Abstract
The ocular surface is naturally covered with a layer of mucus. Along with other functions, this mucus layer serves to trap and eliminate foreign substances, such as allergens, pathogens, and debris. In playing this pivotal role, mucus can also hinder topical delivery of therapeutics to the eye. Recent studies provide evidence that drugs formulated as traditional micro- or nanoparticles are susceptible to entrapment and rapid clearance by ocular mucus. Mucus-penetrating particles (MPPs) is a nanoparticle technology that emerged over the past decade. With a muco-inert surface and a particle size smaller than the mucus mesh size, MPPs can diffuse in ex vivo mucus essentially freely. Preclinical studies have shown that, compared with particles lacking the mucus-penetrating attributes, MPPs can improve the uniformity of drug particle distribution on mucosal surfaces and enhance drug delivery to ocular tissues.
Keywords: drug delivery, mucoadhesive, mucus-penetrating particles, nanoparticles, ocular surface, ocular mucus
Introduction
Efficient topical drug delivery to the eye is a notoriously difficult challenge. Eye drops represent close to 90% of the marketed ophthalmic formulations.1,2 In particular, eye drops in the form of solutions and suspensions remain the preferred dosage form due to localized action, relative ease of administration, and patient comfort (over gels, ointments, and emulsions, which can be associated with blurred vison and sticky sensation).3,4 However, only a small portion of the drug administered as a conventional eye drop reaches the anterior ocular tissues (typically, in the range of 0.1%–5% of the total dispensed dose).1,3,5
The inefficient drug delivery is a result of the well-recognized static and dynamic barriers of the eye, such as poor corneal permeability, reflex blinking, tear secretion, dose spillage, and nasolacrimal drainage.3,6,7 In addition, the ocular surface is protected with a layer of mucus, which spreads over the cornea and conjunctiva. Multiple lines of evidence indicate that mucus in the pulmonary, gastrointestinal (GI), and cervicovaginal tracts may significantly hinder penetration of therapeutics to the underlying tissues, especially for lipophilic compounds, large biologic molecules, or drugs formulated as micro- and nanoparticles.8–14 In contrast, the current understanding of barrier effects of ocular mucus on topical drug delivery is rudimentary. A comprehensive review published in 2015 by Ruponen and Urtti described the barrier role of ocular mucus in the context of topical drug delivery as “undefined.”15 In particular, the authors cited a lack of relevant experimental data and suggested that careful studies were “needed to dissect the role of mucus barrier, epithelial cellular barriers, and contributions of mucoadhesion on ocular drug bioavailability.”
Recent reviews detail a plethora of approaches to enhancing topical delivery to the eye, including mucoadhesive formulations, nanoparticles, permeation enhancers, and solubilizers.5,7,16 However, a few efforts have deliberately compared the effects of mucoadhesion or altering corneal permeability versus the effect of a drug being able to freely penetrate through the mucus layer. Indeed, such comparisons are difficult to perform, as they are often conflated with differences in formulation factors (vehicle viscosity, drug particle size, or drug solubility). Hence, the question remains: Do barrier properties of ocular mucus undermine the efficiency of topical drug delivery to the eye? A novel drug delivery technology known as mucus-penetrating particles (MPPs) may offer an insight into this question. The MPPs are nanoparticles specifically engineered to avoid mucoadhesion and, therefore, are uniquely positioned to serve as comparators to otherwise identical mucoadhesive particles.
In this review, we will discuss recent studies comparing topical delivery of mucoadhesive and mucus-penetrating nanoparticles to the eye and the resulting evidence that ocular mucus plays an important role as a barrier for micro- and nanosuspensions. To facilitate this discussion, we will first present a brief overview of mucus (with a focus on ocular mucus) and introduce the principles of MPP technology.
Mucus and Mucins
Mucus is a complex biological fluid present at apical epithelial surfaces in all organs of the human body that are exposed to or communicate with the outside environment, such as the ocular surface and luminal linings of GI, cervicovaginal, and respiratory tracts. Macroscopically, mucus is often described as a sticky, viscoelastic gel.17,18 Microscopically, it is characterized by the ability to form a heterogenous 3-dimensional network (mucus mesh) riddled with pores (Fig. 1).19–21 Mucus is comprised predominantly of water (∼95%, i.e., can be classified as hydrogel); the other components include mucins (∼1%–5%), lipids, salts, and various non-mucin proteins (enzymes, growth factors, and immunoglobulins).17,22–24 Among these components, mucins are largely responsible for the structure and functions of mucus. Chemically, mucins are a family of glycosylated proteins (glycoproteins) distinguished by several common features: (1) a very high molecular weight (0.5–40 MDa); (2) a polypeptide backbone with repeat domains that are rich in proline, threonine, and serine (PTS domains); and (3) a high degree of glycosylation (as a “rule of thumb,” at least 50% by weight, but can be as high as 90% w/w).22–25
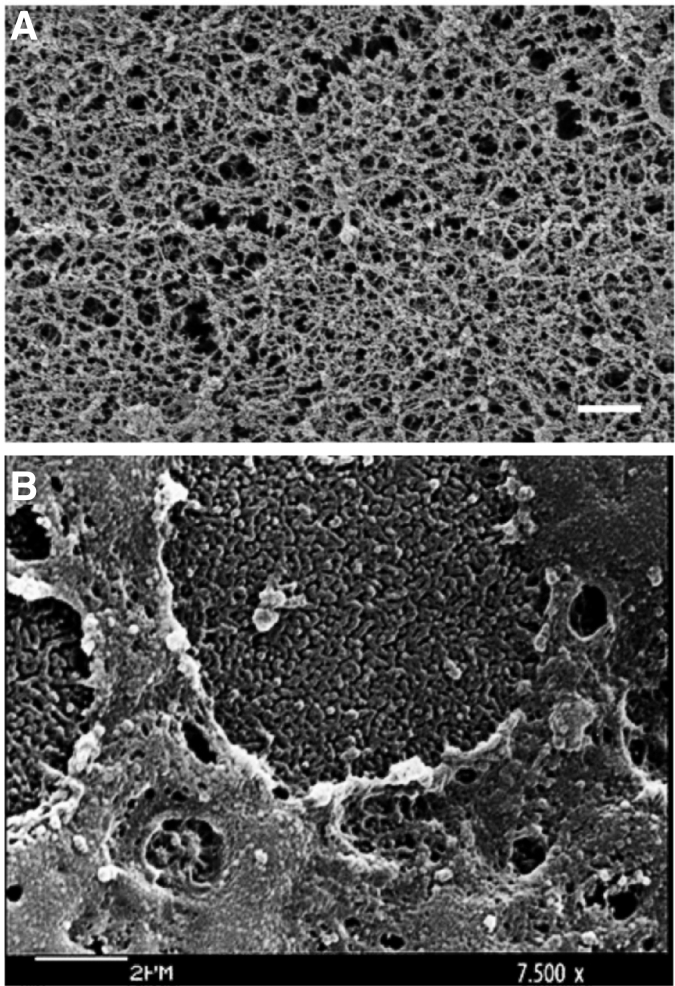
FIG. 1.
Scanning electron micrograph images of human mucus. (A) Human airway mucus. Scale bar represents 500 nm. Adapted from Schuster et al., Copyright 2013, with permission from Elsevier19; (B) Microvilli and mucus at the surface of human corneal epithelium. Adapted from Forte et al., Copyright © 2010, with permission from Wolters Kluwer Health.20
Mucins are conventionally named as MUC1, MUC2, MUC3, etc. according to the genes encoding their expression, with the number assigned in the order of discovery (although the appropriateness of this nomenclature in capturing the mucin diversity is debated).25 To date, 21 different human glycoproteins have been assigned to the MUC family.26 Within that family, mucins are generally subdivided into 2 classes: (1) secreted mucins and (2) membrane-associated mucins (also referred to as cell-associated, epithelium-tethered, membrane-tethered, or transmembrane mucins).
