Abstract
Topic
Systematic review of risk factors for nonadherence and nonpersistence to intravitreal anti–vascular endothelial growth factor (VEGF) injection therapy for neovascular age-related macular degeneration (nAMD).
Clinical Relevance
Lack of adherence (nonadherence) or undertreatment (nonpersistence) with respect to evidence from clinical trials remains a significant barrier to optimizing real-world outcomes for patients with nAMD. Contributing factors and strategies to address this are poorly understood.
Methods
Studies that reported factors for nonadherence and nonpersistence to anti-VEGF therapy as well as studies examining strategies to improve this were included. Trial eligibility and data extraction were conducted according to Cochrane review methods. Risk of bias was assessed using the Mixed Method Assessment Tool and certainty of evidence evaluated according to the GRADE Confidence in the Evidence from Reviews of Qualitative Research tool. Data were collated descriptively.
Results
Of the 1284 abstract results screened, 124 articles were assessed in full and 37 studies met the inclusion criteria. Definitions of nonadherence and nonpersistence varied or were not reported. Nonpersistence occurred early, with up to 50% of patients stopping treatment by 24 months. High rates of nonadherence were similarly reported, occurring in 32% to 95% of patients. Certainty of this finding was downgraded to a moderate level because of the heterogeneity in definitions used across studies. Multiple factors determine nonadherence and nonpersistence, including at the condition, therapy, patient, social/economic, and health systems/healthcare team levels. Moderate quality evidence points to lower baseline vision and poorer response to treatment as condition-related variables. The effects of other factors were of lower certainty, predominantly due to small numbers and potential biases in retrospective assessment. Although many factors are not modifiable (e.g., patient comorbidity), other factors are potentially correctable (e.g., lack of transport or mismatched patient expectations). Evidence on strategies to improve adherence and persistence is limited, but where available, these have proven effective.
Conclusions
Awareness of factors related to poor patient adherence and persistence in nAMD could help identify at-risk populations and improve real-world outcomes. Further work is required to develop uniform definitions and establish high-quality evidence on interventions that can be easily implemented.
Keywords: Adherence, persistence, nonadherence, nonpersistence, compliance, intravitreal, anti-VEGF, age-related macular degeneration
Abbreviations and Acronyms: nAMD, neovascular age-related macular degeneration; PRN, pro re nata; T&E, treat-and-extend; VEGF, vascular endothelial growth factor
Intravitreal anti–vascular endothelial growth factor (VEGF) injections have revolutionized the treatment of neovascular age-related macular degeneration (nAMD).1 , 2 Landmark clinical trials have demonstrated that anti-VEGF injections stabilize disease, and initial and prolonged visual gains are common.3 , 4
Treatment of nAMD requires frequent intravitreal injections. Although the early pivotal trials used a monthly injection regimen, given the difficulties with such intensive treatment, other more flexible regimens have since been developed, with pro re nata (PRN) and treat-and-extend (T&E) protocols the most commonly used.2
Real-world evidence suggests that even with these “less taxing” alternate dosing regimens, outcomes seen in practice mostly do not reach the levels achieved in trial settings, with the discrepancy possibly due to lack of adherence to clinical trial regimens (defined in this article as nonadherence) or lack of persistence with following recommended clinical trial regimens over time (defined as nonpersistence). For example, a recent meta-analysis of real-world observational data based on approximately 26 000 patients reported a mean visual gain of only +5.0 Early Treatment of Diabetic Retinopathy Study letters after 12 months of treatment, with a mean number of 5.4 injections over 8.3 visits.5 This is well below the +11.3 letters seen in the ANCHOR trial with monthly intravitreal ranibizumab6 and +8.9 letters in VIEW1/VIEW 2 studies7 with intravitreal aflibercept every 8 weeks. Long-term results from both clinical trials and registry data also confirm this finding, with more frequent injections consistently showing better visual outcomes.8 , 9
Given the importance of encouraging ongoing and frequent injections, there is a relative lack of awareness among physicians and the health community of the barriers that lead to the inter-related phenomenon of nonadherence and nonpersistence of anti-VEGF treatment in nAMD in the real world. Terminology and agreed definitions may not exist. There is even less discussion on strategies to correct or counteract these barriers. Previous studies have attempted to look at this from a local practice level or focused only on the patient experience.10 , 11 However, a comprehensive analysis has not been performed to date. The purpose of this systematic review is to assess the factors affecting treatment nonadherence and nonpersistence to intravitreal anti-VEGF injections in nAMD.
Methods
This systematic review was conducted in accordance with the principles set out in the Cochrane Handbook for Systematic Reviews of Interventions.12 The protocol for this systematic review was registered with the international PROSPERO database (ID: 172653) before data extraction. Our results and methods are presented in reference to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (http://www.prisma-statement.org, accessed 26 May 2019). All work adhered to the principles of the Declaration of Helsinki.
Eligibility Criteria for Considering Studies for This Review
Studies were eligible to be included in this systematic review based on the following criteria as set out in the Patient, Intervention, Comparator, and Outcome paradigm (Table 1 ). No eligibility restrictions were placed on the basis of the type of anti-VEGF used or the treatment regimen used. The minimum definitions of nonadherence and nonpersistence were not set in advance to allow for maximal inclusion of studies examining this topic. However, it was accepted that the term “nonadherence” was synonymous with “noncompliance.” Likewise, the term “nonpersistence” was interchangeable with “discontinuation,” “cessation,” “lost to follow-up,” or “dropout.” Both quantitative and qualitative studies were eligible for inclusion to comprehensively address all aspects of the research question.
Table 1.
Eligibility Criteria Based on the PICO Strategy
| PICO Component | Inclusion Criteria | Exclusion Criteria | |
|---|---|---|---|
| P | Patients | Studies including patients diagnosed with nAMD | Studies not reporting outcomes separately for patients with nAMD |
| I | Intervention | Patients received at least 1 intravitreal injection of ranibizumab and/or bevacizumab and/or aflibercept | Studies with patients receiving intravitreal injections other than anti-VEGF (e.g., triamcinolone) or other treatment (e.g., photodynamic therapy) |
| C | Comparison | Not applicable | Not applicable |
| O | Outcomes |
|
No specific exclusions |
NA = nonadherence; nAMD = neovascular age-related macular degeneration; NP = nonpersistence; VEGF = vascular endothelial growth factor.
Studies were excluded if they assessed retinal conditions other than nAMD or evaluated interventions for nAMD other than intravitreal anti-VEGF injections. Conference abstracts were also excluded because of the inability to critically assess findings.
The primary outcome measure for this review was reasons or risk factors for treatment nonadherence and nonpersistence after at least 1 intravitreal anti-VEGF injection. Secondary outcome measures included efficacy of strategies to improve treatment adherence and persistence, as well as the rates of nonadherence and nonpersistence. Further assessments were made for factors that may be identified as general barriers to treatment.
Search Methods for Identifying Studies
The following databases were searched: MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), clinicaltrials.gov online database, and Google Scholar. Databases were last searched and results updated on March 19, 2020. In addition, the reference lists from eligible studies were also reviewed to identify any additional suitable reports. No language restrictions were imposed, but if the report was not in English, the text was translated to allow for data extraction and full analysis of the risk of bias. There were no limits placed on publication date, but all studies had to be original and available in full. The search string for each database is provided in the Supplementary Material (Appendix and Table S1, available at www.aaojournal.org). References from the search results were imported into a reference management program (Zotero v5.0.66, open-source software, https://www.zotero.org).
Study Selection
After the database search, the studies were screened by appraising title and abstracts. Those studies that were considered to be consistent with the search criteria were analyzed in full text to confirm their eligibility. Two reviewers assessed the search results independently (M.O. and C.H.), and consensus was reached if there were any differences.
Data Collection and Quality of Evidence Assessment
Data from each eligible study were extracted and collected in a standardized Word (Microsoft Office, Microsoft Corp, Redmond, WA) document form (Table S2, available at www.aaojournal.org). Study origins and treatment setting (e.g., country, hospital clinic), patient demographics (e.g., age and baseline visual acuity), and treatment details including type of anti-VEGF and regimen used were recorded. Factors or correlates reported in the study relating to treatment nonadherence or nonpersistence were evaluated. Additionally, any strategies evaluated to improve the adherence or persistence were also extracted. The methodological quality of each study was assessed according to the Mixed Methods Appraisal Tool version 2018 because it can be used across qualitative, quantitative, and mixed-methods studies.13 The overall quality and certainty of the evidence in the systematic review were evaluated using a modified GRADE approach to include qualitative evidence synthesis—the GRADE Confidence in the Evidence from Reviews of Qualitative Research tool.
