Abstract
Objectives
To investigate the relationship between coronavirus disease 2019 (COVID-19) and obesity in critically ill patients admitted to the intensive care unit (ICU).
Methods
We systematically searched PubMed, SCOPUS, Embase, LILACS, and Web of Science for studies published up to April 27, 2020. The outcome of interest was composite poor outcome, comprising mortality and severe COVID-19. We used a standardized data extraction form to collect information from published reports of eligible studies. Heterogeneity and publication bias were assessed using I2 statistic and funnel plots, respectively.
Results
Nine studies including 6577 patients were selected for evaluation. The COVID-19 patients were 59.80% male and had comorbidities such as hypertension (51.51%), diabetes (30.3%), cardiovascular disease (16.66%), lung disease (15.99%), renal disease (7.49%), cancer (5.07%), and immunosuppression (1.8%). For patients with severe complications, the overall pooled event rates were 56.2% (random; 95% CI: 35.3–75.1; p = 0.015; I2 = 71.461) for obesity, 23.6% (random; 95% CI: 17.9–30.5; p = 0.000; I2 = 87.705) for type 2 diabetes, 45.9% (random; 95% CI: 38.0–53.9; p = 0.000; I2 = 90.152) for hypertension, 20.0% (random; 95% CI: 7.9–42.0; p = 0.000; I2 = 94.577) for smoking, 21.6% (random; 95% CI: 14.1–31.4%; p = 0.000, I2 = 92.983) for lung diseases, and 20.6% (random; 95% CI: 15.2–27.5; p = 0.000, I2 = 85.735) for cardiovascular diseases.
Discussion
This systematic review indicated the relationship between obesity, ICU admission, severe COVID-19, and disease progression in patients with COVID-19. Obese patients with hypertension, type 2 diabetes, smoking habit, lung disease, and/or cardiovascular disease should be cared for with increased attention.
Abbreviations: BMI, body mass index; CI, confidence interval; COVID-19, coronavirus disease 2019; ICU, intensive care unit; IMV, invasive mechanical ventilation; RR, risk ratio; aRR, adjusted risk ratio
Keywords: COVID-19, SARS-CoV-2, ICU, Hospitalization, Intubation, Obesity
Introduction
Coronavirus disease 2019 (COVID-2019)—caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus—was declared a pandemic by the World Health Organization on March 11, 2020. With the outbreak reaching a global spread, concerns about the possible effects of the infection on immunocompromised patients has increased [1].
Modeling studies have aided the understanding of COVID-19 dynamics from the first announcement of the epidemic and publication of the genetic sequence of the causative virus [2]. The severity of COVID-19 symptoms can range from very mild to severe. Older people or those who have existing chronic medical conditions, such as heart disease, lung disease, diabetes, severe obesity, chronic kidney or liver disease, or compromised immune systems may be at higher risk of serious illness [3]. COVID-19 can cause serious respiratory illnesses such as pneumonia and lung failure, which may lead to death [4].
The mortality rate of SARS-CoV-2 (3.8%) [5] is lower than that of SARS-CoV (10%) [6] or MERS-CoV (37.1%) [7], but the number of relative infection cases is more than 10 times higher [3]. Several reports have revealed that SARS-CoV-2 can be transmitted from asymptomatic individuals or those with mild infections [[8], [9], [10]]. These features may explain the sudden spread of the virus epidemic.
There is a lack of systematic review and meta-analysis reporting COVID-19 severity in obese and overweight individuals. This systematic review and meta-analysis investigated the relationship between severity of COVID-19 and obesity for critically ill patients requiring intensive care unit (ICU) care (PICO question). In addition, to improve outcomes of patients with severe COVID-19, we determined the relative effectiveness and certainty of evidence among protocols to treat obese individuals.
Methods
This meta-analysis was reported according to the the National Health Service Centre for Reviews and Dissemination [11] and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA Statement) guidelines [12].
The protocol for the review was registered on the International Prospective Register of Systematic Reviews (PROSPERO) database under the number CRD42020183216. Eligibility criteria were defined in relation to PICOS (participants, interventions, comparisons, outcomes, and study design) as recommended by the PRISMA Statement. This systematic review asked the following questions: (i) “Is obesity associated with higher levels of COVID-19 incidence, prevalence, and risk factors?”; and (ii) “Is obesity associated with higher levels of severe medical complications and does it lead to critical illness and ICU admission?”.
Search strategy and selection criteria
MEDLINE database, EMBASE, Web of Science, BVS/LILACS, SCIELO, SCOPUS, and Google Scholar were systematically searched using the following key Medical Subject Headings terms: “obesity” AND “covid-19” AND “mortality”; “obesity” AND “covid-19” AND “ICU admitted”; obesity” AND “covid-19” AND “ARDS”; obesity” AND “severe covid-19”. Moreover, we reviewed the references from eligible articles to identify other potentially relevant studies. The last search was conducted on May 3, 2020.
