Abstract
Background
During antiretroviral treatment (ART) with plasma HIV RNA below the limit of quantification, HIV RNA can be detected in genital or rectal secretions, termed discordant shedding (DS). We hypothesized that proliferating cells produce virions without HIV replication.
Methods
ART-naive Peruvians initiating ART were observed for DS over 2 years. HIV env and pol genomes were amplified from DS. Antiretrovirals and cytokines/chemokines concentrations were compared at DS and control time points.
Results
Eighty-two participants had ART suppression. DS was detected in 24/82 (29%) participants: 13/253 (5%) cervicovaginal lavages, 20/322 (6%) seminal plasmas, and 6/85 (7%) rectal secretions. HIV RNA in DS specimens was near the limit of quantification and not reproducible. HIV DNA was detected in 6/13 (46%) DS cervicovaginal lavages at low levels. Following DNase treatment, 5/39 DS specimens yielded HIV sequences, all without increased genetic distances. Women with and without DS had similar plasma antiretroviral levels and DS in 1 woman was associated with inflammation.
Conclusions
HIV RNA and DNA sequences and therapeutic antiretroviral plasma levels did not support HIV replication as the cause of DS from the genital tract. Rather, our findings infer that HIV RNA is shed due to proliferation of infected cells with virion production.
Keywords: genital HIV shedding, HIV phylogenetics, drug levels, inflammation
Findings suggest that, during ART suppression, discordant shedding of HIV in genital secretions arises from HIV-infected cells producing virions near the limit of quantification in association with infected cell proliferation. HIV replication isolated to the genital tract was not detected.
Discordant shedding (DS) is defined as episodic detection of human immunodeficiency virus (HIV) RNA in the genital tract when not detected in the plasma due to antiretroviral therapy (ART) suppression of viral replication. While suppression of HIV replication in the plasma successfully reduces the risk of HIV transmission, understanding the mechanisms causing DS remains relevant because DS, which has been observed in 0%–50% of HIV-infected people [1–13], has been attributed to virus replication [12, 14]. HIV replication during ART can select drug-resistant variants, leading to virologic failure [15–17]. Factors associated with DS include suboptimal tissue levels of antiretrovirals [18, 19], low pre-ART CD4+ cell counts, high pre-ART plasma HIV RNA, genital tract coinfections, and genital ulceration [8, 10, 12, 20–22], inflammation [9, 14], or trauma [23, 24]. Given that genetically identical HIV variants have been observed in longitudinal specimens from the uterine cervix during ART suppression [25, 26], we hypothesized that clones of infected cells that express RNA from integrated proviruses can produce DS. However, infection of additional cells by these virions, and hence full cycles of viral replication, would be blocked in individuals with adequate levels of antiretrovirals (ie, entry, reverse transcriptase, and integrase inhibitors). To evaluate whether DS is due to expression of virions from clones of infected cells, which is without known adverse consequences in adequately ART-suppressed individuals, or is from full cycles of HIV replication, which could lead to selection of drug-resistant variants and therapeutic failure, we prospectively followed ART-naive men and women initiating first-line nonnucleoside inhibitor-based ART in Lima, Peru for DS. Their specimens were analyzed to assess infected cell proliferation vs full cycles of virus replication, antiretroviral levels, and inflammation.
METHODS
Study Population and Design
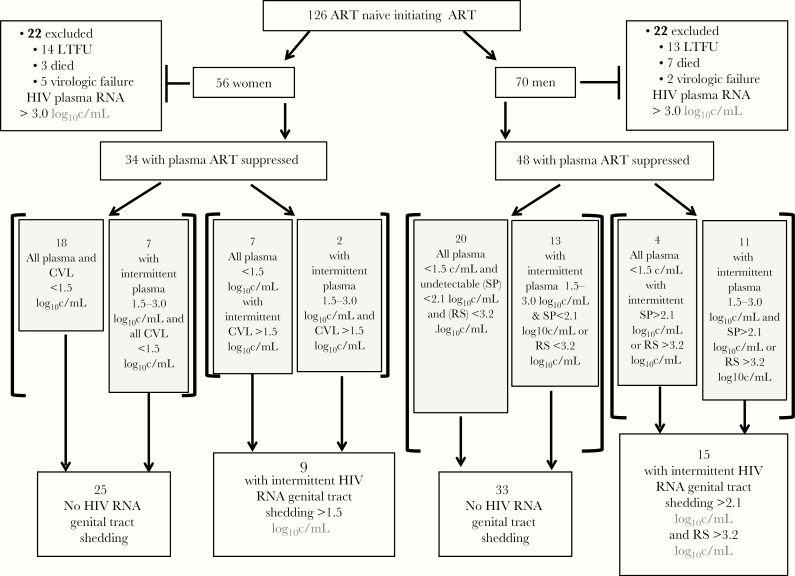
Blood, genital tract, and rectal secretion samples were collected from ART-naive HIV-infected men and women prior to and during ART. All participants provided written informed consent, as approved by Institutional Review Boards in Lima, Peru and Seattle, WA [27]. DS was assessed quarterly in participants completing 18–24 months with ART suppression. DS was defined as a cervicovaginal lavage (CVL), seminal plasma, or rectal secretions with HIV RNA levels above the limit of quantification, that is >1.48 log10 copies/mL, >2.08 log10 copies/mL, and >3.18 log10 copies/mL, respectively (Figure 1 and Supplementary Table 1). ART suppression was defined as a median plasma HIV RNA below the limit of quantification (<1.48 log10 copies/mL). Low-level plasma viremias (LLV) were defined as transiently detectable plasma HIV RNA between 1.5 and 3.0 log10 copies/mL in participants who met the definition of ART suppression. Virologic failure was defined as plasma HIV RNA >3.0 log10 copies/mL. At each study visit, participants completed a questionnaire that asked about genital symptoms and a genital examination was performed with findings recorded. Genital lesions or discharges were diagnosed and treated by participants’ clinicians and were not part of this study.
