Summary
Polysubstituted bicyclic acetals are a class of privileged pharmacophores with a unique 3D structure and an adjacent pair of hydrogen bond acceptors. The key, fused acetal functionality is often assembled, via intramolecular cyclization, from linear substrates that are not readily available. Herein, we report a formal cycloaddition between cinnamyl alcohols and cyclic enol ethers under ambient photoredox catalysis conditions. Polysubstituted bicyclic acetals can be prepared in one step from readily available building blocks. Employment of sugar-derived enol ethers allows easy access to a library of scaffolds with intriguing conformation and medicinal chemistry potential.
Subject Areas: Chemistry, Catalysis, Organic Synthesis
Graphical Abstract
Highlights
-
•
Synergistic catalysis of photocatalyst and HAT reagent
-
•
5/5, 5/6, 5/7, 6/6 bicyclic acetals and polysubstituted THF formed in one step
-
•
High stability of bicyclic acetal in SIF and SGF
Chemistry; Catalysis; Organic Synthesis
Introduction
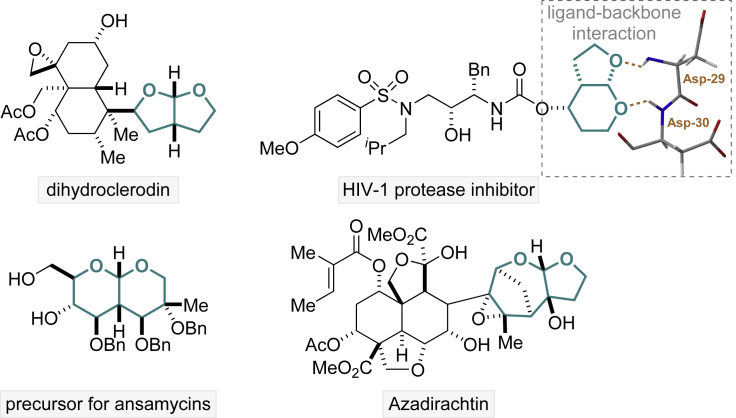
Bicyclic acetals are prevalent structural motifs in natural products, pharmaceuticals, and agrochemicals (Pirrung et al., 1989; Brady et al., 2004; Alonso et al., 2005; Henry and Townsend, 2005; Zhou and Corey, 2005; Baird et al., 2009; Sastraruji et al., 2010; Mori et al., 2015; Ghosh et al., 2017; Karimov et al., 2018; Pan et al., 2018; Lenci et al., 2019; Bera et al., 2018). Compared with other privileged pharmacophores, such as biaryls, bicyclic acetals possess rigorous conformational rigidity, low molecular weight, and a pair of endogenous hydrogen bonding acceptors (King et al., 2004; Surleraux et al., 2005; Ghosh et al., 2009, 2011). They are strong binders to a number of important enzymes and do not carry the liability of instability at low pH environments like their acyclic counterparts (Evans et al., 1981; Coppola, 1984; Tufariello and Winzenberg, 1986; Stern and Swenton, 1989; Roush and Sciotti, 1994). For instance, the bicyclic acetal interacts tightly with two neighboring amino acid residues in HIV-1 protease (Asp-29 and Asp-30), making them excellent candidates for antiviral agents (Figure 1) (King et al., 2004; Surleraux et al., 2005; Ghosh et al., 2009, 2011).
Figure 1.
Examples of Bioactive Compounds Containing Bicyclic Acetal
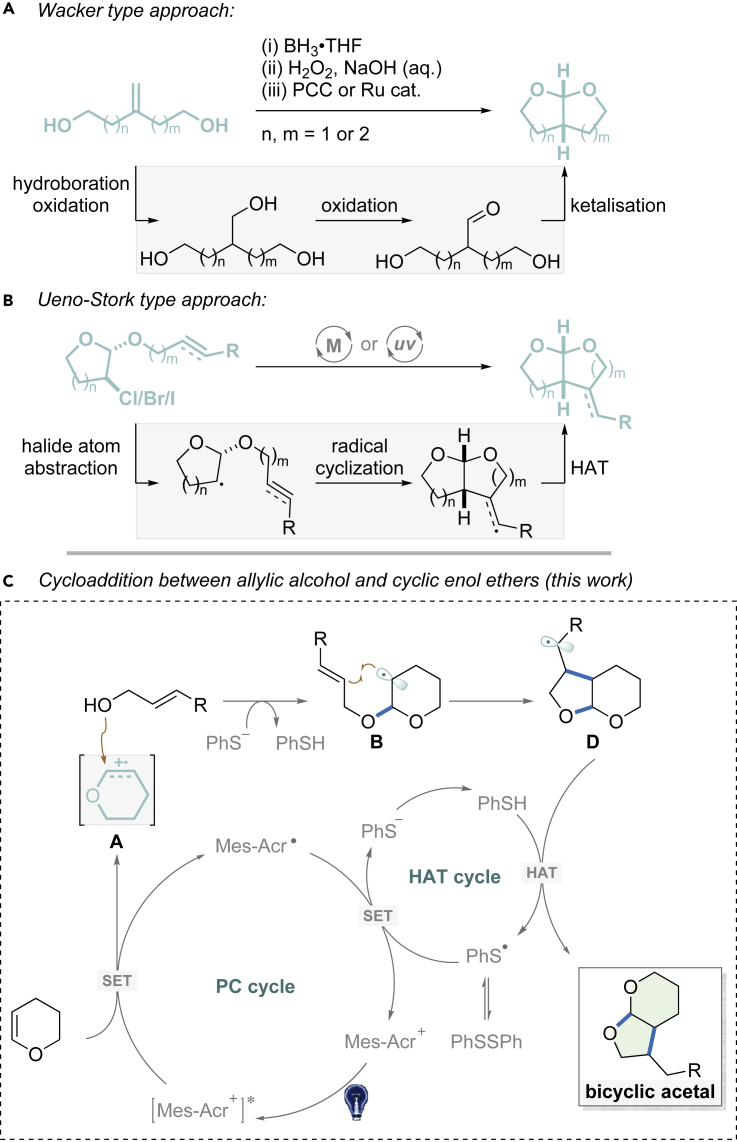
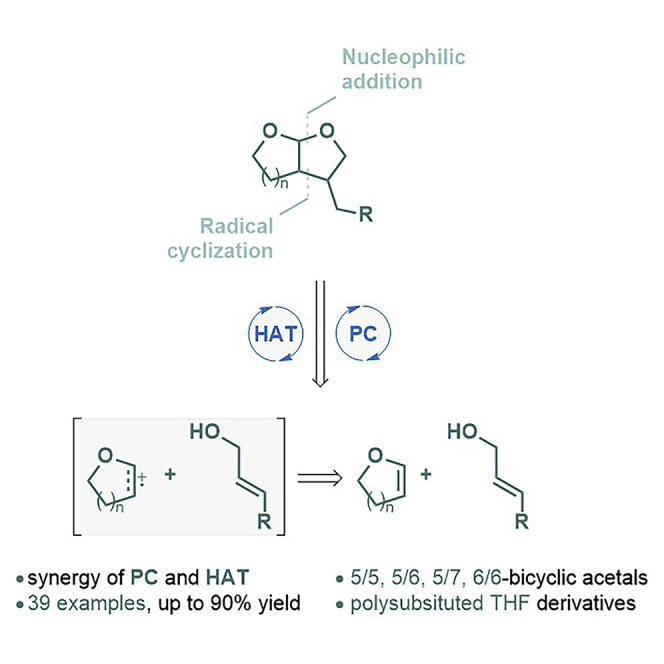
Considerable attention has been drawn to access bicyclic acetals. In general, two synthetic approaches are employed, both of which require linear synthesis of starting materials. The first approach uses gem-disubstituted alkenes bearing two terminal hydroxyl groups that can undergo Wacker-type oxidation, followed by ketalization to furnish the target skeleton (Figure 2A) (Alonso et al., 1998, 2003, 2011; Lorenzo et al., 2000a, 2000b; Roggenbuck et al., 2002; Messerle and Vuong, 2007; Velthuisen et al., 2013). The second option starts from α-halogenated monocyclic acetal. Halide atom