Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- ALT
alanine transferase
- AST
aspartate transferase
- COVID‐19
coronavirus disease 2019
- CSS
cytokine storm syndrome
- DIC
disseminated intravascular coagulation
- GGT
gamma‐glutamyltransferase
- LFT
liver function test
- MOF
multi‐organ failure
- NAFLD
nonalcoholic fatty liver disease
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TMPRSS2
transmembrane serine protease 2
The current pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has led to over 6 million cases and over 370,000 deaths as of June 1, 2020.( 1 ) Although most patients infected with SARS‐CoV‐2 develop only mild symptoms, a minority of patients require hospitalization and intensive care.( 2 ) Based on the available clinical data, abnormal liver function tests (LFTs) are frequently observed in patients with coronavirus disease 2019 (COVID‐19), of which the underlying pathogenesis is incompletely understood. We reviewed the available information on the prevalence, nature, relevance, and the potential pathogenesis of altered LFTs in patients with COVID‐19.
Epidemiology of Abnormal LFTs in Patients With COVID‐19
Liver function tests include measures of hepatocyte injury (aspartate transferase [AST] and alanine transferase [ALT]), bile duct injury or cholestasis (alkaline phosphatase [ALP] and gamma‐glutamyltransferase [GGT]), markers of hepatic clearance/biliary secretion capacity (bilirubin), as well as measures of synthetic capacity (prothrombin time and albumin). LFTs are not necessarily liver‐specific. It has been suggested that elevated aminotransferases in COVID‐19 could also originate from myositis rather than liver injury.( 3 ) In a large descriptive study, the muscle damage marker creatinine kinase was elevated in 14% of patients with COVID‐19.( 4 ) Hypoalbuminemia was reported in 55% of hospitalized patients with COVID‐19( 5 ) and was associated with disease severity.( 6 ) Hypoalbuminemia was an independent predictor of mortality.( 7 ) Lower levels of pre‐albumin in patients with severe COVID‐19 were reported, suggesting decreased hepatic synthesis.( 5 ) In the context of inflammation, hypoalbuminemia may also reflect albumin extravasation as a consequence of increased capillary permeability.( 8 ) Additional factors that could explain the observed hypoalbuminemia in severe COVID‐19 are increased catabolism and malnutrition.
The prevalence of ALT elevations among patients with COVID‐19 ranged between 4% and 33% in Chinese cohorts (weighted average: 19%), but was as high as 39% in a large cohort from the New York City area( 5 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 ) (Fig. 1). ALT elevations were generally mild, defined as less than 5 times the upper reference limit.( 9 , 10 , 14 , 15 , 16 , 17 , 18 , 19 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 ) The prevalence of AST elevations ranged between 4% and 53% in Chinese cohorts (weighted average: 21%) and was 58% in the US cohort( 4 , 5 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 18 , 19 , 21 , 22 , 24 , 25 , 29 , 34 , 35 ) (Fig. 1). AST elevations were similarly less than 5 times the upper reference limit.( 9 , 10 , 14 , 15 , 16 , 18 , 19 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 34 , 36 ) AST and ALT elevations had also been reported in patients with SARS caused by SARS‐CoV.( 37 ) Several case reports have described severe LFT abnormalities( 18 , 38 , 39 ) or acute‐on‐chronic( 40 , 41 ) liver failure in patients with COVID‐19. Zhang et al.( 33 ) reported that 1 of 82 deceased patients with COVID‐19 had a hepatic cause of death, although it was not clear whether this patient had pre‐existing liver disease.
FIG. 1.
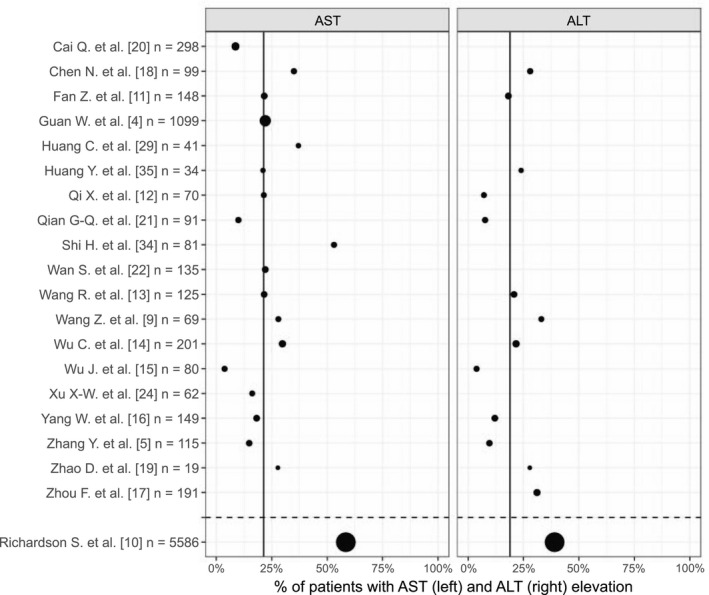
Percentage of hospitalized COVID‐19 patients with AST or ALT elevations on admission. The studies summarized included patients with all levels of disease severity. The size of the dots reflects the relative number of patients included in each study. The upper panels represent Chinese cohorts, whereas the lower panel represents a US cohort. The weighted averages are represented by the vertical black lines and refer to the Chinese cohorts.
