Abstract
Coronavirus disease 2019 (COVID‐19) outbreak is a current global healthcare burden, leading to the life‐threatening severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). However, evidence showed that, even if the prevalence of COVID‐19 damage consists in pulmonary lesions and symptoms, it could also affect other organs, such as heart, liver, and spleen. Particularly, some infected patients refer to the emergency department for cardiovascular symptoms, and around 10% of COVID‐19 victims had finally developed heart injury. Therefore, the use of echocardiography, according to the safety local protocols and ensuring the use of personal protective equipment, could be useful firstly to discriminate between primary cardiac disease or COVID‐19–related myocardial damage, and then for assessing and monitoring COVID‐19 cardiovascular complications: acute myocarditis and arrhythmias, acute heart failure, sepsis‐induced myocardial impairment, and right ventricular failure derived from treatment with high‐pressure mechanical ventilation. The present review aims to enlighten the applications of transthoracic echocardiography for the diagnostic and therapeutic management of myocardial damage in COVID‐19 patients.
Keywords: heart failure, COVID‐19, echocardiography, myocardial injury, myocarditis, SARS‐CoV2
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) pandemic is currently affecting 212 countries throughout the world, with high morbidity and mortality rates. 1 Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection could be easily transmitted through human‐to‐human contact or respiratory droplets, and individuals with underlying cardiovascular disease are at highest risk for severe disease and death, reaching 10.5% fatality rate. 2 Even though the clinical manifestations of COVID‐19 are dominated by respiratory symptoms, 3 some infected patient initially presents typical cardiovascular symptoms (ie, chest discomfort, palpitations, dyspnea). 4 In these cases, it could be challenging for the clinician to establish whether symptoms represent the first expression of SARS‐CoV‐2 cardiac involvement, or they derive from a primary cardiac pathologic condition. Moreover, several cases of COVID‐19–induced myocardial damage has been observed, particularly in critical subjects (in China, 11.8% of dead subjects without underlying cardiovascular disease had myocardial injury 5 ), consisting in acute myocardial injury and myocarditis, cardiac arrest, heart failure (HF) due to pulmonary hypertension (PH), or shock states. 6 This is probably due to coronavirus‐related damage which also involves heart and other organs, provoking degeneration and necrosis of parenchymal cells and formation of hyaline thrombus in small vessels, as shown in a postmortem examination of 3 COVID‐19 victims. 7 In two studies by Shi et al 8 and Guo et al, 9 among 460 and 187 patients hospitalized for COVID‐19, respectively, 20% and 28% had acute myocardial injury, which was associated with higher mortality and incidence of complications, such as acute respiratory distress syndrome (ARDS), malignant arrhythmias, acute renal injury, and coagulopathy. Echocardiography is considered the first‐choice diagnostic technique for the evaluation of myocardial structure and function, due to its high availability and cost‐effectiveness. 10 For this reason, a conscious inhospital application of transthoracic echocardiography (TTE), using a focused and safe approach, according to the latest European Association of Cardiovascular Imaging (EACVI) and American Society of Echocardiography (ASE) recommendations, 11 , 12 could reduce the potential risks of COVID‐19 heart injury, providing early detection and treatment. These documents do not provide strict indications on whether to perform or reject an echocardiographic examination in this period of social distancing, since it should be tailored on the single patient, trying to avoid unnecessary examinations. However, American College of Cardiology (ACC) Clinical Guidance for COVID‐19 suggests that patients demonstrating HF, arrhythmia, electrocardiographic (ECG) changes, or cardiomegaly should undergo echocardiography. 13 Moreover, the evaluation of the right ventricle (RV) could be important in ventilated patients for the early assessment of high positive end‐expiratory pressures (PEEP)‐induced cardiopulmonary overload and in patients with suspected acute cor pulmonale.
The present review discusses the different clinical inhospital applications of echocardiography, from emergency department to COVID wards and ICU, to highlight its usefulness for assisting clinicians in the daily diagnostic and therapeutic management of COVID‐19–affected patients.
2. SUSPECTED ACUTE CORONARY SYNDROMES
As reported by the National Health Commission of China (NHC), some of the confirmed cases of SARS‐CoV‐2 patients first showed cardiovascular rather than respiratory symptoms. 9 After the nasal or pharyngeal swab has done to test COVID‐19 patients' status before the admission 14 , the first step of triaging usually comprises ECG and blood cardiac enzymes dosage; however, evidence has shown that troponin and brain natriuretic peptide (BNP) levels could increase due to COVID‐19 itself, proportionally to the severity of the disease. 9 In fact, a meta‐analysis showed that troponin I values were significantly higher in patients with severe compared to those with mild illness due to SARS‐CoV‐2 infection. 15
He et al conducted a study in critical COVID‐19 patients dividing them into two groups according to the presence (24 patients, 44.4%) or absence (30 patients, 55.6%) of myocardial injury, revealing that the injury group presented significantly higher inhospital mortality (75.0% [18/24] vs. 26.7% [8/30], P = .001), C‐reactive protein (CRP), and N‐terminal pro‐BNP (NT‐pro‐BNP, P < .01). 6 Chen Chen et al also analyzed 150 COVID‐19 subjects and found 22 of them (14.7%) having troponin elevation, which was independently correlated with COVID‐19 critical severity with multivariate regression analysis (odds ratio, OR = 26.909, 95% CI 4.086‐177.226, P = .001). 16
Accordingly, ACC COVID‐19 clinical guidance pointed out that that classic symptoms and presentation of acute myocardial infarction may be unclear in the context of COVID‐19, resulting in underdiagnosis. 13 Moreover, in a small Italian report of 28 COVID‐19 patients with ST‐elevation myocardial infarction (STEMI), 78.6% of them presented with acute chest pain, while 82.1% had regional wall‐motion abnormalities at TTE. 17
In fact, echocardiography could support diagnosis in this setting, revealing suggestive signs of acute myocardial infarction, new‐onset or worsening congestive HF, pericardial effusion or tamponade, and RV overload due to pulmonary embolism or cor pulmonale (Table 1). This would lead to an accurate triaging, ensuring each patient the appropriate treatment.
