Abstract
Different cellular mechanisms have been described as being potentially involved in the progression of neurodegeneration in Parkinson’s disease, although their role is still unclear. The present study aimed to identify in detail, through differentially expressed genes analysis by bioinformatics approaches, the molecular mechanisms triggered after a systemic insult in parkinsonian mice. To address this objective, we combined a dextran sodium sulfate (DSS)-induced ulcerative colitis experimental mice model with an acute 1-methyl-4-phenyl-1,2,3,6-tetradropyridine (MPTP) intoxication. The animals were divided into four experimental groups based on the different treatments: (i) control, (ii) DSS, (iii) MPTP and (iv) MPTP + DSS. The data obtained by microarray and functional enrichment analysis point out the implication of different molecular mechanisms depending on the experimental condition. We see, in the striatum of animals intoxicated only with DSS, dysfunction processes related to the blood. On the other hand, oxidative stress processes are more prominent at the MPTP intoxicated mice. Finally, differentially expressed genes within the MPTP + DSS show functional enrichment in inflammation and programmed cell death. Interestingly, we identify a significant synergistic negative effect of both toxins since the expression of differentially expressed genes (DEGs) related to balanced cellular homeostasis was not enough to prevent processes associated with cell death. This work provides detailed insights into the involvement of systemic inflammation, triggered after an insult in the colon, in the progression of the degeneration in Parkinsonism. In this way, we will be able to identify promising therapeutic targets that prevent the contribution of inflammatory processes in the progression of Parkinson’s disease.
Subject terms: Cellular neuroscience, Parkinson's disease
Introduction
Parkinson’s disease (PD) is the second most common age-related neurodegenerative disorder following Alzheimer’s disease. The main pathological feature of PD is the progressive loss of dopaminergic neurons in the Substantia Nigra pars compacta (SNpc), with the subsequent loss of dopamine (DA) in the striatum. Although much uncertainty still exists about the aetiology of PD, available evidence suggests the implication of numerous processes such as oxidative stress, mitochondrial dysfunction, inflammation and cell apoptosis. Specifically, the deleterious role of systemic inflammation in the onset and progression of PD is becoming evident, so that the interest in its study has increased in the last years1.
In this sense, it is showed that dopaminergic neurons are more vulnerable to oxidative stress and pro-inflammatory cytokines because of their low levels of intracellular glutathione concentrations2. Thus, a sustained systemic or brain inflammation involves activated microglia cells that secrete pro-inflammatory factors that damage neurons3. At the same time, damaged neurons release toxic factors that recruit more glial cells, resulting in a fatal vicious cycle.
To clarify the implication of systemic inflammation in PD, different combinations of experimental animal models have been reported. The most commonly used animal model to approach this issue is based on the systemic administration of lipopolysaccharide (LPS). Extensive research has shown that this endotoxin, from gram-negative bacteria, activates microglia and produces a progressive and cumulative loss of dopaminergic neurons over time4. For example, Qin et al. reported that LPS stimulates cells in the liver to produce TNF-α that is distributed in the blood to the brain to induce the synthesis of more TNF-α and, consequently, damaging dopaminergic neurons5. In another study, the systemic administration of LPS was combined with the induction of ulcerative colitis by the oral ingestion of dextran sulphate sodium (DSS). The results from this work showed an exacerbation of LPS-induced damage in the nigrostriatal system6. Furthermore, García-Domínguez and colleagues combined LPS with the 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP)-based model of PD. The obtained data reinforces the activation of microglial related-events together with the exacerbation of the dopaminergic neurodegeneration7. Together with these observations, in our previous study, we showed an exacerbation of the dopaminergic neuronal death and the glial activation both in the SNpc and striatum when combining MPTP and DSS intoxications8.
Despite the published literature, further analyses are yet required to elucidate in depth the cellular and molecular mechanisms triggered after a systemic insult in Parkinsonian mice. In the present study, we performed microarray and bioinformatics analysis to identify differentially expressed genes (DEGs) of mice treated with MPTP and/or DSS. This work’s aim was to provide a better understanding of the effect of systemic inflammation on the development of PD.
Material and methods
Animals
The study was carried out on 16 three-months-old male C57BL/6J mice acquired from Charles River (Janvier, Le Genest Saint Isle, France). Animals were housed in a special room under regulated temperature (21 ± 1 °C) and 12-h light/dark cycles. The “Three R’s principle” was carefully applied in our study. All procedures related to animal maintenance, care and experimentation were conducted in accordance with the European Community Council Directive (2010/63/UE) for animals to be used in preclinical studies, and were approved by the Institutional Committee on Animal Ethics of the University of Murcia (REGA ES300305440012).
