Abstract
Myelin is a multilayer lipid membrane structure that wraps and insulates axons, allowing for the efficient propagation of action potentials. During developmental myelination of the CNS, oligodendrocyte progenitor cells proliferate and migrate to their final destination, where they terminally differentiate into mature oligodendrocytes and myelinate axons. Lineage progression and terminal differentiation of oligodendrocyte lineage cells are under tight transcriptional and post-transcriptional control. The characterization of a number of recently identified regulatory factors that govern these processes, which are the focus of this review, has greatly increased our understanding of oligodendrocyte development and function. These insights are critical to facilitate efforts to enhance oligodendrocyte progenitor cell differentiation in neurological disorders that disrupt CNS myelin.
Keywords: Oligodendrocytes, myelin, transcription factors, oligodendrocyte lineage progression
Transcriptional and post-transcriptional control of oligodendrocyte formation
During development, oligodendrocyte progenitor cells (OPCs) terminally differentiate and mature into myelinating oligodendrocytes. This differentiation is a tightly controlled process in which extracellular and intrinsic signals regulate transcriptional and physiological changes in the oligodendrocyte lineage cells. Over the past several decades, considerable insight has been gained into the molecular control of oligodendrocyte development, including the identification of myriad molecular cues that control these processes. These include extrinsic extracellular signals [1] as well as oligodendrocyte-intrinsic transcription factors [2], epigenetic modulators [3–5], microRNAs (miRNAs) [6], and signaling pathways [7].
In addition to the critical role that oligodendrocyte differentiation plays during development, newly formed myelinating oligodendrocytes are also generated in adults (see Box 1). Importantly, demyelinated axons are remyelinated by oligodendrocytes derived from adult OPCs, and remyelination failure results from a diminished capacity to produce myelinating oligodendrocytes [8]. In demyelinating diseases such as multiple sclerosis (MS), insufficient remyelination correlates with disease progression [9]. A full understanding of the factors that control oligodendrocyte differentiation during development and in adults is therefore of utmost importance. Here we review the most recent findings regarding the molecular control of oligodendrocyte development at the transcriptional, post-transcriptional, translational and post-translational levels.
Box 1. Non-developmental myelination in adults.
Myelination is a developmental process that begins around birth in rodents and is mainly completed by early adulthood. Therefore, myelination has been long thought to be a primarily developmental process, and myelin considered a nearly static structure in adults. Nevertheless, newly formed oligodendrocytes in the adult brain, derived from adult OPCs, can remyelinate demyelinated axons [9]. In addition, it has recently become increasingly evident that adult-born oligodendrocytes and myelin are crucial for motor-skill learning [94,95], and that myelin is a fairly dynamic structure that undergoes changes and remolding in adulthood [96]. These changes occur at least in part in response to neuronal activity, and are observed in behavioral settings such as skilled learning and social isolation [97]. The unique molecular cues that govern non-developmental CNS myelination, however, remain largely unknown.
Transcriptional control of oligodendrocyte development
Transcriptional control of oligodendrocyte development by transcription factors
During development, oligodendrocyte lineage cells arise from neuroepithelial cells in the ventricular zone. Once the lineage of each oligodendrocyte is specified, tightly-controlled feedback loops involving several transcription factors work to either maintain proliferating OPCs in their progenitor state and prevent their premature differentiation, or release them for differentiation into mature oligodendrocytes (reviewed thoroughly in [2]).
Newly arisen oligodendrocyte lineage cells express the transcription factors NK6 homeobox 1 (NKX6.1), NK6 homeobox 2 (NKX6.2), NK2 Homeobox 2 (NKX2.2) and Oligodendrocyte transcription factor 2 (OLIG2). OLIG2 and NKX2.2 are important determinants of oligodendrocyte differentiation and must be co-expressed as a precondition for differentiation to occur. In the developing neural tube, however, they are expressed in adjacent domains of the ventral ventricular zone and repress one another [10,11].
OLIG2 directly induces the expression of SRY-box 10 (SOX10), a transcription factor that is critical for oligodendrocyte maturation [12] (see Figure 1, Key Figure). In turn, the positive feedback loop between SOX10 and OLIG2 maintains OLIG2 expression in SOX10-expressing cells [13]. The calcineurin-mediated activation of the nuclear factor of activated T cells (NFAT) protein NFATC2 represses the inhibitory effect of OLIG2 on SOX10-mediated NKX2.2 expression and vice versa; NFATC2 also relieves the inhibitory effect of NKX2.2 on SOX10-mediated OLIG2 expression. Hence, calcineurin-mediated activation of NFATC2 allows for co-expression of OLIG2 and NKX2.2, which in turn results in the initiation of oligodendrocyte differentiation [14].
Figure 1, Key Figure: Main axes of SOX10-mediated oligodendrocyte differentiation.
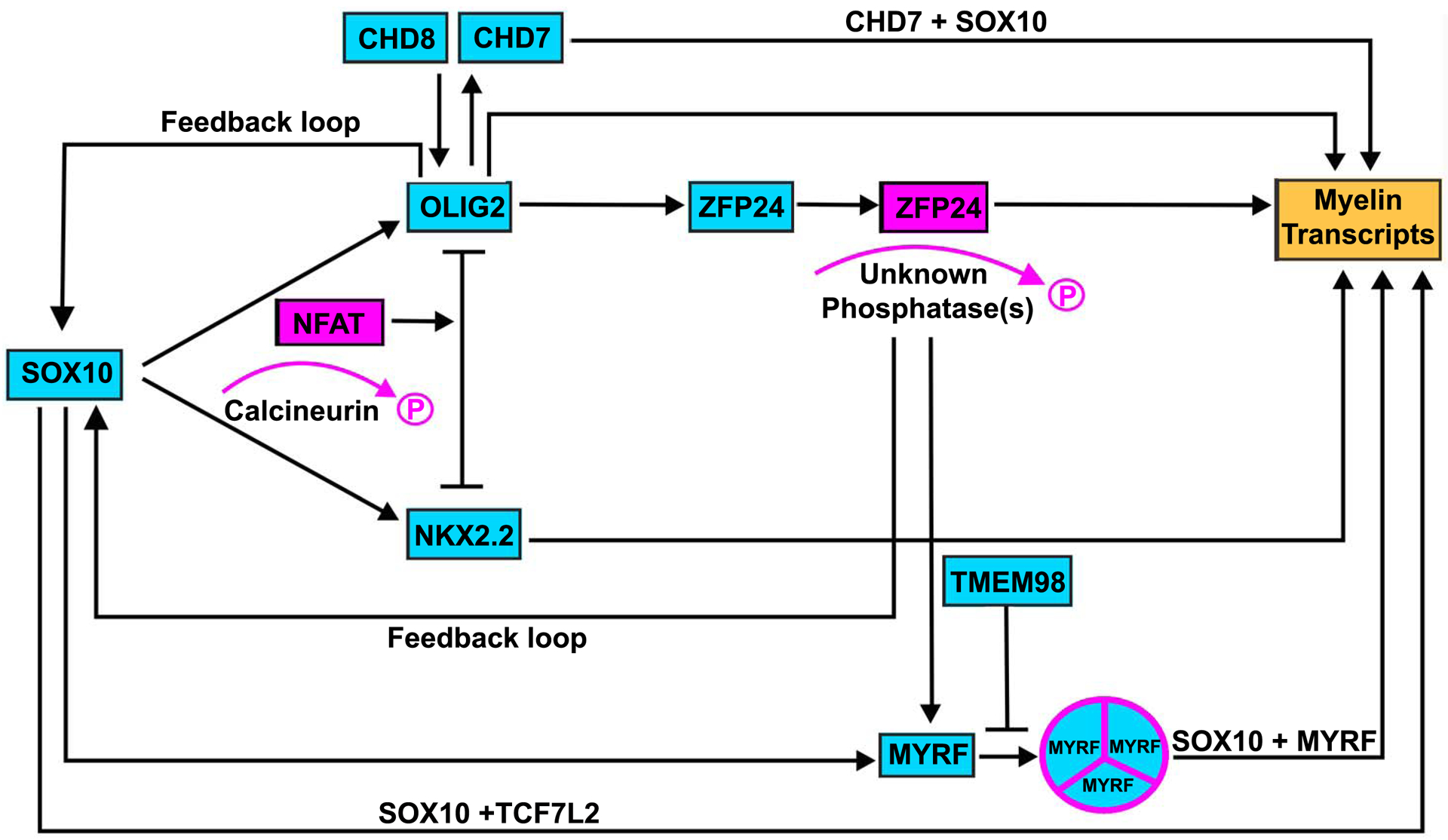
Four main SOX10 axes have been recently characterized: the CHD8-BRG1-OLIG2 axis, the Calcineurin-NFAT axis, the MYRF axis and the TCF7L2 axis. The proteins that are involved in this circuit are marked in cyan, myelin transcripts are marked in yellow, and post-translational modifications (de-phosphorylation of NFAT and ZFP24 and formation of MYRF-homotrimer) are marked in magenta.