abstraction generates an alkyl radical that is captured intramolecularly by a double or triple bond. This Ueno-Stork-type annulation has often been adopted to synthesize the bicyclic acetal core structures (Figure 2B) (Ueno et al., 1982; Stork et al., 1983; Cossy et al., 1994; Yanada et al., 2004; Hayashi et al., 2005; Fukuyama et al., 2016; Hwang et al., 2016; Kyne et al., 2018; Thapa et al., 2017; Venning et al., 2017). Both protocols feature intramolecular cyclization via pre-assembly start materials, which decreases structure versatility, especially when it comes to multi-substituted bicyclic acetals. We envisioned a formal cycloaddition, from readily available olefinic substrates, might present an alternative and modular approach to this type of structures. Herein, we report visible-light-mediated, formal [3 + 2] cycloadditions between commercially available allylic alcohols and cyclic enol ethers (Figure 2C). This transformation delivers the desired bicyclic acetals in a single step and polysubstituted analogs can be accessed.
Figure 2.
Strategies for Constructing Bicyclic Acetals
(A) Wacker type approach.
(B) Ueno-Stork type approach.
(C) Cycloaddition of allylic alcohol and cyclic enol ethers.
In recent years, we developed a series of formal cycloaddition reactions involving styrenes catalyzed by strong photooxidants, acridinium salts (Riener and Nicewicz, 2013; Wang et al., 2017; Wu et al., 2018). Based on the work before, we envisioned that, if the styrene substrates bear a free hydroxy group, an intramolecular event might occur to deliver cyclic acetals. If cyclic enol ethers are employed, the desired bicyclic acetal products might be obtained.
Result and Discussion
Our investigation began using cinnamyl alcohol and dihydropyran (DHP) as substrates. Preliminary trials, using PS-A and PhSSPh as catalysts, afforded the target bicyclic acetal (1) in 25% yield (Table 1, entry 1). The [5, 6] fused ring scaffold is in cis-configuration and moderate dr was observed for the pendant benzyl group (dr = 1.8:1). Side products of DHP were also observed concomitant with product formation. It appears that the addition of DHP to its radical cation A (Figure 2C) might be a competing major side reaction, giving rise to polymeric side products. Increasing the concentration of DHP effectively improves conversion, and high yields were obtained when 4 equiv. of DHP was used (entry 2). Additional acridinium salts were examined, and it was determined that the identity of both the counter ion and N-substituent are important for this reaction (entries 3 and 4). Transition metal-based photosensitizers are incompetent photooxidants, and no desired cycloadduct 1 was detected under these conditions (entries 5 and 6). A survey of additional solvents confirms 1, 2-dicholoethane is the preferred reaction medium (entries 7–9). When either photocatalyst or light is omitted, the cycloadduct is not observed and PhSSPh is also essential to product formation.
Table 1.
