Summary
The disease course of COVID‐19 varies from asymptomatic infection to critical condition leading to mortality. Identification of prognostic factors is important for prevention and early treatment. We aimed to examine whether obesity is a risk factor for the critical condition in COVID‐19 patients by performing a meta‐analysis. The review protocol was registered onto PROSPERO (CRD42020185980). A systematic search was performed in five scientific databases between 1 January and 11 May 2020. After selection, 24 retrospective cohort studies were included in the qualitative and quantitative analyses. We calculated pooled odds ratios (OR) with 95% confidence intervals (CIs) in meta‐analysis. Obesity was a significant risk factor for intensive care unit (ICU) admission in a homogenous dataset (OR = 1.21, CI: 1.002‐1.46; I2 = 0.0%) as well as for invasive mechanical ventilation (IMV) (OR = 2.05, CI: 1.16‐3.64; I2 = 34.86%) in COVID‐19. Comparing body mass index (BMI) classes with each other, we found that a higher BMI always carries a higher risk. Obesity may serve as a clinical predictor for adverse outcomes; therefore, the inclusion of BMI in prognostic scores and improvement of guidelines for the intensive care of patients with elevated BMI are highly recommended.
Keywords: COVID‐19, intensive care, mechanical ventilation, obesity
Abbreviations
- ARDS
acute respiratory distress syndrome
- BMI
body mass index
- COVID‐19
Coronavirus Disease‐19
- ICU
intensive care unit
- IL
interleukin
- IMV
invasive mechanical ventilation
- MCP
monocyte chemoattractant protein
- OR
Odds ratio
- PMG
Cochrane Prognosis Methods Group
- PRISMA
Preferred Reporting in Systematic Reviews and Meta‐analyses
- QUIPS
Quality in Prognostic Studies
- SARS‐CoV‐2
Severe Acute Respiratory Syndrome Coronavirus 2
- TNF
tumour necrosis factor
- WHO
World Health Organization
- CI
confidence interval
- RAS
renin‐angiotensin system,
1. INTRODUCTION
In December 2019, a series of pneumonia cases of unknown origin emerged in Wuhan, China. On 7 January 2020, a novel coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) was identified as the source of infection. 1 As of 14 June 2020, Coronavirus Disease‐19 (COVID‐19) infection has been reported in more than seven million cases. 2
The disease course of COVID‐19 varies in a broad spectrum, from asymptomatic infection to influenza‐like symptoms as well as to severe pneumonia leading to acute respiratory distress syndrome (ARDS). 2 Recognition of prognostic factors associated with adverse clinical outcomes is essential for prevention and early treatment. Certain diseases, including diabetes mellitus, hypertension, cardiovascular diseases or different types of cancer, have been already identified as predisposing factors to adverse outcomes in COVID‐19. 3 As these diseases are frequently associated with excessive body fat mass resulting in various hormonal, metabolic and inflammatory changes, adipose tissue may play an important role in the mechanism of the progression of COVID‐19 and obesity might be an important risk factor as well. 4 , 5 , 6 , 7 Patients with obesity constantly have higher leptin and lower adiponectin levels, as well as they have higher concentrations of pro‐inflammatory cytokines such as tumour necrosis factor (TNF)‐alpha, monocyte chemoattractant protein (MCP)‐1 and interleukin (IL)‐6 produced mainly by adipose tissue, which may contribute to the impaired immune response. 8 , 9 These conditions may influence inflammatory and immune responses. Another contributor could be the sedentary lifestyle alone or together with insulin resistance that influences the immune response to microbial agents by impaired macrophage differentiation and modulation of proinflammatory cytokine levels giving way to the invasion of infectious pathogens. 10 , 11
Interestingly, the obesity survival paradox has been described in certain diseases such as community‐acquired pneumonia, where despite the increased risk of developing pneumonia, an inverse association could be observed between obesity and mortality. 6 To the contrary, in the 2009 H1N1 pandemic, obesity was identified as an independent risk factor of severe disease, hospitalization and death. 5 , 12 , 13 Recent data have suggested that obesity, as seen in H1N1, can also be a disadvantageous factor to COVID‐19. 14 , 15
Although several articles discussing the impact of the body mass index (BMI) on the need for intensive care unit (ICU) admission or invasive mechanical ventilation (IMV) in COVID‐19 are available, a definite conclusion has not been drawn yet. Therefore, we aimed to clarify the association between the patients' BMI and ICU admission and IMV requirement.