Regimen of intoxication for DSS and MPTP
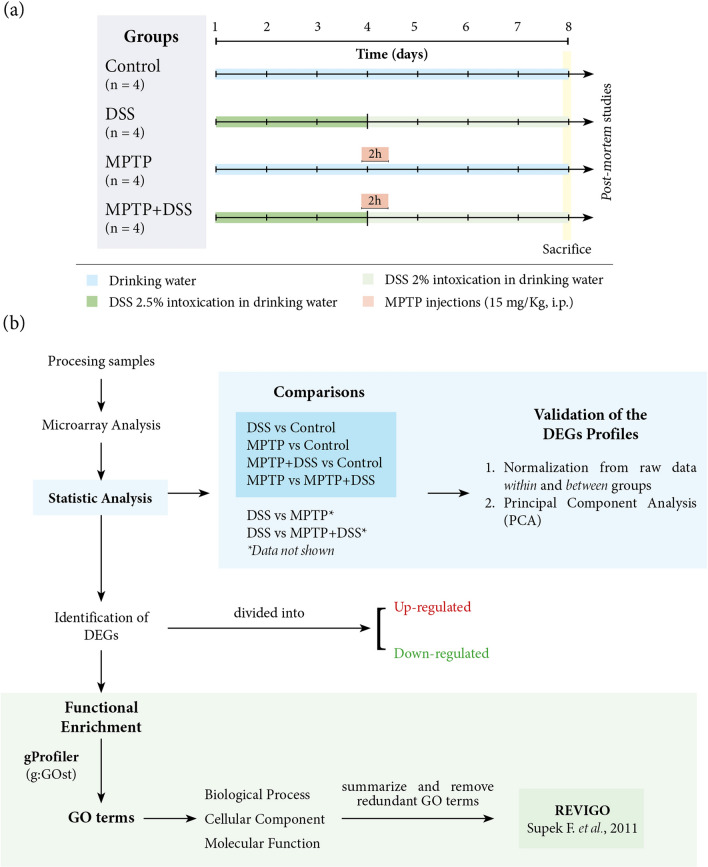
Ulcerative colitis intoxication was induced for 8 days by oral administration of 2–2.5% of DSS (molecular weight, 36–50 kDa, MP Biomedicals LLC, OH, USA) in tap water. On day fourth, MPTP + HCl was intraperitoneal administrated with two injections at 2-h intervals in 1 day dissolved in 0.9% saline (15 mg/Kg, Sigma Aldrich, St Quentin). Mice were distributed in four experimental groups: (a) Control (n = 4), (b) DSS (n = 4), (c) MPTP (n = 5) and (d) MPTP + DSS (n = 5) (Fig. 1a). On day 8, mice were sacrificed by decapitation and brains were immediately removed and dissected into striatum and midbrain8. Samples were correctly stored depending on their future use.
Figure 1.
(a) Experimental design and mice distribution in the different groups. (b) Scheme of the main steps for obtaining and analyzing the results.
RNA extraction and purification for microarray analysis
Striatums were homogenated using QIAzol from the miRNeasy Mini Kit (Qiagen, Hilden, Germany) and stored at – 80 °C until RNA extraction. Total RNA was extracted using the miRNeasy Mini Kit according to the manufacturer’s instructions. RNA samples were quantitated on a NanoDrop 2000 (Thermo Fisher Scientific, Whaltham, MA). RNA quality was examined on an Agilent 2100 Bioanalyzer (Agilent Technologies Inc., Palo Alto, CA) using the RNA 6000 Nano Kit. RNA integrity numbers (RINs) of the samples ranged between 9.2 and 9.7. Samples were stored at – 80 °C until microarray experiments.
RNA labelling, microarray hybridization and feature extraction
RNA samples were thawed and labeled using Agilent Two Color Quick Amp Labeling and RNA Spike-In kits (Agilent), according to the manufacturer's protocol. Experimental samples were labeled with cyanine 5-CTP and used as tests. An Agilent Universal Mouse Reference RNA was labeled with cyanine 3-CTP and used as reference. Each of the labeled test cRNAs was mixed together with labeled reference cRNA. Then, mixes were hybridized onto SurePrint G3 Mouse Gene Expression v2 8 × 60 K microarrays targeting 27,122 Entrez Gene RNAs and 4,578 lncRNAs, using the Agilent Gene Expression Hybridization kit. After hybridization, the microarray slides were washed and scanned in an Agilent G2565CA DNA Microarray Scanner. Images were analyzed with the Agilent Feature Extraction software to automatically generate the datasets. Log10 ratios (test vs reference) were computed after normalization correction performed by linear and Lowess methods.
Analysis of the array expression
The raw data obtained was analyzed with the statistical language R9 and limma package was used10. Intra-array normalization was performed with the method of Loess and inter-array normalization with the Aquantil method. The expression levels of the signals belonging to the same gene were grouped, and the final expression value corresponds to the mean value, as described by Limma instructions. The comparisons between the groups was: D (DSS) vs Ctrl (Control); M (MPTP) vs Ctrl; MD (MPTP + DSS) vs Ctrl; D vs M; D vs MD and M vs MD. DEGs were obtained based on the false discovery rate (FDR, < 0.05) for each comparison.
Enrichment analysis
The enrichment analyses for the up and down DEGs were carried out with gProfileR11 that brings together enrichments from several databases, those of our interest were the annotations from Gene Ontology (GO) and Reactome. From the obtained GO annotations, we carefully summarize and remove redundant terms using Revigo12. These results were illustrated in a network diagram using Cytoscape13 for each ontology for comparisons: biological process (BP), molecular function (MF) and cellular component (CC) (Fig. 1b). The relationships between the nodes are based on the hierarchical association between the terms of the ontology. The brightness of the nodes refers to the level of significance.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Results
Differential gene expression profiles
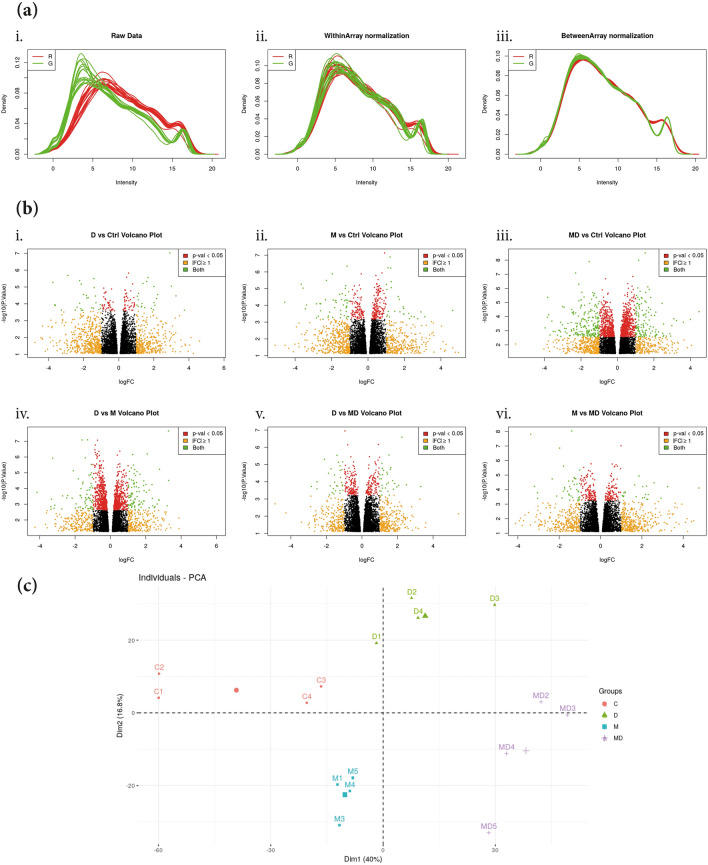
Firstly, normalization from the raw data (Fig. 2a.i) was made within (Fig. 2a.ii) and between groups (Fig. 2a.iii). To avoid any supervised analysis, volcano plots from all the comparisons between the experimental groups showed the distributions of the intensities (Fig. 2b). Moreover, samples from the four experimental groups were distinctly separated in the principal component analysis (PCA) plot, indicating a differential gene expression profile caused by both intoxications (Fig. 2c).