Condition Survey for [3 + 2] Cycloaddition Leading to Bicyclic Acetals
 | ||||
|---|---|---|---|---|
| Entry | Photosensitizer (PS) | Solvent | DHP (Eq.) | Yield (%) |
| 1 | PS-A | DCE | 1 | 25 |
| 2 | PS-A | DCE | 4 | 95 (81) |
| 3 | PS-B | DCE | 4 | 10 |
| 4 | PS-C | DCE | 4 | 5 |
| 5 | Ru(bpy)3Cl2 | DCE | 4 | 0 |
| 6 | Ir[dF(CF3)ppy]2(dtbbpy)PF6 | DCE | 4 | 0 |
| 7 | PS-A | MeCN | 4 | 35 |
| 8 | PS-A | toluene | 4 | 0 |
| 9 | PS-A | DMF | 4 | 0 |
Unless otherwise specified, reactions were performed using photosensitizer (0.01 mmol, 5 mol%), PhSSPh (0.04 mmol, 20 mol%), cinnamyl alcohol (0.2 mmol), dihydropyran (DHP) in 3 mL of solvent. Yield of 1 was determined by GC using n-decane as internal standard. Number in parentheses is isolated yield.
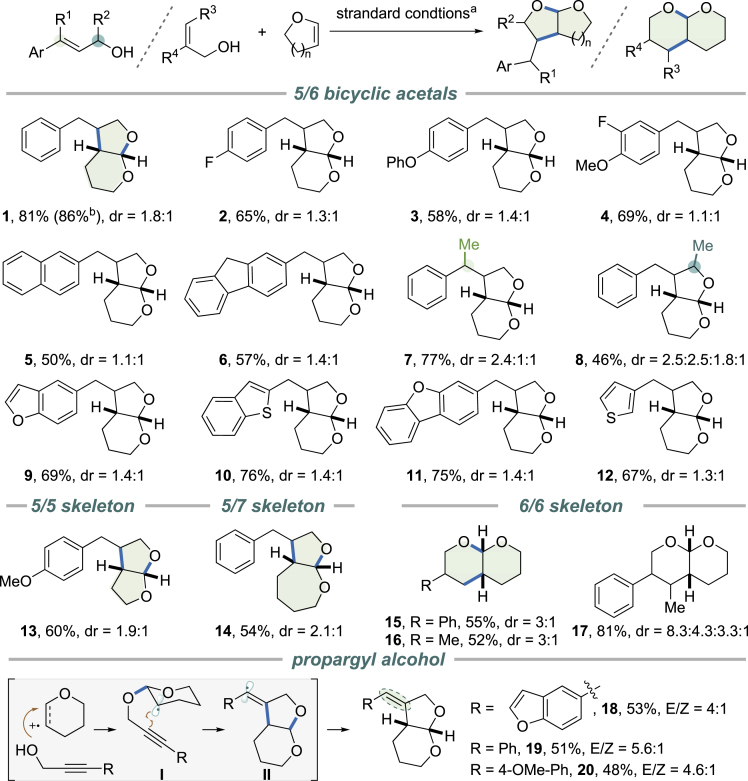
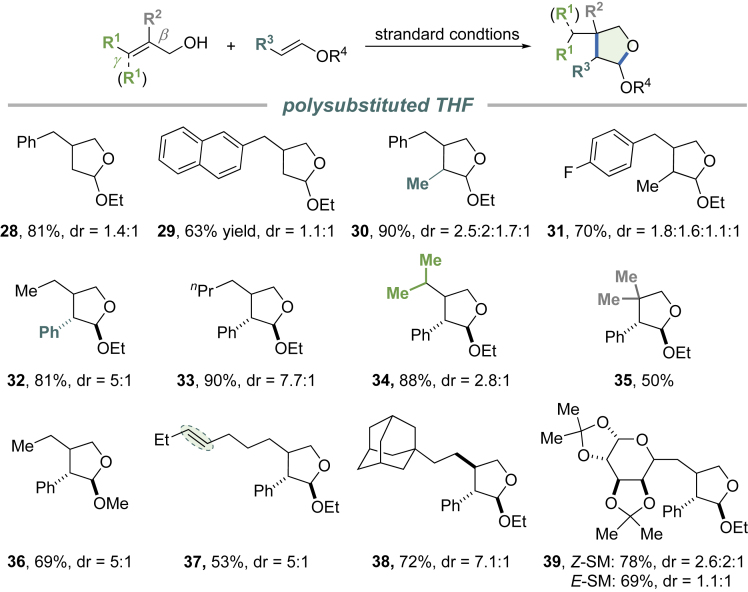
The divergent aspect of this transformation was evaluated using various allylic alcohols and cyclic enol ethers (Figure 3). Cinnamyl alcohols show broad tolerance for substitution and electronic perturbation (products 1–4). Fused aryl substituents can be accommodated by the standard reaction conditions (products 5 and 6). Both α- and γ-alkyl-substituted cinnamyl alcohols undergo smooth cycloaddition to yield bicyclic acetals bearing a vicinal stereogenic center (products 7 and 8). Heteroaryl analogs react with DHP in good efficiency (products 9–12). Besides DHP, cyclic enol ethers with different ring size show similar efficiency in forming the radical cation intermediate. The one-step protocol affords 5/5-7 fused cyclic acetals in moderate to good yield, some of which are undocumented scaffolds. In addition to cinnamyl alcohol, 2-aryl substituted allylic alcohols undergo formal [4 + 2] cycloaddition with DHP to give cyclic acetals with 6/6 ring juncture (products 15, 17). Alkyl allylic alcohols are also applicable to this formal cycloaddition reactions. However, we experienced isolation difficulties for certain substrates. For example, 2-ethylprop-2-en-1-ol reacted smoothly to give the corresponding [6,6] bicyclic acetal 16. On the other hand, the reaction using 3-methylbut-2-en-1-ol suffered from inseparable side products. NOE experiments confirmed formal cis-cycloaddition. Although the diastereoselectivity of the described reaction is moderate, it can be particularly valuable for drug screening, when biologically preferred stereochemistry is unknown. Alkynes can also be intercepted by the radical ring closure. Propargyl alcohols react with DHP to yield bicyclic acetals with an exocyclic double bond (products 18, 19, 20).
