Abstract
BACKGROUND
The social environment shapes human health, producing strong relationships between social factors, disease risk, and survival. The strength of these links has drawn attention from researchers in both the social and natural sciences, who share common interests in the biological processes that link the social environment to disease outcomes and mortality risk. Social scientists are motivated by an interest in contributing to policy that improves human health. Evolutionary biologists are interested in the origins of sociality and the determinants of Darwinian fitness. These research agendas have now converged to demonstrate strong parallels between the consequences of social adversity in human populations and in other social mammals, at least for the social processes that are most analogous between species. At the same time, recent studies in experimental animal models confirm that socially induced stress is, by itself, sufficient to negatively affect health and shorten life span. These findings suggest that some aspects of the social determinants of health—especially those that can be modeled through studies of direct social interaction in nonhuman animals— have deep evolutionary roots. They also present new opportunities for studying the emergence of social disparities in health and mortality risk.
ADVANCES
The relationship between the social environment and mortality risk has been known in humans for some time, but studies in other social mammals have only recently been able to test for the same general phenomenon. These studies reveal that measures of social integration, social support, and, to a lesser extent, social status independently predict life span in at least four different mammalian orders. Despite key differences in the factors that structure the social environment in humans and other animals, the effect sizes that relate social status and social integration to natural life span in other mammals align with those estimated for social environmental effects in humans. Also like humans, multiple distinct measures of social integration have predictive value, and in the taxa examined thus far, social adversity in early life is particularly tightly linked to later-life survival.
Animal models have also been key to advancing our understanding of the causal links between social processes and health. Studies in laboratory animals indicate that socially induced stress has direct effects on immune function, disease susceptibility, and life span. Animal models have revealed pervasive changes in the response to social adversity that are detectable at the molecular level. Recent work a. in mice has also shown that socially induced stress shortens natural life spans owing to multiple causes, including atherosclerosis. This result echoes those in humans, in which social adversity predicts increased mortality risk from almost all major causes of death.
OUTLOOK
Although not all facets of the social determinants of health in humans can be effectively modeled in other social mammals, the strong evidence that some of these determinants are shared argues that comparative studies should play a frontline role in the effort to understand them. Expanding the set of species studied in nature, as well as the range of human populations in which the social environment is well characterized, should be a priority. Such studies have high potential to shed light on the pathways that connect social experience to life course outcomes as well as the evolutionary logic that accounts for these effects. Studies that draw on the power and tools afforded by laboratory model organisms are also crucial because of their potential for identifying causal links. Important research directions include understanding the predictors of interindividual and intersocietal differences in response to social adversity, testing the efficacy of potential interventions, and extending research on the physiological signatures of social gradients to the brain and other tissues. Path-breaking studies in this area will not only integrate results from different disciplines but also involve cross-disciplinary efforts that begin at study conception and design.
The social environment, both in early life and adulthood, is one of the strongest predictors of morbidity and mortality risk in humans. Evidence from long-term studies of other social mammals indicates that this relationship is similar across many species. In addition, experimental studies show that social interactions can causally alter animal physiology, disease risk, and life span itself. These findings highlight the importance of the social environment to health and mortality as well as Darwinian fitness—outcomes of interest to social scientists and biologists alike. They thus emphasize the utility of cross-species analysis for understanding the predictors of, and mechanisms underlying, social gradients in health.
Graphical Abstract
A comparative perspective on the social determinants of health. Social adversity is closely linked to health and mortality outcomes in humans, across the life course. These observations have recently been extended to other social mammals, in which social integration, social status, and early-life adversity have been shown to predict natural life spans in wild populations and molecular, physiological, and disease outcomes in experimental animal models.
In social mammals, including our own species, social conditions powerfully shape the environment that individuals experience from day to day. Adverse social experiences, in particular, elicit biological responses across social species that influence health and aging across the life span (1). It is therefore unsurprising that dimensions of the social environment—particularly measures of socioeconomic status, social integration, and early-life adversity—are among the strongest and most consistent predictors of health and survival outcomes (Fig. 1). For example, differences in socioeconomic status in the United States (as measured by income) can translate to differences of a decade or more of life expectancy (2), and low occupational status translates to ~2 years of reduced life span across seven high-income countries (3). Similarly, low social integration predicts a ~50% increase in all-cause mortality risk in humans, an effect that rivals or exceeds mortality risk associated with obesity, alcoholism, moderate smoking, or sedentary living (4).
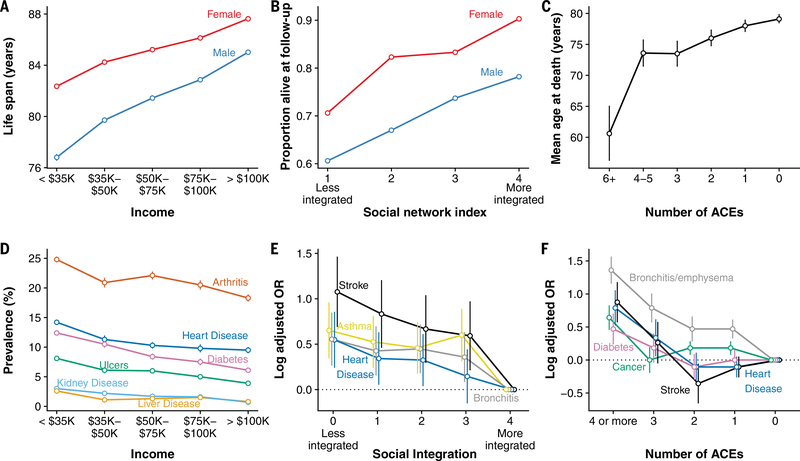
Fig. 1. Social adversity predicts morbidity and mortality in humans.
(A to F) The largest data sets on the health correlates of social adversity come from human populations. Together, they demonstrate that high social adversity is a major predictor of [(A) to (C)] life expectancy and [(D) to (F)] susceptibility to a broad range of diseases. (A) Expected life span at age 40 for men and women in the United States as a function of income at age 40 (n = 1.4 billion person-years) (2). (B) Proportion of study subjects alive after a 9-year follow up, for adult men and women in Alameda County, California, as a function of a composite index of social relationships (n = 6298 individuals) (46). (C) Mean age at death as a function of early adversity in the ACEs study on adult patients at the Kaiser Permanente San Diego Health Appraisal Clinic (n = 17,337 individuals, n = 1539 who had died by follow up) (173). (D) Disease prevalence among adult Americans by income based on the 2015 Centers for Disease Control National Health Interview Survey (n = 242,501 individuals) (174). (E) Disease risk (log odds ratios adjusted for age, sex, and race) as a function of a composite measure of social integration for adult men and women in the United States in the National Health and Nutrition Examination Survey III (n = 18,716 individuals) (31). (F) Disease risk (log odds ratios adjusted for age, sex, race, and educational attainment) by number of ACEs for patients visiting Kaiser Permanente’s San Diego Health Appraisal Clinic (n = 9508 individuals) (9).
These observations raise a natural question: What are the biological processes that account for the strong association between the social environment, disease, and mortality risk? This question is relevant to improving disease prediction, prevention, and targeting interventions; understanding the causes and consequences of social inequality; and investigating the evolution of social group living and its relevance to health. It is also timely. In the past two decades, socioeconomic disparities in mortality have become steeper in the United States (5,6). Aging populations have also highlighted the negative effects of social isolation in the elderly (7,8); in response, the United Kingdom appointed its first Minister of Loneliness in 2018, and the World Health Organization has launched initiatives to focus attention on the social determinants of health. Prospective studies have placed early-life conditions at the root of some of these observations (9,10). The increasing concern about social disparities in health indicates that the current array of measures being used to study and mitigate social gradients are incomplete. Understanding the biology underlying social environmental effects on health—especially physiological changes that precede disease itself—promises to provide new opportunities for effective intervention.
Addressing this question has been challenging for at least two reasons. First, considerable evidence, drawn almost entirely from animal models, supports the hypothesis that social interactions directly affect health outcomes (the “social causation” hypothesis) (11, 12). However, social gradients in human health can also be explained by other environmental mediators (such as diet, smoking, and health care access) (13–15), and in some cases, poor health can cause individuals to experience more adverse social exposures (“health selection”). In many studies of humans, including some that have been foundational for characterizing the effects of social adversity, considerable uncertainty surrounds the relative contribution of social causation versus health selection (14, 16–19). This challenge arises because experimental studies of exposure to many sources of social adversity are nearly impossible in humans. The problem is further compounded by the absence of information about social and biological conditions before the start of many key studies and by the interdependence between social gradients and health over time. Longitudinal datasets that include baseline measures partially address these challenges (16, 20, 21) but still cannot unambiguously disentangle causal pathways because of the difficulty of excluding the effects of correlated or confounding variables (such as time-varying confounders) (6, 22). However, some quasi-experimental studies have found that modest increases in measures of socioeconomic status (income and/or neighborhood conditions) can positively affect physical and mental health, emphasizing the need for further study (23–25).
Second, associations between the social environment and health pose a challenge to typical strategies for studying the biological mechanisms of disease. Social adversity is linked to a remarkably broad set of conditions, including diseases as distinct as tuberculosis, diabetes, cardiovascular disease, and cancer (Fig. 1, D to F). The fact that so many different physiological systems are socially patterned makes choosing an appropriate animal, tissue, or cellular model difficult. This problem is further complicated by the fact that studies of the social environment minimally require social interaction in groups or communities, meaning that social cues cannot be readily modeled in individually housed organisms or cell lines. Even assuming a social causation model, the health consequences of social adversity fit poorly into classical host-agent-environment models, which represent the typical biological approach to studying disease causation (26,27). Studies have instead tended to discuss the social environment in terms of a general “predisposing risk factor,” “social exposure,” or source of “accumulated wear and tear” (28–30). These are useful conceptual models but provide little guidance for traditional studies of biological mechanisms.
Thus, despite broad interest in the biological correlates and consequences of social adversity, the mechanisms, processes, and pathways through which they arise have remained unclear. However, new evidence has been crucial for moving toward clarity on these questions. First, research in other social mammals indicate that social gradients in human health are part of a long evolutionary legacy of social living, at least at the level of local social interactions among coresident individuals (Figs. 2 and 3 and Box 1). These findings suggest that the consequences of social adversity transcend the effects of the modern human environment and point to evolutionary comparative studies as a source of important insight. Second, emerging data sets, especially controlled experimental studies in other social mammals, strongly support direct social environmental effects on physiological function (social causation). Together with the release of unprecedentedly large, integrated data sets from human populations (2, 3, 31), these findings lay the groundwork for understanding how social adversity makes us vulnerable.