2. METHODS
We report our systematic review and meta‐analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) Statement (Table S1). 16 The review protocol was registered on the PROSPERO International Prospective Register of Systematic Reviews (CRD42020185980). We did not deviate from the protocol except for expanding the exclusion criteria. We decided to perform additional meta‐regressions to assess the correlation between BMI values and rate of ICU admission/IMV. These analyses had their own additional eligibility criteria (detailed in Section 2.2.).
2.1. Search strategy
A systematic search was performed in five scientific databases—Medline (via PubMed), Embase, Cochrane Central Register of Controlled Trials (CENTRAL), Scopus and Web of Science—for studies published between 1 January 2020 and 11 May 2020. The following search key was used in all databases without any other filters or restrictions: (‘covid 19') OR (‘Wuhan virus') OR (‘coronavirus') OR (‘2019 nCoV') OR (‘SARS‐cov‐2'). Reference lists of the eligible articles and the citing articles (via Google Scholar search engine) were also screened to capture all relevant studies.
2.2. Selection and eligibility criteria
After the removal of duplicates using a reference management software (EndNote X9, Clarivate Analytics), three pairs of review authors independently screened titles, abstracts and then full‐texts against predefined eligibility criteria (each pair screened one‐third of the pool of records) to accelerate the selection process. A third review author resolved the conflicts.
There were no restrictions on the study designs eligible for inclusion. The inclusion criteria specified any peer‐reviewed studies that reported on BMI classes of patients with confirmed SARS‐CoV‐2 infection comparing the proportion of ICU admission or IMV requirement between each group. We excluded studies with fewer than 10 patients.
Regarding meta‐regression, those studies were eligible that contained information on mean/median BMI and event rate of IMV and ICU in the study population.
From both meta‐analyses and meta‐regression, we excluded studies with preselected population, specifically SARS‐CoV‐2‐infected patients with certain coexisting diseases or conditions that potentially cause malnutrition (e.g., cystic fibrosis) or significantly influence body weight (pregnancy, paediatric population).
2.3. Data extraction
Two independent review authors extracted data from the eligible studies into a standardized data collection form. All disagreements were resolved by an independent third author. The following data were extracted from each included study: authors, publication year, digital object identifier, study site, study design, protocol number, age, gender distribution, number of patients in each reported BMI range, number of patients with IMV in each reported BMI range, number of patients with ICU admission in each reported BMI range, odds ratios for IMV and ICU admission regarding BMI groups.
2.4. BMI categories
We defined BMI classes based on the World Health Organization (WHO) guidelines. 17 We included Asian and Caucasian populations in the same analysis—with their corresponding BMI cut off—only in meta‐analyses comparing patients with obesity and patients without obesity. We applied different cut‐off values for obesity in Asian‐Pacific (obesity >25 kg/m2) and Caucasian (obesity >30 kg/m2) population. 17
2.5. Risk of bias assessment
Based on the recommendations of Cochrane Prognosis Methods Group (PMG), a modified version of the Quality in Prognostic Studies (QUIPS) tool was used by the two independent review authors to assess the quality of the studies included. 18 Any disagreement was resolved by a third party.
2.6. Statistical analysis
We performed all meta‐analytic calculations with Comprehensive Meta‐Analysis (Version 3) statistical software (Biostat, Inc., Engelwood, MJ, USA). For data synthesis, we used the methods recommended by the working group of the Cochrane Collaboration. Pooled odds ratios (ORs) with their 95% confidence intervals (CIs) were calculated from raw data of the articles except for one article, 19 where the given OR and the CI were used. The random effect model with the estimation of DerSimonian and Laird was used in all cases. 20 Heterogeneity was tested using Cochrane's Q and the I 2 statistics. I 2 statistic represents the percentage of the total variability across studies: 30% to 60%, 50% to 90% and 75% to 100% corresponded to moderate, substantial and considerable degrees of heterogeneity, based on the Cochrane's handbook for Systematic Reviews of Interventions. 21 We considered the Q test significant if P < 0.1. Publication bias was examined by visual inspection of funnel plots.
To examine the effect of BMI on the event rate of IMV, meta‐regression was applied. We examined if the regression coefficient is equal to zero. We assessed R2 analogue, which is the explained variances of the models. We calculated corresponding P values and I 2‐test values.
