Abstract
Approximately 60% of all human pathogens and 75% of emerging infectious diseases are zoonotic (of animal origin). Camel zoonotic diseases can be encountered in all camel‐rearing countries. In this article, all studies carried out on camel zoonotic diseases in Iran are reviewed to show the importance of camels for public health in this country. More than 900 published documents were systematically searched to find relevant studies from 1,890 until late 2018. The collected articles were classified according to the aetiological agents. In this study, 19 important zoonotic diseases were reported among Iranian camels including listeriosis, leptospirosis, plague, Q fever, brucellosis, campylobacteriosis, tuberculosis, pasteurellosis, clostridiosis, salmonellosis, Escherichia coli infections, rabies, camelpox, Middle East respiratory syndrome coronavirus, Crimean‐Congo haemorrhagic fever, echinococcosis, cryptosporidiosis, toxoplasmosis and dermatophytosis, most of which belong to bacterial, viral, parasitic and fungal pathogens, respectively. Results show that camels are one of the most important sources of infections and diseases in human; therefore, continuous monitoring and inspection programs are necessary to prevent the outbreak of zoonotic diseases caused by this animal in humans.
Keywords: dromedary camel, epidemiology, Middle East, zoonoses
The importance of zoonotic disease is not hidden, and these diseases have had a tremendous effect on human population throughout history as more than 60% of human diseases are zoonotic. Also the importance of camel farming as a source of milk and meat in world market and especially in the Middle East is developing. So camel zoonotic disease can have a significant role in public health in all around the world including Iran. Indirect zoonoses by cross transmission of camel diseases through other livestock or vice versa do require attention.

1. INTRODUCTION
A zoonosis is any disease or infection that is naturally transmissible from vertebrate animals to humans. More than 60% of human infectious diseases are caused by zoonotic pathogens which have been responsible for some of the most fatal diseases such as Ebola and severe acute respiratory syndrome (SARS) in recent years (Belay et al., 2017). Zoonotic diseases may be obtained or transmitted in a variety of ways including direct contact, through the air (aerosol), contact with an inanimate object that harbours the disease (fomite transmission), oral ingestion and arthropod bite (Hersom, Irsik, & Thrift, 2008). According to studies, camels are one of the important carriers and sources of infection for human, livestock and wildlife in Iran and other parts of the world where camel lives. Some camel diseases and infections can pose a significant threat to public health, including Middle East respiratory syndrome coronavirus (MERS‐CoV; Alkhamis et al., 2018). The first case of this disease was reported in Saudi Arabia in 2012. Subsequently, the disease caused more than 1,500 confirmed human infections with over 580 deaths (Alkhamis et al., 2018). Several studies implicated that dromedary camel is the primary intermediate host and the source of zoonotic inaugurations (Du & Han, 2016). The most important camel zoonotic diseases and infections reported worldwide are actinomycosis (Kilic & Kirkan, 2004), anthrax (Musa, Shomein, Abd el Razig, Meki, & Hassan, 1993), borreliosis (Helmy, 2000), chlamydiosis (Elzlitne & Elhafi, 2016), clostridial diseases (Wernery, Ul‐Haq, Joseph, & Kinne, 2004; Younan & Gluecks, 2007), balantidiasis (Tajik, Farda, paidar, Anousheh, & Dehghani, 2013), melioidosis (Bergin & Torenbeeck, 1991), Staphylococcus aureus (Jaradat, Al Aboudi, Shatnawi, & Ababneh, 2013), Corynebacterium ulcerance (Tejedor, Martin, Lupiola, & Gutierrez, 2000), mycoplasma (Mederos‐Iriarte et al., 2014), streptococcal (Heller, Anderson, & Silveira, 1998), trypanosomiasis (Bennoune, Adili, Amri, Bennecib, & Ayachi, 2013), fascioliasis (Haridy & Morsy, 2000), schistosomiasis (Singh, Borah, Dadhich, & Sharma, 2013), sarcopticosis (Sk, Tuteja, & Sena, 2009), hepatitis(Woo et al., 2014), influenza A virus (Yamnikova et al., 1993), rift valley fever (Swai & Sindato, 2015), West Nile fever (El‐Harrak et al., 2011), leptospirosis, plague, Q fever, brucellosis, campylobacteriosis, tuberculosis, pasteurellosis, clostridiosis, salmonellosis, Escherichia coli, glanders, rabies, camelpox, Middle East respiratory syndrome coronavirus (MERS‐CoV) infection, Crimean‐Congo haemorrhagic fever (CCHF), cysticercosis, toxocariasis, echinococcosis, giardiasis, surra, leishmaniasis, trichinellosis, cryptosporidiosis, toxoplasmosis and dermatophytosis (Wernery, Kinne, & Schuster, 2014). Some of these diseases are very common in camels, while others are rare. Some of these pathogens cause clinical diseases, whereas others are subclinical.
The ability of camels to survive in arid and semiarid areas of the world, endurance in prolonged drought and above all, a high potential to convert the scant resources of the desert into milk and meat, making them more important for raising in order to compensate for more food demand in the future because of globally growing human population and climate change (Al‐Jassim & Sejian, 2015). Camel raising is not only socially acceptable but also economically relevant; therefore, authorities of camel farming are focusing on increasing camel number and, consequently, the farmer income (Mirzaei, 2012). In this situation, control of camel pathogens will continue to be a highly important component of efficient food production and become associated more overtly with the food security agenda (Al‐Jassim & Sejian, 2015). In some regions, camel milk and liver are consumed raw without any heat treatment. There are reports on human plague outbreaks due to eating raw camel liver and meat in Libya, Saudi Arabia, Jordan and Afghanistan in 1976, 1994, 1997 and 2007, respectively (Leslie et al., 2011). In addition, there is close contact between a herdsman and camels on several occasions during watering, riding, grooming and milking. For instance, senile, debilitated or sick animals are often well‐nursed and hand‐fed for long periods (Abbas, Zubeir, & Yassin, 1987); therefore, it may increase the contact between the animal and human and contribute to the transmission of some zoonotic diseases as well. In countries raising camels (e.g. Iran), there is considerable direct contact between farmers and camels, and also meat and milk consumption, which are among the sources of infection. Improper importation or smuggling of camels from neighbouring countries, such as Afghanistan, Pakistan and the United Arab Emirates (UAE), can also account for a source of new zoonotic diseases import.
Despite the huge and rising impacts of zoonotic diseases on human health, there are still gaps in our knowledge of how some zoonotic infections develop and spread in different populations. Accordingly, gathering this information can be helpful to predict and prevent future outbreaks. Public health authorities should focus on detection, investigation and control of these threats with a health‐based approach.
Since the use of camel products in Iran has increased in recent years, and the movement of these livestock between the provinces and neighbouring countries is carried out without special restrictions, these animals and their products can be a source of transmission of some diseases to human (Figure 1). Hence, having more information about the health status of camels is important for health stakeholders and healthcare providers in the country. In this study, the camel zoonotic diseases and infections in Iran are outlined by focusing on the aetiology of diseases and infections, clinical signs, methods of diagnosis, routes of transmission and determining their distribution pattern in order to show camel's public health importance in Iran. It is expected that information from this study be used by relevant authorities in the field of medicine and veterinary medicine.
Figure 1.

Iranian camel's numbers categorized by provinces according to the census of Ministry of Jihad –e‐ Agriculture, 2017 (Ebadzadeh et al., 2018). (1) Alborz (2) Ardabil (3) Azerbaijan, East (4) Azerbaijan, West (5) Bushehr (6) Chahar Mahaal and Bakhtiari (7) Fars (8) Gilan (9) Golestan (10) Hamadan (11) Hormozgān (12) Ilam (13) Isfahan (14) Kerman (15) Kermanshah (16) Khorasan North (17) Khorasan, Razavi (18) Khorasan South (19) Khuzestan (20) Kohgiluyeh and Boyer‐Ahmad (21) Kurdistan (22) Lorestan (23) Markazi (24) Mazandaran (25) Qazvin (26) Qom (27) Semnan (28) Sistan and Baluchestan (29) Tehran (30) Yazd (31) Zanjan
2. MATERIALS AND METHODS
2.1. Search strategy
To review relevant studies, Medline (PubMed), ISI Web of Knowledge, Science Direct, Embase, Scopus and Google Scholar were systematically searched to find all publications from Iran using the keywords of "camel" and "Iran". The retrieved papers that reported camel zoonotic diseases with major public health importance were included in the study.
More than 900 published documents were systematically searched to find related studies from 1890 until late 2018. The included papers were written in English, French and Persian. Iranian search engines such as Scientific Information Database (SID), MEDLIB, Magiran, IranMedex and proceeding of the first national congress of camel were also searched to find the related papers.
Moreover, in order to maximize the sensitivity of the search, bibliographies of the identified studies were screened for additional relevant studies. All the resultant titles and abstracts for the given disease were included in the review. In addition, this review assessed all full‐text articles and incorporated related documents. The collected studies were classified according to the infectious agent (viral, bacterial, parasitic and fungal diseases).
Two authors (RM and HM) independently screened the title and abstract of all obtained studies and then reviewed the full texts of the retrieved studies that met research criteria. The authors were not blinded to the names of the studies' authors and journals. All disagreements between the authors about the final selection of studies were resolved by negotiation with a third author (CH). The agreement rate of the two authors was more than 90%. The variables that were extracted for data analysis included: study design, year and location of the study, disease type, transmission method and the signs of disease in camel.
Six items from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement checklist were selected and used for assessing the quality of reporting (Vandenbroucke et al., 2007).
3. RESULTS
This review covers 19 important zoonotic diseases reported in camels of Iran, most of which belonged to bacterial, viral, parasitic and fungal pathogens in order of prevalence.
3.1. Bacterial zoonotic diseases
In this study, listeriosis, leptospirosis, plague, Q fever, brucellosis, campylobacteriosis, tuberculosis, pasteurellosis, clostridiosis, salmonellosis and E. coli infections were reported among the camels (Table 1).
Table 1.
Summary of zoonotic bacterial diseases of camels reported in Iran; 1890–2018
| A. Bacterial diseases | |||||
|---|---|---|---|---|---|
| Disease | Year | Location | Techniques | No. positive/ No. tested (%) | Ref |
| Leptospirosis | 1959 | Various area of Iran | – | 1/5 (20%) | (Rafyi & Maghami, 1959) |
| 1996 | South Khorasan (Tabas) | Serological test | 1/14 (7%) | (Hadian, 1996) | |
| 2008–2011 | Yazd | Microscopic agglutination test | 22/8% No Numeral data | (Sazmand, 2012) | |
| 2010–2011 | Various area of Iran | Bacterial culture, multiplex PCR | 7/35 (20%), 8/35 (22.85%) | (Safarpoor Dehkordi et al., 2012; Safarpoor Dehkordi & Taghizadeh 2012) | |
| 2012 | Isfahan | PCR | 19/130 (14.61%) | (Doosti et al., 2012) | |
| 2012 | Isfahan | Bacterial culture, Multiplex PCR | 7/49 (14.29%), 8/49 (6.33%) | (Dehkordi et al., 2012; Dehkordi and Taghizadeh 2012) | |
| 2013 | Yazd | Microscopic agglutination test | 30/128 (32.4%) | (Hajikolaei et al., 2013) | |
| 2013 | Qom | Microscopic agglutination test | 51/183 (27.87%) | (Talebi, 2007) | |
| 2014 | Ardabil | Microscopic agglutination test | 12/60 (20%) | (Afkhamnia, Avagyan, Khaki, Bidhendi, & Mostafaey, 2014) | |
| Q fever | 1959 | – | ELISA | 2/12 (16.66%) | (Moghaddas, 2012 2012) |
| 2011 | Isfahan | PCR | 14/130 (10.76%) | (Doosti et al., 2014) | |
| 2015 | North, South and Razavi Khorasan | ELISA | 48/168 (28.7%) | (Janati Pirouz et al., 2015) | |
| 2016 | Khorasan ( North, South, Razavi) | PCR | 4/167 (2.4%) | (Pirouz, Mohammadi, Mehrzad, & Aziz zadeh, 2016) | |
| Brucellosis | 1987 | Fars | Tube agglutination, RBPT | 2/238 (0.84%) | (Motamedi, 1987) |
| 1986–1987 | Qazvin | RBPT, SAT, 2ME, CFT | 77/935 (8%) | (Zowghi & Ebadi, 1991) | |
| 1994 | Isfahan (Najaf Abad) | RBPT | 5/100 (0.05%) | (Miranzade, 1994) | |
| 1999 | Bushehr | RBPT, SAT, 2ME, CFT | 5/258 (1.93%) | (Khadjeh, Zowghi, & Zarif‐fard, 1999) | |
| 2005 | Isfahan (Najaf Abad) | RBPT, Wright, 2ME | 11/384 (2.84%) | (Pourjafar et al. 2005) | |
| 2007 | Hormozgan | RBPT | 3/103 (2.91%) | (Garib, 2011) | |
| 2011 | Sistan and Baluchestan | RBPT, SAT, 2ME | 17/500 (3.4%) | (Sargaz, 2011) | |
| 2008–2011 | Yazd | RBPT | 149/395 (37.83%) | (Sazmand, Rasooli, et al., 2012) | |
| 2012 | Isfahan (Najaf Abad) | (RBPT, mRB, Wright, 2ME), PCR, Culture | 39/310 (12.58%) mRB; 27/310 (8.71%) RBPT; 7/310 (2.26%) Wright; 6/310 (1.94%) 2ME; 18.6/310 (6%) PCR | (Ghorbani et al., 2013) | |
|
2012 |
Isfahan | Multiplex PCR, Culture | 4/35 (11.42%) aborted fetus | (Dehkordi et al., 2012; Dehkordi and Taghizadeh 2012) | |
| 2012 | Various part of Iran | Conventional PCR, real‐time PCR assays | 201/618 (32.52%) C‐PCR, 143/201 (71%), RT‐PCR | (Dehkordi et al., 2012; Dehkordi and Taghizadeh 2012) | |
| 2014 | Isfahan | PCR, blood sample, lymph node | 14/123 (11.38%) blood sample; 16/123 (13.01%) lymph node samples | (Khamesipour, Doosti, Mobarakeh, & Komba, 2014; Khamesipour, Rahimi, Shakerian, Doosti, & Momtaz, 2014) | |
| 2015 | Isfahan (Najaf Abad) | RBPT, Tube agglutination, 2ME, PCR | 18/150 (12%) RBPT; 12/150 (8%) TG, 9/150 (6%) 2ME; 3/150 (1.3%) PCR | (Mahzunieh & Salami, 2015) | |
| Campylobacteriosis | 2006–2006 | Fars, Bushehr | Culture PreT‐KB method | 3/145 (2%) | (Baserisalehi et al., 2007) |
| 2007–2008 | Isfahan (Najaf‐Abad) | Bacteriological examination | 5/94 (2%) | (Rahimi, Momtaz, & Nozarpour, 2010) | |
| 2008–2009 | Isfahan, Yazd | Microbiological analysis | 1/107 (0.9%) | (Rahimi, Momtaz, et al., 2010) | |
| 2010 | Chaharmahal & Bakhtiari, Khuzestan | PCR | 3/130 (2.3%) | (Rahimi et al., 2013) | |
| Plague | 1960 | Iran | – | – | (McGrane & Higgins, 1985) |
| 1974 | Iran | – | – | (Fedorov, 1960) | |
| Tuberculosis | – | Khorasan Razavi | PCR | 4/100 (4%) | (Hashemi et al., 2002) |
| 2009–2010 | Khorasan Razavi (Mashhad) | PCR | 17/102 (16.66%) | (Soleymani Babadi et al., 2012) | |
| Pasteurellosis | 2010 | Fars (Larestan) | Morphological, cultural, biomedical characterization | 53/100 (53%) | (Esmaeili et al., 2010) |
| 2014 | Iran | DNA extraction, PCR assay | 104/971 (10.7%) | (Chitgar et al., 2014) | |
| 2014–2015 | Fars (Larestan) | Morphological, cultural, biomedical characterization | 4/5 (80%) dead camels, 31/48 (64.58%) clinical camels, 8/109 (7.34%) healthy camels | (Tahamtan et al., 2017) | |
| Salmonellosis | 1992 | North East of Iran | Bacteriological examination Serological test | 14/113 (12.39%), 38/102 (37.25) | (Moghaddas, 2012 2012) |
| 1994 | Isfahan (Najaf Abad) | Agar gel immunodiffusion test (AGIT) | 1/59 (1.69%) | (Miranzade, 1994) | |
| 2007–2008 | Isfahan (Najaf Abad) | Bacteriological examination | Case report | (Rahimi et al., 2012) | |
| 2010 | Isfahan (Najaf Abad) | Agar gel immunodiffusion test | 3/384 (0.78%) | (Pourjafar, Mahzounieh, Zahraei Salehi, & Habibollahi Khorasgani, 2010) | |
| 2010 | Tehran | Microbiology test, Histopathology, Gross Pathology, Clinical Pathology | 1/94 (1.1%) | (Nour‐Mohammadzadeh et al., 2010) | |
| 2010–2011 | West Azerbaijan (Urmia region) | Bacteriological examination, PCR | 4/100 (4%) | (Ahmadi, 2012) | |
| 2011–2012 | Fars and Khuzestan | Bacteriological examination, PCR | 1/50 (2.0%) | (Rahimi et al., 2012) | |
| 2012 | Razavi Khorasan | Microbiology | 10% | (Gholami et al., 2014) | |
| 2012 | Various parts of Iran | Microbiology | 84/196 (43%) | (Sepehr, 2012) | |
| 2013 | Kerman | PCR | 8% | (Zavarshani, Kownani, Estabraghi, & Yarahmadi, 2015) | |
| 2015 | Golestan | Quantitative agglutination test | 9/9 (100%) | (Rabbani Khorasani, Eatemadifar, Azadbakht, Emami, & Khormali, 2015) | |
| 2016 | Razavi Khorasan (Mashhad) | Microbiology | 2/10 (20%) | (Mohammady & Najafi Mosleh, 2017) | |
| Listeriosis | 2007–2008 | Isfahan Najaf Abad | Bacteriological test | 9/94 (9.6%) | (Rahimi, Momtaz, et al., 2010) |
| 2011 | Sistan and Baluchestan (Sistan) | Bacteriological examination | 3/80 (3.75%) | (Safdari & Jahantigh, 2014) | |
| 2011 | Various parts of Iran | Culture, PCR, Technique Real‐Time PCR, | 6/101 (5.94%) Milk; 3/100 (3%) Faeces; 10/79 (13.92%) Vaginal Swab; 8/95 (9.47%) Urine | (Dehkordi, Barati, Momtaz, Ahari, & Dehkordi, 2013; Dehkordi, Haghighi Borujeni, Rahimi, & Abdizadeh, 2013) | |
| 2011–2012 | Tehran | Microbiological tests | 6/24 (25%) Frozen meat, 12/24 (50%) Fresh meat | (Mashak et al., 2015) | |
3.1.1. Leptospirosis
Leptospirosis is a disease that causes intensive clinical illness in humans and animals. The causative agent develops directly within its hosts and indirectly in the environment. More than 250 serovars of Leptospira are recognized as pathogenic agents (Ellis, 2015; Ningal et al., 2015). Leptospirosis has been reported from all parts of the world but it is more common in tropical and subtropical areas with high rainfall periods. Annually, about 7–10 million people are infected by Leptospira spp. worldwide (Hartskeerl, Collares‐Pereira, & Ellis, 2011).
