Abstract
Background
Access to healthcare delivery programs and systems is a primary correlate to the overall health and well-being of Veterans and the general population. Participation in clinical research is a gateway to novel therapies that are intended to address current global health issues. Meeting or exceeding recruitment goals in clinical research is one of the key determinants of the timely and successful completion of a study. The travel and time burdens experienced by study participants are often considered barriers to their enrollment into clinical research. The Department of Veterans Affairs (VA) Cooperative Studies Program (CSP) established a consortium of nine VA medical centers (VAMCs) called the Network of Dedicated Enrollment Sites (NODES). The NODES program provides study site-level expertise and innovative approaches that address challenges to clinical research execution. In alignment with our mission, our program developed an approach to increase study participant access to clinical research through implementing “Mobile Recruitment (MoRe)” units. This manuscript describes the utility and challenges associated with employing this strategy to address three common barriers to clinical research participation: 1) research participant travel burden, 2) participant access to study opportunities, and 3) low participant enrollment.
Methods
A plan to introduce the Mobile Recruitment (MoRe) unit as a recruitment strategy was piloted for a high-volume, observational cohort study and mega biobank in the VA health care system, the “Million Veteran Program (MVP)”. MoRe is a recruitment strategy for CSP research integrating mobile technology and atypical research recruitment locations. Recruitment locations include primary or main VA hospitals and their assigned VA Community-Based Outpatient Clinics (CBOCs). Each Node site (n = 9) received components of the MoRe unit including a laptop, printer, portable cart with storage space, cooler/ice packs for specimen storage and transport. Each site's usage of these components varied based on its respective needs. Activities focused on both VA main facilities and CBOC facilities for recruitment.
Results
Seven of the nine Node sites compared the effectiveness of the MoRe unit on MVP study enrollment outcomes over three-time points: pre-intervention period, intervention period, and post-intervention period. The utilization of MoRe in the intervention period demonstrated a 36.9% increase in enrollment compared to the previous six months (pre-intervention period). There was a 2% enrollment increase at the six-month post-intervention period as compared to the intervention period. When comparing the pre-intervention period to the post-intervention period (duration of eighteen months), enrollment increased by 38.9%.
Conclusion
Five of the seven sites experienced an increase in enrollment during the intervention and post-intervention periods. The two sites without an increase in enrollment experienced various extenuating factors. Characteristics of sites using MoRe included the ability to utilize a smaller, unconventional space, i.e. not a traditional clinical research exam space for recruitment. MoRe was utilized in hospital laboratory space, CBOCs, primary care clinics, and other subspecialty clinics that allowed recruitment activities but did not have dedicated space to offer the research teams for that purpose. This initiative successfully demonstrated the benefit of deploying the unit, proving its utility in cases in where there was a lack of space or alternative workstations for research activities. The implementation of MoRe by NODES as a recruitment strategy for MVP may be transferable to other VA clinical research studies, as well as to other healthcare settings executing similar clinical research activities.
Keywords: Mobile recruitment, Recruitment barriers, Veteran research, Access to research, Innovative enrollment strategies
1. Introduction
Access to comprehensive and quality healthcare services is a primary correlate to the overall health and well-being of our nation [3,22]. Clinical care emphasizes the positive effects patients receive from medical interventions, while research advances clinical care by directly addressing questions and needs relevant to daily clinical practice [8,17]. The Department of Veterans Affairs (VA), which runs the nation's largest integrated healthcare system, utilizes clinical research as a key source of comparative evidence on effective clinical treatments that are employed within this health care system [29].
Successful recruitment into clinical research is a key determinant for timely introduction of novel treatment modalities or more effective therapies in clinical practice [7,16]. However, recruitment into these studies often encounters hurdles that prolong the study completion timeline, and therefore impede the progression of advances in care and adoption of research findings [21,32]. Travel and time burden on trial participants has been reported to cause significant delays on study execution and completion, although leveraging electronic technologies to address travel burden on participants has been suggested as a feasible solution in clinical research practice [4,10,11,25].
