Abstract
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a progressive and potentially life-threatening disease characterized by complement-mediated thrombotic microangiopathy. Patients with aHUS may experience fatigue, which can negatively impact their lives, but there is a knowledge gap regarding disease burden in these patients.
Methods
In this longitudinal study, patients with aHUS from the Global aHUS Registry who completed patient-reported outcome assessments (Functional Assessment of Chronic Illness Therapy-Fatigue scale [FACIT-Fatigue], general health status, and work status) at ≥2 time points were assessed relative to treatment status: (i) never treated with eculizumab; (ii) on eculizumab at registry enrollment and continued therapy; and (iii) started eculizumab after registry enrollment.
Results
Patients who started eculizumab after the baseline visit (n = 23) exhibited improvements in fatigue (nearly 75% achieved clinically meaningful improvement), improved general health status (55%), and 25% to 30% rate reduction in symptoms of fatigue, weakness, irritability, nausea/vomiting, and swelling at last follow-up. Among patients already on eculizumab at registry enrollment (n = 295) and those never treated (n = 233), these parameters changed minimally relative to the baseline. Emergency room visits and hospital admissions were similar between groups. The number of health care provider visits and work days missed were higher in patients who started eculizumab after registry enrollment.
Conclusion
These real-world findings confirm the detrimental effects of aHUS on patients’ daily lives, including high levels of fatigue and impairments in general health status. The results suggest clinically meaningful improvement in fatigue, other patient-reported outcomes, and symptoms with eculizumab initiation after enrollment into the aHUS registry.
Keywords: aHUS, atypical hemolytic uremic syndrome, complement, FACIT-Fatigue, fatigue, patient-reported outcomes
Graphical abstract
See Commentary on Page 1123
Atypical hemolytic uremic syndrome (aHUS) is secondary to uncontrolled complement activation, and can cause severe progressive organ damage or death.1,2 aHUS affects both children and adults, and is characterized by microangiopathic hemolytic anemia, thrombocytopenia, and organ dysfunction, most commonly affecting the kidney.1,3 Management of aHUS may include plasma exchange/plasma infusion, inhibition of the alternative pathway of complement with eculizumab or ravulizumab, supportive care (e.g., dialysis and treatment of hypertension), and kidney transplantation in patients who progress to end-stage renal disease.
Since the introduction of eculizumab, a humanized, monoclonal antibody that blocks terminal complement activation at C5, a paradigm shift has occurred in the management of patients with aHUS, together with a corresponding change in the clinical course of the disease.4
Patient perspectives on diseases and their associated treatments have been a major focus of the US Food and Drug Administration, with an emphasis on the need for patient-reported outcomes and generation of real-world evidence and data outside of clinical trials.5 Recent reports of real-world data have revealed that the overall efficacy and safety of eculizumab remains consistent with findings reported in prior clinical trials6, 7, 8; however, there is a lack of understanding regarding the impact of aHUS on patients’ daily lives, as well as a paucity of real-world, patient-reported outcome data in patients with aHUS outside of the clinical trial setting.
A global aHUS patients’ research agenda using input from patients, patient advocacy groups, and caregivers was developed.9 The research agenda identified several knowledge gaps, including the impact of aHUS-related treatments on clinical, psychological, and socioeconomic patient-reported outcomes.
Previous cross-sectional analyses have illustrated the detrimental effects of aHUS on fatigue and associated functional impairment.10,11 The objective of the present longitudinal study was to evaluate change in fatigue levels over time and other real-world, patient-reported outcomes to better describe the impact of this disease, such as general health and work status, among patients enrolled in the Global aHUS Registry. A plain language summary of this study is provided in Supplementary Figure S1.
Methods
Patient Selection
The Global aHUS Registry is an ongoing, observational, noninterventional, multicenter, multinational study (NCT01522183) designed to retrospectively and prospectively collect information on the long-term outcomes and health status of patients with aHUS, as well as the long-term safety and effectiveness of eculizumab in this population.12 Methodology for registry enrollment has been reported previously.12 Briefly, patients with a preexisting diagnosis of aHUS were included, and those with evidence of Shiga toxin-producing Escherichia coli infection and those with ADAMTS13 activity ≤5%, if performed, were excluded. Written informed consent was obtained from all enrolled patients or their parents/guardians; assent was obtained when appropriate.
This analysis included all pediatric (≥5 to <18 years) and adult (≥18 years) patients with aHUS enrolled in the Global aHUS Registry who completed the patient-reported outcomes assessments (Functional Assessment of Chronic Illness Therapy-Fatigue scale [FACIT-Fatigue], general health status, and work status forms) at a minimum of 2 time points. The data cutoff for this analysis was July 8, 2019. The population selected for this analysis was divided into 3 mutually exclusive groups of patients who were (i) never treated with eculizumab; (ii) on eculizumab at registry enrollment and continued therapy; and (iii) started eculizumab after registry enrollment. All comparisons were made between 2 data points for each patient (baseline and last follow-up). Baseline was defined as the date of enrollment for patients who were not treated with eculizumab and those who were on eculizumab at the time of enrollment; for the group who started eculizumab after registry enrollment, baseline was defined as the closest date before the start of eculizumab.
Patient-Reported Outcomes Assessments
Fatigue was assessed using the FACIT-Fatigue scale, a validated tool for measuring health-related quality of life.11 The FACIT-Fatigue scale for adults is a 13-item, self-completed questionnaire assessing fatigue and its impact on daily activities and function, expressing fatigue as a score ranging from 0 to 52.11 The PedsFACIT-Fatigue is an 11-item questionnaire that expresses fatigue as a score ranging from 0 to 44.13 Low FACIT-Fatigue and PedsFACIT-Fatigue scores are associated with greater functional impairment,13, 14, 15, 16 whereas higher scores indicate better quality of life.17 The FACIT-Fatigue scale for adults has been validated extensively in the literature for reliability and clinical validity in measuring health-related quality of life.18 Although the PedsFACIT-Fatigue scale shares some aspects of the FACIT-Fatigue scale, it was developed for the pediatric population to measure fatigue in cancer and customized based on children’s literacy levels and perceptions of fatigue.13
A systematic review of minimum clinically important differences derived from patients with cancer, systemic lupus erythematosus, and rheumatoid arthritis found that minimum clinically important differences for FACIT-Fatigue score improvement varied from 2.8 to 6.8.19 Other studies have reported minimum clinically important differences of 3.0 for the FACIT-Fatigue score (patients with rheumatoid arthritis)20 and 4.7 for the PedsFACIT-Fatigue score (pediatric patients with cancer).13 Here, we defined the minimum clinically important difference indicating improvement as an increase in FACIT-Fatigue and PedsFACIT-Fatigue score of >3 units from baseline. FACIT-Fatigue scores were calculated for subgroups based on clinical status (dialysis, transplant, recent plasma exchange/plasma infusion, and recent hospitalization).
