Abstract
Chronic ethanol consumption is a leading cause of mortality worldwide, with higher risks to develop pulmonary infections, including Aspergillus infections. Mechanisms underlying increased susceptibility to infections are poorly understood. Chronic ethanol consumption induced increased mortality rates, higher Aspergillus fumigatus burden and reduced neutrophil recruitment into the airways. Intravital microscopy showed decrease in leukocyte adhesion and rolling after ethanol consumption. Moreover, downregulated neutrophil activation and increased levels of serum CXCL1 in ethanol-fed mice induced internalization of CXCR2 receptor in circulating neutrophils. Bone marrow-derived neutrophils from ethanol-fed mice showed lower fungal clearance and defective reactive oxygen species production. Taken together, results showed that ethanol affects activation, recruitment, phagocytosis and killing functions of neutrophils, causing susceptibility to pulmonary A. fumigatus infection. This study establishes a new paradigm in innate immune response in chronic ethanol consumers.
Research organism: Mouse, Other
eLife digest
Alcoholism is a chronic disease that has many damaging effects on the body. Over long periods, excessive alcohol intake weakens the immune system, putting consumers at increased risk of getting lung infections such as pneumonia.
Some forms of pneumonia can be caused by the fungus Aspergillus fumigatus. This microbe does not tend to be a problem for healthy individuals, but it can be fatal for those with impaired immune systems. Here, Malacco et al. wanted to find out why excessive alcohol consumers are more prone to pneumonia.
To test this, the researchers used two groups of mice that were either fed plain water or water containing ethanol. After 12 weeks, both groups were infected with Aspergillus fumigatus. The results showed that alcohol-fed mice were more susceptible to the infection caused by strong inflammation of the lungs.
Normally, the immune system confronts a lung infection by activating a group of defense cells called neutrophils, which travel through the blood system to the infection site. Once in the right spot, neutrophils get to work by releasing toxins that kill the fungus. Malacco et al. discovered that after chronic alcohol consumption, neutrophils were less reactive to inflammatory signals and less likely to reach the lungs. They were also less effective in dealing with the infection. Neutrophil released fewer toxins and were thus less able to kill the microbial cells.
These findings demonstrate for the first time how alcohol can affect immune cells during infection and pave the way for new possibilities to prevent fatal lung infections in excessive alcohol consumers. A next step would be to identify how alcohol acts on other processes in the body and to find a way to modulate or even revert the changes it causes.
Introduction
Ethanol abuse is a leading cause of mortality worldwide (World Health Organisation, 2014). Chronic alcohol consumption has been correlated, as a comorbidity, to a wide range of health conditions, including alcoholic liver diseases, cirrhosis and cancers (Szabo and Saha, 2015; Pritchard and Nagy, 2005; Szabo, 1999; Bautista, 1999; Luján et al., 2010). Moreover, alcohol abusers are prone to develop severe lung inflammatory and infectious diseases, including acute respiratory distress syndrome (ARDS) (Liang et al., 2012), pneumonia caused by Streptococcus pneumoniae (Trevejo-Nunez et al., 2015; Tsuchimoto et al., 2015; Boé et al., 2001), Klebsiella pneumoniae (Yeligar et al., 2014; Ohama et al., 2015), Respiratory Syncytial Virus (RSV) infection (Meyerholz et al., 2008) and aspergillosis (Blum et al., 1978; Gustot et al., 2014). The mechanisms associated with this increased risk of disease and death are poorly understood, however studies have suggested that certain aspects of immune function may be altered by chronic ethanol intake (D'Souza El-Guindy et al., 2007; Lippai et al., 2013; Yen et al., 2017; Zhang et al., 2015; Gurung et al., 2009).
Aspergillus fumigatus is a ubiquitous and saprophytic fungus whose conidia are inhaled by humans on a daily basis (Latgé, 1999). Immunocompromised individuals are considered the risk group to develop the pulmonary invasive aspergillosis (IA) (Latgé, 2001) and mortality rates reach up to 95% (Brown et al., 2012). In normal conditions, inhaled conidia are cleared through mucociliary actions. However, if conidia pass through the initial barrier, alveolar macrophage (AM) phagocytosis takes place, resulting in a cascade of cytokine and chemokine release to recruit neutrophils to prevent fungal development (Dagenais and Keller, 2009; Caffrey-Carr et al., 2017). In all these circumstances, an altered leukocyte function may be a major risk factor for IA. Despite all advance in diagnosis and treatment, aspergillosis’ morbidity and mortality remain very high. Mildly immunocompromised conditions such as diabetes mellitus, low-dose corticosteroid therapy and alcoholism has been considered as predisposing factors (Blum et al., 1978; Baddley, 2011; Kousha et al., 2011).
Neutrophils have been shown to be essential to control fungal and bacterial burden in the site of infection and avoid the spread of these microbes and consequently, survival of the host (Gazendam et al., 2016; Romani, 2000; Robertson et al., 2008; Ng et al., 2019). During a pathogen-triggered inflammatory response, neutrophils are the earliest immune effector cells recruited to a site of infection (Kolaczkowska and Kubes, 2013; Sun et al., 2014; Rios-Santos et al., 2007). Neutrophil migration starts with the tethering and rolling of these cells on endothelial cells, a process mediated by selectins and their carbohydrate ligands on neutrophils and endothelial venules (Soethout et al., 2002). These interactions, together with chemokines signals such as CXCL1 and CXCL2 lead neutrophils to a crawling state through the endothelial vase (Kolaczkowska and Kubes, 2013; Phillipson and Kubes, 2011). G protein-coupled receptor on rolling neutrophils binds to the chemokines and changes β2 integrins conformation on the leukocyte surface, allowing a high affinity interaction with endothelial cells (Zemans et al., 2009).
Here, we describe that chronic ethanol consumption facilitates pulmonary infection by both A. fumigatus in mice. Mechanistically, chronic ethanol consumption impairs the normal neutrophil migration to the site of infection via release of high levels of circulating chemokine CXCL1 after infection, followed by downregulation of its receptor 2 (CXCR2). Additionally, ethanol consumption is responsible for an impaired neutrophil function characterized by less phagocytosis, killing, and oxidative burst leading to elevated lung pathology in mice and accentuated mortality rates after infection.
Results
Chronic ethanol consumption did not induce systemic inflammation
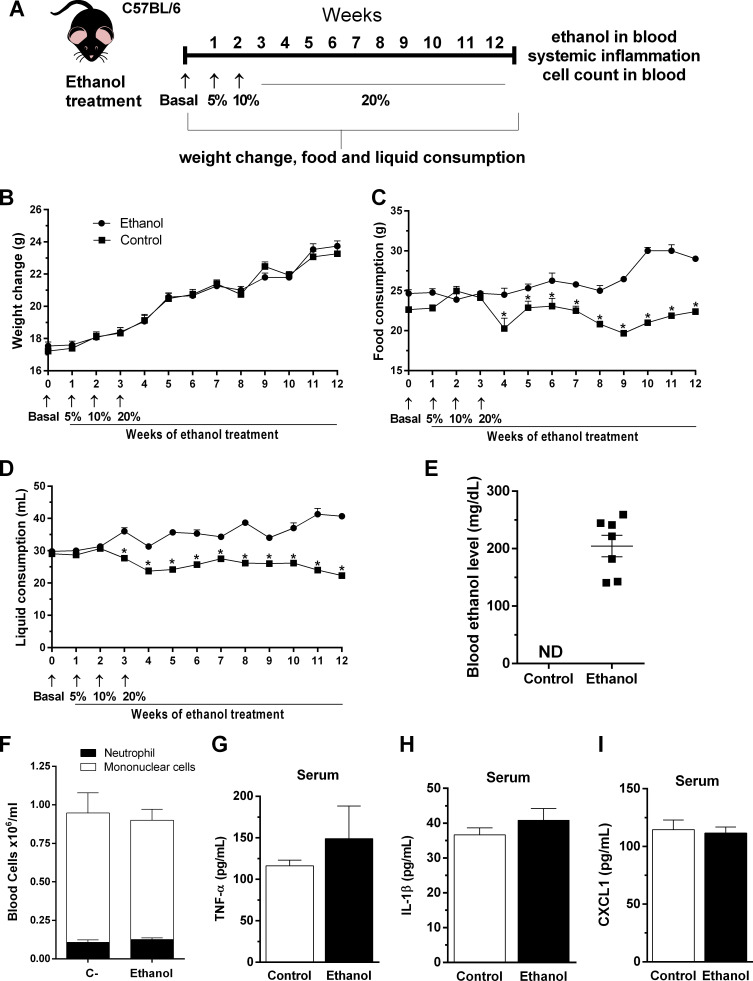
To assess the relevance of ethanol chronic consumption, we characterized several parameters during chronic ethanol consumption on host before the infection (Figure 1A). First, we checked weight change, food and liquid consumption during ethanol treatment. We found that there was no weight change in mice during the treatment with ethanol (Figure 1B). Moreover, food and liquid consumption were diminished in ethanol-treated mice compared with control group (Figure 1C and D). Second, blood ethanol levels in mice were about 200 mg of ethanol per deciliter of whole blood while control group ethanol level were not detectable, measured by gas chromatography after 12 weeks of ethanol treatment, (Figure 1E). In order to verify if ethanol consumption affected hematopoiesis we analyzed bone marrow precursors and blood cell counts. Mononuclear and neutrophil counts in peripheral blood did not show differences between groups (Figure 1F). Chronic ethanol consumption did not affect granulocyte progenitors in bone marrow (Supplementary file 1). In addition, no changes were observed in ALT levels in mice serum after the ethanol treatment compared to the control mice group (data not shown). Finally, serum levels of inflammatory mediators, such as TNF-α, IL-1β and CXCL1, have not been changed in ethanol-fed mice (Figure 1G–I).
Figure 1. Outcome of chronic ethanol treatment in mice.
(A) Experimental design: C57BL/6 mice received ethanol 5% (v/v) in the first week, followed by 10% (v/v) in the second week (to help mice acclimate with this intervention) and were treated during 10 weeks with ethanol 20% (v/v) in their drinking water. Control group received water. (B–D) During treatment, weight change, food and liquid consumption were measured. Data are presented as Mean ± SD (15 mice per group) *Significantly different (p<0.05) in t test. After ethanol treatment, blood was collected to evaluate (E) blood ethanol levels, (F) differential cell count and (G) TNF-α, (H) IL-1β and (I) CXCL1 in serum. Data are presented as Mean ± SD (4 to 9 mice per group) and analyzed with ANOVA test. Please, also see Supplementary file 1 and Figure 1—source data 1.
