Abstract
Severed CNS axons fail to regenerate in adult mammals and there are no effective regenerative strategies to treat patients with CNS injuries. Several genes, including phosphatase and tensin homolog (PTEN) and Krüppel-like factors, regulate intrinsic growth capacity of mature neurons. The Lin28 gene is essential for cell development and pluripotency in worms and mammals. In this study, we evaluated the role of Lin28a in regulating regenerative capacity of diverse populations of CNS neurons in adult mammals. Using a neuron-specific Thy1 promoter, we generated transgenic mice that overexpress Lin28a protein in multiple populations of projection neurons, including corticospinal tracts and retinal ganglion cells. We demonstrate that upregulation of Lin28a in transgenic mice induces significant long distance regeneration of both corticospinal axons and the optic nerve in adult mice. Importantly, overexpression of Lin28a by post-injury treatment with adeno-associated virus type 2 (AAV2) vector stimulates dramatic regeneration of descending spinal tracts and optic nerve axons after lesions. Upregulation of Lin28a also enhances activity of the Akt signaling pathway in mature CNS neurons. Therefore, Lin28a is critical for regulating growth capacity of multiple CNS neurons and may become an important molecular target for treating CNS injuries.
Keywords: Lin28, neuron growth capacity, transgenic mice, CNS lesion, spinal cord injury, optic nerve injury, axon regeneration, AAV vector, gene delivery therapy
Graphical Abstract
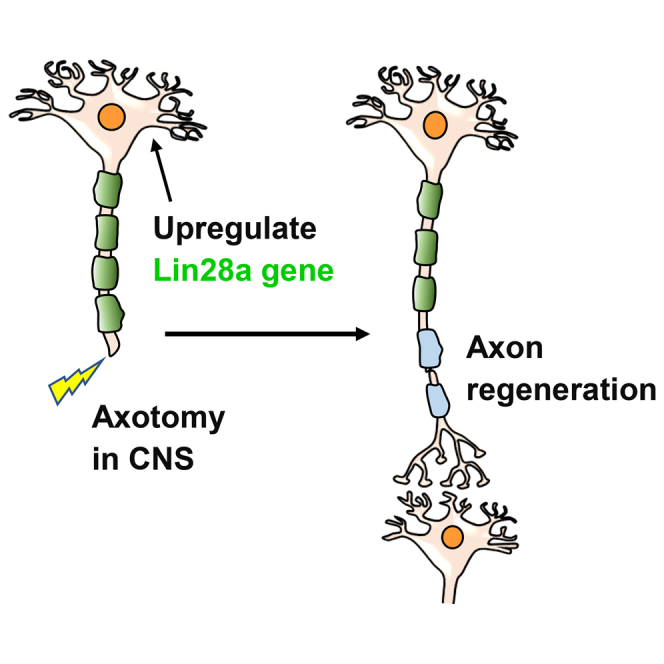
Severed CNS axons fail to regenerate in adult mammals, and there are no effective regenerative strategies to treat patients with CNS injuries. Nathan et al. demonstrate that upregulating Lin28a by either a transgenic or viral approach induces significant long distance regeneration of both corticospinal axons and optic nerves in adult mice.
Introduction
During development, neurons lose growth ability to extend their axons gradually, and the reduced intrinsic growth capacity of mature neurons substantially contributes to repair failure of injured CNS neurons.1, 2, 3, 4, 5 Several cell-autonomous molecules, including phosphatase and tensin homolog (PTEN), Krüppel-like factors (KLFs), c-myc, and SOX11, have been reported to control neuronal growth ability.6, 7, 8, 9, 10, 11 Among them, PTEN is particularly important for regulating the regenerative capacity of mature neurons. So far, none of these gene targets has been translated to clinical use. Because targeting each of those molecules partially enhances growth capability of mature neurons and only achieves a degree of regeneration of injured CNS axons, other genes unidentified may play critical roles for controlling growth failure of mature neurons. It is thus required to identify better targets that may affect multiple pathways for cell growth and provide a more effective strategy for CNS regeneration.
Lin28 is an RNA-binding protein that enhances translation of multiple genes, including insulin-like growth factors (IGFs) and metabolic enzymes for increasing glycolysis and oxidative phosphorylation.12, 13, 14 Lin28 regulates glucose homeostasis by activating insulin-phosphatidylinositol 3-kinase (PI3K)-mammalian target of rapamycin (mTOR) signaling in mammals.15 Lin28 blocks generation of mature let-7 miRNA (microRNA) by binding the terminal loop of let-7 pre-miRNA in embryonic stem cells and prevents its processing by Dicer,16 although it may function by let-7-independent mechanisms.12,17 Lin28 is highly expressed during early embryogenesis and is critical for controlling self-renewal, development, pluripotency, and metabolism of stem cells.16,18 In Caenorhabditis elegans, Lin28 regulates early cell proliferation independent of let-7 and promotes later cell differentiation by suppressing let-7.19 In contrast to one Lin28 gene in worms, vertebrates have two Lin28 paralogs (Lin28a and Lin28b), which are central regulators of growth-related signaling pathways and metabolic enzymes in multiple cells.12,20 Overexpression of Lin28 delays puberty onset, causes gigantism, and improves regrowth of many injured tissues in transgenic (Tg) mice, including hair, cartilage, bone, and mesenchyme.12,21 Lin28 activation is crucial for controlling dedifferentiation of Müller glia and expression of regeneration genes in retina of zebrafish22,23 and proliferation of Müller glia in mice.24 Ectopic expression of Lin28 or other pluripotency-inducing genes (such as Oct4, SOX2, KLF4, and c-myc) could reprogram somatic mammalian cells to become pluripotent stem cells.25,26
Because Lin28 is a gatekeeper molecule to control switching between pluripotency and committed cells, and its reactivation stimulates repair of several tissue systems,27 we hypothesized that reprogramming mature CNS neurons with Lin28 would influence their growth capacity after axotomy. For the first time, we identified the crucial role of Lin28 in controlling the regenerative ability of corticospinal tracts (CSTs) in adult mammals, which are essential for controlling voluntary movements,28 but are particularly refractory to regeneration.29, 30, 31 Out of two subtypes in vertebrates, Lin28a regulated cell maturation32,33 and its upregulation reprogramed human somatic cells into induced pluripotent stem cells26 and enhanced repair of several non-neural tissues in adult mice by improving cellular bioenergetics.12 We thus targeted Lin28a in this study and found that its overexpression by either the Tg or viral vector approach resulted in long distance regeneration of severed spinal cord and optic nerve axons in adult mice. Upregulating Lin28a activated the Akt signaling pathway in both cortex and retina of adult mice. We therefore demonstrate the critical role of Lin28a for controlling growth capacity of mature CNS neurons in adult mammals.
Results
Lin28 Protein Is Downregulated in the Cortex and Retina of Developing Mice
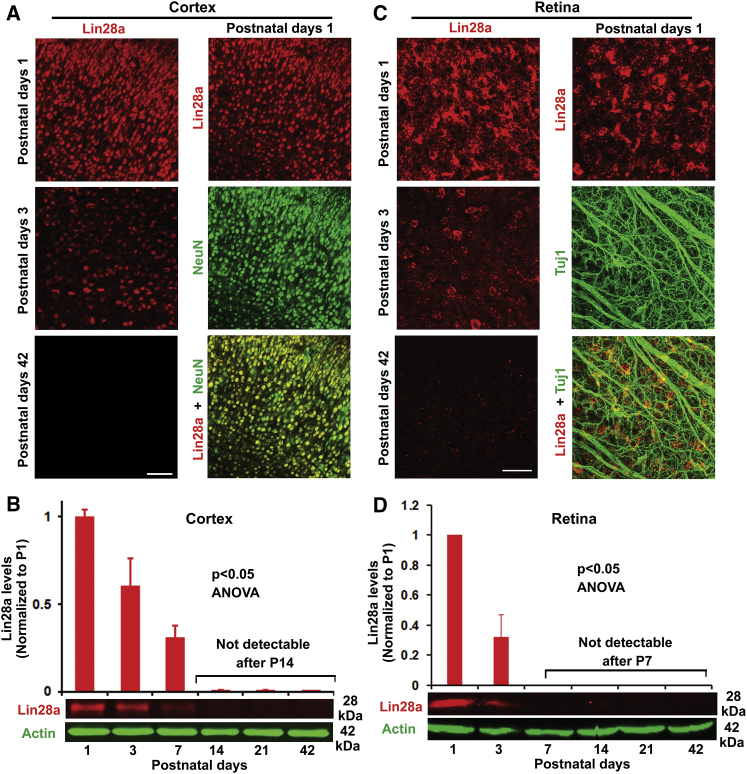
Because Lin28 determines onset of early larval stages of developmental events in C. elegans by regulating self-renewal of nematode stem cells as a heterochronic gene and is highly expressed in mouse embryonic stem cells during early development,34,35 we assessed expression changes of the Lin28a signal in developing mouse CNS by measuring its expression levels in the sensorimotor cortex and retina at different ages, that is, postnatal day 1 (P1), P3, P7, P14, P21, P42, and P56, by immunohistochemistry and western blotting. Lin28a signals were detected from the cortex and retina of P1–P3 mice, especially P1, but they were downregulated during development, becoming undetectable levels from P7 in retina and from P14 in cortex until adult (Figure 1). Immunostaining indicated that Lin28a signals were principally colocalized with neuronal marker NeuN in cortex and partially with retinal ganglion cells (RGCs) marker Tuj1 in retina of P1 (Figures 1A and 1C) and P3 mice, although they also were present in non-RGCs. Because Lin28a controls the growth program of multiple cell types,12,21,27 decreased Lin28a levels in the brain and retina of adult mammals may contribute to the reduction in growth ability of mature neurons during development or after injury.
Figure 1.
Downregulation of Lin28a Protein in the Cerebral Cortex and Retina of C57BL/6 Mice During Development
(A and B) Levels of Lin28a protein were measured from the cerebral cortex at postnatal day 1 (P1), P3, and P42 by immunostaining (A) and at P1, P3, P7, P14, P21, and P42 by western blots (B). The cortical areas within 1.5 mm (for P1-7 mice) or 2 mm (for P14-42 mice) surrounding bregma were collected. We detected age-dependent downregulation of Lin28a, especially in the cortex of mice >2 weeks old. Scale bar, 50 μm. (C and D) Levels of Lin28a protein were determined from the retina of mice at P1, P3, and P42 by immunostaining (C) and at P1, P3, P7, P14, P21, and P42 by western blots (D). Lin28a levels were downregulated with ages, especially in the retina of mice >1 week old. Scale bar, 25 μm. n = 3–4 mice per group in (B) and (D); repeated-measures one-way ANOVA, ∗p < 0.05.