3. RESULTS
3.1. Results of search and selection
We described the selection process in detail in the PRISMA flow chart (Figure 1). We identified a total of 33,987 records, 24 of which were included in the qualitative and quantitative syntheses. Nine studies were included in the meta‐analyses, and 20 articles in the meta‐regression. Five studies contained data for both the meta‐analysis and the meta‐regression.
FIGURE 1.
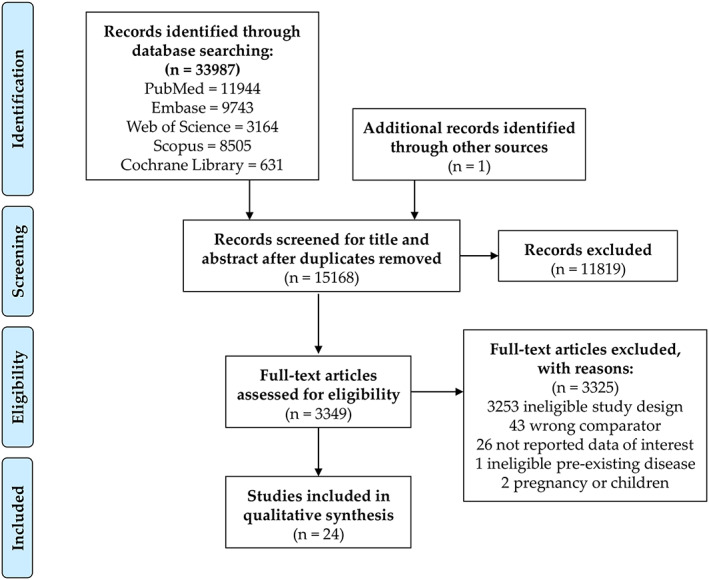
Preferred Reporting in Systematic Reviews and Meta‐analyses (PRISMA) flowchart showing the selection process
3.2. Characteristics of studies included
3.2.1. Meta‐analysis
The characteristics of the studies included in the meta‐analyses are presented in Table 1 and Table S2. Six studies with 2,770 patients reported on ICU admission, the proportion of which ranged from 9% to 43%. Five studies with 509 patients reported on IMV requirement, the proportion of which ranged from 58% to 78%.
TABLE 1.
Characteristics of the included studies
| Study (country) | Cohort type | N0 of patients (female %) | Age b | BMI categories (N0) | Event | Event N0 (%) | ||
|---|---|---|---|---|---|---|---|---|
| Normal (<25) | Overweight (25‐30) | Obese (>30) | ||||||
| Nonobese vs. obese a | ||||||||
| Hu L et al (China) | Retrospective | 294 (49%) | 61 | 229 | 65 | ICU admission | 25 (9%) | |
| Itelman E et al (Israel) | Retrospective | 162 (35%) | 52 | 131 | 31 | ICU admission | 26 (16%) | |
| Kalligeros M et al (USA) | Retrospective | 103 (38%) | 60 | 44 | 59 | ICU admission | 44 (43%) | |
| Lighter J et al (USA) | Retrospective | 1759 (NR) | NR | 1,010 | 749 | ICU admission | 431 (25%) | |
| Lodigiani C et al (Italy) | Retrospective | 361 (32%) | 66 | 274 | 87 | ICU admission | 57 (16%) | |
| Ong S et al (Singapore) | Retrospective |
91 (NR) 27 (NR) |
55 |
51 12 |
40 15 |
ICU admission IMV need |
27 (30%) 16 (59%) |
|
| Normal weight vs. overweight or obese | ||||||||
| Bhatraju PK et al (USA) | Retrospective | 23 (38%) | 64 | 3 | 7 | 13 | IMV need | 18 (78%) |
| Caussy C et al (France) | Retrospective | 291 (NR) | NR | 74 | 121 | 96 | IMV need | 170 (58%) |
| Kalligeros M et al (USA) | Retrospective | 44 (34%) | 60 | 5 | 14 | 25 | IMV need | 29 (66%) |
| Simonnet A et al (France) | Retrospective | 124 (27%) | 60 | 17 | 48 | 59 | IMV need | 89 (72%) |
Note. All studies were conducted in 2020.
Abbreviations: ICU, intensive care unit; IMV, invasive mechanical ventilation; NR = not reported.
Different cut‐off values were used to define obesity in Asian and Caucasian population.
Mean or median.
3.2.2. Meta‐regression
The characteristics of the studies included in the meta‐regression analysis are presented in Table S3.
