Summary
Plastic pollution is entering the world's oceans at alarming rates and is expected to outweigh fish populations by 2050. This plastic waste originates from land-based applications, like consumer product packaging, and is composed of high-durability polyolefins. These conventional plastics possess desirable properties, including high chemical stability, moisture barrier, and thermoplastic characteristics. Unfortunately, if these materials reach marine environments, they fragment into microplastics that cannot be biologically assimilated. The aim of this review is to investigate commercial polymers that are biodegradable in marine environments but have comparable product stability and moisture barrier properties to polyolefins. Among commercially available biopolymers, thermoplastic starches (TPS) and polyhydroxyalkanoates (PHAs) have been shown to biodegrade in marine environments. Moreover, these biopolymers are thermoplastics and possess similar thermoforming properties to polyolefins. At present, TPS and PHAs have limitations, including chemical instability, limited moisture barrier properties, and high production costs. To replace conventional polymers with PHAs and TPS, these properties must be improved.
Subject Areas: Industrial Chemistry, Polymer Chemistry, Environmental Science, Materials Science
Graphical Abstract
Industrial Chemistry; Polymer Chemistry; Environmental Science; Materials Science
Introduction
It is estimated that 20 million tons of plastic has entered the world's oceans (Landon-Lane, 2018) and will outweigh fish populations by 2050 (MacArthur, 2017). Most of the floating plastic debris has been characterized as high-durability conventional polymers that fragment over time into pieces less than 1 cm (Cózar et al., 2014; Haider et al., 2019; Ter Halle et al., 2017; Pauli et al., 2017; Li et al., 2016; Andrady, 2011). Scientists are beginning to understand the scale of this anthropogenic waste and the harm it poses to ecosystems, communities, and the global economy (Nazareth et al., 2019; Cózar et al., 2014; Hartley et al., 2018; Deroiné et al., 2014b; Haider et al., 2019; Vijayaraman et al., 2020; Liu et al., 2020; Schneiderman and Hillmyer, 2017). In response, cities, countries, and retailers have imposed levies, bans, or strict packaging guidelines on single-use plastics (Haider et al., 2019; Xanthos and Walker, 2017; Vlachogianni et al., 2018; Chatain, n.d.). For example, the French multinational retailer Carrefour established stringent requirements on their suppliers to ensure optimized packaging and recyclability following customer use (Group, n.d.).
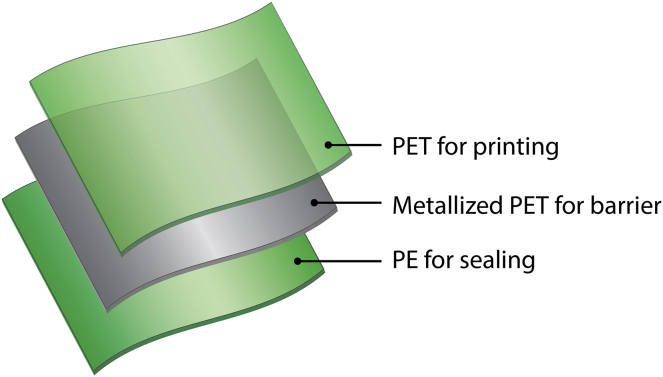
However, complete elimination of single-use plastic packaging may have deleterious socioeconomic consequences. In certain areas, it is cost-prohibitive to purchase large plastic bottles or metal cans of products, like shampoo or coffee (Paler et al., 2019; Jnr et al., 2018). To increase accessibility in these regions, consumer packaged goods (CPGs) companies supply single dosages of these products in flexible plastic pouches called sachets (Paler et al., 2019; Jnr et al., 2018). Sachets can be in the form of monolayer films, like low-density polyethylene (LDPE), or multilayer laminates, including materials like LDPE, polyethylene terephthalate, and/or polypropylene (Figure 1; Paler et al., 2019; Jnr et al., 2018; Mensitieri et al., 2011). Although these materials are inexpensive, lightweight, and compatible with existing packaging converters, they are not recyclable or biodegradable (Nazareth et al., 2019; Paler et al., 2019; Jnr et al., 2018; Arrieta et al., 2017; Deroiné et al., 2014a; Scaffaro et al., 2019; Schneiderman and Hillmyer, 2017). Imposing single-use plastic bans in these regions would necessitate alternative packaging materials.
Figure 1.
Consumer Packaged Goods Companies Supply Single Dosages of Products in Multilayered Flexible Pouches, Called Sachets
This example sachet structure is composed (from outer to inner layer) of polyethylene terephthalate (PET) for printing, vacuum metallized PET for improved barrier properties, and polyethylene (PE) as a heat-sealable layer for high-speed manufacturing.
In an effort to make conventional plastics less environmentally persistent, companies and researchers have incorporated degradable additives during polymer compounding. A prevalent technique is to blend molecules that are susceptible to oxidative degradation, such as rutile nano-titania, with polyolefins to form oxo-degradable polymers. After they are exposed to UV light, oxo-degradable plastics fragment more readily than unmodified polyolefins (Nikolić et al., 2017; Portillo et al., 2016; Li et al., 2016; Kyrikou and Briassoulis, 2007; Dussud et al., 2018). Polyolefins have also been copolymerized or blended with known biodegradable materials, like starch, in hopes of developing biodegradable blends (Panahi et al., 2020; Obasi et al., 2020; DeMarco et al., 2020; Schneiker et al., 2006). These semi-degradable blends can fragment and show signs of biodegradation in aerobic and anaerobic conditions, but the residual polyolefins are resistant to microbial attack and biological assimilation (Portillo et al., 2016; Leja and Lewandowicz, 2010; Kyrikou and Briassoulis, 2007; Al-Salem et al., 2019). Oxo- and semi-degradable polymers streamline the creation of microplastics rather than accelerating biodegradation (Obasi et al., 2020; Rigotti et al., 2020; Portillo et al., 2016). If oxo- and semi-degradable polyolefins were to be regarded as sustainable replacements for today's single-use packaging, these materials would persist in marine environments. Consequently, oxo- and semi-degradable materials are often discouraged by governments, foundations, and research institutions (Obasi et al., 2020; Commissie, 2018). As such, these polyolefin-based blends will not be included in this review.
To minimize the adverse effects of polyolefin-based packaging on marine ecosystems, these materials need to be replaced with marine-degradable polymers. The ideal approach is to develop flexible films that possess polyolefin material properties, but rapidly biodegrade if they enter marine environments. Although extensive research has been done to develop and commercialize biodegradable polymers, these materials still have limitations (Deroiné et al., 2014a, 2014b, 2015; Laycock et al., 2017; Scaffaro et al., 2019; Schneiderman and Hillmyer, 2017). They are sensitive to moisture, a critical problem with both liquid and dry products such as lotion and coffee (Jnr et al., 2018; Arrieta et al., 2017; Deroiné et al., 2014a). Moreover, these materials must have a balance between stability and biodegradability to maximize the shelf-life and quality of the enclosed product (Dilkes-Hoffman et al., 2019; Cinelli et al., 2019; Vähä-Nissi et al., 2015). To enable high-speed manufacturing and decrease production costs, these materials must also be compatible with thermoforming processes (Bugnicourt et al., 2014; Scaffaro et al., 2019; Deroiné et al., 2014a). Finally, these materials must degrade into biologically safe materials if they reach marine environments.
