Abstract
Background
Although telomerase has potential for age-related disease intervention, the overexpression of telomerase in about 90% of cancers, and in HIV virus reservoirs, cautions against se in anti-aging telomerase therapeutics. While multiple reviews document the canonical function of telomerase for maintenance of telomeres, as well as an increasing numbers of reviews that reveal new non-canonical functions of telomerase, there was no systematic review that focuses on the array of associates of the subunit of Telomerase Reverse transcriptase protein (TERT) as pieces of the puzzle to assemble a picture of the how specific TERT complexes uniquely impact aging and age-related diseases and more can be expected.
Methods
A structured search of bibliographic data on TERT complexes was undertaken using databases from the National Center for Biotechnology Information Pubmed with extensive access to biomedical and genomic information in order to obtain a unique documented and cited overview of TERT complexes that may uniquely impact aging and age-related diseases.
Results
The TERT associations include proper folding, intracellular TERT transport, metabolism, mitochondrial ROS (Reactive Oxygen Species) regulation, inflammation, cell division, cell death, and gene expression, in addition to the well-known telomere maintenance. While increase of cell cycle inhibitors promote aging, in cancer, the cell cycle check-point regulators are ambushed in favor of cell proliferation, while cytoplasmic TERT protects a cell cycle inhibitor in oxidative stress. The oncogene cMyc regulates gene expression for overexpression of TERT, and reduction of cell cycle inhibitors-the perfect storm for cancer promotion. TERT binds with the oncogene RMRP RNA, and TERT-RMRP function can regulate levels of that oncogene RNA, and TERT in a TBN complex can regulate heterochromatin. Telomerase benefit and novel function in neurology and cardiology studies open new anti- aging hope. GV1001, a 16 amino acid peptide of TERT that associates with Heat Shock Proteins (HSP’s), bypasses the cell membrane with remarkable anti disease potential.
Conclusions
TERT “associates” are anti-cancer targets for downregulation, but upregulation in antiaging therapy. The overview revealed that unique TERT associations that impact all seven pillars of aging identified by the Trans-NIH Geroscience Initiative that influence aging and urge research for appropriate targeted telomerase supplements/ stimulation, and inclusion in National Institute on Aging Intervention Testing Program. The preference for use of available “smart drugs”, targeted to only cancer, not off-target anti- aging telomerase is implied by the multiplicity of TERT associates functions.
Keywords: Aging, TERT, associates, cancer, oncogenes, cell cycle, diseases, viral infection
1. INTRODUCTION
The promoter of the reverse transcription TERT gene, is a common non-coding mutation in cancer [1-3] and these mutations in the regulation of TERT expression represent a tumorigenic mechanism [2]. Regulation of telomerase by epigenetics results in TERT overexpression [4] and TERT function [5]. The discovery of the role of telomerase as a telomere ribonucleoprotein terminal transferase, i.e., the TERT reverse transcriptase protein with TERC (Telomerase RNA component), heralded a new area in telomere biology and is considered the canonical role of telomerase [6, 7]. Damage to telomeres can trigger DNA damage response, apoptosis and aging [8]. The association of telomere-dysfunction with diseases was recently reviewed [9]. Here, the non-canonical telomerase functions include the discovery that TERT expression regulates tolerance to oxidative stress in mitochondria linking telomerase, mitochondria, oxidative stress, aging diseases, and longevity [8-12]. Telomerase’s ability to reverse tissue degeneration in aged mice provides evidence for the potential for therapeutic TERT intervention in aging [13]. Examples of telomerase benefits emerge in immunology [14], cardiology [15], chemotherapy-induced damage [16] and neurology [17, 18]. Epigenetics [19, 20] and splice variants of TERT RNA can regulate telomerase activity in cancer, and in different cells and tissues [21]. The recently discovered ability to target telomerase, and thereby also selectively target cancer cells selectively, allows the elimination of cancer cells without downregulation of the positive roles of telomerase in other age-related diseases [22, 23]. The TERT peptide GV1001, promises to be an intervention drug in multiple diseases [24].
Stress, especially oxidative stress, impacts multiple age-related diseases (over 15,000 references on aging and oxidative stress are cited in the National library of Medicine National Institutes in 2019). Oxidative stress age-related diseases include cancer, cardiac aging, cardiovascular diseases, skeletal muscle aging, Alzheimer’s Disease, Parkinson’s Disease, Hearing Loss, insulin resistance, diabetes [25], immunosenescence [26], frailty [27] and age-related vascular dysfunction [28] linked to TERT, since oxidative mitochondrial stress is regulated by mitochondrial TERT [8-12]. The associations of TERT with chaperones, mitochondrial DNA, mTor pathway and brain mitochondria, glucose kinase, antioxidant pathways, inflammatory regulators, glucose and fat metabolism, oncogenes, generation of micro and interfering RNA gene regulators, cell cycle regulators of cancer, and evidence of critical roles in cardiovascular and neurological diseases are documented below with the promise of GV1001 in disease intervention. The extensive non-canonical participation of Telomerase subunit TERT with associations (Table 1) that impact aging and age-related signals the need for appropriate telomerase age-related intervention therapy.
Table 1.
Telomerase, subunits, and associates regulate age-related functions.
| Function | Associates | Section |
|---|---|---|
| Nuclear telomere biology Mitochondrial Antioxidant Functions | Chaperones HSP90, TCA | 1, 2 |
| Intracellular transport | Motor 90 Dynein, FKB’S | 3 |
| Oxidative stress protection |
Mitochondrial DNA,
mTOR, NrF2 |
4.2 |
| Inflammation | Nf κB, NrF2 | 5.1-5.3 |
| Metabolism | NrF2 Glucose Fatty acids EZH2 |
5.4-5.5 |
| Gene Regulation | RMRP-RdRP, TBN, siRNA, microRNA | 6.1-6.2 7 |
| Immortality vs. Aging | P16 INKA, | 8 |
| Cytoplasmic Antioxidation Cancer | P15 RNA, TERT-TIA1 | 8 |
| TERT | cMyc oncogene | 9 |
| TERC | cMyc oncogene | 9.1 |
| Neuronal Health | TELOMERASE | 10 |
| Heart Health | TELOMERASE | 11 |
| Intervention | GV1001 | 12 |
| Supplements | - | 13 |
2. TERT CHAPERONES
Tert Chaperones: Required For Proper Protein Shapes For Telomerase Activity: Proteostasis, Macromolecular Functions And Tert-Terc-Telomere Functions [29-31].
2.1. TERT-Hsp90- 23-TERT
The unique associations between heat-shock protein 90 chaperones, and stress-inducible HSP23, dictate TERT proper assembly with the RNA ligand template TERC. Components of the Hsp90 chaperone of telomerase assembly, remain with active enzyme and are required for telomerase activity [29].
2.2. TCAB1-TERT
TCAB1-TERT (Telomerase Cajal Body protein 1 RNA splicers). Depletion of TRiC (TCP-1 Ring Complex Chaperone) is required for TCAB1 folding, or TCAB mutations, impair the control of telomerase activity for telomere elongation [30]. Telomerase depends upon the holoenzyme protein TCAB1, a target for cancer therapeutics [31].
3. INTRACELLULAR NUCLEAR
Intracellular Nuclear- mitochondrial travel. required for tert location to mitochondrial for functions in aging [32-35].