Chronic ethanol consumption increased lethality and impaired pulmonary fungal clearance after A. fumigatus infection
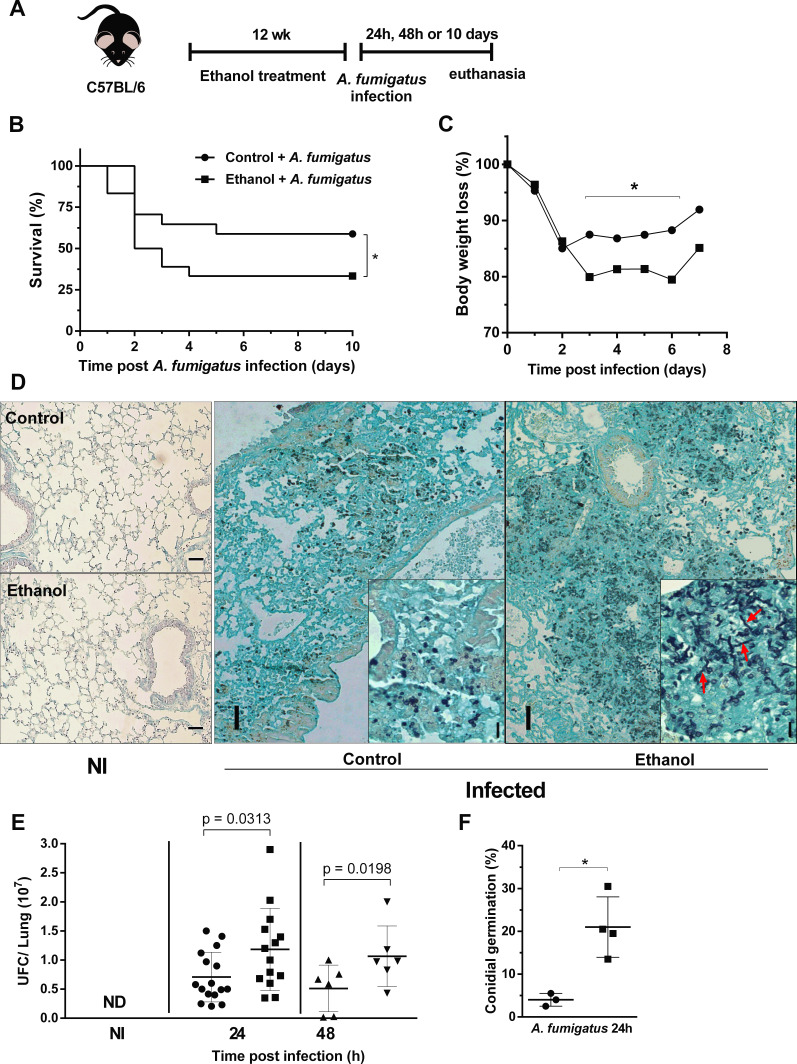
After ethanol treatment, mice were intranasally infected with A. fumigatus conidia (Figure 2A). Ethanol-treated mice showed an increased lethality compared to control mice group (Figure 2B). In addition, weight loss was significantly higher in ethanol treated mice from days 3 to 7 after infection (Figure 2C). These clinical signs were accompanied by higher pulmonary fungal burden in ethanol-fed mice, demonstrating an impaired fungal clearance. Moreover, we were also able to identify hyphae into the airways of infected ethanol-treated mice, showed by red arrows at 24 hr after infection compared to control group (Figure 2D–F). Altogether, these results demonstrate that chronic ethanol consumption increased susceptibility to pulmonary A. fumigatus infection along with an impaired ability to clear the pathogen.
Figure 2. Chronic ethanol consumption leads to susceptibility associated with increased fungal load after A. fumigatus infection.
(A) Experimental design: C57BL/6 mice were treated for 12 weeks with ethanol. The next day after the end of the treatment, mice were intranasally infected with 3 × 108 conidia of A. fumigatus. (B) Comparative lethality curves with P = <0.0001 in Log-rank (Mantel-Cox) test. (C) Comparative weight change curves of ethanol-fed group and control group were performed (18 mice per group). Right lungs were collected 24 and 48 hr after the infection. Homogenate from right lungs were plated and CFUs were quantified. Left lungs were fixed with formaldehyde 4% and embedded in paraffin. Sections were stained with GMS and the percentage of germlings was counted (6–16 mice per group) P value indicated in the figure in t test. (D) Representative slides of GMS staining. The insets in 24 hr images represent magnification to show germlings (red arrows) into lung tissue. (E) Fungal load and (F) fungal germination in lungs p=0.0389 in t test. Bars represent 100 µm.
Ethanol consumption altered cytokine release in airways in mice after A. fumigatus infection
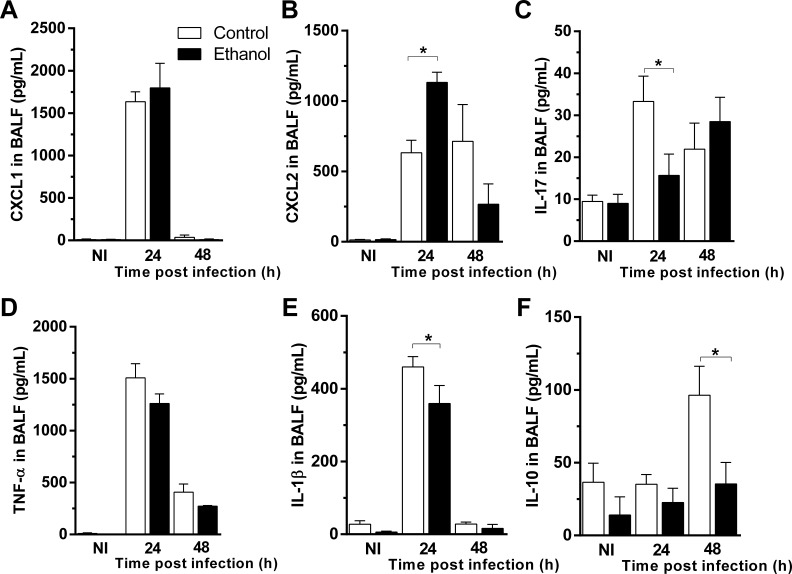
Next, we assessed cytokine and chemokine levels by ELISA after A. fumigatus infection to determine whether levels of inflammatory mediators in BALF supernatants were altered after ethanol consumption. We found no significant differences in the levels of neutrophil chemotactic mediator CXCL1 after A. fumigatus infection in BALF of ethanol-treated mice compared to control group (Figure 3A). Interestingly, another CXCR2 agonist and neutrophil chemotactic mediator, CXCL2 were increased at 24 hr of infection in BAL fluid of ethanol-treated mice compared to control mice (Figure 3B). Alcohol consumption declined IL-17 levels after 24 hr of infection (Figure 3C). We also observed no differences in TNF levels between control and ethanol-fed mice group (Figure 3D). Moreover, ethanol consumption was able to down modulate IL-1β and IL-10 levels after fungal infection (Figure 3E and F). These results suggest that chronic ethanol consumption dysregulate cytokine and chemokine release post A. fumigatus infection.
Figure 3. Ethanol consumption altered cytokine release in airways in mice after A. fumigatus infection.
BALF supernatants were harvested at 24 and 48 hr after infection and used for ELISA assay. (A) CXCL1, (B) CXCL2, (C) IL-17, (D) TNF, (E) IL-1β and (F) IL-10 levels in BALF. Experiments were assayed in triplicate. Data are presented as Mean ± SD (3 to 9 mice per group). *p<0.0247 in ANOVA test. Please, also see Figure 3—source data 1.
Ethanol consumption impaired neutrophil and lymphocyte recruitment into the airways during A. fumigatus infection
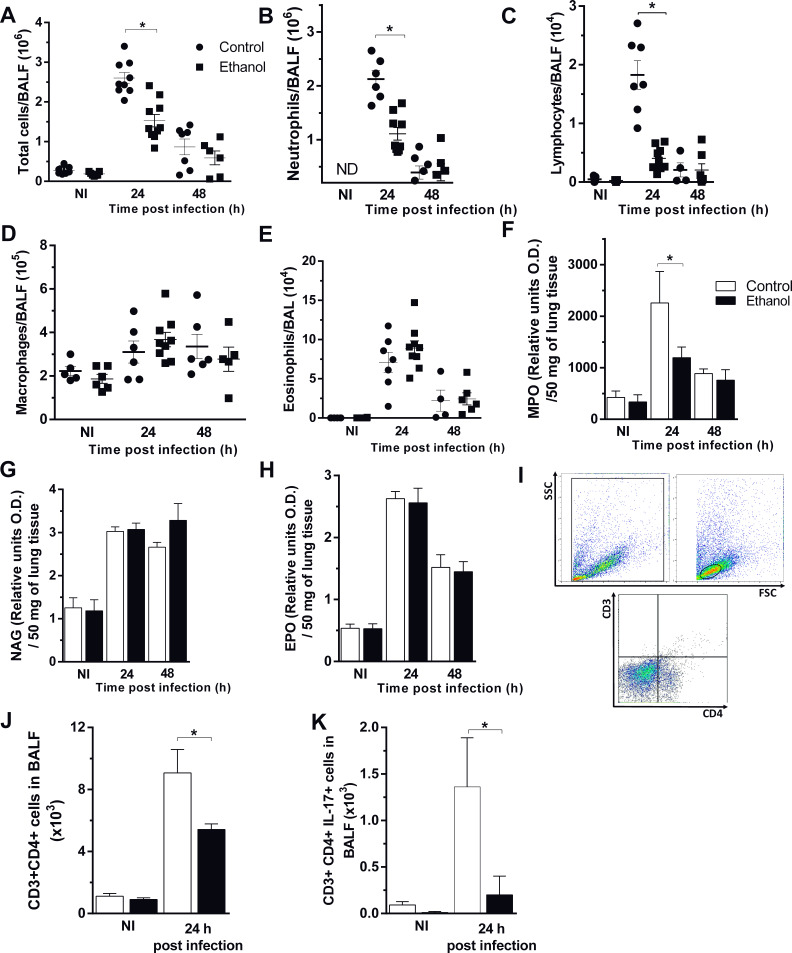
To clarify whether the diminished fungal clearance is due to an impaired inflammatory response, we next observed cell influx into the site of A. fumigatus infection. Although both groups exhibited a large recruitment of total cells into the airways, ethanol-treated mice had a significant reduction of recruited cells, compared to control group 24 hr after infection. However, at 48 hr after infection, there was a similar cell migration into the site of infection in both groups (Figure 4A). We demonstrate that almost all cells migrated to the airways represent neutrophils, one of the most important cells involved in killing and control of A. fumigatus infection (Erwig and Gow, 2016). Neutrophils and lymphocytes into airways were significantly decreased in ethanol-treated mice at 24 hr post infection (Figure 4B and C). In contrast, there were no differences in macrophages and eosinophils recruited to the site of infection (Figure 4D and E). Regarding to inflammatory cells recruited to lung tissue, we also observed a diminished neutrophils migration into the lung parenchyma in ethanol-treated mice, by MPO measurement, at 24 hr after infection (Figure 4F). Similar to alveoli, eosinophils and macrophages in pulmonary tissue exhibited no differences after A. fumigatus infection (Figure 4G and H). In addition, both ethanol-treated and control groups exhibited similar number of leukocytes in blood (Figure 4I). Besides, we characterized the populations of lymphocyte migrating to the airways of infected mice. We assessed CD3+CD4+IL17+ cells and results demonstrate that chronic ethanol consumption strongly reduced lymphocytes and IL-17 production in the site of infection after 24 hr of infection (Figure 4J–L). These results indicate that chronic ethanol consumption mostly affected specifically neutrophils recruitment to airways after A. fumigatus infection.