Secreted mucins are among the largest glycoproteins known; their contour lengths, measured as individual molecules, have been reported to range from hundreds of nanometers to more than 3 μm (depending on the organ and mucin “number”).17,27 Membrane-associated mucins are lower in molecular weight, with the extracellular domain spanning 200–500 nm and a short cytoplasmic tail anchored into the cellular surface.28,29
In both secreted and membrane-associated mucins, the “core” section of the polypeptide backbone comprises PTS domains and is densely grafted via O-linkage at the threonine and serine residues with oligosaccharides (O-glycosylated). These side chains contain 2–22 sugar units and can be can linear or moderately branched, conferring mucins with a brush-like structure.22,30 Due to the high prevalence of sialic acid (pKa 2.6) and the presence of sulfated sugars (pKa <1) as the outermost units of the oligosaccharide chains, PTS domains carry a strong negative charge.18,22 In the case of membrane-associated mucins, the N-terminus of the PTS region extends into the extracellular space whereas the C-terminus is attached to a cleavable domain that allows release from the cellular surface in response to various triggers (e.g., mechanical stress).
In secreted mucins, the PTS region is flanked at both ends with shorter, lightly glycosylated, cysteine-enriched domains. These “naked” terminal regions provide sites for intra- and intermolecular cross-linking via disulfide bonds and allow polymerization of mucin molecules into large homomultimeric assemblies, leading to the formation of long mucin fibers and the mucus mesh. In some secreted mucins, the PTS domains within the central region are also interspersed with cysteine-rich domains, resulting in a relatively hydrophobic region that provides additional sites for physical cross-linking.23 Owing to this structure, mucins have a strong propensity for polyvalent interactions through hydrogen bonding, disulfide bonding, electrostatic interaction, and hydrophobic forces.
The ability of mucins to assemble into a cross-linked network and form strong polyvalent interactions through a variety of mechanisms (including disulfide bonding, electrostatic/ionic interactions, hydrogen bonding, and hydrophobic forces) is essential to one of the key functions of mucus: protection of the underlying tissues from foreign particles. Two mechanisms are believed to be involved in stopping particles from readily penetrating through mucus mesh: (1) size filtering, a mechanism in which particles' diffusion is sterically hindered by the pore size of the mucus mesh; (2) interaction filtering, a mechanism that relies on adhesive polyvalent interactions between the particle and mucin fibers constituting the mucus mesh. Olmstead and Cone first suggested the importance of both mechanisms. In their pioneering work, they demonstrated that although some viruses and antibodies (provided they were smaller than the mucus mesh size) diffused essentially freely in human mucus, similarly sized polystyrene nanoparticles (even those as small as 59 nm) were completely immobilized.31 Ribbeck and coworkers further showed that, although size filtering has little to no effect for objects smaller than the mucus mesh size, interaction filtering (especially, electrostatic interaction) impacts the diffusion of objects of any size and can be attenuated by environmental conditions such as pH and salt content.21,30
Although the entrapment by mucus evolved as a defense against pathogens, it presents a formidable challenge for topical delivery of drugs formulated as micro- and nanoparticles. Since the mucus mesh size is typically less than 1 μm, mucus is impermeable to microparticles due to size filtering. Moreover, virtually all synthetic nanoparticles, even those smaller than 500 nm and not intentionally designed to be mucoadhesive, present multiple sites for polyvalent interactions with mucins and, therefore, will be immobilized in mucus by interaction filtering (unless they are specifically engineered to escape retention by mucus).9,21 As mucus is continuously shed and renewed, such trapped particles are cleared from the mucosal surface. Factors affecting the entrapment/clearance efficiency, such as mucin composition and concentration, mucus mesh size, and turnover rate, vary from organ to organ. In the next section, we will focus on mucus at the ocular surface.
Ocular Mucus
Cornea and conjunctiva are naturally covered with a layer of mucus. This layer is the densest at the epithelial apex and becomes more dilute as it extends outward into the tear fluid. The overall thickness of the ocular mucus layer ranges from 3 to 40 μm (depending on the measurement method used), suggesting that mucus is a major structural component of the tear film.22,32–35 Similar to mucus in other organs, ocular mucus serves many important physiological functions, including hydration, lubrication, cell signaling, and protection of the underlying epithelia from external noxious agents.15,22–24,28,29,36
Two types of secreted mucins are present at the ocular surface: large gel-forming mucins (MUC2, MUC5AC, MUC5B, and MUC6) and smaller soluble mucins (MUC7 and MUC9).22,28,29,35,36 They are produced predominantly by goblet cells in the conjunctiva epithelium and released into the tear film. MUC5AC is the most prevalent secreted ocular mucin; it is present in healthy tear fluid at concentrations as high as 200 μg/mL (MUC2, MUC5B, MUC6, MUC7, and MUC9 are secreted at levels that are orders of magnitude lower).22,37–39 Unlike in other mucus-lined organs, MUC5AC expressed at the ocular surface undergoes a post-secretion reduction of the molecular weight and does not aggregate into large multimers.37 As a result, ocular mucins do not form a continuous viscous gel layer over the cornea but move with the tear film. Assisted by reflex blinking and tear drainage, secreted mucins are cleared from the ocular surface within minutes.9,14 The rapid turnover of secreted mucins in the eye, coupled with their adhesive fibrous structure, is key for one of their primary functions: clearance of allergens, pathogens, and debris from the ocular surface.36
Among membrane-associated mucins at the ocular surface, MUC1, MUC4, and MUC16 are the most abundant.28,29,37 They are expressed at the corneal and conjunctival epithelia and are present in detectable quantities in tears. Membrane-associated MUC13, MUC15, MUC17, MUC20, MUC21, and MUC22 are also expressed by the ocular epithelia but at much lower levels and are undetectable in tear fluid. Together, the membrane-associated mucins form a dense glycocalyx layer extending up to 500 nm from the microvilli of the conjunctival and corneal epithelia.28 Since the primary function of ocular membrane-associated mucins is a reduction of abrasive stress on the underlying epithelia, they are renewed less rapidly than the secreted mucins. However, MUC1 and MUC16 also have a signaling function and form a barrier to pathogens by preventing their adhesion to the epithelial cells.28,36
The role of ocular mucus as a barrier to topical drug delivery to the eye was discussed in a number of recent reviews.8,15,24 The most comprehensive of them, a 2015 review by Ruponen and Urtti, identified only 4 relevant studies.15 In one of those studies, Dursun et al. attempted to investigate the effects of mucus by removing the tear film in vivo from healthy volunteers.40 The authors reported that the precorneal tear layer served as a permeability barrier for fluorescein. The 3 other studies were performed in vitro by Gipson and colleagues; they observed that the expression of mucins by human corneal–limbal epithelial cells reduced the internalization of rose bengal dye and cell surface binding of cationized ferritin.41–43 Therefore, the authors suggested a role for membrane-associated mucins in the protection of ocular surface epithelia.