Figure 1.
Study schema and outcomes. A total of 126 HIV-infected ART-naive participants with AIDS-defining illness or a CD4+ T lymphocytes <250 cells/μL were enrolled into this observational cohort study to evaluate DS. Forty-four participants (22 women and 22 men) were excluded from the analyses of DS from the genital tract due to LTFU, death, or confirmed virologic failure (plasma HIV RNA ≥3.00 log10 c/mL). Eighty-nine of 126 participants (71%) who initiated first-line nevirapine-based ART completed 18–24 study months, and 82/89 (92%) had their median plasma HIV RNA suppressed to <1.48 log10 c/mL (ART suppressed). During the 2-year study, 24/82 (29%) ART suppressed participants had DS, defined as detectable HIV RNA either in CVL fluid at ≥1.48 log10 c/mL, or in SP ≥2.07 log10 c/mL, or in RS ≥3.18 log10 c/mL. Abbreviations: ART, antiretroviral treatment; c/mL, copies/mL; CVL, cervicovaginal lavage; DS, discordant shedding; HIV, human immunodeficiency virus; RS, rectal secretions; LTFU, loss to follow up; SP, seminal plasma.
Specimen Processing
Blood plasma and peripheral blood mononuclear cells (PBMC) were separated using Accuspin tubes (Sigma-Aldrich). Cell pellets and plasma fractions were stored at −80°C until nucleic acids were extracted. Genital specimens were collected at each study visit in the following order: cervical secretions with a swab (Copan) inserted in the os and rotated 360°; and CVL by washing the uterine cervix and vaginal walls with 10 mL 1 × phosphate-buffered saline and aspirating the fluid from the vaginal fornix, pelleting cells, then aliquoting supernatant [26]. Semen was collected by masturbation, with separation of seminal plasma and seminal cells after liquification [28], and rectal secretions by filter-paper strips (TearFlo; HUB Pharmaceuticals) held against the rectal mucosa absorbing fluid to the shoulder [28].
HIV RNA and DNA Extraction and Quantification
Nucleic acids were extracted from plasma, CVL, and seminal plasma after 1:5 dilution with Roswell Park Memorial Institute medium (RPMI; MilliporeSigma), and from rectal secretions diluted 1:21 using silica-based methods (Supplementary Table 1) [29]. DNA was extracted from PBMC and from seminal cell pellets, and CVL supernatants when DS was detected using a Gentra 5 PRIME kit (Gentra Systems).
HIV RNA was quantified in duplicate by a real-time polymerase chain reaction (rtPCR) amplifying HIV gag [28] without DNAse treatment. To assess the reproducibility of detection of DS by HIV RNA PCR, CVL samples were tested by 2 additional methods: (1) gag rtPCR and (2) RealTime HIV-1 Viral Load Assay (Abbott). HIV DNA was quantified by rtPCR of the HIV long terminal repeat (LTR) [30] in pre-ART and ART-suppressed PBMC, and in CVL specimens using rtPCR of gag [31] (Supplementary Table 2).
Single-Genome Amplification and Phylogenetic Analysis
Single-genome amplification (SGA) of HIV env C2-V5 region and pol [25] was performed after treatment with DNase (Ambion Turbo DNA-free; Life Technologies) on pre-ART PBMC and DS specimens (cervical swab, seminal plasma, and rectal secretions). Sequences were aligned in MUSCLE to reference sequences and submitted to DIVEIN [32] for construction of maximum likelihood trees with bootstrapping and analyzed by approximate likelihood ratio test [26, 33]. Sequences were submitted to GenBank (KU740361-KU743103 and KX148403-KX148462; Supplementary Material).
Nevirapine and Efavirenz Levels
A reversed-phase liquid chromatography tandem mass spectrometry assay was used to quantify nevirapine (NVP) and efavirenz (EFV) levels in plasma and CVL (dynamic range, 10–15 000 ng/mL) [34]. Therapeutic drug levels were >3000 ng/mL for NVP [35] and >1000 ng/mL for EFV [36].
Immune Biomarkers
Inflammatory cytokines and chemokines were measured in 50 μL of CVL (Bio-Plex Pro Human Chemokines; BioRad). Inflammatory cytokines included: interleukin-1β (IL-1β), IL-2, IL-4, IL-6, IL-8, IL-10, granulocyte-macrophage colony stimulating factor (GM-CSF), tumor necrosis factor-α (TNF-α), interferon-γ (IFN- γ), IFN-γ induced protein-10 (IP-10), macrophage inflammatory protein-1α (MIP-1α), and MIP-1β. Chemokines included: eotaxin, fractalkine, CXCL-1 (GRO), monocyte chemoattractant protein-1 (MCP-1), MCP-3, macrophage-derived chemokine (MDC).
Statistical Analysis
Mann-Whitney test was used to evaluate continuous variables and Fisher exact test for categorical variables (Prism GraphPad QuickCalcs). Spearman correlation coefficient was used to compare HIV RNA to DNA within CVL. Drug levels (log10 transformed) in plasma and CVL, and cytokines and chemokines in CVL, were compared between participants with and without DS using generalized estimating equations to account for multiple comparisons (Stata SE V12.1; StataCorp). All tests were 2-sided with P values < .05 considered significant.
RESULTS
DS Rates, Correlates, and Reproducibility of Detection
The study enrolled 126 participants; 89 completed 18–24 study months, with 82 participants (34 women/48 men) achieving ART suppression. Prior to initiating ART, HIV RNA was detected in CVL of 25/34 (74%) women and in the seminal plasma of 43/48 (90%) and rectal secretions of 15/17 (88%) men. A median of 9 genital tract specimens per participant were analyzed (interquartile range [IQR], 8–9 study visits, including pre-ART at study entry). DS was detected in 24/82 (29%) participants (9 women/15 men) at 39/575 (7%) time points. The frequency of study visits with DS detected was similar across specimens: CVL 13/253 (5%), seminal plasma 20/322 (6%), and rectal secretions 6/85 (7%).