Figure 4. Chronic ethanol consumption affects cellular influx after A. fumigatus infection.
After ethanol treatment, mice were infected with A. fumigatus. BALF and lungs were harvested at 24 and 48 hr after infection for inflammatory cell infiltrates determination. (A) Total cells, (B) Neutrophil, (C) Macrophage, (D) Lymphocyte, (E) Eosinophil counts in BALF. (F) MPO, (G) NAG and (H) EPO assays in lungs. (I) Blood leukocyte count/mL. BALF cells were labeled with specific antibodies for flow cytometry. (J) Gate strategy for CD3+CD4+ T cells. (K) CD3+CD4+ cells and (L) CD3+CD4+IL-17+ cells in BALF. Experiments were assayed in triplicate. Data are presented as mean ± SD (4 to 7 mice per group). *Significantly different (p<0.01) in ANOVA test. Please, also see Figure 4—source data 1.
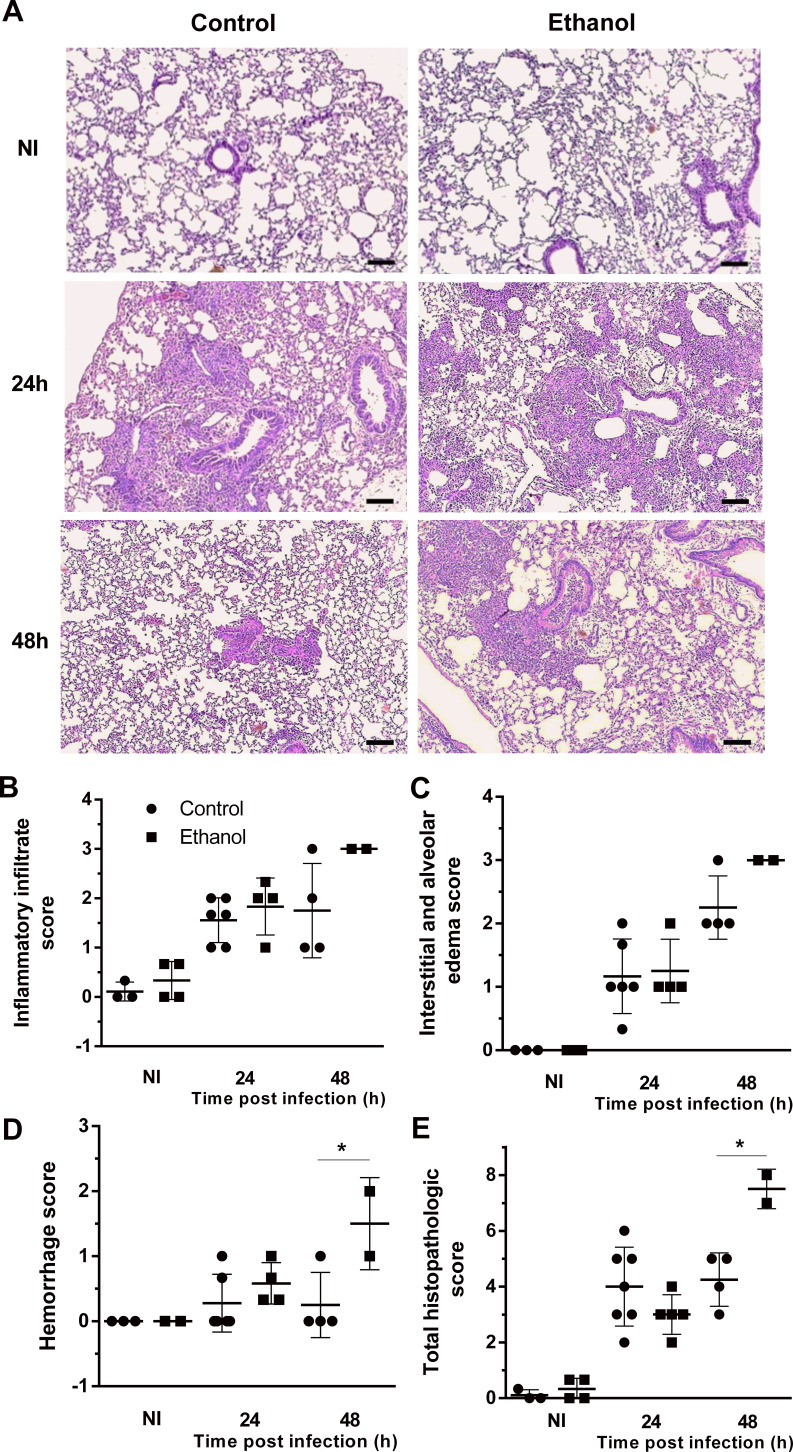
Chronic ethanol consumption increased lung pathology after A. fumigatus infection
We assessed histopathology to determine the effect of ethanol consumption in pulmonary tissue after infection. Tissue sections of infected mice revealed a massive leukocyte recruitment into the lungs at 24 hr after infection, in which the inflammatory infiltrate covers a large part of the pulmonary parenchyma structure, including alveoli and perivascular regions that decreases after 48 hr of infection in the control group. The cellular infiltrate tissue remains more prominent after 48 hr of infection only in ethanol-fed mice (Figure 5A). Histopathology score results showed that both ethanol-treated and control groups had similar levels of inflammatory infiltrate, interstitial and alveolar edema and hemorrhage scores at 24 hr of infection. However, after 48 hr of infection, ethanol consumption showed a remaining cellular infiltrate, higher edema and hemorrhage, which increased the total pathology scores (Figure 5B–D).
Figure 5. Lung histopathology is affected by ethanol treatment in mice.
Lungs were harvested at 24 and 48 hr after infection fixed with formaldehyde 4% and embedded in paraffin. (A) Sections were stained with Hematoxylin and Eosin. Samples were graded on a 0 to 5-point scale score for (B) inflammatory infiltrate, (C) interstitial and alveolar edema, (D) hemorrhage and (E) total histopathologic score. Experiments were assayed in triplicate. Data are presented as mean ± SD (with 2 to 6 mice per group). *p<0.0142 in ANOVA test. Bars represent 100 µm.
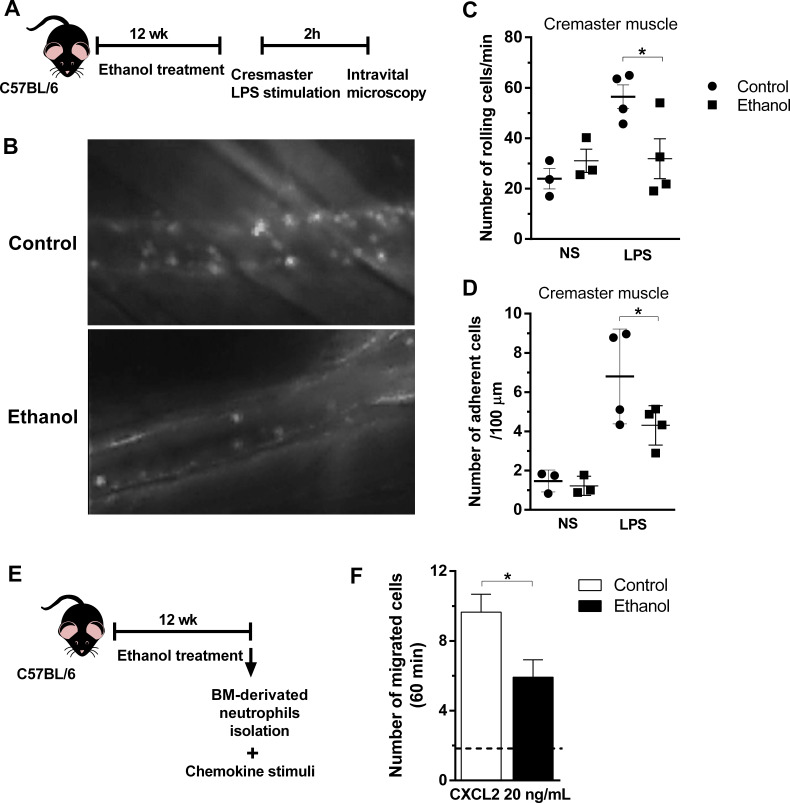
Chronic ethanol consumption reduced leukocyte rolling, adhesion, and chemotaxis in mouse neutrophils
In order to investigate the transmigration process of neutrophils upon chemotaxis in vivo, we first performed an intravital microscopy to visualize migratory cells (Figure 6—source data 1) (Figure 6-rich media videos). Intracrostal administration of LPS was not able to induce a strong increase in rolling and adherence of cells to post capillary venules in ethanol-fed group after 2 hr of stimulation, compared to stimulated control group (Figure 6A–D). In addition, to confirm the impaired migratory ability of neutrophils was caused by ethanol consumption in mice, we performed ex vivo chemotaxis assay towards CXCL2, with mouse bone marrow-derived neutrophils (Figure 6E). As we expected because of previously results showed in Figure 4B, neutrophils from ethanol-treated mice had an impaired migration towards the chemokine stimuli compared to neutrophils from control non-treated mice (Figure 6F). Taken together, these results demonstrate that chronic ethanol ingestion can affect neutrophil rolling, adhesion and recruitment in different tissues.
