Abstract
Introduction
Pregnancy-associated malaria (PAM) is a health problem with serious clinical, epidemiological and economic effects.
Purpose
To analyze the microeconomic evaluations of PAM reported in the world scientific literature.
Methods
Systematic review with 15 different search strategies in PubMed, ScienceDirect, Scielo, Google Scholar and Malaria in Pregnancy (MiP) Library. A search, selection and extraction protocol was applied, which guaranteed completeness and reproducibility in accordance with preferred reporting items for systematic reviews and meta-analysis guidelines. The methodological quality was evaluated using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guide. The analysis were based on frequencies, costs and average and incremental cost-effectiveness ratios in 2018 US dollars adjusted for purchasing power parity.
Results
Twenty-two evaluations published between 1990 and 2018 were analyzed, of which 82% addressed cost-effectiveness in Africa. Twelve interventions were studied; of these, intermittent preventive treatment in pregnant women with sulfadoxine-pyrimethamine (IPTp-SP) was the most frequent strategy. The main outcomes were low birth weight, anaemia and DALYs avoided. The best average cost-effectiveness ratio was reported in IPTp-SP with a cost of US$ 2 per DALY avoided, followed by the administration of IPTp-SP in pregnant women with HIV (US$ 14.2).
Conclusions
The studies focus on Africa with a high heterogeneity in the interventions, outcomes, resources and populations studied. All the interventions were highly cost-effective, which demonstrates the importance of including prevention, care and control resources for PAM as a priority in health sector budgets. This is especially true considering the importance of its intervention for social progress and overcoming poverty in endemic areas.
Keywords: Malaria, Pregnancy, Economic evaluations, Cost-effectiveness, Costs, Systematic review, Public health, Infectious disease, Women's health, Evidence-based medicine, Economics
Malaria; Pregnancy; Economic evaluations; Cost-effectiveness; Costs; Systematic review; Public health; Infectious disease; Women's health; Evidence-based medicine; Economics.
1. Introduction
Malaria affects 40% of the world population. The World Health Organization (WHO) estimates 300–500 million people at risk, of which 109 live in America, with approximately one million deaths caused by paludism [1, 2]. Among the most vulnerable groups are pregnant women; in this group, the disease causes 15% of severe anaemia and 35% of low birth weight (LBW) [3, 4]. Pregnancy-associated malaria (PAM) affects the health of pregnant women, fetuses, newborns and children, causing anaemia, cerebral malaria, severe malaria, intrauterine growth retardation, premature birth, abortions, LBW, congenital malaria, higher risk of coinfections and malnutrition, and maternal, intrauterine, neonatal and infant mortality [4, 5, 6, 7, 8, 9].
With respect to the investigation, there are several meta-analysis available on the efficacy of different drugs to prevent PAM and LBW [10, 11, 12]; on intermittent preventive treatment in pregnant women (IPTp) and the use of mosquito nets with insecticides [13]; the burden of PAM and its effects on maternal and infant health [14] or about specific outcomes such as the risk of abortion and stillbirth [15]. A meta-analysis estimated that IPTp reduces the risk of severe PAM 38%, prophylaxis with chloroquine or IPTp decreases the risk of LBW by up to 43% and prophylaxis with antimalarials, IPTp or insecticide-treated mosquito nets (ITMs) reduces perinatal mortality 27% [16].
In addition, some experts have indicated that investigation on PAM should focus on four areas: i) vaccines and malaria in the first trimester of pregnancy, ii) prevention, control and combination of actions in populations with different transmission risks, iii) increased coverage of existing interventions and iv) immunology, pathology, physiology, diagnosis, burden of disease and economic impact of PAM [17].
Consistent with the last recommendation, microeconomic health assessment studies involve technologies or interventions that not only are effective and safe but also present feasible costs for health systems, that is, they show an optimal relationship between the resources used and benefits obtained [18]. However, in case of PAM, this type of studies seems to be restricted to Africa, and there is marked heterogeneity of designs, interventions, costing types, evaluated outcomes, among other relevant items of the health economy that are dispersed in the scientific literature. In this order of ideas, cost-effectiveness studies are available for IPTp with sulfadoxine-pyrimethamine (SP), the use of ITMs [19, 20], the costs of diagnosis and management PAM [21] and direct and indirect treatment costs [22].
Although subject reviews [23] or systematic reviews [24] of malaria prevention have been published, they exclude partial economic evaluations associated with gestational malaria. In addition, because they are not circumscribed only in PAM, these reviews do not include all available economic studies in this field [21, 22] and did not perform a methodological evaluation of the studies included in the review, which inhibit the determination of internal and external validity of each economic evaluation analyzed.
The purpose of this research was to analyze the economic evaluations of PAM carried out at the global level through a systematic review. The advantages of this type of research include drawing conclusions with a greater extrapolation framework, more precise estimates and a higher level of evidence, allowing for the orientation of subsequent health or investigative actions [25] or public policies based on the evidence, which maximize the efficient in the use of health resources.
2. Materials and methods
2.1. Type of study
Systematic review of the literature.
2.2. Question PICO-RT: population intervention comparator outcome (Outcome) resources and time (Time frame of the economic evaluation)
Population: Pregnant women with malaria or at risk of infection. PAM includes gestational malaria, placental malaria or the presence of Plasmodium in placental tissue, and congenital malaria that affects the product of pregnancy in the uterus or up to 30 days of age, ruling out the fact that it was acquired by anopheline bite or blood transfusion.
Intervention: Pharmacological alternatives with chloroquine (CQ), mefloquine (MQ), IPTp with SP and ITMs.
Comparator: Pharmacological measures other than intervention, with or without educational messages.
Outcome: LBW, anaemia, clinical malaria, years of life lost (YLL) and disability-adjusted life years (DALYs) avoided.
Resources: The costing items included in the partial and full economic evaluations.
Time frame: At least one semester of pregnancy.
2.3. Research protocol according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guide
Identification: Nine search strategies were used combining the MeSH (Medical Subject Headings) terms ‘malaria’, ‘paludism’ and ‘Plasmodium’ with the Boolean ‘AND &’ ‘pregnancy’, ‘placenta’ and ‘gestation’ in PubMed, ScienceDirect, Scielo and Google Scholar. They were complemented by a search in the Malaria in Pregnancy (MiP) Library using the terms ‘economic evaluation’, ‘costs’, ‘cost-minimisation’, ‘cost-effectiveness’, ‘cost-utility’ and ‘cost-benefit’. All searches were conducted in English, Spanish and Portuguese.
Screening: The selection of studies was conducted independently and in duplicate by the two authors. After eliminating the duplicate articles in Zotero, screening was performed with three criteria; first, studies that were original thus filtering the secondary studies as reviews, books, editorials and meta-analysis; second, research in which the population was pregnant women with malaria thus filtering other issues as malaria in children, in general population or in patients of health care institutions different to pregnant, this second criterion also allowed to exclude other populations as coinfections with Human immunodeficiency virus (HIV), helminths and other; and third, manuscripts that did not include economic outcomes such as clinical cases, observational studies, clinical trials. The search was conducted without restrictions by year of publication; the time delimitation was made based on the oldest publication and the last application of the search and selection protocol that was made in October 2018 (with update on March 2019 without new economic evaluations that fulfill the protocol).
