Abstract
Targeted delivery of oligonucleotides to liver hepatocytes using N-acetylgalactosamine (GalNAc) conjugates that bind to the asialoglycoprotein receptor has become a breakthrough approach in the therapeutic oligonucleotide field. This technology has led to the approval of givosiran for the treatment of acute hepatic porphyria, and there are another seven conjugates in registrational review or phase 3 trials and at least another 21 conjugates at earlier stages of clinical development. This review highlights some of the recent chemical and preclinical advances in this space, leading to a large number of clinical candidates against a diverse range of targets in liver hepatocytes. The review focuses on the use of this delivery system for small interfering RNAs (siRNAs) and antisense molecules that cause downregulation of target mRNA and protein. A number of other approaches such as anti-microRNAs and small activating RNAs are starting to exploit the technology, broadening the potential of this approach for therapeutic oligonucleotide intervention.
Graphical Abstract
Targeted delivery of oligonucleotides to hepatocytes using GalNAc conjugates has become a breakthrough approach in the therapeutic oligonucleotide field. Debacker et al. highlight recent chemical and preclinical advances in this space, leading to a large number of clinical candidates against a diverse range of targets in liver hepatocytes.
Main Text
Oligonucleotide therapeutics are an emerging class of drugs that have tremendous potential for treating a wide range of diseases.1 There are now nine marketed oligonucleotide products,2 and nusinersen (Biogen/Ionis), which is used to treat spinal muscular atrophy, generated more than $2 billion in sales in 2019. They are designed based on Watson-Crick base pairing and the sequence of the RNA associated with the disease. Modifications to internucleotide linkages and the ribose sugar are introduced to improve drug-like properties, including serum stability, protein binding, potency, and lower immunogenicity.3,4 A major challenge that has held back the therapeutic exploitation of oligonucleotides is their delivery to the diseased cells because the therapeutic RNA target is inside the cell. Oligonucleotides are large, generally negatively charged molecules that do not freely diffuse across cell membranes, unlike lipophilic small molecule drugs.5
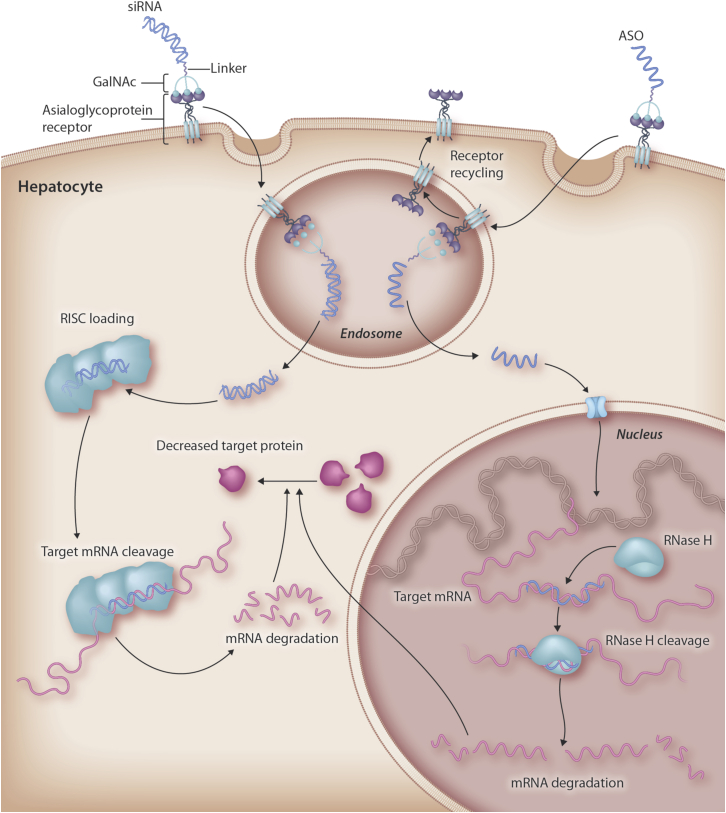
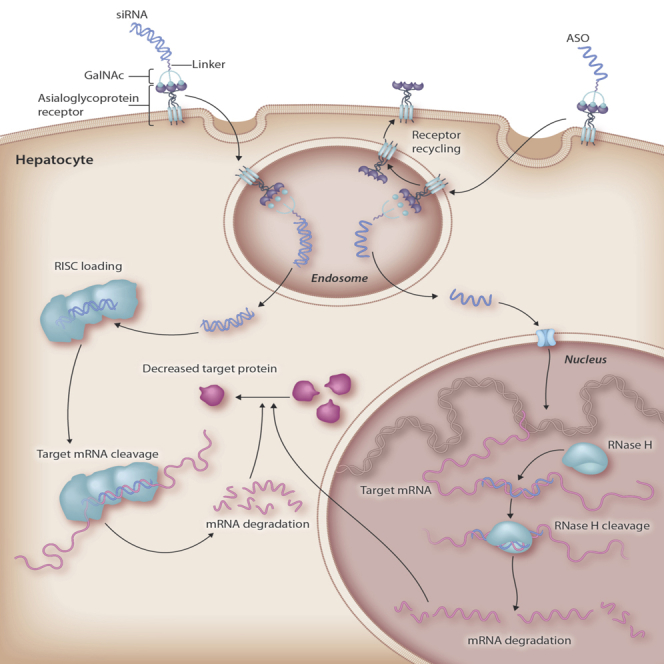
This review focuses on a delivery solution termed N-acetylgalactosamine (GalNAc) and its exploitation by small interfering RNA (siRNA) and antisense oligonucleotides (ASOs), the two leading classes of oligonucleotide therapeutics.1 The approach relies on the fact that liver hepatocytes express the asialoglycoprotein receptor (ASGPR), which binds and clears circulating glycoproteins in which the sialic acid residue has been removed to expose sugar residues.6,7 The ASGPR is a high-capacity, rapidly internalizing receptor with approximately 500,000 copies per hepatocyte. Trimeric GalNAc ligands for the ASGPR have been developed, and these were first utilized to deliver oligonucleotides to the liver more than 20 years ago.8,9 GalNAc conjugates bind to the ASGPR and are taken up in endosomes, where the conjugate dissociates from the receptor. Then, the GalNAc sugars and branches are very quickly lysed from the oligonucleotide before the oligonucleotide escapes to the cytoplasm by a still poorly understood mechanism. The approach for delivering ASOs and siRNAs is highlighted in Figure 1. Several excellent reviews have covered the early history of the field, and the reader is referred to these for more background details.3,10,11
Figure 1.
Targeted Delivery of siRNA and ASOs by GalNAc to Achieve Target mRNA and Protein Knockdown
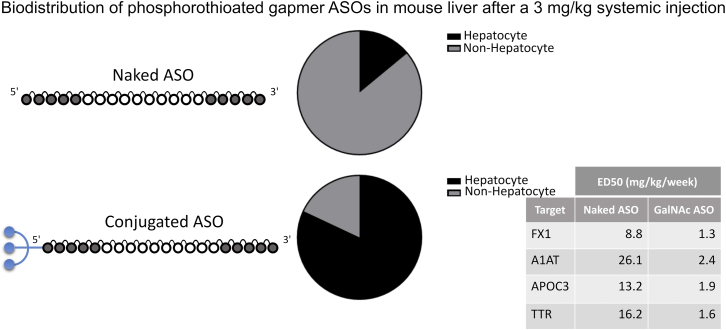
Following systemic administration, unconjugated ASOs distribute to the liver and other tissues,12 so this raises the question regarding why has GalNAc made such a big impact on ASOs being developed for treating liver disease. A key paper was published in 2014 in which the cellular distribution in the liver in mice after treatment with either a non-conjugated or GalNAc-conjugated ASO to SRB1 was determined.13 The unconjugated ASO was predominantly (>70%) taken up by the non-parenchymal cellular faction of the liver while, in contrast, the GalNAc-SRB1 ASO was predominantly (>80%) taken up by the hepatocyte fraction of the liver. Thus, the attachment of the GalNAc moiety to the ASO led to targeted delivery to the hepatocytes and increased ASO drug levels in the hepatocytes by about 6- to 7-fold at equivalent doses (Figure 2). This increased delivery to the hepatocytes significantly contributed to the approximately 7-fold increase in potency in vivo (50% knockdown of liver SRB1 mRNA) compared to non-conjugated SRB1 ASO. Other ASO-GalNAc conjugates to hepatocyte targets, that is, FX1, A1AT, APOC3, and transthyretin (TTR), showed similar or slightly greater potency improvements up to 11-fold,13 demonstrating that this was a general property of GalNAc-ASO conjugates. This improvement in potency in mice has translated very well to the clinic, with typically 10- to 30-fold lower GalNAc-ASO doses being required for functional target knockdown compared to non-targeted ASOs.14
Figure 2.
Biodistribution of ASOs in Mouse Liver after Systemic Injection
Ratios are based on data from Prakash et al.13 and corroborated by data from Watanabe et al.15
For siRNAs, which are double-stranded RNA and typically at least twice the size of ASOs and contain more negative charge, in vivo delivery has been achieved using lipid nanoparticles (LNPs).16 Indeed, patisiran, developed for the treatment of hereditary TTR amyloidosis, was the first siRNA drug to receive regulatory approval in 2018 and utilizes an LNP for liver delivery.17 GalNAc technology has been developed for therapeutic siRNAs and has now largely replaced LNP delivery for liver disease targets.10,11 Similar to ASO hepatocyte delivery, the importance of having a multivalent GalNAc to deliver siRNAs was reported by Nair et al.18 in 2014 where they demonstrated very effective knockdown in the livers of mice of both APOB100 and TTR mRNA by GalNAc-siRNA conjugates. Most siRNAs in the clinic for treatment of liver disease now use the GalNAc-targeting strategy, and Alnylam Pharmaceuticals, who have pioneered siRNA therapy, recently had their first GalNAc US Food and Drug Administration (FDA) drug approval, givosiran, for acute hepatic porphyria in November 2019. As of February 2020, based on their website, they had another 10 GalNAc conjugates in various stages of clinical development and no additional LNP candidates, demonstrating the impact of GalNAc targeting on the field.19
This review summarizes some of the advances in GalNAc chemistry, preclinical models used to evaluate GalNAc conjugates, and pharmacokinetic (PK) and safety data, and it provides more details on the clinical experience with GalNAc conjugates, with a focus on information that has emerged during the last 2–3 years.
