Abstract
1-kestose is a structural component of fructo-oligosaccharides and is composed of 2 fructose residues bound to sucrose through β2-1 bonds. In the present study, the influence of the ingestion of 1-kestose on the intestinal microbiota was investigated in cats. Six healthy cats were administered 1 g/day of 1-kestose for 8 weeks followed by a 2-week wash-out period. Fecal samples were collected from cats after 0, 4, 8, and 10 weeks. The intestinal microbiota was examined by a 16S rRNA gene metagenomic analysis and real-time PCR. Short-chain fatty acids were measured by GC/MS. The results suggested that the intestinal bacterial community structure in feline assigned to this study was divided into 2 types: one group mainly composed of the genus Lactobacillus (GA) and the other mainly composed of the genus Blautia with very few bacteria of Lactobacillus (GB). Furthermore, the number of Bifidobacterium slightly increased after the administration of 1-kestose (at 4 and 8 weeks) (P<0.1). The administration of 1-kestose also increased the abundance of Megasphaera, the butyric acid-producing bacteria, at 4 and 8 weeks (P<0.1). Furthermore, an increase in butyric acid levels was observed after the administration of 1-kestose for 4 weeks (P<0.1). These results suggest that 1-kestose activated butyrate-producing bacteria as well as bifidobacteria and propose its potential as a new generation prebiotic.
Keywords: Bifidobacterium, butyrate, 1-kestose, Megasphaera, prebiotics
Fructo-oligosaccharides (FOS) are oligosaccharides composed of one or more fructose residues bound to sucrose (GF) through β2-1 bonds; those with 1 and 2 fructose residues bound to sucrose are termed 1-kestose (GF2) and nystose (GF3), respectively. Commercially available FOS are mixtures of these components (FOS products), and GF3 is the most abundant FOS component; however, GF2 used in this study is not the dominant FOS component in these mixtures. For example, the percent ratios of GF2, GF3, and fructofuranosyl-nystose (GF4) in a representative synthetic FOS product, Meioligo (Meiji Co., Ltd., Tokyo, Japan) are 32.0, 53.6, and 9.8%, respectively. FOS products are representative oligosaccharides currently being used in Japan and other countries.
One of the dominant biological effects of FOS is its promotion of the growth of Bifidobacterium (in vitro [24, 36], animals [28], and humans [5, 20, 40]). Furthermore, the administration of FOS has been associated with a shortened defecation time and improved lipid metabolism [8,9,10]. Anti-inflammatory activity in the intestines [11] and senescence-associated mental disabilities, delays in aging, including learning disability and memory impairment in senescence-accelerated mice [31], have also been reported in mice.
These beneficial effects of FOS are mainly exerted by the following mechanisms: 1) the activation of Bifidobacterium and Lactobacillus, and 2) increases in the levels of short-chain fatty acids (SCFA). SCFA, such as acetate, which are produced by these beneficial bacteria using FOS, function not only as an energy source in large intestinal mucosal cells [8], but also a substrate of energy metabolism in white adipose tissue, skeletal muscle, and liver [7, 13, 27]. Consequently, SCFA promote peristalsis in the intestines and also exert significant effects on systemic metabolism in the host.
The component of FOS products that stimulates the growth of bifidobacteria and production of SCFA by bacteria has not yet been identified. Suzuki et al. [42] examined differences in cultures to which FOS, 1-kestose, or nystose was added, and found that bifidobacteria exhibited the most rapid growth with the greatest production of metabolites, including lactic acid and acetic acid, in the culture with 1-kestose. Previous studies on the assimilation of FOS using 17 strains of Lactobacillus spp. and 15 strains of Bifidobacterium spp. also demonstrated that the assimilation of 1-kestose was the highest among FOS components [16, 33]. Furthermore, a recent study showed that 1-kestose activated not only Bifidobacterium spp., but also butyric acid-producing bacteria in the intestines [26]. Based on these findings, not only the genera Lactobacillus and Bifidobacterium, but also butyric acid-producing bacteria exhibit strong 1-kestose-assimilating activities, indicating that 1-kestose is the dominant FOS component responsible for prebiotic effects.
Regarding the composition of intestinal microbiota in cats, Firmicutes was identified as the most dominant phylum [3, 19, 35, 41], together with Proteobacteria, Bacteroidetes, Fusobacteria, and Actinobacteria [3, 19, 35, 41]. Previous studies examined the prebiotic effects of FOS and galacto-oligosaccharides (GOS) in cats. Barry et al. [4] fed healthy cats cellulose as a control or a diet containing FOS, and investigated the influence of FOS on the intestinal microbiota. The findings obtained showed that Bifidobacterium spp. levels were higher, whereas Escherichia coli levels were lower in feces in the FOS group than in the cellulose group. Regarding SCFA, butyric acid increased in the FOS group. Kanakupt et al. [23] fed cats diets containing short chain (sc) FOS, GOS, or scFOS + GOS, and then investigated their influence on intestinal bacteria. The findings obtained revealed that Bifidobacterium spp. levels were significantly higher in all groups than in cats fed the diet containing no prebiotics. In a study in which FOS + inulin was administered, Lactobacillales (mostly Lactobacillus spp.) increased in some cats [17]. However, the effects of administering 1-kestose to cats have not yet been examined. Therefore, we herein investigated the influence of 1-kestose on the feline intestinal microbiota.
MATERIALS AND METHODS
Animals
All experiments were reviewed and approved by the Institutional Animal Welfare Committee (Approval No. NBC-54-008). According to the body condition score (BCS) [2], six healthy adult cats with a normal weight were obtained from Kitayama Labes Co., Ltd. (Chiba, Japan) and then used in the present study. BCS was assessed on a 5-point scale as follows: 1, very thin; 2, thin; 3, ideal; 4, overweight; and 5, obese. The profiles of these cats were shown in Table 1. No markedly abnormal symptoms were observed in the hematology or blood chemistry of the cats at the initiation of the study, as shown in Supplementary Tables 1 and 2.