Figure 3.
Substrate Scope for Bicyclic Acetals
Reaction conditions: photosensitizer (0.01 mmol, 5 mol%), PhSSPh (0.04 mmol, 20 mol%), allylic alcohol (0.2 mmol), dihydropyran (0.8 mmol) in 3 mL of DCE. Yield in parenthesis was for 1 mmol preparative-scale reaction. aReaction conditions: photosensitizer (0.01 mmol, 5 mol%), PhSSPh (0.04 mmol, 20 mol%), allylic alcohol (0.2 mmol), dihydropyran (0.8 mmol) in 3 mL of DCE. bYield in parenthesis was for 1 mmol preparative-scale reaction.
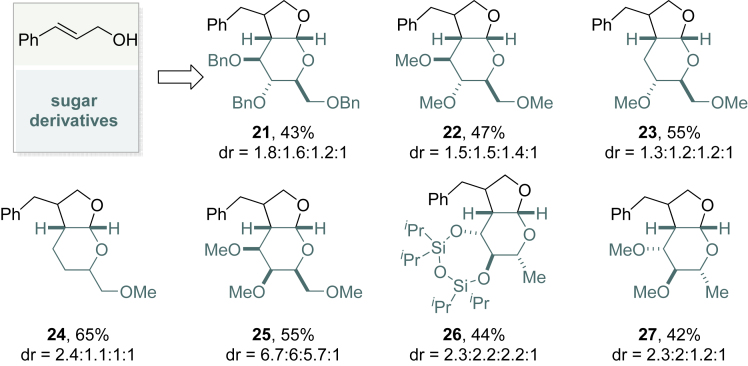
Further substitution of those bicyclic acetals can be accomplished using readily available enol ethers derived from monosaccharides (Figure 4). Natural product-like, polyoxy-substituted bicyclic acetals can be prepared in moderate yield (Jiang et al., 2016). The stereochemistry of the substituents is predefined by the type of sugar starting materials used. Biological evaluation of this intriguing compound library is currently underway.
Figure 4.
Structurally Sophisticated Bicyclic Acetals Derived from Saccharides
Reaction conditions: photosensitizer (0.01 mmol, 5 mol%), PhSSPh (0.04 mmol, 20 mol%), allylic alcohol (0.2 mmol), monosaccharides (0.8 mmol) in 3 mL of DCE.
This cycloaddition can also be extended beyond cyclic enol ethers, as substituted vinyl ethers were also investigated against the standard reaction conditions (Figure 5). The reactions maintain comparable efficiency and selectivity. Polysubstituted tetrahydrofuran (THF) analogs can be accessed in good yield. Ethyl vinyl ether (EVE) reacts smoothly with cinnamyl alcohols to give 2, 4-substitued THFs (products 28 and 29). β-Substituted EVEs lead to 2,3,4-trisubstituted products (30–39). Interestingly, alkyl allylic alcohols are good substrate for β-phenyl EVEs. The relatively high redox potential of the unactivated double bond in these alcohols does not support single electron (SET) oxidation by the excited photosensitizer (Romero and Nicewicz, 2014). The olefin moiety of the enol ether is more prone to SET oxidation (for β-phenyl EVE, Ep/2 = +1.20 V versus SCE). As a consequence, β, β-disubstituted allylic alcohols can react with β-phenyl EVE to deliver a product bearing a quaternary carbon center (35) with complete anti-selectivity. Isolated double bonds in substrates do not interfere with the photoredox process (37). A D-galactopyranose-derived allylic alcohol was also applied to the formal cycloaddition to give product with an additional sugar moiety (39).
Figure 5.
Synthesis of Polysubstituted THF Derivatives
Reaction conditions: photosensitizer (0.01 mmol, 5 mol%), PhSSPh (0.04 mmol, 20 mol%), allylic alcohol (0.2 mmol), vinyl ether (0.8 mmol) in 3 mL of DCE.
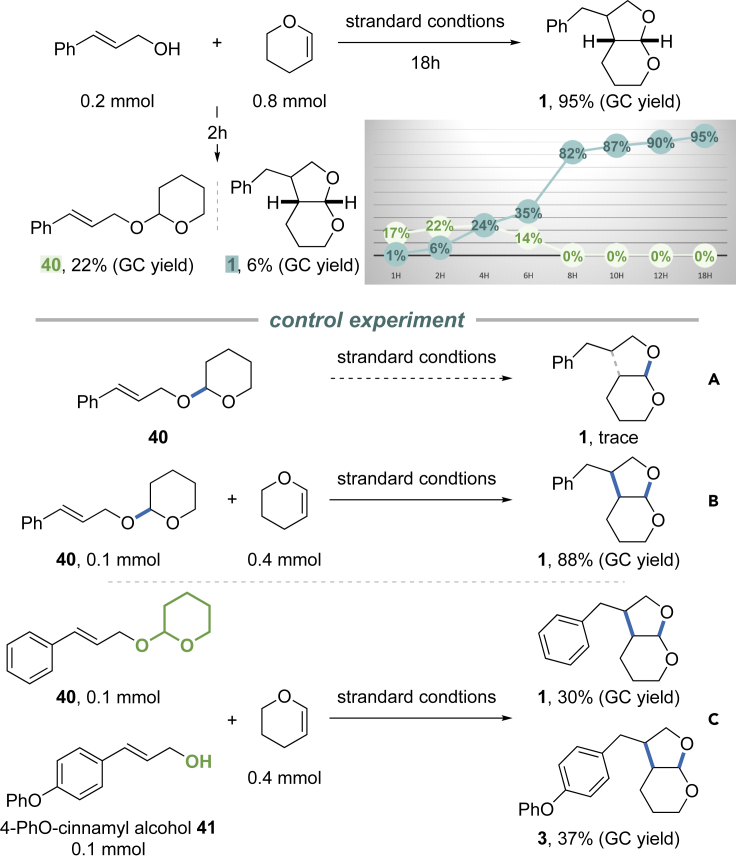
The reaction between cinnamyl alcohol and DHP was monitored closely using GC. We found that acetal 40 was formed immediately upon mixing. It is intriguing that the concentration of 40 remained nearly consistent, at ca. 22%, for the first half of the reaction period, and bicyclic acetal product 1 was gradually formed (Figure 6).