Technological revolution has provided opportunities for integrating digital resources into clinical research activities [1,24,26]. Some studies have incorporated electronic technologies into research using internet and mobile services for clinical research recruitment and retention, electronic consent processes, internet-based interventions, and for web-based data collection and study result dissemination [26].
The VA Cooperative Studies Program (CSP) is a clinical research infrastructure embedded within the nation's largest integrated healthcare system [15]. The program, a division of the VA Office of Research and Development (ORD), was established to provide coordination for and enable cooperation on multi-site clinical trials and epidemiological studies that fall within the purview of VA and its infrastructure is comprised of a number of Coordinating Centers that are responsible for the planning and conduct of large multi-site clinical trials in the VA [27]. CSP also established a consortium of nine VA medical centers (VAMCs) called the Network of Dedicated Enrollment Sites (NODES) that have teams (Nodes) to provide site-level expertise and innovative approaches in addressing challenges to clinical trial execution [2,9,18,30]. NODES incorporated the Mobile Recruitment (MoRe) unit as a recruitment strategy to expand patient access to clinical research and enhance study enrollment. MoRe utilized digital technology that allowed research teams to meet participants in areas of their preference and convenience, as opposed to putting the onus on participants to travel to the research team to participate in research. This manuscript discusses the use of mobile recruitment as a strategy, which employed mobile technologies to potentially reduce study participant travel and time burden, improve access to clinical research, and thus enhance enrollment for Veterans interested in participating in a VA-sponsored, observational cohort study and mega bio-bank, the “Million Veteran Program (MVP)” [13].
2. Methods
2.1. MoRe plan development
In 2014, the NODES program was tasked with the development of several strategies that would improve study enrollment across CSP's portfolio of clinical trials and epidemiologic studies. This effort resulted in the creation of several workgroups, each with a distinct focus on a specific phase of the overall clinical research study cycle. The workgroups examined the current processes in their assigned phases of the study cycle to determine the gaps/weaknesses that served as barriers to study execution and the overall enrollment of participants. One workgroup focused on the development of innovative strategies that would increase access to clinical research for study participants. Mobile Recruitment (MoRe) was proposed as a recruitment strategy for CSP research that would utilize MoRe units as its primary approach to executing that strategy. The MoRe unit utilizes mobile technology for recruitment in a pop-up mobile research recruitment location. This strategy can be used across VA healthcare facilities including the primary or main VA hospital, and their satellite clinics which are called VA Community-Based Outpatient Clinics (CBOCs) [14]. VA CBOCs were established to increase access to primary and other subspecialty care services for Veterans [23]. The establishment and utilization of these clinics to improve access to these services has increased over time with 64% of Veterans receiving their care in these locations [12,20]. The workgroup's efforts centered on creating the MoRe unit and developing standard operating procedures for its use at each Node site (n = 9).
The workgroup identified and selected the Million Veterans Program (MVP), an observational research study, to pilot the MoRe recruitment strategy, based on the following considerations: 1) the study sponsor agreed to the proposal; 2) all Node sites participated as MVP study sites; 3) it was an observational study with simple inclusion criteria (i.e., Veterans using the VA healthcare system for their medical care); and 4) study participants typically completed enrollment in a single visit. It was proposed that the recruitment strategy would be explored for VA clinical trials following the outcome of this pilot.
2.2. Implementation
CSP purchased and distributed components of the MoRe unit for study recruitment efforts. Each MoRe unit included the components listed in Table 1, pictured in Fig. 1, and each site's usage of these components varied based on its respective needs. The cost of each MoRe unit with all components was approximately $2040 in 2014. Utilization of the MoRe unit did not require local institutional approval because most of its components were classified as standard office supplies. The laptops were the only component of the MoRe unit that required local approval from each site's Office of Information and Technology (OI&T) because of the need to interface with the VA Networks and Infrastructure Domain. Compliance with the VA's Information Security policies, specifically, enhanced data security encryption requirements, ensured the highest level of protection of patient data and sensitive information [28].
Table 1.