Table 1. Profiles of cats used in the present study.
| ID No | Strain | Sex | Age (year) | Body weight (kg) | BCSa) | Groupb) |
|---|---|---|---|---|---|---|
| 1 | Narc:Catus | M | 10 | 3.76 | 3 | A |
| 2 | Narc:Catus | M | 10 | 2.97 | 3 | A |
| 3 | Narc:Catus | F | 12 | 2.31 | 3 | B |
| 4 | Narc:Catus | M | 13 | 3.02 | 3 | B |
| 5 | Narc:Catus | F | 13 | 2.56 | 3 | B |
| 6 | NIBS | F | 10 | 3.53 | 3 | B |
a) Body condition score (BCS); assessed on a 5-point scale (1, very thin; 2, thin; 3, ideal; 4, overweight; 5, obese). b) Group; Grouping was performed according to the type of intestinal microbiota composition, as shown in Fig. 1.
Study design
The total period of the study was 10 weeks. During the whole study period, cats were fed a diet manufactured by Nisshin Petfood Inc. (Tochigi, Japan). The diet was only supplemented with 1-kestose in the first 8 weeks and this was followed by a 2-week wash-out period. The diet consisted of 3.9% moisture, 37.6% protein, 13.6% fat, 6.6% ash, and 0.4% crude fiber. Each cat was fed a specific amount of the diet to maintain a constant body weight. Feeding was performed between 9 AM and 5 PM, and water was given ad libitum all day. In addition to the daily diet, 1 g/day of 1-kestose was added to the diet every day during the first 8-week period at 9 AM. The maximum permissive dose of 1-kestose in cats was calculated from the maximum permissive dose of 1-kestose in humans [32] according to the report of Reagan-Shaw et al. [34]. As a result, it was found that the maximum permissive dose of the cat (average body weight: 3.0 kg) used in the present study was 1.89 kg/day. For this reason, about a half amount, 1 g, was set as the daily dose. Fresh fecal samples (within 15 min of defecation) were collected from cats after 0, 4, 8, and 10 weeks and stored immediately at −80°C. Blood samples were also collected from cats after 0, 4, 8, and 10 weeks and analyzed at the LSI Medience Corp. (Tokyo, Japan).
DNA extraction
Genomic DNA was extracted from feces according to the method described by Takahashi et al [43]. Frozen fecal samples were thawed on ice, and 100 mg of each sample was suspended in 4 M guanidium thiocyanate, 100 mM Tris-HCl (pH 9.0), and 40 mM EDTA and beaten with zirconia beads using a FastPrep FP100A instrument (MP Biomedicals, Santa Ana, CA, USA). DNA was extracted from bead-treated suspensions using the Magtration System 12GC and GC series MagDEA DNA 200 (Precision System Science, Chiba, Japan). Following estimations of the DNA concentrations of each sample by spectrophotometry using ND-1000 (NanoDrop Technologies, Wilmington, DE, USA), the final concentration of DNA in samples was adjusted to 10 ng/µl.
Bacterial 16S rRNA gene sequencing and sequence data analysis
Fecal bacterial 16S rRNA gene sequence was analyzed by the MiSeq system (Illumina, San Diego, CA, USA) as previously described [43]. The V3-V4 hypervariable regions of 16S rRNA were PCR amplified from microbial genomic DNA using universal primers for bacteria (341f/R806) [12, 30] and the dual-index method [21]. Barcoded amplicons were sequenced using the paired-end method with a 2 × 284-bp cycle run on the MiSeq system by MiSeq Reagent kit v.3 (600 Cycles) (Illumina, San Diego, CA, USA). After the alignment, overlapping regions within paired-end reads were merged and primer regions were omitted, which resulted in a 430-bp sequence. Only reads with more than 99% of its sequence having quality value scores of ≥20 were extracted for further analyses [43]. Chimeric sequences detected by Usearch6.1.544_i86 were precluded [15]. Based on these sequences, species were identified with an 97% confidence threshold using Metagenome@KIN analysis software (World Fusion, Osaka, Japan) and the TechnoSuruga Lab Microbial Identification database DB-BA 10.0 (TechnoSuruga Laboratory, Shizuoka, Japan) [21, 25]. The abundance of each taxon was calculated at both the phylum and genus levels (Supplementary Tables 3–6).
Quantitative analysis of intestinal microbiota in cat feces using real-time PCR
Using the same extracted DNA sample as that in bacterial 16S rRNA gene sequencing, a quantitative analysis of the following intestinal organisms was performed by real-time PCR (qPCR) detecting specific gene sequence in each 16S rRNA: all bacteria [30], Bifidobacterium spp. [18], Lactobacillus spp. [39], Clostridium cluster XIV [38], and Clostridium perfringens [44]. A list of the primers used is shown in Table 2.
Table 2. List of primers used for real-time PCR.
| Target | Primer name | Oligonucleotide sequence |
|---|---|---|
| All bacteria | 341f | CCTACGGGAGGCAGCAG |
| 534r | ATTACCGCGGCTGCTGG | |
| Bifidobacterium spp. | BifiLM26F | GATTCTGGCTCAGGATGAACGC |
| Bif228R | CTGATAGGACGCGACCCCAT | |
| Lactobacillus spp. | LactoR’F | CACAATGGACGMAAGTCTGATG |
| LBFR | CGCCACTGGTGTTCTTCCAT | |
| Clostridium cluster XIV | CXIV-F1 | GAWGAAGTATYTCGGTATGT |
| CXIV-R2 | CTACGCWCCCTTTACAC | |
| Clostridium perfringens | Cperf 165F | CGCATAACGTTGAAAGATGG |
| Cperf269R | CCTTGGTAGGCCGTTACCC | |
Measurement of SCFA
The measurement of SCFA, including acetate, propionate, and butyrate was performed by GC/MS (Shimadzu, Kyoto, Japan) on Rtx-1701 columns (Restec, Bellefonte, PA, USA). GC/MS samples were prepared as follows: 50 mg (wet weight) of fecal samples were suspended in 400 µl pure water. The suspension was stirred for 10 min and centrifuged at 15,000 × g at 4°C for 5 min. Twenty microliters of 20 mM 2-ethyl butyrate, 80 µl of 3N HCl, and 400 µl of diethylether were then added to the supernatant.
