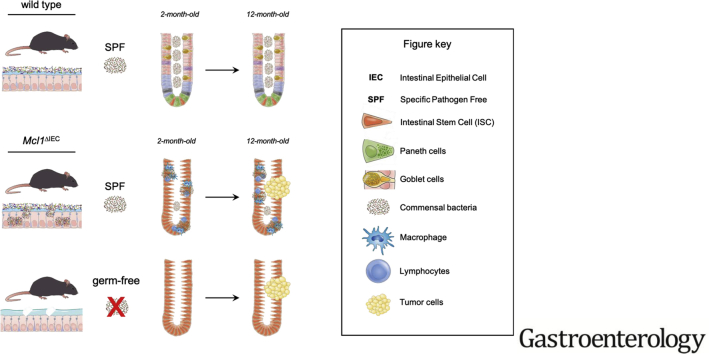
Abstract
Background & Aims
Intestinal epithelial homeostasis depends on a tightly regulated balance between intestinal epithelial cell (IEC) death and proliferation. While the disruption of several IEC death regulating factors result in intestinal inflammation, the loss of the anti-apoptotic BCL2 family members BCL2 and BCL2L1 has no effect on intestinal homeostasis in mice. We investigated the functions of the antiapoptotic protein MCL1, another member of the BCL2 family, in intestinal homeostasis in mice.
Methods
We generated mice with IEC-specific disruption of Mcl1 (Mcl1ΔIEC mice) or tamoxifen-inducible IEC-specific disruption of Mcl1 (i-Mcl1ΔIEC mice); these mice and mice with full-length Mcl1 (controls) were raised under normal or germ-free conditions. Mice were analyzed by endoscopy and for intestinal epithelial barrier permeability. Intestinal tissues were analyzed by histology, in situ hybridization, proliferation assays, and immunoblots. Levels of calprotectin, a marker of intestinal inflammation, were measured in intestinal tissues and feces.
Results
Mcl1ΔIEC mice spontaneously developed apoptotic enterocolopathy, characterized by increased IEC apoptosis, hyperproliferative crypts, epithelial barrier dysfunction, and chronic inflammation. Loss of MCL1 retained intestinal crypts in a hyperproliferated state and prevented the differentiation of intestinal stem cells. Proliferation of intestinal stem cells in MCL1-deficient mice required WNT signaling and was associated with DNA damage accumulation. By 1 year of age, Mcl1ΔIEC mice developed intestinal tumors with morphologic and genetic features of human adenomas and carcinomas. Germ-free housing of Mcl1ΔIEC mice reduced markers of microbiota-induced intestinal inflammation but not tumor development.
Conclusion
The antiapoptotic protein MCL1, a member of the BCL2 family, is required for maintenance of intestinal homeostasis and prevention of carcinogenesis in mice. Loss of MCL1 results in development of intestinal carcinomas, even under germ-free conditions, and therefore does not involve microbe-induced chronic inflammation. Mcl1ΔIEC mice might be used to study apoptotic enterocolopathy and inflammatory bowel diseases.
Keywords: Colorectal Carcinoma, CRC, Cell Death, Tumorigenesis
Abbreviations used in this paper: ABX, antibiotic treatment; CRC, colorectal cancer; GSEA, gene set enrichment analysis; IBD, inflammatory bowel disease; IEC, intestinal epithelial cell; IL, interleukin; ILC, innate lymphoid cell; ISC, intestinal stem cell; RNASeq, RNA-sequencing; smFISH, single-molecule fluorescence in situ hybridization
Graphical abstract
What You Need to Know.
Background and Context
Intestinal epithelial homeostasis depends on a tightly regulated balance between intestinal epithelial cell (IEC) death and proliferation. While the disruption of several IEC death regulating factors result in intestinal inflammation, the loss of the anti-apoptotic BCL2 family members BCL2 and BCL2L1 has no effect on intestinal homeostasis in mice.
New Findings
The BCL2 family member MCL1 is required for maintenance of intestinal homeostasis and prevention of carcinogenesis in mice. Loss of MCL1 results in intestinal carcinogenesis—even under germ-free conditions, and therefore does not involve microbe-induced chronic inflammation.
Limitations
This study was performed in mice. Studies are needed to determine whether MCL1 function is disrupted in patients with inflammatory bowel diseases or cancer.
Impact
Mcl1ΔIEC mice might be used to study apoptotic enterocolopathy and inflammatory bowel diseases.
Intestinal homeostasis is dependent on a tightly regulated interaction among the epithelium, the immune system, and luminal commensal bacteria. The intestinal epithelium compartment is composed of different cell types, including proliferative stem cells as well as highly specialized absorptive (enterocytes) and secretory cells (goblet, Paneth, and neuroendocrine cells).1,2 These intestinal epithelial cells (IECs) provide a physical barrier between the intestinal mucosa and potentially pathogenic microorganisms. IECs display a high cell turnover, necessitating a strictly regulated balance of proliferation and cell death to guarantee intestinal epithelial homeostasis3; however, excessive IEC death is a well-known clinical hallmark of apoptotic enterocolopathy and inflammatory bowel disease (IBD).3, 4, 5
In recent years, there has been great scientific interest in determining the roles played by cell death regulating molecules in maintaining tissue homeostasis and preventing tumorigenesis. Transgenic mice, generated with intestinal-specific knockouts of particular IEC circuits of regulated cell death, FADD, Caspase 8, or RIPK1, display excessive IEC turnover, epithelial defects and IBD-like inflammation.6, 7, 8, 9, 10, 11 In contrast, mice harboring intestinal-specific deletions of other regulated cell death molecules, BCL2 or BCL2L1, display surprisingly little pathology under steady-state conditions. Although capable of modulating intestinal carcinogenesis in tumor models, both are dispensable for maintaining intestinal homeostasis.12,13 Interestingly, another pro-survival BCL2 family member, MCL1, is essential for maintaining homeostasis in several tissues with regenerative capacity.14, 15, 16 The indispensable role for MCL1 in maintaining liver homeostasis15,16 prompted us to study its function in the intestinal tract.
Materials and Methods
Mice
Housing and experimental procedures of all animals were performed in accordance with the Cantonal Veterinary Office (Zurich, Switzerland) under the license numbers ZH217/12 and ZH166/15 or the UK Home Office regulations (licence 70/8646) according to ARRIVE guidelines. Animals were maintained under specific pathogen free (SPF) conditions at the University of Zurich or at the CRUK Beatson Institute, Glasgow (Supplementary Table 1). Germ-free housing of mice was performed within the Clean Mouse Facility of the University of Bern.17 A detailed description of the generation of each mouse line is provided in the Supplementary Material.
Antibiotic (ABX) Treatment
Ciprofloxacin (200 mg/L) (Fluka; Buchs, Switzerland), ampicillin sodium (1 g/L) (Sigma; St. Louis, MO), metronidazole (1 g/L) (Fluka), and vancomycin HCl (500 mg/L) (Fluka) were added to the drinking water of cohoused, 1-month-old wild-type control and Mcl1ΔIEC mice for 4 weeks.18
Innate Lymphoid Cell (ILC) Depletion
Two-month-old Mcl1ΔIECRag1−/− mice were treated with α-Thy1.2–depleting antibody (200 μg) (BioxCell – clone:30H12) or appropriate isotype control via intraperitoneal injection, 3 times per week for 4 weeks.
WNT Signaling Inhibition
i-Mcl1ΔIEC mice or wild-type controls received 5 mg/kg of WNT974 (provided via an material transfer agreement (MTA) with Novartis) orally, twice per day, in a 0.5% methylcellulose, 0.5% tween 80 vehicle.
BrdU Proliferation Analysis
BrdU (Amersham Biosciences; Buckinghamshire, UK) was administered via intraperitoneal injection 2 hours before mice were killed. The number of BrdU-positive crypts was determined by calculating the average number of BrdU-positive crypt cells per half crypt from 50 individual crypts.
Intestinal Epithelial Barrier Permeability
Intestinal epithelial barrier permeability was measured using fluorescein isothiocyanate–labeled dextran (FD-4) as previously described.19 Serum expression of FD-4 was determined using a BioTek Microplate Reader (BioTek; Winooski, VT).
Endoscopy
Direct visualization of colonic mucosal damage of wild-type and Mcl1ΔIEC mice was performed via high-resolution endoscopy using a “Coloview system” (Karl Storz, Tuttlingen, Germany). Mice were provided with food and water as normal until the endoscopy was performed. Isoflurane inhalation (2.0%–2.5%) in oxygen was used to anesthetize the mice for the duration of the procedure.
Calprotectin Analysis
Calprotectin (S100A8 and S100A9) levels were determined from homogenates of colon tissue (real-time polymerase chain reaction) or feces (enzyme-linked immunosorbent assay). Calprotectin primer sequences: S100A8 - Forward AAATCACCATGCCCTCTACAAG and Reverse CCCACTTTTATCACCATCGCAA, S100A9 – Forward ATACTCTAGGAAGGAAGGACACC and Reverse TCCATGATGTCATTTATGAGGGC. Reference gene (Gapdh) - Forward CCACCCCAGCAAGGAGACT and Reverse GAAATTGTGAGGGAGATGCT. Protein levels of Calprotectin (S100A8 and S100A9) were determined by enzyme-linked immunosorbent assay (Immune Diagnostik, #K6936; Bensheim, Germany).