LLV were detected in 33/82 (40%) participants [33] at 49/575 (9%) study visits, with a median HIV RNA of 1.86 log10 copies/mL (IQR, 1.60–2.14 log10 copies/mL). Episodes of LLV (n = 49) occurred at an increased frequency at time points of specimens with vs without DS (7/39 [18%] vs 42/536 [8%)], respectively; P = .04). Among 24 participants with DS, concomitant DS and LLV were detected in 7/24 (29%) of participants on 1 occasion (female participant number 89; male participant numbers 21, 39, 56, 71, 78, and 91) (Supplementary Figure 1).
Analysis of pre-ART samples found that female participants with vs without DS had higher HIV RNA in CVL and male participants had higher HIV RNA in rectal secretions. Those with vs without DS were younger in both sexes (Table 1).
Table 1.
CD4+ T-Cell Counts and HIV Load in Participants Prior to Initiation of ART by Whether Discordant Shedding Was or Was Not Detected During ART Suppression
| Characteristic | With DS, median (IQR)(Women n = 9; Men n = 15)a | Without DS, median (IQR)(Women n = 25; Men n = 33)a | P Value |
|---|---|---|---|
| Women | |||
| Age, y | 29 (24–32) | 35 (30–41) | .02b |
| Plasma HIV RNA, log10 copies/mL | 5.18 (4.62–5.80) | 5.09 (4.62–5.53) | .51 |
| CD4+, T cells/μL | 132 (83–228) | 133 (42–206) | .56 |
| HIV DNA in PBMC, log10 copies/106 cells | 3.02 (2.67–3.28) | 3.33 (3.08–3.49) | .07 |
| Cervicovaginal lavage HIV RNA, log10 copies/mL | 3.27 (1.79–4.22) | 1.96 (1.00–2.72) | .03 |
| Men | |||
| Age, y | 29 (27–37) | 37 (31–41) | .03 |
| CD4+, T cells/μL | 110 (48–204) | 123 (41–196) | .99 |
| Plasma HIV RNA, log10 copies/mL | 5.34 (4.84–5.87) | 5.43 (4.87–5.95) | .38 |
| HIV DNA in PBMC, log10 copies/106 cells | 3.44 (3.15–3.62) | 3.20 (2.80–3.50) | .55 |
| Seminal plasma HIV RNA, log10 copies/mL | 4.08 (3.43–5.08) | 4.24 (3.64–5.12) | .51 |
| Rectal secretions HIV RNA, log10 copies/mL | 5.08 (4.18–5.38) | 6.00 (6.00–6.00) | .04 |
Abbreviations: ART, antiretroviral treatment; DS, discordant shedding; HIV, human immunodeficiency virus; LTR, long terminal repeat; PBMC, peripheral blood mononuclear cells.
aMen without DS contributed fewer specimens to the following: HIV DNA PBMC log10 copies/106 cells n = 33; seminal plasma HIV RNA n = 32; TearFlow secretions n = 11; and for those with DS: HIV DNA PBMC LTR log10 copies/106 cells n = 13; seminal plasma HIV RNA n = 13; rectal secretions n = 3.
bP value that are considered statistically significant are indicated in bold.
During ART suppression, HIV RNA in the 39 DS specimens were at or near the limit of quantification. Repeat testing of the 13 DS CVL for reproducibility detected HIV RNA in the second test of 9/13 (69%) specimens by the gag rtPCR, and significantly fewer (2/13 [16%]; P < .001) by the RealTime HIV-1 assay (Supplementary Table 2). Among women with DS detected during the study, abnormal genital tract findings included a whitish discharge in 3 and a genital ulcer in a fourth participant. These findings were observed more frequently at time points with vs without DS (4/13 vs 0/51; P = .002). Volumes of seminal plasma and rectal secretions were insufficient to assess reproducibility of HIV RNA levels. Abnormal findings among men with DS detected during the study included hemorrhoids in 2 and warts in a third. These abnormalities were not associated with their specimens with vs without DS (1/26 [4%] vs 2/85 [2.4%], respectively; P = .55).
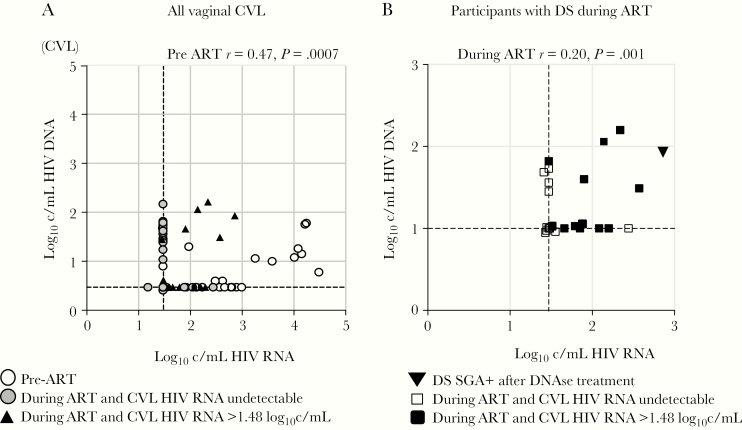
Because neither the gag rtPCR or RealTime HIV-1 assays include DNase, HIV DNA present in these specimens would be quantified along with HIV RNA and could result in erroneously detected or inflated HIV RNA levels. Testing of DS CVL detected HIV DNA in 6/13 (46%) specimens at low levels (median, 1.8 log10 copies/mL; IQR, 1.6–2.1) (Supplementary Table 2). CVL HIV DNA correlated with HIV RNA prior to starting ART (r = 0.47; P < .001) and during ART (r = 0.20; P = .001; Figure 2).
Figure 2.
