Summary
Healthcare workers are at an increased risk of infection, harm and death from COVID‐19. Close and prolonged exposure to individuals infectious with SARS‐CoV‐2 leads to infection. A person’s individual characteristics (age, sex, ethnicity and comorbidities) then influence the subsequent risk of COVID‐19 leading to hospitalisation, critical care admission or death. While relative risk is often reported as a measure of individual danger, absolute risk is more important and dynamic, particularly in the healthcare setting. Individual risk interacts with exposure and environmental risk‐factors, and the extent of mitigation to determine overall risk. Hospitals are a unique environment in which there is a significantly increased risk of infection for all healthcare workers. Anaesthetists and intensivists particularly are at high risk of exposure to SARS‐CoV‐2 infected patients due to their working environments and exposure to certain patient groups. However, the available evidence suggests that the risk for this group of individuals is not currently increased. This review examines factors associated with increased risk of infection with SARS‐CoV‐2, increasing severity of COVID‐19 and death. A risk tool is proposed that includes personal, environmental and mitigating factors, and enables an individualised dynamic ‘point‐of‐time’ risk assessment.
Keywords: COVID‐19, healthcare worker, intensive care, mortality, pandemic, risk
Introduction
Healthcare workers are at particularly high risk of exposure to patients infected with SARS‐CoV‐2 and development of COVID‐19. Among healthcare workers, anaesthetists and intensivists particularly are at a high risk of exposure to patients with severe COVID‐19 and this poses unique challenges.
The aim of this review is to examine factors associated with increased risk of infection with SARS‐CoV‐2, increasing severity of COVID‐19 and death for those working in anaesthesia and intensive care medicine.
Disease transmission
Exposure to respiratory secretions by contact, droplet or airborne routes is the primary mode of transmission of SARS‐CoV‐2, with contact and droplet transmission judged the predominant modes [1]. Airborne transmission (via infected aerosols) likely plays a part, but its extent is uncertain. Most viral shedding and transmission occur before or during the first week of symptomatic illness and while viral RNA may be detected for prolonged periods in severe disease [2], the duration of cellular viral reproduction (and hence infectivity) may be considerably shorter [3].
Transmission of infection is dependent on proximity and duration of contact, so risk of transmission is decreased by reducing time of contact, increasing distance and adding physical barriers, including facemasks and shields, transparent screens or sheets. High‐risk settings include the environment of extensive coughing or sneezing, areas where standard infection control precautions or personal protective equipment (PPE) is inadequate and locations where aerosol‐generating procedures are undertaken.
Facemasks are designed to reduce the risk of droplet dispersal from the wearer to the environment [4]. They protect those around the wearer. Filtered face pieces (FFP2/FFP3/NP95 masks) are designed to protect the wearer [5]. Masks with an expiratory valve should be avoided, but if worn to comply with the universal facemask policy, a facemask should be worn over the filtered face piece to protect protect those around the wearer [6].
Hospitals as high‐risk locations for staff
In hospitals, the presence of infected patients (not all of whom are identified as such), the nature of care procedures undertaken and the need for proximity all inevitably increase the exposure of staff to infection. Standard infection control and specific transmission‐based precautions are designed to mitigate risk [7].
Anaesthetists and intensivists are likely to be exposed to patients at all phases of the illness, but disproportionately to those with severe illness. Patients with severe COVID‐19 who require admission to hospital or to intensive care may have relatively high and possibly sustained respiratory viral shedding [8]. Importantly, patients and hospital staff who are asymptomatic or pre‐symptomatic also shed virus and pose a risk to attending medical staff [2], particularly if transmission‐based precautions are relaxed.
Anaesthetists and intensivists may spend prolonged periods of time indoors and near the airways of infected patients. These are the key characteristics of settings where infection is more likely to be acquired. Many clinical procedures that anaesthetists undertake are aerosol‐generating procedures, creating infectious airborne respiratory aerosols [9]. Tracheal intubation is an aerosol‐generating procedure and despite the debate over the mechanism, there is robust evidence of an association between tracheal intubation and transmission of infection during previous outbreaks of severe corona virus disease [10].
