Abstract
As COVID‐19 infections wreak havoc across the globe, attention has rightly been focused on the vital organ systems (lung, kidney and heart) that are vulnerable to viral attack and contribute to the acute pathology associated with this disease. However, we should not lose sight of the fact that COVID‐19 will attack any cell type in the body expressing ACE2 ‐ including human spermatozoa. These cells possess the entire repertoire of receptors (AT1R, AT2R, MAS) and ligand processing enzymes (ACE1 and ACE2) needed to support the angiotensin signalling cascade. The latter not only provides COVID‐19 with a foothold on the sperm surface but may also promote integration, given the additional presence of a range of proteases (TMPRSS2, TMPRSS11B, TMPRSS12, furin) capable of promoting viral fusion. This article reviews the roles played by these various cellular constituents in maintaining the vitality of human spermatozoa and their competence for fertilization. The reproductive consequences of a viral attack on these systems, in terms of fertility and the risk of sexual transmission, are currently unknown. However, we should be alive to the possibility that there may be reproductive consequences of COVID‐19 infection in young males that go beyond their capacity to survive a viral attack.
Keywords: ACE2, COVID‐19, male infertility, spermatozoa, TMPRSS2, viral fusion
The COVID‐19 (SARS‐CoV‐2) virus has created a global pandemic responsible for over 500 000 deaths and untold human and economic suffering. Attention has rightly focused on the respiratory system, because this is where a life‐and‐death battle is waged between the viral horde and the host's immune system. However, other tissues are also susceptible to viral attack including the kidney, heart, and brain. The purpose of this communication is to highlight the evidence that the male reproductive system, particularly spermatozoa, constitutes another vulnerable target, raising the possibility that COVID‐19 might ultimately induce male infertility and/or facilitate the sexual transmission of this virus, depending on the level of infection.
A recent report published in JAMA Network Open revealed that in an analysis 38 semen samples from COVID‐19 patients, 6 (four at the acute stage of infection and, alarmingly, two who were recovering) tested positive for the virus by RT‐PCR. 1 Importantly, at this point, we have no idea whether the actual virus was viable and infectious. Nevertheless, the possibility that this coronavirus could have a pathophysiological impact on the testes was suggested by additional data indicating that active COVID‐19 infection dramatically reduced the testosterone‐to‐LH ratio, suggesting a significant impact on the responsiveness of Leydig cells to LH stimulation. 2 In many ways, we should not be surprised by these observations because the blood‐testes barrier is known to offer little defense against viral invasion, given the wide range of pathogenic viruses (HIV, hepatitis, mumps, papilloma) that are known to be capable of damaging the testes and rendering the host infertile. Furthermore, the spike protein that gives the COVID‐19 virus its corona is known to target ACE2 (angiotensin‐converting enzyme 2), which is highly expressed by several cell types in the testes including Leydig cells, Sertoli cells, and the germ line. As a result of these factors, several opinion pieces have been published already, raising the possibility of testicular damage and infertility consequent to COVID‐19 infection. 2 , 3 , 4 However, it is also possible that the virus could gain access to male germ cells once they leave the testes, either in the epididymis or following ejaculation. In this Opinion Article, I shall be focusing on this post‐testicular route of infection pointing out, for the first time, that the mature spermatozoon has all of the machinery needed to bind this virus, fuse with it, and even achieve reverse transcription of the viral RNA into proviral DNA. Such considerations raise the possibility that spermatozoa could act as potential vectors of this highly infectious disease. This happens in insects 5 —why not us?
It has been known for many years that the human sperm surface expresses ACE. Indeed, an examination of existing proteomic databases 6 , 7 , 8 as well as surveys of the sperm surface with monoclonal antibodies, 9 demonstrates that these cells, quite literally, hold all of the ACEs. They have been known for some time to express a testicular variant of ACE1, which converts the inactive decapeptide hormone, angiotensin I, to the active octapeptide, angiotensin II (Figure 1). Testicular ACE corresponds to the ancestral non‐duplicated form of the ACE gene; it lacks multiple 5' exons and has a distinct N‐terminus: biochemically however, it performs exactly the same function as somatic ACE1. 10 Spermatozoa also express ACE2, which converts angiotensin II to angiotensin (1‐7). Reference to the human sperm proteome also indicates that these cells possess the two known receptors for angiotensin II: angiotensin II type‐1 receptor (AT1R) and (angiotensin II type‐2 receptor) (AT2R). Furthermore, a recent publication has revealed that human spermatozoa also express the angiotensin (1‐7) MAS receptor. 9 These cells therefore possess the complete repertoire of ligand‐processing enzymes and receptors needed to support angiotensin signaling pathways, raising questions about the physiological roles these pathways play and how they might intersect with COVID‐19 (Figure 1).
