ABSTRACT
Background
Hong Kong faces several public health problems including malnutrition and osteoporosis. Considering the typical Chinese diet and overall low physical activity levels of Chinese adults, timely interventions to improve nutritional status and bone health are needed.
Objectives
We examined the effects of a nutrition plus exercise intervention on serum vitamin B-12 and 25-hydroxyvitamin D [25(OH)D], bone turnover markers, and parathyroid hormone (PTH) concentrations in apparently healthy Chinese middle-aged and older adults.
Methods
In this 24-wk randomized controlled trial, 180 Chinese adults (85 women, mean ± SD age: 61 ± 6 y) were randomly assigned to receive a fortified milk supplement (2 × 30 g/d) and an exercise program (2 × 1 h/wk including resistance, balance, and aerobic training) or no intervention. The primary outcome was physical performance. In this article we analyzed the secondary outcomes serum vitamin B-12 and 25(OH)D concentrations, assessed at baseline, 12 wk, and 24 wk. Also, bone turnover markers and PTH concentrations were studied. Linear mixed models evaluated group differences over time.
Results
A significant time × group interaction (P < 0.001) was found for serum vitamin B-12 and 25(OH)D concentrations and the bone turnover markers, but not for serum PTH concentrations (P = 0.09). The intervention increased mean ± SD vitamin B-12 concentrations from baseline (345 ± 119 pmol/L) to 24 wk (484 ± 136 pmol/L), whereas concentrations remained stable within the control. For 25(OH)D concentrations, the intervention group had a greater increase from baseline (54.7 ± 14.2 nmol/L) to 24 wk (80.1 ± 19.2 nmol/L) than the control (60.6 ± 15.2 compared with 65.6 ± 14.6 nmol/L). The ratio of the net effect of bone formation and resorption was greater in the intervention group, suggesting less bone remodeling, irrespective of sex.
Conclusions
A fortified milk supplement and exercise intervention successfully improved vitamin B-12 and 25(OH)D concentrations as well as the balance of bone turnover markers of Chinese middle-aged and older adults.
This trial was registered at trialregister.nl as NTR6214.
Keywords: nutrition, exercise, vitamin B-12, 25-hydroxyvitamin D, 25(OH)D, bone turnover, middle-aged adults, older adults
Introduction
Approximately 31% of the population of Hong Kong is expected to be 65 y or older in 2036 (1). Being one of the fastest-aging populations in the world, Hong Kong faces several public health problems (2), including osteoporosis and hip fracture incidence (3, 4). In addition, there is a significant burden of malnutrition (5). The onset or progress of aging-related pathologies may be delayed with nutrition and physical activity.
For optimal bone health, it is recommended to have a sufficient intake of calcium, vitamin D, and protein (6, 7). However, the typical Chinese diet is low in calcium and due to the cultural habit of avoiding direct exposure to sunlight, vitamin D deficiency is highly prevalent among Chinese older adults (≥65 y) (8, 9). Furthermore, older adults have a greater need for protein than younger healthy adults (10). An inadequate intake of these key bone nutrients can increase the risk of falls and fractures and the prevalence of osteoporosis (7, 10, 11).
Vitamin B-12 deficiency is a common problem worldwide and its prevalence tends to increase with age (12). However, data are missing on the vitamin B-12 status in middle-aged and older adults living in Hong Kong. Deficiency is often caused by inadequate dietary intake and/or malabsorption of vitamin B-12 (12, 13). Dietary sources of vitamin B-12 are mostly animal-related such as milk. However, the ongoing cohort survey “China Health and Nutrition Survey” reported that milk intake was low in Chinese people aged 60 y and older (14). This might lead to a low vitamin B-12 status, which in turn has been associated with a low bone mineral density (BMD), increased bone turnover, and increased fracture risk (15, 16).
Multiple trials have investigated the effect of nutritional supplementation on nutritional status and bone health, but these are mainly in Western populations. The Asian dietary pattern is different from Western diets, which may require other nutritional strategies. A limited number of studies in Chinese women have shown that milk supplementation for 24 mo increased calcium intake and led to less bone loss (17–19). In addition, men should not be overlooked. Because although BMD decreases at a faster rate in postmenopausal women than in men, the latter group tend to have a higher mortality risk after a fracture (20, 21). Besides nutrition, exercise is beneficial for bone health and a stronger effect may be expected from a combined nutrition and exercise intervention (22, 23). A survey conducted in 2014 showed low physical activity levels among the adult population of Hong Kong (24). Therefore, addressing physical activity levels is of interest. To the best of our knowledge, the current study is the first study targeting both Asian male and female middle-aged and older adults with a combined nutritional and exercise intervention to improve nutritional status and bone health.
This study is part of a large trial investigating the health impact of a nutrition and exercise program in Chinese adults. The aim of the present study was to assess whether a 24-wk multidomain lifestyle intervention including a fortified milk supplement and an exercise program had an effect on serum vitamin B-12 and 25-hydroxyvitamin D [25(OH)D] concentrations and markers of bone turnover in apparently healthy community-dwelling Chinese middle-aged and older adults, compared with a control group without intervention.
Methods
Study population
A 24-wk randomized controlled trial (RCT) was conducted in 180 apparently healthy community-dwelling Chinese middle-aged and older adults aged 50 y or older living in Hong Kong (latitude 22.3°N). Exclusion criteria included recent (i.e., past 3 mo) or concurrent participation in any lifestyle intervention program, BMI ≥ 27.5 kg/m2, self-reported allergy or intolerance to ingredients of the fortified milk supplement, poorly controlled or unstable chronic obstructive pulmonary disease/cardiovascular disease/hypertension, recent illnesses or fractures, undergoing treatment of cancer, and regular use of calcium or vitamin D supplements and traditional Chinese medicines. Eligible participants were randomly assigned, stratified by sex and age (50–64 compared with ≥65 y), to the intervention or control group. Further details concerning participant recruitment, study design, and methods have been described elsewhere (25). The study was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong (reference no. 2016.532) and registered at the Dutch Trial Registry (NTR6214). All participants provided written informed consent.
Treatment
The intervention consisted of a nutrition and an exercise component, both lasting for 24 wk. Participants in the intervention group had to drink 2 glasses of a fortified milk supplement (i.e., 2 sachets of 30 g each of unbranded OPTIMEL 60+ Diamond powder produced by FrieslandCampina, Netherlands; commercially available) daily providing 13.6 g protein, 1008 mg Ca, 30 μg cholecalciferol (vitamin D3), 2.9 μg vitamin B-12, and 212 kcal (Supplemental Table 1 provides a complete overview of the nutritional composition). Cholecalciferol was provided at 300% and 200% of the Chinese recommended nutrient intake (RNI) for persons aged 50–64 and ≥65 y, respectively; this was still below the tolerable upper limit (26). Vitamin B-12 was provided at 121% of the Chinese RNI (26). Participants also received a brief healthy lifestyle kit which highlighted the importance of a balanced diet and daily physical activity.