Advances in Chemistry Enabling GalNAc Delivery
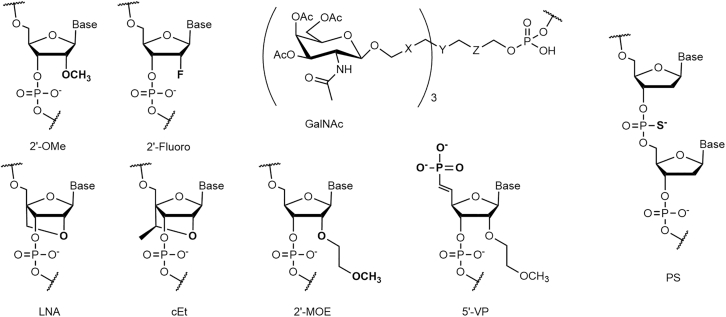
The key breakthrough for the use of GalNAc as a delivery moiety for oligonucleotides was to apply extensive chemical modifications at the 2′ position of the nucleotides and to replace phosphodiester bonds with phosphorothioate (PS) bonds in order to achieve in vivo activity.20,21 These modifications give the conjugates enough nuclease stability to reach the liver after intravenous (i.v.) or subcutaneous injection. Muthiah (Mano) Manoharan was pivotal in the development of GalNAc conjugates.22 He received the 2019 Oligonucleotide Therapeutics Society lifetime achievement award for his many contributions to oligonucleotide chemistry, including leading the team at Alnylam that developed the first human therapeutic applications of GalNAc-conjugated siRNA. The structures of the key chemical modifications used in GalNAc conjugates are shown in Figure 3.
Figure 3.
Schemes of the Most Common Chemical Modifications Found in GalNAc Conjugates
X and Z are commonly ethylene glycol or alkyl spacers. Y is a multifunctional moiety allowing branching of the cluster.
siRNA Optimization
GalNAc-siRNA conjugates are generally made up of patterns of alternating of 2′-O-methyl and 2′-fluoro nucleotides with insertion of PS bonds at the extremities of the strands (Figure 3).4 In the first generation, siRNA GalNAc conjugates were only partially modified; however, more extensive modifications showed a higher potency and duration of action when tested in vivo.21,23 Alnylam estimated the human annual dose required for siRNAs targeting TTR to be 280-fold lower for vutrisiran, a second-generation siRNA that is fully modified and stabilized with PS bonds (called enhanced stabilization chemistry [ESC]), compared to the first-generation chemistry (called standard template chemistry [STC]) for revusiran. The modification of the 5′ end of the antisense strand of siRNA using a stable phosphate analog, vinyl phosphonate, brought even more stability and potency for siRNA conjugates.24 The 5′ vinyl phosphonate protects the end of the siRNA from degradation while removing the need for the cell to phosphorylate the double strand prior insertion into the RNA-induced silencing complex (RISC). The latter benefit increased the potency of certain siRNAs conjugates by up to 10-fold.25,26 With the siRNA nucleotide content moving away from “natural” nucleotides, and the rise in potency and duration of action, a potential problem of safety came to mind. One of the concerns was that the 2′-fluoro nucleotide contained in the oligonucleotides could cause toxicity due to possible incorporation into genomic DNA and non-specific protein interaction.27,28 However, this has not been observed in vivo.29 Furthermore, Foster et al.30 showed that it was possible to reduce the content of 2′-fluoro nucleotides in siRNA to less than 20% for some sequences while maintaining siRNA activity in vivo. Another approach was chosen by Silence Therapeutics involving screening hundreds of modification patterns to identify patterns that used as little modification as possible while maintaining high nuclease stability.31 The higher potency achieved by the modified siRNA also decreases the chance of off-target toxicity by lowering the required dose. Alnylam has offered an elegant approach to off-target seed interaction by incorporating a glycerol nucleic acid (GNA) and named this new siRNA design ESC+. GNAs have lower affinity to the RNA target than do standard nucleotides, and introducing one at a specific position in the seed region was able to dramatically decrease the off-target toxicity of the sequence while maintaining high potency.32,33 The long-lasting efficacy of the modified siRNAs, up to 3 months after a single-dose injection in non-human primates,30 are a boon for the patient but also a safety concern if toxicity is encountered due to the drug. Chemists at Alnylam developed a siRNA “antidote” to increase the safety profile of siRNA drugs termed Reversir. These are short 9-mer LNA-PS-modified oligonucleotides conjugated to a GalNAc cluster designed to recognize and bind to the complementary, RISC-bound antisense strand of the siRNA. Injection of the Reversir 7 days after injection of GalNAc-siRNA to TTR was able to fully reverse the silencing of TTR back to normal in 4 days.34
ASO Conjugate Optimization
Prior to GalNAc conjugation, ASOs were already delivered “naked” in vivo to achieve liver mRNA knockdown. Therefore, the modifications required for good in vivo potency for GalNAc conjugates were similar to unconjugated ASOs. ASO designs depend on their mechanism of action. If the ASO is used to achieve splice switching, the oligonucleotide will be fully modified with 2′-OMe or morpholinos with a high number of PS bonds. In contrast, if the ASO is designed to recruit RNase H to silence mRNA, they are called gapmers and have a central part of just PS-modified DNA and wings on either side containing both PS and modifications at the 2′ position typically using locked nucleic acids (LNAs, cEt) or 2′-OMe/2′-MOE nucleotides (Figures 3 and 4). ASOs are taken up by cells without the need for transfection, and one of the identified mechanisms of uptake of PS ASOs is actually by interacting with the ASGPR.35, 36, 37 Furthermore, while the unconjugated ASOs are mainly delivered to the liver when injected systematically, the conjugation of a GalNAc cluster to the 5′ end of an antisense increases the potency of 2′-OMe and 2′-MOE gapmer ASOs by 10-fold for hepatocyte targets in rodents.13 These advances have translated to improvements in potency up to 30-fold in the clinic.38 A more recent study by Prakash et al.39 in mice showed that GalNAc conjugation improved potency about 20-fold when LNA and cEt gapmers were used.
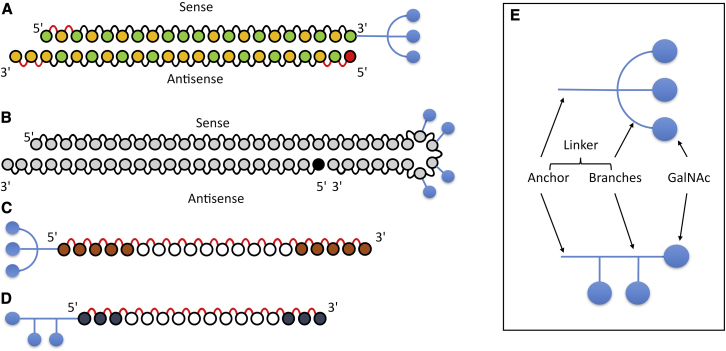
Figure 4.
Examples of GalNAc Clusters in Use in Preclinical and Clinical Studies
Blue branches and circles are GalNAc clusters, green circles are 2′-fluoro nucleotides, yellow circles are 2′-O-methyl nucleotides, brown circles are 2′-methoxyethyl nucleotides, dark gray circles are bridged nucleic acids (LNA/cEt), white circles are DNA nucleotides, red circles are 5′-vinyl phosphonate, and light gray circles are either 2′-fluoro or 2′-O-methyl nucleotides. (A) siRNA GalNAc conjugate with a triantennary cluster (Alnylam). (B) siRNA GalNAc conjugate with sequential GalNAc on a tetraloop (Dicerna). (C) MOE Gapmer GalNAc conjugate with a triantennary cluster (IONIS, second generation). (D) LNA Gapmer GalNAc conjugate with a sequential cluster (Yamamoto). (E) Anatomy of a GalNAc cluster.
Cluster Optimization
The ASGPR is formed of two subunits, ASGR1 and ASGR2, assembled in a hetero-oligomer at different ratios.40 The avidity of the receptor is dependent on the number of ligands attached to the receptor. The affinity of the ASGPR for a trimer of GalNAc is 1,000-fold higher than a dimer and 1,000-fold higher than a monomer, while a tetramer has just a slightly higher affinity for the receptor than a trimer.41 For this reason, the initial work on GalNAc-conjugated oligonucleotides focused on using a trivalent cluster with the presentation distance between sugar thought to be optimal at 15–20 Å between each GalNAc (Figure 4E).42
In order to achieve this structure, two main strategies have been chosen. One way is to synthesize a trivalent cluster and then link it to the oligonucleotide either by post-synthesis conjugation (e.g., amide coupling, phosphoramidite coupling, or click chemistry) or by coupling the cluster to the solid support prior to the oligonucleotide synthesis (Figures 4A and 4C). Details of synthesis pathways and conjugation conditions can be found in several publications.13,18,43, 44, 45, 46, 47, 48 The second approach to build the cluster is to add monomeric GalNAc sequentially during the oligonucleotide synthesis (Figures 4B and 4D). This latter approach offers more flexibility and is favored for non-trivalent cluster, and it benefits from the easy availability of the monomers. Both approaches have seen comparable activity in vivo, and the main concern when choosing one or the other is manufacturing. GalNAc clusters are preferentially attached to the 3′ end of the sense strand for siRNA and to the 5′ end of the ASOs. 5′ GalNAc-conjugated ASOs show a slight potency advantage compare to 3′ conjugation.46,49 However, overall GalNAc conjugates have shown flexibility on the positioning of the cluster while maintaining either ASO or siRNA activity.44,49
The flexibility observed in vivo for the placement and the shape of the cluster has also been observed for cluster valency. Dicerna’s GalNAc platform, GalXC, uses a tetravalent cluster built up sequentially in a tetraloop.50 Sharma et al.48 showed that sequential tetravalent and trivalent clusters have the same tissue accumulation and activity in mice, but that the potency of siRNA conjugates with a divalent cluster was lower. However, Silence Therapeutics have presented data showing comparable in vivo potency for divalent GalNAc siRNA conjugates to tri-antennary GalNAc by changing the spacing of the GalNAc sugars.51 Double-stranded ASOs with two divalent clusters also show a higher potency than conjugation to a single tri-antennary cluster.35 For phosphorothioated ASOs, several studies found that divalent and trivalent GalNAc have comparable potency.35,52 The study on ASO conjugates published by Schmidt et al.35 suggests that the high affinity of ASO with one GalNAc sugar on each end could be due to the conjugates binding two different receptors on the cell membrane. In short, while most conjugates use a trivalent GalNAc, it seems that in many cases a divalent GalNAc might be acceptable.