Figure 2.
Validation of the DEGs profiles. (a) Normalization graphs from the raw data (i) was performed within (ii) and between (iii) groups. (b) Volcano plots comparing the levels of genes expressions between the experimental groups (i) DSS vs control; (ii) MPTP vs control; (iii) MPTP + DSS vs control; (iv) DSS vs MPTP; (v) DSS vs MPTP + DSS and (vi) MPTP vs MPTP + DSS. (c) PCA plot for the four experimental groups of interest (control, DSS, MPTP and MPTP + DSS).
Overall, a total of 2,413 genes were found to be differentially expressed, with an absolute fold change set at > 2 and p-value < 0.05 in all possible comparisons between the experimental groups [see Supplementary file 1]. Of the total DEGs, different number of genes were specifically identified based on the experimental group: DSS (132 genes), MPTP (345 genes) and MPTP + DSS (1,357 genes) compared with the control group, and MPTP compared with MPTP + DSS (281 genes) suggesting different effects in the striatum (Table 1). Moreover, in the independent hierarchical clustering represented as heat-maps, it was confirmed the DEGs within and between groups [see Supplementary file 1], specifically exacerbated in the comparisons between MPTP + DSS and control groups.
Table 1.
Total number of DEGs in the different experimental groups [see Supplementary file 2].
| Comparisons | Total DEGs | Down-regulated | Up-regulated |
|---|---|---|---|
| DSS vs ctrl | 132 | 63 | 69 |
| MPTP vs ctrl | 345 | 150 | 195 |
| MPTP + DSS vs ctrl | 1,357 | 634 | 723 |
| MPTP vs MPTP + DSS | 281 | 124 | 157 |
GO enrichment analysis
Based on the high amount of differential expressed genes between groups, we aimed to perform a functional enrichment analysis using g:GOSt from gProfiler which provides the most enriched GO terms together with other data sources (data shown in the Supplementary Files) associated with our gene lists.
Specifically, the data from the GO enrichment analysis is organized into three categories: (i) biological process, (ii) cellular component and (iii) molecular function. All the GO terms lists obtained in each category were summarize removing redundant terms using REVIGO. The output data was used to generate the GO-terms network graphs in Cytoscape. The setting threshold for the false discovery rate (FDR at 0.05).
Biological process
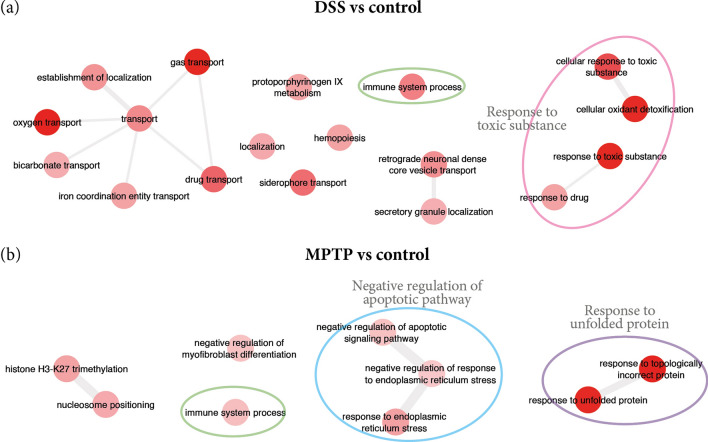
Gene ontology enrichment analysis revealed different profiles depending on the experimental groups. Specifically, the results that concern biological process mainly involved GO terms for response to toxic substance/stimulus, detoxification and immune system but in different way depending on the experimental groups. Thus, up-regulated DEGs, related to response to toxic substance/stimulus, were found in groups treated with DSS (DSS and MPTP vs control; and, MPTP + DSS vs MPTP, Figs. 3 and 4). Interestingly, we found up-regulated DEGs related to detoxification mechanisms in animals treated with DSS compared to control mice (Table 2, Fig. 3a) and MPTP + DSS, clearly observed when comparing MPTP + DSS vs MPTP (Table 3, Fig. 4).
Figure 3.
Summary of the main GO terms found for biological process in the comparisons for (a) DSS vs control and (b) MPTP vs control. The color of the circles help to better identify the groups of terms for specific processes: green for immune system process, pink for response to toxic substance, blue for apoptotic pathway and purple for response to unfolded protein.