Table 1.
Useful echocardiographic findings to aid early diagnosis of acute myocardial involvement in COVID‐19 patients
| Suggested diagnosis | Echocardiographic findings |
|---|---|
| Acute coronary syndromes |
|
| Acute heart failure |
|
| Cardiac tamponade |
|
| Pulmonary embolism or acute cor pulmonale |
|
Abbreviations: IVC = inferior vena cava; LV = left ventricle; PAP = pulmonary artery pressure; PEEP = positive end‐expiratory pressures; PWD = pulsed wave Doppler; TDI = tissue Doppler imaging.
McConnell's sign: depressed contractility of RV free wall compared to RV apex. Common finding in case of pulmonary embolism.
3. ACUTE MYOCARDITIS AND ARRHYTHMIAS
Various degrees of myocardial injury (defined as raised troponin levels over the 99th percentile of reference range) have been recently shown in patients with COVID‐19. 18 , 19 In a clinical study involving 138 patients with COVID‐19, 10 patients (7.2%) had acute myocardial injury 20 and 23 (16.7%) had arrhythmia, the majority of them during hospitalization in intensive care unit (ICU). There are many possible causes of acute myocardial injury in critically ill patients, including acute coronary syndrome, HF, myocarditis, hypotension or shock, sepsis, and infection. To date, the mechanism responsible of myocardial injury in COVID‐19 is uncertain; however, hypothesis has focused on local or systemic immune response, possibly causing cardiomyocytes degeneration and/or microvascular thrombosis. 21 Accordingly, current reports suggest that the majority of COVID‐19 patients with myocardial injury without evidence of epicardial coronary artery thrombosis, show imaging data supporting the diagnosis of acute myocarditis 21 , 22 ; also, cases of fulminant myocarditis and fatal arrhythmias have been described. 23 , 24 Even if a direct cardiotropic localization SARS‐CoV‐2 into myocytes has never been demonstrated, some authors showed autoptic findings (eg, lymphocyte infiltrates and macrophagic response) compatible with viral myocarditis. 25 , 26 , 27
Moreover, in a retrospective study by Ruan et al evaluating factors associated with mortality in 150 COVID‐19 subjects, patients who died showed higher levels of troponin, myoglobin, C‐reactive protein, serum ferritin, and interleukin‐6, suggesting a high inflammatory burden in COVID‐19 with a possible rise in myocarditis‐related cardiac events. 28 For acute myocarditis, a combination of cardiac magnetic resonance (CMR) and myocardial biopsy is the reference diagnostic method, 29 preceded by coronary angiography to rule out acute coronary syndromes. This is also valid for COVID‐19 patients. Accordingly, Inciardi et al presented a case of a 53‐year COVID‐19 woman who developed acute myocarditis diagnosed, after exclusion of coronary disease and TTE findings consistent with acute myocarditis (increased wall thickness, diffuse echo‐bright myocardial appearance and diffuse LV hypokinesis, with LVEF 40%), by CMR as increased wall thickness with diffuse biventricular hypokinesis and signs of marked biventricular myocardial interstitial edema by T2‐mapping sequences and late gadolinium enhancement. 22
However, in critical patients and in this reduced healthcare services emergency status, CMR and myocardial biopsy could not be promptly available and coronary angiography would put unstable patients at higher risks. Therefore, an echocardiographic study could be used as the first investigation tool to orient diagnosis with high‐sensitive but less specific findings, that are listed in Table 2. 30 Additionally, LV longitudinal strain proved to correlate with myocardial edema detected by CMR in patients with acute myocarditis 31 and its bull's eye representation shows the localization of myocardial damage, with GLS typically reducing from endocardial to epicardial layer (Figure 1).
Table 2.
Possible echocardiographic characteristics of acute myocarditis
| Echocardiographic parameters | Typical findings |
|---|---|
| LV dimensions | Either normal or increased |
| LV septal thickness | Either normal or increased (transient LV pseudohypertrophy) |
| LV systolic function |
|
| LV diastolic function | Common LV diastolic dysfunction (↓ E/A, ↑ E/E′) |
| Right ventricle | Sometimes RV global systolic dysfunction with or without RV dilation |
| Pericardium |
Pericardial effusion Brightness of myo‐pericardium |
Abbreviations: EF = ejection fraction; LV = left ventricle; STE = speckle tracking echocardiography.