Mobile recruitment (MoRe) unit.
| MoRe Equipment | MoRe Blood Draw |
|---|---|
| 1. Laptop | 1. Portable [Phlebotomy] Arm Stand |
| 2. Laptop Bag | 2. Phlebotomy Lockable Supply Tote |
| 3. Portable printer/copier/scanner | 3. Portable Privacy Screen |
| 4. Collapsible cart | 4. Insulated Specimen Cooler |
Fig. 1.
Nodes mobile recruitment (MoRe) unit.
Each Node site developed a process that was conducive to recruitment of participants from either their main VA medical facility or one of their VA CBOCs. It was based on each site's respective clinical infrastructure and research operational model. NODES, MVP site teams, and the CSP Coordinating Center submitted an amendment request to the VA Central Institutional Review Board (CIRB) for approval to conduct research activities at the CBOCs that were aligned with each VA medical facility participating as an MVP study site. Since some VA CBOCs are contract facilities i.e. these facilities are not VA-operated clinics but are VA-funded or reimbursed health care facilities that are separate from the main VA medical facility, these sites were not approved by the CIRB to serve as locations for research participant recruitment [6]. Once the approval to recruit at the VA CBOCs was obtained, meetings were scheduled with CBOC Clinical Managers to discuss the operational logistics of conducting research activities at their clinics before research patient visits were scheduled. These clinical managers were receptive because using MoRe required minimal resources from the CBOC. The MoRe unit enabled study teams to conduct all research activities within an unconventional area in the clinic e.g. conference room, office area, etc. The MoRe unit components provided participant privacy and confidentiality during the recruitment process; the ability to provide printed and signed copies of the informed consent and the Health Insurance Portability and Accountability Act of 1996 (HIPAA) documents to the study participant; supplies to collect and store blood samples; and a mechanism to secure original research documents for authorized transport to the main VA medical facility.
One Node site did not use the MoRe unit at the VA CBOCs as these satellite clinics only provided primary care services to its patients, and these patients visited the main VA medical facility for their specialty care. As a result, the patient pool at the CBOCs was not unique and these patients were approached for the study when they came to the main facility for their specialty care needs. This site used the same procedure for recruitment at their main VA medical facility. The study team at this site leveraged the mobile technology to recruit from different clinics (e.g., specialty clinics, outpatient laboratory, etc.) within the main hospital where recruitment was not otherwise possible due to lack of dedicated research space. Other Node sites used the MoRe unit to recruit in the main medical facility when dedicated space was unavailable, and when not recruiting study participants from the CBOCs.
Once the recruitment strategy was employed, the recruitment data was analyzed prior to and after implementation of the MoRe to determine its impact on study recruitment. It was believed that the recruitment strategy utilizing the MoRe unit could be easily translated to clinical trials within the VA healthcare system if proven effective during its use on the MVP.
3. Results
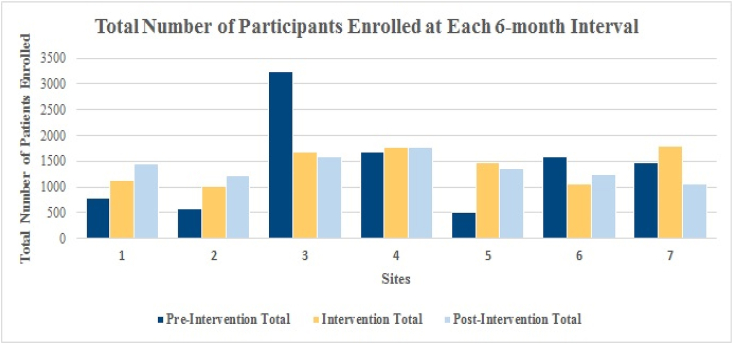
Node sites implemented the MoRe unit as a recruitment strategy to enroll Veterans into the MVP. Two of the nine Node sites were not included in the outcome analysis as they had previously utilized a similar approach to MoRe, including the use of comparable equipment. These two sites also executed research participant recruitment activities at CBOCs prior to the launch of the recruitment strategy utilizing the MoRe unit. Enrollment outcomes were examined over three-time points during an eighteen-month period at the seven participating sites: pre-intervention, intervention and post-intervention. Fig. 2 demonstrates the number of participants enrolled during the pre-intervention, intervention and post-intervention periods. Across these sites, the intervention period of MoRe integration demonstrated a 36.9% increase in enrollment compared to the previous six months (pre-intervention period). There was a 2% enrollment increase at the six-month post-intervention period as compared to the intervention period. When comparing the pre-intervention period to the post-intervention period (eighteen-months), overall enrollment increased by 38.9% (Table 2).
