. Peptide stapling techniques based on different macrocyclisation chemistries. Chem Soc Rev. 2015;44:91–102. [DOI] [PubMed] [Google Scholar]
- 32.Hillman RA, Nadraws JW, Bertucci MA.The Hydrocarbon Staple & Beyond: Recent Advanced Towards Stapled Peptide Therapeutics that Target Protein-Protein Interactions. Curr Top Med Chem. 2018;18:611–624. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Chou DHC. A Thiol–Ene Coupling Approach to Native Peptide Stapling and Macrocyclization. Angew Chem. 2015;127:11081–11084. [DOI] [PubMed] [Google Scholar]
- 34.Zhao B, Zhang Q, Li Z.Constructing thioether-tethered cyclic peptides via on-resin intra-molecular thiol–ene reaction. J Pept Sci. 2016;22: 540–544. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Bruno BJ, Cornillie S, et al. Application of Thiol–yne/Thiol–ene Reactions for Peptide and Protein Macrocyclizations. Chem Eur J. 2017;23:7087–7092. [DOI] [PubMed] [Google Scholar]
- 36.Tian Y, Li J, Zhao H, et al. Stapling of unprotected helical peptides via photoinduced intramolecular thiol-yne hydrothiolation. Chem Sci. 2016;7:3325–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spokoyny AM, Zou Y, Ling JJ, Yu H, Lin YS, Pentelute BL. A Perfluoroaryl-Cysteine SNAr Chemistry Approach to Unprotected Peptide Stapling. J Am Chem Soc. 2013;135:5946–5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fadzen CM, Wolfe JM, Cho CF, Chiocca A, Lawler SE, Pentelute BL. Perfluoroarene–Based Peptide Macrocycles to Enhance Penetration Across the Blood–Brain Barrier. J Am Chem Soc. 2017;139:15628–15631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinogradova EV, Zhang C, Spokoyny AM, Pentelute BL, Buchwald SL. Organometallic Palladium Reagents for Cysteine Bioconjugation. Nature. 2016;526:687–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rojas AJ, Pentelute BL, Buchwald SL. Water-Soluble Palladium Reagents for Cysteine S-Arylation under Ambient Aqueous Conditions. Org Lett. 2017;19:4263–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rojas AJ, Zhang C, Vinogradova EV, et al. Divergent unprotected peptide macrocyclisation by palladium-mediated cysteine arylation. Chem Sci. 2017;8:4257–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang C, Dai P, Spokoyny AM, Pentelute BL. Enzyme-Catalyzed Macrocyclization of Long Unprotected Peptides. Org Lett. 2014;16:3652–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu H, Acharyya A, Wu Y, et al. Design of a Short Thermally Stable a-Helix Embedded in a Macrocycle. Chem Bio Chem. 2018;19:902–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi X, Zhao R, Jiang Y, et al. Reversible stapling of unprotected peptides via chemoselective methionine bis-alkylation/ dealkylation. Chem Sci. 2018;9:3227–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dantas de Araujo A, Perry SR, Fairlie DP. Chemically Diverse Helix-Constrained Peptides Using Selenocysteine Crosslinking. Org Lett. 2018;20:1453–1456. [DOI] [PubMed] [Google Scholar]
- 46.Cistrone PA, Silvestri AP, Hintzen JC, Dawson PE. Rigid Peptide Macrocycles from On-Resin Glaser Stapling. ChemBioChem. 2018;19:1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lautrette G, Touti F, Lee HG, Dai P, Pentelute BL. Nitrogen Arylation for Macrocyclization of Unprotected Peptides. J Am Chem Soc. 2016; 138: 8340–8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee HG, Lautrette G, Pentelute BL, Buchwald SL. Palladium-Mediated Arylation of Lysine in Unprotected Peptides. Angew Chem Int Ed. 2017;56:3177–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vinogradov AA, Choo ZN, Totaro KA, Pentelute BL. Macrocyclization of Unprotected Peptide Isocyanates. Org Lett. 2016;18:1226–1229. [DOI] [PubMed] [Google Scholar]