Figure 6. Chronic ethanol consumption reduced leukocyte rolling and adhesion in vivo and neutrophils chemotaxis ex vivo.
(A) Experimental design: after the chronic ethanol treatment, mice received an intracrostal injection of LPS. After 2 hr, mice cremaster from mouse was exposed to examine the microcirculation by intravital microscopy. Post capillaries venules were recorded. (B) Representative images from the recorded videos (please see the rich media). (C) Number of rolling cells and (D) Number of adherent cells were counted in the videos. (E) Ex vivo neutrophil chemotaxis. Experimental design: after ethanol treatment, BM-derived neutrophils were separated by density gradient and a chemotaxis assay in a Boyden chamber towards CXCL2 was performed. (F) Number of migrated neutrophils in 60 min. Data are presented as Mean ± SD (3 to 5 mice per group) *Significantly different (p<0.0028) in ANOVA test. Please, also see Figure 6—source data 1 and Figure 6-rich media videos.
Figure 6—video 1. Leukocyte rolling and adhesion in vivo-control mice + vehicle.
Figure 6—video 2. Leukocyte rolling and adhesionin vivo-ethanol-treated mice + vehicle.
Figure 6—video 3. Leukocyte rolling and adhesionin vivo-control mice + LPS.
Figure 6—video 4. Leukocyte rolling and adhesionin vivo- ethanol-treated mice + LPS.
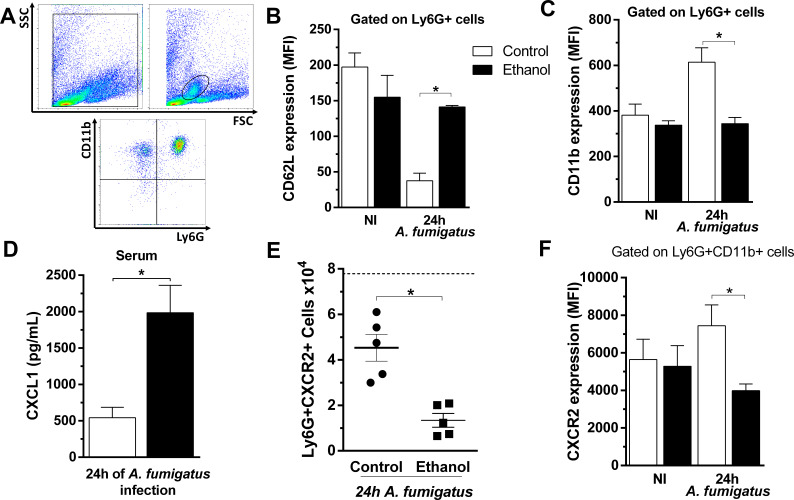
Chronic ethanol consumption impairs neutrophil’s activation and recruitment by modulation of CD11b, CD62L and down regulation of CXCR2 in these cells during A. fumigatus infection
Neutrophils chemotaxis and recruitment is a complex process that requires leukocyte-endothelial interactions as well as inflammatory mediators’ release (Kolaczkowska and Kubes, 2013). Moreover, the main regulator of neutrophil migration in acute inflammation is CXCR2. To further investigate whether the impaired neutrophil migration is due a deficient neutrophil activation status during infection, we accessed the expression of the markers CD11b, CD62L and CXCR2 in circulating neutrophils after A. fumigatus infection using flow cytometry. While CD11b is mobilized from specific granules to the cell surface, enzymatic shedding in activated polymorphonuclear (PMN) rapidly down regulates CD62L. Both markers have their constitutive expression in resting PMNs. We found that neutrophils activation status was compromised, with CD62L up regulation and CD11b down regulation, in the peripheral blood neutrophils of infected ethanol-treated mice compared to infected non-treated mice (Figure 7A–C). In sepsis, the reduction of neutrophils migration is related to the down regulation of CXCR2 protein expression on circulating neutrophils surface (Alves-Filho et al., 2009). Indeed, CXCL1 serum levels were strongly augmented in ethanol-treated mice compared to the control group at 24 hr post infection (Figure 7D). We next analyzed the role of chronic ethanol intake in regulating CXCR2 expression in circulating neutrophils. At 24 hr after A. fumigatus infection, ethanol-treated mice exhibited significantly fewer circulating neutrophils expressing CXCR2 in the surface compared to the non-treated group (Figure 7E and F).
Figure 7. Chronic ethanol consumption impaired neutrophils activation and downregulated CXCR2 in a sepsis-like manner after A. fumigatus infection.
After ethanol treatment and A. fumigatus, blood was harvested at 24 hr post infection. Neutrophils were labeled with specific antibodies for flow cytometry. (A) Gate strategy to analyze neutrophils. Neutrophils were gated by size and cellular complexity and then gated again as Ly6G+CD11b+ cells. (B) CD62L (*p=0.001) and (C) CD11b expression in circulating neutrophils (*p=0.0479). (D) Serum levels of CXCL1 were measured by ELISA assay after 24 hr of infection (*p = 0.0001). (E) Ly6G+CXCR2+ cells (*p=0.0489) and (F) MFI of CXCR2 expression in blood (*p=0.007). Experiments were done at least twice. Data are presented as mean ± SD (3 to 8 mice per group). Analysis were made by ANOVA test. Dashed line represents basal levels of non-infected groups. Please, also see Figure 7—source data 1.
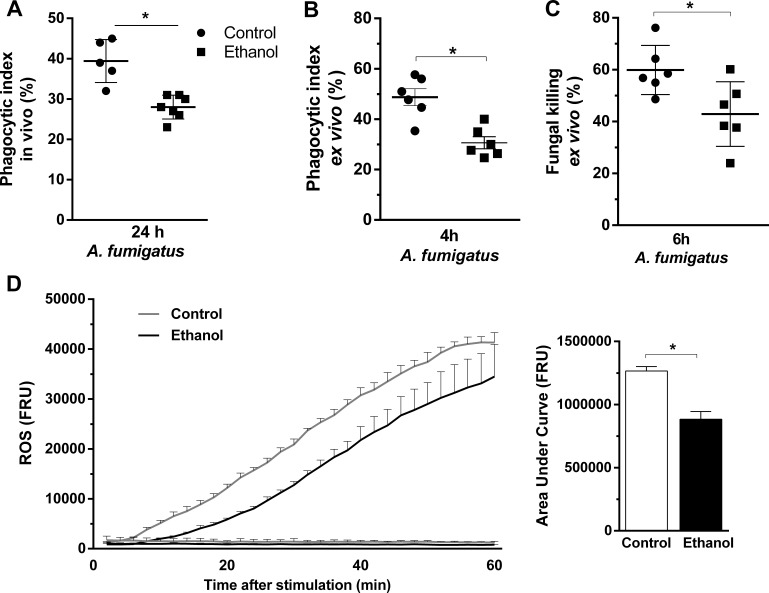
Ethanol consumption decreases neutrophil functions of phagocytosis, killing and oxidative burst after A. fumigatus challenge
For the proper killing of A. fumigatus, fungal phagocytosis and ROS production by neutrophils are key events (Philippe et al., 2003). In this sense, we accessed phagocytosis and killing of A. fumigatus conidia by bone marrow-derived neutrophils from ethanol-treated and non-treated mice. We found that phagocytosis was significantly reduced in ethanol-treated mice either in vivo, evaluated by BALF recruited cells, or ex vivo, in bone marrow neutrophils, compared to control group (Figure 8A and B). Fungal killing was also reduced in ethanol intake mouse neutrophils (Figure 8C). Moreover, to evaluate the effect of chronic ethanol consumption in the promotion of respiratory burst of bone marrow-derived neutrophils we performed chemiluminescence experiments using luminol, which served as a probe for superoxide (O2●−)49. We observed that neutrophils from ethanol-fed mice produced lower levels of ROS triggered by A. fumigatus conidia compared to neutrophils from non-treated mice (Figure 8D). These results suggest that phagocytosis, killing and ROS production functions in neutrophils were affected by chronic ethanol consumption.
Figure 8. Ethanol consumption decreased neutrophils phagocytosis, killing and ROS production after A. fumigatus stimuli.
(A) Conidial phagocytosis was determined in BALF (*p=0.007). (B–D) After ethanol treatment, BM-derived neutrophils were separated by density gradient and incubated with A. fumigatus conidia. (B) Ex vivo phagocytosis was assessed by cytospin preparations from BALFs (*p=0.0013). (C) Killing assay was evaluated by cell lysis with water, the diluted samples were plated in fungal medium and colony-forming units (CFU) were determined after overnight incubation (*p=0.0238). (D) Luminometry assay was performed to evaluate neutrophil-mediated ROS production and area under curve analysis. (*p=0.057). Data are presented as mean ± SD (3 to 6 mice per group). Analysis were made by t student test.
Discussion
For several centuries, chronic ethanol consumption has been associated to increased susceptibility to infections as well as increased morbidity and mortality after injury (Lieber, 2005; Molina et al., 2010). Numerous studies have shown the effects of acute or chronic exposure to ethanol in inflammatory infections such as in models of K. pneumoniae infection and gut bacteria-associated sepsis (Ohama et al., 2015), intravenous Escherichia coli challenge (Bagby et al., 1998), S. pneumoniae (Trevejo-Nunez et al., 2015; Boé et al., 2001) and a few studies reporting this effect in Aspergillus infection (Blum et al., 1978).
In the present study, we demonstrate how chronic ethanol consumption alters immune and inflammatory pulmonary response after A. fumigatus infection. This involves several immunological phenomena, including defective leukocytes rolling and adhesion, impaired neutrophil migration by down regulation of CXCR2, failed neutrophil activation, impaired neutrophil effector functions (phagocytosis and killing), and reduction of polarized-Th17 innate response. Those features are responsible for an increased susceptibility of mice to A. fumigatus infection.
Chronic ethanol consumption did not alter the basal levels of inflammatory cytokines and chemokines before infection. Ethanol ingestion had an important role in modulating immune response after infection, which is in accordance with previous findings (D'Souza El-Guindy et al., 2007; Bhatty et al., 2011). Ethanol consumption also alters IL-6 and TNF expression of LPS-stimulated Kupffer cells (Maraslioglu et al., 2014). In the present work, we found reduction in IL-1β and IL-17 production after A. fumigatus infection in ethanol-fed mice, suggesting that the immunomodulatory effects caused by chronic ethanol consumption may contribute to the impaired inflammatory response against A. fumigatus.