Fig. 2.
Total number of participants enrolled.
*All sites (n = 7) collected 18-months of enrollment data, in 6-month intervals.
Table 2.
Mobile Recruitment (MoRe) Unit Effectiveness on Study Enrollment Outcomes.
| NODE Site | % Improvement |
Factors Influencing Enrollment |
||
|---|---|---|---|---|
| INTVa | Post-INTVb | INTVa | Post-INTVb | |
| Site 1 | 41.8% | 28.9% |
|
|
| Site 2 | 73.8% | 19.8% |
|
|
| Site 3 | −48.0% | −6.5% |
|
|
| Site 4 | 5.6% | 0.7% |
|
|
| Site 5 | 196.4% | −7.7% |
|
|
| Site 6 | −33.4% | 19.0% | ||
| Site 7 | 21.8% | −40.6% | ||
NOTE: Seven of nine Node sites participated in the MoRe intervention evaluation. Initiation of MoRe and time point dates varied by site. All sites (n = 7) collected 18-months of data, in 6-month intervals.
INTV = Intervention, the 6-month period when the intervention was completed, and data were collected.
Post-INTV = Post-Intervention, the 6-month period of data immediately after the intervention period.
FTE = Full-time equivalent (FTE), a unit that indicates the workload of an employed person. E.g., 3.5 = 3 fulltime staff +1 part-time staff.
CBOC = Community Based Outpatient Clinic, which became additional study recruitment/enrollment sites within each respective VA Medical Center or Health Care System.
M − F = Monday through Friday.
WOC = Without compensation employee – VA Research Administration approved volunteer, unpaid.
4. Discussion
Participant recruitment for clinical research is difficult across various types of institutions and medical conditions. The burden of time and travel on participants has been reported as a common barrier to recruitment [4,5,11,19,31]. Although the impact of mobile technology on participant time and travel burden was not directly measured using the MoRe unit, the recruitment outcome utilizing this recruitment strategy is demonstrated in this paper. The MoRe unit expanded MVP recruitment opportunities to 27 additional locations outside the main VA medical facility. MoRe provided flexibility and convenience to the participants by allowing Veterans an opportunity to join MVP without adding extra burden of searching for the research team or location. The MoRe unit offered an intervention that was correlated with improved study participant recruitment in MVP by facilitating research activities in an efficient manner at multiple locations, as opposed to a single, stationary location, that were convenient to the study participants.
The recruitment outcomes of employing the MoRe unit for MVP at seven sites over a period of eighteen months are illustrated in this paper. Five of the seven sites (71%) experienced an increase in enrollment after the initiation of MoRe through the end of the intervention. Four of the seven sites (57%) continued to show an increase from the intervention period through the six-month follow-up period, post intervention (Fig. 2). The sites that did not demonstrate an increase had other confounding factors that contributed to recruitment disruption (Table 2) including but not limited to, reduction in staffing support.