The role of SOX10-interacting proteins in oligodendrocyte differentiation
Full differentiation will not occur, however, without the interaction of SOX10 with a number of other proteins, such as myelin regulatory factor (MYRF) [15,16]. Only very recently has SOX10 been recognized as a major determinant in the terminal differentiation of oligodendrocytes. Four main axes of SOX10-mediated oligodendrocyte differentiation have been characterized: the MYRF axis, the chromodomain helicase DNA binding protein 8 (CHD8)-SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily A, member 4 (BRG1)-OLIG2 axis, the transcription factor 7 like 2 (TCF7L2) axis, and the Calcineurin/NFAT axis (see figure 1).
SOX10 was established early on as a required protein for the maturation of oligodendrocytes and as a direct activator of several genes critical to the myelination process [17], including the transcription factor Myrf [16]. Once induced, MYRF mediates the progression of pre-myelinating oligodendrocytes to a mature, myelinating state [18]. Both MYRF and SOX10 bind many of the same transcriptional control regions in proximity to genes critical to the myelination process, though studies have shown that they are also able to bind individual enhancers independently [16,19]. SOX10 binds to the active enhancer of the pro-myelinating transcription factor zinc finger protein 24 (Zfp24, formerly known as Zfp191) [20]. In turn ZFP24 binds to the enhancer regions of Sox10 and Myrf and increases their expression [21].
The chromatin remodeler CHD8 activates the expression of BRG1-associated SWI/SNF complexes [22]. BRG1, together with OLIG2, activates the expression of ZFP24 [23] and initiates the expression of the downstream chromatin remodeler chromodomain helicase DNA binding protein 7 (CHD7) [24]. In turn, CHD7 cooperates with SOX10 and regulates the onset of CNS myelination and remyelination [25].
At the onset of differentiation, TCF7L2 interacts with transcriptional co-repressor zinc finger and BTB domain-containing protein 33 (ZBTB33) to block β-catenin signaling, which impedes myelination [26]. During oligodendrocyte lineage progression, TCF7L2 cooperates with SOX10 to induce the expression of other genes crucial for oligodendrocyte differentiation, such as Zfp24, Oligodendrocyte transcription factor 1 (Olig1) and Myrf. In addition, TCF7L2 directly activates genes important for myelination and cholesterol biosynthesis [27].
Despite the ongoing discovery of numerous factors and extracellular signals that control oligodendrocyte differentiation, the full integration of this data into a clear understanding of the extracellular factors that are upstream of SOX10 activation in the CNS remains incomplete. In the PNS, Neuregulin on the surface of the axon signals through the ErbB receptor tyrosine kinases on Schwann cells to control myelination [28]. Neuregulin-ErbB signaling results in an increase in intracellular calcium levels, which activates Calcineurin, a calcium- and calmodulin-dependent phosphatase, to mediate the dephosphorylation of NFATC4. Once dephosphorylated, NFATC4 cooperates with SOX10 to drive the expression of the pro-myelination factor early growth response 2 (KROX20) which subsequently leads to PNS myelination [29]. A significant step in our understanding of CNS myelination was recently achieved by the discovery that calcineurin-dependent dephosphorylation of NFATC2 results in its activation [14]. In turn, activated NFATC2 cooperates with SOX10 to release the repression of OLIG2 on NKX2.2, and vice versa [14]. Nevertheless, the triggering event that leads to the intracellular calcium rise that activates calcineurin in oligodendrocyte lineage cells still needs to be determined.
As described above, the major determinants of oligodendrocyte differentiation and myelination are the transcription factors OLIG2, SOX10, NKX2.2, ZFP24 and MYRF. Together they comprise the core regulatory network that controls oligodendrocyte differentiation. This network is summarized in figure 1. Nonetheless, many other transcription factors that play a role in oligodendrocyte lineage cells have been identified over the years; their role in oligodendrocyte lineage cells, myelination and remyelination is summarized in Table 1.
Table 1:
Transcription factors that play a role in oligodendrocyte development and a brief summary of their role in oligodendrocyte lineage cells, myelination, and remyelination.
| Protein | Role in OL development | Effect of its ablation | Role in remyelination |
|---|---|---|---|
| ASCL | Plays a role in OPC formation [104] and OL differentiation [105]. | Reduced number of OPCs [104] and reduced number of OLs [105] | Required for proper remyelination [106] |
| HES1 | Maintains cells as OPCs and inhibits differentiation [107]. | ND | ND |
| HES5 | Maintains cells as OPCs and inhibits differentiation [107]. | Premature OPC differentiation [108] | ND |
| ID2 | Maintains cells as OPCs and inhibits differentiation [109] | Premature OPC differentiation [110] | ND |
| ID4 | Maintains cells as OPCs and inhibits differentiation [109] | Premature OPC differentiation [111] | ND |
| KLF9 | Promotes OPC differentiation in vitro [112]. | No effect [112]. | Important for remyelination [112]. |
| MYRF | Crucial for OPC differentiation [18] | Normal OPCs and loss of OLGs [18]. | Important for remyelination [113] |
| MYT1 | Plays a role in OPC proliferation and OL differentiation [114] | ND | ND |
| NFAT proteins | Promotes OPC differentiation [14] | OL lineage specific ablation of Calcineurin inhibits OPC differentiation [14] | ND |
| NFIA | Controls OPC formation [115] | ND | Inhibits OPC differentiation during remyelination [116] |
| NKX2.2 | Plays a role in OPC differentiation [117]. | No effect on OPCs, loss of OLs [117]. | ND |
| NKX6.1 | Play a role in OPC formation [118] | Reduced number of OL lineage cells [119] | ND |
| NKX6.2 | Plays a role in OPC formation [118] | Paranodal defects [120] | ND |
| OLIG1 | Delayed OPC differentiation [121] | Depends on the experimental system: Normal numbers of OPCs and reduced number of OLs [122] or no change in OL numbers [121] | Important for remyelination [123] |
| OLIG2 | Important for OL lineage cells [124] | Ablation at the OPC stage results in hypomyelination, while ablation at premature OL stage expedites myelination [125] | Olig2 gain of function at the OPC stage promotes remyelination [126] |
| SOX2 and SOX3 | Play a role in OL differentiation [127] | Impairs OL differentiation [127] | ND |
| SOX4 | Promote OPC differentiation [128] | Lethal, however no overt CNS defects in the embryo [129] | ND |
| SOX5 and SOX6 | Inhibit OPC differentiation [130] | Premature OPC differentiation [130] | ND |
| SOX8 | Controls gliogenesis [131] | Loss of OPCs [131]. | ND |
| SOX9 | Controls gliogenesis [132]. | Loss of OPCs [132]. | ND |
| SOX10 | Plays a role in OL differentiation [133] | Normal OPCs, loss of OLs [133] | ND |
| SOX17 | Plays a role in OPC differentiation [134,135] | ND | Overexpression increases the number of OLs and improves remyelination [136] |
| SREBP proteins | Promote OPC differentiation [137] | No change in OPCs, educed number of OLs [36] | ND |
| TCF7L2 | Promotes OPC differentiation [27,43,138] | Reduced number of OLs [27,43,138] | ND |
| YY1 | Plays a role in OL differentiation [139]. | Prevents OL differentiation [139]. | ND |
| ZBTB7A | Modulates the expression of transcripts important for myelination [140]. | Normal numbers of OPCs and OLs [140]. | Important for remyelination [140]. |
| ZEB2 | Plays a role in OPC differentiation [141]. | Reduced number of OLs [141]. | ND |
| ZFP24 | Plays a role in OL differentiation [21] | Hypomyelination with normal numbers of OPCs and OLs [77] | ND |
| ZFP488 | Promotes OPC differentiation [142] | ND | Promotes OPC differentiation during remyelination [143] |
Legend: OL, oligodendrocyte; ND, not determined.
