Abstract
Six isolates of Campylobacter with similar non-standard colonial morphologies were identified during studies isolating Campylobacter from bird faeces and rivers in New Zealand. Genomic (16S rRNA gene sequencing and whole genome analysis) and phenotypic (MALDI-TOF analysis and conventional biochemical tests) showed that the isolates form a monophyletic clade with genetic relationships to Campylobacter coli / Campylobacter jejuni and Campylobacter peloridis /Campylobacter amoricus. They may be distinguished from other Campylobacter by their MALDI-TOF spectral pattern, their florid α-haemolysis, their ability to grow anaerobically at 37 °C, and on 2 % NaCl nutrient agar, and their lack of hippuricase. This study shows that these isolates represent a novel species within the genus Campylobacter for which the name Campylobacter novaezeelandiae sp. nov. is proposed. The presence of C. novaezeelandiae in water may be a confounder for freshwater microbial risk assessment as they may not be pathogenic for humans. The type strain is B423bT (=NZRM 4741T=ATCC TSD-167T).
Keywords: Campylobacter, birds, genomics, New Zealand
The genus Campylobacter was described by Sebald and Veron [1] while reclassifying the genus Vibrios. Since then, 32 Campylobacter species with nine subspecies have been identified [2]. Campylobacteriosis is one of the most common bacterial gastrointestinal illnesses of humans in the developed world [3], including in New Zealand, which has had a high notification rate [4].
Campylobacter are frequently associated with the gastrointestinal tracts of birds [5], particularly chickens [6], but have also been detected in wild birds ranging from albatross [7] to zebra finch [8]. In New Zealand, Campylobacter has also been isolated frequently from waterways, with the majority of isolates being attributed to wild bird sources [9, 10].
Isolation and ecology
As part of source attribution and population structure studies on Campylobacter [11, 12], six isolates with dark-field microscopy characteristics of Campylobacter but non-standard agar-plate morphology were isolated from Bolton broth enrichments (LabM) subcultured onto modified charcoal cefoperazone (mCCDA) agar (Fort Richard Laboratories) after 48 h of incubation. Two isolates were from rivers (100 ml water was filtered through a 0.45 µm filter, and the filter was placed in Bolton broth). Four isolates were from wild non-native bird faeces (three starlings and one duck) collected from deposited droppings with a swab which was placed in Bolton broth with subculture to mCCDA agar after 48 h incubation. Initial enrichment and growth were performed in a microaerobic atmosphere (10 % CO2, 5 % O2, 85 % N2) in a variable atmosphere incubator (Don Whitley) at 42 °C. Single colonies were subcultured to Columbia horse blood agar (Fort Richard Laboratories) before storage in 15 % glycerol in nutrient broth no. 2 (Oxoid) at −80 °C. Interestingly, we have not found similar isolates from chickens [12], ruminants [13], dogs and cats [14, 15] or, most importantly, from human cases of campylobacteriosis [12], which suggests that these isolates have a restricted host range and low virulence.
In New Zealand, freshwater quality standards are based around the use of E. coli counts as an indicator relating to Campylobacter presence [16] and thus human health risk. The presence of Campylobacter novaezeelandiae in river water may act as a confounder for this assumption, requiring modification of the quantitative microbial risk assessments.
Characteristics of the isolates are described in Table 1.
Table 1.
Characteristics of the Campylobacter novaezeelandiae sp. nov. isolates described in this study
|
Isolate |
Source |
Location |
Isolation date |
16S rRNA gene accession |
Genome accession |
|---|---|---|---|---|---|
|
B423bT |
Mallard duck |
Palmerston North, New Zealand |
Aug 2008 |
||
|
B571b |
Starling |
Palmerston North, New Zealand |
Sept 2008 |
– |
|
|
B716b |
Starling |
Palmerston North, New Zealand |
Nov 2008 |
– |
|
|
B1491 |
Starling |
Palmerston North, New Zealand |
May 2009 |
||
|
W441b |
Oroua River |
Feilding, New Zealand |
Oct 2008 |
– |
|
|
W677a |
Pareora River |
South Canterbury, New Zealand |
Apr 2011 |
16S rRNA gene phylogeny
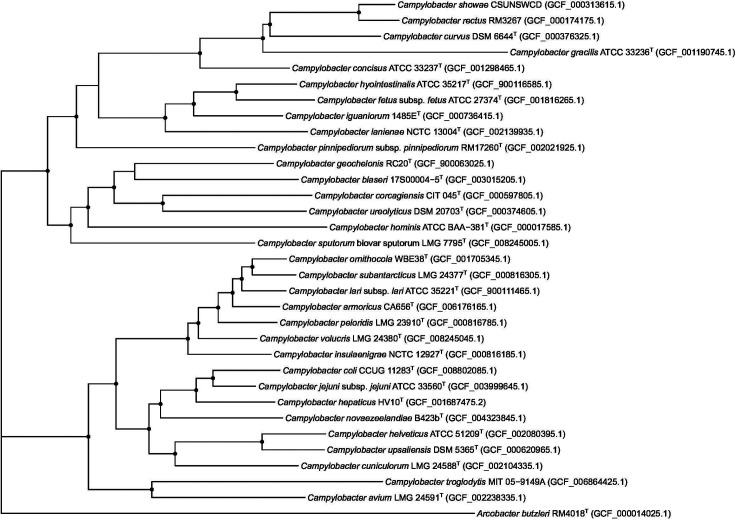
16S rRNA gene PCR analyses were performed using crude DNA extracted by boiling [17], the primers and conditions described by Lane [18] with Sanger sequencing of the products at the Massey Genome Service (Massey University). Comparison of the sequences suggested that all the isolates were related and formed a single distinct species previously described as ‘ Campylobacter sp. nov. 3’ [10]. A 16S rRNA gene-based phylogeny was reconstructed from the 16S rRNA loci extracted from the whole genome sequenced isolates [19] using barrnap (https://github.com/tseemann/barrnap), and aligned using mafft version 7 [20]. The resulting phylogenetic tree (Fig. 1) shows that the isolates (henceforth called Campylobacter novaezeelandiae sp. nov.) clustered together and were closest to Campylobacter amoricus and Campylobacter peloridis . Sequence alignment showed the 16S rRNA genes of the C. novaezeelandiae sp. nov. isolates to be on average 97.6 % similar to those of C. amoricus and 97.4 % similar to those of C. peloridis . As a check of the quality of the genome sequence [21], the sequences of the Sanger sequenced 16S rRNA ene PCR products and the those extracted from the whole genome sequences were compared. The whole genome 16S genes all contained a thymidine insertion at the same position compared to the 16S rRNA PCR products. Campylobacter species contain three copies of the 16S rRNA genes [22] and the 16S gene assembled is a combination of the three, whilst PCR targets only one of the genes, suggesting that one or both of the other 16S genes contain this insertion and explaining the differences between the 16S rRNA gene sequences. Apart from this insertion, the 16S rRNA gene sequences generated by PCR and whole genome sequencing were identical.
