Abstract
Purpose
Thymic stromal lymphopoietin (TSLP) is a pro-allergic cytokine that initiates allergic inflammatory reaction between epithelial and dendritic cells (DCs). miR-19b was reported to suppress TSLP expression. The present study aimed to examine miR-19b expression, regulation, and function in allergic conjunctivitis (AC).
Methods
A murine model of experimental AC was induced in BALB/c mice by short ragweed pollen. The serum, eye balls, conjunctiva, and cervical lymph nodes (CLN) were used for the study. Gene expression was determined by RT-PCR, whereas protein production and activation were evaluated by immunostaining, ELISA, and Western blotting.
Results
In the murine AC model, miR-19b was aberrantly downregulated, whereas the levels of TSLP and p-STAT3, as well as the number of CD11c+ pSTAT3+ DCs were increased. Moreover, Th2 inflammatory cytokine expression was significantly increased. These severe phenotypes could be counteracted by either applying exogenous miR-19b mimic microRNAs or the JAK/STAT inhibitor CYT387. Moreover, overexpression of miR-19b repressed p-STAT3 expression and the number of CD11c+ cells in AC eye and CLN tissues.
Conclusions
These findings suggested that miR-19b reduced ocular surface inflammation by inhibiting Stat3 signaling via TSLP downregulation in a murine AC model. Moreover, the present study further demonstrated the clinical potential of applying miR-19b and anti-JAK/STAT therapies in the treatment of AC.
Keywords: allergic conjunctivitis, miR-19b, inflammation
Allergic conjunctivitis (AC) is a group of allergic diseases affecting the ocular surface and has become more common in children and adolescents. This disease currently affects more than 20% of the population in the United States.1 The symptoms of AC include itching, eyelid swelling, chemosis, tearing, and redness, which negatively impact the quality of daily life.2,3 AC is primarily caused by both type I and type IV hypersensitivity and characterized by aberrant T helper type 2 (Th2) inflammatory responses, which involve an intricate interplay between various cells including dendritic cells (DCs), T cells, B cells, eosinophils, basophils, and mast cells4.
Th2 inflammatory response is a hallmark of allergic disorders.5 Before this disease, Th2 cell differentiation is modulated by higher expression of the OX40 ligand (OX40L) on the cell surface of DCs. This upregulation of OX40L is achieved by stimulating a novel pro-allergic cytokine that is mainly produced by damaged epithelial cells. This cytokine is termed thymic stromal lymphopoietin (TSLP) and acts as a major factor initiating allergic inflammation between epithelial cells (ECs) and DCs at the interface, thus contributing significantly to the progression of AC.
TSLP exerts its signaling effects by binding to the TSLP receptor (TSLPR), which forms a heterodimer with the IL-7Rα chain and has been found on the surface of DCs.6 TSLP is recognized by the extracellular portion of TSLPR, while its intracellular portion harbors a Box 1 motif and a single tyrosine residue involved in signal transduction following TSLP binding, which in turn activates Janus kinase (JAK) phosphorylation.7 STAT3 is a member of the signal transducer and activation of transcription (STAT) family of proteins and is activated by a number of cytokines including IL-6 and IL-23.8 The activation of JAK regulates the phosphorylation and activation of multiple STAT factors including STAT3.9 In addition, TSLPR ligation has been found to phosphorylate STAT3 in human subjects.10 Previous reports indicated that DC maturation was significantly inhibited by the JAK/STAT pathway inhibitor (CYT387) in allergic diseases11 and that STAT3 was required for Th2 cell development and cytokine production following activation.12 In the present study, we speculated that the JAK/STAT pathway in DCs was important in regulating AC downstream of TSLP.
MiR-19b is a primary component of the miR-17-92 cluster, whose expression has been negatively associated with the expression of TSLP mRNA and protein to influence T-cell development.13 Recent studies demonstrated that miR-19b could target TSLP, specifically by binding to the TSLP-3′-UTR region, which directly downregulated TSLP expression at the posttranscriptional level.14 Due to the regulation of TSLP, several reports have examined the involvement of the miR-17-92 cluster in regulating immunity.15,16 However, whether miR-19b is involved in the regulation of TSLP in DCs and in the induction of a Th2 cell response in AC has not been investigated.
In the present study, we aimed to examine the role of miR-19 in regulating TSLP expression and function in the progress of AC in vivo. We hypothesized that miR-19 is critical in regulating TSLP expression by activating TSLPR and the downstream JAK/STAT signaling pathways in DCs and promoting DC maturation. Overexpression of miR-19b or inhibition of the JAK/STAT pathway resulted in an alleviated inflammatory response and attenuation of AC progression. The results of the present study can shed light on future targeting therapies for the treatment of AC.
Method
Animals
Female BalB/c mice (6-8 weeks of age) were supplied by the Institute of Genetics and Developmental Biology at the Chinese Academy of Sciences (Beijing, China). The animal studies were approved by the Institutional Animal Care and Use Committee of the Tianjin Eye Hospital. All mice were maintained in a specific pathogen-free facility and received sterilized food and water ad libitum. Throughout the study, we followed the protocol of the Association for Research in Vision and Ophthalmology statement regarding the experimental use of animals.
Mouse Models for AC
The murine AC model was well established as previously described.17 Briefly, BALB/c mice were sensitized with a mixture of 50 µg of short ragweed (SRW) pollen (Greer Laboratories, Lenoir, NC, USA) in 5 mg of Imject Alum (Thermo Fisher, No. 77161) through footpad injection on day 0. The sensitization procedure was repeated on day 5 to improve the allergic reaction. The experiments were initiated from day 10 to 14 and the mice were subsequently challenged with 1.5 mg of SRW pollen in 10 µL of PBS (pH 7.2). The solution was administered into each eye once a day. The PBS eye drop-treated normal mice were used as controls. A total of 15 mice were used for each group.