We measured change over time in general health status (poor, fair, good, very good, excellent), work status, patient symptoms (anxiety, chest pain, confusion, diarrhea, easy bruising/abdominal bleeding, headache, irritability, nausea/vomiting, shortness of breath, swelling, weakness, jaundice, other), and resource utilization, as captured in the registry patient questionnaire. Resource utilization was calculated as the total number of events (health care provider [HCP] visits, emergency room visits, hospital admissions, and missed days of work due to illness) between baseline and last follow-up divided by the time of exposure during this period (i.e., number of completed forms multiplied by 0.5 years, as forms are collected every 6 months). Patients ≥65 years old were excluded from analyses of resource utilization.
Statistical Analysis
The findings reported in this study are reported as descriptive statistics: median change over time between the first (i.e., baseline) and last data points (including the range), and proportion of patients with clinically meaningful changes in FACIT-Fatigue score and other patient-reported outcomes. Missing dates were imputed where necessary. We performed statistical comparisons for change from baseline to last visit in each of the 3 patient groups; between-group comparisons were not performed because the main interest was to assess changes in patient-reported outcomes in patients who initiated eculizumab; the other 2 groups served as reference groups and we did not prespecify any hypothesis testing for between-group comparisons. For the 2 cohorts never treated with eculizumab and on eculizumab at the time of enrollment, baseline was defined as the first score after enrollment and was compared with that at last follow-up. If the patient discontinued eculizumab, the first score after enrollment was compared with that at the last evaluation point while on eculizumab. For the group that started eculizumab after enrollment, baseline was defined as the before eculizumab score closest to start of eculizumab and was compared with the last score while on eculizumab. Predefined subgroup analyses included by dialysis (yes/no), kidney transplant (yes/no), and recent (within 6 months) prior hospitalization (yes/no).
Results
Baseline Patient Demographics and Disease Characteristics
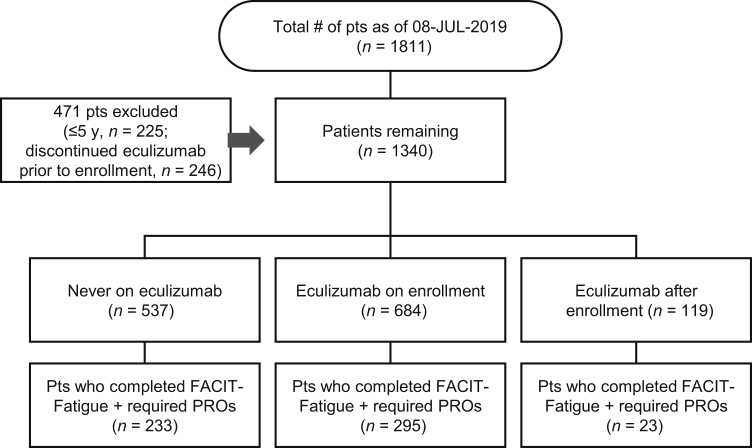
A total of 551 patients with aHUS were included in this analysis (never treated with eculizumab: n = 233; on eculizumab at enrollment: n = 295; and started eculizumab after enrollment: n = 23) (Figure 1). Overall, the registry included 1811 patients as of July 8, 2019, but 471 were excluded, and of the 1340 who remained, 551 completed the FACIT-Fatigue questionnaire and patient-reported outcomes assessments at a minimum of 2 timepoints.
Figure 1.
Total study population. FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; PROs, patient-reported outcomes; pts, patients; y, years old; #, number.
Patient demographics, disease characteristics, and genetics for the overall population and by cohort are shown in Table 1. Median patient age was 28.6 years at aHUS diagnosis and 32.5 years at registry enrollment. More than half (59.7%) of the patients were female. Among patients treated with eculizumab, median time from aHUS diagnosis to eculizumab initiation was 0.06 years. The proportion of patients on acute versus chronic dialysis was 2.0% and 16.2%, respectively. At baseline, the proportion of patients with renal, cardiovascular, gastrointestinal, neurologic, and pulmonary signs and symptoms was similar between patients already on eculizumab at the time of registry enrollment and those who started eculizumab after enrollment, and was relatively higher in these groups than in those never treated with eculizumab.
Table 1.