Fig. 1.
Maximum-likelihood tree based on 16S rRNA gene sequences. The dots on the nodes represent nodes with bootstrap support >90%.
Genome features
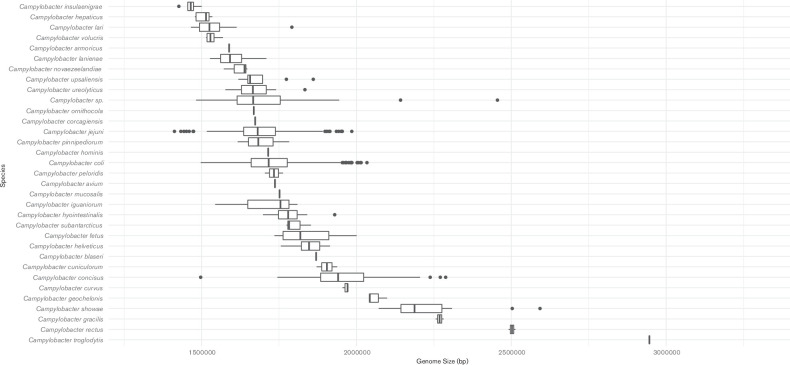
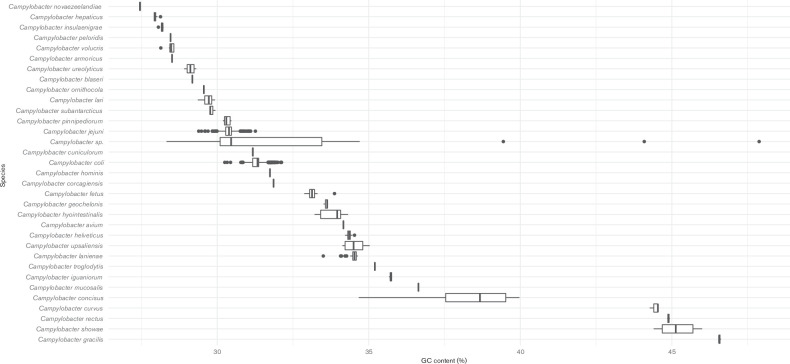
Whole genomes of three of the isolates were sequenced, assembled and examined as described previously [19]. The genomic DNA G+C content of the strains was estimated at 27.5 mol% using the Geneious R10 software package [23]. Comparisons of genome size and G+C content were performed relative to 3210 genomes available in the NCBI RefSeq database that are assigned to the genus ‘ Campylobacter ’. Genomes were downloaded using the NCBI genome download tool (available at https://github.com/kblin/ncbi-genome-download). NCBI-assigned species taxon identifiers were used to group the data. Analysis and visualizations were performed in RStudio. The genome sizes of the C. novaezeelandiae isolates fell within the range of Campylobacter (Fig. 2), however the C. novaezeelandiae isolates possessed the lowest G+C content of all current members of the genus Campylobacter [24, 25] (Fig. 3).
Fig. 2.
Comparison of the genome size of Campylobacter novaezeelandiae sp. nov. and other Campylobacter species
Fig. 3.
Comparison of the G+C content of Campylobacter novaezeelandiae sp. nov. and other Campylobacter species.
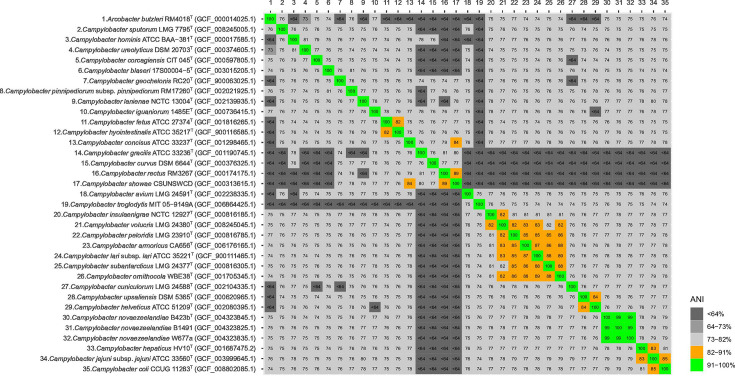
Genomic average nucleotide identity (ANI) values were calculated using an ANI calculator [26] showing that the values between the isolates of C. novaezeelandiae were 99.0–100 % while the most similar of the other Campylobacter species was Campylobacter jejuni with 79.7 % ANI similarity (Fig. 4).
Fig. 4.
Average nucleotide identity (ANI) values (%) comparing Campylobacter novaezeelandiae sp. nov. and other Campylobacter taxa. The list of accession numbers is in Table S1 (available in the online version of this article).