Concerns about deaths among healthcare staff, which now number approximately 200, have led to independent reports and concerns about risk, mitigation and avoidability [11, 12].
Relative and absolute risk
It is important to consider both relative and absolute risk. Relative risk describes the extent to which one individual or group is at risk compared with a comparator individual or group. Absolute risk describes a specific numerical risk of an event, for instance as a proportion or fraction.
Relative risk is highly dependent on the reference group chosen. Of note, risk estimates generally relate to populations and do not describe individual risk. A risk estimate may explain to an individual the degree to which those with similar characteristics are likely to be affected as a group, but it does not predict individual outcome. An individual’s outcome – be it infection, hospital admission, intensive care unit admission, death or survival – will always be dichotomous, that is, for the individual, they 100% do or 100% do not happen.
In this pandemic, risk is dynamic and this can dramatically affect absolute risk without altering relative risk. A rebound or second surge in SARS‐CoV‐2 community transmission may have a minor impact on absolute risk for some individuals and a far greater risk increase for individuals at higher relative risk (Box 1). Conversely, widespread use of dexamethasone for severe COVID‐19 or the availability of an effective vaccine will have the greatest benefit for those with the highest pre‐existing relative risk. [13]
Box 1. Relative and absolute risk.
Consider two anaesthetists, Anaesthetist A has a 1% risk of critical illness if she is infected with SARS‐CoV‐2, while Anaesthetist B has a 10% risk (10‐fold higher relative risk) if he is. In a low risk phase after the epidemic surge, the risk of infection is 1 in 50 for each anaesthetist. The risk of critical illness for Anaesthetist A is 1 in 5000 (0.02%) and for Anaesthetist B, 1 in 500 (0.2%). When a local surge in community infection happens, there is more activity and a shortage of PPE. The risk of infection rises, therefore, to 1 in 5. The risk of critical illness for Anaesthetist A is now 1 in 500 (0.2%) and for Anaesthetist B is 1 in 50 (2%). Their relative risk has remained unchanged but the increase in absolute risk is 10‐fold higher for Anaesthetist B than for Anaesthetist A, 1.8% vs. 0.18%.
Deaths from COVID‐19
During a surge, anaesthetists and intensivists predominantly care for patients who are admitted to hospital with severe COVID‐19 and who become critically ill or require intensive care admission. This is a select group in terms of age, comorbidity and risk. It is therefore useful to add some context.
By the end of May 2020, approximately 7% of the UK population had evidence of a previous SARS‐CoV‐2 infection [14]. If infected with COVID‐19, the risk of requiring hospital admission is unknown, but may be as high as 15%. Among hospitalised patients, approximately 17% required intensive care admission [15]. Mortality for those admitted to intensive care is approximately 41% [16, 17].
On 5 June 2020, there had been 46,104 deaths from COVID‐19 registered in England and Wales [18]. Of these deaths, 67% had occurred in hospital and 31% in care homes [19]. At the same time, the Intensive Care National Audit and Research Centre (ICNARC) reported 3615 deaths in intensive care patients, suggesting that only approximately 10% of all COVID‐19 deaths occur in intensive care (Fig. 1) [20]. More than 90% of COVID‐19 deaths occur in those aged > 65 y and almost half in those aged > 85 y [21]. The characteristics of patients dying in hospital but outside intensive care, and outside hospital, differ significantly from those dying in intensive care, where the median age of those dying is 61 y [15].
Figure 1.