Statistical analysis
Statistical analyses were performed using SPSS Statistics 26 software package (SPSS Inc., Chicago, IL, USA) and Microsoft Excel (Excel version in Microsoft Office 2010 for Windows). The normality of data was examined by the Shapiro-Wilk test. And data were analyzed using Wilcoxon signed-rank test followed by the correction with FDR method (the Benjamini & Hochberg method for adjustments with FDR (BH method)). Differences were considered as significant at P<0.05 and tendency at P<0.1.
RESULTS
Body weight, food intake, hematology, and biochemistry
The administration of 1-kestose (1 g/day) did not significantly influence body weight (Supplementary Fig. 1) or food intake. The number of platelets was significantly decreased in the period of 1-kestose administration. Most of hematological and blood biochemical parameters were not altered by 1-kestose administrartion (Supplementary Tables 1 and 2). And no abnormal symptoms including the petechia caused by decreasing the number of platelets were observed throughout the 10-week study period in any cat.
16S rRNA gene metagenomic analysis of intestinal microbiota
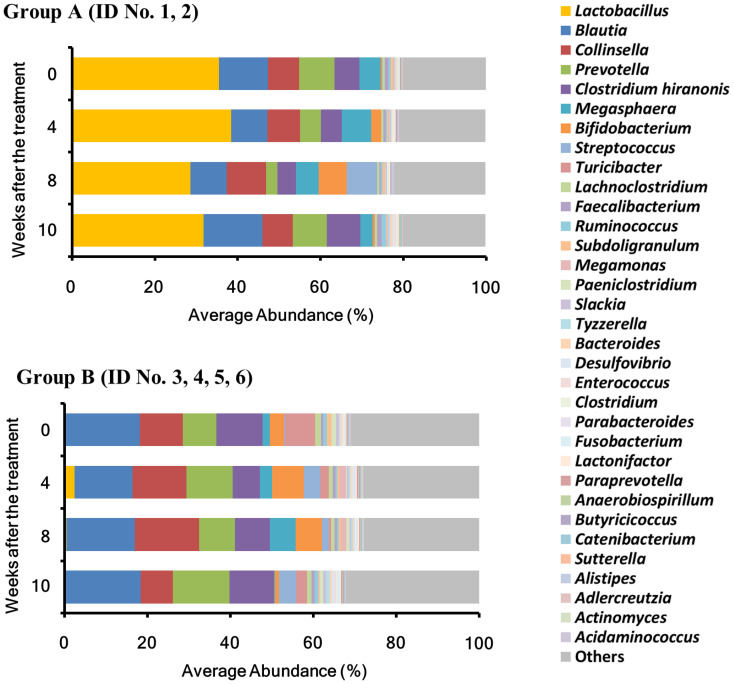
The total numbers of reads analyzed for each sample at the different time points (0, 4, 8, and 10 weeks) were 81,938 ± 22,246, 31,913 ± 4,332, 36,532 ± 5,761, and 36,386 ± 5,770 (AVE ± SE), respectively. The microbiota compositions of 6 animals were classified into 2 types based on the analysis at the genus level: Two animals in which the abundance of Lactobacillus was the highest were classified as Group A (GA), and 4 in which the abundance of Blautia was the highest with very few Lactobacillus were classified as Group B (GB) (Fig. 1, Table 1). At the beginning of the experiment (0 weeks), the abundance of Lactobacillus (phylum Firmicutes) was high (34.4%) in GA. Although its abundance slightly increased and decreased at 4 (38.4%) and 8 (28.6%) weeks after the treatment, respectively, almost no change (31.7%) was noted at 2 weeks after the termination of the 1-kestose treatment (10 weeks). In GB, Blautia accounted for 18.1% at 0 weeks, and its abundance also showed no evident changes after the 1-kestose treatment; 14.0, 16.5% and 18.4% at 4, 8, and 10 weeks, respectively. The abundance of Lactobacillus was markedly lower in GB (0.1%) than in GA (34.4%) at 0 weeks, while the administration of 1-kestose transiently increased Lactobacillus to 2.4% at 4 weeks. However, its level decreased to 0.1% close to the baseline at 10 weeks.
Fig. 1.
Intestinal microbiota composition analysis at the genus level. The mean abundance of the top 34 taxa, with values of 0.1% or higher during the study period, in all samples at each time point (0, 4, 8, and 10 weeks). The group in which the abundance of the genus Lactobacillus at the study initiation was the highest was designated as Group A (GA) and the group in which the abundance of the genus Blautia was the highest with few Lactobacillus was designated as Group B (GB).
Among the genera representing >0.1% of the total microbiota at 0 weeks, the abundance of four genera including Bifidobacterium (phylum Actinobacteria), Megasphaera (phylum Firmicutes), and Collinsella (phylum Actinobacteria) were slightly increased by the administration of 1-kestose (Table 3). The abundance of the genus Megasphaera, a butyric acid-producing bacterium, was 2.9% at 0 week, and significantly increased to 4.3 and 5.9% at 4 and 8 weeks, respectively (P<0.1).
Table 3. Changes in the abundance of taxa exhibiting a slight increase in both Group A and Group B.
| Genus | Average abundance (%) (Minimum%–Maximum%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 week | 4 weeks | 8 weeks | 10 weeks | |||||
| Lactobacillus | 11.9a) | (0.0–43.2)b) | 14.4 | (0.1–48.3) | 9.8 | (0.1–39.5) | 10.6 | (0.0–52.7) |
| Bifidoabcterium | 2.4 | (0.2–8.1) | 5.9 | (0.2–26.9) | 6.5 | (1.3–20.6) | 0.8 | (0.1–3.5) |
| Megasphaera | 2.9 | (0.1–9.4) | 4.3* | (0.6–11.3) | 5.9* | (2.3–12.0) | 1.1 | (0.0–5.4) |
| Collinsella | 9.4 | (7.1–12.6) | 11.3 | (6.8–18.4) | 13.5 | (7.7–21.9) | 7.6 | (3.97–13.7) |
a) Average, b) Minimum–Maximum. *P<0.1 (versus 0 week).