The GalNAc sugar is cleaved from the cluster branches in less than an hour after reaching the endosome by glycosidases, and the clusters arms are cleaved from the siRNA in 4 h (Figure 1).13 Therefore, a bio-cleavable linker for a GalNAc conjugate may not be required for optimal intracellular activity and could lead to reduced plasma stability of the conjugate. Yamamoto et al.49 found that using an unstable phosphodiester link between the oligonucleotide and GalNAc resulted in better activity than using stable PS bonds. However, Prakash et al.43 did not observe any benefit by adding an additional deoxynucleotide linked with a phosphodiester cleavable moiety compared to a more stable hexylamine in ASO-GalNAc conjugates. Further work is required to address any benefits of cleavable over stable linkers.
Preclinical Evaluation of New GalNAc Conjugates
Recent preclinical development has built on and expanded from the early efforts of therapeutics for chronic hepatitis virus infection and rare genetic diseases (for reviews of early ASO and siRNA conjugates, see Springer and Dowdy10 and Huang11).
Hepatitis B Virus Targeting
Current ASO and siRNA conjugates in the clinic targeting hepatitis B virus (HBV) sequences are described later, but new approaches continue to be developed. Roche recently reported development of a next-generation LNA ASO-GalNAc conjugate targeting viral mRNA.53 Treatment with this conjugate in a mouse adeno-associated virus (AAV)-HBV model reduced expression of hepatitis B surface antigen below the limit of detection at the highest dose of 7 mg/kg injected subcutaneously and was sustained throughout the 15-day follow-up period. It remains to be seen how long beyond 15 days this effect will last and how the use of LNA chemistry will compare to the ASO and siRNA conjugates already in the clinic.
Genetic Disease
Genetic diseases continue to provide unique liver targets for rapid preclinical development due to well-defined genetics and pathways. By targeting the liver-specific glycogen synthase GYS2 with a GalNAc-siRNA conjugate, a Dicerna study was able to show reduced accumulation of glycogen in wild-type and glycogen storage disease (GSD) mouse models.54 Weekly subcutaneous injections of 10 mg/kg normalized circulating liver enzymes and liver pathology in a GSD type III mouse model, including reversal of hepatomegaly and α-smooth muscle actin and sirius red fibrotic staining. Knockdown of GYS2 in a GSD type Ia mouse model also showed therapeutic benefit in restoring normal liver morphology, although the siRNA was delivered by an LNP in that experiment. By downregulating TMPRSS6, a negative regulator of hepcidin, which in turn negatively regulates iron absorption and recycling, Silence Therapeutics sought to treat iron overload disorders.55, 56, 57 Preclinical results showed a dose-dependent increase of hepcidin and reduction of serum iron after a single administration of 1 or 3 mg/kg GalNAc conjugate targeting TMPRSS6 in a mouse model of HFE−/− hereditary hemochromatosis. Normalization of erythropoiesis and anemia was also demonstrated in a mouse model of β-thalassemia intermedia, and they also aim to use the same conjugate to treat transfusional iron overload associated with myelodysplastic syndrome. These examples show the potential for a single GalNAc conjugate to treat multiple genetic disorders by targeting a common factor involved in disease pathology. The use of already established mouse models of genetic diseases provides opportunities to rapidly evaluate the effects of these new therapeutics.
Non-alcoholic Fatty Liver Disease
Recent efforts have shown there is promise for GalNAc conjugate treatments for non-alcoholic fatty liver disease (NAFLD), the most common liver condition worldwide.58 GalNAc-siRNA or ASO conjugates targeting TAZ and STK25, factors shown to contribute to non-alcoholic steatohepatitis (NASH) progression, have been evaluated in mouse models of NASH.59,60 Doses of 12.5 mg/kg/week or less showed decreases in liver fat deposition and inflammation in both cases. An effort led by AstraZeneca used an ASO targeting a known genetic variant of the gene PNPLA3, which has been shown to contribute to NAFLD progression.61 This approach will allow for ease of identifying the target patient population by screening for the genetic variant. There has been concern that reduced ASGR expression in advanced liver disease will decrease the potency of GalNAc conjugates in NASH and hepatocellular cancer (HCC). Despite a reduction in ASGR expression greater than 50%, both GalNAc-siRNA and ASO conjugates have been shown to retain potency in mice models of HCC and fibrosis.62, 63, 64 With 40% of NASH patients developing fibrosis and up to 15% progressing to HCC, we would expect to see many more GalNAc conjugate approaches to treat advanced liver disease and HCC in the coming years.58
Secreted Protein Modulation
Another active area of preclinical development takes advantage of the liver as a secretory organ, aiming to treat diseases that do not primarily affect the liver by modulating proteins secreted by hepatocytes. GalNAc-siRNA conjugates downregulating components of the complement system are already in late-stage trials, and more continue to be developed or tested in additional disease models. siRNA conjugates targeting MASP serine proteases have shown efficacy with 10 mg/kg dosing in mouse models of rheumatoid arthritis.65,66 The Alnylam complement C5 siRNA conjugate in the clinic has also been evaluated in rat models of myasthenia gravis, and an another targeting factor XII has been tested in models of hereditary angioedema.67,68 These conjugates have the advantage of strong target validation in the clinic from established inhibitors or antibody treatments. The hope is that the relatively infrequent subcutaneous dosing of GalNAc conjugates will provide a benefit over treatments that require infusions or more frequent dosing.
Preclinical Pharmacokinetics
The pharmacokinetic properties of unconjugated oligonucleotides have been discussed in recent reviews, so this section focuses on the effect of GalNAc conjugation on the pharmacokinetic characteristics of oligonucleotide-based drugs.12,69 Subcutaneous delivery of GalNAc conjugates results in rapid absorption, with a Tmax between 0.25 h and 1 h in mice, 1 and 4 h in monkeys, and 0.5 and 5 h in humans.70, 71, 72 The plasma half-life of GalNAc conjugates is relatively short due to rapid plasma clearance.71,73 GalNAc conjugation remains quite stable in the blood, but there is evidence that loss of GalNAc monomers occurs, and unconjugated oligonucleotide can be measured in the plasma.
The main driver of plasma clearance is tissue distribution, which increases moderately between doses of 1 and 3 mg/kg. However, at higher doses between 12 and 40 mg/kg, clearance decreases 3- to 5-fold.70 Some data suggest that the reduced clearance at higher doses could be caused by saturation of the ASGPR and reduced hepatocyte-mediated clearance.74 However, ASGPR saturation alone likely does not explain the full dynamics of plasma clearance. GalNAc-ASO conjugates may have increased liver uptake via stabilin receptors on nonparenchymal cells, and GalNAc-siRNA conjugates may have increased kidney clearance due to reduced plasma protein binding compared to ASOs.75 It will be important for the field to understand how much these variables contribute to drug clearance and how they are affected by various diseases.
GalNAc-conjugated ASOs have considerably increased levels of plasma clearance when compared to unconjugated ASOs, leading to a 50-fold reduction in maximum plasma concentration (Cmax) and area under the curve (AUC) exposures at 10% of the dose.70 This improved targeting of the liver delivers substantial improvements in potency, allowing reduced dosing frequency at lower doses. The maximum pharmacodynamic (PD) effect is achieved up to 15 days after dosing and is durable, meaning that infrequent dosing regiments can be used.76,77 There is emerging evidence that the duration of effect is species-specific, with longer effects and increased potency in humans compared to non-human primates.
In addition to the liver, GalNAc-conjugated oligonucleotides also distribute to the kidney. At doses below 3 mg/kg, GalNAc conjugation increases the liver-to-kidney ratio. At higher doses, saturation of the ASGPR diminishes liver uptake, and kidney uptake is proportionally increased. Cellular uptake results in removal of GalNAc, and unconjugated oligonucleotide is the major component found in tissues.71,73
An understanding of GalNAc-conjugated oligonucleotide pharmacokinetics is vital to the design of studies with these molecules.
Safety Considerations of GalNAc
In general, toxicity of oligonucleotides will be caused by on-target exaggerated pharmacology, hybridization-mediated off-target effects, chemical modifications, or tissue accumulation of the drug. Hybridization-mediated off-target effects can be mediated by partial or seed region binding to off-target RNAs, with partial complementarity to the antisense strand.33,78 These compounds can be identified in early, high-dose toxicity screens and eliminated as candidate drugs. Systematic analysis of GalNAc-siRNA hepatotoxicity eliminated chemical modification as a cause of toxicity and indicated that seed region-mediated RNAi effects of the antisense strand were a major driver of hepatotoxicity.33
Non-clinical toxicology has identified a number of pathological findings associated with subcutaneous administration of GalNAc-conjugated siRNAs to rats and monkeys that include hepatocellular vacuolation and hepatocellular single-cell necrosis. These reversible findings do not affect the no observed adverse effect level (NOAEL) unless they are severe and associated with elevated liver enzymes.77, 78, 79 Subcutaneous delivery of GalNAc siRNA results in drug accumulation in a number of tissues and is indicated by basophilic granules in proximal renal tubular cells of rats and hepatic Kupffer cells in monkeys, or vacuolation of lymph node macrophages and injection site mononuclear cells.77,78 These findings often show partial recovery.
Immunogenicity has not been seen for GalNAc siRNA but has been observed with GalNAc ASO. The anti-drug antibodies have a very limited effect on pharmacokinetics and appear to be binding rather than neutralizing.70,72,77
GalNAc-conjugated oligonucleotides have demonstrated a favorable safety profile both preclinically and clinically, and the lower clinical doses required to deliver therapeutically beneficial doses of GalNAc conjugates improve the therapeutic window when compared to unconjugated oligonucleotides. One exception is revusiran, a GalNAc-conjugated siRNA targeting TTR. The phase 3 ENDEAVOUR trial of revusiran was terminated early because of an imbalance of deaths.80 Although there was no direct evidence that this imbalance was related to revusiran, it was not possible to rule out a drug-mediated effect as the cause. Non-clinical toxicology of revusiran was unremarkable, with no dose-limiting toxicology in monkeys and reversible microscopic changes in rat liver that correlated with elevated clinical chemistry. The NOAEL was set at 30 mg/kg in rats and 200 mg/kg in monkeys.79 Post hoc analysis of the ENDEAVOR study revealed that patients who died on treatment were older (≥75 years of age) and had more severe disease. Revusiran is a first-generation GalNAc conjugate with reduced stability and potency compared with second-generation compounds with enhanced stabilization chemistry. Patients treated with revusiran received 28 g of compound per year compared to vutrisiran (new TTR siRNA-GalNAc conjugate in phase 3), which achieves similar pharmacodynamic effects with 100 mg/year. This 280-fold lower drug exposure may improve safety with second-generation chemistry, and this seems to be borne out by the clinical experience described below.80
Clinical Experience with GalNAc Conjugates and the First Product Registration
A summary of the clinical status of GalNAc conjugates with either ASOs or siRNAs is shown in Table 1. The information was extracted from pipeline data on the websites of Alnylam, Ionis, Dicerna, Arrowhead Pharmaceuticals, Silence Therapeutics, and Arbutus Biopharma (accessed March 30, 2020). Based on this analysis, there are at least 29 different GalNAc conjugates in clinical development, of which about 55% are RNAi based and about 45% are ASO based. Arrowhead Pharmaceuticals have four additional siRNA conjugates in clinical development focused on liver-related targets using their targeted RNAi molecule (TRIM) platform. They have not publicly revealed the hepatocyte targeting ligand for all of these, although it seems very likely that they are using GalNAc targeting the ASGPR. The clinical trial for AMG-890 (ARO-LPA) describes it as a GalNAc conjugate. Additionally, in a presentation on their HBV drug using the platform TRIM in a phase 2 clinical trial (ARO-HBV/JNJ-3989), the drug was described as two siRNA triggers delivered with GalNAc.81, 82, 83 Indeed, GalNAc conjugates targeting HBV proteins and thus HBV inactivation/depletion are being widely explored, with Ionis/GSK, Alnylam, and Dicerna/Roche also having candidates in phase 1 or 2 clinical development (Table 1), with encouraging results being published.53,84,85
Table 1.