3.3. Association between BMI and ICU admission
3.3.1. Qualitative and quantitative synthesis comparing BMI classes
Lodigiani et al reported 15.4% versus 15.9% ICU admission ratio among patients with BMI less than 25 and with BMI greater than or equal to 25, respectively. 22 In another study, a higher ICU admission ratio was observed among patients with BMI values greater than or equal to 25 compared with patients with a BMI of 25 or lower (46.4% vs. 26.3%, respectively). 19
Our pooled analysis of six studies showed that COVID‐19 patients with obesity have a significantly higher risk for ICU admission (OR = 1.21, CI: 1.002‐1.46; I 2 = 0.0%). 19 , 22 , 23 , 24 , 25 , 26 This result is depicted in Figure 2.
FIGURE 2.
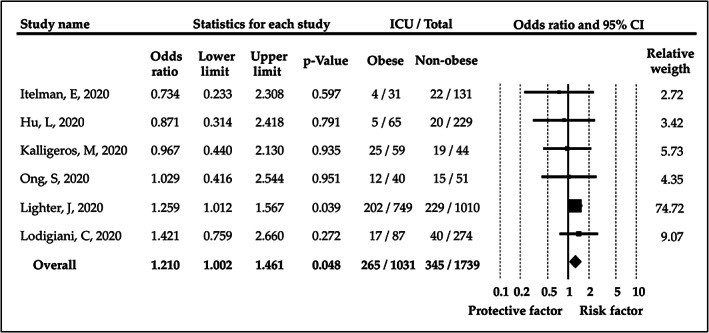
Odds ratios for intensive care unit admission in patients with obesity versus patients without obesity. BMI, body mass index; CI, confidence interval; ICU, intensive care unit
There were not enough studies to compare ICU admission ratios between different BMI ranges.
3.3.2. Multivariate analyses
Kalligeros et al evaluated the association between different BMI ranges and ICU admission with multivariate logistic regression analysis. They found that BMI greater than or equal to 35 carries a six times higher risk for ICU admission compared with BMI lower than 25 (OR = 6.16, CI: 1.42‐26.66). 19 Their results are summarized in Table S4.
3.3.3. Meta‐regression
Because of insufficient data, we could not perform meta‐regression to assess the correlation between BMI and ICU admission. The BMI values of the eligible studies and the corresponding ICU admission ratios are presented in Table S2.
3.4. Association between BMI and IMV requirement
3.4.1. Qualitative and quantitative synthesis comparing BMI classes
We found that IMV is significantly more likely to occur in patients with BMI greater than or equal to 25 compared with those with BMI lower than 25 (OR = 2.63, CI: 1.64‐4.22; I 2 = 0.0%). 15 , 27 , 28 The result of this analysis is shown in Figure S1.
The comparison of patients with obesity and without obesity revealed an increased risk for IMV among patients with obesity (OR = 2.05, CI: 1.16‐3.64; I 2 = 34.86%). 15 , 19 , 25 , 27 , 28 The forest plot displaying this result is found in Figure 3.
FIGURE 3.
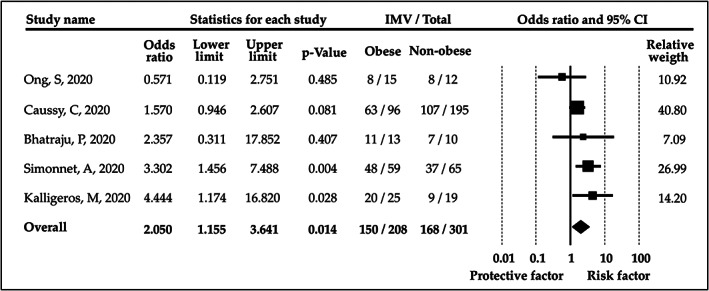
Odds ratios for invasive mechanical ventilation (IMV) in patients with obesity versus patients without obesity. CI, confidence interval
There were enough studies to statistically compare the reported BMI ranges (<25, 25‐30, 30‐35 and ≥35) with each other. We found that the higher BMI ranges always carry a significantly higher risk for IMV compared with the categories with a lower range. The BMI subgroup analyses are shown in Figure 4.
FIGURE 4.