A class of transcription factors that has been gaining attention in this context is nuclear receptors. These are ligand-activated transcription factors that work through homo dimerization or dimerization with each other and by interacting with co-repressors or co-activators [30]. Nuclear receptors including the retinoid X receptors (RXR), vitamin D receptors, liver X receptors (LXR), Thyroid hormone receptors (THR), peroxisome proliferator-activated receptors (PPAR), and the chicken ovalbumin upstream promoter-transcription factors (COUP-TF), are known to play a role in oligodendrocyte development and myelination (see table 2). In addition to their role in oligodendrocyte lineage cell development RXR and LXR play a fundamental role in myelin debris clearance following demyelination, which is a limiting factor in remyelination of aged animals [31,32].
Table 2:
Nuclear receptors that play a role in oligodendrocyte development and a brief summary of their ligands and their roles in oligodendrocyte lineage cells, myelination, and remyelination.
| Nuclear Receptor Family | Ligand | Family member name | Effect of its ablation | Role in OL development | Role in remyelination |
|---|---|---|---|---|---|
| Chicken ovalbumin upstream promoter-transcription factor | Orphan receptor | COUP-TFI | Hypomyelination [144] | Important for OPC differentiation [144] | ND |
| Liver X receptors | Oxysterols | LXRα | Accumulation of myelin debris following demyelination [32] and hypomyelination [145] | ND | Important for remyelination [32,145] |
| LXRβ | Hypomyelination [145,146] | Important for formation of OL lineage cells [145,146] | Important for remyelination [145,146] | ||
| Peroxisome proliferator-activated receptors | 15-Deoxy-delta-12,14-prostaglandin J2 | PPARγ | ND | Important for OPC differentiation [147] | Important for remyelination [148] |
| PPARδ | ND | Important for OPC differentiation [149] | ND | ||
| Retinoid X receptors | 9-cis-retinoic acid | RXRα | Accumulation of myelin debris following demyelination [31] | ND | Important for remyelination [31] |
| RXRγ | Impaired OPC differentiation [150] | ND | Important for remyelination [150] | ||
| Thyroid hormone receptors | T3 | THRα and THRβ | Myelin defects and sustained elevated numbers of OPCs in adults [151] | Important for OPC differentiation [151] | ND |
| Vitamin D receptor | Vitamin D | VDR | ND | Induces OPC differentiation [152] | Important for remyelination [152] |
Legend: OL, oligodendrocyte; ND, not determined.
Signaling pathways and metabolic control of oligodendrocyte development
Myelin is a lipid-rich structure. It is therefore not surprising that myelination relies heavily on lipid synthesis [33], and that hypomyelination is characterized by reduced expression of transcripts important for lipid synthesis [34]. Interestingly, lipids may play a greater role in oligodendrocyte development beyond simply serving as the building blocks of the multilayered myelin sheath. High-throughput pharmacological screens have identified myriad small molecules that have the capacity to enhance oligodendrocyte maturation and CNS myelination. Unexpectedly, it was recently discovered that a number of these “hits” function by targeting the cholesterol biosynthesis pathway, resulting in the accumulation of 8,9-unsaturated sterols in oligodendrocytes [35]. The mechanisms by which 8,9-unsaturated sterols exert their function, however, remain unknown.
The expression of genes involved in fatty acid and cholesterol metabolism is controlled by the transcription factors sterol regulatory element binding proteins (SREBPs), which are encoded by the genes Srebf1 and Srebf2. Recent studies suggest that these transcription factors have a cell autonomous role in both oligodendrocytes, where they function in myelin lipid synthesis, and in astrocytes, to supply myelin building blocks for the oligodendrocytes through transcellular transport [36]. Lipid synthesis and the expression of SREBPs is controlled by the mammalian target of rapamycin (mTOR) pathway, which plays a fundamental role in oligodendrocyte development, myelination, and remyelination (for a detailed review of the function of mTOR in myelination, see [37]).
The Raf-MAPK-ERK1/2 pathway is also known to control oligodendrocyte differentiation and myelination. Ablation of both extracellular signal-regulated kinase 1 and 2 (ERK1 and ERK2) results in hypomyelination, but characterization of the upstream mediators of this pathway was until recently lacking [38,39]. A recent finding has helped clarify this process: the G protein-coupled receptor GPR37 was shown to be a mediator of the Raf-MAPK-ERK1/2 pathway in oligodendrocytes [40]. By tightly controlling ERK phosphorylation and nuclear translocation in oligodendrocyte lineage cells, GPR37 was found to inhibit the premature differentiation of oligodendrocytes.
The recently identified factors that govern the transcriptional control of oligodendrocyte differentiation expand our knowledge of the tight transcriptional control governing lineage progression and gene expression in oligodendrocyte lineage cells. This data is crucial for our attempts to intervene and enhance the myelination and remyelination processes in the CNS (see Box 2).
Box 2. Pushing oligodendrocyte progenitor cells toward terminal differentiation as a therapeutic possibility.
The CNS has a robust potential to remyelinate demyelinated axons, as exemplified in diseases such as MS. During the remyelination process, OPCs in the vicinity of the demyelinated areas migrate to the lesion site, differentiate into mature oligodendrocytes, and remyelinate the demyelinated axons. With increasing age, however, the capacity for myelin repair is diminished, resulting in severe progression of the demyelinating disease [9]. The currently available MS therapies focus on modulation of the immune system. Nevertheless, the brains of MS patients with remyelination failure are characterized by the presence of oligodendrocytes at the pre-myelinating stage in the vicinity of demyelinated axons [8]. These oligodendrocytes send processes to the axons, but fail to fully differentiate and myelinate the axons. Large pharmacological screens have therefore been used to find small molecules that can “push” oligodendrocyte lineage cells toward terminal differentiation in demyelinating diseases. Surprisingly, as mentioned above, many of the identified drugs share a common mechanism in which they inhibit enzymes in the cholesterol biosynthesis pathway, resulting in accumulation of 8,9-unsaturated sterol in the oligodendrocyte lineage cells [35]. The underlying mechanism by which the accumulation of this lipid leads to enhance oligodendrocyte maturation remains unknown. The drug clemastine fumarate that enhances OPC maturation is, so far, the only drug that has been shown to be effective in inducing remyelination in human patients [98].
In addition to MS, other neurological conditions would likely benefit from therapeutic approaches that enhance oligodendrocyte maturation. For example, hypoxic brain injury results in focal damage to white matter tracts. OPCs are recruited to areas of injury but fail to fully remyelinate the demyelinated lesions [99]. Therefore, driving oligodendrocyte maturation would likely be therapeutic [100,101]. Pushing oligodendrocyte progenitor cells toward terminal differentiation may also be beneficial following neuronal injury, where remyelination of regenerated axons appears to be a limiting factor [102].
Epigenetic regulation of oligodendrocyte development
Epigenetic regulation drives oligodendrocyte lineage progression and myelination [4]. The chromatin of OPCs, which are dividing cells, is accessible for transcription factors, co-activators or co- repressors and histone-modifying enzymes. At the OPC stage, extracellular cues drive mainly inhibitory pathways that prevent differentiation and promote proliferation. At the onset of the differentiation process, histone deacetylase activity is associated with formation of the heterochromatin and down regulation of the inhibitors that prevent differentiation [41–43]. Epigenetic regulation of oligodendrocyte maturation by DNA and histone modifications and ATP-dependent chromatin remodeling complexes has been recently reviewed thoroughly [22].
