Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes serious acute respiratory diseases including pneumonia and bronchitis with approximately 2.3% fatality occurrence.
Main body
This study argues the main concepts that need to be considered for the gradual reopening of dental offices include treatment planning approaches, fundamental elements needed to prevent transmission of SARS-CoV-2 virus in dental healthcare settings, personal protection equipment (PPE) for dental health care providers, environmental measures, adjunctive measures, and rapid point of care tests in dental offices.
Conclusion
This article seeks to provide an overview of existing scientific evidence to suggest a guideline for reopening dental offices.
Keywords: SARS-CoV-2, COVID-19, Dental clinics, Dentistry
Background and history
Coronaviruses (CoVs) are the largest group of known positive-sense RNA viruses with a variety of hosts in nature [1]. In the beginning, coronaviruses were thought to cause only enzootic infections in several animals, including a community of birds and mammals. However, current studies have shown that these viruses are infectious in humans [2, 3]. Seven major coronaviruses (CoVs) were recognized by 2020, including SARS-CoV-2. Within these 7 viruses, three of them (SARS-CoV, MERS-CoV, SARS-CoV-2) lead to serious respiratory syndromes with considerable death rates [4–6]. In 2002, SARS-CoV extended over five continents with a 10% death rate, and in 2012, MERS-CoV emerged with a 35% fatality rate in the Arabian Peninsula [7]. A novel coronavirus (SARS-CoV-2) causes serious acute respiratory diseases including pneumonia and bronchitis with approximately 2.3% fatality occurrence [8, 9]. The most prevalent signs and symptoms are cough (76%), fever (98%), myalgia or fatigue (44%), and dyspnea (55%). The SARS-CoV-2 incubation period has been reported to be 1–14 days, and asymptomatic individuals may also involve in the spread of this virus [10–13]. Due to the significant human-to-human route of contamination of these coronaviruses, dentists according to their close contact with patients are at danger of SARS-CoV-2 in dental procedures [5]. Even though all routine dental treatment in countries with SARS-CoV-2 infection has been postponed during the pandemic era, the need for emergency care provided by teams with sufficient personal protective equipment takes priority [14].
Treatment planning approaches
As a general principle, all non-emergent dental care for all individuals should be deferred during the pandemic crisis [15]. However, since the social demand for emergency care services even during this situation will be crucial [16], in situations that are considered an emergency according to the therapist’s judgment, the patient should be called to the office for the most possible conservative treatment [17, 18]. Dentists must be available to their patients for emergency care. Therefore, during pandemics, considering the infection control principles, our approach to treatments can be classified into four main categories:
Treatments that can be accomplished using their standard conventional approach.
Treatments that can be accomplished using a modified approach.
Interim treatments that are performed only to eliminate severe pain or potentially serious patient’s life- or health-threatening conditions so that definitive treatment can be accomplished later.
Treatments that should be avoided; instead, available alternative treatment options should be applied.
Recommendations for clinical interventions
If basic personal protection equipment (PPE), such as facemask and gloves, are not available, regardless of the urgency of the condition, any treatment cannot be proceeding.
For patients who are incapable of mouthrinsing (such as children), the application of a rubber dam for aerosol-generating procedures is recommended. In addition, cotton rolls soaking is a suitable substitute for the pre-procedural rinsing.
Avoid or minimize procedures that may induce coughing, such as taking intraoral x-rays. Instead, extra oral radiographs, such as panoramic and CBCT, are proper alternatives because they will not induce coughing.
Aerosol-generating treatments are strongly recommended to be avoided.
Manual instruments are an appropriate alternative to minimize the generation of aerosols.
Sometimes, for patients with a serious emergency condition, it may be an inevitable option to extract highly infectious teeth with a questionable prognosis that under normal circumstances could have had their prognosis improved by being treated.
Also, when definitive treatment is not possible due to infection control considerations, pulpotomy using MTA can be a good alternative for root canal therapy (RCT) for the management of symptomatic mature permanent teeth.
To minimize the generation of droplets and aerosols in cases that aerosol-generating treatments are irreplaceable, four-handed operation (Fig. 1), high evacuation ejector, and rubber dam can be beneficial.
Spatter-generating treatments are advised to be scheduled as the last appointments of the shift.
As a serious source of cross-infection, dental health care providers should know that backflow may occur during the use of a saliva ejector.
To minimize the need for recall appointments, the use of resorbable sutures is recommended.
Consider avoiding the application of instruments that are not easily disinfected (Table 1) [19–29].
Fig. 1.
Four-handed dentistry. Zones of activity for right-handed and left-handed dentists
Table 1.
Factors related to aerosol contamination
| Device, instruments, measures | Description |
|---|---|
| Ultrasonic and sonic scalers |
• They generate the highest rate of aerosols in a 6–12-in diameter from the operator. • The application of high volume evacuators reduces airborne contamination by 95%. |
| Air polishing |
• Airborne contamination of air polishing is almost equal to ultrasonic scalers. • The application of high-volume evacuator and/or aerosol reduction device reduces airborne contaminations up to 95%. |
| Air-water syringe |
• Airborne contamination of air-water syringes is almost equal to ultrasonic scalers. • However, the application of high-volume evacuators can reduce airborne contamination by nearly 99%. |
| High speed air turbine handpiece |
• An air-driven handpiece is powered by compressed air to spin the air-driven turbine. • Air-driven handpieces reach speeds of up to 400,000 rpm in a variety of torques. • The water flow speeds for turbines with one, two, and three coolant apertures are 42.38, 34.31, and 30.44 mL/min, respectively, or about 1.0 ml/s. • High-speed dental handpieces without anti-retraction valves aspirate the debris and fluids and contaminate the air and water systems of the unit which may lead to cross-infection. |
| Slow speed handpieces |
• The speed of their inbuilt motor can reach up to 80,000 rpm. • The average pressure for air and external water in these handpieces are about 0.25–0.3 (Mpa) and 198 (Kpa), respectively. • The average water flow in these handpieces is about 90–110 (min/ml). |
| Anti-retraction high-speed dental handpiece | • These handpieces reduce the microbial backflow into the tubes of the handpieces and dental units. |
| Electric motor handpieces | • Self-contained internal gearings in an electric motor handpiece enable it to function at a stable torque and speeds up to 200,000 rpm. |
| Tooth preparation with air abrasion | • Extensive microbial contamination with abrasive particles has been demonstrated. |
| Aerosol reduction device | • Aerosol reduction devices such as Jet Shield (DENTSPLY SIRONA INC., USA) can reduce the contamination up to 97% during air polishing. |
| Rubber dam |
• Rubber dams minimize the formation of the blood- and saliva-contaminated aerosols. • The application of rubber dam reduces airborne particles in ~ 1-meter diameter of the source of particle production by 70%. |
| High-volume evacuator |
• High-volume evacuators can efficiently reduce the number of microorganisms, blood, and material released into the air. • Since a small-bore saliva ejector is not an adequate substitute, when a four-handed operation is not an option, utilization of a high-volume evacuator attached to the instrument is necessary. • The use of these types of evacuators when the utilization of a rubber dam is impractical can be highly beneficial. •Researches indicated that the application of high-velocity evacuators with air polishers can reduce CFUs about 94.8%. |
| Ultraviolet radiation |
• Ultra-violet radiation can be considered as a highly fungicidal, viricidal, and bactericidal agent via damaging DNA and denaturation of proteins. • The International Ultraviolet Association (IUVA) stated that UV disinfection can reduce the transmission of the SARS-CoV-2 in air, water, and on surfaces. |
| Patient and dentist position | • The patient in the supine position enables the dental team to stay away from the patient’s breathing way. |
| Ventilation and air-conditioning system | • To reduce environmental contamination and prevent contaminated air circulation |
| Microbiological control of unit water system | • Periodic disinfection of the unit water system via application of chemicals and distilled water. |
| Preprocedural mouth-rinse |
• The efficiency of chlorhexidine on SARS-CoV-2 has not yet been studied. However, the use of 1% hydrogen peroxide or 0.2% povidone-iodine is recommended. • Prophylactic administration of mouthwash reduces the microbial load in the oral cavity. |
| Manual instruments | • Manual instruments are recommended to minimize the aerosol generation. |
Fundamental elements needed to prevent transmission of SARS-CoV-2 virus in dental healthcare settings
Administrative measures
Administrative measures are all infrastructures to implement, guide, support, and monitor adherence to standard and Transmission-Based Precautions. A key administrative measure is provision of necessary and sufficient fiscal and human resources for maintaining infection control (IC) programs. Specific components include adequate staff, inclusion of infection control practices (ICP) in dental facility construction and design decisions, adequate supplies, equipment, and compliance monitoring [30].