The search strategies included are shown in Table 1.
Table 1.
Syntax used in each database.
| Database | Syntax |
|---|---|
| PubMed | 1. (Malaria [Title/Abstract]) AND pregnancy [Title/Abstract] |
| 2. (Malaria [Title/Abstract]) AND placenta [Title/Abstract] | |
| 3. (Malaria [Title/Abstract]) AND gestation [Title/Abstract] | |
| 4. (Paludism [Title/Abstract]) AND pregnancy [Title/Abstract] | |
| 5. (Paludism [Title/Abstract]) AND placenta [Title/Abstract] | |
| 6. (Paludism [Title/Abstract]) AND gestation [Title/Abstract] | |
| 7. (Plasmodium [Title/Abstract]) AND pregnancy [Title/Abstract] | |
| 8. (Plasmodium [Title/Abstract]) AND placenta [Title/Abstract] | |
| 9. (Plasmodium [Title/Abstract]) AND gestation [Title/Abstract] | |
| Science Direct | 10. Title, abstract, keywords: malaria pregnancy |
| 11. Title, abstract, keywords: malaria placenta | |
| 12. Title, abstract, keywords: malaria gestation | |
| 13. Title, abstract, keywords: paludism pregnancy | |
| 14. Title, abstract, keywords: paludism placenta | |
| 15. Title, abstract, keywords: paludism gestation | |
| 16. Title, abstract, keywords: Plasmodium pregnancy | |
| 17. Title, abstract, keywords: Plasmodium placenta | |
| 18. Title, abstract, keywords: Plasmodium gestation | |
| Scielo | 19. (ti:((ab:(malaria pregnancy)))) |
| 20. (ti:((ab:(malaria placenta)))) | |
| 21. (ti:((ab:(malaria gestation)))) | |
| 22. (ti:((ab:(paludism pregnancy)))) | |
| 23. (ti:((ab:(paludism placenta)))) | |
| 24. (ti:((ab:(paludism gestation)))) | |
| 25. (ti:((ab:(Plasmodium pregnancy)))) | |
| 26. (ti:((ab:(Plasmodium placenta)))) | |
| 27. (ti:((ab:(Plasmodium gestation)))) | |
| Google Scholar | 28. allintitle: malaria pregnancy |
| 29. allintitle: malaria placenta | |
| 30. allintitle: malaria gestation | |
| 31. allintitle: paludism pregnancy | |
| 32. allintitle: paludism placenta | |
| 33. allintitle: paludism gestation | |
| 34. allintitle: Plasmodium pregnancy | |
| 35. allintitle: Plasmodium placenta | |
| 36. allintitle: Plasmodium gestation | |
| MiP Library | 37. Search (current: economic evaluation AND Bibliographic reference: RECORDTYPE) |
| 38. Search (current: cost AND Bibliographic reference: RECORDTYPE) | |
| 39. Search (current: cost-minimisation AND Bibliographic reference: RECORDTYPE) | |
| 40. Search (current: cost-effectiveness AND Bibliographic reference: RECORDTYPE) | |
| 41. Search (current: cost-utility AND Bibliographic reference: RECORDTYPE) | |
| 42. Search (current: cost-benefit AND Bibliographic reference: RECORDTYPE) |
Note: These same searches that appear in English, were applied in Spanish and Portuguese.
In the databases, only the title, abstract or keywords filter was applied; no other filters were applied (for example, type of publication, humans, etc.), since they decreased the exhaustiveness and sensitivity of the search. Besides, these filters are different in each database, which would affect reproducibility to the extent that some criteria such as “human studies” could be filtered automatically in PubMed but in the other sources it would have to be done manually, using reading of the title- abstract. For this reason, it was decided to apply selection criteria manually in the references manager, where a common source was configured for the titles obtained in all the strategies.
Eligibility: In this phase, a full text reading was performed to apply two exclusion criteria; first, research that was not carried out in humans thus filtering preclinical studies in animals, in vitro models or models whose unit of analysis was cell cultures, and modeling studies; second, articles which were not available, the latter corresponds to articles removed from the databases or without the full text, and for which no response was obtained to the message sent to the authors.
Review of studies: Studies that met the above criteria were characterised based on the following variables: title, authors, year of publication, country, type of economic evaluation, alternatives analyzed, outcomes included, costs, primary and secondary result (such as costs or average and incremental ratios of cost-effectiveness) and conclusion.
2.4. Reproducibility and methodological quality assessment
The reproducibility of the search and selection was guaranteed, as was the extraction of the study variables, through the independent application by the two researchers; in the event of disagreement a priori, it was determined that these would be resolved by consensus between the two researchers. Kappa index for qualitative data and intraclass correlation coefficient for the quantitative variables, was calculate in the selection of manuscripts, and data extraction.
The methodological quality was evaluated using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guide [26].
2.5. Data analysis
The qualitative synthesis was based on frequencies and was performed for the DALYs avoided with different interventions. For cost comparison, deflation was made to 2018 international dollars, and they were then unified with the Parity of Purchasing Power (PPP) conversion factor of the World Bank's International Comparison Program's database, which accounts for the monetary units a country would need to acquire the same units with US dollars on the national market. In the cost-effectiveness studies, the analysis were based on WHO recommendations that define an alternative as highly cost-effective for incremental ratios less than a gross domestic product (GDP) per capita and cost-effective for values less than three times the GDP per capita [27].
3. Results
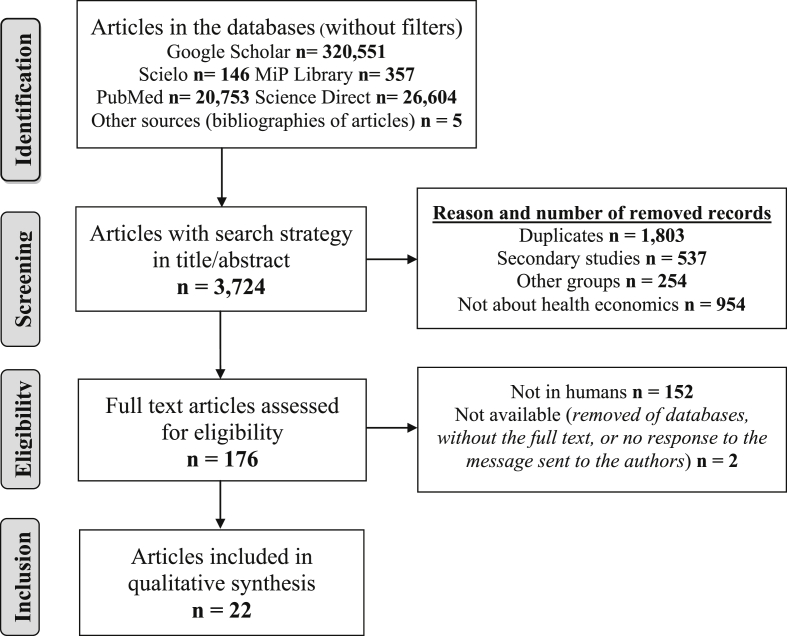
In total, 368,411 studies were identified and 3,724 abstracts were screened, of which 1,128 were eligible, and only 22 studies were included (Figure 1). The intraclass correlation coefficient between the two researchers was 1.0 for the number of studies analyzed at each stage of the search and selection process; for each one of the extracted variables, a kappa index of 1.0 was found for the qualitative variables and the intraclass correlation coefficient was also 1.0 for the quantitative variables.
