Abstract
Objectives
To compare clinical outcomes for stage IIIC and IV ovarian cancer patients receiving neoadjuvant chemotherapy and interval cytoreductive surgery followed by up to three versus more cycles of post-operative chemotherapy.
Methods
We conducted a multi-institution retrospective cohort study of patients treated from January 2005 to February 2016 with neoadjuvant platinum-based therapy followed by interval surgery and post-operative chemotherapy. The following were exclusion criteria: more than four cycles of neoadjuvant chemotherapy, bevacizumab with neoadjuvant chemotherapy, nonplatinum therapy, prior chemotherapy, and elevated CA125 values after three post-operative chemotherapy cycles. Progression-free and overall survival and toxicity profiles were compared between groups receiving up to three cycles versus more that three cycles post-operatively.
Results
A total of 100 patients met inclusion criteria: 41 received up to three cycles and 59 received more than three cycles. The groups were similar in terms of age, body mass index, performance status, tumor histology, optimal cytoreduction rates, and median number of neoadjuvant chemotherapy cycles. Median progression-free survival was 14 vs 16.6 months in those receiving up to three cycles versus more than three cycles, respectively (HR 0.99, 95% CI 0.58 to 1.68, p=0.97). Similarly, median overall survival was not different at 47.1 vs 69.4 months, respectively (HR 1.96, 95% CI 0.87 to 4.42, p=0.10). There were no differences in grade 2 or higher chemotherapyrelated toxicities.
Conclusions
Extending post-operative chemotherapy beyond three cycles in patients receiving neoadjuvant chemotherapy and interval cytoreductive surgery with normalization of CA125 levels was not associated with improved survival or greater toxicity. Future study in a larger cohort is warranted to define optimal length of cytotoxic treatment.
Introduction
Standard management of advanced ovarian cancer has historically included primary cytoreductive surgery followed by adjuvant chemotherapy. More recently, the use of neoadjuvant chemotherapy prior to interval cytoreductive surgery followed by completion of chemotherapy has been shown to be non-inferior to primary cytoreductive surgery in terms of progression-free and overall survival in randomized prospective trials.1,2 This strategy may also reduce peri-operative morbidity associated with cytoreductive surgery and is being used with increasing frequency in the United States in selected patients, such as those deemed unable to be optimally cytoreduced primarily or those with complex medical co-morbidities.2,3
Most patients undergoing neoadjuvant chemotherapy receive three or four cycles of pre-operative chemotherapy in accordance with treatment protocols used in randomized trials.1,2,4,5 However, practice patterns for post-operative chemotherapy following interval cytoreductive surgery are more variable. The number of post-operative chemotherapy cycles generally ranges from three to six cycles, potentially resulting in more chemotherapy for patients receiving the neoadjuvant chemotherapy approach compared with patients receiving upfront surgery. For these patients, the optimal number of chemotherapy cycles following interval cytoreductive surgery that balances treatment efficacy and safety has not been defined.6 In contrast, following primary cytoreductive surgery, most women receive six adjuvant chemotherapy cycles based on prior studies demonstrating that treatment beyond six cycles increases toxicity without improvement in outcomes.7–9 It is unclear whether six post-operative chemotherapy cycles following a maximal surgical cytoreductive effort at interval cytoreductive surgery after neoadjuvant chemotherapy also optimizes patient outcomes.
Our objective was to compare clinical outcomes for stage IIIC and IV ovarian cancer patients receiving neoadjuvant chemotherapy and interval cytoreductive surgery followed by up to three cycles versus more than three cycles of post-operative chemotherapy. We were specifically interested in evaluating outcomes in the subset of patients whose CA125 was normal by the time they completed three cycles of post-operative chemotherapy to determine if any additional chemotherapy after that was beneficial. This subgroup was selected because these patients present clinically challenging decision-making about when to stop chemotherapy. In contrast, many providers would choose to continue chemotherapy in patients with persistently elevated CA125 values at this time point.
Our primary outcome was progression-free survival. Secondary outcomes included overall survival and chemotherapy-related toxicities. We hypothesized that additional chemotherapy beyond three post-operative cycles would not significantly improve progression-free survival and would be associated with greater treatment-related toxicity.