Infection control practice landscape in dental settings
Studies show that in general, knowledge and attitudes regarding infection control are good; however, the compliance and practice levels regarding the same are low [31–36]. Findings indicate that lack of compliance with ICP is multifactorial, and compliance with recommended IC guidelines is challenging, and the results of some studies indicate that compliance is achievable, even in medium and large group practices [32, 37–39].
Organizational and individual factors that affect ICP compliance
A review of the literature concluded that variations in organizational factors (e.g., safety climate, policies and procedures, education and training, adequate resourcing, innovation culture, staff education, and adequate highly trained and experienced staff) and individual factors (e.g., knowledge, perceptions of risk, and past experience) were determinants of adherence to ICP for protection against SARS and other respiratory pathogens [40–43].
Education of dental health care providers (DHCP)
A study on Hong Kong hospital workers demonstrated that the likelihood of SARS infection was strongly associated with having less than 2 h of infection control (IC) training and not understanding infection control procedures [44]. It is important to realize that IC education and training goal are not a simple memorization of protocols. Further attempts to fill the gap between knowledge and practice change should be made [43]. Implementing problem-based learning, evidence-based practice methods, practical demonstration and participation actions, incorporating individual experience, and hands-on training is associated with decreased healthcare-associated infections (HAI) and hand hygiene compliance [45–47]. Strong recurrent HCP education and training with the aim to reduce specific types of infections is effective for guideline implementation [47].
Personal protection equipment (PPE) for DHCP
Personal protection equipment (PPE) reduces the risk of contamination, and healthcare workers should take this issue seriously [48]. For SARS-CoV-2, recommendations for PPE are masks, respirators, gloves, goggles or face shields, and long gowns [49, 50]. More body coverage leads to better protection. Donning and doffing of PPE should be easy [51] since the complexity of use leads to an increased risk of self-contamination especially during doffing [52]. The correct sequence of donning and doffing is depicted in Figs. 2, 3, 4, and 5.
Fig. 2.
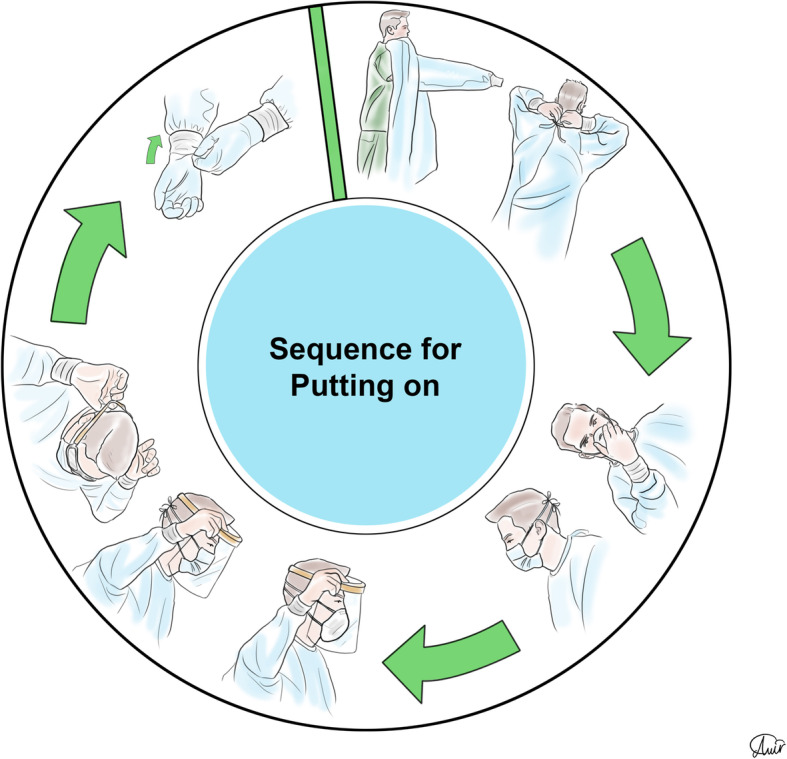
Personal protection equipment donning order
Fig. 3.
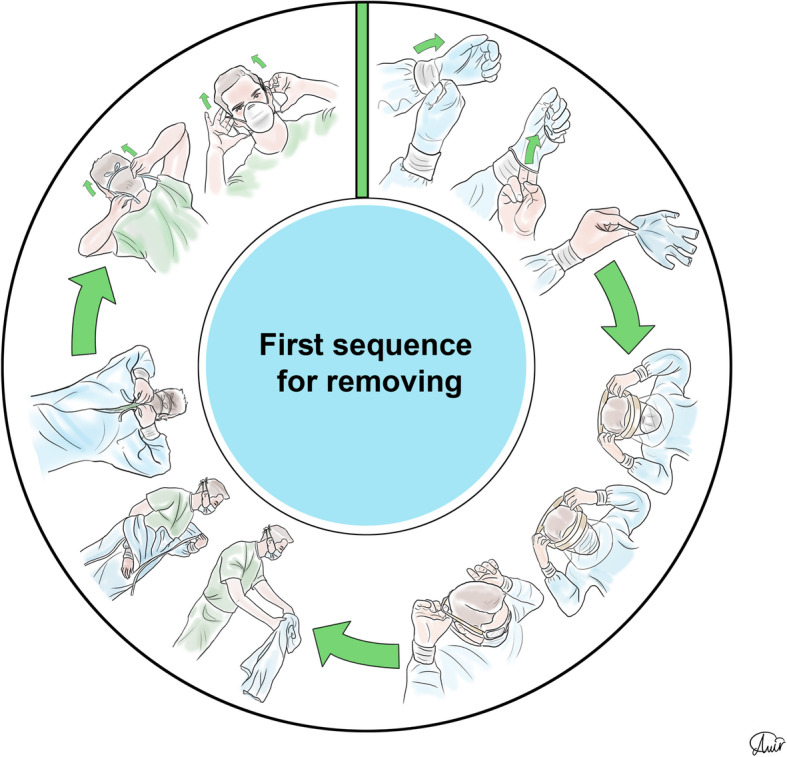
Personal protection equipment doffing first order
Fig. 4.