Figure 1.
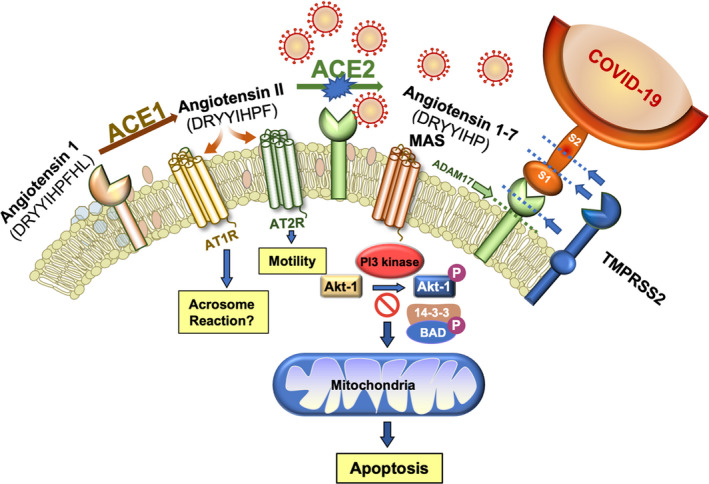
The angiotensin system plays a critical role in the survival and functionality of human spermatozoa but also creates a vulnerability to COVID‐19 attack. Angiotensin 1 is a biologically inactive decapeptide that is cleaved by ACE1 to create angiotensin II, which in turn activates the AT1R and AG2R receptors, both of which are present in these cells. Angiotensin II is further processed by ACE2 to generate angiotensin 1‐7 which binds the MAS receptor activating PI3K. The latter then phosphorylates AKT, which maintains cell viability by phosphorylating key regulators of sperm apoptosis such as BAD. As long as BAD is phosphorylated, it is held in abeyance by a 14‐3‐3 keeper protein. However, if the PI3/AKT pathway becomes compromised, BAD dephosphorylates, is released from its association with 14‐3‐3, and moves to the mitochondria where it inactivates anti‐apoptotic factors and promotes the intrinsic apoptotic cascade. The spike protein on COVID‐19 specifically targets ACE2 and in so doing removes an important stimulus for PI3K/AKT, thereby compromising sperm viability. Subsequent to COVID‐19 binding, the ectodomain of ACE2 may be removed by ADAM proteases and shed from the sperm surface. Alternatively proteases from the TMPRSS‐family, either as intrinsic components of the sperm plasma membrane or delivered by seminal prostasomes, can facilitate fusion between the virus and the sperm surface by cleaving ACE2 and the viral spike proteins (S1 and S2) at the sites indicated by dashed lines, thereby completing the transformation of this cell from procreating gamete to viral vector
ACE1 is a very ancient protein the appearance of which precedes the evolution of the angiotensin system. As a consequence, functions other than angiotensin processing are likely. Indeed, spermatozoa are a good example of the multiple roles of ACE1. This enzyme is quite independent of ACE2 (the gene is located on chromosome 17, while ACE2 is transcribed from the X chromosome); however, there is considerable sequence homology (~41%) between these peptidases in terms of their primary amino acid sequences. Notwithstanding such similarities, COVID‐19 does not interfere with the functions of ACE1 and, as a result, one of the consequences of infection with this virus is to generate an increase in the availability of angiotensin II relative to angiotensin (1‐7). What does this mean in terms of sperm cell biology?