Histology
Formalin-fixed tissue was embedded in paraffin. Paraffin sections were rehydrated and heat-induced antigen retrieval was performed in either Tris/EDTA/BORAT or citrate buffer. Incubation in Ventana buffer and staining was performed on a BenchmarkUltra immunohistochemistry robot (Ventana Instruments) using Chromo Map or Optiview Dab Detection Kits (Ventana) or on a Bond MAX (Leica, Wetzlar, Germany). All primary antibodies used for immunohistochemistry are listed in Supplementary Table 2. A detailed description of the criteria used for the histological scoring is available in the Supplementary Material (Supplementary Table 3).
Tumor Classification
Hematoxylin and eosin–stained slides of intestinal lesions, blinded for genotype and housing conditions, were independently evaluated by 2 pathologists. Tumor classification was based on established nomenclature and criteria for the histologic assessment of intestinal tumors in mice.20 According to the criteria for the classification of human intestinal tumors, a small bowel neoplasm with infiltrative growth in the mucosa was classified as a carcinoma.21 In cases in which mice developed multiple tumors, classification was performed according to the highest lesion.
In Situ Hybridization
In situ hybridization was performed on formalin-fixed paraffin-embedded (FFPE) tissues according to the manufacturer`s protocol using 4 commercially available probes. A detailed description of all probes used in this study can be found in the Supplementary Material.
Ex Vivo Cytokine Expression Analysis
Ex vivo cytokine analysis was performed using supernatant recovered from colon cultures as previously described.22 Supernatant was collected after 48 hours, and cytokine expression was determined using Multiplex analysis kits (Bio-Rad Laboratories, Hercules, CA) and read using Bio-Plex MAGPIX Multiplex Reader powered by Luminex XMAP Technology (Bio-Rad Laboratories).
Sequencing
Genomic DNA was isolated from FFPE tissue blocks using Macherey & Nagel’s (Duren, Germany) NucleoSpin FFPE Kit and analyzed by Sanger sequencing or whole exome sequencing. A detailed description of the sequencing protocols used are available in the Supplementary Material.
RNA-sequencing (RNASeq) analysis was performed on RNA extracted from whole small intestine tissue of i-Mcl1ΔIEC mice (4 days post deletion) or i-ApcΔIEC and i-ApcΔIECMcl1ΔIEC mice (3 days post deletion). A detailed description of the RNASeq protocol is provided in the Supplementary Tables 4–9. Gene set enrichment analysis (GSEA) was performed using the GSEA v2.0 software (Broad Institute). The comparison gene sets were obtained from published sources; genes upregulated following APC loss23 and WNT target genes commonly upregulated in human colorectal cancer.24
Single Molecule Fluorescence In Situ Hybridization (smFISH)
smFISH was performed as previously described.25 A comprehensive description of the protocol, including quantification and probes used (Supplementary Tables 10–12), is provided in the Supplementary Material.
Statistical Analysis
Statistical analyses were conducted as indicated within each figure legend. For all analyses, ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001. Statistical analyses were conducted using GraphPad Prism 5/7 software (La Jolla, CA).
Results
MCL1 Is a Key Regulator of Intestinal Homeostasis
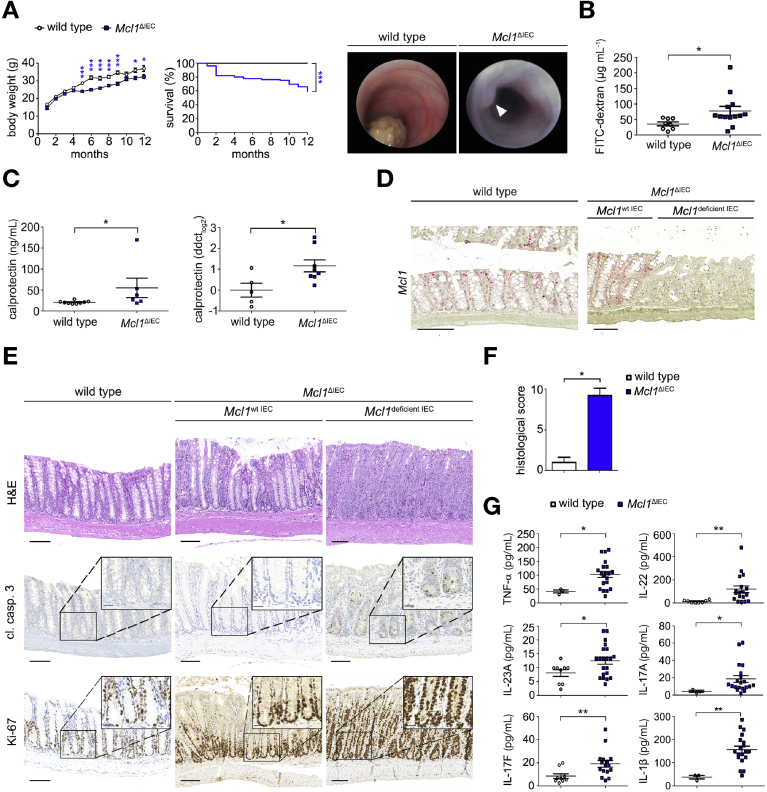
To unravel the physiological role of MCL1 within the intestinal tract, we generated mice with an IEC-specific deletion of Mcl1 (Mcl1ΔIEC mice) (Supplementary Figure 1A). Compared with littermate controls, Mcl1ΔIEC mice developed extensive diarrhea, reduced weight gain, and significantly increased mortality (Figure 1A). Colonoscopies performed on 4-month-old Mcl1ΔIEC mice revealed extensive thickening and whitening of the colonic wall and distinct polyp formation (Figure 1A). Furthermore, 2-month-old Mcl1ΔIEC mice displayed an impaired epithelial barrier function, as demonstrated by increased permeability of orally administered fluorescein isothiocyanate–labeled dextran (Figure 1B). Elevated levels of S1008/S1009 (calprotectin), a biomarker of intestinal inflammation, were also detected in stool samples and tissue homogenates from Mcl1ΔIEC mice (Figure 1C).
Figure 1.
MCL1 is essential for maintaining intestinal homeostasis. (A) Mcl1ΔIEC mice show reduced weight gain (n = 50) and survival (n = 126) compared with littermate controls (n = 34 and n = 61, respectively). Representative colonoscopy images from 4-month-old mice. (B) Two-month-old Mcl1ΔIEC mice display increased serum levels of FITC dextran (n = 13) compared with littermate controls (n = 8) 4 hours after oral administration. (C) Calprotectin (S100A8/S100A9) levels of 2-month-old Mcl1ΔIEC mice and age-matched controls by enzyme-linked immunosorbent assay (ELISA) (n = 6 Mcl1ΔIEC mice, n = 9 wild type) or real-time polymerase chain reaction (n = 8 Mcl1ΔIEC mice, n = 5 wild type). (D) Representative images illustrating Mcl1 expression in the colon (red stain) as demonstrated using in situ hybridization (scale bars: 100 μm). (E) Representative images from the colon of two-month-old Mcl1ΔIEC mice compared with age-matched controls (scale bars: 100 μm , 25 μm-inserts). (F) Blinded histological scores comparing the colon of 2-month-old Mcl1ΔIEC mice with littermate controls (n = 5). (G) Colon cultures established from 2-month-old mice and analyzed using multiplex analysis (minimum n = 10 per group). Data presented as either bar charts or scatter plot graph show mean values ± SEM. Statistical analyses were conducted by 1-way analysis of variance with Bonferroni correction (A [body weight], G), log rank (Mantel-Cox test) (A, survival), Student t test (B and C), or Mann-Whitney test (F), where ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001.
Interestingly, not all IECs within Mcl1ΔIEC mice underwent Mcl1 recombination and subsequently Mcl1ΔIEC mice displayed areas that retained Mcl1 expression alongside areas of Mcl1 deficiency. Consequently, areas of severe mucosal damage appeared alongside histologically normal regions of both the small intestine and colon. Interestingly, IECs in histologically normal mucosal regions retained Mcl1 messenger RNA expression, whereas IECs from damaged mucosal regions lacked Mcl1 (Figure 1D). Furthermore, S1008/S1009 levels were inversely correlated with Mcl1 expression, indicating the severity of intestinal damage was closely correlated with Mcl1 deficiency (Supplementary Figure 1B). Histopathological analysis of 2-month-old Mcl1ΔIEC mice showed architectural disarray with crypt hyperplasia, villous atrophy (small intestine), increased IEC apoptosis (rather than necroptosis) (cleaved caspase 3), and hyperproliferative crypts (Ki-67) throughout both the colon and small intestine (Figure 1E and F, Supplementary Figure 1C–F). Notably, deleting a single copy of the Mcl1 gene was not sufficient to induce the histopathological damage observed in Mcl1ΔIEC mice (Supplementary Figure 2A).
