Abstract
Purpose
To evaluate the evolution of chemosensation via extended psychophysical testing in patients who suffered from sudden chemosensory loss due to coronavirus disease 2019 (COVID-19). Additionally, this study sought to determine whether odor threshold testing provided additional information on olfactory loss due to COVID-19 compared to the more common odor identification testing.
Methods
Prospective cohort study of patients with sudden chemosensory loss since February 2020 and confirmed COVID-19 infection via RT-PCR or serology testing. Olfactory function was tested extensively using the “Sniffin Sticks” test battery. In addition, we screened gustatory perception and nasal cooling sensations using psychophysical tests.
Results
Seventy-two patients completed the study. After a mean of 37 days, 37% of patients showed olfactory dysfunction, 7% were dysgeusic, and 48% showed signs of low sensitivity for cooling sensation. A longer duration of anosmia before smell improvement was correlated with lower olfactory function at 5 weeks. Odor threshold detection was more affected by COVID-19 compared to odor identification.
Conclusion
Five weeks after developing sudden chemosensory loss due to COVID-19, a high proportion of patients were dysosmic and showed signs of low nasal cooling sensitivity, whereas most of them had normal taste function. SARS-CoV-2 affected mainly odor thresholds, possibly suggesting that the major cause of loss of smell lies at the level of the olfactory neuroepithelium, rather than in the central nervous system.
Keywords: Chemosensory loss, Psychophysical test, COVID-19, SARS-CoV-2
Introduction
Since the outbreak of the coronavirus disease 2019 (COVID-19) in December 2019 in China, sudden chemosensory loss (SCL) as a presenting clinical symptom was slow to emerge. Mao et al. [1] reported smell and taste impairment in only 12 out of 214 hospitalized patients between January and mid-February. In contrast, as the disease progressed in Europe, reports of SCL accumulated rapidly with most of them based on surveys [2, 3]. Only a limited number of preliminary quantitative studies started evaluating olfactory and gustatory dysfunction (e.g., [4, 5]). Most of these studies relied solely on screening tests based on odor identification tasks because they are very easy to administer. However, it has been hypothesized that odor detection thresholds represent the function of the peripheral olfactory system to a higher degree than the central nervous processing of olfactory information. Although central nervous damage has also been described, respiratory viruses are expected to produce major damage at the level of the olfactory epithelium [6, 7]. Hence, evaluating detection thresholds may provide specific information on the olfactory loss due to SARS-CoV-2. Moreover, so far studies focused almost entirely on smell and taste and did not investigate the potential loss of trigeminal chemosensation (TCS). Although much less studied than olfaction and gustation, TCS plays a major role in daily life conveying sensations such as airflow, cooling, tingling, burning, or stinging.
As of today, evolution of SCL due to COVID-19 has not been investigated yet by extended psychophysical testing. In this prospective cohort study, we sought to evaluate the evolution of the chemosensory modalities after SCL. We also sought to determine whether testing odor threshold detection provides additional information regarding olfactory loss due to COVID-19 compared to odor identification.
Materials and methods
Study design, setting, and participants
This prospective cohort study was conducted between February 1 and May 30, 2020. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Adult patients with SCL since February 2020 were recruited via public call from our institution. RT-PCR analysis on nasopharyngeal swab or serologic testing for IgG antibodies were performed depending on the date of onset of SCL, ≤ 14 days or > 14 days respectively. Eligible patients were asked to come to the clinic for psychophysical testing at least 2 weeks after the onset of their symptoms. This was necessary to minimize the risk of transmission for our study personnel as psychophysical testing is a 30-min procedure requiring close proximity and access to nostrils and mouth of the participant.
Study population data
Patient demographics, including age, sex, body mass index, and known respiratory allergies were collected. A second questionnaire focused on chemosensory loss: date of onset, duration of anosmia (i.e., the interval between date of onset and beginning of recovery), self-assessment of complete recovery (yes/no), presence of parosmia and/or phantosmia. During patient interview, close-ended questions concerning trigeminal chemosensation were asked and included absent lacrimation while cutting onions, loss of nasal cooling sensation while inhaling eucalyptol/menthol, loss of nasal or oral burning/tingling sensation while eating spicy food, mustard, or wasabi.