Figure 6.
Control Experiments
Subjecting 40 alone to the standard reaction conditions failed to yield product 1 (Figure 6A), suggesting intramolecular cyclization is not operative. However, mixing 40 with DHP under the same conditions generates product 1 in 88% yield (GC, Figure 6B). This result indicates 40 might be a resting state, which helps prevent homodimerization of cinnamyl alcohols (Hamilton and Nicewicz, 2012; Grandjean and Nicewicz, 2013; Nicewicz and Nguyen, 2014; Perkowski et al., 2015; Margrey and Nicewicz, 2016). A competition experiment using 40 and substrate 41, was carried out (Figure 6C). Comparable yield of 1 and 3 was obtained. These results suggest acetal 40 might also serve as a competitive nucleophile that reacts with DHP radical cation. It is also plausible that the combination of 40, DHP, and the acridinium salt generates catalytic amounts of acid that cause retro acetal formation to free up cinnamyl alcohol.
Unlike previous cycloaddition reactions involving styrene, DHP (Ep/2 = +1.51 V vs. SCE) is more prone, than cinnamyl alcohol (Ep/2 = +1.77 V vs. SCE), to single electron oxidation. Presumably, the acridinium salt is first excited to a highly oxidizing state (Mes-Acr+∗ Ep/2 = +2.06 V versus SCE) by blue LED irradiation. In contrast to nucleophilic addition to styrene-derived radical cation, which features exclusive anti-Markovnikov selectivity, the DHP radical cation reacts with the allylic alcohol at its α-carbon, likely due to oxonium stabilization (Schmittel, 1994). DHP is oxidized by Mes-Acr+∗ to generate a key DHP radical cation (Roth et al., 2016), Subsequently, cinnamyl alcohol, or acetal intermediate 40 formed during the reaction, acts as a nucleophile to trap the DHP radical cation, which is followed by a Ueno-Stork-type annulation (Alonso et al., 2011; Velthuisen et al., 2013; Ueno et al., 1982; Stork et al., 1983; Cossy et al., 1994; Yanada et al., 2004; Hayashi et al., 2005; Fukuyama et al., 2016; Hwang et al., 2016; Kyne et al., 2018). Subsequent HAT process, catalyzed by PhSSPh, closes the catalytic cycle.
Several cyclic acetals were evaluated for their preliminary metabolic stability. As shown in Table 2 (see also Tables S1–S6), all four products are stable after incubation with simulated intestinal fluid (SIF, pH 6.8) for 3 h. Stability after 1-h incubation with simulated gastric fluid (SGF, pH 1.2) varies, depending on structure. Bicyclic acetals 15, 1, and 27 are more stable than monocyclic product 35. The 6, 6-bicyclic product 15 is more stable than its corresponding 6, 5-bicyclic analog 1. Substituents on the six-membered ring have impacts on stability as tri-substitution of 1 leads to compound 27, which is the most stable scaffold tested. This exercise demonstrates that these bicyclic acetals are fairly stable in stimulated intestinal fluids, indicating degradation of these molecules might be very low at major human absorption sites. Furthermore, by modulating ring size and substitution pattern, bicyclic acetals can accomplish good stability in SGF.
Table 2.
Stability of Cyclic Acetals in Simulated GI Fluids
| 35 | 15 (Two Isomers) | 1 | 27 (Four Isomers) | |
|---|---|---|---|---|
| SGF, 1 h | ||||
| Degradation (%) | 99.4 | 4.4 | 12.4 | 1.3 |
| Classification | Unstable | Fairly stable | Unstable | Fairly stable |
| SIF, 3 h | ||||
| Degradation (%) | −2.6 | 0.1 | 0.1 | 0.5 |
| Classification | Fairly stable | Fairly stable | Fairly stable | Fairly stable |
Conclusion
In summary, we developed an efficient strategy to prepare libraries of bicyclic acetals from allylic alcohols and cyclic enol ethers. This reaction pathway is distinct from reported [2 + 2], [4 + 2], and linear coupling involving styrenes. Under synergistic catalysis of an acridinium salt and PhSSPh, structurally intriguing scaffolds involving 5/5, 5/6, 5/7, and 6/6 bicyclic acetals are synthesized in one step. This approach can be applied to monosaccharide-derived substrates, gaining access to previously unattainable polysubstituted analogs. Additionally, use of acyclic vinyl ethers offers a straightforward entry to poly-substituted THF derivatives. Preliminary stability studies in simulated gastric fluids demonstrate bicyclic acetals are a promising class of chemical modality for medicinal chemistry.