ACE1 is most heavily expressed on the surface of viable, functional spermatozoa and is concentrated in the neck and mid‐piece region of the cell. 11 The expression of ACE1 appears to be important for sperm function because patients exhibiting complete absence of this enzyme are incapable of fertilization. 12 The precise role played by ACE1 is not certain but a synthesis of the data suggests a role in cell‐cell interaction. ACE1 is important for the expression of testis‐expressed gene 101 (TEX101) which is, in turn, linked to the stabilization of disintegrin and metallopeptidase domain 3 (ADAM3) on sperm plasma membrane. 13 Mice lacking ADAM3 possess motile spermatozoa that are incapable of negotiating the utero‐tubal junction and exhibit an impaired capacity to bind to the zona pellucida. Interestingly, the importance of ACE1 in mediating sperm transport and interaction with the oocyte may not necessarily be related to its angiotensin‐processing function but rather to its ability to catalyze the release of glycosylphosphatidylinositol (GPI)‐anchored proteins from the sperm surface. 14
This observation does not, however, preclude the possibility that ACE1 also plays an important role in spermatozoa as a component of the renin‐angiotensin system. By analogy with somatic cells, a COVID‐19 attack on human spermatozoa would be expected to impact ACE2 activity leading to a buildup of angiotensin II (Figure 1). An increased local availability of angiotensin II would be expected to enhance sperm phagocytosis by neutrophils and possibly increase the local availability of reactive oxygen species (ROS) generated during the phagocytic process. 15 Excessive stimulation of AT1R by angiotensin II might also enhance the generation of ROS in association with the induction of apoptosis and cell senescence, given its apoptosis‐inducing role in other cell types. 16 Curiously, AT1R has been identified as one of a handful of proteins that are thought to be actively generated in the mature gamete as a result of de novo translation on mitochondrial‐type ribosomes, suggesting that this receptor must play a critical role in the lead‐up to fertilization. 17 A role in the induction of the acrosome reaction has been suggested on the basis of the ability of the AT1R antagonist, losartan, to inhibit the induction of acrosomal exocytosis in bovine spermatozoa. 18 As angiotensin II also stimulates the acrosome reaction in human spermatozoa, it is possible that prolonged exposure to elevated levels of angiotensin II as a consequence of COVID‐19 infection might lead to premature acrosomal exocytosis and sperm senescence in our species. 19 Such effects might be related to the ability of angiotensin II to stimulate nitric oxide generation via the AT1R pathway in a losartan‐inhibitable manner. 20 This reactive nitrogen radical would be expected to stimulate capacitation and the acrosome reaction in the short term but generate a state of oxidative stress and senescence if the exposure is prolonged. 21 The AT2R receptor, on the other hand, appears to be important for the regulation of sperm motility in response to angiotensin II generated by ACE1. 22 , 23 The stimulatory impact of the angiotensin II/AT2R axis on sperm movement may, in turn, relate to its capacity to enhance cAMP generation and tyrosine phosphorylation. 24
The importance of ACE2 has recently been highlighted by discovery of the MAS receptor on human spermatozoa. 9 This receptor is localized in the principal piece of the sperm tail and the acrosomal domain of the sperm head, in exactly the same place as phosphoinositide 3‐kinase (PI3K). 25 Activation of the MAS receptor by angiotensin (1‐7) leads to PI3K phosphorylation, which in turn leads to the phosphorylation of AKT. 25 While AKT is phosphorylated, downstream phosphorylation targets such as the apoptosis regulator, BCL2‐associated agonist of cell death (BAD), are also phosphorylated. Phospho‐BAD forms a heterodimer with its 14‐3‐3 keeper protein, leaving Bcl‐2 free to inhibit Bax‐triggered apoptosis, thereby maintaining spermatozoa a viable motile state (Figure 1). However, if PI3K activity is disrupted, AKT and its downstream targets become dephosphorylated, thereby initiating a truncated apoptotic cascade characterized by rapid motility loss, mitochondrial ROS generation, caspase activation in the cytosol, annexin V binding to the cell surface, cytoplasmic vacuolization and oxidative DNA damage. 25 Thus, ACE2 plays an essential role in sperm biology; by stimulating PI3K/AKT phosphorylation, it actively prevents spermatozoa from engaging the intrinsic apoptotic pathway (Figure 1).