Psychophysical measurements
Orthonasal olfactory function was tested using the extended “Sniffin’ Sticks” test battery (Burghart GmbH, Wedel, Germany) based on odor-containing felt tips. Three olfactory tasks were performed: threshold detection (T), discrimination (D), and identification (I). These scores ranged from 1 to 16 (T) or 0 to 16 (D and I). The total olfactory score (TDI score) is the sum of all three subtests and range from 1 to 48. Normative data were used to establish that TDI scores of > 30.5, 16–30.5, and < 16 indicated normosmia, hyposmia, and (functional) anosmia, respectively [8]. Odor threshold (T score) was evaluated following the single-staircase technique with three-alternative forced choice from 16 triplets of stepwise dilutions. In each triplet, two pens were odorless and one was impregnated with n-butanol (cheese-like smell) that the participant had to identify. The final score was the mean of the last four turning points in the staircase. The odor discrimination task (D score) consisted of the identification of the pen that smelled different than two other pens with identical odor. A total of 16 triplets of pens were administered. Finally, for the identification task (I score), the participant was asked to identify each of 16 pens with common odors using a four-alternative forced choice.
To screen for intranasal trigeminal dysfunction, the patient was asked to identify the one pen among the 16 scents that produced a cooling sensation as one of them contained levo-menthol. Screening of taste function was assessed with taste strips for the four basic tastes (sweet, salty, bitter, and sour) at suprathreshold concentrations (Burghart GmbH, Wedel, Germany). Participants were asked to identify the correct taste (four-alternative forced choice). Scores ranged from 0 to 4. Scores of ≥ 3, 2, and ≤ 1 were interpreted as normogeusia, hypogeusia, and ageusia, respectively, (adapted from [9]).
Statistical analysis
The relationship between clinical data and chemosensory scores was analyzed using Spearman correlation for scale data and Fisher’s exact test for categorical data. Comparisons of continuous variables were performed using Kruskal–Wallis H test for three independent groups. Analysis of variance by ranks between the three olfactory task scores were done using Friedman test and post hoc pairwise comparisons with degrees of freedom adjusted according to Bonferroni. Individual Wilcoxon Signed Rank Test (using Bonferroni adjusted alpha value) was used for post hoc comparisons between related samples. All analyses were performed using the Statistical Package for the Social Sciences for Mac OS (SPSS version 25; IBM Corp, Armonk, NY, USA).
Results
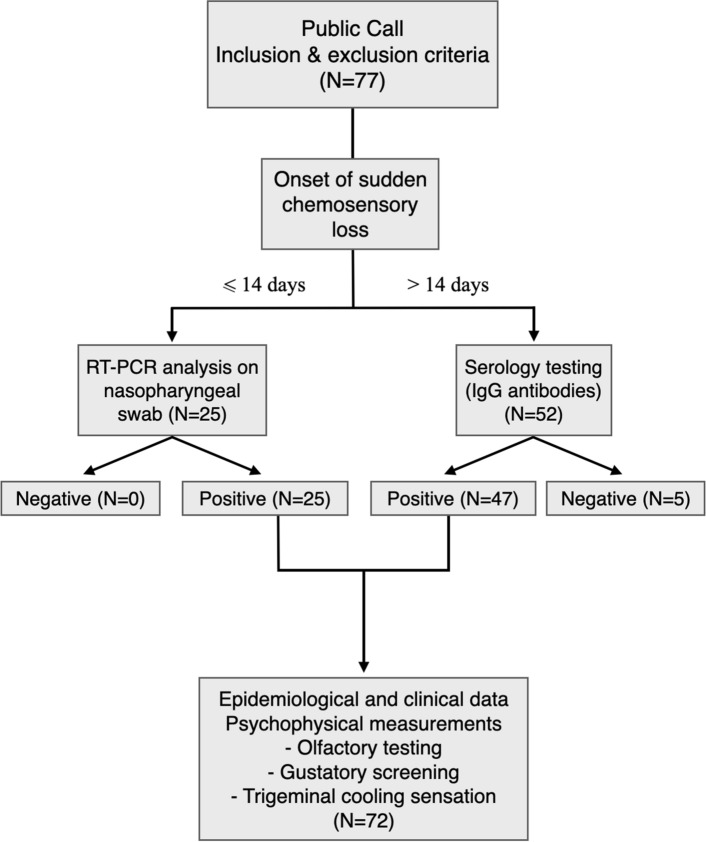
A total of 72 patients were enrolled and completed the study (Fig. 1): 25 patients had a positive RT-PCR analysis on nasopharyngeal swab and 47 had developed IgG antibodies for SARS-CoV-2. Characteristics of study participants are summarized in Table 1.