METHODS
We conducted a multi-institution retrospective cohort study of women diagnosed with and treated for stage IIIC and IV epithelial ovarian/fallopian tube/primary peritoneal cancer between January 2005 and February 2016 at five academic institutions in three cities. Treatment included platinum-based neoadjuvant chemotherapy followed by interval cytoreductive surgery and post-operative chemotherapy. Exclusion criteria were as follows: more than four cycles of neoadjuvant chemotherapy, bevacizumab administration with the neoadjuvant chemotherapy regimen, non-platinum-based therapy, prior history of chemotherapy administration, elevated CA125 values (>35 U/mL) following three cycles of post-operative chemotherapy, other evidence of disease progression during primary treatment (by exam or imaging), maintenance chemotherapy, and insufficient treatment data (lack of chemotherapy start date or number of neoadjuvant chemotherapy or post-operative chemotherapy cycles). Both dose dense and standard every 3 week dosing schedules were included. Patients with post-operative chemotherapy regimens using intraperitoneal chemotherapy were included. Patients were not excluded based on a maximum number of post-operative cycles. The decision to proceed with neoadjuvant chemotherapy as opposed to primary surgery as well as whether to administer up to three cycles versus more than three cycles of post-operative chemotherapy was per physician discretion. Selection criteria were not standardized across all sites given the retrospective nature of this study. Institutional review board approval was obtained at all sites.
Demographic variables, clinical, pathologic, and survival outcome data were collected. Patients were stratified into two groups for analysis: those receiving up to three cycles and those receiving more than three cycles of post-operative chemotherapy. Our primary outcome variable was progression-free survival, defined as time from cycle 1 day 1 of neoadjuvant chemotherapy to the date of first documented recurrence, death, or the date of most recent contact. Recurrence was defined by imaging confirmation with or without a rise in CA125 level, or treatment for a recurrence if a rise in CA125 level alone occurred. Overall survival was defined as time from cycle 1 day 1 of neoadjuvant chemotherapy to the date of death or the date of most recent contact.
Descriptive statistics were reported as median and range or mean±SD for continuous variables, or frequencies and percentages for categorical variables. Data were compared between groups receiving up to three versus more than three post-operative chemotherapy cycles using Student’s t-test (parametric) or Wilcoxon test (non-parametric) for continuous variables and Chi-square test or Fisher’s exact test for categorical variables. Survival analyses were calculated using the Kaplan-Meier method with differences between survival curves calculated by log-rank test. Statistical analyses were performed using Statistical Analysis Software (SAS) 9.3 (SAS Inc., Cary, NC, USA). Statistical significance was determined using an α of 0.05.
RESULTS
Some 225 patients were identified who received neoadjuvant chemotherapy for advanced ovarian cancer. Of these, 125 patients did not meet inclusion criteria leaving 100 evaluable patients. The most common reasons for exclusion were persistent CA125 elevation following three post-operative chemotherapy cycles or disease progression during upfront treatment, receipt of more than four cycles of neoadjuvant chemotherapy, or insufficient treatment data. Forty-one patients received up to three post-operative chemotherapy cycles; three patients received two post-operative chemotherapy cycles; and the remaining 38 patients received three cycles. Fifty-nine patients received more than three post-operative cycles. The rationale for number of post-operative cycles given was only clearly documented for 10 patients. For two patients, three versus six post-operative cycles was discussed and the number given was based on patient preference. In eight patients, suboptimal cytoreduction or delayed normalization of CA125 was used as a rationale for the number of cycles. Baseline demographic characteristics and tumor characteristics are shown in Table 1. Patients in the two groups were similar in terms of age, body mass index, performance status, tumor histology (over 70% serous carcinoma in both groups), stage at presentation, and rates of optimal cytoreduction. The median follow-up time was longer in the group receiving more than three cycles of post-operative chemotherapy (31.4 vs 21.7 months, p=0.005).
Table 1.