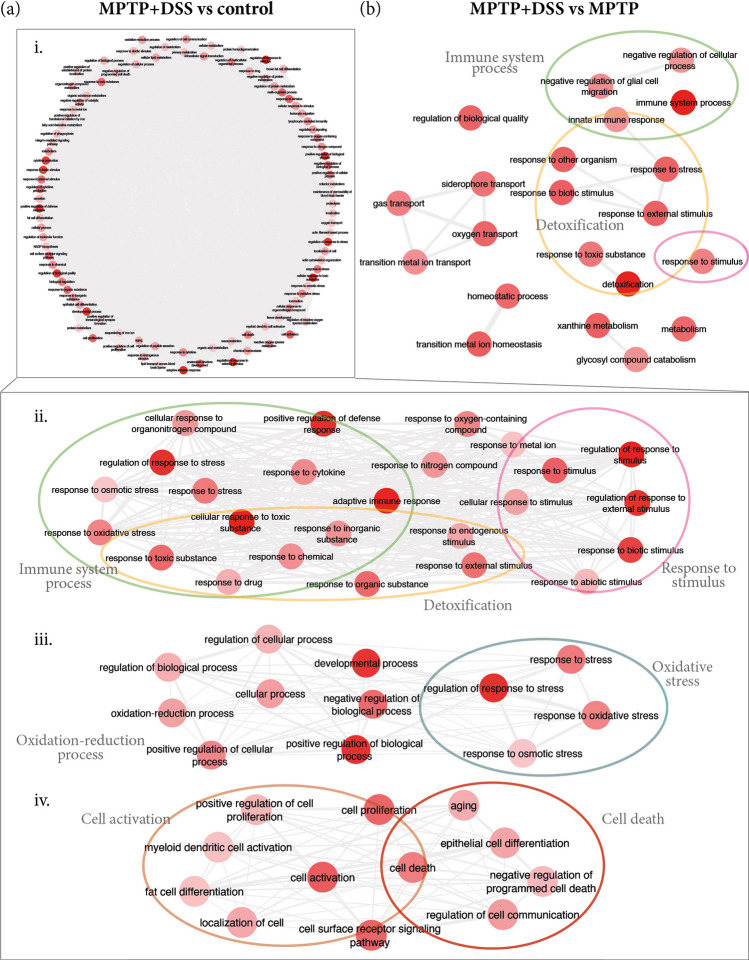
Figure 4.
Summary of the main GO terms found for biological process in the comparisons for MPTP + DSS vs control (a) and MPTP (b, i–iv). The color of the circles help to better identify the groups of terms for specific processes: green for immune system process (ii, b), pink for response to toxic substance/stimulus (ii, b), aquamarine for oxidative stress (iii), orange for cell activation (iv) and red for cell death (iv).
Table 2.
The enriched GO terms for the up-regulated DEGs of DSS and MPTP vs control, respectively.
| GO Term | Description | Corrected p-value | DEGs count |
|---|---|---|---|
| DSS vs control | |||
| GO:0098869 | Cellular oxidant detoxification | 1.08E−04 | 6 |
| GO:1990748 | Cellular detoxification | 1.10E−04 | 6 |
| GO:0098754 | Detoxification | 1.44E−04 | 6 |
| GO:0097237 | Cellular response to toxic substance | 1.00E−03 | 7 |
| GO:0015893 | Drug transport | 2.71E−03 | 6 |
| GO:0002376 | Immune system process | 8.49E−03 | 17 |
| GO:0042493 | Response to drug | 3.23E−02 | 10 |
| GO:0030097 | Hemopoiesis | 3.23E−02 | 9 |
| GO:0046501 | Protoporphyrinogen IX metabolic process | 3.23E−02 | 2 |
| GO:0048534 | Hematopoietic or lymphoid organ development | 4.23E−02 | 9 |
| MPTP vs control | |||
| GO:0035966 | Response to topologically incorrect protein | 7.61E−09 | 11 |
| GO:0006986 | Response to unfolded protein | 1.67E−06 | 10 |
| GO:0035967 | Cellular response to topologically incorrect protein | 6.57E−05 | 8 |
| GO:0034620 | Cellular response to unfolded protein | 2.64E−04 | 7 |
| GO:0030968 | Endoplasmic reticulum unfolded protein response | 1.55E−03 | 6 |
| GO:0034976 | Response to endoplasmic reticulum stress | 6.22E−03 | 9 |
| GO:0002376 | Immune system process | 2.88E−02 | 31 |
| GO:2001234 | Negative regulation of apoptotic signaling pathway | 3.29E−02 | 8 |
| GO:1904761 | Negative regulation of myofibroblast differentiation | 3.31E−02 | 2 |
| GO:1903573 | Negative regulation of response to endoplasmic reticulum stress | 4.80E−02 | 4 |
Table 3.
The enriched GO terms for the up-regulated DEGs of MPTP + DSS vs control and MPTP, respectively.
| GO Term | Description | Corrected p-value | DEGs count |
|---|---|---|---|
| MPTP + DSS vs controla | |||
| GO:0070887 | Cellular response to chemical stimulus | 3.70E−13 | 155 |
| GO:0098754 | Detoxification | 3.36E−11 | 24 |
| GO:0002376 | Immune system process | 5.01E−11 | 135 |
| GO:0006950 | Response to stress | 6.10E−11 | 173 |
| GO:1990748 | Cellular detoxification | 1.07E−09 | 21 |
| GO:0002682 | Regulation of immune system process | 1.65E−09 | 86 |
| GO:0009605 | Response to external stimulus | 3.46E−09 | 135 |
| GO:0006954 | Inflammatory response | 5.40E−09 | 53 |
| GO:0050896 | Response to stimulus | 2.14E−08 | 345 |
| GO:0097237 | Cellular response to toxic substance | 3.37E−08 | 29 |
| GO:0080134 | Regulation of response to stress | 3.37E−08 | 78 |
| GO:0009636 | Response to toxic substance | 3.37E−08 | 45 |
| GO:0042127 | Regulation of cell population proliferation | 4.34E−08 | 93 |
| GO:0006952 | Defense response | 4.34E−08 | 88 |
| GO:0031347 | Regulation of defense response | 4.34E−08 | 48 |
| GO:0098869 | Cellular oxidant detoxification | 4.52E−08 | 18 |
| GO:0042592 | Homeostatic process | 7.16E−08 | 98 |
| GO:0032502 | Developmental process | 1.14E−07 | 238 |
| GO:0032101 | Regulation of response to external stimulus | 1.14E−07 | 60 |
| GO:0001775 | Cell activation | 1.39E−07 | 65 |
| GO:0048583 | Regulation of response to stimulus | 6.53E−07 | 167 |
| GO:0001816 | Cytokine production | 8.61E−07 | 49 |
| GO:0048856 | Anatomical structure development | 9.28E−07 | 220 |
| MPTP + DSS vs MPTPb | |||
| GO:0098754 | Detoxification | 5.56E−04 | 8 |
| GO:0002376 | Immune system process | 5.56E−04 | 34 |
| GO:1990748 | Cellular detoxification | 2.93E−03 | 7 |
| GO:0055076 | Transition metal ion homeostasis | 8.11E−03 | 7 |
| GO:0097577 | Sequestering of iron ion | 9.03E−03 | 2 |
| GO:0051707 | Response to other organism | 9.03E−03 | 21 |
| GO:0046916 | Cellular transition metal ion homeostasis | 9.03E−03 | 6 |
| GO:0046110 | Xanthine metabolic process | 9.03E−03 | 2 |
| GO:0098869 | Cellular oxidant detoxification | 9.03E−03 | 6 |
| GO:0043207 | Response to external biotic stimulus | 9.03E−03 | 21 |
| GO:0009607 | Response to biotic stimulus | 9.03E−03 | 21 |
| GO:0009605 | Response to external stimulus | 9.44E−03 | 32 |
See complete tables in the Supplementary Files.