TERT requires p90 dynein-dynactin motor (cytoplasmic motor for intracellular nuclear-mitochondrial travel to mitochondria) and FKBP52 (FKB506-binding protein immunophilins) and FKBP52-hTERT-Hsp90-Dynein-dynactin complex provides the motor for cytoplasmic transport of hTERT [32]. FKB51and FKB52 proteins stimulate TERT in oxidative stress and thus a target for downregulation of cancer cells [32]. Hsp90-binding immunophilin FKBP51 forms complexes with hTERT enhancing telomerase activity and overexpression of the immunophilin is associated with resistance to induce cell death [33]. FK506-Binding Proteins (FKBPs) are co-receptors for immunosuppressants, and FKBPs inhibitors intervene in antimalarial, antileginonellal, and antichlamydial properties [34]. Conversely, approaches are necessary to intervene in protein misfolding in neurological diseases [35].
4. MITOCHONDRIAL TERT AND OXIDATIVE STRESS
Required for tert antioxidant function and macromolecular protection, and stress resistance [10, 36-48].
4.1. TERT- mDNA (Mitochondrial DNA)
TERT binds to mDNA and protects mDNA from oxidative damage [10]. Telomerase protects mitochondria under oxidative stress [36]. Telomerase affects mitochondria DNA replication [37]. Oxidative DNA damage stalls the human mitochondrial replisome [38]. Telomerase overexpression protects cancer cells from apoptosis [39]. Telomerase affects the cellular response to oxidative stress by autophagy [40].
4.2. mTOR-TERT (Target of Rapamycin, Member of Phosphatidylinositol 3 Kinase)
Diet Restriction (DR) and rapamycin treatment (inhibitor of mTOR) stimulate TERT localization in rodent brain mitochondria, reduce Reactive Oxygen Species (ROS) as an antioxidant, and improve mitochondrial function [41, 42]. Signals from mTOR expression in aging and neuro-regeneration affect both metabolism and autophagy [43]. Inhibition of mTOR expression with rapamycin in a mouse model of Downs’s syndrome intervenes in a cognitive loss by the improvement of autophagy and insulin signaling [44]. The mTor inhibition modulates Aβ plaques deposition and tau tangle aggregation in Alzheimer’s disease [45] and promote inhibition in neurological disease and longevity [46] likely due to improvement in mitochondrial health [41, 42]. However, the PI3K/AKT/mTOR AKT kinase is central to glucose metabolism signaling pathway, as well as the promotion of TERT over expression of stem-like cancer cells suggests targeting both in cancer therapy [47]. The presence of TERT in the signaling complex implies that mTOR- mediates the control of telomerase activity [48]. Telomerase activity is inhibited by various phytochemicals such as isoprenoids, genistein, curcumin, epigallocatechin-3-gallate, and resveratrol [48].
5. METABOLISM, INFLAMMATION, IMMUNITY
Required for energy, anti inflammatory disease, infection resistance, and tumor progression [49-54].
5.1. NrF2-TERT
NrF2 (nuclear Factor erythroid 2 related factor that controls ARE antioxidant response element). Inhibition of human TERT reduces NrF2 and induces glioma cell apoptosis, while Nrf2 overexpression increases TERT [49]. TERT inhibition results in a reduction in pentose phosphate intermediates and stimulation of glycogen [49]. In glucose deficit, both Nrf2 and autophagy support breast cancer progression [50]. In cancer, NrF2 protects against ROS stress, inflammasome assembly, and regulates cancer promotion microRNAs [51]. While TERT and NrF2 are targets for downregulation in cancer, upregulation is desirable for intervention in ROS mediated diseases [52]. Nrf2 activating compounds show down regulation of inflammasomes and inflammation, in non-cancer disease treatments [52]. Nrf2 regulates activation of inflammasomes via regulation of the Trx1/TXNIP (thioredoxin interacting compound) complex [53] and up regulation of TXNIP may be anti-aging and anti-HIV, by down regulation of inflammation found in HIV immune cells [54].
5.2. TERT- NF-κB (Nuclear Factor Kappa Light Chain Enhancer of Activated B Cells)
The ability of NF-κB to alter cell functions reflects multiple gene activations or repressions [55]. In mice, telomerase binds to the NF-κB p65 [56].
5.3. TERT- NF- κB -STAT3 (Signal Transducer and Activator of Transcription)
In humans, STAT3- STAT1-NF-κB physically interact and stimulate cytokines IL6 (interleukin 6) and TNF α (tumor necrosis factor) inflammatory agents that increase telomerase activity and stem-like cancers [57]. In atherosclerosis, inflammation activates TERT [58]. STAT regulates inflammation and immunity [59]. The oriental anti-inflammatory agent, Withania somnifera is a likely drug candidate for human clinical trials in cancer [60]. Over- the counter anti-inflammatory agents are supplements suggested for cancer-induced inflammation treatment [61]. Telomerase supplements for arthritis have conflicting responses, likely due to the activation of different telomerase-associate pathways [15].
5.4. Telomerase-Glucose Hexokinase Telomerase
Telomerase-Glucose and Hexokinase. Telomerase down-regulates glycolytic pathway genes, decreases glucose consumption and lactate production. Inhibition of telomerase RNA expression reduces metastasis, glucosemetabolism, lactate [62]. Hexokinases, also known as glucokinases, catalyze the first step in glucose metabolism [63]. Deletion of hexokinase intervenes in cancer [64]. Hexokinase has a role in growth or death, via starvation induced autophagy by TORC [65]. The TOS binding site for TORC substrates is consered in verebrates [66]. Telomerase links cell death, hexokinase, and autophagy [67]. The role of telomerase in glucose metabolism links telomerase to glucose metabolism in innate immunity [68] and immune CD4+ and CD8+ cells [69]. Recently, a protein was found to code from the RNA ligand of telomerase Tert, called TERP, [70] whose role in biology of cancer is unnown.
5.5. TERT-EZH2
TERT-EZH2 (also known as Zeste homolog 2, histone H3K27 methyltransferase) is required for epigenetic methylation control in lipid metabolism.
Downregulation of TERT decreases EZH2, and correlates with an excess of lipids, and stimulation of ATM damage response in glioma model [70]. TERT in association with EZH2 is a stress responder to DNA damage [71].
6. TERT-RMRP RMRP
(RNA component of mitochondrial RNA Polymerase, ligand mitochondrial exonuclease, also known as RNase MRP RNA, RNA component of Mitochondrial RNA Processing RNA required for regulation of gene expression and RNA dependent RNA polymerase function.
6.1. TERT-RMRP
TERT-RMRP generates RdRP (RNA dependent RNA Polymerase). TERT interacts with the same RNA ligand component as RNase MRP (RNA component of the mitochondrial RNA endonuclease, RMRP, that self-regulates RMRP level by siRNA [72] and generates short RNAs in cancer cell lines [73]). Endogenous siRNAs other than RMRP are also found with the ability of sequence-specific inhibition of micro RNAs [74]. TERT protein levels correlate with RdRP activity in cancer and identify RdRP as a target for anticancer therapeutics [73-75]. In a study, knockdown of RMRP by shRNA inhibited mammary cancer cell replication [75]. The ability of RdRP to generate siRNA was independently confirmed [76] though attempts to generate siRNA to control of RNase MRP RNA with siRNA of RMRP were unsuccessful or complicated by pleiotropic unintended target effects [77]. Using the selective gene scissors, Crisper-Cas9 (Clusters of regularly interspaced palindromic repeats for gene editing) the RMRP locus, results in the deadly accumulation of preribosomal RNA in human HELA cells [78].
