Summary
During the early stages of the coronavirus disease 2019 (COVID-19) pandemic, EASL and ESCMID published a position paper to provide guidance for physicians involved in the care of patients with chronic liver disease. While some healthcare systems are returning to a more normal routine, many countries and healthcare systems have been, or still are, overwhelmed by the pandemic, which is significantly impacting on the care of these patients. In addition, many studies have been published focusing on how COVID-19 may affect the liver and how pre-existing liver diseases might influence the clinical course of COVID-19. While many aspects remain poorly understood, it has become increasingly evident that pre-existing liver diseases and liver injury during the disease course must be kept in mind when caring for patients with COVID-19. This review should serve as an update on the previous position paper, summarising the evidence for liver disease involvement during COVID-19 and providing recommendations on how to return to routine care wherever possible.
Keywords: Liver, Cirrhosis, Cancer, COVID-19, NAFLD, Telemedicine, Transplantation
Abbreviations: ACE2, angiotensin-converting enzyme 2; ACLF, acute-on-chronic liver failure; COVID-19, coronavirus disease 2019; ERC, endoscopic retrograde cholangiography; HCC, hepatocellular carcinoma; IL-6, interleukin-6; LT, liver transplant; MELD, model for end-stage liver disease; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; OGD, oesophagogastroduodenoscopy; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ULN, upper limit of normal
Pre-existing liver disease as a risk factor for COVID-19
Patients with chronic liver diseases per se do not appear to be over-represented in cohorts of patients with coronavirus disease 2019 (COVID-19) where they make up less than 1% of reported cases.1,2 These observations suggest that patients with chronic liver disease are not at increased risk of contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, the risk of infection and/or the risk of a severe course of COVID-19 may be different depending on the nature of the chronic liver disease and the presence or absence of advanced fibrosis or cirrhosis. We will therefore summarise current evidence on the risk of infection and of a severe COVID-19 course in patients with different liver diseases.
Non-alcoholic fatty liver disease
Obesity represents a significant risk factor for a severe course of COVID-193,4 with severe pneumonia being particularly increased in obese men.3 While the precise mechanisms driving this association remain unclear, it has been postulated that adipose tissue may serve both as a viral reservoir and also an immunological hub for the inflammatory response.5 Similarly, other elements of the metabolic syndrome such as hypertension and diabetes are commonly observed in patients with severe COVID-19.6 As non-alcoholic fatty liver disease (NAFLD, or metabolic dysfunction-associated fatty liver disease)7 and non-alcoholic steatohepatitis (NASH) are closely associated with these metabolic comorbidities, identifying whether the presence of NAFLD specifically predisposes to a more severe course of COVID-19 is of clinical relevance. A retrospective cohort of 202 patients with COVID-19 demonstrated an association between NAFLD and disease progression defined as deteriorating dyspnoea, hypoxia or radiological findings whilst in hospital.8 This additional risk has been observed even in younger patients with NAFLD9 and in the absence of type 2 diabetes10 and interestingly, patients with NAFLD also appear to have a longer duration of viral shedding.8 Within patients with NAFLD, non-invasive fibrosis scores appear to correlate with a higher likelihood of developing severe COVID-19 illness, irrespective of metabolic comorbidities,11 however, genetic polymorphisms implicated in the development and progression of NASH do not appear to be associated with severe disease.12,13 In addition, the transcriptional activity of genes relevant for SARS-CoV-2 infection is not increased in liver tissues from patients with NAFLD.14 Larger analyses are needed to determine whether NAFLD is an independent risk factor for a poor prognosis in COVID-19 or whether the reported effects are due to the presence of confounding factors.
Chronic viral hepatitis
In contrast to metabolic liver disease, little or no evidence has emerged to suggest that the presence of chronic viral hepatitis affects the COVID-19 disease course. Data from both an international registry and from a multicentre cohort study in Italy on COVID-19 outcomes in patients with chronic liver disease include patients with viral hepatitis (23–37%). However, despite both studies demonstrating associations between severity of liver disease and poor outcome, it remains unknown whether the presence of chronic viral hepatitis influences prognosis.15,16
Autoimmune hepatitis
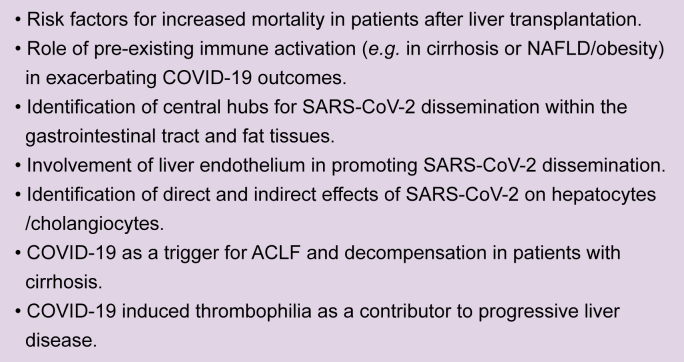
In the previous position paper, we advised against the withdrawal of established immunosuppressive therapy in patients with autoimmune liver disease17 and a panel of experts on autoimmune liver disease have subsequently given similar recommendations.18 While there is still little evidence to demonstrate that immunosuppressive therapy per se predisposes to SARS-CoV-2 infection, a handful of observational studies have suggested an association between corticosteroid use and a more severe COVID-19 disease course.[19], [20], [21], [22], [23] The potential implications of these observations are discussed below in more detail. Further data are needed to determine whether the specific risk of COVID-19 is increased in patients with autoimmune hepatitis and the influence of steroids and/or other immunosuppressive medications on outcome (see Box 1).
Box 1. Open questions for liver-related basic/translational research regarding COVID-19.