The importance of membrane-associated mucins as a barrier to dye penetrance into human corneal epithelial cells was reiterated in several additional studies.44–46 However, Ruponen and Urtti argued that the molecular features of rose bengal (lyophilic anionic molecule) and cationized ferritin (cationic protein) are different from the topical ocular drugs and called for additional mechanistic studies to clarify the role of mucus in ocular drug delivery.15 It should also be noted that rose bengal dye, fluorescein, and even cationized ferritin are very small entities with respect to the pore size in the mucus mesh, and their diffusion through mucus is not expected to be sterically hindered.22 Contrarily, particle-based formulations such as microsuspensions and nanosuspensions, in which the drug particle size is greater than or comparable to the mucus mesh size, are likely to be impacted by both size and interaction filtering.15 Further, several of the aforementioned studies were performed with cell models, lacking the dynamic components of the ocular mucus barrier such as lacrimation and blinking (although efforts to incorporate these components into in vitro models by mimicking the movements of the tear film on the ocular surface are underway).46
Testing the barrier properties of ocular mucus ex vivo is also challenging for practical reasons: Unlike cervicovaginal and respiratory mucus that can be obtained from human subjects relatively easily, ocular mucus is difficult to collect in sufficient quantities without altering its key properties (degree of hydration, mucin composition, etc.). Most ex vivo studies, therefore, must rely on non-ocular mucus as a surrogate. Due to these factors, relevant experimental data on the impact of ocular mucus on topical delivery of drugs (in particular, formulated as micro- and nanosuspensions) had been sparse.
Recent studies on topical ocular delivery of the so-called MPP nanoparticles may help broaden the understanding of barrier properties of ocular mucus, while approaching it from a unique perspective: by comparing the delivery of particles designed to be mucus-penetrating with those known to be mucoadhesive. Before discussing data generated in these studies, we must first introduce the principles of MPP technology and explain the concept of enhanced drug delivery through mucopenetration (not mucoadhesion).
MPP Technology
Many efforts to enhance drug delivery though mucosal surfaces, including those of the eye, focused on mucoadhesive formulations (polymer-based solutions, viscous gels, and mucoadhesive micro- and nanoparticles).47–49 Indeed, compared with conventional formulations that are often almost immediately “washed away” (e.g., due to dose spillage and tear drainage), mucoadhesives may offer a benefit of prolonged residence time. However, this benefit is limited by the shed-off rate of the mucus layer. As schematically illustrated in Fig. 2, mucoadhesive formulations (as well as virtually all micro- and nanoparticles not specifically designed to be mucus-penetrating) become trapped in the outermost layer of secreted mucins due to size and/or interaction filtering. As a result, their ability to spread through the mucus layer toward the underlying tissue is limited and their residence time is largely dictated by the mucin turnover time. Therefore, mucoadhesive formulations do not offer a solution for the limited penetration of drugs (or their carriers) across the mucus layer, especially in organs with aggressive mucus clearance mechanisms.47
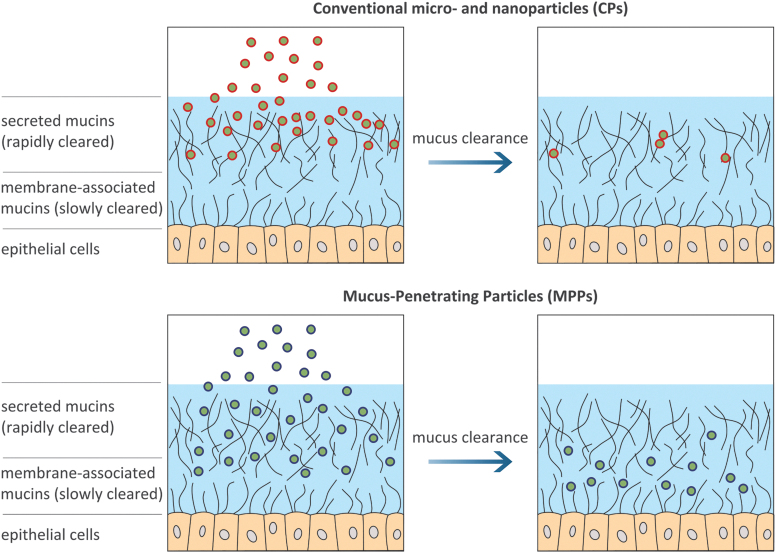
FIG. 2.
Generalized graphical depiction of mucus layer and proposed fate of different types of particles on topical administration: microparticles and conventional (mucoadhesive) nanoparticles are trapped in the outer (secreted) mucus layer and eliminated by mucus clearance; MPPs avoid adhesion in the rapidly cleared outer mucus layer, achieve a more uniform distribution, and penetrate toward the slowly cleared membrane-associated layer. MPPs, mucus-penetrating particles.
Approximately a decade ago, it was first hypothesized that nanoparticles capable of avoiding the adhesive and steric interactions with the mucus mesh could escape entrapment in the most rapidly cleared outer layer of secreted mucins and penetrate toward the less rapidly cleared layer of membrane-associated mucins, thereby delivering the payload closer to the underlying tissue and achieving a prolonged residence time compared with mucoadhesive formulations (Fig. 2).9,50 To confirm this hypothesis, Hanes and coworkers pioneered the concept of MPPs by using polymeric nanoparticles selectively sized to allow for diffusion into mucus pores and having a coating specifically designed to minimize adhesive interactions with mucin fibers.9,50–53 They have shown that polymeric nanoparticles as big as 500 nm in diameter, when coated covalently with extremely dense coatings of low-molecular-weight (2–5 kDa) polyethylene glycol or non-covalently with certain poloxamers, could diffuse in human mucus nearly as rapidly as they diffuse in water, whereas similarly sized uncoated conventional particles (CPs) were essentially immobilized. As an example, Fig. 3 shows velocity distribution histograms and representative particle trajectories in ex vivo human cervicovaginal mucus for 200 nm polystyrene nanoparticles and similarly sized nanoparticles that were surface-treated by using the MPP technology.54
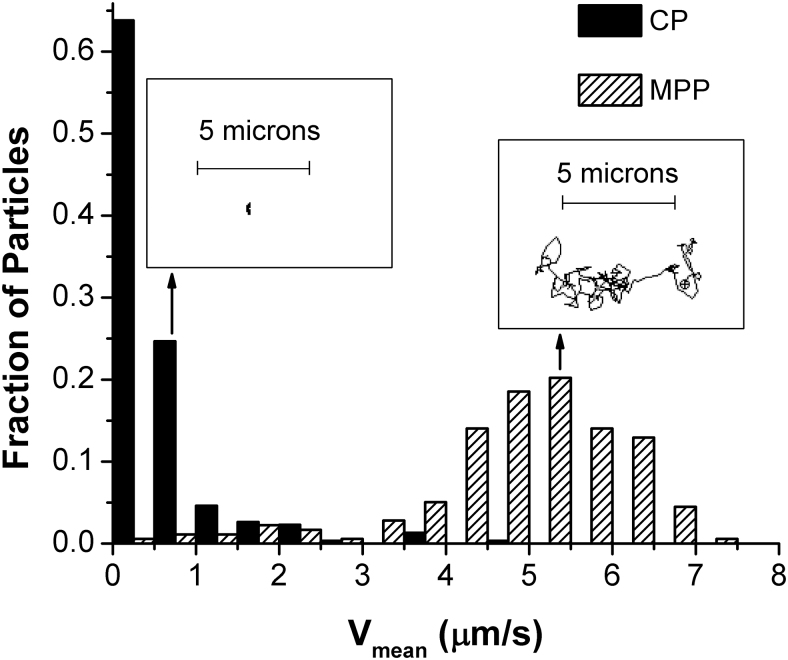
FIG. 3.