Summary of GalNAc-siRNA or GalNAc-ASO Conjugates in the Clinic from the Leading Oligonucleotide Platform Companies
| Clinical Phase | Company | Modality | Drug Name | Target | Lead Indication |
|---|---|---|---|---|---|
| Registered | Alnylam | siRNA | Givlaari | d-aminolevulinate synthase 1 | acute hepatic porphyria |
| Submitted for registration | Alnylam | siRNA | lumasiran | glycolate oxidase 1 | hyperoxaluria type 1 |
| Submitted for registration | Alnylam/ Novartis | siRNA | inclisiran | PCSK9 | hypercholesterolemia |
| Phase 3 | Akcea/Ionis | ASO | AKCEA-TTR-LRx | transthyretin | TTR amyloidosis |
| Phase 3 | Akcea/Ionis/Novartis | ASO | AKCEA-APO(a)-LRx | apolipoprotein(a) | cardiovascular disease |
| Phase 3 | Alnylam/ Sanofi | siRNA | fitusiran | antithrombin | hemophilia/bleeding disorders |
| Phase 3 | Alnylam | siRNA | vutrisiran | transthyretin | TTR amyloidosis |
| Phase 3 | Dicerna | siRNA | nedosiran | lactate dehydrogenase | primary hyperoxaluria |
| Phase 2 | Alnylam | siRNA | cemdisiran | complement C5 | complement-mediated diseases |
| Phase 2 | Akcea/Ionis | ASO | AKCEA-AOPCIII-LRx | apoC-III | cardiovascular disease |
| Phase 2 | Ionis | ASO | IONIS-GHR-LRx | growth hormone receptor | acromegaly |
| Phase 2 | Ionis | ASO | IONIS-PKK-LRx | prekallikrein | hereditary angioedema |
| Phase 2 | Ionis | ASO | IONIS-TMPRSS6- LRx | transmembrane protease, serine 6 | β-thalassemia |
| Phase 2 | Akcea/Pfizer/Ionis | ASO | AKCEA-ANGPTL3-LRx | angiopoietin-like 3 protein | multiple lipid disorders |
| Phase 2 | Ionis | ASO | IONIS-AGT-LRx | angiotensinogen | resistant hypertension |
| Phase 2 | Ionis/Roche | ASO | IONIS-FB-LRx | complement factor B | Immunoglobulin A (IgA) neuropathy/age-related macular degeneration |
| Phase 2 | Ionis/GSK | ASO | IONIS-HBV-LRx | HBV viral proteins | hepatitis B infection |
| Phase 2 | Arrowhead/JNJ | siRNA | JNJ-3989(ARO-HBV) | HNV viral proteins | hepatitis B infection |
| Phase 1/2 | Alnylam | siRNA | ALN-AAT02 | AAT | α1 liver disease |
| Phase 1/2 | Alnylam | siRNA | ALN-HBV02 | HBV viral proteins | hepatitis B virus infection |
| Phase 1 | Arrowhead/Amgen | siRNA | AMG-890 (ARO-LPA) | lipoprotein(a) | cardiovascular disease |
| Phase 1 | Alnylam | siRNA | ALN-AGT | AGT | hypertension |
| Phase 1 | Dicerna/Roche | siRNA | DCR-HBVS(RG6346) | HNV viral proteins | hepatitis B virus |
| Phase 1 | Dicerna | siRNA | DCR-A1AT | SERPINA1 | α1 anti-trypsin deficiency liver disease |
| Phase 1 | Ionis/Bayer | ASO | IONIS-FX1-LRx | factor X1 | thrombosis |
| Phase 1 | Ionis/AstraZeneca | ASO | IONIS-AZ4-2.5LRx | not reported | cardiovascular disease |
| Phase 1 | Ionis/AstraZeneca | ASO | ION839 | not reported | NASH |
| Phase 1 | Arbutus | siRNA | AB-729 | viral protein | hepatitis B infection |
| Phase 1 | Silence | siRNA | SLN-124 | TMPRSS6 | β-thalassaemia and MDS |
Source: Alnylam, Ionis, Dicerna, Arbutus, Silence, and Roche pipeline website data accessed March 2020. Arrowheads ARO-AAT, ARO-APOC3, ARO-ANG3, and ARO-HSD use their TRIM (targeted RNAi molecule) technology but are not included since the exact nature of the liver-targeting conjugates for these molecules are not yet published but are assumed to be GalNAc targeting.
The first GalNAc conjugate to gain registration was Alnylam’s Givlaari (givosiran) based on their ENVISION phase 3 data; it gained approval in the United States on November 20, 2019 and in the European Union on March 3, 2020. The siRNA in givosiran uses Alnylam’s advanced ESC GalNAc technology (see Advances in Chemistry Enabling GalNAc Delivery) and targets aminolevulinate synthase 1 (ALAS1), which leads to downregulation of ALAS1 and prevents accumulation of neurotoxic δ-aminolevulinic acid and porphobilinogen that are associated with acute hepatic porphyria (AHP) attacks.86 The ENVISION trial recruited 94 patients with AHP randomized 1:1 to givosiran (2.5 mg/kg quarterly for 6 months) or placebo and demonstrated a 74% mean reduction in rates of porphyria attacks compared to placebo. Half of the patients on givosiran were attack-free during the 6-month treatment period compared to just 16.3% in the placebo group. There was a small increase in adverse events in the givosiran group, including seven patients with liver enzyme (alanine aminotransferase [ALT]) rises ≥3-fold the upper limit of normal (ULN). However, 93 out of 94 of the patients in the trial continued into the open-label extension period of the study where all patients received givosiran, indicating a manageable toxicity profile in this setting.87
Two additional GalNAc conjugates from Alnylam have been submitted for registration. First, lumasiran is being developed for primary hyperoxaluria type 1 (PH1). This is a rare life-threatening disease that is characterized by the pathologic overproduction of oxalate by the liver that then accumulates in the kidneys, forming toxic calcium oxalate crystals that can lead to kidney failure.88 Lumasiran targets HAO1 mRNA encoding glycolate oxidase in the liver, which is the key enzyme in the pathway of hepatic oxalate production. The ILLUMINATE-A phase 3 study for lumasiran was a randomized, double-blind, placebo-controlled study on approximately 30 patients with PH1. Patients were randomized 2:1 to lumasiran (3 mg/kg monthly for 3 months, followed by quarterly maintenance doses). Lumasiran met its primary endpoint, achieving a significant (p < 0.0001) reduction in 24-h urinary oxalate excretion averaged across months 3–6. There were no serious or severe adverse events, which is encouraging, although detailed data are still to be reported.89 In January 2020, Alnylam reported that they had initiated a rolling submission for a new drug application (NDA) in the United States.90
Second, inclisiran was developed by Alnylam and licensed to The Medicines Company, who led the clinical development. Following release of positive phase 3 data, an NDA was submitted in the United States in December 2019 and The Medicines Company was acquired by Novartis for $9.7 billion, with acquisition completed in January.91 Inclisiran targets proprotein convertase subtilisin/kexin type 9 (PCSK9) mRNA, leading to reduction of hepatic PCSK9 production. PCSK9 is a serine protease that binds to low-density lipoprotein (LDL) receptors and targets the receptor for lysosomal degradation. Blocking this pathway leads to increased LDL receptors and increased clearance of LDL cholesterol (LDL-C), thus reducing cardiovascular risks associated with elevated LDL-C.92 Three phase 3 trials (Orion 9, 10, and 11) were carried out and the data were published online in March 2020.93,94 In all three trials, after two doses (284 mg) spaced 3 months apart, twice-yearly subcutaneous dosing with inclisiran resulted in durable and potent LDL-C reductions versus placebo. The larger Orion 10 and 11 studies treated more than 3,000 patients with atherosclerotic cardiovascular disease who had elevated LDL-C. Patients were randomized in a 1:1 ratio to either receive inclisiran or a placebo during 540 days. At day 510, inclisiran reduced LDL-C levels by approximately 50% in both Orion 10 and Orion 11 clinical trials (p < 0.001 versus placebo in both trials). Given that there are approximately 40 million patients in the United States who have been diagnosed with atherosclerotic cardiovascular disease (ASCVD) or familial hypercholesterolemia (FH), the patient population in Orion 9, inclisiran has the potential to benefit a very large patient population. While therapeutic antibodies are also available to block PCSK9 activity,92 the infrequent dosing of inclisiran alongside its good safety profile should enable it to complete effectively in this lipid-lowering market.
The most advanced ASO-GalNAc conjugates in phase 3 trials (AKCEA-TTR-LRx and AKCEA-APO(a)-LRx) were developed initially by Ionis and then by Akcea Therapeutics. Focusing on AKCEA-APO(a)-LRx, this GalNAc-ASO targets apolipoprotein(a), which when elevated is an independent, genetic risk factor for cardiovascular disease. Positive phase 2 trial data were published in January 2020 from a randomized, double-blind, placebo-controlled trial involving 286 patients with established cardiovascular disease and raised lipoprotein(a).95 Patients received a range of different doses and schedules of the drug. There was a dose-dependent decrease in lipoprotein(a) levels, and at 60 mg of AKCEA-APO(a)-LRx given every 4 weeks, a 72% reduction in lipoprotein(a) levels was seen versus just 6% seen in the placebo group (p < 0.001). There were no significant differences between any APO(a)-LRx dose and placebo with respect to platelet counts, liver and renal measures, or flu-like symptoms. The most common drug-related adverse events were injection-site reactions. In February 2019, Novartis exercised its option to license AKCEA-APO(a)-LRx (new name TQJ230), took over responsibility for worldwide development and commercialization, and have initiated a phase 3 trial giving 80 mg monthly by SC injection (Lp(a)HORIZON phase 3).96 It is estimated that 20%–30% of people who suffer from cardiovascular disease have elevated lipoprotein(a), and so, similar to inclisiran, this drug may benefit a large patient population if the phase 3 trials are successful.