COVID-19, coronavirus disease 2019; NAFLD, non-alcoholic fatty liver disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Cirrhosis
Patients with cirrhosis are at increased risk of infections and associated complications due to cirrhosis-associated immune dysfunction, which is particularly important for patients with decompensated cirrhosis. A recent case series from China reported that in 21 consecutive patients with pre-existing cirrhosis, 5 did not survive SARS-CoV-2-infection24 and, specifically, patients with Child-Pugh class C cirrhosis were more likely to suffer a fatal course of COVID-19.24,25 Another case series from Italy documented 50 patients with cirrhosis and COVID-19; 26% of these patients presented with a model for end-stage liver disease (MELD) score ≥15, increasing from 13% at the last documented visit prior to SARS-Cov-2 infection. The 30-day mortality was 34%, with end-stage-liver disease considered as the cause of death in only 5 patients (29%) whilst respiratory failure due to COVID-19 accounted for death in 12 patients (71%).15 These data are in line with observations from an International registry that reported outcomes in 103 patients with cirrhosis – nearly 40% died, while patients with Child-Pugh class C cirrhosis were at the highest risk of a fatal course of COVID-19 (63%, n = 27).16 Similarly, multicentre hospital coding data in the USA demonstrated a significantly higher risk of mortality from COVID-19 in patients with chronic liver disease compared to those without, with the highest risk found in those with cirrhosis.26 However, these data did not have a contemporaneous comparison group of patients with cirrhosis presenting with acute decompensation without COVID-19. Recently, a prospective multicentre study compared outcomes between patients with cirrhosis and COVID-19 (n = 37), cirrhosis alone (n = 127) and COVID-19 alone (n = 108). Although rates of mortality or transfer to hospice in patients with cirrhosis and COVID-19 were greater than in those with COVID-19 alone (30% vs. 13%, p = 0.03) there was no significant difference compared to those with cirrhosis alone (30% vs. 20%; p = 0.11). The presence of acute-on-chronic liver failure (ACLF) was also similar in the 2 cirrhosis groups (29.7% vs. 22.8%) as was mortality in patients with ACLF (55% vs. 36%; p = 0.25) although the number of cases was small.27 Taken together, we cannot currently conclude that COVID-19 increases the risk of ACLF or mortality in patients with cirrhosis more than other causes of decompensation. However, in patients with COVID-19, mortality is markedly greater in those with cirrhosis than in those without cirrhosis.
Liver transplantation recipients
The clinical course of COVID-19 in immunosuppressed transplant recipients may differ from that in non-immunosuppressed patients.28 Indeed, while hepatocellular injury, as characterised by elevated serum aminotransferases, appears to be relatively less prevalent, acute kidney injury is more common in transplant recipients with COVID-19, possibly due to the use of calcineurin inhibitors.28 These findings will need confirmation in larger case series; however, in line with the general risk factors for severe COVID-19, elderly patients with comorbidities are among those with the highest risk within the cohort of transplant recipients.28,29 Early reports from Italy described low mortality rates in transplant recipients <5%,20,30 however subsequent analyses have reported mortality rates in liver and other solid organ transplant recipients at around 25%.28,[31], [32], [33], [34] The results of a prospective European study from 19 transplant centres were recently reported,35 including 57 liver transplant (LT) recipients with confirmed SARS-CoV-2 infection. Overall and in-hospital case-fatality rates were 12% and 17%, respectively, which are similar to the expected mortality for patients with severe COVID-19 infection. Five of the 7 patients who died had an underlying history of cancer. Taken together the currently available data do not support the notion that transplantation or specific immunosuppressive regimens significantly affect the risk of a severe disease course, but those with underlying cancer may require special attention.28,31,35
Liver injury secondary to COVID-19
Deranged liver biochemistry of varying degrees is common in patients with COVID-19, having been reported in 19–76% of cases.[36], [37], [38], [39], [40], [41] Most of these studies report a predominantly hepatocellular pattern of liver injury with elevated serum aminotransferases (rarely >5 × the upper limit of normal) although cholestatic or mixed patterns of liver injury have also been reported. Importantly, this appears to occur to a similar degree in patients with and without pre-existing liver disease26 and has also been documented in pregnant women in association with increased levels of pro-inflammatory cytokines.42 To what extent this liver injury is derived from the direct effect of SARS-CoV-2, as opposed to a secondary phenomenon caused by the broader COVID-19 disease course remains to be elucidated. SARS-CoV-2 infection of hepatocytes with subsequent mitochondrial disturbance and apoptosis has been suggested,41 but requires confirmatory testing, particularly since single cell RNA sequencing has shown relatively sparse hepatocyte expression of the receptors necessary for viral uptake.43 Similarly, direct infection of cholangiocytes via angiotensin-converting enzyme 2 (ACE2) has been posited as a potential mechanism for intrinsic liver injury,44 but requires further investigation. Given the profound multi-systemic involvement of COVID-19, particularly in the severe and critical forms of disease, liver injury is likely to be multifactorial with contributions from systemic inflammation, intrahepatic immune activation, microvascular thrombosis, hepatic congestion, perturbations of the gut-liver-axis, as well as drug toxicity.[45], [46], [47], [48] The prognostic significance of deranged biochemistry in COVID-19 remains unresolved49; some groups have demonstrated a strong correlation with duration of hospitalisation, organ failure and intensive care unit admission37,41,50 whilst others have failed to observe any significant associations with outcome.39,40