FIGURE 1.
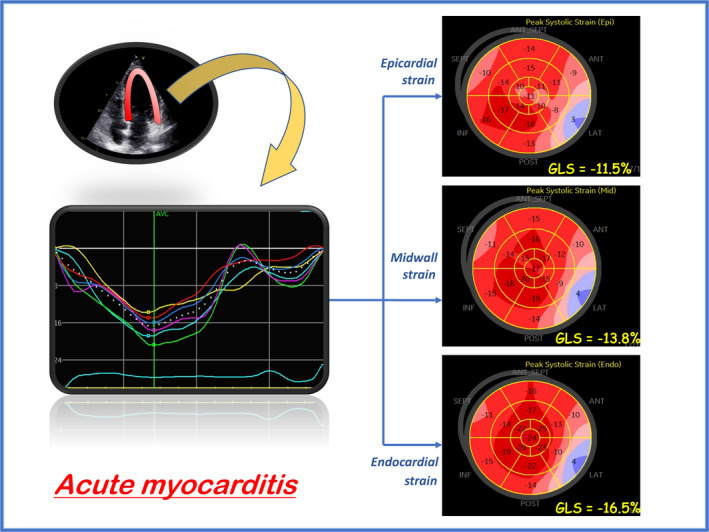
Example of speckle tracking analysis in acute myocarditis, showing a regional area of severe reduction of global longitudinal strain (GLS), and a typical three‐layer variation (GLS worsening from endocardial to epicardial layer)
4. ACUTE HEART FAILURE
In patients with COVID‐19, cardiovascular involvement leading to cardiac dysfunction and failure is not uncommon, probably due to systemic inflammatory response, innate immune‐related myocardial damage, or respiratory‐induced hypoxemia during COVID‐19 progression. 16 , 32 This also affects patients without history of chronic HF, which could rapidly develop severe HF and die for sudden cardiac death after COVID‐19 infection. In fact, the most likely mechanism of HF in these patients is consequent to lung disease, leading to oxygen supply/demand imbalance, high pulmonary vascular resistance, and PH, eventually causing acute RV overload and failure. 33
Therefore, in patients hospitalized for COVID‐19, LV and RV structure and function changes and cardiac filling pressures should be assessed. For this purpose, beyond basic echocardiographic parameters, additive tools could represent a strong support for clinicians: Firstly, lung ultrasound (LU) offers important information on acute hemodynamic changes in HF, through dynamic signs (ie, homogenous and diffuse B‐lines and bilateral pleural effusion), allowing a quick diagnosis and monitoring of acute pulmonary edema. 34 In this setting, it is important to differentiate between the typical LU signs of pulmonary edema and of COVID‐19 leading to ARDS (ie, congestion, coalescent B‐lines, the so‐called “white lung,” air bronchogram) 35 : Even if clinical presentation are crucial for differential diagnosis, there are some notable differences between cardiogenic and COVID‐19 B‐lines: unlike the first, B‐lines have a patchy and nongravity‐related distribution, are commonly separated and coalescent, and present well‐defined spared areas in COVID‐19 pneumonia 36 . However, sometimes these patients could show overlap patterns; thus, LU results should be taken cautiously. 37
In addition, offline speckle tracking analysis provides noninvasive early detection of myocardial damage in patients with HF, studying the deformation of all cardiac chambers, which revealed to have a correlation with LV filling pressures. 38 , 39 , 40 , 41
A comprehensive evaluation of these patients, including clinical, basic, and advanced ultrasonographic parameters, could be useful for an accurate prognostic assessment: In fact, it has been demonstrated that, in patients with acute HF, underlying pathologies, changes in renal function and TTE findings are associated with mortality. 42 Moreover, a recent study by Li et al investigating inhospital mortality in 120 patients with COVID‐19 has shown RV dilation and dysfunction in nonsurvivors and found global right ventricular longitudinal strain (RVLS) < 23% to be an independent predictor of mortality with multivariate analysis (AUC = 0.87, P < .001). 43
As regards treatment, ACC guidance for COVID19 advises the clinicians to be careful in using intravenous fluid therapy in patients with HF or volume overload conditions, since fluid administration for viral infection should be used cautiously and attentively monitored. 13 , 44 Furthermore, the response to HF treatment should be continuously assessed since, in case of failing pharmacological therapy for HF, a timely transition to more aggressive treatment with extracorporeal membrane oxygenation (ECMO) or circulatory assistance devices, such as intra‐aortic balloon pump, could be necessary to assist hemodynamics and improve outcome. 9 Therefore, focused but thorough and, if necessary, repeat ultrasound examination is important in COVID‐19 patients with possible or overt HF not only for diagnosis and prognosis, but also to assess patients' clinical status and response to therapy (Figure 2).
FIGURE 2.