Odds ratios for invasive mechanical ventilation (IMV) between patient groups with different BMI ranges (<25, 25‐30, 30‐35 and ≥35). BMI = body mass index, CI = confidence interval
3.4.2. Multivariate analyses
Kalligeros et al assessed the association between different BMI ranges and IMV with multivariate logistic regression analysis. They found that BMI greater than or equal to 35 carries a six times higher risk for IMV compared with BMI lower than 25. 19 Simonnet et al reported an increased risk for IMV among patients with higher BMI using multivariate logistic regression analysis. 15 They found that BMI ranges of 25 to 30, 30 to 35 and BMI greater than or equal to 35 carry a significantly higher risk for IMV compared to patients with BMI lower than 25. The results of these studies are summarized in Table S4.
3.4.3. Meta‐regression
Meta‐regression scatter plot representing the correlation between BMI and IMV is shown in Figure S2. 15 , 28 , 29 , 30 , 31 , 32 , 33 , 34 No correlation was found (P = 0.8890, Q = 0.02, I 2 = 63.39%, p heterogeneity = 0.0078). However, the analysis provides information only for a narrow BMI range.
3.5. Risk of bias assessment and publication bias
Funnel plots indicating the possibility of publication bias are found in Figures S3 and S4. Egger's tests could not be performed to detect publication bias due to the number of included studies in each hypothesis. Visual assessment of the funnel plots did not imply asymmetry.
The results of the risk of bias assessment of individual studies are shown in Table S5.
3.6. Assessment of heterogeneity
There was no statistical heterogeneity in the meta‐analyses except for comparison of patients with obesity and without obesity regarding IMV requirement (I 2 = 34.86%, moderate level of statistical heterogeneity). The P values related to I 2 were over 0.01.
4. DISCUSSION
According to our meta‐analysis and systematic review, patients with higher BMI have a greater risk for ICU admission and especially for IMV in all comparisons.
The knowledge about the role of obesity in the disease course of respiratory tract infections is limited. Previous studies suggested that obesity might be associated with poor prognosis in COVID‐19. 35 , 36 , 37 Subsequent data have called attention to the higher need for intensive care and mechanical ventilation. 15 , 19 , 28 However, there is no previous meta‐analysis in this topic, and the available studies cannot lead to definitive conclusions because of the low sample sizes and their retrospective nature. Nevertheless, gathering knowledge of the clinical characteristics of these patients is of utmost importance as this could facilitate adequate management and resource allocation. 38
4.1. Obesity and ICU admission
Due to scarce data, we could not perform a conclusive meta‐regression to assess the correlation between BMI and ICU admission, although it is well known that obesity carries enhanced risk of chronic diseases that could contribute to the increased need for ICU admission and unfavourable outcomes in COVID‐19 patients. Unfortunately, initial studies neglected the anthropometric data of patients. 39
Nevertheless, we found in our pooled analysis that obesity is associated with a higher risk for ICU admission in COVID‐19 patients. Since the lower endpoint of the CI in this analysis is close to the zero effect, we feel that it needs confirmation by further studies. This would be particularly important because limited ICU capacity has caused great concern worldwide. Indeed, leading officials of intensive care societies worldwide have proposed to increase ICU capacity because health care should provide intensive care to everyone who needs it. 40
The association between obesity and poor clinical outcomes is certainly multifactorial since obesity itself is widely associated with several prognostic factors. 7 In COVID‐19 patients, hypertension, diabetes and cardiovascular problems are the most common comorbidities 19 that may share similar pathways with obesity related to the renin‐angiotensin system (RAS). 41 Obesity modulates the RAS activity, 42 which can lead to pathological processes in COVID‐19. 43 In this connection, another interesting point was proposed by Lighter et al, namely that a BMI of 35 and above is a risk factor for ICU admission in patients aged less than 60 years, but not in the elderly. 26 Ong et al presented similar findings on IMV requirement. 25 According to the previous studies, obesity seems to increase general mortality risk at older ages, but to a lesser extent than at younger ages. 44 , 45
4.2. Obesity and the need for IMV
Among COVID‐19 patients admitted to the ICU, 40% to 100% of patients require IMV. 33 , 46 , 47 Excessive body mass and obstructive sleep apnea might complicate different forms of respiratory support and endotracheal intubation. 48 , 49 Prolonged endotracheal intubation time, which is more frequently present in patients with obesity, also increases the risk of infection of the medical staff. 50 These are of particular importance considering that we have shown a greater risk for IMV in patients with higher BMI in all comparisons.