Recommendations for the management of patients with chronic liver disease
General recommendations
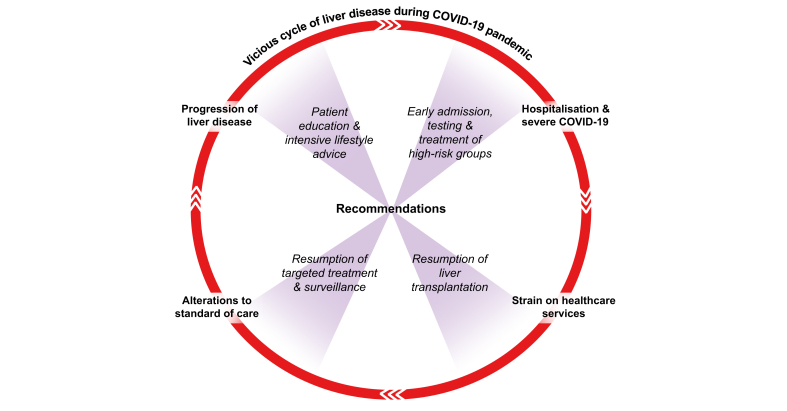
In the aftermath of the COVID-19 peak, there was an urgent need to anticipate and plan for the wave of liver disease yet to come. This will be characterised by emergent hepatic decompensation, increased dropouts from transplant waiting lists and a vast back-log of deferred hospital visits and testing.51 Clinicians and their institutions should therefore be proactive in structuring their services to tackle these challenges and strive to resume standard of care for patients with liver disease wherever possible. Equally, it is important to embrace innovative technologies and methods of practice developed during the pandemic which may continue to be of benefit to patients (e.g. telemedicine use, remote monitoring).52 Combining standard of care with novel ideas will help to mitigate against longer term consequences of the pandemic including missed diagnoses, incomplete hepatocellular carcinoma (HCC) screening, and progressive liver disease. Furthermore, in light of accumulating evidence that baseline liver disease severity is associated with poor outcomes from COVID-19,15,16 treatment of underlying liver disease may represent one of the most important strategies to protect patients from the adverse effects of any future SARS-CoV-2 infection. This in turn will further reduce the burden on healthcare systems and allow more rapid return towards gold standard hepatology practice (Fig. 1). The epidemiology of COVID-19 has proven unpredictable, but the burden of disease is likely to expand and shrink episodically within populations for some time to come. The approach to patient care must therefore be personalised and flexible, balancing national dynamics of SARS-CoV-2 infection, the local resource availability, and the type and severity of each individual patient's underlying liver disease (Fig. 2). Lastly, with time, it will be important to resume clinical trial enrolment wherever possible to allow the field to advance despite unprecedented global events.
Fig. 1.
Liver disease progression and poor outcomes from severe acute respiratory syndrome coronavirus 2 infection are closely associated.
There must therefore be a concerted effort to resume standard of care and restore hepatology/transplantation services in order to improve patient outcomes.
Fig. 2.
Summary of recommendations.
ACLF, acute-on-chronic liver failure; COVID-19, coronavirus disease 2019; HCC, hepatocellular carcinoma; LT, liver transplant; MELD, model for end-stage liver disease; NASH, non-alcoholic steatohepatitis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Specific recommendations
NAFLD
-
•
Patients should be made aware of potential adverse metabolic and hepatic consequences of social isolation, including more sedentary lifestyles and increased consumption of processed foods.
-
•
Preventing liver disease progression through intensive lifestyle intervention, including nutritional guidance, weight loss advice, and diabetes management may help prevent the development of a severe disease course with future SARS-CoV-2 infection.
-
•
Treatment of arterial hypertension should continue in accordance with existing guidelines. There is currently no evidence showing that angiotensin-converting enzyme inhibitors or angiotensin receptor blockers increase the risk of SARS-CoV-2 infection or the risk of developing severe complications or death from COVID-19.53
-
•
Early admission should be considered for all patients with NAFLD who become infected with SARS-CoV-2.
Viral hepatitis
-
•
Continue treatment of chronic HCV and chronic HBV if already receiving treatment.
-
•
Use telemedicine/local laboratory testing for follow-up visits in patients receiving antiviral therapy, send follow-up prescriptions by mail and supply extended prescription supplies including full courses of direct-acting antiviral medications to complete HCV treatment if this has been initiated. However, patients with poor compliance with medications should be considered for directly observed treatment protocols.
-
•
In patients without COVID-19, treatment for HCV and HBV should be initiated according to general guidelines.54,55
-
•
Given the unknown impact of interferon alpha on systemic inflammation associated with COVID-19, alternative agents should be considered when initiating treatment for patients with HBV during the COVID-19 pandemic.
-
•
In patients with COVID-19, initiation of treatment for HBV and HCV is usually not warranted and should be deferred until recovery from COVID-19.
-
•
In patients with COVID-19 in whom there is evidence of high disease activity (flare) or clinical suspicion for severe acute HBV hepatitis, a decision to initiate antiviral therapy should be made on a case-by-case basis in consultation with a specialist.
-
•
In patients with chronic, occult or resolved HBV and COVID-19 receiving corticosteroids, tocilizumab or other immunosuppressive agents, consider the use of antiviral therapy to prevent viral flare or reactivation.
-
•
Continue to work towards the World Health Organization goal of eliminating viral hepatitis by 2030 by trying to adapt the cascade-of-care to the new coronavirus situation and make modifications for safe delivery of services according to local requirements.
Alcohol-related liver disease
-
•
Chronic alcohol consumption may increase an individual's susceptibility to acute respiratory distress syndrome secondary to SARS-CoV-2 infection.56
-
•
Social isolation can lead to new or increased alcohol consumption57; an increase in alcohol-related admissions including new hepatic decompensation should be anticipated during and after periods of physical distancing.
-
•
Clinicians and institutions should therefore implement pre-emptive strategies such as patient outreach and telephone alcohol liaison and cessation services.
-
•
Whilst corticosteroid treatment has shown promise for hospitalised patients with COVID-19 requiring respiratory support,58 concerns remain that patients who are already taking higher doses of corticosteroids may be more susceptible to severe COVID-19.19,59 These concerns must be considered when initiating corticosteroids as a treatment for patients with severe alcoholic hepatitis.