Prokka version 1.14.5 [27] was used to annotate the three draft genomes. Roary version 3.11.2 [28] was used to cluster the annotated sequences and investigate the pan-genome, a clustering similarity threshold of 70 % was specified in this analysis in order to allow clustering of gene sequences that were divergent at the genus level. The three C. novaezeelandiae isolates contained between 1485 and 1591 coding-DNA sequences (CDSs). They shared a pan-genome of 1781 CDSs: 1395 (78 %) of which were core and 386 (21 %) of which were accessory. Even though this is a small sample size, it demonstrates significant variation in gene content between isolates of this species.
ariba version 2.14.1 [29] was used to investigate if the three genomes contained antimicrobial resistance genes using the ResFinder database [30], plasmids using the PlasmidFinder database [31], or virulence genes using the VFDB core database [32]. No AMR genes or plasmids were identified amongst the genomes, but one virulence gene (Cj1427c) was found within the genome of the type strain (B423bT). This gene encodes a sugar-nucleotide epimerase/dehydratase involved in capsular formation [33]. The reference databases used for this analysis consist of genes and genetic markers from described bacterial species, C. novaezeelandiae may contain novel or distantly related AMR genes, plasmids and virulence factors not yet described in these databases. For phylogenetic analyses, genome sequences derived from species type strains (as specified by https://lpsn.dsmz.de/genus/campylobacter) were used where available. If unavailable, RefSeq representative genomes were used. Genomes from a total of 32 Campylobacter species were used. Campylobacter mucosalis was excluded from the analysis, as RefSeq indicated that the only available genome from this species contained significant contamination. Gene clustering in Roary identified 79 core genes (and 188 soft-core genes [occurring in 95 % of the genomes]) that were conserved across the genus Campylobacter .
Phylogenies from alignments of the core gene sequences were generated in mega-X [34], using the maximum-likelihood method and the general time-reversible model. A discrete gamma distribution was used to model evolutionary rate differences among sites (five categories (+G, parameter=0.7929)). The rate variation model allowed for some sites to be evolutionarily invariable ([+I], 19.23 % sites). Trees with the highest log likelihood were chosen for display, and 100 bootstrap replicates were performed to estimate node support. Trees were visualized using ‘ggtree’ [35] in RStudio [36]. In contrast to the 16S rRNA gene sequence similarities (Fig. 1), and in accordance with whole genome ANI analysis (Fig. 4), phylogenetic analysis based on core gene contents supported a close relationship to C. cuniculorum , C. upsaliensis , C. helveticus , C. hepaticus , C. coli and C. jejuni (Fig. 5).
Fig. 5.
Maximum-likelihood tree based on the core genome. The dots on the nodes represent nodes with bootstrap support >90%.
Phenotypic and chemotaxonomic characterization
MALDI-TOF protein spectra were obtained as described by Koziel et al. [37] and discriminant peaks between species were identified using the ‘MALDIquant’ [38] ‘sda’ [39] and ‘crossval’ [40] R packages. C. jejuni subsp. jejuni NCTC 11168, C. coli ATCC 33559T, C. hyointestinalis ATCC 33560T, C. fetus subsp fetus ATCC 27374T and C. upsaliensis ATCC 43954T (ESR Culture Collection) were used as controls. Representative averaged MALDI-TOF spectra and significantly discriminant peaks for different species are shown in Fig. S1.
Electron microscopy was performed as described in Wheeler et al. [41] using an FEI Quanta 200 scanning electron microscope. All isolates were spiral-shaped rods (Fig. 6).
Fig. 6.
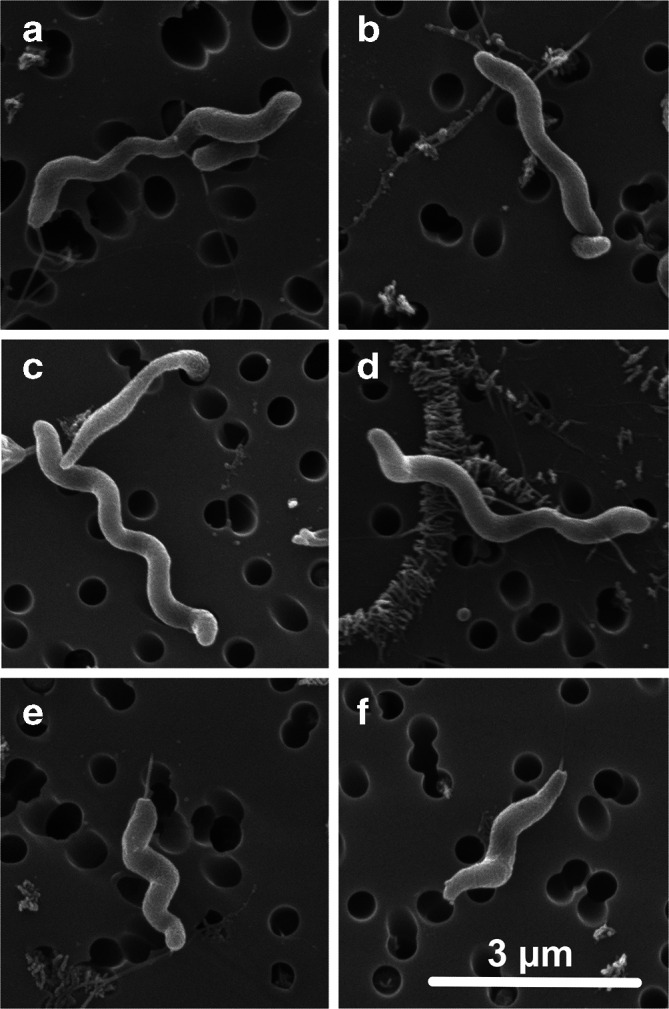
Electron micrograph of Campylobacter novaezeelandiae sp. nov.
