Abstract
Obesity, particularly excess visceral fat accumulation, is highly associated with the development of metabolic syndrome and atherosclerotic cardiovascular disease. Adipose tissue produces a variety of secreted proteins, referred to as adipocytokines, which directly affect nearby or remote organs. Dysregulation of adipocytokines caused by obese conditions contributes to the pathogenesis of various metabolic and cardiovascular disorders. This review focuses on the significance of several adipocytokines that potentially exert beneficial actions on obesity-related diseases, including atherosclerosis and ischemic heart disease.
Keywords: Adipocytokine, Adiponectin, Omentin, Adipolin, Inflammation
Introduction
Obesity, particularly excessive visceral fat accumulation, is causally linked with the cluster of type 2 diabetes, hypertension and dyslipidemia, also known as metabolic syndrome1–3). This results in the progression of atherosclerotic cardiovascular diseases1–3). Adipose tissue has been considered as a long-term energy storage organ; however, it is now recognized that fat tissue produces numerous secretory factors, referred to as adipocytokines or adipokines, which directly influence nearby or remote tissues4–6). Considering this, the adipose tissue functions as an endocrine organ that regulates various pathophysiological processes in obese complications. The adipocytokines comprise a large number of pro-inflammatory mediators that can promote disease progression. In contrast, there are a small number of anti-inflammatory adipocytokines that protect against obese complications. Thus, an imbalance of anti- and pro-inflammatory adipocytokines under conditions of obesity contributes to the pathogenesis of metabolic and cardiovascular diseases. In this review, we provide an overview of our research, determining the significance of crucial adipocytokines that exert beneficial actions on cardiovascular and metabolic diseases.
Adiponectin
Adiponectin, also known as ACRP30 and ADI-POQ, has been identified as an adipose-specific adipocytokine, which is abundantly present in human plasma at concentrations ranging from 3 to 30 µg/ml4, 7). This protein contains a collagen-like domain followed by a globular domain similar to compliment factor C1q. Clinical studies demonstrated that plasma adiponectin levels are negatively associated with body mass index (BMI) and visceral fat area in both men and women7, 8), suggesting that circulating adiponectin is downregulated by obese states. Circulating adiponectin level is associated with the presence of metabolic disorders, and measurement of visceral fat accumulation and adiponectin levels is valuable for evaluation of the clustering of metabolic abnormalities9). In addition, adiponectin levels are reported to associate with the atherogenic lipoprotein profiles10). We have demonstrated that plasma adiponectin concentrations are significantly lower in patients with coronary artery disease (CAD) than those in age- and BMI-adjusted control subjects11). Hypoadiponectinemia (plasma adiponectin concentrations < 4.0 µg/ml in male patients) is independently associated with CAD using multiple logistic regression analysis with confounding factors12). In addition, adiponectin is a crucial indicator of plasma remnant lipoprotein levels, which are linked with coronary plaque vulnerability13, 14). It has also been shown that high plasma adiponectin levels are associated with a reduced risk of myocardial infarction in healthy men15) and CAD in diabetic men16). Furthermore, a high adiponectin level has been found to be a significant predictor of CAD in men initially free of CAD17). Thus, it is plausible that adiponectin may be a useful biomarker for assessment of CAD.
Experimental studies indicate that adiponectin plays a protective role in obesity-linked vascular diseases. Our initial observations demonstrated that physiological concentrations of adiponectin dose-dependently reduced monocyte attachment to TNF-α-stimulated human aortic endothelial cells11). Adiponectin also attenuates TNF-α-stimulated expression of endothelial adhesion molecules, including vascular cell adhesion molecule-1 (VCAM-1) in human endothelial cells through its ability to suppress NF-κB activation18). Similarly, adiponectin suppresses TNF-α-induced expression of IL-8 in human vascular endothelial cells by reducing NF-κB activity19). Adiponectin also inhibits high glucose-induced production of reactive oxygen species in endothelial cells20). Furthermore, adiponectin prevents endothelial cell apoptosis under conditions of serum starvation and promotes migration and angiogenic response of endothelial cells21, 22). These data suggest that adiponectin suppresses endothelial cell activation and injury in vitro (Fig. 1).
Fig. 1.
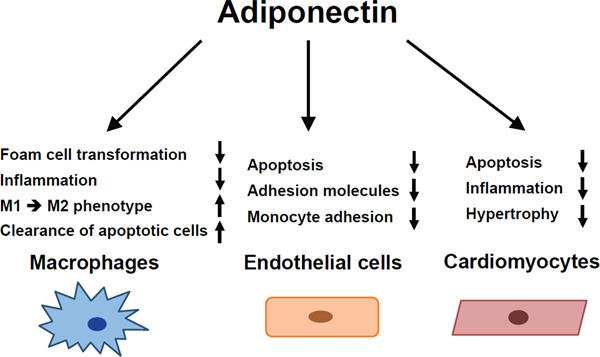
Cardiovascular protection by adiponectin
Adiponectin exerts protective actions on the cardiovascular system. Adiponectin reduces foam cell transformation of macrophages and attenuates inflammatory response in macrophages. Adiponectin switches the macrophage phenotype from inflammatory M1 to the anti-inflammatory M2 and promotes clearance of early apoptotic cells. Adiponectin also reduces apoptosis, expression of adhesion molecules, and monocyte adhesion in endothelial cells. Furthermore, adiponectin attenuates apoptosis, inflammation, and hypertrophic response in cardiomyocytes.