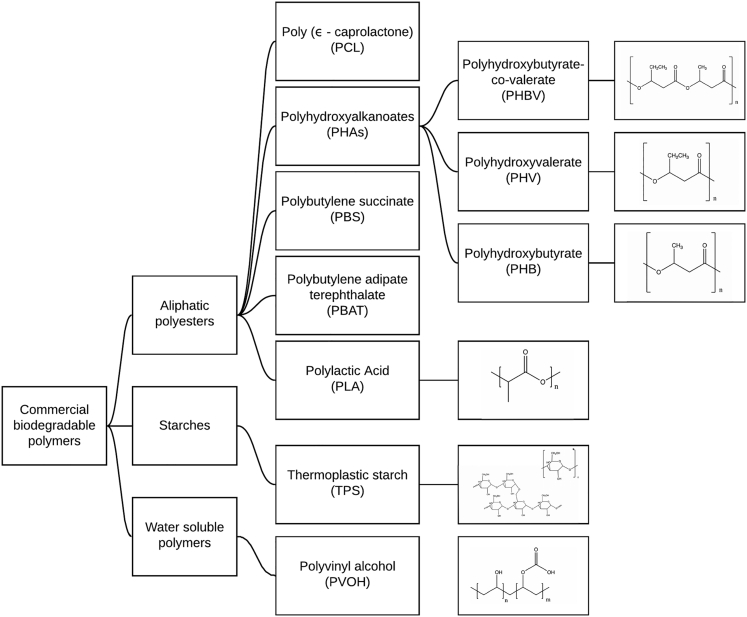
For companies to create effective marine-degradable packaging, a comprehensive review of marine-degradable polymers and their properties is needed. The aim of the present work is to investigate polymers that (1) biodegrade in marine environments, (2) are compatible with industrial thermoforming techniques, (3) will be stable in the presence of product formulations, and (4) possess a moisture barrier or retain biodegradation characteristics when combined with a barrier layer. The following biodegradable thermoplastics, which are commercial or in advanced stages of development, will be given the primary focus: aliphatic polyesters, polyvinyl alcohol (PVOH), and thermoplastic starch (TPS) (Shruti and Kutralam-Muniasamy, 2019; Sekiguchi et al., 2011; Gross and Kalra, 2002; Cho et al., 2011; Narancic and O'Connor, 2018) (Figure 2).
Figure 2.
These Biodegradable Thermoplastics Are Commercial or in Advanced Stages of Development and Will Be Given the Primary Focus in this Review: Aliphatic Polyesters, Polyvinyl Alcohol (PVOH), and Thermoplastic Starch (TPS)
Review
Are Biodegradable Plastics the Answer to Ocean Pollution?
Although biodegradable plastics are often heralded as the solution to ocean plastic, the terms “degradable” and “biodegradable” are frequently used as synonyms. Degradation is an imperative step in the biodegradation process, but only refers to polymer deterioration from abiotic reactions, like hydrolysis, oxidation, and photo-oxidation (Scaffaro et al., 2019; Haider et al., 2019). On the other hand, biodegradation occurs when the polymer fragments are in the presence of appropriate enzymes that can convert the residuals into benign by-products, like carbon dioxide, methane, nitrogen, and water (Haider et al., 2019; Emadian et al., 2017; Vert, 2005).
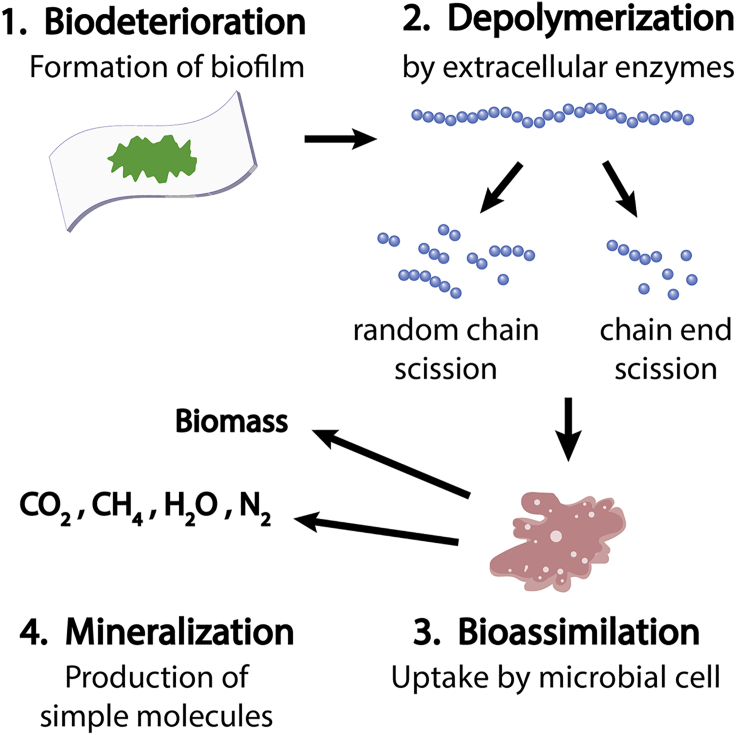
Haider et al. (Haider et al., 2019) described the biodegradation process of polymers in four steps (Figure 3). First, microorganisms form biofilms on the substrate's surface, leading to the biodeterioration of the polymer. To consume the polymer, microorganisms then release extracellular enzymes to depolymerize the substrate, forming small molecules, like dimers and oligomers. These smaller molecules can then be bioassimilated by microorganisms and converted into carbon dioxide, nitrogen, methane, and water (Scaffaro et al., 2019). As these materials deteriorate, increased surface area becomes available for further weathering and, ultimately, mineralization (Haider et al., 2019). We hereafter refer to degradation as the physical and chemical deterioration preceding biodegradation.
Figure 3.
Polymer Biodegradation Process
(1) Biodeterioration: microorganisms form biofilms and deteriorate the substrate surface. (2) Depolymerization: microorganisms release extracellular enzymes to depolymerize the substrate. (3) Bioassimilation: small molecules are consumed as metabolites by cells. (4) Mineralization: molecules are converted into benign products, such as carbon dioxide, nitrogen, methane, and water. Redrawn from (Haider et al., 2019).
Both degradation and biodegradation are dependent on the polymer's chemical and physical attributes. Characteristics such as functional groups present, chain length, degree of crystallinity, morphology, and thickness affect the rate and extent of degradation (Scaffaro et al., 2019; Deroiné et al., 2014b; Haider et al., 2019; Volova et al., 2010; Emadian et al., 2017). For example, polymer backbones composed of heteroatoms, like polyesters, generally have faster degradation rates than polymers with carbon backbones because the ester functional groups are susceptible to hydrolysis. Furthermore, the ester groups serve as linkages in the main chain and undergo random chain scission as hydrolysis proceeds. Note, the presence of reactive functional groups does not immediately signal that a polymer will rapidly degrade. Other chemical and physical characteristics influence the diffusion of moisture and microorganisms into the polymer. For example, polyesters with higher molecular weights, crystallinity, and/or thicknesses impede diffusion and, ultimately, exhibit slower hydrolysis rates (Deroiné et al., 2014b; Haider et al., 2019; Scaffaro et al., 2019; Vert, 2005; Le Duigou et al., 2009; Gross and Kalra, 2002). A more detailed review of physical and chemical degradation processes is presented by Lucas et al. (Lucas et al., 2008) and will not be further discussed in this review.