Galleria mellonella (Biosuppliers) larvae were challenged with the C. novaezeelandiae isolates W441b and W667a as described by Champion et al. [42] using 31G insulin syringes (Becton Dickinson) for inoculation, with incubation at 37 °C in a variable atmosphere incubator with H2 (5 % H2, 10 % CO2, 3 % O2, 82 % N2). Nine isolates of C. jejuni isolated from invasive campylobacteriosis in humans and described in Wheeler et al. [41] were used as positive controls, phosphate-buffered saline inoculated and un-inoculated caterpillars were included as negative controls. Analyses were performed in RStudio, using the ‘survival’ (https://github.com/therneau/survival) and ‘survminer’ (https://github.com/kassambara/survminer) packages. Larvae inoculated with C. novaezeelandiae showed lower mortality than those inoculated with the C. jejuni controls (P=0.0089). The observed relative hazard ratio of 0.53 suggested that isolates of C. novaezeelandiae are approximately half as virulent as isolates of C. jejuni in the wax moth caterpillar model. This ability to kill larvae is discordant with our observation that C. novaezeelandiae has not been isolated from cases of campylobacteriosis and suggest that C. novaezeelandiae may be a useful model organism for exploring the G. mellonella virulence model.
Time taken for transformation to coccoidal forms was performed as described [43] using Columbia horse blood agar in a microaerobic atmosphere at 42 °C. After 3 days of incubation most (80–90 %) of the bacteria are rod-shaped, but after 6 days of incubation the proportion of rod-shaped bacteria has decreased to between 10 to 30 % with the remainder of the bacteria being coccoidal. Gram staining was performed as described by Chapin and Lauderdale [44]. Motility was observed by dark-field microscopy. Tests for oxidase, catalase, motility, hippurate hydrolysis and nitrate reduction were performed as described by MacFaddin [45]. Alkaline phosphatase activity was tested using an API Campy kit (bioMérieux). Indoxyl acetate hydrolysis was tested with the modification described by On and Holmes [46]. Hydrogen sulphide production was tested as described by Ma et al. [47]. Cephalothin and nalidixic acid sensitivity assays were performed using eucast zone cut-offs [48] on both sheep and horse blood-containing Mueller–Hinton agar (Fort Richard Laboratories). Temperature and atmosphere requirements were examined as described by On and Holmes [49] on Columbia horse blood agar and using atmospheres provided by variable atmosphere incubators or gas-generating envelopes (Oxoid). Growth on MacConkey agar (Fort Richard Laboratories), nutrient agar no. 2 (Oxoid), nutrient agar plus 1 % w/v glycine and nutrient agar plus 2 % w/v NaCl was as described by On and Holmes [49]. Growth on and reduction of 0.1 % w/v selenite and 0.04 % w/v 2,3,5 triphenyltetrazolium (TTC) in nutrient agar no. 2 were as described by On and Holmes [49]. Unless otherwise mentioned, tests were performed at 37 °C in a variable atmosphere incubator with H2 (5 % H2, 10 % CO2, 3 % O2, 82 % N2). Control organisms were C. jejuni subsp. jejuni NCTC 11168, C. jejuni NCTC 11351T, C. coli ATCC 33559T, C. hyointestinalis ATCC 33560T, C. fetus subsp fetus ATCC 27374T, C. upsaliensis ATCC 43954T, C. lanienae LR283 and C. helveticus CCUG 30683. Characteristics are given in Table 2, and phenotypic descriptions are given below.
Table 2.
Phenotypic characteristics of Campylobacter novaezeelandiae sp. nov. as compared to other Campylobacter taxa
Taxa: 1, Campylobacter novaezeelandiae sp. nov. (n=6); 2, C. armoricus ; 3, C. blaseri ; 4, C. avium ; 5, C. canadensis ; 6, C. coli ; 7, C. concisus ; 8, C. corcagiensis ; 9, C.cuniculorum; 10, C. curvus ; 11, C. fetus subsp. fetus ; 12, C. fetus subsp. testudinum ; 13, C. fetus subsp. venerealis ; 14, C. geochelonis ; 15, C. gracilis ; 16, C. helveticus ; 17, C. hepaticus ; 18, C. hominis ; 19, C. hyointestinalis subsp. hyointestinalis ; 20, C. hyointestinalis subsp. lawsonii ; 21, C. iguaniorum ; 22, C. insulaenigrae ; 23, C.jejuni subsp. doylei; 24, C. jejuni subsp. jejuni ; 25, C. lanienae ; 26, C. lari subsp. concheus ; 27, C. lari subsp. lari ; 28, C. mucosalis ; 29, C. ornithocola ; 30, C. peloridis ;31, C. pinnipediorum subsp. caledonicus ; 32, C. pinnipediorum subsp. pinnipediorum ; 33, C. rectus ; 34, C. showae ; 35, C. sputorum ; 36, C. subantarcticus ; 37, C.upsaliensis; 38, C. ureolyticus ; 39, C. volucris . +, All strains examined give a positive result; –, strains examined give a negative result; U, unknown; F, 7–28 % strains positive; V, 29–69 % strains positive; M, 70–95 % strains positive; D, unable to be determined; w, weak.