Distributions of the trajectory-average velocity Vmean observed in fresh undiluted cervicovaginal mucus over 15 s for CP (200 nm polystyrene nanoparticles) and MPP (200 nm polystyrene nanoparticles modified with polyethylene glycol, MW 2 kDa). The insets show representative 15-s trajectories corresponding to the ensemble-average values for Vmean. The CPs were completely immobilized in mucus, whereas similarly sized MPPs remained mobile and exhibited trajectories that resemble freely diffusive Brownian motion. Reprinted from Popov et al., Copyright 2016, with permission from Elsevier.54 CPs, conventional particles.
Using imaging techniques and fluorescently labeled particles, Hanes et al. showed that the ability of the MPPs to penetrate mucus in vitro translated into significantly improved distribution and prolonged particle retention in vivo on mucosal surfaces in various organs in animal models, including GI, respiratory, and cervicovaginal tracts.10–12 As illustrated in Fig. 4, nanoparticles formulated as MPPs achieved a uniform surface coverage of the mucus and penetrated deeply into tissue folds; whereas the CPs became clumped together and were unable to penetrate between the tissue folds.10–12 Among a number of studies focused on evaluating the pharmacokinetic implications of MPPs, Popov et al. demonstrated prolonged local exposure in vivo by using MPPs for pulmonary delivery.55 They observed that, after an intratracheal instillation in mice, a biodegradable polymeric MPP formulation encapsulating fluticasone propionate (a small-molecule drug) sustained higher drug levels in the mouse lungs, resulting in a 60% increase in the area under the curve compared with an otherwise similar mucoadhesive formulation (Fig. 5).
FIG. 4.
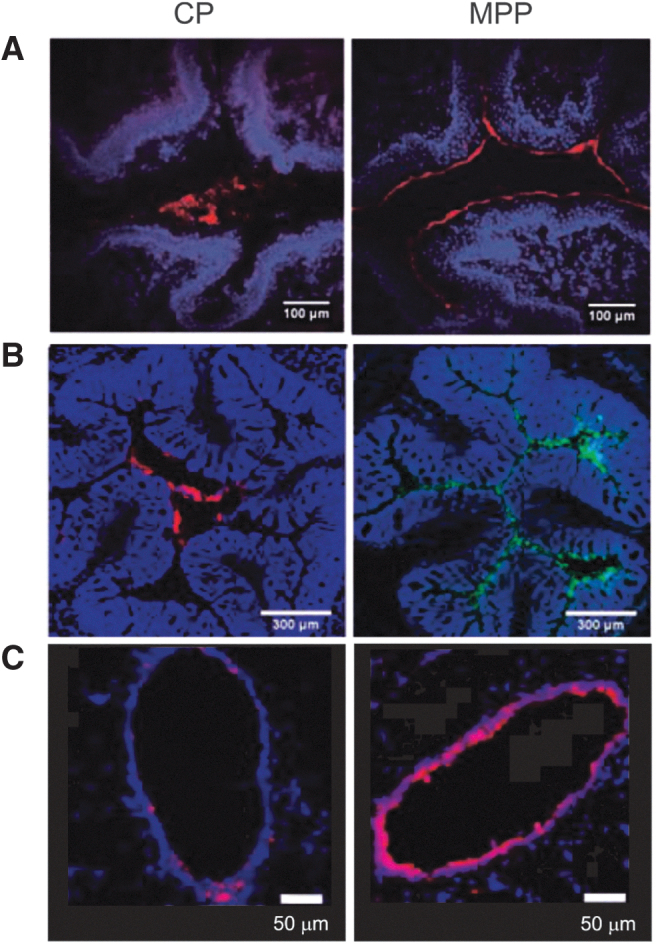
Distributions of MPPs and CPs in the mucus layer of various organs of the mouse model (obtained by fluorescent imaging of tissue cross-sections collected after administration of CP and MPP suspensions; red or green represents fluorescence from the nanoparticles; blue represents fluorescence from the stained tissue). (A) MPPs (particle diameter ∼110 nm) and CPs (particle diameter ∼90 nm) in mouse vagina. Reprinted from Ensign et al., Copyright © 2012, with permission from American Association for the Advancement of Science10. (B) MPPs and CPs (both ∼100 nm in diameter) in mouse colorectum. Reprinted from Maisel et al., Copyright 2015, with permission from Elsevier11. (C) MPPs (particle diameter ∼60 nm) and CPs (particle diameter ∼50 nm) in mouse large airway. Reprinted from Suk et al., Copyright 2014, with permission from Elsevier.12
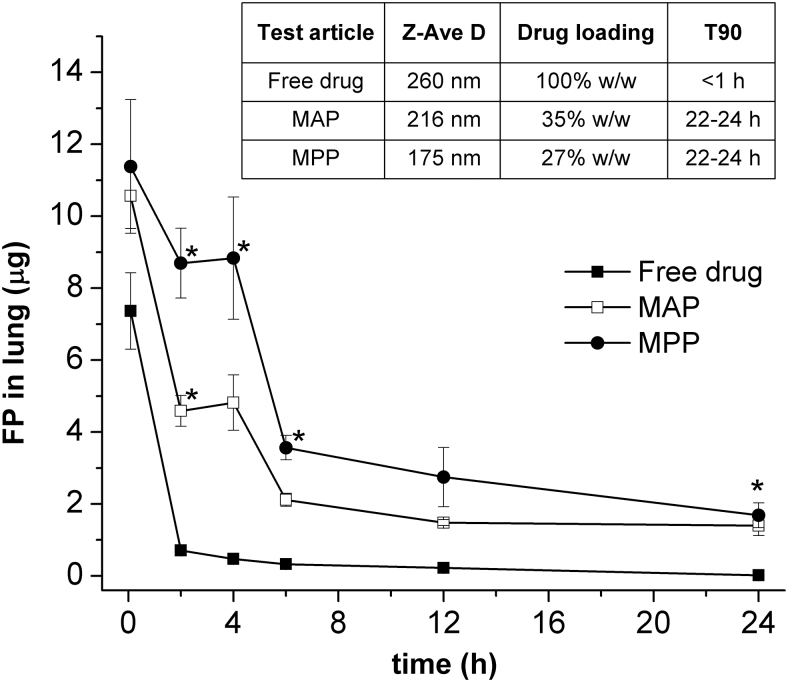
FIG. 5.
Total amount of FP measured in mouse lung after a single intratracheal instillation of 10 μg of FP formulated as free drug, MAPs or MPPs with similar particle size, drug loading, and in vitro drug release profile. Error bars indicate standard error of the mean (n = 6). Symbol (*) indicates statistically significant difference from the free drug group (P < 0.05; ANOVA). The inset shows test article characteristics: hydrodynamic diameter (Z-Ave D), drug loading, and time to release 90% of the drug load in an in vitro release test (T90). Reprinted from Popov et al., Copyright 2016, with permission from Elsevier.55 FP, fluticasone propionate; MAPs, mucoadhesive particles.