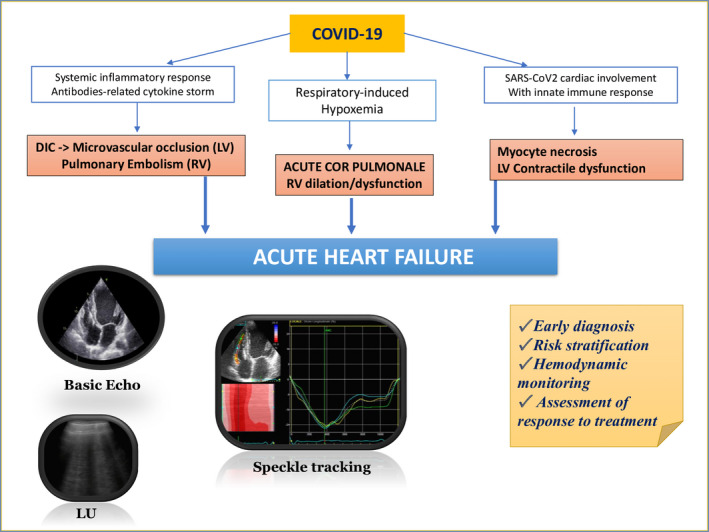
Pathophysiologic hypotheses and usefulness of a comprehensive diagnostic and prognostic approach [basic and advanced echocardiography + lung ultrasound] for acute heart failure in COVID‐19 patients. DIC = disseminated intravascular coagulation; LU = lung ultrasound; LV = left ventricle; RV = right ventricle
5. SEPSIS
In a recent study by Zhou et al involving 191 COVID‐19 subjects, a half of their patients finally developed sepsis at a median of 9 days. In particular, sepsis was the most frequently observed complication, followed by respiratory failure, ARDS, HF, and septic shock. 45
Sepsis is caused by exaggerate host response to infection leading to life‐threatening multiorgan failure (MOF), recognized with altered mental status, difficult or fast breathing, low oxygen saturation, reduced urine output, fast heart rate, weak pulse, cold extremities, or low blood pressure. This could lead to septic shock, that is persisting hypotension despite volume resuscitation and hemodynamic instability requiring vasopressor treatment. 46 The first diagnostic approach could be done with sequential organ failure assessment (SOFA) score, which is a good diagnostic marker for sepsis and septic shock, reflecting the degree of MOF. 47
However, several authors investigated the role of echocardiography for the study of septic shock, which could offer important information on cardiac loss of function due to sepsis. It has been shown that in these patients a certain grade of diastolic dysfunction could be detected by power and tissue Doppler imaging (TDI): The most used parameter is transmitral E/E′ ratio, with a lack of defined cutoff value; however, a higher proportion of diastolic dysfunction with values of E′ < 8‐10 cm/s was found to be independently associated with higher risk of death 48 (odds ratio, OR 7.7 of ICU mortality in a study by Mourad et al 49 ). Moreover, advanced echocardiography has shown good results for the evaluation of patients with septic shock: Orde et al demonstrated the superior value of STE over basic echocardiography in 60 patients with septic shock, detecting LV strain and RV strain impairment in 69% and 72% of patients, respectively, with only 33% patients having reduced LV ejection fraction (EF) and 32% having RV dysfunction based on conventional echocardiography (tricuspid annular plane systolic excursion, TDI s′ wave, RV fractional area change); moreover, they found RV‐free wall strain to be moderately associated with 6‐month mortality (OR 1.1, AUC 0.68). 50 Afterward, Chang et al proposed a cutoff of GLS absolute value <13% as the best marker of ICU mortality in septic shock. 51
The use of echocardiography in this clinical setting could help clinicians in early recognizing myocardial damage due to COVID‐derived sepsis.
6. CRITICAL PATIENTS: FOCUS ON THE RIGHT VENTRICLE
According to common ARDS management, 52 WHO indications emphasize treatment with mechanical ventilation with high PEEP and prone positioning for COVID‐19 patients who finally develop ARDS. 53 Nevertheless, the potential harmful effect of high PEEP, 54 in means of end‐inspiratory overdistension leading to lung injury and higher pulmonary vascular resistance, should not be overlooked 55 ; in fact, WHO recommends limiting this therapeutic strategy to moderate or severe ARDS and to responders. 53 Owing to the close lung‐heart interaction, this effect could also lead to acute cor pulmonale, causing RV systolic and diastolic overload. 56 This is clearly shown by TTE as new RV dilation, end‐systole paradoxical septal movement, and reduced RV overall function 57 , 58 and is characterized by poor prognosis due to circulatory failure. 59 , 60 In the retrospective study by Zhou et al investigating the clinical course of 191 SARS‐CoV‐2 patients, 17% of patients required mechanical ventilation, 97% of whom died. 45 Whether it was due to the end‐stage disease or to ventilation‐induced heart and/or lung complications is not known.
However, further evidence on this topic is timely needed in order improve the therapeutic management of COVID‐19 patients.