-
•
Clinicians should be aware of misinformation circulating online regarding the protective effects of alcohol against SARS-CoV-2, leading to previous instances of deliberate excess consumption.60
Autoimmune liver disease
-
•
In patients with autoimmune liver disease, we currently advise against reducing immunosuppressive therapy to prevent SARS-CoV-2 infection. Reductions should only be considered under special circumstances (e.g. medication-induced lymphopenia, or bacterial/fungal superinfection in cases of severe COVID-19) after consultation with a specialist.
-
•
Whilst corticosteroid treatment has shown promise for hospitalised patients with COVID-19 requiring respiratory support,58 concerns remain that patients who are already taking higher doses of corticosteroids may be more susceptible to SARS-CoV-2 infection and severe COVID-19.19,59 To minimise systemic glucocorticoid exposure we therefore recommend considering budesonide as a first-line agent to induce remission in patients without cirrhosis who have a flare of autoimmune hepatitis.61
-
•
In patients treated with corticosteroids who develop COVID-19, corticosteroid dosing should be sufficient to prevent adrenal insufficiency. Addition of, or conversion to, dexamethasone should only be considered in patients with COVID-19 who require hospitalisation and respiratory support.58
-
•
There remains a paucity of data to make specific recommendations for patients with primary biliary cholangitis, primary sclerosing cholangitis or IgG4-related disease.
-
•
All patients should receive vaccination for Streptococcus pneumoniae and influenza.
Cirrhosis
-
•
Patients with cirrhosis are particularly vulnerable to both the consequences of SARS-CoV-2 infection and to the adverse effects of delayed or altered standard of care during the COVID-19 pandemic.
-
•
Every effort should be made to resume the best standard of care for patients with cirrhosis according to guidelines62 wherever possible.
-
•
Patients with cirrhosis who are infected with SARS-CoV-2 are at high risk of new or worsening hepatic decompensation, severe COVID-19 and death.15,16
-
•
All patients with new or worsening hepatic decompensation or ACLF should be prioritised for SARS-CoV-2 testing even in the absence of respiratory symptoms.16
-
•
In those with cirrhosis who are admitted for reasons other than COVID-19, particular effort should be made to manage these patients in a designated non-COVID-19 ward, preferably in a side-room, in order to reduce the risk of nosocomial SARS-CoV-2 infection.
-
•
Guidelines on prophylaxis of spontaneous bacterial peritonitis, gastrointestinal haemorrhage, and hepatic encephalopathy should be closely followed to prevent decompensation and avoid admission.62
-
•
Early admission should be considered for all patients with cirrhosis who become infected with SARS-CoV-2.
-
•
Because of the link between COVID-19 and circulatory dysfunction, in particular of the pulmonary circulation,63 the use of vasoconstrictor therapy, which is known to increase pulmonary pressure and decrease cardiac output, should be considered with great caution among critically ill patients with cirrhosis and COVID-19.
-
•
Rapid clinical deterioration in patients with advanced liver disease and COVID-19 should prompt consideration of a symptoms-based approach using palliative care guidelines.64
-
•
All patients should receive vaccination for Streptococcus pneumoniae and influenza.
Liver transplant candidates
-
•
Patients on the LT waiting list with decompensated cirrhosis are at high risk of severe COVID-19 and death following SARS-CoV-2 infection.
-
•
We therefore recommend that LT centres aim to restore transplantation services following the peak of the COVID-19 epidemic wherever possible.
-
•
In centres with ongoing resource limitations, LT should be prioritised for patients with poor short-term prognosis including those with acute liver failure, ACLF, high MELD score (including exceptional MELD points) and HCC at the upper limits of the Milan criteria.
-
•
The risk of SARS-CoV-2 transmission via LT remains unknown65; therefore, we currently recommend testing all donors for SARS-CoV-2 infection by reverse transcription PCR and recommend against using livers from SARS-CoV-2-infected donors.66
-
•
We encourage LT centres to develop and improve local and global risk stratification pathways for LT donors and recipients, incorporating a combination of clinical history, chest radiology, and SARS-CoV-2 testing,67 as well as ethical considerations regarding transplantation activities and allocation.68
-
•
Measures should be taken to reduce the risk of SARS-CoV-2 infection in the peri-transplantation period. In areas of high disease burden, a COVID-19 free pathway through transplantation should be implemented, including strict social isolation for waiting list patients, telephone screening for symptoms and exposures before admission, and perioperative management in a designated clean intensive care unit and post-LT ward.69
-
•
Consent for diagnostic and therapeutic procedures related to transplantation should include the potential risk of nosocomial COVID-19.
-
•
LT candidates should be made aware that infection with SARS-CoV-2 in patients undergoing major surgery is associated with an increased risk of severe COVID-19 and death.70
-
•
Living-donor transplantations should be considered on a case-by-case basis and include careful risk stratification of donor and recipient, incorporating a combination of clinical history, chest radiology, and SARS-CoV-2 testing.
Liver transplant recipients
-
•
We advise against reduction of immunosuppressive therapy to prevent SARS-CoV-2 infection. Reduction should only be considered under special circumstances (e.g. medication-induced lymphopenia, or bacterial/fungal superinfection in case of severe COVID-19) after consultation with a specialist.
-
•
Clinicians must be aware of the high reported rates of fear and anxiety regarding COVID-19 in LT recipients and the barrier this may pose to compliance with immunosuppressive medication and attendance at scheduled medical visits.71
-
•
Drug levels of calcineurin inhibitors and mechanistic target of rapamycin inhibitors should be closely monitored when they are administered together with drugs such as hydroxychloroquine, protease inhibitors or alongside new trial drugs for COVID-19.
-
•
Early admission should be considered for all LT recipients who develop COVID-19.
-
•
Risk factors for a severe course of COVID-19 in LT recipients may include underlying malignancy, sarcopenia, graft dysfunction and metabolic comorbidities. However, the individual contributions of these factors require further clarification.