For transfection of MicroRNA (miRNA), miR-19b agomir and its negative control were obtained from GenePharma (Shanghai, China). The agomir of miR-19b that was used for overexpression experiments was double stranded with the following sequence: 5′-UGUGCAAAUCCAUGCAAAACUGA-3′ (forward), and 5′-AGUUUUGCAUGGAUUUGCACAUU-3′ (reverse). Negative control, 5′-UUCUCCGAACGUGUCACGUTT-3′ (forward) and 5′-ACGUGACACGUUCGGAGAATT-3′ (reverse). A total of 50 nM of negative control miRNA or miR-19b agomir were used. We delivered miRNA to mice using a tail vein injection 30 minutes before the SRW pollen challenge daily from day 10 to 14. To enhance the therapeutic effect, we applied miRNA to AC mice on days 10, 12, and 14 by subconjunctival injection in both eyes. A total of 6 mice were used for each group.
To induce inhibition of the JAK/STAT signaling pathway, we sensitized AC mice intraperitoneally with CYT387 (30 mg/kg) in the AC+CYT387 group. PBS was used as the control (n = 6 per group).
Clinical Evaluation
AC clinical scoring was recorded and evaluated under a SL500 Shin Nippon Slit Lamp (Ajinomoto Trading Inc., Tokyo, Japan) on the day before the first sensitization and daily from day 10 to 14 following 15 to 30 minutes of challenge with pollen. The severity of eye symptoms was examined microscopically by two blinded researchers based on clinical scorings of four independent parameters including chemosis, lid edema, redness, and tearing. A 0 to 3 grading system was used according to previous studies18,19 as follows: 0 = no severity; 1 = mild; 2 = moderate; 3 = severe.
Vascular permeability assays were detected by injection of 0.1% Evans blue dye solution in PBS (12 mL/kg) through tail vein in vivo. The mice were sacrificed 60 minutes following injection. Evans blue dye was extracted following incubation for 24 hours from the removed eyelid and conjunctival tissue. A microplate reader was used to measure the extract at 620 nm.
Measurement of Total Immunoglobulin E Levels in the Serum
Blood samples were collected at 20 hours following the last topical challenge with SRW pollen or PBS. The collection was conducted by the tail vein in each group. Following coagulation and centrifugation, the serum was isolated and analyzed using an ELISA kit (Abcam, ab157718) for the determination of total immunoglobulin E (IgE) concentration according to the manufacturer's protocol.
Histology
The eyes were harvested from the animals together with eyelids and the cervical lymph nodes (CLNs) in each group at 24 hours following the last challenge. The eyes were fixed in 4% paraformaldehyde and embedded in paraffin and subsequently cut into 4-µm-thick horizontal sections. To measure the number of infiltrated inflammatory cells in the ocular surface, the tissue sections were processed with hematoxylin and eosin staining for histological evaluation following deparaffinization and rehydration. The number of infiltrating cellular components and eosinophils in the ocular surface was estimated by two blinded researchers. Each group was composed of five representative fields and six sections.
The eyeballs and the CLNs in each group were embedded in OCT immediately following collection and cut into 5-µm-thick sections for immunofluorescent (IF) staining. The sections were cut in a horizontal axis by a cryostat (Leica, CM1850) and subsequently fixed with cold acetone at -20°C for 10 minutes. The sections were then washed with PBS 3 times for 2 minutes each. Following hydration in 100%, 95%, 90%, 80%, and 70% ethanol and PBS, the sections were sequentially blocked with 5% BSA (Roche Diagnostics, Basel, Switzerland) for 1 hour at room temperature. Subsequently, the sections were incubated with rabbit anti-mouse antibodies against TSLP (Abcam, ab188766, 5 µg/mL), or a mixture of rabbit anti-mouse antibodies against TSLP (4 µg/mL), anti-CD11c (5 µg/mL), anti-phospho-STAT3 (Abcam, ab30647, 5 µg/mL), and mouse polyclonal antibodies against CD11c (Abcam, ab11029, 5 µg/mL) in 5% BSA overnight at 4°C. Following extensive washing, the sections were incubated with secondary antibodies conjugated with Alexa Fluor 488 or 594 (1:200 dilution; Thermo Fisher Scientific, Inc.) for 2 hours. DAPI (Beijing Solarbio Science & Technology Co., Ltd., cat. no. S2110) was used for unclear counterstaining. Isotype IgG served as a negative control. The images were photographed using an epifluorescence microscope (Eclipse 400; Nikon).
Total RNA Extraction and RT Quantitative PCR (qPCR)
Total RNA was extracted from conjunctiva, cornea and CLN of mice using the TRIzol reagent (Sigma-Aldrich). All operational steps were strictly carried out following the manufacturer's instructions and subsequently reverse-transcribed into cDNA using the Prime-Script RT reagent kit (Takara Biotechnology Co.). The miRNA molecules were reverse-transcribed by the miRNA reverse transcription kit (TaqMan). Specific primers were designed for the qPCR analysis (Table). qPCR was performed using the SYBR premix Ex Taq kit (Takara Biotechnology Co.). The cycling parameters were set as follows: initial denaturation at 95°C for 3 minutes, 45 cycles of 95°C for 12 seconds, and 62°C for 40 seconds, and final extension at 95°C for 15 seconds. All the melting curves exhibited a single distinct peak, which is shown in the Supplementary files (Figure S3). The relative quantity of mRNA and miRNA-19b with regard to the expression of GAPDH or U6 was estimated using the 2−ΔΔCq method.
Table.