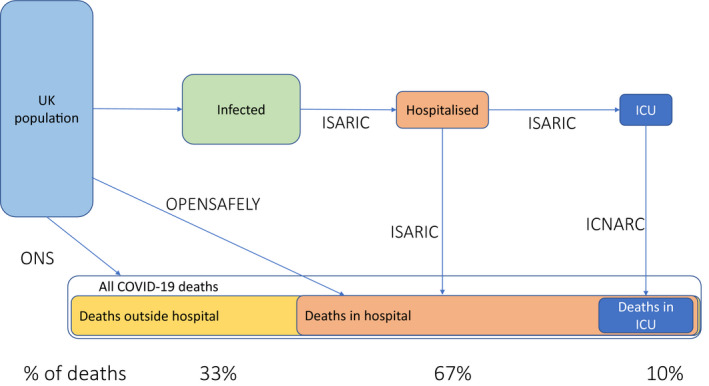
Relationship between COVID‐19 disease progression, locations of deaths in the UK and data sources. ISARIC, International Severe Acute Respiratory and Emerging Infection Consortium [15]; ICNARC, The Intensive Care National Audit and Research Centre [16]; ONS, Office for National statistics [18], OpenSAFELY [23]. [Colour figure can be viewed at wileyonlinelibrary.com]
Risk from SARS‐CoV‐2 infection
Risk can be broken down into four types: risk of infection with SARS‐CoV‐2 due to environmental factors; risk of infection with SARS‐CoV‐2 due to individual factors; risk of hospitalisation or critical illness from COVID‐19; or risk of death from critical illness due to COVID‐19. Together, these create an ‘overall risk of death’ from COVID‐19. Increasingly useful information has become available to understand risk associated with COVID‐19. The Office for National Statistics and Public Health England provide regular updates on the risk of infection and crude data about deaths.
Numerous observational studies have reported univariate associations with outcomes. As many risk factors are linked, these analyses may be misleading. Multivariate analyses are of considerably more value, as they are more likely to exclude confounding covariates and more accurately delineate the relative importance of individual variables.
The International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) was established in 2011 to undertake patient‐based research in the event of epidemic infections. Their study of 20,000 patients admitted to UK hospitals with COVID‐19 provides information about progression to hospital and intensive care admission, and a multivariate analysis explores factors associated with mortality in hospitalised patients [15]. Additionally, ICNARC provides a well‐established audit process for UK adult intensive care units and produces weekly COVID‐19‐related reports that, from June 2020, include multivariate analysis of the impact of pre‐existing personal characteristics on risk of death in patients admitted to intensive care [16]. A limitation of the ICNARC dataset is that past medical history data are limited and include only very severe comorbidities.
General practice has detailed information on most of the UK population. Linking these data with datasets on hospital admission, intensive care admission or death enables examination of risk factors for these events. The two predominant clinical computer systems in general practice in England cover approximately 90% of the English population [22]. These are SystemOne (approximately 24 million patients) and Egton Medical Information Systems EMIS (approximately 35 million patients). The OpenSAFELY study [23] linked more than 17 million anonymised SystemOne patient records with records of death in hospital from COVID‐19 reported to the COVID‐19 Patient Notification System [24]. Multivariate analysis explores factors in the general population associated with in‐hospital mortality. In June 2020, the Chief Medical Officer for England commissioned development of a new risk prediction model for COVID‐19, supported by NHS Digital using data from EMIS’s QResearch database [25]. This has the potential to identify risk for outcomes beyond mortality (Table 1 and Fig. 1).
Table 1.
Main data sources describing populations and related death rates from within UK
| Geographic breadth | Population studied | Patients studied | Proportion of target population studied | Number of deaths | Location of deaths | Last date of data inclusion | Mortality rate a | |
|---|---|---|---|---|---|---|---|---|
| Office for National Statistics [18] | England and Wales | All population | Approximately 59 million | 100% of the population | 48,218 | All locations | 12/06/2020 | 0.08% |
| OpenSAFELY [23] | England | General practice patients | 17,425,445 | Approximately 31% of English population | 5683 | Hospital | 25/04/2020 | 0.03% |
| ISARIC [15] | England, Wales and Scotland | Patients admitted to hospital | 20,133 hospital admissions with 3001 intensive care admissions | 34% of hospital admissions | 5165 | Hospital | 19/04/2020 | 33% |
| ICNARC [16] | England, Wales and Northern Ireland | Patients in intensive care | 9505 intensive care admissions | 74% of intensive care admissions | 3883 | Intensive care | 26/06/2020 | 41% |
ONS, Office for National Statistics;
Mortality of those with completed outcome. Many patients remain in hospital or ICU at the time of reporting.