Elevated ALP was reported in 2%‐5% of patients,( 5 , 11 , 25 , 42 ) and elevated GGT was reported in 13%‐54% of patients (weighted average: 23%).( 5 , 11 , 19 , 42 ) The prevalence of total bilirubin elevations ranged between 1% and 18% of patients with COVID‐19 on admission.( 4 , 5 , 15 , 16 , 18 , 25 , 35 , 43 ) It should be realized, however, that pre‐existing liver disease was not comprehensively described in any of these studies.
Stratification of patients with COVID‐19 according to disease severity, including the extent of respiratory distress and the need for intensive care unit (ICU) admission, indicated that plasma ALT and AST were elevated more frequently and to a greater extent in patients with severe COVID‐19 compared to those with mild disease.( 4 , 5 , 9 , 14 , 22 , 23 , 29 , 31 , 43 , 44 , 45 , 46 , 47 ) This was also the case for patients with SARS during the 2002‐2004 SARS outbreak.( 37 )
The prognostic value of abnormal LFTs in COVID‐19 is unclear. Some studies found that abnormal LFTs, particularly elevated AST and (peak) ALT, are associated with increased disease severity and mortality,( 17 , 20 , 46 , 47 ) whereas other studies did not find an association with mortality,( 48 ) disease progression,( 5 ) ICU admission,( 27 , 48 ) or length of hospital stay.( 11 )
Potential Pathogenesis of Abnormal Liver Function Tests in Patients With COVID‐19
Hepatic Infection With SARS‐CoV‐2
SARS‐CoV‐2 uses the angiotensin‐converting enzyme 2 (ACE2) as docking and entry receptor on host cells.( 49 ) Transmembrane serine protease 2 (TMPRSS2) is also involved in its cellular entry.( 50 )
Theoretically, direct virus‐induced cytopathic effects could play a role in LFT abnormalities in COVID‐19.( 51 ) To determine whether SARS‐CoV‐2 is able to infect the liver, ACE2 expression has been studied in liver cells. Single‐cell RNA‐sequencing approaches indicated that ACE2 mRNA is expressed in a subpopulation of cholangiocytes, not or minimally in hepatocytes, and not in any other liver cell type.( 52 , 53 , 54 , 55 ) In line with this, at the protein level, ACE2 was visualized by immunohistochemistry in a subset of cholangiocytes, but not in hepatocytes.( 56 , 57 ) TMPRSS2 mRNA expression was found in a subset of hepatocytes and cholangiocytes.( 58 ) Zhao et al. infected human liver ductal organoids with SARS‐CoV‐2 and showed increased expression of viral mRNA at 24 hours after infection.( 59 ) In these organoids, 3% of cells co‐expressed ACE2 and biliary markers, and of these, 68% co‐expressed TMPRSS2. ACE2 expression in endothelial cells is debated.( 56 , 57 )
ACE2 is highly expressed on the brush border of small intestinal enterocytes.( 56 , 57 ) Accordingly, SARS‐CoV‐2 infection was observed in human small intestine organoids,( 60 ) and SARS‐CoV‐2 nucleocapsid was detected in the cytoplasm of intestinal biopsies of a patient with COVID‐19.( 61 ) Patients with COVID‐19 with gastrointestinal symptoms were not more likely to have abnormal LFTs.( 62 ) In a minority (<15%) of patients with COVID‐19, viral RNA was detected in blood by PCR in low amounts.( 29 , 63 , 64 ) Assuming brisk viral replication in the intestine, it appears plausible that viruses could enter the portal circulation to reach the liver. Hepatic Kupffer cells would attempt to clear the virus and initiate an inflammatory response. It is also possible that inflammatory mediators from the intestine could enter the portal system and sinusoids.