MCL1 Deficiency Results in Microbiota-induced Chronic Inflammation
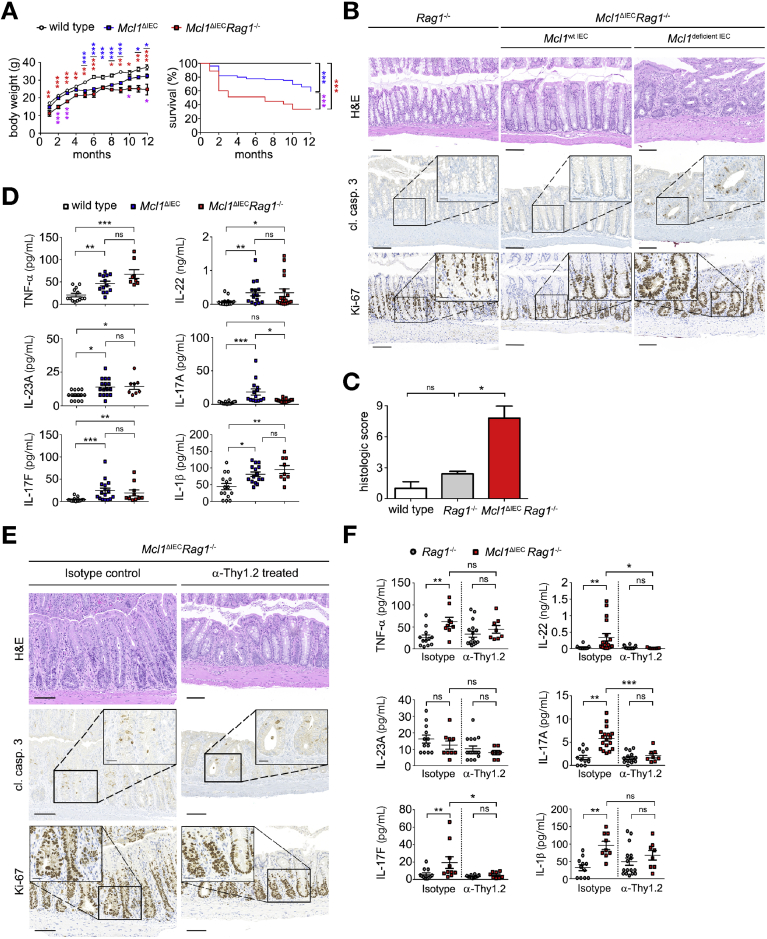
In addition to the impaired epithelial barrier function described previously, we also observed a marked decrease in the protective mucus layer at the intestinal mucosal barrier as well as extensive bacterial translocation from the lumen into the mucosa in Mcl1ΔIEC mice (Supplementary Figure 3A). Taken together, we concluded that MCL1 deficiency resulted in major epithelial cell dysfunction and chronic intestinal inflammation. Indeed, large lymphoid follicles, comprising mainly B and T cells, as well as an increased number of infiltrating immune cells, were detected within the mucosa of Mcl1ΔIEC mice (Supplementary Figure 4A). In addition, proinflammatory cytokines TNF-α, interleukin (IL)-22, IL-23A, IL-17A, IL-17F and IL-1β were significantly in Mcl1ΔIEC mice (Figure 1G and Supplementary Figure 4B). To determine the contribution of adaptive immune cells to mucosal inflammation and subsequent pathology following Mcl1 deletion, Mcl1ΔIEC mice were inter-crossed with Rag1−/− mice (Mcl1ΔIECRag1−/− mice). Unexpectedly, Mcl1ΔIECRag1−/− mice exhibited significantly reduced weight gain and shorter survival compared with both wild-type and Mcl1ΔIEC mice (Figure 2A). In addition, Mcl1ΔIECRag1−/− mice also retained increased IEC apoptosis, hyperproliferative crypts, extensive mucosal damage (Figure 2B and C, Supplementary Figure 5A and B), and elevated levels of proinflammatory cytokines despite lacking conventional T cells (Figure 2D, Supplementary Figure 5C and D). Given the observed cytokine profile, we postulated that ILCs26 were driving chronic intestinal inflammation, at least in the absence of conventional T cells. To test this hypothesis, ILCs were depleted in 2-month-old Mcl1ΔIECRag1−/− mice following a 4-week treatment with α-Thy1.2 antibody. Thy1 (CD90) positive cell depletion (Supplementary Figure 6A) improved MCL1 deficiency–associated damage and resulted in the significant reduction of proinflammatory cytokines IL-22 and IL-17F (Figure 2E and F, Supplementary Figure 6B); however, increased IEC apoptosis and hyperproliferation persisted after ILC depletion (Figure 2E). Thus, by showing that MCL1 is indispensable for intestinal homeostasis, we identified a novel function for MCL1, not observed for family members BCL2 and BCL2L1.12,13
Figure 2.
MCL1 deficiency–associated chronic inflammation is mediated by ILCs. (A) Genetic depletion of Rag1 within Mcl1ΔIEC mice significantly reduced weight gain and survival (n = 19). Blue asterisk: Mcl1ΔIEC mice compared with littermate controls, red asterisk: Mcl1ΔIECRag1−/− mice compared with littermate controls, purple asterisk: Mcl1ΔIEC mice compared with Mcl1ΔIECRag1−/− mice. (B) Representative images from the colon of 2-month-old Rag1−/− control mice compared with Mcl1ΔIECRag1−/− mice showing impaired intestinal architecture (hematoxylin and eosin [H&E]), increased IEC apoptosis (cl. casp. 3) and hyperproliferation (Ki-67) (scale bars: 100 μm, 25 μm-inserts). (C) Blinded histological scores comparing 2-month-old Mcl1ΔIECRag1−/− mice with Rag1−/− mice (n = 5). (D) Colon cultures established from 2-month-old mice and analyzed using multiplex analysis (minimum n = 8 per group). (E) Representative images from colons of 3-month-old Mcl1ΔIECRag1−/− mice following 4 weeks of α-Thy1.2 depleting antibody or corresponding isotype administration (scale bars: 100 μm, 25 μm-inserts). (F) Colon cultures established from 3-month-old mice and analyzed for TNF-α, IL-22, IL-23A, IL-17A, IL-17F, and IL-1β expression using multiplex analysis (minimum n = 8 per group). Data presented as either bar charts or scatter plot graph show mean values ± SEM. Statistical analyses were conducted by 1-way analysis of variance with Bonferroni correction (A [body weight], D and F), log rank (Mantel-Cox test) (A, survival) or Mann-Whitney test (C) where ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001.
Increased IEC Apoptosis and Hyperproliferation Are a Direct Consequence of Mcl1 Deletion
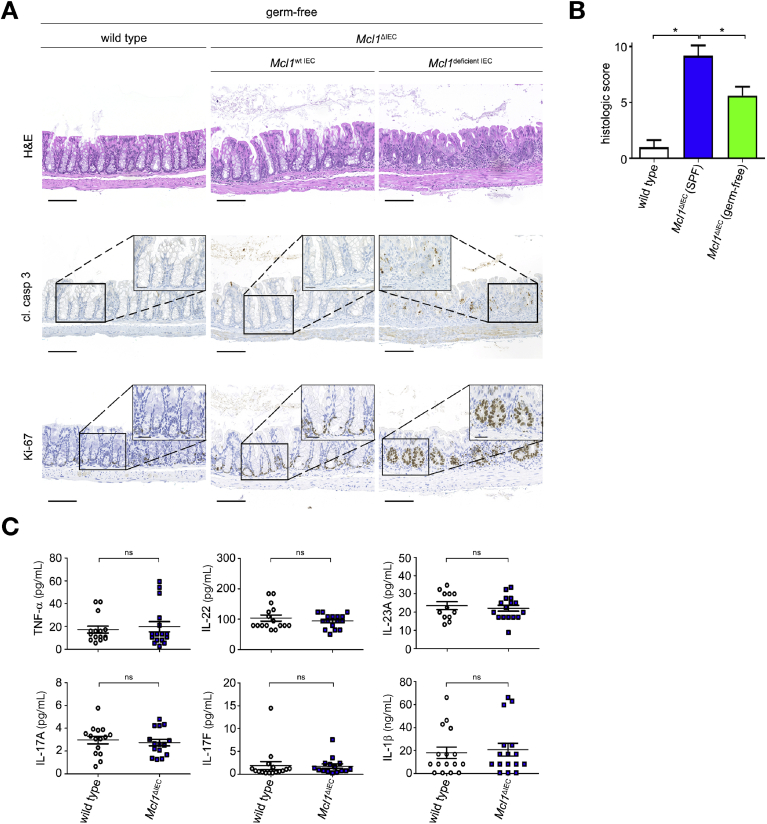
To delineate whether the increased IEC apoptosis and hyperproliferation observed in Mcl1ΔIEC mice were caused by the aforementioned chronic inflammation, or a direct result of MCL1 deficiency, Mcl1ΔIEC mice were either placed under antibiotic treatment (ABX),18 or re-derived under germ-free conditions17 (Supplementary Figure 7A and B). Following 4 weeks of ABX treatment, 2-month-old Mcl1ΔIEC mice showed attenuated damage and significantly reduced intestinal inflammation compared with untreated Mcl1ΔIEC mice (Supplementary Figure 8A); however, ABX-treated Mcl1ΔIEC mice retained increased IEC apoptosis and hyperproliferative crypts (Supplementary Figure 8A). Similarly, germ-free Mcl1ΔIEC mice displayed increased mucosal healing and a distinct lack of the immune cell infiltration observed in SPF Mcl1ΔIEC mice (Figure 3A and B, Supplementary Figures 8B and C and 9A and B). Mcl1 deletion under germ-free conditions did not result in increased proinflammatory cytokine production when compared with littermate controls, despite evidence suggesting an impaired intestinal epithelial barrier (Figure 3C, Supplementary Figures 8D and 10A); however, ameliorating chronic intestinal inflammation did not prevent the increased IEC apoptosis or hyperproliferation associated with Mcl1 deletion (Figure 3A, Supplementary Figure 8B). Collectively, these data show that whereas MCL1 deficiency–related chronic inflammation was due to an uncontrolled immune response against disseminating microbiota, increased IEC apoptosis and hyperproliferation directly result from MCL1 deficiency.