The extent of degradation and mineralization is also determined by the temperature, relative humidity, oxygen levels, and population and diversity of microorganisms. The contribution of these environmental factors is often overlooked, as biodegradable plastics continue to be mistaken as biodegradable in any natural environment. Most often, polymers that are certified to be biodegradable and/or compostable are tested at elevated temperatures (greater than 30°C), in aerobic conditions, and in the presence of diverse microorganisms. These conditions are not representative of the ocean, where temperatures, oxygen levels, and microorganisms are a function of depth, season, and location. Even in highly biotic regions of the ocean, biodegradable polymers show slower biodegradation rates than those tested in terrestrial or managed environments (Haider et al., 2019; Narancic et al., 2018; Schneiderman and Hillmyer, 2017; Emadian et al., 2017; Deroiné et al., 2014a; Tokiwa et al., 2009; Suyama et al., 1998; Shogren, 1997). In other words, polymers that biodegrade in aerobic and biotic environments cannot be assumed to have the same extent of biodegradation in the ocean.
Marine Biodegradation Evaluations
To develop non-persistent packaging, rigorous biodegradation tests must be completed to account for the inherent variability found in ocean environments. There is a myriad of available tests to evaluate the degradation and biodegradation of these polymers. Bench-top methods for quantifying physical and enzymatic degradation in aquatic environments have been published by the International Organization of Standardization (ISO), Organization for Economic Co-operation and Development (OECD), American Society for Testing and Materials (ASTM), and TUV Austria. These testing schemes describe one or more of the following: disintegration, biodegradation, and/or ecotoxicity tests at one temperature.
-
•
Disintegration compares the initial sample mass to the residual sample that is caught on a sieve after 12 weeks of agitation.
-
•
Biodegradation is defined as the percentage of elemental carbon converted to carbon dioxide during the respiration of seawater microorganisms consuming the substrate.
-
•
A common ecotoxicity test investigates changes to the reproduction and morbidity rates of Daphnia (water fleas) after being exposed to the diluted sample for several months (TUV Austria, 2019; American Society for Testing and Materials, 2017).
While the majority of these standards detail the experimental setup for these tests and provide unclear success criteria, TUV Austria is the only standard specifying clear pass/fail criteria for disintegration, biodegradation, and ecotoxicity testing (Table 1) (American Society for Testing and Materials, 2017; 2005; International Organization for Standardization ISO, 2001; International Organization for Standardization ISO, 2016; TUV Austria, 2019).
Table 1.
International Standards Providing Clear Pass/Fail Criteria for Polymer Disintegration, Biodegradation, and/or Ecotoxicity in Marine Environments
| Standard | Disintegration | Biodegradation | Ecotoxicity | Test Considers Depth, Location, & Season |
|---|---|---|---|---|
| ISO 16221 | ✓ | |||
| ISO 18830 | ✓ | ✓ | ||
| OECD TG 306 | ✓ | |||
| OECD 202 | ✓ | |||
| ASTM D6691 | ✓ | |||
| ASTM D7081 | ✓ | ✓ | ||
| TUV Austria “OK Marine” | ✓ | ✓ | ✓ |
Many scientists and engineers contend that these bench-top standards are limited in scope and do not effectively replicate the ocean's dynamic conditions and ecosystems. They argue the standards' methods expose the sample to one temperature, oxygen level, and a single microorganism consortium—effectively concluding biodegradation based on a single environmental data point. To increase the confidence in marine biodegradation results, samples should be tested at multiple temperatures, oxygen levels, and in the presence of diversified microorganism populations.
Another method for evaluating the end-of-life characteristics of degradable polymers is to test them in oceanic field tests. Implementing field tests in marine environments exposes the samples to complex factors, synergies, and competing reactions, thereby providing a more accurate representation of the polymer's degradation in the ocean. Unlike bench-top studies, marine field tests incorporate biotic and abiotic factors, like microorganisms, light, the mechanical action of waves, and seasonal temperature fluctuations (Haider et al., 2019; Deroiné et al., 2014a, 2014b, 2015; Sekiguchi et al., 2011; Beltrán-Sanahuja et al., 2020). However, field tests do not have the benefit of measuring the evolution of carbon dioxide while the polymer samples are submerged. As such, it is uncertain whether the samples mineralized into benign by-products or if they fragmented into microplastics. The other challenge is that these field tests are very expensive and do not scale with the iterative requirements of the industry, requiring a low-cost screening method.
In the absence of representative marine biodegradation standards and accessible field testing, researchers have developed economical degradation evaluations. Degradation is manifested macroscopically by mass loss, mechanical property loss, and surface erosion and can be readily characterized (Scaffaro et al., 2019; Ter Halle et al., 2016). It is common for researchers to submerge polymer samples in seawater and complete gravimetric and thermal analysis, tensile testing, spectroscopy, and electron microscopy at the conclusion of the test. In addition to monitoring the deterioration of material properties, scientists often investigate biofilm formation and the enzymes present on the polymer surface (Dussud et al., 2018; Rummel et al., 2017; Oberbeckmann et al., 2016; Eich et al., 2015; Sekiguchi et al., 2011). These adaptations provide quantitative information on polymer degradation, yet these tests do not quantify the extent of bioassimilation and biodegradation.
Therefore, the most robust holistic biodegradation evaluation would include subjecting the sample specimens to a battery of bench-top respirometric tests and then conducting field tests for validation. Currently, ASTM and TUV Austria define samples surpassing 90% biodegradation within 6 months to be biologically assimilated in marine environments. Materials that do not meet this requirement form microplastics that will not be readily bioassimilated and mineralized. Completing bench-top biodegradation tests is more conducive to iterative development due to lower costs associated with evaluating carbon dioxide evolution (Haider et al., 2019). If the sample results are reproducible and meet these requirements over a series of environmental conditions, researchers could then execute field tests.
Unfortunately, the integrated bench-top and field test approach is rarely demonstrated in the literature. Deroiné et al. (Deroiné et al., 2014a) evaluated the marine degradation of PLA by simultaneously running non-respirometric bench-top degradation tests alongside a seawater field test for 180 days. Beltrán-Sanahuja et al. (Beltrán-Sanahuja et al., 2020) developed a year-long degradation test to mimic the ocean's complex biotic and abiotic conditions in a laboratory setting. In their PLA degradation study, they simulated water columns and seafloors, sunlight, temperatures, and oceanic microorganisms. Although Deroiné and Beltrán-Sanahuja et al. observed mass and morphology changes in their test environments, they did not evaluate if these changes were dominated by degradation or biodegradation. Previous studies report that PLA-degrading microorganisms are not widely found in nature, suggesting bioassimilation was unlikely to have occurred in these bench-top and field tests (Narancic et al., 2018; Tokiwa et al., 2009; Suyama et al., 1998; Shogren, 1997).
Narancic et al. (Narancic et al., 2018) definitively evaluated commercial biodegradable polymers and their blends according to TUV Austria's biodegradation test. Their study included neat polymers and blends of aliphatic polyesters and TPS. After 56 days, polyhydroxybutyrate (PHB) (ENMATY 1000 TiTAN) and TPS (Bioplast TPS BIOTEC) films were the only polymers that passed the biodegradation criteria. These findings provide a clear starting place for companies and researchers to evaluate possible marine biodegradable packaging materials.