Transesophageal echocardiography (TOE) has been widely used for monitoring ventilated patients in the last years. In fact, in these patients TTE is often challenging, due to the position of patients with lower mobility, and the poor acoustic window due to hyperinflated lungs. However, the development of new indices for the assessment of LV systolic/diastolic function and filling pressures by TDI, and of RV dimension and function, have led to reconsider the use of serial TTE for noninvasive monitoring of ventilated patients. 61
Thanks to the widespread use of echocardiography in ICU, RV dimension and function could be closely monitored in these patients. 62 , 63 As Repessé et al suggested, a RV‐driven adjustment of PEEP levels could help intensivists to find a balance between risks and benefits of this therapeutic approach (ie, lung recruitment and overdistension), 56 thus preventing early mortality for ventilation‐induced RV failure. LU could be an important ally also in this context. 64 In addition, RV myocardial performance index (RV MPI), also known as “Tei index” determined on trans‐tricuspid velocities by pulsed wave or tissue Doppler imaging, is a high‐sensitive index for the diagnosis of RV dysfunction. 65 It could be used for monitoring high‐PEEP response in intubated patients, since it has shown to predict RV damage caused by mechanical ventilation 66 and high PEEPs. 67
Moreover, it seems that severe COVID‐19 infection could precipitate predisposition to acute venous thromboembolism, as shown in recent case reports, 68 , 69 and highlighted by Dolhnikoff et al on autoptic evidence, 70 which could lead to further deterioration of patients′ clinical status. Beyond clinical suspicion, echocardiographic signs of RV overload (ie, pulmonary ejection acceleration time < 60 ms with a peak systolic tricuspid valve gradient < 60 mm Hg, or with depressed contractility of RV free wall compared to RV apex, the so‐called McConnell's sign) could suggest the diagnosis of acute PE with high positive predictive value. 71 This should aid in providing early full anticoagulation therapy to these patients, avoiding long waiting times due to computed tomography (CT) scarce availability, and repeat CT scan with further radiation exposure.
Therefore, serially performing TTE in critical patients could represent a useful tool to guide airway management and to early recognize possible COVID‐19 thromboembolic complications.
7. THE CHOICE OF THE RIGHT DEVICE AND TECHNIQUE
Due to the need of balancing between risks of contagion for and benefits for patients, the common indications and modalities to perform echocardiography should be reconsidered in COVID‐19 patients; therefore, the choices for the use of portable devices and transesophageal echocardiography should be tailored on the single patients depending on his clinical status and cardiovascular conditions.
Portable machines have the advantage to be easier to clean and to cover than common echocardiographic machines and could be preferred for a basic assessment of biventricular function, valvular disease, and pericardial effusion. 13 However, in patients with suspected or known cardiac impairment or in uncertain clinical cases, the quality of the TTE evaluation could be sacrificed using portable echocardiographers. As an alternative, we propose the use of a dedicated echocardiographic machine in COVID units which should also be sanitized after use, thus combining safety and effectiveness. In addition, for difficult cases or severe cardiac dysfunction, we suggest performing a comprehensive image acquisition with offline measurement of complex and advanced parameters in a safe environment and at clinician time discretion, in order to obtain a complete echocardiographic examination reducing the time of exposure to SARS‐CoV‐2.
As regards TOE, there are several clinical settings in which this would provide more accurate information than TTE, such as a better anatomic insight into valvular heart disease, exclusion of vegetations on valvular structures or central venous catheters, or intracardiac shunts, assessment of RV function in ventilated patients, poor quality of bedside TTE images.
However, TOE might be stressful to COVID‐19 patients and, as suggested by EACVI consensus document, it should be avoided in most patients with ongoing COVID‐19. Moreover, the risk of contamination of healthcare workers and surfaces is very high during the procedure due to droplets and aerosols containing virus, therefore, in each case of performing TOE to suspected or known COVID‐19 patients, wearing advanced personal protective equipment (filtering face piece particulate class‐3 (FFP3) respirator, gown, gloves, and eye protection) is compulsory, and a dedicated transoesophageal probe in COVID units would be required. All this given, we suggest to carefully consider each indication for TOE and procrastinate them if inappropriate.
Usefulness of echocardiography for ECMO monitoring is a widely discussed topic in the recent times. In fact, TTE or TOE have a pivotal role not only for the evaluation of biventricular function pre‐, during, and post‐ECMO and a serial assessment of patient's loading conditions, which could help therapeutic management and decision‐making, but also for anatomic assessment of the correct positioning of the device, and the presence of complications such as intracardiac thrombotic formations. The latter application could be of great importance in COVID‐19 patients for their known pro‐thrombotic state, as shown by Schmiady et al in a case‐report describing a young patient with severe COVID‐19 respiratory consequences requiring veno‐venous ECMO assistance, who developed multiple thrombi, including in inferior vena cava (IVC) and right atrium, and underwent TOE‐guided percutaneous thrombectomy. TOE would possibly offer most reliable information for this purpose, but the previous discussed concerns should be considered and the use of TTE should be preferred when applicable. 72
8. CONCLUSIONS
Echocardiography is a precious tool in the hands of an expert operator to improve diagnostic procedures and therapeutic management of patients with COVID‐19, aiding clinicians in early recognizing subtle cardiac damage and providing adequate treatment for SARS‐CoV‐2–infected subjects. The use of TTE could change the diagnostic workup of acute coronary syndromes, myocarditis, acute left or right ventricular failure, and secondary myocardial damage due to sepsis or mechanical ventilation, allowing noninvasive assessment and monitoring.
However, the prevention of COVID‐19 spread should not be forgotten; therefore, it is crucial to bear in mind the mandatory use of personal protective equipment (with the use of FFP3 respirator, gown, gloves, and eye protection to face COVID‐19 ill), 12 and the safety recommendations to perform echocardiography at the time of COVID outbreak. Figure 3 resumes the parameters to assess for different suitable applications of echocardiography in COVID‐19 patients.