aResults shown for MPTP + DSS vs control with corrected p-value ≤ 1E-07.
bResults shown for MPTP + DSS vs MPTP with corrected p-value ≤ 1E-03.
Moreover, the data revealed that genes related to immune system processes are up-regulated in DSS (Fig. 3a), MPTP (Fig. 3b) and MPTP + DSS (Fig. 4a) groups compared to the control group; and, when comparing MPTP + DSS with MPTP group (Fig. 4b). However, these genes are especially exacerbated when comparing MPTP + DSS with control and MPTP mice (Fig. 4a.ii and Table 3). Together with the exacerbation of the immune system process, we also observed in this group oxidative stress processes (Fig. 4a.iii and Table 3), cell activation and cell death (Fig. 4a.iii and Table 3).
Cellular component
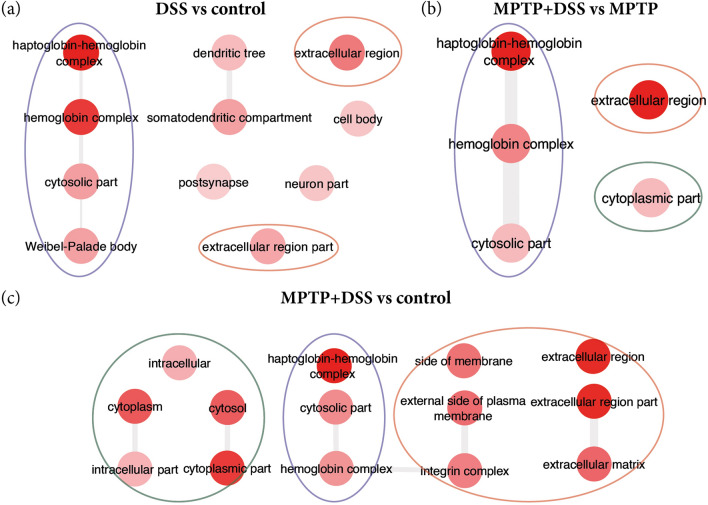
Unlike in the category of biological process where in all groups came out significant up-regulation of the DEGs; in the cellular component were significantly observed only in the comparisons: DSS vs control, MPTP + DSS vs control and MPTP + DSS vs MPTP (Fig. 5). Interestingly, in the three comparisons (animals treated with DSS) the groups of genes related to haptoblobin-hemoglobin complex and the extracellular region stand out. In addition, when animals are treated together with MPTP (Fig. 5b and c.i, Table 4), changes are also observed in the intracellular part.
Figure 5.
Summary of the main GO terms obtained for cellular component in the comparisons for DSS vs control (a), MPTP + DSS vs MPTP (b) and MPTP + DSS vs control (c). The color of the circles correspond to different groups of annotations: purple for haptoblobin-hemoglobin complex, orange for extracellular region and green for intracellular part.
Table 4.
The enriched GO terms for the DEGs of DSS and MPTP vs control, respectively.
| GO Term | Description | Corrected p-value | DEGs count |
|---|---|---|---|
| DSS vs control | |||
| GO:0031838 | Haptoglobin–hemoglobin complex | 7.02E−07 | 4 |
| GO:0005833 | Hemoglobin complex | 1.46E−06 | 4 |
| GO:0005576 | Extracellular region | 1.86E−04 | 20 |
| GO:0044445 | Cytosolic part | 2.26E−03 | 6 |
| GO:0036477 | Somatodendritic compartment | 2.77E−03 | 11 |
| GO:0033093 | Weibel-palade body | 2.77E−03 | 2 |
| GO:0005615 | Extracellular space | 2.77E−03 | 13 |
| GO:0044421 | Extracellular region part | 3.42E−03 | 14 |
| MPTP + DSS vs MPTP | |||
| GO:0005576 | Extracellular region | 2.42E−06 | 39 |
| GO:0031838 | Haptoglobin–hemoglobin complex | 2.42E−06 | 4 |
| GO:0044421 | Extracellular region part | 3.43E−06 | 31 |
| GO:0005615 | Extracellular space | 3.43E−06 | 28 |
| GO:0005833 | Hemoglobin complex | 2.15E−03 | 3 |
| MPTP + DSS vs MPTP | |||
| GO:0044421 | Extracellular region part | 6.98E−07 | 104 |
| GO:0005615 | Extracellular space | 6.98E−07 | 96 |
| GO:0005576 | Extracellular region | 1.73E−06 | 122 |
| GO:0044444 | Cytoplasmic part | 1.73E−06 | 302 |
| GO:0005737 | Cytoplasm | 3.19E−05 | 360 |
| GO:0005829 | Cytosol | 1.01E−04 | 147 |
| GO:0031838 | Haptoglobin–hemoglobin complex | 3.64E−04 | 5 |
| GO:0031012 | Extracellular matrix | 6.95E−04 | 30 |
| GO:0009986 | Cell surface | 7.29E−04 | 53 |
| GO:0008305 | Integrin complex | 2.41E−03 | 7 |
| GO:0044445 | Cytosolic part | 3.07E−03 | 20 |
| GO:0098636 | Protein complex involved in cell adhesion | 3.58E−03 | 7 |
| GO:0005833 | Hemoglobin complex | 5.81E−03 | 4 |
| GO:0009897 | External side of plasma membrane | 7.66E−03 | 30 |