- 50.Haney CM, Loch MT, Horne WS. Promoting peptide a-helix formation with dynamic covalent oxime side-chain cross-links. Chem Commun. 2011;47:10915–10917. [DOI] [PubMed] [Google Scholar]
- 51.Smith JM, Frost JR, Fasan R. Designer macrocyclic organo-peptide hybrids inhibit the interaction between p53 and HDM2/X by accommodating a functional a-helix. Chem Commun. 2014;50:5027–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu C, Hoang HN, Liu L, Fairlie DP. Glucuronic acid as a helix-inducing linker in short peptides. Chem Commun. 2018;54:2162–2165. [DOI] [PubMed] [Google Scholar]
- 53.Nevola L, Martin-Quiros A, Eckelt K, et al. Light-Regulated Stapled Peptides to Inhibit Protein–Protein Interactions Involved in Clathrin-Mediated Endocytosis. Angew Chem Int Ed. 2013;52:7704–7708. [DOI] [PubMed] [Google Scholar]
- 54.Demizu Y, Nagoya S, Shirakawa M, et al. Development of stapled short helical peptides capable of inhibiting vitamin D receptor (VDR)–coactivator interactions. Bioorganic Med Chem Lett. 2013;23:4292–4296. [DOI] [PubMed] [Google Scholar]
- 55.Madden MM, Rivera Vera CI, Song W, Lin Q. Facile synthesis of stapled, structurally reinforced peptide helices via a photoinduced intramolecular 1,3-dipolar cycloaddition reaction. Chem Commun. 2009;0:5588–5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawamoto SA, Coleska A, Ran X, Yi H, Yang CY, Wang S. Design of Triazole-Stapled BCL9 α-Helical Peptides to Target the β-Catenin/B-Cell CLL/lymphoma 9 (BCL9) Protein–Protein Interaction. J Med Chem. 2012;55:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lau YH, de Andrade P, Quah ST, et al. Functionalised staple linkages for modulating the cellular activity of stapled peptides. Chem Sci. 2014; 5:1804–1809. [Google Scholar]
- 58.Lau Y, de Andrade P, Sköld N, et al. Investigating peptide sequence variations for ‘double-click’ stapled p53 peptides. Org Biomol Chem. 2014;12:4074–4077. [DOI] [PubMed] [Google Scholar]
- 59.Wu Y, Olsen LB, Lau Y, et al. Development of a Multifunctional Benzophenone Linker for Peptide Stapling and Photoaffinity Labelling. Chem Bio Chem. 2016;17:689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lau Y, Wu Y, de Andrade P, Galloway WR, Spring DR. A two-component ‘double-click’ approach to peptide stapling. Nat Protocols. 2015;10:585–594. [DOI] [PubMed] [Google Scholar]
- 61.Lau Y, de Andrade P, McKenzie GJ, Venkitaraman AR, Spring DR. Linear Aliphatic Dialkynes as Alternative Linkers for Double-Click Stapling of p53-Derived Peptides. Chem Bio Chem. 2014;15:2680–2683. [DOI] [PubMed] [Google Scholar]
- 62.Wu Y, Villa F, Maman J, et al. Targeting the Genome-Stability Hub Ctf4 by Stapled-Peptide Design. Angew Chem Int Ed. 2017;56:12866–12872. [DOI] [PubMed] [Google Scholar]
- 63.Lau YH, de Andrade P, Wu Y, Spring DR. Peptide stapling techniques based on different macrocyclisation chemistries. Chem Soc Rev. 2015:91–102. [DOI] [PubMed] [Google Scholar]
- 64.Lau Y, Wu Y, Rossmann M, et al. Double Strain-Promoted Macrocyclization for the Rapid Selection of Cell-Active Stapled Peptides. Angew Chem Int Ed. 2015;54:5410–15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown SP, Smith III AB. Peptide/Protein Stapling and Unstapling: Introduction of sTetrazine, Photochemical Release, and Regeneration of the Peptide/Protein. J Am Chem Soc. 2015;137:4034–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grison CM, Burslem GM, Miles JA, et al. Double quick, double click reversible peptide “stapling”. Chem Sci. 2017;8:5166–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Speltz TE, Fanning SW, Mayne CG, et al. Stapled Peptides with gamma-Methylated Hydrocarbon Chains for the Estrogen Receptor/Coactivator Interaction. Angew Chem Int Ed. 2016;55:4252–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Y, Li YH, Li X, et al. A novel peptide stapling strategy enables the retention of ring-closing amino acid side chains for the Wnt/b-catenin signalling pathway. Chem Sci. 2017;8,:7368–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu K, Geng H, Zhang Q, et al. An In-tether Chiral Center Modulates the Helicity, Cell Permeability, and Target Binding Affinity of a Peptide. Angew Chem. 2016;55:8145–8149. [DOI] [PubMed] [Google Scholar]