HIV RNA and DNA levels in CVL of female participants. A, HIV RNA and DNA quantified in all CVL by gag rtPCR, including 34 pre-ART (white) and first value from 253 CVL collected during ART classified as DS (black) or not DS (grey). HIV DNA correlated with RNA levels across all pre-ART (ρ, .47; P = .0007) and during ART (ρ, .20; P = .001). B, HIV RNA and DNA levels in CVL from 9 participants with 13 episodes of DS (black squares and triangle) and without DS (white squares). Triangle indicates the single DS specimen from participant 86 that following DNase treatment yielded 17 HIV RNA env sequences. HIV RNA and DNA below the limit of quantification from multiple specimens are stacked in the lower left corner of the graph. HIV DNA correlated with RNA levels in these 13 DS specimens (r = 0.16; P = .02). The correlation between HIV RNA and HIV DNA values in DNase-untreated specimens, and the inability to amplify HIV RNA sequences following DNase treatment of CVL, suggest that HIV DNA detected in 6 of the specimens accounted for a significant fraction of nucleic acids quantified as “HIV RNA” by the real-time gag assay, and that these episodes of DS could have been false positives. Abbreviations: ART, antiretroviral treatment; c/mL, copies/mL; CVL, cervicovaginal lavage; DS, discordant shedding; HIV, human immunodeficiency virus; rtPCR, real-time polymerase chain reaction; SGA, single-genome amplification.
Single Genome Amplification of HIV env and pol
Sequences were derived by SGA from pre-ART genital tract specimens with detectable HIV RNA at approximately the rate estimated by rtPCR (Supplementary Table 2).
DS specimens yielded HIV env and/or pol SGA sequences from 5/39 (13%) specimens from 4/24 participants, as follows: 0/13 (0%) CVL, 1/13 (8%) cervical swabs, 2/20 (10%) seminal plasma, and 2/6 (17%) rectal secretions. The 5 specimens yielding sequences were from participant 54 (seminal plasma, pol n = 1, env n = 0), 2 time points from participant 55 (rectal secretions pol n = 1 from 1 time point, env n = 1 from a separate time point), from participant 71 (seminal plasma, pol n = 33, env n = 14), and participant 86 (endocervical swab pol n = 2, env n = 17); genital tract HIV RNA values were 2.78, 3.26, 4.40, 3.15, and 2.86 log10 copies/mL, respectively. HIV RNA in plasma corresponding to these DS specimens was undetectable (participants 54 and 55), below the limit of quantification (participant 86), or low (1.98 log10 copies/mL, participant 71).
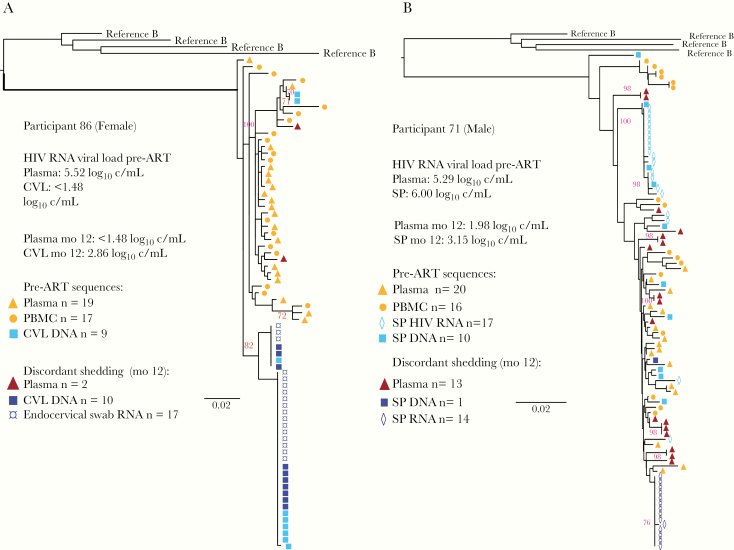
HIV RNA env sequences (n = 17) from participant 86 DNase-treated DS cervical swab included 2 clades, with identical sequences from both cervical swab RNA and CVL DNA (Figure 3); all were hypermutated with multiple stop codons, did not diverge from her most recent common ancestor of infection, and were identical to pre-ART CVL DNA sequences. The pol sequences (n = 2) did not have mutations associated with drug resistance. DS DNase-treated seminal plasma HIV env sequences (n = 14) from participant 71 were identical (Figure 3), and the pol sequences (n = 33) clustered in a monotypic clade that did not diverge from pre-ART seminal plasma sequences, with no drug-resistance mutations. Single pol sequences from participants 54 and 55 DNase-treated specimens had no drug-resistance–associated mutations.
Figure 3.
Phylogenetic analyses of HIV env sequences from pre-ART and time of DS from genital tract. HIV SGA sequences from the blood and genital tract specimens collected pre-ART and during episodes of DS are shown for female participant 86 (A), and male participant 71 (B), whose DS specimens yielded multiple HIV RNA sequences after DNAse treatment. In both cases the HIV RNA sequences derived from cervical swab or seminal plasma during DS episodes were identical to sequences from pre-ART specimens and did not diverge from the most recent common ancestor of infection compared to sequences from pre-ART specimens; this suggests that a proliferating cell clone may have produced these virions. HIV DNA sequences derived from seminal cell pellet and cervicovaginal lavage collected at time of DS were monotypic in the female participant 86 and too few to assess from the male participant 71. All HIV RNA and DNA sequences from participant 86 samples were hypermutated and most likely were not replication competent. HIV RNA was detected in participant 71 plasma at a low level, a “blip” of 1.98 log10 copies/mL, concurrent with detection of genital shedding, whereas in participant 86 plasma viral load was undetectable at the time of DS. Sequences were rooted using representative HIV-1 subtype B sequences from the GenBank database (clade B: B.US.83.RF, B.US.90.WEAU160, B.FR.83.HXB2, B.US.86.JRFL). Maximum likelihood trees were generated in DIVEIN with bootstrap values estimated by approximate likelihood ratio test. Bootstrap values ≥70 are indicated in red. Scale bars indicate number of substitutions per site. Abbreviations: ART, antiretroviral treatment; c/mL, copies/mL; CVL, cervicovaginal lavage; DS, discordant shedding; HIV, human immunodeficiency virus; mo, month after ART initiated; PBMC, peripheral blood mononuclear cells; SGA, single-genome amplification.