Baseline demographic, tumor, and neoadjuvant chemotherapy treatment characteristics
| Variables | ≤3 cycles postoperatively (n=41) | >3 cycles postoperatively (n=59) | P value |
|---|---|---|---|
| Demographic characteristics | |||
| Follow-up time (months) | 25.3±14.3 (21.7) | 36.2±23.7 (31.4) | 0.005 |
| Age (years) | 65.3±10.9 | 63.9±11.5 | 0.55 |
| Body mass index (kg/m2) | 27.5±6.1 | 26.5±6.1 | 0.42 |
| Race | |||
| Caucasian | 28 (68.3%) | 44 (78.0%) | 0.13 |
| African American | 6 (14.6%) | 1 (1.7%) | |
| Hispanic | 3 (7.3%) | 3 (5.1%) | |
| Asian | 3 (7.3%) | 5 (8.5%) | |
| Other | 1 (2.4%) | 4 (6.9%) | |
| Current smoker | 1 (2.4%) | 4 (6.8%) | 0.45 |
| ECOG performance status | |||
| 0 | 14 (34.1%) | 23 (39.0%) | 0.46 |
| 1 | 13 (31.7%) | 13 (22.0%) | |
| 2 | 4 (9.8%) | 4 (6.8%) | |
| 3 | 1 (2.4%) | 0 (0.0%) | |
| Missing | 9 (22.0%) | 19 (32.2%) | |
| ASA score | |||
| 2 | 7 (17.1%) | 16 (27.1%) | 0.48 |
| 3 | 20 (48.8%) | 23 (39.0%) | |
| Missing | 14 (34.1%) | 20 (33.9%) | |
| Tumor characteristics | |||
| Disease status after interval cytoreductive surgery | |||
| R0 (no visible residual disease) | 28 (68.3%) | 31 (52.5%) | 0.10 |
| <1 cm residual disease | 12 (29.3%) | 16 (27.1%) | |
| Optimal not otherwise specified | 1 (2.4%) | 5 (8.5%) | |
| Suboptimal | 0 (0.0%) | 6 (10.2%) | |
| Missing | 0 (0.0%) | 1 (1.7%) | |
| Histology | |||
| Serous | 29 (70.7%) | 44 (74.6%) | 0.24 |
| Clear cell | 1 (2.4%) | 4 (6.8%) | |
| Adenocarcinoma not otherwise specified/epithelial/mixed | 6 (14.6%) | 10 (16.9%) | |
| Complete pathologic response | 4 (9.8%) | 1 (1.7%) | |
| Missing | 1 (2.4%) | 0 (0.0%) | |
| Grade | |||
| 2 | 2 (4.9%) | 2 (3.4%) | 0.40 |
| 3 | 34 (82.9%) | 54 (91.5%) | |
| Missing | 5 (12.2%) | 3 (5.1%) | |
| Stage | |||
| IIIC | 25 (61.0%) | 42 (71.2%) | 0.47 |
| IVA | 6 (14.6%) | 5 (8.5%) | |
| IVB | 10 (24.4%) | 12 (20.3%) | |
| Neoadjuvant treatment characteristics | |||
| Regimen | |||
| Carboplatin/paclitaxel every 21 or 28 days | 18 (43.9%) | 31 (52.5%) | 0.38 |
| Carboplatin/weekly paclitaxel | 20 (48.8%) | 27 (45.8%) | |
| Single-agent carboplatin | 3 (7.3%) | 1 (1.7%) | |
| Neoadjuvant cycles (n) | |||
| 2 | 0 (0.0%) | 1 (1.7%) | 0.72 |
| 3 | 24 (58.5%) | 38 (64.4%) | |
| 4 | 17 (41.5%) | 20 (33.9%) | |
Values shown as mean ± SD (median) or n (%).
ASA, American Society of Anesthesiologists; ECOG, Eastern Cooperative Oncology Group.
There were no significant differences in surgical complication rates, nodal dissection rates, or estimated blood loss between the groups (data not shown). In addition, length of hospital stay, post-operative intensive care unit admissions, and 30-day post-operative complication rates were similar between the two groups. There were more bowel resections performed in patients receiving more than three post-operative cycles compared with those receiving up to three cycles (21.0% vs 4.9%, p=0.04).
Neoadjuvant chemotherapy treatment characteristics are summarized in Table 1. The median number of neoadjuvant chemotherapy cycles received was three for both groups. Administration of granulocyte colony stimulating factor, rates of dose delays, and rates of dose reductions were similar between the two groups. There were no differences in grade 2 or higher chemotherapy-related toxicities between those receiving up to three versus more than three post-operative chemotherapy cycles (data not shown).
Post-operative chemotherapy treatment characteristics are shown in Table 2. The median number of post-operative chemotherapy cycles given in the group receiving more than three post-operative cycles was five (range of four to nine cycles). As anticipated, this group received a higher median number of total chemotherapy cycles when including neoadjuvant chemotherapy and post-operative chemotherapy (8 vs 6 cycles, p<0.0001). There were no differences in granulocyte colony stimulating factor administration, dose delays, dose reductions, or grade 2 or higher toxicities between the two groups. However, limited documentation resulted in several missing data points for these toxicities (Table 2).
Table 2.