Adiponectin inhibits the transformation of human macrophages into foam cells by suppressing the expression of class A scavenger receptor (SR-A)23). Adiponectin also suppresses lipopolysaccharide (LPS)-stimulated production of TNF-α in cultured macrophages24, 25). The accumulation of lipid-laden foam cells and macrophage activation in atherosclerotic lesions are crucial events in atherogenesis. Thus, it is plausible that adiponectin is anti-atherogenic. Consistent with these in vitro findings, overproduction of circulating adiponectin suppresses atherosclerotic lesion formation and reduces expression of SR-A, TNF-α, and VCAM-1 in the aorta of a mouse model of atherosclerosis26, 27). Conversely, adiponectin deficiency exacerbates atherosclerotic lesion formation and T-lymphocyte accumulation in the vascular wall in an atherosclerosis model28). Therefore, adiponectin can prevent the development of atherosclerosis by directly affecting the behavior of vascular component cells, including endothelial cells and macrophages.
Several studies, including ours, have demonstrated that adiponectin is protective against obesity-related heart diseases. Disruption of adiponectin exacerbates myocardial injury in response to ischemia-reperfusion in mice, with an accompanying increase in myocardial apoptosis and TNF-α production29). Adiponectin inhibits apoptosis of cultured cardiac cells under conditions of hypoxia-reoxygenation via activation of the AMPK signaling cascade29). Adiponectin also reduces LPS-induced secretion of TNF-α from cardiac cells through its ability to modulate COX-2 expression and prostaglandin E2 (PGE2) synthesis. Collectively, adiponectin can protect against ischemic injury in the heart through the AMPK-dependent anti-apoptotic and COX-2-dependent anti-inflammatory mechanisms. Likewise, adiponectin has been reported to ameliorate myocardial ischemia-reperfusion damage through reduction of oxidative/nitrative stress30). Adiponectin also inhibits cardiac hypertrophy and dysfunction in vivo following pressure overload or angiotensin II infusion31–33). The beneficial actions of adiponectin on pathological cardiac remodeling are dependent, at least in part, on activation of AMPK signaling pathways. Moreover, adiponectin improves myocardial fibrosis and systolic dysfunction after myocardial infarction34). Loss of adiponectin leads to enhanced left ventricular hypertrophy and diastolic heart failure in mice following aldosterone infusion35). Conversely, overexpression of adiponectin improves left ventricular hypertrophy, diastolic dysfunction and myocardial oxidative stress in response to aldosterone infusion36). We have also demonstrated that adiponectin prevents doxorubicin-induced cardiotoxicity through its ability to stimulate myocyte survival37). Overall, adiponectin acts as an adipocytokine that protects against the development of various heart diseases.
Obesity causes chronic low grade inflammation, thereby resulting in the initiation and progression of pathological conditions, including insulin resistance, type 2 diabetes and atherosclerotic cardiovascular disease6, 38, 39). Plasma adiponectin levels are negatively correlated with plasma levels of an established inflammatory marker, high-sensitive C-reactive protein (CRP)40, 41). There is an inverse correlation between plasma adiponectin and interleukin-6 levels, and body weight reduction through lifestyle changes is associated with a reduction in CRP and interleukin-6 levels and an increase in adiponectin levels42). Thus, the reciprocal association of adiponectin and the inflammatory mediators may contribute to the development of obese complications. In support of this notion, several experimental studies indicate that adiponectin exerts anti-inflammatory actions through modulation of the macrophage phenotype. We have shown that adiponectin can switch macrophage polarization towards an anti-inflammatory phenotype43). Macrophages in obese adipose tissue mainly express markers of the M1 or “classically activated” macrophages, which cause exacerbation of inflammation and tissue destruction44, 45). In contrast, macrophages from lean adipose tissue express markers of the M2 or “alternatively activated” macrophages, which lead to resolution of inflammation and metabolic dysfunction45). Adiponectin deficiency results in increased expression of M1 markers and decreased expression of M2 markers in peritoneal macrophages and the stromal vascular fraction cells of adipose tissue in mice43). Adiponectin also increases the expression of M2 markers in cultured human and mouse macrophages43, 46). The effects of adiponectin on M2 macrophage polarization are mediated through modulation of multiple signaling pathways including AMPK, PPARα and IL-4/STAT646, 47). We have also shown that adiponectin promotes macrophage- mediated removal of early apoptotic bodies, which is important for prevention of inflammatory response48). Moreover, the ability of adiponectin to facilitate the phagocytosis of apoptotic cells by macrophages is dependent on its interactions with calreticulin and its adaptor protein CD91 on the cell surface. Enhanced clearance of early apoptotic debris by macrophages can lead to the M2 phenotype49). Collectively, adiponectin can promote the anti-inflammatory macrophage phenotype, thereby contributing to protection against various obesity-linked diseases (Fig. 1).