Topical Delivery of MPPs to the Eye and the Barrier Effect of Ocular Mucus
Although the benefits of MPPs as a drug delivery modality had been demonstrated in GI, respiratory, and vaginal tract models, translation of these benefits to the ophthalmic route was not certain due to the unique static and dynamic barriers present at the ocular surface (such as the rapid clearance of tear film mucins and a thinner mucus layer compared with other mucus-lined organs). Recent studies, however, have shown the ability of MPP technology to enhance topical ocular delivery in a number of animal models, including rats, rabbits, and minipigs, by using polymeric MPP particles as well as steroids and tyrosine kinase inhibitors formulated as MPPs.56,57
Figure 6 shows the distribution and retention of polymeric MPPs and CPs on the ocular surface of live rats after a single topical instillation.58 In this experiment, both types of particles were 200 nm near-IR-labeled polystyrene core nanoparticles; the MPPs were surface-modified with a muco-inert coating, whereas the CPs remained untreated. As can be seen in the bottom panel of Fig. 6, MPPs spread over the ocular surface uniformly and achieved prolonged retention. In contrast, CPs were rapidly cleared and virtually undetectable after 2 h. These findings with polymeric MPPs confirmed the potential of MPP technology for ocular delivery. However, there are certain limitations associated with the polymeric nanoparticles, such as relatively low drug loading, potential storage stability issues for nanoparticles comprising a biodegradable core, and manufacturing complexity. These limitations may present pharmaceutical development challenges for MPPs containing a polymeric core.
FIG. 6.
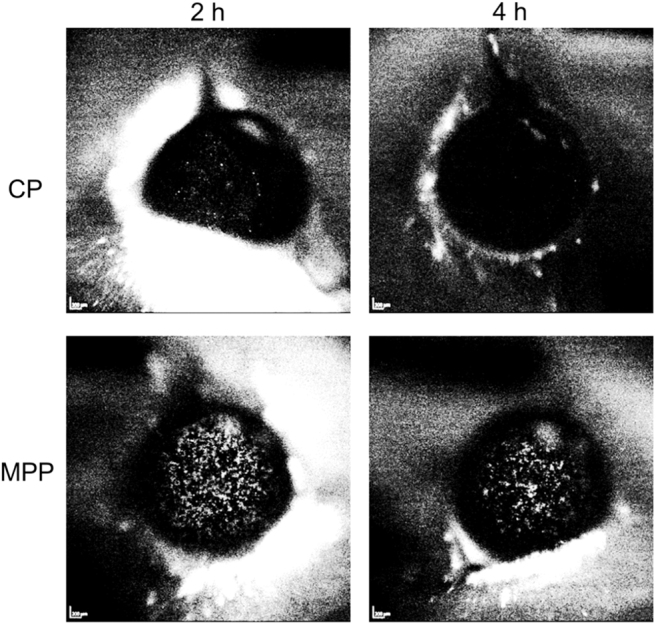
Distribution and retention of near-IR-labeled polystyrene core particles formulated as CP and MPP (both 200 nm in diameter) on the ocular surface of rats after a single topical instillation of equivalent doses (5 μL of 0.9% suspension). Images were collected by using a Heidelberg camera at 2 and 4 h post-instillation. The bright specs on the dark background (ocular surface) represent the particles. The CPs were rapidly eliminated from the ocular surface. The MPPs achieved a uniform spreading over the ocular surface and prolonged retention. Adapted from Weinstock et al.58
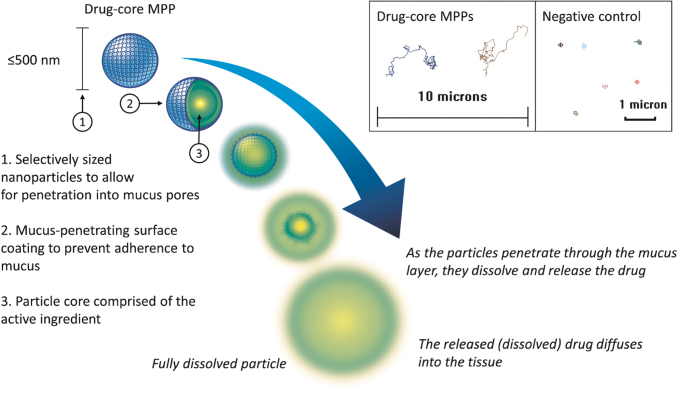
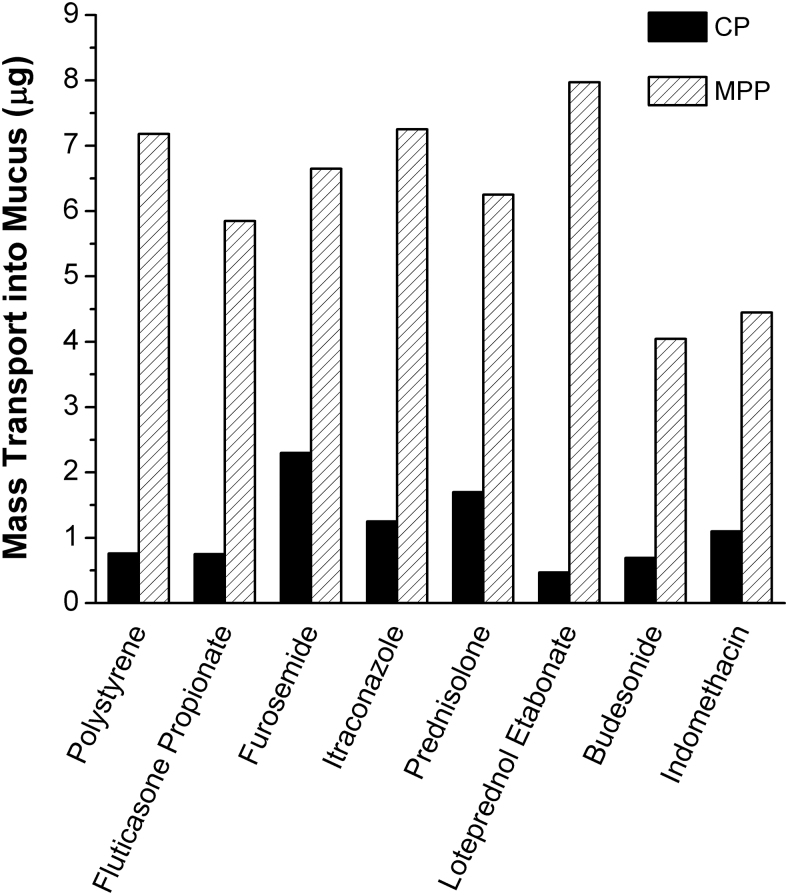
To overcome these challenges and further advance MPP technology, MPPs that do not require encapsulation of drugs into a polymeric matrix as the core particle have been devised.48 These new-generation MPPs, hereafter referred to as drug-core MPPs, comprise mostly pure drug as the core particle and have the required MPP attributes such as a particle size appropriate for penetration into mucus pores and the mucus-penetrating surface coating to prevent adherence to mucus (as illustrated in Fig. 7). Drug-core MPPs offer improved physical and chemical stability over polymeric nanoparticles and can be shelf stable in a ready-to-use form as aqueous suspensions. Moreover, MPPs with a drug core can allow utilization of excipients generally recognized as safe without covalent modifications of the drug or excipients for delivery. Specifically for the topical ophthalmic route, such MPPs can be formulated with excipients previously approved by the FDA for ophthalmic use. The ability of the resulting drug-core MPPs to permeate mucus ex vivo has been demonstrated with drugs from multiple therapeutic classes, including the examples shown in Fig. 8.59–61
FIG. 7.
Schematic depiction of a drug-core MPP. The inset is showing 15-s trajectories of drug-core MPPs (particle size of ∼270 nm in this example) and a negative control (200 nm polystyrene nanoparticles) in human cervicovaginal mucus as observed by fluorescence microscopy. The inset is adapted from Cu et al.61
FIG. 8.