Baseline demographics and disease characteristics
| Characteristic | All (N = 551) | Never on eculizumab (n = 233) | Eculizumab on enrollment (n = 295) | On eculizumab after enrollment (n = 23) |
|---|---|---|---|---|
| Median age at enrollment, yr (range) | 32.5 (5.0–82.6) | 32.1 (5.0–82.6) | 32.5 (5.1–80.8) | 36.2 (11.4–68.6) |
| Pediatric, n (%) | 128 (23.2) | 58 (24.9) | 67 (22.7) | 3 (13.0) |
| Adult, n (%) | 423 (76.8) | 175 (75.1) | 228 (77.3) | 20 (87.0) |
| Age at aHUS diagnosis, yr (range) | 28.7 (0.1–82.6) | 25.7 (0.2–82.6)a | 29.7 (0.1–80.6)b | 32.9 (0.1–68.6) |
| Gender | ||||
| Female, n (%) | 329 (59.7) | 138 (59.2) | 179 (60.7) | 12 (52.2) |
| Male, n (%) | 222 (40.3) | 95 (40.8) | 116 (39.3) | 11 (47.8) |
| Time from aHUS diagnosis to initiation of eculizumab, mo (range) | 0.72 (−1.2 to 357.6) | N/A | 0.6 (1.2–357.6)c | 0.84 (0.0–266.4) |
| Dialysis, n (%) | ||||
| Not on dialysis | 437 (79.3) | 178 (76.4) | 243 (82.4) | 16 (69.6) |
| Acute (≤3 mo) | 11 (2.0) | 2 (0.9) | 8 (2.7) | 1 (4.3) |
| Chronic (>3 mo) | 89 (16.2) | 48 (20.6) | 35 (11.9) | 6 (26.1) |
| Missing | 14 (2.5) | 5 (2.1) | 9 (3.1) | 0 |
| Had kidney transplant before or at baseline, n (%) | 157 (28.5) | 67 (28.8) | 85 (28.8) | 5 (21.7) |
| Renal functiond (eGFR, ml/min per 1.73 m2), n (%) | ||||
| ≥90 | 57 (12.6) | 35 (19.1)e | 21 (8.3)f | 1 (6.3)g |
| <90 to ≥60 | 57 (12.6) | 21 (11.5) | 35 (13.9) | 1 (6.3) |
| <60 to ≥30 | 76 (16.9) | 24 (13.1) | 50 (19.8) | 2 (12.5) |
| <30 to ≥15 | 26 (5.8) | 6 (3.3) | 15 (6.0) | 5 (31.3) |
| <15 | 22 (4.9) | 6 (3.3) | 14 (5.6) | 2 (12.5) |
| Missing | 213 (47.2) | 91 (49.7) | 117 (46.4) | 5 (31.3) |
| Extrarenal manifestation related to aHUS within the past 6 mo, n (%) | ||||
| Cardiovascular | 174 (31.6) | 42 (18.0) | 124 (42.0) | 8 (34.8) |
| Gastrointestinal | 206 (37.4) | 55 (23.6) | 141 (47.8) | 10 (43.5) |
| Neurologic | 165 (29.9) | 44 (18.9) | 115 (39.0) | 6 (26.1) |
| Pulmonary | 77 (14.0) | 18 (7.7) | 55 (18.6) | 4 (17.4) |
| Hospitalization in the past 6 mo, n (%) | ||||
| Yes | 197 (35.8) | 66 (28.3) | 113 (38.3) | 18 (78.3) |
| PE/PI in the past 6 mo, n (%) | ||||
| Yes | 123 (22.3) | 27 (11.6) | 86 (29.2) | 10 (43.5) |
| Genetics, n (%) | ||||
| Tested for ≥5 pathogenic variants with no pathogenic variant identified | 153 (27.8) | 69 (29.6) | 75 (25.4) | 9 (39.1) |
| Tested for <5 pathogenic variants | 40 (7.3) | 21 (9.0) | 17 (5.8) | 2 (8.7) |
| Any pathogenic variant found | 203 (36.8) | 73 (31.3) | 122 (41.4) | 8 (34.8) |
| Anti-CFH‒antibody positive | 78 (14.2) | 29 (12.4) | 45 (15.3) | 4 (17.4) |
| Anti-CFH‒antibody negative | 279 (50.6) | 118 (50.6) | 149 (50.5) | 12 (52.2) |
| Any pathogenic variant found or anti-CFH‒antibody positive | 245 (44.5) | 92 (39.5) | 142 (48.1) | 11 (47.8) |
aHUS, atypical hemolytic uremic syndrome; anti-CFH, anti-complement factor H; eGFR, estimated glomerular filtration rate; N/A, not applicable; PE, plasma exchange; PI, plasma infusion.
Baseline is defined as the date of enrollment for never on eculizumab and eculizumab on enrollment groups, and the closest date before eculizumab start for the third group (on eculizumab after enrollment).
n = 226.
n = 293.
n = 288.
Patients on dialysis at baseline excluded.
n = 183.
n = 252.
n = 16.
Changes in Patient-Reported Outcomes
The median duration of follow-up time was 29.1 months in patients never treated with eculizumab, 23.0 months in those already treated with eculizumab at the time of registry enrollment, and 17.5 months in patients who started eculizumab after registry enrollment. Among patients who were already treated with eculizumab at the time of registry enrollment, the median (range) duration of eculizumab treatment before enrollment was 5.85 (0.03–92.68) months.
FACIT-Fatigue
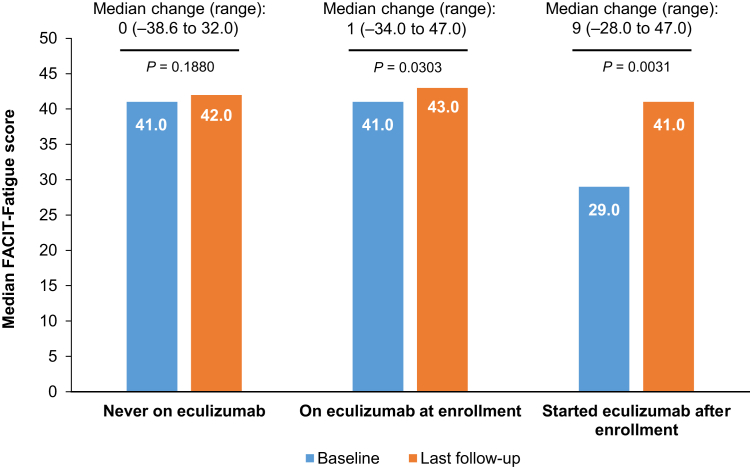
The greatest increase in median FACIT-Fatigue score was observed in patients who started eculizumab after registry enrollment (change of +9.0 from baseline [P = 0.0031] vs. 0.0 [P = 0.1880] and +1.0 [P = 0.0303] in patients who never received eculizumab and those on eculizumab at the time of enrollment, respectively; Figure 2). The proportion of patients who reported clinically meaningful improvements in fatigue (i.e., increase of >3 in FACIT-Fatigue score) was also greatest in patients who started eculizumab after registry enrollment (73.9% vs. 32.6% and 37.3% in never treated and those on eculizumab at the time of registry enrollment, respectively). Among those who started eculizumab after enrollment, all pediatric patients (n = 3, ≥5 to <18 years) and 70% (n = 14) of adult patients reported a clinically meaningful improvement in fatigue, with median changes in FACIT-Fatigue scores of 12.3 and 8.0, respectively.