Adipolin/CTRP12
Recently we performed screening of predicted adipocytokines that were regulated by obese states, identified C1q/TNF-related protein (CTRP) 12 as a novel adipocytokine, and designated this adipocytokine as adipolin (adipose-derived insulin-sensitizing factor) to indicate its potential function50). Adipolin is a member of CTRPs, conserved adiponectin paralogs that contains a collagen-like domain and C1q-like domain. Adipolin is mainly expressed in adipose tissue, particularly in adipocytes. Adipolin expression in adipose tissue and plasma is decreased in mouse models of obesity. Adipolin expression is also reduced in cultured 3T3L1 adipocytes by treatment with various stimuli that mimic obese conditions, such as the proinflammatory cytokine TNF-α or the inducer of endoplasmic reticulum and oxidative stress50). We also demonstrated that the transcriptional factor Krüppel-like factor (KLF) 15 enhances adipolin expression in cultured adipocytes51). TNF-α attenuates mRNA expression of KLF15 and adipolin in adipocytes, partly through modulation of c-Jun N-terminal kinase. Moreover, KLF15 expression is decreased in fat tissue of obese mice. Thus, it is plausible that adipose tissue inflammation caused by obesity suppresses adipose KLF15 expression, leading to reduction of adipolin transcription in adipose tissue (Fig. 2). Experimental studies show that adipolin improves glucose intolerance in obesity. Systemic administration of adipolin to diet-induced obese mice results in improvement of glucose intolerance and insulin resistance, with an accompanying reduction of macrophage accumulation and expression of pro-inflammatory genes in fat tissue50). In cultured macrophages, adipolin suppresses the expression of pro-inflammatory mediators in response to inflammatory stimuli. Thus, it is conceivable that adipolin acts as an anti-inflammatory adipocytokine that promotes insulin sensitivity, at least in part, by suppressing macrophage activation in fat tissue. Similarly, adipolin ameliorates glucose tolerance and insulin sensitivity in obese and diabetic mice, partly through enhancement of insulin signaling in the liver and adipose tissue52). Therefore, adipolin can serve as an insulin sensitizing adipocytokine. It has been shown that adipolin exists in plasma as two isoforms (full and cleaved forms)52, 53). It has also been reported that the endopeptidase furin cleaves adipolin protein between 91-K and 92-S, resulting in the generation of the cleaved form of adipolin54). We have shown that obese mice have reduced levels of plasma full and total (full and cleaved) adipolin with an increase in the ratio of cleaved to full isoform53). Furin is upregulated in fat tissue of obese mice, and TNF-α increases furin expression in adipocytes. These observations suggest that adipose tissue inflammation caused by obese states contributes to enhanced cleavage of adipolin, presumably through upregulation of furin (Fig. 2). It has also been reported that the full form of adipolin is more effective in promoting insulin-induced glucose uptake in adipocytes compared with its cleaved form54). Thus, obese conditions may lead to reduced circulating levels of full isoform of adipolin, thereby contributing to the development of metabolic dysfunction (Fig. 2). Taken together, the approaches to enhance the generation of adipolin, particularly its full isoform, at transcriptional and post-translational levels may be beneficial for manipulation of obesity-linked metabolic disorders.
Fig. 2.
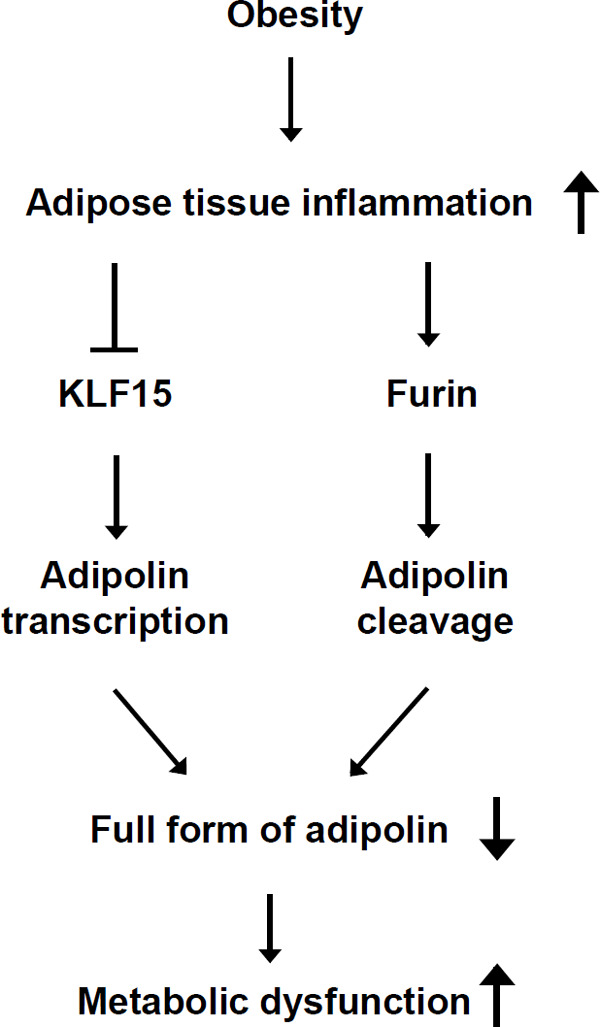
Regulation of adipolin
Obesity is associated with adipose tissue inflammation. These conditions reduce transcript levels of adipolin by suppressing KLF15 expression. Obese states also facilitate the cleavage of adipolin through upregulation of furin in adipose tissue. Thus, obesity leads to reduction of the full form of adipolin, contributing to the progression of metabolic dysfunction.