Antiretroviral Levels in CVL
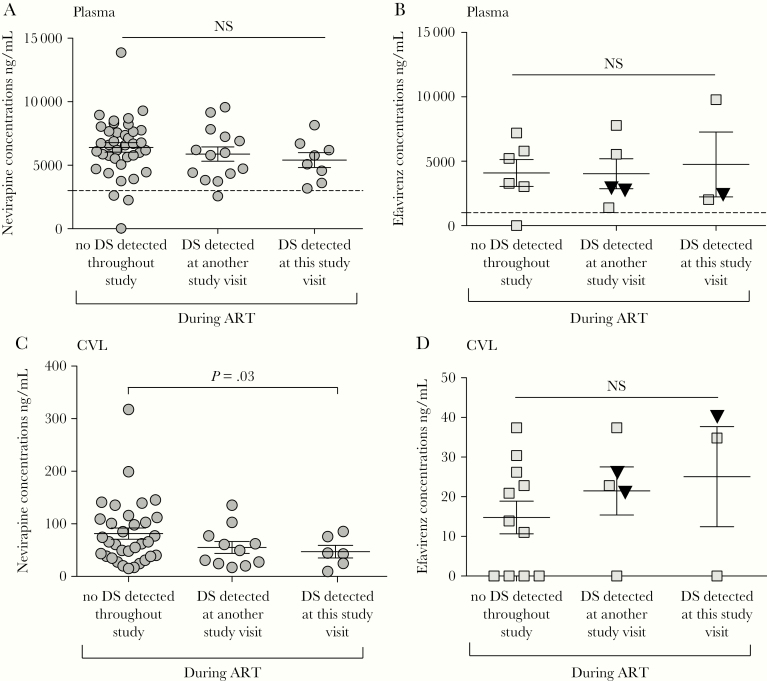
Women with DS included 5 prescribed NVP-based ART and 3 EFV-based ART; 1 of the latter developed a rash and switched to atazanavir/rt. NVP plasma levels in participants with DS did not differ significantly at time points with vs without DS (n = 8 vs 14 time points, respectively), nor compared to specimens from comparable study time points (n = 38) from participants in whom DS was not detected during the study (Figure 4A). Plasma NVP levels were below therapeutic ranges in specimens from 4 women (1 participant with discordant shedding at a study visit when plasma HIV RNA was undetectable and 3 with no DS detected during the study). Plasma EFV levels similarly were not different between participants with vs without DS (Figure 4B). Plasma EFV levels were below the therapeutic range in 1 woman without DS. Of note, all 4 participants with HIV RNA amplified and sequenced from DS specimens had plasma antiretroviral levels in the therapeutic range.
Figure 4.
Comparison of NVP and EFV levels in plasma and CVL of females with vs without DS during ART. NVP (circles) and EFV (squares) levels measured in plasma (A and B) and CVL (C and D) by tandem mass spectrometry are shown relative to lower limit of therapeutic levels for NVP 3000 ng/mL and EFV 1000 ng/mL in the plasma (dashed lines). Median NVP plasma levels did not differ significantly between time points when DS was vs was not detected, nor did levels differ from matched participants without DS detected. Fewer participants took EFV, yielding too few values for a meaningful comparison. Therapeutic EFV plasma levels in female participant 86 with multiple HIV RNA templates amplified from genital tract DS specimen are indicated by inverted triangles. CVL NVP and EFV levels were all below the therapeutic levels of these antiretrovirals due to dilution of cervicovaginal secretions with 10 mL saline. Abbreviations: ART, antiretroviral treatment; CVL, cervicovaginal lavage; DS, discordant shedding; EFV, efavirenz; NS, not significant; NVP, nevirapine.
Nevirapine and EFV CVL levels were all below therapeutic ranges as derived from 10 mL saline irrigations (approximately 10× dilution of cervical secretions; Figure 4C and 4D). However, among those who took NVP-based ART, levels were slightly lower in those with vs without DS.
Immune Biomarkers in CVL
CVL cytokines/chemokines concentrations generally did not significantly differ between women with vs without DS (Supplementary Figure 2). Of note, elevated outliers include cytokines/chemokines in specimens from participant 86 at a study visit with DS detected (her study month 9 results, red symbols) when sequences were amplified, specifically GM-CSF, sCD40L, VEGF, MIP1-α, IL-1β, IL-6, IL-8, IL-10, IL-12p40, and IP-10 (Supplementary Figure 3).
DISCUSSION
This study contributes the following novel findings that reflect on the mechanisms and significance of episodic DS of HIV from the genital tract and rectum: (1) HIV RNA levels detected as DS were primarily just above the limit of quantification, in the range that viruses are stochastically detected or signal is derived from off-target amplification [37]; (2) HIV DNA was detected at higher levels in CVL specimens with vs without DS and were positively associated with HIV RNA levels, suggesting that HIV DNA from lysed cells could be misconstrued as HIV RNA; (3) multiple monotypic HIV RNA env and pol sequences were amplified from a small subset of DS specimens that were identical to monotypic HIV DNA sequences, which is consistent with virion production by proliferating cells; (4) the DS specimen from a female participant yielding the most viral RNA sequences had elevated chemokines and inflammatory cytokines, suggesting that inflammation may contribute to or be caused by DS; (5) DS was associated with concomitant LLV, which is expected if the participant is experiencing episodic viral replication or expansion of a clone of HIV-infected cells; and (6) antiretroviral levels in plasma did not differ between those with vs without DS and were therapeutic in all participants with detectable DS, suggesting that HIV shedding from the genital tract was not due to ART nonadherence; and, lastly, (7) lower antiretroviral levels were found in CVL from those with DS, which may be due to greater dilution of antiretrovirals by increased genital tract secretions associated with inflammation, or simply by chance as CVL were variably diluted as assessed by volume of lavage fluids recovered.