The exercise program consisted of 2 exercise sessions of 1 h each week, conducted in groups of 8–12 participants, for a total of 48 sessions. The exercise sessions consisted of a warm-up (5–10 min), resistance and balance training (20–30 min), an aerobic component (20 min), and a cool-down (5–10 min). Participants were asked to perform against 60%–80% of their estimated 1-repetition maximum load and resistance was progressively increased based on their Rating of Perceived Exertion (RPE) after each week of training. For the aerobic component, the aim was a minimum RPE of 13–15 at the beginning and to gradually proceed to an RPE of 15–18 during the later aerobic sessions. The exercises were designed to include variations and were conducted under the supervision of an exercise instructor.
Participants in the control group were asked to maintain their usual physical activities and dietary habits during the study period and were subjected to the same measurements as the intervention group.
Compliance
Participants were asked to return any unconsumed sachets of the fortified milk supplement every 2 wk. Subsequently, compliance to the nutritional intervention was assessed by dividing the number of sachets of fortified milk supplement consumed by the number of sachets provided. Compliance to the exercise intervention was assessed by dividing the completed exercise sessions by the total number of prescribed sessions.
Assessments
The primary outcome of the trial was physical performance, which was reported in another article (25). In this study we analyzed the secondary outcome of nutritional status including serum vitamin B-12 and 25(OH)D concentrations, and the tertiary outcomes of serum bone turnover markers and parathyroid hormone (PTH) concentrations. For this, blood samples were collected in the morning (from 09:00 to 11:30) after fasting for ≥12 h and after 15-min rest at baseline, 12 wk, and 24 wk. Blood was collected in vacutainers with no added anticoagulant and was kept at room temperature for ∼1 h in order to clot. Hereafter, serum was separated by centrifugation (at 2450 × g for 10–15 min at ambient temperature) and stored at −80°C, according to the instructions of Pathlab Medical Laboratories Ltd and Quest. The laboratory was accredited by the College of American Pathologists. Testing standards were based on the requirements of ISO 15189:2012 and included inspection of policies, procedures, records, internal quality control, and external quality assurance programs.
Serum vitamin B-12 and 25(OH)D concentrations
Serum vitamin B-12 was measured using chemiluminescent immunoassay (IMMULITE 2000; Siemens Healthineers Global). Intra-assay and interassay CVs were 6.7%–7.0% and 6.0%–7.9%, respectively. A cutoff value of <150 pmol/L was used for vitamin B-12 deficiency (13, 27).
Serum 25(OH)D was measured using LC–tandem MS (Quest Diagnostics). This assay measures both 25-hydroxycholecalciferol [25(OH)D3] and 25-hydroxyergocalciferol [25(OH)D2]; total 25(OH)D concentrations were used for the results in the current study. Intra-assay and interassay CVs were between 3.7% and 6.9%. Cutoff values for serum 25(OH)D are <25 nmol/L for deficiency, ≥25 to <50 nmol/L for insufficiency, and ≥50 nmol/L for sufficiency (28, 29).
Bone turnover markers
Serum N-amino terminal propeptide of type I collagen (PINP) (marker of bone formation) was measured using immunoassay (Quest Diagnostics). Intra-assay and interassay CVs were <10%. Serum C-terminal telopeptide of type I collagen (CTX) (β-isomerized; marker of bone resorption) was measured using electrochemiluminescence assay (Quest Diagnostics). Intra-assay and interassay CVs were 1.7%–2.2% and 3.1%–5.7%, respectively.
PTH
Serum concentration of intact PTH was measured using chemiluminescent immunoassay (IMMULITE 2000; Siemens Healthineers Global). Intra-assay and interassay CVs were 4.2%–5.7% and 6.3%–8.8%, respectively.
Dietary intake
All participants were asked to fill in a 3-d dietary record including both weekdays and weekend days at baseline and after 24 wk, which was checked by trained staff for completeness. Data were processed by the Food Processor Nutrition Analysis and Fitness software version 8.0 (ESHA Research), with the addition of composition of local foods based on food composition tables from China and Hong Kong. Protein intake (g/d), calcium intake (mg/d), and vitamin D intake (μg/d) are reported in this article, because these are key nutrients for optimal bone health. Vitamin B-12 intake (μg/d) is reported as well. More elaborate results on dietary changes will be described separately in another article.
Other measurements
Demographic, lifestyle, and medical history data were collected using standardized questionnaires. Physical activity was assessed with a validated Chinese version of the International Physical Activity Questionnaire (IPAQ-C) (30). Anthropometric measurements were done using standardized methods. These additional measurements are described elsewhere (25).
Statistical analysis
The trial was powered based on the outcome of gait speed [primary outcome, described in another article (25)] and the secondary outcome of serum 25(OH)D. Based on a previous trial investigating the effect of milk supplementation on bone turnover markers and vitamin D status in healthy Chinese adults (28), a minimum sample size of 114 (57/group) was needed to detect a mean difference of 8 nmol/L in serum 25(OH)D between the control and intervention groups with an SD of 15 nmol/L, 80% power, and a 5% significance level. Accounting for a noncompliance rate of 20% and a dropout rate of 20%, a final sample size of 180 (90/group) participants was chosen.
Data were analyzed using the intention-to-treat method. Continuous variables are presented as mean ± SD for normally distributed data and as median [IQR] for nonnormally distributed data. Categorical variables are presented as number of subjects (percentage). Independent t test, chi-square test, or Mann–Whitney U test was used to compare values at baseline between groups. All data were checked for normality. In case of a nonnormal distribution, nonparametric tests or log transformations were applied. Extreme outliers in primary dependent variables were retained in final analyses when results including and excluding the outlier were similar.
Differences in serum vitamin concentrations and bone markers between groups over time were analyzed using linear mixed models with subject as a random factor and time (baseline, 12 wk, and 24 wk), group (control and intervention), and time × group as fixed factors. To determine differences in vitamin B-12 deficiency and vitamin D insufficiency between the 2 groups at each time point, a chi-square test was used. Within-group differences compared with baseline were tested by McNemar's test.
In addition to the crude concentrations of the bone turnover markers PINP and CTX, an uncoupling ratio was calculated as the ratio of percentage change from baseline (31):
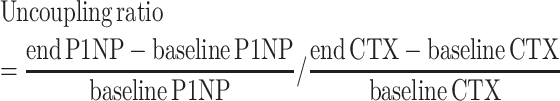 |
(1) |
A ratio <1 indicates net resorption, whereas a ratio >1 indicates net formation (31). A Mann–Whitney U test was used to investigate if the ratio was different between groups.