-
•
All patients should receive vaccination for Streptococcus pneumoniae and influenza.
Hepatocellular carcinoma
-
•
The specific risk of COVID-19 in patients with HCC remains undefined.
-
•
However, mortality from COVID-19 in patients with cancer appears to be determined by age, sex, and comorbidities as opposed to the use of cytotoxic chemotherapy or other anticancer treatment.72
-
•
Care should be maintained according to guidelines including continuing systemic treatments and evaluation for LT.
-
•
Multidisciplinary HCC boards should continue to function remotely and provide treatment recommendations.
-
•
Full HCC surveillance should resume where possible. Where resources remain limited, patients at increased risk, such as those with elevated alpha-fetoprotein levels, advanced cirrhosis, chronic hepatitis B, NASH/diabetes etc. should be prioritised in conjunction with the use of published HCC risk stratification scores.
Liver-related diagnostic procedures
Endoscopy
Endoscopic procedures are associated with an increased risk of disseminating SARS-CoV-2. During oesophagogastroduodenoscopy (OGD) or endoscopic retrograde cholangiography (ERC), spreading of virus-containing droplets can occur. In addition, shedding of the virus in the faeces increases the risk of dissemination during colonoscopy. Thus, depending on the local COVID-19 burden, we recommend SARS-CoV-2 testing prior to endoscopic procedures in all patients. In patients who test negative and in areas with low COVID-19 burden, OGD (to screen for and treat varices) and ERC (for duct dilatation or stent replacement in patients after LT or patients with primary sclerosing cholangitis) should not be delayed.
In patients with COVID-19, indications for endoscopic procedures should be limited to emergencies such as gastrointestinal bleeding and bacterial cholangitis.
Ultrasound (HCC surveillance)
HCC surveillance should only be deferred based on available resources (including availability of therapeutic options in case of HCC diagnosis) at the centre and the individual risk assessment. Patients with increased risk (e.g. patients with elevated alpha-fetoprotein levels, advanced cirrhosis, chronic hepatitis B, HCV-related cirrhosis [even after cure], NASH/diabetes) should be prioritised if resources are limited.
In patients with COVID-19, HCC surveillance can be deferred until after recovery.
Liver biopsy
In areas with low COVID-19 burden, liver biopsies should be performed as indicated, including grading/staging for NAFLD and chronic viral hepatitis and histological assessment of elevated transaminases of unknown aetiology. In areas with high COVID-19 burden or limited availability of resources, biopsy should be prioritised in patients with severely elevated transaminases of unclear cause (e.g. alanine aminotransferase >5× upper limit of normal), suspected transplant rejection and liver masses suspicious of malignancy.
In patients with COVID-19, liver biopsy may be performed based on the individual indication for histological assessment. It must be considered that treatment/care for COVID-19 may outweigh the diagnosis of co-existing liver disease and that systemic inflammation associated with COVID-19 is likely to obscure aetiology-specific histologic characteristics. As discussed earlier, liver function test abnormalities are common in patients with COVID-19, particularly in those with more severe disease, and routine liver biopsy is not required in this context.
Liver-specific considerations in the pharmacological management of COVID-19
The targeted management of COVID-19 is a rapidly evolving field with a plethora of new or repurposed medications constantly shifting in and out of favour. In Europe alone there are currently over 200 registered COVID-19 specific drug trials.73 This number will no doubt continue to increase as we learn more about the pathophysiology of the disease. It is beyond the scope of this updated position paper to comprehensively review the potential therapeutic options for COVID-19. While some interventions, such as infusion of convalescent plasma or favipiravir (recently approved in India) show encouraging signals of efficacy, little is known about their liver-specific side effects or contraindications. However, for some therapeutic agents there are liver-specific considerations which we will discuss. Hepatologists must be mindful of the secondary effects these drugs may have on the liver and continually evaluate the specific risks and benefits conferred to their patients with underlying liver disease.
Remdesivir
Remdesivir is an adenosine-analogue that induces RNA chain termination and was initially developed as an antiviral agent against Ebola. It has emerged as a promising treatment candidate against COVID-19, having been shown to reduce the duration of symptoms when used early in the disease course.74,75 Despite preclinical investigations demonstrating reversible aminotransferase elevations,76 use of remdesivir in controlled trials has not demonstrated a significant impact on liver function tests compared with placebo. In the largest randomised control trial to date, Beigel et al. observed no difference in the rate of aminotransferase elevation between patients taking remdesivir compared with placebo (4% vs. 5.9%).74 Similarly, Wang et al. also reported comparable rates of transaminase elevation in patients receiving remdesivir or placebo.77 Both trials excluded patients with baseline alanine or aspartate aminotransferase >5× upper limit of normal and Wang et al. also excluded patients with cirrhosis. Should remdesivir ultimately move into mainstream use, caution should therefore be exercised in patients with advanced liver disease or with severe baseline derangements in liver biochemistry, but otherwise transaminase elevations do not appear to occur over and above what may be expected as part of the typical disease course of COVID-19. Remdesivir is approved for the treatment of COVID-19 in several European countries.
Tocilizumab
Interleukin-6 (IL-6) appears to be a key driver of the “cytokine storm” leading to significant lung and other organ damage in cases of severe COVID-19. Tocilizumab, a humanised monoclonal antibody targeting IL-6 has therefore been postulated to counter this dysregulated inflammation and has shown promise in retrospective series of COVID-19 by reducing the need for, and duration of, organ support.78 The liver-specific side effect profile of tocilizumab is well established due to its widespread use in rheumatoid arthritis and other auto-inflammatory conditions. Mild serum aminotransferase elevations are common and are usually self-limiting and asymptomatic,79 however, progressive jaundice requiring LT has been reported.80 Rarely, tocilizumab has been associated with HBV reactivation81 and therefore HBV serology should form part of routine pre-treatment work-up.