Success during the intervention period was not solely attributable to the implementation of MoRe. There was a direct correlation between the increase in research staff and the success of MoRe (Table 2). To operate MoRe, adequate staffing at each site was essential. Recruitment at the main facility as well as in CBOCs required two or more staff members. The utilization of MoRe, and the number of staff members working on a team, both played a significant role in increasing enrollment in the MVP. While the specific factors (i.e., people or technology) for increased recruitment cannot be differentiated, it highlights that technology is still reliant upon personnel to carry out the strategy. Consequently, budgeting for efforts cannot simply consider equipment/technical requirements. Personnel were essential in deploying the MoRe unit at multiple locations, thereby creating the perfect conditions for participation in MVP. Both components, personnel and technology, enabled a potential reduction in time and travel burden on the participant, the ability to conduct research in an atypical space, and the ability to enhance enrollment by being available in a variety of settings were more important factors than the cost-savings to a study. Each site that had a staffing increase and concurrently implemented the MoRe unit, demonstrated an increase in participant enrollment. When staffing was reduced, the MoRe unit was less likely to be utilized, and subsequently, participant enrollment decreased. Adequate staffing is an important factor for offering recruitment opportunities at multiple locations which can have a direct correlation to participant time/travel burden.
There are several potential limitations related to the design and methodology of this pilot that may impact the generalizability of the results. Not all sites implemented the MoRe unit at the same time or with the same components of the unit. Some sites implemented it at one CBOC location while others utilized it with varying schedules at multiple CBOCs. Staffing gains and losses were not consistent across all sites and these variations were unpredictable, impacting enrollment during different phases of the eighteen-month period. Utilization of the MoRe unit for MVP, which is an observational study with simple inclusion criteria and not a clinical trial, should also be considered as a limitation of this initiative. The participants' perception on lessening the travel and time burden utilizing this recruitment strategy was not explored in this pilot. Another potential limitation is that while MoRe was unique to NODES, it could be assumed that the research teams’ presence in locations not otherwise utilized for research purposes either at the main VA facilities or in CBOCs may have helped to promote the study among the Veterans. Since 2011, MVP has attempted to reach as many Veterans nationwide as possible through targeted advertisements, letters to many Veterans registered at VA facilities, and through multipronged marketing campaigns nationwide, including the participating study sites. The pilot did not assess the frequency or impact of strategies, including increased awareness, employed by study teams or the National Program in relation to utilizing the MoRe units. This could be considered one of its limitations. The applicability of the MoRe unit for recruitment and for other research procedures in clinical trials remains to be examined.
Despite the limitations, the pilot demonstrated several key strengths. It illustrated that recruitment barriers are mitigated using mobile technology if research is conducted at a place of convenience for participants; if research teams extend coverage to more than one location to increase the pool of eligible participants; and if creative strategies and tactics are developed and implemented when local resources are scarce (e.g., space for research is restricted or when the target population is reduced).
Overall, the implementation of the MoRe unit was considered successful in providing Veterans with additional opportunities to participate in MVP by bringing the study to Community Based Outpatient Clinics, where MVP had not previously been offered. Many Veterans prefer CBOCs due to the proximity to their home, reduced traffic, and/or more available parking than at the main VA medical facility. The concept of the MoRe unit is transferrable to any research center and may be applied to a clinical trial within or outside the Department of Veterans Affairs Healthcare System. It is feasible, cost-effective, and an easily available recruitment strategy that can address barriers to successful recruitment of research participants.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the government of the United States.
Contributor Information
Danielle Beck, Email: danielle.beck@va.gov.