CTRP9
Among CTRPs, CTRP9 shows the highest amino acid identity to adiponectin55, 56). CTRP9 is abundantly expressed in adipose tissue, and circulating CTRP9 levels are reduced in obese mice55, 57). CTRP9 is reported to exert beneficial actions on glucose metabolism55, 58). We have demonstrated that CTRP9 attenuates neointimal hyperplasia in mice following arterial injury59). CTRP9 also suppresses the numbers of proliferating cells in injured arteries and promotes reendothelialization. The in vitro data showed that CTRP9 reduces the proliferation of vascular smooth muscle cells following stimulation with growth factors through the cyclic AMP-protein kinase A (PKA)-dependent pathways59). It has also been shown that CTRP9 induces vascular relaxation through the adiponectin receptor 1/AMPK/eNOS-dependent pathway60). A recent report indicated that CTRP9 attenuates inflammatory responses of endothelial cells via activation of AMPK61). CTRP9 enhances the plaque stability in a mouse model of atherosclerosis through suppression of pro-inflammatory gene expression in macrophages62). Thus, CTRP9 may exert vasculoprotective actions by directly affecting vascular component cells.
Furthermore, we have shown that systemic administration of CTRP9 to mice leads to reduction of myocardial infarct size, apoptosis and pro-inflammatory gene expression following ischemia-reperfusion57, 63). CTRP9 also improves left ventricular dysfunction in mice after injection of LPS63). Although CTRP-9-KO mice are indistinguishable from control mice under physiological conditions, CTRP9-KO mice show exacerbation of myocardial injury and inflammatory response following ischemia-reperfusion or LPS injection57, 63). CTRP9 reduces hypoxia-reoxygenation-induced apoptosis and LPS-stimulated expression of pro-inflammatory cytokines in cardiac myocytes63). The protective actions of CTRP9 in cardiac myocytes are mediated through its ability to modulate AMPK or cyclic AMP signaling pathways. It has also been reported that CTRP9 reduces myocardial infarct size, apoptosis and oxidative stress in diabetic mice after ischemia-reperfusion64). Furthermore, CTRP9 treatment ameliorates pathological cardiac remodeling in vivo following myocardial infarction via activation of PKA65). Therefore, CTRP9 appears to act as an adipocytokine that protects against ischemic heart disease. Collectively, CTRP9 displays a cardiovascular protective function that overlaps with adiponectin, and future research is needed to dissect the similarities and differences in signal transduction cascades between these two adipocytokines.
Omentin
Omentin, also referred to as intelectin-1, was identified as a soluble galactofuranose-binding lectin66). Omentin is abundantly expressed in human visceral adipose tissue, and it exists in human plasma67, 68). Circulating omentin levels are decreased in patients with obesity, impaired glucose tolerance and type 2 diabetes68, 69). Circulating omentin levels negatively correlate with the multiplicity of metabolic risk factors, such as increased waist circumference, dyslipidemia, elevated blood pressure, and glucose intolerance70). Furthermore, plasma omentin levels are decreased in patients with CAD71). It has also been shown that serum omentin levels are negatively associated with the severity of CAD in patients with metabolic syndrome72). In addition, omentin levels inversely correlate with carotid intima-media thickness, which is a marker for atherosclerosis73, 74). Thus, omentin may be a valuable marker for assessment of obesity-linked metabolic and cardiovascular disorders.
A number of experimental studies indicate that omentin plays a crucial role in regulation of cardiovascular disorders. We have shown that omentin promotes ischemia-induced revascularization in vivo in an eNOS-dependent fashion75). Omentin also stimulates endothelial cell survival and angiogenic response in vitro through its ability to promote AMPK/eNOS signaling pathways. Likewise, omentin promotes vasodilation in isolated blood vessels through modulation of endothelium-derived NO76). Omentin also attenuates the inflammatory response to TNF in cultured vascular endothelial cells via AMPK/eNOS-mediated signaling77). Furthermore, we have demonstrated that transgenic mice expressing omentin in fat tissue show reduced neointimal formation in response to injury in vivo78). Omentin suppresses growth of vascular smooth muscle cells through an AMPK-dependent mechanism. Omentin also inhibits growth factor-induced smooth muscle cell migration through the anti-oxidative mechanism79). More recently, we have shown that omentin attenuates atherosclerotic lesion formation in a mouse model of atherosclerosis80). Therefore, omentin acts as an adipocytokine that protects against vascular injury (Fig. 3).
Fig. 3.
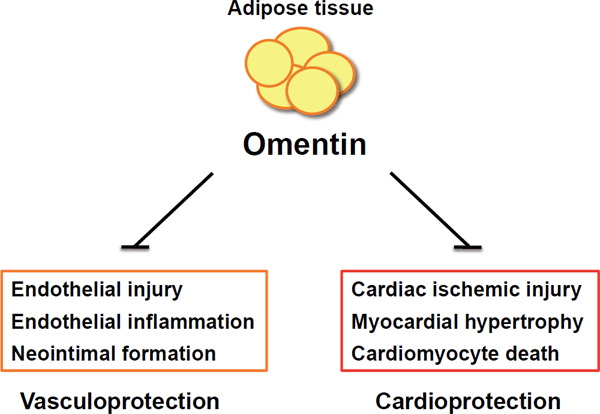
Vasculoprotective and cardioprotective effects of omentin
Omentin attenuates endothelial cell injury, endothelial inflammation, and neointimal formation. Moreover, omentin prevents cardiac ischemic injury, myocardial hypertrophy, and cardiomyocyte death.