In interpreting these data, it is important to note that these databases include patients of all ages and from all walks of life, largely collected during the epidemic surge. They may overestimate risk if data were captured when healthcare systems were not working well, and may underestimate risk because high‐risk patients were shielding. They are unlikely to reflect the underlying characteristics of healthcare workers who will on average be younger and fitter [26]. Doctors have among the lowest rates of mortality of any occupational group in the UK [27]. When relative risk is reported, the reference point is important, for example, in the OpenSAFELY study [23], the reference characteristics are: White ethnicity; female sex; aged 50‐59 years; non‐obese; non‐smoker; living in a non‐deprived location; and without any medical conditions.
Environmental factors and mitigation
Infection is likely affected more by exposure, behaviour and mitigation than individual predisposing factors. Infection transmission is related to the duration and proximity of exposure to infected patients. Risk of transmission is estimated to be 19‐fold higher indoors than outdoors (Nishiura et al., unpublished data https://www.medrxiv.org/content/10.1101/2020.02.28.20029272v2) and exposure in a household is likely the highest risk [28]. Shared transport (e.g. public transport) is considered high risk. Working at home reduces the risk of infection by two‐ to three‐fold compared with working outside the home [14].
Hospitals are high risk for the reasons described above. The likelihood of exposure to infected patients depends on community transmission rates, admission policies, ability to segregate infected from non‐infected patients and work patterns. Risk is increased during periods of high or rising community transmission (reproduction number R0> 1). Staff–staff infection may be an important risk.
In China in January 2020, healthcare workers accounted for more than a quarter of COVID‐19 patients [29], but this fell to < 5% with the implementation of strict infection control practices and mandating PPE when managing COVID‐19 patients [30]. Healthcare workers accounted for approximately 10% of all cases in Italy [31] and 20% in Spain [32].
In June 2020, the UK Office for National Statistics reported for several weeks that through April and May, infection rates among frontline hospital staff in England were four‐ to six‐fold higher than those not employed in these roles [33]. In early June 2020, approximately 1 in 50 hospital staff in patient‐facing roles were infected compared with fewer than 1 in 300 not working in these roles and fewer than 1 in 1000 in the community [33]. Approximately, 7% of the population of England have antibodies to SARS‐CoV‐2 [33], with the proportion higher in regions that experienced high surge levels, for example, approximately 15% in London [34].
Several surveillance studies have explored rates of infection (antigen positive nose and throat swabs) and seropositivity (antibody positive blood test) in UK hospitals. In the SAFER study conducted in London at the peak of the first surge, 21% of frontline healthcare staff were infected and 45% were seropositive [35]. In another London study, just after the first surge peak, 7% of staff were infected and 21% were seropositive [36]. In a Cambridge hospital 4.7% of all staff and 3% of asymptomatic staff were infected [37]. An Oxford study of more than 1000 hospital staff reported an 11% rate of infection, and identified risks of infection from working with COVID‐19 infected patients (OR 2.5), household contact with an infected person (OR 4.6) and working in an acute medical specialty (OR 1.5) but again, those working in intensive care had a low risk (OR 0.46) (Eyre et al., unpublished data https://www.medrxiv.org/content/10.1101/2020.06.24.20135038v2.full.pdf). Junior and non‐White staff had higher risk than consultants and White individuals, respectively.
Among healthcare workers, it is not clear whether anaesthetists and intensivists are at increased (or even decreased) risk of infection. Chinese data indicate low rates of infection in staff undertaking tracheal intubation of the critically ill [38]. In the SAFER study, staff working in intensive care had the lowest rates of SARS‐CoV‐2 infection and seropositivity [35]. Conversely, the intubateCOVID study reported that 10.7% of staff involved in tracheal intubation either tested positive for SARS‐CoV‐2 antigen (3.1%) or developed symptoms consistent with COVID‐19 (8.4%) or were hospitalised with such (0.1%) [39].