Figure 2.
Median Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) score at baseline and follow-up, all groups.
FACIT-Fatigue Scores by Subgroup (Dialysis Status, Kidney Transplant Status, and Recent Hospitalization)
Changes in FACIT-Fatigue scores by subgroup in the overall population are summarized in Table 2. Overall, median FACIT-Fatigue scores were numerically lower among patients on dialysis compared with those who were not on dialysis; within each of these subgroups, there was no substantial change in FACIT-Fatigue score from baseline to last follow-up. At last follow-up, the median FACIT-Fatigue score was similar in the overall population regardless of kidney transplant history. The median FACIT-Fatigue score in patients with a recent hospitalization was numerically lower than in those without a recent hospitalization. In each subgroup, at least 25% of patients reported a clinically meaningful increase in FACIT-Fatigue score between baseline and last follow-up.
Table 2.
Subgroup analyses of FACIT-Fatigue scores in all patients
| Proportion with clinically meaningful change,an (%) |
||||||
|---|---|---|---|---|---|---|
| Variable Median (range), unless otherwise stated |
Baselineb | Last follow-upc | Change (LFU ‒ BL) | Improvement | No change | Worsening |
| Overall on dialysis (n = 67) | 35.0 (5.0–51.0) | 36.0 (1.0–51.0) | 0.0 (−33.0 to 36.0) | 29 (43.3) | 18 (26.9) | 20 (29.9) |
| Overall not on dialysis (n = 423) | 42.0 (0.0–52.0) | 44.0 (3.0–52.0) | 0.0 (−38.6 to 47.0) | 146 (34.5) | 167 (39.5) | 110 (26.0) |
| Overall kidney transplant (n = 157) | 44.0 (5.0–52.0) | 42.0 (3.0–52.0) | −1.0 (−38.6 to 36.0) | 42 (26.8) | 66 (42.0) | 49 (31.2) |
| Overall no kidney transplant (n = 362) | 39.0 (0.0–52.0) | 43.0 (1.0–52.0) | 1.25 (−31.0 to 47.0) | 148 (40.9) | 127 (35.1) | 87 (24.0) |
| Overall with recent hospitalization (n = 197) | 33.0 (0.0–52.0) | 39.0 (1.0–52.0) | 3.0 (−38.6 to 47.0) | 93 (47.2) | 59 (29.9) | 45 (22.8) |
| Overall without recent hospitalization (n = 354) | 44.0 (0.0–52.0) | 44.2 (3.0–52.0) | 0.0 (−31.0 to 47.0) | 112 (31.6) | 143 (40.4) | 99 (28.0) |
BL, baseline; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; LFU, last follow-up.
Clinically meaningful improvement is defined as an increase in FACIT-Fatigue score of more than 3 units from the baseline, worsening is a decrease of more than 3 units from baseline, and no change is any change in FACIT-Fatigue score from baseline of −3, −2, −1, 0, 1, 2, or 3.
Baseline is defined as the date of enrollment for never on eculizumab and eculizumab on enrollment groups; and the closest date before eculizumab start for the group that started eculizumab after enrollment.
If the patient discontinued eculizumab, LFU was the last evaluation point while on eculizumab.
Among patients who initiated treatment with eculizumab following registry enrollment, subgroup analyses showed improvements in median FACIT-Fatigue score regardless of dialysis, kidney transplant, or recent hospitalization status (Table 3). Median (range) increase in the score ranged from 6.0 (−2.0 to 12.3) to 11.5 (−28.0 to 47.0) across the subgroups analyzed. The proportion of patients with a clinically meaningful improvement in FACIT-Fatigue score was >65% regardless of subgroup, and was highest in the subgroups of patients without dialysis, with a kidney transplant, and without a recent hospitalization (Table 3).
Table 3.
Subgroup analyses of FACIT-Fatigue scores in patients starting on eculizumab after enrollment
| Proportion with clinically meaningful change,an (%) |
||||||
|---|---|---|---|---|---|---|
| Variable Median (range), unless otherwise stated |
BLb | LFUc | Change (LFU ‒ BL) | Improvement | No change | Worsening |
| Overall (n = 23) | 29.0 (0.0–49.0) | 41.0 (4.0–52.0) | 9.0 (−28.0 to 47.0) | 17 (73.9) | 2 (8.7) | 4 (17.4) |
| Dialysis (n = 3) | 30.0 (6.0–30.0) | 36.0 (4.0–42.3) | 6.0 (−2.0 to 12.3) | 2 (66.7) | 1 (33.3) | 0 |
| No dialysis (n = 14) | 32.5 (0.0–49.0) | 44.5 (8.0–51.0) | 10.0 (−28.0 to 47.0) | 10 (71.4) | 3 (21.4) | 1 (7.1) |
| Kidney transplant (n = 5) | 31.0 (5.0–47.0) | 40.0 (21.0–51.0) | 9.0 (0.0 to 16.0) | 4 (80.0) | 1 (20.0) | 0 |
| No kidney transplant (n = 16) | 28.5 (0.0–49.0) | 40.1 (4.0–51.0) | 11.5 (−28.0 to 47.0) | 11 (68.8) | 3 (18.8) | 2 (12.5) |
| With recent hospitalization (n = 18) | 29.5 (0.0–49.0) | 39.6 (4.0–52.0) | 10.5 (−28.0 to 40.0) | 13 (72.2) | 4 (22.2) | 1 (5.6) |
| Without recent hospitalization (n = 5) | 25.0 (0.0–35.0) | 40.0 (8.0–47.0) | 11 (−13.0 to 47.0) | 4 (80.0) | 0 | 1 (20.0) |
BL, baseline; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; LFU, last follow-up.
Clinically meaningful improvement is defined as an increase in FACIT-Fatigue score of more than 3 units from the baseline; worsening is a decrease of more than 3 units from baseline; and no change is any change in FACIT-Fatigue score from baseline of −3, −2, −1, 0, 1, 2, or 3. Missing data: dialysis/no dialysis, n = 6; kidney transplant/no kidney transplant, n = 2.