Long noncoding RNAs
Long noncoding RNAs (lncRNAs) are a sub-group of noncoding RNAs that are longer than 200 nucleotides but do not translate to proteins. Over 800 lncRNAs are expressed in the CNS, some of which are expressed in a cell type-specific manner [44]. Recently, a dynamic lncRNAs expression database of different stages of oligodendrocyte development was generated [45]. Of the oligodendrocyte-specific lncRNAs, the functional expression of lncOL1 was further characterized. lncOL1 interacts with a component of polycomb repressive complex 2 (SUZ12) to inhibit the expression of oligodendrocyte differentiation inhibitors, thereby promoting oligodendrocyte differentiation [45]. In addition, lnc158 was found to promote oligodendrocyte differentiation by controlling the levels of its target transcript nuclear factor-IB (NFIB) [46]. The functional roles of the remainder of the oligodendrocyte lineage cell-specific or enriched lncRNAs remains to be determined.
MicroRNAs
MicroRNAs (miRNAs) are a sub-group of noncoding RNAs that are approximately 21–25 nucleotides in length. miRNAs are initially transcribed as part of long transcripts and mature through cleavage by the enzyme Dicer. Oligodendrocyte-specific ablation of Dicer revealed that miR-219 and miR-338 are oligodendrocyte-specific miRNAs that promote oligodendrocyte differentiation [47–49]. Recent systematic characterization of miR-219 targets in oligodendrocyte lineage cells revealed that this miRNA has a cell autonomous role in oligodendrocyte lineage cells. MiR-219 has been found to repress known inhibitors of oligodendrocyte differentiation such as Lingo1, in a stage-specific manner [50]. In addition, these studies have revealed mir-219 targets that were previously not known to play a role in oligodendrocyte differentiation, such as the transcription factor ETS variant 5 (ETV5). This transcription factor was shown to promote oligodendrocyte differentiation in vitro, suggesting that miR-219 exerts its transcriptional activity, at least partially, by mediating ETV5 activity. Oligodendrocyte specific ablation of miR-219 resulted in hypomyelination, but to a lesser extent than ablation of Dicer, suggesting that additional miRNAs are important for CNS myelination. Unlike miR-219, ablation of miR-338 in oligodendrocytes did not result in overt effects on oligodendrocyte differentiation or myelination [50]. Nevertheless, it has been shown in vivo that miR-219 and miR-338 work in collaboration to promote oligodendrocyte differentiation and myelination [50]. Recently, miR-125a-3p was shown to prevent premature differentiation of oligodendrocyte progenitor cells [51]. The predicted miR-125 targets SMAD Family Member 4 (SMAD4), Tyrosine-Protein Kinase Fyn (FYN), Ras homolog family member A (RHOA), Mitogen-Activated Protein Kinase 1 (P38) and Neuregulin 1 (NRG1), which are known to play a role in oligodendrocyte development, suggest that miR-125 indirectly affects the expression of MBP [51].
Recently, it has also become clear that miRNAs have an exciting therapeutic potential; specifically, mir-146 was shown to promotes oligodendrocyte differentiation and remyelination in various models of CNS demyelination [52–54]. The mechanism of miR-146 action may be meditated by the known miR-146 target interleukin-1 receptor-associated kinase 1 (IRAK1) [52–54]. Mir-219 was shown to promote remyelination in the lysolecithin and cuprizone models of toxicant-induced demyelination, as well as in the experimental autoimmune encephalomyelitis (EAE) model of inflammatory demyelination [55,56]. In addition, serum exosomes containing miR-219 and dendritic cell exosomes enriched in miR-219, miR-9 and miR-92–1, which are known to play a role in CNS myelination (see table 3), were shown to improve myelination and remyelination [57,58], suggesting that exosome-mediated delivery of micro-RNAs has therapeutic potential [59,60].
Table 3:
List of miRNAs that play a role in oligodendrocyte development and a brief summary of their targets, and their roles in oligodendrocyte lineage cells, myelination, and remyelination.
| Name | Role in OL lineage cells | Effect of its ablation | Targets/mechanism of action | Role in remyelination |
|---|---|---|---|---|
| miR-219 | Important for OPC differentiation [47–50] | OL specific ablation results in hypomyelination [50] | Inhibits the expression of inhibitors of OPC differentiation, such as Etv5, Lingo-1, NFIA and PDGFRα [50] | Promotes remyelination [50,55,56] |
| miR-338 | Important for OPC differentiation [47–49] | OL specific ablation did not result in overt phenotype [50] | ND | ND |
| miR-138 | Important for OPC differentiation [47–49] | ND | ND | ND |
| miR-23a | Important for OPC differentiation [153,154] | Hypermyelination in miR-23a overexpressing mice [154] | By controlling the expression of its targets lamin B1, phosphatase and tensin homolog on chromosome 10 (PTEN) and the lncRNA 2700046G09Rik miR-23a controls OPC differentiation. [154] | ND |
| miR-17–92 cluster; miR-17, miR18a, miR-19a, miR-19b-1, miR-20a, and miR-92–1 | Regulates OPC proliferation [155] | Hypomyelination [155] | Controls OPC proliferation by regulating Akt signaling, mediated by the predicted target PTEN [155] | ND |
| miR-297c-5p | Important for OPC differentiation [156] | ND | By targeting cyclin T2 (CCNT2) induces OPC differentiation [156] | Promote remyelination [156] |
| miR-146 | Important for OPC differentiation [52,54] | ND | By repressing its target IL-1 receptor-associated kinase 1 (IRAK1) promotes OPCs differentiation. [52,54] | Promotes remyelination [52,53] |
| miR-9 | Important for OPC differentiation [157] | ND | Prevents expression of PMP22 protein in OPCs [157]. | ND |
| miR-125 | Maintains cells as OPCs [51] | ND | The predicted targets Smad4, FYN, RHOA, P38 and NRG1 suggest that mir-125 may indirectly affect MBP expression [51] | ND |
Legend: OL, oligodendrocyte; ND, not determined
A number of other miRNAs that play a role in oligodendrocyte lineage cells have been identified over the recent years; their role in oligodendrocyte lineage cells, myelination, and remyelination is summarized in Table 3.
Translational control of oligodendrocyte development
Global protein translation – the role of eukaryotic translation initiation factors
During developmental myelination or during remyelination, oligodendrocytes produce vast amounts of membrane proteins and lipids that form the myelin sheath through the secretory pathway. The production of such vast amounts of protein makes oligodendrocytes uniquely vulnerable to changes in protein translation homeostasis [61]. During developmental myelination, the eukaryotic translation initiation factor 2B (eIF2B) has an oligodendrocyte cell-autonomous role in maintaining protein translation homeostasis [62]. Mutations in eIF2B inhibit protein translation, delay recovery during the integrated stress response, and cause Vanishing White Matter Disease, an inherited autosomal-recessive CNS hypomyelinating disease [63]. Inhibition of global protein translation mediated by the eukaryotic translation initiation factor 2 alpha (eIF2alpha) phosphorylation protects oligodendrocytes from an inflammatory environment, increases their survival, and enhances myelination [64,65], indicating that oligodendrocytes are specifically sensitive to proper homeostasis of protein translation. Inhibition of global protein translation in order to protect oligodendrocyte lineage cells during remyelination is a potential new direction for treating demyelinating diseases (see Box 3).
Box 3. Protecting oligodendrocytes from CNS inflammation by an enhanced integrated stress response as a therapeutic horizon.
During developmental myelination or during remeylination, oligodendrocytes turn into “protein factory cells” that synthesize vast amounts of protein at an estimated rate of about 105 proteins per minute [103]. This makes oligodendrocytes uniquely vulnerable to changes in protein translation homeostasis. Numerous studies have shown that the inflammatory condition in the brain during demyelinating diseases activates ER stress in oligodendrocytes, which is deleterious for the cells (for review, see [61]). Preclinical work in murine models of demyelination has shown that inhibition of global protein translation and enhancement of the integrated stress response, by either genetic manipulations or pharmacological means, can protect oligodendrocytes and improve their myelination function [64,65].
Spatial and temporal control of protein translation in oligodendrocytes.