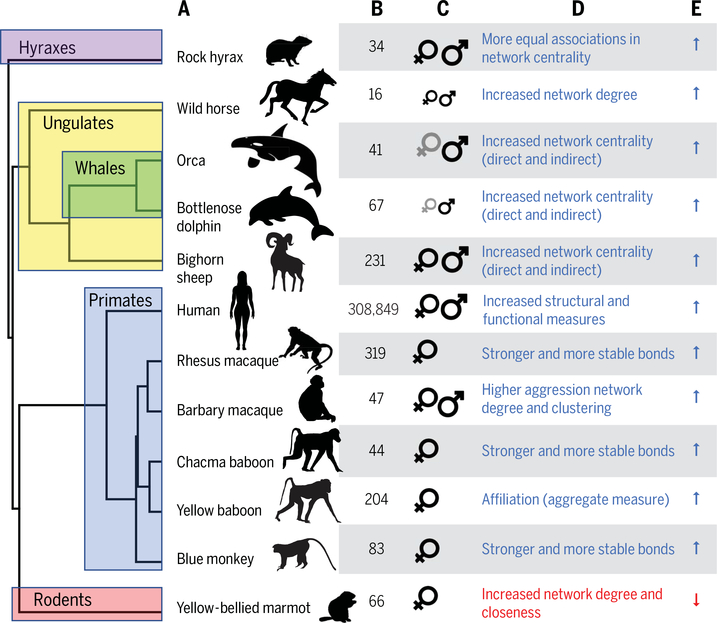
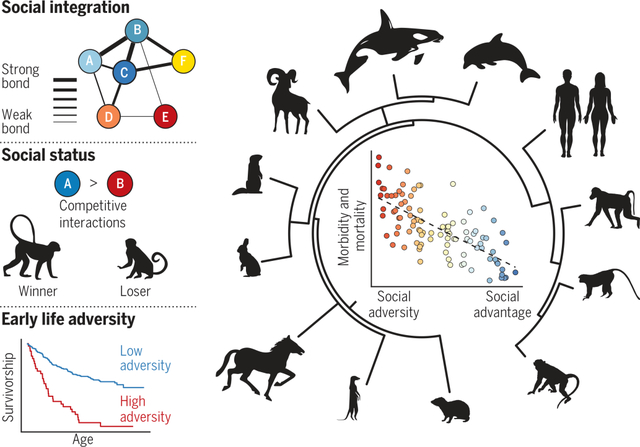
Fig. 2. Social integration and survival in wild social mammals.
All cases shown are based on data from natural populations, with the exception of rhesus macaques (65), for which data are from a provisioned free-ranging population. (A) The social integration-survival relationship has been evaluated in at least 12 species, including humans, which together represent multiple independent transitions to social group living (55). The mammal supertree is from (175). (B) Sample sizes and (C) sex studied. Large symbols indicate adults; small symbols indicate juveniles. Sample size for humans is based on a meta-analysis of 148 studies. Where both sexes were investigated, significant results are shown in black and nonsignificant results in gray. (D) Measure of social integration tested. (E) Direction of the observed effect. Blue arrows correspond to improved survival with greater integration and support; red arrow corresponds to reduced survival with greater integration and support. For Barbary macaques, affiliative networks were unrelated to survival; for orca, social integration predicted survival in males only in limited resource years. We excluded several studies of wild mammals that focused on social group size as the measure of social support and integration [cheetahs (176), wolves (177), voles (178), and bats (179)] because the effects of social factors cannot be disentangled from the effects of other density-dependent factors (such as degree of resource competition and between-group competition). Data are from the following sources: rock hyrax, (180); wild horse, (50); orca, (61); bottlenose dolphin, (49); bighorn sheep, (60); human, (4); rhesus macaque, (65); Barbary macaque, (181); chacma baboon, (47); yellow baboon, (48); blue monkey, (54); yellow-bellied marmot, (53).
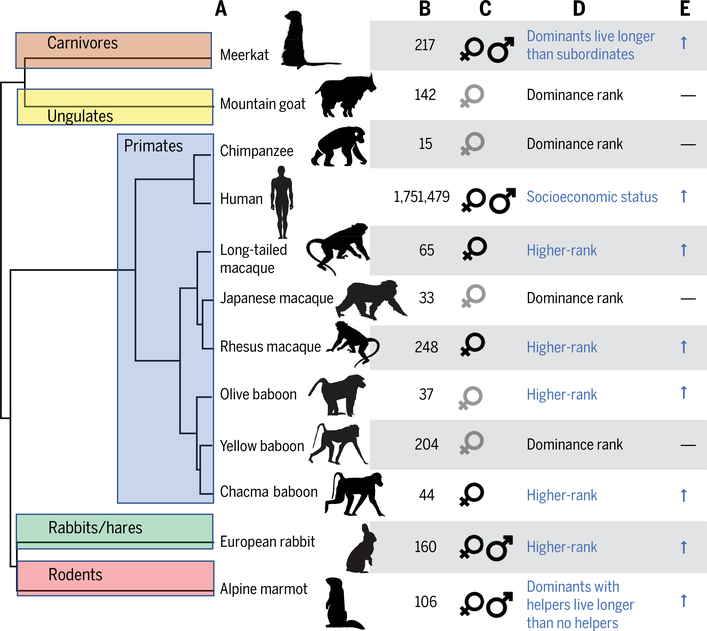
Fig. 3. Social status and survival in wild social mammals.
All cases shown are based on data from natural populations. (A) The social status-survival relationship has been evaluated in at least 12 species, including humans, which together represent multiple transitions from solitary to social living (in carnivores, even-toed ungulates, primates, rabbits and hares, and rodents) (55). The mammal supertree is from (175), with modifications based on (182). (B) Sample sizes and (C) sex studied. Sample size for humans is based on a meta-analysis of 48 studies. Where both sexes were investigated, significant results are shown in black and nonsignificant results in gray. (D) Measure of social status tested. (E) Direction of the observed effect. Blue arrows correspond to improved survival with higher social status or rank; dashes correspond to no relationship between survival and social status or rank, as reported based on the authors’ threshold for statistical significance. Data are from the following sources: meerkat, (79); mountain goat, (183); chimpanzee, (184); human, (3); long-tailed macaque, (82); Japanese macaque, (185); rhesus macaque, (81); olive baboon, (186); yellow baboon, (47); chacma baboon, (80); European rabbit, (78); alpine marmot, (187).
Box 1. Multiple pathways link social factors to health: Evidence from nonhuman primates.
In humans, the social environment is influenced by a complex set of factors, including income, education, occupation, social prestige, and larger cultural and institutional structures. As defined by the World Health Organization, the social determinants of health are “shaped by the distribution of money, power and resources at global, national and local levels” (188). Social status and social integration also intersect with, and can be influenced by, other social identities, such as race, ethnicity, and gender. By comparison, social environments in nonhuman animals are much simpler and are best studied-and probably most relevant to health, reproduction, and survival-at the local level, where coresident individuals directly interact. Social hierarchies can thus often be summarized by using singledimensional measures (189).
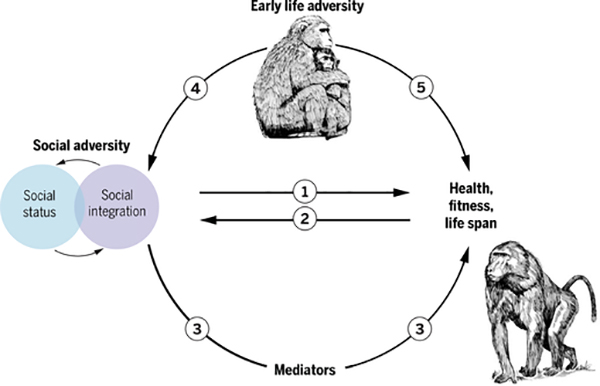
Nevertheless, as in humans, multiple pathways connect social factors to health and Darwinian fitness in other animals. Several of these pathways are analogous to those developed for human populations (16,190–192). Social causation (Fig. 4, arrow 1) is strongly supported by studies that manipulate exposure to chronic social stress while holding other aspects of the environment constant (131,132). By contrast, in species for which social status is determined by physical competition, changes in body condition and physiological measures of endocrine and immune function can precede changes in status (“health selection”) (Fig. 4, arrow 2) (84,193,194). Social environmental links to life span can also be mediated through other environmental exposures (Fig. 4, arrow 3). For example, by influencing huddling behavior, social integration affects winter thermoregulation in Barbary macaques (195). Last, early-life adversity can generate social gradients in adulthood (Fig. 4, arrows 4 and 5). In wild female baboons, for example, early maternal loss predicts reduced social integration in adulthood, lower-than-expected adult social status, and shortened life span (85,99).
As in humans, social status and social relationships can also be interrelated in complex ways (Fig. 4, blue and purple circles). Social status can be relatively independent from social integration, as is the case among wild female baboons (48). Alternatively, social status can structure affiliative social relationships (48, 196, 197); in these cases, high status usually predicts increased social integration, and evidence from captive primates indicates that the effects of status on health-related outcomes may be mediated in part by a path through increased integration (131). Last, developing supportive social relationships can predict subsequent changes in social status. For example, male Assamese macaques that formed stronger social bonds with other males subsequently rose in the dominance hierarchy and also fathered more young (198).
Fig. 4.
Pathways linking social factors to health in nonhuman primates.
Here, we review key themes emerging from this evidence, with an emphasis on recent work that highlights the role of social experience across the life course and findings of shared interest across disciplines. Because this intersection necessarily links to multiple fields, we do not attempt to summarize the full scope of research on either the social determinants of health in humans (which also involve socioeconomic structures not applicable to animal models) or the fitness consequences of social behavior in humans and other animals; instead, we refer readers to excellent reviews, with a within-discipline focus here (6,11,32–36). Our goal in this Review is to emphasize emerging parallels and insights from studies of social mammals, in the context of observations initially made in human populations. We focus on social mammals—particularly those that obligately live in groups—because of their close evolutionary relationship to humans. However, social environmental effects on health and fit ness have also been of interest in other species, especially birds and social insects (37). The degree to which these more distantly related species can be used to understand the social determinants of health in humans remains an important question for future work.
Social adversity and mortality in social mammals
In the social sciences, research on the social determinants of health is motivated by an interest in contributing to policy that reduces health disparities and improves human health span, life span, or life expectancy. This work has a long tradition; social gradients have been described in the sociological literature for at least 120 years (38). In parallel, evolutionary biologists and behavioral ecologists study social interactions with an eye toward understanding the origins of sociality and its consequences for reproductive fitness. This research program is also old; Darwin himself puzzled over the adaptive value of social behavior (39), which is thought to have imparted strong enough selective pressure to drive major morphological and physiological innovations, including advanced cognitive abilities in humans and other primates (40, 41).
Over the past decade, the historically distinct agendas of social science and evolutionary biology have begun to converge. In particular, several long-term studies in wild social mammals now contain enough data to support full life course analyses and have revealed unexpectedly strong links between the social environment and mortality risk that parallel those from long-term studies in humans (Figs. 2 and 3). These findings simultaneously connect to the motivating questions for evolutionary biologists—life span is often the most important predictor of Darwinian fitness (reproductive success, the determinant of an individual genome’s representation in future generations) in long-lived mammals (42)—and place observations in humans on a biological continuum with other species. Together, they illustrate several patterns that consistently shape social gradients in humans and other social mammals and provide crucial justification for studying the biology of social gradients in other species.
Below, we review the evidence for this convergence in connection with three dimensions of the social environment: (i) social integration, defined as an individual’s ability to invest in and maintain affiliative or supportive interactions (whether shaped by intrinsic ability or by the constraints of its environment) (43); (ii) social status, a construct that captures stable or semistable differences in access to resources, whether material (such as food, health care or access to mates) or otherwise (such as psychological capital or social support); and (iii) early-life adversity, with an emphasis on social and familial adversity that occurs during sensitive periods in development. In animals, all three dimensions are captured through observations of direct social interactions. This is an important point of divergence from human studies, in which researchers often measure engagement in larger social, cultural, and economic structures that can knit individuals into a shared socioeconomic framework, even if they never meet. Such structures do not have a clear parallel in animal models; for example, it is difficult to put animals from different geographic locations on a single status scale because they do not interact (although it is possible to ask whether relatively low-status animals in different groups do worse on average, and some researchers have investigated the relative “status” of distinct social groups in relation to one another) (44). The relative simplicity of nonhuman animal societies is thus both an advantage—it rules out some potential confounders and causal pathways that complicate interpretation in humans—and a limitation, as not all aspects of the social determinants of health can be effectively modeled in nonhuman animals. Nevertheless, as in humans, multiple, nonmutually exclusive pathways link social factors to each other and to health and fitness outcomes (Box 1).
Social integration and survival
In humans, the evidence for a link between social isolation and mortality risk is extensive and remarkably consistent across geographically, temporally, and socioeconomically diverse populations (although the current data are largely limited to societies in the developed world) (4, 45). The earliest population-based studies to investigate this relationship estimated that social integration increased the odds of survival by 30 to 80% (odds ratio between 1.3 and 1.8) (46). Recent meta-analyses have included several orders of magnitude more study subjects but nevertheless encompass these original values, with odds ratios ranging from 1.19 to 1.91 depending on measurement approach and inclusion criteria (4, 8).