6.2. RMRP Oncogene
RMRP regulation is altered in multiple cancers [79-82]. RMRP is known as an oncogene upregulated in lung cancers and promotes inhibition of mi RNA-206 [79-80]. MicroRNA 206 modulates cyclin D2, cell division, and invasion in cancers [81-83]. Recent reviews show that RMRP acts as an oncogene that regulates glycolysis, ROS, and apoptosis in humans, and document that RMRP is a target for anticancer therapeutics [84, 85].
7. TBN complex
TERT-BRG1-Nucleostemin (BRG1- ATPase of the SWI/SNF chromatin remodeling complex that remodels chromatin to control gene expression and chromosome centromere) required for regulation of heterochromatin in gene regulation and centromeres epigenetics.
7.1. TERT in TBN
TERT is prominent in cancer and cancer initiating cells; BRG1 interacts with histone deacetylase 2 and alters telom-erase activity in cancer cells [86]. Levels of BRG1 correlate with cancer cell replication levels [87]. Nucleostemin is as-sociated with malignancy in cancer cell line [88]. TBN (TERT-BRG1-Nucleostemin) is prominent in tumor-initiating cells [89]. TERT is involved in heterochromatin maintenance [90], RNA dependent RNA polymerase siRNA production [91], and chromosome segregation [92]. The complex of TERT-BRG1-Nucleostemin (TBN) is reminiscent of heterochromatin complex control in lower organisms and may be potentially useful in targeting cancer [93]. TERT also regulates microRNA [94]. Telomerase intervenes in telomere aneuploid induced replication stress [95]. In summary, TBN directs RNA synthesis, heterochromatin, centromeres, mitosis, siRNA, miRNA, and cell replication targets in cancer therapeutics.
8. TERT-P16 INKA
TERT-P16 INKA aliases CDKN2A, ARF, CDK41, CDKN cyclin-dependent kinase inhibitor 2A,P16-INKA4A: Regulator of cell cycle: required for cell cycle control, brain protection [96-101]. The mammalian INK4a/ARF locus (alternate reading frame) encodes p16INK4a protein and ARF regulators of Retinoblastoma (RB) and p53 pathways that impact and regulate cancer and aging [96, 97]. The interaction of stimulation or inhibition of cell cycle describes the difference in cancer types versus the activation of p16 can intervene in cancer [98]. Cells, with activated p16 INK4a promoter, accumulate p16 and exhibit inflammation, and display senescence [99]. Both TERT and p16 are players in glioblastomas; p16 suppresses hTERT in a human mammary epithelial cell by p16 activation of methylation that irreversibly blocks the hTERT promoter in normal and human breast cancer [100]. Thus, the dual role of p16 includes not only inhibition of cell cycle progression, but also the transcriptional suppression of the TERT promoter in mammalian cells. Bmi-1 (B-cell specific Moloney murine virus integration site 1) is a member of the Polycomb Repressor Complex1 that regulates chromatin structure for renewal l of both normal and cancer stem cells, and lengthens the potential doubling of human cells by inhibition of p16(INK4a [101]. Bmi has the potential for antiaging for self-renewal of normal cells, but, the danger of protection of cancer cells [101].
Modifications of p15, p16 and TERT are inducible; epigallocatechin-3-gallate, found in green tea, inhibits growth and induces apoptosis in cancer cells by activation of the cell cycle inhibitor p16 and downregulation of telomerase [102, 103]. TGF β (transforming growth factor) induces a 30X increase of p15 in stress [104] and the TGFβ pathway stimulation induces aging in glioblastoma cells [105]. The anti-apoptotic role of telomerase involves BCl2 (B-cell lymphoma 2 family of anti-cell death proteins) the over expression of which protects cancer cells from apoptosis [106]. The drug used to target BCL2, Venetoclax in HIV reservoirs, may be effective in cancer to counteract TERT induced anti-apoptosis [107]. The low dose of the drug RG108 methylation inhibitor activates TERT and blocks ROS and inflammation, and at high dose, it activates tumor suppressor p16 INKA [108, 109].
In fully differentiated neurons, the largest pool of cytoplasmic TERT is in the complex (TERT -TIA1- p15INK4b mRNA) and under oxidative stress, p15INKA4b translation occurs and neuronal survival increases [110]. While TERT knockdown promotes apoptosis, TERT induced overexpression reduces apoptosis and TERT exhibits translational control of p15 messenger RNA cell cycle inhibitor expression in oxidative stress and neuronal survival [110]. Studies show mammalian brain with TERT specifically in neurons [111-113].
9. TERT-CMYC
cMyc is a transcription factor protein and oncogene) Required for cancer progression [114-118]. Gliomas show increased TERT mRNA, and telomerase activity [114]. TERT protects cMyc by after-translational regulation of cMyc ubiquitination and thereby protects c Myc from degradation in carcinogenesis [114]. A cMyc-MAX dimer binds to promoters of TERT and cyclin DD that enhance their transcription for TERT overexpression [115], while cMyc represses cyclin-dependent kinase inhibitors p15 and p21 to result in inhibition of cell cycle [116-118]. The upregulation of telomerase and downregulation of cell cycle regulation provide the perfect environment for cancer promotion in post-mitotic cells.
TSC-22 (transcription factor) reacts with c-MYC and prevents the suppression of p15 promoters but enhances overexpression of TERT [119], separating two promoters of cancer for anticancer activity.
9.1. TERC-cMYC
Telomerase RNA component epigenetic control of cancer [120]. cMyc occupies the TERC locus, and mediates excess TERC RNA, while TERC inhibition reduces prostate cancer [120], therefore, both TERT and TERC independently interact with cMyc to modulate cancer pathology. Although TERC has been considered a non-coding RNA, the protein TERP has been found protecting cell viability [70].
10. TERT, STEM CELLS, NEURONAL HEALTH, AND mTOR
Telomerase in neuronal health stem cell neurogenesis. Required for antioxidant and stem cells [121-129]. Adult human neurogenesis was discovered in the dentate gyrus of adult humans throughout life [121]. Retrospective birth dating is possible to measure human cell turnover from by use of the known C14 from nuclear bomb testing generated in the atmosphere as the DNA date mark when a cell duplicates its chromosomes [122]. New human hippocampal cells using 14C, reveal and new neurons are added each day, with minimal aging decline [123] and sparks hope of maintenance of brain functions in aged! Ectopic telomerase expression delays amyotrophic lateral sclerosis [124]. Hippocampal neurogenesis has a potential role in memory and spatial learning [125]. Telomerase is required for the benefits of neurotropic growth factor, Brain-Derived Neurotrophic Factor (BDNF) in early hippocampal brain development [126]. The TERT-mTor association’s role in neuronal mitochondrial health provides hope for intervention in neurological disorders [41-45]. TERT plays a role in the dynamics of neurogenesis, regulated by mitochondria throughout the lifespan [127]. Neural Stem Cells (NSCs) and Neural Progenitor Cells (NPCs), implicate telomerase for an important role in the developing and adult brains of humans and rodents. Recent studies have demonstrated that telomerase in NSCs/NPCs functions in cell proliferation, neuronal differentiation, neuronal survival and neurogenesis [129].