Primers Used for mRNA Analysis
| Primers | Forward | Reverse |
|---|---|---|
| IL-1 | 5′-GCCCACCAAAGAACAAAGTCGG -3′ | 5′-ACAGACTGTCAGCACTTCCC -3′ |
| IL-5 | 5′-CTCTGTTGACAAGCAATGAGCC-3′ | 5′-TCTTCAGTATGTCTAGCCCCTG-3′ |
| IL-6 | 5′-GGAGTGGCTAAGGACCAAGA -3′ | 5′-TCTACCACAGTGAGGAATGTC-3′ |
| IL-8 | 5′-TGCATGGACAGTCATCCACC -3′ | 5′-ATGACAGACCACAGAACGGC -3′ |
| IL-13 | 5′-CCTGGCTCTTGCTTGCCTT-3′ | 5′-GGTCTTGTGTGATGTTGCTCA-3′ |
| IL-33 | 5′-AACAGGCCTTCTTCGTCCTT-3′ | 5′-GAACGCACAGGCGTTTTACT-3′ |
| ST2 | 5′-TGACACCTTACAAAACCCGGA-3′ | 5′-AGGTCTCTCCATAAATGCACA-3′ |
| TSLP | 5′-TCAATCCTATCCCTGGCTGC-3′ | 5′-GCCATTTCCTGAGTACCGTCA-3′ |
| STAT3 | 5′-GAGGAGGCATTCGGAAAG-3′ | 5′-TCGTTGGTGTCACACAGAT-3′ |
| OX40 | 5′-CCCAACCTCGGCAGGACAGC-3′ | 5′-TGTAGGGCGCTGGGTCTCCC-3′ |
| OX40L | 5′-CGGTTGTCATCAAGTGCGATGGG-3′ | 5′-AGCCAAAGAGGCCACCACAGT-3′ |
| CD11C | 5′-GATGGACTGGTGGATCTGGC -3′ | 5′-CACTCAGTGTCTGCTCAGGG -3′ |
Protein Isolation and Western Blot Analysis
The mouse conjunctiva tissues were lysed using the RIPA lysis buffer (Beyotime Institute of Biotechnology) and the protein concentration was measured using the bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.). A total of 30 µg protein was separated by 10% SDS-PAGE and subsequently transferred onto polyvinylidene difluoride membranes. The membranes were then blocked with 5% skimmed milk in TBS containing Tween-20 (TBST) at room temperature for 2 h prior to incubation with the primary antibodies including anti-GAPDH)cat.no.ab181602), anti-TSLP (Abcam, ab188766) and anti-p-STAT3 (dilution 1: 1,000, ab30647) at 4°C overnight. Subsequently, the membranes were washed by TBST and incubated with secondary antibodies (dilution 1:10,000, Abcam, USA) at room temperature for 2 h. Following washing with TBST for three times, the protein bands were visualized by chemiluminescence reagents (EMD, Millipore) and the images were analyzed by the ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
All quantitative data were expressed as mean ± SD. The data were tested for normality with the Shapiro Wilk test or the Kolmogorov-Smirnov test. A Student t-test was used to assess the differences between the two means. The assessment of multiple means was performed by one-way or two-way ANOVA followed by the Bonferroni's post hoc test. In case of a low sample size (low n number) that was not sufficient for normality testing, nonparametric tests (the Mann-Whitney U-testor Kruskal-Wallis test) were used. All statistical analyses were performed using GraphPad Prism (version 8). Statistical significance was defined at P < 0.05. The P values for Figures 1 and 5 are included in the Supplementary Information as Figures S1 and S2A-C, respectively.
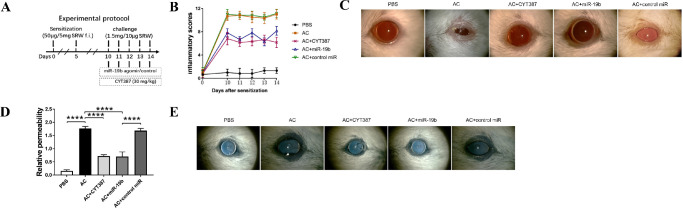
Figure 1.
Inhibition of JAK/STAT or overexpression of miR-19b leads to suppression of phenotypes following induction AC in vivo. (A) Schematic image of establishing the AC)murine model and group treatments with the JAK/STAT inhibitor CYT387, miR-19b agomirs. The image also includes the control miR group. (B) Inflammatory scoring under slit lamp for 15 to 30 minutes following pollen challenge for clinical signs (redness, chemosis, discharge and tearing) from day 10 to day 14 following sensitization of the PBS, AC, AC + CYT387, AC + miR-19b, and AC + control miR groups. (C) Representative images of ocular symptoms under slit lamp for 15 to 30 minutes following the last challenge. (D) Conjunctival vascular permeability was measured by the Evans blue assay. (E) Representative images of eyes in each group following Evans blue injection. n = 6 mice for each group. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
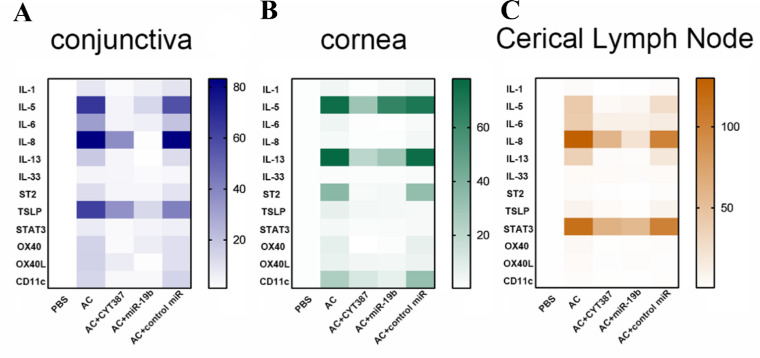
Figure 5.
Inhibition of JAK/STAT or overexpression of miR-19b decreased the expression of DC maturation and of the Th2 inflammatory response genes induced by AC. The mRNA expression levels of IL-1, IL-5, IL-6, IL-8, IL-13, IL-33, ST2, TSLP, STAT3, OX40, OX40L, and CD11c respectively in (A) conjunctiva, (B) cornea, and (C) cervical lymph nodes from mice of the PBS, AC, AC + CYT387, AC + miR-19b, and AC + control miR groups were determined by RT-qPCR. All data were representative of at least 3 independent experiments.