Data Synthesis and Analysis
Rates of treatment nonadherence and nonpersistence, where available, were summarized with the proportion of patients reported for each outcome divided by the total number at risk in the reported study population. Results were reported individually for each study. Meta-analysis was not possible because of the variations in methodology for reporting outcomes across studies (e.g., differences in inclusion criteria with patient death, patient transfer for some studies but not others), differences in time period (total nonpersistence over several years vs. yearly rates), and lack of raw data for some studies to enable reanalysis. As an alternative, rates were tabulated according to each study and the data range provided. Factors for nonadherence and nonpersistence were also extracted from each study. These were broken down into 5 domains based on the standardized World Health Organization multidimensions of adherence: (1) patient related; (2) condition related; (3) therapy related; (4) healthcare team and health system related; and (5) social/economic factors.14 Factors were analyzed qualitatively according to theme, but also quantitatively with an odds ratio or percentage, where reported. Nonpersistence or nonadherence due to patient death or transfer of care was excluded from analysis. If possible, intentional discontinuation by treating physician from disease stability or remission was separated from unintentional nonpersistence in the analysis.
Results
Search Results
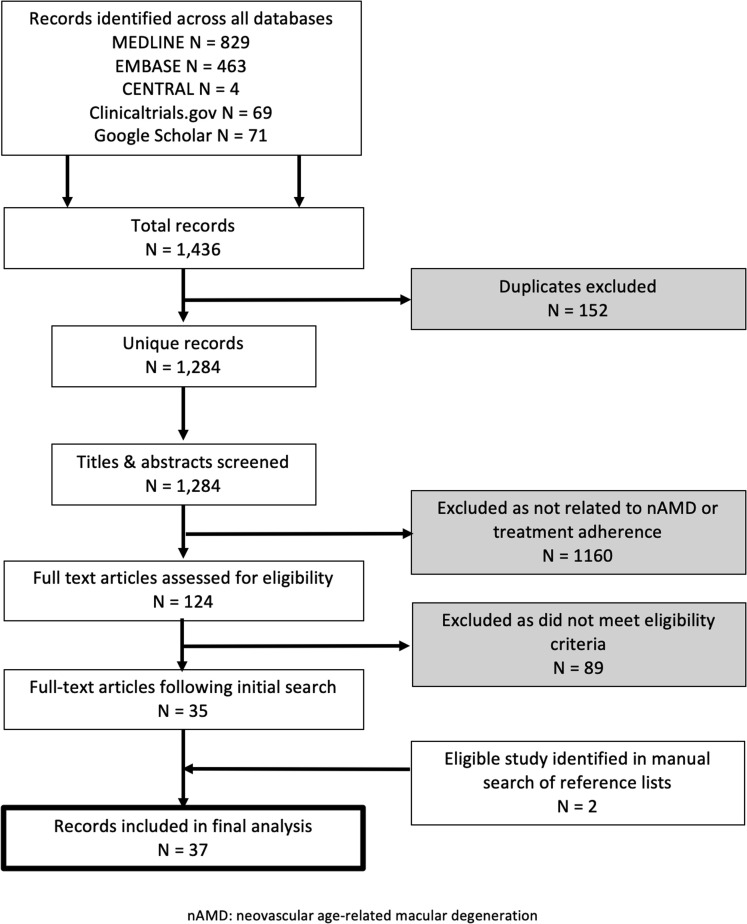
A total of 1436 studies were retrieved from the databases, yielding 1284 unique records after removal of duplicates. Initial screening of the titles and abstracts identified 124 potential studies for full-text review, with 35 remaining eligible after assessment of the full-text report. Two additional studies were identified via a manual search of the reference lists, with a total of 37 reports included in the final analysis. Figure 1 presents a Preferred Reporting Items for Systematic Reviews and Meta-Analyses–based flow diagram showing the number of records identified and excluded at each stage.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses–based flow diagram of screening process. nAMD = neovascular age-related macular degeneration.
Studies were excluded for the following reasons: (1) did not examine nAMD specifically or included other interventions; (2) addressed other domains in health-related quality of life; and (3) cost-effective analysis or modeling of intravitreal injections on visual or quality of life outcomes without any correlation to impact on treatment adherence or persistence.
Study Characteristics
Of the 37 eligible studies, the majority (n = 33) assessed the factors for treatment nonadherence and nonpersistence, and a further 4 studies reported barriers to treatment without additional assessment of adherence and persistence.15 , 16 Only 2 of the final studies explored strategies to improve treatment nonadherence and nonpersistence. Study characteristics are summarized in Table 2 .
Table 2.
Summary Characteristics of Eligible Studies Assessing Treatment Adherence or Persistence
| Study | Country | Clinic Type | Study Design | Methodology | Patients | Drug | Regimen | Planned Frequency of Visits |
|---|---|---|---|---|---|---|---|---|
| Angermann 2019 | Austria | Tertiary | Retrospective observational | Quantitative | 841 | NR | PRN | NR |
| Bobykin 2014 | Russia | NR | Retrospective observational | Mixed | 6 | RBZ | PRN | 4 wks |
| Boulanger-Scemama 2015 | France | Tertiary | Retrospective + telephone survey | Mixed | 60 | RBZ | PRN | 4 wks |
| Boyle 2018 | Australia | Mixed | Cross-sectional survey | Qualitative | 6 | NR | NR | NR |
| Curtis 2012 | United States | NR | Retrospective database | Quantitative | 284 380 | RBZ/BEV/PEG | NR | NR |
| Droege 2013 | Germany | Tertiary | Cross-sectional survey | Qualitative | 77 | RBZ | PRN | 4 wks |
| Ehlken 2018 | Germany | Tertiary | Retrospective observational | Mixed | 466 | RBZ/BEV/AFL | PRN | 4 wks |
| Ehlken 2018a | Germany | Mixed | Retrospective + Prospective | Mixed | 362 | RBZ or BEV | PRN or fixed | 4 wks |
| Heimes 2016 | Germany | NR | Retrospective observational | Mixed | 72 | RBZ | PRN | NR |
| Holz 2015 | Multinational | NR | Retrospective observational | Quantitative | 1514 | RBZ | NR | NR |
| Husler 2013 | Switzerland | Mixed | Cross-sectional survey | Qualitative | 28 | RBZ | NR | NR |
| Jansen 2018 | United States | Local | Self-administered questionnaire | Qualitative | NR | NR | NR | NR |
| Kim 2017 | South Korea | Tertiary | Retrospective observational | Quantitative | 64 | RBZ or BEV | PRN | 4 to 12 wks (8–24 wks if patient refuses additional treatment) |
| Krivosic 2017 | France | NR | Retrospective observational | Quantitative | 163 | NR | NR | NR |
| Kruger Falk 2013 | Denmark | Tertiary | Retrospective observational | Mixed | 399 | RBZ | PRN | 4 to 6 wks (12–24 weeks if no signs of disease activity) |
| Lad 2014 | United States | NR | Retrospective database | Quantitative | 459.237 | RBZ or BEV | NR | NR |
| Massamba 2015 | France | Tertiary | Prospective cohort | Quantitative | 29 | RBZ | PRN | 4 wks |
| McGrath 2013 | Australia | Local | Retrospective + telephone survey | Mixed | 85 | RBZ | T&E | 4–8 wks |
| Nunes 2010 | Brazil | Tertiary | Retrospective + phone interview | Mixed | 19 | BEV | NR | NR |
| Obeid 2018 | United States | Local | Retrospective observational | Quantitative | 2003 | NR | NR | NR |
| Oishi 2011 | Japan | Tertiary | Retrospective + phone interview | Mixed | 86 | RBZ or PEG | PRN | 4–8 wks |
| Ozturk 2018 | United Kingdom | Tertiary | Retrospective observational | Mixed | 21 | AFL | Fixed | 8 wks |
| Polat 2017 | Turkey | NR | Retrospective + telephone survey | Mixed | 125 | RBZ | PRN | 4 wks |
| Ramakrishnan 2020 | United States | Mixed | Secondary analysis of RCT | Quantitative | 1178 | RBZ or BEV | PRN or monthly | 4 wks |
| Rasmussen 2013 | Denmark | Tertiary | Retrospective + telephone survey | Mixed | 381 | RBZ | PRN | 4 wks (8–12 wks if no signs of disease activity) |
| Rasmussen 2017 | Denmark | Tertiary | Retrospective observational | Quantitative | 269 | RBZ or AFL | PRN | 4–6 wks |
| Sii 2018 | United Kingdom | Cross-sectional survey | Qualitative | 53 | RBZ (assumed) | PRN | 4–16 wks | |
| Subhi 2017 | Denmark | Tertiary | Retrospective observational | Quantitative | 59 | RBZ or AFL | PRN | 4 wks (ranibizumab), 8 wks (aflibercept) |
| Varano 2015 | Multinational | NR | Cross-sectional survey | Qualitative | 143 | NR | NR | NR |
| Vaze 2014 | Australia | Local | Retrospective observational | Qualitative | 105 | RBZ | NR | NR |
| Westborg 2018 | Sweden | NR | Retrospective observational | Quantitative | 472 | RBZ or AFL | PRN, T&E, or fixed | NR |
| Wintergerst 2018 | Germany | Tertiary | Retrospective + telephone survey | Mixed | 55 | NR | NR | NR |
AFL = aflibercept; BEV = bevacizumab; NR = not reported; PEG = pegaptanib; PRN = pro re nata; RBZ = ranibizumab; RCT = randomized controlled trial; T&E = treat-and-extend.