Limitations of the Study
A transformation in diastereoselective version cannot be realized under the current reaction conditions.
Resource Availability
Lead Contact
Jiean Chen, chenja@pkusz.edu.cn.
Materials Availability
This study did not generate new unique reagents. All materials used in the work were sourced from public or commercial resources as described under Transparent Methods (see Supplemental Information).
Data and Code Availability
This study did not generate or use any new datasets or machine code.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21801011, 21825101, 21602007), China Postdoctoral Science Foundation (2018M630022), The International Postdoctoral Exchange Fellowship Program (20180033), Guangdong Basic and Applied Basic Research Foundation (2019A1515011641), and Shenzhen Basic Research Funds (JCYJ20170818085510474, JCYJ20170818085438996).
Author Contributions
Methodology, F.W., D.A.N., J.C., and Y.H.; Investigation, F.W., L.W., Y.J., and G.Z.; Writing - Original Draft, F.W. and H.S.; Writing - Review & Editing, D.A.N., J.C., and Y.H.; Supervision, D.A.N., J.C., and Y.H.
Declaration of Interests
The authors declare no competing interests.
Published: August 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101395.
Contributor Information
David A. Nicewicz, Email: nicewicz@unc.edu.
Jiean Chen, Email: chenja@pkusz.edu.cn.
Yong Huang, Email: huangyong@pkusz.edu.cn.
Supplemental Information
References
- Alonso F., Lorenzo E., Melendez J., Yus M. Straight and versatile synthesis of substituted perhydrofuro[2,3-b]pyrans from 2-chloromethyl-3-(2-methoxyethoxy)propene. Tetrahedron. 2003;59:5199–5208. [Google Scholar]
- Alonso F., Lorenzo E., Yus M. 2-chloromethyl-3-(2-methoxyethoxy)propene: Naphthalene-catalysed lithiation and reaction towards electrophiles. Tetrahedron Lett. 1998;39:3303–3306. [Google Scholar]
- Alonso F., Melendez J., Yus M. A new 3-methylidenepentane-1,5-dianion synthon: synthesis of perhydropyrano[2,3-b]pyrans and 1,7-dioxaspiro[4.5]decanes. Tetrahedron Lett. 2005;46:6519–6524. [Google Scholar]
- Alonso F., Rodriguez-Fernandez M., Sanchez D., Yus M. Synthesis of perhydrofuro[2,3-b]furans from isopentenyl alcohol through carbonyl-ene and Wacker-type reactions. Eur. J. Org. Chem. 2011;2011:6459–6469. [Google Scholar]
- Baird M.C., Pyne S.G., Ung A.T., Lie W., Sastraruji T., Jatisatienr A., Jatisatienr C., Dheeranupattana S., Lowlam J., Boonchalermkit S. Semisynthesis and biological activity of stemofoline alkaloids. J. Nat. Prod. 2009;72:679–684. doi: 10.1021/np800806b. [DOI] [PubMed] [Google Scholar]
- Bera S., Chatterjee B., Mondal D. Advances in the asymmetric synthesis of bridged and fused bicyclic acetals. Eur. J. Org. Chem. 2018;2018:5337–5354. [Google Scholar]
- Brady T.P., Kim S.H., Wen K., Theodorakis E.A. Stereoselective total synthesis of (+)-norrisolide. Angew. Chem. Int. Ed. 2004;43:739–742. doi: 10.1002/anie.200352868. [DOI] [PubMed] [Google Scholar]
- Coppola G.M. Amberlyst-15, a superior acid catalyst for the cleavage of acetals. Synthesis. 1984;1984:1021–1023. [Google Scholar]
- Cossy J., Ranaivosata J.L., Bellosta V. Formation of radicals by irradiation of alkyl-halides in the presence of triethylamine. Tetrahedron Lett. 1994;35:8161–8162. [Google Scholar]
- Evans D.A., Tanis S.P., Hart D.J. A convergent total synthesis of (+/-)-colchicine and (+/-)-desacetamidoisocolchicine. J. Am. Chem. Soc. 1981;103:5813–5821. [Google Scholar]
- Fukuyama T., Fujita Y., Rashid M.A., Ryu I. Flow update for a cossy photocyclization. Org. Lett. 2016;18:5444–5446. doi: 10.1021/acs.orglett.6b02727. [DOI] [PubMed] [Google Scholar]
- Ghosh A.K., Chapsal B.D., Baldridge A., Steffey M.P., Walters D.E., Koh Y., Amano M., Mitsuya H. Design and synthesis of potent hiv-1 protease inhibitors incorporating hexahydrofuropyranol-derived high affinity P(2) ligands: structure-activity studies and biological evaluation. J. Med. Chem. 2011;54:622–634. doi: 10.1021/jm1012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A.K., Kulkarni S., Anderson D.D., Hong L., Baldridge A., Wang Y.F., Chumanevich A.A., Kovalevsky A.Y., Tojo Y., Amano M. Design, synthesis, protein-ligand X-ray structure, and biological evaluation of a series of novel macrocyclic human immunodeficiency virus-1 protease inhibitors to combat drug resistance. J. Med. Chem. 2009;52:7689–7705. doi: 10.1021/jm900695w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A.K., Rao K.V., Nyalapatla P.R., Osswald H.L., Martyr C.D., Aoki M., Hayashi H., Agniswamy J., Wang Y.F., Bulut H. Design and development of highly potent HIV-1 protease inhibitors with a crown-like oxotricyclic core as the P2-ligand to combat multidrug-resistant hiv variants. J. Med. Chem. 2017;60:4267–4278. doi: 10.1021/acs.jmedchem.7b00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean J.M.M., Nicewicz D.A. Synthesis of highly substituted tetrahydrofurans by catalytic polar-radical-crossover cycloadditions of alkenes and alkenols. Angew. Chem. Int. Ed. 2013;52:3967–3971. doi: 10.1002/anie.201210111. [DOI] [PubMed] [Google Scholar]