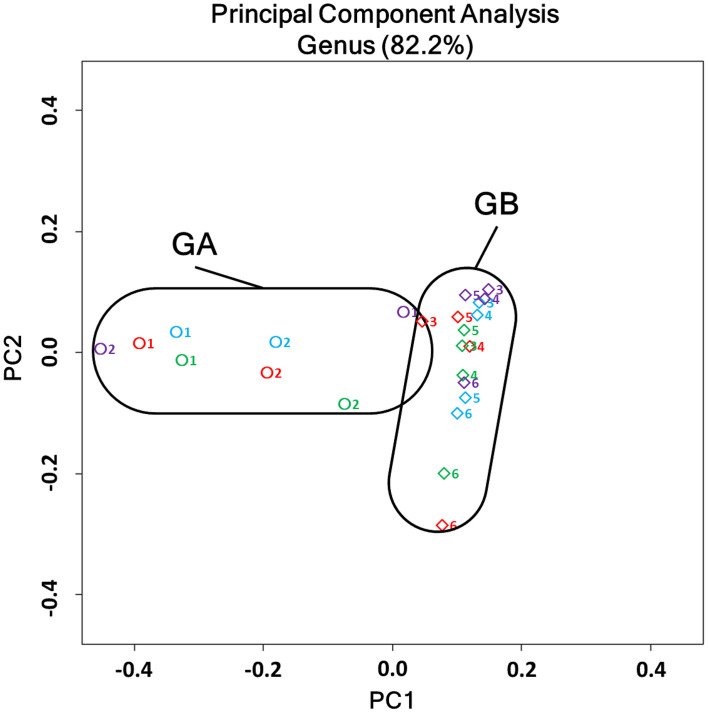
The 2-dimentional display of principal coordinate analysis (PCoA) of the overall structure of the intestinal microbiota is shown in Fig. 2. The clear difference observed in the bacterial community composition between GA and GB, which was shown in Fig. 1, was confirmed in the PCoA analysis. Individual samples at 0 week (shown by blue symbols) were clearly separated into “GA” (ID No. 1, 2) and “GB” (ID No. 3, 4, 5, 6) frames in the figure.
Fig. 2.
Two-dimensional PCoA analysis of microbiota in all samples. A plot of the first primary component (PC1) on the horizontal axis and the second primary component (PC2) on the vertical axis, and each plot represents the microbiota structure of individual samples. Plots were differently colored as follows: 0 weeks, blue; 4 weeks, red; 8 weeks, green; and 10 weeks, purple. The number (1–6) beside the symbol indicates the ID numbers of cats. Circles and diamonds represent Group A (GA) and Group B (GB) samples, respectively.
Moreover, 4 (red), 8 (green), and 10 (purple) weeks after the 1-kestose treatment, the sample symbols for the GA group moved along the horizontal axis, whereas those for the GB group moved along the vertical axis. Samples were all within the original frames of “GA” and “GB”, even after the administration of 1-kestose, and no major change occurred in the basic structure of the bacterial community in either group.
Quantitation of the number of intestinal bacteria by real-time PCR
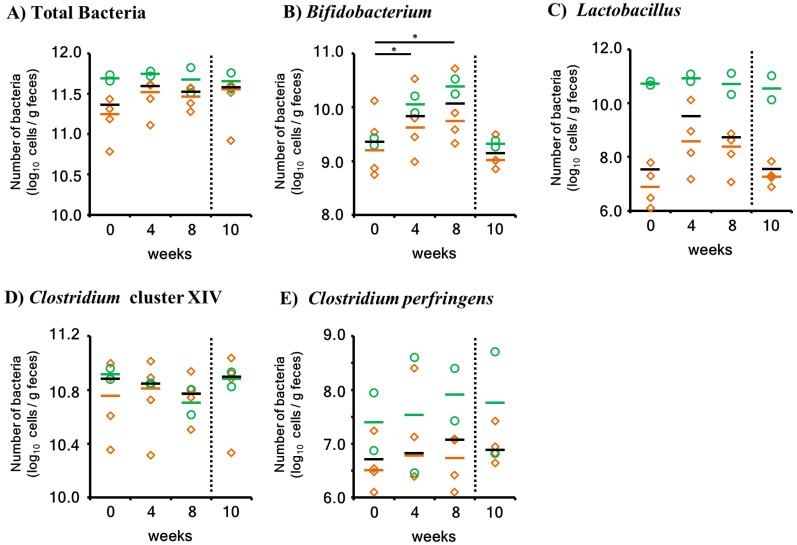
Bifidobacterium and Lactobacillus are efficiently activated by FOS in other animals. Clostridium cluster XIV is a representative butyrate-producing bacterial group. Therefore, to quantitatively assess the effects of 1-kestose on these bacteria, their numbers were measured using real-time PCR (Fig. 3).
Fig. 3.
Quantitation of intestinal bacteria using real-time PCR. Green circles and orange diamonds represent Group A (GA) and Group B (GB) samples, respectively. The short horizontal bars indicate the median. The black bars indicate the median of all samples (GA+GB). To distinguish the 1-kestose administration period (0–8 weeks) and 2 weeks after completion (10 weeks), a vertical broken line was added to the graph. The week in which a slight difference was noted (P<0.1) in the statistical analysis is indicated by “*”.
The total number of bacteria did not change in the GA or GB group after the administration of 1-kestose. The number of Bifidobacterium was slightly higher after the administration of 1-kestose at 4 and 8 weeks than at the initiation of the study (P<0.1) when the statistical analysis was performed using all animals (N=6). Slight increases were observed for both GA and GB. However, the number of bacteria returned to the pretreatment level at 2 weeks after the discontinuation of 1-kestose (10 weeks). In the other bacteria groups, no significant changes were observed even when all animals were included in the statistical analysis. In summary, the 1-kestose treatment slightly increased the bacterial number of Bifidobacterium (P<0.1) in cats. Furthermore, 1-kestose slightly increased the relative abundance of Megasphaera, a butyric acid-producing bacterium (P<0.1).
