Abstract
Idiopathic pulmonary fibrosis (IPF) is a lethal, medically refractory syndrome characterized by intrapulmonary accumulations of extracellular matrix (ECM) proteins produced by fibroblasts. Activation, clonal expansion, and differentiation of lymphocytes are also frequently present in IPF. Activated T cells are known to exert several effects that promote ECM production, but opposing homeostatic actions, wherein T cells can inhibit fibrosis, are less well understood. We found that CD27, a TNF receptor ubiquitously expressed on naive T cells, is downregulated on CD4 T cells of patients with IPF and that CD70, the sole ligand for CD27, is present on human pulmonary fibroblasts. We hypothesized that cognate engagements between lymphocyte CD27 and fibroblast CD70 could have functional consequences. Accordingly, a series of subsequent studies were conducted to examine the possible role of CD27–CD70 interactions in the regulation of fibrogenesis. Using IB, flow cytometry, RT-PCR, and kinomic assays, we found that fibroblast CD70 expression was inversely correlated with cell density and upregulated by TGF-β1 (transforming growth factor-β1). CD70 agonists, including T-cell–derived soluble CD27, markedly diminished fibroblast collagen and fibronectin synthesis, and these effects were potent enough to also inhibit profibrotic actions of TGF-β1 on ECM production in vitro and in two distinct ex vivo human skin models. CD70 activation was mediated by AKT (protein kinase B) and complex interconnected signaling pathways, and it was abated by prior CD70 knockdown. These results show that the CD70–CD27 axis modulates T-cell–fibroblast interactions and may be an important regulator of fibrosis and wound healing. Fibroblast CD70 could also be a novel target for specific mechanistically based antifibrosis treatments.
Keywords: pulmonary fibrosis, fibroblasts, T cells
Clinical Relevance
We found that CD27 is downregulated among highly differentiated T-cell effector–memory cells in patients with chronic diseases, such as idiopathic pulmonary fibrosis, whereas naive T cells ubiquitously express CD27. Other studies have shown that T-cell CD27 interacts with CD70 receptors on pulmonary fibroblasts, causing these mesenchymal cells to decrease their production of extracellular matrix proteins, such as collagen and fibronectin, and this effect is potent enough to antagonize profibrotic effects of TGF-β1 (transforming growth factor-β1). We believe that these findings account for the relative paucity of fibrosis during acute immune responses in which naive CD27+ T cells predominate, whereas chronic immune responses, typically mediated by greater proportions of CD27null, tend to result in more fibrosis due to loss of the CD27–CD70 extracellular matrix inhibition. In addition to having biological implications, these findings illustrate the possibility that stimulation of fibroblast CD70 with activating antibodies or soluble CD27 might be a novel mechanistic approach for treatment of pathological fibrosis.
Disruptions of normal tissue architecture and organ function by excessive accumulation of extracellular matrix (ECM) proteins are a defining feature of human fibrotic diseases (1–3). This pathological fibroproliferation is generally believed to be the end result of disordered or exaggerated responses to recurrent or chronic injuries (1–4). Under normal conditions, and especially in self-limited injuries, fibrotic wound responses are appropriately tempered, which limits the development of scarring. In other cases, however, such as during recurrent or persistent infections, chronic inflammation, or other repetitive tissue injuries, wound repairs may result in dysregulation of activated fibroblasts, resulting in deleterious overproduction of ECM (1, 2, 4, 5).
The regulation of fibrotic responses in both health and disease involves a poorly understood choreography of diverse interactive cell types and control mechanisms. Nonetheless, and despite often wide-ranging differences of clinical phenotypes, a common theme of nearly all pathological fibrotic syndromes is the underlying presence of adaptive immune activation, even if many of the details involved in the immunological modulation of fibroblasts remain undiscovered (1, 2).
The series of investigations reported in this article stemmed from an initial observation that CD27nullCD4+ T cells, corresponding to a repetitively stimulated, highly differentiated T-cell effector–memory (Tem) phenotype, which have previously been shown to have augmented pathologic effector functions (6–10), are significantly increased in the circulation of patients with IPF and several other disease syndromes characterized by adaptive immune system activation (6–10). Moreover, the extent of this T-cell differentiation, as evidenced by the proportions of Tem among the peripheral blood mononuclear cells (PBMNCs) of patients, is correlated with disease severity and mortality in individuals with many disorders, including IPF (6–10). Regardless of the particular disease syndrome in which they arise, Tem cells share several common abnormal phenotypic features, which characteristically include downregulation of costimulatory molecule CD28. Thus, a finding of increased numbers of CD4+CD28null cells is widely used as a specific marker of T-cell pathogenesis (9, 10). Our discovery that CD27 is also coordinately downregulated with CD28 on the CD4 Tem of patients with IPF led us to explore potential consequences of these lymphocyte alterations.
CD27 is a TNF receptor family member that is almost invariably expressed on naive CD4 T cells (11, 12). However, activation of T cells can result in proteolytic cleavage of their cell surface CD27, with resultant extracellular release of functionally active soluble receptor (sCD27) (12). The naturally occurring ligand for CD27 is CD70, a type II transmembrane TNF family glycoprotein that is best known as a transducer for costimulatory signals among leukocytes (11, 13). CD70 also has a limited distribution among other cells and tissues, including human fibroblasts (14) and lung (15).
Cognate interactions between CD27 and CD70 play a critical role in lymphocyte activation and differentiation (11, 13), but the functional significance of mesenchymal CD70 has not been described previously. Accordingly, we conducted an incremental series of investigations to explore the possible participation of the CD27–CD70 axis in the pathogenesis of fibrotic disorders, including IPF. We hypothesized that if the CD70 on the surface of human fibroblasts is functionally active, engagements of this ligand with lymphocyte CD27 might be a heretofore undescribed process by which T cells interact with and affect activities of these mesenchymal cells.
The present study details a previously unknown mechanism for lymphocyte–fibroblast interactions that appears to be involved in the homeostatic regulation of fibrogenesis. In addition to providing fundamental insights into these biological processes, the findings we report may also be actionable in that they indicate that targeting the CD27–CD70 axis (11) could be a novel approach for the development of more effective antifibrotic therapies.
Methods
See also the data supplement for further methodological details and Tables E1–E3 in the data supplement for reagent lists.
Patient Blood Specimens
PBMNCs were isolated from patients with lung diseases, as well as from healthy volunteer control subjects, in the course of previously detailed studies (6, 16–18). Cell surface CD27 expression was determined by flow cytometry in randomly selected subpopulations of these study cohorts (Tables E4–E6).
Fibroblast Cultures
Seed cultures of primary human pulmonary fibroblasts were a generous gift from Dr. Veena Antony (19). Fibroblast cultures were established in Dulbecco’s modified Eagle medium supplemented with 10% heat-inactivated FBS, l-glutamine, penicillin/streptomycin, and amphotericin B at 37°C in a humidified 7% CO2 atmosphere. Fibroblasts at passages 3–9 were inoculated into 24-well plates or 6-well plates (1–2 × 105 cells/ml) and incubated for specified times.
Murine fibroblasts were obtained from outgrowths of mouse lungs freshly harvested from killed C57B/6 mice. These cells were cultured using methods identical to those described for human fibroblasts.
IB, Flow Cytometry, Viability, Cell Cycle Analyses, mRNA Expression and CD70 Knockdown
Details of these procedures are described in the data supplement.
Activation of Fibroblast CD70
Various methods were used to activate CD70, depending on details of the experimental design. Pilot studies established the most facile methods to stimulate adherent plate-bound fibroblasts for many uses by treatment with primary anti-CD70 antibodies or CD27 fusion protein, followed by cross-linking with secondary antibodies (see Tables E1 and E2). In some cases, fibroblasts were seeded onto tissue culture plates that had previously been coated with anti-CD70 antibodies by overnight incubation. In some experiments, TGF-β1 (transforming growth factor-β1) was added to fibroblast culture media (2 ng/ml) at the same time as the CD70 activations.
T-cell Conditioned Media Cultures
CD4 T cells were isolated from human PBMNCs using CD4 immunomagnetic beads. The purified CD4 T cells were stimulated with CD3/CD28 immunomagnetic beads and cultured (5 × 105 cells/200 μl) in RPMI with 10% human AB sera. After 48 hours, the T-cell conditioned medium was collected by centrifugation, and sCD27 was measured by ELISA. Near-confluent fibroblasts were cultured in the presence or absence of 1:10 dilutions of the CD4 T-cell conditioned medium for 24 hours before the cells were lysed for immunoblot analyses. In some experiments, the conditioned medium was heated for 5 minutes at 95°C to denature the sCD27.