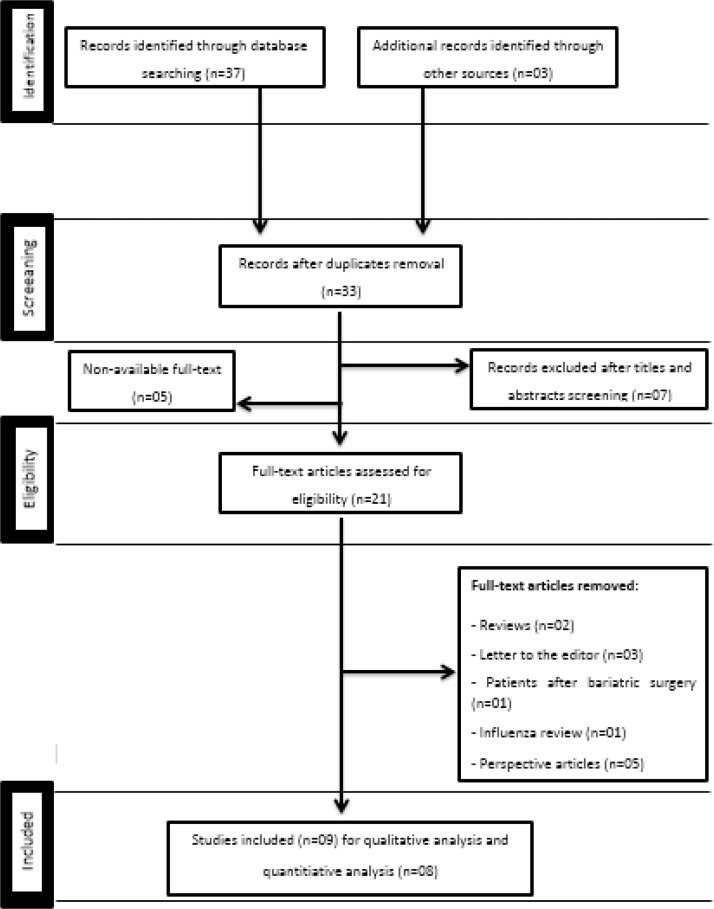
Our eligibility criteria included case reports, case series, clinical trials, and randomized controlled trials published in English, Portuguese, and Spanish in peer-reviewed journals. The studies must that have addressed epidemiological and clinical features of COVID-19 and its association with obesity. Duplicate publications and articles that did not correspond to the objectives of this review were excluded. Similarly, publications without an investigative research or case report, such as descriptive studies, opinion articles, correspondence, editorials, and letters to the editor were excluded. The steps of the literature search are shown in Fig. 1 .
Fig. 1.
PRISMA 2009 flow diagram.
Data extraction
To ensure literature saturation, we examined the references of the included studies or relevant reviews identified through the search. Three investigators independently participated in each phase of the review and screened the titles and abstracts yielded by the search against the inclusion criteria. The investigators screened the full text reports and decided whether they met the inclusion criteria. Discrepancies between them were resolved by discussion and consensus. The final results were reviewed by a senior investigator. The following data were extracted from the included studies: study characteristics, methodology, study design, year of data collection, sample size, outcomes, case definition, exposure and comparators, and participant demographic information (age (mean), prevalence (%), n of death, mortality rate (%) of groups, and quality of score).
Quality appraisal and risk of bias
We appraised cohort, case-control, and cross-sectional studies for the systematic review using the appropriate tools for each study design.
The Newcastle-Ottawa Scale (NOS) and NOS-derived survey were used to assess risk of bias (ROB). in this scale, a maximum of nine points are assigned to quantify the ROB in three domains: (1) selection of study groups (0–4); (2) comparability of groups (0–2); and (3) ascertainment of exposure and outcomes (0–3) for cross-sectional, case–control, and cohort studies [13]. Scoring was undertaken by three reviewers, with a fourth reviewer resolving any disagreements. Studies were considered at low ROB when the overall scores were 9–10, moderate ROB when scores were 6–8, and high ROB when scores were 0–5. Research questions were developed based on the NOS questions covering all three domains so that authors could provide detailed information about their studies. Obesity status for COVID-19 and critically ill patients requiring ICU care were defined as the most important covariates that defined comparability, consistent with the NOS assessment conducted by the reviewers [14].
Summary measures
Quantitative data were grouped by the following variables: prevalence of patients with hypertension, type 2 diabetes, smokers, lung diseases, dyslipidemia, and cardiovascular diseases. The data were analyzed and grouped by 3 researchers, and the tabulation of all data was performed by one researcher. After all tabulations, the data were reviewed by another researcher. This grouped information was evaluated for the event rate considering the 95% confidence interval (CI). The number of patients with COVID-19 was a statistical unit. The contribution weight of each study was also assessed. The Comprehensive Meta-Analysis software (software version 3.0; Biostat, Englewood, NJ, USA) was used to build the forest plot [15].