Figure 1.
Flow gram of search and selection of studies.
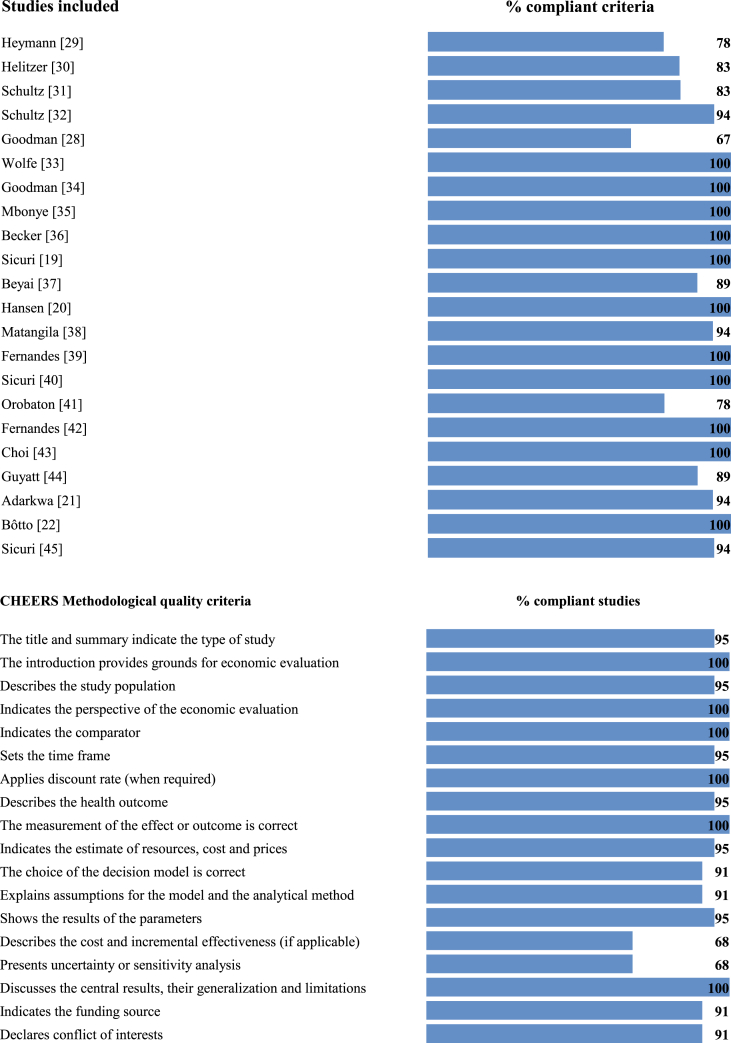
The methodological quality was excellent because they fulfilled >70% of the CHEERS guide criteria, with the exception of Goodman's study [28], which met 67% of them. The least specified criteria were: not choice a decision model [30], not explain assumptions for the model and the analytical method [28, 30], not specify the evaluation's time frame [19, 28, 33], not present uncertainty or sensitivity analysis [21, 28, 29, 30, 41, 45], and not describe the cost and incremental effectiveness [28, 29, 30, 31, 32, 37, 41] (Figure 2).
Figure 2.
Evaluation of methodological quality according to CHEERS criteria.
Publications between 1990 and 2018 were found: 91% were from Africa, one from Brazil and one from Colombia; 80% corresponded to cost-effectiveness studies and 20% to cost studies; the most frequently measured outcome was LBW (65%), followed by DALYs (55%), maternal anaemia (41%) and maternal malaria (29%). The efficacy data were extracted from clinical trials and meta-analysis; 78% of the cost-effectiveness studies evaluated IPTp-SP compared with other drugs or at different doses, and 72% included the health care provider's perspective (Table 2).
Table 2.
Description of studies according to year, country, economic evaluation type, population, compared alternatives and outcomes.
| Author |
Year |
Country |
Population |
Alternatives |
Outcome |
|---|---|---|---|---|---|
| Cost-Effectiveness Analysis | |||||
| Heymann [29] | 1990 | Malawi | Pregnant women | Prophylaxis CQ | Case of malaria avoided |
| Helitzer [30] | 1993 | Malawi | Pregnant women | CQ + MSE, CQ + NMSE, CQCovered + NHEM, CQCovered + OHEM | Compliance with the intervention |
| Schultz [31] | 1995 | Malawi | Women in their 2nd or 3rd pregnancy | SP, SP/CQ, CQ/CQ | LBW |
| Schultz [32] | 1996 | Malawi | Women in their 2nd or 3rd pregnancy | SP, SP/CQ and CQ/CQ | Infant mortality |
| Goodman [28] | 1999 | Sub-Saharan Africa | Primigravida | IPTp-SP and CQ | LBW, DALYs |
| Wolfe [33] | 2001 | Sub-Saharan Africa | Women in their 2nd or 3rd pregnancy | SP2, SPmonth, HIV test + SP, case management | LBW, DALYs |
| Goodman [34] | 2001 | Sub-Saharan Africa | Primigravida | IPTp-SP and CQ | LBW, YLL |
| Mbonye [35] | 2008 | Uganda | Primigravida | Distribution of IPTp-SP by the community | Anaemia, Maternal malaria, LBW, DALYs |
| Becker [36] | 2009 | Congo | Pregnant women | ITM | LBW, Infant mortality, DALYs |
| Sicuri [19] | 2010 | Mozambique | Pregnant women | IPTp-SP | Maternal malaria, Neonatal mortality, DALYs |
| Beyai [37] | 2010 | Gambia | Multiple pregnancies | IPTp-SP | Anaemia, LBW, DALYs |
| Hansen [20] | 2012 | Uganda | Pregnant women (<27 weeks) | IPTp-SP, ITM, IPTp-SP + ITM | Anaemia, LBW, DALYs |
| Matangila [38] | 2014 | Congo | Pregnant women | Microscopy-RDT | Correctly diagnosed |
| Fernandes [39] | 2015 | Sub-Saharan Africa | Pregnant women | IPTp-SP | Maternal malaria, anaemia, LBW, DALYs |
| Sicuri [40] | 2015 | Sub-Saharan Africa | Pregnant women with and without HIV | IPTp-SP/IPTp-MQ, IPTp-MQ + CTX/CTX | Maternal malaria, anaemia, DALYs, non-obstetric hospital admission |
| Orobaton [41] | 2016 | Nigeria | Pregnant women | IPTp-SP distributed by the community | % compliance, head circumference in newborn, Infant mortality |
| Fernandes [42] | 2016 | West Africa | Women in their 1st or 2nd pregnancy | IPTp-SP, ISTp-AL | Maternal malaria, anaemia, LBW, DALYs |
| Choi [43] | 2017 | Sub-Saharan Africa | Pregnant women with HIV | Low 2-IPT, low 3-IPT, high 3-IPT, CTX | Maternal malaria, anaemia, LBW, DALYs |
|
Costs of the disease | |||||
| Guyatt [44] | 2002 | Kenia | Pregnant women | ITM distributed by antenatal program | |
| Adarkwa [21] | 2009 | Ghana | PAM | Description of PAM costs | |
| Bôtto [22] | 2016 | Brazil | PAM and postpartum | Description of PAM costs | |
| Sicuri [45] | 2018 | Colombia | PAM | Description of PAM costs | |
OHEM: Original health educational message; NHEM: New health educational message; CTX: Cotrimoxazole; RDT: Rapid diagnostic test; ISTp-AL: Intermittent Screening and treatment of pregnant women with artemether-lumefantrine; low 2-IPT: IPTp-SP2 (two doses) or CTX; low 3-IPT: IPTp-SP3(three doses) or low coverage CTX; high 3-IPT: IPTp-SP3 or CTX with antiretroviral therapy.