Analyses of bone markers were not adjusted for potential confounders including age, sex, BMI, body weight, energy intake, physical activity, smoking, alcohol use, comorbidities, and fracture history (32), because these variables did not significantly differ at baseline and over time between the groups. For the analysis of serum 25(OH)D, season in which the baseline measurement was performed was added as a confounder to the model. Season was categorized as spring (March to May) and summer (June and July). The aforementioned analyses for serum vitamin concentrations and bone markers were also performed separately for men and women. These sex-specific results should be interpreted with caution, because the power of the study might be inadequate to detect a difference.
Changes in dietary intake over time and within groups were assessed with Wilcoxon's Signed Rank test.
All statistical analyses were performed using IBM SPSS Statistics version 25.0 (IBM Corp.). A 2-sided P value of 0.05 was used for statistical significance.
Results
Participants
In total, 180 people were randomly assigned to either the control or the intervention group. At the end of the study, the control group consisted of 83 participants and the intervention group of 80. Supplemental Figure 1 presents the flowchart of the numbers of participants at different study stages. The dropout rate was low in both groups (intervention: 8%; control: 11%). Furthermore, the intervention group showed a moderate to high compliance to the proposed intervention (80% achieved ≥80% of supplement compliance and 73% attended ≥80% of the exercise program). There were no significant differences in baseline characteristics between participants who were not willing to receive the group allocation, lost to follow-up, or discontinued the study and those who remained in the data analyses (data not shown). The control and intervention groups significantly differed in education level and serum 25(OH)D concentrations at baseline (Table 1). Both groups maintained their weight during the study period.
TABLE 1.
Baseline characteristics of the middle-aged and older Chinese adults in the control and the nutrition plus exercise intervention group1
| Control group | Intervention group | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | All | Men | Women | All | Men | Women | P value2 |
| n | 83 | 38 (45.8) | 45 (54.2) | 80 | 40 (50.0) | 40 (50.0) | |
| Age, y | 60.9 ± 6.0 | 62.9 ± 6.0 | 59.1 ± 6.0 | 61.7 ± 6.3 | 63.1 ± 7.0 | 60.3 ± 6.0 | 0.38 |
| Age group, y | |||||||
| 50–64 | 58 (69.9) | 22 (57.9) | 36 (80.0) | 53 (66.3) | 23 (57.5) | 30 (75.0) | 0.62 |
| ≥65 | 25 (30.1) | 16 (42.1) | 9 (20.0) | 27 (33.8) | 17 (42.5) | 10 (25.0) | |
| Education level | |||||||
| Secondary or below | 65 (78.3) | 29 (76.3) | 36 (80.0) | 49 (61.3) | 18 (45.0) | 31 (77.5) | 0.02 |
| Tertiary or above | 18 (21.7) | 9 (23.7) | 9 (20.0) | 31 (38.8) | 22 (55.0) | 9 (22.5) | |
| Smoking | |||||||
| Never smoke | 77 (92.8) | 32 (84.2) | 45 (100.0) | 71 (88.8) | 32 (80.0) | 39 (97.5) | 0.38 |
| Ex-smoker/current smoker | 6 (7.2) | 6 (15.8) | 0 (0.0) | 9 (11.3) | 8 (20.0) | 1 (2.5) | |
| Alcohol use | |||||||
| Never | 72 (86.7) | 29 (76.3) | 43 (95.6) | 69 (86.3) | 32 (80.0) | 37 (92.5) | 0.93 |
| Ex-user/current user | 11 (13.3) | 9 (23.7) | 2 (4.4) | 11 (13.8) | 8 (20.0) | 3 (7.5) | |
| Weight, kg | 59.1 ± 9.3 | 65.1 ± 8.1 | 54.1 ± 7.1 | 59.5 ± 9.1 | 64.6 ± 6.9 | 54.4 ± 8.1 | 0.78 |
| Height, cm | 160.4 ± 8.4 | 166.7 ± 5.9 | 155.0 ± 6.1 | 162.7 ± 7.6 | 168.7 ± 4.6 | 156.8 ± 4.8 | 0.06 |
| BMI, kg/m2 | 22.9 ± 2.5 | 23.4 ± 2.4 | 22.5 ± 2.6 | 22.4 ± 2.4 | 22.7 ± 2.1 | 22.1 ± 2.6 | 0.18 |
| Self-reported major medical history | |||||||
| Endocrinology diseases | 4 (4.8) | 2 (5.3) | 2 (4.4) | 1 (1.3) | 0 (0.0) | 1 (2.5) | 0.37 |
| Cardiovascular diseases | 16 (19.3) | 10 (26.3) | 6 (13.3) | 14 (17.5) | 10 (25.0) | 4 (10.0) | 0.77 |
| Bone, joint, or muscular problems | 13 (15.7) | 6 (15.8) | 7 (15.6) | 13 (16.3) | 4 (10.0) | 9 (22.5) | 0.92 |
| Gastrointestinal problems | 11 (13.3) | 4 (10.5) | 7 (15.6) | 9 (11.3) | 6 (15.0) | 3 (7.5) | 0.70 |
| Cancer | 4 (4.8) | 2 (5.3) | 2 (4.4) | 2 (2.5) | 1 (2.5) | 1 (2.5) | 0.68 |
| Fracture history | 2 (2.4) | 1 (2.6) | 1 (2.2) | 3 (3.8) | 1 (2.5) | 2 (5.0) | 0.62 |
| Protein intake, g/d | 76.0 [64.0–93.8] | 88.9 [70.9–122] | 70.1 [60.6–81.0] | 85.0 [66.2–106] | 91.4 [80.8–115] | 73.1 [55.6–91.9] | 0.14 |
| Calcium intake, mg/d | 505 [404–692] | 516 [404–656] | 479 [373–692] | 520 [409–692] | 568 [387–808] | 511 [421–648] | 0.66 |
| Energy intake, kcal/d | 1914 ± 531 | 2141 ± 541 | 1722 ± 444 | 1992 ± 475 | 2218 ± 425 | 1766 ± 415 | 0.33 |
| Vitamin D intake, μg/d | 1.9 [1.0–3.1] | 2.3 [1.1–3.5] | 1.6 [1.0–2.9] | 1.9 [1.1–3.1] | 2.7 [1.4–3.8] | 1.7 [1.0–2.6] | 0.74 |
| Vitamin B-12 intake, μg/d | 3.2 [2.2–4.7] | 3.8 [2.4–4.9] | 2.9 [2.2–3.8] | 3.4 [2.2–6.0] | 3.8 [2.6–6.2] | 3.0 [2.0–5.5] | 0.29 |
| Serum vitamin B-12, pmol/L | 343 ± 140 | 299 ± 116 | 378 ± 149 | 345 ± 119 | 345 ± 117 | 346 ± 122 | 0.89 |
| Serum 25(OH)D, nmol/L | 60.6 ± 15.2 | 65.2 ± 15.8 | 56.7 ± 13.7 | 54.7 ± 14.2 | 57.1 ± 14.3 | 52.2 ± 13.7 | 0.01 |
| Serum CTX, pg/mL | 295 [219–372] | 255 [192–315] | 310 [272–418] | 276 [210–400] | 256 [192–288] | 348 [230–492] | 0.55 |
| Serum PINP, μg/L | 41.0 [32.0–51.0] | 34.0 [29.0–41.0] | 44.0 [39.0–55.0] | 41.0 [29.3–51.5] | 32.5 [28.0–40.5] | 49.0 [41.5–65.5] | 0.88 |
| Serum PTH, pmol/L | 4.0 ± 1.7 | 4.2 ± 1.8 | 3.9 ± 1.6 | 4.2 ± 1.7 | 3.9 ± 1.4 | 4.4 ± 2.0 | 0.65 |
Values are n (%), mean ± SD, or median [IQR]. CTX, C-terminal telopeptide of type I collagen; PINP, N-amino terminal propeptide of type I collagen; PTH, parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D.