The studies were mainly European and US based, with a predominantly White population; only 3 reports involved patients from Asian countries.17, 18, 19 The majority of studies assessed patients treated with intravitreal ranibizumab on a PRN dosing regimen, with a few more recent studies including intravitreal aflibercept or ranibizumab on a T&E regimen (Table 2). This reflects the timing of treatment initiation of these patients, with most receiving their first anti-VEGF injection before 2013 (Table S3, available at www.aaojournal.org). Most studies assessed patients treated in a tertiary hospital (university-affiliated hospitals or dedicated retinal clinic) as opposed to a local clinic (general comprehensive clinic) (Table 2).
Definitions
There was significant variation in the terminology and definitions of nonadherence and nonpersistence used across all studies. Definitions were not reported in some studies.16 , 20 , 21 Synonyms used for nonadherence included “noncompliance,”22 , 23 “absenteeism,”24 and “nonattendance.”25 Synonyms for nonpersistence included “treatment discontinuation/cessation”18 , 26 , 27 and “lost to follow-up”
Nonadherence was variably defined as follows:
-
•
No treatment or consultation with a measure of visual acuity and OCT at least every 6 weeks28
-
•
Extreme violation of prescribed treatment22
-
•
Nonattendance of every clinic appointment19
-
•
Receiving less than the recommended 8 injections over 12 months25
-
•
Deviation from treatment recommendations (by patient or physician) with gap in treatment or consultation by more than 8 weeks29
-
•
Visit outside of the prescribed 28 days ± 7 days window
Nonpersistence was variably defined as follows:
-
•
Treatment discontinuation before 12 months,30 study period,26 , 27 or permanently31
-
•
No treatment or visit at clinic for more than 4 months,32 6 months,15 , 33 , 34 or 12 months35 , 36
-
•
No follow-up by any ophthalmologist for 3 months28
-
•
No follow-up within a 12-month period after receiving at least 1 anti-VEGF injection37
-
•
Loss of follow-up of at least 24 months18
In some cases, intentional nonpersistence, either due to assessed treatment futility or treatment success with inactive disease, as agreed to by patient and treating physician, was not explicitly differentiated from patients who were lost to follow-up.
Prevalence of Nonadherence and Nonpersistence
Nonadherence to treatment or monitoring appointments was high with variable rates depending on how strictly it was defined (32%–95%).25 , 28 , 29 In one study, which assessed nonadherence as no treatment or consultation at least every 6 weeks when using a PRN protocol, almost all patients (n = 346, 95.6%) fulfilled this criteria over a 12-month period, with a mean of 2.1 ± 1.1 gaps.28 When determined by self-report however, rates of perceived nonadherence were lower, with patients in another study estimating their rates of nonadherence at 15.7% (n = 143) and caretakers estimating this at a higher 25.8% (n = 230).19 Unsurprisingly, the observed rates of nonadherence were lower in a clinical trial setting, with a secondary analysis of the Comparison of Age-Related Macular Degeneration Treatment Trial reporting only 10.0% of 1060 patients not attending a study visit on time when defined as an average visit interval of 4 weeks ± 7 days over a 24-month period.38 However, when the longest interval between 2 visits was calculated, 83.3% of patients still had at least 1 visit interval that was not on time.
Patients who discontinued treatment due to disease remission or treatment futility as judged by their physician accounted for 3% to 30% of all patients with nAMD who commenced treatment. After excluding these patients, the remaining rates of reported nonintentional treatment nonpersistence varied from 3% to 57% at 12 months, with lower rates when nonpersistence was defined as lack of follow-up visits rather than lack of anti-VEGF treatment.35 Studies of nonpersistence to treatment beyond 24 months were limited, but where available, recorded high rates of nonpersistence (Table 3 ). Within the first 24 months, Kaplan–Meier survival curves demonstrated the onset of nonpersistence for the majority of patients to occur early within the first 6 months.
Table 3.
Reported Rates of Nonpersistence to Treatment or Monitoring Visits over Time (Excluding Patient Death or Transfer of Care)
| Months | Proportion of Patient Nonpersistent with Treatment at Each Time Point N, % |
Curtin (n=284 380) | Lad (n=459 237) | Range (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boluanger (n = 201) | Droege (n = 95) | Kruger Falk (n = 855) | Rasmussen 2013 (n = 555) | Rasmussen 2017 (n = 1027) | Subhi (n = 116) | Heimes (n = 191) | Holz (n = 2227) | Westborg (n = 932) | Ehlken 2018 (n=362) | ||||
| 6 | 174 094 (44.7) | ||||||||||||
| 12 | 37 (3.6) | 16 (13.8) | 532 (23.9) | 503 (50.8) | 68 (18.8) | 104 165 (53.6) | 213 645 (57.4) | 3.6–57.4 | |||||
| 18 | 112 647 (61.7) | 234 239 (65.6) | 61.7 – 65.6 | ||||||||||
| 24 | 10 (7.0) | 16 (13.8) | 60 (31.4) | 1043 (46.8) | 244 743 (71.0) | 7.0–71.0 | |||||||
| 36 | |||||||||||||
| 48 | 36 (4.2) | ||||||||||||
| 60 | 63 (31.3) | 60 (10.8) | 10.8–31.3 | ||||||||||
Factors Affecting Treatment Nonadherence
Risk factors for nonadherence to treatment or monitoring appointments were assessed in 6 studies.19 , 24 , 25 , 28 , 29 Condition-related factors such as worse visual acuity at baseline was associated with an increase (odds ratio, 2.37; P = 0.05) in nonadherence,29 but this was not statistically significant in another study by the same authors.28 Patients whose vision improved with treatment of >3 lines also were more likely to be adherent (19.9% vs. 12.0%, P = 0.04), although the converse (i.e., loss of vision) did not appear to influence adherence pattern in at least 1 study.29
Of all patient-related variables, patient illness (21.0%–42.8%) accounted for a significant cause of nonadherence.19 , 25 Many patients also reported fear of injections as a major barrier to treatment (n = 30, 21%), with a small number also reporting discomfort after injections as a reason for avoidance.19
A substantial cause for nonadherence overall related to health system factors. Patients stated sometimes forgetting their appointment (15%), the appointments were too frequent or inconvenient (10%), or there was insufficient clinic capacity with no available appointments in the time frame the patient required (47%).19 , 25
Social factors play a significant role for most patients, with many citing a lack of caretakers to take them to appointments (25.9%). Only 1 study examined cost factors, with less than 10% reporting financial burden as the primary issue for nonadherence.19 Seasonal factors also may play a role, with 1 study recording almost half of all patients (n = 29, 46%) missing at least 1 visit during the traditional French summer holiday period.24 This may be due to patients weighing the benefits of holidays against their eye health.