11. TELOMERASE HEART HEALTH
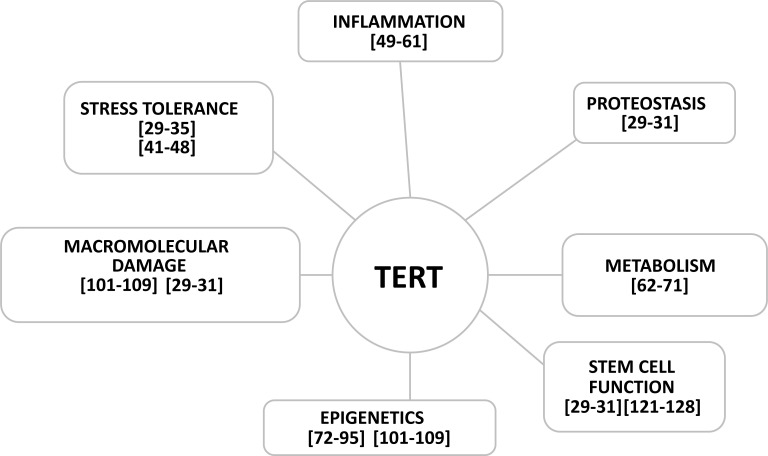
Reviews of the role of telomerase as a therapeutic tissue-specific target relative to cardiovascular health [11] and off- target damage by chemotherapy highlight the importance of telomerase in heart health and drug induced cardiac damage [16]. Telomerase is beneficial in treatment of Coronary Artery Disease (CAD) via protection from ROS [15, 16]. In Ischemia- Reperfusion injury, injury telomerase deficit predisposes heart failure [130]. Telomerase has a critical role in microcirculation since decreased telomerase activity promotes Nitric oxide conversion to peroxide in Coronary Artery Disease (CAD), while telomerase increase restores normal function [131]. Telomerase has potential for intervention in pulmonary hypertension [132]. Telomere attrition is characteristic of cardiac hypertrophy and cardiomyocyte-specific telomere shortening is a human marker of heart failure, and cardiomyocytes with the shortest telomeric lengths are typically correlated with reduced ejection [133] (Fig. 1).
Fig. (1).
Examples of the interactions of the subunit of telomerase TERT protein are cited above and in the text A-H. Each function interacts with the other pillars of the seven pillars of aging identified by the Trans-NIH Geroscience Initiative that identify the major areas that impact aging. Telomerase subunits and associates are major players in pathways that impact aging and the expression of the TERT gene impacts all pillars of aging.
12. GV1001
Telomerase peptide intervention potential in antiaging disease activators -inflammation, oxidative stress related diseases, and amyloid toxicity in neuronal diseases. The16 amino acid peptide of telomerase, used as the cancer vaccine, GV1001, unexpectedly also shows Cell Penetrating Properties, (CPPs), with, ease of cytosolic cargo passage across the plasma membrane for delivery of drug macromolecules via heat shock proteins HSP90 and HSP70 [20, 133]. GV1001 functions properties are anti-inflammatory [134, 135], antioxidant [136, 137], antiviral [138] and anti-amyloid toxicity [139].
13. TERT Supplements
Telomerase supplements were reviewed previously (15). The telomerase activator TA-65 supplement extends telomeres and increases disease free longevity in mice, without cancer promotion [140], and may have potential in telomere attrition found associated with frailty [141]. TA-65 telomerase activator does not increase cancer in mice [142]. Different TERT drugs activate different TERT associated functions (11); i.e., the PGC-1α/TERT (peroxisome proliferator-activated receptor gamma coactivator pathway-1α), activated by drug Catalpol, intervenes in atherosclerosis by downregulation of ROS and inflammation [143] and likely would benefit multiple age-related diseases aggravated by oxidative stress and inflammation. The TERT tissue specific positive effects on endothelial, and pathological effect in vascular smooth muscle and atherosclerosis, are modulated by different supplements yielding conflicting benefit results [15].
14. ANTI CANCER TERT
An unexpected role of telomerase in cancer is impacted by biology of TERT and subsequent chemotherapy [144] that challenges the existing paradigm that indicts telomerase in carcinogenesis, when associates may be the villains.
CONCLUSION
TERT “associates” are anti-cancer targets for downregulation, but upregulation in antiaging therapy. The role of TERT with associates identifies roles for TERT in proteostasis, epigenetics, molecular damage, stress tolerance, metabolism, inflammation, and stem cells, i.e., the seven pillars of aging identified by the Trans-NIH Geroscience Initiative that influence aging and disease. The emerging appreciation of telomerase benefits to health and disease intervention, heart diseases, mental health, and anti-malignancy highlight the urgency for research for targeted telomerase stimulation for improvement of health at any age, delay of age-related pathologies, and encouragement for National Institute on Aging Intervention Testing Program, to research TERT stimulators for treatment without the risk of cancer.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The author declares no conflict of interest, financial or otherwise.
References
- 1.Huang D.S., Wang Z., He X.J., et al. Recurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur. J. Cancer. 2015;51(8):969–976. doi: 10.1016/j.ejca.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiba K., Lorbeer F.K., Shain A.H., et al. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science. 2017;357(6358):1416–1420. doi: 10.1126/science.aao0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich B., Kumar R. TERT promoter mutations in telomere biology. Mutat. Res. 2017;771:15–31. doi: 10.1016/j.mrrev.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Lee D.D., Leão R., Komosa M., et al. DNA hypermethylation within TERT promoter upregulates TERT expression in cancer. J. Clin. Invest. 2019;129(1):223–229. doi: 10.1172/JCI121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis K.A., Tollefsbol T.O. Regulation of the telomerase reverse transcriptase subunit through epigenetic mechanisms. Front. Genet. 2016;7:83. doi: 10.3389/fgene.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greider C.W., Blackburn E.H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 7.Greider C.W., Blackburn E.H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51(6):887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 8.Saretzki G. Telomeres, telomerase and ageing. Subcell. Biochem. 2018;90:221–308. doi: 10.1007/978-981-13-2835-0_9. [DOI] [PubMed] [Google Scholar]
- 9.Amano H, Chaudhury A, Rodriguez-Aguayo C, et al. Telomere dysfunction induces sirtuin repression that drives telomere-dependent disease. 2019. [DOI] [PMC free article] [PubMed]
- 10.Haendeler J., Dröse S., Büchner N., et al. Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler. Thromb. Vasc. Biol. 2009;29(6):929–935. doi: 10.1161/ATVBAHA.109.185546. [DOI] [PubMed] [Google Scholar]
- 11.Haendeler J., Hoffmann J., Brandes R.P., Zeiher A.M., Dimmeler S. Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of tyrosine 707. Mol. Cell. Biol. 2003;23(13):4598–4610. doi: 10.1128/MCB.23.13.4598-4610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saretzki G. Extra-telomeric functions of human telomerase: Cancer, mitochondria and oxidative stress. Curr. Pharm. Des. 2014;20:386–403. doi: 10.2174/1381612820666140630095606. [DOI] [PubMed] [Google Scholar]
- 13.Jaskelioff M., Muller F.L., Paik J.H., et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469(7328):102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Effros R.B. Telomere/telomerase dynamics within the human immune system: Effect of chronic infection and stress. Exp. Gerontol. 2011;46(2-3):135–140. doi: 10.1016/j.exger.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ait-Aissa K., Ebben J.D., Kadlec A.O., Beyer A.M. Friend or foe? Telomerase as a pharmacological target in cancer and cardiovascular disease. Pharmacol. Res. 2016;111:422–433. doi: 10.1016/j.phrs.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quryshi N, Norwood TLE, Ait-Aissa K, Kong A, Beyer AM. 2018. [DOI] [PMC free article] [PubMed]