Personal protection equipment doffing second order
Fig. 5.
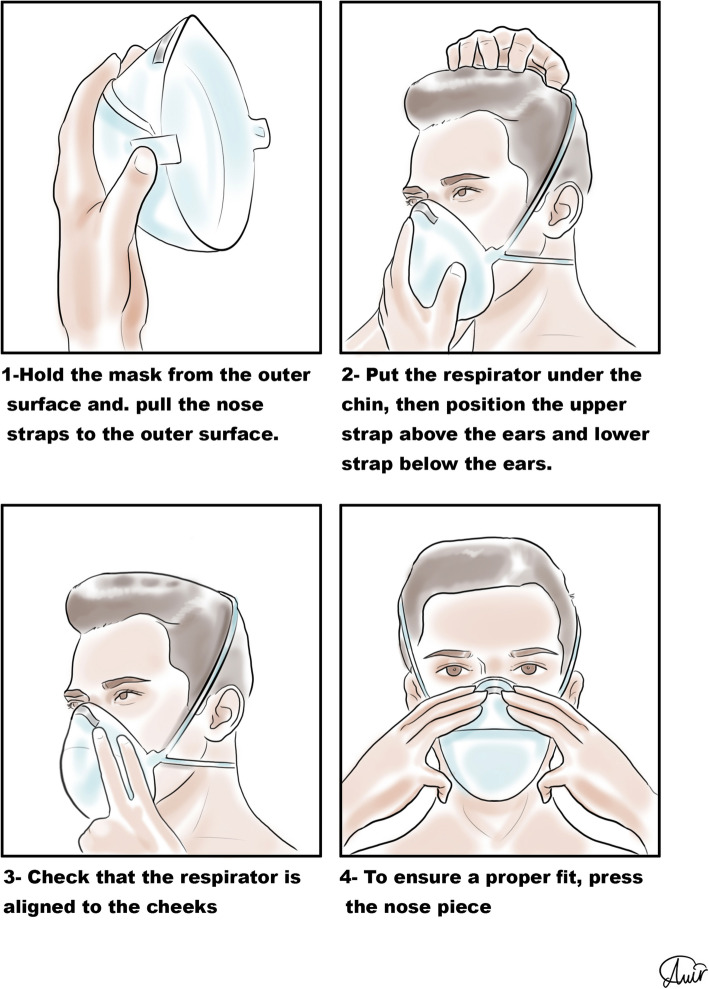
Correct way of putting on and removing a respirator
Masks and respirators
Waterproof surgical masks prevent the spread of respiratory droplets in the environment and protect staff against both infected droplets and contact contamination. Also, they reduce the risk of SARS-CoV-2 contamination by at least 80% [2]. Filtering facepiece respirators (FFRs) including N95 respirators is known as effective and protective equipment that their filtration has been achieved via a network of polypropylene microfibers and electrostatic charges [53]. A meta-analysis revealed that there is no statistically significant difference between surgical masks and facepiece respirators such as FFPs and N95 in terms of protection against airborne viral infections (RR = 0.89, p > 0.05) [54]. Powered air-purifying respirator (PAPR) is also recommended for protection against SARS-CoV-2 [55]. However, due to the electronic nature of this device and the possibility of damage to the electronic parts of it, it is recommended to use it simultaneously with a filtering facepiece respirator [56]. Reusable elastomeric respirators are not commonly used in health care settings and are used widely in the industry and are available in full-face, half-face, and quarter-face models [57]. Comparisons between different masks and respirators are shown in Table 2 [57].
Table 2.
A brief comparison between masks and respirators
| Mask type | Standard | Filtration effectiveness | Re-usability | ||
|---|---|---|---|---|---|
| Single-use medical masks | China: YY/T0969 |
3.0 microns: > 95% 0.1 microns: not effective |
No | ||
| Surgical masks | China : YY 0469 |
3.0 microns: > 95% 0.1 microns: > 30% |
No | ||
| Surgical masks | USA: ASTM F2100 | Level 1 | Level 2, 3 | No | |
|
3.0 microns: > 95% 0.1 microns: > 95% |
3.0 microns: > 95% 0.1 microns: > 95% |
||||
| Surgical masks | Europe: EN 14683 | Type 1 | Type 2,3 | No | |
|
3.0 microns: > 95% 0.1 microns: > 95% |
3.0 microns: > 95% 0.1 microns: > 95% |
||||
| Respirator masks | USA: NIOSH 42 CFR 84 | N95 | N99 | N100 | Yes (under especial conditions) |
| 0.3 microns: > 95% | 0.3 microns: > 99% | 0.3 microns: > 99.97% | |||
| Respirator masks | Europe: EN 149: 2001 | FFP1 | FFP2 | FFP3 | Yes (under especial conditions) |
| 0.3 microns : >80% | 0.3 microns : >94% | 0.3 microns : >99% | |||
| Elastomeric respirators | USA: NIOSH 42 CFR 84 | 10 to 50 APF | Yes | ||
| PAPR | USA: NIOSH 42 CFR 84 | 1000 APF | Yes | ||
PAPR powered air-purifying respirator. APF assigned protection factor
Due to the SARS-CoV-2 pandemic and the reduction in access to face masks and respirators such as the N95, the CDC recommends methods for extended use and reuse of them [58]. For extended use, the CDC recommends using an N95 respirator for up to 8 h; however, it is recommended to follow the manufacturer’s instructions. Based on CDC, it should be noted that FFRs can be reused up to 5 times via the following strategies:
Mask rotation: In this technique, the masks must be numbered and used in turn. The minimum time for not using a used mask should be at least 72 h, as the SARS-CoV-2 loses its viability. However, if a mask is damaged or used in the aerosol-generating process, it should be discarded.
Reprocessing/decontamination: Hydrogen peroxide vaporization can be used on N95 models that do not contain cellulose, such as the 1860 model. Also, methods such as proper UV treatment of N95 masks, moist heat (heating at 60–70 °C and 80–85% relative humidity), and dry heating of the mask at 70 °C for 30 min can be used for decontamination; however, dry and moist heat is not currently recommended for SARS-CoV-2.