Upregulation of Lin28a in the CNS Regions of Tg Mice with Thy1 Promoter
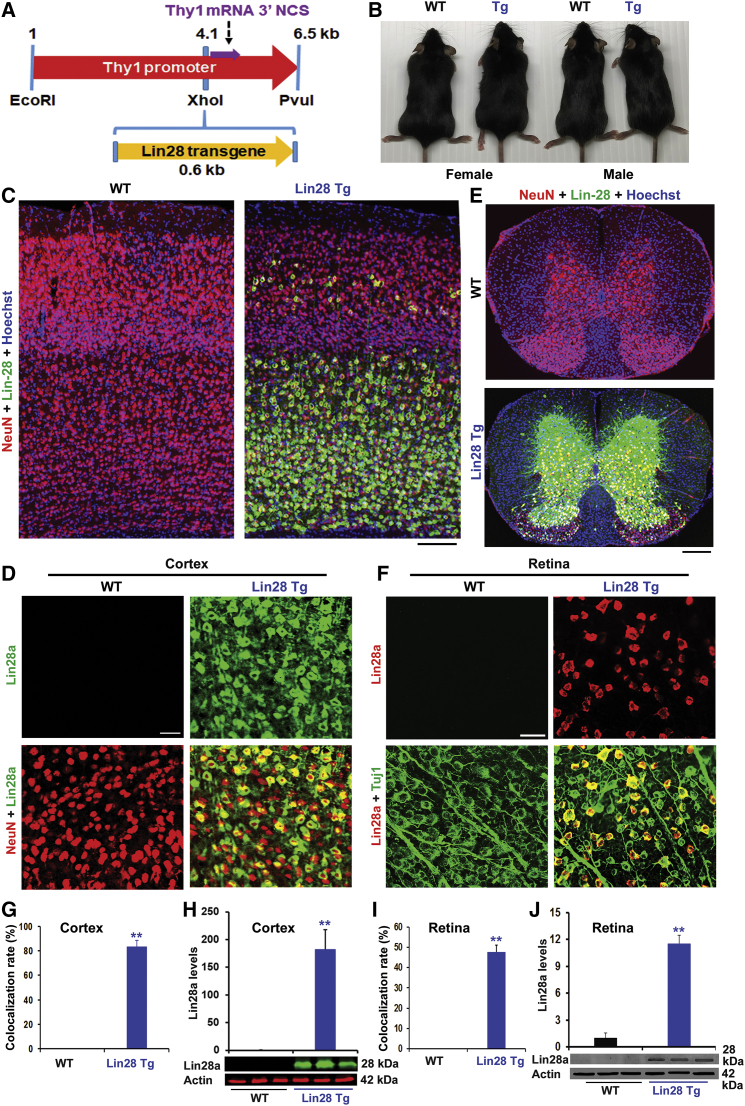
To study function of the Lin28 gene in adult CNS, we generated Thy1 Tg mice that overexpress Lin28a protein. Thy1, a member of the immunoglobulin (Ig) superfamily, is expressed by projection neurons in most areas of the nervous system and also by several types of non-neuronal cells, especially thymocytes.36 Thy1 vector contains 6.5 kb of the murine Thy1.2 gene, extending from the promoter to the intron following exon 4, but lacking exon 3 and its flanking introns.37 This modified Th1.2 cassette contains the sequences required for neuronal expression, but not for non-neural expression.38 It therefore drives transgene expression almost exclusively in the nervous system, but not in the thymus. To target the Lin28 gene, we cloned Lin28a cDNA into an XhoI site of the vector, effectively replacing the deleted sequences (Figure 2A). The gel-purified DNA plasmid was then injected into fertilized oocytes with C57BL6/J background in the Fox Chase Cancer Center. Two founders with the Lin28a transgene were backcrossed to C57BL6 mice for more than seven generations, and the one whose offspring had the higher level of transgene expression in the cortex was used to generate experimental animals in this study. These founders and their offspring survive well and the body sizes of littermate wild-type (WT) and Tg mice are similar (Figure 2B). Because most Tg mice were used for colony maintenance or experiments and sacrificed at ∼12 months of age or younger, we did not monitor their lifespan. We verified upregulation of Lin28a in the sensorimotor cortex, especially layers 4 and 5, spinal cord gray matter, and retina (Figures 2C–2J), by both immunostaining and western blotting assays, in contrast to lack of expression in littermate WT controls. We did not find alteration of Lin28b protein in Tg mice (data not shown). Immunostaining indicates that approximately 80% of NeuN+ neurons in cortex and 50% of Tuj1+ cells in retina show strong signals for Lin28a. In contrast, we did not detect any changes of Lin28a levels outside of the nervous system, such as liver, skeleton muscle, and skin (data not shown).
Figure 2.
Upregulation of Lin28a Protein in the Brain and Retina of Tg Mice with a Thy1 Promoter
(A) Schematic drawing shows the map of the Thy1 Lin28a vector used for generating the Tg mice. To make this vector, the Lin28a coding sequence was subcloned into a Thy1 transgenic construct that drives the transgene expression only in neurons because of its deleted sequence required for expression in non-neural cells. (B) Photographs indicate the littermate WT and Lin28a Tg female and male mice (10 weeks old, C57BL/6 background). (C) Representative immunostaining images at low power indicate layer distribution of Lin28a expression in Tg mice in contrast to lack of Lin28a in littermate WT mice. Scale bar, 125 μm. (D) Representative images at high power indicate colocalization of Lin28a with NeuN in layer 5 sensorimotor cortex of Tg mice in contrast no Lin28a expression in WT mice. Scale bar, 25 μm. (E) Transverse sections of thoracic spinal cord indicate Lin28a expression in the gray matter of Tg mice, which is colocalized with NeuN. Scale bar, 200 μm. (F) Representative images indicate that a portion of Tuj1+ RGCs express Lin28a protein in retina of Tg mice in contrast no Lin28a expression in WT control. Scale bar, 50 μm. (G) Graph indicates ∼80% of NeuN+ neurons expressed high levels of Lin28a protein in layer 5 cortex. (H) Levels of Lin28a were characterized by western blots from cortical tissues of adult mice. Tg mice exhibit high levels of Lin28a expression compared with lack of Lin28a in littermate WT mice. (I) Graph indicates ∼50% of Tuj1+ cells expressed high levels of Lin28a protein in retina of Tg mice. (J) Levels of Lin28a were characterized by western blots from retinal tissues of adult mice. Tg mice exhibit an ∼10-fold increase in Lin28a expression compared with littermate WT mice. n = 3 mice per group in (G)–(J); Student’s t test, ∗∗p < 0.01.
Behavioral tests with the Basso Mouse Scale (BMS) for locomotion, grid walk, grip force, and thermal withdrawal assays indicate that motor and sensory function in Tg mice is normal overall compared with age-matched WT controls (Figure S1). We have also confirmed the overall integrity and normal distribution of several long projection tracts, including CST axons with biotin dextran amine (BDA; molecular mass of 10 kDa) tracing and raphespinal axons with 5-HT staining (data not shown). Evaluation of BDA-traced CST axons in the medulla and neurofilament-stained axons in the optic nerve indicated similar distribution patterns of these tracts in WT and Tg mice (Figure S2).
Upregulating Lin28a Stimulates Robust Regeneration of CSTs in Adult Tg Mice with Spinal Cord Injury (SCI)
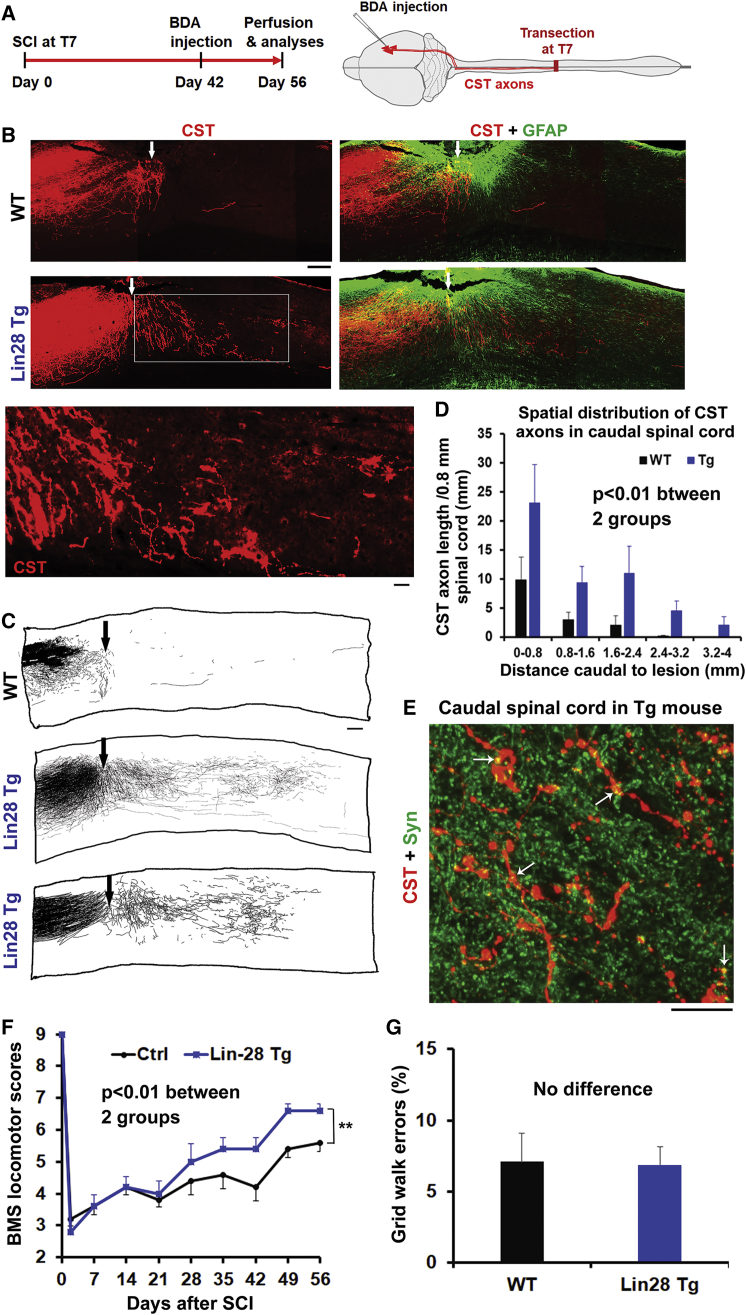
CST axons, the only direct projection fibers that link the cortex to spinal cord, are essential for voluntary motor control of the body and limbs, but the strategies to regenerate them are very limited.39, 40, 41 We thus evaluated regeneration of injured CST axons labeled by tracer BDA in adult Tg mice 8 weeks after dorsal over-hemisection (depth, 1 mm) at T7 (Figures 3A and 3B). T7 spinal cord has a 1.5-mm dorsoventral diameter in adult mice, and this injury typically transects ∼70% of the spinal cord area, including all of the CSTs.42,43 C57BL/6 mice do not display ventral CST axons (Figure S3B). We detected many CST axons that bypassed or crossed the lesion and projected into the caudal spinal cord in Lin28 Tg mice (Figures 3B–3D). Double labeling for CSTs and synapsin 1 (Syn), a widely used pre-synaptic marker, suggested the presence of synaptic structures along the regenerated CST axons in the spinal cord caudal to the lesion (Figure 3E). In some Tg mice, CSTs also appeared in transverse sections 4–7 mm caudal to the lesion. Glial fibrillary acidic protein (GFAP) immunostaining for scar tissues showed similar lesion sizes and reactive scar tissues in all WT and Tg SCI mice. We carefully checked the CST axons in caudal spinal cord and confirmed that they meet the previously defined morphological criteria of regenerating axons.44 We also confirmed the absence of BDA-labeled axons in the original locations of CSTs in the caudal spinal cord and proper intracortical injections of BDA tracer (Figure S3A). PTEN deletion showed similar patterns of CST regeneration after dorsal hemisection in mice.8
Figure 3.