- 17.Zhou Q.G., Hu Y., Wu D.L., et al. Hippocampal telomerase is involved in the modulation of depressive behaviors. J. Neurosci. 2011;31(34):12258–12269. doi: 10.1523/JNEUROSCI.0805-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Q.G., Liu M.Y., Lee H.W., et al. Hippocampal TERT regulates spatial memory formation through modulation of neural development. Stem Cell Reports. 2017;9(2):543–556. doi: 10.1016/j.stemcr.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avin B.A., Umbricht C.B., Zeiger M.A. Human telomerase reverse transcriptase regulation by DNA methylation, transcription factor binding and alternative splicing. Int. J. Oncol. 2016;49(6):2199–2205. doi: 10.3892/ijo.2016.3743. [Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J., Zhao Y., Wang S. Chromatin and epigenetic regulation of the telomerase reverse transcriptase gene. Protein Cell. 2010;1(1):22–32. doi: 10.1007/s13238-010-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X., Wang Y., Chang G., Wang F., Wang F., Geng X. Alternative splicing of hTERT pre-mRNA: A potential strategy for the regulation of telomerase activity. Int. J. Mol. Sci. 2017;18(3):E567. doi: 10.3390/ijms18030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalaydina R.V., Bajwa K., Qorri B., Decarlo A., Szewczuk M.R. Recent advances in “smart” delivery systems for extended drug release in cancer therapy. Int. J. Nanomedicine. 2018;13:4727–4745. doi: 10.2147/IJN.S168053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan M.S., Liu L.S., Leung H.M., Lo P.K. Cancer-cell-specific mitochondria-targeted drug delivery by dual-ligand-functionalized nanodiamonds circumvent drug resistance. ACS Appl. Mater. Interfaces. 2017;9(13):11780–11789. doi: 10.1021/acsami.6b15954. [DOI] [PubMed] [Google Scholar]
- 24.Lee S.A., Kim B.R., Kim B.K., et al. Heat shock protein-mediated cell penetration and cytosolic delivery of macromolecules by a telomerase-derived peptide vaccine. Biomaterials. 2013;34(30):7495–7505. doi: 10.1016/j.biomaterials.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Dai D.F., Chiao Y.A., Marcinek D.J., Szeto H.H., Rabinovitch P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Punder K., Heim C., Wadhwa P.D., Entringer S. Stress and immunosenescence: The role of telomerase. Psychoneuroendocrinology. 2019;101:87–100. doi: 10.1016/j.psyneuen.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soysal P., Isik A.T., Carvalho A.F., et al. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas. 2017;99:66–72. doi: 10.1016/j.maturitas.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Mikhed Y., Daiber A., Steven S. Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. Int. J. Mol. Sci. 2015;16(7):15918–15953. doi: 10.3390/ijms160715918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forsythe H.L., Jarvis J.L., Turner J.W., Elmore L.W., Holt S.E. Stable association of hsp90 and p23, but not hsp70, with active human telomerase. J. Biol. Chem. 2001;276(19):15571–15574. doi: 10.1074/jbc.C100055200. [DOI] [PubMed] [Google Scholar]
- 30.Freund A., Zhong F.L., Venteicher A.S., et al. Proteostatic control of telomerase function through TRiC-mediated folding of TCAB1. Cell. 2014;159(6):1389–1403. doi: 10.1016/j.cell.2014.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L., Roake C.M., Freund A., et al. An activity switch in human telomerase based on RNA conformation and shaped by TCAB1. Cell. 2018;174(1):218–230. doi: 10.1016/j.cell.2018.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong Y.Y., Her J., Oh S.Y., Chung I.K. Hsp90-binding immunophilin FKBP52 modulates telomerase activity by promoting the cytoplasmic retrotransport of hTERT. Biochem. J. 2016;473(20):3517–3532. doi: 10.1042/BCJ20160344. [DOI] [PubMed] [Google Scholar]
- 33.Lagadari M., Zgajnar N.R., Gallo L.I., Galigniana M.D. Hsp90-binding immunophilin FKBP51 forms complexes with hTERT enhancing telomerase activity. Mol. Oncol. 2016;10(7):1086–1098. doi: 10.1016/j.molonc.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Pomplun S., Sippel C., Hähle A., et al. Chemogenomic profiling of human and microbial FK506-binding proteins. J. Med. Chem. 2018;61(8):3660–3673. doi: 10.1021/acs.jmedchem.8b00137. [DOI] [PubMed] [Google Scholar]
- 35.Hekmatimoghaddam S., Zare-Khormizi M.R., Pourrajab F. Underlying mechanisms and chemical/biochemical therapeutic approaches to ameliorate protein misfolding neurodegenerative diseases. Biofactors. 2017;43(6):737–759. doi: 10.1002/biof.1264. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed S., Passos J.F., Birket M.J., et al. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J. Cell Sci. 2008;121(Pt 7):1046–1053. doi: 10.1242/jcs.019372. [DOI] [PubMed] [Google Scholar]
- 37.Balasubramaniam M, Reis RJS, Phil D, et al. Involvement of tRNAs in replication of human mitochondrial DNA and modifying effects of telomerase. 2017. [DOI] [PubMed]
- 38.Stojkovič G., Makarova A.V., Wanrooij P.H., Forslund J., Burgers P.M., Wanrooij S. Oxidative DNA damage stalls the human mitochondrial replisome. Sci. Rep. 2016;6:28942. doi: 10.1038/srep28942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Indran I.R., Hande M.P., Pervaiz S. hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer Res. 2011;71(1):266–276. doi: 10.1158/0008-5472.CAN-10-1588. [DOI] [PubMed] [Google Scholar]
- 40.Green P.D., Sharma N.K., Santos J.H. Telomerase impinges on the cellular response to oxidative stress through mitochondrial ros-mediated regulation of autophagy. Int. J. Mol. Sci. 2019;20:E1509. doi: 10.3390/ijms20061509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miwa S., Czapiewski R., Wan T., et al. Decreased mTOR signalling reduces mitochondrial ROS in brain via accumulation of the telomerase protein TERT within mitochondria. Aging (Albany NY) 2016;8(10):2551–2567. doi: 10.18632/aging.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miwa S., Saretzki G. Telomerase and mTOR in the brain: The mitochondria connection. Neural Regen. Res. 2017;12(3):358–361. doi: 10.4103/1673-5374.202922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perluigi M., Di Domenico F., Butterfield D.A. mTOR signaling in aging and neurodegeneration: At the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol. Dis. 2015;84:39–49. doi: 10.1016/j.nbd.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 44.Tramutola A., Lanzillotta C., Barone E., et al. Intranasal rapamycin ameliorates Alzheimer-like cognitive decline in a mouse model of Down syndrome. Transl. Neurodegener. 2018;7:28. doi: 10.1186/s40035-018-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Domenico F., Tramutola A., Foppoli C., Head E., Perluigi M., Butterfield D.A. mTOR in Down syndrome: Role in Aß and tau neuropathology and transition to Alzheimer disease-like dementia. Free Radic. Biol. Med. 2018;114:94–101. doi: 10.1016/j.freeradbiomed.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson A., Galvan V., Lin A.L., Oddo S. How longevity research can lead to therapies for Alzheimer’s disease: The rapamycin story. Exp. Gerontol. 2015;68:51–58. doi: 10.1016/j.exger.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dogan F., Biray A.C. Correlation between telomerase and mTOR pathway in cancer stem cells. Gene. 2018;641:235–239. doi: 10.1016/j.gene.2017.09.072. [DOI] [PubMed] [Google Scholar]
- 48.Sundin T., Hentosh P. InTERTesting association between telomerase, mTOR and phytochemicals. Expert Rev. Mol. Med. 2012;14:e8. doi: 10.1017/erm.2012.1. [DOI] [PubMed] [Google Scholar]