Figure 3.
IEC apoptosis and hyperproliferation are a direct consequence of Mcl1 deletion. (A) Representative images from the colon of 2-month-old germ-free Mcl1ΔIEC mice compared with littermate controls illustrating increased IEC apoptosis (cl. casp. 3) and hyperproliferation (Ki-67) (scale bars: 100 μm, 25 μm-inserts). (B) Blinded histological scores comparing 2-month-old Mcl1ΔIEC mice with littermate controls under germ-free conditions (n = 5 per group). (C) Colon cultures established from 2-month-old germ-free mice and analyzed using multiplex analysis (minimum n = 15 per group). Data presented as either bar charts or scatter plot graphs represents mean values ± SEM. Statistical analyses were conducted by Mann-Whitney test (B) or by 1-way analysis of variance with Bonferroni correction (C) where ∗P ≤ .05.
MCL1 Is Essential for Intestinal Stem Cell Homeostasis and Differentiation
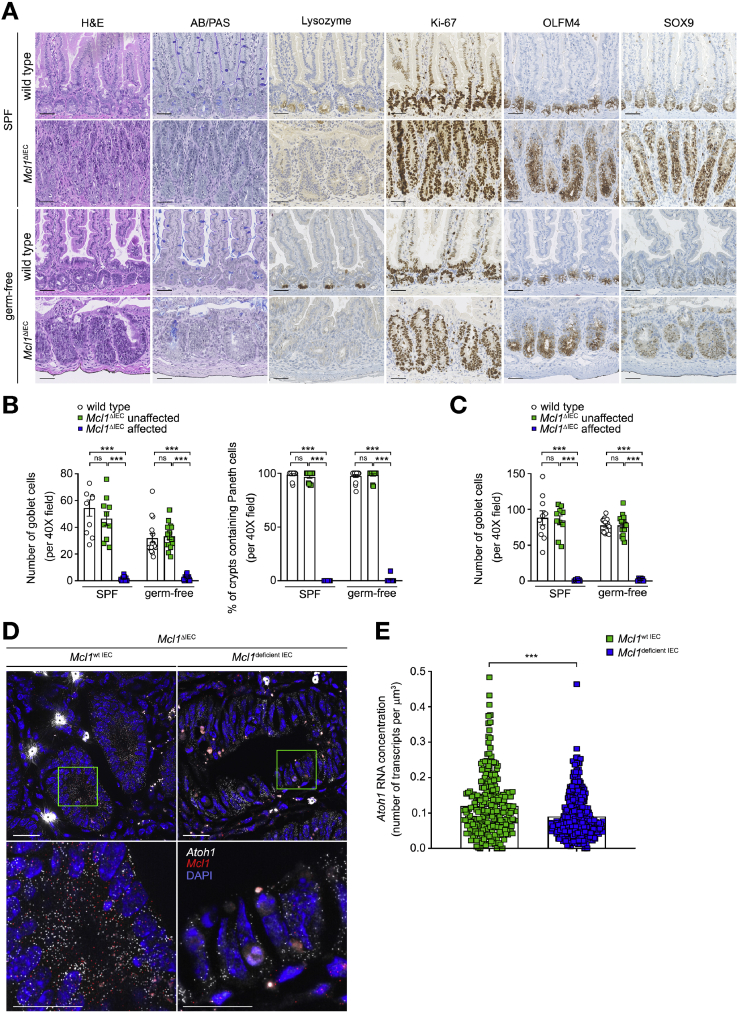
Morphological analysis of the crypt compartments (Figure 1E) suggested that cellular compartmentalization was significantly dysregulated throughout the small intestine and colon of Mcl1ΔIEC mice. Indeed, immunohistochemical analysis revealed that, following Mcl1 deletion, intestinal stem cells (ISCs) were not capable of differentiating toward secretory lineages. Mcl1ΔIEC mice showed a complete loss of both goblet (Alcian blue/periodic acid–Schiff positive) and Paneth cells (Lysozyme positive) in damaged (MCL1 deficient) areas (Figure 4A–C and Supplementary Figure 11A). Of note, in unaffected areas (MCL1 positive), ISCs retained their differentiation capabilities, and produced both goblet and Paneth cells at a comparable rate to control mice (Figure 4B and C). Impaired ISC differentiation was also observed in affected compartments of germ-free Mcl1ΔIEC mice, delineating the necessity for MCL1 in retaining intestinal homeostasis and ISC differentiation from chronic inflammation.
Figure 4.
MCL1 is essential for ISC differentiation. (A) Representative images from the small intestine of 2-month-old Mcl1ΔIEC mice compared with age-matched controls illustrating impaired goblet (Alcian blue/periodic acid–Schiff [AB/PAS]) and Paneth cell (lysozyme) differentiation, increased proliferation (Ki-67) and retention of stem cell markers (OLFM4 and SOX9) (scale bars: 100 μm). (B) Quantification of total goblet (AB/PAS) and Paneth cells (lysozyme) in the small intestine. (C) Quantification of total goblet (AB/PAS) cells in the colon. (D) Representative images from the small intestine of Mcl1ΔIEC mice visualizing Atoh1 expression in Mcl1-positive versus Mcl1-deficient IECs using smFISH. White dots represents single molecules of Atoh1 and red dots represent Mcl1. ∗Indicates overexposed nonspecific signal. Green boxes mark the areas shown in higher magnification below (scale bars: 25 μm-upper, 10 μm-lower). (E) Quantification of Atoh1 smFISH signal (n = 7 per group). Data presented as either bar charts or scatter plot graph show mean values ± SEM. Statistical analyses were conducted by Mann-Whitney test (B, C, and E) where ∗∗∗P ≤ .001.
Interestingly, MCL1-deficient undifferentiated and hyperproliferative cells also retained the ISC markers OLFM4, SOX9, and Lgr5 (restricted to the base of the crypts under steady-state conditions) throughout mid to apical parts of crypts (Figure 4A and Supplementary Figure 11B). To elucidate the molecular basis of this impaired ISC differentiation, we focused on the expression of Atoh1, an essential transcription factor for the differentiation of ISC toward the secretory cell lineages.27 Interestingly, we observed a significant reduction in Atoh1 expression in Mcl1-deficient ISCs compared with Mcl1-positive ISCs within the same mouse using smFISH (Figure 4D and E and Supplementary Figure 12A and B). These results suggest that, in the absence of MCL1, the axis of ISC proliferation and differentiation is constrained and polarized toward a highly proliferative ISC-dominant environment.
Given the well-established connection among hyperproliferation, replicative stress, and DNA damage,16,28 we next sought to look for evidence of DNA damage accumulation. Immunohistochemical staining for the DNA damage response marker γH2AX, revealed strong positivity within the crypts of Mcl1ΔIEC mice. γH2AX positivity was consistently found in Mcl1-deficient IEC but not in Mcl1-expressing IEC or IEC of littermate controls (Supplementary Figure 13A and B). Furthermore, γH2AX positivity was also observed under germ-free conditions, independent of chronic inflammation. Altogether, MCL1 deficiency restricts ISCs to an undifferentiated and hyperproliferative state that makes the ISC susceptible to the accumulation of DNA damage.
MCL1-deficiency–Associated ISC Hyperproliferation Is Dependent on WNT Signaling
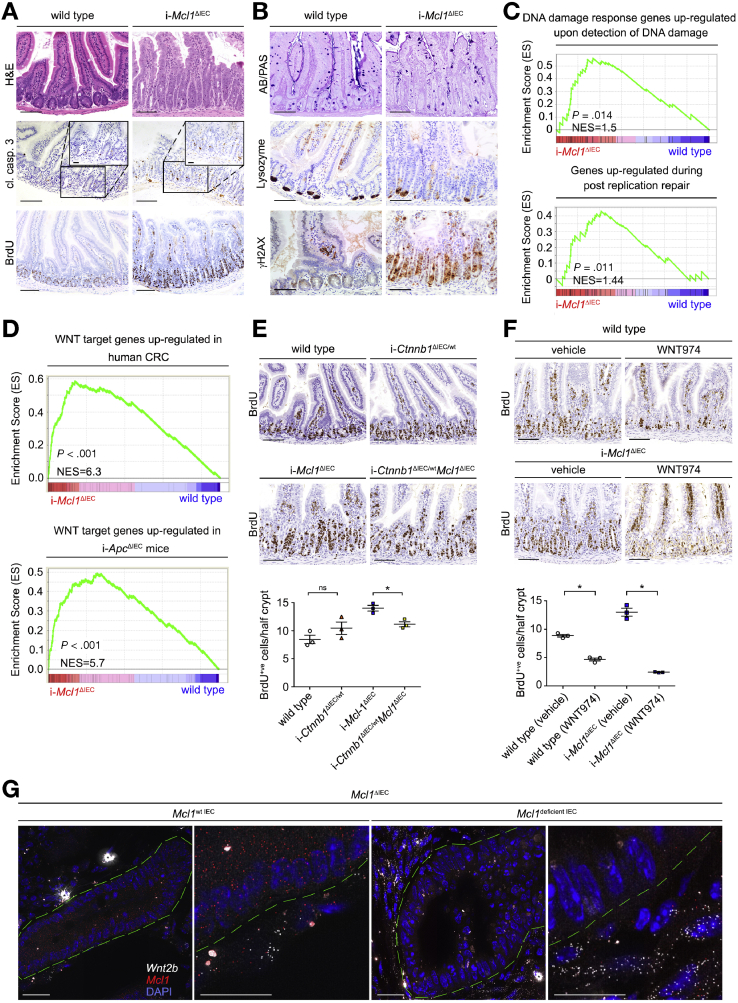
To further investigate the direct consequences of Mcl1 deletion and how it translates to IEC hyperproliferation and loss of differentiation, we generated a tamoxifen-inducible IEC-specific Mcl1 knockout mouse (i-Mcl1ΔIEC mice). Importantly the phenotype of i-Mcl1ΔIEC mice closely recapitulated the phenotype of Mcl1ΔIEC mice. The epithelium of i-Mcl1ΔIEC mice displayed architectural disarray with crypt hyperplasia, villous atrophy (small intestine), increased IEC apoptosis, and IEC hyperproliferation throughout the small intestine and the colon (Figure 5A, Supplementary Figure 14A). i-Mcl1ΔIEC mice also displayed impaired epithelial barrier function and a markedly increased immune infiltration (3–4 days post induction) (Supplementary Figure 14A–C). ISC differentiation to secretory lineages was also significantly reduced in i-Mcl1ΔIEC mice, illustrated by a marked reduction in both goblet and Paneth cells following Mcl1 deletion (Figure 5B). In addition, i-Mcl1ΔIEC mice also displayed strong γH2AX positivity, detectable throughout the small intestine and colon following Mcl1 deletion (Figure 5B). In addition, GSEA (from RNASeq data), indicated a strong enrichment for genes commonly upregulated in response to the detection of DNA damage and postreplication DNA repair in i-Mcl1ΔIEC mice compared with littermate controls (Figure 5C and Supplementary Figure 15A). These data further corroborated that the γH2AX positivity associated with Mcl1 deletion was indeed indicative of DNA damage.