FIGURE 3.
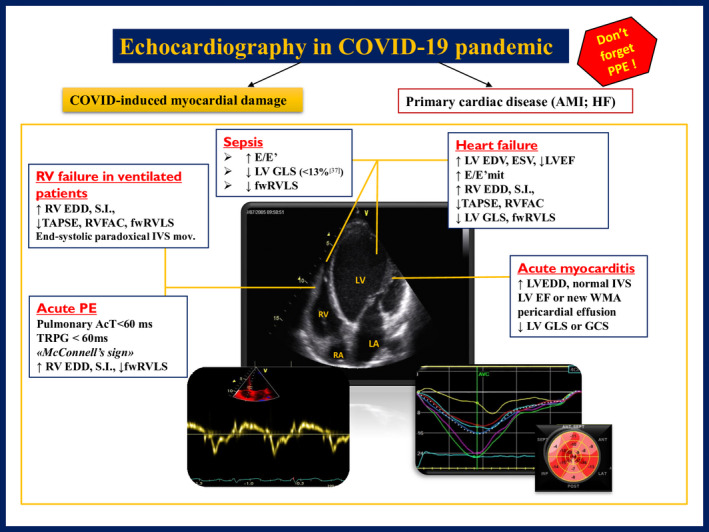
Reference indices for the use of echocardiography in COVID‐19 patients in different possible clinical scenarios. McConnell's sign = echocardiographic evidence of depressed contractility of RV free wall compared to RV apex. AcT = right ventricular outflow Doppler acceleration time; AMI = acute myocardial infarction; EDV = end‐diastolic volume; EF = ejection fraction; ESV = end‐systolic volume; GCS = global circumferential strain; GLS = global longitudinal strain; fwRVLS = free wall right ventricular longitudinal strain; HF = heart failure; IVS = interventricular septum; LA = left atrium; LV = left ventricle; PPE = personal protective equipment; RA = right atrium; RV = right ventricle; RVFAC = right ventricular fractional area change; SI = sphericity index; TAPSE = tricuspid annular plane systolic excursion; TRPG = tricuspid regurgitant pulmonary gradient; WMA = wall‐motion abnormalities
CONFLICT OF INTEREST
No disclosures.
Cameli M, Pastore MC, Soliman Aboumarie H, et al. Usefulness of echocardiography to detect cardiac involvement in COVID‐19 patients. Echocardiography. 2020;37:1278–1286. 10.1111/echo.14779
REFERENCES
- 1. WHO Director‐General's opening remarks at the media briefing on COVID‐19 ‐ 3 May 2020. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200503‐covid‐19‐sitrep‐104.pdf?sfvrsn=53328f46_2. Accessed May 3, 2020.
- 2. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID‐19) — China, 2020. China CDC Weekly. 2020;2(8):113–122. [PMC free article] [PubMed] [Google Scholar]
- 3. Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19). https://www.who.int/docs/default‐source/coronaviruse/who‐china‐joint‐mission‐on‐covid‐19‐final‐report.pdf. Accessed February 24, 2020.
- 4. Tan ZC, Fu LH, Wang DD, Hong K. Cardiac manifestations of patients with COVID‐19 pneumonia and related treatment recommendations. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E005. [DOI] [PubMed] [Google Scholar]
- 5. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He XW, Lai JS, Cheng J, et al. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID‐19 patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E011. [DOI] [PubMed] [Google Scholar]
- 7. Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. [DOI] [PubMed] [Google Scholar]
- 8. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020:e200950. 10.1001/jamacardio.2020.0950. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020:e201017. 10.1001/jamacardio.2020.1017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Popescu BA, Andrade MJ, Badano LP, et al. European Association of Echocardiography recommendations for training, competence, and quality improvement in echocardiogr aphy. Eur J Echocardiogr. 2009;10(8):893–905. [DOI] [PubMed] [Google Scholar]
- 11. Kirkpatrick JN, Mitchell C, Taub C, Kort S, Hung J, Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak. J Am Coll Cardio. 2020;75(24):3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skulstad H, Cosyns B, Popescu BA, et al. COVID‐19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020;21(6):592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maddox TM, Webster SE, Bozkurt B, ACC Science and Quality Committee . COVID‐19 Clinical Guidance for the Cardiovascular Care Team. https://www.acc.org//~/media/Non‐Clinical/Files‐PDFs‐Excel‐MS‐Word‐etc/2020/02/S20028‐ACC‐Clinical‐Bulletin‐Coronavirus.pdf. Accessed March 6, 2020.