|
Characteristic |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
19 |
20 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Motility |
+ |
– |
+ |
+ |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
– |
+ |
+ |
|
|
Oxidase |
+ |
+ |
+ |
+ |
+ |
+ |
V |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
|
Catalase |
+ |
+ |
+ |
w |
V |
+ |
– |
+ |
+ |
– |
+ |
+ |
M |
+ |
V |
– |
+ |
– |
+ |
+ |
|
Urease |
– |
+ |
+ |
– |
V |
– |
– |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
Nitrate reduction |
+ |
– |
+ |
+ |
V |
+ |
F |
M |
+ |
+ |
+ |
+ |
M |
+ |
M |
+ |
V |
– |
+ |
+ |
|
Hippurate hydrolysis |
– |
– |
– |
+ |
– |
– |
– |
– |
– |
F |
– |
– |
– |
+ |
– |
– |
M |
– |
– |
– |
|
Indoxyl acetate hydrolysis |
+ |
– |
+ |
+ |
– |
+ |
– |
V |
+ |
V |
– |
– |
– |
– |
M |
+ |
+ |
– |
– |
– |
|
Alkaline phosphatase |
– |
– |
+ |
– |
– |
– |
M |
+ |
– |
V |
– |
– |
– |
– |
– |
– |
U |
– |
– |
F |
|
H2S production TSI |
– |
U |
+ |
– |
V |
– |
– |
+ |
– |
F |
– |
– |
– |
– |
– |
– |
– |
– |
+ |
+ |
|
Alpha haemolysis |
+ |
– |
– |
– |
– |
F |
F |
– |
+ |
F |
– |
– |
V |
– |
– |
+ |
– |
– |
V |
F |
|
Growth 25 °C microaerobic |
– |
– |
+ |
– |
– |
– |
– |
U |
– |
– |
+ |
+ |
+ |
+ |
– |
– |
– |
– |
F |
– |
|
Growth 37 °C microaerobic |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
V |
+ |
+ |
+ |
+ |
– |
+ |
+ |
– |
+ |
+ |
|
Growth 42 °C microaerobic |
+ |
+ |
+ |
+ |
+ |
+ |
M |
+ |
M |
V |
M |
V |
– |
– |
M |
+ |
+ |
– |
+ |
+ |
|
Growth 37 °C anaerobic |
+ |
+ |
+ |
– |
+ |
– |
+ |
+ |
– |
+ |
F |
+ |
M |
+ |
+ |
– |
– |
+ |
– |
w |
|
Growth 37 °C aerobic |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
V |
– |
– |
– |
– |
– |
– |
– |
– |
|
Growth on nutrient agar |
+ |
U |
U |
– |
– |
+ |
F |
w |
+ |
+ |
M |
U |
M |
U |
+ |
M |
+ |
U |
+ |
+ |
|
Growth on MacConkey |
– |
U |
+ |
– |
+ |
V |
– |
– |
– |
M |
M |
M |
V |
– |
M |
– |
– |
– |
V |
V |
|
R to nalidixic acid (30 µg) |
-* |
– |
– |
– |
V |
– |
M |
+ |
V |
+ |
+ |
+ |
V |
+ |
V |
– |
V |
V |
+ |
+ |
|
R to cephalothin (30 µg) |
+ |
R |
– |
+ |
– |
+ |
– |
– |
M |
– |
– |
U |
– |
– |
– |
– |
M |
– |
F |
– |
|
H2 requirement |
– |
– |
– |
V |
– |
– |
+ |
– |
– |
+ |
– |
– |
– |
– |
+ |
– |
– |
+ |
– |
U |
|
DNA G+C content (mol%) |
27.4 |
28 |
29 |
35 |
U |
31 |
37–41 |
31.9 |
32.4 |
45–46 |
33–35 |
33 |
33–34 |
33.6 |
44–46 |
34 |
27.9 |
32–33 |
34–36 |
31–33 |
|
Selenite reduction |
– |
V |
U |
– |
U |
+ |
F |
U |
– |
– |
M |
U |
F |
– |
– |
– |
U |
– |
+ |
+ |
|
Growth on 2 % NaCl |
+ |
– |
U |
– |
– |
– |
F |
+ |
– |
V |
– |
U |
F |
+ |
– |
F |
– |
U |
– |
– |
|
Growth on 1 % glycine |
+ |
+ |
w |
– |
V |
M |
F |
+ |
– |
+ |
+ |
+ |
F |
+ |
+ |
V |
+ |
+ |
+ |
F |
|
Growth on 0.04 % TTC |
F |
V |
U |
+ |
U |
+ |
– |
– |
V |
+ |
– |
+ |
– |
– |
– |
– |
+ |
– |
F |
– |
|
TTC reduction |
F |
V |
U |
– |
U |
+ |
– |
– |
V |
M |
– |
+ |
– |
– |
– |
– |
U |
– |
F |
– |
|
Characteristic |
21 |
22 |
23 |
24 |
25 |
26 |
27 |
28 |
29 |
30 |
31 |
32 |
33 |
34 |
35 |
36 |
37 |
38 |
39 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Motility |
U |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
U |
U |
U |
U |
+ |
+ |
+ |
+ |
+ |
– |
+ |
|
Oxidase |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
+ |
+ |
V |
+ |
+ |
+ |
+ |
+ |
|
Catalase |
+ |
+ |
M |
+ |
+ |
+ |
+ |
– |
+ |
+ |
– |
+ |
F |
+ |
V† |
+ |
– |
F |
+ |
|
Urease |
– |
– |
– |
– |
– |
– |
– |
– |
+ |
U |
+ |
+ |
– |
– |
V‡ |
U |
– |
+ |
U |
|
Nitrate reduction |
+ |
+ |
– |
+ |
+ |
+ |
+ |
F |
V |
U |
+ |
+ |
+ |
+ |
M |
U |
+ |
+ |
+ |
|
Hippurate hydrolysis |
– |
– |
+ |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
Indoxyl acetate hydrolysis |
– |
– |
+ |
+ |
– |
U |
F |
– |
– |
U |
– |
– |
+ |
V |
– |
– |
+ |
V |
– |
|
Alkaline phosphatase |
U |
U |
– |
– |
+ |
U |
– |
– |
– |
U |
U |
U |
– |
– |
– |
U |
– |
– |
– |
|
H2S production TSI |
+ |
– |
– |
– |
– |
U |
– |
+ |
– |
U |
+ |
+ |
– |
V |
+ |
– |
– |
– |
– |
|
Alpha haemolysis |
+ |
nd |
+ |
+ |
+ |
U |
M |
F |
– |
U |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
V |
U |
|
Growth 25 °C microaerobic |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
U |
+ |
+ |
– |
– |
– |
– |
– |
– |
– |
|
Growth 37 °C microaerobic |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