Baseline defined as the before eculizumab score closest to start of treatment.
Last score while on eculizumab.
General Health
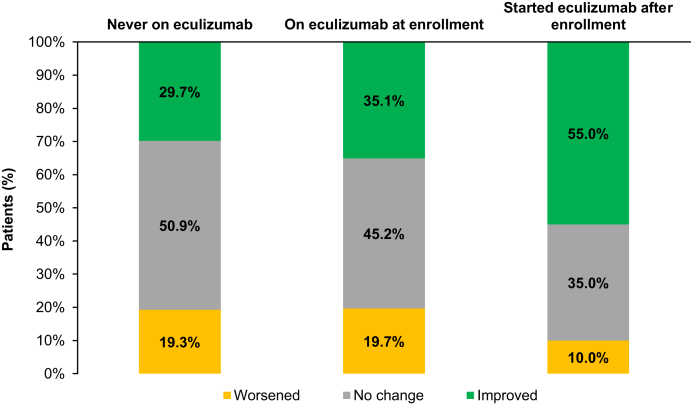
Overall, 69.1% of all patients with aHUS reported excellent (10.0%), very good (23.7%), or good (35.4%) health status at baseline; 30.9% of patients reported fair (23.9%) or poor (7.0%) health status. Findings at last follow-up were similar, with 76.5% reporting excellent (11.2%), very good (28.2%), or good (37.2%) health status, and 23.5% reporting fair (19.2%) or poor (4.3%) health status. The proportion of patients with improved general health at last follow-up compared with baseline was greatest in the cohort of patients who started eculizumab after enrollment (Figure 3).
Figure 3.
General health status, all groups. Shifts in general health calculated by comparing last follow-up to baseline in each group.
Patient Symptoms
In the overall population, fatigue, headache, and weakness were the most commonly reported symptoms at baseline, reported by 61.4% (n = 314), 54.4% (n = 278), and 46.4% (n = 237) of patients, respectively; these remained the top 3 symptoms at last follow-up, reported by 64.4% (n = 329), 55.0% (n = 281), and 42.3% (n = 216) of patients, respectively. Table 4 reports patient symptoms at baseline and last follow-up by treatment group. Consistent with FACIT-Fatigue score results, the greatest reduction in proportion of patients reporting fatigue as a symptom (65%, n = 13, down from 95%, n = 19, at baseline) was observed in the cohort that started eculizumab after enrollment.
Table 4.
Patient-reported symptoms at baseline and last follow-up by patient group
| Characteristic | All patients (N = 511) |
Never on eculizumab (n = 212) |
Eculizumab on enrollment (n = 279) |
On eculizumab after enrollment (n = 20) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | LFU | P value | BL | LFU | P value | BL | LFU | P value | BL | LFU | P value | |
| Patient symptoms, n (%) | ||||||||||||
| Abdominal pain | 127 (24.9) | 110 (21.5) | 0.2077 | 57 (26.9) | 50 (23.6) | 0.4338 | 63 (22.6) | 52 (18.6) | 0.2496 | 7 (35.0) | 8 (40.0) | 0.7440 |
| Anxiety | 167 (32.7) | 163 (31.9) | 0.7890 | 69 (32.5) | 62 (29.2) | 0.4619 | 87 (31.2) | 92 (33.0) | 0.6502 | 11 (55.0) | 9 (45.0) | 0.5271 |
| Chest pain | 81 (15.9) | 66 (12.9) | 0.1812 | 25 (11.8) | 28 (13.2) | 0.6596 | 50 (17.9) | 33 (11.8) | 0.0431 | 6 (30.0) | 5 (25.0) | 0.7233 |
| Confusion | 67 (13.1) | 65 (12.7) | 0.8520 | 20 (9.4) | 22 (10.4) | 0.7451 | 42 (15.1) | 36 (12.9) | 0.4639 | 5 (25.0) | 7 (35.0) | 0.4902 |
| Diarrhea | 147 (28.8) | 117 (22.9) | 0.0320 | 51 (24.1) | 45 (21.2) | 0.4863 | 86 (30.8) | 66 (23.7) | 0.0572 | 10 (50.0) | 6 (30.0) | 0.1967 |
| Easy bruising/abdominal bleeding | 128 (25.0) | 108 (21.1) | 0.1377 | 49 (23.1) | 46 (21.7) | 0.7268 | 72 (25.8) | 57 (20.4) | 0.1320 | 7 (35.0) | 5 (25.0) | 0.4902 |
| Fatigue | 314 (61.4) | 329 (64.4) | 0.3314 | 118 (55.7) | 131 (61.8) | 0.1997 | 177 (63.4) | 185 (66.3) | 0.4780 | 19 (95.0) | 13 (65.0) | 0.0177 |
| Headache | 278 (54.4) | 281 (55.0) | 0.8505 | 110 (51.9) | 113 (53.5) | 0.7705 | 154 (55.2) | 155 (55.6) | 0.9321 | 14 (70.0) | 13 (65.0) | 0.7357 |
| Irritability | 173 (33.9) | 163 (31.9) | 0.5055 | 69 (32.5) | 61 (28.8) | 0.3994 | 91 (32.6) | 95 (34.1) | 0.7194 | 13 (65.0) | 7 (35.0) | 0.0578 |
| Nausea/vomiting | 158 (30.9) | 109 (21.3) | 0.0005 | 63 (29.7) | 44 (20.8) | 0.0336 | 83 (29.7) | 58 (20.8) | 0.0149 | 12 (60.0) | 7 (35.0) | 0.1134 |
| Shortness of breath | 174 (34.1) | 136 (26.6) | 0.0097 | 62 (29.2) | 47 (22.2) | 0.0955 | 101 (36.2) | 81 (29.0) | 0.0709 | 11 (55.0) | 8 (40.0) | 0.3422 |
| Swelling | 138 (27.0) | 94 (18.4) | 0.0010 | 46 (21.7) | 35 (16.5) | 0.1742 | 81 (29.0) | 54 (19.4) | 0.0076 | 11 (55.0) | 5 (25.0) | 0.0528 |
| Weakness | 237 (46.4) | 216 (42.3) | 0.1861 | 92 (43.4) | 83 (39.2) | 0.3747 | 131 (47.0) | 124 (44.4) | 0.5519 | 14 (70.0) | 9 (45.0) | 0.1098 |
| Jaundice | 56 (11.0) | 18 (3.5) | <0.0001 | 21 (9.9) | 11 (5.2) | 0.0660 | 29 (10.4) | 7 (2.5) | 0.0002 | 6 (30.0) | 0 (0) | 0.0079 |
| Other | 59 (11.5) | 39 (7.6) | 0.0336 | 23 (10.8) | 12 (5.7) | 0.0522 | 34 (12.2) | 25 (9.0) | 0.2153 | 2 (10.0) | 2 (10.0) | 1.0000 |
BL, baseline; LFU, last follow-up.