Figure 5.
MCL1 protects the small intestine from activating WNT signaling and DNA damage. (A and B) Representative images from the small intestine of i-Mcl1ΔIEC mice and age-matched controls (4 days post induction) (scale bars: 100 μm, 25 μm-insert). (C) GSEA carried out on RNASeq data from i-Mcl1ΔIEC mice compared with littermate controls. (D) GSEA analysis tested for WNT target genes commonly upregulated in human CRC (upper) or in i-ApcΔIEC mouse models (lower). (E) BrdU staining from the small intestines of wild-type, i-Ctnnb1ΔIEC/wt, i-Mcl1ΔIEC, and i-Ctnnb1ΔIEC/wtMcl1ΔIEC mice, 4 days post induction (scale bars: 100 μm). Quantification of BrdU staining (positive cells/half crypt) is shown below (n = 3). (F) BrdU staining from the small intestines of vehicle-treated wild-type and i-Mcl1ΔIEC mice compared with their WNT974-treated counterparts, 3 days post induction (scale bars: 100 μm). Quantification of BrdU staining (positive cells/half crypt) is shown below (n = 3). (G) Representative images from the small intestine of Mcl1ΔIEC mice visualizing Wnt2b expression in Mcl1-positive versus Mcl1-deficient IECs using smFISH. White dots represents single molecules of Wnt2b and red dots represent Mcl1. ∗Indicates overexposed nonspecific signal. Green dashed lines represent the perimeter of intestinal crypts (scale bars: 25 μm-lower magnification, 10 μm-higher magnification). All data presented as scatter plot graph represents mean values ± SEM. Statistical analysis was conducted by Mann-Whitney test (E and F) where ∗P ≤ .05.
i-Mcl1ΔIEC mice displayed complete Mcl1 recombination throughout the entire intestine (compared with the “patchy” recombination described in Mcl1ΔIEC mice) and so represented a valuable tool by which to more accurately investigate the molecular consequences to Mcl1 deletion (Supplementary Figure 16A). Interestingly, i-Mcl1ΔIEC mice displayed a strong activation of wound-healing programs and increased expression of WNT target genes as determined by RNASeq analysis (Supplementary Tables 13 and 14). Moreover, following Mcl1 deletion, GSEA also indicated an enrichment for genes commonly upregulated in human colorectal cancer (CRC), as well as mice with an IEC-specific deletion of adenomatous polyposis coli (Apc), a prototypical model for intestinal tumorigenesis29 (Figure 5D and Supplementary Figure 14D). To test whether MCL1 deficiency–associated hyperproliferation observed within the ISC compartment was dependent on WNT signaling, we co-deleted a single copy of the canonical WNT pathway mediator Ctnnb1 (β-catenin) in conjunction with Mcl1 (i-Ctnnb1ΔIEC/wtMcl1ΔIEC mice). Deleting a single copy of Ctnnb1 was sufficient to partially rescue the hyperproliferative phenotype associated with Mcl1 deletion (Figure 5E and Supplementary Figure 17A). Subsequently, i-Mcl1ΔIEC mice were treated with the WNT signaling/porcupine inhibitor WNT974. Strikingly, WNT974 treatment completely ameliorated hyperproliferation within the crypts of both the small intestine and colon of i-Mcl1ΔIEC mice (Figure 5F, Supplementary Figures 17B and 18A and B). Collectively, these data suggest that the impaired intestinal homeostasis following Mcl1 deletion inflicts a WNT-induced hyperproliferative state on undifferentiated IECs.
To determine whether increased WNT signaling observed following Mcl1 deletion was a direct or indirect consequence of Mcl1 deletion, we next used smFISH to visualize Wnt2b molecules within the stroma of Mcl1ΔIEC mice. First, Wnt2b expression levels were markedly increased within the stroma of damaged areas (Mcl1-deficient) compared with unaffected areas (Mcl1-expressing) (Figure 5G and Supplementary Figure 19A and B). Second, IECs did not express Wnt2b irrespective of Mcl1 expression. Wnt2b expression was completely restricted to the pool of Mcl1-positive cells within the stroma surrounding the intestinal crypts (Figure 5G and Supplementary Figure 19A and B).
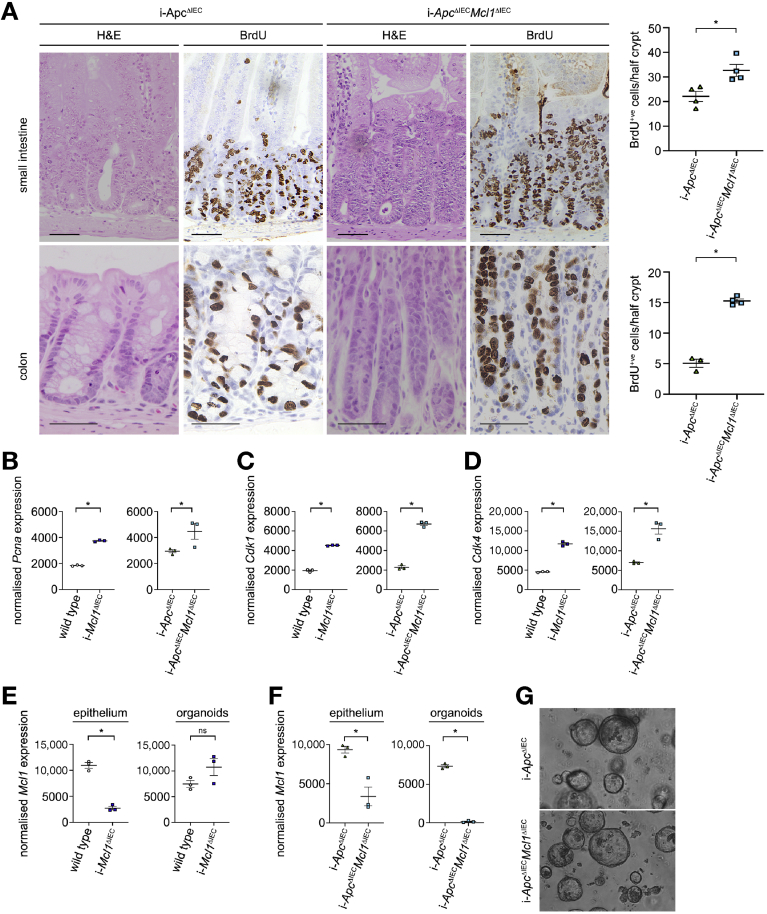
To delineate a potential effect of MCL1 deficiency in driving IEC proliferation from the effect of increased WNT signaling, we next generated a tamoxifen-inducible knockout mouse that facilitated the simultaneously delete both Mcl1 and the essential negative regulator of WNT signaling Apc (i-ApcΔIECMcl1ΔIEC mice). As previously described, Apc deletion (i-ApcΔIEC mice) resulted in increased crypt proliferation and subsequent hyperplasia as a result of constitutive WNT signaling (Figure 6A). Remarkably, co-deletion of Apc and Mcl1 resulted in a further significant increase in crypt proliferation throughout the small intestine and colon (Figure 6A). RNASeq analysis from these mice also showed significantly higher expression of essential proliferation associated genes Pcna, Cdk1, Cdk4, and E2f1, as well as marked increases in Ccnd1 and cMyc, which was not attributable to activated WNT signaling (Figure 6B and D and Supplementary Figure 20A–C). Similar to Mcl1ΔIEC mice, IEC proliferation was intrinsically linked to increased apoptosis irrespective of the increased WNT signaling (Supplementary Figure 20D). These results demonstrate that even in the presence of constitutive WNT signaling, the loss of Mcl1 directly increases IEC proliferation beyond the levels obtained by WNT signaling alone.