– |
V |
+ |
+ |
+ |
+ |
+ |
|
Growth 42 °C microaerobic |
– |
– |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
– |
– |
w |
V |
M |
+ |
M |
V |
+ |
|
Growth 37 °C anaerobic |
w |
– |
– |
– |
w |
U |
– |
+ |
+ |
U |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
|
Growth 37 °C aerobic |
– |
– |
– |
– |
– |
– |
– |
– |
– |
U |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
Growth on nutrient agar |
U |
+ |
+ |
+ |
U |
+ |
+ |
+ |
U |
+ |
U |
U |
F |
V |
M |
– |
+ |
M |
– |
|
Growth on MacConkey |
U |
U |
– |
– |
U |
U |
U |
M |
U |
U |
U |
U |
– |
+ |
V |
F |
– |
V |
w |
|
R to nalidixic acid (30 µg) |
+ |
+ |
– |
– |
+ |
– |
M |
M |
U |
M |
– |
– |
M |
– |
M |
+ |
– |
– |
+ |
|
R to cephalothin (30 µg) |
– |
+ |
– |
+ |
+ |
+ |
+ |
– |
U |
F |
– |
– |
– |
– |
– |
– |
F |
– |
+ |
|
H2 requirement |
– |
– |
– |
– |
U |
U |
– |
+ |
– |
U |
– |
– |
+ |
+ |
– |
U |
– |
+ |
– |
|
DNA G+C content (mol%) |
36 |
U |
28–30 |
30–31 |
36 |
30 |
31–33 |
38–39 |
29.5 |
29 |
31 |
30 |
45–46 |
44–46 |
30–33 |
30 |
32–35 |
28–30 |
29 |
|
Selenite reduction |
U |
U |
– |
M |
U |
U |
V |
F |
U |
U |
U |
U |
– |
– |
U |
– |
+ |
– |
+ |
|
Growth on 2 % NaCl |
U |
– |
– |
– |
– |
+ |
+ |
– |
U |
M |
U |
U |
M |
+ |
+ |
+ |
– |
+ |
– |
|
Growth on 1 % glycine |
+ |
+ |
F |
M |
– |
+ |
+ |
V |
+ |
+ |
– |
V |
M |
V |
+ |
M |
+ |
+ |
– |
|
Growth on 0.04 % TTC |
U |
U |
V |
M |
U |
U |
M |
– |
U |
U |
U |
U |
– |
– |
– |
U |
– |
+ |
– |
|
TTC reduction |
U |
U |
V |
M |
U |
U |
M |
– |
U |
U |
U |
U |
– |
– |
– |
U |
– |
– |
– |
Description of Campylobacter novaezeelandiae sp. nov.
Campylobacter novaezeelandiae (no.vae.zee.lan'di.ae. N.L. gen. n. novaezeelandiae of Nova Zeelandia, pertaining to New Zealand).
Cells are Gram-negative, motile, spiral rods, 3–4 µm long. After 2 days incubation on Columbia horse blood agar at 37 °C in a microaerobic atmosphere containing hydrogen gas, colonies are small (0.5–1 mm), grey, smooth and entire with slightly convex surfaces. Strong, dark α-haemolysis is seen on Columbia horse blood agar. A distinctive ‘ Campylobacter ’ odour [50] is produced. On mCCDA agar, colonies are small and grey with irregular margins. Isolates will grow microaerobically (without hydrogen) at 42 °C, but growth was faster at 37 °C. They grow anaerobically at 37 °C but do not grow in ambient air at 37 °C. They grow on nutrient agar, nutrient agar +1 % w/v glycine and nutrient agar +2 % w/v NaCl, but not on MacConkey agar or on nutrient agar with 0.1 % w/v selenite. Only 1/6 isolates grew on nutrient agar containing 0.04 % w/v tetrazolium chloride, that isolate was also capable of reducing tetrazolium. They grow poorly on Mueller–Hinton agar containing either sheep or horse blood. Isolates are motile and positive for oxidase, catalase, indoxyl acetate hydrolysis and nitrate reduction. They do not hydrolyse hippurate, produce H2S in TSI, show alkaline phosphatase activity nor do they produce urease. All isolates are resistant to cephalothin and sensitive to nalidixic acid. Pathogenicity for vertebrates is unknown and we have not isolated this species from humans or symptomatic animals [12]; however, they were capable of causing death in the Galleria mellonella larval model. The type strain is B423bT (=NZRM 4741T=ATCC TSD-167T). The GenBank accession numbers for the genome assemblies of B423bT, B1491 and W677a are QPGR00000000, QPGQ00000000 and QPGP00000000, respectively, while the read data are deposited under SRA codes SRR8367113, SRR8367114 and SRR8367115 within the BioProject PRJNA480171. The 16S rRNA gene sequences have been deposited in GenBank under the accession numbers MK791738–MK791743.
Supplementary Data
Funding information
Sampling was funded by the Royal Society of New Zealand Marsden Fund (MAU0802), the New Zealand Food Safety Authority project FDI/236/2005 (Enhancing Surveillance of Potentially Food-borne Enteric Diseases in New Zealand: Human Campylobacteriosis in the Manawatu) and a New Zealand Ministry of Health, Aquatic Protozoa Analysis and Advice Services contract, while the phenotypic work was funded by the Massey University Research Fund. Shipping of the isolates to ATCC was funded by mEpiLab, School of Veterinary Science, Massey University.