Leptospirosis is a disease with clinical manifestations such as stillbirth, abortion, haematuria, infertility and death in animals (McGrane & Higgins, 1985; Wernery & Kaaden, 2002). Serological evidence of camel leptospirosis was reported from Iran's neighbouring countries such as Saudi Arabia, UAE, Afghanistan and the former USSR (Hussein & El Nabi, 2009). Leptospirosis may be more important in camels because there are increasing tendency towards camel meat and dairy products in Iran and other neighbouring countries (Doosti, Ahmadi, & Arshi, 2012).
Transmission of leptospirosis to human can occur via several ways such as contact with soil or water contaminated with the urine of infected animals and consumption of unpasteurized milk and dairy products, and people with direct contact (e.g. veterinarians, farmers and abattoir workers) are at high risk of infection (Ningal et al., 2015).
The disease is more prevalent in northern Iran (Rafiei, Hedayati Zadeh Omran, Babamahmoodi, Alizqadeh Navaee, & Valadan, 2012). Leptospirosis in camel was first reported in Iran in 1959. In that report, 20% of serum samples were positive for Leptospira icterohemorrhagic serotype (Hajikolaei, Sazmand, Abdollahpour, & Moghadam, 2013; Mustafa, 1987; Rafyi & Maghami, 1959). In 1996, 41 camels showed signs of recurrent fever, anorexia, severe constipation and jaundice in Tabas (northeastern Iran), and finally, 78% of camels died and 7.14% of serum samples were positive for L. canicola (Hadian, 1996). From 2008 to 2014, leptospiral infection varied from 20% to 32.4% by microscopic agglutination test (Sazmand, 2012), 6.33% to 22.85% by PCR (Doosti et al., 2012) and 14.29% to 20% in bacterial cultures (Safarpoor Dehkordi, Saberian, & Momtaz, 2012; Safarpoor Dehkordi and Taghizadeh 2012) in camels studied from different regions of the country (Table 1). In two serological studies on Leptospira spp. infection in 2013, 2.34% and 27.87% of serum samples from camels of Yazd and Qom provinces, respectively, were infected with at least one of Leptospira spp. serotypes. Among positive sera, L. Pomona (57.9%), L. canicola (23.7%), L. hardjo (10.5%), L. grippotyphosa (5.3%) and L. icterohaemorrhagiae (2.6%) were the most frequent serovars (Hajikolaei et al., 2013).
According to reports on camel leptospirosis, there is a necessity to further study this disease to figure out the clinical signs and its importance in transmitting diseases to humans in Iran and all over the world (Figure 2).
Figure 2.
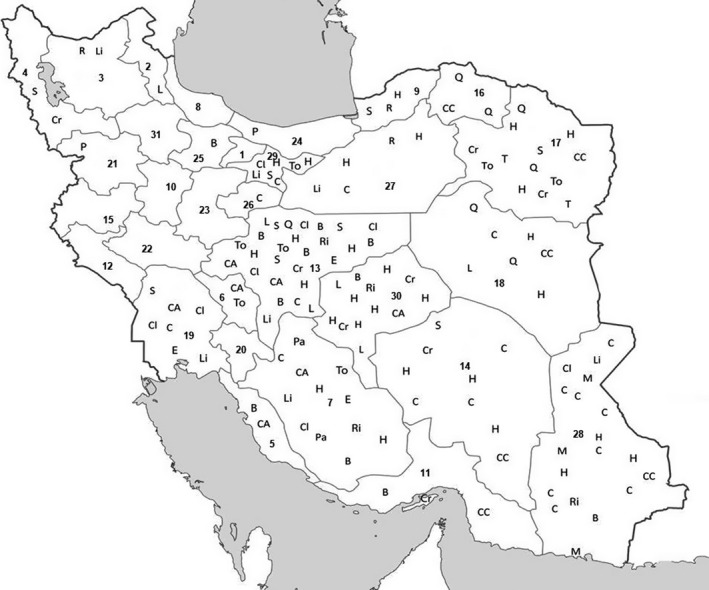
Distribution of camel zoonotic disease in various parts of Iran. Including 22 provinces of report. Abbreviations: B, Brucellosis; C, Camelpox; CA, Campylobacteriosis; CC, Crimean‐Congo Haemorrhagic Fever; Cr, Cryptosporidium spp.; H, Hydatidosis; L, Leptospirosis; Li, Listeriosis; M, MERS‐CoV; P, Plague; Pa, Pasteurellosis; Q, Q fever; R, Rabies; S, Salmonellosis; T, Tuberculosis; To, Toxoplasmosis
3.1.2. Q fever
Q fever is a highly contagious zoonotic disease caused by Coxiella burnetii. A large number of animal species including wild and domestic mammals, birds. If not, please provide clear guidance on where it should be cited in the text. and arthropods, such as ticks, contribute to the transmission of C. burnetii (Maurin & Raoult, 1999). Cattle, sheep, camel and goat are the main sources of the infection (Doosti, Arshi, & Sadeghi, 2014).
Q fever has been reported in all over the world (Angelakis & Raoult, 2010), and recently in the neighbouring countries of Iran, including Oman, Iraq, Afghanistan, UAE, Turkey and Saudi Arabia (Mostafavi, Rastad, & Khalili, 2012). Coxiella burnetii is one of the most widespread infections in camels. Q fever in camel is reported nearly from all parts of North and East Africa and the Middle East. A high prevalence (62%) of Q fever antibodies in camels has been reported from Saudi Arabia, Iran's neighbouring country (Hussein et al., 2015).
Infection in camels is usually subclinical but it can cause late abortion (Janati Pirouz, Mohammadi, Mehrzad, Azizzadeh, & Nazem Shirazi, 2015). Infected camels shed bacteria in urine, faeces, milk, as well as through placenta (Janati Pirouz et al., 2015).
Humans are infected mainly by inhalation of contaminated aerosols (airborne), ingestion of milk or fresh dairy products of infected animals, and exposure to placenta and occasionally ticks (Masala et al., 2004). In human, Q fever is most often asymptomatic but it can appear as an acute or chronic disease (Angelakis & Raoult, 2010; Norlander, 2000).
Q fever is an endemic disease in Iran, which is mostly reported in human, domestic animals and ticks from almost all the provinces of the country (Mostafavi et al., 2012). However, there is little information about the epidemiology of coxiellosis in camels (Janati Pirouz et al., 2015).
In seroepidemiological surveys from 1959 to 2016, samples of camel's blood positive for C. burnetii ranged from 0.0 to 63.6% in Khorasan and Isfahan provinces, respectively (Mogghadass, 2012). Accordingly, considerations for camel hygiene, especially in farms, and restrictions on contact with other domestic animals are important factors to reduce C. burnetii infection among camels.
3.1.3. Brucellosis
Brucellosis is an infectious bacterial disease in human and many other animal species. It is caused by a different genus of Brucella (Corbel, 1997). Brucella melitensis is the most common cause of human disease. Sheep, goat and camel are the main sources of infection (Alavi, Mugahi, Nashibi, & Gharkholu, 2014). Annually, brucellosis causes more than 500,000 infections worldwide (Pappas, Papadimitriou, Akritidis, Christou, & Tsianos, 2006). Brucellosis is more common in areas with poor public health and in countries with the absence or insufficient preventive programs in domestic animals (Capasso, 2002). The disease is widely prevalent and has recently been reported in domestic animals and humans in Oman, Qatar, Kuwait, Saudi Arabia, Iraq, Russia, Turkey, Pakistan and the UAE, which share a vast border with Iran and have close economic relationships (Mohammed & Shigidy, 2013; Wernery, 2014).
Camel brucellosis has been diagnosed in all camel‐rearing countries (Wernery, 2014). Most of the infected camels are asymptomatic carriers of brucellosis. Clinical signs of camel brucellosis are epididymitis and orchitis, lesions of lymph nodes and joint capsules, metritis, abortion and reduced fertility (Esmaeili, 2015; Wernery, 2014).
Human brucellosis is mainly an occupational disease, and the major routes of transmission include contact with animal tissues, blood, urine, vaginal discharge, aborted fetuses, and especially placentas, and consumption of raw milk and other unheated dairy products (Wernery, 2014). Brucellosis is an endemic disease in humans and animals in Iran with an annual incidence of 34 per 100,000 in human (Basiri et al., 2016).
A serological investigation from 1987 to 2014 in various parts of Iran showed that a range of 0.84%–37.83% were positive for Brucella (Sazmand, Hajikolayi, Ghorbanpoor, & Hekmaimoghaddam, 2012). In a study in various parts of Iran in 2012, 32.52% of aborted fetus samples from camels were positive for Brucella detected by conventional PCR (Dehkordi et al., 2012; Dehkordi and Taghizadeh 2012). In another study on camels from Isfahan, central Iran (2012), 11.42% of the studied aborted fetuses of camels were recognized to be infected by brucellosis through culture and multiplex PCR (Dehkordi et al., 2012; Dehkordi and Taghizadeh 2012).
Camel brucellosis seems to be an important endemic zoonotic disease in Iran. For the eradication of brucellosis in camels and further elimination of the disease in human, it is recommended to carry out the ‘test and slaughter' and ‘vaccination' policy. Screening of camels and controlled movement of infected or suspected animals are recommended to control this infection. The control of camel disease will be more successful if debilitated animals are slaughtered, and other animals such as cattle, sheep and goat are vaccinated.
3.1.4. Campylobacteriosis
Campylobacter species are one of the major causes of enteritis in humans and reproductive disease in animals (Salihu, Junaidu, Abubakar, Magaji, & Mohammed, 2009). Campylobacteriosis has been an important infectious disease in both developed and developing countries during the last century (Acheson & Allos 2001).
Studies have indicated that campylobacteriosis is more prevalent in Iran neighbouring countries including Saudi Arabia, Iraq and the UAE (Blaser, 1979; Blaster, Taylor, & Feldman, 1983). Campylobacteriosis can cause abortion, enteritis and lesions in the small and large intestines with typhlocolitis in ruminants, but the clinical presentation in camels has not been described so far (Blaser et al., 1979). The isolation of Campylobacter from camels indicates that camel can serve as a source for both man and animal (Salihu et al., 2009). Many animals shed Campylobacter spp. in their faeces asymptomatically. Ways of transmission are the consumption of undercooked meat and chicken, environmental exposure and direct contact with such farm animals as camels (Kaakoush, Castaño‐Rodríguez, Mitchell, & Man, 2015).
Campylobacteriosis is an endemic disease in Iran, which was reported in human, sheep, cattle, chicken and camel from different areas, especially central provinces of Iran (Pour Reza, 2013).
A survey in Fars and Bushehr provinces, south of Iran, in 2006 showed 2% infection in camel faeces (Baserisalehi, Bahador, & Kapadnis, 2007). Another investigation during 2007–2008 in Najaf Abad, central Iran, showed 5.3% infection in camel meat (Rahimi, Ameri, & Kazemeini, 2010). A microbiological investigation of the raw camel meat in Isfahan and Yazd provinces, central Iran, in 2008 and 2009 revealed infection in 0.9% of samples (Rahimi et al., 2010). In a survey during 2009 and 2010 in Chaharmahal and Bakhtiari and Khuzestan provinces, southwest of Iran, 2.3% of camel meat was positive for C. jejuni and C. coli (Rahimi, Ameri, Alimoradi, Chakeri, & Bahrami, 2013).
This evidence suggests that camel and its meat can be a source of Campylobacter spp. infection in humans in Iran. Hence, monitoring programs and inspections are necessary to prevent outbreaks of such food‐borne diseases. It is also suggested to study more on this disease to figure out the clinical signs and its importance in transmitting diseases to humans in Iran.
3.1.5. Plague
Human plague is an acute and sometimes lethal bacterial disease caused by Yersinia pestis (Butler, 2009). Plague caused three major pandemics during the history all over the world (Mladenova‐Hristova & Tsacheve, 2014). Currently, endemic foci of plague are primarily seen in central and southeast Asia, Africa, and south and northwest of America (Mladenova‐Hristova et al., 2014). Human plague has been reported in camelid and camel playing a very important role in disease transmission to humans (Wernery & Kaaden, 2002). Outbreaks of plague in camels have been reported in Mongolia, China, India, Iran, Iraq, Russia and Africa (Wernery & Kaaden, 2002). Moreover, outbreaks of plague have been reported from Russia through consumption of infected camel milk, and in Afghanistan (2007; Leslie et al., 2011), Saudi Arabia (1994; Saeed, Al‐Hamdan, & Fontaine, 2005; Wernery & Kaaden, 2002), Libya (1977; Misonne, 1977), and Jordan (1997; Arbaji et al., 2005) through consumption of infected camel meat. The disease exists in three clinical forms, namely bubonic, pneumonic and septicemic. The main form of plague in camels is the bubonic form that causes abscesses disseminated over the entire body, pulmonary and cutaneous manifestation, and septicemia (Fedorov, 1960).
Wild rodents are the natural sources of plague. Plague is routinely transmitted to humans and animals by the bite of infected fleas; however, consumption of milk or meat from infected animals can be another rare way of disease transmission in human (Wernery & Kaaden, 2002). Human plague has been reported from all parts of Iran during history, and the country has experienced outbreaks of plague for several centuries (Hashemi Shahraki, Carniel, & Mostafavi, 2016). Besides, there are records of camel plague in Iran in the past (Wernery & Kaaden, 2002). In 1897 and 1907, a few plague‐infected camels were noticed in some areas of Iran (Fedorov, 1960; Mcgrane et al., 1985). In 1974, camel plague transmission was reported in southern areas of the Caspian Sea in the north, and in Kurdistan province (Mesopotamia) in the west of Iran (Fedorov, 1960), the latter is still one of the endemic focus of plague in the world. A serological investigation in an area between Kurdistan and Hamadan in 2011 and 2012 reported that rodents and sheepdogs in this region were positive for plague, emphasizing the area as an active endemic region (Esamaeili et al., 2013).
Yersinia. pestis infection is very critical to health in both humans and animals. According to previous reports, human plague is an endemic disease in western Iran, with incidences of camel plague in the neighbouring countries such as Afghanistan, Jordan and Saudi Arabia. Camel plague, therefore, needs to be considered in both native and imported camels in Iran, necessitating a comprehensive study on plague infection in samples of native and imported camels.
3.1.6. Tuberculosis
Tuberculosis (TB) is one of the major zoonotic diseases in animals and humans caused by the bacterial genus Mycobacterium (Thoen, 2014). Tuberculosis caused millions of human death worldwide when there was no adequate treatment in the past (Ducati, Ruffino‐Netto, Basso, & Santos, 2006). Mycobacterium bovis is an important zoonotic agent (Thoen, LoBue, & De Kantor, 2006). Outbreaks of bovine tuberculosis are a concern for public health authorities (Wernery & Kinne, 2012). There are several reports published on camel tuberculosis in Egypt, Somalia, Ethiopia, UAE, Pakistan, Australia, Dubai, India, Germany, USA, Mauritania and Russia (Mamo et al., 2011; Wernery, 2014; Wernery & Kinne, 2012). Clinical signs of M. bovis in camelids include wasting, anorexia, respiratory distress, enlargement of superficial lymph nodes, recumbency and eventually death (Wernery & Kinne, 2012). Tuberculosis can spread to human through ingestion of raw milk, and sometimes by inhalation of infectious droplets (Wernery & Kinne, 2012).
Tuberculosis exists in all provinces in Iran with the highest incidence and prevalence in Sistan and Baluchestan and Golestan provinces (Metanat, Sharifi‐Mood, Alavi‐Naini, & Aminianfar, 2012). In 2015, a proportion of 12.59 per 100,000 Iranians suffered from tuberculosis. In 2002, a study on camel meats from slaughterhouses in Mashhad, northeastern Iran, showed that tuberculosis was prevalent in camels of the region (Hashemi, Torabi, Darabi, & Shirazi, 2002). An investigation on mediastinal, bronchial and retropharyngeal lymph nodes from 102 dromedary camels slaughtered in Mashhad Abattoir from 2009 to 2010 showed 16.66% infection among samples (Soleymani Babadi et al., 2012).
A program to control tuberculosis in camelids based only on intradermal tuberculin tests will face severe deficiencies. Other than the intradermal test, antemortem tests, such as lymphocyte transformation and ELISA tests, have also failed to be adequately reliable in undomesticated mammals because of false‐negative and false‐positive reactions. This is also true for tuberculosis testing in camelids. However, it is recommended to use several tests to aid in diagnosing tuberculosis in camelids (Fowler, 1999). Using improved strategies to screen camel is suggested to figure out the epidemiology, clinical signs and the importance of this animal in transmitting TB to humans in Iran and all over the world.