One plausible explanation for lower risk in anaesthetists and intensivists is familiarity with infection control precautions and excellent preparedness. Anaesthetists and intensivists are used to working in locations where high‐level infection precautions and the wearing of protective clothing, whether preventing infection transmission to or from patients, are common. Anaesthetists and intensivists have also been aware of the risk their environment and role pose and detailed specialty‐specific guidance [40] has supplemented national advice. It is also possible that anaesthetists and intensivists encounter patients later during their illness and that, despite worsening illness severity, infectiousness may have waned [3].
Individual factors
The risk of infection with SARS‐CoV‐2 varies little with age or sex [14]. There are limited data on which medical conditions increase risk of acquiring COVID‐19: a study of antigen testing in general practice reported positive tests were associated with: older age; male sex; non‐White ethnicity; social deprivation; obesity; and chronic kidney disease [41]. There is a likely significant bias in these results due to variations in who attends for testing.
Risk of severe illness
Jankowski et al. (Jankowski et al., unpublished data https://www.medrxiv.org/content/10.1101/2020.05.05.20091967v3) used data from ISARIC (Docherty et al, unpublished data https://www.medrxiv.org/content/10.1101/2020.04.23.20076042v1) to correlate underlying comorbidities with the risk of requiring hospitalisation (though they describe this as ‘risk of infection’). Conditions associated with increased risk of hospitalisation include: increasing age; male sex; chronic respiratory and cardiac disease; and conditions impacting immune function, including cancers (Table 2).
Table 2.
Health related conditions increasing risk of hospitalisation with COVID‐19. Data from Jankowski et al. (Jankowski et al., unpublished data https://www.medrxiv.org/content/10.1101/2020.05.05.20091967v3)
| Condition | Prevalence in UK population | Prevalence in patients hospitalised with COVID‐19 | Relative risk for hospitalisation with COVID‐19 |
|---|---|---|---|
| Chronic cardiac disease | 14% | 29% | 2.07 |
| Uncomplicated diabetes | 4.8 | 19% | 3.95 |
| Chronic pulmonary disease excluding asthma | 4.5% | 16% | 3.56 |
| Asthma | 8.3 | 14% | 2.15 |
| Malignant neoplasm | 1.5% | 9% | 6.0 |
| Rheumatological disorder | 0.8% | 9% | 11.25 |
| Obesity (BMI > 35 kg.m‐2) | 27.8% | 38.5% | 1.38 |
| Diabetes (with complications) | 1.2% | 6% | 5.0 |
There is no compelling evidence that healthcare work increases the severity of COVID‐19, but there is a paucity of data. In a May 2020 report of Spanish healthcare workers infected with SARS‐CoV‐2, 11% were hospitalised and 1.2% were admitted to intensive care [32]. Healthcare workers without comorbidities accounted for half of those hospitalised, a third admitted to intensive care and close to a quarter of those who died. The frequencies with which UK healthcare workers are admitted to hospital or intensive care with COVID‐19 are not reported, though these data may soon be available from the ISARIC group.
Risk‐factors for death
Pre‐existing patient factors independently associated with increased risk of death in hospitalised patients [15] are shown in Table 3, those associated with death in patients admitted to intensive care [16] in Figure 2 and those associated with death in the general population [23] in Table 4.
Table 3.
Risk of fatality due to COVID‐19 among hospitalised patients as reported by ISARIC [15]. Definitions are available at https://isaric.tghn.org/COVID‐19‐CRF/.
| Risk factor | Relative risk (95%CI) of mortality |
|---|---|
| Age; years | |
| < 50 | 1 |
| 50–59 | 2.63 (2.06–3.35) |
| 60–69 | 4.99 (3.99–6.25) |
| Male sex | 1.23 (1.16–1.33) |
| Chronic cardiac disease | 1.16 (1.08–1.24) |
| Chronic pulmonary disease excluding asthma | 1.17 (1.09–1.27) |
| Malignant neoplasm | 1.13 (1.02–1.24) |
| Obesity (BMI > 35 kg.m‐2) | 1.33 (1.19–1.49) |
| Chronic kidney disease | 1.28 (1.18–1.39) |
| Diabetes | 1.06 (0.99–1.14) |
| Moderate/severe liver disease | 1.51 (1.21–1.88) |
| Chronic neurological disease | 1.17 (1.06–1.29) |
Figure 2.