Kinomic Assays
Pilot studies were conducted to determine the optimal timing of kinase assays. Immunoblots of lysates from CD70-stimulated and unstimulated (isotype) control fibroblast cultures were incubated with PY20 antibody (1:10,000 dilution) overnight at 4°C. The greatest global change of tyrosine phosphorylation occurred approximately 30 minutes after CD70 cross-linking (see Figure E1). Four fibroblast lines were similarly treated, and their lysates were aliquoted for kinomic assays and confirmatory IB. Details of these assays, including analyses of the kinomic data, are found in the data supplement.
Ex Vivo Fibrosis Models
The ex vivo fibrosis models used were as follows:
-
1.
Abdominal skin: Human abdominal skin was obtained from remnants of plastic surgery procedures performed in adult patients. After trimming fat from these specimens, the skin was cut into 1.5-mm sections and cultured in an air–fluid interface (20). Intradermal injections of TGF-β1 (10 ng/skin) and CD27 fusion protein (5 μg/skin) were performed 1 week before harvest, fixation, and hematoxylin and eosin staining. Skin thicknesses at 19 randomly selected cross-sectional areas in each specimen were determined by direct measurements on images and averaged.
-
2.
Human foreskin: Punch biopsies (3-mm diameter) of human foreskin obtained during neonatal circumcisions were incubated in media supplemented with TGF-β1 (10 ng/ml) with or without anti-CD70 monoclonal antibody (mAb) (5 μg/ml) or in media alone (21). After culture for 6 days, the skin specimens were harvested, dried, and weighed. The collagen content was quantitated by hydroxyproline assay and normalized to the total dried weight of each foreskin specimen.
Statistics
Statistical analyses were performed using Prism version 5.0b software (GraphPad Software Inc.). Unpaired continuous variables were compared by Mann-Whitney U test. Paired tests of continuous values before and after (or with and without) a single treatment were carried out with the Wilcoxon signed-rank test. Associations between continuous values were examined by Pearson correlation. A P value less than 0.05 was considered significant.
Study Approval
Written informed consent was obtained from participants before the deidentified specimens used in these studies were obtained under the auspices of an institutional review board–approved protocol (UAB IRB-060815011). Animal studies were conducted under auspices of the University of Pittsburgh Institutional Animal Care and Use Committee (no. 16068267).
Results
Expression of CD27 Is Reduced in Peripheral Blood CD4 T Cells of Patients with IPF, and CD27 Downregulation Is Associated with a Decline in Lung Function
We found that CD27 was coordinately downregulated with CD28 among circulating CD4 T cells of patients with IPF (n = 37; r = 0.92; P < 0.0001) (Figure 1A) and other chronic lung diseases (n = 105; r = 0.83; P < 0.0001) (see Figure E2 and Tables E4–E6). The collinear relationship between CD27 and CD28 expression is also similar in subjects with normal lung function (n = 55; r = 0.77; P < 0.0001), suggesting that CD27 and CD28 coexpression reflects a fundamental process of T cells. Thus, like CD28, CD27 is also expressed on lesser proportions of circulating CD4 T cells in subjects with disease than in healthy control subjects (n = 55; P = 0.03) (Figure 1B). Moreover, CD27 expression was correlated with concurrent forced vital capacities among individual patients with IPF (n = 37; r = 0.39; P < 0.02) (Figure 1C) and subsequent measures of DlCO (n = 22; r = 0.56; P = 0.006) (Figure 1D).
Figure 1.
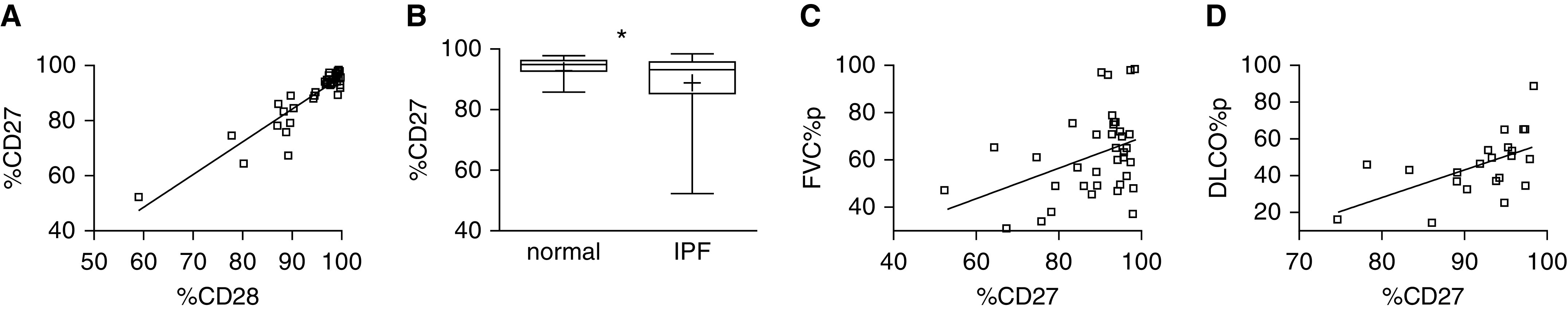
CD27 downregulation on circulating CD4 T cells of patients with idiopathic pulmonary fibrosis (IPF). (A) Correlation between the percentage of CD27+/total CD4+ (%CD27) and CD28+/total CD4+ (%CD28) among patients with IPF (n = 37; r = 0.92; P < 0.0001). Demographic and clinical characteristics of these subjects are detailed in Table E4. (B) %CD27 among these subjects with IPF is reduced compared with healthy control subjects (n = 55; *P = 0.03). The lowest, second lowest, middle, second highest, and highest lines represent 10th, 25th, median, 75th, and 90th percentiles, respectively. Mean is denoted by (+). (C) Forced expiratory volumes, expressed as percent predicted values (FVC%p), correlated with concurrent %CD27 among the patients with IPF (n = 37; r = 0.39; P < 0.02). (D) %CD27 is also correlated with DlCO as percent predicted values (DLCO%p) at subsequent determinations approximately 1 year (11.0 + 3.0 mo) after the T-cell assays (n = 22; r = 0.56; P = 0.006). There were fewer subjects with IPF at these later observations, owing to interval deaths, transplants, or other reasons that precluded DlCO measures. FVC = forced vital capacity.
CD70 Is Expressed on the Surface of Fibroblasts and Regulated by Cell Density and TGF-β1
We first used IB of human fibroblast lysates to test for the presence of CD70 protein. All normal and IPF fibroblast lines evaluated by IB were CD70 positive, although concentrations of the receptor varied considerably among these preparations (Figure 2A). We subsequently isolated and individually tested membrane and cytosol fractions and found that CD70 was located in both compartments (Figure 2B). The presence of cell surface CD70 was also corroborated by flow cytometry (Figure 2C).
Figure 2.