In patients who started eculizumab after enrollment, the proportion reporting the following symptoms decreased by at least 10% at last follow-up compared with baseline: anxiety, diarrhea, easy bruising/abdominal bleeding, fatigue, irritability, nausea/vomiting, shortness of breath, swelling, weakness, and jaundice. The proportion of patients in this group reporting confusion as a symptom increased by 10%.
FACIT-Fatigue scores were also analyzed in the presence or absence of patient symptoms. Overall, patients who reported at least 1 clinical symptom (n = 450) had a lower baseline median (range) FACIT-Fatigue score than those who did not report symptoms (n = 55) (39 [0.0–52.0] vs. 50 [28.0–52.0]). Similar findings were observed at last follow-up (data not shown).
Resource Utilization
Health care resource utilization and work days missed are summarized in Table 5. The rate of emergency room visits and hospital admissions were similar across all groups, whereas patients who started eculizumab after enrollment reported more HCP visits and missed days of work. Overall, patients with aHUS reported a median of 0.38 HCP visits per year, 0.13 emergency room visits per year, 0.17 hospital admissions per year, and 1.58 work days missed per year.
Table 5.
Annual resource utilization rates, all groups (HCP visits, emergency room visits, hospital admissions, and work days missed)
| All | Never on eculizumab | On eculizumab at enrollment | Started eculizumab after enrollment | |
|---|---|---|---|---|
| HCP visits | ||||
| n | 208 | 73 | 121 | 14 |
| Median (range) | 0.38 (0.0–10.67) | 0.33 (0.05–10.67) | 0.40 (0.05–5.33) | 0.88 (0.0–8.40) |
| Emergency room visits | ||||
| n | 114 | 39 | 64 | 11 |
| Median (range) | 0.13 (0.0–0.88) | 0.10 (0.05–0.75) | 0.13 (0.05–0.58) | 0.13 (0.0–0.88) |
| Hospital admissions | ||||
| n | 139 | 47 | 77 | 15 |
| Median (range) | 0.17 (0.0–3.00) | 0.14 (0.05–1.00) | 0.17 (0.05–3.00) | 0.17 (0.0–0.67) |
| Work days missed | ||||
| n | 84 | 30 | 51 | 3 |
| Median (range) | 1.58 (0.06–45.50) | 1.25 (0.08–9.00) | 1.83 (0.06–43.50) | 6.90 (0.56–45.50) |
HCP, health care provider.
Rate calculated as the total number of events between baseline and last follow-up divided by the time of exposure during this period (i.e., number of completed forms multiplied by 0.5 years).
Work Status
Of all patients (N = 511), at baseline 21.1% (n = 108) reported holding a full-time job and 11% (n = 56) worked part-time. In addition, 5.9% (n = 30) and 4.5% (n = 23) were not working or not working full-time, respectively, due to aHUS; 6.1% (n = 31) were not working for pay for reasons unrelated to aHUS. A category of “other” was selected by 23.5% (n = 120; composed primarily of homemakers, self-employed, and individuals on disability), whereas 22.9% (n = 117) were students, and 5.1% (n = 26) were retired. There were no notable differences in work status across the 3 patient groups (data not shown).
Discussion
This analysis of data from the Global aHUS Registry represents the first evaluation of fatigue and other patient-reported outcomes directly from patients enrolled in the registry, including general health status, health care resource utilization, work status, and symptoms, and provides important insights into the burden of aHUS. Previous descriptions of health-related quality of life in patients with aHUS have focused on the effect of eculizumab on health-related quality of life,21 whereas this analysis provides insights into the overall burden and quality of life in patients with aHUS, regardless of treatment.
In the current analysis, there were few differences in any patient-reported outcomes within the groups of patients who were never on eculizumab and those who were already treated with eculizumab at the time of enrollment in the registry. In addition, within those groups, changes from baseline to last follow-up were minimal. Both groups reported baseline median FACIT-Fatigue scores of 41, which is close to the mean score reported in the general population (43.6),22 and thus it is not surprising that the median increases from baseline to last follow-up among these patients were small (0.0 and 1.0, respectively). This may suggest that these groups had less severe disease than those who needed to initiate eculizumab treatment. The cohorts who were never on eculizumab and those who were already treated with eculizumab at the time of enrollment had less frequent dialysis, plasma exchange/plasma infusion, and hospitalization than the cohort that started eculizumab, which is important, as these symptoms have been shown to worsen fatigue in previous, cross-sectional analyses10,11 and thus they likely represent a stable disease course. Furthermore, an analysis of subgroups based on dialysis, kidney transplant, and recent hospitalization status revealed small or no changes in median FACIT-Fatigue score over time regardless of subgroup, and at least 25% of patients across all subgroups reported a clinically meaningful increase in FACIT-Fatigue score.
Differences in patient-reported outcomes between the group on eculizumab at enrollment and those who started eculizumab after registry enrollment are likely attributable to the effects of treatment plateau versus treatment initiation. For example, approximately 40% of patients on eculizumab at enrollment reported a clinically meaningful improvement in fatigue at last follow-up, with a median change of +1 in FACIT-Fatigue score, compared with approximately 75% of patients who started eculizumab after registry enrollment who reported a clinically meaningful change and a median change of +9 points in FACIT-Fatigue score. These findings suggest that the improvements experienced by patients on initiation of eculizumab are sustained over time with continuous treatment.