Figure 6.
MCL1 is an essential regulator of IEC proliferation. (A) Representative images of small intestinal or colonic tissue from i-ApcΔIEC and i-ApcΔIECMcl1ΔIEC mice (3 days post induction), and stained with hematoxylin and eosin (H&E) or for BrdU (scale bar: 50 μm). Quantification of BrdU-positive cells per half crypt from small intestine and colon (right). Normalized expression of Pcna (B), Cdk1 (C), and Cdk4 (D) in whole small intestine tissue from wild-type controls and i-Mcl1ΔIEC mice (4 post induction), or i-ApcΔIEC and i-ApcΔIECMcl1ΔIEC mice (3 post induction) and analyzed by RNASeq (n = 3 per group). (E) Normalized expression of Mcl1 in whole small intestinal tissue at day 4 post induction (left) or small intestine–derived organoids at passage ≥3 (right) from wild-type or i-Mcl1ΔIEC mice as analyzed by RNASeq (n = 3 per group). (F) Relative expression of Mcl1 in whole small intestinal tissue at day 3 post induction (left) or small intestine–derived organoids at passage ≥5 (right) in i-ApcΔIEC or i-ApcΔIECMcl1ΔIEC mice as analyzed by RNASeq (n = 3 per group). (G) Representative images of small intestine–derived organoids from i-ApcΔIEC or i-ApcΔIECMcl1ΔIEC mice (100x). All data presented as scatter plot graph represents mean values ± SEM. Statistical analysis was conducted by 1-tailed Mann-Whitney test where ∗P ≤ .05.
Despite the clear growth advantage observed in MCL1-deficient ISC in vivo, it was surprisingly not possible to establish a viable line of Mcl1-deficient organoids from Mcl1ΔIEC mice. Interestingly, although it was possible to establish a low yield of organoids from the small intestine of i-Mcl1ΔIEC mice, the organoids that formed had escaped recombination and retained Mcl1 expression (Figure 6E). Remarkably, organoids derived from the small intestine of i-ApcΔIECMcl1ΔIEC mice were viable and retained Mcl1 deficiency through at least 5 passages (Figure 6F–G and Supplementary Figure 21A). These observations suggest that the activation of WNT signaling and subsequent WNT saturated environment is essential for the survival of MCL1-deficient ISCs. Collectively, these results not only demonstrate that MCL1 deficiency is intricately linked to an activated WNT signaling pathway, but that a clear proliferation-enhancing effect exists that is directly attributable to MCL1 deficiency.
MCL1 Deficiency Results in Carcinoma Development
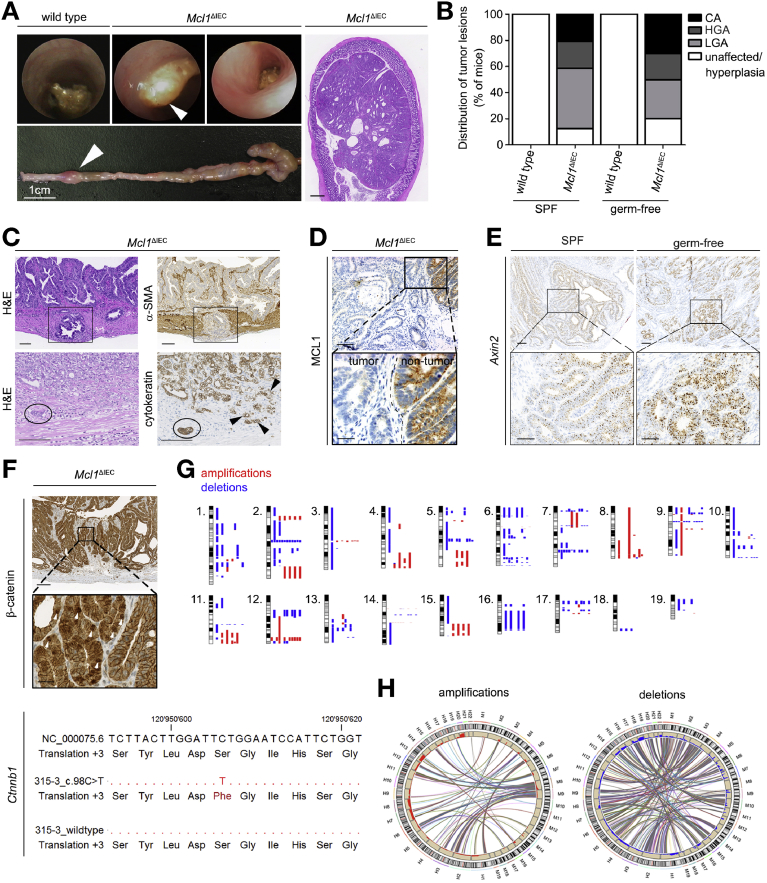
Given the increased proliferation and undifferentiated state of MCL1-deficient ISCs, together with the intestinal polyps observed in 4-month-old Mcl1ΔIEC mice (Figure 1A), we next aimed to monitor the long-term sequelae of MCL1 deficiency. Strikingly, we found that lifelong MCL1 deficiency resulted in intestinal tumorigenesis (Figure 7A). By 1 year, >80% of Mcl1ΔIEC mice displayed intestinal neoplasms within the small intestine and/or colon. Remarkably, intestinal tumors developed with similar incidence under both SPF and germ-free conditions, therefore independently of microbe-induced intestinal inflammation (Figure 7B). Lesions comprised a spectrum ranging from hyperplastic changes over low- and high-grade adenomas to invasive carcinomas,20 thus mirroring Fearon and Vogelstein’s30 adenoma-carcinoma-sequence of human intestinal carcinogenesis (Figure 7C, Supplementary Figure 22A). Carcinoma found in Mcl1ΔIEC mice extended deep into the muscularis propria (black box) and infiltrated intestinal vasculature (black circle) (Figure 7C). Of note, tumors were consistently MCL1 deficient and multiclonal in origin as shown by lineage tracing with the multicolor Cre-reporter R26R-Confetti31 (Figure 7D and Supplementary Figure 22B). MCL1-deficient tumors also displayed inter- and intratumoral heterogeneity with respect to differentiation markers such as CD44, CDX2, and synaptophysin (Supplementary Figure 22C). Furthermore, tumors displayed regional Axin2 positivity and β-catenin nuclear localization indicating responsiveness to active WNT signaling and/or Ctnnb1 mutations (Figure 7E and F and Supplementary Figure 22D).
Figure 7.
MCL1 protects from intestinal carcinoma development. (A) Representative images from colonoscopies (top), macroscopic imaging (bottom), and hematoxylin and eosin (H&E) characterization of colon carcinoma found in 18-month-old Mcl1ΔIEC mouse (scale bar: 500 μm) (right). (B) Percentage and classification of tumor lesions observed in 12-month-old SPF Mcl1ΔIEC mice (n = 42) compared with germ-free Mcl1ΔIEC mice (n = 12). White sections: no tumor lesions or mucosal hyperplasia, light gray sections: low-grade adenomas (LGA), dark gray sections: high-grade adenomas (HGA), black sections: carcinoma (CA). In cases where more than a single lesion was present, individual mice were classified according to the highest tumor lesion. (C) Immunohistochemical (IHC) staining illustrating the invasive growth of carcinoma in Mcl1ΔIEC mice. Carcinoma cells, α-SMA negative (black boxes), and cytokeratin positive (black arrowheads), actively invade the muscularis propria of an 18-month-old Mcl1ΔIEC mouse (scale bar: 100 μm scale bars). Black circle indicates vesicular invasion. (D) IHC analysis showing lack of MCL1 expression in tumors that develop in Mcl1ΔIEC mice (scale bar: 100 μm-upper, 25 μm-lower). (E) ISH for WNT target gene Axin2 (scale bar: 100 μm-upper, 50 μm-lower). (F) Representative IHC staining illustrating nuclear β-catenin localization (white arrowheads) observed in a carcinoma from a 12-month-old Mcl1ΔIEC mouse (scale bar: 100 μm-upper, 25 μm-lower). Below, a heterozygous missense point mutation in a mutational hot spot region of exon 2 of Ctnnb1 (NM_000075.6:c.98C>T - p.Ser33Phe) (3/5 carcinomas sequenced). (G) Array comparative genomic hybridization (aCGH) analysis of tumors isolated from 12-month-old Mcl1ΔIEC mice (from left to right: 8 carcinoma samples, 2 adenoma samples, and 2 unaffected samples from 18-month-old Mcl1ΔIEC mice). (H) Synteny analysis comparing chromosomal aberrations in carcinomas from Mcl1ΔIEC mice with human CRC (murine chromosomes: M1–M19; human chromosomes H1–H22). Statistical analyses for nonrandom distribution of matched copy number gains revealed P < .0001 for both gains and losses.
While progressing through the adenoma-carcinoma-sequence, tumors increasingly displayed genomic alterations in terms of nonrecurrent chromosomal aberrations as determined by array comparative genomic hybridization, as well as single nucleotide variants as determined by whole exome and Sanger sequencing (Figure 7G). Remarkably, Synteny analysis revealed a statistically significant overlap of chromosomal gains and losses with those found in human CRCs (78.3% of gains and 69.8% of losses; Figure 7H).32 Strikingly, single nucleotide variants comprised functionally relevant mutations in genes of the WNT pathway frequently also mutated in human CRC, such as CTNNB1, APC, FBXW7, and ARID130,32,33 (Supplementary Figure 23A).