Upregulation of Lin28a Promotes Robust Regeneration of Injured CST Axons into the Caudal Spinal Cord and Significant Recovery of Locomotor Function in Adult Tg Mice
(A) Schematic drawing indicates the experimental procedures and time protocols in adult Lin28 Tg mice. (B) Neuronal tracer BDA was injected into the sensorimotor cortex 6 weeks after SCI, and BDA-labeled CST axons were evaluated 8 weeks after SCI. Parasagittal sections around the lesion in SCI controls indicated termination of injured dorsal CST axons, and there are no regenerated CST axons in the caudal spinal cord. In contrast, similar sections in Lin28 Tg mice indicated regeneration of a great number of CST axons into the lesion area and caudal spinal cord. Scales bar, 200 μm (low power) and 50 μm (high power). (C) Camera lucida drawings indicate BDA-labeled CST axons from all of the parasagittal sections of three representative mice, one from the WT group and two from the Tg group. CST axons had short sprouting around lesion but terminated without apparent regeneration in SCI control. In contrast, the Tg mice display robust regrowth of CST axons around the lesion and in the caudal spinal cord. Scale bar, 200 μm. (D) BDA-labeled CST fibers were traced from all parasagittal sections of the spinal cord 0–4 mm caudal to the lesion, and the length of CST axons was quantified from every 0.8-mm length of the caudal spinal cord. Most regenerated CST axons reached the spinal cord approximately 2.5 mm caudal to the lesion, and some of them regrew longer than 4 mm caudal to the lesion center, a level close to the lumbar enlargement in this model. (E) The pre-synaptic marker Syn is colocalized to regenerated CST axons (see arrows) in the caudal spinal cord of parasagittal sections. Scale bar, 25 μm. (F) Graph indicates the locomotor BMS scores in adult SCI mice. Lin28 Tg mice exhibit increased BMS scores several weeks after SCI. (G) Graph indicates the grid walk errors in adult mice 8 weeks after SCI. Dorsal is up in all sections. The numbers indicate means ± SEM from five to eight mice per group. Repeated-measures ANOVA in (D) and (F), Student’s t test in (G), ∗∗p < 0.01.
We evaluated functional recovery in Lin28 Tg mice after SCI in a blinded manner. Two days after dorsal over-hemisection SCI, all mice had similar injury severities, with BMS locomotor scores of ∼3 (Figure 3F). Several weeks after injury, WT controls showed partial recovery, but this plateaued by 3–5 weeks. In contrast, BMS scores in Lin 28Tg mice continued to increase 4–8 weeks after SCI, and most of these mice had better coordination than did SCI controls. Therefore, upregulation of Lin28 improves axon regrowth and functional recovery in adult Tg mice with SCI. Notably, evaluation of hindlimb grid walk showed similar numbers of grid walk errors in two groups of mice 8 weeks after SCI (Figure 3G), indicating that greater regeneration may be required for robust functional recovery.
Optic Nerve Axons Regenerate Dramatically in Adult Lin28 Tg Mice after Crush Injury
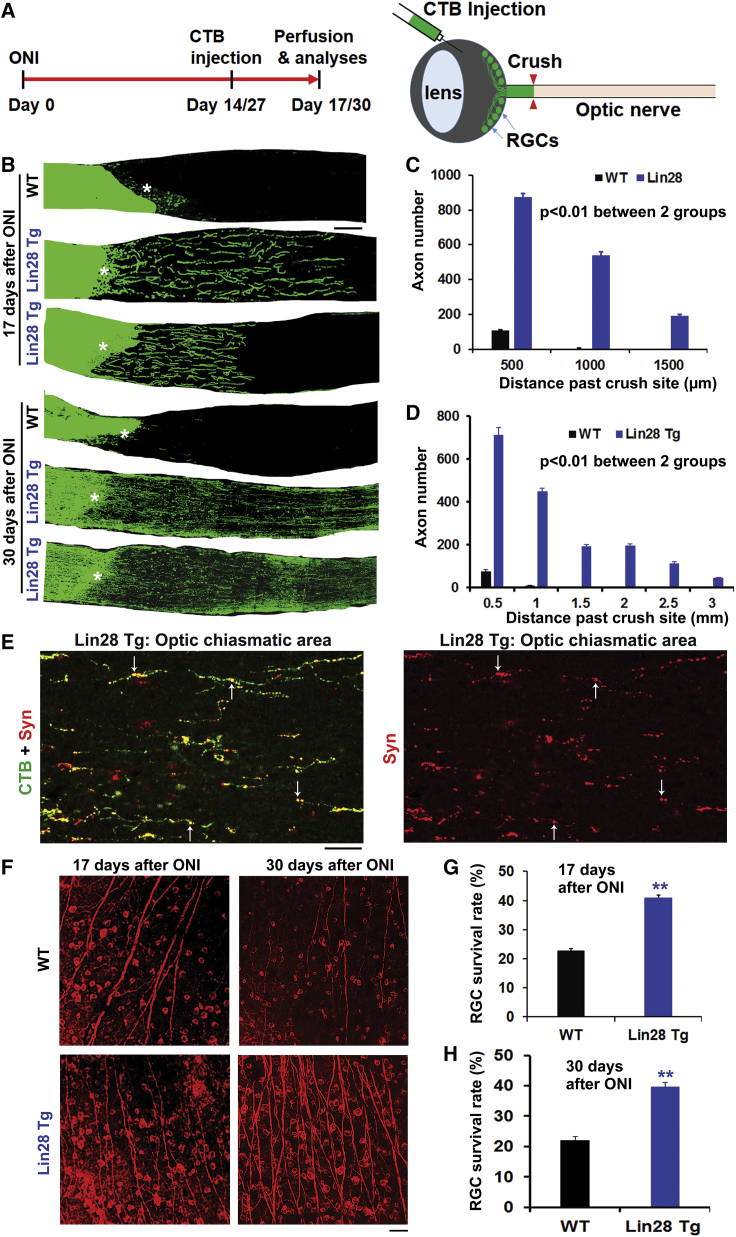
After confirming upregulation of Lin28a in multiple populations of neurons, including the retina, we evaluated regeneration of optic nerve axons 17 days after crush injury in adult WT or Tg mice (8 weeks old). We intravitreally injected cholera toxin subunit B (CTB) tracer conjugated with Alexa Fluor 488 at 14 days after injury and examined the integrity of CTB-traced optic nerve axons 17 days after crush by processing tissue blocks of optic nerves around the lesion (Figure 4A). All of the optic nerve fibers terminated at the lesion site in controls, as reported previously,6,7 although a few axons regrew into the caudal site for a very short distance (usually <500 μm) in a small portion of WT mice. Lin28 Tg mice exhibited robust regeneration of injured optic nerve axons into the caudal site (Figures 4B and 4C), with some >1 mm and others >1.5 mm during a period of 17 days after injury. Quantification of regenerated axons from multiple sections of each animal indicate long distance of regeneration in caudal optic nerve of Lin28 Tg mice. To determine whether Tg overexpression of Lin28a would stimulate sustainable regeneration of injured optic nerve, in a different set of experiments, we examined regrowth of crushed optic nerve at an extended time frame of 30 days after lesion (Figures 4B and 4D). Consistently, Lin28 Tg mice exhibited significant regeneration of injured optic axons, and some of them reached >3 mm past the crush site. Double labeling for CTB and Syn displayed expression of the pre-synaptic marker protein in the optic chiasmatic area (Figure 4E).
Figure 4.
Lin28a Upregulation Enhances Regeneration of Optic Nerve Axons and Survival of RGCs in Adult Transgenic Mice 17 or 30 Days after Injury
(A) Schematic drawing shows the time frames of the major procedures for studying axon regeneration in adult Tg mice with optic nerve crush injury. (B) Representative images indicate that upregulation of Lin28a protein promotes regeneration of optic nerve axons in Tg mice 17 or 30 days after injury. Scale bar, 50 μm. (C) CTB-traced axons were quantified from optic nerve sections at different distances from the lesion. Lin28 Tg mice show dramatic regeneration, and some axons regenerated >1.5 mm past the lesion. (D) Graph indicates dramatic regeneration of injured optic axons in Lin28 Tg mice, and some axons regenerated ∼3 mm past the lesion. (E) The pre-synaptic marker Syn is colocalized to regenerated axons traced by CTB (see arrows) in optic chiasmatic sections. Scale bar, 25 μm. (F) Representative images from retina indicate that upregulation of Lin28a protein increases survival of RGCs in Tg mice 17 or 30 days after injury. Scale bar, 50 μm. (G) Graph indicates that upregulating Lin28a protein enhances survival of RGCs in Tg mice 17 days after injury. (H) Upregulating Lin28a protein enhances survival of RGCs in Tg mice 30 days after injury. n = 6–7 mice in (C), (D), (G), and (H); repeated-measures ANOVA in (C) and (D), Student’s t test in (G) and (H), ∗∗p < 0.01.
Because axotomy of the optic nerve usually induces apoptotic loss of most RGCs a few weeks after traumatic injury, we measured the survival rate of RGCs and demonstrated that upregulating Lin28a significantly increased the number of survived RGCs in Tg mice at either 17 or 30 days after injury (Figure 4F–4H). Deletion of PTEN, a negative regulator of the PI3K/Akt pathway, similarly enhanced survival of RGCs after injury.6,45 Therefore, Tg upregulation of Lin28a protein promoted sustainable optic nerve regeneration and also prevented apoptotic loss of RGCs following axotomy.
Local Injections of AAV2-Lin28a Vector Efficiently Upregulate Target Protein in the Sensorimotor Cortex and Retina
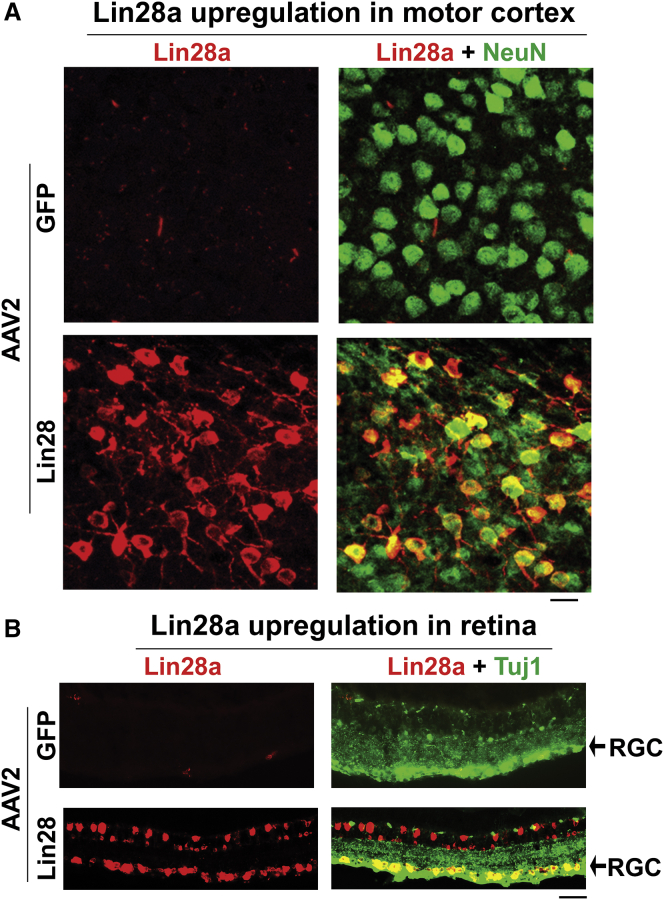
To overexpress Lin28a in mature neurons with a viral vector, we generated adeno-associated virus type 2 (AAV2)-Lin28a and tested whether intracortical and intravitreal injections of this vector would upregulate Lin28a protein in the sensorimotor cortex and retina of adult C57BL/6 mice. Two weeks after viral administration, we measured the expression levels of Lin28a by immunohistochemistry. Lin28a protein was dramatically upregulated in the sensorimotor cortex around injections and in the retina of the injection side, in contrast to lack of expression in the controls treated with AAV2-GFP (Figure 5). Lin28a signals were mainly colocalized with neuronal marker NeuN in the cortex and with RGC marker Tuj1 in the retina, although they also were present in other types of cells. Thus, local application of the AAV2-Lin28a vector efficiently transduces mature neurons in the sensorimotor cortex and retina of adult rodents.
Figure 5.