The prevalence of DS detected in our population (29%) was similar to other studies (0%–50%), as were HIV RNA levels in DS specimens [1–13, 38, 39]. HIV RNA was not reproducibly detected in our DS specimens, which combined with our and others’ mostly failed attempts to sequence HIV RNA from DS specimens [1, 9], suggests stochastic detection of rare HIV RNA templates near the limit of quantification or off target amplification (ie, false-positive reactions) [37]. HIV DNA was detected in a subset of DS specimens. As commercial and the laboratory-validated HIV RNA quantification assays we employed did not include DNase treatment of the specimens prior to quantification, the HIV DNA in the DS specimens likely contributed in part or in full to the “HIV RNA” detected in DS CVL specimens [1, 6, 7, 9, 39, 40]. This is further supported by our finding that HIV RNA correlated with DNA levels across CVL specimens, and could explain why after DNase treatment amplification of HIV RNA templates was rare from DS specimens.
Other studies of DS of HIV from the vagina evaluated specimens for both HIV RNA and DNA [7, 38, 40, 41], as performed in this study, and noted that detection of HIV DNA correlated with white blood cells collected using swabs [40]. In another study [38], HIV DNA was detected less frequently compared to this study, most likely due, at least in part, to collection of cervical secretions using TearFlo filter paper strips, which is less likely to collect cellular material compared to CVL. Of note, CVL in the current study was not clarified of cells by centrifugation after sample collection, which likely contributed to the higher frequency of HIV DNA detection.
While others have attempted to sequence HIV RNA templates from DS specimens [1, 9], our review of the literature suggests we were the first to succeed, albeit modestly. There were only 2 genital specimens from episodes of DS that yielded >10 HIV env and pol sequences. Both bore multiple monotypic HIV RNA and DNA sequences, which supports our hypothesis that cell clones produced virions detected as DS. Although, without sequencing of the associated HIV integration sites to substantiate a clonal population, it could be argued that these sequences came from a burst of viral replication. Importantly, DS HIV RNA sequences from 1 participant were hypermutated, suggesting APOBEC modifications rendered these variants defective and incapable of full cycles of viral replication. The second participant (number 71) had HIV RNA concomitantly detected in both blood and seminal plasma. His blood yielded diverse sequences, consistent with HIV replication, that intermingled with pre-ART plasma sequences [42]. However, neither his seminal plasma env or pol sequences diverged from his pre-ART sequences, nor had drug-resistance mutations. Across participants, LLV were detected at a slightly, but significantly, greater frequency in participants with vs without DS. The occurrence of both LLV and DS concomitantly is suggestive of nonadherence to ART [25, 43–46]. However, as we previously reported [33], these LLV had a low median HIV RNA (1.86 log10 copies/mL), most yielded monotypic sequences, and were associated with elevated plasma levels of high-sensitivity C-reactive protein and soluble CD163; all these factors are consistent with proliferation of HIV-infected clonal cell populations with production of virions. Furthermore, the absence of drug-resistance mutations in any of the 4 participants’ HIV pol sequences and therapeutic plasma antiretroviral levels at the DS specimen time points and consistently during our study, as has been reported by other studies [7, 11, 12], provides evidence against ART nonadherence as the cause of DS.
Low levels of NVP and EFV have been reported in both the male and female genital tract [13, 47]. While we assessed NVP and EFV in CVL, these measurements were compromised by variable dilution of each woman’s secretions. While CVL NVP levels appeared lower in women at study visits when DS was detected, their plasma levels did not differ significantly, which suggests that discordant shedders had less antiretrovirals reaching the genital tract, or more likely that antiretrovirals in the genital tract secretions were more dilute either as a stochastic event or by inflammation increasing the volume of secretions.
The woman whose DS cervical swabs yielded numerous HIV RNA and DNA sequences had proinflammatory markers (IL-1β, IL-6, IL-10, and IL-12p40) and chemotactic factors (IL-8, G-CSF, GM-CSF, IP10, MIP1α, MIP1β, sCD40L, TNF-α, and VEGF) in her CVL that were markedly elevated compared to other participants. These factors promote migration of inflammatory cells [48], and the pronounced increase in innate and T-cell chemoattractants perhaps led HIV-infected cells into the mucosa where these underwent proliferation and released HIV RNA.
Limitations of this study include that seminal plasma and rectal secretion volumes were insufficient to test the reproducibility of HIV RNA quantification, and quantify HIV DNA, antiretrovirals, or cytokines. Rectal secretions were collected from a minority of participants and the small volume collected resulted in a relatively high limit of quantification (Supplementary Table 1). CVL were compromised by variable dilution of cervicovaginal secretions without a standard analyte added for normalization. Quantification of HIV RNA in genital tract specimens without prior DNase treatment precluded accurate estimates of the true prevalence of DS of HIV RNA, which is also likely a limitation of other studies [1–10, 12, 28, 41, 49]. The amplification of HIV RNA sequences from relatively few DS specimens after DNase treatment may be perceived as a methodologic limitation. However, given the lack of reproducible quantification by 2 different HIV RNA quantification assays, and our use of a methods well established in our laboratory [33], suggest a paucity of HIV RNA templates in these specimens. Other limitations include sampling the genital tissues quarterly instead of more frequent sampling. ART adherence was reported to be high in this study and missed study visits infrequent in those with vs without DS (7/182 [4%] vs 7/352 [2%], respectively; P = .25). Lastly, the majority of males in the study refused to provide rectal specimens and often were only able to provide limited semen volumes.