Results
Inhibition of JAK/STAT or Overexpression of miR-19b Leads to Suppression of Phenotypes Induced by AC in Vivo
To evaluate the direct effects of miR-19b overexpression or JAK/STAT pathway inhibition in affecting the progression or outcomes of AC, we used a previously established model17 to induce AC in mice. The regimen used in the experiments is shown in Figure 1A. The mice were sensitized with 50 µg SRW pollen in 5 mg of Imject Alum by footpad injection from day 0 to day 5. From day 10 to day 14, the mice were challenged by applying 1.5 mg of SRW pollen dissolved in 10 µL PBS in the eye. During this challenge period, the mice were subjected to intraperitoneal injection of CYT387 or subconjunctival injection of miR-19b agomir and control miR (Fig. 1A). From day 10 to day 14 postsensitization, PBS control animals indicated low inflammatory scores, whereas pollen sensitized and challenged (AC) animals demonstrated dramatically increased inflammatory scores in the eye (Fig. 1B, Supplementary Figure S1), accompanied by severe eyelid swelling, conjunctival edema, redness, and tearing (Fig. 1C). This suggested that an AC model with clinical manifestations was successfully established in mice. The application of intraperitoneal injection of the JAK/STAT inhibitor, CYT387, reduced the phenotypes and inflammatory scores of AC. Subconjunctival injection of miR-19b could also reduce the AC symptoms and inflammatory scores, whereas the control miR injection revealed similar results with that of the AC group (Figs. 1B and C, Supplementary Figure S1). This confirmed that JAK/STAT was coordinated with miR-19b in regulating inflammatory responses following pollen stimulation in the eye.
Moreover, we evaluated the conjunctival vascular hyperpermeability to determine whether histamine inflammatory mediators played a role in the AC eye (Figs. 1D and E). The Evans blue assay demonstrated that conjunctival vascular hyperpermeability was induced in AC compared with PBS control as determined by extraction of Evans blue in the eye and surrounding tissues (Fig. 1D). Following initial visual observation, additional intense staining of blue color was noted in the eye and surrounding tissues (shown as a wider rim of dark blue) in AC compared with the PBS groups (Fig. 1E). The inhibition of the JAK/STAT pathway could attenuate the vascular hyperpermeability, which was consistent with the aforementioned scoring data. The application of miR-19b agomir could also alleviate the hyperpermeable phenotype of AC, whereas the control miR could not. Taken collectively, these results suggested that the sensitization and challenge with SRW pollens could recapitulate the symptoms of AC, such as elevated inflammatory response and vascular permeability, which could be in turn alleviated by treating the animals with intraperitoneal CYT387 or subconjunctival miR-19b agomir.
Inhibition of JAK/STAT or Overexpression of miR-19b Leads to Weakened Inflammatory Response in AC
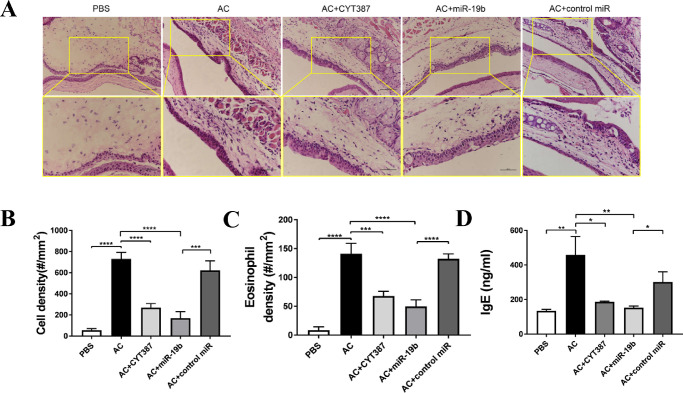
To evaluate the microenvironment of the inflammatory response in AC, we performed histopathological examination of conjunctival tissue (Fig. 2A). Hematoxylin and eosin staining demonstrated clear ocular surface epithelium including corneal epithelial and conjunctival epithelial, subconjunctival tissue, corneal stroma, corneal endothelium, and anterior chamber (data not shown). No apparent edema or infiltrated inflammatory cells were noted in the PBS groups, as shown by cell density (Fig. 2B) and eosinophil density (Fig. 2C). In the AC eye tissue, the cell density was significantly increased compared with that of the PBS control. An increased number of eosinophils was also noted, which was reduced following JAK/STAT inhibition. Similarly, the miR-19 agomir exhibited certain effects with regard to the decrease in the total cellular components and number of eosinophils, while the control miR did not. In parallel, serum IgE levels (secreted by B cells during allergy) among these animals reflected the same trend (Fig. 2D), suggesting that the enhanced IgE-associated inflammatory response by AC could be successfully suppressed either by inhibiting JAK/STAT by CYT387 or by mimicking miR-19b induction in the eye.
Figure 2.
Inhibition of the JAK/STAT pathway or overexpression of miR-19b leads to weakened inflammatory response in AC. (A) Histopathological representative images of hematoxylin and eosin staining of eyeball samples from mice of the PBS, AC, AC + CYT387, AC + miR-19b, and AC + control miR groups. The scale bars represent 200 µm (top) and 500 µm (bottom). (B) Cellular components and (C) eosinophilic granulocytes in the ocular surface tissue of mice in each group were quantified in number/mm2. (D) Total serum IgE levels from mice in each group were measured by ELISA. The data are shown as the mean ± SD of three independent experiments and each experiment was performed in duplicate. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
miR-19b Overexpression Results in Repressed p-STAT3 and CD11c Levels in the AC Eye Tissue and in the CLN Tissue
Given that miR-19b agomir counteracted the pathogenetic features of AC, we hypothesized that miR-19b was dysregulated in AC tissues. To test this hypothesis, we isolated the conjunctival, cornea, and CLN tissue and analyzed the transcriptional levels of miR-19b (Fig. 3A). Among all three types of tissues, the miR-19b levels were extremely low in AC compared with the PBS control. The replenishment of miR-19b returned the miR-19b mRNA levels to the normal levels as expected, whereas the control miR failed to boost miR-19b levels. The inhibition of the JAK/STAT3 pathway did not result in higher miR-19b levels, although an alleviated phenotype was noted in AC mice with CYT387. This could be explained by the fact that JAK/STAT3 functioned downstream of miR-19b, whose expression was not subjective to JAK/STAT3 itself. Subsequently, we analyzed CD11c+ p-STAT3+ cells, which were considered as activated dendritic cells (DCs) on the ocular surface. The levels of CD11c+ p-STAT3+ cells were considerably increased in the AC group compared with those in the PBS group. Following treatment with the miR-19b agomir or CYT387, the number of DCs that were positive for CD11c and p-STAT3 was considerably decreased to a level comparable to that of the PBS control (Fig. 3B). These results indicated that miR-19b acted upstream of the JAK/STAT3 signaling pathway, which was required for preventing the overactivation of DCs on the ocular surface of AC animals.
Figure 3.