P value by independent t test, chi-square, or Mann–Whitney U test where appropriate to analyze differences between the entire control and entire intervention groups.
Serum vitamin B-12 and 25(OH)D concentrations
A significant time × group interaction was found for serum vitamin B-12 concentrations (P < 0.001), independent of sex. The vitamin B-12 concentrations remained stable over time within the control group, whereas in the intervention group, the vitamin B-12 concentrations increased from baseline to 24 wk (Table 2, Supplemental Figure 2A). The mean vitamin B-12 concentration was sufficient at baseline in both groups. The prevalence of vitamin B-12 deficiency (<150 pmol/L) was low and similar in both groups over time (Table 3).
TABLE 2.
Serum biochemistry in middle-aged and older Chinese adults in the control and the nutrition plus exercise intervention group at baseline, 12 wk, and 24 wk1
| Variable | Group | Baseline | 12 wk | 24 wk | P value for time2 | P value for group2 | P value for time × group2 |
|---|---|---|---|---|---|---|---|
| Serum vitamin B-12, pmol/L | Control | 343 ± 140 | 358 ± 138 | 357 ± 134 | <0.001 | <0.001 | <0.001 |
| Intervention | 345 ± 119 | 468 ± 140 | 484 ± 136 | ||||
| Serum 25(OH)D, nmol/L | Control | 60.6 ± 15.2 | 66.7 ± 15.7 | 65.6 ± 14.6 | <0.001 | 0.001 | <0.001 |
| Intervention | 54.7 ± 14.2 | 81.1 ± 17.5 | 80.1 ± 19.2 | ||||
| Serum PINP, μg/L | Control | 41.0 [32.0–51.0] | 42.0 [34.0–50.3] | 40.0 [32.0–52.0] | <0.001 | 0.006 | <0.001 |
| Intervention | 41.0 [29.3–51.5] | 34.0 [27.0–45.0] | 29.0 [24.0–41.3] | ||||
| Serum CTX, pg/mL | Control | 295 [219–372] | 306 [228–394] | 315 [232–428] | 0.006 | 0.001 | <0.001 |
| Intervention | 276 [210–400] | 234 [163–346] | 239 [149–344] | ||||
| Serum PTH, pmol/L | Control | 4.0 ± 1.7 | 4.1 ± 2.2 | 4.1 ± 2.3 | 0.02 | 0.50 | 0.09 |
| Intervention | 4.2 ± 1.7 | 3.8 ± 1.5 | 3.7 ± 1.8 |
n = 83, control group; n = 80, intervention group. Values are means ± SDs or medians [IQRs]. CTX, C-terminal telopeptide of type I collagen; PINP, N-amino terminal propeptide of type I collagen; PTH, parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D.
P values for time, group, and time × group effect were tested by linear mixed models. Logarithmic transformation was applied to the variables PINP and CTX for running the linear mixed model.
TABLE 3.
Prevalence of vitamin B-12 deficiency and vitamin D insufficiency over time in middle-aged and older Chinese adults in the control and the nutrition plus exercise intervention group1
| Variable | Control (n = 83) | Intervention (n = 80) | P value2 |
|---|---|---|---|
| Vitamin B-12 deficiency3 | |||
| Baseline | 2 (2.4) | 4 (5.1) | 0.38 |
| 12 wk | 4 (5.1) | 1 (1.4) | 0.20 |
| 24 wk | 1 (1.4) | 1 (1.4) | 0.98 |
| Vitamin D insufficiency4 | |||
| Baseline | 19 (22.9)a | 30 (37.5)a | 0.042 |
| 12 wk | 11 (14.1)b | 3 (4.1)b | 0.034 |
| 24 wk | 8 (11.0)c | 5 (7.1)c | 0.42 |
Values are n (%) unless indicated otherwise. Labeled percentages in a column without a common letter differ, P < 0.05.
P value by chi-square test to analyze differences between the control and intervention groups.
Defined as <150 pmol/L or <200 pg/mL.
Defined as ≥25 to <50 nmol/L. No vitamin D deficiency was present (<25 nmol/L).
A significant time × group interaction was found for serum 25(OH)D concentrations as well (P < 0.001; adjusted for season), independent of sex. The 25(OH)D concentrations increased in both groups, with a greater increase in the intervention group (Table 2, Supplemental Figure 2B). The mean 25(OH)D concentration was sufficient at baseline in both groups. No vitamin D deficiency (<25 nmol/L) was seen in the study population over time. Prevalence of vitamin D insufficiency (≥25 to <50 nmol/L) decreased by 30% from baseline to 24 wk in the intervention group and in the control group by 12% (Table 3). The prevalence of insufficiency was significantly lower in the intervention group than in the control group at baseline and 12 wk (P = 0.042 and P = 0.034, respectively), but equal at 24 wk (P = 0.42).
Bone turnover markers
The interaction time × group was significant (P < 0.001) for both bone turnover markers PINP and CTX. PINP and CTX values in the control group did not change over time, whereas a decrease for both markers from baseline to 24 wk was seen in the intervention group (Table 2). CTX and PINP values were lower in men than in women, but followed a similar pattern over time (Supplemental Figure 2C, D).