In summary, our study suggests that DS can be falsely diagnosed due to amplification of HIV DNA in genital tract specimens. This is supported by the detection of HIV RNA/DNA at levels near the limit of quantification and that individuals with DS had greater pre-ART HIV RNA levels in CVL or rectal secretions but not in their plasma, suggesting that cells in the vagina or rectum could have produced virions locally or HIV DNA in the cells could be misconstrued as HIV RNA. The following, multiple findings from our study contribute data that refute the hypothesis that HIV replication causes DS and support the hypothesis that DS is the result of HIV-infected cell proliferation with viral RNA transcription leading to the production of virions: (1) rare DS specimens yielded multiple sequences, which were clonal; (2) the DS specimen that yielded the most sequences were all hypermutated and likely noninfectious; (3) and this was associated with elevated levels of CVL chemokines and cytokines that could drive cellular proliferation; (4) amplified sequences did not reveal evolution or new HIV drug-resistance mutations; and (5) individuals with and without DS had similar levels of plasma antiretrovirals in the therapeutic range. Importantly, these findings also suggest that DS poses a negligible, if any, risk of HIV transmission and do not provide data counter to the axiom “undetectable (plasma) equals untransmittable” [50, 51].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge the men and women who participated in this study and the work of the clinical study personnel (Carmela Ganoza, Marcela Rodriguez, and Angie Roldan) who made this study possible; and we acknowledge the technical support from Kelli Kraft, Jillian Legard, and Jennifer McKernan-Mullin.
Financial support. This work was supported by the National Institute of Allergy and Infectious Disease (grant numbers R01 AI071212 and R01 AI091550 to L. M. F.); and the University of Washington Center for AIDS Research (grant numbers P30 AI027757, AI068636, and AI106701 to K. Holmes).
Potential conflicts of interest. C. M. is currently receiving funding from Merck Sharp & Dohme.
All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, WA, 13–16 February 2015.
References
- 1. Mayer KH, Boswell S, Goldstein R, et al. Persistence of human immunodeficiency virus in semen after adding indinavir to combination antiretroviral therapy. Clin Infect Dis 1999; 28:1252–9. [DOI] [PubMed] [Google Scholar]
- 2. Kovacs A, Wasserman SS, Burns D, et al. Determinants of HIV-1 shedding in the genital tract of women. Lancet 2001; 358:1593–601. [DOI] [PubMed] [Google Scholar]
- 3. Cu-Uvin S, Snyder B, Harwell JI, et al. Association between paired plasma and cervicovaginal lavage fluid HIV-1 RNA levels during 36 months. J Acquir Immune Defic Syndr 2006; 42:584–7. [DOI] [PubMed] [Google Scholar]
- 4. Nagot N, Ouedraogo A, Weiss HA, et al. ; Yerelon Study Group Longitudinal effect following initiation of highly active antiretroviral therapy on plasma and cervico-vaginal HIV-1 RNA among women in Burkina Faso. Sex Transm Infect 2008; 84:167–70. [DOI] [PubMed] [Google Scholar]
- 5. Sheth PM, Kovacs C, Kemal KS, et al. ; Toronto Mucosal Immunology Group Persistent HIV RNA shedding in semen despite effective antiretroviral therapy. AIDS 2009; 23:2050–4. [DOI] [PubMed] [Google Scholar]
- 6. Cu-Uvin S, DeLong AK, Venkatesh KK, et al. Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS 2010; 24:2489–97. [DOI] [PubMed] [Google Scholar]
- 7. Launay O, Tod M, Tschöpe I, et al. Residual HIV-1 RNA and HIV-1 DNA production in the genital tract reservoir of women treated with HAART: the prospective ANRS EP24 GYNODYN study. Antivir Ther 2011; 16:843–52. [DOI] [PubMed] [Google Scholar]
- 8. Homans J, Christensen S, Stiller T, et al. Permissive and protective factors associated with presence, level, and longitudinal pattern of cervicovaginal HIV shedding. J Acquir Immune Defic Syndr 2012; 60:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Politch JA, Mayer KH, Welles SL, et al. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS 2012; 26:1535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gianella S, Smith DM, Vargas MV, et al. Shedding of HIV and human herpesviruses in the semen of effectively treated HIV-1-infected men who have sex with men. Clin Infect Dis 2013; 57:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Osborne BJ, Sheth PM, Yi TJ, et al. Impact of antiretroviral therapy duration and intensification on isolated shedding of HIV-1 RNA in semen. J Infect Dis 2013; 207:1226–34. [DOI] [PubMed] [Google Scholar]
- 12. King CC, Ellington SR, Davis NL, et al. Prevalence, magnitude, and correlates of HIV-1 genital shedding in women on antiretroviral therapy. J Infect Dis 2017; 216:1534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kourtis AP, Wiener J, Hurst S, et al. Brief report: HIV shedding in the female genital tract of women on ART and progestin contraception: extended follow-up results of a randomized clinical trial. J Acquir Immune Defic Syndr 2019; 81:163–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spencer LY, Christiansen S, Wang CH, et al. Systemic immune activation and HIV shedding in the female genital tract. J Acquir Immune Defic Syndr 2016; 71:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 2010; 375:2092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eshleman SH, Wilson EA, Zhang XC, et al. Virologic outcomes in early antiretroviral treatment: HPTN 052. HIV Clin Trials 2017; 18:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thompson CG, Cohen MS, Kashuba AD. Antiretroviral pharmacology in mucosal tissues. J Acq Immun Def Synd 2013; 63(Suppl 2):S240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thompson CG, Gay CL, Kashuba ADM. HIV persistence in gut-associated lymphoid tissues: pharmacological challenges and opportunities. AIDS Res Hum Retroviruses 2017; 33:513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. LeGoff J, Weiss HA, Gresenguet G, et al. Cervicovaginal HIV-1 and herpes simplex virus type 2 shedding during genital ulcer disease episodes. AIDS 2007; 21:1569–78. [DOI] [PubMed] [Google Scholar]