Corticosteroids
There seems to be a dichotomous relationship between corticosteroids and COVID-19. Whilst patients already taking corticosteroids may be at increased risk of adverse outcomes from COVID-19, those with established severe disease seem to paradoxically benefit from corticosteroid introduction. In patients with inflammatory bowel disease, the use of corticosteroids has been associated with intensive care unit admission, ventilator requirement and/or death.19 Similarly, patients on maintenance glucocorticoids for rheumatological conditions have an increased rate of hospitalisation following SARS-CoV-2 infection.59 As yet, small case series have been unable to draw definitive conclusions regarding the risks posed by corticosteroid use in patients with autoimmune hepatitis or after LT.31,34 In these patients, the risks of hepatitis flares or graft rejection must be weighed against the potential risks of developing severe COVID-19. Currently, we advise against routine reduction of immunosuppression in patients with autoimmune hepatitis or LT recipients, including the use of steroids if required. Corticosteroids however do appear to be a viable treatment option for patients with severe COVID-19 requiring respiratory support. In June 2020, the RECOVERY58 trial reported that dexamethasone reduced deaths by one-third in ventilated patients and by one-fifth in patients receiving supplemental oxygen. It is likely that this agent will be increasingly used in the management of severe COVID-19 including in patients with pre-existing chronic liver disease.
Anticoagulation
Patients with advanced liver disease are at increased risk of venous thromboembolism.82 Similarly, coagulopathy is a common abnormality in patients with COVID-19 and has emerged as a major driver of morbidity and mortality, particularly in patients with severe disease. Hospitalised patients with COVID-19 have alarmingly high rates of venous thromboembolic disease with an observed incidence of 20% at day 7, and 42% at day 21 despite thromboprophylaxis.83 As well as macro-thrombotic events, COVID-19 is also associated with widespread micro-thrombosis and endothelial dysfunction contributing to multiorgan failure in the terminal phase of the disease.84,85 The role of anticoagulation in patients with COVID-19 has therefore been extensively investigated and has been shown to improve outcomes in severe COVID-19,86 although unified risk stratification models and treatment thresholds have yet to emerge. Given that both advanced liver disease and COVID-19 are both associated with a hypercoagulable state, it reasons that SARS-CoV-2 infection in patients with cirrhosis may yield a cumulative risk of prothrombotic complications. We therefore suggest that in this scenario patients should be deemed at particularly high risk of thromboembolic events. Whilst historically there have been reservations about the use of anticoagulation in patients with cirrhosis and portal hypertension, systematic review has demonstrated no excess of bleeding events in anticoagulated patients with cirrhosis and portal vein thrombosis.87 Furthermore, anticoagulation may have antifibrotic properties88 and even confer a survival advantage in patients with cirrhosis.89 Further reassurance is provided by a recent multicentre Italian study in which 80% of patients with cirrhosis and COVID-19 received thromboprophylaxis without any evidence of major haemorrhagic complications.15 Whilst thromboprophylaxis, typically with low molecular weight heparin, should form part of standard of care for all patients with cirrhosis admitted to hospital, it remains to be determined whether patients with COVID-19 and cirrhosis should receive early treatment with enhanced or therapeutic anticoagulation.
Financial support
P.N.N. was supported by the National Institute of Health Research (NIHR) Birmingham Biomedical Research Centre (BRC). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Authors' contributions
T.Bo. Conceptualization, Supervision, Writing – original draft; T.M. Conceptualization, Visualization, Writing – original draft; P.N.N. Conceptualization, Supervision; M.Mo. Conceptualization, Validation; M.Ma. Validation; E.C. Validation; R.J. Validation, Writing – original draft; R.M. Validation, Writing – original draft; M.C. Conceptualization, Validation; T.Be. Conceptualization, Supervision, Writing – original draft.
Conflict of interest
T.Bo. reports consultancy fees from Gilead. R.J. reports grants from Yaqrit Discovery Limited, other from Yaqrit Discovery Limited, Founder of Hepyx Limited, license of drug, ornithine phenylacetate to Mallinckrodt, Founder of Cyberliver Limited, outside the submitted work. M.C. reports personal fees from Gilead. T.Be. reports grants, personal fees and non-financial support from Gilead. All other authors report no conflicts of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The authors would like to thank Pierre-Emmanuel Rautou, Karine Lacombe, Slim Fourati and Subash C. Sonkar for valuable input on the manuscript.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100169.