Risk of bias in the studies
Heterogeneity was evaluated using the Q method, and the I² value was analyzed [16,17]. A heterogeneity value above 75 (range, 0–100) may reflect higher significance [17,18]; therefore, we adopted random analysis for all meta-analyses to reduce potential heterogeneity because the studies had different characteristics, sample size, group data, and location [19]. The particularities of the sample designs of each study were also evaluated. A funnel plot was included for each analysis.
Synthesis of results
Summary measurements used by each publication included in this study were recorded. The methodological features of all publications were extracted, and an evidence summary was presented for each study. For confounders, studies were categorized according to the variable: if it was statistically controlled for, found to be non-significantly associated with both obesity and critically ill requiring ICU, matched between studies, excluded, or not accounted for in the statistical model. Meta-analysis was performed by combining the results of reported prevalence and incidence of any assessed outcome in comparative studies.
Results
Scientific information database
A flowchart illustrating the selection of studies for inclusion in this systematic review is shown in Fig. 1. The search process in all scientific databases led to 40 articles. After removing unrelated articles, 33 were recorded using the EndNote software. So, after screening, 21 articles were recorded in EndNote. Finally, after deleting duplicates, 9 articles remained for qualitative analysis and 8 for quantitative analysis.
Studies characteristics
Eight articles were entered in the final step of the systematic review and meta-analysis. The death rate was calculated dividing the number of deaths by the number of cases, resulting in the probability (%) of dying if infected by the virus. The quality assessment of studies using NOS indicated moderate quality, with scores ranging from 6 to 8.
All articles assessed the relationship between COVID-19 and obesity in two ways. The first group included healthy exposed (A), patient exposed (B), unhealthy exposed (C), and patient unexposed (D) cases in their studies, from which we extracted odds ratio information using the “case–control OR calculator” function of the Biostat software. The second group included mean obesity and its 95% confidence interval along with the COVID-19 status of critically ill and ICU patients, from which we extracted severe complications information using the “effect size based on mean comparison” function of the Biostat software.
Subgroup and overall summary of the relationship between COVID19 and obesity
A detailed description of the included studies is shown in Table 1 . Of nine articles, five were retrospective cohort studies, one was a prospective cohort study, two were cross-sectional studies, and one was a case series. The association between obesity and severe complications in patients with COVID-19 is expected due to the growing prevalence of both diseases. In the pooled data from all studies (n = 6577), the majority of patients with COVID-19 were male (n = 3796; 59.80%) and the main comorbidities were hypertension (n = 3388; 51.51%), diabetes (n = 1993; 30.3%), cardiovascular disease (n = 1096; 16.66%), lung disease (n = 1052; 15.99%), immunosuppression (n = 119; 1.8%), renal disease (n = 492; 7.49%), and cancer (n = 334; 5.07%) (Table 1). Table 1 summarizes the prevalence of these comorbidities in obese patients with COVID-19 obtained from the available studies.
Table 1.
Characteristics and information of studies included.
| Study | Simmonet et al. (2020) | Abou-Arab O et al. (2020) | Barrasa H et al. (2020) | Zheng et al. (2020) | Hu L et al. (2020) | Kalligeros M et al. (2020) | Richardson et al. (2020) | Garg S et al. (2020) | Piva S et al. (2020) | N total |
|---|---|---|---|---|---|---|---|---|---|---|
| Location | Lille, France | Amiens, France | Vitoria, Spain | Whenzou, China | Wuhan, China | Rhode Island, USA | New York, USA | USA | Brescia, Italy | |
| Type of study | Retrospective Cohort | Case series | Retrospective Cohort | Observational cross-sectional study | Retrospective Cohort | Retrospective Cohort | Cross-sectional study | Retrospective Cohort | Prospective Cohort | |
| New Castle Scale (NOS) | 7 | – | 8 | 6 | 7 | 6 | 6 | 7 | 7 | |
| Total patients (positive for COVID-19) | 124 | 2 | 48 | 66 | 323 | 103 | 5700 | 178 | 33 | 6577 |