- 21. Paz-Bailey G, Sternberg M, Lewis DA, et al. Comparison of lavage and swabs for the collection of genital ulcer specimens to measure HIV RNA shedding. J Clin Virol 2009; 46:165–8. [DOI] [PubMed] [Google Scholar]
- 22. Low AJ, Konate I, Nagot N, et al. Cervicovaginal HIV-1 shedding in women taking antiretroviral therapy in Burkina Faso: a longitudinal study. J Acquir Immune Defic Syndr 2014; 65:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lawn SD, Subbarao S, Wright TC Jr, et al. Correlation between human immunodeficiency virus type 1 RNA levels in the female genital tract and immune activation associated with ulceration of the cervix. J Infect Dis 2000; 181:1950–6. [DOI] [PubMed] [Google Scholar]
- 24. Tobian AA, Kigozi G, Manucci J, et al. HIV shedding from male circumcision wounds in HIV-infected men: a prospective cohort study. PLoS Med 2015; 12:e1001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tobin NH, Learn GH, Holte SE, et al. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J Virol 2005; 79:9625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bull ME, Learn GH, McElhone S, et al. Monotypic human immunodeficiency virus type 1 genotypes across the uterine cervix and in blood suggest proliferation of cells with provirus. J Virol 2009; 83:6020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soria J, Bull M, Mitchell C, et al. Transmitted HIV resistance to first-line antiretroviral therapy in Lima, Peru. AIDS Res Hum Retroviruses 2012; 28:333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zuckerman RA, Whittington WL, Celum CL, et al. Higher concentration of HIV RNA in rectal mucosa secretions than in blood and seminal plasma, among men who have sex with men, independent of antiretroviral therapy. J Infect Dis 2004; 190:156–61. [DOI] [PubMed] [Google Scholar]
- 29. Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol 1990; 28:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arvold ND, Ngo-Giang-Huong N, McIntosh K, et al. Maternal HIV-1 DNA load and mother-to-child transmission. AIDS Patient Care STDS 2007; 21:638–43. [DOI] [PubMed] [Google Scholar]
- 31. Ellis GM, Page LC, Burman BE, Buskin S, Frenkel LM. Increased detection of HIV-1 drug resistance at time of diagnosis by testing viral DNA with a sensitive assay. J Acquir Immune Defic Syndr 2009; 51:283–9. [DOI] [PubMed] [Google Scholar]
- 32. Mullins Lab, University of Washington. DIVEIN https://indra.mullins.microbiol.washington.edu/DIVEIN/diver.html. Accesses 16 April 2020.
- 33. Bull ME, Mitchell C, Soria J, et al. Monotypic low-level HIV viremias during antiretroviral therapy are associated with disproportionate production of X4 virions and systemic immune activation. AIDS 2018; 32:1389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bennetto-Hood C, Johnson VA, King JR, Hoesley CJ, Acosta EP. Novel methodology for antiretroviral quantitation in the female genital tract. HIV Clin Trials 2009; 10:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Vries-Sluijs TE, Dieleman JP, Arts D, et al. Low nevirapine plasma concentrations predict virological failure in an unselected HIV-1-infected population. Clin Pharmacokinet 2003; 42:599–605. [DOI] [PubMed] [Google Scholar]
- 36. Bednasz CJ, Venuto CS, Ma Q, et al. Efavirenz therapeutic range in HIV-1 treatment-naive participants. Ther Drug Monit 2017; 39:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ruiz-Villalba A, van Pelt-Verkuil E, Gunst QD, Ruijter JM, van den Hoff MJ. Amplification of nonspecific products in quantitative polymerase chain reactions (qPCR). Biomol Detect Quantif 2017; 14:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chinula L, Nelson JAE, Wiener J, et al. Effect of the depot medroxyprogesterone acetate injectable and levonorgestrel implant on HIV genital shedding: a randomized trial. Contraception 2018; 98:193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fiore JR, Suligoi B, Saracino A, et al. Correlates of HIV-1 shedding in cervicovaginal secretions and effects of antiretroviral therapies. AIDS 2003; 17:2169–76. [DOI] [PubMed] [Google Scholar]
- 40. Henning TR, Kissinger P, Lacour N, Meyaski-Schluter M, Clark R, Amedee AM. Elevated cervical white blood cell infiltrate is associated with genital HIV detection in a longitudinal cohort of antiretroviral therapy-adherent women. J Infect Dis 2010; 202:1543–52. [DOI] [PubMed] [Google Scholar]
- 41. Graham SM, Holte SE, Peshu NM, et al. Initiation of antiretroviral therapy leads to a rapid decline in cervical and vaginal HIV-1 shedding. AIDS 2007; 21:501–7. [DOI] [PubMed] [Google Scholar]
- 42. Shankarappa R, Gupta P, Learn GH Jr, et al. Evolution of human immunodeficiency virus type 1 envelope sequences in infected individuals with differing disease progression profiles. Virology 1998; 241:251–9. [DOI] [PubMed] [Google Scholar]
- 43. Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. Decreased adherence to antiretroviral therapy observed prior to transient human immunodeficiency virus type 1 viremia. J Infect Dis 2007; 196:1773–8. [DOI] [PubMed] [Google Scholar]
- 44. Li JZ, Gallien S, Ribaudo H, Heisey A, Bangsberg DR, Kuritzkes DR. Incomplete adherence to antiretroviral therapy is associated with higher levels of residual HIV-1 viremia. AIDS 2014; 28:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maggiolo F, Di Filippo E, Comi L, et al. Reduced adherence to antiretroviral therapy is associated with residual low-level viremia. Pragmat Obs Res 2017; 8:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taramasso L, Magnasco L, Bruzzone B, et al. How relevant is the HIV low level viremia and how is its management changing in the era of modern ART? A large cohort analysis. J Clin Virol 2020; 123:104255. [DOI] [PubMed] [Google Scholar]
- 47. Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antivir Ther 2011; 16:1149–67. [DOI] [PubMed] [Google Scholar]
- 48. Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta 2014; 1843:2563–82. [DOI] [PubMed] [Google Scholar]
- 49. Nelson JAE, De Paris K, Ramirez C, et al. Female genital tract shedding of HIV-1 is rare in women with suppressed HIV-1 in plasma. AIDS 2020; 34:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eisinger RW, Dieffenbach CW, Fauci AS. HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA 2019; 321:451–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.