Evidence for direct hepatic infection was provided by showing SARS‐CoV‐2 particles without membrane‐bound vesicles in the cytoplasm of hepatocytes of 2 patients with COVID‐19 with LFT abnormalities.( 45 ) However, no confirmatory PCR testing for viral nucleic acids was performed, leaving the possibility that these “spiked” inclusions could be of different origin.( 65 )
Role of the Host Inflammatory Response to SARS‐CoV‐2 Infection
Following SARS‐CoV‐2 infection, the host immune response can be rapid and controlled, resulting in disease resolution with no or mild symptoms, or delayed and dysregulated, resulting in host‐damaging complications. COVID‐19 complications include acute respiratory distress syndrome, a coagulopathy reminiscent of disseminated intravascular coagulation (DIC) and thrombotic microangiopathy, multi‐organ failure (MOF), and ultimately death.( 66 ) An excessive release of early‐response inflammatory factors, especially IL‐6, IL‐10, IL‐2 and interferon gamma, correlates with disease severity( 67 ) and may reflect cytokine storm syndrome (CSS). CSS is an excessive or uncontrolled release of pro‐inflammatory cytokines that is associated with MOF.( 68 ) The cascade of events leading to MOF includes an early phase of endothelial damage and extravasation of inflammatory cells and release of mediators, and a later phase that includes amplification of inflammation and cell damage, which could affect various organs including the liver (bystander effect).( 68 ) Additionally, CSS may result in DIC.( 69 ) DIC is observed in critical and nonsurvivor patients with COVID‐19, as evidenced by raised D‐dimer levels and prolonged prothrombin time,( 70 ) as well as autopsy findings of pulmonary embolism and thrombotic microangiopathy in multiple organs.( 71 )
Endothelitis was observed in the liver of patients with COVID‐19,( 72 ) and fibrin microthrombi were found in liver sinusoids.( 73 ) The largest series of liver biopsies taken at autopsy (48 cases) reported massive dilation of portal vein branches, luminal thrombosis, portal tract fibrosis, and microthrombi in the sinusoids.( 74 ) The altered LFTs observed in critical patients with COVID‐19 could therefore be related to CSS leading to shock and coagulopathy, which affect liver perfusion and result in cell death. Indeed, several studies reported that patients with severe COVID‐19 had higher plasma aminotransferases on admission compared to those with mild disease, concomitantly with higher inflammatory markers.( 4 , 5 , 9 , 11 , 14 , 22 , 23 , 29 , 31 , 44 , 46 , 75 )
In patients with mild COVID‐19, abnormal LFTs on admission may not be related to general inflammation. Zhao et al.( 19 ) compared patients with mild COVID‐19 pneumonia to patients with non‐COVID‐19 pneumonia and comparable disease severity. There were no differences in C‐reactive protein and IL‐6 between the two groups. While none of the patients with non‐COVID‐19 pneumonia had elevations in AST, ALT, or GGT on admission, elevations were observed in 28%, 28%, and 44% of patients with COVID‐19, respectively, suggesting that patients with mild COVID‐19 may have LFT abnormalities independently of the inflammatory status. One explanation for these findings is that the specific inflammation caused by SARS‐CoV‐2 is more likely to cause LFT abnormalities compared with general inflammation elicited by other pathogens. Whether LFT abnormalities are present in patients with asymptomatic or paucisymptomatic COVID‐19 who do not require hospitalization is unknown.
Drug‐Induced Liver Injury
Alterations in LFTs in patients with COVID‐19 have been reported at hospital admission, implying that patients may develop these before starting drug treatment. However, a comprehensive description of pre‐existing conditions and prior medication use is lacking.
Many medications used for the symptoms or the management of patients with COVID‐19, such as acetaminophen, antivirals, antibiotics, corticosteroids and immune‐modulators, are potentially hepatotoxic.
Fan et al.( 11 ) retrospectively studied the relationship between medication use and LFTs in 148 patients with COVID‐19. Among patients with no LFT abnormalities on admission, 48% developed them about a week after admission. Whereas 58% of those who developed LFT abnormalities after admission had received lopinavir‐ritonavir, only 31% of those with normal LFTs had received it. However, due to the retrospective nature of this study, lack of treatment randomization should be taken into account. Cai et al. reported 7 times higher odds of LFT alterations after the use of lopinavir‐ritonavir.( 20 ) In contrast, in a clinical trial including 199 patients with severe COVID‐19, the AST, ALT, and total bilirubin elevations were not more frequent in the lopinavir‐ritonavir group compared with those given standard care. Patients with severe liver disease were excluded from the trial.
Remdesivir was recently reported to be superior to placebo in shortening the time to recovery of hospitalized patients with COVID‐19.( 76 ) In a trial comparing remdesivir treatment for either 5 or 10 days, severe but not immediately life‐threatening ALT/AST elevations were reported in 4%‐6% of patients, and life‐threatening AST/ALT elevations in 2%‐3% of patients, necessitating treatment discontinuation.( 77 )
Acetaminophen is frequently used for COVID‐19 symptom relief and can cause alterations in aminotransferases even at therapeutic doses.( 78 ) However, no studies have assessed its role in COVID‐19 management specifically.