Omentin also plays an important role in obesity-related heart disease. We have shown that systemic administration of omentin attenuates myocardial infarct size and apoptosis following ischemia-reperfusion in wild-type mice81). Transgenic mice expressing omentin in fat tissue also exhibit reduced myocardial damage in response to ischemia in vivo. The beneficial effects of omentin on acute cardiac injury and apoptosis appear to be mediated through two independent mechanisms involving Akt and AMPK signaling pathways. We have also reported that omentin reduces cardiac hypertrophy and systolic dysfunction after pressure overload82). Omentin suppresses agonist-stimulated hypertrophic response of cultured cardiac myocytes via activation of AMPK. Omentin is also reported to prevent doxorubicin-inducible cardiomyocyte death in vitro via suppression of mitochondrial reactive oxygen species83). Thus, these observations suggest that omentin serves as a cardioprotective adipocytokine (Fig. 3). Taken together, low levels of circulating omentin caused by obese conditions can contribute to the development of cardiovascular disorders. However, the precise mechanism by which omentin affects the cardiovascular system (e.g., receptor-mediated signaling cascades) remains to be elucidated. Furthermore, the clinical significance of omentin is incompletely understood. Resolution of these issues requires future research.
Conclusion
During the last decade, several adipocytokines that potentially exert protective actions on obese complications have been identified. Reduced production of these protective adipocytokines caused by obesity, particularly excess visceral fat accumulation, may lead to development of metabolic dysfunction and cardiovascular disorders. Thus, the approach to enhance the synthesis and secretion of these adipocytokines or to promote their receptor-mediated signaling pathways can be valuable for prevention and treatment of obesity-related metabolic and cardiovascular complications.
Acknowledgements
This work was supported by Grant-in-Aid for Scientific Research, Grant-in-Aid for Challenging Exploratory Research, and grants from the Takeda Science Foundation and Uehara Memorial Foundation. Molecular Cardiovascular Medicine was endowed by Kowa Pharmaceutical Co. Ltd.
Conflict of Interest
Research grants were received from Astellas Pharma Inc. and Daiichi Sankyo Co. Ltd. However, the research topics of these grants were not restricted. Lecture fees were received from Kowa Pharmaceutical Co. Ltd.
References
- 1). Matsuzawa Y, Funahashi T, Kihara S, Shimomura I: Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol, 2004; 24: 29-33 [DOI] [PubMed] [Google Scholar]
- 2). Reilly MP, Rader DJ: The metabolic syndrome: more than the sum of its parts? Circulation, 2003; 108: 1546-1551 [DOI] [PubMed] [Google Scholar]
- 3). Eguchi K, Manabe I: Toll-like receptor, lipotoxicity and chronic inflammation: the pathological link between obesity and cardiometabolic disease. J Atheroscler Thromb, 2014; 21: 629-639 [DOI] [PubMed] [Google Scholar]
- 4). Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K: Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol, 2003; 14: 561-566 [DOI] [PubMed] [Google Scholar]
- 5). Berg AH, Scherer PE: Adipose tissue, inflammation, and cardiovascular disease. Circ Res, 2005; 96: 939-949 [DOI] [PubMed] [Google Scholar]
- 6). Ouchi N, Parker JL, Lugus JJ, Walsh K: Adipokines in inflammation and metabolic disease. Nat Rev Immunol, 2011; 11: 85-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y: Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun, 1999; 257: 79-83 [DOI] [PubMed] [Google Scholar]
- 8). Ryo M, Nakamura T, Kihara S, Kumada M, Shibazaki S, Takahashi M, Nagai M, Matsuzawa Y, Funahashi T: Adiponectin as a biomarker of the metabolic syndrome. Circ J, 2004; 68: 975-981 [DOI] [PubMed] [Google Scholar]
- 9). Takahara M, Katakami N, Kaneto H, Noguchi M, Shimomura I: Contribution of visceral fat accumulation and adiponectin to the clustering of metabolic abnormalities in a Japanese population. J Atheroscler Thromb, 2014; 21: 543-553 [PubMed] [Google Scholar]
- 10). Miyazaki T, Hiki M, Shimada K, Kume A, Kiyanagi T, Sumiyoshi K, Ohmura H, Daida H: The high molecular weight adiponectin level is associated with the atherogenic lipoprotein profiles in healthy Japanese males. J Atheroscler Thromb, 2014; 21: 672-679 [DOI] [PubMed] [Google Scholar]
- 11). Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y: Novel modulator for endothelial adhesion molecules: adipocytederived plasma protein adiponectin. Circulation, 1999; 100: 2473-2476 [DOI] [PubMed] [Google Scholar]
- 12). Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y: Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol, 2003; 23: 85-89 [DOI] [PubMed] [Google Scholar]
- 13). Chan DC, Watts GF, Ng TW, Uchida Y, Sakai N, Yamashita S, Barrett PH: Adiponectin and other adipocytokines as predictors of markers of triglyceride-rich lipoprotein metabolism. Clin Chem, 2005; 51: 578-585 [DOI] [PubMed] [Google Scholar]
- 14). Matsuo N, Matsuoka T, Onishi S, Yamamoto H, Kato A, Makino Y, Kihara S: Impact of Remnant Lipoprotein on Coronary Plaque Components. J Atheroscler Thromb, 2015; 22: 783-795 [DOI] [PubMed] [Google Scholar]