Upregulation of Lin28a Protein in Sensorimotor Cortices and Retinas of Adult C57BL/6 Mice 2 Weeks after Local Injections of AAV2-Lin28a Vector
(A) Images of double staining for Lin28a and neuronal marker NeuN indicate upregulation of Lin28a protein in the sensorimotor cortices of C57BL/6 mice locally treated with AAV2-Lin28a, in contrast to lack of Lin28a signals in the controls treated with AAV2-GFP. Scales bar, 20 μm. (B) Images of double staining for Lin28a and RGC marker Tuj1 indicate upregulation of Lin28a protein in RGCs and other retinal cells of C57BL/6 mice intravitreally treated with AAV2-Lin28a, in contrast to lack of Lin28a signals in the controls treated with AAV2-GFP. Scales bar, 40 μm.
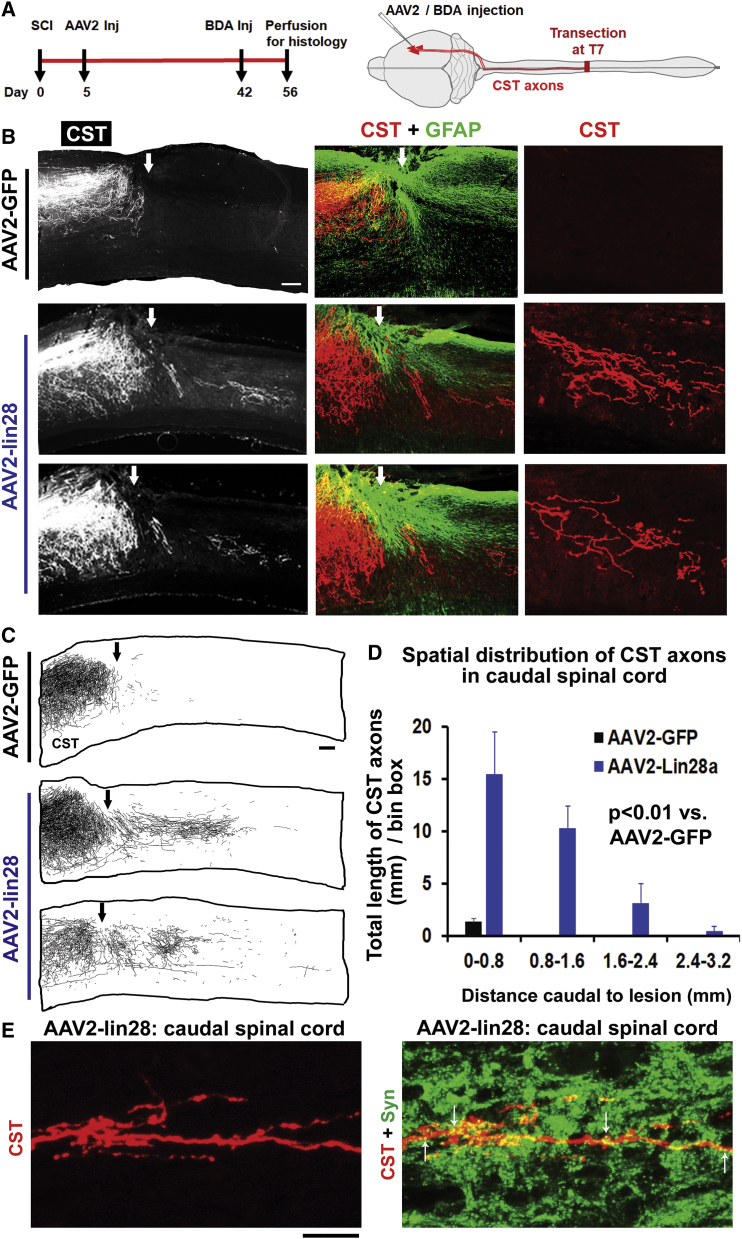
Treatment with AAV2-Lin28a Vector Delivered 5 Days after Injury Promotes Regeneration of CST Axons in Adult Mice
To determine whether upregulating Lin28a after injury promotes axon regeneration in vivo, we performed dorsal over-hemisection at T7 in 8- to 10-week-old C57BL/6 mice and 5 days later injected AAV2 vectors (2 × 1012 genomic copies/m) for GFP (control [Ctrl]) or Lin28a into the left sensorimotor cortex. Because GFP signals were not strong enough to visualize axonal structures following AAV2 infection, we used anterograde tracer BDA to examine regrowth of CST axons 8 weeks after SCI (>4 months old). In contrast to termination of CSTs in SCI controls, animals treated with Lin28a vector displayed remarkable CST regeneration into the lesion area and caudal spinal cord (Figure 6). Many regenerated CST axons typically paralleled the GFAP+ reactive astrocytic processes surrounding the dorsal lesion epicenter and grew into the deeply transected areas close to the ventral spinal cord. CST axons regrew approximately 2 mm into the caudal spinal cord in most mice, but reached >3 mm caudal to the lesion in others. CST axons in the caudal spinal cord displayed meandering courses and branching patterns, the features of regenerated axons.44 Dot-like Syn signals were also colocalized to the regenerated CST axons in the spinal cord caudal to the lesion (Figure 6E). Immunostaining for GFAP indicated that the sizes of the injury and reactive scar tissue areas were similar in both control and Lin28 animals, although it is technically challenging to measure the accurate lesion depth based on GFAP staining. Therefore, our AAV2-Lin28a vector, delivered 5 days after SCI, promoted dramatic regrowth of CST axons in adult rodents. We confirmed upregulation of Lin28a protein in the sensorimotor cortex following local injections of the AAV2-Lin28a vector as shown in Figure 5.
Figure 6.
Local Injections of AAV2-Lin28a into the Sensorimotor Cortex Initiated 5 Days after SCI Stimulate Robust Regeneration of Injured CST Axons into the Caudal Spinal Cord of Adult Mice
(A) Schematic drawing indicates the experimental procedures and time protocols in adult mice intracortically treated with AAV2 vectors. (B) Parasagittal sections around the lesion in SCI controls indicated termination of injured dorsal CST axons, and there are no regenerated CST axons in the caudal spinal cord. In contrast, similar sections in two representative AAV2-Lin28a-treated mice indicated regeneration of a great number of CST axons into the lesion area and caudal spinal cord. Dorsal is up in all sections. Scale bar, 200 μm. (C) Camera lucida drawings indicate BDA-labeled CST axons from all the parasagittal sections of three representative mice, one from the AAV2-GFP group and two from the AAV2-Lin28a group. CST axons terminated without apparent regeneration in the SCI control. In contrast, the animals treated with AAV2-Lin28a display robust regrowth of CST axons around the lesion and in the caudal spinal cord. Scale bar, 200 μm. (D) BDA-labeled CST fibers were traced from all parasagittal sections of the spinal cord 0–3.2 mm caudal to the lesion, and the length of CST axons was quantified from every 0.8-mm length of the caudal spinal cord. Most regenerated CST axons reached the spinal cord approximately 2 mm caudal to the lesion and some of them regrew longer than 3 mm caudal to the lesion center. (E) Parasagittal sections indicate colocalization of pre-synaptic marker Syn (see arrows) to regenerated CST axons in the caudal spinal cord. Scale bar, 25 μm. The numbers indicate means ± SEM from four to five mice per group. Repeated-measures ANOVA in (D).
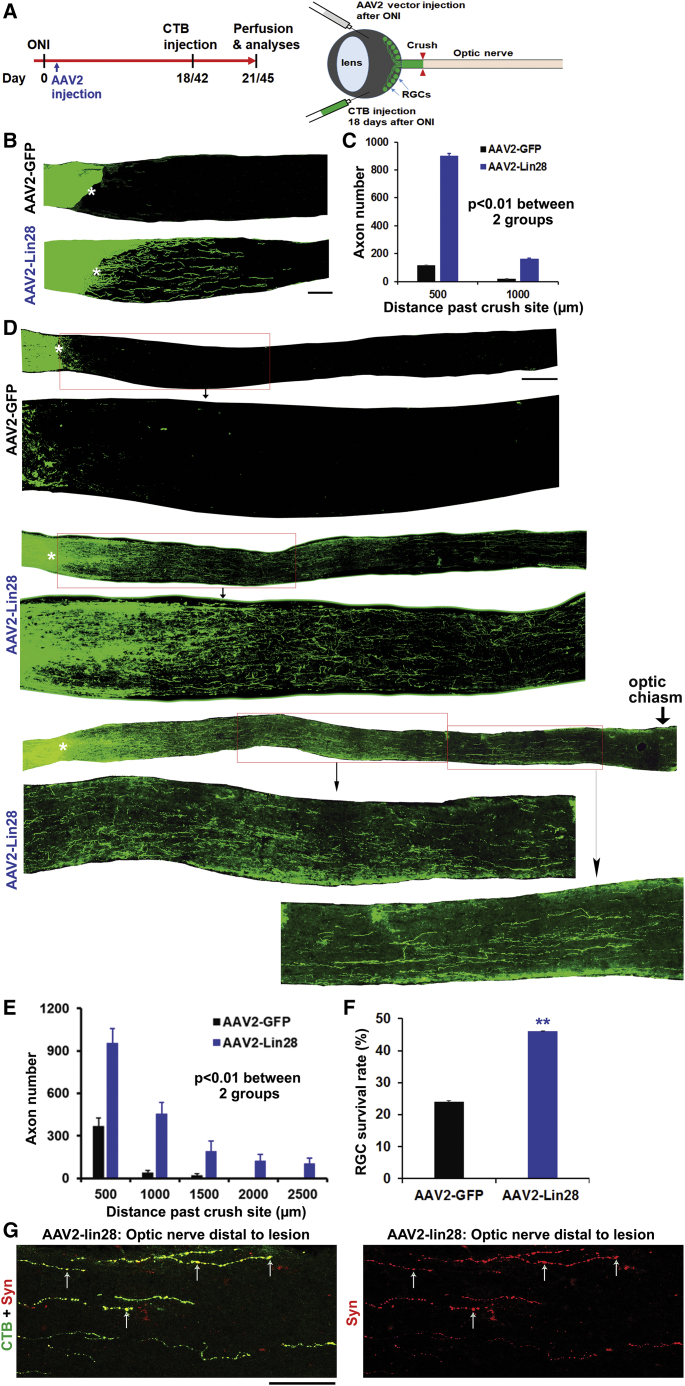
Intravitreal Treatment with AAV2-Lin28a Vector Stimulated Dramatic Regeneration of Optic Nerve Axons and Survival of RGCs
Because overexpression of Lin28a in Tg mice is not feasible for treating patients with CNS axon injury, we injected AAV2-GFP (controls) or AAV2-Lin28a intravitreally into adult WT C57BL/6 mice immediately after optic nerve crush and evaluated regeneration of optic nerve axons 21 days after injury labeled by CTB tracer (Figure 7A). Intravitreal injection of AAV2 viral vector infected most of RGCs, as reported previously.46, 47, 48 Consistently, we detected dramatic regeneration of injured optic axons (Figures 7B and 7C) and increased survival of RGCs in retina (Figure 7F) in AAV2-Lin28a-treated mice compared with AAV2-GFP controls. To further evaluate the therapeutic potential of upregulating Lin28, we then performed separate experiments to inject AAV2 vectors 1 day after injury and examined axon regeneration 45 days after ONI. Consistently, we detected long distance optic nerve regeneration, and some CTB-labeled axons regenerated along the whole length of optic nerve and reached the optic chiasm levels (Figures 7D and 7E). Moreover, regenerated axons in the distal optic nerve displayed dot-like distribution of the Syn protein (Figure 7G). Thus, upregulation of Lin28a by viral infection is also highly effective for stimulating robust and sustained axon regeneration of injured optic nerve, indicating the great therapeutic potential of our AAV viral vector that encodes the Lin28a gene.
Figure 7.