| GO:0098552 | Side of membrane | 7.66E−03 | 37 |
Molecular function
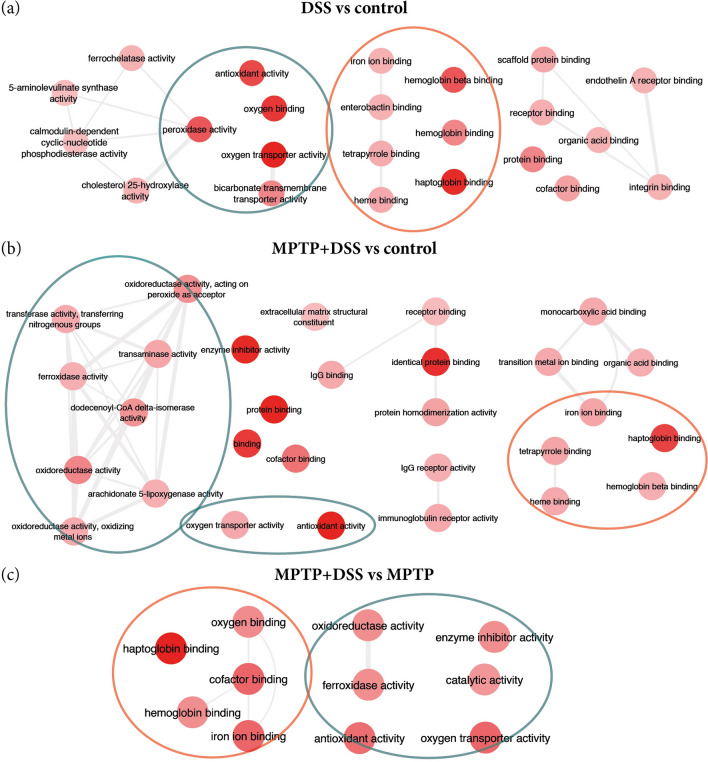
Regarding molecular function GO annotations, we found terms from up-regulated DEGs mainly implicated in antioxidant activity and haptoglobin binding in the comparisons for DSS vs control, MPTP + DSS vs control and MPTP + DSS vs MPTP (Fig. 6, Table 5).
Figure 6.
Summary of the main GO terms obtained for molecular function in the comparisons for DSS vs control (a), MPTP + DSS vs control (b) and MPTP + DSS vs MPTP (c).
Table 5.
The most significant enriched GO terms for the DEGs of DSS and MPTP vs control and MPTP + DSS vs MPTP.
| GO term | Description | Corrected p-value | DEGs count |
|---|---|---|---|
| DSS vs controla | |||
| GO:0031720 | Haptoglobin binding | 9.59E−06 | 4 |
| GO:0005344 | Oxygen carrier activity | 3.37E−06 | 4 |
| GO:0019825 | Oxygen binding | 2.73E−05 | 4 |
| GO:0016209 | Antioxidant activity | 9.96E−05 | 5 |
| GO:0140104 | Molecular carrier activity | 1.63E−04 | 4 |
| GO:0004601 | Peroxidase activity | 2.19E−04 | 4 |
| GO:0031722 | Hemoglobin beta binding | 2.19E−04 | 2 |
| GO:0016684 | Oxidoreductase activity, acting on peroxide as acceptor | 2.52E−04 | 4 |
| GO:0031721 | Hemoglobin alpha binding | 1.02E−03 | 2 |
| GO:0030492 | Hemoglobin binding | 2.78E−03 | 2 |
| GO:0015106 | Bicarbonate transmembrane transporter activity | 2.78E−03 | 2 |
| GO:0005515 | Protein binding | 2.78E−03 | 35 |
| GO:0005452 | Inorganic anion exchanger activity | 7.66E−03 | 2 |
| MPTP + DSS vs controla | |||
| GO:0042802 | Identical protein binding | 7.99E−07 | 97 |
| GO:0016209 | Antioxidant activity | 8.02E−07 | 16 |
| GO:0005515 | Protein binding | 2.33E−06 | 348 |
| GO:0030414 | Peptidase inhibitor activity | 1.78E−05 | 22 |
| GO:0004857 | Enzyme inhibitor activity | 1.93E−05 | 31 |
| GO:0061134 | Peptidase regulator activity | 4.69E−05 | 23 |
| GO:0004866 | Endopeptidase inhibitor activity | 6.02E−05 | 20 |
| GO:0061135 | Endopeptidase regulator activity | 1.28E−04 | 20 |
| GO:0048037 | Cofactor binding | 3.16E−04 | 37 |
| GO:0005488 | Binding | 4.53E−04 | 460 |
| GO:0016491 | Oxidoreductase activity | 5.62E−04 | 46 |
| GO:0003824 | Catalytic activity | 1.12E−03 | 209 |
| GO:0030234 | Enzyme regulator activity | 1.12E−03 | 50 |
| GO:0042803 | Protein homodimerization activity | 1.22E−03 | 48 |
| GO:0004869 | Cysteine-type endopeptidase inhibitor activity | 1.35e−03 | 10 |
| GO:0016722 | Oxidoreductase activity, oxidizing metal ions | 1.50E−03 | 6 |
| GO:0016684 | Oxidoreductase activity, acting on peroxide as acceptor | 1.75E−03 | 9 |
| GO:0005506 | Iron ion binding | 1.75E−03 | 18 |
| GO:0004322 | Ferroxidase activity | 2.04E−03 | 5 |
| GO:0016724 | Oxidoreductase activity, oxidizing metal ions, oxygen as acceptor | 2.04E−03 | 5 |
| GO:1901567 | Fatty acid derivative binding | 2.53E−03 | 7 |
| GO:0031720 | Haptoglobin binding | 3.21E−03 | 4 |
| GO:0046906 | Tetrapyrrole binding | 3.49E−03 | 16 |
| GO:0004165 | Dodecenoyl-coa delta-isomerase activity | 3.73E−03 | 3 |
| GO:0004601 | Peroxidase activity | 4.13E−03 | 8 |
| GO:0020037 | Heme binding | 6.13E−03 | 15 |
| GO:0043177 | Organic acid binding | 6.15E−03 | 17 |
| GO:0046914 | Transition metal ion binding | 6.71E−03 | 50 |
| MPTP + DSS vs MPTPa | |||
| GO:0031720 | Haptoglobin binding | 4.89E−04 | 3 |
aResults shown with corrected p-value ≤ 1E−03.