3.1.7. Pasteurellosis
Pasteurellosis is a zoonotic disease with a tendency for opportunistic infection (Yasutomo & Kazunari, 2005). Mannheimia haemolytica and Pasteurella multocida are well established to be the major aetiological agents of many pasteurellosis outbreaks (Mohamed & Abdelsalam, 2008). Both species are commensal residents in the upper respiratory tract of healthy cattle, camel, sheep, dog, cat, horse, etc. (Mohamed & Abdelsalam, 2008). Carriage rates of the bacteria are quite high in the oral or nasal secretions of animals. Infection in human is a worldwide problem resulting from animal bites or contact with nasal secretions (Mohamed & Abdelsalam, 2008).
The worst outbreaks in camels occur during the rainy season as the animals are in poor physical conditions (transportation over long distances, deficiencies of vitamins and minerals, and heavy parasitic infestations). Pasteurellosis in camels is reported in Algeria, Egypt, India, Libya, Mauritania, North Africa, Somaliland, Soviet Union, Sudan and Chad (Mustafa, 1987). Pasteurella in camels shows a range of pulmonary and septicemic diseases (Momin, Pethkar, Jaiswal, & Jhala, 1987). In Iran, clinical outbreaks of camel pasteurellosis were reported in 1936, 1943 and 1969 (Wernery & Kaaden, 2002). In 2009, 53% of camels got infected, and 10 of them were lost in an outbreak in Larestan, Fars province, south of Iran (Esmaeili et al., 2010). From 2012 to 2013, PCR assay in camels of Tehran abattoir indicated that P. multocida prevalence ranged from 7.1% to 14.5% in lung samples (Chitgar, Haghdost, Jamshidian, & Hesaraki, 2014). In a study in 2014 and 2015 in Larestan, Fars provinces, south of Iran, 80% of dead camels, 64.58% of sick camels and 7.34% of samples from healthy camels were positive for P. multocida (Tahamtan, Amrabadi, Shahriari, & Namavari, 2017 ).
Further studies are needed to clarify the epidemiology and risk factors of camel pasteurellosis in Iran. Vaccination is highly recommended for control of disease in Iran.
3.1.8. Salmonellosis
Salmonella is one of the leading causes of food‐borne gastroenteritis. Over 2,500 identified serovars of Salmonella spp. are responsible for infections in humans and animals globally (Mohamed & Suelam, 2010). Around 40,000 cases of salmonellosis are reported annually. Animals are the principal source of this pathogen. Salmonella spp. are mainly transmitted via the faecal–oral route (Chiu, Su, & Chu, 2004). Foods from animal sources such as beef, poultry meat, egg and milk are the most common sources of human salmonellosis (Oosterom, 1991; Salehi, Mahzounieh, & Saeedzadeh, 2005).
Salmonella infection in camels is reported from Sudan, Palestine, France, North Africa, USA, Somalia, Ethiopia, Egypt, UAE and Iran. In camels, Salmonella disease can cause enteritis, septicemia and abortion (Sepehr, 2012). Chronic salmonellosis in camels is characterized by diarrhoea, weight loss and death within a few weeks (Wernery & Kaaden, 2002). Healthy camels can be the carriers of Salmonella spp. (Salehi et al., 2005). Humans can be infected by the consumption of contaminated foods originated from camels, infected drinking water, or close contact with infected camels (Wernery & Kaaden, 2002).
Serological investigations showed that Salmonella infection in camel faeces samples varied between 4% in 1992 and 12.39% in 2013 (Moghaddas, 2012 2012). Similar studies on slaughtered camels indicated that 37.25% and 1.69% of camel samples were infected by S. Typhi in the north and centre of Iran in 1992 and 1994, respectively (Miranzade, 1994). A microbiological investigation on tissue samples of camels in Tehran, north of Iran, in 2010 showed that 1.1% were contaminated with S. Typhimurium (Nour‐Mohammadzadeh et al., 2010). Cross‐sectional studies on camels revealed that 43% and 100% of camel milk samples were infected by Salmonella spp. in rural areas and Golestan province, Iran, during 2012 and 2015, respectively (Sepehr, 2012).
In 2012, 10% of camel meat samples from Mashhad, northeast of Iran, were reportedly infected by Salmonella spp. (Golami & Seyedin, 2011). In 2016, an outbreak of abortion and diarrhoea was reported in a camel herd in Mashhad, northeast of Iran. The presence of Salmonella in straw and beet pulp sampled from rumen contents of camels was recognized in two of ten cases by microbiological tests (Mohammady & Najafi Mosleh, 2017).
According to the studies in Iran, camels are an important source of Salmonella. It is, therefore, important to control and prevent salmonellosis in these animals and their products to decrease the transmission of this agent to a human.
3.1.9. Escherichia coli
E. coli O157: H7 is food and water‐borne zoonotic agent (García, Fox, & Besser, 2010). Infection caused by this bacterium is usually asymptomatic in domestic animals and wildlife (Rahimi, Kazemeini, & Salajegheh, 2012; Suardana, Widiasih, Mahardika, Pinatih, & Daryono, 2015). Human infection usually occurs due to improper hygiene, ingestion of contaminated food and water, or direct contact with infected animals (Ateba & Mbewe, 2014; Bogard, Fuller, Radke, Selman, & Smith, 2013). Human E. coli O157: H7 infections have been reported in more than 30 countries (Rahimi et al., 2012). E. coli enterotoxaemia is reported in dromedary camels, and sporadic cases have been reported in adult breeding camels in the UAE, Bahrain, Sudan and East Africa (Al‐Ruwaili, Khalil, & Selim, 2012; Wernery & Kaaden, 2002). Affected animals develop a disease with evidence of yellowish watery diarrhoea, sunken eyes, abdominal cavity distention and CNS signs in some dromedaries (Al‐Ruwaili et al., 2012). Sporadic cases of E. coli infection were reported from several parts of Iran (Koochakzadeh, Badouei, Mazandarani, & Madadgar, 2014), but there is limited information regarding the prevalence of E. coli O157: H7 in Iranian camels (Rahimi et al., 2012). In a study on camel meat samples from Isfahan, Shahrekord, Yazd, Fars and Khuzestan from 2006 to 2010, 1.1% to 2% of samples were infected by E. coli O157: H7 (Rahimi et al., 2010).
Due to rare positive reports provided on camels in Iran, it seems that camel meat is not an important source for E. coli O157: H7 infection; however, monitoring and inspection programs on camel meat and its products remain an important strategy to prevent outbreaks of such a food‐borne disease.
3.2. Listeriosis
Listeriosis is one of the major zoonotic food‐borne diseases (Al‐Swailem et al., 2010). Cattle, sheep, camels and humans are the sources of this globally distributed disease (Heymann, 2015) being more common in regions with cold temperatures. The annual incidence of listeriosis in humans varies from 0.1 to 11.3 cases per 1,000,000 in different countries (Swaminathan & Gerner‐Smidt, 2007).
Listeria contains seven species, of which L. monocytogenes infects both humans and animals (Rahimi, Momtaz, Behzadnia, & Baghbadorani, 2014). Listeria monocytogenes is very resistant to dryness and may stay viable in dry soils and faeces for up to 2 years. This microorganism could be found in the soil, vegetables, sewage, genital secretions and nasal mucous membrane of healthy animals (Al‐Swailem et al., 2010; Dehkordi, Barati, Momtaz, Ahari, & Dehkordi, 2013; Dehkordi, Haghighi Borujeni, Rahimi & Abdizadeh, 2013). Listeriosis infects camels due to consuming spoiled forage infected with L. monocytogenes (Safdari & Jahantigh, 2014). Listeriosis causes a disease with signs of meningoencephalitis in new word camels that include circling, trembling of the head, running into objects and fever. Some cases develop unilateral facial nerve paralysis in association with drooping lips, ears, eyelids and paralysis of the jaw and pharynx, which interfere with mastication and swallowing (Wernery & Kaaden, 2002). Human listeriosis is associated with consumption of contaminated milk, soft cheese and undercooked meat from infected animals (Rahimi et al., 2014). Reports of human listeriosis in Iran are uncertain (Lotfollahi et al., 2011).
In 2011, a report from Sistan and Baluchistan province showed that 8.75% of camel raw meat samples were infected by Listeria bacteria, three samples of which were associated with L. monocytogenes and four samples were contaminated with L. unique (Safdari & Jahantigh, 2014).
Camel meat samples showed evidence of Listeria spp. infections in 50% and 9.6% of cases from Tehran (2011) and Isfahan (2007), respectively (Mashak, Zabihi, Sodagari, Noori, & Akhondzadeh Basti, 2015; Rahimi et al., 2010). Another study conducted in various parts of Iran (2011) detected L. monocytogenes infections in milk, urine, faeces and vaginal secretions of camels in 5.94% of camel raw milk, with the highest (15.18%) shedding of L. monocytogenes in camel vaginal secretion (Dehkordi, Barati, et al., 2013; Dehkordi, Haghighi Borujeni, et al., 2013).
The results of studies in Iran show that camel meat is an important source of Listeria infection for humans; thus, continuous monitoring and inspection programs are necessary to prevent outbreaks of listeriosis in humans.
3.3. Viral zoonotic diseases
In this study, rabies, camelpox, MERS‐CoV infection, and CCHF are reported as zoonotic viral infections among camels in Iran (Table 2).
Table 2.
Summary of zoonotic viral diseases of camels reported in Iran; 1890–2018
| B. Viral diseases | |||||
|---|---|---|---|---|---|
| Disease | Year | Location | Techniques | No. positive/ No. tested (%) | Ref |
| Rabies | 1996–2006 | Golestan | Histopathological examination, Fluorescent antibody technique | 3 cases | (Bokaei et al., 2009) |
| 2012 | Semnan (Torod region) | Histopathological examination, Fluorescent antibody technique | 8 cases | (Esmaeili et al., 2012) | |
| 2012 | East Azerbaijan (Khoda Afarin) | Histopathological examination, Fluorescent antibody technique | – | (Nadalian, 2012) | |
| Camlepox | 1957 | Sistan and Baluchestan (Bazman) | – | 1 case | (Moghaddas, 2012 2012) |
| 1979 | Sistan and Baluchestan (Bampur and Zabol) | Electron microscopy | – | (Moghaddas, 2012 2012) | |
| 1984 | Isfahan, Kerman, Fars, Khuzestan, Semnan, Zabol, Bampur | Electron microscopy | Outbreak | (Moghaddas, 2012 2012) | |
| 1993 | Kerman (Zarand) | Electron microscopy | 50/80 (62.5%) | (Rashidi, 1993) | |
| 1996 | Sistan and Baluchestan (Iranshahr(Delgan)) | Electron microscopy | 3/50 (6%) | (Moghaddas, 1998) | |
| 2000 | Tehran(Pishva) | Electron microscopy | 2/110 (1.81%) | (Moghaddas, 2012 2012) | |
| 2014 | Qum, South Khorasan, Sistan and Baluchestan (Khash) | PCR (Bioneer Kit) | (100%) No Numeral data | (Mosadeghhesari et al., 2014) | |
| MERS‐CoV | 2014 | Sistan and Baluchestan (Zabol city) | Serology test | 3/18 (16.66%) | (Khalaj, 2014a) |
| 2014 | Sistan and Baluchestan (Zabol and Mirjaveh) | Serology test | 8/186 (4.30%) | (Khalaj, 2014b) | |
| 2014 | Kerman and West Azerbaijan | RT‐PCR | 7/98 (7.14%) | (Khalili Bagaloy et al., 2017) | |
| CHHF | 2014 | North Khorasan, South Khorasan, Razavi Khorasan | ELISA | 9/136 (5.29%) | (Champour et al., 2014) |
3.3.1. Rabies
Rabies is a severe and widespread zoonotic disease (Blancou, 1988) caused by a group of neurotropic viruses from the genus Lyssavirus of the Rhabdoviridae family, sometimes known as genotype 1 virus to distinguish it from other closely related viruses causing similar illnesses (Hyun et al., 2005; Sacramento, Badrane, Bourhy, & Tordo, 1992). Rabies virus has been isolated from nearly all mammalians. Herbivores and man are the final hosts and fail to normally play a role as vectors. Carnivorous and vampire bats are considered the sources of the virus (Nigg & Walker, 2009; Rupprecht, Hanlon, & Hemachudha, 2002). More than 55 000 people die of rabies annually mostly in Asia and Africa(Chaurasia, 2014). Rabies has been reported in camels from Morocco, Mauritania, Sudan, Yemen, Saudi Arabia, UAE, Niger, Jordan, India, Israel and Iran (Abbas & Omer, 2005; Fassi‐Fehri, 1987). Rabies in dromedary camel occurs in two forms of ‘raging fury’ and ‘silent fury’, the latter, however, is rarely seen in camels. Raging fury includes two of excitative (furious) and paralytic phases (Wernery & Kaaden, 2002). Infected animals transfer the virus to other animals and humans via saliva following a bite or scratch (Gholami, Fayaz, & Farahtaj, 2014).
Iran is highly endemic for rabies, where it can easily circulate in wildlife and livestock (Farahtaj, Fayaz, Howaizi, Biglari, & Gholami, 2014; Janani et al., 2007). This disease is widespread in all provinces, especially in the north, northwest and northeast regions of the country (Simani, Gholami, Farahtaj, Yousefi‐Behzadi, & Fayaz, 2001), occasionally reported in camelids of Iran (Esmaeili, Ghasemi, & Ebrahimzadeh, 2012). Most of the rabid camels were reported from Sistan and Baluchistan region, southeastern Iran (Simani, 2003; Simani et al., 2001).
Three rabid camels were also reported from Golestan province during 1996–2006 Bokaei, Fayaz, Pourmehdi, and Haghdoost (2009). In 2008, an outbreak of camel rabies was reported for the first time in Torod region of Semnan province, central Iran. Eight infected camels were attacked by a rabid wolf. Another camel rabies was reported from Khoda Afarin county in East Azerbaijan province, northwest of Iran, in 2012 (Moghaddas, 2012 2012).
There is a need for more studies on camels in Iran to have a better overview of the rabies situation in these animals. As this virus is neurotropic and highly fragile, the last line of more studies may be deleted, except those reporting the disease in camel/other animals. Further investigations may include rather prompt vaccination of suspected exposed animals and prevention from exposure to rabid and wild animals.
3.3.2. Camelpox
Camelpox is an important contagious skin disease of camelids (Balamurugan, Venkatesan, Bhanuprakash, & Singh, 2013). The causative agent of the disease is the camelpox virus (CMLV) belonging to the family Poxviridae. This disease can be pathogenic for human as well (Prabhu et al., 2015). Camelpox is mostly reported from Asia (Iran, Iraq, Saudi Arabia, UAE, Yemen, Syria, India, Afghanistan and Pakistan), Africa (Algeria, Egypt, Kenya, Mauretania, Niger, Somalia, Morocco, Ethiopia, Oman and Sudan) and the southern parts of former USSR (Wernery, Kaaden, & Ali, 1997). The clinical manifestation of camelpox varies from mild local to severe systemic disease. The disease is characterized by an initial rise in temperature, followed by enlarged lymph nodes, skin lesions (erythematous macules, papules, vesicles and pustules followed by crusts from ruptured pustules), and prostration (Balamurugan et al., 2013; Wernery & Kaaden, 2002; Wernery et al., 1997). Infected camels shed the virus into secretions including saliva, milk, ocular discharge, nasal discharges and dried scab. The route of transmission is via inhalation, skin abrasions and tick bite (Prabhu et al., 2015). The first official report of camelpox was from one camel in Bazman region of Sistan and Baluchistan province in 1957 (Moghaddas, 2012 2012). Camelpox was also reported from Bampur and Zabol in Sistan and Baluchestan province, southeastern Iran, in 1979 (Moghaddas, 2012 2012). Outbreaks of camelpox were reported in camels from Isfahan, Kerman, Fars, Khuzestan, Semnan, Zabol and Bampur regions in 1984 (Moghaddas, 2012 2012). In 1993, 62.5% of camelpox cases were reported among susceptible camels in Zarand city, Kerman province, south of Iran (Rashidi, 1993). In 1996 and 2000, 6% and 0.018% of camels were infected with camelpox in Sistan and Baluchistan province and Tehran province (Pishva), respectively (Moghaddas, 2012 2012). In an outbreak of camelpox in 2014, scabs from skin lesions were collected from infected camels from Qom province (Khash city) in central Iran, and Sistan and Baluchistan province in southeastern Iran, and South Khorasan province in eastern Iran. Samples were nearly 100% identical to each other and to camelpox strains CMS and M‐96. These isolates also had 99% and 95% similarity to CP‐1 strain and FIN/T2000 isolate, respectively (Mosadeghhesari, Oryan, Zibaee, & Varshovi, 2014).
The vaccination program has been developed to prevent and control camelpox in almost any camel raising country and some other countries (e.g., Saudi Arabia, UAE and Morocco) that have camel racing competitions (Higgins, 1992). Besides, the live‐attenuated cell culture camelpox vaccine is currently used in many countries, such as Afghanistan, Bahrain, Iraq, Jordan, Kuwait, Lebanon, Oman, Pakistan, Syria, UAE, Yemen, Egypt, Morocco and Russia (Bhanuprakash et al., 2010), various inactivated camelpox vaccines were used in Saudi Arabia (Khalafalla & El‐Dirdiri, 2003) and UAE (Wernery & Kaaden, 2002). No inactive or live vaccine against camelpox has yet been produced in Iran. Because of the importance of camelpox and the lack of a comprehensive study on this disease in Iran, more monitoring programs and inspections are necessary to prevent outbreaks of this disease and its economic loss.
3.3.3. MERS‐CoV
MERS‐CoV is a novel coronavirus that causes severe acute respiratory disease. First reported in Saudi Arabia in 2012 (Gonzalez Gompf, 2015), the disease has been described primarily in countries of the Middle East including Saudi Arabia, Qatar, UAE, Jordan, Kuwait, Lebanon, Oman, Iran and Yemen. Imported cases have also been reported in Algeria, Austria, China, Egypt, France, Germany, Greece, Italy, Malaysia, Netherlands, Philippines, South Korea, Thailand, Tunisia, Turkey, UK and USA (Ramadan & Shaib, 2019). MERS‐CoV has been found in many dromedary camels (Mackay & Arden, 2015) and is enzootic in camels across the Arabian Peninsula and parts of Africa, causing mild upper respiratory tract disease in its camel host. MERS‐CoV infects dromedary camels and can be transmitted to human through close contact. Camels are infected transiently, and it seems that the virus will be cleared after the acute infection. Camels may also act as intermediate hosts by transmitting the virus from its origin to humans. The exact source that maintains the virus has not yet been identified (Azha et al., 2014).