Emerging results from wild mammals are strikingly similar to those in humans. The first wild animal study to demonstrate a relationship between individual-based measures of social integration and adult survival, in wild baboons, was published a decade ago (47). Since then, similar results have been reported for a variety of other social mammals, including independent replication in a second population of baboons (Fig. 2) (48). In some species, juvenile survival may also be linked to the ability to socially integrate into mixed-age social groups (49,50). An important caveat to these studies is that some are based on very small sample sizes, others do not control for group size or population density (which could affect survival through mechanisms other than the opportunity for affiliative social interactions) (51), and the direction of causation cannot be easily determined. Further, a few exceptions stand out. For example, in yellow- bellied marmots, females who were more well- integrated into a social network died earlier; this difference from other social mammals may be linked to the fact that social group living is not obligate in this species, unlike the others that have been studied (52,53). In other cases, the results depend on specific measures of social integration: In blue monkeys, females who maintained strong and consistent social bonds with the same partners lived longest, but those with strong and inconsistent bonds fared the worst (54). Thus, caution should be exercised in painting a homogeneous picture across all social mammals. Nonetheless, the pattern of greater survival with greater social integration appears relatively consistent in studies of wild mammals thus far and is remarkably close to the effect sizes in humans, with odds ratios in the range of 1.23 to 1.72 (Fig. 2). These studies include representatives from five mammalian orders and capture multiple independent evolutionary transitions to social living (in primates, rodents, odd-toed ungulates, even-toed ungulates, and hyracoids or their ancestors) (55). These observations suggest a convergent relationship between affiliative social interactions and survival that is detectable across tens of millions of years of evolutionary time.
In keeping with studies in humans, this pattern is evident despite substantial variation in measurement approaches. Although all measures are based on direct observation of social interactions, some have relied on social network analyses of affiliative interactions or proximity to “neighbors,” whereas others have focused on pairwise interactions (such as bond strength, consistency, or the relative frequency of interactions). These studies represent a mix of what are called, in studies of humans, “structural” measures (such as the number of social ties or the position of an individual in a network) and “functional” measures (such as the extent to which social ties provide particular resources, including perceived social support in humans). In humans, structural and functional measures are only moderately correlated with each other but have similar associations with survival, and multidimensional measures make the best predictors (4,43,56,57). No study has yet examined the relationship between structural and functional measures in wild mammals, although both types of data have been analyzed. For example, the thermoregulatory benefit of social huddling in Barbary macaques (58) and vervet monkeys (59) is a functional measure; network centrality in bighorn sheep (60) and orcas (61) is a structural measure (centrality is a measure of the contribution an individual makes to a social network’s overall connectivity) (62). However, several studies indicate that measures of affiliative social relationships vary in predictive power (63, 64), and a recent comparative analysis in rhesus macaques points to the particular importance of bond strength and consistency, as opposed to affiliative behavior (such as grooming) per se (65). As the number and power of available studies grow, comparisons of structural and functional measures across species should further refine the aspects of social integration that are most consistently important.
Social status and survival
Like social integration and support, the overall link between socioeconomic status and survival rates in human populations is well established and cuts across cultural and national boundaries (66, 67). The earliest data on this phenomenon, from the United Kingdom starting in 1931, showed that the risk of death from heart disease was more than twofold higher for men in the lowest social class than in the highest (68). Fifty years later, the Whitehall studies of British civil servants revealed more than a threefold difference among white-collar British workers (69). Today, we know that low socioeconomic status is linked to increased mortality risk from nearly all causes, including chronic disease and infectious disease as well as accidents and violent death (Fig. 1) (2, 34, 66, 70, 71). The consistency of this relationship over time and space has motivated some researchers to label socioeconomic status inequalities as a “fundamental cause” of disease (28).
Social status in other social mammals is much simpler. Hierarchies do not extend beyond the members of a coresident social group or population, and a single measure of status—typically dominance rank, which is commonly defined as the ability to win social conflicts or to displace conspecifics from resources (1)—is usually sufficient to capture stable differences in resource access (although within species, dominance rank can be sex specific). However, in other social mammals, too, social status is often linked to survival and can predict physiological differences that strongly parallel those observed in humans (32, 33, 72–74). Despite long-standing interest in its causes and consequences, the relationship between social status and fertility has been more intensively studied than its relationship with survival (75–77), and the literature on social status and life span remains somewhat biased toward long-term studies of primates. Nonetheless, results are generally consistent with those observed in humans; to date, studies of wild rab bits (78), meerkats (79), baboons (47,80), rhesus macaques (81), and long-tailed (cynomolgus) macaques (82) all show a survival advantage to higher social rank (although not always in a linear fashion).
As with studies of social integration and survival, comparative analyses may help identify factors that influence the link between social status and survival. For physiological outcomes, comparative studies in animals already emphasize that the costs of low status are moderated by social context. Low-status animals tend to exhibit higher levels of stress- associated glucocorticoid hormones when they belong to strictly enforced hierarchies and lack access to social support (83), suggesting that social status and social integration may have interrelated effects on health outcomes (Box 1). One study of wild female baboons showed that social status did not directly predict survival, but social affiliation did. However, higherranking females were more socially affiliated to males, suggesting an indirect effect of social status on survival (48). The survival advantage for dominant meerkats is also explained by the effects of social status on social integration: Subordinates were less well-integrated into the group and hence more exposed to extrinsic mortality risks, such as predators (79). Last, studies in social mammals highlight how variation in the nature of social status attainment and maintenance can produce distinct biological outcomes (84). For example, some hierarchies are determined by physical strength and are therefore dynamic over time (such as male baboons and male red deer), whereas others are largely determined by the social status of close kin (such as female baboons and female spotted hyenas). In the latter case, hierarchies can persist over multiple generations (85,86), providing what is perhaps the closest nonhuman analog to structurally embedded social hierarchies in humans.
The long-term effects of early-life adversity
Early development is a period of substantial sensitivity to environmental adversity, including social as well as physical hardship. In humans, extensive evidence supports a relationship between social adversity in early-life and later-life health outcomes, including reproductive timing, cardiovascular disease, viral infection, and premature mortality (87–90). For example, low socioeconomic status in early life is associated with a more than twofold increased probability of early-onset coronary heart disease, even among study subjects who achieved high socioeconomic status as adults (91). Similarly, racial and ethnic minorities who climb the social ladder to higher status in young adulthood nevertheless experience early adversity-associated costs to health (92–95). Such observations suggest that the social roots of later-life health gradients can be established many years earlier and may be refractory to later life change, perhaps because of biological embedding (30).
Early-life effects are also well studied in other animals [including in many nonmammals (96, 97)]. However, although the early- life social environment has long been linked to physiological, growth-associated, and cognitive traits (98), its relationship to adult health and survival—especially after a long intervening period—has only recently been investigated in natural populations. In the first study in animals to use the adverse childhood experiences (ACEs) framework, which tallies the number of discrete insults experienced early in life (an ACE represents a potentially traumatic or developmentally disruptive environmental exposure in early life, such as physical abuse or familial separation in humans), yellow baboon females who experienced more early- life adversity were shown to experience substantially shorter life spans (99). Females who experienced three or more major insults (of six studied, including low social status, maternal social isolation, maternal loss, high resource competition, a short interval until the birth of a younger sibling, and early-life drought) died approximately a decade earlier than those who experienced none, an effect size even larger than those documented in human populations (Fig. 1F). Most of the sources of early adversity had a social component, and the two with the largest predictive effects—maternal loss and the birth of a close-in-age younger sibling— specifically point to the importance of mothers as a source of early-life social support. Recent work in wild spotted hyenas, a highly social carnivore, corroborates these findings (100). In hyenas, a cumulative adversity index incorporating maternal social status, maternal loss in the infant-juvenile period, and an animal’s own deviation from expected social status early in life also strongly predicts life span, again on a time scale of years.
These results both fit with and enrich models of early adversity developed for human populations that attempt to account for ACEs- related results (101). For example, consistent with the accumulation of risks model (102,103), they indicate that sequential deleterious exposures combine to have especially negative effects. However, although sources of early adversity in humans are often correlated—for example, children living in poverty are also likely to live in households with a missing parent (104)—in wild animal populations, correlations between different sources of adversity may be weak or absent altogether (99). This structure facilitates examination of the cumulative effects of early adverse experiences as well as discrimination between the effects of individual exposures. In some cases, longitudinal animal studies can also provide data to test the sensitive-period hypothesis, which posits that early-life social adversity affects later-life health in a manner that is only partially modifiable by later-life experience (105). Strong tests of this hypothesis are difficult to conduct in humans because exposure to early adversity tends to be correlated with later-life exposure to adversity (for example, because of limited social mobility) (106). In animal societies, however, social conditions in adulthood are not always well predicted by social conditions in early life or intergenerationally (99). This decoupling has been leveraged in baboons to show that early adversity in one generation predicts reduced juvenile survival in the next, independently of the juvenile’s own early-life experience (107).
Last, studies in animals support the hypothesis that the effects of early adversity on life span among humans are not fully explained by health care access or health risk behaviors such as smoking, alcoholism, or illicit drug use (because these are distinctly human variables). Instead, these studies highlight alternative mechanisms with potential relevance to human studies. For example, female baboons who experienced high levels of early adversity also tend to be more socially isolated from other females later in life (99). In parallel, orphaned elephants have reduced social contact with high-quality social partners (mature adults) compared with nonorphans (108). Given the strong association between affiliative social relationships and mortality risk, these observations suggest that early social adversity may influence later-life outcomes in part through patterning social interactions in adulthood. Such a model is reminiscent of the pathway model proposed for humans: that childhood circumstances affect adult health risk indirectly by putting individuals on trajectories that structure future exposure to later adversity (87,109).
Biological pathways from social adversity to health
Cross-species comparisons thus suggest that social environments, both in early life and adulthood, are key determinants of life span variation in humans and other social mammals. These parallel findings point to opportunities to draw on data from other social mammals to address outstanding questions about the social determinants of health in humans. Animal models for social gradients in human health can (i) reduce the complexity of human social environments; (ii) open the door to prospective and intergenerational study designs that can be executed on a much faster time scale; and (iii) in some cases, allow for direct experimental manipulation and invasive sampling. Below, we focus on several emerging themes that draw on one or more of these features, beginning with links between social adversity and health across the life course, and then potential proximate (mechanistic) and ultimate (evolutionary) pathways that could account for these observations. Because the relevant literature is broad, we also point interested readers to taxon- and discipline-specific perspectives reviewed elsewhere (33,110–114).
Social adversity and health outcomes across the life course
Studies that relate the social environment to health in humans are larger, better replicated, and more representative than their counterparts in any other species (although studies that investigate non-Western nations are still lacking and may influence the types of social conditions classified as “adverse”). However, because they are largely correlational, questions about causal direction persist that can only be partially addressed by using longitudinal or cohort designs (115). One of the most important contributions of studies of social adversity in other social mammals therefore stems from their interpretive clarity, especially in cases in which the social environment itself can be manipulated in controlled experiments: the “gold standard” for inferring causality.
Such studies have long supported a role for social causation, not only for physiological changes that are precursors to disease but also for disease outcomes themselves. For example, low social status more than doubles the rate of coronary artery atherosclerosis and hyper- insulinemia in diet-controlled female long-tailed (cynomolgus) macaques (116,117). In males, low status and/or social instability also predicts increased prevalence of coronary artery stenosis, and low status (but not social instability) increases susceptibility to experimentally administered adenovirus (118,119). Relevant to cancer outcomes, lower levels of social reciprocity in female rats predict both earlier tumor onset and shortened life span, and social isolation leads to a 30-fold increase in primary tumor metastasis in mice (120). Thus, manipulation of the social environment in captivity recapitulates social gradients in the leading causes of death in humans, including heart disease, diabetes, and respiratory infections (121).