The fate of ACE2 following interaction with COVID‐19 binding is complex and dependent on cell type. If the cells are capable of endocytosis, then ACE may become internalized within the endocytotic vesicle. However, as spermatozoa are generally regarded as non‐endocytotic, the more likely fate of ACE2 is cleavage (shedding) of its ectodomain through the action of surface proteases such as ADAM17. 26 , 27 This may be a protective strategy because shed ACE2 ectodomain should bind the virus and prevent it gaining access to the cell surface. On the other hand, proteases recruited by the virus to facilitate membrane fusion, notably TMPRSS2 (a type II transmembrane serine protease), can also cleave ACE2 at amino acids 697 to 716, resulting in the shedding of 13 kD ACE2 fragment from the cell surface and facilitating viral entry. 28 Cleavage of ACE2 following exposure to COVID‐19 would be expected to induce a decrease in sperm viability and function, leading to a loss of fertility. Furthermore, the processing of ACE2 on the surface of human spermatozoa may effectively turn these cells into viral vectors, capable of sexually transmitting COVID‐19 to other individuals in the community.
Actual fusion between the virus and human spermatozoa requires the presence of the above‐mentioned protease, TMPRSS2, to cleave the viral spike proteins (S) at the S1/S2 boundary or within S2 subunit, thereby removing the structural constraint of S1 on S2 and releasing the internal membrane fusion peptide (Figure 1). This protease is known to be present in prostasomes that are released into seminal fluid from the prostate gland at ejaculation. 29 As one of the major functions of these exosome‐like structures is to transfer their contents, including proteins, to the spermatozoa following ejaculation, the incorporation of TMPRSS2 from this source seems probable. 30 Furthermore, a close examination of the human sperm proteomic databases reveals the presence of related proteases TMPRSS11B and TMPRSS12 7 as well as furin, 6 in these cells, all of which are thought to serve as activating proteases for viral infection including coronaviruses. 31 , 32 , 33 The presence of these activating proteases as well as ACE2 in the sperm plasma membrane would be expected to allow the COVID‐19 virus to bind to the cell surface and ultimately fuse, either in the testes or during the prolonged sojourn of these cells in the epididymis. In contrast, oocytes appear to be completely devoid of TMPRSS2, 33 making infection of the female germ line highly unlikely—unless, of course, they are fertilized by a COVID‐19 carrying spermatozoon. In this context, it should be emphasized spermatozoa have a demonstrable capacity to carry viral infections from the male to the female reproductive tract, as happens during the sexual transmission of the Zika virus, for example. 34 They also have a proven capacity to fuse with enveloped viruses 35 and possess reverse transcriptase activity capable of generating proviral DNA, 36 as is apparently the case for human immunodeficiency virus 1. 37
1. SUMMARY AND FUTURE DIRECTIONS
At present, there have been few opportunities to study the reproductive competence of patients who have recovered from COVID‐19 infection. In order to prepare for the moment that we do, there are pieces of information that we should acquire and studies that we should contemplate. At the moment, we do not know the conditions regulating the relative activation of AT1R vs AT2R by angiotensin II in spermatozoa, nor do we fully understand the biological consequences of activating these specific receptors. Given the current availability of AT1R vs AT2R receptor antagonists exhibiting high levels of target specificity, it may be timely to exploit these compounds to gain a deeper understanding of the possible consequences of angiotensin II upregulation consequent to COVID‐19 infection at different stages of the spermatozoon's journey from the seminiferous epithelium to the ejaculate. 38 We shall also need to assess the extent to which COVID‐19 triggers the anticipated release of ACE2 from the sperm surface and whether the loss of this enzyme leads to an increased rate of sperm senescence and cell death. Most importantly, we shall need to investigate the competence of spermatozoa, at different stages of epididymal maturation and post‐ejaculatory activation, to fuse with this coronavirus and reverse transcribe its genome to create the corresponding DNA. What subsequently happens, when viral RNA and proviral DNA are released into the oocyte at fertilization is a crucial question that will have to be addressed particularly if, as projected, post‐COVID‐19 patients end up requesting assisted reproductive technology to resolve their infertility problems.
We clearly still have much to learn about COVID‐19. Its arrival has been relatively recent and there are still no definitive data to indicate its impact on male fertility or the potential of spermatozoa to serve as vectors for the sexual transmission of this disease. Nevertheless, the evident importance of the renin‐angiotensin and TMPRSS‐family proteases in sperm cell biology, as well as the clear capacity of these cells to fuse with viruses, suggests that we should at least be open to the possibility.