Given that fatigue is associated with impaired productivity, depression, and decreased quality of life, and strongly associated with functional decline,23, 24, 25 the benefits of eculizumab on fatigue are likely to be valued by patients. The change in FACIT-Fatigue scores in patients treated with eculizumab in this current analysis is similar to what has previously been reported in an eculizumab clinical trial in adult patients with aHUS.14 We believe findings from our study are likely more reflective of everyday practice and provide valuable insights for clinicians and patients. The differences in baseline FACIT-Fatigue scores between the groups never treated with eculizumab and those who started eculizumab after registry enrollment also suggest that patients in the registry who never received eculizumab may have a different disease phenotype than those who received eculizumab. This might be expected in a real-world setting and could be indicative of confounding by disease severity.
We also assessed patient-reported general health status, and believe this is the first report of this outcome in patients with aHUS. More than 75% of patients with aHUS reported good, very good, or excellent health status at last follow-up. Among patients who initiated treatment with eculizumab after registry enrollment, most (55%) reported an improvement in general health status from baseline to last follow-up. In addition, this group had the lowest proportion of patients with worsened general health status (10%).
We also believe this is the first analysis to evaluate patient-reported symptoms in patients with aHUS. The top 3 patient-reported symptoms were fatigue, headache, and weakness. Overall, fatigue was the most commonly reported symptom at baseline and at last follow-up. Among patients treated with eculizumab at the time of registry enrollment, the proportion of patients reporting fatigue as a symptom was relatively unchanged; in contrast, among those who initiated eculizumab after registry enrollment, the proportion decreased from 95% to 65%. Taken together, these findings further support the real-world benefits of complement inhibitors such as eculizumab in patients with aHUS. In addition, they emphasize the importance and relevance of patient-reported outcomes, which provide information from the patient perspective and, when used in conjunction with clinical assessments performed by physicians, reflect the full spectrum of disease as well as the overall effectiveness of treatment. A greater understanding of the burden of aHUS on patients could contribute to the body of evidence on the benefit of treating these patients.
Health care resource utilization and work status were similar among all 3 patient groups, with the exception of the number of HCP visits and number of work days missed, which were higher among patients who initiated eculizumab treatment after registry enrollment. The increase in number of HCP visits and number of work days missed in this group was not surprising and is likely secondary to the clinical manifestations (e.g., thrombotic microangiopathy) that led clinicians to initiate eculizumab and the need for more monitoring in a patient beginning a new therapy. This is anticipated to decrease over time and likely to become similar to resource utilization rates in patients who were on eculizumab at registry enrollment, although this should be confirmed in future analyses of data from this registry.
Limitations of these findings include the use of a patient-reported outcomes instrument that is not disease specific and the inherent limitations of using patient-reported outcomes from a global registry (i.e., missing data and underreporting or overreporting of outcomes). Inherent challenges associated with patient questionnaires, such as information bias, can lead to inaccurate outcome estimates.26 Self-reporting bias and recall bias have also been widely reported to impact the results of patient questionnaires, and present a challenge in data analysis when working with real-world outcomes from a patient registry, especially for those with a 6-month recall period.26 The generalizability of our results is also limited due to the small sample size of some of the cohorts studied, but longer follow-up, which should also increase sample sizes, should provide further validation of our results. Last, patients who were not treated with eculizumab were unable to report information on change in outcomes after treatment, and may thus have underestimated their overall level of fatigue.
The median follow-up in the 3 groups in this study ranged from 17.5 to 29.1 months. The Global aHUS Registry continues to collect patient-reported outcomes, and this should enable future reporting of longer follow-up data that may provide new insights into the burden of aHUS and how it interferes with patients’ activities of daily living, working, and health care resource utilization. Moreover, future analyses will provide an opportunity to describe changes in patient-reported outcomes for patients who switch from every-2-week dosing with eculizumab to every-8-week dosing with ravulizumab.
Conclusion
In summary, this analysis of patient-reported outcomes from the Global aHUS Registry provides new insight into the patient burden associated with aHUS. Patients who were not on eculizumab at the time of baseline measurements, but started after enrollment, showed clinically meaningful improvement in fatigue, general health, and other patient-reported outcomes, whereas changes in these outcomes were minimal over time in patients who never received eculizumab and those who were already on eculizumab at registry enrollment. Results of this registry analysis describe the burden of aHUS and support the use of C5 inhibition by treatments such as eculizumab to improve patient-reported outcomes in patients with aHUS.
Disclosures
LAG has received speaker fees, advisory board honoraria, and research support from Alexion Pharmaceuticals, Inc., and has received research funding for Emory University, Atlanta, GA, from Alexion Pharmaceuticals, Inc., for his participation in the Eculizumab in Pediatric Patients and aHUS International Registry clinical studies; has received grant/research support and/or consultancy fees from AbbVie, Inc., Alexion Pharmaceuticals, Inc., Bristol-Myers Squibb, Advicenne Pharmaceuticals, Mallinckrodt Pharmaceuticals, Otsuka America Pharmaceutical, Inc., and Vifor Pharma; has served as a member of a scientific advisory board for Alexion Pharmaceuticals, Inc.; and is a member of data safety monitoring boards for Retrophin, Inc., and Relypsa, Inc. CL is the Chair of the Scientific Advisory Board of the Global aHUS Registry, scientific advisor, and member of speaker’s bureau for Alexion Pharmaceuticals, Inc.; scientific advisor for Achillion, Apellis, Novartis, and Ra Pharmaceuticals; and recipient of an unrestricted research grant from Aurin Biotech, Inc. VN is an employee of PAREXEL International, contracted by Alexion Pharmaceuticals, Inc., for data analysis. IA-D, IT, and BM are employees and stockholders of Alexion Pharmaceuticals, Inc. CSH has received travel grants, advisory board honoraria, and honoraria as a speaker for Alexion Pharmaceuticals, Inc. ERO has received lecture and consultancy fees from Alexion Pharmaceuticals, Inc. LS is the national coordinator for the Global aHUS Registry (Sweden) run by Alexion Pharmaceuticals, Inc., and has received lecture and/or consultancy fees from Fresenius and Baxter. RS is on the speaker board and has received speaker fees, advisory board honoraria, and research support from Alexion Pharmaceuticals, Inc. SRC has received research funding from Alexion Pharmaceuticals, Inc. All the other authors declared no competing interests.