However, these studies have been short term, relying on genetically predisposed strains or environmental manipulations to accelerate disease outcomes. Only recently have animal studies attempted to model the pattern observed in humans: social gradients that lead to poorer health or elevated mortality from multiple causes, manifested over the life course. In one case, researchers aggregated almost a decade’s worth of data to demonstrate that rhesus macaques randomized into an early maternal loss treatment experienced poorer health later in life, despite standardized housing conditions in adulthood (122). Similarly broad effects have been observed in an experimental mouse model of social status. In the first study to investigate the consequences of chronic stress across natural life spans, persistent exposure to socially dominant animals was shown to shorten the median life spans of socially subordinate male mice by 12.4% (12), an effect size comparable with that of dietary restriction in the same strains (123). Low- status animals also experienced earlier onset of multiorgan lesions, including tumors. In a subset of 17-month-old mice, subordinates had elevated p53 and p16Ink4a markers of cellular senescence and, remarkably, 50% prevalence of early-stage atherosclerotic lesions, which generally occur only in genetically predisposed strains exposed to highly atherogenic diets. By contrast, no lesions were observed in dominant mice.
Replication of these findings will be crucial for assessing their generalizability. Nevertheless, they strongly support the idea that chronic social stress can be sufficiently toxic to explain multiple pathological outcomes, including accelerated senescence (124). In the mouse life span study, for instance, subordinates were housed in proximity to, but physically separated from, dominant mice (12). However, subordinates exhibited close to a twofold increase in glucocorticoid levels, suggesting that simple exposure to threat from an aggressive social partner can induce broad physiological changes (12).
Molecular signatures of social adversity
If social causation contributes to the relationship between social adversity, health, and mortality risk, what are the physiological and molecular changes that mediate this relationship? Efforts to identify these mechanisms have historically focused on neuroendocrine signaling, particularly the contribution of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (73,125). Experimental animal models generally support the idea that these pathways are altered by social adversity-induced stress (73,125,126), with some corroborating evidence from studies in wild mammals (83,127). However, the purpose of social adversity-associated changes in neuroendocrine signaling is to communicate a threat to, and regulate the return to, physiological homeostasis. To explain pathophysiology (for example, as a consequence of chronic signaling) (126), social adversity-associated changes must also lead to changes in target cells and tissues. Understanding how social adversity connects to molecular changes within the cell has become an increasing focus of research, building on a broader sociogenomics literature that shows that social interactions can substantially alter gene regulation (128–130).
Thus far, we know the most about social adversity and gene regulation in peripheral blood cells, which are the most commonly collected sample type in humans and other social mammals. These studies yield a rapidly developing picture of how social adversity causally alters the regulation of the immune system in experimental animal models (131,132). The most consistent finding from experimental manipulations of the social environment in nonhuman animals is that increased social adversity drives increased expression of genes linked to inflammation, including those that regulate, code for, or interact with biomarkers of chronic stress [such as interleukin-6 (IL6) and IL-1β]. These changes appear to be shaped by socially patterned differences in the use of immune defense-modulating transcription factors, especially nuclear factor κB (NFκB), a master regulator of inflammation (131). In animal models of early social adversity and social status, predicted DNA binding sites for NFκB are enriched near genes that are more transcriptionally active in socially stressed individuals (131). Further, in rhesus macaques, regions of the genome that are more physically accessible to transcription factor binding in low-status animals also tend to contain NFkB-binding sites (133). Because NFκB can be prevented from interacting with DNA through glucocorticoid signaling, this observation suggests a link between functional genomic studies and previous work on stress neuroendocrinology (125). Glucocorticoid resistance—a hallmark of chronic stress—is also associated with increased expression of proinflammatory transcription factors (126).
These patterns parallel those observed in research on social adversity in humans. Although studies in human populations are necessarily correlational, the animal-model work suggests that socially induced stressors are also likely to causally alter gene regulation and the HPA axis in our own species. A growing body of research supports a link between exposure to social adversity and DNA methylation and gene expression markers associated with glucocorticoid signaling and inflammation (134–136). If so, gene regulatory signatures of social adversity may be broadly conserved in social mammals (137). Because relatively few species have been studied at this point, this hypothesis requires data from a broader range of species to test; even in the species studied thus far, often only one sex has been well characterized. Nevertheless, it is notable that cross-species analyses of other aspects of social behavior, such as territorial aggression and social monogamy, have identified conserved roles for the same gene regulatory pathways in taxa as diverse as rodents, songbirds, frogs, and fish (128,129).
At the same time, social environmental effects on gene regulation are also context dependent. For example, in rhesus macaque females, the effects of experimentally manipulated social status are magnified in immune cells after exposure to lipopolysaccharide, which stimulates the innate immune response against bacteria (131). Consistent with correlative studies in humans, low-status animals up-regulate proinflammatory, NFκB-regulated pathways relative to high-status animals, whereas high status predicts higher expression of antiviral genes. This pattern has been interpreted as a stressmediated trade-off between antibacterial and antiviral defense (137). However, recent work indicates that this pattern is contingent on the local cellular environment: Key regulators of antiviral defense that are positively correlated with social status after exposure to bacterial compounds actually become negatively correlated with social status in the same animals, after challenge with a viral mimic (138). Such studies may provide a window into understanding why the effects of social adversity differ across settings and into the basis of cumulative risk and multiple hit models (102,103). However, they also caution against the idea that there is a simple map between social environmental effects on immune gene expression and differential susceptibility to specific pathogens.
Evolutionary frameworks for the social determinants of health
The studies above focus on the proximate physiological and molecular mechanisms that explain the social determinants of health. However, the congruence between findings in humans and observations in other social mammals not only suggests that nonhuman species can serve as effective models for humans but also that social gradients in health maybe coupled with the evolution of social living itself. Comparative studies can therefore also contribute by highlighting the evolutionary logic that explains social gradients (83,139,140). Such studies have already been key to understanding the evolutionary costs and benefits of transitions to group living (55,141,142).
Social gradients within species arise because social costs and benefits are not equally distributed across individuals coresiding in the same social group. Consistent, species-level differences in the steepness of social hierarchies and the stability of social bonds emerge from the need to resolve this tension, as discussed in a long history of comparative work on the emergence of egalitarian versus “despotic” animal societies (140,143). However, individuals are likely subject to additional selection for sensitivity to the quality of social relationships within social groups (144). For example, the concept of the “dominance behavioral system,” developed in evolutionary psychology, argues that humans and other social animals have evolved finely tuned biological sensors to evaluate their and others’ relative social status (145). In support of this argument, work in mice has identified specific sensory and neural substrates for assessing dominance and social integration (146–149). However, we know of no case to date in which the fitness consequences of variation in social sensitivity has been evaluated in a natural social mammal population. Doing so would require measuring interindividual differences in the response to a common social environment, accurately assessing the “appropriate” social response, and potentially measuring subjective social experience. The increasing availability of life course data from wild mammals as well as new methods for quantifying perceived social stress (such as in captive rhesus macaques) (150) may make such studies feasible in the near future.
By contrast, data from wild social mammals have already brought clarity to evolutionary hypotheses about the long-term health effects of early adversity. For example, an extensive body of theory has been developed to account for observations of such effects in humans (151–154). The most commonly invoked ideas focus on predictive adaptive responses (PARs), which propose that early-life effects evolved because natural selection favors organisms that tailor their later-life phenotype to the environmental cues they experience in early life. PAR models argue that it is the mismatch between early adverse conditions and later, more benign conditions that produces the adverse health effects of early adversity. However, because predictive models assume that early-life environmental cues must be reliable indicators of the later-life environment, theoretical work suggests that PARs are unlikely to evolve in long-lived species (155). In nonhuman animals, the best empirical support for PARs comes from short-lived species (156,157). By contrast, studies in long-lived mammals provide better support for an alternative set of models: developmental constraints (158–161). Developmental constraints models posit that early-life effects evolve because they allow immediate survival, at the expense of optimal development, even if they incur later-life costs; they are the result of natural selection on the ability to “make the best of a bad situation.” If so, individuals who experienced early adversity may perform quite poorly when faced with adverse environments in adulthood—a conclusion with substantially different intervention and policy implications than those of the PAR model.
Tests of more refined PAR models are ongoing (162,163). However, the above work already illustrates the value of studies in nonhuman species for testing evolutionary arguments relevant to social gradients in health (164,165). It also highlights the challenges in clearly discriminating adaptive from nonadaptive responses: Apparently costly responses to social adversity can be favored by natural selection if they are better than no phenotypic adjustment at all (166).
Conclusions and new directions
The available evidence indicates that social impacts on life span are a shared phenomenon across humans and other social mammals and that the health-related outcomes of social adversity in nonhuman animals parallel socially patterned pathologies in humans. To some degree, the mechanisms that underlie these observations are also similar across species: Social conditions that promote chronic stress also predict increased inflammation, HPA axis dysregulation, and changes to sympathetic nervous system signaling (126). These findings suggest a shared biology underlying the influence of social gradients and a coherent evolutionary logic for when these gradients tend to be shallower versus steeper—arguments that have been made in various forms over the years (32). Only recently, however, have they been supported by both experimental tests for causal outcomes and data on natural mortality, with correspondingly refined estimates from very large studies in humans (4,12,99,131).
A shared biology in turn suggests that integrating human and nonhuman animal studies can help address longstanding questions about the social determinants of health. Research at this interface should open several new opportunities. First, the findings outlined here argue that the social determinants of health should be of central interest to biologists as well as social scientists. This is not yet the case for many disciplines; for example, the field of genomics was recently taken to task for ignoring the literature on social gradients in health and, as a consequence, redefining health disparities in terms of population genetic diversity (a genetic explanation) instead of recognizing its fundamental origins in the social environment(167).Research with natural links to the social determinants of health has been similarly limited in other disciplines; for example, recent studies that compare genetic and nonheritable predictors of immune function consider age, sex, and past pathogen exposure as environmental factors but not the social environment (168).Broadening this perspective presents an opportunity to leverage new methodological advances to understand the causes and consequences of social gradients, including scope for potential intervention. Animal model studies may be ideal for testing proposed interventions because they ensure compliance and eliminate other confounding factors.
Second, the parallels between studies highlight untapped opportunities to translate biological outcome measures across fields, especially molecular and physiological markers of social adversity and health. One important gap to fill involves the fact that nearly all of the evidence that social adversity compromises natural life span in social mammals comes from natural populations. By contrast, the best evidence for social causation of specific physiological or health outcomes comes from laboratory studies. Demonstrating that such findings are not artifacts of captivity— for example, by translating these outcome measures to natural populations—is crucial for understanding whether the relationship between social adversity and life span in nature can be explained, at least in part, by the mechanisms being identified in experimental studies. For example, although the prevailing model for social causation in laboratory studies invokes exposure to chronic social stress, some researchers have argued that animals in their natural environments are unlikely to experience chronic stress, or at least not to the degree that it could shorten life span (169).
Last, researchers must expand the set of study systems to other species and tissue types (especially the brain) and to a more diverse set of human populations. Increased diversity will help reveal how variation in social gradients emerge. For example, differences in the routes through which status is attained, the steepness and regularity of hierarchy enforcement, and the availability of coping outlets have all been proposed to modify the severity of social gradients (1, 32,139). In humans and at least six other primates, increased life span equality is positively correlated with increased life expectancy overall, in support of the idea that members of more egalitarian groups tend to have longer survival (170,171). In some species, the canonical direction of social gradients may also be reversed. In species in which competition for high status is energetically demanding, as it is in hierarchies that are based on physical competition (83,127), high-ranking individuals have been shown to exhibit higher glucocorticoid levels, up-regulate inflammation-related pathways, and experience accelerated “biological aging” (based on telomere shortening and epigenetic clock prediction) (79, 84,127,172). Such results stress that different types of social systems can produce different kinds of gradients. Understanding why—for example, by use of evolutionary comparative methods across species—may suggest ways to decouple social environmental variation from its negative health consequences in humans.