| Obese patients | 94 (75.8%) | 2 (100%) | 23 (48%) | 45 (68%) | 13(4%) | 49 (47.5%) | 2528/4170 (60.7%) | 73/151 (48.3%) | 6 (18%) | 2833 (43.6%) |
| BMI ≥ 30 | 59 (47.6%) | 1 (50%) | 15 (31%) | – | 13 (4%) | 22 (21.3%) | 1737 (41.7%) | – | – | |
| BMI 35–39,9 | 17 (13.7%) | – | – | – | – | 27 (26.2%) | – | – | – | |
| BMI ≥ 40 | 18 (14.5%) | 1 (50%) | 7 (14%) | – | – | – | 791 (19%) | – | – | |
| Age (mean) | 60 | 63 | 63,2 | 47 | 61 | 60 | – | – | 64 | – |
| Sex | ||||||||||
| Male | 90 | – | 27 | 13 | 166 | 63 | 3437 | – | 30 | 3796 (59.80%) |
| Female | 34 | 2 | 21 | 32 | 157 | 40 | 2263 | – | 3 | 2552 (40.20%) |
| Other comorbidities | ||||||||||
| Hypertension | 60 (49%) | – | 21 (44%) | 19 (28.8%) | 105 (32.5%) | 66 (64%) | 3026 (56.5%) | 79/159 (49.7%) | 15 (45%) | 3388 (51.51%) |
| Diabetes | 28 (23%) | – | 9 (19%) | 14 (24.2%) | 47 (14.6%) | 38 (36.8%) | 1808 (33.8%) | 47/166 (28.3%) | 2 (6%) | 1993 (30.3%) |
| Cardiovascular Disease | – | – | 5 (10%) | – | 41 (12.7%) | 25 (24.2%) | 966 (18%) | 45/162 (27.8%) | 14 (43%) | 1096 (16.66%) |
| Dyslipidemia | 34 (28%) | – | – | 45 (68.2%) | – | – | – | – | – | 79 (1.2%) |
| Hypothyroidism | – | – | 9 (19%) | – | – | – | – | – | – | 9 (0.13%) |
| Lung disease | – | – | 18 (38%) | – | 35 (10.9%) | 20 (19.4%) | 920 (17.3%) | 55/159 (34.6%) | 4 (12%) | 1052 (15.99%) |
| Immunosuppression | – | – | 3 (6%) | – | – | 2 (1.9%) | 98 (1.8%) | 15/156 (9.6%) | 1 (3%) | 119 (1.8%) |
| Smoker | – | – | 9 (19%) | 8 (12.1%) | 38 (11.8%) | 12 (11.7%) | – | – | – | 67 (1.01%) |
| Gastrointestinal/Liver disease | – | – | – | – | 30 (9.2%) | 3 (2.9%) | 30 (0.6%) | 10/152 (6.6%) | – | 73 (1.1%) |
| Blood disorder | – | – | – | – | – | – | – | 9/156 (5.8%) | – | 9 (0.13%) |
| Renal disease | – | – | – | – | 7 (2.2%) | 11 (10.6%) | 454 (8.5%) | 20/153 (13.1%) | – | 492 (7.48%) |
| Rheumatologic/Autoimmune disease | – | – | – | – | – | – | – | 3/154 (1.9%) | – | 3 (0.04%) |
| Cancer | – | – | – | – | 5 (1.5%) | 9 (8.7%) | 320 (6%) | – | – | 334 (5.07%) |
| Endocrine system disease | – | – | – | – | 15 (4.6%) | – | – | – | – | 15 (0.22%) |
| Nervous system disease | – | – | – | – | 10 (3.1%) | – | – | – | – | 10 (0.15%) |
| Mortality (TOTAL) | 18 (15%) | – | 14 (31%) | – | 35 (10.8%) | – | 553/2634 (21%) | – | 1 (3%) | 621 (9.44%) |
Data are expressed in absolute number of cases and % number in ().
Cardiovascular disease: heart failure, congestive heart failure, coronary artery disease, cardiomyopathy.
Lung Disease: COPD*, asthma, interstitial lung disease, pulmonary hypertension, respiratory system disease.
Immunosuppression: transplant, HIV.
Gastrointestinal/liver disease: chronic liver disease, cirrhosis, digestive system disease, hepatitis B, hepatitis C.
Renal disease: chronic kidney disease, end stage renal disease.
*COPD; Chronic obstructive pulmonary disease.
Severe complications in obese vs. non-obese patients
Three studies involving 463 individuals, 107 obese (50 severe complications) and 356 non-obese (177 severe complications), were assessed. The meta-analysis indicated no significant difference between them (risk ratio [RR] 1.403; 95% CI 0.908–2.167; p = 0.127, Fig. 2 a). The heterogeneity rate in the studies that assessed the Q-value of the relationship between obese and non-obese patients with COVID-19 was 3.231 (p = 0.199; I2 = 38.101; Fig. 2a). An I2 index lower than 25%, between 25% and 75%, and higher than 75% was considered as low, medium, and high heterogeneity, respectively. According to this classification, the heterogeneity rate in obese vs. non-obese patients was medium. Therefore, the random effect model was used to analyze the articles.
Fig. 2.
Forest plots for severe complications in obese and non-obese individuals (a); obese individuals with severe complications (b); hypertension patients (c); type 2 diabetes patients (d); lung disease patients (e); smoker patients (f); cardiovascular disease patients (g).
Event rate for obese individuals with severe complications
In 4 studies involving 130 obese individuals, 73 of them presented severe complications. The overall pooled event rate was 56.2% (random; 95% CI: 35.3%–75.1%; Fig. 2b). The heterogeneity rate for severe complications in obese individuals was considered medium (p = 0.015; I2 = 71.461).
Hypertension patient event rate
In 8 studies involving 6556 patients with COVID-19, 3391 had hypertension. The overall pooled event rate was 45.9% (random; 95% CI: 38.0%–53.9%; Fig. 2c). The heterogeneity of the event rate for hypertension was considered high (p = 0.000; I2 = 90.152).