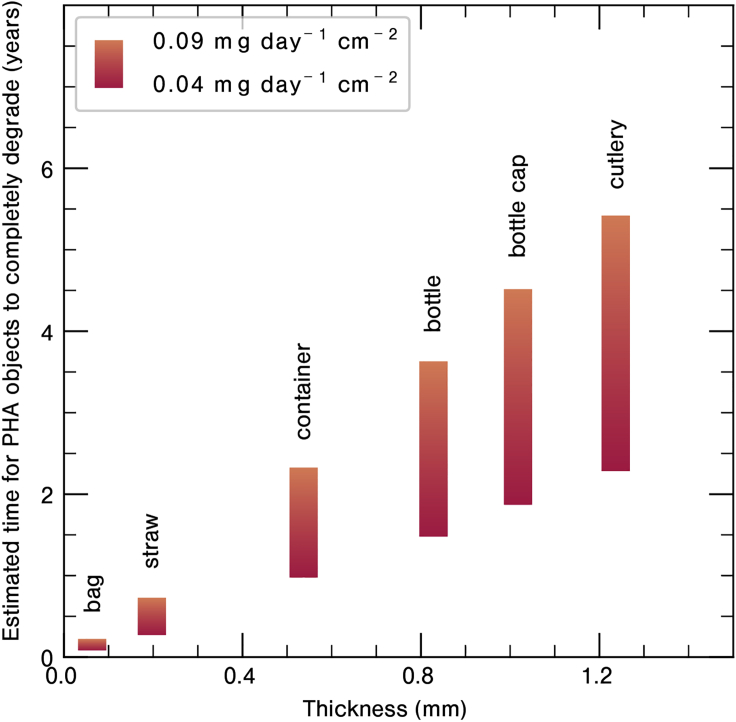
PHA biodegradation in marine environments has been widely tested and confirmed by the scientific community (Dilkes-Hoffman et al., 2019; Bugnicourt et al., 2014; Deroiné et al., 2014b; Volova et al., 2010; Tokiwa et al., 2009). Dilkes-Hoffman et al. (Dilkes-Hoffman et al., 2019) extensively reviewed the marine degradation studies of PHA-based products to estimate the relative time frame in which these polymers could be expected to mineralize (Figure 4). They found that PHA films ≤0.2 mm thick would biodegrade within several months in marine environments. Thin films have the advantage over bulk plastics in that their diffusion lengths are much smaller, facilitating water and microorganism penetration (Dilkes-Hoffman et al., 2019; Scaffaro et al., 2019; Vert, 2005; Gross and Kalra, 2002).
Figure 4.
Estimated Time for Different PHA Objects to Completely Degrade as a Function of Object Thickness with Two Different Degradation Rates
Image redrawn from (Dilkes-Hoffman et al., 2018).
Finally, PVOH has been shown to be biodegradable in municipal wastewater treatment facilities, but due to its carbon backbone it is unlikely to mineralize in isolated environments, like the ocean. PVOH is commonly used as the water-soluble packaging for single-dose laundry and dishwasher detergents. After the polymer dissolves in the water, it is transported to a municipal water treatment facility where it can be consumed by the myriad of microorganisms present. The high concentration and diversity of microorganisms in these facilities are required to biodegrade PVOH's carbon backbone (Halima, 2016; Leja and Lewandowicz, 2010; Gross and Kalra, 2002). In light of these results, we restrict our investigation of commercial marine biodegradable polymers to the PHA family and TPS.
Material Processing
For a polymer to be used on commercial packaging converters, it must be compatible with thermoplastic processing methods. Such processing methods include blow molding, injection molding, casting, co-extrusion, and heat sealing. TPS and PHAs are thermoplastics and have been successfully used in the aforementioned thermoforming techniques (Emblem, 2012; Dilkes-Hoffman et al., 2018). From a materials processing perspective, these materials have the potential to replace conventional polymers in packaging applications.
Conventional multilayered structures are commonly formed by laminating films with a sealant or adhesive. The sealant also serves as a compatibilizer, joining hydrophobic and low-surface-energy materials. Many of the sealants found in multilayer laminates are comprised of ethylene-vinyl alcohol-based (EVOH) copolymers, which also compromises the marine biodegradability of these multilayered materials. To avoid the use of EVOH, Dilkes-Hoffman et al. (Dilkes-Hoffman et al., 2018) developed bilayer TPS/PHB films through the co-extrusion process. After subjecting the bilayers to 75% relative humidity (RH) for several weeks, the TPS and PHB began to delaminate and crack. It is well known that TPS is hydrophilic and PHB has hydrophobic character, suggesting that a compatibilizer is needed during processing (Scaffaro et al., 2019). This topic requires more research as the use of even marine-degradable sealants/adhesives would stagnate the biodegradation rate by increasing the thickness of the package.
In addition to incompatibility, there are other processing shortcomings associated with biodegradable polymers. Unlike polyolefins, aliphatic polyesters and starches are particularly susceptible to thermal degradation and contamination during manufacturing and throughout the supply chain. For example, while on packaging converters, these polymers will experience elevated temperatures from friction. Even in the presence of trace amounts of water vapor, these plastics can begin to hydrolyze, resulting in poor mechanical properties and even packaging failure (Emblem, 2012; Scaffaro et al., 2019). It is likely that these materials will also be exposed to microbial populations and be contaminated during transportation or storage (Kulkarni Vishakha et al., 2012). As a result, polyolefins have more reliable performance and extended shelf-lifes compared to biodegradable polymers.
Stability
Stability is often tested to evaluate the product's physical, chemical, and functional properties during shipping, storage, and use (Helanto et al., 2019; Emblem, 2012). Biopolymers have been extensively studied for applications in food preservation and packaging, especially for fresh produce. Oftentimes, product stability or shelf-life is correlated with aroma, oil, water vapor, and oxygen barrier, as these properties directly influence the esthetics and longevity of food (Helanto et al., 2019; Dilkes-Hoffman et al., 2018; Vähä-Nissi et al., 2015, 2012; Emblem, 2012). There is a myriad of reviews on packaging stability for the food industry (Fabra et al., 2014; Cha and Chinnan, 2004), but no such stability studies are available for biopolymers used in cosmetic or personal care product packaging (Cinelli et al., 2019). The lack of stability studies is likely attributed to stability tests being developed internally according to formulation-specific requirements. The results of these tests are typically proprietary, and subsequently, are not well documented in the literature (Postles, 2019; Cinelli et al., 2019). However, moisture barrier evaluations have been routinely published and can provide important insights into the stability of cosmetics and personal care products. Therefore, we restrict our stability evaluation to the results from moisture barrier tests.
Moisture Barrier
Moisture barrier describes the transmission of water vapor through permeable substrate materials. Barrier types typically fall into two categories: absolute and permeable barriers. Absolute barriers, like metal and glass, are impenetrable to moisture, whereas uncoated paper and plastic films are permeable (Emblem, 2012). Permeable materials constitute most single-use packaging because they are less dense and expensive than absolute barriers. Permeable packaging materials fall on a spectrum of barrier properties. For example, multilayered polyolefin films are more impervious to moisture, transporting water at the rate of < 1 g/m2/day, whereas Cellophane (cellulose) can transmit over 4,000 g/m2/day (Helanto et al., 2019; Vähä-Nissi et al., 2012; Emblem, 2012; Shi et al., 2013).