Fig. 1.
Flow diagram. Flow diagram illustrating cohort selection. COVID-19 coronavirus disease 2019, RT-PCR reverse transcriptase-polymerase chain reaction, IgG immunoglobulin G
Table 1.
Characteristics of study patients
| Characteristic | All patients (N = 72) |
|---|---|
| Age | |
| Mean ± SD, years old | 38.9 ± 12.4 |
| Gender | |
| Female, N (%) | 49 (68.1) |
| Male, N (%) | 23 (31.9) |
| BMI | |
| Mean ± SD, kg/m2 | 24.0 ± 4.3 |
| Allergic patients, N (%) | 11 (15.3) |
| Duration of anosmia | |
| Median (range), days | 17 (3–61) |
| Time interval between SCL onset and psychophysical assessment | |
| Mean ± SD, days | 37.0 ± 10.9 |
BMI body mass index (calculated as weight in kilograms divided by height in meters squared), SCL sudden chemosensory loss, SD standard deviation
Olfaction
After a mean of 37 days after SCL, 6 patients were anosmic (8%), 21 were hyposmic (29%), and 45 were normosmic (63%) with TDI scores (mean ± SD) of 9.8 ± 4.4, 25.7 ± 3.7, and 37.8 ± 3.9, respectively, (Fig. 2 and Table 2). Only 3 patients (4%) considered to have fully recovered their sense of smell, all in the normosmic group. Duration of anosmia was significantly correlated with olfactory subgroup (Table 2). Post hoc comparison showed statistical differences between normosmic and hyposmic groups (p = 0.001), and normosmic and anosmic groups (p = 0.035) but not between anosmic and hyposmic groups.
Fig. 2.
Distribution of patients for each psychophysical test. Distribution of all 72 patients in different categories for each psychophysical test: olfactory (left), gustatory (middle), and trigeminal (right)
Table 2.
Comparison of anosmic, hyposmic, and normosmic patients
| Anosmic (N = 6) | Hyposmic (N = 21) | Normosmic (N = 45) | p value | Test | |
|---|---|---|---|---|---|
| Sociodemographics and clinical data | |||||
| Age (mean ± SD), years old | 42 ± 16.5 | 42.6 ± 15.0 | 37.0 ± 10.0 | 0.391 | KW |
| Sex (F/M) | 5/1 | 13/8 | 31/14 | 0.68 | F |
| BMI (mean ± SD), kg/m2 | 25.8 ± 5.6 | 25.1 ± 4.4 | 23.1 ± 3.9 | 0.134 | KW |
| Allergic patients, N (%) | 1 (17) | 5 (24) | 5 (11) | 0.339 | F |
| Time interval between SCL onset and psychophysical assessment | |||||
| Mean ± SD, days | 41.1 ± 13.3 | 37.1 ± 8.5 | 35.3 ± 11.4 | 0.677 | KW |
| Olfaction | |||||
| Threshold score, (median ± IQR), /16 | 1 ± 0.5 | 3 ± 1.5 | 13 ± 3.00 | ||
| Discrimination score, (median ± IQR), /16 | 4 ± 1.25 | 11 ± 1.25 | 13 ± 1.50 | ||
| Identification score, (median ± IQR), /16 | 5 ± 2.37 | 12 ± 2.00 | 14 ± 1.25 | ||
| TDI score, (mean ± SD), /48 | 9.8 ± 4.4 | 25.7 ± 3.7 | 37.8 ± 3.9 | ||
| Perceived complete recovery, N (%) | 0 (0) | 0 (0) | 3 (7) | 0.651 | F |