In October 2014, three cases of MERS‐CoV‐related coronaviruses were reported among 18 susceptible camels in Zabol city, Sistan and Baluchistan province, southeastern Iran. The source of infection was due to the animals imported illegally from Pakistan (Khalaj, 2014a). In December 2014, eight illegally imported camels from Pakistan to Iran were infected with a coronavirus ‘potentially related’ to MERS‐CoV. The animals were among 186 camels that were tested at two border stations, namely, Zabol and Mirjaveh, in Sistan and Baluchistan province (Khalaj, 2014b).
Six cases of MERS‐CoV infection were reported from Iran, five cases during May–July 2014 and one case in March 2015 in Kerman province, southern Iran. The cases presented epidemiological data related to those who had returned from Hajj (Moniri, Marjani, Tabarsi, Yadegarynia, & Nadji, 2015; Mortazavi, Monavari, Ataei Pirkooh, & Tavakoli, 2014).
In 2014, a molecular study showed pancoronavirus RNA in 7.14% of nasal swab samples from apparently healthy camels. Positive samples (4.08%) belonged to West Azerbaijan province, northwest of Iran, and the remaining were obtained from Kerman province, southeast of Iran (Khalili Bagaloy, Sakhaee, & Khalili, 2017). Following surveillance and monitoring across the country, there have been no reports of further animal and human cases of MERS‐CoV in Iran. Camels imported from the neighbouring countries should be strictly monitored continuously.
3.3.4. Crimean‐Congo haemorrhagic fever
CCHF is a tick‐borne viral zoonotic disease caused by one of the most medically important arboviruses belonging to the genus Orthonairovirus in the family Nairoviridae (Fajs et al., 2014; Shayan, Bokaean, Shahrivar, & Chinikar, 2015). The most common viral sources are domestic livestock including sheep, goat, cattle and camel, which show asymptomatic infections (Schwarz et al., 1996). The virus transmission to humans has been reported through an infected tick bite, direct contact with fresh meat or blood of viraemic animals and nosocomial infection (Schwarz et al., 1996; Shayan et al., 2015).
CCHF is one of the most widespread and common tick‐borne viral diseases (Aslam et al., 2016). The geographical distribution of the disease overlaps with that of Hyalomma ticks (Soares‐Weiser, Thomas, G, & Garner, 2010). CCHF is endemic in some parts of Africa, the Middle East and southeastern Europe (Fajs et al., 2014). Positive serological tests have been reported in Oman (Body et al., 2016), Iran and Niger (Mariner, Morrill, & Ksiazek, 1995) among tested camels.
The disease has been reported from most parts of Iran. Most patients are reported from Sistan and Baluchistan province, southeast of the country, due to a long border with two high‐risk countries, Afghanistan and Pakistan (Chinikar et al., 2012; Telmadarraiy, Chinikar, Vatandoost, Faghihi, & Hosseini‐Chegeni, 2015). CCHF antibodies are found in Iranian sheep (38%), goats (36%) and cattle (18%) (Saidi, Casals, & Faghih, 1975). In 2014, a seroepidemiological study of CCHF virus in camels of Khorasan provinces (North, South and Razavi), northeast of Iran, showed that from 170 camels collected from different regions of Khorasan territory, a total of 5.29% camels were IgG‐positive. Eight of the nine positive samples were taken from female camels (Champour et al., 2014).
As CCHF is a serious infectious disease, imported animals, particularly camels that carry a large number of ticks should be screened more carefully at border points of the country or, alternatively, camels should be slaughtered at border cities allowing the import of processed meat.
3.4. Parasitic zoonotic diseases
Echinococcosis, cryptosporidiosis and toxoplasmosis have been reported as zoonotic parasitic diseases in Iranian camels (Table 3).
Table 3.
Summary of zoonotic parasite diseases of camels reported in Iran; 1890–2018
| C. Parasite infections | |||||
|---|---|---|---|---|---|
| Disease | Year | Location | Techniques | No. positive/No. tested (%) | Ref |
| Echinococcosis | 1970 | Tehran | Direct light microscopic examination | 612/955 (64%) | (Mobedi et al., 1970) |
| 1971 | Shiraz | Direct light microscopic examination | 15/35 (42.8%) | (Afshar et al., 1971) | |
| 1992 | Shiraz | Direct light microscopic examination | 28/40 (70%) | (Moghadar et al., 1992) | |
| 1994 | Isfahan, Yazd | Direct light microscopic examination | 70/100 (70%) | (Mowlavi, 1997) | |
| 2001 | Yazd, Isfahan, Kerman | Direct light microscopic examination | 37/144 (25.7%) | (Anvari et al., 2001) | |
| 2001–2002 | Yazd, Kerman, Zahedan, Mashhad, Isfahan | Direct light microscopic examination | 26.5%, 25.7%, 32.7%, 59.3%, 35.2%, Total: 233/661 (35.5%) | (Ahmadi, 2005) | |
| 2002 | Tehran, Isfahan, Yazd | Molecular, PCR, RFLP | 23 Cases | (Ahmadi & Dalimi, 2002) | |
| 2006–2008 | Golestan | Direct light microscopic examination | 403/1341 (30.1%) | (Mostafalu & Rahchamani, 2016) | |
| 2009 | Kerman | Direct light microscopic examination | 45/217 (20.73%) | (Fathi, Mirzaei Dehaghi, & Radfar, 2012) | |
| 2009 | Razavi Khorasan (Mashhad) | Direct light microscopic examination | 85/207 (41.1%) | (Sharifiyazdi, Oryan, Ahmadnia, & Valinezhad, 2011) | |
| 2009–2010 | Yazd | Serological, IH, CIEP | 18/141 (12.85%) IH, 16/141 (11.3%) CIEP | (Sazmand et al., 2013) | |
| 2010–2011 | Khorasan Razavi, Yazd, South Khorasan, Semnan, Sistan va Baluchestan | Direct light microscopic examination | 54/136 (40.40%), 25/80 (31.20%), 32/120 (26.60%) 6/27 (22.20%), 18/75 (24.00%), Total: 135/438 (30.82%) | (Moghaddas, Borji, Naghibi Aboul, Razmi, & Shayan, 2014) | |
| 2016 | South Khorasan Sistan va Baluchestan | Direct light microscopic examination | 85/207 (41%) | (Valinezhad & Oryan 2017) | |
| 2015–2016 | Semnan | Direct light microscopic examination | 221/475 (46/52%) | (Darebaghi Hosein Abadi, Najmoddin, Keneshlou, & Kordi, 2016) | |
| Cryptosporidium spp | 2009 | Isfahan(Najaf Abad) | Microscopic examination of smears stained by modified Ziehl‐Neelsen technique | 39/103 (37.9%) | (Razavi et al., 2009) |
| 2009 | Razavi Khorasan (Mashhad) | Microscopic examination of smears stained by modified Ziehl‐Neelsen technique | 6/316 (1.9%) | (Borji et al., 2009) | |
| 2010 | Yazd | Microscopic examination of smears stained by modified Ziehl‐Neelsen technique | 61/300 (20.33%) | (Sazmand, Rasooli, et al., 2012) | |
| 2010 | Qeshm Island southern Iran | Microscopic examination of smears stained by modified Ziehl‐Neelsen technique | 11/65 (16.9%) | (Nazifi et al., 2010) | |
| 2012 | West Azerbaijan (Miandoab region) | Microscopic examination of smears stained by modified Ziehl‐Neelsen technique | 17/170 (10%) | (Radfar, Gowhari, & Khalili, 2013) | |
| 2012 | Kerman (Shahr‐e Babak) | Microscopic examination of smears stained by modified Ziehl‐Neelsen technique, ELISA | 4/85 (4.7%) | (Radfar et al., 2013) | |
| 2016 | Razavi Khorasan | Microscopic examination of smears stained by modified Ziehl‐Neelsen technique, PCR | 4/60 (6.6%) | (Sadrbazzaz et al., 2016) | |
| Toxoplasmosis | 2006 | Razavi Khorasan (Mashhad) | Serological screening indirect fluorescent antibody test (IFAT) | 5/120 (4.16%) | (Sadrebazzaz et al., 2006) |
| 2011–2012 | Tehran, Isfahan, Fars | Cell line culture, enzyme‐linked immunosorbent assay (ELISA), PCR | 5/160 (3.12%) | (Dehkordi, Barati, et al., 2013; Dehkordi, Haghighi Borujeni, et al., 2013) | |
| 2013 | Isfahan | PCR | 8/122 (6.6%) 0/36 (0%) | (Khamesipour, Doosti, et al., 2014; Khamesipour, Rahimi, et al., 2014) | |
| 2014–2015 | Razavi Khorasan (Sabzevar) | PCR | 26/40 (65%) | (Aliabadi & Ziaali, 2016) | |
3.4.1. Echinococcosis
Human echinococcosis is a zoonotic infection caused by larval forms (metacestodes) of tapeworms of the genus Echinococcus (Grosso, Gruttadauria, Biondi, Marventano, & Mistretta, 2012; Otero‐Abad & Torgerson, 2013). The most important genera for public health are E. granulosus and E. multilocularis (Grosso et al., 2012). Present estimates suggest that it results in the loss of 1–3 million disability‐adjusted life per year (El Berbri et al., 2015). Typical hosts are dogs and other carnivores, and intermediate hosts are ungulates such as sheep, goats, horses and camels. Echinococcosis in camel usually causes no significant clinical signs, but, there are occasional reports concerning respiratory problems, abdominal distention and death from anaphylaxis (Wernery & Kaaden, 2002). Humans as the accidental hosts are infected by oral ingestion of eggs that contaminated vegetables, water, foods or hands (Eckert & Deplazes, 2004).
The disease is endemic to hyperendemic in agricultural countries of Europe, Africa, America, the Middle East and Asia (Moro & Schantz, 2009). Camel echinococcosis is reported from North and East Africa, Egypt, Sudan, Somalia, Libya, Central Africa, Iraq and Iran (Wernery & Kaaden, 2002). There are few data about the prevalence of echinococcosis in dromedaries from the Middle East countries including Iran.
There are many reports of human echinococcosis cases as a health problem distributed throughout Iran. It is estimated that echinococcosis is responsible for almost 1% of surgical operations in Iran (Rokni, 2009). Information on the epidemiology of echinococcosis in camels in different regions of Iran is very limited (Borji & Parandeh 2010). Cross‐sectional studies and parasitology tests from 1970 to 2016 showed that infected camels with E. granulosus varied from 20.73% to 70% (Afshar, Nazarian, & Baghban‐Baseer, 1971; Mobedi, Madadi, & Arfaa, 1970; Moghadar, Oryan, & Pour, 1992; Mowlavi, 1997; Sazmand & Joachim, 2017) in various parts of Iran (Table 3). A seroepidemiological study from 2009 to 2010 in Yazd province, central Iran, disclosed that 12.8% of camels were positive with indirect haemagglutination (Sazmand, Razi Jalali, Hekmatimoghaddam, & Asadollahi, 2013).
As it can be observed from different studies in Iran, at least 12% of the camels in all parts of the country are infected with echinococcosis, which is a high rate of infection. Thus, camels can play an important role in the transmission of Echinococcus in Iran, and the camel carnivorous cycle should also be targeted in any control program.
3.4.2. Cryptosporidiosis
Cryptosporidiosis is one of the intracellular protozoal diseases caused by the genus Cryptosporidium (Rossle & Latif, 2013) infecting fish, amphibians, reptiles, birds and mammals. It has been identified as the cause of numerous outbreaks of diarrhoeal disease in humans and animals all over the world (Fayer, 2004; Razavi, Oryan, Bahrami, Mohammadalipour, & Gowhari, 2009). Cryptosporidium is transmitted via the faecal–oral route and easily spreads through water, food and contact with infected animals and contaminated environments (Lal, Cornish, Fearnley, Glass, & Kirk, 2015; Sazmand & Joachim, 2017). Cryptosporidiosis is a zoonotic problem and its excreted oocysts could be the sources of human infection and great public health concern (Pieniazek et al., 2003).
Cryptosporidium sp. is reported in dromedary camels in Egypt (Abou‐Eisha, 1994) and Iraq (Hussin, Khalaf, & Ali, 2015). The disease in camel can lead to severe diarrhoea, emaciation, dehydration and death (Wernery & Kaaden, 2002). In Iran, epidemiological studies reported a wide range of infections from 0% to 32% in different human societies (Jafari, Maghsood, & Fallah, 2012). Few data are available on Cryptosporidium prevalence in camels of Iran (Nazifi et al., 2010; Sazmand & Joachim, 2017). From 2008 to 2012, microscopic examination of smears stained by modified Ziehl‐Neelsen showed that 1.9% to 37.9% of tested camels (Camelus dromedarius) were positive for Cryptosporidium oocysts (Borji, Razmi, Movassaghi, Naghibi, & Maleki, 2009; Razavi et al., 2009) in various parts of Iran (Table 3). In 2009 and 2010, a study in Yazd province, central Iran, presented evidence that the rates of infection in faeces and abomasal mucosa of camels were 20.33% and 12%, respectively (Sazmand, Rasooli, Nouri, Hamidinejat, & Hekmatimoghaddam, 2012). In the last reviewed study in Khorasan Razavi province (2016), 6.6% of camel faeces were infected with Cryptosporidium spp., and three of the four samples were C. andersonii, and one of the four samples was C. parvum (Sadrbazzaz et al., 2016).
According to different studies on camels in Iran, camels can be a potential source of cryptosporidiosis infection for human; therefore, slaughterhouse staff, farmers and veterinarians, who have close contact with camels, are at risk of infection and should wash their hands carefully to reduce the risk of infection.
3.4.3. Toxoplasmosis
Toxoplasmosis is a cyst‐forming parasitic disease caused by Toxoplasma gondii (Tenter, Heckeroth, & Weiss, 2000). It is an intestinal coccidial parasite of Felidae, particularly cats, which is a primary host. The parasite also infects humans, ruminants and other mammalians as intermediate hosts (Dubey, 2009). Infection with T. gondii has a worldwide distribution, and at least one‐third of the world population is infected with the parasite (Robert‐Gangneux & Darde, 2012). Toxoplasmosis is considered a food‐borne infection observed in many domesticated or food animals (Sarvi et al., 2015). The overall seroprevalence rate of toxoplasmosis among the general population is 39.3% in Iran. Toxoplasma antibodies in camels are reported in Egypt, Nigeria, Saudi Arabia, Sudan, Turkmenistan, UAE, India and Iran (Hussein Khalil, 2015). Toxoplasmosis in camel causes mild disease with dyspnoea associated with pyothorax and abortion (Wernery & Kaaden, 2002). Human infection may result from ingestion of cysts with bradyzoites, oocysts from cat faeces (e.g. on vegetables) and trans‐placental spread of tachyzoites to the fetus during acute infection in pregnancy. Camels acquire the infection by ingesting food contaminated with oocysts. Cats given camel meat excreted oocysts of Cystoisospora felis, C. rivoltu and T. gondii (Hilali, Fatani, & Al‐Atiya, 1995). Humans acquire the infection from camels by eating raw or undercooked camel meat contaminated with tissue cysts (Hilali & Fahmy, 1993; Wernery & Kaaden, 2002).
In 2004–2005, antibodies against T. gondii were found in 2.5% of 120 camels tested in Mashhad (Sadrebazzaz, Haddadzadeh, & Shayan, 2006). In 2011–2012, T. gondii was shown to be present in 3.12% of camel milk samples in Tehran, Isfahan and Fars provinces (Dehkordi, Barati, et al., 2013; Dehkordi, Haghighi Borujeni, et al., 2013). In 2013 and 2015, studies on T. gondii in camel blood samples showed that 6.6% and 65% of camels were infected in Isfahan and Khorasan Razavi provinces, respectively (Khamesipour Doosti, Mobarakeh, & Komba, 2014; Khamesipour, Rahimi, Shakerian, Doosti, & Momtaz, 2014; Sazmand & Joachim, 2017). Consumption of undercooked camel meat may constitute a risk of infection to humans and should, therefore, be of public concern. The camel–cat cycle should also be targeted in any control program of toxoplasmosis in Iran.
3.5. Mycotic zoonotic diseases
There is only one fungal zoonotic disease, viz., dermatophytosis (ringworm), reported for camels (Table 4).
Table 4.
Summary of zoonotic fungal diseases of camels reported in Iran; 1890–2018
| D. Fungal diseases | |||||
|---|---|---|---|---|---|
| Disease | Year | Location | Techniques | No. positive/No. tested (%) | Ref |
| Ringworm | 1994–1998 | Different areas of Iran | Direct microscopic examination | 14/63 (22.22%) | (Khosravi & Mahmoudi, 2003) |
| 2003–2013 | Different area of Iran | Direct microscopic examination | 74/100 (74%) | (Shokri & Khosravi, 2016) | |
3.5.1. Dermatophytosis
Dermatophytosis is a zoonotic disease caused by a group of fungi named dermatophytes that can infect the skin, hair and nails (Almuzaini, Osman, & Saeed, 2016; Hayette & Sacheli, 2015). The major sources of the fungi are pets, farm and wild animals (Sharma, Kumawat, Sharma, Seth, & Chandra, 2015a; 2015b). The prevalence of dermatophytes varies in different parts of the world (Sharma, Kumawat, Sharma, Seth, & Chandra, 2015a; Sharma et al., 2015b; Vena et al., 2012).
Dermatophytosis is a common disease of camels worldwide (Almuzaini et al., 2016; Tuteja, Patil, Narnaware, Nagarajan, & Dahiya, 2013a; 2013b) more commonly seen in camels under 3 years of age with a peak incidence age between 3 and 12 months (Fadlelmula, Agab, Le Horgne, Abbas, & Abdalla, 1993). Direct and indirect contacts with infected animals and fomites are the modes of infection transmission to human and other animals and vice versa (Tuteja, Patil, Narnaware, Nagarajan, & Dahiya, 2013a; Tuteja et al., 2013b; Wernery & Kaaden, 2002).