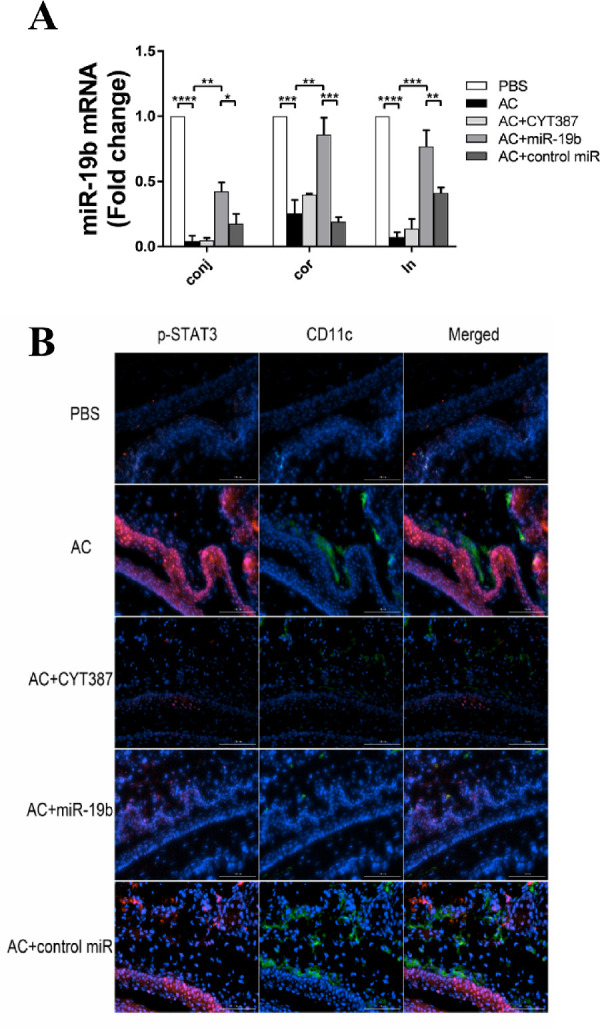
miR-19b overexpression results in repressed p-STAT3 and CD11c levels in AC eye and CLN tissues. (A) Expression levels of miR-19b in conjunctiva, cornea, and CLNs derived from the PBS, AC, AC + CYT387, AC + miR-19b, and AC + control miR groups as determined by qPCR. The results are expressed as mean ± SD from three independent experiments and each experiment was performed in triplicate. (B) Immunofluorescent staining on frozen sections of eyeballs from mice in each group that were probed for p-STAT3 (red) and CD11c (green) with DAPI counterstaining (blue). The scale bars represented 100 µm. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. The experiments were repeated 3 times.
The Induction of TSLP Expression in Conjunctiva, Cornea, and CLN Tissues by AC Is Inhibited by miR-19b Agomir or the JAK/STAT3 Inhibitor
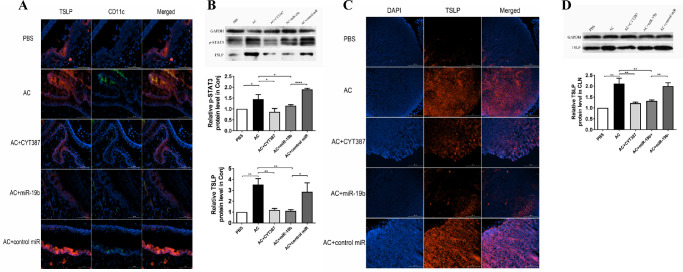
TSLP is a key cytokine that participates in the initiation of the allergic cascade in DC-mediated Th2 differentiation during AC. To explore the influence of miR-19b and the JAK/STAT pathway on TSLP expression and confirm that DCs could produce TSLP in AC mice, we sought to compare the pathology of conjunctiva and cornea among PBS, AC, AC+CYT387, AC+miR-19b, and AC+control miR groups by IF staining. The detection was achieved by probing for double markers. An increase in TSLP expression was noted in both ocular epithelium from both cornea and conjunctiva in AC and AC + control miR groups compared with the corresponding TSLP levels in the PBS group. However, TSLP expression was weak in the AC + miR-19b and in the AC + CYT387 groups at a level comparable to PBS (Fig. 4A). Notably, TSLP immunoreactivity was also detected in CD11c+ DCs, which infiltrated the conjunctival stroma near the epithelial area. The increase in the number of TSLP+ CD11c+ DCs in AC mice was reduced in the AC + miR-19b and in the AC + CYT387 groups. IF analysis in AC mice demonstrated that both p-STAT3 and TSLP were upregulated. Western blot was subsequently used to identify the protein expression dynamics of these two proteins in the conjunctiva (Fig. 4B). The expression levels of p-STAT3 were co-active with TSLP, which was induced by AC and further reduced by administrating miR-19b mimic or the JAK/STAT inhibitor.
Figure 4.
The induced expression of TSLP in conjunctiva, cornea, and CLN tissues by AC was inhibited by miR-19b agomir or the JAK/STAT3 inhibitor. (A) Costaining on frozen sections of eyeballs with TSLP (red) and CD11c (green) with DAPI counterstaining (blue) in the PBS, AC, AC + CYT387, AC + miR-19b, and AC + control miR groups. Scale bars: 100 µm. (B) Total TSLP and phosphorylated STAT3 (p-STAT3) expression levels in conjunctiva as determined by western blot analysis. (C) The TSLP protein production of CLNs from mice in each group was evaluated by IF staining against TSLP (red) and with DAPI (blue) nuclear counterstaining. Scale bar: 200 µm. (D) Total TSLP expression in the different tissues was determined by western blot analysis. GAPDH served as a loading control. *P < 0.05, **P < 0.01, *** P < 0.001, and ****P < 0.0001. All data are representative of at least 3 independent experiments.
In addition to cornea and conjunctiva, the CLN tissue of AC mice further exhibited high expression levels of TSLP compared with those of the PBS control (Fig. 4C). Moreover, AC + miR-19b and AC + CYT387 mice exhibited reduced TSLP staining compared with the AC group, while the AC + control miR group expressed high levels of TSLP, which was further confirmed by western blot analysis (Fig. 4D).