Type 2 diabetes patient event rate
In 8 studies involving 6563 patients with COVID-19, 1995 had type 2 diabetes. The overall pooled event rate was 23.6% (random; 95% CI: 17.9%–30.5%; Fig. 2d). The heterogeneity of the event rate for type 2 diabetes was considered high (p = 0.000; I2 = 87.705).
Lung disease patient event rate
In 5 studies involving 6333 patients with COVID-19, 1048 had lung diseases. The overall pooled event rate was 21.6% (random; 95% CI: 14.1%–31.4%; Fig. 2e). The heterogeneity of the event rate for lung diseases was considered high (p = 0.000; I2 = 92.983).
Smokers event rate
In 4 studies involving 540 patients with COVID-19, 103 were smokers. The overall pooled event rate was 20.0% (random; 95% CI: 7.9%–42.0%; Fig. 2f). The heterogeneity of the event rate for smoking was considered high (p = 0.000, I2 = 94.577).
Cardiovascular disease event rate
In 6 studies involving 6369 patients with COVID-19, 1096 had cardiovascular diseases. The overall pooled event rate was 20.6% (random; 95% CI: 15.2 %–27.5 %; Fig. 2g). The heterogeneity of the event rate for cardiovascular diseases was considered high (p = 0.000; I2 = 85.735).
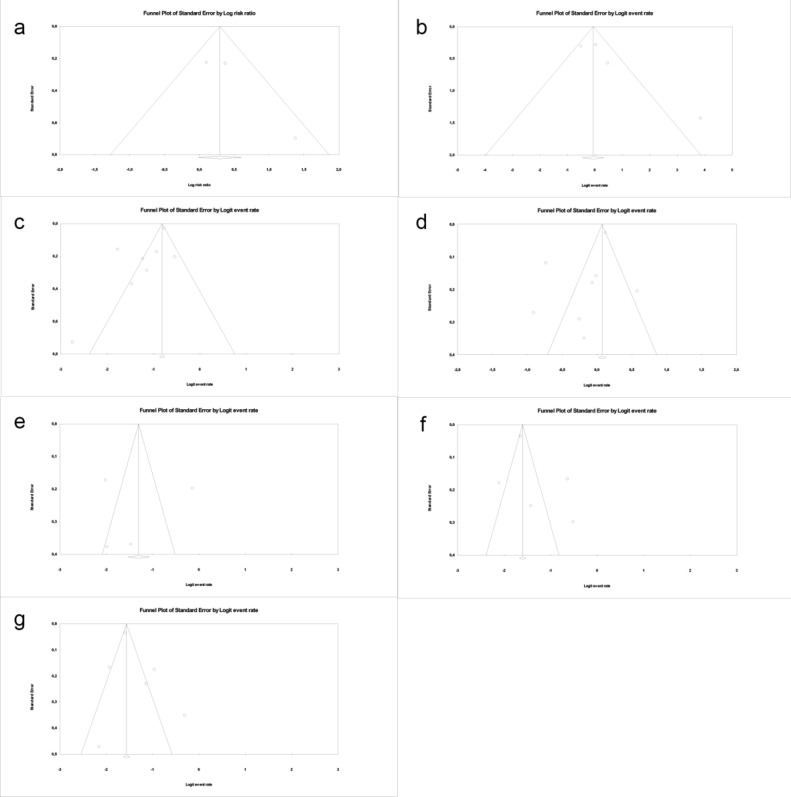
Publication bias
A funnel plot of the relationship between COVID-19 and obesity in studies with extractable RR and event rates were is indicated in Fig. 3 The standard error is plotted against the log event rate and RR. An asymmetric funnel plot indicates a low level of publication or high study bias, further supporting the reliability of the overall findings. Fig. 3 presents the funnel plot graphs for each analysis.
Fig. 3.
Funnel plots of standard error by log risk ratio (X: log risk event; Y: standard error): Severe complications in obese individuals and non-obese individuals (a); severe complications in obese individuals (b); patients with hypertension (c); patients with type 2 diabetes mellitus (d); smoker patients (e); patients with lung diseases (f); patients with cardiovascular diseases (g).
Discussion
This study investigated the relationship between COVID-19 and obesity through a systematic review and meta-analysis. As results indicated, information regarding severely obese COVID-19 patients who were critically ill and required intensive care is limited. The highlight of this research is that severely obese individuals presented a high risk for progression to critical illness and require ICU care. There was a high frequency of obesity among patients admitted to intensive care due to COVID-19. Invasive mechanical ventilation was associated with severe obesity and was independent of age, sex, diabetes, and hypertension [20].
Patients with COVID-19 and associated comorbidities require special care because of increased risk of in-hospital death. The virus causes serious infections, especially in smokers, elderly, obese, hypertensive, and diabetic patients [21]. Being obese not only increases the risk of infection and complications for the individual, but recent evidence indicates that a large obese population increases the chance of appearance of more virulent viral strain, and prolonged virus shedding may increase the mortality rate of an influenza pandemic [22]. The etiology of metabolic syndrome represents a complex interaction between genetic, metabolic, environmental, and dietary factors [23]. Its clinical diagnosis, which has already been established for adults, is based on metabolic abnormalities including abdominal obesity, dyslipidemia, high blood pressure, and hyperglycemia [24].