In summary, the first GalNAc-oligonucleotide conjugate is registered, there are seven other GalNAc-oligonucleotide conjugates either submitted for approval or in phase 3 trials, and there at least a further 20 conjugates in earlier stage clinical trials. So far, the tolerance of these conjugates appears to be good, with occasional liver and injection site reactions appearing to be the most common adverse events. With this number of agents in clinical development it seems very likely that this will result in a robust flow of new oligonucleotide therapeutics reaching the market during the next few years, transforming the field and bringing oligonucleotides to the fore as a new class of therapeutic agents.
Future Prospects
While most GalNAc conjugates in preclinical development and certainly in clinical development are either siRNA or ASO based, other oligonucleotide platforms are also exploiting the technology for modulating hepatocyte targets. For example, Regulus Therapeutics developed GalNAc-conjugated anti-microRNAs (miRs) targeting microRNA-122 for the treatment of chronic hepatitis C virus infection and a microRNA-103/107 oligonucleotide that improved insulin sensitivity and glucose tolerance in animal models of NASH.97 While both conjugates were halted in clinical development, Regulus indicated that they are continuing to exploit GalNAc targeting for liver projects.98
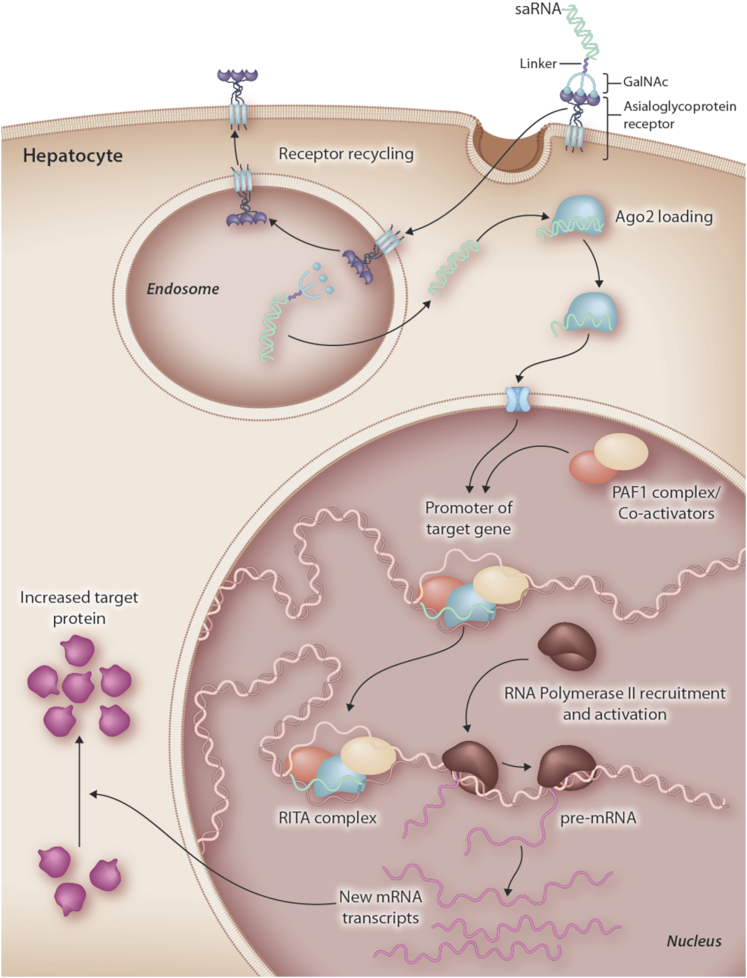
MiNA Therapeutics are exploiting small activating RNAs (saRNAs) for RNA activation.99 saRNAs, similar to siRNAs, are loaded into Ago2 but work in the nucleus, binding at the promoter region of the target gene to generate an RNA-induced transcriptional activation (RITA) complex that leads to new mRNA and increased protein (Figure 5).100 The upregulation of hepatocyte nuclear factor 4-α (HNF4A) in primary rat hepatocytes using a GalNAc-HNF4A saRNA conjugate has been reported.101 Delivery of HNF4A saRNA to the liver using a dendrimer-based delivery vehicle has been shown to have activity in a high-fat diet model of NAFLD, and since GalNAc delivery should focus delivery to the hepatocytes, this could be an interesting opportunity for both fatty liver disease and cirrhosis.102,103
Figure 5.
Targeted Delivery of saRNA by GalNAc to Achieve Target mRNA and Protein Upregulation
A future breakthrough for GalNAc oligonucleotides will be the delivery of these drugs by oral rather than subcutaneous administration, particularly for use in chronic disease and in broad patient populations. Progress on this new mode of delivery has recently been reported by Ionis and Alnylam.104,105
While most ligand-based approaches are utilizing GalNAc for delivery, other ligand-based delivery systems targeting oligonucleotides to extrahepatic tissues are emerging. For example, a GLP1R agonist peptide has been shown to effectively target an ASO to the GLP1R present on pancreatic insulin-secreting β cells, leading to target knockdown. This target knockdown was not seen with the free oligonucleotide or in GLP1R knockout pancreatic β cells.106 This opens up the possibility of utilizing ligand targeting of oligonucleotides to restore functional islet mass in type 2 diabetes. The transferrin receptor is also gathering interest as a promising ligand target, and transferrin receptor (TfR)-antibody-siRNA conjugates have been able to silence their target in skeletal and cardiac muscle after intravenous administration.107
Another focus of research in the oligonucleotide field is to look at approaches to increase endosomal escape, given that this is seen as a rate-limiting step for many oligonucleotides, with less than 1% of drug escaping from the endosome.10,108,109 It has been speculated that the combination of high ASGR expression and rapid turnover in the hepatocyte provides increased opportunities for the rare endosomal membrane destabilization required for oligonucleotide release into the cytoplasm.110 There may be another unknown mechanism by which GalNAc conjugation improves endosomal escape, and deepening our understanding of escape dynamics could potentially increase the potency of GalNAc conjugates and enable the use of other suitable receptor targets for tissue-specific delivery of oligonucleotides.
In summary, the rapid emergence of a robust pipeline of GalNAc-targeted oligonucleotides for a wide range of liver-based diseases is a true game changer for the field of oligonucleotide therapeutics, and the findings from this work are starting to be exploited using other ligand-based delivery systems.
Author Contributions
All authors have contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
All authors are, at the time of writing this review, employees of MiNA Therapeutics.
References
- 1.Levin A.A. Treating disease at the RNA level with oligonucleotides. N. Engl. J. Med. 2019;380:57–70. doi: 10.1056/NEJMra1705346. [DOI] [PubMed] [Google Scholar]
- 2.Wang F., Zuroske T., Watts J.K. RNA therapeutics on the rise. Nat. Rev. Drug Discov. 2020 doi: 10.1038/d41573-020-00078-0. Published online April 27, 2020. [DOI] [PubMed] [Google Scholar]
- 3.Craig K., Abrams M., Amiji M. Recent preclinical and clinical advances in oligonucleotide conjugates. Expert Opin. Drug Deliv. 2018;15:629–640. doi: 10.1080/17425247.2018.1473375. [DOI] [PubMed] [Google Scholar]
- 4.Khvorova A., Watts J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 2017;35:238–248. doi: 10.1038/nbt.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juliano R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016;44:6518–6548. doi: 10.1093/nar/gkw236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steirer L.M., Park E.I., Townsend R.R., Baenziger J.U. The asialoglycoprotein receptor regulates levels of plasma glycoproteins terminating with sialic acid α2,6-galactose. J. Biol. Chem. 2009;284:3777–3783. doi: 10.1074/jbc.M808689200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bocci V., Pacini A., Pessina G.P., Bargigli V., Russi M. The role of sialic acid in determining the survival of circulating interferon. Experientia. 1977;33:164–166. doi: 10.1007/BF02124043. [DOI] [PubMed] [Google Scholar]
- 8.Hangeland J.J., Flesher J.E., Deamond S.F., Lee Y.C., Ts’O P.O.P., Frost J.J. Tissue distribution and metabolism of the [32P]-labeled oligodeoxynucleoside methylphosphonate-neoglycopeptide conjugate, [YEE(ah-GalNAc)3]-SMCC-AET-pUmpT7, in the mouse. Antisense Nucleic Acid Drug Dev. 1997;7:141–149. doi: 10.1089/oli.1.1997.7.141. [DOI] [PubMed] [Google Scholar]
- 9.Biessen E.A., Vietsch H., Rump E.T., Fluiter K., Kuiper J., Bijsterbosch M.K., van Berkel T.J. Targeted delivery of oligodeoxynucleotides to parenchymal liver cells in vivo. Biochem. J. 1999;340:783–792. [PMC free article] [PubMed] [Google Scholar]
- 10.Springer A.D., Dowdy S.F. GalNAc-siRNA conjugates: leading the way for delivery of RNAi therapeutics. Nucleic Acid Ther. 2018;28:109–118. doi: 10.1089/nat.2018.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y. Preclinical and clinical advances of GalNAc-decorated nucleic acid therapeutics. Mol. Ther. Nucleic Acids. 2017;6:116–132. doi: 10.1016/j.omtn.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geary R.S., Norris D., Yu R., Bennett C.F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev. 2015;87:46–51. doi: 10.1016/j.addr.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Prakash T.P., Graham M.J., Yu J., Carty R., Low A., Chappell A., Schmidt K., Zhao C., Aghajan M., Murray H.F. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014;42:8796–8807. doi: 10.1093/nar/gku531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crooke S.T., Baker B.F., Xia S., Yu R.Z., Viney N.J., Wang Y., Tsimikas S., Geary R.S. Integrated assessment of the clinical performance of GalNAc3-conjugated 2′-O-methoxyethyl chimeric antisense oligonucleotides: I. Human volunteer experience. Nucleic Acid Ther. 2019;29:16–32. doi: 10.1089/nat.2018.0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe A., Nakajima M., Kasuya T., Onishi R., Kitade N., Mayumi K. Comparative characterization of hepatic distribution and mRNA reduction of antisense oligonucleotides conjugated with triantennary N-acetyl galactosamine and lipophilic ligands targeting apolipoprotein B. J Pharmacol Exp Ther. 2016;2016 doi: 10.1124/jpet.115.230300. [DOI] [PubMed] [Google Scholar]
- 16.Maier M.A., Jayaraman M., Matsuda S., Liu J., Barros S., Querbes W., Tam Y.K., Ansell S.M., Kumar V., Qin J. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 2013;21:1570–1578. doi: 10.1038/mt.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams D., Gonzalez-Duarte A., O’Riordan W.D., Yang C.C., Ueda M., Kristen A.V., Tournev I., Schmidt H.H., Coelho T., Berk J.L. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 18.Nair J.K., Willoughby J.L.S., Chan A., Charisse K., Alam M.R., Wang Q., Hoekstra M., Kandasamy P., Kel’in A.V., Milstein S. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 19.Alnylam Pharmaceuticals. Alnylam development pipeline of investigational RNAi therapeutics. https://www.alnylam.com/alnylam-rnai-pipeline/.