These results suggested that the expression of TSLP together with that of p-STAT3 was stimulated by AC in multiple tissues including cornea, conjunctiva and cervical lymph node. Loss of miR-19b or the activation of the JAK/STAT3 pathways was important in maintaining its expression.
Inhibition of the JAK/STAT Pathway or Overexpression of miR-19b Decreases the Expression Levels of DC Maturation and of the Th2 Inflammatory Response Genes Induced by AC
To examine the cross-talk among different cytokines during the AC inflammatory response, we evaluated the mRNA expression levels of IL-1, IL-5, IL-6, IL-8, IL-13, IL-33, ST2, TSLP, STAT3, OX40, OX40L, and CD11c separately in the conjunctiva (Fig. 5A, Supplementary Figure S2A), cornea (Fig. 5B, Supplementary Figure S2B), and CLN tissues (Fig. 5C, Supplementary Figure S2C) by qPCR analysis. The results indicated that the mRNA levels of all the aforementioned 12 genes were upregulated in the conjunctival, corneal, and CLN tissue of AC mice compared with the mice of the PBS group. The mice treated with CYT387 and miR-19b agomir exhibited downregulation of the expression of TSLP and STAT3 mRNA. These outcomes further suggested that the activation of TSLP occurred in the three aforementioned tissues of the AC mice, whereas inhibition of the JAK/STAT3 signaling pathway or the increase in the levels of miR-19b efficiently suppressed this process.
Furthermore, the data demonstrated that the expression levels of IL-1, IL-6, and IL-8 mRNAs were decreased in AC mice treated with CYT387 or miR-19b agomir (all P < 0.05). Moreover, we observed that the levels of IL-33 and ST2 were mildly decreased in the AC + CYT387 and AC + miR-19b groups. Therefore, we concluded that the inflammatory reaction was inhibited by upregulation of miR-19b and treatment with the inhibitor of the JAK/STAT3 signaling pathway.
To explore whether DCs or Th2 cells were active, we examined the expression of DC maturation markers, such as OX40/OX40L and CD11c, as well as the mRNA expression levels of the genes corresponding to Th2-secreted cytokines, such as IL-5 and IL-13. The data indicated that these levels were upregulated in the AC group and reduced in mice of the AC + CYT387 and AC + miR-19b groups. These results further demonstrated that AC was dependent on the DC-mediated Th2 cell activation, which was in turn promoted by miR-19b and the JAK/STAT3 signaling pathway.
Discussion
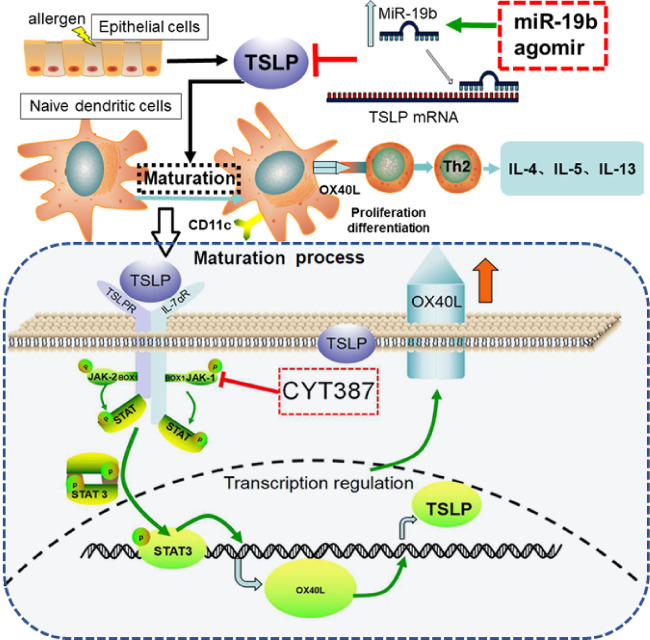
AC is an allergic disease of the ocular surface, which is one of the most common clinical diseases encountered from both ophthalmologists and allergists.20 In the present study, we reported that the pathogenesis of AC involved miR-19b as a regulator of DC maturation. This was promoted via the JAK/STAT signaling pathway and by theTh2 responses. We have established a murine model of AC and treated the animals with miR-19b agomir to increase the levels of miR-19b or with CYT387 to block the JAK/STAT signaling pathway. We observed a significant decrease of STAT3 phosphorylation by overexpression of miR-19b, suggesting that JAK/STAT3 acted downstream within the same signaling pathway. We further noted that the intervention with miR-19b or the JAK/STAT inhibitor could both affect the maturation of DCs and the Th2-associated cytokine expression. We speculated that miR-19b reduced the transcriptional activity of phosphorylated JAK/STAT, thereby inhibiting the expression of OX40L, which is a key factor that acts as a molecular switch in the allergic cascade and in the regulation of the DC-mediated initiation of the Th2 cell response (Fig. 6).
Figure 6.
Hypothetical scheme of the regulatory network underlying miR-19b targeted TSLP. miR-19b is essential in downregulating TSLP and could be stimulated to allergens on the ocular interface of epithelial cells and DCs. Under AC, TSLP elevation induces activation of the JAK/STAT3 pathway in DCs, which leads to surface expression of OX40L and increased expression of TSLP. These effects render DCs in an active and mature form. Subsequently, active DCs promote differentiation of Th2 cells to initiate and magnify the inflammatory response.
miR-19b has been shown to suppress TSLP by binding to its 3′UTR and therefore influencing T-cell development.13 In the present study, we observed a downregulation of TSLP by miR-19b agomir on ocular surfaces and draining CLNs, verifying that miR-19b was indeed a negative regulator of TSLP. In addition, miR-19b levels were extremely low in AC compared with those of the PBS control group. The replenishment of miR-19b by miR-19 agomir injection increased significantly the expression levels of miR-19 compared with those of the negative control miR injection. In the conjunctiva and lymph nodes, it seemed that the control miR exhibited slight protective effects on the loss of miR-19b in the AC model (Fig. 3A). The exact reason why this occurs is unknown. However, there may be two factors that should be considered. One involves the base sequence of the control miR and the other the secondary reaction. Secondary reactions may be caused by the treatment of nucleotide fragments through the tail vein injection combined with conjunctiva injection. The injection of nucleotide fragments may induce inflammation and subsequently increase the expression levels of miR-19. Previous studies have reported that the expression of miR-19 was increased in response to the stimulation including infarction or infection.21,22 Therefore, further research is required to produce a clear conclusion on the protective effects of miR-19b on AC. A higher number of experimental samples and additional time points can be used in future studies. The redesign of the sequence of the control miR can also provide a sufficient control probe. In contrast to miR-19b, we found that the inhibition of the JAK/STAT pathway using CYT378 could also suppress TSLP despite of low levels of miR-19b, implying another regulatory mechanism that was present in addition to the direct targeting by miR-19b.