Factors Affecting Treatment Nonpersistence
In general, the reasons for nonpersistence reflected those seen for nonadherence (Table 4 ). Baseline visual acuity was a strong condition-specific factor, with worse baseline vision in the affected eye conferring higher risk of nonpersistence of 1.4 to 8.1 times. However, there was no consistent threshold level of visual acuity where this risk increased. Poor response to treatment and worse final visual acuity were also associated with higher rates of nonpersistence. There was conflicting evidence as to whether bilateral disease was a risk factor, with some studies suggesting a 3.7-fold increased risk of discontinuation if bilateral injections were required,28 , 30 whereas other studies suggested that bilaterality was a protective factor.37
Table 4.
Risk Factors for Treatment or Visit Nonpersistence
| Risk Factors | Studies | Risk of NP (OR or Primary Reason for NP as % of Responses) | Effect of Risk Factor (Qualitative Studies) |
|---|---|---|---|
| Condition-Related Factors | |||
| Bilaterality | Obeid | −0.69 OR | |
| Ehlken | 3.704 OR | ||
| Rasmussen 2018 | 3.70 OR | ||
| Worse baseline VA | Oishi | 8.1 OR | Increased risk |
| Westborg | 1.42 OR | ||
| Polat | 0 vs. 22.6% | No difference | |
| McGrath | Increased risk | ||
| Bobykin | |||
| Boulanger | 42.5 vs. 51.0 letters | ||
| Ehlken 2018 | OR 2.37 | ||
| Worse baseline VA in fellow eye | Westborg | No difference | |
| Bobykin | Decreased risk | ||
| Worse final VA | Oishi | Increased risk | |
| Sii | Increased risk if in worse eye | ||
| Droege | Increased risk | ||
| Ehlken 2018 | Increased risk | ||
| No change in VA to treatment | Obeid | Increased risk | |
| Polat | Increased risk | ||
| Vaze | Increased risk | ||
| Nunes | Increased risk | ||
| Patient-Related Factors | |||
| Older age | Obeid | 1.58 OR 81–85 | |
| 2.29 OR 86–90 | |||
| 3.31 OR > 90 | |||
| Ehlken 2018 | OR 1.04 | ||
| Rasmussen 2018 | OR 1.05 | ||
| Westborg | No difference | ||
| Subhi | Increased age > 90 yrs | ||
| Rasmussen 2013 | Increased age | ||
| McGrath | No difference | ||
| Bobykin | Increased > 80 yrs | ||
| Wintergerst | Increased | ||
| Husler | 13.0% | ||
| Boulanger | 82.0 vs. 76.6 yrs | ||
| Non-White ethnicity | Obeid | 1.47 OR African American | |
| 2.63 OR Asian American | |||
| 3.07 OR other ethnicity | |||
| Systemic illness | Westborg | 1.27 OR | |
| Oishi | Increased risk | ||
| Droege | 16.7% | ||
| Rasmussen 2013 | 41.5% | ||
| McGrath | 0.48 OR | ||
| Kruger Falk | 5.6% | ||
| Vaze | 42.3% | ||
| Nunes | 15.8% | ||
| Heimes | 26% | ||
| Wintergast | 25% | ||
| Huser | 8.9% | ||
| Ocular comorbidities | |||
| Fear of injections | Polat | 29.6% | |
| Droege | 5.3% | ||
| Kruger Falk | 2.8% | ||
| Vaze | 11.5% | ||
| Wintergast | 25% | ||
| Huser | 8.9% | ||
| Perception injections not helpful or not needed | Polat | 21.6% | |
| Rasmussen 2013 | Increased risk | ||
| McGrath | 10.4% | ||
| Kruger Falk | 8.3% | ||
| Vaze | 23.1% | ||
| Nunes | 42.1% | ||
| Wintergast | 11% | ||
| Huser | 21.7% | ||
| Loss of motivation | Droege | 38% | |
| Heimes | 5% | ||
| Therapy-Related Factors | |||
| Treatment regimen | Hanemoto | Decreased burden for T&E vs. PRN | |
| Droege | Decreased burden for PRN vs. monthly | ||
| Same day injection | Krivosic | Decreased risk | |
| Anti-VEGF drug type | Subhi | No difference | |
| Westborg | 1.45 OR RBZ vs. AFL | ||
| Health Systems and Healthcare Team Factors | |||
| Lack of information | Nunes | 26.3% | |
| Huser | 4.3% | ||
| Varano | Increased risk | ||
| Did not like or trust physician | Kruger Falk | 2.8% | |
| Huser | 8.7% | ||
| Tertiary center | Westborg | 1.30 OR | |
| Rasmussen 2018 | 0.33 OR | ||
| Dissatisfaction with treatment center | Polat | 17.6% | |
| Longer distance from home to treatment center | Polat | R value = −0.227 | |
| Obeid | 1.33 OR 21–30 miles | ||
| 1.55 OR > 30 miles | |||
| Boyle | Increased risk > 50 km | ||
| McGrath | 2.48 OR | ||
| Boulanger | Median distance 18 vs. 40 km | ||
| Follow-up burden | Polat | 16% | |
| Boyle | Increased with shorter intervals | ||
| Subhi | Increased risk | ||
| Droege | 18.9% | ||
| Rasmussen | Increased risk | ||
| Kruger Falk | 8.3% | ||
| Vaze | 7.7% | ||
| Huser | 13.0% | ||
| Fixed appointments | Rasmussen 2018 | 0.44 OR | |
| Difficulties with appointments | Nunes | 10.5% | |
| Social/Economic Factors | |||
| Lower socioeconomic status | Obeid | 1.52 OR | |
| Social isolation or lack of carer | Polat | 16% | |
| Oishi | Increased risk | ||
| Droege | 61.5% | ||
| Wintergerst | Increased risk | ||
| Huser | 8.9% | ||
| Lack of insurance status | McGrath | No difference | |
| Heimes | 3% | ||
| Wintergast | 5% | ||
| Financial burden | Polat | 20.8% | |
| Oishi | Increased risk | ||
| Boyle | Increased risk (indirect costs) | ||
| Droege | 34.8% | ||
| McGrath | 8.1% | ||
| Vaze | 7.7% | ||
| Wintergast | 10% | ||
| Huser | 13% | ||
| Holz | 2% | ||
| Spooner | Increased burden (direct and indirect costs) | ||
| Lack of transport | Droege | 46.3% | |
| Rasmussen | Increased risk | ||
| Vaze | 7.7% | ||
| Nunes | 5.3% | ||
| Heimes | 38% | ||
| Wintergast | 27% |
AFL = aflibercept; NP = nonpersistence; OR = odds ratio; PRN = pro re nata; RBZ = ranibizumab; T&E = treat-and-extend; VA = visual acuity; VEGF = vascular endothelial growth factor.
The therapy regimen may contribute to risk of nonpersistence. In a Japanese study using PRN treatment, patients who were only given a single injection, instead of 3 loading doses at initiation, had a higher risk of nonpersistence.17
Patient-related factors accounted for a significant cause of nonpersistence. In particular, older age was an issue, with the risk increasing per decade of life. The highest risk occurred in those aged more than 90 years with a 3-fold increased risk compared with those younger than 80 years of age. General ill health or presence of other systemic comorbidities was also associated with risk of nonpersistence independent of age. There was no difference due to patient gender or the presence of other ocular comorbidities.