ACKNOWLEDGMENTS
We thank F. Florey Eischen for her invaluable support in organizing this working group, M. Shanahan and J. Buher Kane for their contributions to early discussions, and three anonymous reviewers for constructive feedback on early versions of this manuscript.
Funding: The Triangle Area Social and Biological Determinants of Health Working Group is supported by the Duke Center for Population Health and Aging (with funding from NIH P30AG034424), the Carolina Population Center (with funding from NIH P2C HD050924), the Triangle Center for Evolutionary Medicine, and the Duke Social Sciences Research Institute. This work was also supported by NIH grants R01AG057235, R01HD088558, and R01GM102562 to J.T.; R00AG051764, R01AG060931, and T32AG000139 to N.S.-M.; R01HL087103 to C.A.S.; R01AG057800 to Y.C.Y; R24AG065172 to J.T., A.B., and K.M.H.; R01AG053308 and P01AG031719 to S.C.A.; P01HD031921 and R01HD087061 to K.M.H.; F32HD084117 to L.G.; R01DK102496 to A.B.; R01MD013349, R01MD011728, and T32HD091058 to A.E.A.; a Human Frontier Science Program Research Grant to J.T.; a Minnesota Partnership for Biotechnology and Medical Genomics 18.04 to A.B.; and a Jacobs Foundation Early Career Fellowship to D.W.B.; G.A.N. received support from NIH T32HD091058 and NIH 1K99AG062749-01A1, L.G. received support from NIH T32HD007168, J.R.B. received support from the Bridging Biodiversity and Conservation Science program at the University of Arizona, and G.A.N. and N.S.-M. received support from NIH T32AG000029.
Footnotes
Competing interests: Authors declare no competing interests.
REFERENCES AND NOTES
- 1.Sapolsky RM, Social status and health in humans and other animals. Annu. Rev. Anthropol. 33, 393–418 (2004). doi: 10.1146/annurev.anthro.33.070203.144000 [DOI] [Google Scholar]
- 2.Chetty R et al. , The association between income and life expectancy in the United States, 2001–2014. JAMA 315, 1750–1766 (2016). doi: 10.1001/jama.2016.4226; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stringhini S et al. , Socioeconomic status and the 25 * 25 risk factors as determinants of premature mortality: A multicohort study and meta-analysis of 1–7 million men and women. Lancet 389, 1229–1237 (2017). doi: 10.1016/S0140-6736(16)32380-7; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holt-Lunstad J, Smith TB, Layton JB, Social relationships and mortality risk: A meta-analytic review. PLOS Med 7, e1000316 (2010). doi: 10.1371/journal.pmed.1000316; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marmot M, Health equity in England: The Marmot review 10 years on. BMJ 368, m693 (2020). doi: 10.1136/bmj.m693; [DOI] [PubMed] [Google Scholar]
- 6.O’Rand AM, Lynch SM, Socioeconomic status, health, and mortality in aging populations, in Future Directions for the Demography of Aging, Hayward, Majmundar K, Eds. (National Academies Press, 2018), pp. 67–95. [PubMed] [Google Scholar]
- 7.Hawkley LC, Cacioppo JT, Loneliness matters: A theoretical and empirical review of consequences and mechanisms. Ann. Behav. Med. 40, 218–227 (2010). doi: 10.1007/s12160-010-9210-8; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D, Loneliness and social isolation as risk factors for mortality: A meta-analytic review. Perspect. Psychol. Sci. 10, 227–237 (2015). doi: 10.1177/1745691614568352; [DOI] [PubMed] [Google Scholar]
- 9.Felitti VJ et al. , Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 14, 245–258 (1998). doi: 10.1016/S0749-3797(98)00017-8; [DOI] [PubMed] [Google Scholar]
- 10.Yang YC, Schorpp K, Boen C, Johnson M, Harris KM, Socioeconomic status and biological risks for health and illness across the life course. J. Gerontol. B Psychol. Sci. Soc. Sci. 75, 613–624 (2020). doi: 10.1093/geronb/gby108; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shively CA, Wilson ME, Social Inequalities in Health in Nonhuman Primates: The Biology of the Gradient (Springer, 2016). [Google Scholar]
- 12.Razzoli M et al. , Social stress shortens lifespan in mice. Aging Cell 17, e12778 (2018). doi: 10.1111/acel.12778; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muennig P, Health selection vs. causation in the income gradient: What can we learn from graphical trends? J. Health Care Poor Underserved 19, 574–579 (2008). doi: 10.1353/hpu.0.0018; [DOI] [PubMed] [Google Scholar]
- 14.Kröger H, Pakpahan E, Hoffmann R, What causes health inequality? A systematic review on the relative importance of social causation and health selection. Eur. J. Public Health 25, 951–960 (2015). doi: 10.1093/eurpub/ckv111; [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann R, Kröger H, Geyer S, Social causation versus health selection in the life course: Does their relative importance differ by dimension of SES? Soc. Indic. Res. 141, 1341–1367 (2019). doi: 10.1007/s11205-018-1871-x [DOI] [Google Scholar]
- 16.Kane JB, Harris KM, Morgan SP, Guilkey DK, Pathways of health and human capital from adolescence into young adulthood. Soc. Forces 96, 949–976 (2018). doi: 10.1093/sf/sox079; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson M, Marmot M, The effects of promotions on heart disease: Evidence from Whitehall. Econ. J. (Lond.) 122, 555–589 (2012). doi: 10.1111/j.1468-0297.2011.02472.x [DOI] [Google Scholar]
- 18.Stringhini S et al. , Health behaviours, socioeconomic status, and mortality: Further analyses of the British Whitehall II and the French GAZEL prospective cohorts. PLOS Med. 8, e1000419 (2011). doi: 10.1371/journal.pmed.1000419; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Case A, Paxson C, The long reach of childhood health and circumstance: Evidence from the Whitehall II study. Econ. J. 121, F183–F204 (2011). doi: 10.1111/j.1468-0297.2011.02447.x; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane JB, Harris KM, Siega-Riz AM, Intergenerational pathways linking maternal early life adversity to offspring birthweight. Soc. Sci. Med. 207, 89–96 (2018). doi: 10.1016/j.socscimed.2018.04.049; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sealy-Jefferson S, Giurgescu C, Helmkamp L, Misra DP, Osypuk TL, Perceived physical and social residential environment and preterm delivery in African-American women. Am. J. Epidemiol. 182, 485–493 (2015). doi: 10.1093/aje/kwv106; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harper S, Strumpf EC, Social epidemiology: Questionable answers and answerable questions. Epidemiology 23, 795–798 (2012). doi: 10.1097/EDE.0b013e31826d078d; [DOI] [PubMed] [Google Scholar]
- 23.Ozer EJ, Fernald LCH, Weber A, Flynn EP, VanderWeele TJ, Does alleviating poverty affect mothers’ depressive symptoms? A quasi-experimental investigation of Mexico’s Oportunidades programme. Int. J. Epidemiol. 40, 1565–1576 (2011). doi: 10.1093/ije/dyr103; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costello EJ, Erkanli A, Copeland W, Angold A, Association of family income supplements in adolescence with development of psychiatric and substance use disorders in adulthood among an American Indian population. JAMA 303, 1954–1960 (2010). doi: 10.1001/jama.2010.621; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamad R, Rehkopf DH, Poverty and child development: A longitudinal study of the impact of the earned income tax credit. Am. J. Epidemiol. 183, 775–784 (2016). doi: 10.1093/aje/kwv317; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassel J, The contribution of the social environment to host resistance: The Fourth Wade Hampton Frost Lecture. Am. J. Epidemiol. 104,107–123 (1976). doi: 10.1093/oxfordjournals.aje.a112281; [DOI] [PubMed] [Google Scholar]
- 27.Koch R, An address on bacteriological research. BMJ 2,380–383 (1890). doi: 10.1136/bmj.2.1546.380; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Link BG, Phelan J, Social conditions as fundamental causes of disease. J. Health Soc. Behav. 35, 80–94 (1995). doi: 10.2307/2626958; [DOI] [PubMed] [Google Scholar]
- 29.McEwen BS, Seeman T, Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann. N. Y. Acad. Sci. 896, 30–47 (1999). doi: 10.1111/j.1749-6632.1999.tb08103.x; [DOI] [PubMed] [Google Scholar]
- 30.Hertzman C, Boyce T, How experience gets under the skin to create gradients in developmental health. Annu. Rev. Public Health 31, 329–347, 3p, 347 (2010). doi: 10.1146/annurev.publhealth.012809.103538; [DOI] [PubMed] [Google Scholar]
- 31.Yang YC et al. , Social relationships and physiological determinants of longevity across the human life span. Proc. Natl. Acad. Sci. U.S.A. 113, 578–583 (2016). doi: 10.1073/pnas.1511085112; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sapolsky RM, The influence of social hierarchy on primate health. Science 308, 648–652 (2005). doi: 10.1126/science.1106477; [DOI] [PubMed] [Google Scholar]
- 33.Cavigelli SA, Caruso MJ, Sex, social status and physiological stress in primates: The importance of social and glucocorticoid dynamics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370, 20140103 (2015). doi: 10.1098/rstb.2014.0103; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elo IT, Social class differentials in health and mortality: Patterns and explanations in comparative perspective. Annu. Rev. Sociol. 35, 553–572 (2009). doi: 10.1146/annurev-soc-070308-115929 [DOI] [Google Scholar]
- 35.Harris KM, Schorpp KM, Integrating biomarkers in social stratification and health research. Annu. Rev. Sociol. 44, 361–386 (2018). doi: 10.1146/annurev-soc-060116-053339; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holt-Lunstad J, Why social relationships are important for physical health: A systems approach to understanding and modifying risk and protection. Annu. Rev. Psychol. 69, 437–458 (2018). doi: 10.1146/annurev-psych-122216-011902; [DOI] [PubMed] [Google Scholar]
- 37.Johnson B, Carey JR, Hierarchy and connectedness as determinants of health and longevity in social insects, in Sociality, Hierarchy, Health: Comparative Biodemography: A Collection of Papers (National Academies Press, 2014), pp. 269–293. [PubMed] [Google Scholar]
- 38.Rowntree BS, Poverty: A Study of Town Life (Macmillan, 1901). [Google Scholar]
- 39.Darwin C, On The Origin of Species by Means of Natural Selection, or Preservation of Favoured Races in the Struggle for Life (John Murray, 1859). [PMC free article] [PubMed] [Google Scholar]
- 40.Dunbar RIM, Shultz S, Evolution in the social brain. Science 317, 1344–1347 (2007). doi: 10.1126/science.1145463; [DOI] [PubMed] [Google Scholar]
- 41.Silk JB, The adaptive value of sociality in mammalian groups. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 539–559 (2007). doi: 10.1098/rstb.2006.1994; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clutton-Brock TH, Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems (Univ. Chicago Press, 1988). [Google Scholar]
- 43.Berkman LF, Glass T, Brissette I, Seeman TE, From social integration to health: Durkheim in the new millennium. Soc. Sci. Med. 51, 843–857 (2000). doi: 10.1016/S0277-9536(00)00065-4; [DOI] [PubMed] [Google Scholar]
- 44.Markham AC, Alberts SC, Altmann J, Intergroup conflict: Ecological predictors of winning and consequences of defeat in a wild primate population. Anim. Behav. 82, 399–403 (2012). doi: 10.1016/j.anbehav.2012.05.009; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.House JS, Landis KR, Umberson D, Social relationships and health. Science 241, 540–545 (1988). doi: 10.1126/science.3399889; [DOI] [PubMed] [Google Scholar]