Post-operative chemotherapy treatment characteristics and toxicitiesCaption
| Variables | ≤3 cycles post-operatively (n=41) | >3 cycles post-operatively (n=59) | P value |
|---|---|---|---|
| Treatment characteristics | |||
| Regimen | |||
| Carboplatin/paclitaxel every 21 or 28 days | 17 (41.5%) | 27 (45.8%) | 0.49 |
| Carboplatin/weekly paclitaxel or docetaxel | 19 (46.3%) | 24 (40.7%) | |
| Intraperitoneal chemotherapy | 4 (9.8%) | 5 (8.5%) | |
| Other | 0 (0.0%) | 3 (5.3%) | |
| Missing | 1 (2.4%) | 0 (0.0%) | |
| Post-operative cycles (n) | |||
| 2 | 3 (7.3%) | N/A | <0.0001 |
| 3 | 38 (92.7%) | N/A | |
| 4 | N/A | 22 (37.3%) | |
| 5 | N/A | 10 (16.9%) | |
| 6 | N/A | 23 (39.0%) | |
| 7 | N/A | 3 (5.1%) | |
| 8 | N/A | 0 (0.0%) | |
| 9 | N/A | 1 (1.7%) | |
| Median number of total treatment cycles | 6 | 8 | <0.0001 |
| Granulocyte colony stimulating factor use | |||
| Yes | 12 (29.3%) | 25 (42.4%) | 0.23 |
| No | 20 (48.8%) | 19 (32.2%) | |
| Missing | 9 (22.0%) | 14 (25.4%) | |
| Dose delay ≥7 days | |||
| Yes | 12 (29.3%) | 23 (39.0%) | 0.57 |
| No | 20 (48.8%) | 23 (39.0%) | |
| Missing | 9 (22.0%) | 13 (22.0%) | |
| Dose reduction | |||
| Yes | 12 (29.3%) | 16 (27.1%) | 0.54 |
| No | 13 (31.7%) | 25 (42.4%) | |
| Missing | 16 (39.0%) | 18 (30.5%) | |
| Toxicities* | |||
| Neutropenia | |||
| Yes | 11 (26.8%) | 11 (18.6%) | 0.73 |
| No | 18 (43.9%) | 28 (47.5%) | |
| Missing | 12 (29.3%) | 20 (33.9%) | |
| Thrombocytopenia | |||
| Yes | 6 (14.6%) | 4 (6.8%) | 0.60 |
| No | 23 (56.1%) | 35 (59.3%) | |
| Missing | 12 (29.3%) | 20 (33.9%) | |
| Anemia | |||
| Yes | 4 (9.8%) | 9 (15.3%) | 0.68 |
| No | 25 (61.0%) | 30 (50.8%) | |
| Missing | 12 (29.3%) | 20 (33.9%) | |
| Fatigue | |||
| Yes | 6 (14.6%) | 8 (13.6%) | 0.75 |
| No | 23 (56.1%) | 29 (49.2%) | |
| Missing | 12 (29.3%) | 22 (37.3%) | |
| Nausea/vomiting | |||
| Yes | 1 (2.4%) | 7 (11.9%) | 0.22 |
| No | 28 (68.3%) | 31 (52.5%) | |
| Missing | 12 (29.3%) | 21 (35.6%) | |
| Diarrhea | |||
| Yes | 0 (0.0%) | 1 (1.7%) | 0.62 |
| No | 29 (70.7%) | 36 (61.0%) | |
| Missing | 12 (29.3%) | 22 (37.3%) | |
| Neuropathy | |||
| Yes | 5 (12.2%) | 16 (27.1%) | 0.23 |
| No | 24 (58.5%) | 25 (42.4%) | |
| Missing | 12 (29.3%) | 18 (30.5%) | |
| Allergic reaction | |||
| Yes | 1 (2.4%) | 0 (0.0%) | 0.50 |
| No | 28 (68.3%) | 37 (62.7%) | |
| Missing | 12 (29.3%) | 22 (37.3%) | |
| Other† | |||
| Yes | 4 (9.8%) | 10 (16.9%) | 0.58 |
| No | 25 (61.0%) | 29 (49.2%) | |
| Missing | 12 (29.3%) | 20 (33.9%) | |
Values shown as n (%).
All toxicities grade 2 or higher.
Included acute kidney injury, anorexia, constipation, phlebitis, abdominal pain.
N/A, not applicable.
Median CA125 values at various time points during treatment are shown in Table 3. Normalization of CA125 occurred at a later time point for patients receiving more than three post-operative chemotherapy cycles compared with those receiving up to three post-operative cycles (normalization after median cycle number four total versus cycle number three total, respectively, p=0.03).
Table 3.