Patients also reported fear of injections as a barrier to treatment, although the extent to which this was responsible varied between 3% and 30% overall. However, patient perception that the treatment was not helpful or not required was a strong risk factor, accounting for up to 42% in some series.39 However, the studies did not clarify if this was related to an expectation of improvement with treatment and subsequent disappointment when only stability of vision was achieved. This is in keeping with the finding that patients who ceased treatment often reported lack of information about the treatment plan or the expected outcomes of treatment versus natural history.39 , 40
Health system and socioeconomic-related factors represented a significant cause for treatment nonpersistence as reported by patients. Lack of transport or distance to the treatment center was a key factor in most studies, accounting for 5% to 46% of responses. In contrast, in Denmark, where the government funds transport to the hospital, only 1 of the 4 Danish studies included in the review reported transport as an issue. Follow-up burden was also reported as a significant factor, but the specifics of whether this was regimen related was not explored. The impact of cost or the financial burden on treatment persistence was variable, accounting for as low as 2% in 1 large multi-country study26 and as high as 30% in others.41 Of note, in Sweden, where treatment for nAMD is covered by the Swedish National Insurance, 50.6% of patients reportedly still discontinued treatment during the first year of diagnosis. In some surveys, patients reported that indirect costs, related to transport for example, were more significant than the cost of the treatment itself.42 Government and insurance regulations may play a factor. In a 2013 study from Germany, the authors examined the role of reimbursement and the approval pathway on adherence to treatment.41 At the time, patients in Germany were required to obtain approval from their insurance company before treatment. The cost of the injection was covered by insurance but required up-front payment before obtaining a refund. Patients were only approved for 3 injections at a time before their case was reviewed. Consequently, up to one-third of patients reported some difficulties with the up-front payment, and a small proportion had difficulties obtaining approval or refunds. Likewise, in the real-world multi-national AURA study, researchers found there were significant differences between countries in the number of injections given, ranging from a mean of 3.2 injections over 2 years in Venezuela to 11.0 injections in Ireland.26 Although the correlation to reimbursement or approval process was not directly assessed, the authors suggested the difference in injection frequency may be due to these health system–related policies.
Patients may also express a desire to stop treatment before actually doing so. Three studies surveyed patients who were actively participating in treatment on their willingness or future intentions to stop treatment and visits.40 , 42 , 43 Reasons were similar to those reported by those who had ceased treatment, including failure to notice any improvement in vision,40 , 42 , 43 treatment burden on self and caretakers, and perceptions of being “too old.”40 , 42
Strategies to Improve Treatment Adherence and Persistence
There is limited evidence on the impact of strategies to improve treatment adherence or persistence, with only 2 studies available in the literature.15 , 44 One report examined whether patients on a PRN regimen who performed their monitoring visit at a local clinic with telemedicine capabilities had better treatment adherence and increased injections compared with patients who had both monitoring and injections at the same tertiary reference center. The outcomes suggested that the telemedicine group had greater treatment adherence with a significantly greater number of monitoring visits (telemedicine group 22.8 visits vs. control group 18.4 visits, P < 0.001) and a greater number of injections (telemedicine group 13.9 injections vs. control group 11.1 injections, P = 0.02) over the total monitoring period. Another study examined whether providing a fast-track approach with same-day injection was better than the standard protocol of booking a separate injection visit.44 In this study, the mean time between the date of the monitoring visit to injection was shorter in the fast-track group compared with the standard protocol group (4.1 ± 7.5 vs. 5.6 ± 18.7 days, respectively). All patients surveyed in the fast-track group also reported satisfaction with same-day injection.
Barriers to Treatment
The literature review also identified studies that examined potential barriers to treatment, but these did not specifically address how these barriers correlated to adherence or persistence.41 , 45, 46, 47 Additional risk factors reported in these studies include the differences in perceived treatment burden by patients or their caretakers of the various injection regimens, with lower burden index for patients on T&E regimen compared with PRN,45 and PRN more favorable compared with monthly.48 However, treating ophthalmologists reported difficulties of some patients in understanding the proactive nature of T&E regimens.47 Socioeconomic factors were also prominent in these studies with caretaker productivity loss (both time and financial) a significant burden.45 , 46 Overall, treatment burden, both in terms of visit frequency and travel time, was cited as the most significant barrier.46
Quality Assessment
Quality of each study was rated with the Mixed Methods Appraisal Tool, with individual assessments based on qualitative, quantitative, and mixed criteria (Table S4, available at www.aaojournal.org). Most studies were limited by their retrospective nature with possible ascertainment or recall bias and lack of control group. Another frequent shortcoming was the lack of or inconsistent definitions for both the outcome and contributing factors. The rationale for why some of the assessed factors were selected was usually not recorded. Qualitative studies also demonstrated variability in terms of the breadth of questions asked, the nature of the questioning (closed vs. open-ended), and whether any validation of the questionnaire was performed (Table S5, available at www.aaojournal.org). The overall certainty of the evidence was synthesized according to the GRADE Confidence in the Evidence from Reviews of Qualitative Research assessment (Table 5 ).
Table 5.
Summary of Findings and Certainty of Evidence Using GRADE Confidence in the Evidence from Reviews of Qualitative Research Assessment
| Summary of Review Finding | Contributing Studies | Confidence in the Evidence | Explanation of Confidence in the Evidence from Reviews of Qualitative Research Assessment |
|---|---|---|---|
| High rates of nonadherence and nonpersistence to anti-VEGF treatment is observed, with the onset highest in the first 12 mos. | Lad 2014, Curtis 2012, Droege 2013, Westborg 2018, Subhi 2017, Boulanger-Scemama 2015, Rasmussen 2013, Heimes 2016, Kruger-Falk 2013 | Moderate | Moderate limitations based on variability in methodology, in particular with regard to selection and definition of outcomes; minor concerns regarding coherence across studies |
| Condition-related factors such as worse baseline visual acuity and poor response to treatment are associated with increased risk of both nonadherence and nonpersistence. | Oishi 2011, Polat 2017, Bobykin 2014, Boulanger-Scemama 2015, Ehlken 2018, Droege 2013, Obeid 2018, Vaze 2014, Nunes 2010 | Moderate | Moderate concerns regarding methodological limitations, in particular with regard to assessment of response to treatment; minor concerns with coherence |
| Injection regimens that individualize treatment reduce patient burden and improve adherence and persistence. | Droege 2013, Hanemoto 2017 | Low | Serious concerns regarding data adequacy and relevance to current practice |
| The most significant patient-related factor affecting treatment nonadherence or nonpersistence is significant systemic comorbidities and patient perception of treatment as not helpful or not needed. | Obeid 2018, Ehlken 2018, Rasmussen 2018, Bobykin 2014, Wintergerst 2018, Husler 2013, Droege 2013, Kruger-Falk 2013, Vaze 2014, Nunes 2010, Heimes 2016, Husler 2013 | Low | Serious concerns regarding methodological limitations, in particular with regard to assessment of patient perception of response to treatment; minor concerns regarding coherence across studies |
| Social isolation and lack of carer or transport were prominent factors for treatment nonadherence or nonpersistence. Indirect costs rather than direct costs of treatment were also barriers. | Oishi 2011, Droege 2013, Wintergerst 2018, Husler 2013, Heimes 2016, Polat 2017, McGrath 2015, Vaze 2014, Spooner 2018, Obeid 2018 | Low | Serious concerns regarding methodological limitations and adequacy of data, in particular with regard to the financial burden to patients across different health systems and settings |
| Health systems that allow for reduced visits (1-stop service) or for monitoring in a local center improved treatment adherence. | Heimes 2016, Krivosic 2017 | Low | Serious concerns regarding methodological limitations and adequacy of data given only 2 studies examined strategies to improve adherence |
VEGF = vascular endothelial growth factor.
Discussion
The inter-related factors of nonadherence and nonpersistence to intravitreal anti-VEGF therapy is one of the major challenges in preventing vision loss from nAMD, despite the success of major clinical trials showing its effectiveness. Despite the importance, there is limited understanding of what is nonadherence and nonpersistence and the factors leading to these. There is also lack of awareness of effective methods needed to alter these behaviors that can be inter-related or separate issues for each patient.
This systematic review reveals a lack of consensus definitions and terminology in the literature to identify nonpersistence in patients with nAMD. The World Health Organization defines “adherence” as “the extent to which a person’s behavior corresponds with agreed recommendations from a healthcare provider.”14 Most studies included here followed a PRN treatment protocol, with nonadherence to intravitreal therapy defined as an all-or-nothing outcome of complete adherence to monthly visits or a more relaxed definition allowing deviation by more than a set number of weeks or months. However, no specific definitions for nonadherence in those using a T&E protocol were provided. Future work to develop a consensus on definitions will need to be able to straddle the differences and incorporate all treatment protocols. Likewise, there was no agreement on definitions for nonpersistence, with variable timeframes and lack of differentiation as to whether the discontinuation was intentional or unintentional. For many studies, reasons listed for nonpersistence included patient death, transfer of care, or planned treatment cessation by the physician due to disease remission or treatment futility. Arguably, death or care transfer should be considered under a different context than for traditional causes of nonpersistence, and planned treatment cessation should also be categorized separately.