There are not enough studies about the frequency of camel dermatophytes in Iran. In a study between 1994 and 1998, 22% of camel specimens from different areas of the country were infected with dermatophytosis (Khosravi & Mahmoudi, 2003). In another study during 2003–2013, 74% of camel samples belonging to warm and humid regions of Iran were positive for Trichophyton verrucosum (Shokri & Khosravi, 2016). Routine and regular inspection of camels, isolation of infected camels, disinfection of contaminated stables, vaccination and improved hygiene are useful measured to manage dermatophytosis in camels.
4. DISCUSSION
There are 171,500 dromedary camels distributed across 22 out of 31 provinces of Iran, 70% of which live in Sistan and Baluchestan and Khorasan provinces in the east of Iran. Some camels are smuggled from neighbouring countries, particularly Afghanistan and Pakistan, to Iran with no surveillance and monitoring systems, or maybe the systems are neglected for such animals. Thereby, there is a potential threat of bringing in new non‐endemic pathogenic organisms into Iran that have not already seen in Iranian camels; therefore, such trade and importation must be limited as much as possible (Mogghadass, 2012; Moghaddas, 2012 2012).
Reported camel zoonotic diseases in Iran can be divided into two major categories. The first group is devoted to the diseases that are rare in Iran and received limited studies including plague, MERS‐CoV and CCHF. More attention should be paid to these diseases as they are neglected most of the time. The second category is related to the diseases that are common in Iran but received very little attention in the majority of studies (Yavari, 2013). These should be considered the most important zoonotic diseases of Iranian camels, including leptospirosis, Q fever, brucellosis, campylobacteriosis, tuberculosis, pasteurellosis, salmonellosis, E. coli, listeriosis, rabies, camelpox, echinococcosis, cryptosporidiosis, toxoplasmosis and dermatophytosis. Specific strategies, therefore, should be planned to have a better prevention and control program for each of the above diseases in the country.
The prevention and control of mentioned diseases are dependent on having enough information on the infectious agent, occurrence, transmission method, incubation period, immunity and control programs.
If the mode of transmission is known, precautions can be put in place to prevent outbreaks of diseases. Some disease‐control interventions are directed towards the mode of transmission including direct contact, droplets, a vector such as a tick and food‐borne or air‐borne routes. Diseases such as leptospirosis, brucellosis, salmonellosis, pasteurellosis, campylobacteriosis, MERS‐CoV, CCHF, cryptosporidiosis and dermatophytosis can be transmitted through direct contact with animal tissues or fluids including nasal secretion, saliva, urine, placenta and faeces. In the case of CCHF, the disease can also be transmitted through direct contact with camel fresh meat or the blood of viraemic animals (Kloos & Berhane, 2006). Consumption of raw or half‐raw milk and other unpasteurized dairy products can transmit plague, Q fever, listeriosis, brucellosis, campylobacteriosis and tuberculosis from camel to human (Kloos & Berhane, 2006). Plague, listeriosis and campylobacteriosis can also be transmitted from camel to human by consumption of undercooked meat. Leptospirosis, campylobacteriosis, E. coli infections, salmonellosis, cryptosporidiosis can be transmitted by the ingestion of food or water contaminated with the urine or faeces of infected camels. Diseases such as tuberculosis and Q fever can be transmitted through contaminated aerosols, fluids aerosolized from an animal to a person (e.g. sneezing or coughing), or by inhaled aerosolized materials. Rabies and pasteurellosis can spread by infected, diseased or source animal bites and scratches. Plague, camelpox, MERS‐CoV and CCHF are transmitted by an infected arthropod vector (e.g. fleas or ticks); therefore, these agents can be controlled by appropriate anti‐ectoparasite drugs (Kloos & Berhane, 2006; Murphy, 2008).
Several major steps need to be taken in order to prevent and control camel zoonotic diseases. The most valuable preventive step is vaccination, not only for the protection of individual animal but also to build up a level of population immunity that suffices to break transmission. It seems that vaccination can be an effective way to control diseases such as camelpox and brucellosis among camels.
Because camel is one of the hosts and zoonotic agents circulating in other species (except for MERS‐CoV and camelpox), screening and control of other animals should be followed as well, in addition to monitoring these agents in camels. Moreover, the birth certificate of camels and more accurate monitoring of their movements should be seriously considered to have a better surveillance program among Iranian camels.
There should also be full supervision to prevent the slaughter of camels at homes or public places. Camels should be slaughtered only in standard slaughterhouses under the control and supervision of a veterinarian, who uses personal protective equipment and is aware of endemic diseases in camels and their mode of transmission to human.
Camel meat and dairy products must be purchased from authorized veterinary locations, and consumption of raw or undercooked camel's meat and unpasteurized dairy products should be avoided. Camel and its by‐products (fur, wool and uncleaned head and trotter) should be under the control of veterinary systems during shipment.
5. CONCLUSIONS
In this study, 19 reported camel zoonotic diseases and infections in Iran were reviewed and outlined with referring to reported regions. It seems that camel infections are neglected in Iranian academic researches. Future studies, therefore, should address this topic to determine the exact impacts of camel diseases on public health in Iran.
Ministry of Health, Ministry of Agriculture and Environmental Organization must pay more attention to echinococcosis, brucellosis, salmonellosis and MERS‐COV in order to control these diseases and infections in camels and prevent their transmission to humans. Authorities should focus on herd health management including extended screening, vaccination, and training and paying more attention to water and food safety. As some of camel zoonotic diseases are imported from other countries, monitoring should be seriously considered on the birth certificate of the camels and their movements.
6. ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this is a review article with no original research data
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Mohammadpour R, Champour M, Tuteja F, Mostafavi E. Zoonotic implications of camel diseases in Iran. Vet Med Sci. 2020;6:359–381. 10.1002/vms3.239
REFERENCES
- Abbas, B. , el Zubeir, A. E. A. , & Yassin, T. T. M. (1987). Survey for certain zoonotic diseases in camels in Sudan. Journal of Livestock and Veterinary Medicine in Tropical Countries, 40(3), 231–233. [PubMed] [Google Scholar]
- Abbas, B. , & Omer, O. (2005). Review of infectious diseases of the camel. Veterinary Bulletin, 75(8), 1–16. [Google Scholar]
- Abou‐Eisha, A. (1994). Cryptosporidial infection in man and farm animals in Ismailia Governorate. Veterinary Medical Journal Giza, 42(2), 107–111. [Google Scholar]
- Acheson, D. , & Allos, B. M. (2001). Campylobacter jejuni infections: Update on emerging issues and trends. Clinical Infectious Diseases, 32(8), 1201–1206. 10.1086/319760 [DOI] [PubMed] [Google Scholar]
- Afkhamnia, M. , Avagyan, S. , Khaki, P. , Bidhendi, S. M. , & Mostafaey, M. (2014). The detection of Leptospira in bactrian camels (Camelus bactrianus) in North West Iran. Journal of Camel Practice and Research, 21(2), 215–217. [Google Scholar]
- Afshar, A. , Nazarian, I. , & Baghban‐Baseer, B. (1971). A survey of the incidence of hydatid cyst in camels in South Iran. British Veterinary Journal, 127(11), 544–546. [DOI] [PubMed] [Google Scholar]
- Ahmadi, M. (2012). Detection Salmonella spp. Carriers by using invA gene amplification in Urmia region camels by Polymerase Chain Reaction 17th Iranian Veterinary Congress Tehran, Iran persian. [Google Scholar]
- Ahmadi, N. (2005). Hydatidosis in camels (Camelus dromedarius) and their potential role in the epidemiology of Echinococcus granulosus in Iran. Journal of Helminthology, 79(02), 119–125. [DOI] [PubMed] [Google Scholar]
- Ahmadi, N. , & Dalimi, A. (2002). Molecular characterization of Echinococcus granulosus isolated from sheep and camel in Iran. Archives of Razi Institute, 53(1), 47–56. [Google Scholar]
- Alavi, S. M. , Mugahi, S. , Nashibi, R. , & Gharkholu, S. (2014). Brucellosis risk factors in the southwestern province of Khuzestan, Iran. International Journal of Enteric Pathogens, 2(1), e15610 10.17795/ijep15610 [DOI] [Google Scholar]
- Aliabadi, J. , & Ziaali, P. N. (2016). Survey of Toxoplasma Gondii in livestocks' meat (Sheep, Goat, Camel), using nested PCR method In Sabzavar district. European Online Journal of Natural and Social Sciences, 5(2), 368. [Google Scholar]
- Al‐Jassim, R. , & Sejian, V. (2015). Review paper: Climate change and camel production: Impact and contribution. Journal of Camelid Science, 8, 1–17. [Google Scholar]
- Alkhamis, M. , Fernández‐Fontelo, A. , VanderWaal, K. , Abuhadida, S. , Puig, P. , & Alba‐Casals, A. (2018). Temporal dynamics of Middle East respiratory syndrome coronavirus in the Arabian Peninsula, 2012–2017. Epidemiology & Infection, 147, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almuzaini, A. M. , Osman, S. A. , & Saeed, E. M. A. (2016). An outbreak of dermatophytosis in camels (Camelus dromedaríus) at Qassim Region, Central of Saudi Arabia. Journal of Applied Animal Research, 44(1), 126–129. [Google Scholar]
- Al‐Ruwaili, M. A. , Khalil, O. M. , & Selim, S. A. (2012). Viral and bacterial infections associated with camel (Camelus dromedarius) calf diarrhea in North Province, Saudi Arabia. Saudi Journal of Biological Sciences, 19(1), 35–41. 10.1016/j.sjbs.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Swailem, A. A. , Al‐Dubaib, M. A. , Al‐Ghamdi, G. , Al‐Yamani, A. , Al‐Naeem, A. M. , Al‐Mejali, A. , … Mahmoud, O. M. (2010). Cerebral listeriosis in a she‐camel at Qassim Region, Central Saudi Arabia‐a case report. Veterinarski Arhiv, 80(4), 539–547. [Google Scholar]
- Angelakis, E. , & Raoult, D. (2010). Q fever. Veterinary Microbiology, 140(3), 297–309. 10.1016/j.vetmic.2009.07.016 [DOI] [PubMed] [Google Scholar]
- Anvari, M. , Moubedi, I. , Masoud, J. , Mansoorian, A. , Farahnak, A. , & Mohebali, M. (2001). Camels, Camelus dromedarius intermediate host of Echinococcus granulosus in the central region of Iran. Journal of Shahid Sadoughi University of Medical Sciences, 8(4), 74–79. [Google Scholar]
- Arbaji, A. , Kharabsheh, S. , Al‐Azab, S. , Al‐Kayed, M. , Amr, Z. S. , Abu Baker, M. , & Chu, M. C. (2005). A 12‐case outbreak of pharyngeal plague following the consumption of camel meat, in north‐eastern Jordan. Annals of Tropical Medicine and Parasitology., 99(8), 789–793. 10.1179/136485905X65161 [DOI] [PubMed] [Google Scholar]
- Aslam, S. , Latif, M. S. , Daud, M. , Rahman, Z. U. , Tabassum, B. , Riaz, M. S. , … Husnain, T. (2016). Crimean‐Congo hemorrhagic fever: Risk factors and control measures for the infection abatement. Biomedical Reports, 4(1), 15–20. 10.3892/br.2015.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ateba, C. N. , & Mbewe, M. (2014). Genotypic characterization of Escherichia coli O157: H7 isolates from different sources in the north‐west province, South Africa, using enterobacterial repetitive intergenic consensus PCR analysis. International Journal of Molecular Sciences, 15(6), 9735–9747. 10.3390/ijms15069735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azha, E. I. , El‐Kafrawy, S. A. , Farraj, S. A. , Hassan, A. M. , Al‐Saeed, M. S. , Hashem, A. M. , & Madani, T. A. (2014). Evidence for camel‐to‐human transmission of MERS coronavirus. New England Journal of Medicine, 370(26), 2499–2505. 10.1056/NEJMoa1401505 [DOI] [PubMed] [Google Scholar]
- Balamurugan, V. , Venkatesan, G. , Bhanuprakash, V. , & Singh, R. K. (2013). Camelpox, an emerging orthopox viral disease. Indian Journal of Virology, 24(3), 295–305. 10.1007/s13337-013-0145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserisalehi, M. , Bahador, N. , & Kapadnis, B. (2007). Isolation and characterization of Campylobacter spp. from domestic animals and poultry in the south of Iran. Pakistan Journal of Biological Sciences, 10(9), 1519–1524. [DOI] [PubMed] [Google Scholar]
- Basiri, H. , Akbari, N. , Azizpour, M. , Hosseini, S. , Behrozikhah, A. , & Eskandari, S. (2016). Amplification, cloning, and expression of Brucella melitensis bp26 gene (OMP28) isolated from Markazi province (Iran) and purification of Bp26 Protein. Archives of Razi Institute, 68(2), 111–116. [Google Scholar]
- Belay, E. D. , Kile, J. C. , Hall, A. J. , Barton‐Behravesh, C. , Parsons, M. B. , Salyer, S. , & Walke, H. (2017). Zoonotic disease programs for enhancing global health security. Emerging Infectious Diseases, 23(13), S65–S70. 10.3201/eid2313.170544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennoune, O. , Adili, N. , Amri, K. , Bennecib, L. , & Ayachi, A. (2013). Trypanosomiasis of camels (Camelus dromedarius) in Algeria: First report. Veterinary Research Forum: an International Quarterly Journal, 4(4), 273–275. [PMC free article] [PubMed] [Google Scholar]
- Bergin, T. , & Torenbeeck, L. (1991). Melioidosis in camels [first record; Pseudomonas pseudomallei; Queensland].[Short contribution]. Australian Veterinary Journal, 68(9), 309. (Australia). [DOI] [PubMed] [Google Scholar]
- Bhanuprakash, V. , Prabhu, M. , Venkatesan, G. , Balamurugan, V. , Hosamani, M. , Pathak, K. M. , & Singh, R. K. (2010). Camelpox: Epidemiology, diagnosis and control measures. Expert Review of Anti‐infective Therapy, 8(10), 1187–1201. 10.1586/eri.10.105 [DOI] [PubMed] [Google Scholar]
- Blancou, J. (1988). Ecology and epidemiology of fox rabies. Review of Infectious Diseases, 10(4), S606–S609. 10.1093/clinids/10.Supplement_4.S606 [DOI] [PubMed] [Google Scholar]
- Blaser, M. J. (1979). Campylobacter enteritis: Clinical and epidemiologic features. Annals of Internal Medicine, 91(2), 179–185. 10.7326/0003-4819-91-2-179 [DOI] [PubMed] [Google Scholar]
- Blaster, M. J. , Taylor, D. , & Feldman, R. A. (1983). Epidemiology of Campylobacter jejuni infections. Epidemiologic Reviews, 5(1), 157–176. 10.1093/oxfordjournals.epirev.a036256 [DOI] [PubMed] [Google Scholar]
- Body, M. H. H. , Abdulmajeed, H. A. , Hammad, M. H. , Mohamed, S. A. , Saif, S. A. , Salim, A.‐M. , … Rajamony, S. (2016). Cross‐sectional survey of Crimean‐Congo hemorrhagic fever virus in the sultanate of Oman. Journal of Veterinary Medicine and Animal Health, 8(6), 44–49. 10.5897/JVMAH2016.0472 [DOI] [Google Scholar]
- Bogard, A. K. , Fuller, C. C. , Radke, V. , Selman, C. A. , & Smith, K. E. (2013). Ground beef handling and cooking practices in restaurants in eight states. Journal of Food Protection, 76(12), 2132–2140. 10.4315/0362-028X.JFP-13-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokaei, S. , Fayaz, A. , Pourmehdi, M. , & Haghdoost, A. A. (2009). The prevalence of rabies and animal bites in littoral provinces of Caspian Sea, Iranian. Veterinary Journal, 5(1), 5–14. [Google Scholar]
- Borji, H. , & Parandeh, S. (2010). The abattoir condemnation of meat because of parasitic infection, and its economic importance: Results of a retrospective study in north–eastern Iran. Annals of Tropical Medicine & Parasitology, 104(8), 641–647. 10.1179/136485910X12851868780261 [DOI] [PubMed] [Google Scholar]