- 46.Berkman LF, Syme SL, Social networks, host resistance, and mortality: A nine-year follow-up study of Alameda County residents. Am. J. Epidemiol. 109, 186–204 (1979). doi: 10.1093/oxfordjournals.aje.a112674; [DOI] [PubMed] [Google Scholar]
- 47.Silk JB et al. , Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 20, 1359–1361 (2010). doi: 10.1016/j.cub.2010.05.067; [DOI] [PubMed] [Google Scholar]
- 48.Archie EA, Tung J, Clark M, Altmann J, Alberts SC, Social affiliation matters: Both same-sex and opposite-sex relationships predict survival in wild female baboons. Proc. Biol. Sci. 281, 20141261 (2014). doi: 10.1098/rspb.2014.1261; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanton MA, Mann J, Early social networks predict survival in wild bottlenose dolphins. PLOS ONE 7, e47508 (2012). doi: 10.1371/journal.pone.0047508; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nunez CMV, Adelman JS, Rubenstein DI, Sociality increases juvenile survival after a catastrophic event in the feral horse (Equus caballus). Behav. Ecol. 26,138–147 (2015). doi: 10.1093/beheco/aru163 [DOI] [Google Scholar]
- 51.Dantzer B et al. , Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science 340,1215–1217 (2013). doi: 10.1126/science.1235765; [DOI] [PubMed] [Google Scholar]
- 52.Blumstein DT, Williams DM, Lim AN, Kroeger S, Martin JGA, Strong social relationships are associated with decreased longevity in a facultatively social mammal. Proc. Biol. Sci. 285, 20171934 (2018). doi: 10.1098/rspb.2017.1934; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.A. P. Montero, D. M. Williams, J. G. A. Martin, D. T. Blumstein, More social female yellow-bellied marmots, Marmota flaviventer, have enhanced summer survival. Anim. Behav. 160, 113–119 (2020). doi: 10.1016/j.anbehav.2019.12.013 [DOI] [Google Scholar]
- 54.Thompson NA, Cords M, Stronger social bonds do not always predict greater longevity in a gregarious primate. Ecol. Evol. 8,1604–1614 (2018). doi: 10.1002/ece3.3781; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lukas D, Clutton-Brock TH, The evolution of social monogamy in mammals. Science 341, 526–530 (2013). doi: 10.1126/science.1238677; [DOI] [PubMed] [Google Scholar]
- 56.Yang YC, McClintock MK, Kozloski M, Li T, Social isolation and adult mortality: The role of chronic inflammation and sex differences. J. Health Soc. Behav. 54, 183–203 (2013). doi: 10.1177/0022146513485244; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boen CE et al. , Social relationships, inflammation, and cancer survival. Cancer Epidemiol. Biomarkers Prev. 27, 541–549 (2018). doi: 10.1158/1055-9965.EPI-17-0836; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campbell LAD, Tkaczynski PJ, Lehmann J, Mouna M, Majolo B, Social thermoregulation as a potential mechanism linking sociality and fitness: Barbary macaques with more social partners form larger huddles. Sci. Rep. 8, 6074 (2018). doi: 10.1038/s41598-018-24373-4; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McFarland R et al. , Social integration confers thermal benefits in a gregarious primate. J. Anim. Ecol. 84, 871–878 (2015). doi: 10.1111/1365-2656.12329; [DOI] [PubMed] [Google Scholar]
- 60.Vander Wal E, Festa-Bianchet M, Réale D, Coltman DW, Pelletier F, Sex-based differences in the adaptive value of social behavior contrasted against morphology and environment. Ecology 96, 631–641 (2015). doi: 10.1890/14-1320.1; [DOI] [PubMed] [Google Scholar]
- 61.Ellis S et al. , Mortality risk and social network position in resident killer whales: Sex differences and the importance of resource abundance. Proc. Biol. Sci. 284, 20171313 (2017). doi: 10.1098/rspb.2017.1313; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farine DR, Whitehead H, Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 84, 1144–1163 (2015). doi: 10.1111/1365-2656.12418; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheney DL, Silk JB, Seyfarth RM, Network connections, dyadic bonds and fitness in wild female baboons. R. Soc. Open Sci. 3, 160255 (2016). doi: 10.1098/rsos.160255; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silk JB, Seyfarth RM, Cheney DL, Quality versus quantity: Do weak bonds enhance the fitness of female baboons? Anim. Behav. 140, 207–211 (2018). doi: 10.1016/j.anbehav.2018.04.013 [DOI] [Google Scholar]
- 65.Ellis S, Snyder-Mackler N, Ruiz-Lambides A, Platt ML, Brent LJN, Deconstructing sociality: The types of social connections that predict longevity in a group-living primate. Proc. Biol. Sci. 286, 20191991 (2019). doi: 10.1098/rspb.2019.1991; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hajat A, Kaufman JS, Rose KM, Siddiqi A, Thomas JC, Long-term effects of wealth on mortality and self-rated health status. Am. J. Epidemiol. 173, 192–200 (2011). doi: 10.1093/aje/kwq348; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilkinson RG, Marmot MG, Social Determinants of Health: The Solid Facts (World Health Organization, 2003). [Google Scholar]
- 68.Marmot MG, Adelstein AM, Robinson N, Rose GA, Changing social-class distribution of heart disease. BMJ 2, 1109–1112 (1978). doi: 10.1136/bmj.2.6145.1109; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rose G, Marmot MG, Social class and coronary heart disease. Br. Heart J. 45, 13–19 (1981). doi: 10.1136/hrt.45.1.13; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marmot MG, Wilkinson RG, Social Determinants of Health (Oxford Univ. Press, 1999). [Google Scholar]
- 71.Lantz PM, Golberstein E, House JS, Morenoff J, Socioeconomic and behavioral risk factors for mortality in a national 19-year prospective study of U.S. adults. Soc. Sci. Med. 70,1558–1566 (2010). doi: 10.1016/j.socscimed.2010.02.003; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shively CA, Day SM, Social inequalities in health in nonhuman primates. Neurobiol. Stress 1, 156–163 (2014). doi: 10.1016/j.ynstr.2014.11.005; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kohn J, Panagiotakopoulos L, Neigh GN, The effects of social experience on the stress system and immune function in nonhuman primates, in Developments in Primatology: Progress and Prospects (2016), pp. 49–77. [Google Scholar]
- 74.Bartolomucci A, Social stress, immune functions and disease in rodents. Front. Neuroendocrinol. 28, 28–49 (2007). doi: 10.1016/j.yfrne.2007.02.001; [DOI] [PubMed] [Google Scholar]
- 75.Goymann W, Wingfield JC, Allostatic load, social status and stress hormones: The costs of social status matter. Anim. Behav. 67, 591–602 (2004). doi: 10.1016/j.anbehav.2003.08.007 [DOI] [Google Scholar]
- 76.Abbott DH, McNeilly AS, Lunn SF, Hulme MJ, Burden FJ, Inhibition of ovarian function in subordinate female marmoset monkeys (Callithrix jacchus jacchus). J. Reprod. Fertil. 63, 335–345 (1981). doi: 10.1530/jrf.0.0630335; [DOI] [PubMed] [Google Scholar]
- 77.Strauss ED, Holekamp KE, Social alliances improve rank and fitness in convention-based societies. Proc. Natl. Acad. Sci. U.S.A. 116, 8919–8924 (2019). doi: 10.1073/pnas.1810384116; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.von Holst D, Hutzelmeyer H, Kaetzke P, Khaschei M, Schönheiter R, Social rank, stress, fitness, and life expectancy in wild rabbits. Naturwissenschaften 86, 388–393 (1999). doi: 10.1007/s001140050638; [DOI] [PubMed] [Google Scholar]
- 79.Cram DL et al. , Rank-related contrasts in longevity arise from extra-group excursions not delayed senescence in a cooperative mammal. Curr. Biol. 28, 2934–2939.e4 (2018). doi: 10.1016/j.cub.2018.07.021; [DOI] [PubMed] [Google Scholar]
- 80.Wasser SK, Norton GW, Rhine RJ, Klein N, Kleindorfer S, Ageing and social rank effects on the reproductive system of free-ranging yellow baboons (Papio cynocephalus) at Mikumi National Park, Tanzania. Hum. Reprod. Update 4, 430–438 (1998). doi: 10.1093/humupd/4.4.430; [DOI] [PubMed] [Google Scholar]
- 81.Blomquist GE, Sade DS, Berard JD, Rank-related fitness differences and their demographic pathways in semi-free- ranging rhesus macaques (Macaca mulatta). Int. J. Primatol. 32, 193–208 (2011). doi: 10.1007/s10764-010-9461-z [DOI] [Google Scholar]
- 82.van Noordwijk MA, van Schaik CP, The effects of dominance rank and group size on female lifetime reproductive success in wild long-tailed macaques, Macaca fascicularis. Primates 40,105–130 (1999). doi: 10.1007/BF02557705; [DOI] [PubMed] [Google Scholar]
- 83.Abbott DH et al. , Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm. Behav. 43, 67–82 (2003). doi: 10.1016/S0018-506X(02)00037-5; [DOI] [PubMed] [Google Scholar]
- 84.Lea AJ et al. , Dominance rank-associated gene expression is widespread, sex-specific, and a precursor to high social status in wild male baboons. Proc. Natl. Acad. Sci. U.S.A. 115, E12163–E12171 (2018). doi: 10.1073/pnas.1811967115; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lea AJ, Learn NH, Theus MJ, Altmann J, Alberts SC, Complex sources of variance in female dominance rank in a nepotistic society. Anim. Behav. 94, 87–99 (2014). doi: 10.1016/j.anbehav.2014.05.019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Holekamp KE, Smith JE, Strelioff CC, Van Horn RC, Watts HE, Society, demography and genetic structure in the spotted hyena. Mol. Ecol. 21, 613–632 (2012). doi: 10.1111/j.1365-294X.2011.05240.x; [DOI] [PubMed] [Google Scholar]
- 87.Hayward MD, Gorman BK, The long arm of childhood: The influence of early-life social conditions on men’s mortality. Demography 41, 87–107 (2004). doi: 10.1353/dem.2004.0005; [DOI] [PubMed] [Google Scholar]
- 88.Ferraro KF, Schafer MH, Wilkinson LR, Childhood disadvantage and health problems in middle and later life: Early imprints on physical health? Am. Sociol. Rev 81, 107–133 (2016). doi: 10.1177/0003122415619617; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Rand AM, Hamil-Luker J, Processes of cumulative adversity: Childhood disadvantage and increased risk of heart attack across the life course. J. Gerontol. B Psychol. Sci. Soc. Sci. 60, 117–124 (2005). doi: 10.1093/geronb/60.Special_Issue_2.S117; [DOI] [PubMed] [Google Scholar]
- 90.Meier HCS et al. , Early life socioeconomic position and immune response to persistent infections among elderly Latinos. Soc. Sci. Med. 166, 77–85 (2016). doi: 10.1016/j.socscimed.2016.07.004; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kittleson MM et al. , Association of childhood socioeconomic status with subsequent coronary heart disease in physicians. Arch. Intern. Med. 166, 2356–2361 (2006). doi: 10.1001/archinte.166.21.2356; [DOI] [PubMed] [Google Scholar]
- 92.Gaydosh L, Schorpp KM, Chen E, Miller GE, Harris KM, College completion predicts lower depression but higher metabolic syndrome among disadvantaged minorities in young adulthood. Proc. Natl. Acad. Sci. U.S.A. 115, 109–114 (2018). doi: 10.1073/pnas.1714616114; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miller GE, Yu T, Chen E, Brody GH, Self-control forecasts better psychosocial outcomes but faster epigenetic aging in low-SES youth. Proc. Natl. Acad. Sci. U.S.A. 112, 10325–10330 (2015). doi: 10.1073/pnas.1505063112; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.James SA, Hartnett SA, Kalsbeek WD, John Henryism and blood pressure differences among black men. J. Behav. Med. 6, 259–278 (1983). doi: 10.1007/BF01315113; [DOI] [PubMed] [Google Scholar]
- 95.James SA, Strogatz DS, Wing SB, Ramsey DL, Socioeconomic status, John Henryism, and hypertension in blacks and whites. Am. J. Epidemiol. 126, 664–673 (1987). doi: 10.1093/oxfordjournals.aje.a114706; [DOI] [PubMed] [Google Scholar]