Gowns
Different qualities have been reported for gowns [59]. Most models of isolation gowns often leave the neck exposed, which can be a route of contamination [60]. The most protection is assigned to coveralls followed by long gowns, gowns, and aprons, respectively [51]. According to the studies, modified gowns with attached gloves, cover the wrist area, and gowns that fit tightly at the neck area reduce the risk of contamination in the best way [51]. It is also recommended that the gowns be removed simultaneously with the gloves [51].
Gloves
Adding tabs to the gloves for taking them off from the hands reduces the risk of contamination [51]. Studies showed that the risk of contamination using double or triple gloves is less than single glove. Also, donning three layers of gloves due to the complex doffing process is not suggested due to more risk of self-contamination [61, 62]. Cleaning of gloves with hypochlorite or quaternary ammonium except alcohol-based hand rubs may decrease hand contamination [51]. Dentists should use arm-length surgical gloves (Fig. 6) [63].
Fig. 6.

Arm-length surgical gloves that completely cover the wrist area
Eye protectors
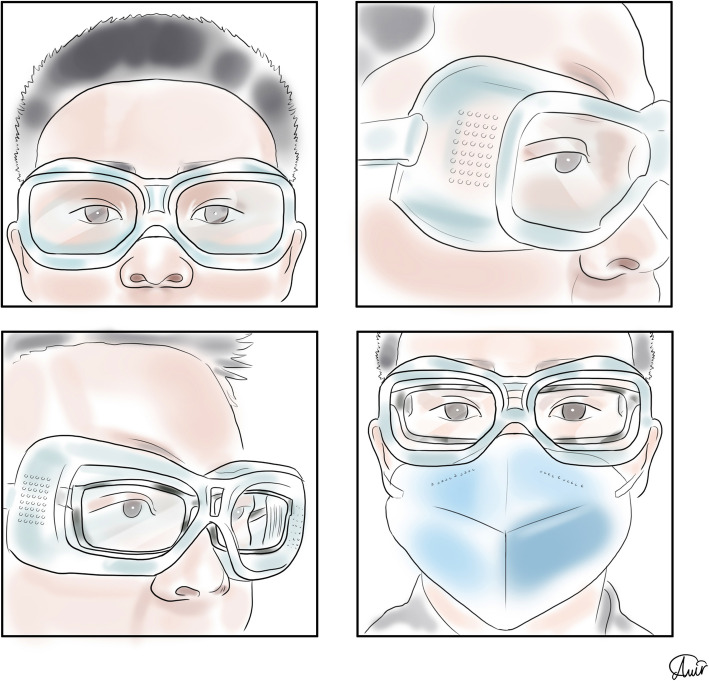
Lindsley et al. used breathing and coughing simulators to determine the efficacy of face shields in reducing contamination. They proved that face shields are effective in reducing the exposure to large infectious particles, but smaller particles are able to remain airborne and flow around a face shield to be inhaled [64]. Face shields are more bulky than goggles and protect the entire face [64]. Figure 7 shows a standard eye protector providing full eye seal.
Fig. 7.
A proper goggles provide a complete eye seal
Hand hygiene
It has been shown that hand hygiene does not provide an adequate defensive response to viruses without the use of face masks [65]. Ethanol is widely used in the world for hand rubbing in various forms including gels and foams [66]. Also, using alcohol-based disinfectants are promising substances to protect healthcare workers against SARS-CoV-2 [67]. The mechanism of alcohol-based sanitizers is denaturing proteins so that enveloped viruses including coronaviruses are removed by using these sanitizers [68]. Reports demonstrated that alcohol-based hand rubs could contain at least 60% ethanol to provide effective protection [69]. In 5 moments, healthcare workers should consider hand rubbing seriously: before touching a patient, before aseptic treatments, after exposure to body fluids, after touching a patient, and after touching the patients’ surroundings (Fig. 8) [70].
Fig. 8.
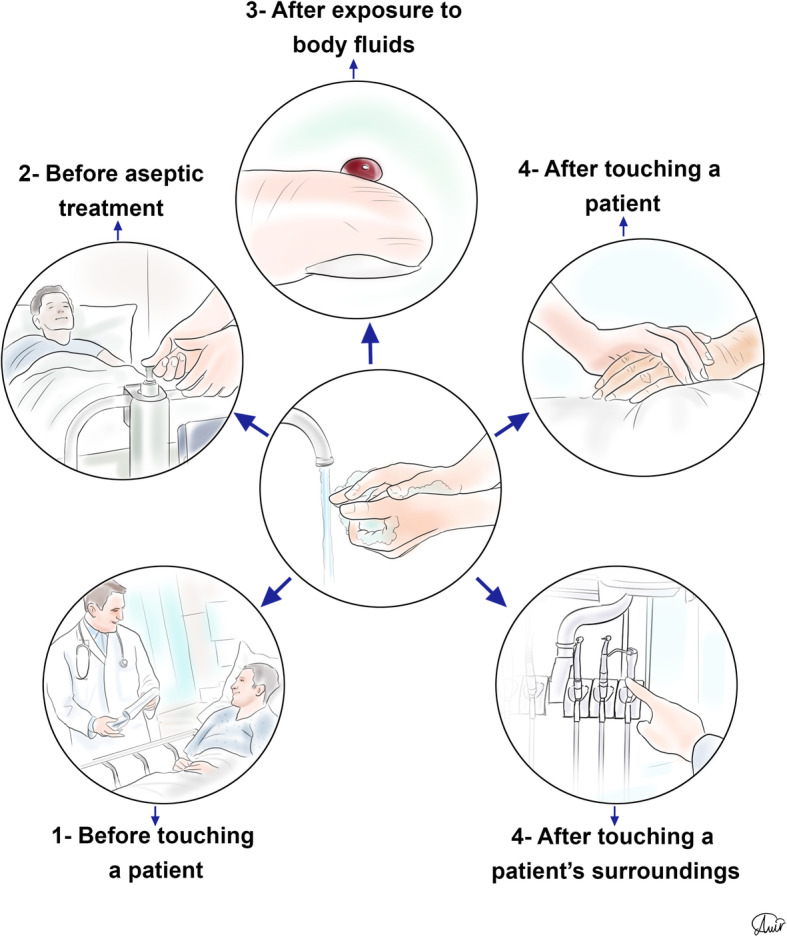
Five main times that hand hygiene should be considered seriously
Environmental measures
Cleaning and disinfecting non-critical surfaces in patient-care areas are part of standard precautions. In general, these procedures do not need to be changed for patients on transmission-based precautions and are appropriate for SARS-CoV-2 in healthcare settings, including those patient-care areas in which aerosol-generating procedures are performed. The cleaning and disinfection of all patient-care areas are important for frequently touched surfaces, especially those closest to the patient, that are most likely to be contaminated (e.g., dental chair, cabinets, doorknobs, desks, elevators, bathroom sinks, surfaces, and equipment in close proximity to the patient). The frequency or intensity of cleaning may need to change based on the patient’s level of hygiene and the degree of environmental contamination and for certain for infectious agents whose reservoir is the intestinal tract [71, 30, 72, 73]. A summary of the substances used for disinfecting and cleaning is presented in Table 3 [74–87].