- 15). Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB: Plasma adiponectin levels and risk of myocardial infarction in men. JAMA : the journal of the American Medical Association, 2004; 291: 1730-1737 [DOI] [PubMed] [Google Scholar]
- 16). Schulze MB, Shai I, Rimm EB, Li T, Rifai N, Hu FB: Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes, 2005; 54: 534-539 [DOI] [PubMed] [Google Scholar]
- 17). Ai M, Otokozawa S, Asztalos BF, White CC, Cupples LA, Nakajima K, Lamon-Fava S, Wilson PW, Matsuzawa Y, Schaefer EJ: Adiponectin: an independent risk factor for coronary heart disease in men in the Framingham offspring Study. Atherosclerosis, 2011; 217: 543-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y: Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation, 2000; 102: 1296-1301 [DOI] [PubMed] [Google Scholar]
- 19). Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, Funahashi T, Takata M, Temaru R, Sato A, Yamazaki K, Nakamura N, Kobayashi M: Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res, 2005; 97: 1245-1252 [DOI] [PubMed] [Google Scholar]
- 20). Ouedraogo R, Wu X, Xu SQ, Fuchsel L, Motoshima H, Mahadev K, Hough K, Scalia R, Goldstein BJ: Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes, 2006; 55: 1840-1846 [DOI] [PubMed] [Google Scholar]
- 21). Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y: Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res, 2004; 94: e27-e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K: Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem, 2004; 279: 1304-1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y: Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation, 2001; 103: 1057-1063 [DOI] [PubMed] [Google Scholar]
- 24). Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y: Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood, 2000; 96: 1723-1732 [PubMed] [Google Scholar]
- 25). Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME: Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun, 2004; 316: 924-929 [DOI] [PubMed] [Google Scholar]
- 26). Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y: Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation, 2002; 106: 2767-2770 [DOI] [PubMed] [Google Scholar]
- 27). Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T: Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem, 2003; 278: 2461-2468 [DOI] [PubMed] [Google Scholar]
- 28). Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, Colvin RA, Kihara S, Funahashi T, Luster AD, Libby P: Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res, 2008; 102: 218-225 [DOI] [PubMed] [Google Scholar]
- 29). Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K: Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med, 2005; 11: 1096-1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL: Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation, 2007; 115: 1408-1416 [DOI] [PubMed] [Google Scholar]
- 31). Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K: Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med, 2004; 10: 1384-1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, Hori M, Matsuzawa Y, Funahashi T, Kitakaze M: Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res, 2005; 67: 705-713 [DOI] [PubMed] [Google Scholar]
- 33). Shimano M, Ouchi N, Shibata R, Ohashi K, Pimentel DR, Murohara T, Walsh K: Adiponectin deficiency exacerbates cardiac dysfunction following pressure over-load through disruption of an AMPK-dependent angiogenic response. J Mol Cell Cardiol, 2010; 49: 210-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, Sam F, Ouchi N, Walsh K: Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol, 2007; 42: 1065-1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Sam F, Duhaney TA, Sato K, Wilson RM, Ohashi K, Sono-Romanelli S, Higuchi A, De Silva DS, Qin F, Walsh K, Ouchi N: Adiponectin deficiency, diastolic dysfunction, and diastolic heart failure. Endocrinology, 151: 322-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Tanaka K, Wilson RM, Essick EE, Duffen JL, Scherer PE, Ouchi N, Sam F: Effects of adiponectin on calcium-handling proteins in heart failure with preserved ejection fraction. Circ Heart Fail, 2014; 7: 976-985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Maruyama S, Shibata R, Ohashi K, Ohashi T, Daida H, Walsh K, Murohara T, Ouchi N: Adiponectin ameliorates doxorubicin-induced cardiotoxicity through Akt protein-dependent mechanism. J Biol Chem, 2011; 286: 32790-32800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Moore KJ, Tabas I: Macrophages in the pathogenesis of atherosclerosis. Cell, 2011; 145: 341-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Donath MY, Shoelson SE: Type 2 diabetes as an inflammatory disease. Nat Rev Immunol, 2011; 11: 98-107 [DOI] [PubMed] [Google Scholar]
- 40). Matsushita K, Yatsuya H, Tamakoshi K, Wada K, Otsuka R, Zhang H, Sugiura K, Kondo T, Murohara T, Toyoshima H: Inverse association between adiponectin and C-reactive protein in substantially healthy Japanese men. Atherosclerosis, 2006; 188: 184-189 [DOI] [PubMed] [Google Scholar]
- 41). Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, Nishizawa H, Maeda N, Kobayashi H, Hiraoka H, Matsuzawa Y: Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation, 2003; 107: 671-674 [DOI] [PubMed] [Google Scholar]