- 96.Cooper EB, Kruuk LEB, Ageing with a silver-spoon: A meta-analysis of the effect of developmental environment on senescence. Evol. Lett. 2, 460–471 (2018). doi: 10.1002/evl3.79; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Monaghan P, Haussmann MF, The positive and negative consequences of stressors during early life. Early Hum. Dev. 91, 643–647 (2015). doi: 10.1016/j.earlhumdev.2015.08.008; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Monaghan P, Organismal stress, telomeres and life histories. J. Exp. Biol. 217, 57–66 (2014). doi: 10.1242/jeb.090043; [DOI] [PubMed] [Google Scholar]
- 99.Tung J, Archie EA, Altmann J, Alberts SC, Cumulative early life adversity predicts longevity in wild baboons. Nat. Commun. 7, 11181 (2016). doi: 10.1038/ncomms11181; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Strauss ED, Shizuka D, Holekamp KE, Juvenile rank acquisition is associated with fitness independent of adult rank. Proc. Biol. Sci. 287, 20192969 (2020). doi: 10.1098/rspb.2019.2969; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang YC, Gerken K, Schorpp K, Boen C, Harris KM, Early-life socioeconomic status and adult physiological functioning: A life course examination of biosocial mechanisms. Biodemography Soc. Biol. 63, 87–103 (2017). doi: 10.1080/19485565.2017.1279536; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Willson AE, Shuey KM, Elder GH Jr., Cumulative advantage processes as mechanisms of inequality in life course health. Am. J. Sociol. 112, 1886–1924 (2007). doi: 10.1086/512712 [DOI] [Google Scholar]
- 103.Ferraro KF, Shippee TP, Aging and cumulative inequality: How does inequality get under the skin? Gerontologist 49, 333–343 (2009). doi: 10.1093/geront/gnp034; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jacobs J, Agho K, Stevens G, Raphael B, Do childhood adversities cluster in predictable ways? A systematic review. Vulnerable Child. Youth Stud. 7, 103–115 (2012). doi: 10.1080/17450128.2012.658886 [DOI] [Google Scholar]
- 105.Ben-Shlomo Y, Kuh D, A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. Int. J.Epidemiol. 31, 285–293 (2002). doi: 10.1093/ije/31.2.285; [DOI] [PubMed] [Google Scholar]
- 106.Chetty R et al. , The fading American dream: Trends in absolute income mobility since 1940. Science 356, 398–406 (2017). doi: 10.1126/science.aal4617; [DOI] [PubMed] [Google Scholar]
- 107.Zipple MN, Archie EA, Tung J, Altmann J, Alberts SC, Intergenerational effects of early adversity on survival in wild baboons. eLife 8, e47433 (2019). doi: 10.7554/eLife.47433; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goldenberg SZ, Wittemyer G, Orphaned female elephant social bonds reflect lack of access to mature adults. Sci. Rep. 7, 14408 (2017). doi: 10.1038/s41598-017-14712-2; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pudrovska T, Anikputa B, Early-life socioeconomic status and mortality in later life: An integration of four life-course mechanisms. J. Gerontol. B Psychol. Sci. Soc. Sci. 69, 451–460 (2014). doi: 10.1093/geronb/gbt122; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McEwen BS, Understanding the potency of stressful early life experiences on brain and body function. Metabolism 57 (Suppl 2), S11–S15 (2008). doi: 10.1016/j.metabol.2008.07.006; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chang SWC et al. , Neuroethology of primate social behavior. Proc. Natl. Acad. Sci. U.S.A. 110 (Suppl 2), 10387–10394 (2013). doi: 10.1073/pnas.1301213110; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davidson RJ, McEwen BS, Social influences on neuroplasticity: Stress and interventions to promote wellbeing. Nat. Neurosci. 15, 689–695 (2012). doi: 10.1038/nn.3093; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Berens AE, Jensen SKG, Nelson CA 3rd, Biological embedding of childhood adversity: From physiological mechanisms to clinical implications. BMC Med. 15, 135 (2017). doi: 10.1186/s12916-017-0895-4; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Snyder-Mackler N, Lea AJ, Functional genomic insights into the environmental determinants of mammalian fitness. Curr. Opin. Genet. Dev. 53, 105–112 (2018). doi: 10.1016/j.gde.2018.08.001; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adler N, Bush NR, Pantell MS, Rigor, vigor, and the study of health disparities. Proc. Natl. Acad. Sci. U.S.A. 109 (suppl. 2), 17154–17159 (2012). doi: 10.1073/pnas.1121399109; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.C. A. Shively, T. C. Register, T. B. Clarkson, Social stress, visceral obesity, and coronary artery atherosclerosis: Product of a primate adaptation. Am. J. Primatol. 71, 742–751 (2009). doi: 10.1002/ajp.20706; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.C. A. Shively, Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biol. Psychiatry 44, 882–891 (1998). doi: 10.1016/S0006-3223(97)00437-X; [DOI] [PubMed] [Google Scholar]
- 118.Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM, Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis 2, 359–368 (1982). doi: 10.1161/01.ATV.2.5.359; [DOI] [PubMed] [Google Scholar]
- 119.Cohen S et al. , Chronic social stress, social status, and susceptibility to upper respiratory infections in nonhuman primates. Psychosom. Med. 59, 213–221 (1997). doi: 10.1097/00006842-199705000-00001; [DOI] [PubMed] [Google Scholar]
- 120.Hermes GL et al. , Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc. Natl. Acad. Sci. U.S.A. 106, 22393–22398 (2009). doi: 10.1073/pnas.0910753106; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.World Health Organization, “World Health Organization, Global Health Estimates 2016: disease burden by cause, age, sex, by country and by region, 2000–2016” (World Health Organization, 2016); http://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html. [Google Scholar]
- 122.Conti G et al. , Primate evidence on the late health effects of early-life adversity. Proc. Natl. Acad. Sci. U.S.A. 109, 8866–8871 (2012). doi: 10.1073/pnas.1205340109; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Swindell WR, Dietary restriction in rats and mice: A meta-analysis and review of the evidence for genotype-dependent effects on lifespan. Ageing Res. Rev. 11, 254–270 (2012). doi: 10.1016/j.arr.2011.12.006; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Epel ES, Lithgow GJ, Stress biology and aging mechanisms: Toward understanding the deep connection between adaptation to stress and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 69 (suppl. 1), S10–S16 (2014). doi: 10.1093/gerona/glu055; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McEwen BS et al. , Mechanisms of stress in the brain. Nat. Neurosci. 18, 1353–1363 (2015). doi: 10.1038/nn.4086; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cohen S et al. , Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. U.S.A. 109, 5995–5999 (2012). doi: 10.1073/pnas.1118355109; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gesquiere LR et al. , Life at the top: Rank and stress in wild male baboons. Science 333, 357–360 (2011). doi: 10.1126/science.1207120; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Young RL et al. , Conserved transcriptomic profiles underpin monogamy across vertebrates. Proc. Natl. Acad. Sci. U.S.A. 116, 1331–1336 (2019). doi: 10.1073/pnas.1813775116; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rittschof CC et al. , Neuromolecular responses to social challenge: Common mechanisms across mouse, stickleback fish, and honey bee. Proc. Natl. Acad. Sci. U.S.A. 111, 17929–17934 (2014). doi: 10.1073/pnas.1420369111; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burmeister SS, Jarvis ED, Fernald RD, Rapid behavioral and genomic responses to social opportunity. PLOS Biol. 3, e363 (2005). doi: 10.1371/journal.pbio.0030363; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Snyder-Mackler N et al. , Social status alters immune regulation and response to infection in macaques. Science 354, 1041–1045 (2016). doi: 10.1126/science.aah3580; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tung J et al. , Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc. Natl. Acad. Sci. U.S.A. 109, 6490–6495 (2012). doi: 10.1073/pnas.1202734109; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Snyder-Mackler N et al. , Social status alters chromatin accessibility and the gene regulatory response to glucocorticoid stimulation in rhesus macaques. Proc. Natl. Acad. Sci. U.S.A. 116, 1219–1228 (2019). doi: 10.1073/pnas.1811758115; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McDade TW et al. , Genome-wide analysis of DNA methylation in relation to socioeconomic status during development and early adulthood. Am. J. Phys. Anthropol. 169, 3–11 (2019). doi: 10.1002/ajpa.23800; [DOI] [PubMed] [Google Scholar]
- 135.Cole SW, Human social genomics. PLOS Genet. 10, e1004601 (2014). doi: 10.1371/journal.pgen.1004601; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Danese A, McEwen BS, Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 106, 29–39 (2012). doi: 10.1016/j.physbeh.2011.08.019; [DOI] [PubMed] [Google Scholar]
- 137.Cole SW, The conserved transcriptional response to adversity. Curr. Opin. Behav. Sci. 28, 31–37 (2019). doi: 10.1016/j.cobeha.2019.01.008; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sanz J et al. , Social history and exposure to pathogen signals modulate social status effects on gene regulation in rhesus macaques. Proc. Natl. Acad. Sci. U.S.A. 201820846 (2019). doi: 10.1073/pnas.1820846116; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marmot MG, Sapolsky R, Of baboons and men: Social circumstances, biology, and the social gradient in health, in Sociality, Hierarchy, Health: Comparative Biodemography: A Collection of Papers (National Academies Press, 2014), pp. 365–388. [PubMed] [Google Scholar]
- 140.Sterck EHM, Watts DP, van Schaik CP, The evolution of female social relationships in nonhuman primates. Behav Ecol. Sociobiol. 41, 291–309 (1997). doi: 10.1007/s002650050390 [DOI] [Google Scholar]
- 141.Shultz S, Opie C, Atkinson QD, Stepwise evolution of stable sociality in primates. Nature 479, 219–222 (2011). doi: 10.1038/nature10601; [DOI] [PubMed] [Google Scholar]
- 142.Kappeler PM, Pozzi L, Evolutionary transitions toward pair living in nonhuman primates as stepping stones toward more complex societies. Sci. Adv. 5, eaay1276 (2019). doi: 10.1126/sciadv.aay1276; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wrangham RW, An ecological model of female-bonded primate groups. Behaviour 75, 262–300 (1980). doi: 10.1163/156853980X00447 [DOI] [Google Scholar]
- 144.Silk JB, Social components of fitness in primate groups. Science 317, 1347–1351 (2007). doi: 10.1126/science.1140734; [DOI] [PubMed] [Google Scholar]
- 145.Johnson SL, Leedom LJ, Muhtadie L, The dominance behavioral system and psychopathology: Evidence from self-report, observational, and biological studies. Psychol. Bull. 138, 692–743 (2012). doi: 10.1037/a0027503; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zelikowsky M et al. , The neuropeptide Tac2 controls a distributed brain state induced by chronic social isolation stress. Cell 173, 1265–1279.e19 (2018). doi: 10.1016/j.cell.2018.03.037; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hurst JL et al. , Individual recognition in mice mediated by major urinary proteins. Nature 414, 631–634 (2001). doi: 10.1038/414631a; [DOI] [PubMed] [Google Scholar]