Evaluating Moisture Barrier
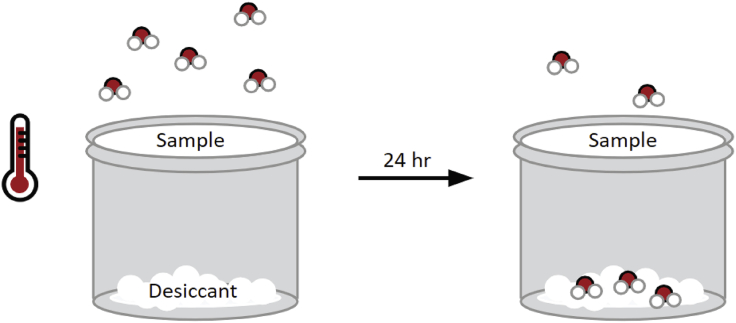
Moisture barrier is evaluated by the water vapor transmission rate (WVTR) and water vapor permeability coefficient (WVPC). WVTR quantifies moisture barrier for non-homogeneous and/or multilayer samples, whereas the WVPC (also called the permeance) generally describes transport through homogeneous and monolayer materials (American Society for Testing and Materials ASTM, 2016). Two common variations for measuring WVTR are the desiccant and water methods described in ASTM E96. In each method, the flexible packaging substrate separates the desiccant or water from the controlled atmosphere for 24-h. Throughout the test, water vapor passes from areas of high to low moisture concentrations through the permeable sample (Figure 5) (American Society for Testing and Materials ASTM, 2016; Dilkes-Hoffman et al., 2019; Shi et al., 2013).
Figure 5.
Moisture Barrier Can be Evaluated by the Water Vapor Transmission Rate (WVTR)
The WVTR is often measured using the desiccant method described in ASTM E96, where the flexible permeable packaging separates the desiccant from the controlled temperature and humidity. Water vapor passes from high to low moisture concentrations through the permeable sample, corresponding to changes in desiccant mass.
The sample's WVTR is defined by
| (Equation 1) |
where G is weight change in grams of desiccant or water before and after the test, t is the testing duration in days, and A is sample surface area in square meters. WVPC is defined by
| (Equation 2) |
where Δp is the water vapor pressure difference.
Testing conditions are internally selected based on simulated usage and shipping conditions. The most rigorous testing condition described in ASTM E96 is 37.8°C (100°F) and 100% RH. Note that WVTR and WVPC values increase with increasing RH and temperature, meaning permeability and transmission rates can only be directly compared with samples tested at the same conditions (Helanto et al., 2019; Koller, 2014).
Moisture Barrier Requirements
Moisture barrier requirements are dictated by the sensitivity of enclosed products to changes in the water content. Products that are minimally affected by water vapor, like baby diapers, do not have rigorous barrier requirements. A moderate barrier is needed for packaging hygroscopic products, like dry laundry detergent, which readily aggregates when exposed to water vapor in the air (Cinelli et al., 2019). However, the most stringent barrier requirements are reserved for aqueous products, like shampoo, where water loss dramatically alters product performance and quality (Cinelli et al., 2019; Dilkes-Hoffman et al., 2019; Helanto et al., 2019).
Petersen et al. (Petersen et al., 1999) described the following acceptable methods for improving barrier properties of packaging for more sensitive products:
-
1.
Coat moisture-sensitive materials with hydrophobic components
-
2.
Laminate two or more biopolymers through co-extrusion
-
3.
Develop blends of biopolymers with different properties
-
4.
Modify biopolymers (chemically or physically) or
-
5.
Develop biopolymer-based micro and nanocomposites.
Even in the non-degradable packaging realm, no single material fits all moisture barrier requirements. Instead, multilayered composites are used with different layers filling key roles such as moisture barrier and thermoforming capability (Helanto et al., 2019; Schneiderman and Hillmyer, 2017; Mensitieri et al., 2011). The barrier properties of bioplastics are typically inferior to those of conventional polymers, requiring at least the same treatment. To improve the moisture barrier of PHAs and TPS, composites composed of inorganic additives, metallized polymers, or lamination must be used.
Moisture Barrier Studies of Biodegradable Polymers
Many of these barrier improvements have been applied to PHA. Dilkes-Hoffmann et al. (Dilkes-Hoffman et al., 2018) developed a biodegradable TPS-PHBV bilayer laminate to improve moisture barrier properties of hydrophilic TPS. PHAs, including PHBV, are the most hydrophobic of the biological aliphatic polyesters (Dilkes-Hoffman et al., 2018). Pairing PHBV with TPS reduced the moisture uptake of the overall film. However, within 5 days of testing in 75% RH, the moisture content was greater than 5 wt% and the bilayer structure started to crack and delaminate. This amount of moisture transfer would be unacceptable for packaging hygroscopic or aqueous product (Cinelli et al., 2019).
Finally, polymer processing methods have influenced moisture barrier properties by changing the amount of free volume in the polymer film. Koller (2014) found that the WVPC decreased for samples that were formed using compression molding while solvent cast films were more permeable to moisture. This suggests the compression molding decreased the amount of free volume of the polymer films. Decreasing the free volume corresponds to minimizing the diffusion pathways, thereby forcing the molecules to take a more tortuous path (Follain et al., 2014).
Another method for improving moisture barrier properties of polymer films is depositing a thin layer of metal, often aluminum, on the substrate via vacuum metallization. Metallization imparts a portion of aluminum's absolute barrier properties and produces plastic films that are generally lightweight and inexpensive. Vacuum metallization is commonly found in multilayered structures, such as snack chip bags, granola bars, and condiments sachets. Unfortunately, vacuum metallization leaves biodegradable polymers with undesirable properties, such as increased thickness, which stagnates biodegradation rates (Garcia et al., 2018; Fabra et al., 2014). These challenges have been addressed by applying thin layers of Al2O3 to biodegradable polymers using atomic layer deposition (ALD).
Vähä-Nissi et al. (Vähä-Nissi et al., 2015) applied Al2O3 via ALD to cellulose and biodegradable polyesters, including PHBV (50 μm BV301050, Goodfellow Ltd). PHBV's transmission rate before ALD was 8.3 g/m2/day, which was the lowest moisture vapor transmission rate (MVTR) of the biopolymers tested. The moisture barrier properties dramatically improved after the ALD process, with PHBV transmitting approximately 1 g/m2/day (Table 2). The stark difference in the moisture barrier properties is attributed to PHBV's ester groups, which likely reacted with the ALD precursors to form a continuous Al2O3 coating. These esters linkages are also the reactive sites for hydrolysis, suggesting that the ALD of Al2O3 may affect PHBV's hydrolytic degradation. Vähä-Nissi et al. selected PLA as a representative polymer and exposed the Al2O3-coated PLA to selected food simulants, including ethanol, acetic acid, and water. Each simulant dissolved the Al2O3 coating, requiring an additional resistant layer to prevent dissolution in packaging applications. As seen in Figure 1, a similar laminate approach has been replicated in industry to protect the metallized layer.
Table 2.
WVTRs for Polymer Films with 25 nm ALD Al2O3Deposited at 100°C(Vähä-Nissi et al., 2015)
| Oxidizing Precursor | WVTR (23°C, 50% RH) g/m2/day |
|---|---|
| A-CEL | 290 |
| A-CEL + ALD | <1.0 |
| CEL | 100 |
| CEL + ALD | 44 |
| C-CEL | 35 |
| C-CEL + ALD | 2 |
| BOPP | <1.0 |
| BOPP + ALD | <1.0 |
| PHB/HV | 8.3 |
| PHB/HV + ALD | 1.0 |
| O-PA | <1.0 |
| O-PA + ALD | <1.0 |
| O-PET | 3.1 |
| O-PET + ALD | <1.0 |
| PLA | 21 |
| PLA + ALD | <1.0 |
A-CEL, anchored cellulose; CEL, cellulose; BOPP, biaxially oriented polypropylene; O-PA, oriented polyamide and polyethylene; O-PET, oriented polyethylene terephthalate.