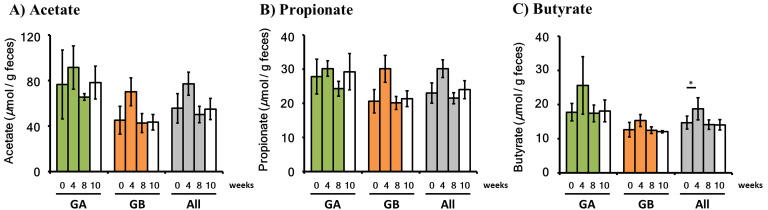
Measurement of SCFA
SCFA in feces were measured using GC/MS (Fig. 4). 1-kestose-induced changes in SCFA levels in all animals (N=6) were subjected to a statistical analysis. No significant changes were noted in acetic acid or propionic acid levels, whereas butyric acid levels were slightly higher after the administration of 1-kestose for 4 weeks than at 0 week (P<0.1). Elevated butyric acid levels returned to the pretreatment level after 8 weeks of administration and at 10 weeks (2 weeks after the discontinuation of administration). No increases were observed in the levels of acetate or propionate, even in the group of all animals.
Fig. 4.
Short-chain fatty acids (SCFA) measurement. The measurement of SCFA was performed using samples from Group A (GA), Group B (GB), and both groups. The 1-kestose administration period (0–8 weeks) is indicated by the following colors: GA, green; GB, orange; All, gray. The follow-up period is shown with white. Values are presented by AVE ± SE. In a statistical analysis of each week versus 0 weeks, the week at which a slight increase was noted (P<0.1) is indicated by “*”.
DISCUSSION
In feline assigned to this study, two kinds of gut microbial community composition were confirmed by the 16S rRNA gene metagenomic analysis; one group mainly composed of the genus Lactobacillus (GA) and the other mainly composed of the genus Blautia with few Lactobacillus (GB). When the smallest fructo-oligosaccharide component, 1-kestose, was administered, no significant changes were observed in the abundance or bacterial number of Lactobacillus, whereas slight changes were noted in Bifidobacterium. In addition, 1-kestose increased the abundance of Megasphaera, a butyrate-producing bacterium, and intestinal butyric acid levels when GA and GB were analyzed together.
In the present study, the microbiota composition of cats were clearly divided into two distinct types including GA and GB. The difference was so evident that was considered the difference of “enterotype”, which had been separated in a human study [1]. The study on the intestinal microbiota in healthy humans revealed that the microbiota may be classified into 3 patterns (enterotypes) with different dominant bacterial groups, and a relationship between these differences in the microbiota and medium- to long-term eating habits has been reported [1]. In addition, a controlled−feeding study of human subjects showed that a minor change of microbiome composition was detected within 24 hr of initiating different diets, but, the enterotype of the subject remained stable during the 10-day study. Therefore, this study also demonstrated the alternative enterotype state was associated with long term (-years) diet [46]. In our study, all cats were fed the same diet during the study period, but the diet up to be enrolled in the present study might be different since each cat had the different background. Therefore, there is a possibility that the difference of the enterotype was formed. Furthermore, marked changes were not observed in the basic composition of the microbiota in GA or GB when the potent prebiotic, 1-kestose, was administered for a relatively short period (8 weeks), which is consistent with the characteristics of “enterotype” proposed in humans. However, this discussion might be weak due to a small number of samples analyzed in the present study.
The administration of 1-kestose did not increase the abundance or number of Lactobacillus further in GA, in which Lactobacillus was already dominant. On the other hand, its administration slightly increased the abundance of Lactobacillus in GB, in which the population size of the genus Lactobacillus was very low. Hidaka et al. reported a similar phenomenon for the genus Bifidobacterium [20] in a study to investigate the influence of FOS on intestinal bacteria in humans. In that study, subjects with a small number of fecal bifidobacteria showed a 10,000-fold higher count after the FOS treatment than in the initial count. In contrast, the bacterial number did not show any significant changes in subjects with a large number of bifidobacteria.
In a real-time PCR assay, the number of Bifidobacterium was slightly higher after the administration of 1-kestose (at 4 and 8 weeks) than at the study initiation (0 week) in all animals (P<0.1). Previous studies reported that the administration of FOS increased the genus Bifidobacterium in cats [4, 23]. Barry et al. [4] fed cats weighing 5.7 kg on average a diet containing 4% FOS for 30 days. In that study, 68.7 g/day diet on average was ingested, and 4% FOS was similar to 2.7 g/day of FOS. The logarithmic number of Bifidobacterium in feces 25–26 days after the initiation of the FOS-containing diet was 11.6/g feces, which was 15.8-fold higher than that in the control cellulose group (10.4/g feces). Therefore, the bifidogenic effect of 1 g of FOS was 5.9- times higher than that of the cellulose group. In the present study, the duration of administration of 1-kestose (1 g/day) was 8 weeks, and the number of Bifidobacterium after 4 and 8 weeks of administration increased by 3.0- and 5.5-fold. This result suggested that the bifidogenic effect of 1-kestose was similar to that of FOS and the administration of 1-kestose was demonstrated to increase bifidobacteria in cats, as was confirmed by the FOS administration.
The abundance of Megasphaera, butyrate-producing bacteria, was slightly higher after the administration of 1-kestose (at 4 and 8 weeks) than at the initiation of the study (0 week) (P<0.1). M. elsdenii, which was identified in this genus by an analysis at the species level in samples, is a lactic acid-assimilating butyric acid-producing bacterium [29]. M. elsdenii is beneficial for ruminants, such as cows and sheep, and its use as a probiotic in rats and pigs has also been reported [22, 47]. A previous study demonstrated that the abundance of the genus Megasphaera (M. elsdenii) in cats aged 18 and 30 weeks was at most 0.1 and 0.2%, respectively, but markedly increased to 8.4% at 42 weeks [14], with the highest (87-fold) increase among all genera. In the present study, the mean abundance of Megasphaera increased approximately 1.5- and 2-fold after the administration of 1-kestose for 4 and 8 weeks, respectively. Butyrate levels also slightly increased (P<0.1) at 4 weeks when analyzed in the all animals group, which is consistent with the increase observed in the abundance of Megasphaera at 4 weeks. Therefore, 1-kestose-induced elevations in intestinal butyric acid levels in the present study were attributed to increases in butyric acid-producing bacteria, including Megasphaera. In addition, in our previous study in which rats were fed diets containing 1-kestose at different concentrations (0.5–5.0%) for 4 weeks, butyric acid levels in cecal contents significantly increased in the groups fed a diet containing 2.5% or more 1-kestose [45]. Since evidence to show that 1-kestose stimulates butyrate-producing bacteria to produce butyrate in the intestines has not yet been obtained for prebiotics other than 1-kestose, the use of 1-kestose as a new generation prebiotic is promising for the well-being of cats.