- 20.Allerson C.R., Sioufi N., Jarres R., Prakash T.P., Naik N., Berdeja A., Wanders L., Griffey R.H., Swayze E.E., Bhat B. Fully 2′-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering RNA. J. Med. Chem. 2005;48:901–904. doi: 10.1021/jm049167j. [DOI] [PubMed] [Google Scholar]
- 21.Nair J.K., Attarwala H., Sehgal A., Wang Q., Aluri K., Zhang X., Gao M., Liu J., Indrakanti R., Schofield S. Impact of enhanced metabolic stability on pharmacokinetics and pharmacodynamics of GalNAc-siRNA conjugates. Nucleic Acids Res. 2017;45:10969–10977. doi: 10.1093/nar/gkx818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oligonucleotide Therapeutics Society 2019 OTS Lifetime Achievement Award winner Muthiah (Mano) Manoharan. 2019. https://www.oligotherapeutics.org/muthiah-mano-manoharan-presented-with-2019-ots-lifetime-achievement-award/
- 23.Hassler M.R., Turanov A.A., Alterman J.F., Haraszti R.A., Coles A.H., Osborn M.F., Echeverria D., Nikan M., Salomon W.E., Roux L. Comparison of partially and fully chemically-modified siRNA in conjugate-mediated delivery in vivo. Nucleic Acids Res. 2018;46:2185–2196. doi: 10.1093/nar/gky037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima W.F., Prakash T.P., Murray H.M., Kinberger G.A., Li W., Chappell A.E., Li C.S., Murray S.F., Gaus H., Seth P.P. Single-stranded siRNAs activate RNAi in animals. Cell. 2012;150:883–894. doi: 10.1016/j.cell.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Parmar R., Willoughby J.L.S., Liu J., Foster D.J., Brigham B., Theile C.S., Charisse K., Akinc A., Guidry E., Pei Y. 5′-(E)-vinylphosphonate: a stable phosphate mimic can improve the RNAi activity of siRNA-GalNAc conjugates. ChemBioChem. 2016;17:985–989. doi: 10.1002/cbic.201600130. [DOI] [PubMed] [Google Scholar]
- 26.Prakash T.P., Kinberger G.A., Murray H.M., Chappell A., Riney S., Graham M.J., Lima W.F., Swayze E.E., Seth P.P. Synergistic effect of phosphorothioate, 5′-vinylphosphonate and GalNAc modifications for enhancing activity of synthetic siRNA. Bioorg. Med. Chem. Lett. 2016;26:2817–2820. doi: 10.1016/j.bmcl.2016.04.063. [DOI] [PubMed] [Google Scholar]
- 27.Shen W., Liang X.H., Sun H., Crooke S.T. 2′-Fluoro-modified phosphorothioate oligonucleotide can cause rapid degradation of P54nrb and PSF. Nucleic Acids Res. 2015;43:4569–4578. doi: 10.1093/nar/gkv298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janas M.M., Jiang Y., Schlegel M.K., Waldron S., Kuchimanchi S., Barros S.A. Impact of oligonucleotide structure, chemistry, and delivery method on in vitro cytotoxicity. Nucleic Acid Ther. 2017;27:11–22. doi: 10.1089/nat.2016.0639. [DOI] [PubMed] [Google Scholar]
- 29.Janas M.M., Zlatev I., Liu J., Jiang Y., Barros S.A., Sutherland J.E., Davis W.P., Liu J., Brown C.R., Liu X. Safety evaluation of 2′-deoxy-2′-fluoro nucleotides in GalNAc-siRNA conjugates. Nucleic Acids Res. 2019;47:3306–3320. doi: 10.1093/nar/gkz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster D.J., Brown C.R., Shaikh S., Trapp C., Schlegel M.K., Qian K., Sehgal A., Rajeev K.G., Jadhav V., Manoharan M. Advanced siRNA designs further improve in vivo performance of GalNAc-siRNA conjugates. Mol. Ther. 2018;26:708–717. doi: 10.1016/j.ymthe.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindholm M.W. Development of novel therapies using advanced GalNAc-siRNA technology. 2018. https://www.silence-therapeutics.com/media/1780/opt-congress-2018-development-of-novel-therapies-using-galnac.pdf
- 32.Schlegel M.K., Foster D.J., Kel’in A.V., Zlatev I., Bisbe A., Jayaraman M., Lackey J.G., Rajeev K.G., Charissé K., Harp J. Chirality dependent potency enhancement and structural impact of glycol nucleic acid modification on siRNA. J. Am. Chem. Soc. 2017;139:8537–8546. doi: 10.1021/jacs.7b02694. [DOI] [PubMed] [Google Scholar]
- 33.Janas M.M., Schlegel M.K., Harbison C.E., Yilmaz V.O., Jiang Y., Parmar R., Zlatev I., Castoreno A., Xu H., Shulga-Morskaya S. Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat. Commun. 2018;9:723. doi: 10.1038/s41467-018-02989-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zlatev I., Castoreno A., Brown C.R., Qin J., Waldron S., Schlegel M.K., Degaonkar R., Shulga-Morskaya S., Xu H., Gupta S. Reversal of siRNA-mediated gene silencing in vivo. Nat. Biotechnol. 2018;36:509–511. doi: 10.1038/nbt.4136. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt K., Prakash T.P., Donner A.J., Kinberger G.A., Gaus H.J., Low A., Østergaard M.E., Bell M., Swayze E.E., Seth P.P. Characterizing the effect of GalNAc and phosphorothioate backbone on binding of antisense oligonucleotides to the asialoglycoprotein receptor. Nucleic Acids Res. 2017;45:2294–2306. doi: 10.1093/nar/gkx060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donner A.J., Wancewicz E.V., Murray H.M., Greenlee S., Post N., Bell M., Lima W.F., Swayze E.E., Seth P.P. Co-Administration of an excipient oligonucleotide helps delineate pathways of productive and nonproductive uptake of phosphorothioate antisense oligonucleotides in the liver. Nucleic Acid Ther. 2017;27:209–220. doi: 10.1089/nat.2017.0662. [DOI] [PubMed] [Google Scholar]
- 37.Seth P.P., Tanowitz M., Bennett C.F. Selective tissue targeting of synthetic nucleic acid drugs. J. Clin. Invest. 2019;129:915–925. doi: 10.1172/JCI125228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viney N.J., van Capelleveen J.C., Geary R.S., Xia S., Tami J.A., Yu R.Z., Marcovina S.M., Hughes S.G., Graham M.J., Crooke R.M. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 39.Prakash T.P., Yu J., Kinberger G.A., Low A., Jackson M., Rigo F., Swayze E.E., Seth P.P. Evaluation of the effect of 2′-O-methyl, fluoro hexitol, bicyclo and morpholino nucleic acid modifications on potency of GalNAc conjugated antisense oligonucleotides in mice. Bioorg. Med. Chem. Lett. 2018;28:3774–3779. doi: 10.1016/j.bmcl.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Meier M., Bider M.D., Malashkevich V.N., Spiess M., Burkhard P. Crystal structure of the carbohydrate recognition domain of the H1 subunit of the asialoglycoprotein receptor. J. Mol. Biol. 2000;300:857–865. doi: 10.1006/jmbi.2000.3853. [DOI] [PubMed] [Google Scholar]
- 41.Lee Y.C., Lee R.T. Interactions of oligosaccharides and glycopeptides with hepatic carbohydrate receptors. In: Ernst B., Hart G.W., Sinaÿ P., editors. Carbohydrates in Chemistry and Biology: A Comprehensive Handbook. Wiley; 2008. pp. 549–561. [Google Scholar]
- 42.Khorev O., Stokmaier D., Schwardt O., Cutting B., Ernst B. Trivalent, Gal/GalNAc-containing ligands designed for the asialoglycoprotein receptor. Bioorg. Med. Chem. 2008;16:5216–5231. doi: 10.1016/j.bmc.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Prakash T.P., Yu J., Migawa M.T., Kinberger G.A., Wan W.B., Østergaard M.E., Carty R.L., Vasquez G., Low A., Chappell A. Comprehensive structure-activity relationship of triantennary N-acetylgalactosamine conjugated antisense oligonucleotides for targeted delivery to hepatocytes. J. Med. Chem. 2016;59:2718–2733. doi: 10.1021/acs.jmedchem.5b01948. [DOI] [PubMed] [Google Scholar]
- 44.Matsuda S., Keiser K., Nair J.K., Charisse K., Manoharan R.M., Kretschmer P., Peng C.G., V Kel’in A., Kandasamy P., Willoughby J.L. siRNA conjugates carrying sequentially assembled trivalent N-acetylgalactosamine linked through nucleosides elicit robust gene silencing in vivo in hepatocytes. ACS Chem. Biol. 2015;10:1181–1187. doi: 10.1021/cb501028c. [DOI] [PubMed] [Google Scholar]
- 45.Farzan V.M., Ulashchik E.A., Martynenko-Makaev Y.V., Kvach M.V., Aparin I.O., Brylev V.A., Prikazchikova T.A., Maklakova S.Y., Majouga A.G., Ustinov A.V. Automated solid-phase click synthesis of oligonucleotide conjugates: from small molecules to diverse N-Acetylgalactosamine clusters. Bioconjug. Chem. 2017;28:2599–2607. doi: 10.1021/acs.bioconjchem.7b00462. [DOI] [PubMed] [Google Scholar]
- 46.Østergaard M.E., Yu J., Kinberger G.A., Wan W.B., Migawa M.T., Vasquez G., Schmidt K., Gaus H.J., Murray H.M., Low A. Efficient synthesis and biological evaluation of 5′-GalNAc conjugated antisense oligonucleotides. Bioconjug. Chem. 2015;26:1451–1455. doi: 10.1021/acs.bioconjchem.5b00265. [DOI] [PubMed] [Google Scholar]
- 47.Migawa M.T., Prakash T.P., Vasquez G., Wan W.B., Yu J., Kinberger G.A., Østergaard M.E., Swayze E.E., Seth P.P. A convenient synthesis of 5′-triantennary N-acetyl-galactosamine clusters based on nitromethanetrispropionic acid. Bioorg. Med. Chem. Lett. 2016;26:2194–2197. doi: 10.1016/j.bmcl.2016.03.070. [DOI] [PubMed] [Google Scholar]
- 48.Sharma V.K., Osborn M.F., Hassler M.R., Echeverria D., Ly S., Ulashchik E.A., Martynenko-Makaev Y.V., Shmanai V.V., Zatsepin T.S., Khvorova A., Watts J.K. Novel cluster and monomer-based GalNAc structures induce effective uptake of siRNAs in vitro and in vivo. Bioconjug. Chem. 2018;29:2478–2488. doi: 10.1021/acs.bioconjchem.8b00365. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto T., Sawamura M., Wada F., Harada-Shiba M., Obika S. Serial incorporation of a monovalent GalNAc phosphoramidite unit into hepatocyte-targeting antisense oligonucleotides. Bioorg. Med. Chem. 2016;24:26–32. doi: 10.1016/j.bmc.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 50.Brown B.D., Dudek H.T., Pursell N., Lai C., Wang W., Storr R., Nazef N., Kim B. Methods and compositions for inhibiting expression of LDHA. Patent application publication WO/2019/075419. 2019. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019075419 published April 18, 2019.