In general, several studies in ICU have shown that patients with higher BMI require IMV more frequently and for a longer period. 51 , 52 This could be partly explained by the reduced pulmonary reserves, anatomical alterations of the chest wall; hence, it is not surprising that these patients have a higher risk for progressing into ARDS. 53 , 54
Moreover, obesity may also alter immune response. Protective immune functions might be disabled; for instance, vaccination success can be poor in the case of obesity. 55 Both hyperinflammation and enhanced coagulation have been reported as frequent finding in both COVID‐19 and obesity. 56 , 57 These features and findings in COVID‐19 patients may explain our results, but until specifically investigated, this remains a hypothesis.
4.3. Strengths
To our knowledge, this is the first meta‐analysis about this topic despite its importance. We followed a rigorous methodology, and besides meta‐analyses, we also performed meta‐regression and the assessment of publication bias. No considerable heterogeneity was detected in any analyses. To assess the risk‐increasing effect of obesity as accurately as possible, we also conducted analyses that compare BMI ranges with each other.
4.4. Limitations
A crucial limitation is that our results are from a sparse number of retrospective studies. In addition, our analysis can be limited by the different strategies of different hospitals regarding ICU admission and an indication of IMV, which has been scarcely defined across the included studies. Besides, the BMI distribution can also be largely different between Asian and Caucasian populations. We sought to correct this in our analysis, but the limitation could not be completely avoided.
4.5. Implications for future practice
Based on our findings, it is highly recommended to measure anthropometric parameters of patients and include BMI in the development of a specific COVID‐19 risk assessment score in the future. As patients with higher BMI have a greater risk for ICU admission and IMV, patients with obesity may need special monitoring and earlier escalation of treatment. Therefore, it is important to amend guidelines for COVID‐19 risk stratification and treatment with special attention to patients with obesity. Besides, prevention might also be important. Returning waves of COVID‐19 pandemic cannot be excluded; therefore, it remains important to support weight loss and regular physical activity and also to elaborate vaccination programmes with special regard to the higher risk groups including individuals with elevated BMI.
4.6. Implications for future research
Because of the limited information on this topic, it should be subject to future studies. Multivariate analyses should be conducted to examine whether obesity is an independent risk factor, as it has been suggested in the case of H1N1 influenza. 13 Anthropometric parameters of patients with COVID‐19 should be included in future studies.
Conducting further basic research would be crucial for a better understanding of the pathogenesis of COVID‐19 in patients with obesity. Literature suggests that adipose tissue may serve as a reservoir for certain pathogens (e.g., Influenza A virus and Mycobacterium tuberculosis ). 58 It would be worth examining this in terms of COVID‐19 as well.
5. CONCLUSION
In summary, our meta‐analysis and systematic review revealed that obesity is a significant risk factor for ICU admission and particularly for IMV requirement in COVID‐19. It may serve as a clinical predictor for risk stratification models; therefore, measurement of anthropometric and metabolic parameters in COVID‐19 would be crucial. Patients with obesity should be closely monitored and might need escalation of therapy earlier to avoid unfavourable clinical outcomes. As returning waves of the pandemic are expected, improvement of guidelines for patients with obesity is highly recommended.
CONFLICTS OF INTEREST
The authors have no conflict of interest to declare.
FUNDING INFORMATION
This study was funded by the Economic Development and Innovation Operational Programme (European Regional Development Fund) within the framework of Programme Széchenyi 2020 (GINOP‐2.3.2‐15‐2016‐00048 – STAY ALIVE) and the Human Resources Development Operational Programme (European Regional Development Fund, EFOP 3.6.2‐16‐2017‐00006 – LIVE LONGER) and the Medical School of University of Pécs.
Supporting information
Figure S1. Forest plot comparing patients with overweight or obesity to patients with normal weight regarding invasive mechanical ventilation. IMV=invasive mechanical ventilation, CI=confidence interval
Figure S2. Meta‐regression assessing the correlation between the body mass index and invasive mechanical ventilation, BMI=body mass index
Figure S3. Funnel plot assessing the publication bias of the meta‐analysis that compares non‐obese and obese patients regarding intensive care unit.
Figure S4. Funnel plot assessing the publication bias of the meta‐analysis that compares non‐obese and obese patients regarding invasive mechanical ventilation.
Table S1. Supporting Information
Table S2. Eligibility criteria in each included study in the meta‐analysis
Table S3. Characteristics of the studies included in the meta‐regression
Table S4. Study‐level data on multivariate analysis
Table S5. Risk of bias assessment with QUIPS tool
ACKNOWLEDGEMENTS
The analysis was conducted on behalf of the Translational Action and Research Group against Coronavirus (KETLAK) Study Group.