Overall, 44% of the studies included primigravidas or women who were pregnant for the second time [28, 31, 32, 33, 34, 35, 36, 42] and 39% did not specify the number of pregnancies [19, 20, 29, 30, 38, 39, 41]; one study conducted an economic evaluation of women who had multiple pregnancies [37] and two included women with HIV [40, 43]. Almost all interventions were performed during the second and third trimesters of pregnancy and those that evaluated LBW and infant death continued follow-up until one month after delivery.
The items most frequently included in costing were medications, the salaries of health personnel, training for workers and the costs of prenatal programmes based on institutional rates, scientific literature or consultation with experts. Expenses for the patient or her family included travel, loss of productivity and costs associated with the disease or its prevention, collected through patient surveys.
The cost studies reported that for each episode of malaria, 24.3% corresponds to direct costs (mainly drugs) and 75.7% to indirect costs, such as for travel time, waiting time and downtime [21]. In Brazil [22], the direct costs for pregnant women under ambulatory care were 35%, whereas for hospitalisation they were 15%. In Colombia, the direct costs of care for hospitalised women [45] represented 38%. Additionally, the costs of distributing ITM during prenatal care in Kenya corresponded to US $ 3.81 [44].
The comprehensive economic evaluations showed that IPTp-SP was more cost-effective than IPTp-CQ in YLL and LBW prevention [34]. In Malawi, IPTp-SP was more cost-effective than IPTp-CQ, and its combination with SP helped in preventing LBW-associated neonatal death [32]. Another study in Malawi indicated that the IPTp-CQ was not cost-effective for cases of PAM which were avoided [29]. Subsequently, different forms of administration of the drug were evaluated in the same country, and it was determined that delivery to pregnant women with an educational message was found to be more cost-effective [30]. In Nigeria, IPTp-SP distributed via the community was cost-effective, and it was related to increases in newborn head circumference and lower probabilities of fetal death [41]. In the Democratic Republic of the Congo, the use of rapid tests for the diagnosis of PAM was more cost-effective [38].
Case management of PAM presented a cost of US$ 6.2 for each DALY avoided, whereas the cost of IPTp-SP ranged from US$ 0.5 to 16.9 and the distribution of ITMs was between US$ 1.5 and 15.1. Avoiding a DALY by prophylaxis with CTX in pregnant women with HIV would cost approximately US$ 12–34.9, whereas using SP with two or three doses would cost between US$ 9.8 and 30.03 depending on the degree of coverage of HIV detection (Table 3).
Table 3.
Average Cost-Effectiveness (ACE) for DALYs avoided with PAM interventions (costs deflated for 2018 and adjusted by PPP).
| Author and Intervention | Costs | DALYs avoided | ACE |
|---|---|---|---|
| Wolfe [33] –Case management | 875 | 140 | 6,2 |
| Fernandes [42]- Detection + Intermittent with arthemeter-lumefantrine | 2.962 | 156 | 19,0 |
| Wolfe [33] - IPTp-SP monthly | 2.687 | 71 | 37,8 |
| Sicuri [40] -IPTp-MQ | 1.081 | 9 | 120,1 |
| Mbonye [35]- IPTp-SP distributed throughout the community | 328.955 | 1.110 | 296,4 |
|
IPTp-SP | |||
| Hansen [20] | 381 | 558 | 0,7 |
| Fernandes [39] (2 doses) | 837 | 151 | 5,5 |
| Fernandes [39] (3 doses) | 994 | 442 | 2,2 |
| Wolfe [33] | 1.687 | 100 | 16,9 |
| Fernandes [42] | 573 | 556 | 1,0 |
| Sicuri [19] | 301 | 571 | 0,5 |
|
ITM | |||
| Becker [36] (R. of the Congo) | 149.380 | 9.897 | 15,1 |
| Hansen [20] (Uganda) | 832 | 541 | 1,5 |
|
IPTp-SP + MTI | |||
| Hansen [20] (Uganda) | 1.202 | 590 | 2,0 |
| Beyai [37] (Gambia) | 15.839 | 58 | 271,2 |
|
Pregnant womenwith HIV | |||
| Sicuri [40] - IPTp-SP + MQ + CTX(HIV) | 3.581 | 603 | 5,9 |
| Wolfe [33] – HIV test + IPTp-SP | 4.374 | 277 | 15,8 |
|
CTX (HIV) | |||
| Choi [43] (Tanzania) | 122.590 | 3.516 | 34,9 |
| Choi [43] (Mozambique) | 182.901 | 15.240 | 12,0 |
| Choi [43] (Kenya) | 184.650 | 5.339 | 34,6 |
| Choi [43] (Malawi) | 144.536 | 11.317 | 12,8 |
| Choi [43] (Ghana) | 138.042 | 9.312 | 14,8 |
|
IPTp-SP2 Low coverage of HIV detection | |||
| Choi [43] (Ghana) | 137.653 | 12.055 | 11,4 |
| Choi [43] (Malawi) | 143.512 | 14.671 | 9,8 |
| Choi [43] (Kenya) | 183.073 | 7.000 | 26,2 |
| Choi [43] (Mozambique) | 183.720 | 16.424 | 11,2 |
| Choi [43] (Tanzania) | 121.512 | 4.364 | 27,8 |
|
IPTp-SP3 Low coverage of HIV detection | |||
| Choi [43] (Malawi) | 143.878 | 14.563 | 9,9 |
| Choi [43] (Kenya) | 183.597 | 6.934 | 26,5 |
| Choi [43] (Mozambique) | 184.850 | 16.022 | 11,5 |
| Choi [43] (Tanzania) | 122.380 | 4.327 | 28,3 |
| Choi [43] (Ghana) | 137.786 | 11.912 | 11,6 |
|
IPTp-SP3 High HIV detection | |||
| Choi [43] (Tanzania) | 122.693 | 4.231 | 29,0 |
| Choi [43] (Malawi) | 145.481 | 12.382 | 11,7 |
| Choi [43] (Kenya) | 185.629 | 6.183 | 30,0 |
| Choi [43] (Mozambique) | 184.957 | 15.997 | 11,6 |
| Choi [43] (Ghana) | 138.265 | 11.434 | 12,1 |
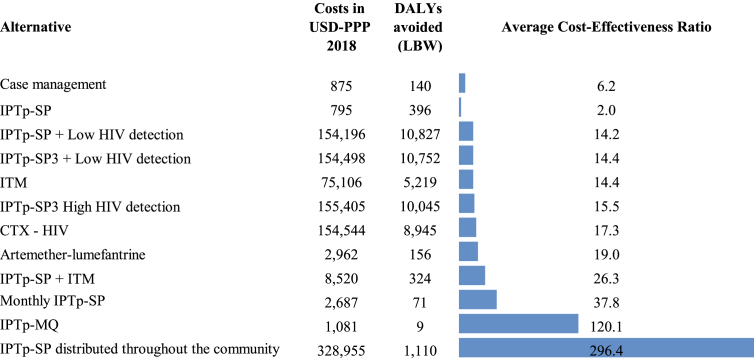
The average cost-effectiveness ratio for IPTp-SP was US$ 2 for each DALY avoided; it was the most cost-effective intervention, followed by the administration of IPTp-SP in pregnant women with HIV. Administration of IPTp-SP throughout the community showed the highest cost per DALY avoided (US$ 296.4), followed by administration of IPTp-MQ (Figure 3).