Preliminary data did not associate hydroxychloroquine treatment with significant LFT abnormalities.( 79 , 80 )
Pre‐existing Liver Diseases and COVID‐19
Abnormal LFTs at admission could result from pre‐existing (chronic) liver diseases. Reported prevalence rates of pre‐existing liver disease in patients with COVID‐19 vary from 1%‐11%.( 42 , 47 , 81 , 82 ) As most studies reporting LFTs are retrospective, the aforementioned numbers are subject to underreporting, but it appears unlikely that pre‐existing liver disease accounts for all observed abnormalities in LFTs.
Whether the presence of pre‐existing liver disease could affect the course of COVID‐19 and vice versa is largely unclear. Plasma inflammatory markers were not more elevated in patients with chronic liver diseases,( 44 , 47 ) and no association was found between pre‐existing liver disease and COVID‐19 severity or mortality.( 83 ) However, chronic liver disease comprises a spectrum of conditions that may differentially affect outcomes. Patients with advanced liver disease are generally at an increased risk of infection due to cirrhosis‐associated immune dysfunction.( 84 ) Another patient category that raises concerns is liver transplant recipients and patients with auto‐immune liver disease receiving immunosuppressant drugs. However, based on currently available data, there is no reason to believe that these patients are at a higher risk of infection or more severe complications compared with the general population.( 85 , 86 ) One study reported high mortality in liver transplant recipients, but these patients also displayed comorbidities.( 87 ) It is speculated that immunosuppression could even be beneficial, as it might reduce the risk of developing a hyper‐inflammatory state and CSS. Conversely, however, it may increase virus‐induced injury and the risk for bacterial or fungal superinfection.
Metabolic Dysfunction–Associated Fatty Liver Disease and COVID‐19 Severity
Several studies have identified obesity as a significant risk factor for the severity of COVID‐19 disease, independent of associated co‐morbidities such as age, type 2 diabetes mellitus, and hypertension.( 51 , 88 , 89 , 90 , 91 , 92 , 93 ) Nonalcoholic fatty liver disease (NAFLD), also known as nonalcoholic associated fatty liver disease, is highly associated with obesity but also observed in lean individuals.( 94 ) In the initial studies characterizing patients with COVID‐19, NAFLD was rarely reported, but as a common (and possibly asymptomatic) hepatic condition, it may account for some of the LFT alterations observed on admission. Moreover, it could explain the differences in ALT/AST prevalence observed between the large US cohort and the Chinese cohorts (Fig. 1). NAFLD prevalence is higher in the United States (24%) than in China (15%).( 95 ) NAFLD is closely related to obesity and other lifestyle‐related metabolic disorders (e.g., type 2 diabetes). In the U.S. COVID‐19 cohort, 42% of patients were obese and 34% had diabetes.( 10 ) In contrast, in the largest Chinese cohort, obesity was not reported, but diabetes prevalence was 15%.( 4 )
Patients with NAFLD displayed more rapid disease progression and longer viral shedding time compared to patients without NAFLD.( 96 ) Increased risk for severe disease was observed if NAFLD was present alongside obesity,( 97 ) in patients without diabetes,( 98 ) in younger patients,( 99 ) and in patients with increased hepatic fibrosis scores.( 100 ) Although it is unclear how obesity and NAFLD could increase COVID‐19 severity, similar pathways relating to alterations in the immune response, macrophage activation and (low‐grade) inflammation, often present in both conditions, are thought to play a key role.( 100 , 101 , 102 , 103 ) NAFLD increases hepatotoxicity of certain drugs, including acetaminophen, which could also aggravate LFT alterations in the course of COVID‐19.( 104 , 105 )
Other Causes of LFT Elevations in Critically Ill Patients
In critical patients with COVID‐19, hepatic injury may be caused by changes in hemodynamics and oxygen delivery. Hypoxic hepatitis can cause sharp increases in aminotransferases in the setting of respiratory failure, shock, or cardiac failure.( 106 ) During acute cardiac failure, which may occur in critical patients with COVID‐19,( 107 ) the systemic arterial pressure suddenly drops, leading to a reduction in hepatic arterial perfusion and hepatocellular hypoxia. The pathogenesis comprises not only hepatic ischemia, but also hepatic venous congestion due to elevated central venous pressure, which may predispose hepatocytes to even more significant hypoxic injury.( 108 ) Similar hemodynamic alterations in the liver may occur in mechanically ventilated patients in response to high positive end‐respiratory pressure (PEEP).( 109 , 110 ) Whether these hemodynamic alterations can alter LFTs is unclear.( 110 ) Importantly, the use of high PEEP is usually unnecessary for the respiratory management of patients with COVID‐19, as lung compliance is relatively high.( 111 )
Conclusions
Mildly abnormal plasma LFTs, especially AST and ALT, are frequently observed in patients with COVID‐19 on admission and are associated with severe disease and increased inflammatory markers. In general, abnormal LFTs in patients with COVID‐19 do not lead to significant liver function impairment or failure, and liver‐directed treatment is unnecessary.