CA125 values during treatment
| Time point | ≤3 cycles post-operatively (n=41) | >3 cycles post-operatively (n=59) | P value |
|---|---|---|---|
| Prior to cycle 1 | 1085 (165.7–23 665.3) | 1145.7 (13.1–26 000.0) | 0.85 |
| Following three neoadjuvant cycles | 26.7 (2.8–102.0) | 50.0 (6.1–3401.0) | 0.27 |
| After interval cytoreductive surgery | 24.5 (7.0–316.0) | 43.0 (6.0–1708.0) | 0.18 |
| Following three post-operative cycles | 9.5 (1.5–36.0) | 13.8 (3.0–34.0) | 0.02 |
| Following completion of all therapy | 8.9 (1.5–36.0) | 10.0 (3.0–29.0) | 0.29 |
| Cycle number after which CA125 normalized | 3 (0–5) | 4 (0–6) | 0.03 |
All values shown as median (range).
Subgroup analyses were performed to investigate whether a particular subset of patients with poor prognostic characteristics might have benefitted from additional chemotherapy cycles. These subgroups included: stage IV disease, optimal cytoreduction with residual disease less than one centimeter or not otherwise specified (Table 1), and delayed normalization of CA125 (later than cycle three). There was no subgroup for which a significant progression-free survival or overall survival benefit was observed when comparing those receiving up to three cycles versus those receiving more than three cycles (data not shown). Suboptimal cytoreduction could not be analyzed as a subgroup due to there being no patients in the group receiving up to three post-operative cycles in this analysis.
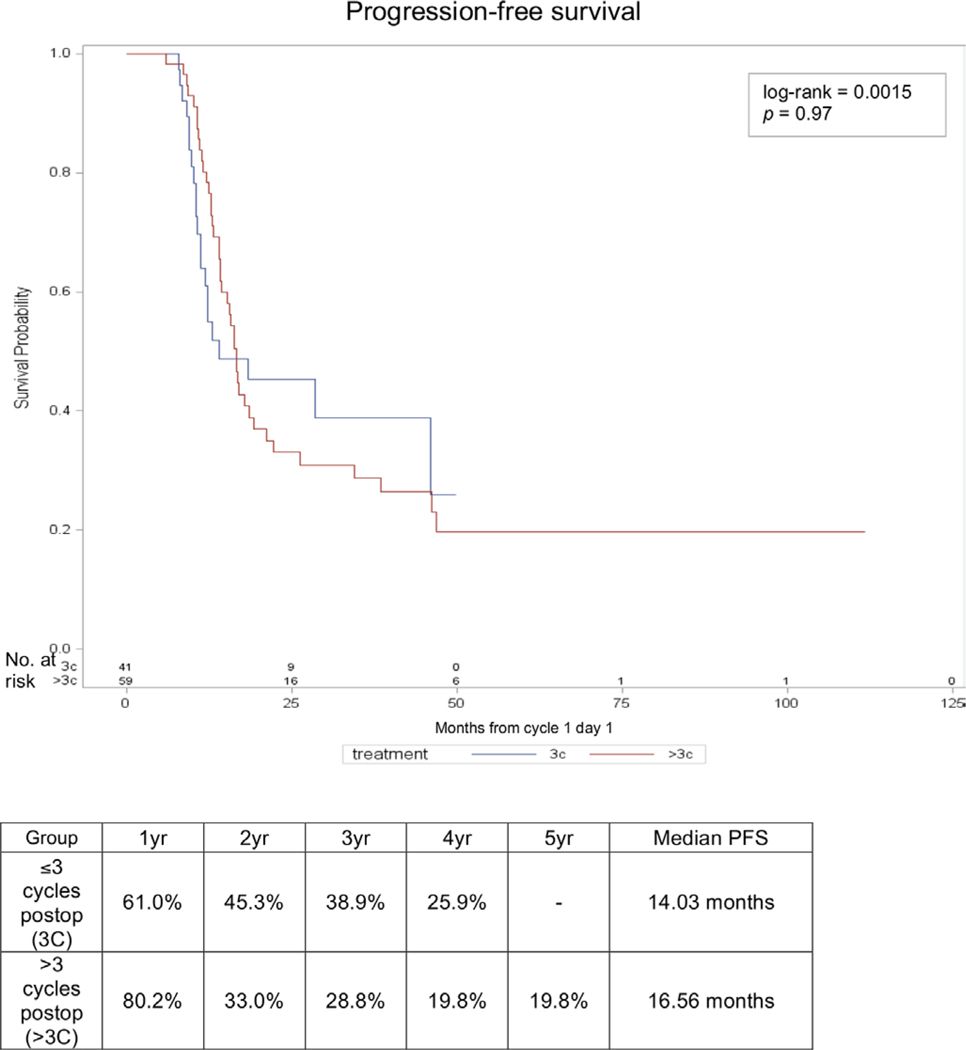
Kaplan-Meier survival analysis curves for progression-free survival and overall survival are shown in Figures 1 and 2, respectively. Median progression-free survival was 14 vs 16.6 months in those receiving up to three post-operative cycles versus those receiving more than three post-operative cycles, respectively, and the progression-free survival curves were not significantly different between the groups (p=0.97). Similarly, median overall survival was 47.1 vs 69.4 months, respectively, and the overall survival curves were not significantly different between the groups (p=0.098).