Lin28a Upregulation by AAV2 Gene Delivery Dramatically Enhances Regeneration of Optic Nerve Axons and Survival of RGCs in Adult WT Mice 21 or 45 Days after Injury
(A) Schematic drawing shows time frames of the major procedures for studying optic axon regeneration in adult mice treated with viral vectors. (B and C) Images (B) and graph (C) indicate that intravitreal AAV2-Lin28a injected immediately after injury promotes regeneration of optic nerve axons in adult mice 21 days after injury. Scale bar, 50 µm. (D and E) Images (D) and graph (E) indicate that intravitreal AAV2-Lin28a treatment injected one day after injury stimulates long distance regeneration of optic nerve axons in adult mice 45 days after nerve crush. Scale bar, 100 µm. (F) Intravitreal AAV2-Lin28a enhances survival of RGCs in adult mice 21 days after injury. (G) Syn is colocalized to the regenerated axons traced by CTB (see arrows) in optic nerve distal to the lesion. Scale bar, 20 μm. n = 5–7 mice in (C), (E), and (F); repeated-measures ANOVA in (C) and (E), Student’s t test in (F), ∗∗p < 0.01.
Akt and S6 Kinase at Least Partially Mediate Actions of Lin28a Signaling in CNS Neurons of Adult Mice
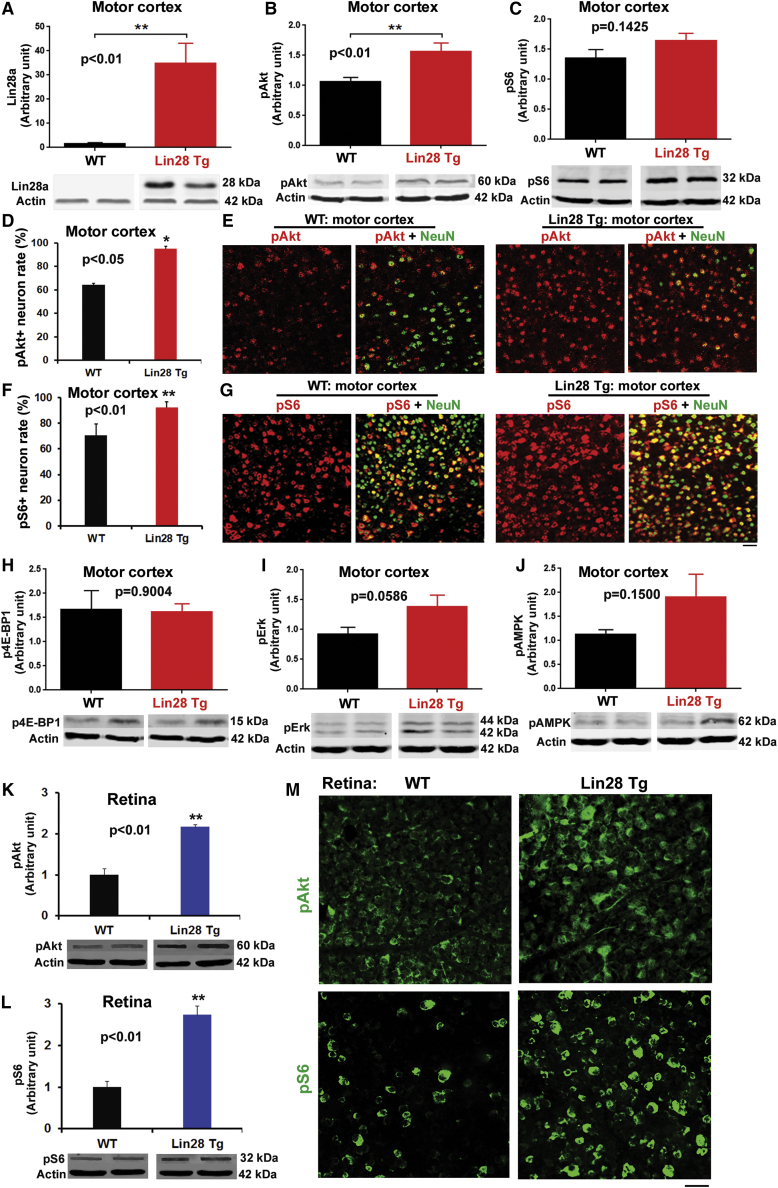
The signaling pathways by which Lin28 controls neuronal growth in adult CNS remain largely unknown. We examined a number of signaling pathways that regulate growth of mammalian cells. Because rapamycin, the mTOR inhibitor, diminished Lin28-mediated insulin sensitivity and enhanced glucose uptake, and the insulin-PI3K-mTOR pathway mediated actions of let-7 in mammalian non-neuronal cells,15 we examined whether upregulating Lin28a in sensorimotor cortex of adult Tg mice would alter kinase activities along the PI3K-mTOR pathway. Particularly, we measured the levels of Ser473 phosphorylation of Akt (pAkt) and Ser235/236 phosphorylation S6 ribosomal protein (pS6) by western blotting. Both pAkt and pS6 indicate the activated forms of these kinases. We detected significantly enhanced levels of pAkt (p < 0.001) and a trend of increased pS6 (p = 0.1425) in the sensorimotor cortex of Tg mice compared with littermate WT controls (Figures 8A and 8C). We confirmed the increased number of cortical neurons immunostained for pAkt and pS6 in Tg mice (Figures 8D–8F). In contrast, the levels of cortical p4E-BP1, another signaling protein downstream of Akt, were unaltered in Lin28 Tg mice (Figure 8H). Moreover, we confirmed increased pAkt and pS6 in the retina of adult Tg mice by both western blotting and immunostaining assays (Figures 8K–8M). Because the Lin28 signal interacts with ERK (extracellular signal-regulated kinase) and AMPK (adenosine 5′-monophosphate-activated protein kinase) pathways in stem and cancer cells,49,50 we also examined their activities by measuring the levels of phosphorylated p44/42 MAPK (mitogen-activated protein kinase) (Erk1/2) at Thr202/Tyr204 and phosphorylated AMPKα (p-AMPKα) at Thr172 in sensorimotor cortex. We detected a trend of increased levels of both p-Erk (p = 0.0586) and p-AMPKα (p = 0.1500) in Tg mice (Figures 8I and 8J), suggesting their moderate roles in mediating Lin28 actions in mature neurons. Therefore, activation of Akt, S6 kinase, and likely Erk and AMPK mediates actions of Lin28 signaling in CNS neurons of adult mice. Indeed, numerous studies reported that targeting individual signals along the PI3K/mTOR6,45,51,52 and Erk53 pathways promoted regeneration of different types of CNS neurons after injury. AMPK signal appears to mediate axon initiation and neuronal polarization during development.54
Figure 8.
Upregulating Lin28a Altered the Levels of Several Signaling Proteins Related to Neuronal Growth in Sensorimotor Cortex and Retina of Adult Tg Mice
(A) Levels of Lin28a protein were determined from the sensorimotor cortex of adult mice by western blotting to confirm its upregulation in Tg mice. (B and C) Levels of phosphorylated (B) Akt and (C) S6 were measured from the sensorimotor cortex of adult mice by western blotting. They were increased in the Tg group, especially the levels of p-Akt. (D and E) Coronal brain sections containing sensorimotor cortex from adult mice were immunostained for phosphorylated Akt and NeuN (E). p-Akt+ cells co-localized with NeuN were significantly increased in Tg group compared with littermate WT controls (D). Scale bar, 50 µm. (F and G) Coronal brain sections containing sensorimotor cortex from adult mice were immunostained for phosphorylated S6 kinase and NeuN (G). p-S6+ cells co-localized with NeuN were increased in Tg group compared with littermate WT controls (F). Scale bar, 50 µm. (H) Levels of phosphorylated 4E-BP1 were measured from the sensorimotor cortex of adult mice by western blotting. They were similar between Tg mice and the WT control group. (I and J) Levels of phosphorylated (I) Erk and (J) AMPKα were measured from the sensorimotor cortex of adult mice by western blotting. Both showed a trend of increase in Tg group. (K and L) Levels of phosphorylated (K) Akt and (L) S6 were measured from the retinas of adult mice by western blotting. They were significantly increased in the Tg group. (M) Immunostaining for phosphorylated Akt and S6 consistently indicated the increased signals for both in retina of Tg mice. Scale bar, 20 μm. n = 3–4 mice per group; Student’s t test, ∗p < 0.05, ∗∗p < 0.01.
Discussion
We reported novel crucial function of the RNA-binding protein Lin28 in controlling regrowth of both motor and sensory neurons in adult mammalian CNS. Upregulating Lin28a in Tg mice or by AAV2 infection stimulated dramatic and long distance regeneration of injured CSTs and optic nerves in adult rodents and also significantly promoted survival of RGCs. In adult mice treated with AAV2-Lin28a vector after injury, some CST axons regenerated >3 mm past the SCI lesion, and a portion of optic axons regrew the entire length of optic nerves and reached the optic chiasm levels. Therefore, we demonstrate that manipulating Lin28a activity represents a very attractive approach for regenerating various types of cells, including different populations of CNS neurons.
It is important to identify new molecular targets for manipulating regeneration of mature CNS neurons, including Lin28 reported in this study. Severed CNS axons fail to regenerate, and neuronal disconnections usually result in severe functional deficits in many patients, including traumatic brain/spinal cord injury, stroke, neurodegenerative diseases, and visual system disorders. Developing successful regenerative strategies for rewiring CNS axons is extremely important for neuroscience research. Both reduced intrinsic growth capability of developed neurons and extrinsic inhibitory environment around the lesion mainly attribute to the regeneration failure in adult mammals.55,56 Various signaling proteins controlled CNS neuronal survival and axon regeneration, including mTOR,6 STAT3,57 SOCS3,45,58 LKB1,43, KLFs,7,59 b-RAF,60 c-myc,10 SOX11,11,61 and osteopontin.62, 63, 64 The strategies that target these genes promoted CNS regeneration, but the overall extent of regrowth achieved so far is still limited, even with combined approaches. Overexpression of certain KLFs, such as KLF6/7, promoted limited axon regeneration, although suppressing others, such as KLF4, had similar effects on neuronal regrowth.7 PTEN deletion to activate mTOR stimulated dramatic regeneration of CST axons and RGCs after injury,6,8 but deleting PTEN combined with SOCS3 deficiency or with overexpressing such genes as osteopontin, IGF, c-myc, SOX11, and b-RAF promoted further regeneration of injured optic nerve axons. However, the combinatorial approaches, such as deleting PTEN plus overexpressing osteopontin and IGF, only promotes regeneration of selective α-RGCs, which cover 6% of RGCs in intact retinas.64,65 In this study, we identified that activating Lin28a alone stimulated robust regeneration of both motor and sensory tracts after CNS injuries.