The pathogenetic mechanisms for abnormal LFTs in COVID‐19 are not fully understood: They are likely multifactorial and, while direct SARS‐CoV‐2 infection in hepatocytes and/or cholangiocytes appears unlikely, microthrombotic endothelialitis, immune dysregulation, drug‐induced liver injury, and hepatic ischemia related to hypoxia and MOF could all play a role.
Author Contributions
A.B.: conceptualization, formal analysis, data curation, investigation, visualization, writing – original draft; I.P.P.: conceptualization, investigation, writing – original draft; F.A.J.A.B., H.M., A.F., F.F., R.F., M.S., H.J.V.: writing – review & editing; G.P.: conceptualization, investigation, writing – review and editing, supervision.
Acknowledgments
We would like to thank Maximilian Agostini for assistance with the graph and Vincent Bloks for assistance with the literature review. We also thank Dr. Dimitris Piliotopoulos, Dr. Federica LoRusso, Dr. Annalisa Barbaglia, and Dr. Federica Fontanella for starting the AIRI CLIP initiative and thereby promoting international collaboration that made the writing of this review possible.
Potential conflict of interest: Dr. Bodewes advises Vertex. Dr. Strazzabosco advises Bayer and Engitix.
References
Author names in bold designate shared co‐first authorship.
- 1. WHO . Coronavirus disease 2019 (COVID‐19) Situation Report‐133. 2020. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200601‐covid‐19‐sitrep‐133.pdf?sfvrsn=9a56f2ac_4. Accessed June 1, 2020.
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China. JAMA 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 3. Bangash MN, Patel J, Parekh D. COVID‐19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol 2020;5:529‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID‐19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int 2020. Apr 2. 10.1111/liv.14455. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6. Aziz M, Fatima R, Lee‐Smith W, Assaly R. The association of low serum albumin level with severe COVID‐19: a systematic review and meta‐analysis. Crit Care 2020;24:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang J, Cheng A, Kumar R, Fang Y, Chen G, Zhu Y, et al. Hypoalbuminemia predicts the outcome of COVID‐19 independent of age and co‐morbidity. J Med Virol 2020. May 25. 10.1002/jmv.26003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. J Parenter Enter Nutr 2019;43:181‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020;71:769‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA 2020;323:2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID‐19‐related liver damage. Clin Gastroenterol Hepatol 2020;18:1561‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qi X, Liu C, Jiang Z, Gu Y, Zhang G, Shao C, et al. Multicenter analysis of clinical characteristics and outcomes in patients with COVID‐19 who develop liver injury. J Hepatol 2020;73:455‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang R, Pan M, Zhang X, Han M, Fan X, Zhao F, et al. Epidemiological and clinical features of 125 hospitalized patients with COVID‐19 in Fuyang, Anhui, China. Int J Infect Dis 2020;95:421‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID‐19) in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis 2020. Feb 29. 10.1093/cid/ciaa199. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID‐19): a multi‐center study in Wenzhou city, Zhejiang, China. J Infect 2020;80:388‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;6736:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao D, Yao F, Wang L, Zheng L, Gao Y, Ye J, et al. A comparative study on the clinical features of coronavirus 2019 (COVID‐19) pneumonia with other pneumonias. Clin Infect Dis 2020. Mar 12. 10.1093/cid/ciaa247. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID‐19: abnormal liver function tests. J Hepatol 2020. Apr 13. 10.1016/j.jhep.2020.04.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qian G‐Q, Yang N‐B, Ding F, Ma AHY, Wang Z‐Y, Shen Y‐F, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID‐19 in Zhejiang, China: a retrospective, multi‐centre case series. QJM An Int J Med 2020;113:474‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, et al. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. J Med Virol 2020;92:797‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu X‐W, Wu X‐X, Jiang X‐G, Xu K‐J, Ying L‐J, Ma C‐L, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ 2020;368:m792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, et al. COVID‐19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy 2020;75:1742‐1752. [DOI] [PubMed] [Google Scholar]
- 26. Cao W, Shi L, Chen L, Xu X, Wu Z. Clinical features and laboratory inspection of novel coronavirus pneumonia (COVID‐19) in Xiangyang, Hubei. medRxiv 2020. Feb 25. 10.1101/2020.02.23.20026963. [Epub ahead of print] [DOI] [Google Scholar]