The uncoupling ratio was greater for the intervention group (median: 0.70, mean: 1.90) than for the control group (median: −0.11, mean: −0.10; U = 1624, P < 0.001) (Figure 1). Results were similar for men (median change: 0.52, U = 403, P = 0.02) and women (median change: 0.85, U = 397, P = 0.002) (Figure 1).
FIGURE 1.
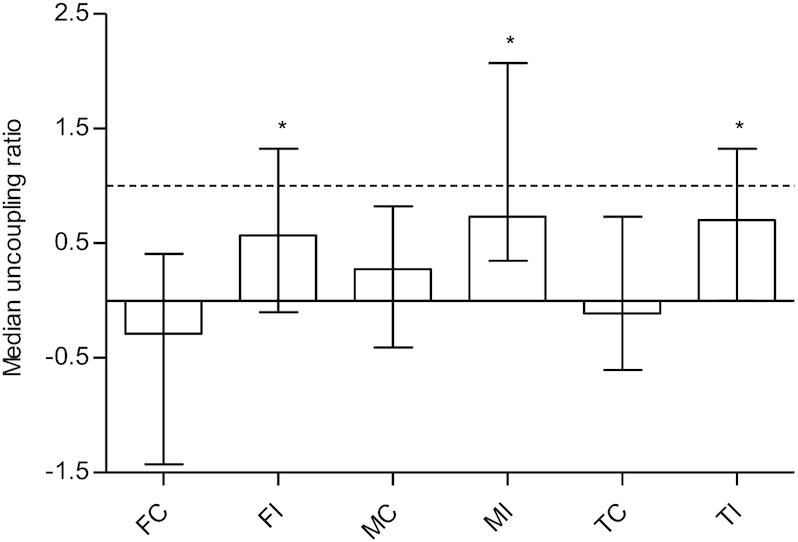
Uncoupling ratio in middle-aged and older Chinese adults in the control (n = 83) and the nutrition plus exercise group (n = 80), separated by sex (FC, n = 45; FI, n = 38; MC, n = 40; MI, n = 40). Values are medians ± IQRs. The horizontal dotted line represents a balance between bone resorption and formation. *Different from corresponding control, P < 0.05. FC, females control; FI, females intervention; MC, males control; MI, males intervention; TC, total control; TI, total intervention.
PTH
The interaction time × group was not significant (P = 0.09) for serum PTH concentrations and there was no group effect (P = 0.50). Similar effects were found for men and women separately. Within the intervention group, PTH concentrations decreased from baseline to 24 wk, whereas they remained stable over time in the control group (Table 2). Thirty participants (36%) in the control group and 36 participants (50%) in the intervention group had elevated PTH concentrations at baseline (defined as >4.0 pmol/L).
Dietary intake
In the intervention group, median [IQR] calcium intake increased from 520 [409–692] mg/d at baseline to 1.39 × 103 [1.30 × 103 to 1.54 × 103] mg/d after 24 wk (P < 0.001), median dietary vitamin D intake increased from 1.9 [1.1–3.1] μg/d to 31 [30–32] μg/d (P < 0.001), and median dietary vitamin B-12 intake increased from 3.4 [2.2–6.0] μg/d at baseline to 5.4 [4.2–7.3] μg/d after 24 wk (P = 0.001). In the control group, there was no significant change in calcium, vitamin D, and vitamin B-12 intake: from 504 [404–692] mg/d to 529 [417–678] mg/d, from 1.9 [1.0–3.1] μg/d to 1.8 [1.4–3.2] μg/d, and from 3.2 [2.2–4.7] μg/d to 2.8 [2.1–4.0] μg/d, respectively.
For protein intake, no statistically significant differences were observed between the 2 groups and over time. Protein intake was ≥0.8 g · kg−1 · d−1 in almost all participants (≥96%), both at baseline and at 24 wk. In addition, protein intakes were ≥1.0 g · kg−1 · d−1 and ≥1.2 g · kg−1 · d−1 in the majority of the participants at 24 wk (in ≥84% and ≥70%, respectively).
Discussion
The aim of this RCT was to investigate whether a 24-wk multidomain lifestyle intervention including a fortified milk supplement and an exercise program influenced serum vitamin B-12 and 25(OH)D concentrations and markers of bone turnover in apparently healthy community-dwelling Chinese middle-aged and older adults, compared with a control group without intervention. Our findings showed that the intervention was successful in improving vitamin B-12 and 25(OH)D concentrations and the balance of bone turnover markers.
Whereas no change was observed in the control group, the intervention group significantly improved in terms of serum vitamin B-12 concentrations. Overall, vitamin B-12 concentrations were adequate (i.e., ≥150 pmol/L) at baseline, with low prevalence of deficiency in both groups, which may be attributed to the fact that the majority of the participants were younger than 65 y old. A difference in results may be expected when studies are conducted in older adults because vitamin B-12 deficiency is more prevalent in people aged 65 y and older (12). The dietary vitamin B-12 intake was in line with the serum vitamin B-12 concentrations (increase in the intervention group and stable values in the control group). Both groups had intakes well above the Chinese RNI for vitamin B-12 of 2.4 μg/d (26). In other parts of China, prevalence of low vitamin B-12 concentrations has ranged from 13.5% in Shanghai (deficiency defined as <139 pmol/L) (33) to 74.5% in rural North China (deficiency defined as <185 pmol/L) (34). To our knowledge, there are currently no data available on the vitamin B-12 status in the general population aged 50 y or older living in Hong Kong. Therefore, the need for improving vitamin B-12 status in middle-aged and older adults in Hong Kong remains to be investigated.
The fortified milk supplement was effective in achieving and maintaining serum 25(OH)D concentrations >50 nmol/L, which is currently recommended for prevention of osteoporosis in postmenopausal women (7). In addition, a serum 25(OH)D concentration of 70–80 nmol/L has been suggested as the optimal level for a lower fracture risk and to support the skeleton (35, 36). The intervention group met this level as well; mean serum concentrations increased to 80 nmol/L. A similar improvement in serum 25(OH)D concentrations after milk supplementation was also found in other studies in postmenopausal Chinese women (17, 18).
The number of participants with vitamin D insufficiency decreased over time in both the intervention and control groups. As there were multiple participants in the control group with a baseline serum 25(OH)D concentration close to the cutoff value for vitamin D insufficiency (<50 nmol/L; 13 participants had a concentration between 50.0 and 55.0 nmol/L), it is likely that even a slight increase in serum concentrations might have caused a shift in vitamin D status classification. In addition, regardless of the treatment group, the participants were informed if they had low serum 25(OH)D concentrations at baseline together with being given very general lifestyle advice to improve their concentrations. It is, however, important to note that the dietary vitamin D intake stayed the same over time (baseline to 24 wk) in the control group. This suggests that although a lifestyle change may influence serum 25(OH)D concentrations, a combination of lifestyle and nutrition is more effective, as seen by the larger impact in the intervention group.