Aitken RJ. COVID-19 and human spermatozoa—Potential risks for infertility and sexual transmission?. Andrology.2021;9:48–52. 10.1111/andr.12859
REFERENCES
- 1. Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease. JAMA Netw Open. 2020;3:e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang S, Zhou X, Zhang T, Wang Z. The need for urogenital tract monitoring in COVID‐19. Nat Rev Urol. 2020;17(6):314‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abobaker A, Raba AA. Does COVID‐19 affect male fertility? World J Urol. 2020;1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verma S, Saksena S, Sadri‐Ardekani H. ACE2 receptor expression in testes: implications in COVID‐19 pathogenesis. Biol Reprod. 2020;ioaa080. 10.1093/biolre/ioaa080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mao Q, Wu W, Liao Z, et al. Viral pathogens hitchhike with insect sperm for paternal transmission. Nat Commun. 2019;10(1):955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker MA, Reeves G, Hetherington L, Müller J, Baur I, Aitken RJ. Identification of gene products present in Triton X‐100 soluble and insoluble fractions of human spermatozoa lysates using LC‐MS/MS analysis. Proteomics Clin Appl. 2007;1(5):524‐532. [DOI] [PubMed] [Google Scholar]
- 7. Castillo J, Jodar M, Oliva R. The contribution of human sperm proteins to the development and epigenome of the preimplantation embryo. Hum Reprod Update. 2018;24(5):535‐555. [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Wan J, Ling X, Liu M, Zhou T. The human sperm proteome 2.0: An integrated resource for studying sperm functions at the level of posttranslational modification. Proteomics. 2016;16(19):2597‐2601. [DOI] [PubMed] [Google Scholar]
- 9. Valdivia A, Cortés L, Beitia M, et al. Role of Angiotensin‐(1–7) via MAS receptor in human sperm motility and acrosome reaction. Reproduction. 2020;159:241‐249. [DOI] [PubMed] [Google Scholar]
- 10. Lattion AL, Soubrier F, Allegrini J, Hubert C, Corvol P, Alhenc‐Gelas F. The testicular transcript of the angiotensin I‐converting enzyme encodes for the ancestral, non‐duplicated form of the enzyme. FEBS Lett. 1989;252(1–2):99‐104. [DOI] [PubMed] [Google Scholar]
- 11. Nikolaeva MA, Balyasnikova IV, Alexinskaya MA, et al. Testicular isoform of angiotensin I‐converting enzyme (ACE, CD143) on the surface of human spermatozoa: revelation and quantification using monoclonal antibodies. Am J Reprod Immunol. 2006;55(1):54‐68. [DOI] [PubMed] [Google Scholar]
- 12. Li LJ, Zhang FB, Liu SY, et al. Human sperm devoid of germinal angiotensin‐converting enzyme is responsible for total fertilization failure and lower fertilization rates by conventional in vitro fertilization. Biol Reprod. 2014;90(6):125. [DOI] [PubMed] [Google Scholar]
- 13. Fujihara Y, Noda T, Kobayashi K, et al. Identification of multiple male reproductive tract‐specific proteins that regulate sperm migration through the oviduct in mice. Proc Natl Acad Sci USA. 2019;116(37):18498‐18506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watanabe H, Kondoh G. Mouse sperm undergo GPI‐anchored protein release associated with lipid raft reorganization and acrosome reaction to acquire fertility. J Cell Sci. 2011;124(15):2573‐2581. [DOI] [PubMed] [Google Scholar]
- 15. Marey MA, Yousef MS, Liu J, et al. Angiotensin II increases sperm phagocytosis by neutrophils in vitro: A possible physiological role in the bovine oviduct. Mol Reprod Dev. 2016;83(7):630‐639. [DOI] [PubMed] [Google Scholar]
- 16. Capettini LS, Montecucco F, Mach F, Stergiopulos N, Santos RA, da Silva RF. Role of renin‐angiotensin system in inflammation, immunity and aging. Curr Pharm Des. 2012;18(7):963‐970. [DOI] [PubMed] [Google Scholar]
- 17. Gur Y, Breitbart H. Protein synthesis in sperm: dialog between mitochondria and cytoplasm. Mol Cell Endocrinol. 2008;282(1–2):45‐55. [DOI] [PubMed] [Google Scholar]