The results of the review indicate that the rate of reported nonadherence and nonpersistence in nAMD is high overall at up to 60% nonpersistence at 24 months follow-up in some series. The majority of nonpersistence occurs within 6 to 12 months, suggesting that the decision is often made early on whether to continue with therapy. Interestingly, the rates of nonpersistence were higher in 1 study when it was defined as no further intravitreal injections, as opposed to no monitoring visit, suggesting that some of this early nonpersistence is intentional cessation of intravitreal injections, rather than lack of follow-up.35 This may be due to perceived or actual suboptimal response to treatment or issues with the injection itself, although data separating these outcomes are limited.
Conversely, once a patient has remained on treatment for several years, he is less likely to be nonadherent or nonpersistent in the future. It may be that once a routine is established and any initial barriers have been overcome, the patient is more invested in continuing his treatment. However, whether these behavioral and environmental influences on patients and their caretakers change over time has not been clearly defined in the literature.
The reasons and risk factors for nonadherence and nonpersistence are multidimensional. As expected, strong correlations exist with patient-related factors such as increased systemic comorbidities, longer distance from home to treatment center, lack of a caretaker to assist with transport, and poorer baseline visual acuity. As expected, treatment efficacy was also a protective factor, with patients who experienced greater visual gains more likely to be both adherent and persistent. However, other factors such as bilaterality showed conflicting results with some studies suggesting poorer adherence with unilateral eye involvement,37 whereas others reported worse outcomes with bilaterally treated disease.28
Cost was not as consistent a risk factor as expected, with financial barriers only accounting for 2% to 30% of causes of nonpersistence and less than 10% for nonadherence. This may be due to the variety of countries included in this review. For example, access to intravitreal injections is relatively easy and mostly funded by the government in places like the United Kingdom and Sweden. In contrast, lower socioeconomic status was significantly associated with higher nonpersistence in a study based in the United States where insurance status significantly affected ability to access health care.37 National health policy also plays a role, because different countries may have different rules on the total number of injections that can be subsidized per patient. For example, in Taiwan, patients can be reimbursed for 3 to 7 doses in each eye of ranibizumab or aflibercept over a 2-year period by an approved ophthalmologist. Switching between intravitreal agents is also restricted, so the decision on anti-VEGF type needs to be made at the initial application, regardless of treatment outcomes. The unique reimbursement rules and need for approval can therefore influence access and persistence in each country. However, even in countries where the cost of the drug itself may be funded, patients reported some financial stress related to indirect costs for treatment, such as the cost of parking10 and productivity losses, with the caregiver needing to take time off work to accompany patients for treatment.46
Physicians’ decision-making, including tolerance to fluid on OCT imaging, may also be influenced by a patient’s request to defer treatment or to influence it. In a recent study comparing real-world clinician versus reading-center assessment of choroidal neovascularization disease activity, 20% of scans were judged active by the reading center, but no injections were given.49 Of these cases, some were assessed by the clinician as chronic degenerative cysts only, whereas others were monitored in a “watch and wait” fashion, reportedly due to patient preference.
Another unexpected finding from the review was that treatment burden did not rank as highly as expected as a cited cause for treatment nonpersistence or nonadherence, accounting for only 7% to 18.9% of all cases. It may be that this terminology, when used in questionnaires, is considered too general or vague for some patients, who prefer to ascribe the difficulties to more specific barriers as “lack of transport” or “ill health.” This is reflected in the studies examining perceived barriers to treatment, with patients identifying visit frequency as a significant burden and preferencing regimens with fewer visits such as the T&E regimen.45 Patients also reported greater satisfaction with same-day injection protocols when using a PRN regimen, suggesting that treatment burden due to visit frequency was an issue.44 Phase III trials are currently underway investigating the use of ranibizumab sustained delivery devices.50 , 51 Future studies looking at the adherence patterns for these longer-acting devices will be helpful in confirming if perception of treatment burden is similarly reduced.
The importance of various risk factors for nonadherence and nonpersistence identified in this systematic review, however, may not necessarily remain static over time. The majority of studies included here assessed patients who were treated before 2013 and may not necessarily reflect modern practices. Almost all were using ranibizumab on a PRN regimen (Table S3, available at www.aaojournal.org), and treatment or monitoring was often discontinued by the physician because of perceived lack of disease activity. Although we were not able to study the effects of different injection regimens on rates of adherence and persistence, there is increasing adoption of T&E as the preferred protocol in many countries. Changes to reimbursement criteria and updated regulatory requirements may also influence accessibility of treatment in some countries. In addition, it is likely that capacity constraints will also exert more pressures on individual clinics as intravitreal injections become more common for other indications and the aging populations continue to increase. Given that some patients in the review already reported difficulties with obtaining clinic appointments, delays due to overbooked clinics are likely to pose a growing problem. In contrast, better population and physician awareness about nAMD may also help identify patients at an earlier stage of disease, and baseline visual acuity may become less important of a factor over time.
Strategies to improve treatment nonadherence or nonpersistence are well established in several systemic chronic diseases. In diabetes mellitus, for example, previous studies have assessed a range of interventions, including modifying the insulin delivery system, simplifying the treatment regimen, improving the safety profile, or providing intensive support services to promote adherence.52 , 53 Within ophthalmology, strategies for adherence to glaucoma topical therapy have been extensively investigated, namely, simplifying regimens, lowering costs, educating patients, and involving the family and caretakers.54 In contrast, there is a surprising lack of data assessing the effectiveness of different strategies on rates of treatment adherence and persistence to intravitreal injection therapy in nAMD. A limited form of telemedicine and organization of a fast-track, same-day injection stream include 2 strategies that have been evaluated thus far. Although there are significant differences between intravitreal injection therapy and other systemic and ocular diseases, lessons learned from management of chronic systemic conditions can still be applied. For example, instead of eliciting information about nonadherence to medication as for glaucoma management,55 asking patients with nAMD about their intentions or willingness to stop treatment may help to identify at-risk patients and to trigger interventions before the event. Further evaluation is required to assess whether providing education for patients and their caretakers at varying time points, for example, at baseline before commencing treatment, after 3 months, and at 1 year, may help to clarify the goals of treatment and encourage persistence into the long term. In addition, text message reminders have been shown to be a simple cost-effective tool in the primary care setting to notify patients of upcoming healthcare appointments. This intervention can be easily applied to remind patients with nAMD of their next clinic or injection visit and may be an effective solution for those 15% of respondents who reported missed appointments because of forgetfulness.19
However, some interventions to improve adherence, although seemingly obvious, may prove more challenging to implement. For example, transportation was identified as a key barrier in this review. Although ideally all patients would be provided transportation to clinic appointments, the attitudes and regulations governing this may differ between healthcare systems. In the United States, offering free transport to Medicare patients may be considered an inappropriate inducement to treatment; therefore, legal review should be considered to understand if it is appropriate. Usually, it is not permitted. In Australia, community volunteer driver programs and Red Cross transport services are currently available initiatives. However, the vast distance between home and treatment location for many rural and regional patients makes this a deterrent. Finally, even in geographically smaller countries such as Sweden, where there is universal free transport to hospitals, some patients still struggle to engage with their treatment,20 which highlights the multifactorial nature of this problem.
In conclusion, the challenges of nonadherence or nonpersistence to intravitreal therapy are important and not only isolated to patients with nAMD. They represent a significant problem in patients receiving anti-VEGF injections for other indications such as diabetic macular edema or retinal vein occlusion.56 However, given the relatively rapid deterioration that can happen in nAMD compared with macular edema from other retinovascular conditions, the effect of nonadherence on visual acuity is potentially more damaging and permanent.29
Studies have consistently shown that results from clinical trials are not necessarily translatable to the real world, with discrepancies likely due to differences in patient population and underuse of treatment. This systematic review reveals moderate level evidence to support the finding that high levels of nonadherence and nonpersistence are observed, particularly in the first 12 months. Indeed, the current novel coronavirus (severe acute respiratory syndrome coronavirus 2) pandemic is a concern, because many patients are likely to have been or continue to be nonadherent during this period. The long-term impact on this population is yet to be known.