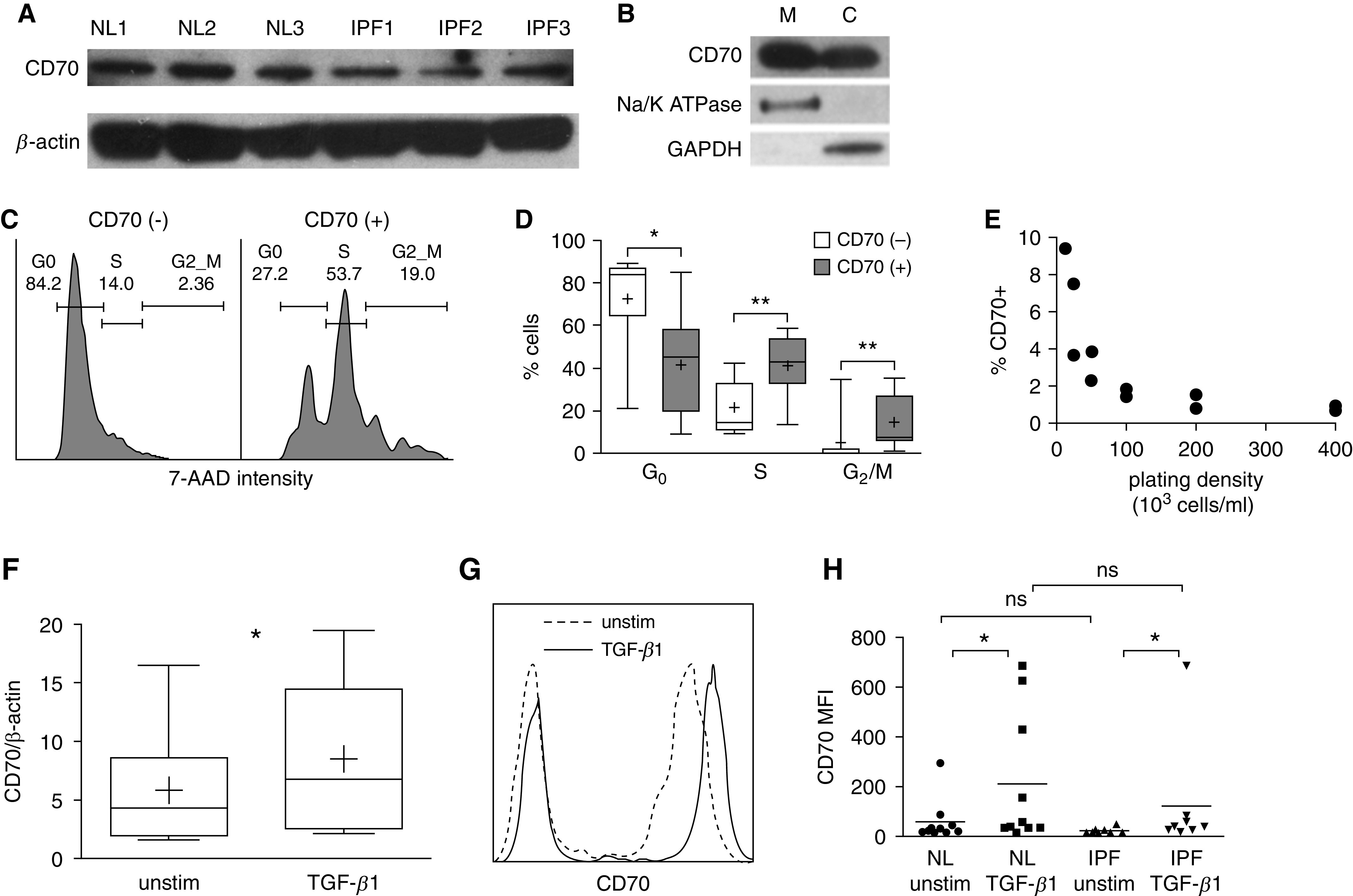
CD70 expression in human pulmonary fibroblasts. (A) Immunoblot analyses of lysates from six healthy (NL) subjects and six patients with IPF showed variable amounts of CD70 (half of these studies are depicted here). (B) Differential immunoblot analyses of fibroblast compartments showed that CD70 is present on both membrane (M) and cytosolic (C) fractions (n = 6). Na+,K+-ATPase was used as a control for the presence of membrane proteins, and GAPDH was used as a control for the cytosolic fractions. (C) Representative cell cycle analysis by flow cytometry shows that CD70null pulmonary fibroblasts within cultures were predominantly in G0 phase, whereas there were greater proportions of S and G2/M cells among the CD70+ fibroblasts. The proportions of cells in each phase are denoted. (D) Compilations and quantitation of these results (n = 9) confirmed that the cell cycle differences were significant: *P < 0.05 and **P < 0.01. The lowest, second lowest, middle, second highest, and highest lines represent 10th, 25th, median, 75th, and 90th percentiles, respectively. Mean is denoted by (+). (E) The proportions of CD70+ fibroblasts after 48 hours in culture were inversely related to cell-plating density (n = 10). (F) CD70 mRNA expression, normalized to β-actin mRNA, was increased from baseline (unstim) by TGF-β1 (transforming growth factor-β1) (2 ng/ml for 24 h; n = 8; *P = 0.05). (G) Representative flow cytometry plot showing that CD70 surface expression was increased 24 hours after TGF-β1 stimulation. (H) Flow cytometry quantitation and compilation showed that TGF-β1 stimulation increased CD70 surface expression, expressed as mean fluorescence intensity (MFI), compared with basal conditions (unstimulated) in primary lung fibroblast lines from 10 healthy human donors and 8 IPF lungs (*P ≤ 0.05 and ns). 7-AAD = 7-aminoactinomycin D; NL = healthy human lungs; ns = not significant; unstim = unstimulated.
We next performed experiments to better understand the observed variability in human fibroblast CD70 expression. Among other influences, CD70 expression was highly associated with the cell cycle, being more prevalent among the fibroblasts in S (n = 9; P < 0.01) and G2/M phases (n = 9; P < 0.01) (Figures 2C and 2D). The proportions of CD70+ fibroblasts within cultures were also inversely related to the extent of cell confluence, whereby the proportion of actively dividing (i.e., S and G2/M) fibroblasts is increased by low plating density (Figure 2E). Isolation and propagation of fibroblasts that had previously been segregated into CD70null subpopulations by cell sorting showed that expression of this surface molecule was facultative and highly fluid; many cells that were initially CD70null later expressed this receptor when passaged again (see Figure E3).
We speculated that if the fibroblast CD70 has physiological relevance, expression of this ligand might also be modified by TGF-β1, a potent profibrotic cytokine that has a singularly important role in adaptive wound responses, as well as by the development of IPF and other fibroproliferative disorders (1–5). Incremental assays confirmed that fibroblast CD70 mRNA and protein expression was upregulated by TGF-β1 among fibroblast cultures (Figures 2F–2H). Given wide dispersion of values, the mean fluorescence intensities (MFIs) of CD70 expressions, determined by flow cytometry, were not significantly different between normal and IPF fibroblasts, either without (59 ± 86 MFI units vs. 23 ± 12 MFI units, normal vs. IPF, respectively; P = 0.57) or with TGF-β1 stimulation (212 ± 265 MFI units vs. 123 ± 129 MFI units, normal vs. IPF, respectively; P = 0.23) (Figure 2H). Similarly, the magnitude of the CD70 MFI increases induced by TGF-β1 did not significantly differ between normal (480 ± 780%) and IPF fibroblasts (660 ± 1,600%).
CD70 Agonism Decreases Fibroblast ECM Production
Additional studies explored potential actions of fibroblast CD70 on ECM production, which is an important function of these mesenchymal cells in health and disease (2–5). Fibroblasts from both normal and IPF lungs (n = 10 each) decreased their production of COL (pro–collagen, type I, α1) and FN (fibronectin) proteins after incubation with function-stimulating anti-CD70 antibodies (P ≤ 0.05 for each) (Figures 3A and 3B). Anti-CD70 antibody treatment also caused reductions of COL mRNA (P < 0.001) and FN mRNA (P < 0.05) (n = 21) (Figure 3C). Furthermore, these effects were potent enough to oppose the profibrotic actions of TGF-β1 in both normal and IPF fibroblasts (n = 10 each; P < 0.05 for each) (Figures 3A and 3B).
Figure 3.