Figure 3.
Average Cost-Effectiveness Ratio (cost per DALY avoided) in the interventions identified from the institutional perspective.
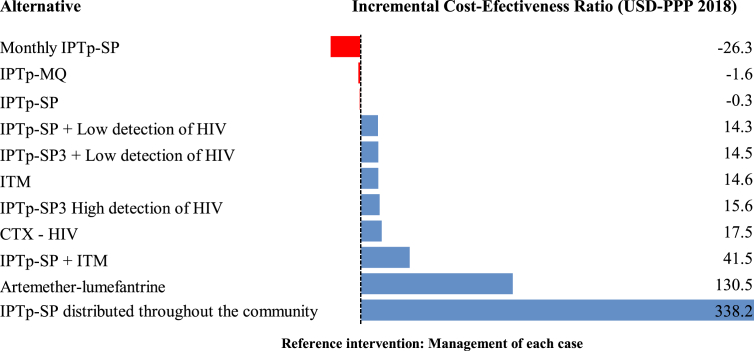
A meta-analysis of the incremental cost-effectiveness ratio (ICER) indicates that the monthly IPTp-SP scheme or administering two doses of SP, with IPTp-MQ, is the dominant strategy to avoid costs for the institution when compared with the management of each case of PAM, whereas treatment with artemether-lumefantrine and distribution by the community of IPTp-SP are interventions that imply the highest costs for each additional DALY avoided, although they were less than one GDP per capita in the countries analyzed (Figure 4).
Figure 4.
Meta-analysis of the Incremental Cost-Effectiveness Ratio (additional cost per DALY avoided) in the interventions identified from the institutional perspective.
4. Discussion
Most economic evaluations are from Africa, which is associated with malaria's global profile, where 90% of cases occur on this continent [1]. The highest academic and scientific output on PAM in Africa is consistent with the findings of this review, demonstrating a knowledge gap regarding the microeconomic impact of PAM in other regions, thus limiting decision-making possibilities based on scientific evidence and using available resources in interventions that maximise health benefits [9].
Partial economic evaluations were found in Brazil and Colombia, which demonstrate the economic burden of PAM, but do not relate the costs of managing it to the health gains, in which case full economic evaluations are more useful [46, 47]. In this sense, cost-effectiveness analysis was most frequently applied for PAM, comparing the monetary costs of different interventions with their effects on morbidity, mortality or disability, allowing the prioritisation of health actions [48].
The above applies to cost-effectiveness studies with good methodological quality. In this review, many studies did not provide an adequate description of the assumptions of the analytical model and did not present the incremental or sensitivity analysis, thus decreasing the internal and external validity of the conclusions, given that the analytical model identifies the health times and states analyzed. ICER informs the additional cost when choosing an intervention other than the one used routinely, and the uncertainty analysis allows to establish the degree of stability of the result in regard to changes in the model's parameters, which are key aspects for public policy decision makers [47, 49]. Furthermore, few studies include a comprehensive description in terms of weeks of pregnancy, number of previous pregnancies, coinfections, prenatal controls, follow-ups of fetal health and the product of the pregnancy, which should be considered for subsequent studies, given that these variables are determinants for the maximisation of the economic resources destined to the prevention and caring for patients with PAM [4, 9, 50].
With respect to the interventions studied, the most frequent was IPTp-SP from the second trimester of pregnancy. This is related to the fact that in most endemic areas for malaria, pregnant women attend prenatal care during this quarter and prophylaxis with SP can only be administered after week 13 owing to its teratogenic effects in the first weeks of pregnancy. These findings show that little is known about the risk of infection, the consequences of malaria during the first trimester and the best interventions for PAM during this period, which should be improved in subsequent studies [4, 17, 51].
Regarding costs, it should be mentioned that in most endemic countries, medicines for the treatment or prevention of PAM are distributed and financed by governments or international organisations [51], and the most expensive items correspond to the implementation of interventions on site and care of pregnant women [20, 36] as well as the indirect costs associated with travel to health centers, downtime, etc. This is worsened when PAM and poverty are intimately related and constitute a vicious circle that exacerbates social inequalities and hinders social development [52]. This calls for state intervention through high-coverage preventive treatment and control measures that would allow pregnant women and the health system to save the costs of treating the disease and its associated complications [9, 37, 45, 53], apart from improving the dynamics of families and the macroeconomies of the affected countries [52].
The most relevant clinical outcomes of PAM were included in this review, highlighting the avoided DALYs as a combined measure of morbidity, mortality and disability, which allows us to differentiate the current health status and an ideal health situation in which the entire population lives up to old age free of disease and disability [54, 55]. Based on this outcome, according to the WHO threshold, all the alternatives were found to be highly cost-effective, although with important differences according to the use of medications, the need for training, new distribution routes, supervision protocols [35, 40], duration of the intervention and the level of adherence that is related to the efficacy and development of parasitic resistance [10, 19, 33] which are key variables for decision makers, given that they would determine the success of health-related actions in the affected areas [47, 49].
It is striking that the simultaneous use of IPTp-SP with ITMs can lower its cost-effectiveness. This has been explained by the reduction in coverage and longer implementation or follow-up time and that and pregnant women may consider using ITMs less important when they receive SP, and this could lead to an incorrect or lower use, thus reducing their effectiveness [20, 56]. However, WHO recommends distributing ITMs together with IPTp-SP because it protects pregnant women from the first trimester, as well as the newborn [51]. Artemether-lumefantrine is recommended in areas of high incidence of malaria and resistance to SP to control malaria during pregnancy, maternal anaemia and LBW [42, 57].