Human bone is continuously remodeled through a process of bone formation and resorption. Biomarkers of bone turnover can be measured to assess bone remodeling rates. A decrease in the serum concentrations of PINP and CTX was seen with the intervention, whereas the concentrations remained stable in the control group. Decreased PINP concentrations do not necessarily mean that there is less bone formation (31). Likewise, decreased CTX concentrations do not automatically imply bone resorption. Therefore, an uncoupling ratio was calculated to arrive at an interpretation of the net effect (31, 37). Although the median value for the uncoupling ratio was <1 for both groups, which would suggest net resorption, the ratio was significantly greater for the intervention group than for the control group. This suggests that there was less bone remodeling in the intervention group. Higher remodeling rates have been associated with an increased fracture risk and bone fragility (37–42). Although CTX and PINP values were lower in men than in women, the uncoupling ratio was similar for both sexes. A reduction in CTX and PINP concentrations after milk supplementation was also found in other studies investigating Chinese postmenopausal women (28, 29, 43). Long-term trials investigating milk supplementation alone confirm the beneficial effect on bone health. In the study of Lau et al. (17), milk supplementation for 2 y in apparently healthy postmenopausal Chinese women aged 55–59 y resulted in less bone loss at different sites. This was also found in the study of Chee et al. (18), who performed a 2-y milk supplementation study in apparently healthy postmenopausal Chinese women aged 50–65 y in Malaysia. Unfortunately, we had only indirect measures of bone health and no information about BMD.
PTH helps the body to maintain stable concentrations of calcium in the blood (44). Suppression of PTH concentrations (by a high protein/calcium intake) may reduce bone resorption and thereby improve bone density (45). In the current study, the interaction time × group for serum PTH concentrations was not significant but concentrations in the intervention group decreased significantly from baseline to 24 wk (mean change: 11%). In contrast, Chee et al. (18) found that serum PTH concentrations of their control group significantly increased over time (figure-derived mean change from baseline: 50%), whereas PTH concentrations in their intervention group did not significantly change over time. This difference in effect could be explained by the lower calcium intakes of participants in this study (46) and lower PTH baseline values. In the current study, 36% of the control group and 50% of the intervention group had elevated PTH concentrations at baseline (defined as >4.0 pmol/L).
The baseline values of PINP and PTH (41.0 μg/L and 4.1 pmol/L, respectively) were in line with the estimated reference concentrations for healthy Chinese adults aged 50–79 y (PINP: 36.9–52.7 μg/L; PTH: 3.7–4.0 pmol/L) (47). However, mean CTX concentrations in our study were lower (320 and 311 pg/mL in the control and intervention, respectively) than the estimated reference concentration (445 pg/mL) (47). This may indicate, keeping in mind interlaboratory variances, a lower rate of bone resorption in our study population, suggesting that a greater effect of the intervention may be expected in age-matched individuals.
To the best of our knowledge, there are no comparable studies looking at the effect of a combined nutrition and exercise intervention on bone turnover markers or BMD. As described above, studies in Chinese women investigating milk supplementation alone have shown a reduction in bone turnover markers (28, 29, 43) and less bone loss (17–19). Multiple meta-analyses have assessed the effect of exercise alone on BMD in older adults (48–52). All suggest that exercise can preserve or increase BMD to some extent, depending on the bone site and the duration, intensity, and type of exercises. There is a lack of studies looking at the effect of long-term exercise on PINP and CTX concentrations in comparable populations. There is no conclusive evidence available on the optimal exercise intervention but it seems likely that resistance training, potentially combined with other forms of exercise, should be included. Because both nutrition alone and exercise alone can affect bone turnover markers or BMD, it remains to be investigated if a combined intervention has synergistic or additive effects.
The calcium intake at baseline was comparable with the mean daily calcium intake in men and women aged 60–84 y in Hong Kong (410 and 420 mg/d, respectively) (53). The intervention increased calcium intake successfully (from 520 to 1.39 × 103 mg/d) but no change was observed for protein intake. The milk supplement provided only an additional 13.6 g protein/d. Furthermore, protein intake (g · kg−1 · d−1) was equal to or above the current RDA of 0.8 g · kg−1 · d−1 in almost all cases. For healthy adults older than 65 y, a protein intake above the RDA may be beneficial for bone health (higher BMD, slower rate of bone loss, reduced bone turnover, and reduced hip fracture risk) (11, 54). Still, the majority of the participants in both groups met the criteria of 1.0 and 1.2 g · kg−1 · d−1. Note that our study population also included middle-aged adults (50–65 y). The already adequate protein intake, combined with the nutritional improvements and improved overall level of physical activity (25), could have contributed to the improved bone turnover (11, 55).
A limitation of this study is the absence of a nutrition or exercise group alone. Consequently, it is not possible to conclude which component or which combination of components of the intervention contributed the most to our positive findings regarding bone turnover. However, as stated before, a stronger effect may be expected when nutrition and exercise interventions are combined (7, 22, 23, 56). Because there is little information available about the nutritional status of the general population aged 50 y or older living in Hong Kong, we are unable to conclude if our study population was a representative sample or that they were healthier. At least in this study population, the supplement was not needed to improve vitamin B-12 concentrations. Lastly, the sex-specific results should be interpreted with caution. The power of the study might be inadequate to detect sex-specific differences in serum vitamin concentrations and bone markers between the control and intervention groups.
Strengths of the current study include the low dropout rate and moderate to high compliance to the proposed intervention, which also indicates that this program may be sustainable over the long term. Furthermore, we were able to see improvements in nutritional status and bone markers in individuals with relatively good baseline values. This also shows the potential of the intervention program for more vulnerable groups, for example, frail older adults. Lastly, previous studies about nutritional and exercise interventions for improvement of bone health have largely focused on (postmenopausal) women. Osteoporosis in men is an underappreciated medical concern, even though it is known that hip fractures in men are associated with greater mortality than in women (57). No remarkable sex differences were seen for the uncoupling ratio in the current study, showing that also male adults can benefit.
In conclusion, this study showed that milk supplementation in combination with exercise is effective in improving vitamin B-12 and 25(OH)D concentrations and bone turnover of apparently healthy community-dwelling Chinese middle-aged and older adults. Therefore, it contributes to the knowledge on how to prevent and reduce malnutrition and osteoporosis in the rapidly aging population of Hong Kong.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—RC, JW, SO, MCEB, and LCPGMdG: designed the research; RC, JW, and SO: conducted the research; JW, PP, and LCPGMdG: periodically reviewed the quality of the data; IG: analyzed the data and wrote the manuscript; JW and LCPGMdG: had primary responsibility for the final content; and all authors: critically reviewed the manuscript and read and approved the final manuscript.