- 42). Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D: Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. Jama, 2003; 289: 1799-1804 [DOI] [PubMed] [Google Scholar]
- 43). Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K: Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem, 2010; 285: 6153-6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Lumeng CN, Bodzin JL, Saltiel AR: Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest, 2007; 117: 175-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Komohara Y, Fujiwara Y, Ohnishi K, Shiraishi D, Takeya M: Contribution of Macrophage Polarization to Metabolic Diseases. J Atheroscler Thromb, 2016; 23: 10-17 [DOI] [PubMed] [Google Scholar]
- 46). Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, Gupta M, Chan L, Al-Omran M, Teoh H, Verma S: Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol, 2010; 299: H656-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE: Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem, 2011; 286: 13460-13469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K: Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest, 2007; 117: 375-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Savill J, Dransfield I, Gregory C, Haslett C: A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol, 2002; 2: 965-975 [DOI] [PubMed] [Google Scholar]
- 50). Enomoto T, Ohashi K, Shibata R, Higuchi A, Maruyama S, Izumiya Y, Walsh K, Murohara T, Ouchi N: Adipolin/C1qdc2/CTRP12 protein functions as an adipokine that improves glucose metabolism. J Biol Chem, 2011; 286: 34552-34558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Enomoto T, Ohashi K, Shibata R, Kambara T, Uemura Y, Yuasa D, Kataoka Y, Miyabe M, Matsuo K, Joki Y, Hayakawa S, Hiramatsu-Ito M, Ito M, Murohara T, Ouchi N: Transcriptional regulation of an insulin-sensitizing adipokine adipolin/CTRP12 in adipocytes by Kruppel-like factor 15. PLoS One, 2013; 8: e83183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, Wong GW: C1q/TNF-related protein- 12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem, 2012; 287: 10301-10315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53). Enomoto T, Shibata R, Ohashi K, Kambara T, Kataoka Y, Uemura Y, Yuasa D, Murohara T, Ouchi N: Regulation of adipolin/CTRP12 cleavage by obesity. Biochem Biophys Res Commun, 2012; 428: 155-159 [DOI] [PubMed] [Google Scholar]
- 54). Wei Z, Lei X, Seldin MM, Wong GW: Endopeptidase cleavage generates a functionally distinct isoform of C1q/tumor necrosis factor-related protein-12 (CTRP12) with an altered oligomeric state and signaling specificity. J Biol Chem, 2012; 287: 35804-35814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, Gimeno R, Lodish HF: Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J, 2009; 23: 241-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Ouchi N, Walsh K: Cardiovascular and metabolic regulation by the adiponectin/C1q/tumor necrosis factorrelated protein family of proteins. Circulation, 2012; 125: 3066-3068 [DOI] [PubMed] [Google Scholar]
- 57). Kambara T, Ohashi K, Shibata R, Ogura Y, Maruyama S, Enomoto T, Uemura Y, Shimizu Y, Yuasa D, Matsuo K, Miyabe M, Kataoka Y, Murohara T, Ouchi N: CTRP9 protects against myocardial injury following ischemia- reperfusion through AMPK-dependent mechanism. The Journal of biological chemistry, 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58). Peterson JM, Wei Z, Seldin MM, Byerly MS, Aja S, Wong GW: CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol, 2013; 305: R522-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59). Uemura Y, Shibata R, Ohashi K, Enomoto T, Kambara T, Yamamoto T, Ogura Y, Yuasa D, Joki Y, Matsuo K, Miyabe M, Kataoka Y, Murohara T, Ouchi N: Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J, 2013; 27: 25-33 [DOI] [PubMed] [Google Scholar]
- 60). Zheng Q, Yuan Y, Yi W, Lau WB, Wang Y, Wang X, Sun Y, Lopez BL, Christopher TA, Peterson JM, Wong GW, Yu S, Yi D, Ma XL: C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arteriosclerosis, thrombosis, and vascular biology, 2011; 31: 2616-2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61). Jung CH, Lee MJ, Kang YM, La Lee Y, Seol SM, Yoon HK, Kang SW, Lee WJ, Park JY: C1q/TNF-related protein-9 inhibits cytokine-induced vascular inflammation and leukocyte adhesiveness via AMP-activated protein kinase activation in endothelial cells. Mol Cell Endocrinol, 2016; 419: 235-243 [DOI] [PubMed] [Google Scholar]
- 62). Li J, Zhang P, Li T, Liu Y, Zhu Q, Chen T, Liu T, Huang C, Zhang J, Zhang Y, Guo Y: CTRP9 enhances carotid plaque stability by reducing pro-inflammatory cytokines in macrophages. Biochem Biophys Res Commun, 2015; 458: 890-895 [DOI] [PubMed] [Google Scholar]