One of the unique features of oligodendrocytes is that axonal myelination depends heavily on the uneven distribution of myelin proteins within the oligodendrocyte. Specifically, myelin basic protein (MBP) mediates membrane compaction [66]. Therefore, MBP translation, which may damage cell membranes at the soma, requires anterograde mRNA transport using the dynein/dynactin motor complex [67] followed by local translation of Mbp mRNA in the cell’s processes, in proximity to the developing myelin sheath [68]. The factors that participate in the spatial and temporal control of MBP translation are largely unknown. The ERK/MAP kinase effector ERK2 has been shown to control the translation, but not the transcription, of MBP [69]. Similarly, the tumor overexpressed gene (TOG) was shown to control the translation, but not the transcription, of MBP, most likely by affecting its mRNA translocation to the cell processes [70]. Several mRNA-binding proteins from the heterogeneous nuclear ribonucleoprotein (hnRNP) protein family have been shown to have a specific role in local translation of MBP. For example, hnRNP-A2 is responsible for MBP mRNA transport, while hnRNP-E1 inhibits its translation and hnRNP-K stimulates its translation [71]. HnRNP-A2 exert its activity by binding to N6-methyladenosine (m6A)-bearing RNAs [72], suggesting that mRNA methylation may play a role in oligodendrocyte development. Nevertheless, the role that the m6A mark plays in oligodendrocyte development and CNS myelination remains unknown (see Outstanding Questions). Surprisingly, transcriptome studies on biochemically-isolated myelin from mouse brains have revealed that myelin is enriched in a large number of mRNA molecules, including transcripts that are important for myelin sheath formation as well as those important for local translation, suggesting that this spatial and temporal control of protein translation may be relevant to many other myelin transcripts [73].
In vivo, the spatial and temporal control of CNS myelination depends on programmed cell death in unmyelinated brain regions [74,75], and on the ability of the surviving cells in myelinated brain regions to properly interact with axons [76]. The lysosomal G protein ras related GTP binding A (RAGA) promotes myelination by inhibiting the expression of the transcription factor EB (TFEB), which acts as a myelination inhibitor [75]. TFEB controls the expression of ER stress and pro-apoptotic genes in premyelinating oligodendrocytes, in a cell autonomous manner. These TFEB induced genes mediate oligodendrocyte programmed cell death in unmyelinated brain regions, thereby shaping CNS myelination [74,75]. Furthermore, proper myelin targeting to axons (and not to the cell soma), membrane wrapping, and internode extension are heavily dependent on proper axon-glia interaction mediated by the neuronal cell adhesion molecules 2 and 3 (CADM2 and3) and on the oligodendrocyte cell adhesion molecule 4 (CADM4) [76].
Post-translational control of oligodendrocyte development
Post-translation modification of proteins, such as phosphorylation, adds another layer of complexity to the molecular control of oligodendrocyte development. De-phosphorylation of NFAT proteins, mediated by Calcineurin, is the key event that initiates both PNS and CNS myelination [14,29].
Dephosphorylation of the transcription factor ZFP24 in its DNA binding domains increases ZFP24 binding to its DNA targets in proximity to genes that are crucial for oligodendrocyte differentiation and myelination [21]. The binding of ZFP24 to its DNA binding targets is important for the expression of numerous genes that are critical for CNS myelination [34] and is a key event in CNS myelination, given that Zfp24 null mice are severely hypomyelinated [77]. Human patients hemizygous for 18q chromosomal deletions that encompass Znf24 display seizures and tremors, suggestive of myelin abnormalities [78]. The enhancement of ZFP24 activity by modulation of its phosphorylation state may induce oligodendrocyte differentiation and therefore may be beneficial to patients suffering from demyelinating diseases (see Box 2). Nonetheless, the kinases and phosphatases that regulate ZFP24 activity remain unknown, as are the upstream modulators of this control.
OLIG1 and OLIG2 also undergo phosphorylation, and the phosphorylation state of OLIG2 has been shown to determine its function. Dephosphorylating serine 147 (S147) of OLIG2, for example, dictates the formation of oligodendrocyte lineage cells [79]. Meanwhile, phosphorylation of OLIG2 on residues S10, S13 and S14 is elevated during the neural progenitor stage and is reduced as the cells differentiate to myelinating oligodendrocytes. Likewise, the kinase glycogen synthase kinase 3 (GSK3) mediates OLIG2 phosphorylation at S10, cyclin-dependent kinases 1 and 2 (CDK1 and CDK2) at S14, and casein kinase 2 (CK2) at S13, to determine OLIG2 functions [79–81]. The phosphatase(s) that mediate OLIG2 dephosphorylation are as yet unknown.
OLIG1 is phosphorylated in oligodendrocyte lineage cells at S149 by protein kinase A (PKA) [82]. Independent of its phosphorylation status, OLIG1 is also acetylated at lysine 150 in vivo [83]. OLIG1 acetylation is regulated by the acetyltransferase CREB-binding protein (CBP), and OLIG1 deacetylation is regulated by histone deacetylases HDAC1, HDAC3, and HDAC10. Acetylation of OLIG1 regulates its chromatin dissociation and subsequent translocation to the cytoplasm, while also increasing its binding to the oligodendrocyte differentiation inhibitor of DNA binding 2 (ID2). The retention of the OLIG1-ID2 complex in the cytoplasm likely relieves the inhibitory effect of ID2 on oligodendrocyte differentiation [83].
MYRF is a membrane-bound transcription factor that is associated with the endoplasmic reticulum (ER). Its activity is controlled by complex post-translational processing – MYRF activation requires self-cleavage, which results in the release of its N-terminal fragment [19]. After cleavage, the N-terminal undergoes homo-trimerization, resulting in the active form of the protein [84]. As a homotrimer, MYRF is then translocated to the nucleus where it acts as a transcription factor that mediates the expression of genes crucial for oligodendrocyte differentiation and myelination [18]. The cleavage of MYRF is controlled by the ER resident transmembrane protein 98 (TMEM98), which binds to the C-terminal of MYRF to prevent its self-cleavage, thereby providing another layer of negative regulation to prevent premature differentiation of oligodendrocyte lineage cells into oligodendrocytes [85].
Concluding Remarks and Future Perspectives
Over the past few decades, numerous factors have been identified that control oligodendrocyte development. These include extrinsic extracellular cues as well as oligodendrocyte-intrinsic transcription factors, epigenetic modulators, DNA methylation, non-coding RNAs and signaling pathways. Many of these factors were originally discovered through large random screens and their functional interactions with each other were poorly understood. Nevertheless, over the last few years, many new components of the regulatory network that controls the development of oligodendrocytes have been uncovered. These findings have revealed integrated, complex transcriptional networks that control oligodendrocyte differentiation and CNS myelination. Among other advances, these findings have led to a new understanding of the factors that cooperate with SOX10 to mediate CNS myelination, including TCF7L2 [27], CHD7 [25], ZFP24 [21], OLIG2 [16], MYRF [86], and NFATC2 [14], as well as new insights into the role of the calcium- and Calcineurin-mediated network in the initiation of both PNS and CNS myelination [14,29] (see figure 1). This latter finding is of particular interest as recent studies have shown that neuronal activity raises intracellular calcium levels in oligodendrocyte lineage cells and that high frequency calcium transients mediate myelin sheath elongation [87,88]. The concept of calcium-dependent intrinsic transcriptional control of oligodendrocyte differentiation may provide a framework to understand the transcriptional control of activity-dependent myelination. Further studies are required to determine whether the newly identified Calcineurin- NFATC2-SOX10 axis activation in oligodendrocytes is downstream of neuronal activity, and whether there is a direct link between neural activity to the intrinsic transcriptional control of oligodendrocyte differentiation.
The work reviewed here demonstrates that highly complex regulatory networks interact to control oligodendrocyte development and CNS myelination. We expect that this information will serve as the basis for the development of a “tool kit” to treat demyelinating diseases. New therapeutic approaches will likely include strategies to protect pre-existing oligodendrocytes from stress (see Box 3), improve myelin clearance [31,32], and enhance oligodendrocyte differentiation (see Box 2). We anticipate that these strategies will improve remyelination and the overall outcome of hypomyelinating and demyelinating diseases of the CNS.