Acknowledgements
We thank Anthony Pita and Sarah Moore for performing the water sampling. We thank Mike Dickison (@adzebill) who gently pointed out that our original name was incorrect and the nomenclature reviewer who corrected the name. We thank the Fonterra Research and Development Centre and Betty Smythe for access to their MALDI-TOF. We thank Stuart Littlejohn for performing the coccoidal transformation microscopy.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: CDSs, coding-DNA sequences; mCCDA, modified charcoal cefoperazone agar; TSI, triple sugar iron agar; TTC, 2,3,5 triphenyltetrazolium.
One supplementary table and one supplementary figure are available with the online version of this article.
Genome assemblies have been deposited in GenBank under the accession numbers QPGR00000000, QPGQ00000000 and QPGP00000000, and read data are deposited under SRA codes SRR8367113, SRR8367114 and SRR8367115 within the BioProject PRJNA480171. 16S rRNA gene sequences have been deposited in GenBank under the accession numbers MK791738 - MK791743.
References
- 1.Sebald M, Veron M. Teneur en bases de l’ADN et classification des vibrions. Ann Inst Pasteur. 1963;105:897–910. [PubMed] [Google Scholar]
- 2.Gilbert MJ, Zomer AL, Timmerman AJ, Spaninks MP, Rubio-García A, et al. Campylobacter blaseri sp. nov., isolated from common seals (Phoca vitulina) Int J Syst Evol Microbiol. 2018;68:1787–1794. doi: 10.1099/ijsem.0.002742. [DOI] [PubMed] [Google Scholar]
- 3.Samuel MC, Vugia DJ, Shallow S, Marcus R, Segler S, et al. Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996–1999. Clin Infect Dis. 2004;38:S165–S174. doi: 10.1086/381583. [DOI] [PubMed] [Google Scholar]
- 4.Baker M, Wilson N, Edwards R. Campylobacter infection and chicken: an update on New Zealand’s largest ‘common source outbreak’. N Z Med J. 2007;120:75–79. [PubMed] [Google Scholar]
- 5.Waldenström J, Broman T, Carlsson I, Hasselquist D, Achterberg RP, et al. Prevalence of Campylobacter jejuni, Campylobacter lari, and Campylobacter coli in different ecological guilds and taxa of migrating birds. Appl Environ Microbiol. 2002;68:5911–5917. doi: 10.1128/AEM.68.12.5911-5917.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newell DG, Fearnley C. Sources of Campylobacter colonization in broiler chickens. Appl Environ Microbiol. 2003;69:4343–4351. doi: 10.1128/AEM.69.8.4343-4351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debruyne L, Broman T, Bergström S, Olsen B, On SLW, et al. Campylobacter subantarcticus sp. nov., isolated from birds in the sub-Antarctic region. Int J Syst Evol Microbiol. 2010;60:815–819. doi: 10.1099/ijs.0.011056-0. [DOI] [PubMed] [Google Scholar]
- 8.Benskin CMH, Rhodes G, Pickup RW, Wilson K, Hartley IR. Diversity and temporal stability of bacterial communities in a model passerine bird, the zebra finch. Mol Ecol. 2010;19:5531–5544. doi: 10.1111/j.1365-294X.2010.04892.x. [DOI] [PubMed] [Google Scholar]
- 9.Carter PE, McTavish SM, Brooks HJL, Campbell D, Collins-Emerson JM, et al. Novel clonal complexes with an unknown animal reservoir dominate Campylobacter jejuni isolates from river water in New Zealand. Appl Environ Microbiol. 2009;75:6038–6046. doi: 10.1128/AEM.01039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French N, Yu S, Biggs P, Holland B, Fearnhead P, et al. Evolution of Campylobacter species in New Zealand. In: Sheppard SK, editor. Campylobacter Ecology and Evolution. Caister Academic Press; 2014. pp. 221–240. editor. [Google Scholar]
- 11.Mohan V, Stevenson M, Marshall J, Fearnhead P, Holland BR, et al. Campylobacter jejuni colonization and population structure in urban populations of ducks and starlings in New Zealand. Microbiologyopen. 2013;2:659–673. doi: 10.1002/mbo3.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullner P, Shadbolt T, Collins-Emerson JM, Midwinter AC, Spencer SEF, et al. Molecular and spatial epidemiology of human campylobacteriosis: source association and genotype-related risk factors. Epidemiol Infect. 2010;138:1372–1383. doi: 10.1017/S0950268809991579. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson DA, O'Donnell AJ, Akhter RN, Fayaz A, Mack HJ, et al. Updating the genomic taxonomy and epidemiology of Campylobacter hyointestinalis . Sci Rep. 2018;8:2393. doi: 10.1038/s41598-018-20889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bojanić K, Midwinter AC, Marshall JC, Biggs PJ, Acke E, et al. Isolation of emerging Campylobacter species in working farm dogs and their frozen home-killed raw meat diets. J Vet Diagn Invest. 2019;31:23–32. doi: 10.1177/1040638718820082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bojanić K, Midwinter AC, Marshall JC, Rogers LE, Biggs PJ, et al. Isolation of Campylobacter spp. from client-owned dogs and cats, and retail raw meat pet food in the Manawatu, New Zealand. Zoonoses Public Health. 2017;64:438–449. doi: 10.1111/zph.12323. [DOI] [PubMed] [Google Scholar]
- 16.McBride G, Till D, Ryan T, Ball A, Lewis G, et al. Pathogen occurence and human health risk assessment analysis. 2002.