Examples of drugs formulated for enhanced mucus-penetration as drug-core MPPs. In an ex vivo assay, nanoparticles of various drugs formulated as drug-core MPPs exhibited increased mass transport through human cervicovaginal mucus as opposed to similarly sized CPs. Polystyrene CPs and MPPs (both 200 nm) were used as negative and positive controls with well-established mucoadhesive and mucus-penetrating behavior, respectively. Adapted from Popov et al.59
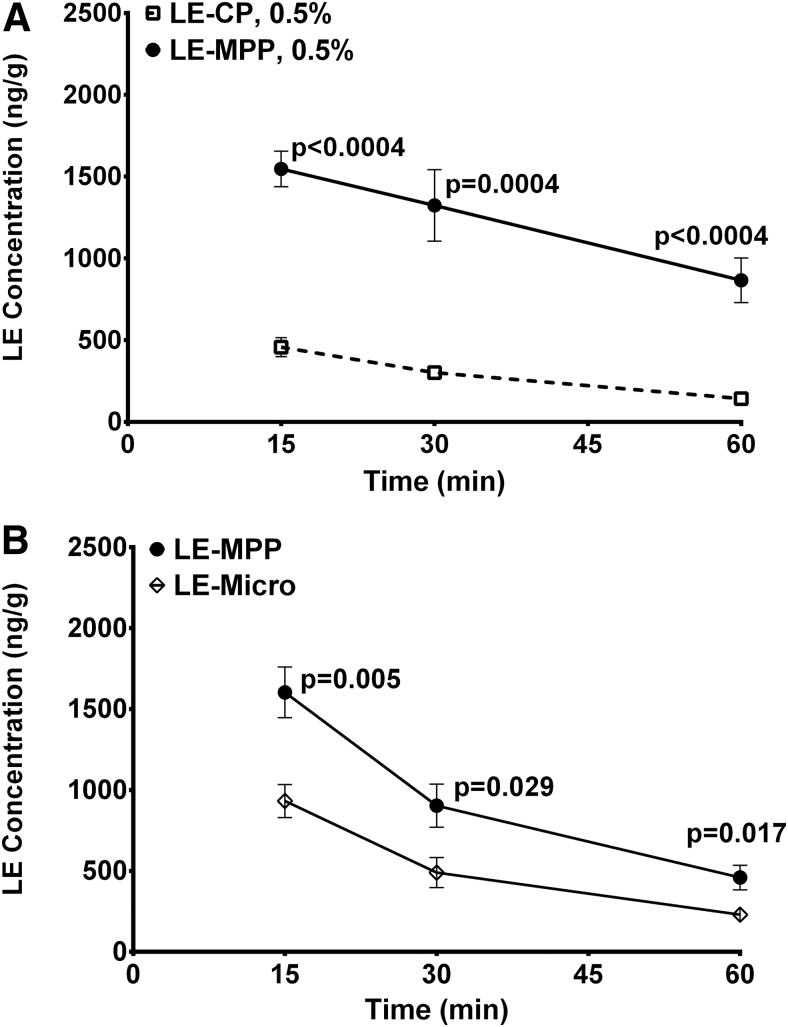
Using loteprednol etabonate (LE, an ester steroid) formulated as drug-core MPPs, Schopf et al. conducted a series of preclinical studies designed to better understand the role of ocular mucus and mucus penetration for the delivery of nano- and microparticles.56,57 To assess the impact of adhesive interactions with ocular mucus, they measured drug levels in the cornea of rabbits after instillation of equivalent doses of mucus-penetrating LE nanoparticles (LE-MPP; 240 nm in size) and similarly sized control particles of LE without the MPP surface modification (LE-CP; also 240 nm in size). As evident from Fig. 9A, LE-MPP nanoparticles produced significantly higher drug levels in the cornea compared with those produced by LE particles of the same size but lacking the mucus-penetrating properties. Since the MPP and CP particles in this experiment were of the same size, it is unlikely that the observed effect was related to any difference in particle dissolution kinetics in vivo. It should also be noted that, not limiting this behavior to LE, a similar effect of the surface coating was confirmed in vivo for other drugs, such as axitinib and other tyrosine kinase inhibitors, formulated as nanoparticles (more specifically, nanocrystals sized in the 200–300 nm range as measured by dynamic light scattering) with and without the MPP coating.57,62
FIG. 9.
Corneal levels of LE after administration of various LE formulations to rabbits as a single topical dose (50 μL of 0.5% suspension). (A) Administration of LE-CP or LE-MPP (both 240 nm in particle size as measured by the average hydrodynamic diameter). (B) Administration of LE-MPP (240 nm) or LE-Micro (particle size >1 μm). All data are shown as mean ± SEM (n = 6 eyes). Statistical analysis was performed by using the Holm-Sidak method and multiple t-tests; P values are shown on graphs. Republished with permission of Schopf et al., Copyright 2012 ARVO; permission conveyed through Copyright Clearance Center, Inc.57 LE, loteprednol etabonate.
In an additional experiment, Schopf et al. compared LE-MPP with a micronized LE suspension (median particle size greater than 1 μm) treated with the same MPP-enabling surface-altering agent to assess the influence of steric interactions with the mucus network. As shown in Fig. 9B, they confirmed that the smaller particle size (e.g., 200 nm vs. greater than 1 μm) contributed to improved ocular exposure, even when the surface modifier was identical.57 Together, these results suggest that mucus at the ocular surface plays an important role as a barrier for delivery of micro- and nanoparticles and that proper particle size and surface modification allow the particles to avoid the mucus entrapment and clearance.
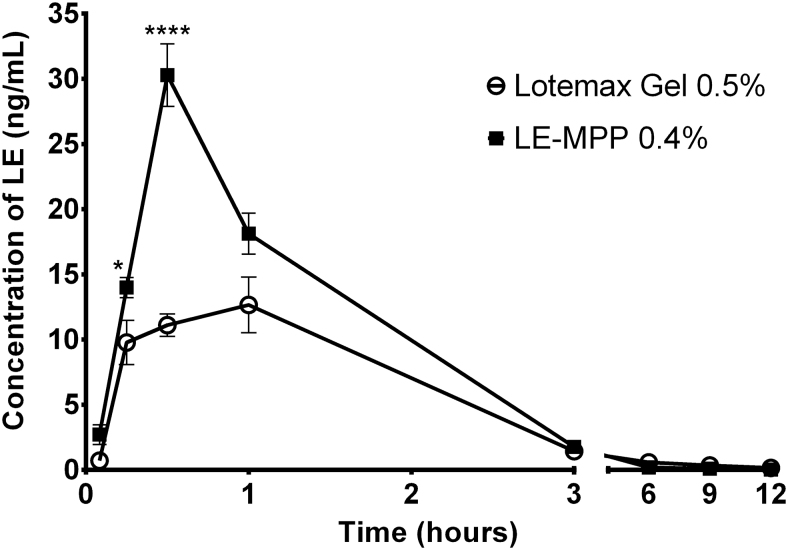
Interestingly, mucus-penetrating formulations were, at least in some cases, able to outperform mucoadhesive gel-based formulations containing micronized drugs. As evident from Fig. 10, a non-viscous suspension of LE formulated as drug-core MPPs produced substantially higher drug levels in the aqueous humor of rabbits compared with those delivered by LOTEMAX® gel, resulting in an ∼60% increase in the area under the curve (AUC0–3h) and a more than 200% increase in the peak concentration.62 This outcome is especially remarkable considering that LE-MPP was administered in this experiment at a 20% lower drug concentration (0.4% vs. 0.5% for LOTEMAX gel). Thus, although mucoadhesive formulations have a place in topical drug delivery to the eye, they do not overcome all the challenges associated with the aggressive precorneal clearance mechanisms. By avoiding interactions with the mucus barrier, mucus-penetrating formulations may offer a new way to enhance topical drug delivery to the eye without the downsides sometimes associated with viscous and sticky mucoadhesive gels.