- 27. Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, et al. Clinical progression of patients with COVID‐19 in Shanghai, China. J Infect 2020;80:e1‐e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in‐hospital death of patients with COVID‐19. Kidney Int 2020;97:829‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo S, Zhang X, Xu H. Don’t overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID‐19). Clin Gastroenterol Hepatol 2020;18:1636‐1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin Infect Dis 2020. Mar 16. 10.1093/cid/ciaa270. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ 2005;172:367‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang B, Zhou X, Qiu Y, Feng F, Feng J, Jia Y, et al. Clinical characteristics of 82 death cases with COVID‐19. medRxiv 2020. Feb 27. 10.1101/2020.02.26.20028191. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20:425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang Y, Tu M, Wang S, Chen S, Zhou W, Chen D, et al. Clinical characteristics of laboratory confirmed positive cases of SARS‐CoV‐2 infection in Wuhan, China: a retrospective single center analysis. Travel Med Infect Dis 2020. Feb 27. 10.1016/j.tmaid.2020.101606. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang Y, Zhou H, Yang R, Xu Y, Feng X, Gong P. Clinical characteristics of 36 non‐survivors with COVID‐19 in Wuhan, China. medRxiv 2020. Mar 5. 10.1101/2020.02.27.20029009. [Epub ahead of print] [DOI] [Google Scholar]
- 37. Cui H‐J, Tong X‐L, Li P, Hao Y‐X, Chen X‐G, Li A‐G, et al. Serum hepatic enzyme manifestations in patients with severe acute respiratory syndrome: retrospective analysis. World J Gastroenterol 2004;10:1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weber S, Mayerle J, Irlbeck M, Gerbes AL. Severe liver failure during SARS‐CoV‐2 infection. Gut 2020;69:1365‐1367. [DOI] [PubMed] [Google Scholar]
- 39. Wander P, Epstein M, Bernstein D. COVID‐19 presenting as acute hepatitis. Am J Gastroenterol 2020;115:941‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qiu H, Wander P, Bernstein D, Satapathy SK. Acute on chronic liver failure from novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Liver Int 2020;40:1590‐1593. [DOI] [PubMed] [Google Scholar]
- 41. Große K, Kramer M, Trautwein C, Bruns T. SARS‐CoV‐2 as an extrahepatic precipitator of acute‐on‐chronic liver failure. Liver Int 2020;40:1792‐1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang C, Shi L, Wang F. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol 2020;5:428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mao R, Qiu Y, He J, Tan J, Li X, Liang J, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID‐19: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol 2020;5:667‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li L, Li S, Xu M, Yu P, Zheng S, Duan Z, et al. Risk factors related to hepatic injury in patients with corona virus disease 2019. medRxiv 2020. Mar 10. 10.1101/2020.02.28.20028514v2. [Epub ahead of print] [DOI] [Google Scholar]
- 45. Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS‐CoV‐2 infection of the liver directly contributes to hepatic impairment in patients with COVID‐19. J Hepatol 2020. May 12. 10.1016/j.jhep.2020.05.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, et al. Acute liver injury in COVID‐19: prevalence and association with clinical outcomes in a large US cohort. Hepatology 2020. May 30. 10.1002/hep.31404. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lei F, Liu Y, Zhou F, Qin J‐J, Zhang P, Zhu L, et al. Longitudinal association between markers of liver injury and mortality in COVID‐19 in China. Hepatology 2020. May 2. 10.1002/hep.31301. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vespa E, Pugliese N, Piovani D, Capogreco A, Danese S, Aghemo A. Liver tests abnormalities in COVID‐19: trick or treat? J Hepatol 2020. May 27. 10.1016/j.jhep.2020.05.033. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou P, Yang X‐L, Wang X‐G, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gao F, Zheng KI, Wang X‐B, Sun Q, Pan K, Wang T, et al. Obesity is a risk factor for greater COVID‐19 severity. Diabetes Care 2020. May. 10.2337/dc20-0682. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 52. Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. medRxiv 2020. Feb 4. 10.1101/2020.02.03.931766 [Epub ahead of print] [DOI] [Google Scholar]
- 53. Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun 2020;526:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med 2020;14:185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. De Smet V, Verhulst S, van Grunsven LA. Single cell RNA sequencing analysis did not predict hepatocyte infection by SARS‐CoV‐2. J Hepatol 2020. May 28. 10.1016/j.jhep.2020.05.030. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hikmet F, Mear L, Uhlen M, Lindskog C. The protein expression profile of ACE2 in human tissues. bioRxiv 2020. Apr 3. 10.1101/2020.03.31.016048. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hamming I, Timens W, Bulthuis M, Lely A, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pirola CJ, Sookoian S. SARS‐CoV‐2 virus and liver expression of host receptors: putative mechanisms of liver involvement in COVID‐19. Liver Int 2020. Apr 30. 