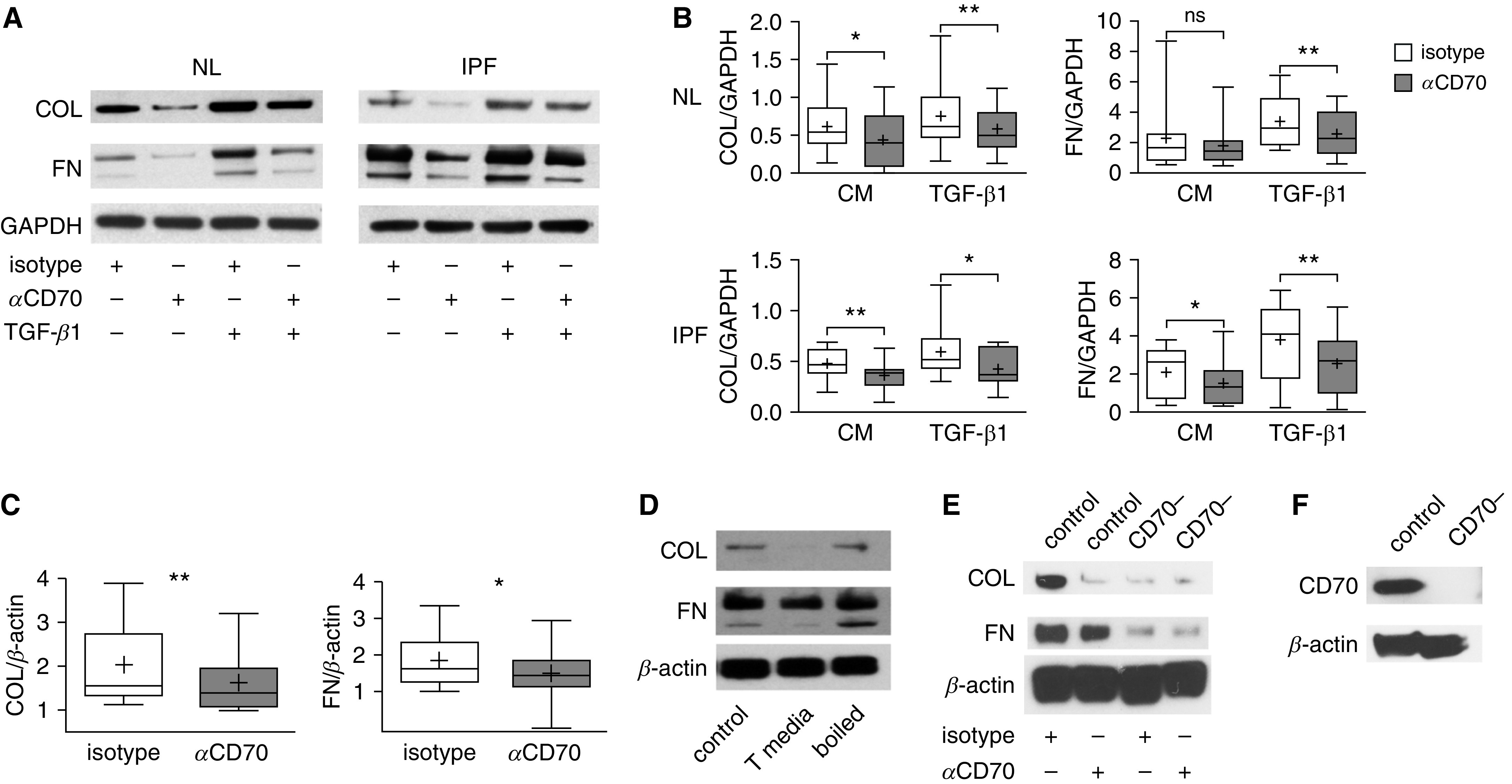
Fibroblast CD70 activation decreases extracellular matrix (ECM) production. (A) Representative immunoblots of fibroblasts from NL or IPF lungs (IPF) after 24 hours of CD70 monoclonal antibody or control isotype treatments (both were cross-linked by secondary antibodies). The anti-CD70 antibody treatment reduced production of pro-COL (pro–collagen I, α1 chain) and FN (fibronectin) compared with the same treatment with control isotype antibody, and it also attenuated the profibrotic effects of TGF-β1 (2 ng/ml). (B) Densitometric quantification of IB from 10 NL donors and 10 IPF lungs (ECM protein concentrations normalized to housekeeping protein) after 24-hour treatment with anti-CD70 antibodies or isotype control antibodies in the presence of TGF-β1 or control unstimulated complete medium (CM). *P < 0.05 and **P < 0.01. The lowest, second lowest, middle, second highest, and highest lines represent 10th, 25th, median, 75th, and 90th percentiles, respectively. Mean is denoted by (+). (C) RT-PCR assay showed decreases in COL and FN mRNA concentrations 12 hours after CD70 activation. (D) T-cell conditioned medium (T media) also reduced fibroblast ECM protein compared with effects of CM (control) or T-cell conditioned medium that had been denatured by boiling. (E) CD70 shRNA-transfected fibroblasts (CD70−) were resistant to the effect of anti-CD70 activation for 24 hours, whereas fibroblasts transfected with a scrambled sequence (control) still exhibited the same ECM reduction as wild-type cells. (F) IB confirmed the efficiency of CD70 knockdown in a stable CD70 shRNA-transfected fibroblast line. Data are representative of three independent experiments.
We corroborated these findings by treating fibroblasts with a CD27 fusion protein, which simulates the in vivo natural ligand of CD70, and found broadly similar effects on ECM production (n = 6; P = 0.06) (see Figure E4). These studies were further extended by testing effects of native sCD27, which is produced during acute T-cell responses (12). We generated sCD27 in vitro by activating healthy control naive CD4 T cells and confirmed the presence of sCD27 in the culture supernatants by ELISA after 72 hours and 96 hours of incubation (P < 0.01 for each) (see Figure E5). Fibroblasts cocultured with these sCD27+ supernatants decreased their production of ECM proteins (Figure 3D), similar to the treatments with anti-CD70 mAb or CD27 fusion protein. ECM production was not altered, however, by incubation with sCD27 that had been denatured by boiling (Figure 3D).
To further validate that our findings were specific consequences of CD70 agonism, we performed a series of analogous experiments using fibroblasts in which CD70 expression was decreased by prior treatment with shRNA. ECM production was markedly diminished in the CD70 antibody–treated fibroblasts that had reduced expression of this receptor compared with autologous cell lines that had instead been transfected with (control) scrambled sequence constructs (Figure 3E). The efficacy of the CD70 knockdown was confirmed by IB (Figure 3F).
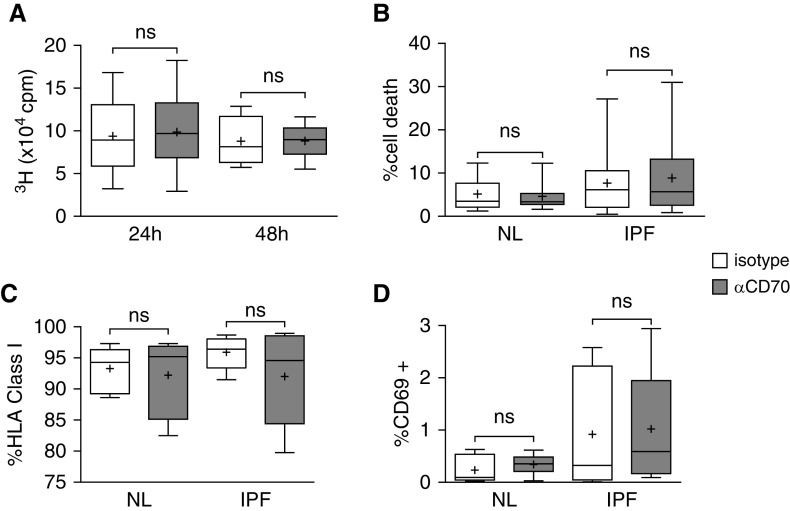
CD70-mediated Inhibition of ECM Production Is Not Due to Fibroblast Growth Inhibition or Cytotoxicity
To further investigate the mechanism(s) by which ECM production is inhibited by CD70 ligation, we evaluated the effect of anti-CD70 antibody treatments on fibroblast proliferation, activation, and viability. Exposure of primary human fibroblasts to varying durations of anti-CD70 did not change their [3H]thymidine incorporation among 11 normal and 5 IPF fibroblast preparations after incubation for 24 and 48 hours (Figure 4A). We also confirmed anti-CD70 treatment had no effect on fibroblast viability in normal and IPF fibroblast cultures (n = 10 each) (Figure 4B). Anti-CD70 antibodies similarly did not alter fibroblast expression of activation marker CD69 in normal fibroblasts (n = 8) and IPF fibroblasts (n = 8). Similarly, human leukocyte antigen class I expression did not change in these preparations (P = 0.64 and P = 0.31 for normal and IPF fibroblasts, respectively) (Figures 4C and 4D).
Figure 4.
CD70 ligation does not alter fibroblast proliferation, viability, or activation. (A) [3H]thymidine (3H) incorporation in cultured normal (n = 11) and IPF (n = 5) fibroblasts was not significantly altered (ns) 24 hours (n = 16) or 48 hours (n = 8) after anti-CD70 antibody treatment compared with incubation with isotype control antibody, each at 2 μg/ml. The lowest, second lowest, middle, second highest, and highest lines represent 10th, 25th, median, 75th, and 90th percentiles, respectively. Mean is denoted by (+). (B) Cell viability 24 hours after similar treatments of fibroblasts from 10 normal lungs and 10 IPF lungs also showed no effect of CD70 activation. Cell death here is defined as the percentage of fibroblasts among the cultures that labeled with 7-AAD and stained with annexin V. (C) Flow cytometry measures of human leukocyte antigen (HLA) class I surface expression were not altered by anti-CD70 treatment among eight normal fibroblast lines or eight lines derived from IPF lungs. (D) Concurrent measures also showed that CD69 expression was not significantly altered by the anti-CD70 in these normal and IPF fibroblasts. cpm = counts per minute.