| Duration of anosmia, median (range), days | 31 (6–61) | 28 (4–47) | 12 (3–47) | < 0.005 | KW |
| Parosmia, N (%) | 3 (50) | 6 (29) | 7 (13) | 0.199 | F |
| Phantosmia, N (%) | 2 (33) | 5 (24) | 8 (15) | 1 | F |
| Gustatory screening, N (%) | 0.821 | F | |||
| Ageusia | 0 (0) | 0 (0) | 1 (2) | ||
| Hypogeusia | 0 (0) | 2 (9) | 2 (4) | ||
| Normogeusia | 6 (100) | 19 (91) | 42 (94) | ||
| Cooling sensation, N (%) | 0.128 | F | |||
| Incorrect/absent identification | 4 (67) | 13 (62) | 14 (31) | ||
| Correct identification | 2 (33) | 8 (38) | 23 (51) | ||
| Not tested | 0 (0) | 0 (0) | 8 (18) |
BMI body mass index, F Fisher’s exact test, F/M female/male, KW Kruskal–Wallis H test, IQR interquartile range, SCL sudden chemosensory loss, SD standard deviation, TCS trigeminal chemosensation, TDI threshold discrimination identification (olfactory score)
Threshold, discrimination, and identification task scores are shown in Table 2. The results of the Friedman test indicated that there was a statistically significant difference in olfactory testing across all olfactory subtests (threshold, discrimination, identification, χ2 = 30.83 (df = 2, N = 72), p < 0.001). Post hoc pairwise comparisons showed that odor detection threshold score was significantly lower than both discrimination (p = 0.001) and identification scores (p < 0.001), whereas discrimination and identification scores were not statistically different. Similarly, post hoc comparisons between each olfactory group confirmed that odor detection threshold was different for anosmic (p = 0.031) and hyposmic patients (p < 0.005), but not different for normosmic patients (p = 0.185) (Fig. 3). Taken together, these results indicate that odor detection was the olfactory task that mostly determined the final TDI score, and, therefore, was the most affected smell ability by COVID-19 in our cohort.
Fig. 3.
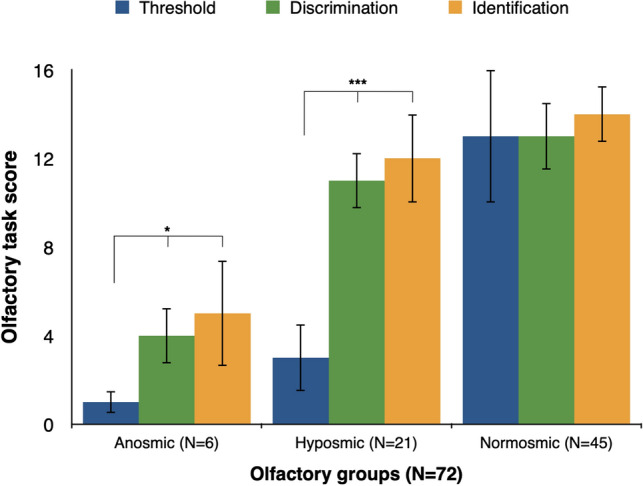
Olfactory subtest scores in each olfactory group. Median olfactory subtest scores across each olfactory group (error bars represent interquartile range). *p < 0.05. ***p < 0.005
Moreover, parosmia and phantosmia were reported in 16 (22%) and 15 (21%) patients, respectively. Their presence was not correlated to lower olfactory score (Table 2). Of note, 6 out of 15 reports of phantosmia were described as a continuous smell of burning or tobacco. We found no correlation between TDI score and patient characteristics, in particular age, sex, and BMI. The relationship between TDI score and reported duration of anosmia was strong (r = − 0.53; p < 0.01) but not with the time interval between onset of olfactory loss and olfactory testing.