10.1111/liv.14500. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, et al. Recapitulation of SARS‐CoV‐2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell 2020. Apr 17. 10.1007/s13238-020-00718-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, et al. SARS‐CoV‐2 productively infects human gut enterocytes. Science 2020. May 1. 10.1126/science.abc1669. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS‐CoV‐2. Gastroenterology 2020;158:1831‐1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, et al. Prevalence and characteristics of gastrointestinal symptoms in patients with SARS‐CoV‐2 infection in the United States: a multicenter cohort study. Gastroenterology 2020. Apr 22. 10.1053/j.gastro.2020.04.045. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang W, Du RH, Li B, Zheng XS, Lou YX, Hu B, et al. Molecular and serological investigation of 2019‐nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect 2020;9:386‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA 2020;323:1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Philips CA, Ahamed R, Augustine P. SARS‐CoV‐2 related liver impairment—perception may not be the reality. J Hepatol 2020. May 23. 10.1016/j.jhep.2020.05.025. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Picchianti Diamanti A, Rosado MM, Pioli C, Sesti G, Laganà B. Cytokine release syndrome in COVID‐19 patients, a new scenario for an old concern: the fragile balance between infections and autoimmunity. Int J Mol Sci 2020;21:3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. EBioMedicine 2020;55:102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med 2008;26:711‐715. [DOI] [PubMed] [Google Scholar]
- 69. Jose RJ, Manuel A. COVID‐19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med 2020;8:e46‐e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xiong M, Liang X, Wei Y. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. Br J Haematol 2020;189:1050‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Postmortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology 2020. May 4. 10.1111/his.14134. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet 2020;395:1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nunes Duarte‐Neto A, de Almeida Monteiro RA, da Silva LFF, Malheiros DMAC, de Oliveira EP, Theodoro Filho J, et al. Pulmonary and systemic involvement of COVID‐19 assessed by ultrasound‐guided minimally invasive autopsy. Histopathology 2020. May 22. 10.1111/his.14160. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sonzogni A, Previtali G, Seghezzi M, Alessio Maria G, Gianatti A, Licini L, et al. Liver histopathology in COVID 19 infection is suggestive of vascular alteration. medRxiv 2020. May 11. 10.1101/2020.05.06.20092718. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Parohan M, Yaghoubi S, Seraj A. Liver injury is associated with severe coronavirus disease 2019 (COVID‐19) infection: a systematic review and meta‐analysis of retrospective studies. Hepatol Res 2020. May 9. 10.1111/hepr.13510. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid‐19—preliminary report. N Engl J Med 2020. May 22. 10.1056/nejmoa2007764. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 77. Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 days in patients with severe Covid‐19. N Engl J Med 2020. May 27. 10.1056/nejmoa2015301. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. National Institute of Diabetes and Digestive and Kidney Diseases . Acetaminophen. In: LiverTox: Clinical and Research Information on Drug‐Induced Liver Injury. Updated Jan 28, 2016. https://www.ncbi.nlm.nih.gov/books/NBK548162/. [Accessed May 31, 2020] [PubMed] [Google Scholar]
- 79. Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID‐19: a living systematic review. Ann Intern Med 2020. May 27. 10.7326/M20-2496. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 80. Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. JAMA Network Open 2020;3:e208857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID‐19 in patients with liver and kidney diseases: an early systematic review and meta‐analysis. Trop Med Infect Dis 2020;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: a meta‐analysis. Liver Int 2020;40:1316‐1320. [DOI] [PubMed] [Google Scholar]
- 83. Lippi G, de Oliveira MHS, Henry BM. Chronic liver disease is not associated with severity or mortality in Coronavirus disease 2019 (COVID‐19). Eur J Gastroenterol Hepatol 2020. Apr 10. 10.1097/meg.0000000000001742. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Albillos A, Lario M, Álvarez‐Mon M. Cirrhosis‐associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 2014;61:1385‐1396. [DOI] [PubMed] [Google Scholar]
- 85. D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transplant 2020;26:832‐834. [DOI] [PubMed] [Google Scholar]