- 49.Ahmad F., Dixit D., Sharma V., et al. Nrf2-driven TERT regulates pentose phosphate pathway in glioblastoma. Cell Death Dis. 2016;7:e2213. doi: 10.1038/cddis.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker A., Singh A., Tully E., et al. Nrf2 signaling and autophagy are complementary in protecting breast cancer cells during glucose deprivation. Free Radic. Biol. Med. 2018;120:407–413. doi: 10.1016/j.freeradbiomed.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh A., Happel C., Manna S.K., et al. Transcription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesis. J. Clin. Invest. 2013;123(7):2921–2934. doi: 10.1172/JCI66353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hennig P., Garstkiewicz M., Grossi S., Di Filippo M., French L.E., Beer H.D. The Crosstalk between Nrf2 and Inflammasomes. Int. J. Mol. Sci. 2018;19(2):E562. doi: 10.3390/ijms19020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hou Y., Wang Y., He Q., et al. Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav. Brain Res. 2018;336:32–39. doi: 10.1016/j.bbr.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 54.Doitsh G., Galloway N.L., Geng X., et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505(7484):509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Q., Lenardo M.J., Baltimore D. 30 years of NF-κB: A Blossoming of relevance to human pathobiology. Cell. 2017;168(1-2):37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghosh A., Saginc G., Leow S.C., et al. Telomerase directly regulates NF-κB-dependent transcription. Nat. Cell Biol. 2012;14(12):1270–1281. doi: 10.1038/ncb2621. [DOI] [PubMed] [Google Scholar]
- 57.Chung S.S., Aroh C., Vadgama J.V. Constitutive activation of STAT3 signaling regulates hTERT and promotes stem cell-like traits in human breast cancer cells. PLoS One. 2013;8(12):e83971. doi: 10.1371/journal.pone.0083971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gizard F., Heywood E.B., Findeisen H.M., et al. Telomerase activation in atherosclerosis and induction of telomerase reverse transcriptase expression by inflammatory stimuli in macrophages. Arterioscler. Thromb. Vasc. Biol. 2011;31(2):245–252. doi: 10.1161/ATVBAHA.110.219808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung S.S., Wu Y., Okobi Q., et al. Proinflammatory cytokines IL-6 and TNF-α increased telomerase activity through NF-κB/STAT1/STAT3 activation, and withaferin a inhibited the signaling in colorectal cancer cells. Mediators Inflamm. 2017;2017:5958429. doi: 10.1155/2017/5958429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y., Kong W., Jiang J. Prevention and treatment of cancer targeting chronic inflammation: Research progress, potential agents, clinical studies and mechanisms. Sci. China Life Sci. 2017;60(6):601–616. doi: 10.1007/s11427-017-9047-4. [DOI] [PubMed] [Google Scholar]
- 62.Bagheri S., Nosrati M., Li S., et al. Genes and pathways downstream of telomerase in melanoma metastasis. Proc. Natl. Acad. Sci. USA. 2006;103(30):11306–11311. doi: 10.1073/pnas.0510085103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robey R.B., Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25(34):4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- 64.Patra K.C., Wang Q., Bhaskar P.T., et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24(2):213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roh J.I., Kim Y., Oh J., et al. Hexokinase 2 is a molecular bridge linking telomerase and autophagy. PLoS One. 2018;13(2):e0193182. doi: 10.1371/journal.pone.0193182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roberts D.J., Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015;22(2):364. doi: 10.1038/cdd.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts D.J., Tan-Sah V.P., Ding E.Y., Smith J.M., Miyamoto S. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol. Cell. 2014;53(4):521–533. doi: 10.1016/j.molcel.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner ML, Cronin JG, Noleto PG, Sheldon IM. 2016.
- 69.Palmer C.S., Hussain T., Duette G., et al. Regulators of glucose metabolism in CD4+ and CD8+ T cells. Int. Rev. Immunol. 2016;35(6):477–488. doi: 10.3109/08830185.2015.1082178. [DOI] [PubMed] [Google Scholar]
- 70.Rubtsova M, Naraykina Y, Vasilkova D, et al. Protein encoded in human telomerase RNA is involved in cell protective pathways. 2018. [DOI] [PMC free article] [PubMed]
- 71.Ahmad F., Patrick S., Sheikh T., et al. Telomerase Reverse Transcriptase (TERT) - Enhancer of Zeste Homolog 2 (EZH2) network regulates lipid metabolism and DNA damage responses in glioblastoma. J. Neurochem. 2017;143(6):671–683. doi: 10.1111/jnc.14152. [DOI] [PubMed] [Google Scholar]
- 72.Maida Y., Yasukawa M., Furuuchi M., et al. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature. 2009;461(7261):230–235. doi: 10.1038/nature08283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maida Y., Yasukawa M.I., Masutomi K. De Novo RNA synthesis by RNA-dependent RNA polymerase activity 33 of telomerase reverse transcriptase. Mol. Cell. Biol. 2016;36:1248–1259. doi: 10.1128/MCB.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maida Y., Kyo S., Lassmann T., Hayashizaki Y., Masutomi K. Off-target effect of endogenous siRNA derived from RMRP in human cells. Int. J. Mol. Sci. 2013;14(5):9305–9318. doi: 10.3390/ijms14059305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maida Y., Masutomi K. Telomerase reverse transcriptase moonlights: Therapeutic targets beyond telomerase. Cancer Sci. 2015;106(11):1486–1492. doi: 10.1111/cas.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mukherjee S., Firpo E.J., Wang Y., Roberts J.M. Separation of telomerase functions by reverse genetics. Proc. Natl. Acad. Sci. USA. 2011;108(50):E1363–E1371. doi: 10.1073/pnas.1112414108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mattijssen S., Hinson E.R., Onnekink C., et al. Viperin mRNA is a novel target for the human RNase MRP/RNase P endoribonuclease. Cell. Mol. Life Sci. 2011;68(14):2469–2480. doi: 10.1007/s00018-010-0568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goldfarb K.C., Cech T.R. Targeted CRISPR disruption reveals a role for RNase MRP RNA in human preribosomal RNA processing. Genes Dev. 2017;31(1):59–71. doi: 10.1101/gad.286963.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song H., Sun W., Ye G., et al. Long non-coding RNA expression profile in human gastric cancer and its clinical significances. J. Transl. Med. 2013;11:225. doi: 10.1186/1479-5876-11-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shao Y, Ye M, Li Q, Sun W, Ye G. LncRNA-RMRP promotes carcinogenesis by acting as a miR-206 sponge and is used as a novel biomarker for gastric cancer. 2016. [DOI] [PMC free article] [PubMed]
- 81.Meng Q., Ren M., Li Y., Song X. LncRNA-RMRP acts as an oncogene in lung cancer. PLoS One. 2016;11(12):e0164845. doi: 10.1371/journal.pone.0164845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keklikoglou I., Hosaka K., Bender C., et al. MicroRNA-206 functions as a pleiotropic modulator of cell proliferation, invasion and lymphangiogenesis in pancreatic adenocarcinoma by targeting ANXA2 and KRAS genes. Oncogene. 2015;34(37):4867–4878. doi: 10.1038/onc.2014.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pan J, Zhang D, Zhang J, Qin P, Wang J. 2018.