Table 3.
Methods of disinfecting non-critical surfaces in patient-care areas
| Disinfecting non-critical surfaces in patient-care areas | |||
|---|---|---|---|
| Vaporized hydrogen peroxide | Disinfectants | ||
| Types | Virucidal efficacy | Hypochlorous acid (HOCl) | Other disinfectants |
| Non-condensing vaporized hydrogen peroxide (VHP) technology (Steris) and condensing search hydrogen peroxide vapour (HPV) technology (Bioquell) | Limited evidence is available for the virucidal activity of condensing HPV systems. Recently, several studies have demonstrated the in vitro activity of condensing HPV systems against individual viruses, including feline calicivirus (FCV), adenovirus, lactococcal bacteriophages6, and MS2 coliphage | Virucidal efficacy | Alkalis, oxidizing agents, alcohols, and aldehydes |
|
• Virucidal ability of solutions containing a high amount of HOCl is better than those containing HCl • Reduction of efficacy after spraying from a distance more than 30 cm • Minimum concentration should be more than 40 ppm for effective virucidal effect • The 100 and 200 ppm concentrated solutions inactivated more than 99.9% of AIV directly after spraying, while the 50 ppm concentration required at least 3 min of contact | |||
Adjunctive measures
Prophylactic medication for dental health care providers
Currently, there is no available and reliable evidence to support the prophylactic use of a medication(s) for dental health care providers, although a handful of trials in the world are being conducted to keep health care providers and vulnerable people safe from SARS-CoV-2 during the pandemic. Until further information, the focus of the dental health care providers should be on maximum application of safety regulations and recommendations by their local dental boards.
Immunization
By far, three different types of coronaviruses (SARS-CoV, MERS-CoV, and SARS-CoV-2) have considerably affected global health in about 20 years; nonetheless, there is still no approved vaccines for these viruses [88]. Although currently numerous preclinical and clinical trials are being conducted with some promising results [88], it is noteworthy that one major drawback is that RNA viruses usually have higher mutation rates compared to DNA viruses, resulting in challenges for vaccine development [89]. Nevertheless, numerous pharmaceutical companies are actively involved in the different stages of vaccine development. In case of the development of an effective vaccine, dental health care providers, unarguably, should be among the first groups of professionals who receive the vaccine.
Rapid point of care tests in dental offices
Currently, two major types of testing are available according to centers for disease control and prevention: viral testing and antibody testing indicating if the person does have a current infection or indicating if there was a previous infection, respectively. American Dental Association newsletter on April 17, 2020 urged dentists to be cautious about using novel coronavirus diagnostic tests before they have been properly evaluated and made available for dentists. Meanwhile, American Dental Association has sought federal recognition that licensed dentists may administer point of service tests authorized by the Food and Drug Administration (FDA) for novel coronavirus (SARS-CoV-2); however, because of the medical demand, currently, the medical suppliers are not planning on providing point-of-care tests to dentists in the very near future. It is noteworthy that at the present time, American Dental Association does not consider SARS-CoV-2 testing to be a scope of practice issue for dental offices according to ADA newsletter abovementioned. Although there is currently no FDA-approved or cleared test to diagnose or detect SARS-CoV-2, findings of some studies [90–93] may direct the future research into cheaper, faster, and more effective testing methods that would be available for the dental practices. A study conducted in a Hong Kong hospital reported consistent detection of coronavirus in the saliva of patients admitted from the first day that they were hospitalized [94]. In order to collect the samples, patients were instructed to cough out saliva from the throat into a sterile container that was later sent to the lab for analysis. This study underscored the advantage of simple and safe saliva sampling in a pandemic situation that can be actually utilized safely not only in the dental offices but also in anywhere including but not limited to busy clinics, airports, etc. [90]. It is of paramount importance that any testing method must strongly reduce the risk of SARS-CoV-2 transmission. Since Saliva may play a crucial role in the human-to-human transmission, salivary diagnostics might be an easy and cost-effective point-of-care platform for SARS-CoV-2 diagnosis because saliva self-collection will likely reduce the risk of SARS-CoV-2 transmission [91]. Additionally, the nasopharyngeal and oropharyngeal collection results in discomfort and possible bleeding especially in infected patients with thrombocytopenia that is potentially dangerous [91]. Our profession needs to emerge from this pandemic situation and probably enter a new world so maybe this testing method would become one of the routines in our daily practices.
Conclusion
This article seeks to provide an overview of existing scientific evidence to suggest a guideline for reopening dental offices. We believe that studying this article and paying attention to its instructions can provide readers with an overview of the arrangements that should be considered for the gradual reopening of dental offices.
Acknowledgements
Not applicable
Abbreviations
- ADA
American Dental Association
- APF
Assigned Protection Factor
- CDC
Centers for Disease Control and Prevention
- CFR
Code of Federal Regulations
- CBCT
Cone-beam computed tomography
- CoVs
Coronaviruses
- DHCP
Dental health care providers
- DNA
Deoxyribonucleic acid
- FCV
Feline calicivirus
- FFRs
Filtering facepiece respirators
- FFP
Filtering facepiece
- FDA
Food and Drug Administration
- HAI
Healthcare-associated infections
- HCP
Health care providers
- HC1
Hydrochloric acid
- HPV
Hydrogen peroxide vapour
- HOC1
Hypochlorous acid
- IC
Infection control
- ICP
Infection control practices
- IUVA
International Ultraviolet Association
- KPa
Kilo pascal
- MPa
Mega pascal
- MERS-CoV
Middle East respiratory syndrome coronavirus
- MTA
Mineral trioxide aggregate
- NIOSH
National Institute for Occupational Safety and Health
- PAPR
Powered air-purifying respirator
- RNA
Ribonucleic acid
- ppm
Part per million
- PPE
Personal protection equipment
- RCT
Root canal therapy
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SARS-CoV
Severe acute respiratory syndrome-related coronavirus
- VHP
Vaporized hydrogen peroxide
- UV
Ultraviolet
Authors’ contributions
SOK, HRF, AM, VK, PM, and OM contributed substantially to the conception and design of the study and the acquisition of data. BC, PF, and BH provided critical revision of the article and provided final approval of the version to publish. BC as the corresponding author verify that all individuals who made contributions to this study are included either as authors or are acknowledged at the end of the paper. The authors read and approved the final manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due but are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Seied Omid Keyhan, Email: keyhanomid@ymail.com.
Hamid Reza Fallahi, Email: dr.hamidrezafallahi@gmail.com.
Amin Motamedi, Email: draminmotamedi@gmail.com.
Vahid Khoshkam, Email: khoshkam@usc.edu.
Paymon Mehryar, Email: Mehryar.paul@gmail.com.
Behzad Cheshmi, Email: Beh.cheshomi@gmail.com.