STAT3 is present in cells with tyrosine-phosphorylated or unphosphorylated isoforms and plays essential roles in several cellular processes. However, it has been well studied in AC. TSLP expression is predominant in epithelial cells at barrier surfaces in response to danger signals and it can directly activate multiple STAT proteins and induce robust and sustained activation of the Janus kinase 1 and 2 enzymes in murine DCs.23 This is mediated because of the heterodimeric TSLPR (TSLP receptor) using JAK-2 in concert with IL-7Rα-associated JAK-1.24 Consistent with these findings, higher phosphorylation levels of STAT3 were noted in the AC group compared with those of the PBS group, whereas the overexpression of miR-19b caused a downregulation of TSLP expression concurrently with the levels of p-STAT3 in the AC + miR-19b group. IL-6 is a cytokine, which is responsive to STAT3 signaling.25 IL-6 expression was higher in the AC group and was reduced in either miR-19b agomir or CYT387 treatment groups. These findings are consistent with the conclusion that miR-19b plays a crucial role in STAT3 activation. A previous study demonstrated that STAT3 phosphorylation promoted the activation and migration of myeloid dendritic cells.6,26 The present study further demonstrated that the increased activation of DCs marked by CD11c+ was inhibited by CYT387.
Th2 cells are hallmarks of AC and their polarization requires DC cell activation.26 It is well known that TSLP-primed DCs produce OX40L,5 a ligand of OX40, which promotes the differentiation of naïve OX40 expressing CD4+ T cells into Th2 cells.27 The results of the present study indicated that the overexpression of miR-19b was able to reduce the expression levels of CD11c+ DCs, as well as the secretion of the TSLP/OX40L pathway signaling cytokines (TSLP, OX40L, OX40) following AC induction. The AC + CYT387 group indicated similar results as the AC + miR-19b group. A recent study supported our findings showing that the expression of OX40L was dramatically downregulated in TSLP-primed DCs by inhibiting the JAK/STAT pathway following CYT387 treatment in an asthmatic cell model.11 In addition, the expression levels of the classic Th2 cytokines, namely IL-5 and IL-13 were markedly increased in the AC group. This alteration was diminished in the AC + miR-19b group and in the AC + CYT387 group. The present study demonstrated that the activation of inflammatory Th2 cells was significantly restrained in AC mice following miR-19b overexpression or JAK/STAT pathway inhibition.
Th2 cells are induced when dendritic cells present peptides of allergens to naïve CD4+ T cells. Th2 cells produce the cytokines IL-3, IL-4, IL-5, IL-9, and IL-13, which are also termed Th2-type cytokines. Th2-type cytokines are important survival signals for mast cells, basophils, and eosinophils. B cells adapt to produce IgE and bind to specific Fcε receptors on mast cells and basophils.28 The binding of IgE influences the release of granule-stored allergic mediators, such as histamine from mast cells.29 In the present study, the administration of miR-19b agomir or CYT387 during the challenge phase decreased IgE production or prevented histamine-induced conjunctival vascular hyperpermeability. The data supported that the inhibition of IgE release and the reduced conjunctival vascular hyperpermeability were mediated by increasing the levels of miR-19b or by inhibiting the JAK/STAT3 signaling pathway, possibly as a result of attenuated Th2 cytokine secretion.
In summary, the present study demonstrated that a two-step strategy composed of a sensitization period from day 0 to day 5 followed by a challenge period from day 10 to day 14 by SRW pollens could effectively generate an AC model in mice, presenting severe inflammatory phenotypes that mimic clinical manifestations. By using this in vivo model, we showed that miR-19b expression was dysregulated. TSLP and p-STAT3 expression as well as CD11c+ pSTAT3+ DCs and Th2 inflammatory cytokine expression were aberrantly enriched during AC pathogenesis. These severe phenotypes could be counteracted by application of either exogenous miR-19b mimic miRNAs or the JAK/STAT inhibitor CYT387. We speculated that miR-19b and JAK/STAT function coordinated with parallel mechanisms to regulate TSLP levels as suggested in Figure 6. Future studies are required to dissect the exact molecular pathways and explore the underlying regulatory networks. The results from the present study have paved the way to study TSLP as a hub gene for mediating AC progression and provide future options of using TSLP as a biomarker to predict the stages of AC or the disease prognosis. Moreover, the present study demonstrated the clinical potential of applying miR-19b and anti-JAK/STAT therapies in the treatment of AC.
Supplementary Material
Acknowledgments
Supported by grants from the National Natural Science Foundation of China (81170828; 81670837), the Tianjin Science & Technology Foundation (15JCZDJC35300), and the Tianjin Health and Family Planning Communication Foundation (14KG133).
Author contributions: XL designed the research; CG, JL, PH, and YW performed the experiments; SS, LW, MY, LL, and RH analyzed the data and prepared figures; CG and XL wrote the manuscript; all authors reviewed the manuscript.