Acknowledgments
The sponsor and authors thank the patients and their families for their participation in and support for this clinical study, as well as global investigators who have contributed patient data for this study. The authors acknowledge Radha Narayan, PhD, of Alexion Pharmaceuticals, Inc., for critical review of the manuscript. This analysis was supported by Alexion Pharmaceuticals, Inc., Boston, MA, USA. Alexion Pharmaceuticals, Inc., was responsible for the collection, management, and analysis of information contained in the Global aHUS Registry. Alexion Pharmaceuticals, Inc., contributed to data interpretation, preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. Medical writing and editorial support were provided by Linda V. Wychowski, PhD, Kersten Reich, MPH, and Corey Eagan, MPH, of Eloquent Scientific Solutions. These services complied with international guidelines for Good Publication Practice and were funded by Alexion Pharmaceuticals, Inc.
Data Sharing
Alexion Pharmaceuticals, Inc. will consider requests for disclosure of clinical study participant-level data provided that participant privacy is ensured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy (http://alexion.com/research-development).
Footnotes
Figure S1. Plain language summary: functional assessment of fatigue and other patient-reported outcomes in patients enrolled in the Global aHUS Registry.
Supplementary Material
References
- 1.Fakhouri F., Zuber J., Fremeaux-Bacchi V. Haemolytic uraemic syndrome. Lancet. 2017;390:681–696. doi: 10.1016/S0140-6736(17)30062-4. [DOI] [PubMed] [Google Scholar]
- 2.Campistol J.M., Arias M., Ariceta G. An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia. 2015;35:421–447. doi: 10.1016/j.nefro.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Noris M., Caprioli J., Bresin E. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuber J., Frimat M., Caillard S. Use of highly individualized complement blockade has revolutionized clinical outcomes after kidney transplantation and renal epidemiology of atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2019;30:2449–2463. doi: 10.1681/ASN.2019040331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration Framework for FDA's Real-World Evidence Program. https://www.fda.gov/media/120060/download Available at: Revised December, 2018. Accessed December 18, 2019.
- 6.Socie G., Caby-Tosi M.P., Marantz J.L. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10-year pharmacovigilance analysis. Br J Haematol. 2019;185:297–310. doi: 10.1111/bjh.15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menne J., Delmas Y., Fakhouri F. Outcomes in patients with atypical hemolytic uremic syndrome treated with eculizumab in a long-term observational study. BMC Nephrol. 2019;20:125. doi: 10.1186/s12882-019-1314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rondeau E., Cataland S.R., Al-Dakkak I. Eculizumab safety: five-year experience from the global atypical hemolytic uremic syndrome registry. Kidney Int Rep. 2019;4:1568–1576. doi: 10.1016/j.ekir.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodward L., Johnson S., Walle J.V. An innovative and collaborative partnership between patients with rare disease and industry-supported registries: the Global aHUS Registry. Orphanet J Rare Dis. 2016;11:154. doi: 10.1186/s13023-016-0537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Licht C., Sartz L., Al-Dakkak I. Patient-reported fatigue amongst children enrolled in the Global aHUS Registry. Pediatr Nephrol. 2018;33:1807–2008. [Google Scholar]
- 11.Sartz L., Greenbaum L.A., Haas C. FACIT-Fatigue scores in adult patients at enrollment into the Global aHUS Registry. Nephrol Dial Transplant. 2018;33 [Google Scholar]
- 12.Licht C., Ardissino G., Ariceta G. The global aHUS registry: methodology and initial patient characteristics. BMC Nephrol. 2015;16:207. doi: 10.1186/s12882-015-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai J.S., Cella D., Kupst M.J. Measuring fatigue for children with cancer: development and validation of the pediatric Functional Assessment of Chronic Illness Therapy-Fatigue (pedsFACIT-F) J Pediatr Hematol Oncol. 2007;29:471–479. doi: 10.1097/MPH.0b013e318095057a. [DOI] [PubMed] [Google Scholar]
- 14.Fakhouri F., Hourmant M., Campistol J.M. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis. 2016;68:84–93. doi: 10.1053/j.ajkd.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Greenbaum L.A., Fila M., Ardissino G. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89:701–711. doi: 10.1016/j.kint.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Noris M., Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 2013;33:479–492. doi: 10.1016/j.semnephrol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallinson T., Cella D., Cashy J. Giving meaning to measure: linking self-reported fatigue and function to performance of everyday activities. J Pain Symptom Manage. 2006;31:229–241. doi: 10.1016/j.jpainsymman.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Webster K., Cella D., Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordin A., Taft C., Lundgren-Nilsson A. Minimal important differences for fatigue patient reported outcome measures-a systematic review. BMC Med Res Methodol. 2016;16:62. doi: 10.1186/s12874-016-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cella D., Eton D.T., Lai J.S. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee A.A., Kandhare A.D., Bodhankar S.L. Evaluation of health-related quality of life in hemolytic uraemic syndrome patients treated with eculizumab:a systematic evaluation on basis of EMPRO. Ren Fail. 2018;40:107–118. doi: 10.1080/0886022X.2018.1427110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cella D., Lai J.S., Chang C.H. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 23.Espahbodi S., Bassett P., Cavill C. Fatigue contributes to work productivity impairment in patients with axial spondyloarthritis: a cross-sectional UK study. Clin Exp Rheumatol. 2017;35:571–578. [PubMed] [Google Scholar]
- 24.Rueda-Lara M., Lopez-Patton M.R. Psychiatric and psychosocial challenges in patients undergoing haematopoietic stem cell transplants. Int Rev Psychiatry. 2014;26:74–86. doi: 10.3109/09540261.2013.866075. [DOI] [PubMed] [Google Scholar]
- 25.Michael K. Fatigue and stroke. Rehabil Nurs. 2002;27:89–94. doi: 10.1002/j.2048-7940.2002.tb01995.x. 103. [DOI] [PubMed] [Google Scholar]
- 26.Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9:211–217. doi: 10.2147/JMDH.S104807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.