FIG. 10.
Pharmacokinetic profiles of LE in rabbit aqueous humor after a single topical instillation of 35 μL of LE-MPP (0.4% LE) or Lotemax™ gel (0.5% LE). All data are shown as mean ± SEM (n = 6 eyes). Symbols * and **** indicate P < 0.05 and P < 0.0001, respectively. Adapted from Langer et al.62
Conclusion
Recent studies comparing topical delivery of mucus-penetrating nanoparticles and conventional formulations indicate that ocular mucus plays an important role as a barrier to micro- and nanosuspensions. The strong adhesive nature and selective permeability, which make mucus so effective in trapping and clearing extraneous pathogens, also undermine the effectiveness of CP-based formulations. Ophthalmic microsuspensions are subject to both size and interaction filtering with the mucus mesh and, as a result, the drug is often largely trapped by secreted mucins in the tear film and eliminated by mucus turnover before it can reach the target tissues. Conventional nanosuspensions are also likely to be rapidly removed from the ocular surface since most nanoparticles are subject to strong polyvalent interactions with the mucins.
Nanoparticles engineered by using MPP technology to evade the mucus entrapment and associated rapid clearance are emerging as a new approach to topical ocular delivery. As demonstrated in in vivo experiments, MPPs can deliver drugs to mucosal tissues more uniformly, achieve higher concentrations at the target tissues, and, in some cases, prolong the drug residence time compared with conventional formulations.56,57
One product utilizing MPP technology (trademarked by Kala Pharmaceuticals, Inc. as AMPPLIFY® Drug Delivery Technology), INVELTYS® (LE ophthalmic suspension 1%), has already been approved by the U.S. FDA.58,63 It is the first twice-daily ocular corticosteroid indicated for the treatment of postoperative inflammation and pain after ocular surgery. Another program (KPI-121 0.25% ophthalmic suspension for temporary relief of the signs and symptoms of dry eye disease) has completed Phase 3 trials and is currently undergoing an NDA review.64
Acknowledgments
The author thanks Hongming Chen and Steven Zhang (employees of Kala Pharmaceuticals, Inc.) for their critical review of this article.
Author Disclosure Statement
The MPP technology described in this publication is being developed by Kala Pharmaceuticals, Inc. The author is an employee of Kala Pharmaceuticals, Inc. and owns company stock.
Funding Information
No specific funding was received for this article.
References
- 1. Gaudana R., Jwala J., Boddu S.H.S., and Mitra A.K.. Recent perspectives in ocular drug delivery. Pharm. Res. 26:1197–1216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel A. Ocular drug delivery systems: an overview. World J. Pharmacol. 2:47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kompella U.B., Kadam R.S., and Lee V.H.L.. Recent advances in ophthalmic drug delivery. Ther. Deliv. 1:435–456, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 57:1595–1639, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Subrizi A., del Amo E.M., Korzhikov-Vlakh V., Tennikova T., Ruponen M., and Urtti A.. Design principles of ocular drug delivery systems: importance of drug payload, release rate, and material properties. Drug Discov. Today. 24:1446–1457, 2019 [DOI] [PubMed] [Google Scholar]
- 6. Yavuz B., and Kompella U.B.. Ocular drug delivery. In: Whitcup S., Azar D., eds. Pharmacologic Therapy of Ocular Disease. Handbook of Experimental Pharmacology. Vol 242. Cham: Springer International Publishing; 2017; p. 57–93 [DOI] [PubMed] [Google Scholar]
- 7. Cholkar K., Patel S.P., Vadlapudi A.D., and Mitra A.K.. Novel strategies for anterior segment ocular drug delivery. J. Ocul. Pharmacol. Ther. 29:106–123, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sigurdsson H.H. Kirch, J., and Lehr, C.-M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. 453:56–64, 2013 [DOI] [PubMed] [Google Scholar]
- 9. Lai S.K., Wang Y.-Y., and Hanes J.. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 61:158–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ensign L.M., Tang B.C., Wang Y.-Y., et al. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci. Transl. Med. 4:138ra79, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maisel K., Ensign L., Reddy M., Cone R., and Hanes J.. Effect of surface chemistry on nanoparticle interaction with gastrointestinal mucus and distribution in the gastrointestinal tract following oral and rectal administration in the mouse. J. Control. Release. 197:48–57, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suk J.S., Kim A.J., Trehan K., et al. Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J. Control. Release. 178:8–17, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dombu C.Y., and Betbeder D.. Airway delivery of peptides and proteins using nanoparticles. Biomaterials. 34:516–525, 2013 [DOI] [PubMed] [Google Scholar]
- 14. Murgia X., Loretz B., Hartwig O., Hittinger M., and Lehr C.M.. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv. Drug Deliv. Rev. 124:82–97, 2018 [DOI] [PubMed] [Google Scholar]
- 15. Ruponen M., and Urtti A.. Undefined role of mucus as a barrier in ocular drug delivery. Eur. J. Pharm. Biopharm. 96:442–446, 2015 [DOI] [PubMed] [Google Scholar]
- 16. Yellepeddi V.K., and Palakurthi S.. Recent advances in topical ocular drug delivery. J. Ocul. Pharmacol. Ther. 32:67–82, 2016 [DOI] [PubMed] [Google Scholar]
- 17. Cone R.A. Mucus. In: Mestecky J., Lamm, M.E., Ogra, P.L., et al., eds. Mucosal Immunology. Burlington, MA: Academic Press; 2005; p. 49–72 [Google Scholar]
- 18. Cone R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 61:75–85, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Schuster B.S., Suk J.S., Woodworth G.F., and Hanes J.. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials. 34:3439–3446, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Forte R., Cennamo G., Del Prete S., Cesarano I., and Del Prete A.. Scanning electron microscopy of corneal epithelium in soft contact lens wearers. Cornea. 29:732–736, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Witten J., Samad T., and Ribbeck K.. Selective permeability of mucus barriers. Curr. Opin. Biotechnol. 52:124–133, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leal J., Smyth H.D.C., and Ghosh D.. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 532:555–572, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bansil R., and Turner B.S.. The biology of mucus: composition, synthesis and organization. Adv. Drug Deliv. Rev. 124:3–15, 2018 [DOI] [PubMed] [Google Scholar]
- 24. Taherali F., Varum F., and Basit A.W.. A slippery slope: on the origin, role and physiology of mucus. Adv. Drug Deliv. Rev. 124:16–33, 2018 [DOI] [PubMed] [Google Scholar]
- 25. Dekker J., Rossen J.W.A., Büller H.A., and Einerhand A.W.C.. The MUC family: an obituary. Trends Biochem. Sci. 27:126–131, 2002 [DOI] [PubMed] [Google Scholar]
- 26. HUGO Gene Nomenclature Committee. Available at: https://www.genenames.org/ (last accessed May19, 2020)