Fibroblast CD70 Signaling
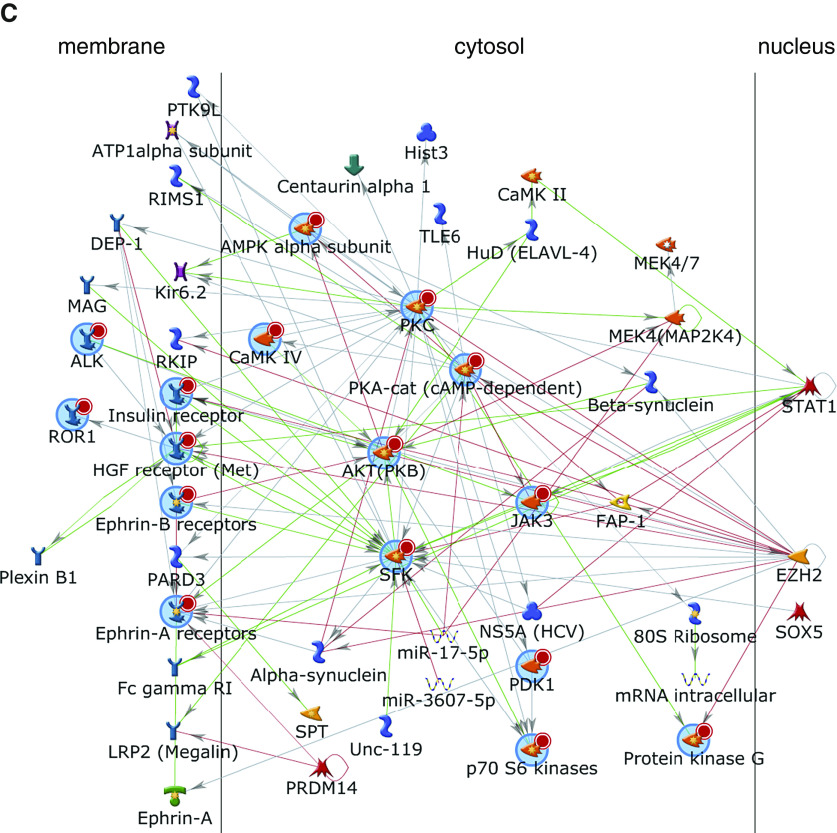
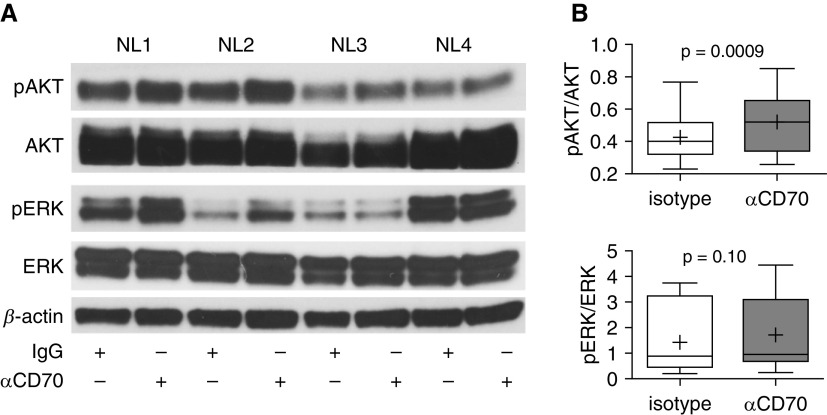
To demonstrate that fibroblast CD70 activation results in specific intracellular signaling events, we examined kinase signaling of CD70-stimulated and isotype control–treated pulmonary fibroblasts by using phosphotyrosine kinase and serine/threonine kinase arrays. We found that CD70 cross-linkage resulted in increases of several kinase activities, including Met; ephrin family kinases; and InSR (insulin receptor), ALK (anaplastic lymphoma kinase), JAK3 (Janus kinase 3), ROR1 (inactive tyrosine-protein kinase transmembrane receptor), and Src kinases (Figures 5A and 5B and Tables E7 and E8). These kinases interacted in a complex interactive network clustered around PKA (protein kinase A) and AKT (protein kinase B) pathways (Figure 5C). Validation by focused IB of these fibroblast lysates (n = 11) confirmed that CD70 activation resulted in significant increases of phosphorylated AKT (pAKT) (P = 0.0009) but not pERK (phosphorylated extracellular signal-regulated kinase) (P = 0.1) (Figure 6), which confirmed the kinomic results. CD70 stimulation also did not affect either Smad or Wnt/β-catenin signaling (see Figure E6).
Figure 5.
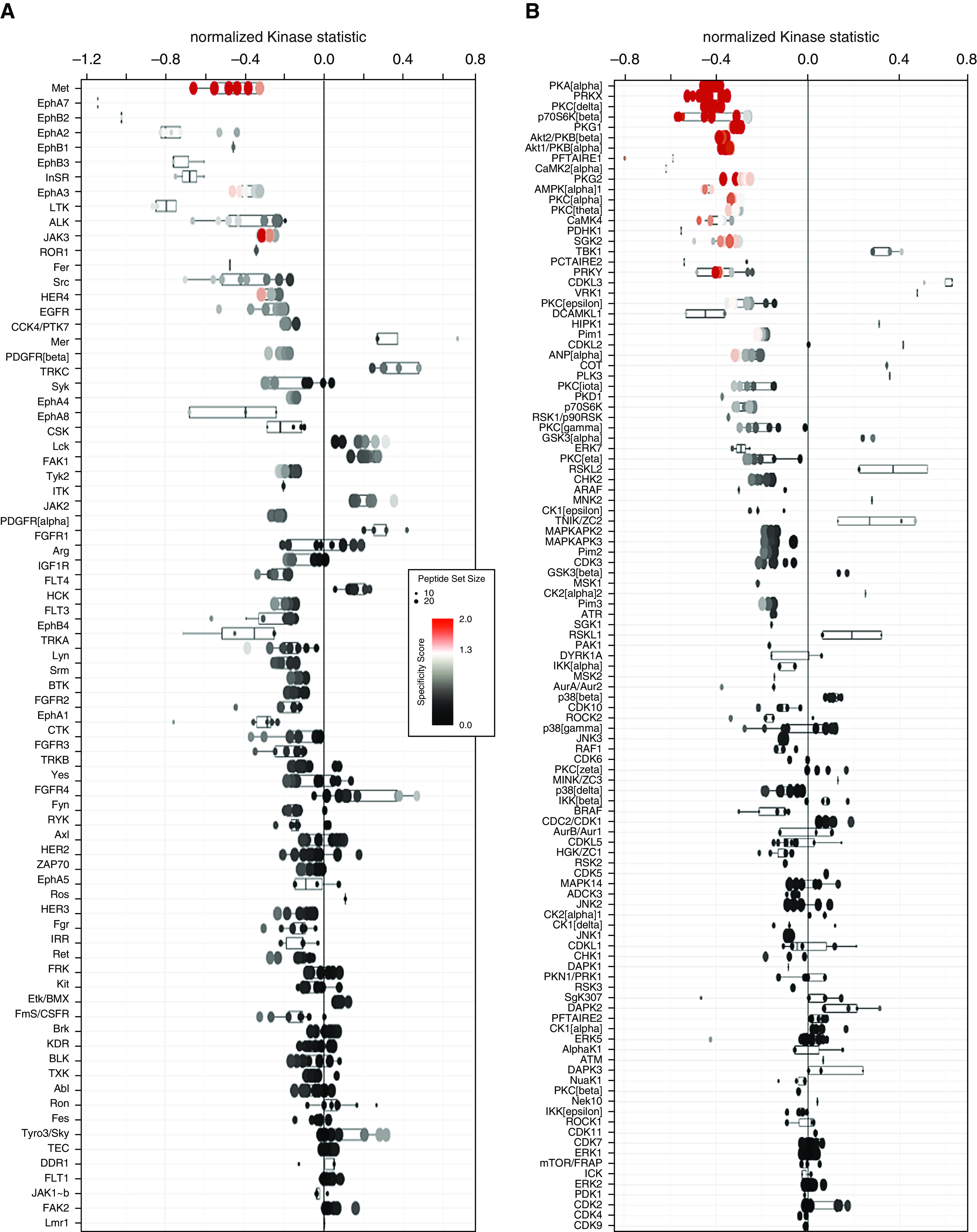
Signal transduction induced by fibroblast CD70 ligation. (A and B) Kinomic profiling using whole-chip peptides showed that CD70 ligation increased activities of a number of tyrosine kinases (A) and serine/threonine kinases (B), as measured by mean kinase statistics (43, 44). A normalized kinase statistic less than 0 indicates an increase in kinase activity in the CD70 antibody–treated group compared with the isotype control group (each consisting of four matched pairs of different primary pulmonary fibroblast lines). See also Tables E7 and E8. (C) Annotated network modeling of CD70 activation signaling effects in fibroblasts. Arrows denote the direction of interaction, and the color of the lines indicates the type of interaction (green = positive; red = inhibitory; gray = complex). A more detailed figure legend for this network can be found in Figure E7.