Gustatory screening
Sixty-four patients (89%) reported having lost their taste concurrently with their loss of smell. After a mean of 37 days, screening revealed that 1 patient was ageusic (1%), 4 were hypogeusic (6%), and 67 were normogeusic (93%) (Fig. 2). In contrast to olfactory scores, there was no relationship between gustatory scores and duration of anosmia.
Cooling sensation
Eight patients (11%) were unable to answer any of the questions about TCS and were, therefore, not included in the analysis. Absence of at least one trigeminal chemosensory function during the period of SCL was reported by 37 patients (58%). Identification of menthol-induced cooling sensation was correct in 33 patients (52%). There was a higher prevalence of incorrect menthol identification among patients reporting at least one impaired trigeminal function compared with those reporting normal trigeminal function (p < 0.001; N = 64).
Discussion
Five weeks on average after the onset of olfactory loss, 37% of patients were still displaying olfactory dysfunction according to olfactory testing. These results suggest that complete recovery of smell may not always happen after a couple of weeks as it has been found in previous studies [4, 10]. This difference was not explained by the time interval between time of symptom onset and time of assessment. In contrast, reported duration of anosmia was inversely correlated with olfactory scores, suggesting that the longer the initial anosmia, the poorer the smell/taste recovery. The duration of initial anosmia may, therefore, be a useful prognostic factor for smell recovery. Interestingly, this correlation was not found in other studies [5]. Furthermore, neither age, sex, nor BMI predicted olfactory outcome, although they are known to be associated with more severe forms of COVID-19 [11].
Moreover, there was a clear mismatch between our proportion of normosmic patients (62%) and of those who reported full smell recovery (6%). An explanation may be that normosmia as defined by psychophysical measurement and population-based normative data, is not equivalent to the assumption that the patient’s ability to smell returned to its normal level. It may be that some patients tested as normosmic are still recovering. On the other hand, it may also partially reflect the known disparity between olfactory self-rating and objective measuring, emphasizing the importance of studies based on psychophysical testing to investigate chemosensory function rather based on subjective rating alone [12]. Similarly, hyposmic and anosmic patients will have to be followed up to investigate the risk for permanent post-viral olfactory dysfunction, a known yet rare complication of upper respiratory viral infections [13]. Surprisingly, Boscolo-Rizzo et al. [14] found that almost half of their 202 patients reported full resolution after 4 weeks of olfactory loss. Unfortunately, their study focused on subjective assessment of olfactory function and not psychophysical testing which makes comparison to our study difficult.
This is the first study to fully explore olfactory loss due to COVID-19 via extended olfactory testing. We found that odor detection ability was the most affected olfactory task by COVID-19. In comparison to odor identification, this subtest is considered to more closely reflect functions of the olfactory neuroepithelium [6]. This appeals, therefore, to the concept that SARS-CoV-2 induces olfactory loss at the level of the peripheral rather than at a more central nervous level. This is consistent with recent studies showing that cells from the olfactory epithelium express two known proteins used by SARS-CoV-2 to infect human cells (ACE2 and TPMRSS2), to imaging studies showing olfactory cleft mucosal thickening during COVID-19 [15–17] and studies reporting olfactory neuritis during COVID-19 [18]. Regarding other human coronaviruses, experiments in animals showed also that viral spread to the central nervous system started at the olfactory neuroepithelium [19].
In our study, most COVID-19 patients quickly reached high identification scores, as soon as they started to recover smell detection ability. Yet, most studies on COVID-19-related olfactory loss have so far relied solely upon screening tests based on smell identification [5, 20]. This is likely the reason why one study found that 38% of their normosmic patients reported being anosmic [5]. We calculated that if we had used only the identification test, 20 patients (28%) would be in a different olfactory category and 12 hyposmic patients (17%) would have been misdiagnosed as normosmic. Our study suggests that the combined assessment of odor detection threshold and odor identification would be the most appropriate way to address olfactory function in COVID-19, rather than the commonly used odor identification task alone.