Figure 1.
Progression-free survival (PFS). Kaplan-Meier curve and percent survival over time by treatment group.
Figure 2.
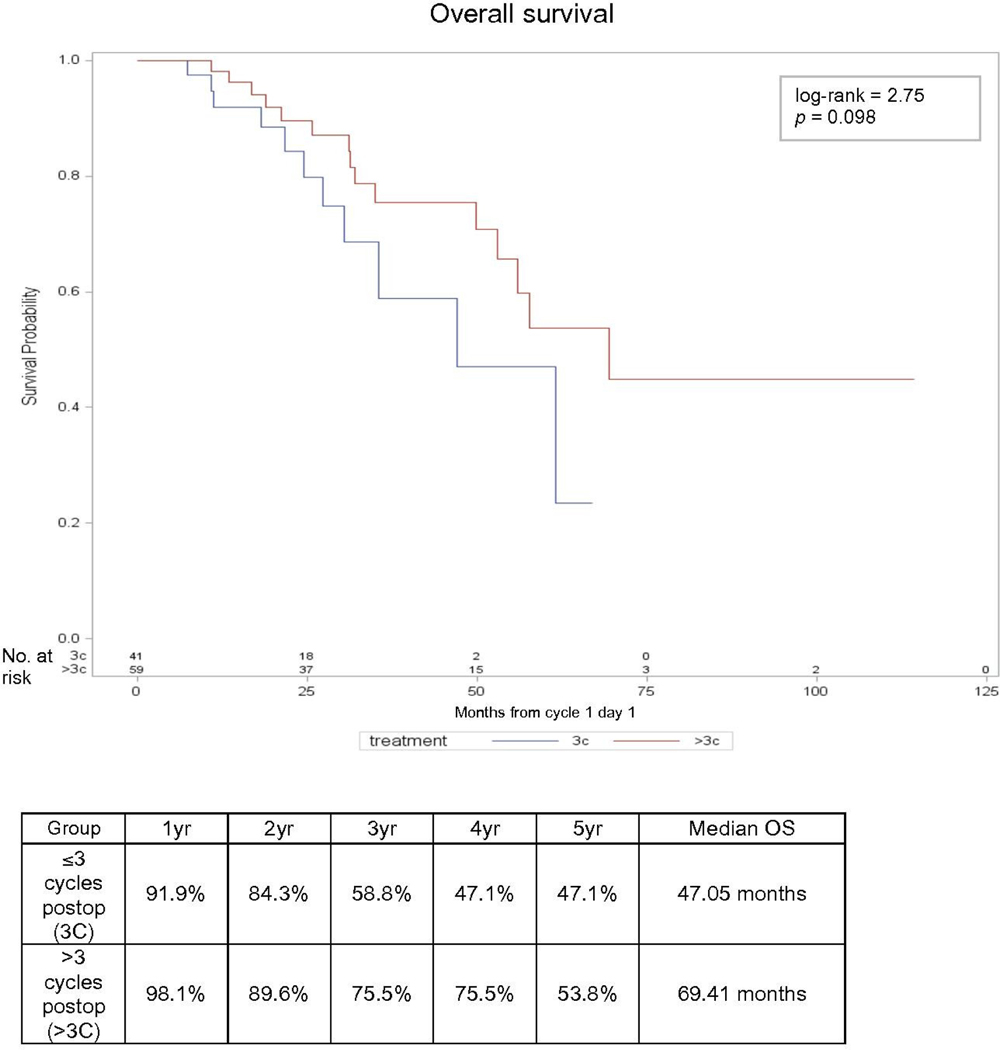
Overall survival (OS). Kaplan-Meier curve and percent survival over time by treatment group.
DISCUSSION
In this hypothesis-generating pilot study, we found that additional chemotherapy beyond three post-operative cycles did not impact progression-free survival or overall survival if CA125 was normalized after cycle three of post-operative chemotherapy, though a trend toward improvement in overall survival with more than three cycles was observed. Importantly, we excluded patients with residual disease by exam, imaging, and CA125 values following three post-operative chemotherapy cycles to address the research question of whether additional chemotherapy was beneficial in patients for whom the timing of stopping chemotherapy is least clear.