SCFA, including acetate, propionate, and butyrate, transiently increased at 4 weeks, but returned to pretreatment levels at 8 weeks in all groups after the 1-kestose treatment. Among these SCFA, only butyrate observed a slight increase (P<0.1) at 4 weeks. These fluctuations in SCFA levels may be explained by “resilience”, which is widely observed in biology. “Resilience” maintains homeostasis in the intestinal microbiota [37]. For example, when a component exerting an influence on the microbiota was administered for the same period as the present study, it temporarily influenced the status of intestinal bacteria. However, this influence did not persist, and the status finally returned to the original level through the “resilience” phenomenon. Therefore, by the administration of 1-kestose with a higher dose, resilience may have broken through into a new constant phase in which increases in SCFA, particularly butyrate, may persist.
Another discussion of fluctuations in SCFA levels may be explained by the incorporation of SCFA through hydrogen-coupled monocarboxylate transporters (MCTs) that is known as a main absorption mechanism in colon epithelial cells [6, 7]. In a previous report, butyric acid increased MCT1 expression and activity in human intestinal epithelial cells [6]. Similarly, increased butyric acid may have activated MCT1 and promoted SCFA absorption in the present study, through which the SCFA level may have returned to the pretreatment level at 8 weeks.
The present study demonstrated that 1-kestose activates not only Bifidobacterium, but also the butyric acid-producing genus, Megasphaera in the intestines of cats. Butyric acid levels increased after 4 weeks of the administration of 1-kestose. It is well known that butyrate exerts significant effects on the integrity of immunity as well as energy supply in intestinal epithelial cells. These results suggest that the administration of 1-kestose to cats activates beneficial intestinal bacteria, particularly butyrate-producing bacteria, and, thus, is expected to promote the well-being of cats by maintaining a healthy intestinal microbiota.
CONFLICTS OF INTEREST
Mikako Shinohara and Takumi Tochio are employees of B Food Science Co., Ltd., which is the producer of 1-kestose used in the present study. Masaharu Kiyosue and Seiji Kimura are employees of Nisshin Petfood Inc.
Supplementary
Acknowledgments
Mikako Shinohara: Methodology, Investigation, Data curation, Visualization, Writing draft preparation. Masaharu Kiyosue: Conceptualization, Methodology, Animal experiment, Investigation, Data curation. Takumi Tochio: Conceptualization, Methodology. Seiji Kimura: Animal experiment, Data curation. Yasuhiro Koga: Writing, Reviewing, and editing, Supervision.This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D. R., Fernandes G. R., Tap J., Bruls T., Batto J. M., Bertalan M., Borruel N., Casellas F., Fernandez L., Gautier L., Hansen T., Hattori M., Hayashi T., Kleerebezem M., Kurokawa K., Leclerc M., Levenez F., Manichanh C., Nielsen H. B., Nielsen T., Pons N., Poulain J., Qin J., Sicheritz-Ponten T., Tims S., Torrents D., Ugarte E., Zoetendal E. G., Wang J., Guarner F., Pedersen O., de Vos W. M., Brunak S., Doré J., Antolín M., Artiguenave F., Blottiere H. M., Almeida M., Brechot C., Cara C., Chervaux C., Cultrone A., Delorme C., Denariaz G., Dervyn R., Foerstner K. U., Friss C., van de Guchte M., Guedon E., Haimet F., Huber W., van Hylckama-Vlieg J., Jamet A., Juste C., Kaci G., Knol J., Lakhdari O., Layec S., Le Roux K., Maguin E., Mérieux A., Melo Minardi R., M’rini C., Muller J., Oozeer R., Parkhill J., Renault P., Rescigno M., Sanchez N., Sunagawa S., Torrejon A., Turner K., Vandemeulebrouck G., Varela E., Winogradsky Y., Zeller G., Weissenbach J., Ehrlich S. D., Bork P. and MetaHIT Consortium. 2011. Enterotypes of the human gut microbiome. Nature 473: 174–180. doi: 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin K., Bartges J., Buffington T., Freeman L. M., Grabow M., Legred J., Ostwald D., Jr.2010. AAHA nutritional assessment guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 46: 285–296. doi: 10.5326/0460285 [DOI] [PubMed] [Google Scholar]
- 3.Barko P. C., McMichael M. A., Swanson K. S., Williams D. A.2018. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 32: 9–25. doi: 10.1111/jvim.14875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry K. A., Wojcicki B. J., Middelbos I. S., Vester B. M., Swanson K. S., Fahey G. C., Jr.2010. Dietary cellulose, fructooligosaccharides, and pectin modify fecal protein catabolites and microbial populations in adult cats. J. Anim. Sci. 88: 2978–2987. doi: 10.2527/jas.2009-2464 [DOI] [PubMed] [Google Scholar]
- 5.Bouhnik Y., Flourié B., Riottot M., Bisetti N., Gailing M. F., Guibert A., Bornet F., Rambaud J. C.1996. Effects of fructo-oligosaccharides ingestion on fecal bifidobacteria and selected metabolic indexes of colon carcinogenesis in healthy humans. Nutr. Cancer 26: 21–29. doi: 10.1080/01635589609514459 [DOI] [PubMed] [Google Scholar]
- 6.Borthakur A., Saksena S., Gill R. K., Alrefai W. A., Ramaswamy K., Dudeja P. K.2008. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: involvement of NF-kappaB pathway. J. Cell. Biochem. 103: 1452–1463. doi: 10.1002/jcb.21532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown A. J., Goldsworthy S. M., Barnes A. A., Eilert M. M., Tcheang L., Daniels D., Muir A. I., Wigglesworth M. J., Kinghorn I., Fraser N. J., Pike N. B., Strum J. C., Steplewski K. M., Murdock P. R., Holder J. C., Marshall F. H., Szekeres P. G., Wilson S., Ignar D. M., Foord S. M., Wise A., Dowell S. J.2003. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278: 11312–11319. doi: 10.1074/jbc.M211609200 [DOI] [PubMed] [Google Scholar]