Notes
Supported by FrieslandCampina, Netherlands, and by Jaap Schouten Foundation, Netherlands award JSF_SU_8_17 (to IG).
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
Abbreviations used: BMD, bone mineral density; CTX, C-terminal telopeptide of type I collagen; PINP, N-amino terminal propeptide of type I collagen; PTH, parathyroid hormone; RCT, randomized controlled trial; RNI, recommended nutrient intake; RPE, Rating of Perceived Exertion; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Inge Groenendijk, Division of Human Nutrition and Health, Wageningen University & Research, Wageningen, Netherlands.
Ruth Chan, Department of Medicine & Therapeutics, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong.
Jean Woo, Department of Medicine & Therapeutics, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong.
Sherlin Ong, FrieslandCampina, Amersfoort, Netherlands.
Panam Parikh, FrieslandCampina, Amersfoort, Netherlands.
Marjolijn C E Bragt, FrieslandCampina, Amersfoort, Netherlands.
Lisette C P G M de Groot, Division of Human Nutrition and Health, Wageningen University & Research, Wageningen, Netherlands.
References
- 1. Census and Statistics Department. Hong Kong population projections, 2017–2066. Hong Kong: Demographic Statistics Section, Census and Statistics Department; 2017. [Google Scholar]
- 2. Woo J. Aging in Hong Kong: a comparative perspective. New York: Springer; 2013. [Google Scholar]
- 3. Dhanwal DK, Cooper C, Dennison EM. Geographic variation in osteoporotic hip fracture incidence: the growing importance of Asian influences in coming decades. J Osteoporos. 2010:757102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheung EYN, Tan KCB, Cheung C-L, Kung AWC. Osteoporosis in East Asia: current issues in assessment and management. Osteoporos Sarcopenia. 2016;2:118–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wei J-M, Li S, Claytor L, Partridge J, Goates S. Prevalence and predictors of malnutrition in elderly Chinese adults: results from the China Health and Retirement Longitudinal Study. Public Health Nutr. 2018;21:3129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lems WF, Post PN, van den Bergh JPW, Cornelder HW, Elders PJM, Geusens PPM, Ginai AZ, Groen M, Hegeman JH, van Helden SH et al. Richtlijn Osteoporose en Fractuurpreventie. Utrecht: CBO; 2011. [Google Scholar]
- 7. Rizzoli R, Stevenson JC, Bauer JM, van Loon LJC, Walrand S, Kanis JA, Cooper C, Brandi M-L, Diez-Perez A, Reginster J-Y. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: a consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas. 2014;79:122–32. [DOI] [PubMed] [Google Scholar]
- 8. Kung AWC, Lee K-K. Knowledge of vitamin D and perceptions and attitudes toward sunlight among Chinese middle-aged and elderly women: a population survey in Hong Kong. BMC Public Health. 2006;6:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Yun C, He Y, Piao J, Yang L, Yang X. Vitamin D status among the elderly Chinese population: a cross-sectional analysis of the 2010–2013 China national nutrition and health survey (CNNHS). Nutr J. 2017;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta D et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14:542–59. [DOI] [PubMed] [Google Scholar]
- 11. Rizzoli R, Biver E, Bonjour J-P, Coxam V, Goltzman D, Kanis JA, Lappe J, Rejnmark L, Sahni S, Weaver C et al. Benefits and safety of dietary protein for bone health–an expert consensus paper endorsed by the European Society for Clinical and Economical Aspects of Osteopororosis, Osteoarthritis, and Musculoskeletal Diseases and by the International Osteoporosis Foundation. Osteoporos Int. 2018;29:1933–48. [DOI] [PubMed] [Google Scholar]
- 12. Baik HW, Russell RM. Vitamin B12 deficiency in the elderly. Annu Rev Nutr. 1999;19:357–77. [DOI] [PubMed] [Google Scholar]
- 13. Wong CW. Vitamin B12 deficiency in the elderly: is it worth screening?. Hong Kong Med J. 2015;21:155–64. [DOI] [PubMed] [Google Scholar]
- 14. Xu X, Hall J, Byles J, Shi Z. Assessing dietary quality of older Chinese people using the Chinese Diet Balance Index (DBI). PLoS One. 2015;10:e0121618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dhonukshe-Rutten RA, Pluijm SM, de Groot LC, Lips P, Smit JH, van Staveren WA. Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. J Bone Miner Res. 2005;20:921–9. [DOI] [PubMed] [Google Scholar]
- 16. Herrmann W, Obeid R, Schorr H, Hübner U, Geisel J, Sand-Hill M, Ali N, Herrmann M. Enhanced bone metabolism in vegetarians – the role of vitamin B12 deficiency. Clin Chem Lab Med. 2009;47:1381–7. [DOI] [PubMed] [Google Scholar]
- 17. Lau EMC, Woo J, Lam V, Hong A. Milk supplementation of the diet of postmenopausal Chinese women on a low calcium intake retards bone loss. J Bone Miner Res. 2001;16:1704–9. [DOI] [PubMed] [Google Scholar]
- 18. Chee WSS, Suriah AR, Chan SP, Zaitun Y, Chan YM. The effect of milk supplementation on bone mineral density in postmenopausal Chinese women in Malaysia. Osteoporos Int. 2003;14:828–34. [DOI] [PubMed] [Google Scholar]
- 19. Chen Y, Xiao Y, Xie B, Zhang Q, Ma X, Li N, Liu M, Zhang Q. Effect of milk powder supplementation with different calcium contents on bone mineral density of postmenopausal women in Northern China: a randomized controlled double-blind trial. Calcif Tissue Int. 2016;98:60–6. [DOI] [PubMed] [Google Scholar]
- 20. Alswat KA. Gender disparities in osteoporosis. J Clin Med Res. 2017;9:382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haentjens P, Magaziner J, Colon-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, Boonen S. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152:380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Daly RM. Exercise and nutritional approaches to prevent frail bones, falls and fractures: an update. Climacteric. 2017;20:119–24. [DOI] [PubMed] [Google Scholar]
- 23. Willems HME, van den Heuvel E, Schoemaker RJW, Klein-Nulend J, Bakker AD. Diet and exercise: a match made in bone. Curr Osteoporos Rep. 2017;15:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Surveillance and Epidemiology Branch, Centre for Health Protection, Department of Health. Behavioural Risk Factor Survey. Hong Kong: Department of Health; 2015. [Google Scholar]