- Hamilton D.S., Nicewicz D.A. Direct catalytic anti-Markovnikov hydroetherification of alkenols. J. Am. Chem. Soc. 2012;134:18577–18580. doi: 10.1021/ja309635w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi N., Shibata I., Baba A. Inter- and intramolecular radical couplings of ene-ynes or halo-alkenes promoted by an InCl3/MeONa/Ph2SiH2 system. Org. Lett. 2005;7:3093–3096. doi: 10.1021/ol051114o. [DOI] [PubMed] [Google Scholar]
- Henry K.M., Townsend C.A. Synthesis and fate of O-carboxybenzophenones in the biosynthesis of aflatoxin. J. Am. Chem. Soc. 2005;127:3300–3309. doi: 10.1021/ja045520z. [DOI] [PubMed] [Google Scholar]
- Hwang J.Y., Baek J.H., Shin T.I., Shin J.H., Oh J.W., Kim K.P., You Y., Kang E.J. Single-electron-transfer strategy for reductive radical cyclization: Fe(CO)5 and phenanthroline system. Org. Lett. 2016;18:4900–4903. doi: 10.1021/acs.orglett.6b02375. [DOI] [PubMed] [Google Scholar]
- Jiang H., Xu L.P., Fang Y., Zhang Z.X., Yang Z., Huang Y. A migratory ether formation route to medium-sized sugar mimetics. Angew. Chem. Int. Ed. 2016;55:14338–14342. doi: 10.1002/anie.201608974. [DOI] [PubMed] [Google Scholar]
- Karimov R.R., Tan D.S., Gin D.Y. Synthesis of the hexacyclic triterpene core of the jujuboside saponins via tandem Wolff rearrangement-intramolecular ketene hetero-Diels-Alder reaction. Tetrahedron. 2018;74:3370–3383. doi: 10.1016/j.tet.2018.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N.M., Prabu-Jeyabalan M., Nalivaika E.A., Wigerinck P., De Bethune M.P., Schiffer C.A. Structural and thermodynamic basis for the binding of TMC114, a next-generation human immunodeficiency virus type 1 protease inhibitor. J. Virol. 2004;78:12012–12021. doi: 10.1128/JVI.78.21.12012-12021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyne S.H., Clemancey M., Blondin G., Derat E., Fensterbank L., Jutand A., Lefevre G., Ollivier C. Elucidating dramatic ligand effects on set processes: Iron hydride versus iron borohydride catalyzed reductive radical cyclization of unsaturated organic halides. Organometallics. 2018;37:761–771. [Google Scholar]
- Lenci E., Menchi G., Saldivar-Gonzalez F.I., Medina-Franco J.L., Trabocchi A. Bicyclic acetals: biological relevance, scaffold analysis, and applications in diversity-oriented synthesis. Org. Biomol. Chem. 2019;17:1037–1052. doi: 10.1039/c8ob02808g. [DOI] [PubMed] [Google Scholar]
- Lorenzo E., Alonso F., Yus M. New trimethylenemethane dianion synthons: application to the preparation of substituted perhydrofuro[2,3-b]furans. Tetrahedron. 2000;56:1745–1757. [Google Scholar]
- Lorenzo E., Alonso F., Yus M. Substituted perhydrofuropyrans: easy preparation from 2-chloromethyl-3-(2-methoxyethoxy)propene through 3-methylene-1,6-diols. Tetrahedron Lett. 2000;41:1661–1665. [Google Scholar]
- Margrey K.A., Nicewicz D.A. A general approach to catalytic alkene anti-Markovnikov hydrofunctionalization reactions via acridinium photoredox catalysis. Acc. Chem. Res. 2016;49:1997–2006. doi: 10.1021/acs.accounts.6b00304. [DOI] [PubMed] [Google Scholar]
- Messerle B.A., Vuong K.Q. Rhodium- and iridium-catalyzed double hydroalkoxylation of alkynes, an efficient method for the synthesis of O,O-acetals: catalytic and mechanistic studies. Organometallics. 2007;26:3031–3040. [Google Scholar]
- Mori N., Kitahara T., Mori K., Watanabe H. Asymmetric formal synthesis of azadirachtin. Angew. Chem. Int. Ed. 2015;54:14920–14923. doi: 10.1002/anie.201507935. [DOI] [PubMed] [Google Scholar]
- Nicewicz D.A., Nguyen T.M. Recent applications of organic dyes as photoredox catalysts in organic synthesis. ACS Catal. 2014;4:355–360. [Google Scholar]
- Pan S., Chen S., Dong G. Divergent total syntheses of enmein-type natural products: (-)-enmein, (-)-isodocarpin, and (-)-sculponin r. Angew. Chem. Int. Ed. 2018;57:6333–6336. doi: 10.1002/anie.201803709. [DOI] [PubMed] [Google Scholar]
- Perkowski A.J., You W., Nicewicz D.A. Visible light photoinitiated metal-free living cationic polymerization of 4-methoxystyrene. J. Am. Chem. Soc. 2015;137:7580–7583. doi: 10.1021/jacs.5b03733. [DOI] [PubMed] [Google Scholar]
- Pirrung M.C., Chang V.K., Deamicis C.V. Mechanism and synthetic applications of the photochemical generation and X-H insertion reactions of oxacarbenes. J. Am. Chem. Soc. 1989;111:5824–5831. [Google Scholar]