WHO also recommends IPTp-SP in pregnant African women to reduce the incidence of PAM, anaemia, complicated malaria, LBW and maternal and infant mortality [37, 51]. Added to this is the review which shows that this intervention saves resources for the health provider, patients and their families and constitutes a dominant strategy in the cost-effectiveness plan. Although the IPTp-MQ scheme is also highly cost-effective, low drug tolerance with multiple side effects does not favour its use as a preventive treatment [40]. Active detection of malaria cases in low endemic regions outside the African continent is recommended [58, 59, 60, 61]; however, research on the efficacy and cost-effectiveness of this strategy should be improved to lower the complications of PAM in the epidemiological, parasitological and economic aspects as well as in each country's health system coverage.
Interventions with IPTp-SP3 and CTX for pregnant women with HIV were highly cost-effective, constituting important evidence considering the negative feedback of PAM-HIV coinfection, whose intervention would reduce the negative effects of both. HIV potentiates the consequences of PAM by increasing the parasitic density, anaemia, cerebral and placental malaria, preterm delivery, LBW and neonatal death; PAM, in turn, increases viraemia, the progression to AIDS and the risk of vertical transmission of HIV [4, 62, 63, 64]. However, the cost-effectiveness of this intervention depends, to a large extent, on close adherence to the daily dose (>80%) [43, 65].
The main limitations of this study which, in turn, become recommendations for subsequent studies or public policy measures, are related to the need to standardize the social and institutional costs of PAM and the measures of effectiveness to increase the number of studies that evaluate interventions other than that involving IPTp-SP mainly for the first trimester of pregnancy and, outside Africa, to perform uncertainty analysis and to increase the number of evaluations from the social perspective. Furthermore, the studies were not exhaustive in the description of the socioeconomic, clinical, and parasitological characteristics of the pregnant women studied, which should be improved in subsequent studies. The fact of not having registered online the search and selection protocol of the studies should also be declared as a limitation of this review; as well as the few data on Francophone Africa.
5. Conclusions
Published studies are scarce and restricted to Africa, which shows lack of knowledge of the economic impact of PAM and more cost-effective interventions in other endemic countries. Despite the high heterogeneity of impact measures, the costing systems used, interventions and populations studied and all alternatives identified were highly cost-effective. This demonstrates the importance of including resources for preventing and controlling PAM and providing care for patients with PAM as a priority in the health budget to reach the goal of eliminating the disease, as demanded by WHO [58], promoting social progress and overcoming poverty in endemic areas.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Colciencias Colombia, Project 111577757447, Contract 755-2017, University of Antioquia.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Suppl 1. PRISMA checklist
References
- 1.OrganizaciónMundial de la Salud [Internet] Paludismo. http://www.who.int/es/news-room/fact-sheets/detail/malaria [Accesojulio de 2018]. Disponible en:
- 2.OrganizaciónPanamericana de la Salud Situación de la Malaria en la Región de las Américas, 2000–2016. https://www.paho.org/hq/index.php?option=com_docman&view=download&category_slug=statistics-data-maps-8109&alias=45344-situation-malaria-region-americas-2000-2016-344&Itemid=270&lang=es [Internet]; [Acceso Julio de 2018]. Disponible en:
- 3.Botero D., Restrepo M. En: Parasitosis Humanas. 5a ed. CIB; Medellín: 2012. Malaria (paludismo) pp. 215–273. [Google Scholar]
- 4.Desai M., Kuile F., Nosten F., McGready R., Asamoa K., Brabin B. Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 5.Brabin B. World Health Organization publications; Geneva: 1991. The Risk and Severity of Malaria in Pregnant Women; pp. 1–67. [Google Scholar]
- 6.Menendez C. Malaria during pregnancy: a priority area of malaria research and control. Parasitol. Today. 1995;11:178–183. doi: 10.1016/0169-4758(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 7.Nosten F., Rogerson S.J., Beeson J.G., Mcgready R., Mutabingwa T.K., Brabin B. Malaria in pregnancy and the endemicity spectrum: what can we learn? Trends Parasitol. 2004;20:425–432. doi: 10.1016/j.pt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Brabin B.J., Romagosa C., Abdelgalil S., Menéndez C., Verhoeff F.H., McGready R. The sick placenta-the role of malaria. Placenta. 2004;25:359–378. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Rogerson S.J., Hviid L., Duff P.E., Leke R.F.G., Taylor D.W. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect. Dis. 2007;7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 10.Muanda F., Chaabane S., Boukhris T., Santos F., Sheehy O., Perreault S. Antimalarial drugs for preventing malaria during pregnancy and the risk of low birth weight: a systematic review and meta-analysis of randomized and quasi-randomized trials. BMC Med. 2015;13:193. doi: 10.1186/s12916-015-0429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGready R., White N., Nosten F. Parasitological efficacy of antimalarials in the treatment and prevention of falciparum malaria in pregnancy 1998 to 2009: a systematic review. BJOG. 2011;118:123–135. doi: 10.1111/j.1471-0528.2010.02810.x. [DOI] [PubMed] [Google Scholar]
- 12.Gutman J., Kovacs S., Dorsey G., Stergachis A., Ter Kuile F. Safety, tolerability, and efficacy of repeated doses of dihydroartemisinin-piperaquine for prevention and treatment of malaria: a systematic review and meta-analysis. Lancet Infect. Dis. 2017;17:184–193. doi: 10.1016/S1473-3099(16)30378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Eijk A., Hill J., Larsen D., Webster J., Steketee R., Eisele T. Coverage of intermittent preventive treatment and insecticide-treated nets for the control of malaria during pregnancy in sub-Saharan Africa: a synthesis and meta-analysis of national survey data, 2009–11. Lancet Infect. Dis. 2013;13:1029–1042. doi: 10.1016/S1473-3099(13)70199-3. [DOI] [PubMed] [Google Scholar]
- 14.Desai M., Cot M. Epidemiology of malaria during pregnancy: burden and impact of Plasmodium falciparum malaria on maternal and infant health. In: Hommel M., Kremsner P., editors. Encyclopedia of Malaria. Springer; New York: 2015. pp. 1–14. [Google Scholar]