Supplementary data
References
- 1.Team C.C.-R. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 — United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson E., Walker A.J., Bhaskaran K.J., Bacon S., Bates C., Morton C.E. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020 doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai Q., Chen F., Wang T., Luo F., Liu X., Wu Q. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43:1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 4.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan P.M., Caplice N.M. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity. 2020;28:1191–1194. doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eslam M., Sanyal A.J., George J., Sanyal A., Neuschwander-Tetri B., Tiribelli C. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 8.Ji D., Qin E., Xu J., Zhang D., Cheng G., Wang Y. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y.-J., Zheng K.I., Wang X.-B., Yan H.-D., Sun Q.-F., Pan K.-H. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: a multicenter preliminary analysis. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao F., Zheng K.I., Wang X.B., Yan H.D., Sun Q.F., Pan K.H. Metabolic associated fatty liver disease increases COVID-19 disease severity in non-diabetic patients. J Gastroenterol Hepatol. 2020 doi: 10.1111/jgh.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Targher G., Mantovani A., Byrne C.D., Wang X.B., Yan H.D., Sun Q.F. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020 doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 12.Valenti L., Jamialahmadi O., Romeo S. Lack of genetic evidence that fatty liver disease predisposes to COVID-19. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., Invernizzi P. Genomewide association study of severe covid-19 with respiratory failure. N Engl J Med. 2020 doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biquard L., Valla D., Rautou P.-E. No evidence for an increased liver uptake of SARS-CoV-2 in metabolic associated fatty liver disease. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iavarone M., D'Ambrosio R., Soria A., Triolo M., Pugliese N., Del Poggio P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon A.M., Webb G.J., Aloman C., Armstrong M.J., Cargill T., Dhanasekaran R. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boettler T., Newsome P.N., Mondelli M.U., Maticic M., Cordero E., Cornberg M. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lleo A., Invernizzi P., Lohse A.W., Aghemo A., Carbone M. Management of patients with autoimmune liver disease during COVID-19 pandemic. J Hepatol. 2020;73:453–455. doi: 10.1016/j.jhep.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenner E.J., Ungaro R.C., Gearry R.B., Kaplan G.G., Kissous-Hunt M., Lewis J.D. Corticosteroids, but not TNF Antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26:832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 21.Di Giorgio A., Nicastro E., Speziani C., De Giorgio M., Pasulo L., Magro B. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monti S., Balduzzi S., Delvino P., Bellis E., Quadrelli V.S., Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020;79:667–668. doi: 10.1136/annrheumdis-2020-217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norsa L., Indriolo A., Sansotta N., Cosimo P., Greco S., D'Antiga L. Uneventful course in IBD patients during SARS-CoV-2 outbreak in northern Italy. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi X., Liu Y., Wang J., Fallowfield J., Wang J., Li X. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut. 2020 doi: 10.1136/gutjnl-2020-321666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi X., Wang J., Li X., Wang Z., Liu Y., Yang H. Clinical course of COVID-19 in patients with pre-existing decompensated cirrhosis: initial report from China. Hepatol Int. 2020;14:478–482. doi: 10.1007/s12072-020-10051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh S., Khan A. Clinical characteristics and outcomes of COVID-19 among patients with pre-existing liver disease in United States: a Multi-Center Research Network study. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajaj J., Garcia-Tsao G., Biggins S., Kamath P., Wong F., McGeorge S. Comparison of mortality risk in patients with cirrhosis and covid-19 compared to cirrhosis alone and covid-19 alone: a Multi-Center Matched Cohort. Gut. 2020 doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee B.T., Perumalswami P.V., Im G.Y., Florman S., Schiano T.D. COVID-19 in liver transplant recipients: an initial experience from the U.S. Epicenter. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao F., Zheng K.I., Gu J.Y., George J., Zheng M.H. COVID-19 and Liver Transplantation: lessons learned from three reported cases. Transpl Infect Dis. 2020;4:e13335. doi: 10.1111/tid.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhoori S., Rossi R.E., Citterio D., Mazzaferro V.S. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belli L.S., Duvoux C., Karam V., Adam R., Cuervas-Mons V., Pasulo L. COVID-19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol. 2020;5:724–725. doi: 10.1016/S2468-1253(20)30183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernández-Ruiz M., Andrés A., Loinaz C., Delgado J.F., López-Medrano F., San Juan R. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20:1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira M.R., Mohan S., Cohen D.J., Husain S.A., Dube G.K., Ratner L.E. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webb G.J., Moon A.M., Barnes E., Barritt A.S., Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;5:643–644. doi: 10.1016/S2468-1253(20)30125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becchetti C., Zambelli M.F., Pasulo L., Donato M.F., Invernizzi F., Detry O. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020 doi: 10.1136/gutjnl-2020-321923. gutjnl-2020-321923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloom P.P., Meyerowitz E.A., Reinus Z., Daidone M., Gustafson J., Kim A.Y. Liver biochemistries in hospitalized patients with COVID-19. Hepatology (Baltimore, Md) 2020 doi: 10.1002/hep.31326. [DOI] [PubMed] [Google Scholar]
- 37.Cai Q., Huang D., Yu H., Zhu Z., Xia Z., Su Y. COVID-19: abnormal liver function tests. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao R., Qiu Y., He J.S., Tan J.Y., Li X.H., Liang J. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi X., Liu C., Jiang Z., Gu Y., Zhang G., Shao C. Multicenter analysis of clinical characteristics and outcomes in patients with COVID-19 who develop liver injury. J Hepatol. 2020;73:455–458. doi: 10.1016/j.jhep.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vespa E., Pugliese N., Piovani D., Capogreco A., Danese S., Aghemo A. Liver tests abnormalities in COVID-19: trick or treat? J Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y., Liu S., Liu H., Li W., Lin F., Jiang L. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng G., Zeng F., Zhang L., Chen H., Chen X., Yin M. Characteristics of pregnant COVID-19 patients with liver injury. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Smet V., Verhulst S., van Grunsven L.A. Single cell RNA sequencing analysis did not predict hepatocyte infection by SARS-CoV-2. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chai X., Hu L., Zhang Y., Han W., Lu Z., Ke A. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 2020.2002.2003.931766. [Google Scholar]