When we analyzed non-obese and obese patients independent of severity, there was no difference between them (Fig. 2a). This may be partially explained by the difference between the number of obese (n = 107) and non-obese (n = 356) patients assessed in the study. For this reason, case-controlled clinical studies should be conducted with a higher proportion of severely obese COVID-19 patients to compare the fatality rate between obese and non-obese patients.
According to analyzed studies, the most common preexisting conditions of COVID-19 patients were hypertension (56 %–64% [25,26], 35.6% [27], 49% [20], 44% [28]), diabetes (33.8 %–36.8 % [26], 31.3% [27], 23% [20], 19% [28]), cardiovascular diseases (24.2% [26], 10% [28]), dyslipidemias (68.8% [27]), and smoking (19% [28]) (Table 1). The meta-analysis data for the proposed individual outcomes demonstrated obesity (Fig. 2b), hypertension (Fig. 2c), type 2 diabetes (Fig. 2d), lung diseases (Fig. 2e), smoking (Fig. 2f), and cardiovascular diseases (Fig. 2g) were associated with severe complications. There was a consistent association between obesity and severe complications across all studies. In particular, the increase in mortality requires serious attention.
Obesity restricts ventilation by impeding diaphragm excursion, impairs immune responses to viral infection [29], promotes inflammation, and induces diabetes and oxidant stress, adversely affecting cardiovascular function [30]. In populations with high prevalence of obesity, COVID-19 affects younger populations more than previously reported. Providing public information to younger adults, reducing the threshold for virus testing in obese individuals, and maintaining greater vigilance for this at-risk population are necessary to reduce the prevalence of severe COVID-19 disease [31].
Obese subjects have chronically higher leptin and lower adiponectin concentrations. This unfavorable hormone also leads to dysregulation of the immune response and can contribute to the pathogenesis of obesity-linked complications [32]. These patients have a higher concentration of several pro-inflammatory cytokines such as alpha-TNF, MCP-1, and IL-6, mainly produced by visceral and subcutaneous adipose tissue, leading to an impaired innate immunity [33].
Luzi and Radaelli [22] identified three factors that make obese individuals infected with COVID-19 more contagious than normoweight individuals: (a) obese individuals with influenza shed the virus for a longer period of time, potentially increasing the chance to spread the virus to others [34]; (b) the obese microenvironment favors the emergence of novel strains due to the reduced and delayed capacity to produce interferons by obese individuals. The delay in interferon production to oppose viral replication allows more viral RNA replication, increasing the chances of the appearance of novel, more virulent viral strains [35]; and (c) the body mass index (BMI) correlates positively with infectious virus in exhaled breath [36].
Obesity-related chronic inflammation with antigen participation causes reduced macrophage activation and blunted pro-inflammatory cytokine production upon macrophage stimulation [37]. B and T cell responses are impaired in obese and obese diabetic patients, and this causes increased susceptibility and delay in the resolution of viral infections. A dysregulated pro-inflammatory response contributes to the severe lung lesions observed in patients during the influenza pandemic [22].
Tracheal intubation, mechanical ventilation, and extracorporeal membrane oxygenation (ECMO) were adopted in severe cases of patients admitted in intensive care for SARS-CoV-2 related severe acute respiratory disease [20]. Researchers from Spain applied the strategies demonstrated in a previous study [27], such as intubation (94%), high-flow oxygen nasal therapy (6%), tracheostomy in ventilated patients (9%), prone position in mechanically ventilated patients (49%), and ECMO (2%) [28].
In a Chinese study with obese and normoweight patients, obese participants (BMI 31.1 kg/m2) required invasive mechanical ventilation. Individuals with a BMI of 30−35 kg/m2 and ≥35 kg/m2 required mechanical ventilation three and six times more often, respectively, than normoweight individuals. In addition, half of the obese group had hypertension and a quarter of them had diabetes [20]. Kalligeros et al. 2020 [26] investigated the association between obesity and other chronic diseases with severe outcomes, such as ICU admission and invasive mechanical ventilation (IMV), in patients hospitalized with COVID-19 in the USA. Among the hospitalized patients (n = 103), 41 (39.8%) were admitted to the ICU and 29 (70.7%) required IMV. The prevalence of obesity was 47.5% (49/103), and severe obesity (BMI ≥ 35 kg/m2) was associated with ICU admission. Patients who required IMV were more likely to have heart disease, obesity (BMI 30–34.9 kg/m2), or severe obesity (BMI ≥ 35 kg/m2).