It has been demonstrated that chronic ethanol consumption causes cellular abnormality in mice lung resident cells. Alveolar macrophages from ethanol-fed mice had deregulation in NADPH oxidase system, impairing phagocytosis and killing against K. pneumoniae (Yeligar et al., 2012). In fact, an impaired NADPH oxidase activity is a well-known risk factor to develop invasive aspergillosis and other life threatening diseases, as seen in Chronic Granulomatous Disease (CGD) patients (Cohen et al., 1981; King et al., 2016; Segal and Romani, 2009). Another study showed both lymphocytes and neutrophils response impaired by ethanol consumption in a model of cutaneous infection by Staphylococcus aureus, in which chronic ethanol-fed mice showed great skin lesions and bacteremia associated with reduced IL-17 and IL-1β production, suggesting that Th17-mediated neutrophilic response was impaired (Parlet et al., 2015).
Neutrophil recruitment to the site of infection is essential for the control of invading extracellular pathogens (Kolaczkowska and Kubes, 2013; Sun et al., 2014; Rios-Santos et al., 2007). Neutrophils are major cell type recruited for A. fumigatus conidia and hyphae killing and neutropenic patients are more susceptible to systemic fungal infections (Dagenais and Keller, 2009; Gazendam et al., 2016). In our data, neutrophil recruitment to infection site, in ethanol-fed mice, was impaired even though we did not observe decrease in both CXCL1 and CXCL2 BALF levels, indicating that ethanol consumption is responsible for compromising neutrophils activation and migration. Moreover, our data showed reduced leukocyte rolling and adhesion after LPS stimuli in ethanol-treated mice outside the airways. CXCL1 and CXCL2 chemokines bind to CXCR2 displaying an essential role in neutrophils activation and ensuing adhesion to endothelium (Kolaczkowska and Kubes, 2013; Phillipson and Kubes, 2011). In fact, it was demonstrated in vitro that chronic ethanol exposure impacted tight junction structures in epithelial cells, leading them vulnerable to endotoxemia (Wood et al., 2013). However, in our study vascular permeability was similar in both ethanol-fed mice and control mice groups (data not shown).
It has been well established that the decrease of CXCR2 expression impairs neutrophil migration (Russo et al., 2009), especially in sepsis (Rios-Santos et al., 2007; Alves-Filho et al., 2009), in which a great amount of inflammatory cytokines and chemokines is released to the blood, causing a complex systemic inflammation (Pierrakos and Vincent, 2010). In a model of severe sepsis, it was found that the migration failure and consequent mortality of individual was associated with the diminished expression of neutrophils CXCR2, which was due to great release of systemic CXCL1 (39). As seen in sepsis, our findings showed decrease CXCR2 expression and great amount of systemic CXCL1 levels in serum of ethanol-fed mice after A. fumigatus infection. To our knowledge, this is the first report establishing a relation between chronic ethanol intake and downregulation of CXCR2 receptor in mouse neutrophils. It is also important to mention that the cooperation between the GPCR receptor CXCR2 and P-selectin ligand, the P-selectin glycoprotein ligand-1 (PSGL-1) is essential to a successful neutrophil migration. The signaling events that ensure the adhesion cascade include the conversion of integrin αLβ2 from a low-affinity conformation to an extended high-affinity conformation that causes arrest and, consequently, perivascular crawling (Yago et al., 2018). Further studies are needed to address the role of chronic ethanol consumption in expression of P-selectin and PSGL-1. Our data indicate that chronic ethanol consumption drives host to a sepsis-like phenotype and this mechanism is responsible for impaired neutrophil migration. The mechanisms whereby CXCR2 is downregulated need further investigation.
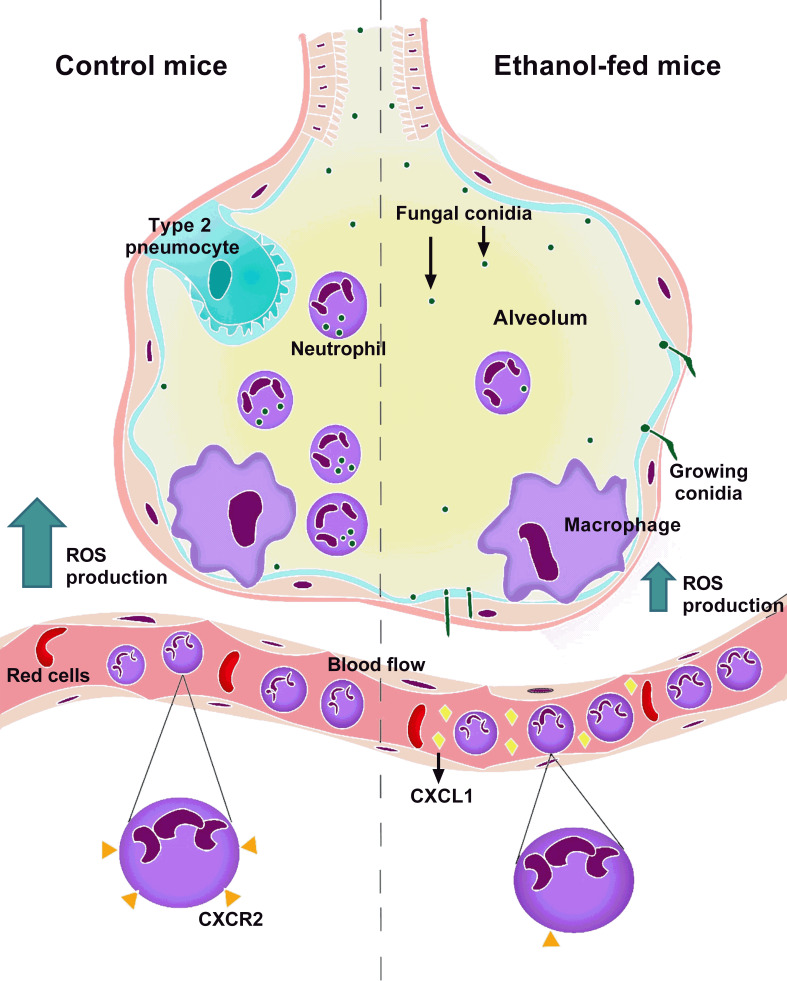
To conclude, the findings presented here indicate a new paradigm in how chronic ethanol consumption strongly impairs neutrophils host pulmonary defense against A. fumigatus infection. This infection in mice causes a great inflammatory response, with release of cytokines and chemokines that act in favor to recruit neutrophils into the alveoli and these neutrophils are able to clear the fungus. In contrast, in a condition of chronic ethanol consumption, despite the correct induction of inflammatory response, neutrophils exhibit failure in activation, through the down regulation of CD11b and up regulation of CD62L in blood neutrophils and by accentuated release of CXCR2 ligands in blood flow. This leads to CXCR2 down regulation, which culminated in impaired neutrophils recruitment, increased fungal load and exacerbated lung pathology in mice. We also associate the lower neutrophils levels into the airways with lower innate polarized-Th17 immune response and reduced phagocytosis and killing of A. fumigatus conidia. In fact, we observed growing conidia and substantial fungal burden in lung from ethanol-fed mice, contributing to the highest susceptibility of ethanol-treated mice to A. fumigatus infection (Figure 9).
Figure 9. Mechanism related to chronic ethanol dysfunction in neutrophils after A. fumigatus infection.
In normal conditions, infection with A. fumigatus in mice causes a huge inflammatory response, characterized by neutrophil chemokines release. The recruited neutrophils clear the infection by phagocytosis and ROS-mediated killing. In contrast, in a condition of chronic ethanol consumption, despite the correct induction of inflammatory response, there is an increase of CXCR2 ligands in blood flow, causing CXCR2 downregulation. This leads to lower neutrophils recruitment, culminating in substantial fungal burden into the lungs from ethanol-fed mice.
Materials and methods
Key resources table.
| Reagent type (species) or resource |
Designation | Source or reference |
Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | C57BL/6J | Multidisciplinary center for Biological Investigation on Laboratory Animal Science (CEMIB) -Unicamp | C57BL/6JUnib | |
| Genetic reagent (Aspergillus fumigatus) | Strain A1163 |
A. fumigatus CEA17 isolate (CEA10 derivative) and converted to pyrG+ via A. niger pyrG gene ectopic insertion Fedorova et al., 2008; Malacco et al., 2019. |
||
| Cell line (M. musculus) | Primary bone marrow neutrophils | This paper | C57BL/6JUnib | Freshly isolated from C57BL/6J (M. musculus) |
| Peptide, recombinant protein | Recombinant murine MIP-2 (CXCL2) | PeproTech | Cat# 250–15 | Chemotaxis (20 ng/ml) |
| Antibody | Purified NA/LE CD16/CD32 Clone 2.4G2 - FC Block (Rat monoclonal) | BD Biosciences | Cat# 553140 | FACS (1:100) |
| Antibody | anti-CD3-FITC (Rat monoclonal) | BD Biosciences | Cat# 555274 | FACS (1:100) |
| Antibody | anti-CD4-APC (Rat monoclonal) | BD Biosciences | Cat# 553051 | FACS (1:200) |
| Antibody | anti-IL-17a-PE (Rat monoclonal) | BD Biosciences | Cat# 559502 | FACS (1:100) |
| Antibody | anti-Ly6G-BV421 (Rat monoclonal) | BD Biosciences | Cat# 562737 | FACS (1:50) |
| Antibody | anti-CXCR2-PE (Rat monoclonal) | R and D Systems | Cat# FAB2164P | FACS (1:10) |
| Antibody | anti-CD62L-APC (Rat monoclonal) | BD Biosciences | Cat# 553152 | FACS (1:100) |
| Antibody | anti-CD11b-FITC (Rat monoclonal) | BD Biosciences | Cat# 553310 | FACS (1:100) |
| Commercial assay or kit | Mouse TNF-a ELISA kit | R and D Systems | Cat# DY410 | |
| Commercial assay or kit | Mouse IL-1b ELISA kit | R and D Systems | Cat# DY401 | |
| Commercial assay or kit | Mouse CXCL1 ELISA kit | R and D Systems | Cat# DY453 | |
| Commercial assay or kit | Mouse CXCL2 ELISA kit | R and D Systems | Cat# DY452 | |
| Commercial assay or kit | Mouse IL-17 ELISA kit | R and D Systems | Cat# DY421 | |
| Commercial assay or kit | Mouse IL-10 ELISA kit | R and D Systems | Cat# DY417 | |
| Software, algorithm | Prism | GraphPad | ||
| Software, algorithm | FlowJo | BD |
Ethics statement and mouse model of chronic ethanol consumption
All animal experiments received prior approval from the Animal Ethics Committee (CEUA) of Universidade Federal de Minas Gerais (UFMG), Brazil (Protocol number: 4/2015). Male C57BL/6J mice were randomly allocated into experimental groups. Mice were maintained in specific pathogen-free conditions. The chronic ethanol consumption model used was previously described (Ceron et al., 2018; Simplicio et al., 2017). Briefly, mice received ethanol 5% (v/v) in the first week, followed by 10% (v/v) in the second week (to help mice acclimate with this intervention) and were treated during 10 weeks with 20% (v/v) of ethanol in their drinking water. Control group received water. This protocol replicates blood ethanol levels after chronic ethanol consumption in human subjects, as described in studies with C57BL/6J or BALB/c mice (Ceron et al., 2018; Simplicio et al., 2017; Urso et al., 1981). Weight change, food and liquid consumption were measured weekly during ethanol treatment.