- 8.Canfora E. E., Jocken J. W., Blaak E. E.2015. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 11: 577–591. doi: 10.1038/nrendo.2015.128 [DOI] [PubMed] [Google Scholar]
- 9.Cani P. D., Neyrinck A. M., Maton N., Delzenne N. M.2005. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like Peptide-1. Obes. Res. 13: 1000–1007. doi: 10.1038/oby.2005.117 [DOI] [PubMed] [Google Scholar]
- 10.Cani P. D., Knauf C., Iglesias M. A., Drucker D. J., Delzenne N. M., Burcelin R.2006. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 55: 1484–1490. doi: 10.2337/db05-1360 [DOI] [PubMed] [Google Scholar]
- 11.Capitán-Cañadas F., Ocón B., Aranda C. J., Anzola A., Suárez M. D., Zarzuelo A., de Medina F. S., Martínez-Augustin O.2016. Fructooligosaccharides exert intestinal anti-inflammatory activity in the CD4+ CD62L+ T cell transfer model of colitis in C57BL/6J mice. Eur. J. Nutr. 55: 1445–1454. doi: 10.1007/s00394-015-0962-6 [DOI] [PubMed] [Google Scholar]
- 12.Caporaso J. G., Lauber C. L., Walters W. A., Berg-Lyons D., Lozupone C. A., Turnbaugh P. J., Fierer N., Knight R.2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 108 Suppl 1: 4516–4522. doi: 10.1073/pnas.1000080107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., Macfarlane G. T.1987. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28: 1221–1227. doi: 10.1136/gut.28.10.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deusch O., O’Flynn C., Colyer A., Swanson K. S., Allaway D., Morris P.2015. A Longitudinal Study of the Feline Faecal Microbiome Identifies Changes into Early Adulthood Irrespective of Sexual Development. PLoS One 10: e0144881. doi: 10.1371/journal.pone.0144881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edgar R. C., Haas B. J., Clemente J. C., Quince C., Knight R.2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27: 2194–2200. doi: 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endo A., Nakamura S., Konishi K., Nakagawa J., Tochio T.2016. Variations in prebiotic oligosaccharide fermentation by intestinal lactic acid bacteria. Int. J. Food Sci. Nutr. 67: 125–132. doi: 10.3109/09637486.2016.1147019 [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Mazcorro J. F., Barcenas-Walls J. R., Suchodolski J. S., Steiner J. M.2017. Molecular assessment of the fecal microbiota in healthy cats and dogs before and during supplementation with fructo-oligosaccharides (FOS) and inulin using high-throughput 454-pyrosequencing. PeerJ 5: e3184. doi: 10.7717/peerj.3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gueimonde M., Tölkkö S., Korpimäki T., Salminen S.2004. New real-time quantitative PCR procedure for quantification of bifidobacteria in human fecal samples. Appl. Environ. Microbiol. 70: 4165–4169. doi: 10.1128/AEM.70.7.4165-4169.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handl S., Dowd S. E., Garcia-Mazcorro J. F., Steiner J. M., Suchodolski J. S.2011. Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol. Ecol. 76: 301–310. doi: 10.1111/j.1574-6941.2011.01058.x [DOI] [PubMed] [Google Scholar]
- 20.Hidaka H., Eida T., Takizawa T., Tokunaga T., Tashiro Y.1986. Effects of Fructooligosaccharides on Intestinal Flora and Human Health. Bifidobact. Microflora 5: 37–50. doi: 10.12938/bifidus1982.5.1_37 [DOI] [Google Scholar]
- 21.Hisada T., Endoh K., Kuriki K.2015. Inter- and intra-individual variations in seasonal and daily stabilities of the human gut microbiota in Japanese. Arch. Microbiol. 197: 919–934. doi: 10.1007/s00203-015-1125-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashizume K., Tsukahara T., Yamada K., Koyama H., Ushida K.2003. Megasphaera elsdenii JCM1772T normalizes hyperlactate production in the large intestine of fructooligosaccharide-fed rats by stimulating butyrate production. J. Nutr. 133: 3187–3190. doi: 10.1093/jn/133.10.3187 [DOI] [PubMed] [Google Scholar]
- 23.Kanakupt K., Vester Boler B. M., Dunsford B. R., Fahey G. C., Jr.2011. Effects of short-chain fructooligosaccharides and galactooligosaccharides, individually and in combination, on nutrient digestibility, fecal fermentative metabolite concentrations, and large bowel microbial ecology of healthy adults cats. J. Anim. Sci. 89: 1376–1384. doi: 10.2527/jas.2010-3201 [DOI] [PubMed] [Google Scholar]
- 24.Kaplan H., Hutkins R. W.2000. Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl. Environ. Microbiol. 66: 2682–2684. doi: 10.1128/AEM.66.6.2682-2684.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasai C., Sugimoto K., Moritani I., Tanaka J., Oya Y., Inoue H., Tameda M., Shiraki K., Ito M., Takei Y., Takase K.2015. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 15: 100. doi: 10.1186/s12876-015-0330-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koga Y., Tokunaga S., Nagano J., Sato F., Konishi K., Tochio T., Murakami Y., Masumoto N., Tezuka J. I., Sudo N., Kubo C., Shibata R.2016. Age-associated effect of kestose on Faecalibacterium prausnitzii and symptoms in the atopic dermatitis infants. Pediatr. Res. 80: 844–851. doi: 10.1038/pr.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Poul E., Loison C., Struyf S., Springael J. Y., Lannoy V., Decobecq M. E., Brezillon S., Dupriez V., Vassart G., Van Damme J., Parmentier M., Detheux M.2003. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278: 25481–25489. doi: 10.1074/jbc.M301403200 [DOI] [PubMed] [Google Scholar]