Neoadjuvant chemotherapy followed by interval cytoreductive surgery and post-operative chemotherapy is a valid approach for the treatment of advanced ovarian cancer in selected individuals.6 The optimal number of chemotherapy cycles – particularly in the post-operative setting following interval cytoreductive surgery – remains unclear. In the setting of upfront surgery followed by post-operative chemotherapy, the concept that more may be better has been explored in various forms: more upfront cycles,7 additional agents,10 maintenance cycles,11 and dose-dense regimens.12,13 Of these investigations, Japanese data support that more chemotherapy may translate into improved survival outcomes.12 In the setting of neoadjuvant chemotherapy followed by interval cytoreductive surgery and post-operative chemotherapy, there is also some suggestion that additional chemotherapy beyond six total cycles may be acceptable or beneficial. National Comprehensive Cancer Network (NCCN) guidelines recommend a minimum of six treatment cycles including at least three post-operative chemotherapy cycles.14 In addition, in the Japan Clinical Oncology Group 0602 trial,5 four cycles of both neoadjuvant chemotherapy and post-operative chemotherapy were given based on phase II feasibility data; the results of this trial are pending. A recent retrospective multi-institution analysis in Canada found no significant differences in overall survival for patients receiving zero to three versus four or more post-operative chemotherapy cycles following neoadjuvant chemotherapy and interval cytoreductive surgery.15 Our study showed similar findings in a selected population and may be better validated in a larger prospective setting.
Although this study was not designed to adequately determine toxicity outcomes, we examined these outcomes as pilot data. We observed no significant differences in grade 2 or higher toxicities between those receiving up to three or more than three post-operative chemotherapy cycles. These data were limited by missing documentation of these values, and further prospective study with a larger cohort would help clarify this issue. Even if additional chemotherapy in this setting would not confer greater immediate toxicity, extending post-operative chemotherapy to beyond three cycles may have detrimental effects – including a higher risk of platinum hypersensitivity,16 decreased bone marrow reserve, and potentially decreased quality of life – when administering chemotherapy at the time of future recurrences. These important considerations can help guide shared decision-making with patients and should be further investigated in a larger cohort.
The group receiving more than three post-operative chemotherapy cycles had a later normalization of their CA125 levels as well as a trend toward more residual disease at the time of interval cytoreductive surgery. This finding was used as a documented rationale for administering additional chemotherapy cycles beyond three post-operative chemotherapy cycles in eight patients. Documentation on the rationale for the number of chemotherapy cycles given was inconsistent and a recognized limitation to our available data. It should again be noted that all patients in our study had normal CA125 values by the completion of three post-operative chemotherapy cycles as we felt that those with persistently elevated values at this time point would represent a group with a poorer prognosis.17–19 The groups were, however, similar with respect to adverse prognostic factors. In addition, subgroup analyses examining factors that may confer a poorer prognosis, including cytoreduction status, did not demonstrate a significant difference in progression-free survival or overall survival between those receiving up to three post-operative cycles and those receiving more than three post-operative cycles.
Additionally, we acknowledge that although not statistically significant, there was a trend observed toward longer overall survival in the group receiving more than three post-operative chemotherapy cycles. It is unclear whether this observation reflects the population of patients who may have received more chemotherapy, both upfront and possibly at future recurrences, due to better treatment tolerance and better baseline health. Further study in a larger cohort is necessary.
Our study has several limitations, including incomplete data with regard to toxicities, selection bias regarding treatment duration given lack of a standard protocol, and the relatively small sample size given the exclusion criteria. The variation in chemotherapy regimens also may have caused some heterogeneity influencing toxicity and survival outcomes. Nonetheless, given the limited sample size, we felt inclusion of multiple standard platinum-based regimens was warranted. Given the paucity of data in this setting when this study was designed, we planned to use these results as pilot data and therefore a statistical sample size calculation was not performed. Another limitation is therefore that the study may not have been adequately powered to detect a significant difference in survival; as such we consider these pilot data hypothesis-generating only. There was notably a significant difference in length of follow-up between the two analyzed groups. Given that the group receiving more than three post-operative cycles which showed a trend toward improved overall survival had a longer follow-up duration, it is possible that longer follow-up in the group receiving up to three post-operative chemotherapy cycles may result in significant findings and is a limitation of our retrospective analysis. Strengths include our multicenter collaboration, increased generalizability due to inclusion of a variety of practice patterns and providers, and use of data from a specific population that presents a clinically challenging dilemma. Future potential areas of study include toxicity comparisons at the time of treatment for recurrent disease, and evaluation of time to development of platinum-resistant disease.