- 84.De Paepe B., Lefever S., Mestdagh P. How long noncoding RNAs enforce their will on mitochondrial activity: Regulation of mitochondrial respiration, reactive oxygen species production, apoptosis, and metabolic reprogramming in cancer. Curr. Genet. 2018;64(1):163–172. doi: 10.1007/s00294-017-0744-1. [DOI] [PubMed] [Google Scholar]
- 85.Zhao Y., Sun L., Wang R.R., Hu J.F., Cui J. The effects of mitochondria-associated long noncoding RNAs in cancer mitochondria: New players in an old arena. Crit. Rev. Oncol. Hematol. 2018;131:76–82. doi: 10.1016/j.critrevonc.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 86.Wu S., Ge Y., Huang L., Liu H., Xue Y., Zhao Y. BRG1, the ATPase subunit of SWI/SNF chromatin remodeling complex, interacts with HDAC2 to modulate telomerase expression in human cancer cells. Cell Cycle. 2014;13(18):2869–2878. doi: 10.4161/15384101.2014.946834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu Q., Lian J.B., Stein J.L., Stein G.S., Nickerson J.A., Imbalzano A.N. The BRG1 ATPase of human SWI/SNF chromatin remodeling enzymes as a driver of cancer. Epigenomics. 2017;9(6):919–931. doi: 10.2217/epi-2017-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang J., Wang L., Ji Q., Zhu H., Han S. Knockdown of nucleostemin in an ovarian cancer SKOV-3 cell line and its effects on cell malignancy. Biochem. Biophys. Res. Commun. 2017;487(2):262–267. doi: 10.1016/j.bbrc.2017.04.046. [DOI] [PubMed] [Google Scholar]
- 89.Okamoto N., Yasukawa M., Nguyen C., et al. Maintenance of tumor initiating cells of defined genetic composition by nucleostemin. Proc. Natl. Acad. Sci. USA. 2011;108(51):20388–20393. doi: 10.1073/pnas.1015171108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maida Y., Yasukawa M., Okamoto N., et al. Involvement of telomerase reverse transcriptase in heterochromatin maintenance. Mol. Cell. Biol. 2014;34(9):1576–1593. doi: 10.1128/MCB.00093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Claycomb J.M., Batista P.J., Pang K.M., et al. The Argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation. Cell. 2009;139(1):123–134. doi: 10.1016/j.cell.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sugiyama T., Cam H., Verdel A., Moazed D., Grewal S.I. RNA-dependent RNA polymerase is an essential component of a self-enforcing loop coupling heterochromatin assembly to siRNA production. Proc. Natl. Acad. Sci. USA. 2005;102(1):152–157. doi: 10.1073/pnas.0407641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lassmann T., Maida Y., Tomaru Y., et al. Telomerase reverse transcriptase regulates microRNAs. Int. J. Mol. Sci. 2015;16(1):1192–1208. doi: 10.3390/ijms16011192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Masutomi K., Possemato R., Wong J.M., et al. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc. Natl. Acad. Sci. USA. 2005;102(23):8222–8227. doi: 10.1073/pnas.0503095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meena J.K., Cerutti A., Beichler C., et al. Telomerase abrogates aneuploidy-induced telomere replication stress, senescence and cell depletion. EMBO J. 2015;34(10):1371–1384. doi: 10.15252/embj.201490070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim W.Y., Sharpless N.E. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127(2):265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 97.He S., Sharpless N.E. Senescence in health and disease. Cell. 2017;169(6):1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Z., Gao J., Zhou J., Liu H., Xu C. Olaparib induced senescence under P16 or P53 dependent manner in ovarian cancer. J. Gynecol. Oncol. 2019;30(2):e26. doi: 10.3802/jgo.2019.30.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu JY, Souroullas GP, Diekman BO, et al. 2019.
- 100.Bazarov A.V., Van Sluis M., Hines W.C., et al. p16 (INK4a) -Mediated suppression of telomerase in normal and malignant human breast cells. Aging Cell. 2010;9(5):736–746. doi: 10.1111/j.1474-9726.2010.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haga K., Ohno S., Yugawa T., et al. Efficient immortalization of primary human cells by p16INK4a-specific short hairpin RNA or Bmi-1, combined with introduction of hTERT. Cancer Sci. 2007;98(2):147–154. doi: 10.1111/j.1349-7006.2006.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meng J., Tong Q., Liu X., Yu Z., Zhang J., Gao B. Epigallocatechin-3-gallate inhibits growth and induces apoptosis in esophageal cancer cells through the demethylation and reactivation of the p16 gene. Oncol. Lett. 2017;14(1):1152–1156. doi: 10.3892/ol.2017.6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moradzadeh M., Hosseini A., Erfanian S., Rezaei H. Epigallocatechin-3-gallate promotes apoptosis in human breast cancer T47D cells through down-regulation of PI3K/AKT and Telomerase. Pharmacol. Rep. 2017;69(5):924–928. doi: 10.1016/j.pharep.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 104.Hannon G.J., Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 105.Kumar R., Gont A., Perkins T.J., Hanson J.E.L., Lorimer I.A.J. Induction of senescence in primary glioblastoma cells by serum and TGFβ. Sci. Rep. 2017;7(1):2156. doi: 10.1038/s41598-017-02380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Del Bufalo D., Rizzo A., Trisciuoglio D., et al. Involvement of hTERT in apoptosis induced by interference with Bcl-2 expression and function. Cell Death Differ. 2005;12(11):1429–1438. doi: 10.1038/sj.cdd.4401670. [DOI] [PubMed] [Google Scholar]
- 107.Cummins N.W., Sainski-Nguyen A.M., Natesampillai S., Aboulnasr F., Kaufmann S., Badley A.D. Maintenance of the HIV reservoir is antagonized by selective BCL2 inhibition. J. Virol. 2017;91(11):e00012–e00017. doi: 10.1128/JVI.00012-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oh Y.S., Jeong S.G., Cho G.W. Anti-senescence effects of DNA methyltransferase inhibitor RG108 in human bone marrow mesenchymal stromal cells. Biotechnol. Appl. Biochem. 2015;62(5):583–590. doi: 10.1002/bab.1393. [DOI] [PubMed] [Google Scholar]
- 109.Brueckner B., Garcia B.R., Siedlecki P., et al. Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA methyltransferases. Cancer Res. 2005;65(14):6305–6311. doi: 10.1158/0008-5472.CAN-04-2957. [DOI] [PubMed] [Google Scholar]
- 110.Iannilli F., Zalfa F., Gartner A., Bagni C., Dotti C.G. Cytoplasmic TERT associates to RNA granules in fully mature neurons: Role in the translational control of the cell cycle inhibitor p15INK4B. PLoS One. 2013;8(6):e66602. doi: 10.1371/journal.pone.0066602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spilsbury A., Miwa S., Attems J., Saretzki G. The role of telomerase protein TERT in Alzheimer’s disease and in tau-related pathology in vitro. J. Neurosci. 2015;35(4):1659–1674. doi: 10.1523/JNEUROSCI.2925-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eitan E., Tichon A., Daniel G., Priel E. Telomerase expression in adult and old mouse Purkinje neurons. Rejuvenation Res. 2012;15(2):206–209. doi: 10.1089/rej.2011.1285. [DOI] [PubMed] [Google Scholar]