Földi M, Farkas N, Kiss S, et al. Obesity is a risk factor for developing critical condition in COVID‐19 patients: A systematic review and meta‐analysis. Obesity Reviews. 2020;21:e13095. 10.1111/obr.13095
REFERENCES
- 1. https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/. Accessed 14 June, 2020.
- 2. Lake MA. What we know so far: COVID‐19 current clinical knowledge and research. Clin Med (Lond). 2020;20(2):124‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huttunen R, Syrjanen J. Obesity and the risk and outcome of infection. Int J Obes (Lond). 2013;37(3):333‐340. [DOI] [PubMed] [Google Scholar]
- 5. Luzi L, Radaelli MG. Influenza and obesity: its odd relationship and the lessons for COVID‐19 pandemic. Acta Diabetol. 2020;57(6):759‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malavazos AE, Corsi Romanelli MM, Bandera F, Iacobellis G. Targeting the adipose tissue in COVID‐19. Obesity (Silver Spring). 2020;28(7):1178–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res. 2017;122:1‐7. [DOI] [PubMed] [Google Scholar]
- 8. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richard C, Wadowski M, Goruk S, et al. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Research & Care. 2017;5(1):e000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reidy PT, Yonemura NM, Madsen JH, et al. An accumulation of muscle macrophages is accompanied by altered insulin sensitivity after reduced activity and recovery. Acta Physiol (Oxf). 2019;226(2):e13251. 10.1111/apha.13251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng Q, Cui G, Chen J, et al. Regular exercise enhances the immune response against microbial antigens through up‐regulation of toll‐like receptor signaling pathways. Cell Physiol Biochem. 2015;37(2):735‐746. [DOI] [PubMed] [Google Scholar]
- 12. Fezeu L, Julia C, Henegar A, et al. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta‐analysis. Obes Rev. 2011;12(8):653‐659. [DOI] [PubMed] [Google Scholar]
- 13. Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis. 2018;218(9):1378‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finer N, Garnett SP, Bruun JM. COVID‐19 and obesity. Clin Obes. 2020;10(3):e12365. 10.1111/cob.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020;28(7):1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Organization WH . The Asia‐Pacific perspective: redefining obesity and its treatment. 2000.
- 18. Hayden JC, P.; Bombardier, C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144(6):427‐437. [DOI] [PubMed] [Google Scholar]
- 19. Kalligeros M, Shehadeh F, Mylona EK, et al. Association of obesity with disease severity among patients with COVID‐19. Obesity (Silver Spring). 2020;28(7):1200–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.N DRL. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177–188, 3. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane. 2019. www.training.cochrane.org/handbook [Google Scholar]
- 22. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan. Italy Thromb Res. 2020;191:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu L, Chen S, Fu Y, Gao Z, Long H, Wang JM, Ren HW, Zuo Y, Li H, Wang J, Xu QB. Risk factors associated with clinical outcomes in 323 COVID‐19 hospitalized patients in Wuhan, China [published online ahead of print, 2020 May 3]. Clin Infect Dis. 2020. 10.1093/cid/ciaa539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Itelman E, Wasserstrum Y, Segev A, et al. Clinical characterization of 162 COVID‐19 patients in Israel: preliminary report from a large tertiary center. Isr Med Assoc J. 2020;22(5):271‐274. [PubMed] [Google Scholar]
- 25. Ong SWX, Young BE, Leo YS, Lye DC. Association of higher body mass index (BMI) with severe coronavirus disease 2019 (COVID‐19) in younger patients. Clin Infect Dis. 2020. 10.1093/cid/ciaa548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for Covid‐19 hospital admission. Clin Infect Dis. 2020. 10.1093/cid/ciaa415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caussy C, Wallet F, Laville M, Disse E. Obesity is associated with severe forms of COVID‐19. Obesity (Silver Spring). 2020;28(7):1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid‐19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382(21):2012‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spiezia L, Boscolo A, Poletto F, et al. COVID‐19‐related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(6):998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S. Pulmonary embolism in COVID‐19 Patients: awareness of an increased prevalence [published online ahead of print 2020 Apr 24], 2020. Circulation 2020. 10.1161/circulationaha.120.047430 [DOI] [PubMed] [Google Scholar]