The mechanism by which SARS-CoV-2 leads to taste impairment is still unclear. It may be via direct damage of the gustatory organ, as ACE2 receptors have been identified in the mouth and in particular on the tongue. In our study, 93% of our cohort was normogeusic 5 weeks on average after SCL which confirms results by Hintschich et al. [21]. This contrast with olfactory recovery may suggest that patients tend to recover taste better than smell. On the other hand, patients may misinterpret loss of retronasal flavor perception as loss of taste. This may have produced an overestimation of the proportion of patients (89%) who considered themselves dysgeusic initially. Lastly, gustatory loss has also been reported as an accompanying symptom in patients with olfactory loss with the explanation that olfaction amplifies gustation (and vice versa) within the chemosensory system through central nervous interactions [22, 23]. A limitation of our study was the use of a basic taste strip test to screen for gustatory function. Future studies should include an extended taste strip test as proposed by Landis et al. [9] to improve precision in assessing gustatory function and help clarify the role of retro-nasal olfaction in the reported loss of taste.
To our knowledge, this study is among the first to psychophysically address trigeminal impairment due to COVID-19. We found that 58% of patients reported the absence of at least one trigeminal sensation and 48% did not identify menthol-induced cooling sensation. This association may suggest that SARS-CoV-2 affects TCS as well as olfaction and gustation, although proper psychophysical testing is required in future studies to support this idea. Parma et al. found a very similar rate of 46% of participants reporting decreased oral chemesthesis [24]. Intranasal trigeminal nerve endings may also be a potential target for respiratory neurotropic viruses, although this has not been shown yet for human coronaviruses [25]. This may agree with observations in patients with non-COVID-associated postviral olfactory loss where decreased TCS is typical [26]. However, our preliminary results on nasal cooling need to be considered carefully. They require rigorous trigeminal testing in future studies, using a lateralization test, or event-related potentials [27]. Future chemosensory studies should also include assessment of retronasal olfactory function and extended gustatory investigation to clarify the relative importance of retronasal olfaction and dysgeusia in reported loss of taste. Such testing is of particular importance when considering the discrepancies between results from subjective ratings and less biased psychophysical testing [12].
Conclusion
Five weeks after developing sudden olfactory loss due to COVID-19, more than a third of patients displayed olfactory dysfunction according to psychophysical testing. Odor detection was the olfactory ability most affected by SARS-CoV-2 compared to odor discrimination or odor identification. This may also indicate that COVID-19-related smell impairment is predominantly due to peripheral rather than central nervous system damage. Moreover, a longer duration of initial anosmia was correlated with a lower olfactory function, suggesting that it may be of clinical utility to predict smell recovery rate. In contrast to the evolution of smell function, the vast majority of patients scored in the normal range when screening their taste function at 5 weeks, casting doubt on the exact physiological mechanism underlying the reported loss of taste. Moreover, this study found that almost half of the patients appeared to exhibit signs of abnormal nasal cooling sensation prompting more systematic investigations of trigeminal sensitivity in COVID-19.
Acknowledgements
We thank Sylvie Desmet, Marine Dawirs, Danielle Degreef and Ikram Laghmari for their excellent work in coordinating the study. We also thank Olivier Nicod, Jerome Pamart, and Alix Maillet for their help in patient recruitment.
Author contributions
SDLB had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization and methodology: SDLB and MH. Acquisition, analysis, or interpretation of data: SDLB, MH, NP, JV, LP, and GC. Original draft preparation: SDLB, MH. Critical revision of the manuscript for important intellectual content: TH, JRL, OLB, DD, and AR. Statistical analysis: SDLB, and OLB. Administrative, technical, or material support: NP, JV, LP, and M-PT.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
Since 2018, Thomas Hummel did research together with and received funding from Sony, Stuttgart, Germany; Smell and Taste Lab, Geneva, Switzerland; Takasago, Paris, France: aspuraclip, Berlin, Germany.
Ethics approval
The study was performed in accordance with the Declaration of Helsinki on Biomedical Studies Involving Human Subjects. The study protocol was approved by the Review Board of CHU Saint-Pierre, Brussels, Belgium (CHUSP200407).