Cytokine and chemokine measurement
Cytokine and chemokine levels (TNF-α, IL-1β, CXCL1, CXCL2, IL-17, IL-10) were quantified in BAL, serum or plasma fluid using DuoSet ELISA kits (R and D Systems), in accordance to the manufacturer’s instructions.
Determination of blood ethanol levels
The determination of blood ethanol levels was made as previously described (Gonzaga et al., 2015). Briefly, mice were anesthetized and 100 μL of blood was collected and transferred into headspace vials. Blood ethanol levels were measured by gas chromatograph using a gas chromatographer as previously described (Ceron et al., 2018).
Mice infection
To determine the impact of chronic alcohol intake in fungal pulmonary infection, male C57BL/6J mice were treated for 12 weeks. After the last day of treatment, mice were infected intranasally with Aspergillus fumigatus A1163 strain (Fedorova et al., 2008). The fungus was grown in complete media for 48 hr at 37°C (Malacco et al., 2019). Fungal conidia were harvested by washing the media with sterile phosphate-buffered saline (PBS). After filtering, conidia were centrifuged at 1400 x g resuspended and counted in Neubauer chamber. Mice were infected with 3 × 108 conidia/animal, prepared in PBS.
BAL and tissue extraction
After 24 or 48 hr of infection, mice were anesthetized with ketamine (100 mg/kg) and xilazine (6 mg/Kg) and blood smear and serum or plasma were collected. After that, mice were euthanized and bronchoalveolar lavage fluid (BALF) was harvested as previously described (Malacco et al., 2019). BALF total cell counts were determined by counting leukocytes in Neubauer chamber. Differential cell count and in vivo phagocytosis count were obtained from cytospin preparations (Shandon III). BALF supernatants were used for cytokines, chemokines and total protein measurements. Protein amounts were quantified in BALF samples using the Bradford assay (Bradford, 1976).
Lung pathology analysis
At the indicated time points, lungs were collected. The right lobes were removed and frozen for subsequent analysis of myeloperoxidase (MPO) (Huang et al., 2016), N-acetyglucosaminidase (NAG) (Reiner et al., 1981), eosinophil peroxidase (EPO) (Strath et al., 1985) or measurement of fungal burden. The left lobes were fixed in formalin 4% (v/v) for histopathological analysis. Formalin-fixed tissue was dehydrated gradually in ethanol, embedded in paraffin, and 4 µm sections were stained with Hematoxylin and Eosin (H and E) or Grocott’s methenamine silver (GMS). The total histopathology score considered inflammatory infiltrate, interstitial and alveolar edema and hemorrhage (Hubbs et al., 1997). The percentage of germination of A. fumigatus conidia was counted in 200 to 300 fungal conidia in GMS-stained slides at x 100 magnification microscope.
Flow cytometry
Leukocytes obtained from BAL or blood samples were subjected to hypotonic lysis to remove residual erythrocytes, as described previously (Russo et al., 2009). Briefly, cells were treated with Fc block (R and D Systems), labeled with relevant antibodies, namely: CD3 - fluorescein isothiocyanate (FITC), CD4 - APC, IL-17 - phycoerythrin (PE), Ly6G - brilliant violet 421 (BV421), CXCR2 - PE, CD62L – APC and CD11b – FITC or isotype control. At least 30,000 events were acquired in a FACScan cytometer, and data were analyzed using FlowJo (Tree Star, Ashland, OR, USA) software. The relevant populations were gated, using accepted criteria for granularity, and sized and evaluated for staining of relevant surface and intracellular markers.
Intravital microscopy
The mouse cremaster preparation was used to study the behavior of leukocytes in the microcirculation and adjacent connective tissue, as previously described (Pinho et al., 2007). Briefly, 2 hr prior the surgery, mouse cremaster muscle were injected with LPS (250 ng/mL) diluted in saline. Then an incision was made in the scrotal skin to expose the cremaster muscle, which was then carefully removed from the associated fascia. A lengthwise incision was made on the ventral surface of the cremaster muscle using a cautery. The testicle and the epididymis were separated from the underlying muscle and were moved into the abdominal cavity. The muscle was then spread out over an optically clear viewing pedestal and was secured along the edges with a 4–0 suture. The exposed tissue was superfused with warm PBS. An intravital microscope (Olympus BX50F4) with x 20 magnification objective lens and x 10 times magnification eyepiece was used to examine the cremasteric microcirculation. A video camera (5100 HS; Panasonic) was used to project the images onto a monitor, and the images were recorded for playback analysis. The numbers of rolling and adherent leukocytes were determined offline during the video playback analyses. Leukocytes were considered adherent to the venular endothelium if they remained stationary for at least 30 s. Rolling leukocytes were defined as white cells moving at a velocity slower than that of the erythrocytes within a given vessel.
BM-derived neutrophils, phagocytosis and killing of fungal conidia
After isolation of mouse bone marrow (BM) from femur and tibia in RPMI 1640 medium, neutrophils were separated by density gradient centrifugation using Histopaque 1077 (density, 1.077 g/ml) in a 15 ml conical tube. Then, erythrocytes were lysed using ACK lysing buffer and the neutrophils were counted. Neutrophils purity was over than 80% (Swamydas and Lionakis, 2013). Phagocytosis and killing assay were performed by incubation of BM-derived neutrophils from mice treated and non-treated with ethanol with A. fumigatus conidia for 4 hr (phagocytosis) or 6 hr (killing) at 37° with 5% of CO2 in the ratio of 5:1. Phagocytosis was evaluated in cytospin preparations. To determine A. fumigatus killing, cells were lysed with distilled water and the diluted samples were plated in fungal medium and colony-forming units (CFU) were determined after overnight incubation at 37°C, and the percentage of killing was calculated as a percentage of the viability after incubation without neutrophils.
ROS detection
Luminometry assays were performed to evaluate the production of ROS by BM-derived neutrophils (Goes et al., 2016). Neutrophils (1 × 106 cells/well) were resuspended in complete RPMI medium without phenol red. Then the cells were plated in 96 well opaque plates (NUNC, Rochester, NY, USA) with 0.05 mM luminol (5-Amino-2,3-dihydro-1,4-phthalazinedione; Sigma-Aldrich) and A. fumigatus conidia in the proportion of 10 conidia to one neutrophil. Measurements were taken for 60 min with 2 min interval. Production of ROS was assayed by the light intensity generated by the reaction between ROS and luminol and expressed as fluorescent relative units.
Ex vivo chemotaxis assay
A modified Boyden chamber assay to examine the neutrophil chemoattractant response to CXCL-2 (kindly provided by Dr. José Carlos Alves-Filho, Universidade de São Paulo, Ribeirão Preto, Brazil) was performed using a 48-well microchamber (Neuro Probe) as previously described (Pinho et al., 2007). Murine bone marrow neutrophils were isolated as above described and resuspended in RPMI. Recombinant mouse CXCL-2 (20 ng/mL) diluted in running buffer (for wells containing neutrophils) or appropriate buffer control was added to the lower chambers of the apparatus. A 5-µm-pore polycarbonate membrane (Neuro Probe) was placed between the upper and lower chambers, and 5 × 104 cells in a volume of 50 µL were added to the top chambers of the apparatus. Cells were allowed to migrate into the membrane for 1 hr per treatment at 37°C with 5% CO2. Following incubation, the chamber was disassembled and the membrane was scraped and washed three times in PBS to remove nonadherent cells before being fixed in methanol and stained using the Diff-Quik system (Dade Behring). Each well-associated membrane area was scored using light microscopy to count the intact cells present in five random fields.
Statistical analysis
All experiments were made at least twice (biological replication) by independent experiments. The sample size estimation was done with G*Power 3.1 Software (Jacob Cohen's A power primer, 1992 in Psychological Bulletin Journal). Statistical analysis was performed with Graph Pad Prism six software (Graph Pad Prism Software, Inc, Sandiego, CA). Data are presented as the mean ± SD and were analyzed using One-way analysis of variance (ANOVA) followed by Tukey post-test to compare different groups. Student’s t test was used to compare two groups. Survival analysis was made by Log Rank test. Statistical significance was set as < 0.05. For more information, please see the transparent reporting form.
Acknowledgements
We would like to thank Universidade Federal de Minas Gerais for the opportunity to develop this work. We are thankful to Ilma Marçal and Rosemeire Oliveira for technical support.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Nathalia Luisa Sousa de Oliveira Malacco, Email: nathalialuisa2@gmail.com.
Frederico Marianetti Soriani, Email: fredsori@gmail.com.
Jos WM van der Meer, Radboud University Medical Center, Netherlands.
Frank L van de Veerdonk, Radboud University Medical Center, Netherlands.
Funding Information
This paper was supported by the following grants:
Fundação de Amparo à Pesquisa do Estado de Minas Gerais APQ-01756-10 to Jessica Amanda Marques Souza, Leda Quercia Vieira, Danielle Glória Souza, Vanessa Pinho, Mauro Martins Teixeira, Frederico Marianetti Soriani.
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior 001 to Nathalia Luisa Sousa de Oliveira Malacco, Jessica Amanda Marques Souza.