Patients with SARS have overwhelming immune and inflammatory responses and high mortality rates from acute respiratory failure [38]. Severe pulmonary inflammatory infiltrate of pulmonary tissue impedes alveolar gas exchange. In addition, 20% of hospitalized patients develop significant cardiovascular morbidity characterized by troponin rise, tachyarrhythmias, and thromboembolic events, which are strongly associated with mortality risk [39]. Alternatively, we should consider the impact of obesity on pulmonary function, which may be a potential cause of worse clinical treatment of obese individuals due to the dynamic of pulmonary ventilation, with decreased diaphragmatic excursions and a relative increase in anatomical death space [22].
Because immunity does not exist yet and a significant group of individuals develop severe disease, the novel COVID-19 is a threat to all populations of the world. SARS-CoV-2 has the capacity to escape innate immune responses, which allows the pathogen to produce large copy amounts in primarily infected tissues. Through the infection of innate immune cells and/or the recruitment of uninfected cells from the circulation to the primary site of infection, massive immune reactions induce hyperinflammation that can result in a cytokine storm and life-threatening complications [39]. It is likely that host characteristics promote the progression of SARS-CoV-2 infection. For this reason, the care of specific groups of patients should be given increased attention.
Our study has several limitations. First, the primary outcome for our meta-analysis was that patients hospitalized with COVID-19 correlated with obesity and different comorbidities. Second, despite our strict inclusion criteria, there were differences in study design and significant heterogeneity between studies for several interventions, probably reflecting the range of ages, settings, and types of studies. However, we undertook analyses of subgroups to provide insights into factors that drive heterogeneity and did sensitivity analyses restricted to studies that measured how the disease contributed to complications, which did not change our inferences. Finally, we identified many gaps in the literature, such as clinical characterization, infection prevention, mortality reduction, and efficient therapeutic overlap, which should be investigated to provide a standardized protocol for treating severe COVID-19.
COVID-19 is a viral inflammatory disease; obesity is a factor in disease severity, having the greatest impact in patients with a BMI ≥ 35 kg/m2. Patients with obesity, especially those with severe obesity, should take extra precautions to avoid COVID-19 contamination during the current pandemic [20]. Future studies should investigate the mechanisms of association between COVID-19 and obesity.
This meta-analysis revealed that patients with severe obesity are at high risk of severe COVID-19 infection, IMV, ICU admission, and mortality, independent of age, race, sex, and comorbidities such as diabetes, hypertension, dyslipidemia, or pulmonary disease. This systematic review showed that obese COVID-19 patients with associated comorbidities required special care due to increased risk of in-hospital death.
PRISMA and PROSPERO
This meta-analysis reported according to the the National Health Service Centre for Reviews and Dissemination and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA Statment) guidelines.
The protocol for the review was registered on the International Prospective Register of Systematic Reviews (PROSPERO) database under the number CRD42020183216. Eligibility criteria were defined in relation to PICOS (participants, interventions, comparisons, outcomes, and study design) as recommended by PRISMA Statment. This systematic review asked the following questions (i) is obesity associated with higher levels of COVID-19 incidence, prevalence and risk factors?; and (ii) is obesity associated with higher levels of severe medical complications and lead for critically illness and ICU admitted?
Funding
This work was supported by São Paulo Research Foundation (FAPESP; grant nº. 18/25934-0) and Coordination for the Improvement of Higher Education Personnel (CAPES - Finance Code 001).
Conflict of interest
All authors have nothing to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful for University of São Paulo and financial support provided by São Paulo Research Foundation (FAPESP) and Coordination for the Improvement of Higher Education Personnel (CAPES).
References
- 1.D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26(6):832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 2.McBryde E. The value of early transmission dynamic studies in emerging infectious diseases. Lancet Infect Dis. 2020;20(5):512–513. doi: 10.1016/S1473-3099(20)30161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Coronavirus disease 2019 (COVID-19). Accessed: April 17, 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html.
- 4.Ahn D.G., Shin H.J., Kim M.H., et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available from https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. [Accessed 2 April 2020].