Consent to participate
Informed written consent was obtained from all participants to participate in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaye R, Chang CWD, Kazahaya K, et al. COVID-19 anosmia reporting tool: initial findings. Otolaryngol Neck Surg. 2020;163:132–134. doi: 10.1177/0194599820922992. [DOI] [PubMed] [Google Scholar]
- 3.Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020 doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck. 2020;42:1252–1258. doi: 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lechien JR, Cabaraux P, Chiesa-Estomba CM, et al. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck. 2020 doi: 10.1002/hed.26279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen DT, Rumeau C, Gallet P, Jankowski R. Olfactory exploration: state of the art. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133:113–118. doi: 10.1016/j.anorl.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 7.Tian J, Pinto JM, Cui X, et al. Sendai virus induces persistent olfactory dysfunction in a murine model of PVOD via effects on apoptosis, cell proliferation, and response to odorants. PLoS One. 2016;11:e0159033. doi: 10.1371/journal.pone.0159033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oleszkiewicz A, Schriever VA, Croy I, et al. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Oto Rhino Laryngol. 2019;276:719–728. doi: 10.1007/s00405-018-5248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landis BN, Welge-Luessen A, Brämerson A, et al. “Taste Strips”—a rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J Neurol. 2009;256:242–248. doi: 10.1007/s00415-009-0088-y. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y, Min P, Lee S, Kim S-W. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 2020 doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landis BN, Hummel T, Hugentobler M, et al. Ratings of overall olfactory function. Chem Senses. 2003;28:691–694. doi: 10.1093/chemse/bjg061. [DOI] [PubMed] [Google Scholar]
- 13.Welge-Lüssen A, Wolfensberger M (2006) Olfactory disorders following upper respiratory tract infections. In: Hummel T, Welge-Lüssen A (eds) Taste and smell. Karger, Basel, pp 125–132 [DOI] [PubMed]
- 14.Boscolo-Rizzo P, Borsetto D, Fabbris C, et al. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol Neck Surg. 2020 doi: 10.1001/jamaoto.2020.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brann DH, Tsukahara T, Weinreb C, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020;6:eabc5801. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem Neurosci. 2020;11:1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spoldi C, Castellani L, Pipolo C, et al. Isolated olfactory cleft involvement in SARS-CoV-2 infection: prevalence and clinical correlates. Eur Arch Oto Rhino Laryngol. 2020 doi: 10.1007/s00405-020-06165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirschenbaum D, Imbach LL, Ulrich S, et al. Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet. 2020;396:166. doi: 10.1016/S0140-6736(20)31525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubé M, Le Coupanec A, Wong AHM, et al. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol. 2018 doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaira LA, Salzano G, Fois AG, et al. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. What we know from the literature. Int Forum Allergy Rhinol. 2020 doi: 10.1111/alr.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hintschich CA, Wenzel JJ, Hummel T, et al. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. Int Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landis BN, Scheibe M, Weber C, et al. Chemosensory interaction: acquired olfactory impairment is associated with decreased taste function. J Neurol. 2010;257:1303–1308. doi: 10.1007/s00415-010-5513-8. [DOI] [PubMed] [Google Scholar]
- 23.Han P, Georgi M, Cuevas M, et al. Decreased electrogustometric taste sensitivity in patients with acquired olfactory dysfunction. Rhinol J. 2018;56:158–165. doi: 10.4193/Rhin17.186. [DOI] [PubMed] [Google Scholar]
- 24.Parma V, Ohla K, Veldhuizen MG, et al. More than smell—COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 2020 doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12:14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frasnelli J, Schuster B, Hummel T. Interactions between olfaction and the trigeminal system: what can be learned from olfactory loss. Cereb Cortex. 2007;17:2268–2275. doi: 10.1093/cercor/bhl135. [DOI] [PubMed] [Google Scholar]
- 27.Hummel T, Frasnelli J (2019) The intranasal trigeminal system. In: Doty R (ed) Handbook of clinical neurology, vol 164, pp 119–134 [DOI] [PubMed]