Conselho Nacional de Desenvolvimento Científico e Tecnológico 474528-2012- 0 and 483184-2011-0 to Frederico Marianetti Soriani.
Instituto Nacional de Ciência e Tecnologia em Dengue e Interações Microrganismo-Hospedeiro to Nathalia Luisa Sousa de Oliveira Malacco, Jessica Amanda Marques Souza, Flavia Rayssa Braga Martins, Leda Quercia Vieira, Danielle Glória Souza, Vanessa Pinho, Mauro Martins Teixeira, Frederico Marianetti Soriani.
Universidade Federal de Minas Gerais 001 to Nathalia Luisa Sousa de Oliveira Malacco, Jessica Amanda Marques Souza, Flavia Rayssa Braga Martins, Milene Alvarenga Rachid, Celso Martins Queiroz-Junior, Grazielle Ribeiro Goes, Leda Quercia Vieira, Danielle Glória Souza, Vanessa Pinho, Mauro Martins Teixeira, Frederico Marianetti Soriani.
Fundação de Amparo à Pesquisa do Estado de Minas Gerais APQ-02198-14 and APQ-03950-17 to Jessica Amanda Marques Souza, Leda Quercia Vieira, Danielle Glória Souza, Vanessa Pinho, Mauro Martins Teixeira, Frederico Marianetti Soriani.
Additional information
Competing interests
No competing interests declared.
Author contributions
Investigation, Methodology, Writing - original draft.
Methodology.
Methodology.
Resources, Methodology.
Methodology.
Resources, Methodology.
Formal analysis.
Methodology.
Methodology.
Resources.
Resources.
Resources, Methodology.
Resources, Writing - review and editing.
Conceptualization, Resources, Supervision, Funding acquisition, Project administration, Writing - review and editing.
Ethics
Animal experimentation: This study was performed in strict accordance with the recommendations of the CONCEA (Conselho Nacional de Controle de Experimentação Animal) from Brazil. All animal experiments received prior approval from the Animal Ethics Committee (CEUA) of Universidade Federal de Minas Gerais (UFMG), Brazil (Protocol number: 4/2015).
Additional files
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files.
References
- Alves-Filho JC, Freitas A, Souto FO, Spiller F, Paula-Neto H, Silva JS, Gazzinelli RT, Teixeira MM, Ferreira SH, Cunha FQ. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial Sepsis. PNAS. 2009;106:4018–4023. doi: 10.1073/pnas.0900196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddley JW. Clinical risk factors for invasive aspergillosis. Medical Mycology. 2011;49:S7–S12. doi: 10.3109/13693786.2010.505204. [DOI] [PubMed] [Google Scholar]
- Bagby GJ, Zhang P, Stoltz DA, Nelson S. Suppression of the granulocyte colony-stimulating factor response to Escherichia coli challenge by alcohol intoxication. Alcoholism: Clinical and Experimental Research. 1998;22:1740–1745. doi: 10.1111/j.1530-0277.1998.tb03974.x. [DOI] [PubMed] [Google Scholar]
- Bautista AP. Impact of alcohol on the ability of kupffer cells to produce chemokines and its role in alcoholic liver disease. Journal of Gastroenterology and Hepatology. 1999;2000:349–356. doi: 10.1046/j.1440-1746.2000.02174.x. [DOI] [PubMed] [Google Scholar]
- Bhatty M, Jan BL, Tan W, Pruett SB, Nanduri B. Role of acute ethanol exposure and TLR4 in early events of Sepsis in a mouse model. Alcohol. 2011;45:795–803. doi: 10.1016/j.alcohol.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J, Reed JC, Thompson WM, Pizzo S. Miliary aspergillosis. Am J Roentgenol. 1978;131:707–709. doi: 10.2214/ajr.131.4.707. [DOI] [PubMed] [Google Scholar]
- Boé DM, Nelson S, Zhang P, Bagby GJ. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. The Journal of Infectious Diseases. 2001;184:1134–1142. doi: 10.1086/323661. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Science Translational Medicine. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- Caffrey-Carr AK, Kowalski CH, Beattie SR, Blaseg NA, Upshaw CR, Thammahong A, Lust HE, Tang Y-W, Hohl TM, Cramer RA, Obar JJ. Interleukin 1α is critical for resistance against highly virulent Aspergillus fumigatus isolates. Infection and Immunity. 2017;85:00661-17. doi: 10.1128/IAI.00661-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceron CS, do Vale GT, Simplicio JA, Ricci ST, De Martinis BS, de Freitas A, Tirapelli CR. Chronic ethanol consumption increases vascular oxidative stress and the mortality induced by sub-lethal Sepsis: potential role of iNOS. European Journal of Pharmacology. 2018;825:39–47. doi: 10.1016/j.ejphar.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Isturiz RE, Malech HL, Root RK, Wilfert CM, Gutman L, Buckley RH. Fungal infection in chronic granulomatous disease the importance of the phagocyte in defense against fungi. The American Journal of Medicine. 1981;71:59–66. doi: 10.1016/0002-9343(81)90259-x. [DOI] [PubMed] [Google Scholar]
- D'Souza El-Guindy NB, de Villiers WJ, Doherty DE. Acute alcohol intake impairs lung inflammation by changing pro- and anti-inflammatory mediator balance. Alcohol. 2007;41:335–345. doi: 10.1016/j.alcohol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clinical Microbiology Reviews. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwig LP, Gow NAR. Interactions of fungal pathogens with phagocytes. Nature Reviews Microbiology. 2016;14:163–176. doi: 10.1038/nrmicro.2015.21. [DOI] [PubMed] [Google Scholar]
- Fedorova ND, Khaldi N, Joardar VS, Maiti R, Amedeo P, Anderson MJ, Crabtree J, Silva JC, Badger JH, Albarraq A, Angiuoli S, Bussey H, Bowyer P, Cotty PJ, Dyer PS, Egan A, Galens K, Fraser-Liggett CM, Haas BJ, Inman JM, Kent R, Lemieux S, Malavazi I, Orvis J, Roemer T, Ronning CM, Sundaram JP, Sutton G, Turner G, Venter JC, White OR, Whitty BR, Youngman P, Wolfe KH, Goldman GH, Wortman JR, Jiang B, Denning DW, Nierman WC. Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLOS Genetics. 2008;4:e1000046. doi: 10.1371/journal.pgen.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazendam RP, van Hamme JL, Tool AT, Hoogenboezem M, van den Berg JM, Prins JM, Vitkov L, van de Veerdonk FL, van den Berg TK, Roos D, Kuijpers TW. Human neutrophils use different mechanisms to kill Aspergillus fumigatus conidia and hyphae: evidence from phagocyte defects. The Journal of Immunology. 2016;196:1272–1283. doi: 10.4049/jimmunol.1501811. [DOI] [PubMed] [Google Scholar]
- Goes GR, Rocha PS, Diniz AR, Aguiar PH, Machado CR, Vieira LQ. Trypanosoma cruzi needs a signal provided by reactive oxygen species to infect macrophages. PLOS Neglected Tropical Diseases. 2016;10:e0004555. doi: 10.1371/journal.pntd.0004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzaga NA, Mecawi AS, Antunes-Rodrigues J, De Martinis BS, Padovan CM, Tirapelli CR. Ethanol withdrawal increases oxidative stress and reduces nitric oxide bioavailability in the vasculature of rats. Alcohol. 2015;49:47–56. doi: 10.1016/j.alcohol.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Gurung P, Young BM, Coleman RA, Wiechert S, Turner LE, Ray NB, Waldschmidt TJ, Legge KL, Cook RT. Chronic ethanol induces inhibition of antigen-specific CD8+ but not CD4+ immunodominant T cell responses following Listeria monocytogenes inoculation. Journal of Leukocyte Biology. 2009;85:34–43. doi: 10.1189/jlb.0208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustot T, Maillart E, Bocci M, Surin R, Trépo E, Degré D, Lucidi V, Taccone FS, Delforge ML, Vincent JL, Donckier V, Jacobs F, Moreno C. Invasive aspergillosis in patients with severe alcoholic hepatitis. Journal of Hepatology. 2014;60:267–274. doi: 10.1016/j.jhep.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Huang J, Milton A, Arnold RD, Huang H, Smith F, Panizzi JR, Panizzi P. Methods for measuring myeloperoxidase activity toward assessing inhibitor efficacy in living systems. Journal of Leukocyte Biology. 2016;99:541–548. doi: 10.1189/jlb.3RU0615-256R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbs AF, Castranova V, Ma JY, Frazer DG, Siegel PD, Ducatman BS, Grote A, Schwegler-Berry D, Robinson VA, Van Dyke C, Barger M, Xiang J, Parker J. Acute lung injury induced by a commercial leather conditioner. Toxicology and Applied Pharmacology. 1997;143:37–46. doi: 10.1006/taap.1996.8053. [DOI] [PubMed] [Google Scholar]
- King J, Henriet S, Warris A. Aspergillosis in chronic granulomatous disease. Journal of Fungi. 2016;2:15–16. doi: 10.3390/jof2020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature Reviews Immunology. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. European Respiratory Review. 2011;20:156–174. doi: 10.1183/09059180.00001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latgé J. Aspergillus fumigatus and aspergillosis Aspergillus fumigatus and aspergillosis. Clinical Microbiology Reviews. 1999;12:310–350. doi: 10.1128/CMR.00140-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latgé JP. The pathobiology of Aspergillus fumigatus. Trends in Microbiology. 2001;9:382–389. doi: 10.1016/S0966-842X(01)02104-7. [DOI] [PubMed] [Google Scholar]
- Liang Y, Yeligar SM, Brown LAS. Chronic-Alcohol-Abuse-Induced oxidative stress in the development of acute respiratory distress syndrome. The Scientific World Journal. 2012;2012:1–9. doi: 10.1100/2012/740308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS. Metabolism of alcohol. Clinics in Liver Disease. 2005;9:1–35. doi: 10.1016/j.cld.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, Szabo G. Alcohol-induced IL-1β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. Journal of Leukocyte Biology. 2013;94:171–182. doi: 10.1189/jlb.1212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luján M, Gallego M, Belmonte Y, Fontanals D, Vallès J, Lisboa T, Rello J. Influence of pneumococcal serotype group on outcome in adults with bacteraemic pneumonia. European Respiratory Journal. 2010;36:1073–1079. doi: 10.1183/09031936.00176309. [DOI] [PubMed] [Google Scholar]