- 28.Mao B., Li D., Zhao J., Liu X., Gu Z., Chen Y. Q., Zhang H., Chen W.2015. Metagenomic insights into the effects of fructo-oligosaccharides (FOS) on the composition of fecal microbiota in mice. J. Agric. Food Chem. 63: 856–863. doi: 10.1021/jf505156h [DOI] [PubMed] [Google Scholar]
- 29.Marounek M., Fliegrova K., Bartos S.1989. Metabolism and some characteristics of ruminal strains of Megasphaera elsdenii. Appl. Environ. Microbiol. 55: 1570–1573. doi: 10.1128/AEM.55.6.1570-1573.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muyzer G., de Waal E. C., Uitterlinden A. G.1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59: 695–700. doi: 10.1128/AEM.59.3.695-700.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura S., Kondo N., Yamaguchi Y., Hashiguchi M., Tanabe K., Ushiroda C., Kawahashi-Tokuhisa M., Yui K., Miyakoda M., Oku T.2014. Daily feeding of fructooligosaccharide or glucomannan delays onset of senescence in SAMP8 mice. Gastroenterol. Res. Pract. 2014: 303184. doi: 10.1155/2014/303184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oku T., Nakamura M., Hashiguchi-Ishiguro M., Tanabe K., Nakamura S.2009. Bioavailability and Laxative Threshold of 1-kestose in Human Adults. Dyn. Biochem. Process Biotechnol. Mol. Biol. 3: 90–95. [Google Scholar]
- 33.Ose R., Hirano K., Maeno S., Nakagawa J., Salminen S., Tochio T., Endo A.2018. The ability of human intestinal anaerobes to metabolize different oligosaccharides: Novel means for microbiota modulation? Anaerobe 51: 110–119. doi: 10.1016/j.anaerobe.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 34.Reagan-Shaw S., Nihal M., Ahmad N.2008. Dose translation from animal to human studies revisited. FASEB J. 22: 659–661. doi: 10.1096/fj.07-9574LSF [DOI] [PubMed] [Google Scholar]
- 35.Ritchie L. E., Steiner J. M., Suchodolski J. S.2008. Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis. FEMS Microbiol. Ecol. 66: 590–598. doi: 10.1111/j.1574-6941.2008.00609.x [DOI] [PubMed] [Google Scholar]
- 36.Rossi M., Corradini C., Amaretti A., Nicolini M., Pompei A., Zanoni S., Matteuzzi D.2005. Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study of pure and fecal cultures. Appl. Environ. Microbiol. 71: 6150–6158. doi: 10.1128/AEM.71.10.6150-6158.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sommer F., Anderson J. M., Bharti R., Raes J., Rosenstiel P.2017. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 15: 630–638. doi: 10.1038/nrmicro.2017.58 [DOI] [PubMed] [Google Scholar]
- 38.Song Y., Liu C., Finegold S. M.2004. Real-time PCR quantitation of clostridia in feces of autistic children. Appl. Environ. Microbiol. 70: 6459–6465. doi: 10.1128/AEM.70.11.6459-6465.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Songjinda P., Nakayama J., Tateyama A., Tanaka S., Tsubouchi M., Kiyohara C., Shirakawa T., Sonomoto K.2007. Differences in developing intestinal microbiota between allergic and non-allergic infants: a pilot study in Japan. Biosci. Biotechnol. Biochem. 71: 2338–2342. doi: 10.1271/bbb.70154 [DOI] [PubMed] [Google Scholar]
- 40.Souza D. D. S., Tahan S., Weber T. K., Araujo-Filho H. B., de Morais M. B.2018. Randomized, Double-Blind, Placebo-Controlled Parallel Clinical Trial Assessing the Effect of Fructooligosaccharides in Infants with Constipation. Nutrients 10: E1602. doi: 10.3390/nu10111602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suchodolski J. S., Foster M. L., Sohail M. U., Leutenegger C., Queen E. V., Steiner J. M., Marks S. L.2015. The fecal microbiome in cats with diarrhea. PLoS One 10: e0127378. doi: 10.1371/journal.pone.0127378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki N., Aiba Y., Hiroyuki T., Fukumori Y., Koga Y.2006. Superiority of 1-kestose, the Smallest Fructo-oligosaccharide, to a Synthetic Mixture of Fructo-oligosaccharides in the Selective Stimulating Activity in Bifidobacteria. Biosci. Microflora 25: 109–116. doi: 10.12938/bifidus.25.109 [DOI] [Google Scholar]
- 43.Takahashi S., Tomita J., Nishioka K., Hisada T., Nishijima M.2014. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS One 9: e105592. doi: 10.1371/journal.pone.0105592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi S., Yoshida Y., Nakanishi N., Tsukahara T., Ushida K.2008. Quantitative real-time PCR monitoring of Escherichia coli and Clostridium perfringens with oral administration of Lactobacillus plantarum strain Lq80 to weaning piglets. Anim. Sci. 79: 737–744. doi: 10.1111/j.1740-0929.2008.00588.x [DOI] [Google Scholar]
- 45.Tochio T., Kitaura Y., Nakamura S., Sugawa C., Takahashi M., Endo A., Shimomura Y.2016. An alteration in the cecal microbiota composition by feeding of 1-kestose results in a marked increase in the cecal butyrate content in rats. PLoS One 11: e0166850. doi: 10.1371/journal.pone.0166850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu G. D., Chen J., Hoffmann C., Bittinger K., Chen Y. Y., Keilbaugh S. A., Bewtra M., Knights D., Walters W. A., Knight R., Sinha R., Gilroy E., Gupta K., Baldassano R., Nessel L., Li H., Bushman F. D., Lewis J. D.2011. Linking long-term dietary patterns with gut microbial enterotypes. Science 334: 105–108. doi: 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida Y., Tsukahara T., Ushida K.2009. Oral administration of Lactobacillus plantarum Lq80 and Megasphaera elsdenii iNP-001 induces efficient recovery from mucosal atrophy in the small and the large intestines of weaning piglets. Anim. Sci. J. 80: 709–715. doi: 10.1111/j.1740-0929.2009.00692.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.