- 18. Gur Y, Breitbart H, Lax Y, Rubinstein S, Zamir N. Angiotensin II induces acrosomal exocytosis in bovine spermatozoa. Am J Physiol. 1998;275(1):E87‐E93. [DOI] [PubMed] [Google Scholar]
- 19. Köhn F‐M, Müller C, Drescher D, et al. Effect of angiotensin converting enzyme (ACE) and angiotensins on human sperm functions. Andrologia. 1998;30(4–5):207‐215. [DOI] [PubMed] [Google Scholar]
- 20. Vedantam S, Atreja SK, Garg M. Angiotensin‐II induced nitric oxide production during buffalo sperm capacitation and acrosome reaction. Res Vet Sci. 2012;92(2):207‐212. [DOI] [PubMed] [Google Scholar]
- 21. Aitken RJ. The capacitation‐apoptosis highway: oxysterols and mammalian sperm function. Biol Reprod. 2011;85(1):9‐12. [DOI] [PubMed] [Google Scholar]
- 22. Gianzo M, Muñoa‐Hoyos I, Urizar‐Arenaza I, et al. Angiotensin II type 2 receptor is expressed in human sperm cells and is involved in sperm motility. Fertil Steril. 2016;105(3):608‐616. [DOI] [PubMed] [Google Scholar]
- 23. Vinson GP, Mehta J, Evans S, et al. Angiotensin II stimulates sperm motility. Regul Pept. 1996;67(2):131‐135. [DOI] [PubMed] [Google Scholar]
- 24. Mededovic S, Fraser LR. Angiotensin II stimulates cAMP production and protein tyrosine phosphorylation in mouse spermatozoa. Reproduction. 2004;127(5):601‐612. [DOI] [PubMed] [Google Scholar]
- 25. Koppers AJ, Mitchell LA, Wang P, Lin M, Aitken RJ. Phosphoinositide 3‐kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J. 2011;436(3):687‐698. [DOI] [PubMed] [Google Scholar]
- 26. Palau V, Pascual J, Soler MJ, Riera M. Role of ADAM17 in kidney disease. Am J Physiol Renal Physiol. 2019;317(2):F333‐F342. [DOI] [PubMed] [Google Scholar]
- 27. Frayne J, Hurd EA, Hall L. Human tMDC III: a sperm protein with a potential role in oocyte recognition. Mol Hum Reprod. 2002;8(9):817‐822. [DOI] [PubMed] [Google Scholar]
- 28. Heurich A, Hofmann‐Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88(2):1293‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Y‐W, Lee M‐S, Lucht A, et al. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am J Pathol. 2010;176(6):2986‐2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saez F, Sullivan R. Prostasomes, post‐testicular sperm maturation and fertility. Front Biosci. 2016;21:1464‐1473. [DOI] [PubMed] [Google Scholar]
- 31. Yun B, Zhang Y, Liu Y, et al. TMPRSS12 is an activating protease for subtype b avian metapneumovirus. J Virol. 2016;90(24):11231‐11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ji HL, Zhao R, Matalon S, Matthay MA. Elevated Plasmin(ogen) as a Common Risk Factor for COVID‐19 Susceptibility. Physiol Rev. 2020;100(3):1065‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh M, Bansal V, Feschotte C. A single‐cell RNA expression map of human coronavirus entry factors. BioRxiv. 2020;2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Joguet G, Mansuy J‐M, Matusali G, et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis. 2017;17(11):1200‐1208. [DOI] [PubMed] [Google Scholar]
- 35. Nussbaum O, Laster J, Loyter A. Fusion of enveloped viruses with sperm cells: interaction of Sendai, influenza, and Semliki Forest viruses with bull spermatozoa. Exp Cell Res. 1993;206(1):11‐15. [DOI] [PubMed] [Google Scholar]
- 36. Sciamanna I, Barberi L, Martire A, et al. Sperm endogenous reverse transcriptase as mediator of new genetic information. Biochem Biophys Res Commun. 2003;312(4):1039‐1046. [DOI] [PubMed] [Google Scholar]
- 37. Bagasra O, Farzadegan H, Seshamma T, Oakes JW, Saah A, Pomerantz RJ. Detection of HIV‐1 proviral DNA in sperm from HIV‐1‐infected men. AIDS. 1994;8(12):1669‐1674. [DOI] [PubMed] [Google Scholar]
- 38. Jackson L, Eldahshan W, Fagan SC, Ergul A. Within the brain: the renin angiotensin system. Int J Mol Sci. 2018;19(3):876. [DOI] [PMC free article] [PubMed] [Google Scholar]