- 63). Kambara T, Shibata R, Ohashi K, Matsuo K, Hiramatsu-Ito M, Enomoto T, Yuasa D, Ito M, Hayakawa S, Ogawa H, Aprahamian T, Walsh K, Murohara T, Ouchi N: C1q/Tumor Necrosis Factor-Related Protein 9 Protects against Acute Myocardial Injury through an Adiponectin Receptor I-AMPK-Dependent Mechanism. Mol Cell Biol, 2015; 35: 2173-2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Su H, Yuan Y, Wang XM, Lau WB, Wang Y, Wang X, Gao E, Koch WJ, Ma XL: Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFalpha-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice. Basic Res Cardiol, 2013; 108: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65). Sun Y, Yi W, Yuan Y, Lau WB, Yi D, Wang X, Wang Y, Su H, Gao E, Koch WJ, Ma XL: C1q/tumor necrosis factor-related protein-9, a novel adipocyte-derived cytokine, attenuates adverse remodeling in the ischemic mouse heart via protein kinase A activation. Circulation, 2013; 128: S113-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Tsuji S, Uehori J, Matsumoto M, Suzuki Y, Matsuhisa A, Toyoshima K, Seya T: Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J Biol Chem, 2001; 276: 23456-23463 [DOI] [PubMed] [Google Scholar]
- 67). Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, Shuldiner AR, Fried SK, McLenithan JC, Gong DW: Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab, 2006; 290: E1253-1261 [DOI] [PubMed] [Google Scholar]
- 68). de Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, Yu DZ, Pray J, Ndubuizu K, Patil S, Schwartz A, Kligman M, Fried SK, Gong DW, Shuldiner AR, Pollin TI, McLenithan JC: Omentin plasma levels and gene expression are decreased in obesity. Diabetes, 2007; 56: 1655-1661 [DOI] [PubMed] [Google Scholar]
- 69). Pan HY, Guo L, Li Q: Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract, 2010; 88: 29-33 [DOI] [PubMed] [Google Scholar]
- 70). Shibata R, Ouchi N, Takahashi R, Terakura Y, Ohashi K, Ikeda N, Higuchi A, Terasaki H, Kihara S, Murohara T: Omentin as a novel biomarker of metabolic risk factors. Diabetol Metab Syndr, 2012; 4: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71). Shibata R, Ouchi N, Kikuchi R, Takahashi R, Takeshita K, Kataoka Y, Ohashi K, Ikeda N, Kihara S, Murohara T: Circulating omentin is associated with coronary artery disease in men. Atherosclerosis, 2011; 219: 811-814 [DOI] [PubMed] [Google Scholar]
- 72). Shang FJ, Wang JP, Liu XT, Zheng QS, Xue YS, Wang B, Zhao LY: Serum omentin-1 levels are inversely associated with the presence and severity of coronary artery disease in patients with metabolic syndrome. Biomarkers, 2011; 16: 657-662 [DOI] [PubMed] [Google Scholar]
- 73). Yoo HJ, Hwang SY, Hong HC, Choi HY, Yang SJ, Seo JA, Kim SG, Kim NH, Choi KM, Choi DS, Baik SH: Association of circulating omentin-1 level with arterial stiffness and carotid plaque in type 2 diabetes. Cardiovasc Diabetol, 2011; 10: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74). Shibata R, Takahashi R, Kataoka Y, Ohashi K, Ikeda N, Kihara S, Murohara T, Ouchi N: Association of a fat-derived plasma protein omentin with carotid artery intima-media thickness in apparently healthy men. Hypertens Res, 2011; 34: 1309-1312 [DOI] [PubMed] [Google Scholar]
- 75). Maruyama S, Shibata R, Kikuchi R, Izumiya Y, Rokutanda T, Araki S, Kataoka Y, Ohashi K, Daida H, Kihara S, Ogawa H, Murohara T, Ouchi N: Fat-derived factor omentin stimulates endothelial cell function and ischemia- induced revascularization via endothelial nitric oxide synthase-dependent mechanism. J Biol Chem, 2012; 287: 408-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76). Yamawaki H, Tsubaki N, Mukohda M, Okada M, Hara Y: Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem Biophys Res Commun, 2010; 393: 668-672 [DOI] [PubMed] [Google Scholar]
- 77). Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M, Hara Y: Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun, 2011; 408: 339-343 [DOI] [PubMed] [Google Scholar]
- 78). Uemura Y, Shibata R, Kanemura N, Ohashi K, Kambara T, Hiramatsu-Ito M, Enomoto T, Yuasa D, Joki Y, Matsuo K, Ito M, Hayakawa S, Ogawa H, Murohara T, Ouchi N: Adipose-derived protein omentin prevents neointimal formation after arterial injury. FASEB J, 2015; 29: 141-151 [DOI] [PubMed] [Google Scholar]
- 79). Kazama K, Okada M, Yamawaki H: A novel adipocytokine, omentin, inhibits platelet-derived growth factor-BB-induced vascular smooth muscle cell migration through antioxidative mechanism. Am J Physiol Heart Circ Physiol, 2014; 306: H1714-1719 [DOI] [PubMed] [Google Scholar]
- 80). Hiramatsu-Ito M, Shibata R, Ohashi K, Uemura Y, Kanemura N, Kambara T, Enomoto T, Yuasa D, Matsuo K, Ito M, Hayakawa S, Ogawa H, Otaka N, Kihara S, Murohara T, Ouchi N: Omentin attenuates atherosclerotic lesion formation in apolipoprotein E-deficient mice. Cardiovasc Res, 2015; [DOI] [PubMed] [Google Scholar]
- 81). Kataoka Y, Shibata R, Ohashi K, Kambara T, Enomoto T, Uemura Y, Ogura Y, Yuasa D, Matsuo K, Nagata T, Oba T, Yasukawa H, Numaguchi Y, Sone T, Murohara T, Ouchi N: Omentin prevents myocardial ischemic injury through AMP-activated protein kinase- and Akt-dependent mechanisms. J Am Coll Cardiol, 2014; 63: 2722-2733 [DOI] [PubMed] [Google Scholar]
- 82). Matsuo K, Shibata R, Ohashi K, Kambara T, Uemura Y, Hiramatsu-Ito M, Enomoto T, Yuasa D, Joki Y, Ito M, Hayakawa S, Ogawa H, Kihara S, Murohara T, Ouchi N: Omentin functions to attenuate cardiac hypertrophic response. J Mol Cell Cardiol, 2015; 79: 195-202 [DOI] [PubMed] [Google Scholar]
- 83). Kazama K, Okada M, Yamawaki H: Adipocytokine, omentin inhibits doxorubicin-induced H9c2 cardiomyoblasts apoptosis through the inhibition of mitochondrial reactive oxygen species. Biochem Biophys Res Commun, 2015; 457: 602-607 [DOI] [PubMed] [Google Scholar]