In addition, reasons for patient nonadherence and nonpersistence to intravitreal injections are complex and multifactorial, although the evidence for specific factors is of low to medium level in quality. Further work is currently under way to standardize and better define these terms. We need effective tools to identify and triage the patients at risk to develop meaningful interventions best suited to the individual patient at the appropriate moment in their treatment course. Current efforts to improve drug duration and efficacy may help to reduce treatment burden, but it is likely coordinated solutions that target other domains, such as transport and access to care, will be required to optimize the long-term visual outcomes for all patients.
Manuscript no. D-19-00539.
Footnotes
Supplemental material available atwww.aaojournal.org.
Disclosure(s): All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): B.E.: Consultant – Bayer, Novartis, Allergan.
R.F.: Grant – Novartis, CentreVue, Heidelberg Engineering, Zeiss; Consultant – Novartis, Bayer, Roche/Genentech, Ellex, Alimera, Allergan, Santhera, Inositec, Opthea; Support for travel – Novartis.
S.J.T.: Support for travel – Bayer; Board member – Bayer, Novartis; Grants – Bayer, Novartis, Roche; Travel expenses – Bayer and Novartis.
T.Y.W.: Clinical trial grants – Allergan, Bayer, Boehringer-Ingelheim, Genentech, Merck, Novartis, Oxurion (formerly ThromboGenics), Roche, Samsung Bioepis, NMRC Singapore, Novartis Singapore; Consulting fees/travel support/review fees/consultant fees – Allergan, Bayer, Boehringer-Ingelheim, Genentech, Merck, Novartis, Oxurion (formerly ThromboGenics), Roche, Samsung Bioepis; Stock – Plano, EyRIS.
C.H.: Employee – Bayer.
A.L.: Consultant – Allergan, Bayer Health Care, Beyeonics, Forsight Labs, Notal Vision, Novartis, Roche.
L.P.: Consultant – Kantar.
P.M.: Consultant – Bayer, Allergan, Abbott.
M.O.: Travel support – Bayer Health Care; Honorarium for lectures – Bayer, Allergan.
Financial Support: Development of this manuscript for publication, specifically assistance with parts of the literature review, language translation, and initial data extraction support, was provided by Kantar Health, with the financial support of Bayer Pharmaceuticals. However, the study design, final data extraction, data analysis and manuscript writing were performed by the primary author (M.O.).
This work is a joint initiative of the AMD Barometer and the Vision Academy, of which all authors are members. The Vision Academy is a global group of ophthalmologists, supported by Bayer Pharmaceuticals, who share best practice and knowledge and lead the wider community in the drive toward optimized patient care.
HUMAN SUBJECTS: No human subjects were included in this study. All research adhered to the tenets of the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of the study.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Okada, Mitchell, Finger, Eldem, Talks, Barratt, Wong, Loewenstein
Data collection: Okada, Hirst, Paladini
Analysis and interpretation: Okada, Mitchell, Finger, Eldem, Talks, Barratt, Wong, Loewenstein
Obtained funding: Okada, Mitchell, Finger, Eldem, Talks, Hirst, Paladini, Barratt, Wong, Loewenstein
Overall responsibility: Okada, Mitchell, Finger, Eldem, Talks, Barratt, Wong, Loewenstein
Supplementary Data
References
- 1.Lim L.S., Mitchell P., Seddon J.M., et al. Age-related macular degeneration. Lancet. 2012;379:1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P., Liew G., Gopinath B., Wong T.Y. Age-related macular degeneration. Lancet. 2018;392:1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 3.Solomon S.D., Lindsley K., Vedula S.S., et al. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2019;3:CD005139. doi: 10.1002/14651858.CD005139.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakri S.J., Thorne J.E., Ho A.C., et al. Safety and efficacy of anti-vascular endothelial growth factor therapies for neovascular age-related macular degeneration: a report by the American Academy of Ophthalmology. Ophthalmology. 2019;126:55–63. doi: 10.1016/j.ophtha.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Kim L.N., Mehta H., Barthelmes D., et al. Meta-analysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2016;36:1418–1431. doi: 10.1097/IAE.0000000000001142. [DOI] [PubMed] [Google Scholar]
- 6.Brown D.M., Kaiser P.K., Michels M., et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 7.Heier J.S., Brown D.M., Chong V., et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Gillies M.C., Campain A., Barthelmes D., et al. Long-term outcomes of treatment of neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 2015;122:1837–1845. doi: 10.1016/j.ophtha.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Rofagha S., Bhisitkul R.B., Boyer D.S., et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 10.Boyle J., Vukicevic M., Koklanis K., Itsiopoulos C. Experiences of patients undergoing anti-VEGF treatment for neovascular age-related macular degeneration: a systematic review. Psychol Health Med. 2015;20:296–310. doi: 10.1080/13548506.2014.936886. [DOI] [PubMed] [Google Scholar]
- 11.Senra H., Ali Z., Balaskas K., Aslam T. Psychological impact of anti-VEGF treatments for wet macular degeneration-a review. Graefes Arch Clin Exp Ophthalmol. 2016;254:1873–1880. doi: 10.1007/s00417-016-3384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J.P., Green S. The Cochrane Collaboration; 2011. Cochrane Handbook for Systematic Reviews of Interventions.http://handbook.cochrane.org Accessed June 1, 2018. [Google Scholar]
- 13.Hong Q.N., Fàbregues S., Bartlett G., et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ Inf. 2018;34:285–291. [Google Scholar]
- 14.Anonymous . World Health Organization; Geneva, Switzerland: 2003. Adherence to Long-Term Therapies: Evidence for Action. [Google Scholar]
- 15.Heimes B., Gunnemann F., Ziegler M., et al. [Compliance of age related macular degeneration patients undergoing anti-VEGF therapy: analysis and suggestions for improvement] Ophthalmology. 2016;113:925–932. doi: 10.1007/s00347-016-0275-z. [DOI] [PubMed] [Google Scholar]
- 16.Chang A.A., Stokes J., Priestman L., et al. Persistence to aflibercept therapy in wet AMD patients engaged with the Smartsight Support program. Clin Exp Ophthalmol. 2018;46:92–93. [Google Scholar]
- 17.Oishi A., Mandai M., Nishida A., et al. Remission and dropout rate of anti-VEGF therapy for age-related macular degeneration. Eur J Ophthalmol. 2011;21:777–782. doi: 10.5301/EJO.2011.7430. [DOI] [PubMed] [Google Scholar]
- 18.Kim J.H., Chang Y.S., Kim J.W. Natural course of patients discontinuing treatment for age-related macular degeneration and factors associated with visual prognosis. Retina. 2017;37 doi: 10.1097/IAE.0000000000001494. [DOI] [PubMed] [Google Scholar]
- 19.Varano M., Eter N., Winyard S., et al. Current barriers to treatment for wet age-related macular degeneration (wAMD): findings from the wAMD patient and caregiver survey. Clin Ophthalmol. 2015;9:2243–2250. doi: 10.2147/OPTH.S92548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subhi Y., Sorensen T.L. Neovascular age-related macular degeneration in the very old (>/=90 years): epidemiology, adherence to treatment, and comparison of efficacy. J Ophthalmol. 2017;2017:7194927. doi: 10.1155/2017/7194927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wintergerst M.W.M., Bouws J., Loss J., et al. Reasons for delayed and discontinued therapy in age-related macular degeneration. Ophthalmology. 2018;115:1035–1041. doi: 10.1007/s00347-017-0610-z. [DOI] [PubMed] [Google Scholar]
- 22.Bobykin E. The influence of patient compliance with antiangiogenic therapy on its efficacy for neovascular age-related macular degeneration. Vestn Oftalmol. 2014;130:88–96. [PubMed] [Google Scholar]
- 23.Polat O., Inan S., Ozcan S., et al. Factors affecting compliance to intravitreal anti-vascular endothelial growth factor therapy in patients with age-related macular degeneration. Turk J Ophthalmol. 2017;47:205–210. doi: 10.4274/tjo.28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massamba N., Dirani A., Knoeri J., et al. Evaluating the impact of summer vacation on the visual acuity of AMD patients treated with ranibizumab. Eye. 2015;29:1453–1457. doi: 10.1038/eye.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozturk M., Harris M.L., Nguyen V., et al. Real-world visual outcomes in patients with neovascular age-related macular degeneration receiving aflibercept at fixed intervals as per UK licence. Clin Exp Ophthalmol. 2018;46:407–411. doi: 10.1111/ceo.13085. [DOI] [PubMed] [Google Scholar]