- 113.Eitan E., Braverman C., Tichon A., et al. Excitotoxic and radiation stress increase TERT levels in the mitochondria and cytosol of cerebellar purkinje neurons. Cerebellum. 2016;15(4):509–517. doi: 10.1007/s12311-015-0720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koh C.M., Khattar E., Leow S.C., et al. Telomerase regulates MYC-driven oncogenesis independent of its reverse transcriptase activity. J. Clin. Invest. 2015;125(5):2109–2122. doi: 10.1172/JCI79134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu K.J., Grandori C., Amacker M., et al. Direct activation of TERT transcription by c-MYC. Nat. Genet. 1999;21(2):220–224. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- 116.Bouchard C., Thieke K., Maier A., et al. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J. 1999;18(19):5321–5333. doi: 10.1093/emboj/18.19.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Staller P., Peukert K., Kiermaier A., et al. Repression of p15INK4b expression by Myc through association with Miz-1. Nat. Cell Biol. 2001;3(4):392–399. doi: 10.1038/35070076. [DOI] [PubMed] [Google Scholar]
- 118.Claassen G.F., Hann S.R. A role for transcriptional repression of p21CIP1 by c-Myc in overcoming transforming growth factor β -induced cell-cycle arrest. Proc. Natl. Acad. Sci. USA. 2000;97(17):9498–9503. doi: 10.1073/pnas.150006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zheng L., Suzuki H., Nakajo Y., Nakano A., Kato M. Regulation of c-MYC transcriptional activity by transforming growth factor-beta 1-stimulated clone 22. Cancer Sci. 2018;109(2):395–402. doi: 10.1111/cas.13466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baena-Del V.J.A., Zheng Q., Esopi D.M., et al. MYC drives overexpression of telomerase RNA (hTR/TERC) in prostate cancer. J. Pathol. 2018;244(1):11–24. doi: 10.1002/path.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eriksson P.S., Perfilieva E., Björk-Eriksson T., et al. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 122.Spalding K.L., Bhardwaj R.D., Buchholz B.A., Druid H., Frisén J. Retrospective birth dating of cells in humans. Cell. 2005;122(1):133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 123.Spalding K.L., Bergmann O., Alkass K., et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eitan E., Tichon A., Gazit A., Gitler D., Slavin S., Priel E. Novel telomerase-increasing compound in mouse brain delays the onset of amyotrophic lateral sclerosis. EMBO Mol. Med. 2012;4(4):313–329. doi: 10.1002/emmm.201200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lieberwirth C., Pan Y., Liu Y., Zhang Z., Wang Z. Hippocampal adult neurogenesis: Its regulation and potential role in spatial learning and memory. Brain Res. 2016;1644:127–140. doi: 10.1016/j.brainres.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fu W., Killen M., Culmsee C., Dhar S., Pandita T.K., Mattson M.P. The catalytic subunit of telomerase is expressed in developing brain neurons and serves a cell survival-promoting function. J. Mol. Neurosci. 2000;14:3–15. doi: 10.1385/JMN:14:1-2:003. [DOI] [PubMed] [Google Scholar]
- 127.Khacho M., Slack R.S. Mitochondrial dynamics in the regulation of neurogenesis: From development to the adult brain. Dev. Dyn. 2018;247(1):47–53. doi: 10.1002/dvdy.24538. [DOI] [PubMed] [Google Scholar]
- 128.Liu M.Y., Nemes A., Zhou Q.G. The emerging roles for telomerase in the central nervous system. Front. Mol. Neurosci. 2018;11:160. doi: 10.3389/fnmol.2018.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Beyer A.M., Freed J.K., Durand M.J., et al. Critical role for telomerase in the mechanism of flow-mediated dilation in the human microcirculation. Circ. Res. 2016;118(5):856–866. doi: 10.1161/CIRCRESAHA.115.307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ait-Aissa K., Heisner J.S., Norwood T.L.E., et al. Telomerase deficiency predisposes to heart failure and ischemia-reperfusion injury. Front. Cardiovasc. Med. 2019;6:31. doi: 10.3389/fcvm.2019.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mouraret N., Houssaïni A., Abid S., et al. Role for telomerase in pulmonary hypertension. Circulation. 2015;131(8):742–755. doi: 10.1161/CIRCULATIONAHA.114.013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sharifi-Sanjani M., Oyster N.M., Tichy E.D., et al. Cardiomyocyte-specific telomere shortening is a distinct signature of heart failure in humans. J. Am. Heart Assoc. 2017;7:6. doi: 10.1161/JAHA.116.005086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim H., Seo E.H., Lee S.H., Kim B.J. The telomerase-derived anticancer peptide vaccine GV1001 as an extracellular heat shock protein-mediated cell-penetrating peptide. Int. J. Mol. Sci. 2016;17(12):2054. doi: 10.3390/ijms17122054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Choi J., Kim H., Kim Y., et al. The anti-inflammatory effect of GV1001 mediated by the down regulation of ENO1-induced pro-inflammatory cytokine production. Immune Netw. 2015;15(6):291–303. doi: 10.4110/in.2015.15.6.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ko Y.J., Kwon K.Y., Kum K.Y., et al. The anti-inflammatory effect of human telomerase-derived peptide on P. gingivalis lipopolysaccharide-induced inflammatory cytokine production and its mechanism in human dental pulp cells. Mediators Inflamm. 2015;2015:385127. doi: 10.1155/2015/385127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koo T.Y., Yan J.J., Yang J. Protective effect of peptide GV1001 against renal ischemia-reperfusion injury in mice. Transplant. Proc. 2014;46(4):1117–1122. doi: 10.1016/j.transproceed.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 137.Lee S.A., Kim J., Sim J., et al. A telomerase-derived peptide regulates reactive oxygen species and hepatitis C virus RNA replication in HCV-infected cells via heat shock protein 90. Biochem. Biophys. Res. Commun. 2016;471(1):156–162. doi: 10.1016/j.bbrc.2016.01.160. [DOI] [PubMed] [Google Scholar]
- 138.Kim H., Choi M.S., Inn K.S., Kim B.J. Inhibition of HIV-1 reactivation by a telomerase-derived peptide in a HSP90-dependent manner. Sci. Rep. 2016;6:28896–28906. doi: 10.1038/srep28896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Park H.H., Lee K.Y., Kim S., et al. Novel vaccine peptide GV1001 effectively blocks β-amyloid toxicity by mimicking the extra-telomeric functions of human telomerase reverse transcriptase. Neurobiol. Aging. 2014;35(6):1255–1274. doi: 10.1016/j.neurobiolaging.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 140.Chen W., Shin K.H., Kim S., et al. hTERT peptide fragment GV1001 demonstrates radio protective and antifibrotic effects through suppression of TGF-β signaling. Int. J. Mol. Med. 2018;41(6):3211–3220. doi: 10.3892/ijmm.2018.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jakob S., Haendeler J. Molecular mechanisms involved in endothelial cell aging: Role of telomerase reverse transcriptase. Z. Gerontol. Geriatr. 2007;40(5):334–338. doi: 10.1007/s00391-007-0482-y. [DOI] [PubMed] [Google Scholar]
- 142.Bernardes de Jesus B., Schneeberger K., Vera E., Tejera A., Harley C.B., Blasco M.A. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell. 2011;10(4):604–621. doi: 10.1111/j.1474-9726.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang Y., Wang C., Jin Y., et al. Activating the PGC-1α/TERT pathway by catalpol ameliorates atherosclerosis via modulating ROS production, DNA damage, and telomere function: Implications on mitochondria and telomere link. Oxid. Med. Cell. Longev. 2018;2018:2876350. doi: 10.1155/2018/2876350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Smith S.J. How telomerase biology affects cancer biology, chemotherapy aging.; Proceedings of the 36th World Cancer Conference; Oct 11-13 2018; Zurich Switzerland. [Google Scholar]