Parsa Firoozi, Email: Parsafir2@gmail.com.
Parisa Yousefi, Email: dr.parisa_ysf@yahoo.com.
Behzad Houshmand, Email: info@drbhoushman.com.
References
- 1.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (2020) Infection prevention and control during health care when COVID-19 is suspected: interim guidance, 19 March 2020. [https://www.who.int/publications-detail-redirect/10665-331495]
- 3.Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry M, Gamieldien J, Fielding BC. Identification of new respiratory viruses in the new millennium. Viruses. 2015;7(3):996–1019. doi: 10.3390/v7030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020;12(1):9. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 7.Tartaglia GM, Kumar S, Fornari CD, Corti E, Connelly ST. Mouthwashes in the 21(st) century: a narrative review about active molecules and effectiveness on the periodontal outcomes. Expert Opin Drug Deliv. 2017;14(8):973–982. doi: 10.1080/17425247.2017.1260118. [DOI] [PubMed] [Google Scholar]
- 8.Eurosurveillance Editorial T. Note from the editors: novel coronavirus (2019-nCoV) Eurosurveillance. 2020;25(3):2001231. doi: 10.2807/1560-7917.ES.2020.25.3.2001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao WM, Song SH, Chen ML, Zou D, Ma LN, Ma YK, et al. The 2019 novel coronavirus resource. Yi Chuan. 2020;42(2):212–221. doi: 10.16288/j.yczz.20-030. [DOI] [PubMed] [Google Scholar]
- 10.Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Euro Surveill. 2020;25(5):2000062. doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng VCC, Wong SC, Chen JHK, Yip CCY, Chuang VWM, Tsang OTY, et al. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol. 2020;41(5):493–498. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.W-j G, Ni Z-y HY, Liang W-h, C-q O, He J-x, et al. Clinical characteristics of 2019 novel coronavirus infection in China. MedRxiv. 2020;2020(2002):2006.20020974. [Google Scholar]
- 13.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coulthard P. Dentistry and coronavirus (COVID-19) - moral decision-making. Br Dent J. 2020;228(7):503–505. doi: 10.1038/s41415-020-1482-1. [DOI] [PubMed] [Google Scholar]
- 15.Fallahi HR, Keyhan SO, Zandian D, Kim S-G, Cheshmi B. Being a front-line dentist during the Covid-19 pandemic: a literature review. Maxillofac Plast Reconstr Surg. 2020;42:1–9. doi: 10.1186/s40902-020-00256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alharbi A, Alharbi S, Alqaidi S. Guidelines for dental care provision during the COVID-19 pandemic. Saudi Dent J. 2020;32(4):181–186. doi: 10.1016/j.sdentj.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dave M, Seoudi N, Coulthard P. Urgent dental care for patients during the COVID-19 pandemic. Lancet. 2020;395(10232):1257. doi: 10.1016/S0140-6736(20)30806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo H, Zhou Y, Liu X, Tan J (2020) The impact of the COVID-19 epidemic on the utilization of emergency dental services. J Dent Sci. 10.1016/j.jds.2020.02.002 [DOI] [PMC free article] [PubMed]
- 19.International Ultraviolet Association (IUVA) (2020) IUVA Fact Sheet on UV Disinfection for COVID-19 http://www.iuva.org/. [http://www.iuva.org/IUVA-Fact-Sheet-on-UV-Disinfection-for-COVID-19#]
- 20.Barnes JB, Harrel SK, Rivera-Hidalgo F. Blood contamination of the aerosols produced by in vivo use of ultrasonic sealers. J Periodontol. 1998;69(4):434–438. doi: 10.1902/jop.1998.69.4.434. [DOI] [PubMed] [Google Scholar]
- 21.Bentley CD, Burkhart NW, Crawford JJ. Evaluating spatter and aerosol contamination during dental procedures. J Am Dent Assoc. 1994;125(5):579–584. doi: 10.14219/jada.archive.1994.0093. [DOI] [PubMed] [Google Scholar]
- 22.Cavalcanti BN, Serairdarian PI, Rode SM. Water flow in high-speed handpieces. Quintessence Int. 2005;36(5):361–364. [PubMed] [Google Scholar]
- 23.FMC, Hertford House (2018) A practice manager’s guide to dental handpieces www.dentistry.co.uk. [https://www.dentistry.co.uk/2018/08/22/practice-managers-guide-dental-handpieces/]
- 24.Harrel SK, Barnes JB, Rivera-Hidalgo F. Aerosol reduction during air polishing. Quintessence Int. 1999;30(9):623–628. [PubMed] [Google Scholar]
- 25.Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135(4):429–437. doi: 10.14219/jada.archive.2004.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller R, Micik R. Air pollution and its control in the dental office. Dent Clin North Am. 1978;22(3):453–476. [PubMed] [Google Scholar]
- 27.Muzzin KB, KING TB, BERRY CW. Assessing the clinical effectiveness of an aerosol reduction device for the air polisher. J Am Dent Assoc. 1999;130(9):1354–1359. doi: 10.14219/jada.archive.1999.0407. [DOI] [PubMed] [Google Scholar]
- 28.Pîrvu C, Pătraşcu I, Pîrvu D, Ionescu C. The dentist’s operating posture–ergonomic aspects. J Med Life. 2014;7(2):177. [PMC free article] [PubMed] [Google Scholar]
- 29.Bureau of Naval Personnel (1963) Advanced Speeds in Operative Dentistry. [https://books.google.com/books?id=HxCVxwEACAAJ]
- 30.Jane D, Siegel M, Rhinehart E, Jackson M, Chiarello L. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. 2007. Am J Infect Control. 2007;35:S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Maweri SA, Tarakji B, Shugaa-Addin B, Al-Shamiri HM, Alaizari NA, AlMasri O (2015) Infection control: Knowledge and compliance among Saudi undergraduate dental students. GMS Hyg Infect Control 10:Doc10 [DOI] [PMC free article] [PubMed]
- 32.Alharbi G, Shono N, Alballaa L, Aloufi A. Knowledge, attitude and compliance of infection control guidelines among dental faculty members and students in KSU. BMC Oral Health. 2019;19(1):7. doi: 10.1186/s12903-018-0706-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binalrimal S, AlDrees A, AlWehaibi M, AlAsmary M, AlShammery A, AlHaidri E, et al. (2019) Awareness and compliance of dental students and interns toward infection control at Riyadh Elm University. GMS Hyg Infect Control 14:Doc10 [DOI] [PMC free article] [PubMed]
- 34.Ghimire B, Chandra S. Awareness of infection control among dental students and interns. JNMA J Nepal Med Assoc. 2018;56(210):598–601. [PMC free article] [PubMed] [Google Scholar]
- 35.Mutters NT, Hägele U, Hagenfeld D, Hellwig E, Frank U (2014) Compliance with infection control practices in an university hospital dental clinic. GMS Hyg Infect Control 9(3):Doc18 [DOI] [PMC free article] [PubMed]