Reactome enriched analysis
The data obtained from the Reactome annotations divided the comparisons into different events in the same scenario (striatum level). Firstly, DSS compared to control stand out mechanisms related to heme dysfunction (as scavenging of heme from plasma or heme biosynthesis) possibly due to the bleeding produced by the DSS administration. Since free heme promotes the conversion of low density lipoproteins into cytotoxic products, make it toxic, it is also observed immune system processes up-regulation (Table 6).
Table 6.
Functional enriched Reactome annotations for all the comparisons.
| Reactome code | Description | Corrected p-value | DEGs count |
|---|---|---|---|
| DSS vs control | |||
| 1247673 | Erythrocytes take up oxygen and release carbon dioxide | 7.33E−08 | 5 |
| 1237044 | Erythrocytes take up carbon dioxide and release oxygen | 1.92E−07 | 5 |
| 1480926 | O2/CO2 exchange in erythrocytes | 1.92E−07 | 5 |
| 2168880 | Scavenging of heme from plasma | 8.25E−05 | 5 |
| 2173782 | Binding and uptake of ligands by scavenger receptors | 1.52E−04 | 5 |
| 189451 | Heme biosynthesis | 1.08E−02 | 2 |
| 189445 | Metabolism of porphyrins | 2.54E−02 | 2 |
| 216083 | Integrin cell surface interactions | 3.02E−02 | 3 |
| 168256 | Immune system | 3.02E−02 | 13 |
| MPTP vs control | |||
| 140342 | Apoptosis induced DNA fragmentation | 1.45E−05 | 5 |
| 2559584 | Formation of senescence-associated heterochromatin foci (SAHF) | 4.68E−05 | 5 |
| 2559586 | DNA damage/telomere stress induced senescence | 4.58E−03 | 6 |
| 75153 | Apoptotic execution phase | 5.18E−03 | 5 |
| MPTP + DSS vs control | |||
| 168256 | Immune system | 1.48E−07 | 111 |
| 168249 | Innate immune system | 1.14E−06 | 73 |
| 6798695 | Neutrophil degranulation | 2.75E−06 | 47 |
| 1247673 | Erythrocytes take up oxygen and release carbon dioxide | 2.91E−04 | 4 |
| 198933 | Immunoregulatory interactions between a lymphoid and a Non-lymphoid cell | 2.12E−03 | 19 |
| 5686938 | Regulation of TLR by endogenous ligand | 2.12E−03 | 6 |
| 804914 | Transport of fatty acids | 5.34E−03 | 4 |
| 425397 | Transport of vitamins, nucleosides, and related molecules | 8.93E−03 | 8 |
| 8978868 | Fatty acid metabolism | 1.33E−02 | 19 |
| 109582 | Hemostasis | 3.06E−02 | 39 |
| 216083 | Integrin cell surface interactions | 4.19E−02 | 10 |
| 1236975 | Antigen processing-cross presentation | 4.45E−02 | 7 |
| 2142688 | Synthesis of 5-eicosatetraenoic acids | 4.68E−02 | 3 |
| MPTP + DSS vs MPTP | |||
| 174577 | Activation of C3 and C5 | 7.20E−03 | 3 |
| 1247673 | Erythrocytes take up oxygen and release carbon dioxide | 1.21E−02 | 3 |
| 1480926 | O2/CO2 exchange in erythrocytes | 1.79E−02 | 3 |
| 1237044 | Erythrocytes take up carbon dioxide and release oxygen | 1.79E−02 | 3 |
Regarding MPTP compared to control, we observed two main pathways: (i) programmed cell death by apoptosis induced DNA fragmentation, and (ii) cellular responses to external stimuli that implicated DNA damage/telomerase stress induced senescence (Table 6).
On the other hand, the last events are exacerbated when comparing MPTP + DSS with both control and MPTP mice, which are the immune system processes. Specifically, it is observed: (i) the innate immune system response, with the up-regulation of the neutrophil degranulation and the toll-like receptor cascade (regulation of TLR by endogenous ligand) and, (ii) the adaptive immune system response, with the Class I MHC mediated antigen processing and presentation (antigen processing cross presentation) and immunoregulatory interactions between a lymphoid and a non-lymphoid cell (Table 6).
Discussion
Prior work have remarked the involvement of systemic inflammation in the development of PD. The findings from the present work extend those published reports through a bioinformatics approach. We aimed to elucidate in depth the molecular and cellular mechanisms triggered after a systemic insult in the striatum of mice untreated and treated with MPTP. The importance of specifically studying the effect of both toxins in the striatum is based on previous data obtained by immunohistochemical techniques in which we observed exacerbation of the inflammatory events when combining both toxins8.
The results from this study provide novel information about the DEGs in the striatum of mice intoxicated only with DSS. It is clear to observe the up-regulation of processes related to detoxification and inflammatory mechanisms (Fig. 3, Table 3) mainly triggered by the bleeding and lesion of the mucosal surface caused by the administration of DSS14. This is the first study to our knowledge to describe the molecular and cellular mechanisms activated in the striatum after a local insult in the colon.