Figure 6.
CD70 ligation activated pAKT (phosphorylated protein kinase B) but not pERK (phosphorylated extracellular signal-regulated kinase) in pulmonary fibroblasts. (A) Immunoblot analyses of the same protein lysates (n = 4) used in kinomic profiling (Figure 5) showed increases of pAKT (Ser473) after CD70 stimulation but variable results with pERK (Thr202/Tyr204). (B) Densitometric analyses of images from IB of 14 fibroblast lines treated with either anti-CD70 antibody or isotype control IgG showed significant increases in pAKT (P = 0.0009) but not pERK (P = 0.1).
CD70 Agonism Decreases Fibrosis Induced by TGF-β1 Ex Vivo
The effects of CD70 agonism on fibrosis cannot be tested in mice, because CD70 is not expressed on murine fibroblasts (22). To examine effects of CD70 agonism in assays that more closely approximate in vivo conditions, we used two different ligands of this receptor in two distinct ex vivo models of human skin fibrosis (20, 21). CD27 fusion protein treatment significantly reduced dermal thickness in three distinct human abdominal skin samples stimulated with TGF-β1 (P < 0.001) (Figures 7A and 7B). Treatment with anti-CD70 antibodies also significantly decreased hydroxyproline (a measure of collagen production) in 20 human foreskin cultures similarly stimulated with TGF-β1 (P = 0.003) (Figure 7C).
Figure 7.
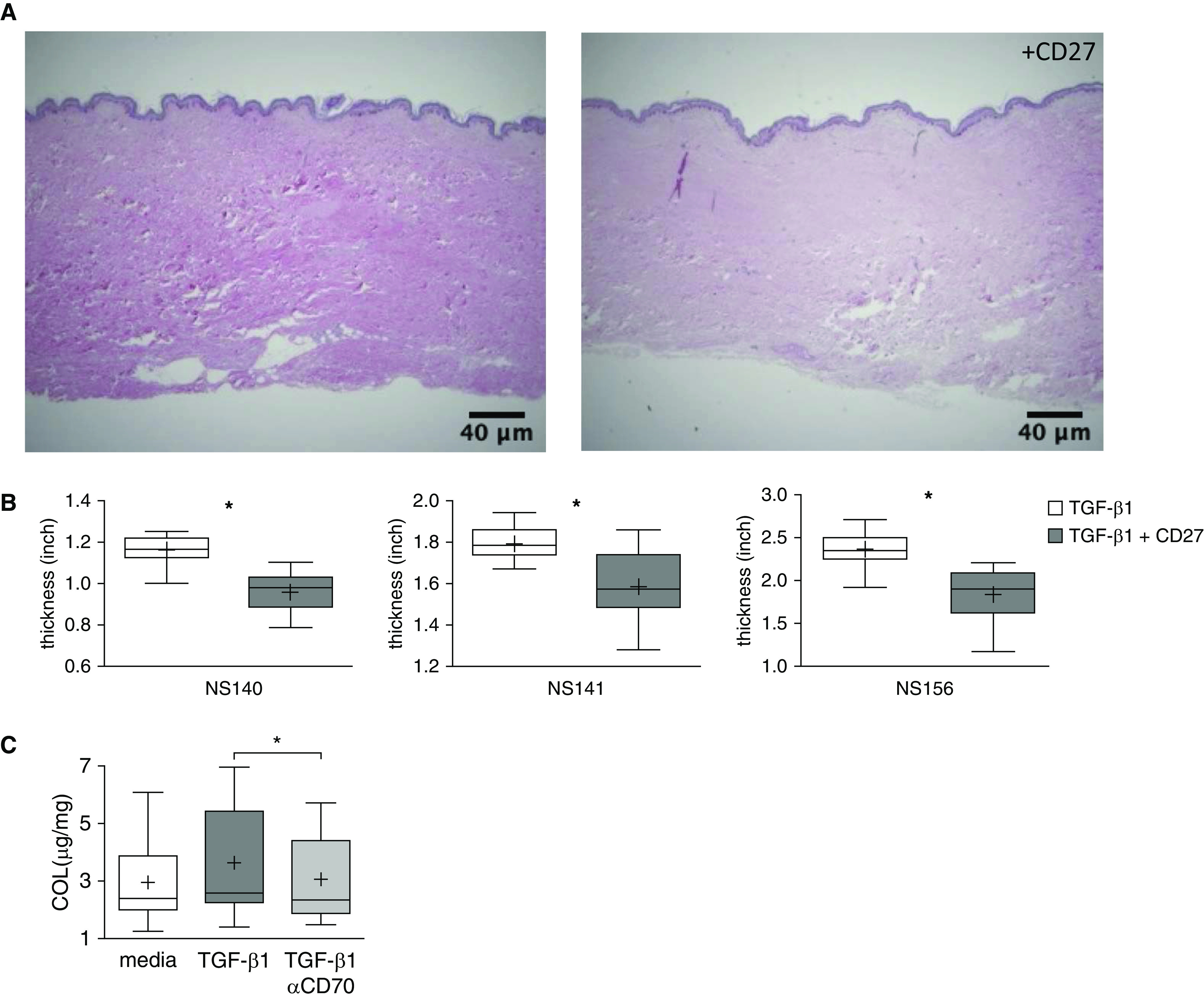
CD70 activation decreases ex vivo fibrosis. (A) Representative cross-sectional images of cultured human abdominal skin tissues injected with TGF-β1 (10 ng/skin sample), with or without concomitant injections of CD27 fusion protein (5 μg/skin sample) (hematoxylin and eosin stain). These preparations were harvested and imaged after treatment for 1 week. Scale bars, 40 μm. (B) Dermal thicknesses were quantified by averaging 19 random vertical measurements of histological cross-sections on each image. Each graph represents one set of these measures in each of the abdominal skin samples from three distinct individuals (NS140, NS141, and NS156). *P < 0.001. The lowest, second lowest, middle, second highest, and highest lines represent 10th, 25th, median, 75th, and 90th percentiles, respectively. Mean is denoted by (+). (C) Human foreskin punch biopsy samples from 20 distinct subjects were cultured in media supplemented with TGF-β1 (10 ng/ml), with or without anti-CD70 antibody (5 μg/ml), for 6 days. Hydroxyproline content, normalized to the dried weight of each skin specimen, was diminished in the specimens that received anti-CD70 treatment. *P = 0.003.
Discussion
The studies described in this article stemmed from an initial finding that CD27 expression on circulating CD4 T cells among patients with IPF is coordinately regulated with CD28 (6–10) (Figure 1). CD28, which is a near-ubiquitous costimulatory molecule on surfaces of naive or early differentiated T cells, becomes downregulated (together with CD27) on human T lymphocytes that have undergone repeated antigen stimulation and proliferation, as seen among patients with a variety of chronic immunological syndromes, including IPF (6–10). Subsequent studies to examine the potential significance of the CD27 downregulation included our confirmation of a previous report that CD70, the unique ligand for CD27, is present on human primary human lung fibroblasts (14). Incremental investigations then showed that human fibroblast expression of CD70 is upregulated by TGF-β1 and downregulated by increased cell density (Figure 2). Functional assays demonstrated that fibroblast CD70 ligation inhibited production of ECM proteins that are central in the development of fibrosis (Figures 3 and 7). We also showed that the reduction of fibroblast ECM production attributable to CD70 ligation is specifically dependent on the presence of this receptor (Figure 3E) and that CD70 stimulation results in activation of discrete, complex signaling pathways (Figures 5 and 6).