Additives, such as inorganic clays, have shown immense improvements to biopolymer-based moisture barriers. Sanchez-Garcia et al. (Sanchez-Garcia et al., 2010) incorporated 5 wt% organo-modified clays into PHBV, ultimately decreasing the WVPC by 76%. Research has also shown that these clays can form interpenetrating layers, which have been shown to improve moisture barrier properties (Follain et al., 2014; Koller, 2014).
Many studies, including those highlighted here, have improved the moisture barrier properties using these techniques. However, these improvements still do not meet the requirements for moisture-sensitive materials like shampoo and powdered detergents (Helanto et al., 2019; Follain et al., 2014; Koller, 2014). The present challenge is that TPS and PHA, even in multilayers, do not have the barrier properties needed to enclose hygroscopic or aqueous products. This deficiency requires that improvement if CPG packaging is to be marine biodegradable.
Conclusion
TPS and PHAs have the potential to replace conventional polyolefins in single-use flexible packaging. These materials meet established biodegradation requirements and have degraded in marine field tests. Although both these polymers are commercially available, they still have limitations. Despite PHAs possessing more hydrophobicity than other bio-based polyesters, their moisture barriers are not currently sufficient to contain liquid or dry products, like shampoo or coffee. There are opportunities for more research into the relative chemical stabilities of these materials and how to maximize the shelf-life without negatively impacting biodegradability. Finally, despite TPS being readily renewable and abundant, PHAs are at present produced at small volumes and are 5–10 times more expensive than polyethylene (Gross and Kalra, 2002; Bugnicourt et al., 2014). The properties of PHAs and TPS, namely, chemical stability, moisture barrier, and cost, must be improved to enable commercialization.
Acknowledgments
Author Contributions
Conceptualization, A.B. and T.D.S.; Writing – Original Draft, A.B.; Writing – Review and Editing, A.B. and T.D.S.; Visualization, A.B. and T.D.S.; Supervision, T.D.S.
Declarations of Interests
The authors declare no competing interests.
References
- Al-Salem S., Al-Hazza’a A., Karam H., Al-Wadi M., Al-Dhafeeri A., Al-Rowaih A. Insights into the evaluation of the abiotic and biotic degradation rate of commercial pro-oxidant filled polyethylene (pe) thin films. J. Environ. Manage. 2019;250:109475. doi: 10.1016/j.jenvman.2019.109475. [DOI] [PubMed] [Google Scholar]
- American Society for Testing and Materials (ASTM) ASTM International; 2016. ASTM E96 Standard Test Method for Water Vapor Transmission of Materials. [Google Scholar]
- American Society for Testing and Materials (ASTM) 2017. ASTM D6691 - 17 Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in the Marine Environment by a Defined Microbial Consortium or Natural Sea Water Inoculum. [Google Scholar]
- American Society for Testing and Materials (ASTM) 2005. ASTM D7081-05 Standard Specification for Non-floating Biodegradable Plastics in the Marine Environment (Withdrawn 2014) [Google Scholar]
- Andrady A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011;62:1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Arrieta M.P., Samper M.D., Aldas M., López J. On the use of pla-phb blends for sustainable food packaging applications. Materials. 2017;10:1008. doi: 10.3390/ma10091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán-Sanahuja A., Casado-Coy N., Simó-Cabrera L., Sanz-Lázaro C. Monitoring polymer degradation under different conditions in the marine environment. Environ. Pollut. 2020;259:113836. doi: 10.1016/j.envpol.2019.113836. [DOI] [PubMed] [Google Scholar]
- Bugnicourt E., Cinelli P., Lazzeri A., Alvarez V.A. Express Polymer Letters; 2014. Polyhydroxyalkanoate (Pha): Review of Synthesis, Characteristics, Processing and Potential Applications in Packaging. [Google Scholar]
- Cha D.S., Chinnan M.S. Biopolymer-based antimicrobial packaging: a review. Crit. Rev. Food Sci. Nutr. 2004;44:223–237. doi: 10.1080/10408690490464276. [DOI] [PubMed] [Google Scholar]
- Chatain B. Parliament seals ban on throwaway plastics by 2019. https://www.europarl.europa.eu/news/en/press-room/20190321IPR32111/parliament-seals-ban-on-throwaway-plastics-by-2021
- Cho H., Moon H., Kim M., Nam K., Kim J. Biodegradability and biodegradation rate of poly (caprolactone)-starch blend and poly (butylene succinate) biodegradable polymer under aerobic and anaerobic environment. Waste Manag. 2011;31:475–480. doi: 10.1016/j.wasman.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Cinelli P., Coltelli M.B., Signori F., Morganti P., Lazzeri A. Cosmetic packaging to save the environment: future perspectives. Cosmetics. 2019;6:26. [Google Scholar]
- Commissie E. European Commission; 2018. Report from the Commission to the European Parliament and the Council on the Impact of the Use of Oxo-Degradable Plastic, Including Oxo-Degradable Plastic Carrier Bags, on the environment. [Google Scholar]
- Cózar A., Echevarría F., González-Gordillo J.I., Irigoien X., Úbeda B., Hernández-León S., Palma Á.T., Navarro S., García-de Lomas J., Ruiz A. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. U S A. 2014;111:10239–10244. doi: 10.1073/pnas.1314705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco, A., Wideman, G.J., Pickett, A.N., and Mleziva, M.M. Soft thermoplastic injection molded and flushable materials, February 20 2020. US Patent App. 16/344,594.