Collectively, these findings demonstrate that carcinogenesis in Mcl1ΔIEC mice morphologically and genetically closely recapitulates human intestinal carcinogenesis.30,33 Furthermore, because tumors develop under germ-free conditions, MCL1 deficiency–related tumorigenesis is uncoupled from microbiota-induced chronic inflammation, a well-established promoter of intestinal carcinogenesis.34
Discussion
In this study, we have identified an essential role for MCL1 in preserving intestinal homeostasis and preventing carcinoma formation, previously undescribed among BCL2 antiapoptotic family members. These findings, in particular the microbiota-independency of MCL1 deficiency–associated tumorigenesis, clearly demonstrate an essential role for MCL1 in preserving intestinal homeostasis. These results may have important implications for understanding the pathogenesis of apoptotic enterocolopathy, IBD, and CRC development.
Disturbances in epithelial barrier integrity due to dysfunctional IEC–intrinsic molecular circuits that control tissue homeostasis, renewal, and the repair of IECs are cardinal features of both apoptotic enterocolopathy and IBD. Remarkably, intestinal pathology due to IEC-specific MCL1 deficiency shared hallmark features with IBD, including barrier dysfunction, chronic inflammation, increased IEC apoptosis, hyperproliferation, and impaired ISC differentiation.1,3,5 Thus, Mcl1ΔIEC mice may represent a pertinent preclinical model of both apoptotic enterocolopathy and IBD, while also opening up the possibility that (even a temporally restricted) dysregulation of MCL1 may contribute to the development of these, and similar, conditions.
Given the well-established increased risk for patients with IBD to develop intestinal carcinomas, which increases parallel to the duration and severity of their disease,35 it was interesting to discover that Mcl1ΔIEC mice also spontaneously developed intestinal carcinomas. Remarkably, however, although chronic inflammation has been established as a major driver of intestinal carcinoma,35 MCL1 deficiency–related carcinoma formation was independent of microbe-induced chronic inflammation.
Given the multifaceted and crucial roles of MCL1,36 various mechanisms are conceivable to explain MCL1 deficiency–related tumorigenesis. Of note, tumor lesions observed in Mcl1ΔIEC mice exhibited the full spectrum of human CRC development described by Fearon and Vogelstein,30 ranging from dysplastic crypts and low-grade adenoma to high-grade adenoma and carcinoma. Interestingly, tumors that developed as a result of MCL1 deficiency not only bear a striking similarity to human CRC, but revealed significant correlations with the subgroup of CRC characterized by active WNT signaling. These results indicate a potentially direct and immediate function for MCL1 in regulating IEC proliferation and suppressing tumor formation, the functional consequence of which becomes obvious in the presence of constitutive WNT signaling. This scenario not only explains the mutational spectrum observed in MCL1-deficient tumors, with clear enrichment of genes associated with WNT signaling,32 but also explains the differential viability observed in ex vivo organoid cultures derived from the intestines of i-Mcl1ΔIEC mice compared with those derived from i-ApcΔIECMcl1ΔIEC mice. Ex vivo culture of Mcl1-deficient organoids was only possible with the added deletion of Apc, indicating that the survival of Mcl1 deficient ISC is dependent on the cell-intrinsic activation of WNT signaling. At the same time, we clearly show that in the presence of constitutive WNT signaling, Mcl1 has a direct role in regulating ISC proliferation.
MCL1-deficient ISCs, which retain OLFM4, Lgr5, and SOX9 positivity, did not differentiate into the secondary secretory lineages, as illustrated by a complete loss of both Paneth and goblet cells. This loss of differentiation is restricted to Mcl1-deficient (phenotypic) regions of Mcl1ΔIEC mice. Interestingly, Mcl1-deficient ISCs showed a significant reduction of the transcription factor Atoh1, essential for the differentiation to secondary secretory lineages.27 However, ISC from Mcl1-expressing (nonphenotypic) areas retain high Atoh1 expression levels and differentiate to Paneth and goblet cells at a rate similar to wild-type ISCs. The inability of MCL1-deficient ISCs to differentiate to secretory cells was also observed under germ-free conditions, removing the potential influence of chronic inflammation. Whether or not this reduced Atoh1 expression is a direct result of MCL1 deficiency or is a secondary effect of a WNT signaling saturated environment deserves further investigation. These data suggest that MCL1 is essential for maintaining the strictly controlled balance between ISC proliferation and differentiation, and the polarization of ISCs toward a hyperproliferative state in the absence of MCL1 may facilitate genetic alterations predisposing to tumorigenesis.
In addition, the strong positivity of γH2AX following Mcl1 deletion within Mcl1ΔIEC and i-Mcl1ΔIEC mice also may be important in the context of tumor formation, and further studies are required to determine the significance of this observation. These γH2AX-positive cells potentially represent IECs that had accumulated DNA damage but escaped apoptotic programming. DNA damage is a well-established hallmark of tumorigenesis,28 and following Mcl1 deletion, may originate from the increased replicative stress. The potential role of MCL1 in maintaining DNA replication integrity is very intriguing, and our findings support recent publications describing increased replicative stress and higher susceptibility to DNA double-strand breaks37,38 and the strong synergistic effects of combining MCL1 and PARP-1 inhibitors to treat certain tumors.37,39 Collectively, these recent findings strongly suggest a nonredundant role for MCL1 in maintaining DNA replication integrity, the absence of which may result in DNA damage and potentially tumor initiation, as observed in Mcl1ΔIEC mice.
Conceptually, these findings show that, depending on the context, MCL1 dysregulation can promote tumorigenesis by different means. On one hand, although very rare in human cancers,40 it is conceivable that early steps of carcinogenesis may be induced by severely impaired intestinal homeostasis resulting from, even transient, gene alterations and loss of MCL1 function. On the other hand, amplifications of chromosome 1 resulting in overexpression of MCL1,36,41 a recurrent finding in a variety of cancers including CRC, is well known to confer a survival advantage within tumors.42 Thus, pharmacologic inhibition of MCL1 recently has emerged as an attractive target to increase the susceptibility of different tumors to conventional antitumor therapies.39,43,44 Although recently published MCL1 inhibitors have been shown to be tolerable and functional in a number of different cancer models,45,46 intestinal pathology due to pharmacological inhibition of MCL1 has not yet been extensively addressed. A complete lack of adverse effects within the intestinal epithelium would be surprising given the MCL1 dependency of IECs shown in this study.
In summary, we not only demonstrate the crucial role for MCL1 in maintaining intestinal homeostasis, but also uncover an unexpected tumor suppressor capacity for MCL1 within the intestinal tract. These findings may contribute to unravelling the early stages of intestinal cancer development, and may have important implications for current strategies aimed at pharmacologically targeting MCL1.45, 46, 47
Transcript Profiling
Sequencing data have been deposited at the European Nucleotide Archive under accession numbers PRJEB20295, PRJEB20400, PRJEB38846, PRJEB38847, and PRJEB38848.
Acknowledgments
We thank Marion Bawohl, Renaud Maire, Christiane Mittmann, Fabiola Prutek, Marcel Glönkler, and André Fitsche for excellent technical assistance, as well as Massimo Lopes, Ruaidhri Jackson, Gerhard Rogler, Martin Hausmann, and Andreas Diefenbach for sharing reagents and helpful discussion.
CRediT Authorship Contributions
Marc E Healy, PhD (Conceptualization: Equal; Data curation: Equal; Formal analysis: Equal; Writing – original draft: Lead). Yannick Boege, PhD (Conceptualization: Equal; Data curation: Equal; Formal analysis: Equal). Michael Hodder, PhD (Conceptualization: Equal; Data curation: Equal; Formal analysis: Equal). Friederike Böhm, PhD (Data curation: Supporting; Formal analysis: Supporting). Mohsen Malehmir, PhD (Data curation: Supporting; Formal analysis: Supporting). Anna-Lena Scherr, PhD (Data curation: Supporting; Formal analysis: Supporting). Jasna Jetzer, MSc (Data curation: Supporting; Formal analysis: Supporting). Lap Kwan Chan, PhD (Data curation: Supporting; Formal analysis: Supporting; Writing – review & editing: Supporting). Rossella Parrotta, PhD (Data curation: Supporting; Formal analysis: Supporting; Writing – review & editing: Supporting) Kurt Jacob, PhD (Data curation: Supporting; Formal analysis: Supporting). Laure-Alix Clerbaux, PhD (Data curation: Supporting; Formal analysis: Supporting). Susanne Kreutzer, PhD (Data curation: Supporting; Formal analysis: Supporting). Andrew Campbell, PhD (Data curation: Supporting; Formal analysis: Supporting). Ella Gilchrist, BSc (Data curation: Supporting; Formal analysis: Supporting). Kathryn Gilroy, BSc (Formal analysis: Supporting; Writing – review & editing: Supporting). Ann-Katrin Rodewald, MD (Data curation: Supporting; Formal analysis: Supporting). Hanna Honcharova-Biletska, MD (Data curation: Supporting; Formal analysis: Supporting). Roman Schimmer, MSc (Data curation: Supporting; Formal analysis: Supporting). Karelia Vélez, (Data curation: Supporting; Formal analysis: Supporting). Simone Büeler, MSc (Data curation: Supporting; Formal analysis: Supporting). Patrizia Cammareri, PhD (Data curation: Supporting; Formal analysis: Supporting). Gabriela Kalna, PhD (Data curation: Supporting; Formal analysis: Supporting). Anna S. Wenning, MSc (Data curation: Supporting; Formal analysis: Supporting). Kathy D. McCoy, PhD (Conceptualization: Supporting; Methodology: Supporting). Mercedes Gomez de Agüero, PhD (Data curation: Supporting). Henning Schulze-Bergkamen, MD (Conceptualization: Supporting; Methodology: Supporting; Resources: Supporting). Christoph Klose, PhD (Conceptualization: Supporting; Data curation: Supporting). Kristian Unger, PhD (Data curation: Supporting; Resources: Supporting). Andrew J. Macpherson, PhD (Conceptualization: Supporting; Resources: Supporting). Andreas E Moor, MD (Data curation: Supporting; Formal analysis: Supporting; Resources: Supporting; Supervision: Supporting; Writing – review & editing: Supporting). Bruno Köhler, MD (Conceptualization: Supporting; Data curation: Supporting; Resources: Supporting; Supervision: Supporting). Owen J. Sansom, PhD (Conceptualization: Equal; Formal analysis: Equal; Funding acquisition: Equal; Resources: Equal; Supervision: Equal; Writing – review & editing: Equal). Mathias Heikenwälder, PhD (Conceptualization: Equal; Formal analysis: Equal; Funding acquisition: Equal; Resources: Equal; Supervision: Equal; Writing – review & editing: Equal). Achim Weber, MD (Conceptualization: Equal; Formal analysis: Equal; Methodology: Equal; Project administration: Equal; Resources: Equal; Supervision: Equal; Writing – original draft: Supporting; Writing – review & editing: Lead).