- 26.Holz F.G., Tadayoni R., Beatty S., et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99:220–226. doi: 10.1136/bjophthalmol-2014-305327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruger Falk M., Kemp H., Sorensen T.L. Four-year treatment results of neovascular age-related macular degeneration with ranibizumab and causes for discontinuation of treatment. Am J Ophthalmol. 2013;155:89–95.e3. doi: 10.1016/j.ajo.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 28.Ehlken C., Wilke T., Bauer-Steinhusen U., et al. Treatment of neovascular age-related macular degeneration patients with vascular endothelial growth factor inhibitors in everyday practice: identification of health care constraints in Germany—the PONS study. Retina. 2018;38 doi: 10.1097/IAE.0000000000001681. [DOI] [PubMed] [Google Scholar]
- 29.Ehlken C., Helms M., Bohringer D., et al. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2018;12:13–20. doi: 10.2147/OPTH.S151611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen A., Sander B., Larsen M., et al. Neovascular age-related macular degeneration treated with ranibizumab or aflibercept in the same large clinical setting: visual outcome and number of injections. Acta Ophthalmol (Copenh) 2017;95:128–132. doi: 10.1111/aos.13233. [DOI] [PubMed] [Google Scholar]
- 31.Vaze A., Fraser-Bell S., Gillies M. Reasons for discontinuation of intravitreal vascular endothelial growth factor inhibitors in neovascular age-related macular degeneration. Retina. 2014;34:1774–1778. doi: 10.1097/IAE.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 32.Westborg I., Rosso A. Risk factors for discontinuation of treatment for neovascular age-related macular degeneration. Ophthalmic Epidemiol. 2018;25:176–182. doi: 10.1080/09286586.2017.1397701. [DOI] [PubMed] [Google Scholar]
- 33.Boulanger-Scemama E., Querques G., About F., et al. Ranibizumab for exudative age-related macular degeneration: a five-year study of adherence to follow-up in a real-life setting. J Fr Ophtalmol. 2015;38:620–627. doi: 10.1016/j.jfo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Angermann R., Rauchegger T., Nowosielski Y., et al. Treatment compliance and adherence among patients with diabetic retinopathy and age-related macular degeneration treated by anti-vascular endothelial growth factor under universal health coverage. Graefes Arch Clin Exp Ophthalmol. 2019;257:2119–2125. doi: 10.1007/s00417-019-04414-y. [DOI] [PubMed] [Google Scholar]
- 35.Lad E.M., Hammill B.G., Qualls L.G., et al. Anti-VEGF treatment patterns for neovascular age-related macular degeneration among Medicare beneficiaries. Am J Ophthalmol. 2014;158:537–543.e2. doi: 10.1016/j.ajo.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Curtis L.H., Hammill B.G., Qualls L.G., et al. Treatment patterns for neovascular age-related macular degeneration: analysis of 284 380 Medicare beneficiaries. Am J Ophthalmol. 2012;153:1116–1124.e1. doi: 10.1016/j.ajo.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 37.Obeid A., Gao X., Ali F.S., et al. Loss to follow-up among patients with neovascular age-related macular degeneration who received intravitreal anti-vascular endothelial growth factor injections. JAMA Ophthalmol. 2018;136:1251–1259. doi: 10.1001/jamaophthalmol.2018.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramakrishnan M.S., Yu Y., VanderBeek B.L. Association of visit adherence and visual acuity in patients with neovascular age-related macular degeneration: secondary analysis of the comparison of age-related macular degeneration treatment trial. JAMA Ophthalmol. 2020;138:237–242. doi: 10.1001/jamaophthalmol.2019.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nunes R.P., Nobrega M.J., De Novelli F.J., et al. Causes of interruption of bevacizumab therapy in age-related macular degeneration. Arq Bras Oftalmol. 2010;73:146–149. doi: 10.1590/s0004-27492010000200009. [DOI] [PubMed] [Google Scholar]
- 40.Hüsler S., Schmid H. Coping with wet age-related macular degeneration—a study from Switzerland. Klin Monatsbl Augenheilkd. 2013;230:1251–1256. doi: 10.1055/s-0033-1351029. [DOI] [PubMed] [Google Scholar]
- 41.Droege K.M., Muether P.S., Hermann M.M., et al. Adherence to ranibizumab treatment for neovascular age-related macular degeneration in real life. Graefes Arch Clin Exp Ophthalmol. 2013;251:1281–1284. doi: 10.1007/s00417-012-2177-3. [DOI] [PubMed] [Google Scholar]
- 42.Boyle J., Vukicevic M., Koklanis K., et al. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol Health Med. 2018;23:127–140. doi: 10.1080/13548506.2016.1274040. [DOI] [PubMed] [Google Scholar]
- 43.Sii S., Aspinall P., Borooah S., Dhillon B. Exploring factors predicting changes in patients’ expectations and psychosocial issues during the course of treatment with intravitreal injections for wet age-related macular degeneration. Eye. 2018;32:673–678. doi: 10.1038/eye.2017.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krivosic V., Philippakis E., Couturier A., et al. [A “fast track” to improve management of neovascular age related macular degeneration] J Fr Ophtalmol. 2017;40:642–647. doi: 10.1016/j.jfo.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Hanemoto T., Hikichi Y., Kikuchi N., Kozawa T. The impact of different anti-vascular endothelial growth factor treatment regimens on reducing burden for caregivers and patients with wet age-related macular degeneration in a single-center real-world Japanese setting. PloS One. 2017;12 doi: 10.1371/journal.pone.0189035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spooner K.L., Mhlanga C.T., Hong T.H., et al. The burden of neovascular age-related macular degeneration: a patient’s perspective. Clin Ophthalmol Auckl NZ. 2018;12:2483–2491. doi: 10.2147/OPTH.S185052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iida T., Ishii K. Physician, patient, and caregiver experience of different wet age-related macular degeneration anti-VEGF treatment regimens in Japan: a qualitative assessment. Clin Ophthalmol. 2016;10:2505–2513. doi: 10.2147/OPTH.S120803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Droege K.M., Caramoy A., Kersten A., et al. Patient preference of ranibizumab treatment regimen for neovascular age-related macular degeneration—monthly injections versus pro re nata. Graefes Arch Clin Exp Ophthalmol. 2014;252:31–34. doi: 10.1007/s00417-013-2412-6. [DOI] [PubMed] [Google Scholar]
- 49.Liakopoulos S., Spital G., Brinkmann C.K., et al. ORCA study: real-world versus reading centre assessment of disease activity of neovascular age-related macular degeneration (nAMD) Br J Ophthalmol. 2020 doi: 10.1136/bjophthalmol-2019-315717. bjophthalmol-2019-315717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.A Phase III study to evaluate the port delivery system with ranibizumab compared with monthly ranibizumab injections in participants with wet age-related macular degeneration (Archway). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03677934. Accessed June 28, 2020.
- 51.Extension Study for the Port Delivery System with Ranibizumab (Portal). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03683251. Accessed June 28, 2020.
- 52.García-Pérez L.-E., Alvarez M., Dilla T., et al. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4:175–194. doi: 10.1007/s13300-013-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guerci B., Chanan N., Kaur S., et al. Lack of treatment persistence and treatment nonadherence as barriers to glycaemic control in patients with type 2 diabetes. Diabetes Ther. 2019;10:437–449. doi: 10.1007/s13300-019-0590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Budenz D.L. A clinician’s guide to the assessment and management of nonadherence in glaucoma. Ophthalmology. 2009;116:S43–47. doi: 10.1016/j.ophtha.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 55.Hahn S.R. Patient-centered communication to assess and enhance patient adherence to glaucoma medication. Ophthalmology. 2009;116:S37–42. doi: 10.1016/j.ophtha.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 56.Best A.-L., Fajnkuchen F., Nghiem-Buffet S., et al. Treatment efficacy and compliance in patients with diabetic macular edema treated with ranibizumab in a real-life setting. J Ophthalmol. 2018;2018:4610129. doi: 10.1155/2018/4610129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.