- Deroiné M., Le Duigou A., Corre Y.-M., Le Gac P.-Y., Davies P., César G., Bruzaud S. Accelerated ageing of polylactide in aqueous environments: comparative study between distilled water and seawater. Polym. Degrad. Stab. 2014;108:319–329. [Google Scholar]
- Deroiné M., Le Duigou A., Corre Y.-M., Le Gac P.-Y., Davies P., César G., Bruzaud S. Seawater accelerated ageing of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) Polym. Degrad. Stab. 2014;105:237–247. [Google Scholar]
- Deroiné M., César G., Le Duigou A., Davies P., Bruzaud S. Natural degradation and biodegradation of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) in liquid and solid marine environments. J. Polym. Environ. 2015;23:493–505. [Google Scholar]
- Dilkes-Hoffman L.S., Pratt S., Lant P.A., Levett I., Laycock B. Polyhydroxyalkanoate coatings restrict moisture uptake and associated loss of barrier properties of thermoplastic starch films. J. Appl. Polym. Sci. 2018;135:46379. [Google Scholar]
- Dilkes-Hoffman L.S., Lant P.A., Laycock B., Pratt S. The rate of biodegradation of pha bioplastics in the marine environment: a meta-study. Mar. Pollut. Bull. 2019;142:15–24. doi: 10.1016/j.marpolbul.2019.03.020. [DOI] [PubMed] [Google Scholar]
- Le Duigou A., Davies P., Baley C. Seawater ageing of flax/poly (lactic acid) biocomposites. Polym. Degrad. Stab. 2009;94:1151–1162. [Google Scholar]
- Dussud C., Hudec C., George M., Fabre P., Higgs P., Bruzaud S., Delort A.-M., Eyheraguibel B., Meistertzheim A.-L., Jacquin J. Colonization of non-biodegradable and biodegradable plastics by marine microorganisms. Front. Microbiol. 2018;9:1571. doi: 10.3389/fmicb.2018.01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich A., Mildenberger T., Laforsch C., Weber M. Biofilm and diatom succession on polyethylene (pe) and biodegradable plastic bags in two marine habitats: early signs of degradation in the pelagic and benthic zone? PLoS One. 2015;10:1–13. doi: 10.1371/journal.pone.0137201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emadian S.M., Onay T.T., Demirel B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017;59:526–536. doi: 10.1016/j.wasman.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Emblem A. Elsevier; 2012. Packaging Technology: Fundamentals, Materials and Processes. [Google Scholar]
- Fabra M., López-Rubio A., Lagaron J. Smart Polymers and Their Applications. Elsevier; 2014. Biopolymers for food packaging applications; pp. 476–509. [Google Scholar]
- Follain N., Chappey C., Dargent E., Chivrac F., Crétois R., Marais S. Structure and barrier properties of biodegradable polyhydroxyalkanoate films. J. Phys. Chem. C. 2014;118:6165–6177. [Google Scholar]
- Garcia C.V., Shin G.H., Kim J.T. Metal oxide-based nanocomposites in food packaging: applications, migration, and regulations. Trends Food Science Technol. 2018;82:21–31. [Google Scholar]
- Gross R.A., Kalra B. Biodegradable polymers for the environment. Science. 2002;297:803–807. doi: 10.1126/science.297.5582.803. [DOI] [PubMed] [Google Scholar]
- Group C. Anti-waste: reducing packaging waste. http://www.carrefour.com/combating-waste/anti-waste-reducing-packaging-waste
- Haider T.P., Völker C., Kramm J., Landfester K., Wurm F.R. Plastics of the future? the impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 2019;58:50–62. doi: 10.1002/anie.201805766. [DOI] [PubMed] [Google Scholar]
- Halima N.B. Poly (vinyl alcohol): review of its promising applications and insights into biodegradation. RSC Adv. 2016;6:39823–39832. [Google Scholar]
- Ter Halle A., Ladirat L., Gendre X., Goudounèche D., Pusineri C., Routaboul C., Tenailleau C., Duployer B., Perez E. Understanding the fragmentation pattern of marine plastic debris. Environ. Sci. Technol. 2016;50:5668–5675. doi: 10.1021/acs.est.6b00594. [DOI] [PubMed] [Google Scholar]
- Ter Halle A., Ladirat L., Martignac M., Mingotaud A.F., Boyron O., Perez E. To what extent are microplastics from the open ocean weathered? Environ. Pollut. 2017;227:167–174. doi: 10.1016/j.envpol.2017.04.051. [DOI] [PubMed] [Google Scholar]
- Hartley B.L., Pahl S., Holland M., Alampei I., Veiga J.M., Thompson R.C. Turning the tide on trash: empowering european educators and school students to tackle marine litter. Mar. Policy. 2018;96:227–234. [Google Scholar]
- Helanto K.E., Matikainen L., Talja R., Rojas O.J. Bio-based polymers for sustainable packaging and biobarriers: a critical review. BioResources. 2019;14:4902–4951. [Google Scholar]
- International Organization for Standardization (ISO) ISO; 2001. ISO 16221:2001 Water Quality – Guidance for Determination of Biodegradability in the Marine Environment. [Google Scholar]
- International Organization for Standardization (ISO) ISO; 2016. ISO 18830:2016 Plastics – Determination of Aerobic Biodegradation of Non-floating Plastic Materials in a Seawater/sandy Sediment Interface – Method by Measuring the Oxygen Demand in Closed Respirometer. [Google Scholar]
- Jnr A.K.-L., Yunana D., Kamsouloum P., Webster M., Wilson D.C., Cheeseman C. Recycling waste plastics in developing countries: use of low-density polyethylene water sachets to form plastic bonded sand blocks. Waste Manag. 2018;80:112–118. doi: 10.1016/j.wasman.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Koller M. Applied Food Biotechnology; 2014. Poly (Hydroxyalkanoates) for Food Packaging: Application and Attempts towards Implementation. [Google Scholar]
- Kulkarni Vishakha S., Butte Kishor D., Rathod Sudha S. Natural polymers–a comprehensive review. Int. J. Res. Pharm. Biomed. Sci. 2012;3:1597–1613. [Google Scholar]
- Kyrikou I., Briassoulis D. Biodegradation of agricultural plastic films: a critical review. J. Polym. Environ. 2007;15:125–150. [Google Scholar]
- Landon-Lane M. Corporate social responsibility in marine plastic debris governance. Mar. Pollut. Bull. 2018;127:310–319. doi: 10.1016/j.marpolbul.2017.11.054. [DOI] [PubMed] [Google Scholar]
- Laycock B., Nikolić M., Colwell J.M., Gauthier E., Halley P., Bottle S., George G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017;71:144–189. [Google Scholar]
- Leja K., Lewandowicz G. Polymer biodegradation and biodegradable polymers-a review. Polish J. Environ. Stud. 2010;19 [Google Scholar]
- Li W.C., Tse H., Fok L. Plastic waste in the marine environment: a review of sources, occurrence and effects. Sci. Total Environ. 2016;566:333–349. doi: 10.1016/j.scitotenv.2016.05.084. [DOI] [PubMed] [Google Scholar]
- Liu T., Zhao Y., Zhu M., Liang J., Zheng S., Sun X. Seasonal variation of micro-and meso-plastics in the seawater of jiaozhou bay, the yellow sea. Mar. Pollut. Bull. 2020;152:110922. doi: 10.1016/j.marpolbul.2020.110922. [DOI] [PubMed] [Google Scholar]
- Lucas N., Bienaime C., Belloy C., Queneudec M., Silvestre F., Nava-Saucedo J.-E. Polymer biodegradation: mechanisms and estimation techniques–a review. Chemosphere. 2008;73:429–442. doi: 10.1016/j.chemosphere.2008.06.064. [DOI] [PubMed] [Google Scholar]
- MacArthur E. Science; 2017. Beyond Plastic Waste. [DOI] [PubMed] [Google Scholar]
- Mensitieri G., Di Maio E., Buonocore G.G., Nedi I., Oliviero M., Sansone L., Iannace S. Processing and shelf life issues of selected food packaging materials and structures from renewable resources. Trends Food Sci. Technol. 2011;22:72–80. [Google Scholar]