- 6.WHO Summary of probable SARS cases with ons et of illness from 1 November 2002 to 31 July 2003. Available from https://www.who.int/csr/sars/country/table2004_04_21/en/. [Accessed 27 April 2020]
- 7.WHO Middle East respiratory syndrome coronavirus (MERS-CoV) monthly summary, November 2019. Available from https://www.who.int/emergencies/mers-cov/en/. [Accessed 27 April 2020]
- 8.Pan X., Chen D., Xia Y., Wu X., Li T., Ou X., et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20(April (4)):410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [published online ahead of print, 2020 Feb 21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Health Service (NHS) Centre for Reviews and Dissemination . York Publishing Services; University of York: 2001. Report number 4. Undertaking systematic reviews of research on effectiveness.www.york.ac.uk/inst/crd/crdrep.htm Available at: [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta- analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., et al. Ottawa Hospital Research Institute; Ottawa, ON: 2011. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [webpage on the Internet] Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Accessed 27 April 2020] [Google Scholar]
- 14.Mertz D., Kim T.H., Johnstone J., Lam P., Science M., Kuster S.P., et al. Populations at risk for severe or complicated influenza illness: a systematic review and meta-analysis. BMJ. 2012;347 doi: 10.1136/bmj.f5061. f506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borenstein M., Hedges L., Higgins J., Rothstein H. Wiley; Chichester, UK: 2009. Introduction to meta-analysis. [DOI] [Google Scholar]
- 16.Carvalho M.V., de Moraes S.L.D., Lemos C.A.A., Santiago Júnior J.F., Vasconcelos B.C.D.E., Pellizzer E.P. Surgical versus non-surgical treatment of actinic cheilitis: a systematic review and meta-analysis. Oral Dis. 2019;25(4):972–981. doi: 10.1111/odi.12916. [DOI] [PubMed] [Google Scholar]
- 17.de Medeiros F.C.F.L., Kudo G.A.H., Leme B.G., Saraiva P.P., Verri F.R., Honório H.M., et al. Dental implants in patients with osteoporosis: a systematic review with meta-analysis. Int J Oral Maxillofac Surg. 2018;47(4):480–491. doi: 10.1016/j.ijom.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Annibali S., Bignozzi I., Cristalli M.P., Graziani F., La Monaca G., Polimeni A. Periimplant marginal bone level: a systematic review and meta-analysis of studies comparing platform switching versus conventionally restored implants. J Clin Periodontol. 2012;39:1097–1113. doi: 10.1111/j.1600-051X.2012.01930.x. [DOI] [PubMed] [Google Scholar]
- 19.Deeks J.J., Higgins J.P.T., Altman D.G., et al. In: Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019) Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., editors. Cochrane; 2019. Chapter 10: analysing data and undertaking meta-analyses.www.training.cochrane.org/handbook Available from. [Google Scholar]
- 20.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. High prevalence of obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cure E., Cumhur Cure M. Comment on “Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19”. J Med Virol. 2020;(April) doi: 10.1002/jmv.25937. [published online ahead of print, 2020 Apr 8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luzi L., Radelli M.G. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020;57(June (6)):759–764. doi: 10.1007/s00592-020-01522-8. Epub 2020 Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esposito K., Marfella R., Ciotola M., Di Palo C., Giugliano F., D’Armiento M., et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 24.Alberti K.G., Zimmet P., Shaw J. Metabolic syndrome a new world-wide definition. A consensus statement from the lnternational Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 25.Richardson S., Hirsh J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [published online ahead of print, 2020 Apr 22] [published correction appears in doi: 10.1001/jama.2020.7681] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalligeros M., Shehadeh F., Mylona E.K., Benitez G., Beckwith C.G., Chan P.A., et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity (Silver Spring, MD) 2020;28(7):1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng K.I., Gao F., Wang X., Sun Q., Pan K., Wang T., et al. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrasa H., Rello J., Tejada S., Martín A., Balziskueta G., Vinuesa C., et al. SARS-CoV-2 in Spanish Intensive Care Units: Early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med. 2020;(April) doi: 10.1016/j.accpm.2020.04.001. 2020;S2352-5568(20)30064-3. [published online ahead of print, 2020 Apr 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honce R., Schultz-Cherry S. Impact of obesity on influenza a virus pathogenesis, immune response, and evolution. Front Immunol. 2019;10(May):1071. doi: 10.3389/fimmu.2019.01071. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GBD 2015 Obesity Collaborators, Afshin A., Forouzanfar M.H., et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kass D.A., Duggal P., Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395(10236):1544–1545. doi: 10.1016/S0140-6736(20)31024-2. Epub 2020 May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richard C., Wadowski M., Goruk S., Cameron L., Sharma A.M., Field C.J. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res Care. 2017;5(1) doi: 10.1136/bmjdrc-2016-000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maier H., Lopez R., Sanchez N., Ng S., Gresh L., Ojeda S., et al. Obesity increased the duration of influenza A virus shedding in adults. J Infect Dis. 2018;218(9):1372–1382. doi: 10.1093/infdis/jiy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honce R., Karlsson E.A., Wohlgemuth N., Estrada L.D., Meliopoulos V.A., Yao J., et al. Obesity-related microenvironment promotes emergence of virulent influenza virus strains. mBio. 2020;11(March (2)) doi: 10.1128/mBio.03341-19. pii: e03341-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan J., Grantham M., Pantelic J., de Mesquita P.J.B., Albert B., Liu F., et al. Infectious virus in exhaled breath of syntomatic seasonal influenza cases from a college community. PNAS. 2018;115(5):1081–1086. doi: 10.1073/pnas.1716561115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahn S.Y., Sohn S.H., Lee S.Y., Park H.L., Park Y.W., Kim H., et al. The effect of lipopolysaccharide-induced obesity and its chronic inflammation on influenza virus-related pathology. Environ Toxicol Pharmacol. 2015;40(3):924–930. doi: 10.1016/j.etap.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 38.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;(April) doi: 10.1093/cvr/cvaa106. cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felsenstein S., Herbert J.A., McNamara P.S., Hedrichb C.M. COVID-19: Immunology and treatment options. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108448. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]