the VA Cooperative Studies Program (CSP) Network of Dedicated Enrollment Sites (NODES):
Terence M. Keane, Pantel S. Vokonas, Dan Darroch, James LePage, Jennifer Compton, David Leehey, Conor McBurney, Stephanie Keen, Panagiotis Kougias, Sarah Perusich, Michael E. DeBakey, Timothy Morgan, Karyn Isip, Selcuk Adabag, Marti Donaire, Debra K. Johnson, Trisha Suppes, Karen Bratcher, Ann N. Roseman, Merritt Raitt, Daniel Clegg, Jennifer Romesser, Kandi Velarde, Cicilia Velarde, Christina Nessler, Sunder Mudaliar, Murray Stein, and Catherine DeLue
References
- 1.Andriesen J., Bull S., Dietrich J., Haberer J.E., Van Der Pol B., Voronin Y.…Priddy F. Using digital technologies in clinical HIV research: real world applications and considerations for future work. J. Med. Internet Res. 2017;19(7):e274. doi: 10.2196/jmir.7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakaeen F.G., Reda D.J., Gelijns A.C., Cornwell L., Omer S., Al Jurdi R., Huang G.D. Department of veterans Affairs cooperative studies program Network of dedicated enrollment sites: implications for surgical trials. JAMA Surg. 2014;149(6):507–513. doi: 10.1001/jamasurg.20134150. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt J., Bathija P. Ensuring access to quality health care in vulnerable communities. Acad. Med.: journal of the Association of American Medical Colleges. 2018;93(9):1271–1275. doi: 10.1097/ACM.0000000000002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borno H.T., Zhang L., Siegel A., Chang E., Ryan C.J. At what cost to clinical trial enrollment? A retrospective study of patient travel burden in cancer clinical trials. Oncol. 2018;23(10):1242–1249. doi: 10.1634/theoncologist.2017-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang B.H., Hendricks A.M., Slawsky M.T., Locastro J.S. Patient recruitment to a randomized clinical trial of behavioral therapy for chronic heart failure. BMC Med. Res. Methodol. 2004;4:8. doi: 10.1186/1471-2288-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapko M.K., Hedeen A., Maciejewski M., Fortney J., Borowsky S. CBOC performance evaluation, program implications and future performance measures. 2000. https://www.research.va.gov/resources/pubs/docs/cboc_imp.pdf
- 7.Chin Feman S.P., Nguyen L.T., Quilty M.T., Kerr C.E., Nam B.H., Conboy L.A., …. Davis R.B. Effectiveness of recruitment in clinical trials: an analysis of methods used in a trial for irritable bowel syndrome patients. Contemp. Clin. Trials. 2008;29(2):241–251. doi: 10.1016/j.cct.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen P.D., Herman L., Jedlinski S., Willocks P., Wittekind P. Ethical issues in clinical neuroscience research: a patient's perspective. Neurotherapeutics. 2007;4(3):537–544. doi: 10.1016/j.nurt.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Condon D.L., Beck D., Kenworthy-Heinige T., Bratcher K., O'Leary M., Asghar A.…Huang G.D. vol. 6. The Department of Veterans Affairs' Network of Dedicated Enrollment Sites (NODES) model. Contemporary Clinical Trials Communications; 2017. A Cross-Cutting Approach to Enhancing Clinical Trial Site Success; pp. 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fogel D.B. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemporary clinical trials communications. 2018;11:156–164. doi: 10.1016/j.conctc.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford J.G., Howerton M.W., Lai G.Y., Gary T.L., Bolen S., Gibbons M.C., Bass E.B. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008 Jan 15;112(2):228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 12.Fortney J., Enderle M., McDougall S., Clothier J., Otero J., Altman L., Curran G. Implementation outcomes of evidence-based quality improvement for depression in VA community based outpatient clinics. Implement. Sci.: ISCUS. 2012;7:30. doi: 10.1186/1748-5908-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaziano J.M., Concato J., Brophy M., Fiore L., Pyarajan S., Breeling J.…O'Leary T.J. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J. Clin. Epidemiol. 2016;70:214–223. doi: 10.1016/j.jclinepi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Hedeen A.N., Heagerty P.J., Fortney J.C., Borowsky S.J., Walder D.J., Chapko M.K. VA community-based outpatient clinics: quality of care performance measures. Med. Care. 2002;40(7):570–577. doi: 10.1097/00005650-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Huang G.D., Ferguson R.E., Peduzzi P.N., O'Leary T.J. Scientific and organizational collaboration in comparative effectiveness research: the VA Cooperative Studies Program model. Am. J. Med. 2010;123(12 Suppl 1):e24–31. doi: 10.1016/j.amjmed.