In conclusion, we found that in patients receiving neoadjuvant chemotherapy followed by interval cytoreductive surgery and post-operative chemotherapy, receipt of more than three cycles of post-operative chemotherapy was neither associated with improved survival nor with greater toxicity compared with receipt of up to three post-operative cycles. Our retrospective findings should be considered hypothesis-generating and prompt future study in a larger cohort with a longer follow-up period.
Highlights.
We included only patients with normal CA125 after three post-operative chemotherapy cycles.
No survival benefit was observed with more than three cycles compared with up to three post-operative chemotherapy cycles.
No subgroup was identified for which more chemotherapy is clearly beneficial.
Acknowledgements
The authors thank Chloe Krasnoff and Kaavya Raman for assistance with data abstraction.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Footnotes
Competing interests None declared.
Patient consent for publication Not required.
ethics approval Institutional Review Boards: NorthShore University HealthSystem (EH16–172), University of Chicago (13372B), University of California, Los Angeles (16–000350-CR-00002), University of California, Irvine (HS#2016–3058), CedarsSinai Medical Center (45573).
Provenance and peer review Not commissioned; externally peer reviewed.
data availability statement Data are available upon reasonable request.
ReFeRenCeS
- 1.Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943–53. [DOI] [PubMed] [Google Scholar]
- 2.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015;386:249–57. [DOI] [PubMed] [Google Scholar]
- 3.Melamed A, Hinchcliff EM, Clemmer JT, et al. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecol Oncol 2016;143:236–40. [DOI] [PubMed] [Google Scholar]
- 4.Fagotti A, Ferrandina G, Vizzielli G, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): final analysis of peri-operative outcome. Eur J Cancer 2016;59:22–33. [DOI] [PubMed] [Google Scholar]
- 5.Onda T, Satoh T, Saito T, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/ IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer 2016;64:22–31. [DOI] [PubMed] [Google Scholar]
- 6.Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:3460–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dizon DS, Weitzen S, Rojan A, et al. Two for good measure: six versus eight cycles of carboplatin and paclitaxel as adjuvant treatment for epithelial ovarian cancer. Gynecol Oncol 2006;100:417–21. [DOI] [PubMed] [Google Scholar]
- 8.Kim HS, Park N-H, Chung HH, et al. Are three additional cycles of chemotherapy useful in patients with advanced-stage epithelial ovarian cancer after a complete response to six cycles of intravenous adjuvant paclitaxel and carboplatin? Jpn J Clin Oncol 2008;38:445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertelsen K, Jakobsen A, Strøyer I, et al. A prospective randomized comparison of 6 and 12 cycles of cyclophosphamide, adriamycin, and cisplatin in advanced epithelial ovarian cancer: a Danish Ovarian Study Group trial (DACOVA). Gynecol Oncol 1993;49:30–6. [DOI] [PubMed] [Google Scholar]
- 10.Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the Gynecologic Cancer Intergroup. J Clin Oncol 2009;27:1419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei L, Chen H, Wei DM, et al. Maintenance chemotherapy for ovarian cancer. Cochrane Database Syst Rev 2013;(6):CD007414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dosedense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol 2013;14:1020–6. [DOI] [PubMed] [Google Scholar]
- 13.Chan JK, Brady MF, Penson RT, et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N Engl J Med 2016;374:738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. Version 2, 2018.
- 15.Altman AD, McGee J, May T, et al. Neoadjuvant chemotherapy and chemotherapy cycle number: a national multicentre study. Gynecol Oncol 2017;147:257–61. [DOI] [PubMed] [Google Scholar]
- 16.Markman M, Kennedy A, Webster K, et al. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol 1999;17:1141. [DOI] [PubMed] [Google Scholar]
- 17.Zeng J, Huang H, Shan Y, et al. The effect of CA125 nadir level on survival of advanced-stage epithelial ovarian carcinoma after interval debulking surgery. J Cancer 2017;8:3410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaznik-Wikiel ME, Sukumvanich P, Beriwal S, et al. Possible use of CA-125 level normalization after the third chemotherapy cycle in deciding on chemotherapy regimen in patients with epithelial ovarian cancer: brief report. Int J Gynecol Cancer 2011;21:1013–7. [DOI] [PubMed] [Google Scholar]
- 19.Gadducci A, Menichetti A, Guiggi I, et al. Correlation between CA125 levels after sixth cycle of chemotherapy and clinical outcome in advanced ovarian carcinoma. Anticancer Res 2015;35:1099–104. [PubMed] [Google Scholar]