Our findings, together with recent reports,46,66 support the essential role of Lin28 in reprograming diverse populations of mature CNS neurons in mammals. Lin28 is an RNA-binding protein that uniquely contains a cold shock domain and a pair of CCHC (cysteine cysteine histidine cysteine) zinc finger domains. Lin28a is predominantly present in the cytoplasm and can transport to and from nucleus in contrast to the primary distribution of Lin28b in nucleolus.67,68 Because Lin28a and Lin28b are highly conserved and have similar structure and functions, it will be interesting to study the role of Lin28b in the CNS and whether targeting both would stimulate additional axon regeneration after CNS injuries. Lin28 is a heterochronic gene to regulate the timing of developmental events in C. elegans, including the cell fates specific to later development and terminal differentiation of some cell types.27 Lin28 is essential for mediating transition from pluripotency to a committed cell lineage,69 and it can reprogram mammalian somatic cells to pluripotent cells by increasing their divisions.26,70 Lin28 has diverse biological functions in vivo because its overexpression increases both the organ and body size and delays the onset of puberty, and its loss causes embryonic lethality by reducing cell growth, fat accumulation, and brain size.71,72 Lin28 is essential for controlling proliferation of neural precursor cells in vitro and also in developing brain.71,73 Moreover, Lin28 is a critical molecular target for regenerating different types of peripheral tissues, including digit, epidermal hair, and pinna.12
Lin28 may regulate cell growth by let-7-dependent and -independent pathways. Regulating let-7 function is the best known mechanism for Lin28 in worms and mammals. Let-7 encodes a miRNA in the heterochronic pathway that controls cell differentiation during development.74 Lin28a binds to an evolutionarily conserved motif (GGAG) within both non-coding let-7 primary transcripts in the nucleus and let-7 precursors (pre-let-7) in the cytoplasm and blocks processing them into mature let-7, thus modulating cell functions.75,76 Consistently, let-7 inhibits regeneration of anterior ventral microtubule neurons in C. elegans.77 Lin28 may also function by directly binding mRNAs and regulating their expression via let-7-independent mechanisms.27 Lin28 controls L3 cell fates in C. elegans through let-7, but it promotes L2 cell fates independently of let-7.19 Selective knockout of Lin28 in skeletal muscle of mice impaired glucose tolerance and insulin resistance, but it did not alter let-7 levels.15 Let-7 repression is required but is insufficient to promote tissue repair in Tg mice.12 Whether Lin28 regulates regrowth of mature CNS neurons by the let-7 pathway remains unknown.
Lin28 activation may promote regrowth of RGCs indirectly by targeting presynaptic inhibitory neurons. Lin28 activation promotes growth of various types of targeted cells by reprogramming them,12,33 but a recent study suggests that Lin28 overexpression in retina promotes RGC regeneration mainly by targeting amacrines hyperactivated after RGC injury.66 Injecting AAV2-Lin28 into retina overcame presynaptic inhibition of amacrines and potentiated responsiveness of injured RGCs to IGF1 because selective Lin28 expression in RGCs only induced modest regeneration. Either Lin28 expression in amacrines or blocking synaptic inhibition of amacrines promoted RGC survival and IGF1-induced regeneration, but combinatorial Lin28 upregulation in amacrines and widespread IGF1 expression in retina are necessary for inducing robust RGC regeneration. Notably, we found that intra-retinal AAV2-Lin28a alone stimulated robust and long distance RGC regeneration, reaching ∼900 axons at 0.5 mm past the lesion (Figure 7, versus ∼600–900 axons by Lin28 + IGF1 in Zhang et al.66). Consistently, Lin28a facilitated neuronal formation in mammalian retina likely mediated by IGF signaling.78 We also observed a similar extent of RGC regeneration in Lin28 Tg mice (Figure 4) and confirmed the results in both Tg and viral experiments by two independent scientists (F.M.N. and S.W.). In our Lin28 Tg mice with Thy1 promoter that mainly targets projection neurons, Lin28a was also possibly expressed in amacrines because some Thy1 Tg lines expressed transgene in this cell type.37 It is interesting to study whether Lin28 controls regrowth of other types of neurons (e.g., CSTs) also by a presynaptic regulation.
We demonstrate that that the mTOR-Akt pathway at least partially mediates actions of Lin28, although other signals, such as Erk and AMPK, probably play moderate roles. As an RNA-binding protein, Lin28 enhances translation of a great number of mRNAs,27 including the genes in the IGF-PI3K-mTOR signaling axis and others regulating cell cycle (e.g., Myc, Ras, and Hmga2) by suppressing let-7 signaling.20 Particularly, upon activating PI3K signaling by Lin28, phosphorylated Akt activates mTOR signaling by phosphorylating the GTPase-activating tuberous sclerosis 1/2 complex, which consequently phosphorylates ribosomal S6 kinases and/or 4-EB-P1/2. We identified that Lin28 regulated neuronal growth by activating S6 kinase, but not affecting 4-EB-P1. Other signals, especially Erk and AMPK (also the signals downstream of IGF), appear also to play moderate roles in mediating regrowth of mature CNS neurons.
We demonstrate that Lin28 is an important target molecule for CNS repair and that its upregulation has a great therapeutic potential for neurological disorders. Because Lin28 actions on development are evolutionarily conserved from worms to rodents to humans,27 our research results may translate into application to humans, especially to those with CNS axonal damages, such as brain/spinal cord injury, stroke, and glaucoma. Because AAV vectors have favorable safety profiles, efficiently transduce a wide range of cell types, and are frequently used to treat neurological disorders,79,80 this study may help us identify innovative, practical strategies to treat CNS disorders by enhancing neuronal survival as well as regeneration and reconnections among neurons. Given that multiple factors contribute to CNS regenerative failure,55 it is interesting to combine Lin28 activation with other effective approaches, such as suppressing scar-sourced inhibitors around the lesion, for synergistic actions.
Materials and Methods
Characterization of Lin28 Protein Expression in the Cortex and Retina
All of the experimental procedures with animals were approved by the Institutional Animal Care and Use Committee at Temple University. We evaluated the expression level of Lin28a protein in the cortices and retinas of developmental C57BL/6 mice by western blots. For sample preparation, mice were perfused with ice-cold PBS for 5 min, and fresh tissue blocks of both sides of cortices and retinas were collected immediately and then prepared in lysis buffer (50 mM Tris-HCl [pH 7.2], 10 mM MgCl2, 300 mM NaCl, and 1.5% IGEPAL) containing various protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 2 mM orthovanadate, 10 μg/mL leupeptin, and 10 μg/mL aprotinin). The supernatants of tissue lysates containing equal amounts of total protein were loaded onto Tris-glycine gels and transferred to nitrocellulose membranes. Membranes were blocked with 5% blotting grade milk (Bio-Rad), blotted with rabbit anti-Lin28a antibody (catalog no. 8641S, Cell Signaling Technology) and mouse anti-actin clone C4 antibody (MP Biomedicals, for loading control), and then incubated with secondary antibodies conjugated to IRDye 800CW goat anti-mouse IgG (LI-COR Biosciences) or IRDye 680RD goat anti-rabbit IgG (LI-COR Biosciences). The western blot bands were visualized and quantified with the LI-COR Odyssey scanner and Odyssey ImagingStudio software. At least three to four separate experiments were performed. To verify neuronal expression of Lin28a in the CNS, coronal brain sections and whole-mount retina from C57BL/6 mice of various ages were immunostained for Lin28a and for the neuronal marker NeuN or βIII-tubulin (Tuj1, MMS-435P, BioLegend). Because of low levels of Lin28a expression in the developmental retina of WT mice, anti-rabbit biotin-conjugated secondary antibody and tyramide signal amplification (TSA) systems (PerkinElmer) were used to detect Lin28a signals in retinas.
Generation and Characterization of Lin28a Tg Mice
To generate Tg mice expressing Lin28a in the nervous system, mouse Lin28a cDNA (plasmid 21147, Addgene) was subcloned into the XhoI site of the Thy1 promoter construct (plasmid 26357, Addgene).37 Plasmid was digested with EcoRI and PvuI and the resultant 6.5-kb fragment was injected into fertilized oocytes of C57BL/6 mice in the Fox Chase Cancer Center at Temple University. The founder mice were crossed with C57BL/6 WT mice for more than seven generations, and their offspring were used for this study. For genotyping, DNAs were extracted from the tail samples, and PCR was performed with the primers 5′-TCTGAGTGGCAAAGGACCTTAGG-3′ (forward) and 5′-CCCACCGCAGTTGTAGCACC-3′ (reverse), which amplify the sequence at the junction of the Thy1 promoter and Lin28a gene. DNA amplification was performed using GoTaq (Promega), and PCR conditions were as follows: 35 cycles of 94°C for 30 s (denaturation), 57°C for 1 min (annealing), and 72°C for 1 min (extension). The size of the PCR product was 600 bp.
We evaluated the expression level of Lin28a protein in the sensorimotor cortex and retina of Tg mice by both immunohistochemistry and western blots. For the former, the brain and retina derived from Tg mice or WT littermates were fixed in 4% paraformaldehyde (PFA) for 2 h and immersed in 30% sucrose solution overnight. The coronal brain sections containing the sensorimotor cortex and the whole-mount retina were immunostained with a rabbit anti-Lin28a antibody and an Alexa Fluor 594-conjugated secondary antibody. Cross-sections of optic nerve in some WT and Tg mice were immunostained for neurofilament (catalog no. MAB1615, Sigma). For western blotting, mice were perfused with ice-cold PBS for 5 min, and fresh brain and retinal tissues were collected immediately and then prepared in lysis buffer. The samples were used to detect the levels of Lin28 (catalog no. 8641S for Lin28a and catalog no. 5422S for Lin28b, Cell Signaling Technology) as described above. The levels of Lin28a in sensorimotor cortical areas (1.5 and 2 mm surrounding bregma in P1–P7 and P14–P42 mice, respectively) were measured (Figures 1A and 2D).
The overall motor and sensory function in Lin28 Tg mice was evaluated with multiple behavioral tests. The BMS scores were evaluated while the mouse was walking in an open field and confirmed from digital video records. The grid walk errors were counted from videotapes played at a slow speed (four separate trials per test) and averaged from different trials. For grasping analysis, the mouse was pulled steadily until it lost hindlimb grip, and then the peak force was measured by the force meter. Thermal withdrawal was tested by dipping each of the hindpaws into a hot water bath at 50°C and counting the seconds of paw withdrawal from the hot water.
Production of AAV2-GFP or AAV2-Lin28 Vectors and Evaluation of Their Transduction in the Cortex and Retina
To generate AAV2-based vectors, we inserted GFP (control) or GFP-Lin28a into the pAM/chicken β-actin/woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) vector and packaged viral vectors in HEK293 cells using helper and packaging vectors. The AAV2 vector plasmids contain the expression cassettes, including chicken β-actin promoter, cDNA encoding GFP (control) or GFP-Lin28a, WPRE, and SV40 poly(A) between inverted terminal repeats (ITRs). The recombinant GFP-Lin28a plasmid was cloned and generated by PCR in our laboratory. The vector, pAAV-RC2 (AAV2 rep and cap expression plasmid), and helper plasmid were co-transfected into HEK293 cells by polyethyleneimine.81 The prepared AAV2 vectors were purified by double centrifugation with cesium chloride and then dialyzed overnight. The vector titers used in this study were initially determined by infecting fibroblast cells and confirmed in transfected adult dorsal root ganglion cultures by immunostaining for GFP or Lin28a.
To evaluate efficiency of our AAV2-Lin28a in transducing mature neurons, we injected AAV2-GFP or AAV2-Lin28a (2 × 1012 genomic copies/mL) into five sites of the sensorimotor cortex (see the coordinates described below) and into vitreous of adult C57BL/6 mice. Two weeks after the AAV2 vector injections, mice were perfused with 4% PFA and the fixed brain and eyeball were collected and prepared for immunohistochemistry as described above.
Spinal Cord Injury, Intracortical Treatments with AAV2-GFP or AAV2-Lin28a Vectors, and Tracer Injections to Label CST Axons in Adult Mice
We performed dorsal over-hemitransection SCI at T7 in Lin28a Tg mice (littermate WT as controls) and in C57BL/6J mice for viral experiments. To lesion spinal cords in adult mice (8–10 weeks old), we exposed the dorsal spinal cord by T6–T7 laminectomy. A dorsal over-hemisection (1 mm in depth and approximately 1.5 mm in dorsoventral diameter) was performed at T7 with a 30-gauge needle and microscissors to completely sever the dorsal CST. The lesion depth of 1 mm was ensured by passing a marked 30-gauge needle at least five times across the dorsal spinal cord. To upregulate Lin28a in cortical motor neurons of adult C57BL/6 mice in viral experiments, 5 days after SCI, we used a nanoinjector to inject AAV2-GFP or AAV2-Lin28a (2 × 1012 genomic copy/mL) into five sites within the left sensorimotor cortex (anterior-posterior coordinates from bregma, 1.0, 0.5, 0, −0.5, and −1.0 mm, all at 1.0 mm lateral and at a depth of 1.0 mm).