Despite our increasingly detailed understanding of oligodendrocyte maturation in the context of developmental myelination, our understanding of oligodendrocyte development in adulthood and disease is still lacking. Specifically, in the context of disease, oligodendrocyte lineage cells can give rise to Schwann cells [89,90] or become antigen presenting cells [91]. In addition, post-mitotic oligodendrocytes have recently been shown to participate in CNS remyelination [92]. Moreover, for unknown reasons, adult-born myelin sheaths are shorter and thinner than myelin sheaths that are deposited during development [93]. The transcriptional and post-transcriptional controls of these phenomena remain poorly understood, and represent one of many fascinating areas for future work (see Outstanding Questions).
Acknowledgments
Relevant work in the Popko laboratory has been supported by grants from the NIH (NS034939, NS109372, NS067550), the National Multiple Sclerosis Society (RG-1501-02797, PP-1603-08106), the Myelin Repair Foundation, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. In this review the authors focused primarily on recently published work; we apologize to all the colleagues whose work was not cited due to space limitations.
References
- 1.Wheeler NA and Fuss B (2016) Extracellular cues influencing oligodendrocyte differentiation and (re)myelination. Exp. Neurol 283, 512–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Küspert M and Wegner M (2016) SomethiNG 2 talk about-Transcriptional regulation in embryonic and adult oligodendrocyte precursors. Brain Res. 1638, 167–182 [DOI] [PubMed] [Google Scholar]
- 3.Emery B and Lu QR (2015) Transcriptional and epigenetic regulation of oligodendrocyte development and myelination in the central nervous system. Cold Spring Harb. Perspect. Biol 7, a020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J et al. (2016) Epigenetic control of oligodendrocyte development: adding new players to old keepers. Curr. Opin. Neurobiol 39, 133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez M and Casaccia P (2015) Interplay between transcriptional control and chromatin regulation in the oligodendrocyte lineage. Glia 63, 1357–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galloway DA and Moore CS (2016) miRNAs As Emerging Regulators of Oligodendrocyte Development and Differentiation. Front. Cell Dev. Biol 4, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaesser JM and Fyffe-Maricich SL (2016) Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp. Neurol 283, 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang A et al. (2002) Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N. Engl. J. Med 346, 165–173 [DOI] [PubMed] [Google Scholar]
- 9.Franklin RJM and Ffrench-Constant C (2017) Regenerating CNS myelin - from mechanisms to experimental medicines. Nat. Rev. Neurosci 18, 753–769 [DOI] [PubMed] [Google Scholar]
- 10.Sun T et al. (2001) Olig bHLH proteins interact with homeodomain proteins to regulate cell fate acquisition in progenitors of the ventral neural tube. Curr. Biol 11, 1413–1420 [DOI] [PubMed] [Google Scholar]
- 11.Sun T et al. (2003) Cross-repressive interaction of the Olig2 and Nkx2.2 transcription factors in developing neural tube associated with formation of a specific physical complex. J. Neurosci 23, 9547–9556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Küspert M et al. (2011) Olig2 regulates Sox10 expression in oligodendrocyte precursors through an evolutionary conserved distal enhancer. Nucleic Acids Res. 39, 1280–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weider M et al. (2015) Elevated in vivo levels of a single transcription factor directly convert satellite glia into oligodendrocyte-like cells. PLoS Genet. 11, e1005008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weider M et al. (2018) Nfat/calcineurin signaling promotes oligodendrocyte differentiation and myelination by transcription factor network tuning. Nat. Commun 9, 899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z et al. (2007) Induction of oligodendrocyte differentiation by Olig2 and Sox10: evidence for reciprocal interactions and dosage-dependent mechanisms. Dev. Biol 302, 683–693 [DOI] [PubMed] [Google Scholar]
- 16.Hornig J et al. (2013) The transcription factors Sox10 and Myrf define an essential regulatory network module in differentiating oligodendrocytes. PLoS Genet. 9, e1003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H et al. (2007) Olig1 and Sox10 interact synergistically to drive myelin basic protein transcription in oligodendrocytes. J. Neurosci 27, 14375–14382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emery B et al. (2009) Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell 138, 172–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bujalka H et al. (2013) MYRF is a membrane-associated transcription factor that autoproteolytically cleaves to directly activate myelin genes. PLoS Biol. 11, e1001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Anido C et al. (2015) Differential Sox10 genomic occupancy in myelinating glia. Glia 63, 1897–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elbaz B et al. (2018) Phosphorylation state of ZFP24 controls oligodendrocyte differentiation. Cell Rep. 23, 2254–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koreman E et al. (2018) Chromatin remodeling and epigenetic regulation of oligodendrocyte myelination and myelin repair. Mol. Cell. Neurosci 87, 18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Y et al. (2013) Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell 152, 248–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao C et al. (2018) Dual requirement of CHD8 for chromatin landscape establishment and histone methyltransferase recruitment to promote CNS myelination and repair. Dev. Cell 45, 753–768.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He D et al. (2016) Chd7 cooperates with Sox10 and regulates the onset of CNS myelination and remyelination. Nat. Neurosci 19, 678–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fancy SPJ et al. (2009) Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 23, 1571–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao C et al. (2016) Dual regulatory switch through interactions of Tcf7l2/Tcf4 with stage-specific partners propels oligodendroglial maturation. Nat. Commun 7, 10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birchmeier C and Nave K-A (2008) Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 56, 1491–1497 [DOI] [PubMed] [Google Scholar]
- 29.Kao S-C et al. (2009) Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science 323, 651–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lonard DM and O’Malley BW (2012) Nuclear receptor coregulators: modulators of pathology and therapeutic targets. Nat. Rev. Endocrinol 8, 598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Natrajan MS et al. (2015) Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain 138, 3581–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantuti-Castelvetri L et al. (2018) Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 359, 684–688 [DOI] [PubMed] [Google Scholar]
- 33.Saher G et al. (2005) High cholesterol level is essential for myelin membrane growth. Nat. Neurosci 8, 468–475 [DOI] [PubMed] [Google Scholar]
- 34.Aaker JD et al. (2016) Transcriptional fingerprint of hypomyelination in zfp191null and shiverer (mbpshi) mice. ASN Neuro 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hubler Z et al. (2018) Accumulation of 8,9-unsaturated sterols drives oligodendrocyte formation and remyelination. Nature 560, 372–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camargo N et al. (2017) Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol. 15, e1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Figlia G et al. (2018) Myelination and mTOR. Glia 66, 693–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishii A et al. (2012) ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J. Neurosci 32, 8855–8864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fyffe-Maricich SL et al. (2011) The ERK2 mitogen-activated protein kinase regulates the timing of oligodendrocyte differentiation. J. Neurosci 31, 843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang H-J et al. (2016) G protein-coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination. Nat. Commun 7, 10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marin-Husstege M et al. (2002) Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J. Neurosci 22, 10333–10345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L et al. (2016) Hdac3 Interaction with p300 Histone Acetyltransferase Regulates the Oligodendrocyte and Astrocyte Lineage Fate Switch. Dev. Cell 36, 316–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye F et al. (2009) HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat. Neurosci 12, 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y et al. (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci 34, 11929–11947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He D et al. (2017) lncRNA Functional Networks in Oligodendrocytes Reveal Stage-Specific Myelination Control by an lncOL1/Suz12 Complex in the CNS. Neuron 93, 362–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y et al. (2018) A novel long noncoding RNA lnc158 promotes the differentiation of mouse neural precursor cells into oligodendrocytes by targeting nuclear factor-IB. Neuroreport 29, 1121–1128 [DOI] [PubMed] [Google Scholar]