Otherwise, when comparing MPTP mice with the control group, we found biological processes related to the response to unfolded protein (Fig. 3b, Table 2). Interestingly, these results are in accordance with the observations of Fornai and colleagues who described that the administration of MPTP reduces the protein degradation function of the striatal ubiquitin–proteasome system15. Later on, it was added that this could lead the accumulation of unfolded proteins and consequently, activates stress-induced cell death mechanisms16. Moreover, it was demonstrated that oxidative stress processes may participate in the formation of cross-linked protein aggregates17. In these animals, we also noticed DEGs associated with an attempt to regulate negatively the apoptotic signalling pathway and positively the activation of the inflammatory response (Table 3). Interestingly, the data from Reactome analysis showed annotations related to the execution of apoptosis mechanisms induced by DNA fragmentation and the induction of cellular senescence. These results are in line with those reported to another study that suggests that the nature and severity of DNA damage may determine the cellular response18. More research is needed to clarify how is the interplay of both routes after a DNA insult19.
Most notably were the observations that resulted from the comparison between MPTP + DSS and control animals. As this work indicates, we obtained an exacerbation of the up-regulated DEGs in all the analysis related to detoxification, oxidative stress and immune system processes.
Our results not only reinforce those results reported in the literature but also extend detailed information on the contribution of systemic inflammation to the progression of the neurodegeneration associated with Parkinson’s disease. Although our aim was highly achieved, we are aware of some intrinsic limitations such as the number of animals used in this type of studies20,21 or the focus in one brain area. The relevance of this lies in the contribution of novel and detailed information that describes different expression profiles that can be used as a guide for further and specific analysis. We advise that future work should evaluate upregulated pathways in different brain areas over time. In this way, we will be able to identify promising therapeutic targets that prevent the contribution of inflammatory processes in the progression of Parkinson’s disease.
Conclusions
Altogether, we provide functional and comprehensive bioinformatics analyses of the deleterious effect of the systemic inflammation in the striatum of MPTP intoxicated mice. Interestingly, the data showed in this study describes a scenario that becomes more complex when combining both treatments. Thus, the processes related to inflammation and oxidative stress are exacerbated resulting in a significant up-regulation of the cell death mechanisms.
Supplementary information
Acknowledgements
Research work of the authors was supported by the Spanish Ministry of Science and Innovation (FIS PI13 01293); Fundación Séneca (19540/PI/14) and “Prediction of cognitive properties of new drug candidates for neurodegenerative diseases in early clinical development” (European Community’s Seventh Framework Programme (FP7/2007-2013) for the Innovative Medicine Initiative under Grant Agreement No. 115009) to MTH.
Author contributions
M.T.H. and A.L.G.M. designed the research; A.L.G.M. and C.S.R. performed the research; A.L.G.M. analyzed the data and designed the figures; L.C.B., A.G.C., A.P., S.V. and E.F.V. contributed to the discussion of the in vivo results; A.L.G.M. and M.T.H. discussed all the results and wrote the paper.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
E. Fernandez-Villalba, Email: emiliano.fernandez@carm.es
M. T. Herrero, Email: mtherrer@um.es
Supplementary information
is available for this paper at 10.1038/s41598-020-69695-4.
References
- 1.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 2.Sian J, et al. Alterations in glutathione levels in parkinson’s disease and other in neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 3.Collins LM, Toulouse A, Connor TJ, Nolan YM. Contributions of central and systemic inflammation to the pathophysiology of Parkinson’s disease. Neuropharmacology. 2012;62:2153–2167. doi: 10.1016/j.neuropharm.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Lehnardt S, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin L, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villaran RF, et al. Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: potential risk factor in Parkinson`s disease. J. Neurochem. 2010;114:1687–1700. doi: 10.1111/j.1471-4159.2010.06879.x. [DOI] [PubMed] [Google Scholar]
- 7.García-Domínguez I, et al. Peripheral inflammation enhances microglia response and nigral dopaminergic cell death in an in vivo MPTP model of Parkinson’s disease. Front. Cell. Neurosci. 2018;12:1–16. doi: 10.3389/fncel.2018.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gil-Martínez A-L, et al. Local gastrointestinal injury exacerbates inflammation and dopaminergic cell death in parkinsonian mice. Neurotox. Res. 2019;35:913–935. doi: 10.1007/s12640-019-0010-z. [DOI] [PubMed] [Google Scholar]
- 9.RC Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 10.Ritchie ME, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;e43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reimand, J., Kolde, R. GProfileR: Interface to the ’G:Profiler’ Toolkit. (2018). https://cran.r-project.org/package=gProfileR.
- 12.Supek F, Bošnjak M, Škunca N, Šmuc T. Revigo summarizes and visualizes long lists of gene ontology terms. PLoS ONE. 2011;6:e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J. Biomed. Biotechnol. 2012;2012:1–13. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fornai F, et al. Parkinson-like syndrome induced by continuous MPTP infusion: Convergent roles of the ubiquitin-proteasome system and α-synuclein. Proc. Natl. Acad. Sci. USA. 2005;102:3413–3418. doi: 10.1073/pnas.0409713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egawa N, et al. The endoplasmic reticulum stress sensor, ATF6α, protects against neurotoxin-induced dopaminergic neuronal death. J. Biol. Chem. 2011;286:7947–7957. doi: 10.1074/jbc.M110.156430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoerndli F, David DC, Götz J. Functional genomics meets neurodegenerative disorders: part II: application and data integration. Prog. Neurobiol. 2005;76:169–188. doi: 10.1016/j.pneurobio.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Vousden KH, Lane DP. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 19.Childs BG, Baker DJ, Kirkland JL, Campisi J, Van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014;15:1139–1153. doi: 10.15252/embr.201439245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirnics K, et al. Molecular signatures of neurodegeneration in the cortex of PS1/PS2 double knockout mice. Mol. Neurodegener. 2008;3:1–11. doi: 10.1186/1750-1326-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Ganini D, Mason RP. Role of cytochrome c in α-synuclein radical formation: Implications of α-synuclein in neuronal death in Maneb- and paraquat-induced model of Parkinson’s disease. Mol. Neurodegener. 2016;11:1–12. doi: 10.1186/s13024-016-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].