Six weeks after SCI, the mice received BDA (molecular mass of 10 kDa) tracer injections into five sites of the sensorimotor cortex at the same coordinates as for AAV2 vector injections. Mice were perfused 2 weeks after BDA injection and fixed spinal cords were dissected for histology. To compare axon numbers in the caudal spinal cord between different groups, we determined the length of BDA-labeled CST axons in all parasagittal sections of spinal cord from 0 to 4 mm caudal to the lesion epicenter in each animal. The injury center was determined as the midpoint of histological abnormalities produced by lesion cavitation, reactive astrocytes, and morphological changes of injured axons. The CST axons caudal to the lesion were traced manually in each of the parasagittal sections, and their total length inside of several bin boxes at 0.8, 1.6, 2.4, 3.2, and 4.0 mm caudal to the lesion center was measured using Photoshop and ImageJ software. Some parasagittal sections were doubly stained for Syn (catalog no. 5297S, Cell Signaling Technology) and BDA-traced CST axons.
To determine functional recovery in Lin28 Tg mice, we evaluated locomotion alterations during 8 weeks of survival by measuring locomotor BMS scores at 2 days after SCI and weekly thereafter. The grid walk performance was also evaluated 8 weeks after SCI by counting the walk errors from videotapes of different trials. The behavioral tests were performed by two persons who were unaware of animal identifications.
Optic Nerve Crush Injury, Tracer Injection, and Evaluation of Axon Regeneration and RGC Survival
To access the optic nerve, we used microscissors and dull/angled forceps to gently push away tissues near the eye and avoid damaging the orbital venous sinus. Fine-angled forceps were used to crush optic nerve for 10 s with a consistent pressure at ∼1 mm behind the optic disc. To preserve retinal blood supply, we were careful not to damage underlying ophthalmic artery. To upregulate Lin28a in the retina of adult C57BL/6 mice, immediately or 1 day after optic nerve crush, we injected AAV2-GFP or AAV2-Lin28a (2 × 1012 genomic copies/mL) intravitreally at the crush side. At 18 or 42 days after injury, we labeled regenerating axons by anterogradely injecting Alexa Fluor 488-conjugated CTB tracer (2 μL per mouse, 2.5 μg/μL) into vitreous with a micropipette. Three days after CTB injection, mice were perfused with 4% PFA and optic nerves and retinas were collected. Fixed optic nerves containing an injury site were sectioned longitudinally (10 μm) and five representative sections were selected from each optic nerve by visualizing the most regenerating axons along the sections. We counted regenerating axons crossing several locations past the lesion and calculated their number by dividing axon number by the nerve size at each distance. Selected longitudinal sections in the optic nerve and optic chiasm were immunostained for Syn (catalog no. 5297S, Cell Signaling Technology). Following 2-h post-fixation in the same PFA and overnight incubation in 30% sucrose, we immunostained the whole-mounted retina with an antibody for Tuj1 and examined the number of surviving RGCs in retina ipsilateral to injury. After quantifying the average number of Tuj1-postive cells per field, we obtained the total number of viable RGCs by multiplying the figure by retinal area, as reported previously.6,45
Characterization of Signaling Proteins Potentially Downstream of Lin28a in the Sensorimotor Cortex and Retina
To study the signals downstream of Lin28a in mature cortical neurons and RGCs, we perfused Lin28 Tg mice and littermate WTs with cold PBS and collected fresh tissues of the sensorimotor cortex and retina for western blotting assay. Because of limited tissue size in mouse retina, we first evaluated potential changes of signaling proteins in the sensorimotor cortex with the following primary antibodies: rabbit Lin28a (catalog no. 8641S, Cell Signaling Technology), rabbit p-AMPKα (Thr172; 40H9), mouse monoclonal antibody (mAb) pAkt (Ser473, 587F11), rabbit mAb pS6 ribosomal protein (Ser235/236), rabbit mAb p-4E-BP1 (eukaryotic initiation factor 4E-binding protein 1, Thr37/46, 236B4), rabbit mAb p-Erk1/2 (p44/42), and rabbit or mouse β-actin. All of these antibodies were purchased from Cell Signaling Technology. We then confirmed the alterations of pAkt Ser473 and pS6 Ser235/236 in sensorimotor cortex by immunohistochemistry. We also measured the changes of pAkt Ser473 and pS6 Ser235/236 in the retinas by both western blotting and immunostaining assays.
Statistical Analysis
GraphPad Prism software was used for statistical analysis. Data in graphs are shown as means ± SEM. Developmental changes of Lin28a were analyzed with repeated-measures one-way ANOVA. Comparisons between two groups at multiple time points were analyzed with a repeated-measures ANOVA. The experiments comparing a single determination of means between two independent groups were analyzed with Student’s t test. Differences between groups with p < 0.05 were considered significant (∗p < 0.05, ∗∗p < 0.01), and a Bonferroni correction was used for multiple comparisons. During the experimental procedures, including surgery, histology, behavioral test, and axon quantification, the evaluating researchers were blinded to animal genotypes and viral vector treatments.
Author Contributions
F.M.N., Y.O., and S.L. contributed to experimental designs, data analyses, figure creation, and writing the manuscript. S.W., X.J., A.S., and G.H. contributed to experimental designs and data collection. Z.-Q.F. contributed to experimental designs.
Conflict of Interest
The authors declare no competing interests.
Acknowledgments
We thank Dr. George Smith in the Shriners Hospitals Pediatric Research Center for technical support. This work was supported by research grants to S.L. from NIH (R01NS105961, 1R01NS079432, and 1R01EY024575) and from the Shriners Research Foundation (SHC-85100, SHC-86200-PHI-16, and 85112-PHI-18).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2020.04.010.
Supplemental Information
References
- 1.Goldberg J.L., Klassen M.P., Hua Y., Barres B.A. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- 2.Chen D.F., Jhaveri S., Schneider G.E. Intrinsic changes in developing retinal neurons result in regenerative failure of their axons. Proc. Natl. Acad. Sci. USA. 1995;92:7287–7291. doi: 10.1073/pnas.92.16.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu K., Tedeschi A., Park K.K., He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu. Rev. Neurosci. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- 4.Benowitz L.I., He Z., Goldberg J.L. Reaching the brain: advances in optic nerve regeneration. Exp. Neurol. 2017;287:365–373. doi: 10.1016/j.expneurol.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Sun F., He Z. Neuronal intrinsic barriers for axon regeneration in the adult CNS. Curr. Opin. Neurobiol. 2010;20:510–518. doi: 10.1016/j.conb.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park K.K., Liu K., Hu Y., Smith P.D., Wang C., Cai B., Xu B., Connolly L., Kramvis I., Sahin M. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore D.L., Blackmore M.G., Hu Y., Kaestner K.H., Bixby J.L., Lemmon V.P., Goldberg J.L. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu K., Lu Y., Lee J.K., Samara R., Willenberg R., Sears-Kraxberger I., Tedeschi A., Park K.K., Jin D., Cai B. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurimoto T., Yin Y., Omura K., Gilbert H.Y., Kim D., Cen L.P., Moko L., Kügler S., Benowitz L.I. Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion. J. Neurosci. 2010;30:15654–15663. doi: 10.1523/JNEUROSCI.4340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belin S., Nawabi H., Wang C., Tang S., Latremoliere A., Warren P., Schorle H., Uncu C., Woolf C.J., He Z., Steen J.A. Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomics. Neuron. 2015;86:1000–1014. doi: 10.1016/j.neuron.2015.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norsworthy M.W., Bei F., Kawaguchi R., Wang Q., Tran N.M., Li Y., Brommer B., Zhang Y., Wang C., Sanes J.R. Sox11 expression promotes regeneration of some retinal ganglion cell types but kills others. Neuron. 2017;94:1112–1120.e4. doi: 10.1016/j.neuron.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shyh-Chang N., Zhu H., Yvanka de Soysa T., Shinoda G., Seligson M.T., Tsanov K.M., Nguyen L., Asara J.M., Cantley L.C., Daley G.Q. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell. 2013;155:778–792. doi: 10.1016/j.cell.2013.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lozier A.M., Rich M.E., Grawe A.P., Peck A.S., Zhao P., Chang A.T., Bond J.P., Sholler G.S. Targeting ornithine decarboxylase reverses the LIN28/Let-7 axis and inhibits glycolytic metabolism in neuroblastoma. Oncotarget. 2015;6:196–206. doi: 10.18632/oncotarget.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polesskaya A., Cuvellier S., Naguibneva I., Duquet A., Moss E.G., Harel-Bellan A. Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev. 2007;21:1125–1138. doi: 10.1101/gad.415007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu H., Shyh-Chang N., Segrè A.V., Shinoda G., Shah S.P., Einhorn W.S., Takeuchi A., Engreitz J.M., Hagan J.P., Kharas M.G., DIAGRAM Consortium. MAGIC Investigators The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147:81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rybak A., Fuchs H., Smirnova L., Brandt C., Pohl E.E., Nitsch R., Wulczyn F.G. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat. Cell Biol. 2008;10:987–993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 17.Balzer E., Heine C., Jiang Q., Lee V.M., Moss E.G. LIN28 alters cell fate succession and acts independently of the let-7 microRNA during neurogliogenesis in vitro. Development. 2010;137:891–900. doi: 10.1242/dev.042895. [DOI] [PubMed] [Google Scholar]
- 18.Mayr F., Heinemann U. Mechanisms of Lin28-mediated miRNA and mRNA regulation—a structural and functional perspective. Int. J. Mol. Sci. 2013;14:16532–16553. doi: 10.3390/ijms140816532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vadla B., Kemper K., Alaimo J., Heine C., Moss E.G. lin-28 controls the succession of cell fate choices via two distinct activities. PLoS Genet. 2012;8:e1002588. doi: 10.1371/journal.pgen.1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jun-Hao E.T., Gupta R.R., Shyh-Chang N. Lin28 and let-7 in the metabolic physiology of aging. Trends Endocrinol. Metab. 2016;27:132–141. doi: 10.1016/j.tem.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Reddien P.W. Lin28: time for tissue repair. Cell. 2013;155:738–739. doi: 10.1016/j.cell.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandran R., Fausett B.V., Goldman D. Ascl1a regulates Müller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat. Cell Biol. 2010;12:1101–1107. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorsuch R.A., Lahne M., Yarka C.E., Petravick M.E., Li J., Hyde D.R. Sox2 regulates Müller glia reprogramming and proliferation in the regenerating zebrafish retina via Lin28 and Ascl1a. Exp. Eye Res. 2017;161:174–192. doi: 10.1016/j.exer.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao K., Qiu S., Tian L., Snider W.D., Flannery J.G., Schaffer D.V., Chen B. Wnt regulates proliferation and neurogenic potential of Müller glial cells via a Lin28/let-7 miRNA-dependent pathway in adult mammalian retinas. Cell Rep. 2016;17:165–178. doi: 10.1016/j.celrep.2016.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 27.Tsialikas J., Romer-Seibert J. LIN28: roles and regulation in development and beyond. Development. 2015;142:2397–2404. doi: 10.1242/dev.117580. [DOI] [PubMed] [Google Scholar]