- 47.Zhao X et al. (2010) MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 65, 612–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dugas JC et al. (2010) Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron 65, 597–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin D et al. (2009) Dicer ablation in oligodendrocytes provokes neuronal impairment in mice. Ann. Neurol 66, 843–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H et al. (2017) miR-219 Cooperates with miR-338 in Myelination and Promotes Myelin Repair in the CNS. Dev. Cell 40, 566–582.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lecca D et al. (2016) MiR-125a-3p timely inhibits oligodendroglial maturation and is pathologically up-regulated in human multiple sclerosis. Sci. Rep 6, 34503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu XS et al. (2017) MicroRNA-146a Promotes Oligodendrogenesis in Stroke. Mol. Neurobiol 54, 227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J et al. (2017) MiR-146a promotes remyelination in a cuprizone model of demyelinating injury. Neuroscience 348, 252–263 [DOI] [PubMed] [Google Scholar]
- 54.Santra M et al. (2014) Thymosin β4 up-regulation of microRNA-146a promotes oligodendrocyte differentiation and suppression of the Toll-like proinflammatory pathway. J. Biol. Chem 289, 19508–19518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan H-B et al. (2017) Transplanted miR-219-overexpressing oligodendrocyte precursor cells promoted remyelination and improved functional recovery in a chronic demyelinated model. Sci. Rep 7, 41407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu S et al. (2017) miR-219 attenuates demyelination in cuprizone-induced demyelinated mice by regulating monocarboxylate transporter 1. Eur. J. Neurosci 45, 249–259 [DOI] [PubMed] [Google Scholar]
- 57.Pusic AD and Kraig RP (2014) Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia 62, 284–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pusic AD et al. (2014) IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J. Neuroimmunol 266, 12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pusic KM et al. (2016) Environmental Enrichment Stimulates Immune Cell Secretion of Exosomes that Promote CNS Myelination and May Regulate Inflammation. Cell Mol. Neurobiol 36, 313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pusic AD et al. (2014) What are exosomes and how can they be used in multiple sclerosis therapy? Expert Rev Neurother 14, 353–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clayton BLL and Popko B (2016) Endoplasmic reticulum stress and the unfolded protein response in disorders of myelinating glia. Brain Res. 1648, 594–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin Y et al. (2014) Impaired eukaryotic translation initiation factor 2B activity specifically in oligodendrocytes reproduces the pathology of vanishing white matter disease in mice. J. Neurosci 34, 12182–12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moon SL and Parker R (2018) EIF2B2 mutations in vanishing white matter disease hypersuppress translation and delay recovery during the integrated stress response. RNA 24, 841–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Way SW et al. (2015) Pharmaceutical integrated stress response enhancement protects oligodendrocytes and provides a potential multiple sclerosis therapeutic. Nat. Commun 6, 6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Way SW and Popko B (2016) Harnessing the integrated stress response for the treatment of multiple sclerosis. Lancet Neurol. 15, 434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Snaidero N et al. (2017) Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Rep. 18, 314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herbert AL et al. (2017) Dynein/dynactin is necessary for anterograde transport of Mbp mRNA in oligodendrocytes and for myelination in vivo. Proc. Natl. Acad. Sci. USA 114, E9153–E9162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Müller C et al. (2013) Making myelin basic protein -from mRNA transport to localized translation. Front. Cell Neurosci 7, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Michel K et al. (2015) Translational control of myelin basic protein expression by ERK2 MAP kinase regulates timely remyelination in the adult brain. J. Neurosci 35, 7850–7865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maggipinto MJ et al. (2017) Conditional knockout of TOG results in CNS hypomyelination. Glia 65, 489–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Torvund-Jensen J et al. (2014) Transport and translation of MBP mRNA is regulated differently by distinct hnRNP proteins. J. Cell Sci 127, 1550–1564 [DOI] [PubMed] [Google Scholar]
- 72.Alarcón CR et al. (2015) HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell 162, 1299–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thakurela S et al. (2016) The transcriptome of mouse central nervous system myelin. Sci. Rep 6, 25828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun LO et al. (2018) Spatiotemporal Control of CNS Myelination by Oligodendrocyte Programmed Cell Death through the TFEB-PUMA Axis. Cell DOI: 10.1016/j.cell.2018.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meireles AM et al. (2018) The Lysosomal Transcription Factor TFEB Represses Myelination Downstream of the Rag-Ragulator Complex. Dev. Cell 47, 319–330.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elazar N et al. (2018) Axoglial adhesion by cadm4 regulates CNS myelination. Neuron DOI: 10.1016/j.neuron.2018.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Howng SYB et al. (2010) ZFP191 is required by oligodendrocytes for CNS myelination. Genes Dev. 24, 301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cody JD et al. (2015) Consequences of chromsome18q deletions. Am. J. Med. Genet. C, Semin. Med. Genet 169, 265–280 [DOI] [PubMed] [Google Scholar]
- 79.Li H et al. (2011) Phosphorylation regulates OLIG2 cofactor choice and the motor neuron-oligodendrocyte fate switch. Neuron 69, 918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh SK et al. (2016) Post-translational Modifications of OLIG2 Regulate Glioma Invasion through the TGF-β Pathway. Cell Rep. 16, 950–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou J et al. (2017) A sequentially priming phosphorylation cascade activates the gliomagenic transcription factor olig2. Cell Rep. 18, 3167–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Niu J et al. (2012) Phosphorylated olig1 localizes to the cytosol of oligodendrocytes and promotes membrane expansion and maturation. Glia 60, 1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dai J et al. (2015) Olig1 acetylation and nuclear export mediate oligodendrocyte development. J. Neurosci 35, 15875–15893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim D et al. (2017) Homo-trimerization is essential for the transcription factor function of Myrf for oligodendrocyte differentiation. Nucleic Acids Res. 45, 5112–5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang H et al. (2018) Interactive repression of MYRF self-cleavage and activity in oligodendrocyte differentiation by TMEM98 protein. J. Neurosci DOI: 10.1523/JNEUROSCI.0154-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Emery B (2013) Playing the field: Sox10 recruits different partners to drive central and peripheral myelination. PLoS Genet. 9, e1003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baraban M et al. (2018) Ca 2+ activity signatures of myelin sheath formation and growth in vivo. Nat. Neurosci 21, 19–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krasnow AM et al. (2018) Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo. Nat. Neurosci 21, 24–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ulanska-Poutanen J et al. (2018) Injury-induced perivascular niche supports alternative differentiation of adult rodent CNS progenitor cells. Elife 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zawadzka M et al. (2010) CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 6, 578–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Falcão AM et al. (2018) Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med DOI: 10.1038/s41591-018-0236-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duncan ID et al. (2018) The adult oligodendrocyte can participate in remyelination. Proc. Natl. Acad. Sci. USA DOI: 10.1073/pnas.1808064115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duncan ID et al. (2017) Thin myelin sheaths as the hallmark of remyelination persist over time and preserve axon function. Proc. Natl. Acad. Sci. USA 114, E9685–E9691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McKenzie IA et al. (2014) Motor skill learning requires active central myelination. Science 346, 318–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xiao L et al. (2016) Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci 19, 1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hughes EG et al. (2018) Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci 21, 696–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jensen SK and Yong VW (2016) Activity-Dependent and Experience-Driven Myelination Provide New Directions for the Management of Multiple Sclerosis. Trends Neurosci. 39, 356–365 [DOI] [PubMed] [Google Scholar]
- 98.Green AJ et al. (2017) Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet 390, 2481–2489 [DOI] [PubMed] [Google Scholar]
- 99.Billiards SS et al. (2008) Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 18, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cree BAC et al. (2018) Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain 141, 85–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang F et al. (2018) Enhancing Oligodendrocyte Myelination Rescues Synaptic Loss and Improves Functional Recovery after Chronic Hypoxia. Neuron 99, 689–701.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bei F et al. (2016) Restoration of visual function by enhancing conduction in regenerated axons. Cell 164, 219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pfeiffer SE et al. (1993) The oligodendrocyte and its many cellular processes. Trends Cell Biol. 3, 191–197 [DOI] [PubMed] [Google Scholar]