These data highlight a mechanism that links fibrotic wound healing with T-cell responses. According to this paradigm, fibroblast ECM production would be comparatively inhibited during acute inflammation due to fibroblast CD70 activation, in turn a consequence of high CD27 expression and sCD27 secretion by the newly activated CD4 T cells that predominate in these particular immune responses. Conversely, however, CD70 inhibition of ECM production would be less operative during chronic inflammation, which is characterized by greater proportions of well-differentiated CD4 Tem cells that do not express or secrete CD27. A relative paucity of fibroblast CD70-mediated ECM inhibition because of the CD27 downregulation on Tem cells could also contribute to maladaptive consequences in other afflictions that are accompanied by persistent or recurrent T-cell activation and differentiation, such as obesity, chronic infections, and various autoimmune syndromes (1–4, 23). ECM production might also be enhanced by the CD70 downregulation that occurs with crowding (Figure 2E) among the dense collections of fibroblasts that reside in the defining lesions (fibroblast foci) of IPF and several other fibrotic diseases (5).
Earlier characterizations of CD70 focused on the expression and function of this receptor among activated leukocytes, especially those in germinal centers, tonsils, skin, and gut (24); in hematological malignancies (25–27); and in autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis (28, 29). CD70 molecules contain a putative casein kinase I substrate motif (-SXXS-) that can initiate intracellular signaling cascades in common with other TNF members that transduce signals (30) and as we observed in assays in the present study (Figures 5 and6). Various studies have shown that leukocyte CD70 activation may regulate cell cycle entry, alter B-cell IgG production (31), and augment natural killer and T-cell–mediated cytotoxicity (32, 33). However, our findings indicate that effects of CD70 agonism are very cell specific in that this receptor does not mediate cycling or activation of pulmonary fibroblasts (Figure 4). Moreover, ERK signaling appears to be a prominent consequence of CD70 engagement in leukocytes (31–33), whereas this pathway was not significantly involved in CD70 signaling among the fibroblasts in the present study (Figure 6).
Human pulmonary fibroblast CD70 was recently noted to be a costimulatory molecule for T-cell activation (via engagements with CD27 on T-cell surfaces) (14). However, neither the regulation of CD70 expression nor effects mediated by this receptor in fibroblasts have been explored. To the best of our knowledge, the present study is a novel demonstration that CD70 is functionally active in fibroblasts and, in particular, involved in their regulation of ECM production. Other unique demonstrations in the present study include showing that T-cell expression of CD27 and CD28 is tightly linked in IPF (Figure 1A) and other chronic inflammatory lung diseases (Figure E2), as well as the finding that CD27 expression is a correlate of current and future pulmonary function parameters in patients with IPF (Figures 1C and 1D).
The importance of T-cell–fibroblast interactions has long been recognized in conditions of homeostasis, as well as in disease states (2, 4, 34). Soluble factors secreted by fibroblasts, such as indolamine, fibroblast growth factor, prostaglandin E2, type I IFN, IL-6, IL-7, and chemokines that include CCL21, CXCL12, and CXCL13 influence T-cell responses by altering lymphocyte signaling and/or promoting T-cell trafficking to loci of inflammation (35–37).
Moreover, lymphocyte–fibroblast interactions are bidirectional, and several T-cell functions that influence these mesenchymal cells have also been described (37, 38). Depending on circumstances, CD4 T cells can produce soluble cytokines that variously inhibit fibroblast ECM production (e.g., IFN-γ and IL-10) (39, 40) or are profibrotic (e.g., IL-4, IL-13, and TGF-β1) (1, 2, 4). Direct contact with activated T-cell membranes (41) or T-cell CM (42) has also previously been found to inhibit fibroblast production of collagen. Of note, however, these preceding studies did not identify a particular ligand/receptor mechanism that accounted for these effects, in contrast to the present findings that show the CD27–CD70 axis is a potent and specific mediator of T-cell–fibroblast interactions (Figure 3).
In contrast, IB and flow cytometry studies of mouse fibroblasts failed to detect CD70 expression (data not shown), and these results are substantiated by other reports (22). As a consequence, CD27–CD70 effects cannot easily be tested in vivo in a facile laboratory animal. The presence of a negative feedback mechanism that limits fibrogenesis during acute and transient adaptive immune responses (i.e., CD27–CD70 interactions that inhibit ECM production) seems likely to be highly adaptive in a long-lived organism that has delayed reproductive capacity (i.e., humans) to prevent needless accumulation over several years of potentially deleterious fibrotic lesions. However, the evolutionary development of processes that limit immune-associated fibrosis may be less imperative in organisms with shorter lifespans and much earlier development of reproductive potential (and greater fecundity), which might account for the lack of functional, ECM-inhibiting CD70 on mouse fibroblasts. We circumvented the absence of an applicable rodent in vivo fibrosis model by use of redundant human skin ex vivo assays (Figure 7). In addition to these and/or perhaps other analogous ex vivo models, future studies requiring demonstration of in vivo effects (e.g., for preclinical tests of therapeutic CD70 agonists [11]) could possibly use human–murine chimeras, transgenic animals, or higher-level organisms (e.g., primates) whose fibroblasts express CD70 (22).
We believe that these are seminal descriptions of a homeostatic mechanism that plays an important role in the regulation of human fibrosis and wound healing. The T-cell–fibroblast interactions shown in the present study are also almost certainly not limited just to IPF, but are probably operative, too, in myriad fibroproliferative syndromes that are associated with inflammation (1) and cause untold morbidity and mortality in modern societies (2–4, 23). Moreover, the findings of the present study are likely not merely arcane, but could be actionable by illuminating the potential for targeting CD70 to decrease (or perhaps even reverse) ECM production and fibrosis (Figure 7). Antihuman CD70 mAb preparations are in early clinical trials for malignant diseases (11), and it may be possible to make relatively minor modifications of those agents to repurpose them for antifibrosis treatments.
Footnotes
Supported by U.S. National Institutes of Health grants 1R01 HL119960 (S.R.D.) and 1U01 HL133232 (S.R.D.).
Author Contributions: T.K.T.-N. and J.X. performed the assays and analyzed the results. C.F.-B. conducted the ex vivo human skin fibrosis experiments. F.C.S. and D.J.K. acquired the specimens and/or collected clinical data. T.K.T.-N. and S.R.D. conceived the study and wrote the paper. All authors participated in proofreading and/or editing the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0450OC on April 22, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wynn TA. Fibrotic disease and the TH1/TH2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockey DC, Bell PD, Hill JA. Fibrosis: a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 3.Thannickal VJ, Zhou Y, Gaggar A, Duncan SR. Fibrosis: ultimate and proximate causes. J Clin Invest. 2014;124:4673–4677. doi: 10.1172/JCI74368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garrett SM, Baker Frost D, Feghali-Bostwick C. The mighty fibroblast and its utility in scleroderma research. J Scleroderma Relat Disord. 2017;2:69–134. doi: 10.5301/jsrd.5000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis. Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilani SR, Vuga LJ, Lindell KO, Gibson KF, Xue J, Kaminski N, et al. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS One. 2010;5:e8959. doi: 10.1371/journal.pone.0008959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herazo-Maya JD, Noth I, Duncan SR, Kim S, Ma SF, Tseng GC, et al. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci Transl Med. 2013;5:205ra136. doi: 10.1126/scitranslmed.3005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mack DG, Lanham AM, Palmer BE, Maier LA, Fontenot AP. CD27 expression on CD4+ T cells differentiates effector from regulatory T cell subsets in the lung. J Immunol. 2009;182:7317–7324. doi: 10.4049/jimmunol.0804305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004;10:119–124. doi: 10.1016/j.molmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Dumitriu IE. The life (and death) of CD4+ CD28null T cells in inflammatory diseases. Immunology. 2015;146:185–193. doi: 10.1111/imm.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wajant H. Therapeutic targeting of CD70 and CD27. Expert Opin Ther Targets. 2016;20:959–973. doi: 10.1517/14728222.2016.1158812. [DOI] [PubMed] [Google Scholar]