- 51.Hauptmann J., Weingärtner A., Samarsky D., Bethge L., Frauendorf C., Gallafent A. Ligand modified double-stranded nucleic acids. Patent application publication 2970801. 2018. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2018185253 published June 23, 2016.
- 52.Kinberger G.A., Prakash T.P., Yu J., Vasquez G., Low A., Chappell A., Schmidt K., Murray H.M., Gaus H., Swayze E.E., Seth P.P. Conjugation of mono and di-GalNAc sugars enhances the potency of antisense oligonucleotides via ASGR mediated delivery to hepatocytes. Bioorg. Med. Chem. Lett. 2016;26:3690–3693. doi: 10.1016/j.bmcl.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 53.Javanbakht H., Mueller H., Walther J., Zhou X., Lopez A., Pattupara T., Blaising J., Pedersen L., Albæk N., Jackerott M. Liver-targeted anti-HBV single-stranded oligonucleotides with locked nucleic acid potently reduce HBV gene expression in vivo. Mol. Ther. Nucleic Acids. 2018;11:441–454. doi: 10.1016/j.omtn.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pursell N., Gierut J., Zhou W., Dills M., Diwanji R., Gjorgjieva M., Saxena U., Yang J.S., Shah A., Venkat N. Inhibition of glycogen synthase II with RNAi prevents liver injury in mouse models of glycogen storage diseases. Mol. Ther. 2018;26:1771–1782. doi: 10.1016/j.ymthe.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Altamura S., Altamura S., Muckenthaler M.U., Dames S., Frauendorf C., Schubert S. SLN124, a Galnac-siRNA conjugate targeting TMPRSS6, for the treatment of iron overload and ineffective erythropoiesis such as in beta-thalassemia. Blood. 2018;132(Suppl 1):2340. [Google Scholar]
- 56.Altamura S., Schaeper U., Dames S., Löffler K., Eisermann M., Frauendorf C., Müdder K., Neves J., Muckenthaler M.U. SLN124, a GalNAc-siRNA conjugate targeting TMPRSS6, efficiently prevents iron overload in hereditary haemochromatosis type 1. HemaSphere. 2019;3:e301. doi: 10.1097/HS9.0000000000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffmann T. Case study: identification and development of SLN124, a conjugated GalNAc-siRNA therapeutic for the treatment of iron overload disorders. 2018. https://www.silence-therapeutics.com/media/1781/opt-congress-2018-identification-and-development-of-sln124.pdf
- 58.Marjot T., Moolla A., Cobbold J.F., Hodson L., Tomlinson J.W. Nonalcoholic fatty liver disease in adults: current concepts in etiology, outcomes, and management. Endocr. Rev. 2020;41:66–117. doi: 10.1210/endrev/bnz009. [DOI] [PubMed] [Google Scholar]
- 59.Wang X., Sommerfeld M.R., Jahn-Hofmann K., Cai B., Filliol A., Remotti H.E., Schwabe R.F., Kannt A., Tabas I. A therapeutic silencing RNA targeting hepatocyte TAZ prevents and reverses fibrosis in nonalcoholic steatohepatitis in mice. Hepatol. Commun. 2019;3:1221–1234. doi: 10.1002/hep4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cansby E., Nuñez-Durán E., Magnusson E., Amrutkar M., Booten S.L., Kulkarni N.M., Svensson L.T., Borén J., Marschall H.U., Aghajan M., Mahlapuu M. Targeted delivery of Stk25 antisense oligonucleotides to hepatocytes protects mice against nonalcoholic fatty liver disease. Cell. Mol. Gastroenterol. Hepatol. 2019;7:597–618. doi: 10.1016/j.jcmgh.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindén D., Ahnmark A., Pingitore P., Ciociola E., Ahlstedt I., Andréasson A.C., Sasidharan K., Madeyski-Bengtson K., Zurek M., Mancina R.M. Pnpla3 silencing with antisense oligonucleotides ameliorates nonalcoholic steatohepatitis and fibrosis in Pnpla3 I148M knock-in mice. Mol. Metab. 2019;22:49–61. doi: 10.1016/j.molmet.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willoughby J.L.S., Chan A., Sehgal A., Butler J.S., Nair J.K., Racie T., Shulga-Morskaya S., Nguyen T., Qian K., Yucius K. Evaluation of GalNAc-siRNA conjugate activity in pre-clinical animal models with reduced asialoglycoprotein receptor expression. Mol. Ther. 2018;26:105–114. doi: 10.1016/j.ymthe.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim Y., Jo M., Schmidt J., Luo X., Prakash T.P., Zhou T., Klein S., Xiao X., Post N., Yin Z., MacLeod A.R. Enhanced potency of GalNAc-conjugated antisense oligonucleotides in hepatocellular cancer models. Mol. Ther. 2019;27:1547–1557. doi: 10.1016/j.ymthe.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanowitz M., Hettrick L., Revenko A., Kinberger G.A., Prakash T.P., Seth P.P. Asialoglycoprotein receptor 1 mediates productive uptake of N-acetylgalactosamine-conjugated and unconjugated phosphorothioate antisense oligonucleotides into liver hepatocytes. Nucleic Acids Res. 2017;45:12388–12400. doi: 10.1093/nar/gkx960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banda N.K., Desai D., Scheinman R.I., Pihl R., Sekine H., Fujita T., Sharma V., Hansen A.G., Garred P., Thiel S. Targeting of liver mannan-binding lectin-associated serine protease-3 with RNA interference ameliorates disease in a mouse model of rheumatoid arthritis. Immunohorizons. 2018;2:274–295. doi: 10.4049/immunohorizons.1800053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holers V.M., Borodovsky A., Scheinman R.I., Ho N., Ramirez J.R., Dobó J., Gál P., Lindenberger J., Hansen A.G., Desai D. Key components of the complement lectin pathway are not only required for the development of inflammatory arthritis but also regulate the transcription of factor D. Front. Immunol. 2020;11:201. doi: 10.3389/fimmu.2020.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kusner L.L., Yucius K., Sengupta M., Sprague A.G., Desai D., Nguyen T., Charisse K., Kuchimanchi S., Kallanthottathil R., Fitzgerald K. Investigational RNAi therapeutic targeting C5 is efficacious in pre-clinical models of myasthenia gravis. Mol. Ther. Methods Clin. Dev. 2019;13:484–492. doi: 10.1016/j.omtm.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J., Qin J., Borodovsky A., Racie T., Castoreno A., Schlegel M., Maier M.A., Zimmerman T., Fitzgerald K., Butler J., Akinc A. An investigational RNAi therapeutic targeting factor XII (ALN-F12) for the treatment of hereditary angioedema. RNA. 2019;25:255–263. doi: 10.1261/rna.068916.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin W., Rogge M. Targeting RNA: a transformative therapeutic strategy. Clin. Transl. Sci. 2019;12:98–112. doi: 10.1111/cts.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y., Yu R.Z., Henry S., Geary R.S. Pharmacokinetics and clinical pharmacology considerations of GalNAc3-conjugated antisense oligonucleotides. Expert Opin. Drug Metab. Toxicol. 2019;15:475–485. doi: 10.1080/17425255.2019.1621838. [DOI] [PubMed] [Google Scholar]
- 71.Yu R.Z., Graham M.J., Post N., Riney S., Zanardi T., Hall S., Burkey J., Shemesh C.S., Prakash T.P., Seth P.P. Disposition and pharmacology of a GalNAc3-conjugated ASO targeting human lipoprotein(a) in Mice. Mol. Ther. Nucleic Acids. 2016;5:e317. doi: 10.1038/mtna.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agarwal S., Simon A.R., Goel V., Habtemariam B.A., Clausen V.A., Kim J.B., Robbie G.J. Pharmacokinetics and pharmacodynamics of the small interfering ribonucleic acid, givosiran, in patients with acute hepatic porphyria. Clin. Pharmacol. Ther. 2020;108:63–72. doi: 10.1002/cpt.1802. [DOI] [PubMed] [Google Scholar]
- 73.Yu R.Z., Gunawan R., Post N., Zanardi T., Hall S., Burkey J., Kim T.W., Graham M.J., Prakash T.P., Seth P.P. Disposition and pharmacokinetics of a GalNAc3-conjugated antisense oligonucleotide targeting human lipoprotein(a) in monkeys. Nucleic Acid Ther. 2016;26:372–380. doi: 10.1089/nat.2016.0623. [DOI] [PubMed] [Google Scholar]
- 74.Bon C., Hofer T., Bousquet-Mélou A., Davies M.R., Krippendorff B.F. Capacity limits of asialoglycoprotein receptor-mediated liver targeting. MAbs. 2017;9:1360–1369. doi: 10.1080/19420862.2017.1373924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller C.M., Donner A.J., Blank E.E., Egger A.W., Kellar B.M., Østergaard M.E., Seth P.P., Harris E.N. Stabilin-1 and stabilin-2 are specific receptors for the cellular internalization of phosphorothioate-modified antisense oligonucleotides (ASOs) in the liver. Nucleic Acids Res. 2016;44:2782–2794. doi: 10.1093/nar/gkw112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zimmermann T.S., Karsten V., Chan A., Chiesa J., Boyce M., Bettencourt B.R., Hutabarat R., Nochur S., Vaishnaw A., Gollob J. Clinical Proof of Concept for a Novel Hepatocyte-Targeting GalNAc-siRNA Conjugate. Mol Ther. 2017;25:71–78. doi: 10.1016/j.ymthe.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cichocki J. Nonclinical safety evaluation of GalNAc-siRNA conjugates. 2018. https://www.alnylam.com/wp-content/uploads/2018/05/ABS_Capella-Slides_FINAL05092018.pdf
- 78.Janas M.M., Harbison C.E., Perry V.K., Carito B., Sutherland J.E., Vaishnaw A.K., Keirstead N.D., Warner G. The nonclinical safety profile of GalNAc-conjugated RNAi therapeutics in subacute studies. Toxicol. Pathol. 2018;46:735–745. doi: 10.1177/0192623318792537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sutherland J.E., Hettinger J.L., Chan A., Gilbert J., Warner G.L., Davis W.P. Nonclinical safety profile of revusiran, a 1st-generation GalNAc-siRNA conjugate for treatment of hereditary transthyretin-mediated amyloidosis. Nucleic Acid Ther. 2020;30:33–49. doi: 10.1089/nat.2019.0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Judge D.P., Kristen A.V., Grogan M., Maurer M.S., Falk R.H., Hanna M., Gillmore J., Garg P., Vaishnaw A.K., Harrop J. Phase 3 multicenter study of revusiran in patients with hereditary transthyretin-mediated (hATTR) amyloidosis with cardiomyopathy (ENDEAVOUR) Cardiovasc. Drugs Ther. 2020;34:357–370. doi: 10.1007/s10557-019-06919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amgen Randomized study to evaluate efficacy, safety, and tolerability of AMG 890 in subjects with elevated lipoprotein(a) 2020. https://clinicaltrials.gov/ct2/show/NCT04270760
- 82.Arrowhead Pharmaceuticals Platforms that accelerate drug discovery. 2020. https://arrowheadpharma.com/science/
- 83.Gane E., Locarnini S.A., Lim T.H., Strasser S., Sievert W., Cheng W., Thompson A., Given B., Schluep T., Hamilton J. First results with RNA interference (RNAi) in chronic hepatitis B (CHB) using ARO-HBV. 2018. http://www.natap.org/2018/AASLD/AASLD_155.htm
- 84.Martinez M.G., Villeret F., Testoni B., Zoulim F. Can we cure hepatitis B virus with novel direct-acting antivirals? Liver Int. 2020;40:27–34. doi: 10.1111/liv.14364. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 85.Oligonucleotide Therapeutics Society. To (Hep)B or not to (Hep)B? Hepatitis B cure in reach for oligonucleotide therapeutics. https://www.oligotherapeutics.org/to-hepb-or-not-to-hepb/.