- 104.Parras CM et al. (2007) The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J. Neurosci 27, 4233–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sugimori M et al. (2008) Ascl1 is required for oligodendrocyte development in the spinal cord. Development 135, 1271–1281 [DOI] [PubMed] [Google Scholar]
- 106.Nakatani H et al. (2013) Ascl1/Mash1 promotes brain oligodendrogenesis during myelination and remyelination. J. Neurosci 33, 9752–9768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kondo T and Raff M (2000) Basic helix-loop-helix proteins and the timing of oligodendrocyte differentiation. Development 127, 2989–2998 [DOI] [PubMed] [Google Scholar]
- 108.Liu A et al. (2006) A molecular insight of Hes5-dependent inhibition of myelin gene expression: old partners and new players. EMBO J. 25, 4833–4842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Samanta J and Kessler JA (2004) Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development 131, 4131–4142 [DOI] [PubMed] [Google Scholar]
- 110.Wang S et al. (2001) A role for the helix-loop-helix protein Id2 in the control of oligodendrocyte development. Neuron 29, 603–614 [DOI] [PubMed] [Google Scholar]
- 111.Marin-Husstege M et al. (2006) Multiple roles of Id4 in developmental myelination: predicted outcomes and unexpected findings. Glia 54, 285–296 [DOI] [PubMed] [Google Scholar]
- 112.Dugas JC et al. (2012) The T3-induced gene KLF9 regulates oligodendrocyte differentiation and myelin regeneration. Mol. Cell. Neurosci 50, 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Duncan GJ et al. (2017) Myelin regulatory factor drives remyelination in multiple sclerosis. Acta Neuropathol. 134, 403–422 [DOI] [PubMed] [Google Scholar]
- 114.Nielsen JA et al. (2004) Myelin transcription factor 1 (Myt1) modulates the proliferation and differentiation of oligodendrocyte lineage cells. Mol. Cell. Neurosci 25, 111–123 [DOI] [PubMed] [Google Scholar]
- 115.Deneen B et al. (2006) The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron 52, 953–968 [DOI] [PubMed] [Google Scholar]
- 116.Fancy SPJ et al. (2012) Evidence that nuclear factor IA inhibits repair after white matter injury. Ann. Neurol 72, 224–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qi Y et al. (2001) Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development 128, 2723–2733 [DOI] [PubMed] [Google Scholar]
- 118.Cai J et al. (2005) Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron 45, 41–53 [DOI] [PubMed] [Google Scholar]
- 119.Liu R et al. (2003) Region-specific and stage-dependent regulation of Olig gene expression and oligodendrogenesis by Nkx6.1 homeodomain transcription factor. Development 130, 6221–6231 [DOI] [PubMed] [Google Scholar]
- 120.Southwood C et al. (2004) CNS myelin paranodes require Nkx6–2 homeoprotein transcriptional activity for normal structure. J. Neurosci 24, 11215–11225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paes de Faria J et al. (2014) New Olig1 null mice confirm a non-essential role for Olig1 in oligodendrocyte development. BMC Neurosci. 15, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xin M et al. (2005) Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J. Neurosci 25, 1354–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arnett HA et al. (2004) bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 306, 2111–2115 [DOI] [PubMed] [Google Scholar]
- 124.Lu QR et al. (2002) Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109, 75–86 [DOI] [PubMed] [Google Scholar]
- 125.Mei F et al. (2013) Stage-specific deletion of Olig2 conveys opposing functions on differentiation and maturation of oligodendrocytes. J. Neurosci 33, 8454–8462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wegener A et al. (2015) Gain of Olig2 function in oligodendrocyte progenitors promotes remyelination. Brain 138, 120–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hoffmann SA et al. (2014) Stem cell factor Sox2 and its close relative Sox3 have differentiation functions in oligodendrocytes. Development 141, 39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Potzner MR et al. (2007) Prolonged Sox4 expression in oligodendrocytes interferes with normal myelination in the central nervous system. Mol. Cell. Biol 27, 5316–5326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheung M et al. (2000) Roles of Sox4 in central nervous system development. Brain Res. Mol. Brain Res 79, 180–191 [DOI] [PubMed] [Google Scholar]
- 130.Stolt CC et al. (2006) SoxD proteins influence multiple stages of oligodendrocyte development and modulate SoxE protein function. Dev. Cell 11, 697–709 [DOI] [PubMed] [Google Scholar]
- 131.Stolt CC et al. (2005) Impact of transcription factor Sox8 on oligodendrocyte specification in the mouse embryonic spinal cord. Dev. Biol 281, 309–317 [DOI] [PubMed] [Google Scholar]
- 132.Stolt CC et al. (2003) The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 17, 1677–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stolt CC et al. (2002) Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 16, 165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sohn J et al. (2006) Identification of Sox17 as a transcription factor that regulates oligodendrocyte development. J. Neurosci 26, 9722–9735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fauveau M et al. (2018) SOX17 transcription factor negatively regulates oligodendrocyte precursor cell differentiation. Glia 66, 2221–2232 [DOI] [PubMed] [Google Scholar]
- 136.Ming X et al. (2013) Transgenic overexpression of Sox17 promotes oligodendrocyte development and attenuates demyelination. J. Neurosci 33, 12528–12542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Monnerie H et al. (2017) Reduced sterol regulatory element-binding protein (SREBP) processing through site-1 protease (S1P) inhibition alters oligodendrocyte differentiation in vitro. J. Neurochem 140, 53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hammond E et al. (2015) The Wnt effector transcription factor 7-like 2 positively regulates oligodendrocyte differentiation in a manner independent of Wnt/β-catenin signaling. J. Neurosci 35, 5007–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.He Y et al. (2007) The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron 55, 217–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Davidson NL et al. (2017) Leukemia/lymphoma-related factor (LRF) exhibits stage- and context-dependent transcriptional controls in the oligodendrocyte lineage and modulates remyelination. J. Neurosci. Res 95, 2391–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Weng Q et al. (2012) Dual-mode modulation of Smad signaling by Smad-interacting protein Sip1 is required for myelination in the central nervous system. Neuron 73, 713–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang S-Z et al. (2006) An oligodendrocyte-specific zinc-finger transcription regulator cooperates with Olig2 to promote oligodendrocyte differentiation. Development 133, 3389–3398 [DOI] [PubMed] [Google Scholar]
- 143.Soundarapandian MM et al. (2011) Zfp488 promotes oligodendrocyte differentiation of neural progenitor cells in adult mice after demyelination. Sci. Rep 1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yamaguchi H et al. (2004) The nuclear orphan receptor COUP-TFI is important for differentiation of oligodendrocytes. Dev. Biol 266, 238–251 [DOI] [PubMed] [Google Scholar]
- 145.Meffre D et al. (2015) Liver X receptors alpha and beta promote myelination and remyelination in the cerebellum. Proc. Natl. Acad. Sci. USA 112, 7587–7592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu P et al. (2014) Liver X receptor β is essential for the differentiation of radial glial cells to oligodendrocytes in the dorsal cortex. Mol. Psychiatry 19, 947–957 [DOI] [PubMed] [Google Scholar]
- 147.De Nuccio C et al. (2011) Peroxisome proliferator-activated receptor γ agonists accelerate oligodendrocyte maturation and influence mitochondrial functions and oscillatory Ca(2+) waves. J. Neuropathol. Exp. Neurol 70, 900–912 [DOI] [PubMed] [Google Scholar]
- 148.Feinstein DL et al. (2002) Peroxisome proliferator-activated receptor-gamma agonists prevent experimental autoimmune encephalomyelitis. Ann. Neurol 51, 694–702 [DOI] [PubMed] [Google Scholar]
- 149.Saluja I et al. (2001) PPAR delta agonists stimulate oligodendrocyte differentiation in tissue culture. Glia 33, 191–204 [PubMed] [Google Scholar]
- 150.Huang JK et al. (2011) Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci 14, 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Baas D et al. (2002) Persistence of oligodendrocyte precursor cells and altered myelination in optic nerve associated to retina degeneration in mice devoid of all thyroid hormone receptors. Proc. Natl. Acad. Sci. USA 99, 2907–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.de la Fuente AG et al. (2015) Vitamin D receptor-retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J. Cell Biol 211, 975–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lin S-T and Fu Y-H (2009) miR-23 regulation of lamin B1 is crucial for oligodendrocyte development and myelination. Dis. Model. Mech 2, 178–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lin S-T et al. (2013) MicroRNA-23a promotes myelination in the central nervous system. Proc. Natl. Acad. Sci. USA 110, 17468–17473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Budde H et al. (2010) Control of oligodendroglial cell number by the miR-17–92 cluster. Development 137, 2127–2132 [DOI] [PubMed] [Google Scholar]
- 156.Kuypers NJ et al. (2016) Remyelinating Oligodendrocyte Precursor Cell miRNAs from the Sfmbt2 Cluster Promote Cell Cycle Arrest and Differentiation. J. Neurosci 36, 1698–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lau P et al. (2008) Identification of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J. Neurosci 28, 11720–11730 [DOI] [PMC free article] [PubMed] [Google Scholar]