- 86. Lleo A, Invernizzi P, Lohse AW, Aghemo A, Carbone M. Management of patients with autoimmune liver disease during COVID‐19 pandemic. J Hepatol 2020;73:453‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lee BT, Perumalswami PV, Im GY, Florman S, Schiano TD. COVID‐19 in liver transplant recipients: an initial experience from the U.S. Gastroenterology 2020. May 20. 10.1053/j.gastro.2020.05.050. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med 2020;382:1859‐1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, et al. Association of obesity with disease severity among patients with COVID‐19. Obesity 2020. Apr 30. 10.1002/oby.22859. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zheng KI, Gao F, Wang X‐B, Sun Q‐F, Pan K‐H, Wang T‐Y, et al. Letter to the Editor: Obesity as a risk factor for greater severity of COVID‐19 in patients with metabolic associated fatty liver disease. Metabolism 2020;108:154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity 2020;28:1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, et al. Obesity and COVID‐19 severity in a designated hospital in Shenzhen, China. Diabetes Care 2020;43:1392‐1398. [DOI] [PubMed] [Google Scholar]
- 93. Hajifathalian K, Kumar S, Newberry C, Shah S, Fortune B, Krisko T, et al. Obesity is associated with worse outcomes in COVID‐19: analysis of early data from New York City. Obesity 2020. May 29. 10.1002/oby.22923. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero‐Gomez M, et al. A new definition for metabolic dysfunction‐associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202‐209. [DOI] [PubMed] [Google Scholar]
- 95. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11‐20. [DOI] [PubMed] [Google Scholar]
- 96. Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, et al. Non‐alcoholic fatty liver diseases in patients with COVID‐19: a retrospective study. J Hepatol 2020;73:451‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zheng KI, Gao F, Wang X‐B, Sun Q‐F, Pan K‐H, Wang T‐Y, et al. Letter to the Editor: Obesity as a risk factor for greater severity of COVID‐19 in patients with metabolic associated fatty liver disease. Metabolism 2020;108:154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gao F, Zheng KI, Wang X, Yan H‐D, Sun Q‐F, Pan K‐H, et al. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol 2020. May 21. 10.1111/jgh.15112. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhou Y, Zheng KI, Wang X‐B, Yan H, Sun Q, Pan K‐H, et al. Younger patients with MAFLD are at increased risk of severe COVID‐19 illness: a multicenter preliminary analysis. J Hepatol 2020. Apr 26. 10.1016/j.jhep.2020.04.027. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Targher G, Mantovani A, Byrne CD, Wang X‐B, Yan H‐D, Sun Q‐F, et al. Risk of severe illness from COVID‐19 in patients with metabolic dysfunction‐associated fatty liver disease and increased fibrosis scores. Gut 2020;69:1545‐1547. [DOI] [PubMed] [Google Scholar]
- 101. Michalakis K, Ilias I. SARS‐CoV‐2 infection and obesity: common inflammatory and metabolic aspects. Diabetes Metab Syndr Clin Res Rev 2020;14:469‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chiappetta S, Sharma AM, Bottino V, Stier C. COVID‐19 and the role of chronic inflammation in patients with obesity. Int J Obes 2020;44:1790‐1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lefere S, Tacke F. Macrophages in obesity and non‐alcoholic fatty liver disease: crosstalk with metabolism. JHEP Rep 2019;1:30‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Michaut A, Moreau C, Robin MA, Fromenty B. Acetaminophen‐induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int 2014;34:e171‐e179. [DOI] [PubMed] [Google Scholar]
- 105. Massart J, Begriche K, Moreau C, Fromenty B. Role of nonalcoholic fatty liver disease as risk factor for drug‐induced hepatotoxicity. J Clin Transl Res 2017;3:212‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Newton JM, Aronsohn A, Jensen DM. Liver dysfunction in critically ill patients. In: Rajendram R, Preedy VR, Patel VB, eds. Diet and Nutrition in Critical Care. New York: Springer; 2015:35‐48. [Google Scholar]
- 107. Akhmerov A, Marbán E. COVID‐19 and the heart. Circ Res 2020;126:1443‐1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kavoliuniene A, Vaitiekiene A, Cesnaite G. Congestive hepatopathy and hypoxic hepatitis in heart failure: a cardiologist’s point of view. Int J Cardiol 2013;166:554‐558. [DOI] [PubMed] [Google Scholar]
- 109. Kotzampassi K, Paramythiotis D, Eleftheriadis E. Deterioration of visceral perfusion caused by intra‐abdominal hypertension in pigs ventilated with positive end‐expiratory pressure. Surg Today 2000;30:987‐992. [DOI] [PubMed] [Google Scholar]
- 110. Kredel M, Muellenbach RM, Johannes A, Brederlau J, Roewer N, Wunder C. Hepatic effects of lung‐protective pressure‐controlled ventilation and a combination of high‐frequency oscillatory ventilation and extracorporeal lung assist in experimental lung injury. Med Sci Monit 2011;17:BR275‐BR281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Roesthuis L, van den Berg M, van der Hoeven H. Advanced respiratory monitoring in COVID‐19 patients: use less PEEP! Crit Care 2020;24:230. [DOI] [PMC free article] [PubMed] [Google Scholar]