- 17.Nohra A, Grinberg A, Midwinter AC, Marshall JC, Collins-Emerson JM, et al. Molecular epidemiology of Campylobacter coli strains isolated from different sources in New Zealand between 2005 and 2014. Appl Environ Microbiol. 2016;82:AEM.00934-16. doi: 10.1128/AEM.00934-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic Acid Techniques in Bacterial Systematics. Chicester: John Wiley & Sons; 1991. pp. 115–175. [Google Scholar]
- 19.Wilkinson DA, Midwinter AC, Kwan E, Bloomfield SJ, French NP, et al. Draft whole-genome sequences of three isolates of a novel strain of a Campylobacter sp. isolated from New Zealand birds and water. Microbiol Resour Announc. 2019;8:1–2. doi: 10.1128/MRA.00258-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol. 2018;68:461–466. doi: 10.1099/ijsem.0.002516. [DOI] [PubMed] [Google Scholar]
- 22.Taylor DE, Eaton M, Yan W, Chang N. Genome maps of Campylobacter jejuni and Campylobacter coli . J Bacteriol. 1992;174:2332–2337. doi: 10.1128/JB.174.7.2332-2337.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, et al. Geneious
- 24.Debruyne L, Gevers D, Vandamme P. Taxonomy of the family Campylobacteraceae. In: Nachamkin I, Szymanski C, Blaser M, editors. Campylobacter. Washington, DC, USA: ASM Press; 2008. pp. 3–26. [Google Scholar]
- 25.Van TTH, Elshagmani E, Gor MC, Scott PC, Moore RJ. Campylobacter hepaticus sp. nov., isolated from chickens with spotty liver disease. Int J Syst Evol Microbiol. 2016;66:4518–4524. doi: 10.1099/ijsem.0.001383. [DOI] [PubMed] [Google Scholar]
- 26.Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, et al. DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 2007;57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 27.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 28.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt M, Mather AE, Sánchez-Busó L, Page AJ, Parkhill J, et al. ARIBA: rapid antimicrobial resistance genotyping directly from sequencing reads. Microb Genom. 2017;3:1–11. doi: 10.1099/mgen.0.000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Yang J, Yu J, Yao Z, Sun L, et al. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res. 2005;33:D325–D328. doi: 10.1093/nar/gki008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong A, Lange D, Houle S, Arbatsky NP, Valvano MA, et al. Role of capsular modified heptose in the virulence of Campylobacter jejuni . Mol Microbiol. 2015;96:1136–1158. doi: 10.1111/mmi.12995. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu G, Smith DK, Zhu H, Guan Y, Lam Tommy Tsan‐Yuk, Lam TT. ggtree: an r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol. 2017;8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- 36.RStudio Team RStudio: integrated development environment for R. 2015.
- 37.Koziel M, O'Doherty P, Vandamme P, Corcoran GD, Sleator RD, et al. Campylobacter corcagiensis sp. nov., isolated from faeces of captive lion-tailed macaques (Macaca silenus) Int J Syst Evol Microbiol. 2014;64:2878–2883. doi: 10.1099/ijs.0.063867-0. [DOI] [PubMed] [Google Scholar]
- 38.Gibb S, Strimmer K. MALDIquant: a versatile R package for the analysis of mass spectrometry data. Bioinformatics. 2012;28:2270–2271. doi: 10.1093/bioinformatics/bts447. [DOI] [PubMed] [Google Scholar]
- 39.Ahdesmäki M, Strimmer K. Feature selection in omics prediction problems using cat scores and false nondiscovery rate control. Ann Appl Stat. 2010;4:503–519. doi: 10.1214/09-AOAS277. [DOI] [Google Scholar]
- 40.Strimmer K. Package ‘crossval’ Title Generic Functions for Cross Validation. 2015.
- 41.Wheeler NE, Blackmore T, Reynolds AD, Midwinter AC, Marshall J, et al. Genomic correlates of extraintestinal infection are linked with changes in cell morphology in Campylobacter jejuni . Microb Genom. 2019;5:1–12. doi: 10.1099/mgen.0.000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Champion OL, Karlyshev AV, Senior NJ, Woodward M, La Ragione R, et al. Insect infection model for Campylobacter jejuni reveals that O-methyl phosphoramidate has insecticidal activity. J Infect Dis. 2010;201:776–782. doi: 10.1086/650494. [DOI] [PubMed] [Google Scholar]
- 43.Moran AP, Upton ME. Effect of medium supplements, illumination and superoxide dismutase on the production of coccoid forms of Campylobacter jejuni ATCC 29428. J Appl Bacteriol. 1987;62:43–51. doi: 10.1111/j.1365-2672.1987.tb02379.x. [DOI] [PubMed] [Google Scholar]
- 44.Atlas R, Snyder J. Reagents, stains and media: Bacteriology. In: Jorgensen JH, Pfaller MA, editors. Manual of Clinical Microbiology. American Society for Microbiology; 2015. [Google Scholar]
- 45.MacFaddin JF. Biochemical Tests for Identification of Medical Bacteria. 3rd ed. Philadelphia, USA: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 46.On SL, Holmes B. Assessment of enzyme detection tests useful in identification of campylobacteria. J Clin Microbiol. 1992;30:746–749. doi: 10.1128/jcm.30.3.746-749.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma M, Amano T, Enokimoto M, Yano T, Moe KK, et al. Influence of pH of TSI medium on the detection of hydrogen sulfide production by Campylobacter hyointestinalis . Lett Appl Microbiol. 2007;44:544–549. doi: 10.1111/j.1472-765X.2006.02097.x. [DOI] [PubMed] [Google Scholar]
- 48.Sifré E, Salha BA, Ducournau A, Floch P, Chardon H, et al. EUCAST recommendations for antimicrobial susceptibility testing applied to the three main Campylobacter species isolated in humans. J Microbiol Methods. 2015;119:206–213. doi: 10.1016/j.mimet.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 49.On SL, Holmes B. Reproducibility of tolerance tests that are useful in the identification of campylobacteria. J Clin Microbiol. 1991;29:1785–1788. doi: 10.1128/jcm.29.9.1785-1788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Edany AA, Khudor MH, Radhi LY. Isolation, identification and toxigenic aspects of Campylobacter jejuni isolated from slaughtered cattle and sheep at Basrah city. Basrah J Vet Res. 2015;14:316–327. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.