- Malacco NLSO, Souza JAM, Mendes AC, Rachid MA, Kraemer LR, Mattos MS, Lima GN, Sousa LP, Souza DG, Pinho V, Teixeira MM, Russo RC, Soriani FM. Acute lung injury and repair induced by single exposure of Aspergillus fumigatus in immunocompetent mice. Future Microbiology. 2019;14:1511–1525. doi: 10.2217/fmb-2019-0214. [DOI] [PubMed] [Google Scholar]
- Maraslioglu M, Oppermann E, Blattner C, Weber R, Henrich D, Jobin C, Schleucher E, Marzi I, Lehnert M. Chronic Ethanol Feeding Modulates Inflammatory Mediators, Activation of Nuclear Factor- κ B, and Responsiveness to Endotoxin in Murine Kupffer Cells and Circulating Leukocytes. Mediators of Inflammation. 2014;2014:1–16. doi: 10.1155/2014/808695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Edsen-Moore M, McGill J, Coleman RA, Cook RT, Legge KL. Chronic alcohol consumption increases the severity of murine influenza virus infections. The Journal of Immunology. 2008;181:641–648. doi: 10.4049/jimmunol.181.1.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, Happel KI, Zhang P, Kolls JK, Nelson S. Focus on: alcohol and the immune system. Alcohol Res Health. 2010;33:97–108. [PMC free article] [PubMed] [Google Scholar]
- Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nature Reviews. Immunology. 2019;19:255–265. doi: 10.1038/s41577-019-0141-8. [DOI] [PubMed] [Google Scholar]
- Ohama H, Asai A, Ito I, Suzuki S, Kobayashi M, Higuchi K, Suzuki F. M2b macrophage elimination and improved resistance of mice with chronic alcohol consumption to opportunistic infections. The American Journal of Pathology. 2015;185:420–431. doi: 10.1016/j.ajpath.2014.09.022. [DOI] [PubMed] [Google Scholar]
- Parlet CP, Kavanaugh JS, Horswill AR, Schlueter AJ. Chronic ethanol feeding increases the severity of Staphylococcus aureus skin infections by altering local host defenses. Journal of Leukocyte Biology. 2015;97:769–778. doi: 10.1189/jlb.4A0214-092R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe B, Ibrahim-Granet O, Prévost MC, Gougerot-Pocidalo MA, Sanchez Perez M, Van der Meeren A, Latgé JP. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infection and Immunity. 2003;71:3034–3042. doi: 10.1128/IAI.71.6.3034-3042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nature Medicine. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Critical Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho V, de Castro Russo R, Amaral FA, de Sousa LP, Barsante MM, de Souza DG, Alves-Filho JC, Cara DC, Hayflick JS, Rommel C, Ruckle T, Rossi AG, Teixeira MM. Tissue- and Stimulus-Dependent role of phosphatidylinositol 3-Kinase isoforms for neutrophil recruitment induced by chemoattractants in vivo. The Journal of Immunology. 2007;179:7891–7898. doi: 10.4049/jimmunol.179.11.7891. [DOI] [PubMed] [Google Scholar]
- Pritchard MT, Nagy LE. Ethanol-Induced liver injury: potential roles for Egr-1. Alcoholism: Clinical & Experimental Research. 2005;29:146S–150. doi: 10.1097/01.alc.0000189286.81943.51. [DOI] [PubMed] [Google Scholar]
- Reiner RG, Tanner AR, Keyhani AH, Wright R. Tanner a R, keyhani a H, Wright R. A comparative study of lysosomal enzyme activity in monocytes and kupffer cells isolated simultaneously in a rat model of liver injury. Clinical and Experimental Immunology. 1981;43:376–380. [PMC free article] [PubMed] [Google Scholar]
- Rios-Santos F, Alves-Filho JC, Souto FO, Spiller F, Freitas A, Lotufo CM, Soares MB, Dos Santos RR, Teixeira MM, Cunha FQ. Down-regulation of CXCR2 on neutrophils in severe Sepsis is mediated by inducible nitric oxide synthase-derived nitric oxide. American Journal of Respiratory and Critical Care Medicine. 2007;175:490–497. doi: 10.1164/rccm.200601-103OC. [DOI] [PubMed] [Google Scholar]
- Robertson CM, Perrone EE, McConnell KW, Dunne WM, Boody B, Brahmbhatt T, Diacovo MJ, Van Rooijen N, Hogue LA, Cannon CL, Buchman TG, Hotchkiss RS, Coopersmith CM. Neutrophil depletion causes a fatal defect in murine pulmonary Staphylococcus aureus clearance. Journal of Surgical Research. 2008;150:278–285. doi: 10.1016/j.jss.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L. Innate and adaptive immunity in Candida Albicans infections and saprophytism. Journal of Leukocyte Biology. 2000;68:175–179. doi: 10.1189/jlb.68.2.175. [DOI] [PubMed] [Google Scholar]
- Russo RC, Guabiraba R, Garcia CC, Barcelos LS, Roffê E, Souza AL, Amaral FA, Cisalpino D, Cassali GD, Doni A, Bertini R, Teixeira MM. Role of the chemokine receptor CXCR2 in bleomycin-induced pulmonary inflammation and fibrosis. American Journal of Respiratory Cell and Molecular Biology. 2009;40:410–421. doi: 10.1165/rcmb.2007-0364OC. [DOI] [PubMed] [Google Scholar]
- Segal BH, Romani LR. Invasive aspergillosis in chronic granulomatous disease. Medical Mycology. 2009;47:S282–S290. doi: 10.1080/13693780902736620. [DOI] [PubMed] [Google Scholar]
- Simplicio JA, Gonzaga NA, Nakashima MA, De Martinis BS, Cunha TM, Tirapelli LF, Tirapelli CR. Tumor necrosis factor-α receptor 1 contributes to ethanol-induced vascular reactive oxygen species generation and hypertension. Journal of the American Society of Hypertension. 2017;11:684–696. doi: 10.1016/j.jash.2017.07.008. [DOI] [PubMed] [Google Scholar]
- Soethout EC, Müller KE, Rutten VP. Neutrophil migration in the lung, general and bovine-specific aspects. Veterinary Immunology and Immunopathology. 2002;87:277–285. doi: 10.1016/S0165-2427(02)00053-3. [DOI] [PubMed] [Google Scholar]
- Strath M, Warren DJ, Sanderson CJ. Detection of eosinophils using an eosinophil peroxidase assay its use as an assay for eosinophil differentiation factors. Journal of Immunological Methods. 1985;83:209–215. doi: 10.1016/0022-1759(85)90242-X. [DOI] [PubMed] [Google Scholar]
- Sun B, Hu X, Liu G, Ma B, Xu Y, Yang T, Shi J, Yang F, Li H, Zhang L, Zhao Y. Phosphatase Wip1 negatively regulates neutrophil migration and inflammation. The Journal of Immunology. 2014;192:1184–1195. doi: 10.4049/jimmunol.1300656. [DOI] [PubMed] [Google Scholar]
- Swamydas M, Lionakis MS. Isolation, purification and labeling of mouse bone marrow neutrophils for functional studies and adoptive transfer experiments. Journal of Visualized Experiments. 2013;10:e50586. doi: 10.3791/50586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defence. Alcohol and Alcoholism. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Szabo G, Saha B. Alcohol’s Effect on Host Defense. Alcohol Research : Current Reviews. 2015;37:159–170. doi: 10.35946/arcr.v37.2.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevejo-Nunez G, Chen K, Dufour JP, Bagby GJ, Horne WT, Nelson S, Kolls JK. Ethanol impairs mucosal immunity against Streptococcus pneumoniae infection by disrupting interleukin 17 gene expression. Infection and Immunity. 2015;83:2082–2088. doi: 10.1128/IAI.02869-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimoto Y, Asai A, Tsuda Y, Ito I, Nishiguchi T, Garcia MC, Suzuki S, Kobayashi M, Higuchi K, Suzuki F. M2b monocytes provoke bacterial pneumonia and gut Bacteria-Associated Sepsis in alcoholics. The Journal of Immunology. 2015;195:5169–5177. doi: 10.4049/jimmunol.1501369. [DOI] [PubMed] [Google Scholar]
- Urso T, Gavaler JS, Van Thiel DH. Blood ethanol levels in sober alcohol users seen in an emergency room. Life Sciences. 1981;28:1053–1056. doi: 10.1016/0024-3205(81)90752-9. [DOI] [PubMed] [Google Scholar]
- Wood S, Pithadia R, Rehman T, Zhang L, Plichta J, Radek KA, Forsyth C, Keshavarzian A, Shafikhani SH. Chronic alcohol exposure renders epithelial cells vulnerable to bacterial infection. PLOS ONE. 2013;8:e54646. doi: 10.1371/journal.pone.0054646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation . Global Status Report on Alcohol and Health 2014. WHO; 2014. http://www.who.int/substance_abuse/publications/global_alcohol_report/msbgsruprofiles.pdf [Google Scholar]
- Yago T, Liu Z, Ahamed J, McEver RP. Cooperative PSGL-1 and CXCR2 signaling in neutrophils promotes deep vein thrombosis in mice. Blood. 2018;132:1426–1437. doi: 10.1182/blood-2018-05-850859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar SM, Harris FL, Hart CM, Brown LA. Ethanol induces oxidative stress in alveolar macrophages via upregulation of NADPH oxidases. The Journal of Immunology. 2012;188:3648–3657. doi: 10.4049/jimmunol.1101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar SM, Harris FL, Hart CM, Brown LAS. Glutathione attenuates ethanol-induced alveolar macrophage oxidative stress and dysfunction by down-regulating NADPH oxidases. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2014;4:429–441. doi: 10.1152/ajplung.00159.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CH, Ho PS, Yeh YW, Liang CS, Kuo SC, Huang CC, Chen CY, Shih MC, Ma KH, Sung YF, Lu RB, Huang SY. Differential cytokine levels between early withdrawal and remission states in patients with alcohol dependence. Psychoneuroendocrinology. 2017;76:183–191. doi: 10.1016/j.psyneuen.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. American Journal of Respiratory Cell and Molecular Biology. 2009;40:519–535. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhang F, Zhu Z, Luong D, Meadows GG. Chronic alcohol consumption enhances iNKT cell maturation and activation. Toxicology and Applied Pharmacology. 2015;282:139–150. doi: 10.1016/j.taap.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]