- 31. Piva S, Filippini M, Turla F, et al. Clinical presentation and initial management critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in Brescia. Italy J Crit Care. 2020;58:29‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020. 10.1111/jth.14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cardoso FS, Pereira R, Germano N. Liver injury in critically ill patients with COVID‐19: a case series. Crit Care. 2020;24(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alattar R, Ibrahim TBH, Shaar SH, et al. Tocilizumab for the treatment of severe coronavirus disease. J Med Virol. 2019;2020. 10.1002/jmv.25964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cai Q, Huang D, Ou P, et al. COVID‐19 in a Designated Infectious Diseases Hospital Outside Hubei Province. Allergy: China; 2020. [DOI] [PubMed] [Google Scholar]
- 36. Peng YD, Meng K, Guan HQ, et al. Clinical features and outcomes of 112 patients with cardiovascular disease infected with novel coronavirus pneumonia. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;2(48):E0048 [DOI] [PubMed] [Google Scholar]
- 37. Zheng KI, Gao F, Wang XB, et al. Letter to the editor: obesity as a risk factor for greater severity of COVID‐19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID‐19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020. 10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 40. Cecconi M. Challenges and management in Italy and lessons learned. ICU Management & Practice. 2020;20(1). https://healthmanagement.org/c/icu/issuearticle/challenges-and-management-in-italy-and-lessons-learned [Google Scholar]
- 41. Hanff TC, Harhay MO, Brown TS, Cohen JB, Mohareb AM. Is there an association between COVID‐19 mortality and the renin‐angiotensin system—a call for epidemiologic investigations. Clin Infect Dis. 2020. 10.1093/cid/ciaa329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramalingam L, Menikdiwela K, LeMieux M, et al. The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1106‐1114. [DOI] [PubMed] [Google Scholar]
- 43. Danser AHJ, Epstein M, Batlle D. Renin‐angiotensin system blockers and the COVID‐19 pandemic: at present there is no evidence to abandon renin‐angiotensin system blockers. Hypertension. 2020;75(6):1382‐1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thorpe RJ Jr, Ferraro KF. Aging, obesity, and mortality: misplaced concern about obese older people? Res Aging. 2004;26(1):108‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life‐table analysis. Ann Intern Med. 2003;138(1):24‐32. [DOI] [PubMed] [Google Scholar]
- 46. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington State. JAMA. 2020;323(16):1612‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yakushiji H, Goto T, Shirasaka W, et al. Associations of obesity with tracheal intubation success on first attempt and adverse events in the emergency department: an analysis of the multicenter prospective observational study in Japan. PLoS One. 2018;13(4):e0195938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nowbar S, Burkart KM, Gonzales R, et al. Obesity‐associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Ann Intern Med. 2004;116(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 50. Cook TM, El‐Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID‐19: guidelines from the difficult airway society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020;75(6):785‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hogue CW Jr, Stearns JD, Colantuoni E, et al. The impact of obesity on outcomes after critical illness: a meta‐analysis. Intensive Care Med. 2009;35(7):1152‐1170. [DOI] [PubMed] [Google Scholar]
- 52. O'Brien JM Jr, Phillips GS, Ali NA, Lucarelli M, Marsh CB, Lemeshow S. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med. 2006;34(3):738‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65(1):44‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anzueto A, Frutos‐Vivar F, Esteban A, et al. Influence of body mass index on outcome of the mechanically ventilated patients. Thorax. 2011;66(1):66‐73. [DOI] [PubMed] [Google Scholar]
- 55. Honce R, Schultz‐Cherry S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol. 2019;10:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bourgeois C, Gorwood J, Barrail‐Tran A, et al. Specific biological features of adipose tissue, and their impact on HIV persistence. Front Microbiol. 2019;10:2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Forest plot comparing patients with overweight or obesity to patients with normal weight regarding invasive mechanical ventilation. IMV=invasive mechanical ventilation, CI=confidence interval
Figure S2. Meta‐regression assessing the correlation between the body mass index and invasive mechanical ventilation, BMI=body mass index
Figure S3. Funnel plot assessing the publication bias of the meta‐analysis that compares non‐obese and obese patients regarding intensive care unit.
Figure S4. Funnel plot assessing the publication bias of the meta‐analysis that compares non‐obese and obese patients regarding invasive mechanical ventilation.
Table S1. Supporting Information
Table S2. Eligibility criteria in each included study in the meta‐analysis
Table S3. Characteristics of the studies included in the meta‐regression
Table S4. Study‐level data on multivariate analysis
Table S5. Risk of bias assessment with QUIPS tool