- 14. Valente S, Anselmi F, Cameli M. Acute coronary syndromes during COVID‐19. Eur Heart J. 2020;41(22):2047–2049. 10.1093/eurheartj/ehaa457. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lippi G, Lavie CJ, Sanchis‐Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID‐19): evidence from a meta‐analysis. Prog Cardiovasc Dis. 2020. 10.1016/j.pcad.2020.03.001. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen C, Chen C, Yan JT, Zhou N, Zhao JP, Wang DW. Analysis of myocardial injury in patients with COVID‐19 and association between concomitant cardiovascular diseases and severity of COVID‐19. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E008. [DOI] [PubMed] [Google Scholar]
- 17. Stefanini GG, Montorfano M, Trabattoni D, et al. ST‐Elevation myocardial infarction in patients with COVID‐19: clinical and angiographic outcomes. Circulation. 2020;141(25):2113–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peng F, Tu L, Yang Y, et al. Management and treatment of COVID‐19: the chinese experience. Can J Cardiol. 2020;36(6):915–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China[J/OL]. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med. 2020;14(2):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hendren NS, Drazner MH, Bozkurt B, Cooper LT Jr. Description and Proposed Management of the Acute COVID‐19 Cardiovascular Syndrome. Circulation. 2020;141(23):1903–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020. 10.1001/jamacardio.2020.1096. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020:ehaa190. 10.1093/eurheartj/ehaa190. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeng JH, Liu YX, Yuan J, et al. First case of COVID‐19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020:1–5. 10.1007/s15010-020-01424-5. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako‐Tsubo syndrome in a patient with SARS‐CoV‐2 respiratory infection. Eur Heart J. 2020;41(19):1861–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med; 2020;46(5):846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pollack A, Kontorovich AR, Fuster V, et al. Viral myocarditis—diagnosis, treatment options and current controversies. Nat Rev Cardiol. 2015;12(11):670–680. [DOI] [PubMed] [Google Scholar]
- 30. Sinagra G, Anzini M, Pereira NL, et al. Myocarditis in clinical practice. Mayo Clin Proc. 2016;91(9):1256–1266. [DOI] [PubMed] [Google Scholar]
- 31. Løgstrup BB, Nielsen JM, Kim WY, Poulsen SH. Myocardial oedema in acute myocarditis detected by echocardiographic 2D myocardial deformation analysis. Eur Heart J Cardiovasc Imaging. 2016;17(9):1018–1026. [DOI] [PubMed] [Google Scholar]
- 32. Madjid M, Safavi‐Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020. 10.1001/jamacardio.2020.1286. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33. Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus Disease 2019 (COVID‐19) and Cardiovascular Disease. Circulation. 2020;141(20):1648–1655. [DOI] [PubMed] [Google Scholar]
- 34. Gargani L. Ultrasound of the lungs: more than a room with a view. Heart Fail Clin. 2019;15(2):297–303. [DOI] [PubMed] [Google Scholar]
- 35. Vetrugno L, Bove T, Orso D, et al. Our Italian experience using lung ultrasound for identification, grading and serial follow‐up of severity of lung involvement for management of patients with COVID‐19. Echocardiography. 2020;37(4):625–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gargani L, Soliman‐Aboumarie H, Volpicelli G, Corradi F, Pastore MC, Cameli M. Why, when, and how to use lung ultrasound during the COVID‐19 pandemic: enthusiasm and caution. Eur Heart J Cardiovasc Imaging. 2020. 10.1093/ehjci/jeaa163. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vetrugno L, Bove T, Orso D, et al. Lung ultrasound and the COVID‐19 "Pattern": not all that glitters today is gold tomorrow. J Ultrasound Med. 2020. 10.1002/jum.15327. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pastore MC, Mandoli GE, Aboumarie HS, et al. Working Group of Echocardiography of the Italian Society of Cardiology. Basic and advanced echocardiography in advanced heart failure: an overview. Heart Fail Rev. 2019. 10.1007/s10741-019-09865-3. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 39. Cameli M, Pastore MC, Mandoli GE, et al. Prognosis and risk stratification of patients with advanced heart failure (from PROBE). Am J Cardiol. 2019;124(1):55–62. [DOI] [PubMed] [Google Scholar]
- 40. Cameli M, Pastore MC, Henein MY, et al. (2019) The left atrium and the right ventricle: two supporting chambers to the failing left ventricle. Heart Fail Rev. 2019;24(5):661–669. [DOI] [PubMed] [Google Scholar]
- 41. Badano LP, Kolias TJ, Muraru D, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two‐dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19(6):591–600. [DOI] [PubMed] [Google Scholar]
- 42. Cameli M, Pastore MC, De Carli G, et al. ACUTE HF score, a multiparametric prognostic tool for acute heart failure: a real‐life study. Int J Cardiol. 2019;296:103–108. [DOI] [PubMed] [Google Scholar]
- 43. Li Y, Li H, Zhu S, et al. Prognostic Value of Right Ventricular Longitudinal Strain in Patients with COVID‐19. J Am Coll Cardiol Img. 2020. 10.1016/j.jcmg.2020.04.014. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Franchi F, Vetrugno L, Scolletta S. Echocardiography to guide fluid therapy in critically ill patients: check the heart and take a quick look at the lungs. J Thorac Dis. 2017;9(3):477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. WHO Interim guidance 13th March 2020 . Clinical management of severe acute respiratory infection (SARI) when COVID‐19 disease is suspected. WHO/2019‐nCoV/clinical/2020.4. Accessed March 13, 2020.