- 27. Round A.N., Berry M., McMaster T.J., et al. Heterogeneity and persistence length in human ocular mucins. Biophys. J. 83:1661–1670, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Govindarajan B., and Gipson I.K.. Membrane-tethered mucins have multiple functions on the ocular surface. Exp. Eye Res. 90:655–663, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fini M.E., Jeong S., Gong H., et al. Membrane-associated mucins of the ocular surface: new genes, new protein functions and new biological roles in human and mouse. Prog. Retin. Eye Res. 75:100777, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lieleg O., and Ribbeck K.. Biological hydrogels as selective diffusion barriers. Trends Cell Biol. 21:543–551, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olmsted S.S., Padgett J.L., Yudin A.I., Whaley K.J., Moench T.R., and Cone R.A.. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys. J. 81:1930–1937, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nichols B.A., Chiappino M.L., and Dawson C.R.. Demonstration of the mucous layer of the tear film by electron microscopy. Invest. Ophthalmol. Vis. Sci. 26:464–473, 1985 [PubMed] [Google Scholar]
- 33. Prydal J.I., Artal P., Woon H., and Campbell F.W.. Study of human precorneal tear film thickness and structure using laser interferometry. Invest. Ophthalmol. Vis. Sci. 33:2006–2011, 1992 [PubMed] [Google Scholar]
- 34. Prydal J.I., and Campbell F.W.. Study of precorneal tear film thickness and structure by interferometry and confocal microscopy. Invest. Ophthalmol. Vis. Sci. 33:1996–2005, 1992 [PubMed] [Google Scholar]
- 35. Gipson I.K., and Inatomi T.. Cellular origin of mucins of the ocular surface tear film. In: Sullivan D.A., Dartt, D., Meneray, M.A., eds. Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2. Advances in Experimental Medicine and Biology. Boston, MA: Springer; 1998; p. 221–227 [DOI] [PubMed] [Google Scholar]
- 36. Mantelli F., and Argüeso P.. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 8:477–483, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spurr-Michaud S., Argüeso P., and Gipson I.. Assay of mucins in human tear fluid. Exp. Eye Res. 84:939–950, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao H., Jumblatt J.E., Wood T.O., and Jumblatt M.M.. Quantification of MUC5AC protein in human tears. Cornea. 20:873–877, 2001 [DOI] [PubMed] [Google Scholar]
- 39. McKenzie R.W., Jumblatt J.E., and Jumblatt M.M.. Quantification of MUC2 and MUC5AC transcripts in human conjunctiva. Investig. Ophthalmol. Vis. Sci. 41:703–708, 2000 [PubMed] [Google Scholar]
- 40. Dursun D., Monroy D., Knighton R., et al. The effects of experimental tear film removal on corneal surface regularity and barrier function. Ophthalmology. 107:1754–1760, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Argüeso P., Tisdale A., Spurr-Michaud S., Sumiyoshi M., and Gipson I.K.. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Invest. Ophthalmol. Vis. Sci. 47:113–119, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sumiyoshi M., Ricciuto J., Tisdale A., Gipson I.K., Mantelli F., and Argüeso P.. Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 49:197–203, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blalock T.D., Spurr-Michaud S.J., Tisdale A.S., et al. Functions of MUC16 in corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 48:4509–4518, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Argüeso P., Guzman-Aranguez A., Mantelli F., Cao Z., Ricciuto J., and Panjwani N.. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J. Biol. Chem. 284:23037–23045, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gipson I.K., Spurr-Michaud S., Tisdale A., and Menon B.B.. Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. Batra S.K., ed. PLoS One. 9:e100393, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hampel U., Garreis F., Burgemeister F., Eßel N., and Paulsen F.. Effect of intermittent shear stress on corneal epithelial cells using an in vitro flow culture model. Ocul. Surf. 16:341–351, 2018 [DOI] [PubMed] [Google Scholar]
- 47. Schattling P., Taipaleenmäki E., Zhang Y., and Städler B.. A polymer chemistry point of view on mucoadhesion and mucopenetration. Macromol. Biosci. 17:1700060, 2017 [DOI] [PubMed] [Google Scholar]
- 48. Mansuri S., Kesharwani P., Jain K., Tekade R.K., and Jain N.K.. Mucoadhesion: a promising approach in drug delivery system. React. Funct. Polym. 100:151–172, 2016 [Google Scholar]
- 49. Cuggino J.C., Blanco E.R.O., Gugliotta L.M., Alvarez Igarzabal C.I., and Calderón M.. Crossing biological barriers with nanogels to improve drug delivery performance. J. Control. Release. 307:221–246, 2019 [DOI] [PubMed] [Google Scholar]
- 50. Wang Y.Y., Lai S.K., Suk J.S., Pace A., Cone R., and Hanes J.. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew. Chem. Int. Ed. 47:9726–9729, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tang B.C., Dawson M., Lai S.K., et al. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc. Natl. Acad. Sci. U. S. A. 106:19268–19273, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang M., Lai S.K., Wang Y.-Y., et al. Biodegradable nanoparticles composed entirely of safe materials that rapidly penetrate human mucus. Angew. Chem. Int. Ed. 50:2597–2600, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yu T., Wang Y.Y., Yang M., et al. Biodegradable mucus-penetrating nanoparticles composed of diblock copolymers of polyethylene glycol and poly(lactic-co-glycolic acid). Drug Deliv. Transl. Res. 2:124–128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Popov A., Enlow E., Bourassa J., and Chen H.. Mucus-penetrating nanoparticles made with “mucoadhesive” poly(vinyl alcohol). Nanomedicine. 12:1863–1871, 2016 [DOI] [PubMed] [Google Scholar]
- 55. Popov A., Schopf L., Bourassa J., and Chen H.. Enhanced pulmonary delivery of fluticasone propionate in rodents by mucus-penetrating nanoparticles. Int. J. Pharm. 502:188–197, 2016 [DOI] [PubMed] [Google Scholar]
- 56. Schopf L., Enlow E., Popov A., Bourassa J., and Chen H.. Ocular pharmacokinetics of a novel loteprednol etabonate 0.4% ophthalmic formulation. Ophthalmol. Ther. 3:63–72, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schopf L.R., Popov A.M., Enlow E.M., et al. Topical ocular drug delivery to the back of the eye by mucus-penetrating particles. Transl. Vis. Sci. Technol. 4:11, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weinstock R.J., Rowen S.L., Sood P., and Trattler W. Introducing a new ocular corticosteroid powered by AMPPLIFY technology. Cataract Refract.Surg.Today. Available at: https://crstoday.com/?p=15478 (last accessed May19, 2020)
- 59. Popov A., Enlow E.M., Bourassa J.L., Gardner C.R., Chen H., and Ensign L.M. inventors; Kala Pharmaceuticals, Inc., Johns Hopkins University, assignees. Pharmaceutical nanoparticles showing improved mucosal transport. World patent application WO/2013/166385; November 7, 2013
- 60. Ong W., Nowak P., Cu Y., et al. Sustained pulmonary delivery of a water-soluble antibiotic without encapsulating carriers. Pharm. Res. 33:563–572, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cu Y., Nowak P., Ong W., Bourassa J., Popov A., and Chen H.. Mucus-penetrating antibiotic prodrug nanosuspension for treatment of infections at the mucosal surface. Poster presented at 2014 AAPS Annual Meeting; November 2–6, 2014; San Diego, CA [Google Scholar]
- 62. Langer R., Chen H., and Hanes J. The nano state. Available at: https://theophthalmologist.com/business-profession/the-nano-state (last accessed May19, 2020)
- 63. Kim T., Sall K., Holland E.J., Brazzell R.K., Coultas S., and Gupta P.K.. Safety and efficacy of twice daily administration of KPI-121 1% for ocular inflammation and pain following cataract surgery. Clin. Ophthalmol. 13:69–86, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kala Pharmaceuticals, Inc. Safety and efficacy of KPI-121 in subjects with DED (STRIDE 3). Available at https://clinicaltrials.gov/ct2/show/record/NCT03616899 (last accessed May19, 2020)