- Borji, H. , Razmi, G. R. , Movassaghi, A. R. , Naghibi, A. , & Maleki, M. (2009). Prevalence of Cryptosporidium and Eimeria infections in dromedary (Camelus dromedarius) in abattoir of Mashhad, Iran. Journal of Camel Practice and Research, 16(2), 1–4. [Google Scholar]
- Butler, T. (2009). Plague into the 21st century. Clinical Infectious Diseases, 49(5), 736–742. 10.1086/604718 [DOI] [PubMed] [Google Scholar]
- Capasso, L. (2002). Bacteria in two‐millennia‐old cheese, and related epizoonoses in Roman populations. Journal of Infection, 45(2), 122–127. 10.1053/jinf.2002.0996 [DOI] [PubMed] [Google Scholar]
- Champour, M. , Mohammadi, G. , Chinikar, S. , Razmi, G. , Shah‐Hosseini, N. , Khakifirouz, S. , … Jalali, T. (2014). Seroepidemiology of Crimean‐Congo hemorrhagic fever virus in one‐humped camels (Camelus dromedarius) population in northeast of Iran. Journal of Vector Borne Diseases, 51(1), 62. [PubMed] [Google Scholar]
- Chaurasia, R. (2014). Rabies review. European Journal of Biomedical and Pharmaceutical Sciences, 1(2), 281–310. [Google Scholar]
- Chinikar, S. , Ghiasi, S. M. , Naddaf, S. , Piazak, N. , Moradi, M. , Razavi, M. R. , … Bouloy, M. (2012). Serological evaluation of Crimean‐Congo hemorrhagic fever in humans with high‐risk professions living in enzootic regions of Isfahan Province of Iran and genetic analysis of circulating strains. Vector‐Borne and Zoonotic Diseases, 12(9), 733–738. 10.1089/vbz.2011.0634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitgar, A. , Haghdost, R. S. , Jamshidian, M. , & Hesaraki, S. (2014). Prevalence of Pasteurella multocida and Mycoplasma Arginini and in dromedary camel (Camelus dromedarius) in Iran: The effect of season on P. multocida prevalence. Advances in Environmental Biology, 8(9), 792–796. [Google Scholar]
- Chiu, C.‐H. , Su, L.‐H. , & Chu, C. (2004). Salmonella enterica serotype Choleraesuis: Epidemiology, pathogenesis, clinical disease, and treatment. Clinical Microbiology Reviews, 17(2), 311–322. 10.1128/CMR.17.2.311-322.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbel, M. J. (1997). Brucellosis: An overview. Emerging Infectious Diseases, 3(2), 213–221. 10.3201/eid0302.970219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darebaghi Hosein Abadi, A. , Najmoddin, A. , Keneshlou, A. , & Kordi, H. (2016). A Survey on hydatid cyst infection rate in slaughtered camel in Semnan abattoir, Iran. The Second National Congress of Camel in Iran, Hormozgan persian. [Google Scholar]
- Dehkordi, F. S. , Barati, S. , Momtaz, H. , Ahari, S. N. H. , & Dehkordi, S. N. (2013). Comparison of shedding, and antibiotic resistance properties of listeria monocytogenes isolated from milk, feces, urine, and vaginal secretion of bovine, ovine, caprine, buffalo, and camel species in Iran. Jundishapur Journal of Microbiology, 6(3), 284–294. [Google Scholar]
- Dehkordi, F. S. , Haghighi Borujeni, M. R. , Rahimi, E. , & Abdizadeh, R. (2013). Detection of Toxoplasma gondii in raw caprine, ovine, buffalo, bovine, and camel milk using cell cultivation, cat bioassay, capture ELISA, and PCR methods in Iran. Foodborne Pathogens and Disease, 10(2), 120–125. [DOI] [PubMed] [Google Scholar]
- Dehkordi, F. S. , Saberian, S. , & Momtaz, H. (2012). Detection and segregation of Brucella abortus and Brucella melitensis in aborted bovine, ovine, caprine, buffaloes and camelid fetuses by application of conventional and real‐time polymerase chain reaction. The Thai Journal of Veterinary Medicine, 42(1), 13. [Google Scholar]
- Dehkordi, F. , & Taghizadeh, F. (2012). Prevalence and some risk factors associated with brucellosis and leptospirosis in aborted fetuses of ruminant species. Research Opinions in Animal & Veterinary Sciences, 2(4), 275–281. [Google Scholar]
- Doosti, A. , Ahmadi, R. , & Arshi, A. (2012). PCR detection of leptospirosis in Iranian camels. Bulgarian Journal of Veterinary Medicine, 15(3), 178–183. [Google Scholar]
- Doosti, A. , Arshi, A. , & Sadeghi, M. (2014). Investigation of Coxiella burnetii in Iranian camels. Comparative Clinical Pathology, 23(1), 43–46. 10.1007/s00580-012-1567-6 [DOI] [Google Scholar]
- Du, L. , & Han, G.‐Z. (2016). Deciphering MERS‐CoV evolution in dromedary camels. Trends in Microbiology, 24(2), 87–89. 10.1016/j.tim.2015.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey, J. (2009). History of the discovery of the life cycle of Toxoplasma gondii . International Journal for Parasitology, 39(8), 877–882. 10.1016/j.ijpara.2009.01.005 [DOI] [PubMed] [Google Scholar]
- Ducati, R. G. , Ruffino‐Netto, A. , Basso, L. A. , & Santos, D. S. (2006). The resumption of consumption: A review on tuberculosis. Memórias do Instituto Oswaldo Cruz, 101(7), 697–714. 10.1590/S0074-02762006000700001 [DOI] [PubMed] [Google Scholar]
- Ebadzadeh, H. R. , Ahmadi, K. , Mohammadnia Afroozi, S. , Dehghani, R. A. , Abbasi, M. , & Yari, S. (2018). Agricultural statistics in 2017. Tehran: Ministry of Agriculture‐Jahad. [Google Scholar]
- Eckert, J. , & Deplazes, P. (2004). Biological, epidemiological, and clinical aspects of Echinococcosis, a zoonosis of increasing concern. Clinical Microbiology Reviews, 17(1), 107–135. 10.1128/CMR.17.1.107-135.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Berbri, I. , Petavy, A. F. , Umhang, G. , Bouslikhane, M. , Fassi Fihri, O. , Boué, F. , & Dakkak, A. (2015). Epidemiological investigations on cystic echinococcosis in North‐West (Sidi Kacem Province) Morocco: Infection in ruminants. Advances in Epidemiology, 2015, 9 10.1155/2015/104025 [DOI] [Google Scholar]
- El‐Harrak, M. , Martín‐Folgar, R. , Llorente, F. , Fernández‐Pacheco, P. , Brun, A. , Figuerola, J. , & Jiménez‐Clavero, M. Á. (2011). Rift valley and West nile virus antibodies in camels, North Africa. Emerging Infectious Diseases, 17(12), 2372–2374. 10.3201/eid1712.110587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, W. A. (2015). Animal Leptospirosis. Leptospira and Leptospirosis (pp. 99–137). Berlin, Heidelberg: Springer. [Google Scholar]
- Elzlitne, R. , & Elhafi, G. (2016). Seroprevalence of Chlamydia abortus in camel in the western region of Libya. Journal of Advanced Veterinary and Animal Research, 3(2), 178–183. 10.5455/javar.2016.c151 [DOI] [Google Scholar]
- Esamaeili, S. , Azadmanesh, K. , Naddaf, S. R. , Rajerison, M. , Carniel, E. , & Mostafavi, E. (2013). Serologic survey of plague in animals, Western Iran. Emerging Infectious Diseases, 19(9), 1549–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili, H. (2015). Brucellosis in Islamic republic of Iran. Journal of Medical Bacteriology, 3(3–4), 47–57. [Google Scholar]
- Esmaeili, H. , Ghasemi, E. , & Ebrahimzadeh, H. (2012). An outbreak of camel rabies in Iran. Journal of Camel Practice and Research, 19(1), 19–20. [Google Scholar]
- Esmaeili, H. , Hamedi, M. , Hamidiya, Z. , Amrabadi, O. , Khaji, L. , Gholami, H. , … Noori, H. (2010). Bacterial –an outbreak of camel pasteurellosis in the Larestan region of Iran. 4th Iranian Congress of Clinical Microbiology, Isfahan / Iran. [Google Scholar]
- Fadlelmula, A. , Agab, H. , Le Horgne, J. , Abbas, B. , & Abdalla, A. (1993). First isolation of Trichophyton verrucosum as the aetiology of ringworm in the Sudanese camels (Camelus dromedarius). Revue D'elevage Et De Medecine Veterinaire Des Pays Tropicaux (France), 47(2), 184–187. [PubMed] [Google Scholar]
- Fajs, L. , Jakupi, X. , Ahmeti, S. , Humolli, I. , Dedushaj, I. , & Avšič‐Županc, T. (2014). Molecular epidemiology of Crimean‐Congo hemorrhagic fever virus in Kosovo. PLOS Neglected Tropical Diseases, 8(1), e2647 10.1371/journal.pntd.0002647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahtaj, F. , Fayaz, A. , Howaizi, N. , Biglari, P. , & Gholami, A. (2014). Human rabies in Iran. Tropical Doctor, 44(4), 226–229. 10.1177/0049475514528174 [DOI] [PubMed] [Google Scholar]
- Fassi‐Fehri, M. (1987). Diseases of camels. Revue Scientifique Et Technique (International Office of Epizootics), 6(2), 337–354. [DOI] [PubMed] [Google Scholar]
- Fathi, S. , Mirzaei Dehaghi, M. , & Radfar, M. H. (2012). Occurrence of hydatidosis in camels (Camelus dromedarius) and their potential role in the epidemiology of Echinococcus granulosus in Kerman area, southeast of Iran. Comparative Clinical Pathology, 21(5), 921–927. 10.1007/s00580-011-1200-0 [DOI] [Google Scholar]
- Fayer, R. (2004). Cryptosporidium: A water‐borne zoonotic parasite. Veterinary Parasitology, 126(1–2), 37–56. 10.1016/j.vetpar.2004.09.004 [DOI] [PubMed] [Google Scholar]
- Fedorov, V. (1960). Plague in camels and its prevention in the USSR. Bulletin of the World Health Organization, 23(2–3), 275. [PMC free article] [PubMed] [Google Scholar]
- Fowler, M. E. (1999). Medicine and surgery of South American camelids: Llama, Alpaca, Vicuna, Guanaco. Arnes, AI:, Iowa State University Press. [Google Scholar]
- García, A. , Fox, J. G. , & Besser, T. E. (2010). Zoonotic enterohemorrhagic Escherichia coli: A One Health perspective. ILAR Journal, 51(3), 221–232. 10.1093/ilar.51.3.221 [DOI] [PubMed] [Google Scholar]
- Garib, Z. , et al. (2011). Prevalence of brucellosis in camels in Hormozgan province, Iran. The 4th National Iranian Congress of Brucellosis persian. [Google Scholar]
- Gholami, A. , Fayaz, A. , & Farahtaj, F. (2014). Rabies in Iran: Past, present and future. Journal of Medical Microbiology and Infectious Diseases, 2(1), 1–10. [Google Scholar]
- Ghorbani, A. , Rabbani Khorasgani, M. , Zarkesh‐Esfahani, H. , Sharifiyazdi, H. , Kashani, A. D. , & Emami, H. (2013). Comparison of serology, culture, and PCR for detection of brucellosis in slaughtered camels in Iran. Comparative Clinical Pathology, 22(5), 913–917. 10.1007/s00580-012-1499-1 [DOI] [Google Scholar]
- Golami, S. , & Seyedin, S. M. (2011). Detection Salmonella antigens in camel meat with a specific culture and agglutination test in Mashhad. Twentieth National Congress of Food Science and Technology, Tehran ‐ Sharif University of Technology.persian. [Google Scholar]
- Gonzalez Gompf, S. (2015). Middle east respiratory syndrome coronavirus infection (MERS‐CoV Infection). Medicinenet. [Google Scholar]
- Grosso, G. , Gruttadauria, S. , Biondi, A. , Marventano, S. , & Mistretta, A. (2012). Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World Journal of Gastroenterology, 18(13), 1425–1437. 10.3748/wjg.v18.i13.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadian, M. (1996). leptospiros disease in camel herd of Tabas county, Iran. Iranian Veterinary Journal, 2(4), 36–37. [Google Scholar]
- Hajikolaei, M. , Sazmand, A. , Abdollahpour, G. , & Moghadam, S. (2013). Serological study on leptospiral infection in camels (Camelus dromedarius): A provincial study. Journal of Veterinary Research, 68(2), 121–125. [Google Scholar]
- Haridy, F. M. , & Morsy, T. A. (2000). Camel: A new Egyptian host for Fasciola gigantica . Journal of the Egyptian Society of Parasitology, 30(2), 451–454.eng. [PubMed] [Google Scholar]
- Hartskeerl, R. , Collares‐Pereira, M. , & Ellis, W. (2011). Emergence, control and re‐emerging leptospirosis: Dynamics of infection in the changing world. Clinical Microbiology and Infection, 17(4), 494–501. 10.1111/j.1469-0691.2011.03474.x [DOI] [PubMed] [Google Scholar]
- Hashemi, A. , Torabi, M. , Darabi, E. , & Shirazi, N. (2002). Isolation and Characterization of tuberclosis in camels from Slaughterhouse to Mashhad, Iran. Razavi Khorasan Provincial Veterinary Service. [Google Scholar]
- Hashemi Shahraki, A. , Carniel, E. , & Mostafavi, E. (2016). Plague in Iran: Past and current situation. Epidemiology Health, 38, e2016033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayette, M.‐P. , & Sacheli, R. (2015). Dermatophytosis, trends in epidemiology and diagnostic approach. Current Fungal Infection Reports, 9(3), 164–179. 10.1007/s12281-015-0231-4 [DOI] [Google Scholar]
- Heller, M. , Anderson, D. , & Silveira, F. (1998). Streptococcal peritonitis in a young dromedary camel. Australian Veterinary Journal, 76(4), 253–254. 10.1111/j.1751-0813.1998.tb10152.x [DOI] [PubMed] [Google Scholar]
- Helmy, N. (2000). Seasonal abundance of Ornithodoros (O.) savignyi and prevalence of infection with Borrelia spirochetes in Egypt. Journal of the Egyptian Society of Parasitology, 30(2), 607–619. [PubMed] [Google Scholar]
- Hersom, M. , Irsik, M. , & Thrift, T. (2008). Biosecurity and biological risk management for livestock enterprises. UF/IFASExtension. AN19., Department of Animal Sciences [Google Scholar]
- Heymann, D. L. ; A. American Public Health . (2015). Control of communicable diseases manual : An official report of the American Public Health Association. Washington, DC: APHA Press, An Imprint of The American Public Health Association. [Google Scholar]
- Higgins, E. A. (1992). Epidemiology and control of camel pox in Bahrain. The First International Camel Confernce, Dubai. [Google Scholar]
- Hilali, M. , & Fahmy, M. (1993). Trypanozoon‐like epimastigotes in the larvae of Cephalopina titillator (Diptera: Oestridae) infesting camels (Camelus dromedarius) infected with Trypanosoma evansi . Veterinary Parasitology, 45(3–4), 327–329. 10.1016/0304-4017(93)90087-4 [DOI] [PubMed] [Google Scholar]
- Hilali, M. , Fatani, A. , & Al‐Atiya, S. (1995). Isolation of tissue cysts of Toxoplasma, Isospora, Hammondia and Sarcocystis from camel (Camelus dromedarius) meat in Saudi Arabia. Veterinary Parasitology, 58(4), 353–356. 10.1016/0304-4017(94)00727-T [DOI] [PubMed] [Google Scholar]
- Hussein Khalil, N. E. A. (2015). Pathological and bacteriological studies on condemned lungs of camels at Tamboul Abattoir, Sudan. B.V.Sc., University of Khartoum. [Google Scholar]
- Hussein, M. F. , Alshaikh, M. A. , Al‐Jumaah, R. S. , GarelNabi, A. , Al‐Khalifa, I. , & Mohammed, O. B. (2015). The Arabian camel (Camelus dromedarius) as a major reservoir of Q fever in Saudi Arabia. Comparative Clinical Pathology, 24(4), 887–892. 10.1007/s00580-014-2002-y [DOI] [Google Scholar]
- Hussein, M. F. , & El Nabi, A. G. (2009). Serological evidence of leptospirosis in camels in Saudi Arabia. Journal of Animal and Veterinary Advances, 8(5), 1010–1012. 10.3923/javaa.2009.1010.1012 [DOI] [Google Scholar]
- Hussin, A. , Khalaf, J. , & Ali, H. (2015). Detection of intestinal protozoa in camels and their breeders in Najef, Iraq. Research Journal for Veterinary Practitioners, 3(3), 53–57. 10.14737/journal.rjvp/2015/3.3.53.57 [DOI] [Google Scholar]
- Hyun, B.‐H. , Lee, K.‐K. , Kim, I.‐J. , Lee, K.‐W. , Park, H.‐J. , Lee, O.‐S. , … Lee, J.‐B. (2005). Molecular epidemiology of rabies virus isolates from South Korea. Virus Research, 114(1), 113–125. 10.1016/j.virusres.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Jafari, R. , Maghsood, A. H. , & Fallah, M. (2012). Prevalence of cryptosporidium infection among livestock and humans in contact with livestock in hamadan district, Iran, 2012. Journal of Research in Health Sciences, 13(1), 86–89. [PubMed] [Google Scholar]
- Janani, A. , Fayaz, A. , Simani, S. , Farahtaj, F. , Eslami, N. , Howaizi, N. , … Sabetghadam, M. (2007). Epidemiology and control of rabies in Iran. Developments in Biologicals, 131, 207–211. [PubMed] [Google Scholar]
- Janati Pirouz, H. , Mohammadi, G. , Mehrzad, J. , Azizzadeh, M. , & Nazem Shirazi, M. H. (2015). Seroepidemiology of Q fever in one‐humped camel population in northeast Iran. Tropical Animal Health and Production, 47(7), 1293–1298. 10.1007/s11250-015-0862-z [DOI] [PubMed] [Google Scholar]
- Jaradat, Z. , Al Aboudi, A. , Shatnawi, M. , & Ababneh, Q. (2013). Staphylococcus aureus isolates from camels differ in coagulase production, genotype and methicillin resistance gene profiles. The Journal of Microbiology, Biotechnology and Food Sciences, 2(6), 2455. [Google Scholar]
- Kaakoush, N. O. , Castaño‐Rodríguez, N. , Mitchell, H. M. , & Man, S. M. (2015). Global epidemiology of Campylobacter infection. Clinical Microbiology Reviews, 28(3), 687–720. 10.1128/CMR.00006-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadjeh, G. , Zowghi, E. , & Zarif‐fard, M. R. (1999). Incidence of Brucellosis in one‐humped camels of Boushehr. Archive of Razi Institute, 50(1), 83–86. [Google Scholar]