- 36.Porter S, El-Maaytah M, Afonso W, Scully C, Leung T. Cross-infection compliance of UK dental staff and students. Oral Dis. 1995;1(4):198–200. doi: 10.1111/j.1601-0825.1995.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 37.Anders PL, Townsend NE, Davis EL, McCall W., Jr Observed infection control compliance in a dental school: A natural experiment. Am J Infect Control. 2016;44(9):153–156. doi: 10.1016/j.ajic.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 38.Harte JA, Charlton DG. Characteristics of infection control programs in US Air Force dental clinics: a survey. J Am Dent Assoc. 2005;136(7):885–892. doi: 10.14219/jada.archive.2005.0289. [DOI] [PubMed] [Google Scholar]
- 39.Porteous NB, Bizra E, Cothron A, Yeh C-K. A survey of infection control teaching in US dental schools. J Dent Educ. 2014;78(2):187–194. [PubMed] [Google Scholar]
- 40.Barker AK, Brown K, Siraj D, Ahsan M, Sengupta S, Safdar N. Barriers and facilitators to infection control at a hospital in northern India: a qualitative study. Antimicrob Resist Infect Control. 2017;6(1):35. doi: 10.1186/s13756-017-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dimick JB, Swoboda SM, Pronovost PJ, Lipsett PA. Effect of nurse-to-patient ratio in the intensive care unit on pulmonary complications and resource use after hepatectomy. Am J Crit Care. 2001;10(6):376. [PubMed] [Google Scholar]
- 42.Garland KV. A survey of United States dental hygienists' knowledge, attitudes, and practices with infection control guidelines. J Am Dent Hyg Assoc. 2013;87(3):140–151. [PubMed] [Google Scholar]
- 43.Munksgaard Danmark (2006) En revurdering af demens: personen kommer i første række. [https://munksgaard.dk/products/en-revurdering-af-demens-bog-16147-9788762804401]
- 44.Lau JT, Fung KS, Wong TW, Kim JH, Wong E, Chung S, et al. SARS transmission among hospital workers in Hong Kong. Emerg Infect Dis. 2004;10(2):280. doi: 10.3201/eid1002.030534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berta W, Ginsburg L, Gilbart E, Lemieux-Charles L, Davis D. What, why, and how care protocols are implemented in Ontario nursing homes. Can J Aging. 2013;32(1):73–85. doi: 10.1017/S0714980813000081. [DOI] [PubMed] [Google Scholar]
- 46.Dekker M, Caris MG, Van Gunsteren AM, Van Mansfeld R, Lucas C, Vandenbroucke-Grauls CM. Effectiveness of a behavioral approach to improve healthcare worker compliance with hospital dress code. Infect Control Hosp Epidemiol. 2017;38(12):1435–1440. doi: 10.1017/ice.2017.233. [DOI] [PubMed] [Google Scholar]
- 47.Storr J, Twyman A, Zingg W, Damani N, Kilpatrick C, Reilly J, et al. Core components for effective infection prevention and control programmes: new WHO evidence-based recommendations. Antimicrob Resist Infect Control. 2017;6(1):6. doi: 10.1186/s13756-016-0149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cook T. Personal protective equipment during the coronavirus disease (COVID) 2019 pandemic – a narrative review. Anaesthesia. 2020;75(7):920–927. doi: 10.1111/anae.15071. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization (2020) Advice on the use of masks in the community, during home care and in healthcare settings in the context of the novel coronavirus ( 2019-nCoV) outbreak: interim guidance, 29 January 2020. [https://apps.who.int/iris/handle/10665/330987]
- 50.World Health Organization (2020) Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages: interim guidance, 6 April 2020. [https://apps.who.int/iris/handle/10665/331695]
- 51.Verbeek JH, Rajamaki B, Ijaz S, Sauni R, Toomey E, Blackwood B, et al. (2020) Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev 4(4):Cd011621 [DOI] [PMC free article] [PubMed]
- 52.Roberge RJ. Evaluation of the rationale for concurrent use of N95 filtering facepiece respirators with loose-fitting powered air-purifying respirators during aerosol-generating medical procedures. Am J Infect Control. 2008;36(2):135–141. doi: 10.1016/j.ajic.2007.04.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Respiratory protective devices — Filtering halfmasks to protect against particles —Requirements, testing,marking (2001). [http://www.nobelcert.com/DataFiles/FreeUpload/EN%20149-2001%20plus%20A1-2009.pdf]
- 54.Long Y, Hu T, Liu L, Chen R, Guo Q, Yang L, et al. Effectiveness of N95 respirators versus surgical masks against influenza: a systematic review and meta-analysis. J Evid Based Med. 2020;13(2):93–101. doi: 10.1111/jebm.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saadi RA, Bann DV, Patel VA, Goldenberg D, May J, Isildak H. A commentary on safety precautions for otologic surgery during the COVID-19 pandemic. Otolaryngol Head Neck Surg. 2020;162(6):797–799. doi: 10.1177/0194599820919741. [DOI] [PubMed] [Google Scholar]
- 56.Roberge MR, Vojtko MR, Roberge RJ, Vojtko RJ, Landsittel DP. Wearing an N95 respirator concurrently with a powered air-purifying respirator: effect on protection factor. Respir Care. 2008;53(12):1685–1690. [PubMed] [Google Scholar]
- 57.National Academies of Sciences. Reusable elastomeric respirators in health care: considerations for routine and surge use. Washington (DC): National Academies Press (US); 2018 Dec 6. 3, Implementing Reusable Elastomeric Respirators in Health Care Settings: Routine and Surge Use (2019). [https://www.ncbi.nlm.nih.gov/books/NBK540080/] [PubMed]
- 58.Centers for Disease Control Prevention: Recommended guidance for extended use and limited reuse of N95 filtering facepiece respirators in healthcare settings (2014). [https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html]
- 59.Balci FSK. Isolation gowns in health care settings: Laboratory studies, regulations and standards, and potential barriers of gown selection and use. Am J Infect Control. 2016;44(1):104–111. doi: 10.1016/j.ajic.2015.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zamora JE, Murdoch J, Simchison B, Day AG. Contamination: a comparison of 2 personal protective systems. Can Med Assoc J. 2006;175(3):249–254. doi: 10.1503/cmaj.060094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Casanova LM, Rutala WA, Weber DJ, Sobsey MD. Effect of single-versus double-gloving on virus transfer to health care workers’ skin and clothing during removal of personal protective equipment. Am J Infect Control. 2012;40(4):369–374. doi: 10.1016/j.ajic.2011.04.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osei-Bonsu K, Masroor N, Cooper K, Doern C, Jefferson KK, Major Y, et al. Alternative doffing strategies of personal protective equipment to prevent self-contamination in the health care setting. Am J Infect Control. 2019;47(5):534–539. doi: 10.1016/j.ajic.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 63.Bell T, Smoot J, Patterson J, Smalligan R, Jordan R. Ebola virus disease: the use of fluorescents as markers of contamination for personal protective equipment. IDCases. 2015;2(1):27–30. doi: 10.1016/j.idcr.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lindsley WG, Noti JD, Blachere FM, Szalajda JV, Beezhold DH. Efficacy of face shields against cough aerosol droplets from a cough simulator. J Occup Environ Hyg. 2014;11(8):509–518. doi: 10.1080/15459624.2013.877591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong VW, Cowling BJ, Aiello AE. Hand hygiene and risk of influenza virus infections in the community: a systematic review and meta-analysis. Epidemiol Infect. 2014;142(5):922–932. doi: 10.1017/S095026881400003X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kampf G. Efficacy of ethanol against viruses in hand disinfection. J Hosp Infect. 2018;98(4):331–338. doi: 10.1016/j.jhin.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health Organization (2020) Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected: interim guidance, January 2020. [https://apps.who.int/iris/handle/10665/330674]