Disclosure: C. Guo, None; J. Liu, None; P. Hao, None; Y. Wang, None; S. Sui, None; L. Li, None; M. Ying, None; R. Han, None; L. Wang, None; X. Li, None
References
- 1. Ono SJ, Abelson MB. Allergic conjunctivitis: update on pathophysiology and prospects for future treatment. J Allergy Clin Immunol. 2005; 115: 118–122. doi: 10.1016/j.jaci.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 2. Wei CC, Kung YJ, Chen CS, et al.. Allergic conjunctivitis-induced retinal inflammation promotes myopia progression. EBioMedicine. 2018; 28: 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fernandes SSC, Andrade CR, Alvim CG, Camargos PAM, Ibiapina CDC. Epidemiological trends of allergic diseases in adolescents. J Bras Pneumol. 2017; 43: 368–372. doi: 10.1590/S1806-37562016000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel DS, Arunakirinathan M, Stuart A, Angunawela R. Allergic eye disease. BMJ. 2017; 2: 200–202. [DOI] [PubMed] [Google Scholar]
- 5. Pattarini L, Trichot C, Bogiatzi S, et al.. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J Exp Med. 2017; 214: 1529–1546. doi: 10.1084/jem.20150402. Epub 2017 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bell BD, Kitajima M, Larson RP, et al.. The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses. Nat Immunol. 2013; 14: 364–371. doi: 10.1038/ni.2541. Epub 2013 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rochman Y, Kashyap M, Robinson GW, et al.. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7–induced signaling. Proc Natl Acad Sci USA. 2010; 107: 19455–19460. Epub 2010 Oct 25 doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hillmer EJ, Zhang H, Li HS, Watowich SS. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016; 31: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wohlmann A, Sebastian K, Borowski A, Krause S, Friedrich K. Signal transduction by the atopy-associated human thymic stromal lymphopoietin (TSLP) receptor depends on Janus kinase function. Biol Chem. 2010; 391: 181–186. doi: 10.1515/BC.2010.029. [DOI] [PubMed] [Google Scholar]
- 10. Levin SD, Koelling RM, Friend SL, et al.. Thymic stromal lymphopoietin: a cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol. 1999; 162: 677–683. [PubMed] [Google Scholar]
- 11. Shi Z, Jiang W, Wang M, et al.. Inhibition of JAK/STAT pathway restrains TSLP-activated dendritic cells mediated inflammatory T helper type 2 cell response in allergic rhinitis. Mol Cell Biochem. 2017; 430: 161–169. doi: 10.1007/s11010-017-2963-7. Epub 2017 Feb 18. [DOI] [PubMed] [Google Scholar]
- 12. Stritesky GL, Muthukrishnan R, Sehra S, et al.. The transcription factor STAT3 is required for T helper 2 cell development. Immunity. 2011; 34: 39–49. doi: 10.1016/j.immuni.2010.12.013. Epub 2011 Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Z, Chen Y, Xu S, et al.. Aberrant decrease of microRNA19b regulates TSLP expression and contributes to Th17 cells development in myasthenia gravis related thymomas. J Neuroimmunol. 2015; 288: 34–39. [DOI] [PubMed] [Google Scholar]
- 14. Ye L, Mou Y, Wang J, Jin ML. Effects of microRNA-19b on airway remodeling, airway inflammation and degree of oxidative stress by targeting TSLP through the Stat3 signaling pathway in a mouse model of asthma. Oncotarget. 2017; 8: 47533–47546. doi: 10.18632/oncotarget.17258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geng XR, Qiu SQ, Yang LT, et al.. Allergen-specific immune response suppresses interleukin 10 expression in B cells via increasing micro-RNA-17-92 cluster. Cell Biochem Funct. 2016; 34: 449–454. doi: 10.1002/cbf.3207. Epub 2016 Aug 4. [DOI] [PubMed] [Google Scholar]
- 16. Simpson LJ, Patel S, Bhakta NR, et al.. A microRNA upregulated in asthma airway T cells promotes TH2 cytokine production. Nat Immunol. 2014; 15: 1162–1170. doi: 10.1038/ni.3026. Epub 2014 Nov 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Su W, Wan Q, Huang J, et al.. Culture medium from TNF-alpha-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms.J Allergy Clin Immunol. 2015; 136: 423–432. [DOI] [PubMed] [Google Scholar]
- 18. Magone MT, Chan CC, Rizzo LV, Kozhich AT, Whitcup SM. A novel murine model of allergic conjunctivitis. Clin Immunol Immunopathol. 1998; 87: 75–84. [DOI] [PubMed] [Google Scholar]
- 19. Groneberg DA, Bielory L, Fischer A, Bonini S, Wahn U. Animal models of allergic and inflammatory conjunctivitis. Allergy. 2003; 58: 1101–1113. [DOI] [PubMed] [Google Scholar]
- 20. Leonardi A, Motterle L, Bortolotti M. Allergy and the eye. Clin Exp Immunol. 2008; 1: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao F, Kataoka M, Liu N, et al.. Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction. Nat Commun. 2019; 10: 1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ashraf U, Zhu B, Ye J, et al.. MicroRNA-19b-3p modulates Japanese encephalitis virus-mediated inflammation via targeting RNF11. J Virol. 2016; 90: 4780–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arima K, Watanabe N, Hanabuchi S, Chang M, Sun SC, Liu YJ. Distinct signal codes generate dendritic cell functional plasticity. Sci Signal. 2010; 3: ra4. doi: 10.1126/scisignal.2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rochman Y, Kashyap M, Robinson GW, et al.. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci USA. 2010; 107: 19455–19460. doi: 10.1073/pnas.1008271107. Epub 2010 Oct 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu H, Ren G, Wang T, et al.. Aberrantly expressed Fra-1 by IL-6/STAT3 transactivation promotes colorectal cancer aggressiveness through epithelial-mesenchymal transition. Carcinogenesis. 2015; 36: 459–468. doi: 10.1093/carcin/bgv017. Epub 2015 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agrawal K, Arora N, Serine protease allergen favours Th2 responses via PAR-2 and STAT3 activation in murine model. Allergy. 2018; 73: 569–575. doi: 10.1111/all.13315. Epub 2017 Oct 16. [DOI] [PubMed] [Google Scholar]
- 27. Li F, Wang Y, Lin L, et al.. Mast cell-derived exosomes promote Th2 cell differentiation via OX40L-OX40 ligation. J Immunol Res. 2016; 3623898: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sallmann E, Reininger B, Brandt S, et al.. High-affinity IgE receptors on dendritic cells exacerbate Th2-dependent inflammation. J Immunol. 2011; 187: 164–171. doi: 10.4049/jimmunol.1003392. Epub 2011 May 27. [DOI] [PubMed] [Google Scholar]
- 29. Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002; 2: 773–786. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.