- 15.Moore K., Simpson J., Scoullar M., McGready R., Fowkes F. Quantification of the association between malaria in pregnancy and stillbirth: a systematic review and meta-analysis. Lancet Glob. Health. 2017;5:1101–1112. doi: 10.1016/S2214-109X(17)30340-6. [DOI] [PubMed] [Google Scholar]
- 16.Garner P., Gulmezoglu A. Drugs for preventing malaria in pregnant women. Cochrane Database Syst. Rev. 2006:CD000169. doi: 10.1002/14651858.CD000169.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Greenwood B., Alonso P., terKuile F., Hill J., Steketee R. Malaria in pregnancy: priorities for research. Lancet Infect. Dis. 2007;7:169–174. doi: 10.1016/S1473-3099(07)70028-2. [DOI] [PubMed] [Google Scholar]
- 18.Zarate V. Evaluaciones económicas en salud: conceptos básicos y clasificación. Medicina Chile. 2010;138(Suppl 2):93–97. [Google Scholar]
- 19.Sicuri E., Bardajı A., Nhampossa T., Maixenchs M., Nhacolo A., Nhalungo D. Cost-effectiveness of intermittent preventive treatment of malaria in pregnancy in Southern Mozambique. PloS One. 2010;5 doi: 10.1371/journal.pone.0013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen K.S., Ndyomugyenyi R., Magnussen P., Clarke S.E. Cost-effectiveness analysis of three health interventions to prevent malaria in pregnancy in an area of low transmission in Uganda. Int. Health. 2012;4:38–46. doi: 10.1016/j.inhe.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adarkwa M. Kwame Nkrumah University of Science and Technology; 2009. The Economic burden of Malaria in Pregnancy in the Sunyani Municipality. [Tesis Doctoral] [Google Scholar]
- 22.Bôtto-Menezes C., Bardají A., Dos Santos C., Fernandes S., Hanson K., Martínez F. Costs associated with malaria in pregnancy in the Brazilian Amazon, a low endemic area where Plasmodium vivax predominates. PLoS Neglected Trop. Dis. 2016;31(10) doi: 10.1371/journal.pntd.0004494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worrall E., Morel C., Yeung S., Borghi J., Webster J., Hill J. The economics of malaria in pregnancy--a review of the evidence and research priorities. Lancet Infect. Dis. 2007;7(2):156–168. doi: 10.1016/S1473-3099(07)70027-0. [DOI] [PubMed] [Google Scholar]
- 24.Desai M., Hill J., Fernandes S., Walker P., Pell C., Gutman J. Prevention of malaria in pregnancy. Lancet Infect. Dis. 2018;18(4):119–132. doi: 10.1016/S1473-3099(18)30064-1. [DOI] [PubMed] [Google Scholar]
- 25.Cardona J.A., Higuita L.F., Rios L. Medellín: Universidad Cooperativa de Colombia; 2016. Revisiones sistemáticas de a literatura científca. [Google Scholar]
- 26.Husereau D., Drummond M., Petrou S., Carswell C., Moher D., Greenberg D. Consolidated health economic evaluation reporting standards (CHEERS) statement. Int. J. Technol. Assess. Health Care. 2013;29:117–122. doi: 10.1017/S0266462313000160. [DOI] [PubMed] [Google Scholar]
- 27.Edejer T., Baltussen R., Adam T., Hutubessy R., Acharya A., Evans D. World Health Organization publications; Geneva: 2003. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis; p. 329. [Google Scholar]
- 28.Goodman C.A., Coleman P.G., Mills A.J. Cost-effectiveness of malaria control in sub-Saharan Africa. Lancet. 1999;354(9176):378–385. doi: 10.1016/s0140-6736(99)02141-8. [DOI] [PubMed] [Google Scholar]
- 29.Heymann D.L., Steketee R.W., Wirima J.J., McFarland D.A., Khoromana C.O., Campbell C.C. Antenatal chloroquine chemoprophylaxis in Malawi: chloroquine resistance, compliance, protective efficacy and cost. Trans. R. Soc. Trop. Med. Hyg. 1990;84:496–498. doi: 10.1016/0035-9203(90)90011-3. [DOI] [PubMed] [Google Scholar]
- 30.Helitzer-Allen D.L., McFarland D.A., Wirima J., Macheso A.P. Malaria chemoprophylaxis compliance in pregnant women: a cost-effectiveness analysis of alternative interventions. Soc. Sci. Med. 1993;36:403–407. doi: 10.1016/0277-9536(93)90402-p. [DOI] [PubMed] [Google Scholar]
- 31.Schultz L.J., Steketee R.W., Chitsu L., Wirima J.J. Antimalarials during pregnancy: a cost-effectiveness analysis. Bull. World Health Organ. 1995;73:207–214. [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz L.J., Steketee R.W., Chitsulo L., Macheso A., Kazembe P., Wirima J.J. Evaluation of maternal practices, efficacy, and costeffectiveness of alternative antimalarial regimens for use in pregnancy: chloroquine and sulfadoxinepyrimethamine. Am. J. Trop. Med. Hyg. 1996;55(Suppl 1):87–94. doi: 10.4269/ajtmh.1996.55.87. [DOI] [PubMed] [Google Scholar]
- 33.Wolfe E.B., Parise M.E., Haddix A.C., Nahlen B.L., Ayisi J.G., Misore A. Cost-effectiveness of sulfadoxine-pyrimethamine for the prevention of malaria-associated low birth weight. Am. J. Trop. Med. Hyg. 2001;64(3,4):178–186. doi: 10.4269/ajtmh.2001.64.178. [DOI] [PubMed] [Google Scholar]
- 34.Goodman C.A., Coleman P.G., Mills A.J. The cost-effectiveness of antenatal malaria prevention in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2001;64(Suppl 1–2):45–56. doi: 10.4269/ajtmh.2001.64.45. [DOI] [PubMed] [Google Scholar]
- 35.Mbonye A.K., Hansen K.S., Bygbjerg I.C., Magnussen P. Intermittent preventive treatment of malaria in pregnancy: the incremental cost-effectiveness of a new delivery system in Uganda. Trans. R. Soc. Trop. Med. Hyg. 2008;102:685–693. doi: 10.1016/j.trstmh.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Becker-Dreps S.I., Biddle A.K., Pettifor A., Musuamba G., Imbie D.N., Meshnick S. Cost-effectiveness of adding bed net distribution for malaria prevention to antenatal services in Kinshasa, Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2009;81:496–502. [PubMed] [Google Scholar]
- 37.Beyai P.L. London School of Hygiene & Tropical Medicine; 2010. The Cost-Effectiveness of Intermittent Preventive Treatment for Malaria in Gambian Multigravidae Including Examination of Indirect Costs. [Tesis Doctoral] [Google Scholar]
- 38.Matangila J.R., Lufuluabo J., Ibalanky A.L., Inocencio da Luz R.A., Lutumba P., Van Geertruyden J.P. Asymptomatic Plasmodium falciparum infection is associated with anaemia in pregnancy and can be more cost-effectively detected by rapid diagnostic test than by microscopy in Kinshasa, Democratic Republic of the Congo. Malar. J. 2014;2(13):132. doi: 10.1186/1475-2875-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandes S., Sicuri E., Kayentao K., van Eijk A.M., Hill J., Webster J. Cost-effectiveness of two versus three or more doses of intermittent preventive treatment for malaria during pregnancy in sub-Saharan Africa: a modelling study of meta-analysis and cost data. Lancet Glob. Health. 2015;3:e143–e145. doi: 10.1016/S2214-109X(14)70385-7. [DOI] [PubMed] [Google Scholar]