- 86.Scott L.J. Givosiran: first approval. Drugs. 2020;80:335–339. doi: 10.1007/s40265-020-01269-0. [DOI] [PubMed] [Google Scholar]
- 87.Balwani M., Gouya L., Rees D., Stein P., Stölzel U., Aguilera P., Bissell D.M., Bonkovsky H., Keel S., Parker C. ENVISION, a phase 3 study to evaluate efficacy and safety of givosiran, an investigational RNAi therapeutic targeting aminolevulinic acid synthase 1, in acute hepatic porphyria patients. 2019. https://www.journal-of-hepatology.eu/article/S0618-8278(19)30142-2/fulltext
- 88.Weigert A., Martin-Higueras C., Hoppe B. Novel therapeutic approaches in primary hyperoxaluria. Expert Opin. Emerg. Drugs. 2018;23:349–357. doi: 10.1080/14728214.2018.1552940. [DOI] [PubMed] [Google Scholar]
- 89.Alnylam Pharmaceuticals Alnylam reports positive topline results from ILLUMINATE-A phase 3 Study of lumasiran for the treatment of primary hyperoxaluria type 1. 2019. https://investors.alnylam.com/press-release?id=24376
- 90.Alnylam Pharmaceuticals Alnylam initiates rolling submission of new drug application (NDA) to U.S. Food and Drug Administration (FDA) 2020. https://investors.alnylam.com/press-release?id=24431
- 91.Novartis Novartis successfully completes acquisition of The Medicines Company, adding a potentially first-in-class, investigational cholesterol-lowering therapy inclisiran. 2020. https://www.novartis.com/news/media-releases/novartis-successfully-completes-acquisition-medicines-company-adding-potentially-first-class-investigational-cholesterol-lowering-therapy-inclisiran
- 92.Kosmas C.E., Muñoz Estrella A., Sourlas A., Silverio D., Hilario E., Montan P.D., Guzman E. Inclisiran: a new promising agent in the management of hypercholesterolemia. Diseases. 2018;6:63. doi: 10.3390/diseases6030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ray K.K., Wright R.S., Kallend D., Koenig W., Leiter L.A., Raal F.J., Bisch J.A., Richardson T., Jaros M., Wijngaard P.L.J., Kastelein J.J.P., ORION-10 and ORION-11 Investigators Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N. Engl. J. Med. 2020;382:1507–1519. doi: 10.1056/NEJMoa1912387. [DOI] [PubMed] [Google Scholar]
- 94.Raal F.J., Kallend D., Ray K.K., Turner T., Koenig W., Wright R.S., Wijngaard P.L.J., Curcio D., Jaros M.J., Leiter L.A., Kastelein J.J.P., ORION-9 Investigators Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N. Engl. J. Med. 2020;382:1520–1530. doi: 10.1056/NEJMoa1913805. [DOI] [PubMed] [Google Scholar]
- 95.Tsimikas S., Karwatowska-Prokopczuk E., Gouni-Berthold I., Tardif J.C., Baum S.J., Steinhagen-Thiessen E., Shapiro M.D., Stroes E.S., Moriarty P.M., Nordestgaard B.G., AKCEA-APO(a)-LRx Study Investigators Lipoprotein(a) reduction in persons with cardiovascular disease. N. Engl. J. Med. 2020;382:244–255. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 96.Novartis Novartis to pursue transformational therapy to reduce risk of cardiovascular disease in people living with elevated levels of inherited lipoprotein(a) 2020. https://www.novartis.com/news/media-releases/novartis-pursue-transformational-therapy-reduce-risk-cardiovascular-disease-people-living-elevated-levels-inherited-lipoproteina
- 97.Regulus Therapeutics RG-125(AZD4076), a microRNA Therapeutic Targeting microRNA-103/107 Being Developed for the Treatment of NASH in Patients with Type 2 Diabetes/Pre-Diabetes, Enters Phase I Clinical Development. 2020. http://ir.regulusrx.com/news-releases/news-release-details/rg-125azd4076-microrna-therapeutic-targeting-microrna-103107
- 98.Regulus Therapeutics Regulus platform technology. 2020. http://regulusrx.com/platform/platform-technology/
- 99.Voutila J., Reebye V., Roberts T.C., Protopapa P., Andrikakou P., Blakey D.C., Habib R., Huber H., Saetrom P., Rossi J.J., Habib N.A. Development and mechanism of small activating RNA targeting CEBPA, a novel therapeutic in clinical trials for liver cancer. Mol. Ther. 2017;25:2705–2714. doi: 10.1016/j.ymthe.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Portnoy V., Lin S.H.S., Li K.H., Burlingame A., Hu Z.H.H., Li H., Li L.C. saRNA-guided Ago2 targets the RITA complex to promoters to stimulate transcription. Cell Res. 2016;26:320–335. doi: 10.1038/cr.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Voutila J., Purdie K., Debacker A., Reebye V., Huang K.-W., Catley M., Blakey D., Habib R., Saetrom P., Rossi J., Habib N. Development of GalNAc-conjugated saRNA targeting HNF4A for treatment of metabolic disease. 2019. https://minatx.com/wp-content/uploads/2019/10/191008-OTS-2019-poster-HNF4A-final.pdf
- 102.Huang K.W., Reebye V., Czysz K., Ciriello S., Dorman S., Reccia I., Lai H.S., Peng L., Kostomitsopoulos N., Nicholls J. Liver activation of hepatocellular nuclear factor-4α by small activating RNA rescues dyslipidemia and improves metabolic profile. Mol. Ther. Nucleic Acids. 2020;19:361–370. doi: 10.1016/j.omtn.2019.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nishikawa T., Bell A., Brooks J.M., Setoyama K., Melis M., Han B., Fukumitsu K., Handa K., Tian J., Kaestner K.H. Resetting the transcription factor network reverses terminal chronic hepatic failure. J. Clin. Invest. 2015;125:1533–1544. doi: 10.1172/JCI73137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jadhav V. Advances in RNAi therapeutics platform. 2019. https://www.alnylam.com/wp-content/uploads/2019/06/2019.06.22_Platform-Advances_FINAL.pdf
- 105.Crooke, S.T., Monia, B.P., Bennett, C.F., and Swayze, E.E. The creation of RNA targeting technology. https://ir.ionispharma.com/news-releases/news-release-details/ionis-pharmaceuticals-hold-technology-webcast.
- 106.Ämmälä C., Drury W.J., 3rd, Knerr L., Ahlstedt I., Stillemark-Billton P., Wennberg-Huldt C., Andersson E.-M., Valeur E., Jansson-Löfmark R., Janzén D. Targeted delivery of antisense oligonucleotides to pancreatic β-cells. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aat3386. eaat3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sugo T., Terada M., Oikawa T., Miyata K., Nishimura S., Kenjo E., Ogasawara-Shimizu M., Makita Y., Imaichi S., Murata S. Development of antibody-siRNA conjugate targeted to cardiac and skeletal muscles. J. Control. Release. 2016;237:1–13. doi: 10.1016/j.jconrel.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 108.Gilleron J., Querbes W., Zeigerer A., Borodovsky A., Marsico G., Schubert U., Manygoats K., Seifert S., Andree C., Stöter M. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013;31:638–646. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- 109.Juliano R.L. Intracellular trafficking and endosomal release of oligonucleotides: what we know and what we don’t. Nucleic Acid Ther. 2018;28:166–177. doi: 10.1089/nat.2018.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dowdy S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017;35:222–229. doi: 10.1038/nbt.3802. [DOI] [PubMed] [Google Scholar]