- Khalafalla, A. I. , & El‐Dirdiri, G. A. (2003). Laboratory and field investigation of live attenuated and inactivated camelpox vaccines. Camel Practice and Research, 10(2), 191–200. [Google Scholar]
- Khalaj, M. (2014a). Infection with coronavirus in camels, Iran. Retrieved from http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapEventSummary&reportxml:id=16747. [Google Scholar]
- Khalaj, M. (2014b). Infection with coronavirus in camels. Retrieved from http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportxml:id=16411 [Google Scholar]
- Khalili Bagaloy, H. , Sakhaee, E. , & Khalili, M. (2017). Detection of pancoronavirus using PCR in Camelus dromedarius in Iran (first report). Comparative Clinical Pathology, 26(1), 193–196. 10.1007/s00580-016-2368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamesipour, F. , Doosti, A. , Mobarakeh, H. I. , & Komba, E. V. (2014). Toxoplasma gondii in cattle, camels and sheep in Isfahan and Chaharmahal va Bakhtiary provinces, Iran. Jundishapur Journal of Microbiology, 7(6), e17460 10.5812/jjm.17460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamesipour, F. , Rahimi, E. , Shakerian, A. , Doosti, A. , & Momtaz, H. (2014). Molecular study of the prevalence of brucella abortus and brucella melitensis in the blood and lymph node samples of slaughtered camels by polymerase chain reaction (PCR) in Iran. Acta Veterinaria Belgrade, 64(2), 245–256. 10.2478/acve-2014-0023 [DOI] [Google Scholar]
- Khosravi, A. R. , & Mahmoudi, M. (2003). Dermatophytes isolated from domestic animals in Iran. Mycoses, 46(5–6), 222–225. [DOI] [PubMed] [Google Scholar]
- Kilic, N. , & Kirkan, S. (2004). Actinomycosis in a one‐humped camel (Camelus dromedarius). Journal of Veterinary Medicine Series A, 51(7–8), 363–364. 10.1111/j.1439-0442.2004.00654.x [DOI] [PubMed] [Google Scholar]
- Kloos, H. , & Berhane, Y. (2006). Zoonotic diseases of public health importance In Berhane Y., Hailemariam D., & Kloos H. (Eds.), Epidemiology and ecology of health and diseases in Ethiopia (692–700). 1st edition. Addis Ababa: Shama books. [Google Scholar]
- Koochakzadeh, A. , Badouei, M. A. , Mazandarani, E. , & Madadgar, O. (2014). Survey on O157: H7 enterohemorrhagic Escherichia coli (EHEC) in cattle in Golestan province, Iran. Iranian Journal of Microbiology, 6(4), 276. [PMC free article] [PubMed] [Google Scholar]
- Lal, A. , Cornish, L. M. , Fearnley, E. , Glass, K. , & Kirk, M. (2015). Cryptosporidiosis: A disease of tropical and remote areas in Australia. PLOS Neglected Tropical Diseases, 9(9), e0004078 10.1371/journal.pntd.0004078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie, T. , Whitehouse, C. A. , Yingst, S. , Baldwin, C. , Kakar, F. , Mofleh, J. , … Kakar, R. (2011). Outbreak of gastroenteritis caused by Yersinia pestis in Afghanistan. Epidemiology & Infection, 139(5), 728–735. [DOI] [PubMed] [Google Scholar]
- Lotfollahi, L. , Nowrouzi, J. , Irajian, G. , Masjedian, F. , Kazemi, B. , Falahat, L. E. A. , & Ramez, M. (2011). Prevalence and antimicrobial resistance profiles of Listeria monocytogenes in spontaneous abortions in humans. African Journal of Microbiology Research, 5(14), 1990–1993. 10.5897/AJMR11.498 [DOI] [Google Scholar]
- Mackay, I. M. , & Arden, K. E. (2015). MERS coronavirus: Diagnostics, epidemiology, and transmission. Virology Journal, 12(1), 1 10.1186/s12985-015-0439-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahzunieh, M. R. , & Salami, M. (2015). Serological and molecular survey on camel brucellosis in Najaf Abad, Iran. Biological Journal of Microorganism, 4(14), 167–174. [Google Scholar]
- Mamo, G. , Bayleyegn, G. , Tessema, T. S. , Legesse, M. , Medhin, G. , Bjune, G. , … Ameni, G. (2011). Pathology of camel tuberculosis and molecular characterization of its causative agents in pastoral regions of Ethiopia. PLoS ONE, 6(1), e15862 10.1371/journal.pone.0015862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariner, J. C. , Morrill, J. , & Ksiazek, T. (1995). Antibodies to hemorrhagic fever viruses in domestic livestock in Niger: Rift Valley fever and Crimean‐Congo hemorrhagic fever. The AmericanJournal of Tropical Medicine and Hygiene, 53(3), 217–221. 10.4269/ajtmh.1995.53.217 [DOI] [PubMed] [Google Scholar]
- Masala, G. , Porcu, R. , Sanna, G. , Chessa, G. , Cillara, G. , Chisu, V. , & Tola, S. (2004). Occurrence, distribution, and role in abortion of Coxiella burnetii in sheep and goats in Sardinia, Italy. Veterinary Microbiology, 99(3), 301–305. 10.1016/j.vetmic.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Mashak, Z. , Zabihi, A. , Sodagari, H. , Noori, N. , & Akhondzadeh Basti, A. (2015). Prevalence of Listeria monocytogenes in different kinds of meat in Tehran province, Iran. British Food Journal, 117(1), 109–116. [Google Scholar]
- Maurin, M. , & Raoult, D. (1999). Q Fever. Clinical Microbiology Reviews, 12(4), 518–553. 10.1128/CMR.12.4.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrane, J. , & Higgins, A. (1985). Infectious diseases of the camel: Viruses, bacteria, and fungi. British Veterinary Journal, 141(5), 529–547. [DOI] [PubMed] [Google Scholar]
- Mederos‐Iriarte, L. E. , Poveda, J. B. , Poveda, C. G. , Vega‐Orellana, O. M. , Gutierrez, C. , Corbera, J. A. , & Ramirez, A. S. (2014). Mycoplasma detection and isolation from one‐humped camels (Camelus dromedarius). Tropical Animal Health and Production, 46(7), 1317–1320. [DOI] [PubMed] [Google Scholar]
- Metanat, M. , Sharifi‐Mood, B. , Alavi‐Naini, R. , & Aminianfar, M. (2012). The epidemiology of tuberculosis in recent years: Reviewing the status in south‐eastern Iran. Zahedan Journal of Research in Medical Sciences, 13(9), 1–7. [Google Scholar]
- Miranzade, H. (1994). A survey of disease and pathological conditions in slaughtered camels at Najafabad slaughter houses, Tehran University of Veterinary Medicine. [Google Scholar]
- Mirzaei, F. (2012). Production and trade of camel products in some Middle East countries. Journal of Agricultural Economics & Development, 1(6), 153–160. [Google Scholar]
- Misonne, X. (1977). A natural focus of plague in Libya. Annales De La Societe Belge De Medecine Tropicale, 57(3), 163–168. [PubMed] [Google Scholar]
- Mladenova‐Hristova, I. , & Tsachev, I. (2014). Yersinia pestis old and new challenges in humans and in animals. Trakia Journal of Sciences, 12(2), 211. [Google Scholar]
- Mobedi, I. , Madadi, H. , & Arfaa, F. (1970). Camel, Camelus dromedarius, as intermediate host of Echinococcus granulosus in Iran. Journal of Parasitology, 56(6), 1255 10.2307/3277581 [DOI] [PubMed] [Google Scholar]
- Mogghadass, E. (2012). Comprehensive guide for camel disease. Tehran, Nilubarg. [Google Scholar]
- Moghadar, N. , Oryan, A. , & Pour, M. (1992). Helminths recovered from the liver and lungs of camel with special reference to their incidence and pathogenesis in Shiraz, islamic‐republic‐of‐Iran. Indian Journal of Animal Sciences, 62(11), 1018–1023. [Google Scholar]
- Moghaddas, E. E. A. (1998). A case report of camel pox in Iranshahr city, Iran. Iranian Journal of Health, 3, 26–28. [Google Scholar]
- Moghaddas, E. , Borji, H. , Naghibi Aboul, G. , Razmi, G. , & Shayan, P. (2014). Epidemiological study of hydatidosis in the dromedaries (Camelus dromedarius) of different regions of Iran. Asian Pacific Journal of Tropical Biomedicine, 4(1), S148–S151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddas, E. (2012). Mogghadass, E. (2012). Comprehensive guide for camel disease. Tehran, Nilubarg. [Google Scholar]
- Mohamed, M. , & Suelam, I. (2010). Isolation of non‐typhoid Salmonella from humans and camels with reference to its survival in abattoir effluents. Global Veterinaria, 5(6), 356–361. [Google Scholar]
- Mohamed, R. , & Abdelsalam, E. (2008). A review on pneumonic pasteurellosis (respiratory mannheimiosis) with emphasis on pathogenesis, virulence mechanisms and predisposing factors. Bulgarian Journal of Veterinary Medicine, 11(3), 139–160. [Google Scholar]
- Mohammady, G. R. , & Najafi Mosleh, S. (2017). Salmonellosis outbreak in a herd of camel. The Second National Congress of Camel in Iran, Hormozgan. Persian. [Google Scholar]
- Mohammed, M. A. , & Shigidy, M. T. (2013). Sero‐prevalence and epidemiology of brucellosis in camels, sheep and goats in Abu Dhabi emirate. International Journal of Animal and Veterinary Advances, 5(2), 82–86. [Google Scholar]
- Momin, R. , Pethkar, D. , Jaiswal, T. , & Jhala, V. (1987). An outbreak of pasteurellosis in camels. Indian, Indian Veterinary Journal., 64, 896–897. [Google Scholar]
- Moniri, A. , Marjani, M. , Tabarsi, P. , Yadegarynia, D. , & Nadji, S. A. (2015). Health care associated middle east respiratory syndrome (MERS): A case from Iran. Tanaffos, 14(4), 262. [PMC free article] [PubMed] [Google Scholar]
- Moro, P. , & Schantz, P. M. (2009). Echinococcosis: A review. International Journal of Infectious Diseases, 13(2), 125–133. 10.1016/j.ijid.2008.03.037 [DOI] [PubMed] [Google Scholar]
- Mortazavi, H. S. , Monavari, S. H. , Ataei Pirkooh, A. , & Tavakoli, A. (2014). Middle east respiratory syndrome coronavirus (MERS‐CoV): A review article. Iranian Journal of Virology, 8(2), 59–68. 10.21859/isv.8.2.3.59 [DOI] [Google Scholar]
- Mosadeghhesari, M. , Oryan, A. , Zibaee, S. , & Varshovi, H. R. (2014). Molecular investigation and cultivation of camelpox virus in Iran. Archives of Virology, 159(11), 3005–3011. 10.1007/s00705-014-2169-1 [DOI] [PubMed] [Google Scholar]
- Mostafalu, Y. , & Rahchamani, R. (2016). A Survey on hydatid cyst infection rate in slaughtered camel in Golestan abattoir from 1385 to 1387. The Second National Congress of Camel in Iran, Hormozgan. Persian. [Google Scholar]
- Mostafavi, E. , Rastad, H. , & Khalili, M. (2012). Q fever: An emerging public health concern in Iran. Asian Journal of Epidemiology, 5(3), 66 10.3923/aje.2012.66.74 [DOI] [Google Scholar]
- Motamedi, G. R. (1987). Seroprevalence of camel brucellosis in Fars region Shahid Chamran University. [Google Scholar]
- Mowlavi, G. (1997). Hydatidosis and testicular filariasis (D.evansi) in camel (C.dromedarious) in central part of Iran Tehran University of Medical Sciences. [Google Scholar]
- Murphy, F. A. (2008). Emerging zoonoses: The challenge for public health and biodefense. Preventive Veterinary Medicine, 86(3), 216–223. 10.1016/j.prevetmed.2008.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa, M. T. , Shomein, A. M. , Abd el Razig, Y. M. , Meki, N. T. , & el Hassan, S. M. (1993). Anthrax in humans and camels in the Sudan with reference to the disease in the country. Journal of Livestock and Veterinary Medicine in Tropical Countries, 46(3), 438–439. [PubMed] [Google Scholar]
- Mustafa, I. (1987). Bacterial diseases of dromedaries and Bactrian camels(anthrax, brucellosis, haemorrhagic septicaemia, plague, salmonellosis, tuberculosis, paratuberculosis, leptospirosis, clostridial infections). Revue Scientifique Et Technique De l'OIE, 6(2), 391–405. [DOI] [PubMed] [Google Scholar]
- Nadalian, M. G. (2012). Rabies in camel The First National Congress of Camel in Iran Mashhad, Iran Persian. [Google Scholar]
- Nazifi, S. , Behzadi, M. , Haddadi, S. , Jahromi, A. R. , Mehrshad, S. , & Tamadon, A. (2010). Prevalence of cryptosporidium isolated from dromedary camels (Camelus dromedarius) in Qeshm Island, Southern Iran. Comparative Clinical Pathology, 19(3), 311–314. 10.1007/s00580-009-0862-3 [DOI] [Google Scholar]
- Nigg, A. J. , & Walker, P. L. (2009). Overview, prevention, and treatment of rabies. Pharmacotherapy, 29(10), 1182–1195. 10.1592/phco.29.10.1182 [DOI] [PubMed] [Google Scholar]
- Ningal, S. P. , Kothule, M. B. , Jadhav, N. Y. , Kadam, S. D. , Katare, Y. S. , & Hapse, S. A. (2015). A review on leptospirosis. Epidemiology, 3(4), 5–8. [Google Scholar]
- Norlander, L. (2000). Q fever epidemiology and pathogenesis. Microbes and Infection, 2(4), 417–424. 10.1016/S1286-4579(00)00325-7 [DOI] [PubMed] [Google Scholar]
- Nour‐Mohammadzadeh, F. , Baradaran Seyed, Z. , Hesaraki, S. , Yadegari, Z. , Alidadi, N. , & Sattari Tabrizi, S. (2010). Septicemic salmonellosis in a two‐humped camel calf (Camelus bactrianus). Tropical Animal Health and Production, 42(8), 1601–1604. 10.1007/s11250-010-9626-y [DOI] [PubMed] [Google Scholar]
- Oosterom, J. (1991). Epidemiological studies and proposed preventive measures in the fight against human salmonellosis. International Journal of Food Microbiology, 12(1), 41–51. 10.1016/0168-1605(91)90046-R [DOI] [PubMed] [Google Scholar]
- Otero‐Abad, B. , & Torgerson, P. R. (2013). A systematic review of the epidemiology of echinococcosis in domestic and wild animals. PLoS Neglected Tropical Diseases, 7(6), e2249 10.1371/journal.pntd.0002249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas, G. , Papadimitriou, P. , Akritidis, N. , Christou, L. , & Tsianos, E. V. (2006). The new global map of human brucellosis. The Lancet Infectious Diseases, 6(2), 91–99. 10.1016/S1473-3099(06)70382-6 [DOI] [PubMed] [Google Scholar]
- Pieniazek, N. J. , Knight, R. , Grimes, B. H. , Veal, D. A. , Da silva, A. J. , & Graczyk, T. K. (2003). Detection of cryptosporidium parvum and Giardia lamblia carried by synanthropic flies by combined fluorescent in situ hybridization and a monoclonal antibody. The American Journal of Tropical Medicine and Hygiene, 68(2), 228–232. 10.4269/ajtmh.2003.68.228 [DOI] [PubMed] [Google Scholar]
- Pirouz, M. H. J. , Mohammadi, G. R. , Mehrzad, J. , & Aziz zadeh M.. (2016). Investigation of Coxiella burnetii infection in camel population of northeast of Iran with qPCR. The Second National Congress of Camel in Iran, Hormozgan. Persian. [Google Scholar]
- Pour Reza, A. (2013). Campylobacter Jujeni. Iranian Association of Clinical Laboratory Doctors, 21(5), 37–40. [Google Scholar]
- Pourjafar, M. et al. (2005). Serological study of brucellosis in slaughtered camels at Najaf Abad slaughter houses. The Natoinal Iranian Congress of Brucellosis 5–6 July 2005, Tehran Kamalolmolk, Tehran. [Google Scholar]
- Pourjafar, M. , Mahzounieh, M. , Zahraei Salehi, T. , & Habibollahi Khorasgani, A. (2010). The serological survey of salmonella infection in camels (Camelus dromedarius) of Najafabad, Iran. Iranian Journal of Veterinary Clinical Sciences, 2(2), 23–28. [Google Scholar]
- Prabhu, M. , Yogisharadhya, R. , Pavulraj, S. , Suresh, C. , Sathish, G. , & Singh, R. (2015). Camelpox and buffalopox: Two emerging and re‐emerging orthopox viral diseases of India. Advances in Animal and Veterinary Sciences, 3(10), 527–541. 10.14737/journal.aavs/2015/3.10.527.541 [DOI] [Google Scholar]
- Rabbani Khorasani, M. , Eatemadifar, Z. , Azadbakht, E. , Emami, H. , & Khormali, M. (2015). Detection of Anti‐Salmonella, Shigella, Escherichia, Pseudomonas and Staphylococcus Antibodies incamel milk. The Regional Conference of Camel Management and Production under Open Range System (RCCMPR), Khartoum‐Sudan Khartoum‐Sudan. [Google Scholar]
- Radfar, M. H. , Gowhari, M. A. , & Khalili, M. (2013). Comparison of capture ELISA and modified Ziehl‐Neelsen for detection of Cryptosporidium parvum in feces of camel (Camelus dromedarius) in Iran. Scientia Parasitologica, 14(3), 147–152. [Google Scholar]
- Rafiei, A. , Hedayati Zadeh Omran, A. , Babamahmoodi, F. , Alizqadeh Navaee, R. , & Valadan, R. (2012). Review of leptospirosis in Iran. Journal of Mazandaran University of Medical Sciences, 22(94), 102–110. [Google Scholar]
- Rafyi, A. , & Maghami, G. (1959). Sur la fréquence de la leptospirose en Iran. Bulletin De La Société De Pathologie Exotique, 52(5), 592–596. [PubMed] [Google Scholar]