- 40.Sicuri E., Fernandes S., Macete E., González R., Mombo-Ngoma G., Massougbodgi A. Economic evaluation of an alternative drug to sulfadoxine-pyrimethamine as intermittent preventive treatment of malaria in pregnancy. PloS One. 2015;10 doi: 10.1371/journal.pone.0125072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orobaton N., Austin A.M., Abegunde D., Ibrahim M., Mohammed Z., Abdul-Azeez J. Scaling-up the use of sulfadoxine-pyrimethamine for the preventive treatment of malaria in pregnancy: results and lessons on scalability, costs and programme impact from three local government areas in Sokoto State, Nigeria. Malar. J. 2016;15:533. doi: 10.1186/s12936-016-1578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes S., Sicuri E., Halimatou D., Akazili J., Boiang K., Chandramohan D. Cost effectiveness of intermittent screening followed by treatment versus intermittent preventive treatment during pregnancy in West Africa: analysis and modelling of results from a non-inferiority trial. Malar. J. 2016;15:493. doi: 10.1186/s12936-016-1539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi S.E., Brandeau M.L., Bendavid E. Cost-effectiveness of malaria preventive treatment for HIV-infected pregnant women in sub-Saharan Africa. Malar. J. 2017;16:403. doi: 10.1186/s12936-017-2047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guyatt H.L., Gotink M.H., Ochola S.A., Snow R.W. Free bednets to pregnant women through antenatal clinics in Kenya: a cheap, simple and equitable approach to delivery. Trop. Med. Int. Health. 2002;7(5):409–420. doi: 10.1046/j.1365-3156.2002.00879.x. [DOI] [PubMed] [Google Scholar]
- 45.Sicuri E., Bardají A., Sanz S., Alonso S., Fernandes S., Hanson K. Patients’ costs, socio-economic and health system aspects associated with malaria in pregnancy in an endemic area of Colombia. PLoS Neglected Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardona J. A brief guide of health economics. Curare. 2016;3 [Google Scholar]
- 47.Drummond M., O’Brien B., Sculpher M., Stoddart G., Torrance G. 3 ed. Oxford Medical Publications; Oxford: 2005. Methods for the Economic Evaluation of Health Care Programmes; pp. 1–156. [Google Scholar]
- 48.Prieto L., Sacrist J., Antoñanzas F., Rubio T. Analisis coste-efectividad en la evaluacion economica de intervenciones sanitarias. Med. Clínica. 2004;122:505–510. doi: 10.1016/s0025-7753(04)74288-8. [DOI] [PubMed] [Google Scholar]
- 49.Carrasquilla G., Pulido A., De-La-Hoz A., Mieth K., Muñoz O. Guía Metodológica para la elaboración de Guías de Práctica Clínica con Evaluación Económica en el Sistema General de Seguridad Social en Salud Colombiano. minsalud. Bogotá. 2014:1–312. [Google Scholar]
- 50.Kibusi S.M., Kimunai E., Hines C.S. Predictors for uptake of intermittent preventive treatment of malaria in pregnancy (IPTp) in Tanzania. BMC Publ. Health. 2015;15:540. doi: 10.1186/s12889-015-1905-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHO WHO Policy Brief for the Implementation of Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine (IPTp-SP) https://www.who.int/malaria/publications/atoz/iptp-sp-updated-policy-brief-24jan2014.pdf?ua=1 [Internet]; [Acceso Nov de 2018]. Disponible en:
- 52.Sachs J., Malaney P. The economic and social burden of malaria. Nature. 2002;415(6872):680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 53.Ruiz W., Kroeger A. The socioeconomic impact of malaria in Colombia and Ecuador. Health Pol. Plann. 1994;9:144–154. doi: 10.1093/heapol/9.2.144. [DOI] [PubMed] [Google Scholar]
- 54.WHO . Metrics: Disability-Adjusted Life Year (DALY) 2018. Health statistics and information systems.http://www.who.int/healthinfo/global_burden_disease/metrics_daly/en/ [Internet] [Acceso Nov 2018]. Disponible en: [Google Scholar]
- 55.Gonzáles T. Entendiendo el uso y resultados del indicador. años de vida ajustados por discapacidad. REMAP. 2015;4:195–210. [Google Scholar]
- 56.Yukich J., Tediosi F., Lengeler C. Swiss Tropical Institute; Basel: 2007. Operations, Costs, and Costeffectiveness of Five Insecticide-Treated Net Programs (Eritrea, Malawi, Tanzania, Togo,Senegal) and Two Indoor Residual Spraying Programs (Kwa-Zulu Natal, Mozambique) [Google Scholar]
- 57.Esu E., Berens-Riha N., Pritsch M., Nwachuku N., Loescher T., Meremikwu M. Intermittent screening and treatment with artemether-lumefantrine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in pregnancy: a facility-based, open-label, non-inferiority trial in Nigeria. Malar. J. 2018;17:251. doi: 10.1186/s12936-018-2394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Global W.H.O. World Health Organization publications; Geneva: 2015. Technical Strategy for Malaria 2016–2030; pp. 1–29. [Google Scholar]
- 59.Organización Panmericana de la Salud, Instituto Nacional de Saluyd de Colombia [Internet] Guía. Protocolo para la vigilancia en salud pública de la malaria. https://www.paho.org/col/index.php?option=com_docman&view=download&category_slug=publicaciones-ops-oms-colombia&alias=1223-protocolo-para-la-vigilancia-en-salud-publica-de-malaria&Itemid=688 [Accesonoviembre de 2018]. Disponible en:
- 60.ProgramaNacional de Prevención y Control de la Malaria [Internet] Malria y Embarazo. http://www.bvs.hn/Honduras/Malaria/Informe%20definitivo%20Consultoria%20sobre%20Malaria%20y%20Embarazo%20L%20Brutus%202006/Informe%20definitivo.pdf Disponible en:
- 61.Phillips R.S. Current status of malaria and potential for control. Clin. Microbiol. Rev. 2001;14:208–226. doi: 10.1128/CMR.14.1.208-226.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuile F.O., Parise M.E., Verhoeff F.H., Udhayakumar V., Newman R.D., Van Eijk A.M. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2004;71(suppl):41–54. [PubMed] [Google Scholar]
- 63.Johnbull O.S., Uche A.P., Kesiena A.J., Francis F.A., Oyemocho A. Prevalence and risk factors of malaria in HIV-infected pregnant women on anti-retroviral therapy in Enugu, South East Nigeria. J. AIDS Clin. Res. 2014;5:321. [Google Scholar]
- 64.Njunda A.L., Njumkeng C., Nsagha S.D., Assob J.C., Kwenti T.E. The prevalence of malaria in people living with HIV in Yaounde, Cameroon. BMC Publ. Health. 2016;16:964. doi: 10.1186/s12889-016-3647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Srivastava K., Arora A., Kataria A., Cappelleri J.C., Sadosky A., Peterson A.M. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer. Adherence. 2013;7:419–434. doi: 10.2147/PPA.S44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl 1. PRISMA checklist