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Huang G.D., Bull J., Johnston McKee K., Mahon E., Harper B., Roberts J.N., CTTI recruitment project team. Clinical trials recruitment planning: a proposed framework from the clinical trials transformation initiative. Contemp. Clin. Trials. 2018;66:74–79. doi: 10.1016/j.cct.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Institute of Medicine (US) In: Patients Charting the Course: Citizen Engagement and the Learning Health System: Workshop Summary. Olsen L.A., Saunders R.S., McGinnis J.M., editors. vol. 2. National Academies Press (US); Washington (DC): 2011. https://www.ncbi.nlm.nih.gov/books/NBK92063/ Clinical Research, Patient Care, and Learning That Is Real-Time and Continuous. [PubMed] [Google Scholar]
- 18.Johnson M.R., Kenworthy-Heinige T., Beck D.J., Asghar A., Broussard E., Bratcher K.…Planeta B. Research site mentoring: a novel approach to improving study recruitment. Contemporary Clinical Trials Communications. 2018;9:172–177. doi: 10.1016/j.conctc.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlawish J., Cary M.S., Rubright J., Tenhave T. How redesigning AD clinical trials might increase study partners' willingness to participate. Neurology. 2008;71(23):1883–1888. doi: 10.1212/01.wnl.0000336652.05779.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maciejewski M.L., Perkins M., Li Y.F., Chapko M., Fortney J.C., Liu C.F. Utilization and expenditures of veterans obtaining primary care in community clinics and VA medical centers: an observational cohort study. BMC Health Serv. Res. 2007;7:56. doi: 10.1186/1472-6963-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald A.M., Knight R.C., Campbell M.K., Entwistle V.A., Grant A.M., Cook J.A.…Snowdon C. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Office of Disease Prevention and Health Promotion Access to health services. 2019. https://www.healthypeople.gov/2020/topics-objectives/topic/Access-to-Health-Services
- 23.Panangala S.V., Mendez B.H.P. Congressional Research Service; 2010. Veterans Health Administration: Community-Based Outpatient Clinics. 2010. R41044-Ref Type: Report. [Google Scholar]
- 24.Perez M.V., Mahaffey K.W., Hedlin H., Rumsfeld J.S., Garcia A., Ferris T.…Apple Heart Study Investigators Large-scale Assessment of a smartwatch to identify atrial fibrillation. N. Engl. J. Med. 2019;381(20):1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds T. Clinical trials: can technology solve the problem of low recruitment? BMJ. 2011;342:d3662. doi: 10.1136/bmj.d3662. [DOI] [PubMed] [Google Scholar]
- 26.Rosa C., Campbell A.N., Miele G.M., Brunner M., Winstanley E.L. Using e-technologies in clinical trials. Contemp. Clin. Trials. 2015;45(Part A):41–54. doi: 10.1016/j.cct.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Department of Veterans Affairs . 2014. Veterans Health Administration. VHA Directive 1205: VHA Cooperative Studies Program. April 28. [Google Scholar]
- 28.U.S. Department of Veterans Affairs . VA Secretary Unveils Data Security Encryption Program; 2018. Office of Public and Intergovernmental Affairs.https://www.va.gov/opa/pressrel/pressrelease.cfm?id=1169 [Google Scholar]
- 29.U.S. Department of Veterans Affairs Veterans health administration. About VHA. 2019. https://www.va.gov/health/aboutvha.asp
- 30.Velarde K., Romesser J., Johnson M.R., Clegg D.O., Efimova O.V., Oostema S.J.…Huang G.D. An initiative using informatics to facilitate clinical research planning and recruitment in the VA health care system. Contemporary Clinical Trials Communications. 2018 September;11:107–112. doi: 10.1016/j.conctc.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voils C.I., Yancy W.S., Jr., Weinberger M., Bolton J., Coffman C.J., Jeffreys A.…Bosworth H.B. The trials and tribulations of enrolling couples in a randomized, controlled trial: a self-management program for hyperlipidemia as a model. Patient Educ. Counsel. 2011;84(1):33–40. doi: 10.1016/j.pec.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Zimmerman L.P., Goel S., Sathar S., Gladfelter C.E., Onate A., Kane L.L.…Kho A.N. A novel patient recruitment strategy: patient selection directly from the community through linkage to clinical data. Appl. Clin. Inf. 2018;9(1):114–121. doi: 10.1055/s-0038-1625964. [DOI] [PMC free article] [PubMed] [Google Scholar]