- 28.Deumens R., Koopmans G.C., Joosten E.A. Regeneration of descending axon tracts after spinal cord injury. Prog. Neurobiol. 2005;77:57–89. doi: 10.1016/j.pneurobio.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Pearse D.D., Pereira F.C., Marcillo A.E., Bates M.L., Berrocal Y.A., Filbin M.T., Bunge M.B. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 30.Raineteau O., Schwab M.E. Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- 31.Blesch A., Tuszynski M.H. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32:41–47. doi: 10.1016/j.tins.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Vogt E.J., Meglicki M., Hartung K.I., Borsuk E., Behr R. Importance of the pluripotency factor LIN28 in the mammalian nucleolus during early embryonic development. Development. 2012;139:4514–4523. doi: 10.1242/dev.083279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shyh-Chang N., Daley G.Q. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell. 2013;12:395–406. doi: 10.1016/j.stem.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss E.G., Lee R.C., Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–646. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- 35.Yang D.H., Moss E.G. Temporally regulated expression of Lin-28 in diverse tissues of the developing mouse. Gene Expr. Patterns. 2003;3:719–726. doi: 10.1016/s1567-133x(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 36.Gordon J.W., Chesa P.G., Nishimura H., Rettig W.J., Maccari J.E., Endo T., Seravalli E., Seki T., Silver J. Regulation of Thy-1 gene expression in transgenic mice. Cell. 1987;50:445–452. doi: 10.1016/0092-8674(87)90498-3. [DOI] [PubMed] [Google Scholar]
- 37.Feng G., Mellor R.H., Bernstein M., Keller-Peck C., Nguyen Q.T., Wallace M., Nerbonne J.M., Lichtman J.W., Sanes J.R. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 38.Vidal M., Morris R., Grosveld F., Spanopoulou E. Tissue-specific control elements of the Thy-1 gene. EMBO J. 1990;9:833–840. doi: 10.1002/j.1460-2075.1990.tb08180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Case L.C., Tessier-Lavigne M. Regeneration of the adult central nervous system. Curr. Biol. 2005;15:R749–R753. doi: 10.1016/j.cub.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 40.McGee A.W., Strittmatter S.M. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- 41.Lang B.T., Cregg J.M., DePaul M.A., Tran A.P., Xu K., Dyck S.M., Madalena K.M., Brown B.P., Weng Y.L., Li S. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature. 2015;518:404–408. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohtake Y., Park D., Abdul-Muneer P.M., Li H., Xu B., Sharma K., Smith G.M., Selzer M.E., Li S. The effect of systemic PTEN antagonist peptides on axon growth and functional recovery after spinal cord injury. Biomaterials. 2014;35:4610–4626. doi: 10.1016/j.biomaterials.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohtake Y., Sami A., Jiang X., Horiuchi M., Slattery K., Ma L., Smith G.M., Selzer M.E., Muramatsu S.I., Li S. Promoting axon regeneration in adult CNS by targeting liver kinase B1. Mol. Ther. 2019;27:102–117. doi: 10.1016/j.ymthe.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steward O., Zheng B., Tessier-Lavigne M. False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J. Comp. Neurol. 2003;459:1–8. doi: 10.1002/cne.10593. [DOI] [PubMed] [Google Scholar]
- 45.Sun F., Park K.K., Belin S., Wang D., Lu T., Chen G., Zhang K., Yeung C., Feng G., Yankner B.A., He Z. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–375. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X.W., Li Q., Liu C.M., Hall P.A., Jiang J.J., Katchis C.D., Kang S., Dong B.C., Li S., Zhou F.Q. Lin28 signaling supports mammalian PNS and CNS axon regeneration. Cell Rep. 2018;24:2540–2552.e6. doi: 10.1016/j.celrep.2018.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin K.R., Klein R.L., Quigley H.A. Gene delivery to the eye using adeno-associated viral vectors. Methods. 2002;28:267–275. doi: 10.1016/s1046-2023(02)00232-3. [DOI] [PubMed] [Google Scholar]
- 48.Pernet V., Joly S., Jordi N., Dalkara D., Guzik-Kornacka A., Flannery J.G., Schwab M.E. Misguidance and modulation of axonal regeneration by Stat3 and Rho/ROCK signaling in the transparent optic nerve. Cell Death Dis. 2013;4:e734. doi: 10.1038/cddis.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsanov K.M., Pearson D.S., Wu Z., Han A., Triboulet R., Seligson M.T., Powers J.T., Osborne J.K., Kane S., Gygi S.P. LIN28 phosphorylation by MAPK/ERK couples signalling to the post-transcriptional control of pluripotency. Nat. Cell Biol. 2017;19:60–67. doi: 10.1038/ncb3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y., Liu Y., Lin L., Huang Q., He W., Zhang S., Dong S., Wen Z., Rao J., Liao W., Shi M. The lncRNA MACC1-AS1 promotes gastric cancer cell metabolic plasticity via AMPK/Lin28 mediated mRNA stability of MACC1. Mol. Cancer. 2018;17:69. doi: 10.1186/s12943-018-0820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park K.K., Liu K., Hu Y., Kanter J.L., He Z. PTEN/mTOR and axon regeneration. Exp. Neurol. 2010;223:45–50. doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 52.Grider M.H., Park D., Spencer D.M., Shine H.D. Lipid raft-targeted Akt promotes axonal branching and growth cone expansion via mTOR and Rac1, respectively. J. Neurosci. Res. 2009;87:3033–3042. doi: 10.1002/jnr.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hollis E.R., 2nd, Jamshidi P., Löw K., Blesch A., Tuszynski M.H. Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation. Proc. Natl. Acad. Sci. USA. 2009;106:7215–7220. doi: 10.1073/pnas.0810624106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amato S., Liu X., Zheng B., Cantley L., Rakic P., Man H.Y. AMP-activated protein kinase regulates neuronal polarization by interfering with PI 3-kinase localization. Science. 2011;332:247–251. doi: 10.1126/science.1201678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu Y., Belin S., He Z. Signaling regulations of neuronal regenerative ability. Curr. Opin. Neurobiol. 2014;27:135–142. doi: 10.1016/j.conb.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma K., Selzer M.E., Li S. Scar-mediated inhibition and CSPG receptors in the CNS. Exp. Neurol. 2012;237:370–378. doi: 10.1016/j.expneurol.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bareyre F.M., Garzorz N., Lang C., Misgeld T., Büning H., Kerschensteiner M. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc. Natl. Acad. Sci. USA. 2011;108:6282–6287. doi: 10.1073/pnas.1015239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith P.D., Sun F., Park K.K., Cai B., Wang C., Kuwako K., Martinez-Carrasco I., Connolly L., He Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blackmore M.G., Wang Z., Lerch J.K., Motti D., Zhang Y.P., Shields C.B., Lee J.K., Goldberg J.L., Lemmon V.P., Bixby J.L. Krüppel-like factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proc. Natl. Acad. Sci. USA. 2012;109:7517–7522. doi: 10.1073/pnas.1120684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Donovan K.J., Ma K., Guo H., Wang C., Sun F., Han S.B., Kim H., Wong J.K., Charron J., Zou H. B-RAF kinase drives developmental axon growth and promotes axon regeneration in the injured mature CNS. J. Exp. Med. 2014;211:801–814. doi: 10.1084/jem.20131780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z., Reynolds A., Kirry A., Nienhaus C., Blackmore M.G. Overexpression of Sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J. Neurosci. 2015;35:3139–3145. doi: 10.1523/JNEUROSCI.2832-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bei F., Lee H.H.C., Liu X., Gunner G., Jin H., Ma L., Wang C., Hou L., Hensch T.K., Frank E. Restoration of visual function by enhancing conduction in regenerated axons. Cell. 2016;164:219–232. doi: 10.1016/j.cell.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y., Wang X., Li W., Zhang Q., Li. Y., Zhang Z., Zhu J., Chen B., Williams P.R., Zhang Y. A sensitized IGF1 treatment restores corticospinal axon-dependent functions. Neuron. 2017;95:817–833.e4. doi: 10.1016/j.neuron.2017.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duan X., Qiao M., Bei F., Kim I.J., He Z., Sanes J.R. Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mTOR signaling. Neuron. 2015;85:1244–1256. doi: 10.1016/j.neuron.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanes J.R., Masland R.H. The types of retinal ganglion cells: current status and implications for neuronal classification. Annu. Rev. Neurosci. 2015;38:221–246. doi: 10.1146/annurev-neuro-071714-034120. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y., Williams P.R., Jacobi A., Wang C., Goel A., Hirano A.A., Brecha N.C., Kerschensteiner D., He Z. Elevating growth factor responsiveness and axon regeneration by modulating presynaptic inputs. Neuron. 2019;103:39–51.e5. doi: 10.1016/j.neuron.2019.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piskounova E., Polytarchou C., Thornton J.E., LaPierre R.J., Pothoulakis C., Hagan J.P., Iliopoulos D., Gregory R.I. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell. 2011;147:1066–1079. doi: 10.1016/j.cell.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balzer E., Moss E.G. Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA Biol. 2007;4:16–25. doi: 10.4161/rna.4.1.4364. [DOI] [PubMed] [Google Scholar]
- 69.Faas L., Warrander F.C., Maguire R., Ramsbottom S.A., Quinn D., Genever P., Isaacs H.V. Lin28 proteins are required for germ layer specification in Xenopus. Development. 2013;140:976–986. doi: 10.1242/dev.089797. [DOI] [PubMed] [Google Scholar]
- 70.Hanna J., Saha K., Pando B., van Zon J., Lengner C.J., Creyghton M.P., van Oudenaarden A., Jaenisch R. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang M., Yang S.L., Herrlinger S., Liang C., Dzieciatkowska M., Hansen K.C., Desai R., Nagy A., Niswander L., Moss E.G., Chen J.F. Lin28 promotes the proliferative capacity of neural progenitor cells in brain development. Development. 2015;142:1616–1627. doi: 10.1242/dev.120543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu H., Shah S., Shyh-Chang N., Shinoda G., Einhorn W.S., Viswanathan S.R., Takeuchi A., Grasemann C., Rinn J.L., Lopez M.F. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat. Genet. 2010;42:626–630. doi: 10.1038/ng.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cimadamore F., Amador-Arjona A., Chen C., Huang C.T., Terskikh A.V. SOX2-LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc. Natl. Acad. Sci. USA. 2013;110:E3017–E3026. doi: 10.1073/pnas.1220176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mondol V., Pasquinelli A.E. Let’s make it happen: the role of let-7 microRNA in development. Curr. Top. Dev. Biol. 2012;99:1–30. doi: 10.1016/B978-0-12-387038-4.00001-X. [DOI] [PubMed] [Google Scholar]
- 75.Newman M.A., Thomson J.M., Hammond S.M. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008;14:1539–1549. doi: 10.1261/rna.1155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Viswanathan S.R., Daley G.Q., Gregory R.I. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zou Y., Chiu H., Zinovyeva A., Ambros V., Chuang C.F., Chang C. Developmental decline in neuronal regeneration by the progressive change of two intrinsic timers. Science. 2013;340:372–376. doi: 10.1126/science.1231321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xia X., Teotia P., Ahmad I. Lin28a regulates neurogliogenesis in mammalian retina through the Igf signaling. Dev. Biol. 2018;440:113–128. doi: 10.1016/j.ydbio.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Ojala D.S., Amara D.P., Schaffer D.V. Adeno-associated virus vectors and neurological gene therapy. Neuroscientist. 2015;21:84–98. doi: 10.1177/1073858414521870. [DOI] [PubMed] [Google Scholar]
- 80.McCown T.J. Adeno-associated virus (AAV) vectors in the CNS. Curr. Gene Ther. 2011;11:181–188. doi: 10.2174/156652311795684759. [DOI] [PubMed] [Google Scholar]
- 81.Liu Y., Keefe K., Tang X., Lin S., Smith G.M. Use of self-complementary adeno-associated virus serotype 2 as a tracer for labeling axons: implications for axon regeneration. PLoS ONE. 2014;9:e87447. doi: 10.1371/journal.pone.0087447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.