- Rahimi, E. , Ameri, M. , Alimoradi, M. , Chakeri, A. , & Bahrami, A. R. (2013). Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from raw camel, beef, and water buffalo meat in Iran. Comparative Clinical Pathology, 22(3), 467–473. 10.1007/s00580-012-1434-5 [DOI] [Google Scholar]
- Rahimi, E. , Ameri, M. , & Kazemeini, H. R. (2010). Prevalence and antimicrobial resistance of campylobacter species isolated from raw camel, beef, lamb, and goat meat in Iran. Foodborne Pathogens and Disease, 7(4), 443–447. [DOI] [PubMed] [Google Scholar]
- Rahimi, E. , Kazemeini, H. R. , & Salajegheh, M. (2012). Escherichia coli O157:H7/NM prevalence in raw beef, camel, sheep, goat, and water buffalo meat in Fars and Khuzestan provinces, Iran. Veterinary Research Forum, 3(1), 15–17. [PMC free article] [PubMed] [Google Scholar]
- Rahimi, E. , Momtaz, H. , Behzadnia, A. , & Baghbadorani, Z. T. (2014). Incidence of Listeria species in bovine, ovine, caprine, camel and water buffalo milk using cultural method and the PCR assay. Asian Pacific Journal of Tropical Disease, 4(1), 50–53. 10.1016/S2222-1808(14)60313-3 [DOI] [Google Scholar]
- Rahimi, E. , Momtaz, H. , & Nozarpour, N. (2010). Prevalence of Listeria spp., campylobacter spp. and Escherichia coli o157:h7 isolated from camel carcasses during processing. Bulgarian Journal of Veterinary Medicine, 13(3), 179–185. [Google Scholar]
- Ramadan, N. , & Shaib, H. (2019). Middle East respiratory syndrome coronavirus (MERS‐CoV): A review. Germs, 9(1), 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidi . (1993). Report of occurrence camel pox disease in camel herd of Zarand city of the Kerman province. 2nd Conventional of Iranian Veterinary Clinicians, Tehran. Persian. [Google Scholar]
- Razavi, S. , Oryan, A. , Bahrami, S. , Mohammadalipour, A. , & Gowhari, M. (2009). Prevalence of Cryptosporidium infection in camels (Camelus dromedarius) in a slaughterhouse in Iran. Tropical Biomedicine, 26(3), 267–273. [PubMed] [Google Scholar]
- Robert‐Gangneux, F. , & Darde, M.‐L. (2012). Epidemiology of and diagnostic strategies for toxoplasmosis. Clinical Microbiology Reviews, 25(2), 264–296. 10.1128/CMR.05013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokni, M. (2009). Echinococcosis/hydatidosis in Iran. Iranian Journal of Parasitology, 4(2), 1–16. [Google Scholar]
- Rossle, N. F. , & Latif, B. (2013). Cryptosporidiosis as threatening health problem: A review. Asian Pacific Journal of Tropical Biomedicine, 3(11), 916–924. 10.1016/S2221-1691(13)60179-3 [DOI] [Google Scholar]
- Rupprecht, C. E. , Hanlon, C. A. , & Hemachudha, T. (2002). Rabies re‐examined. The Lancet Infectious Diseases, 2(6), 327–343. 10.1016/S1473-3099(02)00287-6 [DOI] [PubMed] [Google Scholar]
- Sacramento, D. , Badrane, H. , Bourhy, H. , & Tordo, N. (1992). Molecular epidemiology of rabies virus in France: Comparison with vaccine strains. Journal of General Virology, 73(5), 1149–1158. 10.1099/0022-1317-73-5-1149 [DOI] [PubMed] [Google Scholar]
- Sadrbazzaz, A. , Valinezhad, A. , Zibaee, S. , Farhudi, M. , Seyedin, M. , Kermani, H. , & Hoseinion, H. (2016). Survey of Cryptosporidium infection in some herd of camel in Razavi Khorasan The Second National Congress of Camel in Iran, Hormozgan. Persian. [Google Scholar]
- Sadrebazzaz, A. , Haddadzadeh, H. , & Shayan, P. (2006). Seroprevalence of Neospora caninum and Toxoplasma gondii in camels (Camelus dromedarius) in Mashhad, Iran. Parasitology Research, 98(6), 600–601. 10.1007/s00436-005-0118-3 [DOI] [PubMed] [Google Scholar]
- Saeed, A. A. B. , Al‐Hamdan, N. A. , & Fontaine, R. E. (2005). Plague from eating raw camel liver. Emerging Infectious Diseases Journal, 11(9), 1456–1457. 10.3201/eid1109.050081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safarpoor Dehkordi, F. , & Taghizadeh, F. (2012). Prevalence and some risk factors associated with brucellosis and leptospirosis in aborted fetuses of ruminant species. Research Opinions in Animal & Veterinary Sciences., 2(4), 275–281. [Google Scholar]
- Safdari, A. , & Jahantigh, M. (2014). Evaluation of raw meat contamination of listeria species in camel of sistan region. Indian Journal of Fundamental and Applied Life Sciences, 4(3), 537–540. [Google Scholar]
- Saidi, S. , Casals, J. , & Faghih, M. (1975). Crimean hemorrhagic fever‐Congo (CHF‐C) virus antibodies in man, and in domestic and small mammals, in Iran. The American Journal of Tropical Medicine and Hygiene, 24(2), 353–357. 10.4269/ajtmh.1975.24.353 [DOI] [PubMed] [Google Scholar]
- Salehi, T. Z. , Mahzounieh, M. , & Saeedzadeh, A. (2005). The isolation of antibiotic‐resistant Salmonella from intestine and liver of poultry in Shiraz province of Iran. International Journal of Poultry Science, 4(5), 320–322. 10.3923/ijps.2005.320.322 [DOI] [Google Scholar]
- Salihu, M. , Junaidu, A. , Abubakar, M. , Magaji, A. , & Mohammed, L. (2009). Isolation and characterization of Campylobacter species from camel (Camelus dramedarius) in Sokoto State, Northwestern Nigeria. International Journal of Animal and Veterinary Advances, 1(1), 25–27. [Google Scholar]
- Sargaz, G. R., et al. (2011). A report of occurrence of brucellosis in Sistan region camels. 4th National Iranian Congress of Brucellosis Tehran. Persian.** [Google Scholar]
- Sarvi, S. , Daryani, A. , Rahimi, M. T. , Aarabi, M. , Shokri, A. , Ahmadpour, E. , … Sharif, M. (2015). Cattle toxoplasmosis in Iran: A systematic review and meta–analysis. Asian Pacific Journal of Tropical Medicine, 8(2), 120–126. 10.1016/S1995-7645(14)60301-1 [DOI] [PubMed] [Google Scholar]
- Sazmand, A. (2012). A review of infectious disease of camels in yazd province of Iran( epizootiology study). First National Congress of Camel in Iran Mashhad, Iran Persian. [Google Scholar]
- Sazmand, A. , Hajikolayi, M. R. , Ghorbanpoor, M. , & Hekmaimoghaddam, S. H. (2012). Seroprevalence of brucellosis in camels (Camelus dromedarius) in center of Iran. First National Congress of Camel in Iran, Mashhad, Iran. Persian. [Google Scholar]
- Sazmand, A. , & Joachim, A. (2017). Parasitic diseases of camels in Iran (1931–2017) – a literature review. Parasite, 24, 1931–2017. 10.1051/parasite/2017024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazmand, A. , Rasooli, A. , Nouri, M. , Hamidinejat, H. , & Hekmatimoghaddam, S. (2012). Prevalence of Cryptosporidium spp. in camels and involved people in Yazd Province, Iran. IranianJournal of Parasitology, 7(1), 80. [PMC free article] [PubMed] [Google Scholar]
- Sazmand, A. , Razi Jalali, M. , Hekmatimoghaddam, S. , & Asadollahi, Z. (2013). Seroprevalence of hydatidosis in camels of Yazd province, Iran. Journal of Veterinary Laboratory Research, 5(2), 121–128. [Google Scholar]
- Schwarz, T. F. , Nsanze, H. , Longson, M. , Nitschko, H. , Gilch, S. , Shurie, H. , … Jager, G. (1996). Polymerase chain reaction for diagnosis and identification of distinct variants of Crimean‐Congo hemorrhagic fever virus in the United Arab Emirates. The American Journal of Tropical Medicine and Hygiene, 55(2), 190–196. 10.4269/ajtmh.1996.55.190 [DOI] [PubMed] [Google Scholar]
- Sepehr, S. (2012). Prevalence of Salmonella enterica Contamination of camel milk in Iran. Research Journal of Biological Sciences, 7(4), 195–199. 10.3923/rjbsci.2012.195.199 [DOI] [Google Scholar]
- Sharifiyazdi, H. , Oryan, A. , Ahmadnia, S. , & Valinezhad, A. (2011). Genotypic characterization of Iranian camel (Camelus dromedarius) isolates of Echinoccocus granulosus . The Journal of Parasitology, 97(2), 251–255. 10.1645/GE-2642.1 [DOI] [PubMed] [Google Scholar]
- Sharma, V. , Kumawat, T. K. , Sharma, A. , Seth, R. , & Chandra, S. (2015a). Dermatophytes: Diagnosis of dermatophytosis and its treatment. African Journal of Microbiology Research, 9(19), 1286–1293. 10.5897/AJMR2015.7374 [DOI] [Google Scholar]
- Sharma, V. , Kumawat, T. K. , Sharma, A. , Seth, R. , & Chandra, S. (2015b). Distribution and prevalence of dermatophytes in semi‐arid region of India. Advances in Microbiology, 5(02), 93 10.4236/aim.2015.52010 [DOI] [Google Scholar]
- Shayan, S. , Bokaean, M. , Shahrivar, M. R. , & Chinikar, S. (2015). Crimean‐Congo hemorrhagic fever. Laboratory Medicine, 46(3), 180–189. 10.1309/LMN1P2FRZ7BKZSCO [DOI] [PubMed] [Google Scholar]
- Shokri, H. , & Khosravi, A. (2016). An epidemiological study of animals dermatomycoses in Iran. Journal of Medical Mycology, 26(2), 170–177. 10.1016/j.mycmed.2016.04.007 [DOI] [PubMed] [Google Scholar]
- Simani, S. (2003). Rabies situation in Iran. Journal of Veterinary Medicine Faculty, University of Tehran, 58(3), 275–278. [Google Scholar]
- Simani, S. , Gholami, A. , Farahtaj, F. , Yousefi‐Behzadi, M. , & Fayaz, A. (2001). Epidemiological survey of different rabies virus strains in Iran. Journal of Sciences Islamic Republic of Iran, 12(4), 315–320. [Google Scholar]
- Singh, T. , Borah, M. K. , Dadhich, H. , & Sharma, G. D. (2013). Hepatic schistosomiasis in camel (Camelus dromedarius). Comparative Clinical Pathology, 22(5), 989–991. 10.1007/s00580-012-1515-5 [DOI] [Google Scholar]
- Sk, D. , Tuteja, F. , & Sena, D. (2009). Sarcopticosis in dormedary camel ‐clinical observations and its therapeutic management. Indian Journal of Animal Science, 79(3), 139–242. [Google Scholar]
- Soares‐Weiser, K. , Thomas, S. , G, G. T. , & Garner, P. (2010). Ribavirin for Crimean‐Congo hemorrhagic fever: Systematic review and meta‐analysis. BMC Infectious Diseases, 10(1), 207 10.1186/1471-2334-10-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleymani Babadi, M. , Kioomars, T. , Nader, A. , Keyvan, M. T. , Reza, S. , Mohammad, S. G. , … Mohammad Hasan, A. (2012). Mycobacterium isolated from tuberculosis‐like lesions in slaughtered dromedary camels in Northeast Iran. First National Congress of Camel in Iran, Mashhad Persian. [Google Scholar]
- Suardana, I. W. , Widiasih, D. A. , Mahardika, I. G. N. K. , Pinatih, K. J. P. , & Daryono, B. S. (2015). Evaluation of zoonotic potency of Escherichia coli O157: H7 through arbitrarily primed PCR methods. Asian Pacific Journal of Tropical Biomedicine, 5(11), 915–920. 10.1016/j.apjtb.2015.07.023 [DOI] [Google Scholar]
- Swai, E. S. , & Sindato, C. (2015). Seroprevalence of Rift Valley fever virus infection in camels (dromedaries) in northern Tanzania. Tropical Aanimal Health and Production, 47(2), 347–352. 10.1007/s11250-014-0726-y [DOI] [PubMed] [Google Scholar]
- Swaminathan, B. , & Gerner‐Smidt, P. (2007). The epidemiology of human listeriosis. Microbes and Infection, 9(10). 10.1016/j.micinf.2007.05.011 [DOI] [PubMed] [Google Scholar]
- Tahamtan, Y. , Amrabadi, O. R. , Shahriari, R. , & Namavari, M. (2017). Analysis and identification the causative agent of hemorrhagic septicemia and respiratory disease in camel in larestan. Applied Animal Science Research Journal, 6(24), 63–68. [Google Scholar]
- Tajik, J. , Farda, S. R. N. , paidar, A. , Anousheh, S. , & Dehghani, E. (2013). Balantidiasis in a dromedarian camel. Asian Pacific Journal of Tropical Disease, 3(5), 409–412. 10.1016/S2222-1808(13)60093-6 [DOI] [Google Scholar]
- Talebi, A. (2007). Sero_epidemiological Study of Leptospirosis in slaughtered Camels in Iran. Phd. Tehran, Iran: University of Tehran. [Google Scholar]
- Tejedor, M. T. , Martin, J. L. , Lupiola, P. , & Gutierrez, C. (2000). Caseous lymphadenitis caused by Corynebacterium ulcerans in the dromedary camel. The Canadian Veterinary Journal, 41(2), 126. [PMC free article] [PubMed] [Google Scholar]
- Telmadarraiy, Z. , Chinikar, S. , Vatandoost, H. , Faghihi, F. , & Hosseini‐Chegeni, A. (2015). Vectors of Crimean Congo hemorrhagic fever virus in Iran. Journal of Arthropod‐Borne Diseases, 9(2), 137. [PMC free article] [PubMed] [Google Scholar]
- Tenter, A. M. , Heckeroth, A. R. , & Weiss, L. M. (2000). Toxoplasma gondii: From animals to humans. International Journal or Parasitology, 30(12), 1217–1258. 10.1016/S0020-7519(00)00124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen, C. O. (2014). Overview of tuberculosis and other mycobacterial Infections. The Merck Veterinary Manual. [Google Scholar]
- Thoen, C. , LoBue, P. , & De Kantor, I. (2006). The importance of Mycobacterium bovis as a zoonosis. Veterinary Microbiology, 112(2), 339–345. 10.1016/j.vetmic.2005.11.047 [DOI] [PubMed] [Google Scholar]
- Tuteja, F. , Patil, N. , Narnaware, S. , Nagarajan, G. , & Dahiya, S. (2013a). Camel dermal mycoses caused by dermatophytes. Journal of Camel Practice and Research, 20(2), 157–165. [Google Scholar]
- Tuteja, F. , Patil, N. , Narnaware, S. , Nagarajan, G. , & Dahiya, S. (2013b). Primarily human pathogenic fungi causing dermatophytosis in camel. Journal of Camel Practice and Research, 20(2), 151–155. [Google Scholar]
- Valinezhad, A. , & Oryan, A. (2017). Prevalance of hydati in camels slaughtered in North East of Iran. Animal Science Reasearch Journal, 6(22), 15–20. [Google Scholar]
- Vandenbroucke, J. P. , von Elm, E. , Altman, D. G. , Gotzsche, P. C. , Mulrow, C. D. , Pocock, S. J. , … Egger, M. (2007). Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Annals of Internal Medicine, 147(8), W163–W194. 10.1097/EDE.0b013e3181577511 [DOI] [PubMed] [Google Scholar]
- Vena, G. A. , Chieco, P. , Posa, F. , Garofalo, A. , Bosco, A. , & Cassano, N. (2012). Epidemiology of dermatophytoses: Retrospective analysis from 2005 to 2010 and comparison with previous data from 1975. Microbiologica‐Quarterly Journal of Microbiological Sciences, 35(2), 207. [PubMed] [Google Scholar]
- Wernery, U. (2014). Camelid brucellosis: A review. Revue Scientifique Et Technique (International Office of Epizootics), 33(3), 839–857. 10.20506/rst.33.3.2322 [DOI] [PubMed] [Google Scholar]
- Wernery, U. , & Kaaden, O. R. (2002). Infectious diseases in camelids. Tokyo, Japan: Georg Thieme Verlag. [Google Scholar]
- Wernery, U. , Kaaden, O. , & Ali, M. (1997). Orthopox virus infections in dromedary camels in United Arab Emirates (UAE) during winter season. Journal of Camel Practice and Research, 4(1), 51–55. [Google Scholar]
- Wernery, U. , & Kinne, J. (2012). Tuberculosis in camelids: A review. Revue Scientifique Et Technique (International Office of Epizootics), 31(3), 899–906. 10.20506/rst.31.3.2161 [DOI] [PubMed] [Google Scholar]
- Wernery, U. , Kinne, J. , Schuster, R. K. (2014). Camelid infectious disorders. Paris, France: OIE; (World Organisation for Animal Health). [Google Scholar]
- Wernery, U. , Ul‐Haq, A. , Joseph, M. , & Kinne, J. (2004). Tetanus in a camel (Camelus dromedarius) – A case report. Tropical Animal Health and Production, 36(3), 217–224. 10.1023/B:TROP.0000016835.02928.28 [DOI] [PubMed] [Google Scholar]
- Woo, P. C. Y. , Lau, S. K. P. , Teng, J. L. L. , Tsang, A. K. L. , Joseph, M. , Wong, E. Y. M. , … Yuen, K.‐Y. (2014). New hepatitis E virus genotype in camels, the Middle East. Emerging Infectious Diseases, 20(6), 1044–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamnikova, S. S. , Mandler, J. , Bekh‐Ochir, Z. H. , Dachtzeren, P. , Ludwig, S. , Lvov, D. K. , & Scholtissek, C. (1993). A reassortant H1N1 influenza a virus caused fatal epizootics among camels in mongolia. Virology, 197(2), 558–563. 10.1006/viro.1993.1629 [DOI] [PubMed] [Google Scholar]
- Yasutomo, A. , & Kazunari, K. (2005). Pasteurellosis as zoonosis. Internal Medicine, 44(7), 692–693. 10.2169/internalmedicine.44.692 [DOI] [PubMed] [Google Scholar]
- Yavari, P. (2013). Epidemiology textbook on prevalent disease in Iran. Tehran, Iran: Gap Publication. [Google Scholar]
- Younan, M. , & Gluecks, I. (2007). Clostridium perfringens type B enterotoxaemia in a Kenyan camel. Journal of Camel Practice and Research, 14(1), 65. [Google Scholar]
- Zavarshani, M. , Kownani, M. , Estabraghi, E. , & Yarahmadi, E. (2015). Detection Salmonella spp. carriers in camel using polymerase chain reaction and cultural methods. Biological Forum, Research Trend. [Google Scholar]
- Zowghi, E. , & Ebadi, A. (1991). "Brucellosis in camels in Iran. Razi State Vaccine and Serum Institute, 40(2), 111–115. [Google Scholar]