- 45.Morgan K., Samuel K., Vandeputte M., Hayes P.C., Plevris J.N. SARS-CoV-2 infection and the liver. Pathogens. 2020;9:430. doi: 10.3390/pathogens9060430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Assante G., Williams R., Youngson N.A. Is the increased risk for MAFLD patients to develop severe COVID-19 linked to perturbation of the gut-liver axis? J Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fraga M., Moradpour D., Artru F., Romailler E., Tschopp J., Schneider A. Hepatocellular type II fibrinogen inclusions in a patient with severe COVID-19 and hepatitis. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonzogni A., Previtali G., Seghezzi M., Alessio M.G., Gianatti A., Licini L. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020 doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jothimani D., Venugopal R., Abedin M.F., Kaliamoorthy I., Rela M. COVID-19 and liver. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tapper E.B., Asrani S.K. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serper M., Shaked A., Olthoff K.M., Hoteit M., Appolo B., Reddy K.R. A local response to COVID-19 for advanced liver disease: current model of care, challenges and opportunities. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.The European Society for Cardiology . 2020. ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic.https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance [cited (Last update: 10 June 2020)]; Available at: [Google Scholar]
- 54.Lampertico P., Agarwal K., Berg T., Buti M., Janssen H.L.A., Papatheodoridis G. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Pawlotsky J.-M., Negro F., Aghemo A., Berenguer M., Dalgard O., Dusheiko G. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 56.Crews F.T., Bechara R., Brown L.A., Guidot D.M., Mandrekar P., Oak S. Cytokines and alcohol. Alcohol Clin Exp Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 57.Da B.L., Im G.Y., Schiano T.D. COVID-19 hangover: a rising tide of alcohol use disorder and alcohol-associated liver disease. Hepatology (Baltimore, Md) 2020 doi: 10.1002/hep.31307. [DOI] [PubMed] [Google Scholar]
- 58.Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. 2020 2020.2006.2022.20137273. [Google Scholar]
- 59.Gianfrancesco M., Hyrich K.L., Al-Adely S., Carmona L., Danila M.I., Gossec L. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alcohol and COVID-19: what you need to know. https://www.euro.who.int/en/health-topics/disease-prevention/alcohol-use/data-and-statistics/fact-sheet-alcohol-and-covid-19-what-you-need-to-know [cited June 24th, 2020]; Available at:
- 61.Manns M.P., Woynarowski M., Kreisel W., Lurie Y., Rust C., Zuckerman E. Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis. Gastroenterology. 2010;139:1198–1206. doi: 10.1053/j.gastro.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 62.Angeli P., Bernardi M., Villanueva C., Francoz C., Mookerjee R.P., Trebicka J. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 63.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Introduction to the ESMO COVID-19 palliative care pathways. https://www.esmo.org/covid-19-and-cancer/covid-19-full-coverage/covid-19-useful-resources/covid-19-palliative-care-pathways [cited June 17th 2020]; Available at:
- 65.Kates O.S., Fisher C.E., Rakita R.M., Reyes J.D., Limaye A.P. Use of SARS-CoV-2-infected deceased organ donors: should we always “just say no?”. Am J Transplant. 2020;20:1787–1794. doi: 10.1111/ajt.16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Di Maira T., Berenguer M. COVID-19 and liver transplantation. Nat Rev Gastroenterol Hepatol. 2020 doi: 10.1038/s41575-020-0347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galvan N.T.N., Moreno N.F., Garza J.E., Bourgeois S., Hemmersbach-Miller M., Murthy B. Donor and transplant candidate selection for solid organ transplantation during the COVID-19 pandemic. Am J Transplant. 2020 doi: 10.1111/ajt.16138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chew C.A., Iyer S.G., Chieh Kow A.W., Madhavan K., Teng Wong A.S., Halazun K.J. An international multicentre study of protocols for liver transplantation during a pandemic: a case for quadripartite equipoise. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lembach H., Hann A., McKay S.C., Hartog H., Vasanth S., El-Dalil P. Resuming liver transplantation amid the COVID-19 pandemic. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet (London, England) 2020 doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reuken P.A., Rauchfuss F., Albers S., Settmacher U., Trautwein C., Bruns T. Between fear and courage: attitudes, beliefs, and behavior of liver transplantation recipients and waiting list candidates during the COVID-19 pandemic. Am J Transplant. 2020 doi: 10.1111/ajt.16118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee L.Y.W., Cazier J.B., Starkey T., Turnbull C.D., Kerr R., Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.EU Clinical Trials Register - Clinical trials for covid-19. https://www.clinicaltrialsregister.eu/ctr-search/search?query=covid-19 [cited June 21st, 2020]; Available at:
- 74.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of covid-19 — preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 75.Goldman J.D., Lye D.C.B., Hui D.S., Marks K.M., Bruno R., Montejano R. Remdesivir for 5 or 10 days in patients with severe covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Remdesivir . National Library of Medicine (US); Bethesda (MD): 2006. Drugs and Lactation Database (LactMed) [Google Scholar]
- 77.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:E474–E484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maini R.N., Taylor P.C., Szechinski J., Pavelka K., Bröll J., Balint G. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817–2829. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- 80.Anger F., Wiegering A., Wagner J., Lock J., Baur J., Haug L. Toxic drug-induced liver failure during therapy of rheumatoid arthritis with tocilizumab subcutaneously: a case report. Rheumatology (Oxford, England) 2017;56:1628–1629. doi: 10.1093/rheumatology/kex221. [DOI] [PubMed] [Google Scholar]
- 81.Chen L.-F., Mo Y.-Q., Jing J., Ma J.-D., Zheng D.-H., Dai L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: a prospective clinical observation. Int J Rheum Dis. 2017;20:859–869. doi: 10.1111/1756-185X.13010. [DOI] [PubMed] [Google Scholar]
- 82.Søgaard K.K., Horváth-Puhó E., Grønbaek H., Jepsen P., Vilstrup H., Sørensen H.T. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol. 2009;104:96–101. doi: 10.1038/ajg.2008.34. [DOI] [PubMed] [Google Scholar]
- 83.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McGonagle D., O'Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loffredo L., Pastori D., Farcomeni A., Violi F. Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and meta-analysis. Gastroenterology. 2017;153:480–487.e1. doi: 10.1053/j.gastro.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 88.Turco L., de Raucourt E., Valla D.C., Villa E. Anticoagulation in the cirrhotic patient. JHEP Rep. 2019;1:227–239. doi: 10.1016/j.jhepr.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Villa E., Cammà C., Marietta M., Luongo M., Critelli R., Colopi S. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253–1260.e4. doi: 10.1053/j.gastro.2012.07.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.