- 25. Woo J, Chan R, Ong S, Bragt M, Bos R, Parikh P, de Groot LCPGM. Randomized controlled trial of exercise and nutrition supplementation on physical and cognitive function in older Chinese adults aged 50 years and older. J Am Med Dir Assoc. 2020;21:395–403. [DOI] [PubMed] [Google Scholar]
- 26. Chinese Nutrition Society. Chinese Dietary Reference Intakes summary: 2013. Beijing, China: People's Medical Publishing House; 2017. [Google Scholar]
- 27. de Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr Bull. 2008;29:S238–44. [DOI] [PubMed] [Google Scholar]
- 28. Kruger MC, Ha PC, Todd JM, Kuhn-Sherlock B, Schollum LM, Ma J, Qin G, Lau E. High-calcium, vitamin D fortified milk is effective in improving bone turnover markers and vitamin D status in healthy postmenopausal Chinese women. Eur J Clin Nutr. 2012;66:856–61. [DOI] [PubMed] [Google Scholar]
- 29. Kruger MC, Chan YM, Kuhn-Sherlock B, Lau LT, Lau C, Chin YS, Todd JM, Schollum LM. Differential effects of calcium- and vitamin D-fortified milk with FOS-inulin compared to regular milk, on bone biomarkers in Chinese pre- and postmenopausal women. Eur J Nutr. 2016;55:1911–21. [DOI] [PubMed] [Google Scholar]
- 30. Macfarlane DJ, Lee CC, Ho EY, Chan KL, Chan DT. Reliability and validity of the Chinese version of IPAQ (short, last 7 days). J Sci Med Sport. 2007;10:45–51. [DOI] [PubMed] [Google Scholar]
- 31. Thorpe MP, Evans EM. Dietary protein and bone health: harmonizing conflicting theories. Nutr Rev. 2011;69:215–30. [DOI] [PubMed] [Google Scholar]
- 32. US Department of Health and Human Services. Bone health and osteoporosis: a report of the Surgeon General. Rockville, MD: Office of the Surgeon General (US); 2004. [Google Scholar]
- 33. Wang Y, Zheng Y, Yan F, Zhang W. Status of vitamin B12 deficiency in the elderly Chinese community people. Health (N Y). 2015;7:7. [Google Scholar]
- 34. Zhang W, Li Y, Wang TD, Meng H-X, Min G-W, Fang Y-L, Niu X-Y, Ma L-S, Guo J-H, Zhang J et al. Nutritional status of the elderly in rural North China: a cross-sectional study. J Nutr Health Aging. 2014;18:730–6. [DOI] [PubMed] [Google Scholar]
- 35. Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–6. [DOI] [PubMed] [Google Scholar]
- 36. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- 37. Hlaing TT, Compston JE. Biochemical markers of bone turnover – uses and limitations. Ann Clin Biochem. 2014;51:189–202. [DOI] [PubMed] [Google Scholar]
- 38. Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18:1051–6. [DOI] [PubMed] [Google Scholar]
- 39. Heaney RP. Is the paradigm shifting?. Bone. 2003;33:457–65. [DOI] [PubMed] [Google Scholar]
- 40. Chapurlat RD, Palermo L, Ramsay P, Cummings SR. Risk of fracture among women who lose bone density during treatment with alendronate. Osteoporos Int. 2005;16:842–8. [DOI] [PubMed] [Google Scholar]
- 41. Khosla S, Melton LJ 3rd, Wermers RA, Crowson CS, O'Fallon W, Riggs B. Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res. 1999;14:1700–7. [DOI] [PubMed] [Google Scholar]
- 42. Bjarnason NH, Sarkar S, Duong T, Mitlak B, Delmas PD, Christiansen C. Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos Int. 2001;12:922–30. [DOI] [PubMed] [Google Scholar]
- 43. Kruger MC, Chan YM, Lau LT, Lau CC, Chin YS, Kuhn-Sherlock B, Todd JM, Schollum LM. Calcium and vitamin D fortified milk reduces bone turnover and improves bone density in postmenopausal women over 1 year. Eur J Nutr. 2018;57:2785–94. [DOI] [PubMed] [Google Scholar]
- 44. Song L. Calcium and bone metabolism indices. Adv Clin Chem. 2017;82:1–46. [DOI] [PubMed] [Google Scholar]
- 45. Gaffney-Stomberg E, Insogna KL, Rodriguez NR, Kerstetter JE. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J Am Geriatr Soc. 2009;57:1073–9. [DOI] [PubMed] [Google Scholar]
- 46. Souberbielle JC, Brazier F, Piketty ML, Cormier C, Minisola S, Cavalier E. How the reference values for serum parathyroid hormone concentration are (or should be) established?. J Endocrinol Invest. 2017;40:241–56. [DOI] [PubMed] [Google Scholar]
- 47. Hu W-W, Zhang Z, He J-W, Fu W-Z, Wang C, Zhang H, Yue H, Gu J-M, Zhang Z-L. Establishing reference intervals for bone turnover markers in the healthy Shanghai population and the relationship with bone mineral density in postmenopausal women. Int J Endocrinol. 2013:513925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martyn-St James M, Carroll S. A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med. 2009;43:898–908. [DOI] [PubMed] [Google Scholar]
- 49. Hamilton CJ, Swan VJD, Jamal SA. The effects of exercise and physical activity participation on bone mass and geometry in postmenopausal women: a systematic review of pQCT studies. Osteoporos Int. 2010;21:11–23. [DOI] [PubMed] [Google Scholar]
- 50. Marques EA, Mota J, Carvalho J. Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age (Dordr). 2012;34:1493–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao R, Zhao M, Xu Z. The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: a meta-analysis. Osteoporos Int. 2015;26:1605–18. [DOI] [PubMed] [Google Scholar]
- 52. Zhao R, Zhang M, Zhang Q. The effectiveness of combined exercise interventions for preventing postmenopausal bone loss: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2017;47:241–51. [DOI] [PubMed] [Google Scholar]
- 53. Centre for Food Safety. The first Hong Kong Total Diet Study: minerals. Report No. 9 Hong Kong: Food and Environmental Hygiene Department of The Government of the Hong Kong Special Administrative Region; 2014. [Google Scholar]
- 54. Groenendijk I, den Boeft L, van Loon LJC, de Groot L. High versus low dietary protein intake and bone health in older adults: a systematic review and meta-analysis. Comput Struct Biotechnol J. 2019;17:1101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bonjour J-P. The dietary protein, IGF-I, skeletal health axis. Horm Mol Biol Clin Investig. 2016;28:39–53. [DOI] [PubMed] [Google Scholar]
- 56. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznaric Z, Nair KS et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ebeling P. Osteoporosis in men: why change needs to happen. Nyon, Switzerland: International Osteoporosis Foundation; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