- Riener M., Nicewicz D.A. Synthesis of cyclobutane lignans via an organic single electron oxidant-electron relay system. Chem. Sci. 2013;4:2625–2629. doi: 10.1039/C3SC50643F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggenbuck R., Schmidt A., Eilbracht P. Synthesis of furo[2,3b]furans and furo[2,3b]pyrans via rhodium-catalyzed tandem hydroformylation/acetalization. Org. Lett. 2002;4:289–291. doi: 10.1021/ol017083o. [DOI] [PubMed] [Google Scholar]
- Romero N.A., Nicewicz D.A. Mechanistic insight into the photoredox catalysis of anti-Markovnikov alkene hydrofunctionalization reactions. J. Am. Chem. Soc. 2014;136:17024–17035. doi: 10.1021/ja506228u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth H.G., Romero N.A., Nicewicz D.A. Experimental and calculated electrochemical potentials of common organic molecules for applications to single-electron redox chemistry. Synlett. 2016;27:714–723. [Google Scholar]
- Roush W.R., Sciotti R.J. Enantioselective total synthesis of (-)-chlorothricolide. J. Am. Chem. Soc. 1994;116:6457–6458. [Google Scholar]
- Sastraruji K., Sastraruji T., Pyne S.G., Ung A.T., Jatisatienr A., Lie W. Semisynthesis and acetylcholinesterase inhibitory activity of stemofoline alkaloids and analogues. J. Nat. Prod. 2010;73:935–941. doi: 10.1021/np100137h. [DOI] [PubMed] [Google Scholar]
- Schmittel M. Umpolung of ketones via enol radical cations. Top. Curr. Chem. 1994;169:183–230. [Google Scholar]
- Stern A.J., Swenton J.S. The unusually slow hydrolysis rate of silyl methyl ketals in benzoquinone systems - the question of siloxy stabilization of an adjacent positive charge and stereoelectronic effects on ketal hydrolysis. J. Org. Chem. 1989;54:2953–2958. [Google Scholar]
- Stork G., Mook R., Biller S.A., Rychnovsky S.D. Free-radical cyclization of bromo acetals. Use in the construction of bicyclic acetals and lactones. J. Am. Chem. Soc. 1983;105:3741–3742. [Google Scholar]
- Surleraux D.L., Tahri A., Verschueren W.G., Pille G.M., De Kock H.A., Jonckers T.H., Peeters A., De Meyer S., Azijn H., Pauwels R. Discovery and selection of tmc114, a next generation HIV-1 protease inhibitor. J. Med. Chem. 2005;48:1813–1822. doi: 10.1021/jm049560p. [DOI] [PubMed] [Google Scholar]
- Thapa S., Basnet P., Giri R. Copper-catalyzed dicarbofunctionalization of unactivated olefins by tandem cyclization/cross-coupling. J. Am. Chem. Soc. 2017;139:5700–5703. doi: 10.1021/jacs.7b01922. [DOI] [PubMed] [Google Scholar]
- Tufariello J.J., Winzenberg K. A nitrone-based synthesis of the pyrrolizidine alkaloid croalbinecine. Tetrahedron Lett. 1986;27:1645–1648. [Google Scholar]
- Ueno Y., Chino K., Watanabe M., Moriya O., Okawara M. Homolytic carbocyclization by use of heterogeneous supported organotin catalyst - a new synthetic route to 2-alkoxytetrahydrofurans and γ-butyrolactones. J. Am. Chem. Soc. 1982;104:5564–5566. [Google Scholar]
- Velthuisen E.J., Baughman T.M., Johns B.A., Temelkoff D.P., Weatherhead J.G. Synthesis and pharmacokinetic profile of highly deuterated brecanavir analogs. Eur. J. Med. Chem. 2013;63:202–212. doi: 10.1016/j.ejmech.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Venning A.R.O., Kwiatkowski M.R., Pena J.E.R., Lainhart B.C., Guruparan A.A., Alexanian E.J. Palladium-catalyzed carbocyclizations of unactivated alkyl bromides with alkenes involving auto-tandem catalysis. J. Am. Chem. Soc. 2017;139:11595–11600. doi: 10.1021/jacs.7b06794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.F., Wu F.J., Chen J.A., Nicewicz D.A., Huang Y. Visible-light-mediated [4+2] cycloaddition of styrenes: synthesis of tetralin derivatives. Angew. Chem. Int. Ed. 2017;56:6896–6900. doi: 10.1002/anie.201702940. [DOI] [PubMed] [Google Scholar]
- Wu F.J., Wang L.F., Chen J.A., Nicewicz D.A., Huang Y. Direct synthesis of polysubstituted aldehydes via visible-light catalysis. Angew. Chem. Int. Ed. 2018;57:2174–2178. doi: 10.1002/anie.201712384. [DOI] [PubMed] [Google Scholar]
- Yanada R., Koh Y., Nishimori N., Matsumura A., Obika S., Mitsuya H., Fujii N., Takemoto Y. Indium-mediated atom-transfer and reductive radical cyclizations of iodoalkynes: synthesis and biological evaluation of hiv-protease inhibitors. J. Org. Chem. 2004;69:2417–2422. doi: 10.1021/jo035482m. [DOI] [PubMed] [Google Scholar]
- Zhou G., Corey E.J. Short, enantioselective total synthesis of aflatoxin B2 using an asymmetric [3+2]-cycloaddition step. J. Am. Chem. Soc. 2005;127:11958–11959. doi: 10.1021/ja054503m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate or use any new datasets or machine code.