- 47. Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–2175. [DOI] [PubMed] [Google Scholar]
- 48. Gonzalez C, Begot E, Dalmay F, et al. (2016) Prognostic impact of left ventricular diastolic function in patients with septic shock. Ann Intensive Care. 2016;6(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mourad M, Chow‐Chine L, Faucher M, et al. Early diastolic dysfunction is associated with intensive care unit mortality in cancer patients presenting with septic shock. Br J Anaesth. 2014;112(1):102–109. [DOI] [PubMed] [Google Scholar]
- 50. Orde SR, Pulido JN, Masaki M, et al. Outcome prediction in sepsis: speckle tracking echocardiography based assessment of myocardial function. Crit Care. 2014;18:R149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chang W‐T, Lee W‐H, Lee W‐T, et al. Left ventricular global longitudinal strain is independently associated with mortality in septic shock patients. Intensive Care Med. 2015;41:1791–1799. [DOI] [PubMed] [Google Scholar]
- 52. Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial Investigators , Cavalcanti AB, Suzumura ÉA, et al. Effect of lung recruitment and titrated positive end‐expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (2019‐nCoV) infection is suspected: Interim guidance. World Health Organization; 2020. https://apps.who.int/iris/handle/10665/330893. Accessed January 28, 2020. [Google Scholar]
- 54. Gage A, Higgins A, Lee R, Panhwar MS, Kalra A. Reacquainting Cardiology with Mechanical Ventilation in Response to the COVID‐19 Pandemic. JACC Case Rep. 2020. 10.1016/j.jaccas.2020.03.007. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goligher EC, Kavanagh BP, Rubenfeld GD, et al. Oxygenation response to positive end‐expiratory pressure predicts mortality in acute respiratory distress syndrome. A secondary analysis of the LOVS and ExPress trials. Am J Respir Crit Care Med. 2014;190:70–76. [DOI] [PubMed] [Google Scholar]
- 56. Repessé X, Charron C, Vieillard‐Baron A. Acute cor pulmonale in ARDS: rationale for protecting the right ventricle. Chest. 2015;147(1):259–265. [DOI] [PubMed] [Google Scholar]
- 57. Jardin F, Dubourg O, Bourdarias JP. Echocardiographic pattern of acute cor pulmonale. Chest. 1997;111(1):209–217. [DOI] [PubMed] [Google Scholar]
- 58. Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1725–1733. [DOI] [PubMed] [Google Scholar]
- 59. Franchi F, Faltoni A, Cameli M, et al. Influence of positive end‐expiratory pressure on myocardial strain assessed by speckle tracking echocardiography in mechanically ventilated patients. Biomed Res Int. 2013;2013:918548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lhéritier G, Legras A, Caille A, et al. Prevalence and prognostic value of acute cor pulmonale and patent foramen ovale in ventilated patients with early acute respiratory distress syndrome: a multicenter study. Intensive Care Med. 2013;39(10):1734–1742. [DOI] [PubMed] [Google Scholar]
- 61. Vieillard‐Baron A, Prin S, Chergui K, Dubourg O, Jardin F. Echo‐Doppler demonstration of acute cor pulmonale at the bedside in the medical intensive care unit. Am J Respir Crit Care Med. 2002;166(10):1310–1319. [DOI] [PubMed] [Google Scholar]
- 62. Salem R, Vallee F, Rusca M, Mebazaa A. Hemodynamic monitoring by echocardiography in the ICU: the role of the new echo techniques. Curr Opin Crit Care. 2008;14(5):561–568. [DOI] [PubMed] [Google Scholar]
- 63. Pastore MC, De Carli G, Mandoli GE, et al. The prognostic role of speckle tracking echocardiography in clinical practice: evidence and reference values from the literature. Heart Fail Rev. 2020. 10.1007/s10741-020-09945-9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 64. Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung Ultrasound for Critically Ill Patients. Am J Respir Crit Care Med. 2019;199(6):701–714. [DOI] [PubMed] [Google Scholar]
- 65. Miller D, Farah MG, Liner A, et al. The relation between quantitative right ventricular ejection fraction and in dices of tricuspid annular motion and myocardial performance index. J Am Soc Echocardiogr. 2004;17:443–447. [DOI] [PubMed] [Google Scholar]
- 66. Cherpanath TGV, Simonis FD, Bouma BJ, et al. Myocardial function during low versus intermediate tidal volume ventilation in patients without acute respiratory distress dyndrome. Anesthesiology. 2020;132(5):1102–1113. [DOI] [PubMed] [Google Scholar]
- 67. Gernoth C, Wagner G, Pelosi P, Luecke T. Respiratory and haemodynamic changes during decremental open lung positive end‐expiratory pressure titration in patients with acute respiratory distress syndrome. Crit Care. 2009;13(2):R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID‐19 pneumonia: a random association? Eur Heart J. 2020;41(19):1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xie Y, Wang X, Yang P, Zhang S. COVID‐19 complicated by acute pulmonary embolism. Radiology. 2020;2(2):e200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dolhnikoff M, Duarte‐Neto AN, de Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID‐19. J Thromb Haemost. 2020;18(6):1517–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J, 2020;41(4):543–603. [DOI] [PubMed] [Google Scholar]
- 72. Schmiady MO, Sromicki J, Kucher N, Ouda A. Successful percutaneous thrombectomy in a patient with COVID‐19 pneumonia and acute pulmonary embolism supported by extracorporeal membrane oxygenation. Eur Heart J. 2020:ehaa403. 10.1093/eurheartj/ehaa403 [DOI] [PMC free article] [PubMed] [Google Scholar]