Footnotes
Conflict of interest The authors disclose no conflicts.
Funding Achim Weber was supported by grants from the Swiss National Science Foundation (SNF 310030_146940), from the Krebsliga Zurich, and the Vontobel Stiftung Zurich, Switzerland. Henning Schulze-Bergkamen was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG, SCHU 1443 4–1). Mathias Heikenwälder was supported by an ERC consolidator grant (Hepatometabopath), an ERC Horizon 2020 (HEP-CAR) grant, the SFBTR 179, 209, 1335 and a grant by the Wilhelm-Sander Stiftung. Owen J. Sansom was supported by an ERC starting grant (311301-ColonCan). Owen J. Sansom and Patrizia Cammareri were supported by a Cancer Research UK core grant (A21139 and A17196). Michael C. Hodder was supported by an MRC doctoral training grant (MR/J50032x/1). Bruno Köhler was supported by grants DKH70113426 and DFG KO 5205/1–1, and Brigitte und Dr. Konstanze Wegener-Stiftung.
Author names in bold designate shared co-first authorship.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.03.017.
Contributor Information
Michael C. Hodder, Email: achim.weber@usz.ch.
Owen J. Sansom, Email: o.sansom@beatson.gla.ac.uk.
Mathias Heikenwälder, Email: m.heikenwaelder@dkfz.de.
Achim Weber, Email: achim.weber@usz.ch.
Supplementary Material
References
- 1.Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 2.van der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 3.Gunther C., Neumann H., Neurath M.F. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062–1071. doi: 10.1136/gutjnl-2011-301364. [DOI] [PubMed] [Google Scholar]
- 4.Iwamoto M., Koji T., Makiyama K. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol. 1996;180:152–159. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Karamchandani D.M., Chetty R. Apoptotic colopathy: a pragmatic approach to diagnosis. J Clin Pathol. 2018;71:1033–1040. doi: 10.1136/jclinpath-2018-205388. [DOI] [PubMed] [Google Scholar]
- 6.Dannappel M., Vlantis K., Kumari S. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–94. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin J., Kumari S., Kim C. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature. 2016;540:124–128. doi: 10.1038/nature20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunther C., Martini E., Wittkopf N. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newton K., Wickliffe K.E., Maltzman A. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature. 2016;540:129–133. doi: 10.1038/nature20559. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi N., Vereecke L., Bertrand M.J. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513:95–99. doi: 10.1038/nature13706. [DOI] [PubMed] [Google Scholar]
- 11.Welz P.S., Wullaert A., Vlantis K. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 12.Scherr A.L., Gdynia G., Salou M. Bcl-xL is an oncogenic driver in colorectal cancer. Cell Death Dis. 2016;7:e2342. doi: 10.1038/cddis.2016.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Heijden M., Zimberlin C.D., Nicholson A.M. Bcl-2 is a critical mediator of intestinal transformation. Nat Commun. 2016;7:10916. doi: 10.1038/ncomms10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opferman J.T., Iwasaki H., Ong C.C. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 15.Weber A., Boger R., Vick B. Hepatocyte-specific deletion of the antiapoptotic protein myeloid cell leukemia-1 triggers proliferation and hepatocarcinogenesis in mice. Hepatology. 2010;51:1226–1236. doi: 10.1002/hep.23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boege Y., Malehmir M., Healy M.E. A dual role of caspase-8 in triggering and sensing proliferation-associated DNA damage, a key determinant of liver cancer development. Cancer Cell. 2017;32:342–359.e10. doi: 10.1016/j.ccell.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith K., McCoy K.D., Macpherson A.J. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19:59–69. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Grivennikov S.I., Wang K., Mucida D. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fredenburgh L.E., Velandia M.M., Ma J. Cyclooxygenase-2 deficiency leads to intestinal barrier dysfunction and increased mortality during polymicrobial sepsis. J Immunol. 2011;187:5255–5267. doi: 10.4049/jimmunol.1101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boivin G.P., Washington K., Yang K. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 21.WHO Classification of Tumors Editorial Board. Digestive system tumours. Lyon (France): International Agency for Research on Cancer; 2019. (WHO classification of tumours series: 5th ed.; vol. 1). http://publications.iarc.fr/579.
- 22.Zenewicz L.A., Yancopoulos G.D., Valenzuela D.M. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sansom O.J., Reed K.R., Hayes A.J. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van der Flier L.G., Sabates-Bellver J., Oving I. The intestinal Wnt/TCF signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 25.Moor A.E., Harnik Y., Ben-Moshe S. Spatial reconstruction of single enterocytes uncovers broad zonation along the intestinal villus axis. Cell. 2018;175:1156–1167.e15. doi: 10.1016/j.cell.2018.08.063. [DOI] [PubMed] [Google Scholar]
- 26.Artis D., Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 27.Yang Q., Bermingham N.A., Finegold M.J. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 28.Zeman M.K., Cimprich K.A. Causes and consequences of replication stress. Nat Cell Biol. 2014;16:2–9. doi: 10.1038/ncb2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackstadt R., Sansom O.J. Mouse models of intestinal cancer. J Pathol. 2016;238:141–151. doi: 10.1002/path.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 31.Schepers A.G., Snippert H.J., Stange D.E. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., Sethi N.S., Hinoue T. Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell. 2018;33:721–735.e8. doi: 10.1016/j.ccell.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi C.R., Bakir I.A., Hart A.L. Clonal evolution of colorectal cancer in IBD. Nat Rev Gastroenterol Hepatol. 2017;14:218–229. doi: 10.1038/nrgastro.2017.1. [DOI] [PubMed] [Google Scholar]
- 36.Perciavalle R.M., Opferman J.T. Delving deeper: MCL-1's contributions to normal and cancer biology. Trends Cell Biol. 2013;23:22–29. doi: 10.1016/j.tcb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen G., Magis A.T., Xu K. Targeting Mcl-1 enhances DNA replication stress sensitivity to cancer therapy. J Clin Invest. 2018;128:500–516. doi: 10.1172/JCI92742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattoo A.R., Pandita R.K., Chakraborty S. MCL-1 depletion impairs DNA double-strand break repair and reinitiation of stalled DNA replication forks. Mol Cell Biol. 2017;37(3) doi: 10.1128/MCB.00535-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Annunziato S., de Ruiter J.R., Henneman L. Comparative oncogenomics identifies combinations of driver genes and drug targets in BRCA1-mutated breast cancer. Nat Commun. 2019;10:397. doi: 10.1038/s41467-019-08301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beroukhim R., Mermel C.H., Porter D. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juin P., Geneste O., Gautier F. Decoding and unlocking the BCL-2 dependency of cancer cells. Nat Rev Cancer. 2013;13:455–465. doi: 10.1038/nrc3538. [DOI] [PubMed] [Google Scholar]
- 42.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Pan R., Ruvolo V.R., Wei J. Inhibition of Mcl-1 with the pan-Bcl-2 family inhibitor (-)BI97D6 overcomes ABT-737 resistance in acute myeloid leukemia. Blood. 2015;126:363–372. doi: 10.1182/blood-2014-10-604975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hird A.W., Tron A.E. Recent advances in the development of Mcl-1 inhibitors for cancer therapy. Pharmacol Ther. 2019;198:59–67. doi: 10.1016/j.pharmthera.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Kotschy A., Szlavik Z., Murray J. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538:477–482. doi: 10.1038/nature19830. [DOI] [PubMed] [Google Scholar]
- 46.Tron A.E., Belmonte M.A., Adam A. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat Commun. 2018;9:5341. doi: 10.1038/s41467-018-07551-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiang W., Yang C.Y., Bai L. MCL-1 inhibition in cancer treatment. Onco Targets Ther. 2018;11:7301–7314. doi: 10.2147/OTT.S146228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.