- 68.Nicolle L. SARS safety and science. Can J Anaesth. 2003;50(10):983–985. doi: 10.1007/BF03018360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lotfinejad N, Peters A, Pittet D (2020) Hand hygiene and the novel coronavirus pandemic: the role of healthcare workers. J Hosp Infect. 10.1016/j.jhin.2020.03.017 [DOI] [PMC free article] [PubMed]
- 70.Lotfinejad N, Assadi R, Aelami MH, Pittet D. Emojis in public health and how they might be used for hand hygiene and infection prevention and control. Antimicrob Resist Infect Control. 2020;9(1):1–6. doi: 10.1186/s13756-020-0692-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheung KS, Hung IF, Chan PP, Lung K, Tso E, Liu R et al (2020) Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 10.1053/j.gastro.2020.03.065 [DOI] [PMC free article] [PubMed]
- 72.Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuen K-S, Ye Z-W, Fung S-Y, Chan C-P, Jin D-Y. SARS-CoV-2 and COVID-19: The most important research questions. Cell Biosci. 2020;10(1):1–5. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barbut F, Yezli S, Otter J. Activity in vitro of hydrogen peroxide vapour against Clostridium difficile spores. J Hosp Infect. 2012;80(1):85–87. doi: 10.1016/j.jhin.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Bentley K, Dove B, Parks S, Walker J, Bennett A. Hydrogen peroxide vapour decontamination of surfaces artificially contaminated with norovirus surrogate feline calicivirus. J Hosp Infect. 2012;80(2):116–121. doi: 10.1016/j.jhin.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 76.Berrie E, Andrews L, Yezli S, Otter J. Hydrogen peroxide vapour (HPV) inactivation of adenovirus. Lett Appl Microbiol. 2011;52(5):555–558. doi: 10.1111/j.1472-765X.2011.03033.x. [DOI] [PubMed] [Google Scholar]
- 77.Clifford White G (1999) The handbook of chlorination and alternative disinfectants. 8678-8679 p.
- 78.Hao X, Li B, Zhang Q, Lin BZ, Ge L, Wang C, et al. Disinfection effectiveness of slightly acidic electrolysed water in swine barns. J Appl Microbiol. 2013;115(3):703–710. doi: 10.1111/jam.12274. [DOI] [PubMed] [Google Scholar]
- 79.Heckert R, Best M, Jordan L, Dulac G, Eddington D, Sterritt W. Efficacy of vaporized hydrogen peroxide against exotic animal viruses. Appl Environ Microbiol. 1997;63(10):3916–3918. doi: 10.1128/aem.63.10.3916-3918.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maillard J. Viricidal activity and mechanisms of action of biocides. Sci Prog. 1997;80:287–315. [PubMed] [Google Scholar]
- 81.Otter J, Yezli S, Perl TM, Barbut F, French G. The role of ‘no-touch’automated room disinfection systems in infection prevention and control. J Hosp Infect. 2013;83(1):1–13. doi: 10.1016/j.jhin.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Pottage T, Richardson C, Parks S, Walker J, Bennett A. Evaluation of hydrogen peroxide gaseous disinfection systems to decontaminate viruses. J Hosp Infect. 2010;74(1):55–61. doi: 10.1016/j.jhin.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 83.Quinn PJ, Markey BK (2001) Disinfection and disease prevention in veterinary medicine. Disinfection, sterilization and preservation 5th edition Philadelphia: Lippincott, Williams & Wilkins:1069-1103
- 84.Rice EW, Adcock NJ, Sivaganesan M, Brown JD, Stallknecht DE, Swayne DE. Chlorine inactivation of highly pathogenic avian influenza virus (H5N1) Emerg Infect Dis. 2007;13(10):1568. doi: 10.3201/eid1310.070323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Russell AD. Factors influencing the efficacy of antimicrobial agents. Principles and Practice of Disinfection, Sterilization and Preservation A Fraise. P Lambert y JY Maillard (Edts) Cap. 2004;3:98–128. [Google Scholar]
- 86.Tamaki S, Bui VN, Ngo LH, Ogawa H, Imai K. Virucidal effect of acidic electrolyzed water and neutral electrolyzed water on avian influenza viruses. Arch Virol. 2014;159(3):405–412. doi: 10.1007/s00705-013-1840-2. [DOI] [PubMed] [Google Scholar]
- 87.Zhao Y, Xin H, Zhao D, Zheng W, Tian W, Ma H, et al. Free chlorine loss during spraying of membraneless acidic electrolyzed water and its antimicrobial effect on airborne bacteria from poultry house. Ann Agric Environ Med. 2014;21(2):249–255. doi: 10.5604/1232-1966.1108585. [DOI] [PubMed] [Google Scholar]
- 88.Ahn D-G, Shin H-J, Kim M-H, Lee S, Kim H-S, Myoung J, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duffy S. Why are RNA virus mutation rates so damn high? PLoS Bio. 2018;16(8):e3000003. doi: 10.1371/journal.pbio.3000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khurshid Z, Asiri FYI, Al Wadaani H. Human saliva: non-invasive fluid for detecting Novel Coronavirus (2019-nCoV) Int J Environ Res Public Health. 2020;17(7):2225. doi: 10.3390/ijerph17072225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sabino-Silva R, Jardim ACG, Siqueira WL. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin Oral Investig. 2020;24(4):1619–1621. doi: 10.1007/s00784-020-03248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma S, Kumar V, Chawla A, Logani A. Rapid detection of SARS-CoV-2 in saliva: Can an endodontist take the lead in point-of-care COVID-19 testing? Int Endod J. 2020;53(7):1017–1019. doi: 10.1111/iej.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.To KK-W, Tsang OT-Y, Yip CC-Y, Chan K-H, Wu T-C, Chan JM-C et al (2020) Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 10.1093/cid/ciaa149 [DOI] [PMC free article] [PubMed]
- 94.Ogden G, Bahrami M, Sivarajasingam V, Phillips G. Dental students' knowledge and compliance in cross infection control procedures at a UK dental hospital. Oral Dis. 1997;3(1):25–30. doi: 10.1111/j.1601-0825.1997.tb00005.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due but are available from the corresponding author on reasonable request.