- 12.Hintzen RQ, de Jong R, Hack CE, Chamuleau M, de Vries EF, ten Berge IJ, et al. A soluble form of the human T cell differentiation antigen CD27 is released after triggering of the TCR/CD3 complex. J Immunol. 1991;147:29–35. [PubMed] [Google Scholar]
- 13.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutton AJ, Polak ME, Spalluto CM, Wallington JC, Pickard C, Staples KJ, et al. Human lung fibroblasts present bacterial antigens to autologous lung Th cells. J Immunol. 2017;198:110–118. doi: 10.4049/jimmunol.1600602. [DOI] [PubMed] [Google Scholar]
- 15.Genotype-Tissue Expression (GTEx) project. GTEx version 7. 2019 2020 [accessed 2020 Apr 4]. Available from: https://www.gtexportal.org/
- 16.Feghali-Bostwick CA, Tsai CG, Valentine VG, Kantrow S, Stoner MW, Pilewski JM, et al. Cellular and humoral autoreactivity in idiopathic pulmonary fibrosis. J Immunol. 2007;179:2592–2599. doi: 10.4049/jimmunol.179.4.2592. [DOI] [PubMed] [Google Scholar]
- 17.Kahloon RA, Xue J, Bhargava A, Csizmadia E, Otterbein L, Kass DJ, et al. Patients with idiopathic pulmonary fibrosis with antibodies to heat shock protein 70 have poor prognoses. Am J Respir Crit Care Med. 2013;187:768–775. doi: 10.1164/rccm.201203-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu X, Gadgil AS, Givelber R, George MP, Stoner MW, Sciurba FC, et al. Peripheral T cell functions correlate with the severity of chronic obstructive pulmonary disease. J Immunol. 2009;182:3270–3277. doi: 10.4049/jimmunol.0802622. [DOI] [PubMed] [Google Scholar]
- 19.Surolia R, Li FJ, Wang Z, Li H, Dsouza K, Thomas V, et al. Vimentin intermediate filament assembly regulates fibroblast invasion in fibrogenic lung injury. JCI Insight. 2019;4:123253. doi: 10.1172/jci.insight.123253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasuoka H, Larregina AT, Yamaguchi Y, Feghali-Bostwick CA. Human skin culture as an ex vivo model for assessing the fibrotic effects of insulin-like growth factor binding proteins. Open Rheumatol J. 2008;2:17–22. doi: 10.2174/1874312900802010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson CL, Kojima S, Getsios S. RNA interference in keratinocytes and an organotypic model of human epidermis. Methods Mol Biol. 2010;585:127–146. doi: 10.1007/978-1-60761-380-0_10. [DOI] [PubMed] [Google Scholar]
- 22.Park YM, Squizzato S, Buso N, Gur T, Lopez R. The EBI search engine: EBI search as a service-making biological data accessible for all. Nucleic Acids Res. 2017;45:W545–W549. doi: 10.1093/nar/gkx359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueha S, Shand FH, Matsushima K. Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis. Front Immunol. 2012;3:71. doi: 10.3389/fimmu.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hintzen RQ, Lens SM, Koopman G, Pals ST, Spits H, van Lier RA. CD70 represents the human ligand for CD27. Int Immunol. 1994;6:477–480. doi: 10.1093/intimm/6.3.477. [DOI] [PubMed] [Google Scholar]
- 25.Lens SM, Drillenburg P, den Drijver BF, van Schijndel G, Pals ST, van Lier RA, et al. Aberrant expression and reverse signalling of CD70 on malignant B cells. Br J Haematol. 1999;106:491–503. doi: 10.1046/j.1365-2141.1999.01573.x. [DOI] [PubMed] [Google Scholar]
- 26.Gruss HJ, Kadin ME. Pathophysiology of Hodgkin’s disease: functional and molecular aspects. Baillieres Clin Haematol. 1996;9:417–446. doi: 10.1016/s0950-3536(96)80019-9. [DOI] [PubMed] [Google Scholar]
- 27.Ho AW, Hatjiharissi E, Ciccarelli BT, Branagan AR, Hunter ZR, Leleu X, et al. CD27-CD70 interactions in the pathogenesis of Waldenstrom macroglobulinemia. Blood. 2008;112:4683–4689. doi: 10.1182/blood-2007-04-084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee WW, Yang ZZ, Li G, Weyand CM, Goronzy JJ. Unchecked CD70 expression on T cells lowers threshold for T cell activation in rheumatoid arthritis. J Immunol. 2007;179:2609–2615. doi: 10.4049/jimmunol.179.4.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han BK, White AM, Dao KH, Karp DR, Wakeland EK, Davis LS. Increased prevalence of activated CD70+CD4+ T cells in the periphery of patients with systemic lupus erythematosus. Lupus. 2005;14:598–606. doi: 10.1191/0961203305lu2171oa. [DOI] [PubMed] [Google Scholar]
- 30.Eissner G, Kolch W, Scheurich P. Ligands working as receptors: reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor Rev. 2004;15:353–366. doi: 10.1016/j.cytogfr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Arens R, Nolte MA, Tesselaar K, Heemskerk B, Reedquist KA, van Lier RA, et al. Signaling through CD70 regulates B cell activation and IgG production. J Immunol. 2004;173:3901–3908. doi: 10.4049/jimmunol.173.6.3901. [DOI] [PubMed] [Google Scholar]
- 32.Al Sayed MF, Ruckstuhl CA, Hilmenyuk T, Claus C, Bourquin JP, Bornhauser BC, et al. CD70 reverse signaling enhances NK cell function and immunosurveillance in CD27-expressing B-cell malignancies. Blood. 2017;130:297–309. doi: 10.1182/blood-2016-12-756585. [DOI] [PubMed] [Google Scholar]
- 33.García P, De Heredia AB, Bellón T, Carpio E, Llano M, Caparrós E, et al. Signalling via CD70, a member of the TNF family, regulates T cell functions. J Leukoc Biol. 2004;76:263–270. doi: 10.1189/jlb.1003508. [DOI] [PubMed] [Google Scholar]
- 34.Murakami S, Okada H. Lymphocyte-fibroblast interactions. Crit Rev Oral Biol Med. 1997;8:40–50. doi: 10.1177/10454411970080010201. [DOI] [PubMed] [Google Scholar]
- 35.Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- 36.Roozendaal R, Mebius RE. Stromal cell-immune cell interactions. Annu Rev Immunol. 2011;29:23–43. doi: 10.1146/annurev-immunol-031210-101357. [DOI] [PubMed] [Google Scholar]
- 37.Tan J, Tedrow JR, Nouraie M, Dutta JA, Miller DT, Li X, et al. Loss of Twist1 in the mesenchymal compartment promotes increased fibrosis in experimental lung injury by enhanced expression of CXCL12. J Immunol. 2017;198:2269–2285. doi: 10.4049/jimmunol.1600610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wick G, Backovic A, Rabensteiner E, Plank N, Schwentner C, Sgonc R. The immunology of fibrosis: innate and adaptive responses. Trends Immunol. 2010;31:110–119. doi: 10.1016/j.it.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Granstein RD, Flotte TJ, Amento EP. Interferons and collagen production. J Invest Dermatol. 1990;95(Suppl):75S–80S. doi: 10.1111/1523-1747.ep12874789. [DOI] [PubMed] [Google Scholar]
- 40.Moroguchi A, Ishimura K, Okano K, Wakabayashi H, Maeba T, Maeta H. Interleukin-10 suppresses proliferation and remodeling of extracellular matrix of cultured human skin fibroblasts. Eur Surg Res. 2004;36:39–44. doi: 10.1159/000075073. [DOI] [PubMed] [Google Scholar]
- 41.Chizzolini C, Rezzonico R, Ribbens C, Burger D, Wollheim FA, Dayer JM. Inhibition of type I collagen production by dermal fibroblasts upon contact with activated T cells: different sensitivity to inhibition between systemic sclerosis and control fibroblasts. Arthritis Rheum. 1998;41:2039–2047. doi: 10.1002/1529-0131(199811)41:11<2039::AID-ART20>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 42.Mikko M, Fredriksson K, Wahlström J, Eriksson P, Grunewald J, Sköld CM. Human T cells stimulate fibroblast-mediated degradation of extracellular matrix in vitro. Clin Exp Immunol. 2008;151:317–325. doi: 10.1111/j.1365-2249.2007.03565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson JC, Taylor RB, Fiveash JB, de Wijn R, Gillespie GY, Willey CD. Kinomic alterations in atypical meningioma. Med Res Arch. 2015;2015(3) doi: 10.18103/mra.v0i3.104. 10.18103/mra.v0i3.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbert AN, Shevin RS, Anderson JC, Langford CP, Eustace N, Gillespie GY, et al. Generation of microtumors using 3D human biogel culture system and patient-derived glioblastoma cells for kinomic profiling and drug response testing J Vis Exp 2016(11254026. [DOI] [PMC free article] [PubMed] [Google Scholar]