- 148.Matthews GA et al. , Dorsal raphe dopamine neurons represent the experience of social isolation. Cell 164, 617–631 (2016). doi: 10.1016/j.cell.2015.12.040; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhou T, Sandi C, Hu H, Advances in understanding neural mechanisms of social dominance. Curr. Opin. Neurobiol. 49, 99–107 (2018). doi: 10.1016/j.conb.2018.01.006; [DOI] [PubMed] [Google Scholar]
- 150.Capitanio JP, Hawkley LC, Cole SW, Cacioppo JT, A behavioral taxonomy of loneliness in humans and rhesus monkeys (Macaca mulatta). PLOS ONE 9, e110307 (2014). doi: 10.1371/journal.pone.0110307; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nettle D, Frankenhuis WE, Rickard IJ, The evolution of predictive adaptive responses in human life history. Proc. Biol. Sci. 280, 20131343 (2013). doi: 10.1098/rspb.2013.1343; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gluckman PD, Hanson MA, Spencer HG, Predictive adaptive responses and human evolution. Trends Ecol. Evol. 20, 527–533 (2005). doi: 10.1016/j.tree.2005.08.001; [DOI] [PubMed] [Google Scholar]
- 153.Lea AJ, Tung J, Archie EA, Alberts SC, Developmental plasticity: Bridging research in evolution and human health. Evol. Med. Public Health 2017, 162–175 (2018). doi: 10.1093/emph/eox019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gluckman PD et al. , Towards a new developmental synthesis: Adaptive developmental plasticity and human disease. Lancet 373, 1654–1657 (2009). doi: 10.1016/S0140-6736(09)60234-8; [DOI] [PubMed] [Google Scholar]
- 155.Botero CA, Weissing FJ, Wright J, Rubenstein DR, Evolutionary tipping points in the capacity to adapt to environmental change. Proc. Natl. Acad. Sci. U.S.A. 112, 184–189 (2015). doi: 10.1073/pnas.1408589111; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Laforsch C, Beccara L, Tollrian R, Inducible defenses: The relevance of chemical alarm cues inDaphnia. Limnol. Oceanogr. 51, 1466–1472 (2006). doi: 10.4319/lo.2006.51.3.1466 [DOI] [Google Scholar]
- 157.Uller T, Nakagawa S, English S, Weak evidence for anticipatory parental effects in plants and animals. J. Evol. Biol. 26, 2161–2170 (2013). doi: 10.1111/jeb.12212; [DOI] [PubMed] [Google Scholar]
- 158.Lea AJ, Altmann J, Alberts SC, Tung J, Developmental constraints in a wild primate. Am. Nat. 185, 809–821 (2015). doi: 10.1086/681016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Douhard M et al. , Fitness consequences of environmental conditions at different life stages in a long-lived vertebrate. Proc. Biol. Sci. 281, 20140276 (2014). doi: 10.1098/rspb.2014.0276; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hayward AD, Rickard IJ, Lummaa V, Influence of early-life nutrition on mortality and reproductive success during a subsequent famine in a preindustrial population. Proc. Natl. Acad. Sci. U.S.A. 110, 13886–13891 (2013). doi: 10.1073/pnas.1301817110; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hayward AD, Lummaa V, Testing the evolutionary basis of the predictive adaptive response hypothesis in a preindustrial human population. Evol. Med. Public Health 2013, 106–117 (2013). doi: 10.1093/emph/eot007; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Berghanel A, Heistermann M, Schulke O, Ostner J, Prenatal stress effects in a wild, long-lived primate: Predictive adaptive responses in an unpredictable environment. Proc. Biol. Sci. 283, 20161304 (2016). doi: 10.1098/rspb.2016.1304; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Douhard M et al. , The influence of weather conditions during gestation on life histories in a wild Arctic ungulate. Proc. Biol. Sci. 283, 20161760 (2016). doi: 10.1098/rspb.2016.1760; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nesse RM et al. , Evolution in health and medicine Sackler colloquium: Making evolutionary biology a basic science for medicine. Proc. Natl. Acad. Sci. U.S.A. 107 (suppl. 1), 1800–1807 (2010). doi: 10.1073/pnas.0906224106; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stearns SC, Nesse RM, Govindaraju DR, Ellison PT, Evolution in health and medicine Sackler colloquium: Evolutionary perspectives on health and medicine. Proc. Natl. Acad. Sci. U.S.A. 107 (suppl. 1), 1691–1695 (2010). doi: 10.1073/pnas.0914475107; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nettle D, Bateson M, Adaptive developmental plasticity: What is it, how can we recognize it and when can it evolve? Proc. Biol. Sci 282, 20151005 (2015). doi: 10.1098/rspb.2015.1005; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.West KM, Blacksher E, Burke W, Genomics, health disparities, and missed opportunities for the nation’s research agenda. JAMA 317,1831–1832 (2017). doi: 10.1001/jama.2017.3096; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brodin P et al. , Variation in the human immune system is largely driven by non-heritable influences. Cell 160, 37–47 (2015). doi: 10.1016/j.cell.2014.12.020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Beehner JC, Bergman TJ, The next step for stress research in primates: To identify relationships between glucocorticoid secretion and fitness. Horm. Behav 91, 68–83 (2017). doi: 10.1016/j.yhbeh.2017.03.003; [DOI] [PubMed] [Google Scholar]
- 170.Colchero F et al. , The emergence of longevous populations. Proc. Natl. Acad. Sci. U.S.A. 113, E7681–E7690 (2016). doi: 10.1073/pnas.1612191113; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sapolsky RM, Share LJ, A pacific culture among wild baboons: Its emergence and transmission. PLOS Biol. 2, E106 (2004). doi: 10.1371/journal.pbio.0020106; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Anderson JA et al. , The costs of competition: high social status males experience accelerated epigenetic aging in wild baboons. bioRxiv 961052 [Preprint] 24 February 2020. doi: 10.1101/2020.02.22.961052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Brown DW et al. , Adverse childhood experiences and the risk of premature mortality. Am. J. Prev. Med. 37, 389–396 (2009). doi: 10.1016/j.amepre.2009.06.021; [DOI] [PubMed] [Google Scholar]
- 174.National Center for Health Statistics, National Health Interview Study, 2015 (2015); ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS.
- 175.Fritz SA, Bininda-Emonds ORP, Purvis A, Geographical variation in predictors of mammalian extinction risk: Big is bad, but only in the tropics. Ecol. Lett 12, 538–549 (2009). doi: 10.1111/j.1461-0248.2009.01307.x; [DOI] [PubMed] [Google Scholar]
- 176.Durant SM, Factors affecting life and death in Serengeti cheetahs: Environment, age, and sociality. Behav. Ecol. 15, 11–22 (2004). doi: 10.1093/beheco/arg098 [DOI] [Google Scholar]
- 177.Almberg ES et al. , Social living mitigates the costs of a chronic illness in a cooperative carnivore. Ecol. Lett 18, 660–667 (2015). doi: 10.1111/ele.12444; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Getz LL, McGuire B, Pizzuto T, Hofmann JE, Frase B, Social organization of the prairie vole (Microtus ochrogaster). J. Mammal. 74, 44–58 (1993). doi: 10.2307/1381904 [DOI] [Google Scholar]
- 179.Gager Y, Gimenez O, O’Mara MT, Dechmann DKN, Group size, survival and surprisingly short lifespan in socially foraging bats. BMC Ecol 16, 2 (2016). doi: 10.1186/s12898-016-0056-1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Barocas A, Ilany A, Koren L, Kam M, Geffen E, Variance in centrality within rock hyrax social networks predicts adult longevity. PLOS ONE 6, e22375 (2011). doi: 10.1371/journal.pone.0022375; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lehmann J, Majolo B, McFarland R, The effects of social network position on the survival of wild Barbary macaques, Macaca sylvanus. Behav. Ecol. 27, 20–28 (2016). doi: 10.1093/beheco/arv169 [DOI] [Google Scholar]
- 182.Rogers J et al. , The comparative genomics and complex population history of Papio baboons. Sci. Adv. 5, eaau6947 (2019). doi: 10.1126/sciadv.aau6947; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Panagakis A, Hamel S, Côté SD, Influence of early reproductive success on longevity and late reproductive success in an alpine ungulate. Am. Nat. 189, 667–683 (2017). doi: 10.1086/691388; [DOI] [PubMed] [Google Scholar]
- 184.Pusey A, Williams J, Goodall J, The influence of dominance rank on the reproductive success of female chimpanzees. Science 277, 828–831 (1997). doi: 10.1126/science.277.5327.828; [DOI] [PubMed] [Google Scholar]
- 185.Fedigan LM, Fedigan L, Gouzoules S, Gouzoules H, Koyama N, Lifetime reproductive success in female Japanese macaques. Folia Primatol. (Basel) 47, 143–157 (1986). doi: 10.1159/000156271; [DOI] [PubMed] [Google Scholar]
- 186.Packer C, Collins DA, Sindimwo A, Goodall J, Reproductive constraints on aggressive competition in female baboons. Nature 373, 60–63 (1995). doi: 10.1038/373060a0; [DOI] [PubMed] [Google Scholar]
- 187.Berger V, Lemaître J-F, Allainé D, Gaillard J-M, Cohas A, Early and adult social environments shape sex-specific actuarial senescence patterns in a cooperative breeder. Am. Nat. 192, 525–536 (2018). doi: 10.1086/699513; [DOI] [PubMed] [Google Scholar]
- 188.World Health Organization, About social determinants of health (2017); www.who.int/social_determinants/sdh_definition/en.
- 189.Sanchez-Tojar A, Schroeder J, Farine DR, A practical guide for inferring reliable dominance hierarchies and estimating their uncertainty. J. Anim. Ecol. 87, 594–608 (2018). doi: 10.1111/1365-2656.12776; [DOI] [PubMed] [Google Scholar]
- 190.Braveman P, Gottlieb L, The social determinants of health: It’s time to consider the causes of the causes. Public Health Rep. 129 (suppl. 2), 19–31 (2014). doi: 10.1177/00333549141291S206; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Taylor SE, Repetti RL, Seeman T, Health psychology: What is an unhealthy environment and how does it get under the skin? Annu. Rev. Psychol 48, 411–447 (1997). doi: 10.1146/annurev.psych.48.1.411; [DOI] [PubMed] [Google Scholar]
- 192.Marmot M, Wilkinson RG, Social organization, stress, and health, in Social Determinants of Health (2005), pp. 6–30. [Google Scholar]
- 193.Beehner JC, Bergman TJ, Cheney DL, Seyfarth RM, Whitten PL, Testosterone predicts future dominance rank and mating activity among male chacma baboons. Behav. Ecol. Sociobiol. 59, 469–479 (2006). doi: 10.1007/s00265-005-0071-2 [DOI] [Google Scholar]
- 194.Archie EA, Altmann J, Alberts SC, Social status predicts wound healing in wild baboons. Proc. Natl. Acad. Sci. U.S.A. 109, 9017–9022 (2012). doi: 10.1073/pnas.1206391109; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.McFarland R, Majolo B, Coping with the cold: Predictors of survival in wild Barbary macaques, Macaca sylvanus. Biol. Lett. 9, 20130428 (2013). doi: 10.1098/rsbl.2013.0428; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Seyfarth RM, A model of social grooming among adult female monkeys. J. Theor. Biol. 65, 671–698 (1977). doi: 10.1016/0022-5193(77)90015-7; [DOI] [PubMed] [Google Scholar]
- 197.Snyder-Mackler N et al. , Social status drives social relationships in groups of unrelated female rhesus macaques. Anim. Behav. 111, 307–317 (2016). doi: 10.1016/j.anbehav.2015.10.033; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schulke O, Bhagavatula J, Vigilant L, Ostner J, Social bonds enhance reproductive success in male macaques. Curr. Biol. 20, 2207–2210 (2010). doi: 10.1016/j.cub.2010.10.058; [DOI] [PubMed] [Google Scholar]