- Narancic T., O’Connor K.E. Plastic waste as a global challenge: are biodegradable plastics the answer to the plastic waste problem? Microbiology. 2018;165:129–137. doi: 10.1099/mic.0.000749. [DOI] [PubMed] [Google Scholar]
- Narancic T., Verstichel S., Reddy Chaganti S., Morales-Gamez L., Kenny S.T., De Wilde B., Babu Padamati R., O’Connor K.E. Biodegradable plastic blends create new possibilities for end-of-life management of plastics but they are not a panacea for plastic pollution. Environ. Sci. Technol. 2018;52:10441–10452. doi: 10.1021/acs.est.8b02963. [DOI] [PubMed] [Google Scholar]
- Nazareth M., Marques M.R., Leite M.C., Castro Í.B. Commercial plastics claiming biodegradable status: is this also accurate for marine environments? J. Hazard. Mater. 2019;366:714–722. doi: 10.1016/j.jhazmat.2018.12.052. [DOI] [PubMed] [Google Scholar]
- Nikolić M.A., Gauthier E., Colwell J.M., Halley P., Bottle S.E., Laycock B., Truss R. The challenges in lifetime prediction of oxodegradable polyolefin and biodegradable polymer films. Polym. Degrad. Stab. 2017;145:102–119. [Google Scholar]
- Obasi H.C., Egeolu F., Ezenwajiaku H. Effects of starch content and compatibilizer on the mechanical, water absorption and biodegradable properties of potato starch filled polypropylene blends. Quan. J. Environ. Stud. 2020;1:32–43. [Google Scholar]
- Oberbeckmann S., Osborn A.M., Duhaime M.B. Microbes on a bottle: substrate, season and geography influence community composition of microbes colonizing marine plastic debris. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paler M.K.O., Malenab M.C.T., Maralit J.R., Nacorda H.M. Plastic waste occurrence on a beach off southwestern luzon, Philippines. Mar. Pollut. Bull. 2019;141:416–419. doi: 10.1016/j.marpolbul.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Panahi L., Gholizadeh M., Hajimohammadi R. Investigating the degradability of polyethylene using starch, oxo-material, and polylactic acid under the different environmental conditions. Asia-Pacific J. Chem. Eng. 2020;15:e2402. [Google Scholar]
- Pauli N.-C., Petermann J.S., Lott C., Weber M. Macrofouling communities and the degradation of plastic bags in the sea: an in situ experiment. R. Soc. Open Sci. 2017;4:170549. doi: 10.1098/rsos.170549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K., Nielsen P.V., Bertelsen G., Lawther M., Olsen M.B., Nilsson N.H., Mortensen G. Potential of biobased materials for food packaging. Trends Food Sci. Technol. 1999;10:52–68. [Google Scholar]
- Portillo F., Yashchuk O., Hermida É. Evaluation of the rate of abiotic and biotic degradation of oxo-degradable polyethylene. Polym. Test. 2016;53:58–69. [Google Scholar]
- Postles A. Bournemouth University; 2019. Factors Affecting the Measurement of Stability and Safety of Cosmetic products. PhD Thesis. [Google Scholar]
- Rigotti D., Dorigato A., Pegoretti A. Thermo-mechanical behavior and hydrolytic degradation of linear low density polyethylene/poly (3-hydroxybutyrate) blends. Front. Mater. 2020;7:31. [Google Scholar]
- Rummel C.D., Jahnke A., Gorokhova E., Kühnel D., Schmitt-Jansen M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017;4:258–267. [Google Scholar]
- Sanchez-Garcia M.D., Lopez-Rubio A., Lagaron J.M. Natural micro and nanobiocomposites with enhanced barrier properties and novel functionalities for food biopackaging applications. Trends Food Sci. Technol. 2010;21:528–536. [Google Scholar]
- Scaffaro R., Maio A., Sutera F., Gulino E.F., Morreale M. Degradation and recycling of films based on biodegradable polymers: a short review. Polymers. 2019;11:651. doi: 10.3390/polym11040651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman D.K., Hillmyer M.A. 50th anniversary perspective: there is a great future in sustainable polymers. Macromolecules. 2017;50:3733–3749. [Google Scholar]
- Schneiker S., dos Santos V.A.M., Bartels D., Bekel T., Brecht M., Buhrmester J., Chernikova T.N., Denaro R., Ferrer M., Gertler C. Genome sequence of the ubiquitous hydrocarbon-degrading marine bacterium alcanivorax borkumensis. Nat. Biotechnol. 2006;24:997–1004. doi: 10.1038/nbt1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi T., Saika A., Nomura K., Watanabe T., Watanabe T., Fujimoto Y., Enoki M., Sato T., Kato C., Kanehiro H. Biodegradation of aliphatic polyesters soaked in deep seawaters and isolation of poly (É›-caprolactone)-degrading bacteria. Polym. Degrad. Stab. 2011;96:1397–1403. [Google Scholar]
- Shi C., Zhang S., Li M., Sun W., Fan G., Jin Y., Yang J., Dong T. Barrier and mechanical properties of biodegradable poly (ε-caprolactone)/cellophane multilayer film. J. Appl. Polym. Sci. 2013;130:1805–1811. [Google Scholar]
- Shogren R. Water vapor permeability of biodegradable polymers. J. Environ. Polym. Degrad. 1997;5:91–95. [Google Scholar]
- Shruti V., Kutralam-Muniasamy G. Bioplastics: missing link in the era of microplastics. Sci. Total Environ. 2019;697:134139. doi: 10.1016/j.scitotenv.2019.134139. [DOI] [PubMed] [Google Scholar]
- Suyama T., Tokiwa Y., Ouichanpagdee P., Kanagawa T., Kamagata Y. Phylogenetic affiliation of soil bacteria that degrade aliphatic polyesters available commercially as biodegradable plastics. Appl. Environ. Microbiol. 1998;64:5008–5011. doi: 10.1128/aem.64.12.5008-5011.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokiwa Y., Calabia B.P., Ugwu C.U., Aiba S. Biodegradability of plastics. Int. J. Mol. Sci. 2009;10:3722–3742. doi: 10.3390/ijms10093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUV Austria . 2019. OK BIODEGRADABLE MARINE : Initial Acceptance Tests. [Google Scholar]
- Vähä-Nissi M., Sundberg P., Kauppi E., Hirvikorpi T., Sievänen J., Sood A., Karppinen M., Harlin A. Barrier properties of al2o3 and alucone coatings and nanolaminates on flexible biopolymer films. Thin Solid Films. 2012;520:6780–6785. [Google Scholar]
- Vähä-Nissi M., Pitkänen M., Salo E., Sievänen-Rahijärvi J., Putkonen M., Harlin A. Atomic layer deposited thin barrier films for packaging. Cellulose Chem. Technol. 2015;49:575–585. [Google Scholar]
- Vert M. Aliphatic polyesters: great degradable polymers that cannot do everything. Biomacromolecules. 2005;6:538–546. doi: 10.1021/bm0494702. [DOI] [PubMed] [Google Scholar]
- Vijayaraman S., Mondal P., Nandan A., Siddiqui N.A. 57–65. Springer; 2020. Presence of microplastic in water bodies and its impact on human health. (Advances in Air Pollution Profiling and Control). [Google Scholar]
- Vlachogianni T., Fortibuoni T., Ronchi F., Zeri C., Mazziotti C., Tutman P., Varezić D.B., Palatinus A., Trdan Š., Peterlin M. Marine litter on the beaches of the adriatic and ionian seas: an assessment of their abundance, composition and sources. Mar. Pollut. Bull. 2018;131:745–756. doi: 10.1016/j.marpolbul.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Volova T., Boyandin A., Vasiliev A., Karpov V., Prudnikova S., Mishukova O., Boyarskikh U., Filipenko M., Rudnev V., Xuân B.B. Biodegradation of polyhydroxyalkanoates (phas) in tropical coastal waters and identification of pha-degrading bacteria. Polym. Degrad. Stab. 2010;95:2350–2359. [Google Scholar]
- Xanthos D., Walker T.R. International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): a review. Mar. Pollut. Bull. 2017;118:17–26. doi: 10.1016/j.marpolbul.2017.02.048. [DOI] [PubMed] [Google Scholar]