- 70.Perell GT, Staebell RL, Hairani M, Cembran A, Pomerantz WC. Tuning Sulfur Oxidation States on Thioether-Bridged Peptide Macrocycles for Modulation of Protein Interactions. ChemBioChem. 2107;18:1836–1844. [DOI] [PubMed] [Google Scholar]
- 71.Lin H, Jiang Y, Zhang Q, Hu K, Li Z. (An in-tether sulfilimine chiral center induces helicity in short peptides. Chem Commun. 2016;52:10389–10391. [DOI] [PubMed] [Google Scholar]
- 72.Hu K, Sun C, Yu M, et al. Dual In-Tether Chiral Centers Modulate Peptide Helicity. Bioconjugate Chem. 2017;28:1537–1543. [DOI] [PubMed] [Google Scholar]
- 73.Serrano JC, Sipthorp J, Xu W, Itzhaki LS, Ley SV. A New Methodology for Incorporating Chiral Linkers into Stapled Peptides. ChemBioChem. 2017;18:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang PY, Zou H, Lee C, et al. Stapled, Long-Acting Glucagon-like Peptide 2 Analog with Efficacy in Dextran Sodium Sulfate Induced Mouse Colitis Models. J Med Chem. 2018;61:3218–3223. [DOI] [PubMed] [Google Scholar]
- 75.Zhao H, Liu QS, Geng H, et al. Crosslinked Aspartic Acids as Helix-Nucleating Templates. Angew Chem. 2016;128:12267–12272. [DOI] [PubMed] [Google Scholar]
- 76.Xie M, Zhao H, Liu Q, et al. (Structural Basis of Inhibition of ERα-Coactivator Interaction by High- Affinity N-Terminus Isoaspartic Acid Tethered Helical Peptides. J Med Chem. 2017;60:8731–8740. [DOI] [PubMed] [Google Scholar]
- 77.Joy ST, Arora PS. An optimal hydrogen-bond surrogate for a-helices. Chem Commun. 2016; 52: 5738–5741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hilinkski GJ, Kim YW, Hong J, et al. Stitched αHelical Peptides via Bis Ring-Closing Metathesis. J Am Chem Soc. 2014;136:12314–12322. [DOI] [PubMed] [Google Scholar]
- 79.de Araujo A D, Lim J, Wu KC, et al. Bicyclic Helical Peptides as Dual Inhibitors Selective for Bcl2A1 and Mcl1 Proteins. J Med Chem. 2018;61:2962–2972. [DOI] [PubMed] [Google Scholar]
- 80.Speltz TE, Mayne CG, Fanning SW, et al. A “cross-stitched” peptide with improved helicity and proteolytic stability. Org Biomol Chem. 2018;16:3702–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Araujo AD, Hoang HN, Kok W, et al. Comparative a-Helicity of Cyclic Pentapeptides in Water. Angew Chem Int Ed. 2014;53:6965–6969. [DOI] [PubMed] [Google Scholar]
- 82.Tian Y, Jiang Y, Li J, Wang D, Zhao H, Li Z. Effect of Stapling Architecture on Physiochemical Properties and Cell Permeability of Stapled a-Helical Peptides: A Comparative Study. ChemBioChem. 2017;18:2087–2093. [DOI] [PubMed] [Google Scholar]
- 83.Stewart ML, Fire E, Keating AE, Walensky LD. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat Chem Bio. 2010;6:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bird GH, Crannell WC, Walensky LD. Chemical Synthesis of Hydrocarbon-Stapled Peptides for Protein Interaction Research and Therapeutic Targeting. Curr Protoc Chem Biol. 2011;3:99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shoichet BK. Interpreting Steep Dose-Response Curves in Early Inhibitor Discovery. J Med Chem. 2006;49:7274–7277. [DOI] [PubMed] [Google Scholar]
- 86.Bhattacharya S, Zhang H, Cowburn D, Debnath AK. Novel Structures of Self-Associating Stapled Peptides. Biopolymer. 2012;97:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chu Q, Moellering RE, Hilinski GJ, Kim Y-W, Grossmann TN, Yeh JT-H, Verdine GL. Towards understanding cell penetration by stapled peptides. Med Chem Comm. 2015;6:111–119 [Google Scholar]