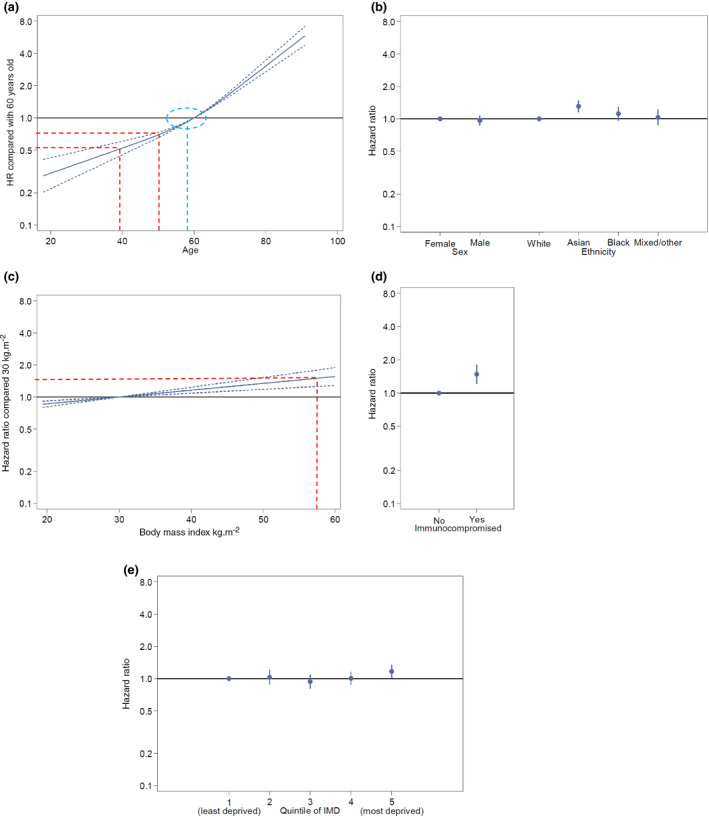
Figure from the Intensive Care National Audit and Research Centre (ICNARC) [16] showing hazard ratios for death after admission to intensive care. (a) age modified to indicate an approximately 50% increase in risk for an increase in age of 10 years (red lines) and the inflection point at just below 60 years age (blue circle). (b) Sex and ethnicity. (c) Body mass index modified to show approximately 50% risk increase at above 55 kg.m‐2 (red lines). (d) Severe immunocompromise. (e) Quintiles of social deprivation. © ICNARC 2020. These data derive from the ICNARC report on COVID‐19 in critical care (26 June 2020) in the Case Mix Programme Database ICNARC, London, UK. The Case Mix Programme is the national clinical audit of patient outcomes from adult critical care co‐ordinated by the Intensive Care National Audit and Research Centre (ICNARC). For more information on the representativeness and quality of these data, please contact ICNARC. [Colour figure can be viewed at wileyonlinelibrary.com]
Table 4.
Risk of fatality due to COVID‐19 amongst the UK population as reported in the OpenSAFELY study [23].
| Factor | Hazard ratio (95% CI) for death |
|---|---|
| Age; years | |
| < 40 | 0.06 (0.04‐0.08) |
| 40–49 | 0.3 (0.25‐0.36) |
| 50–59 | 1.0 |
| 60–69 | 2.40 (2.16‐2.66) |
| Male sex | 1.99 (1.88–2.10) |
| Ethnicity | |
| White | 1.0 |
| Mixed | 1.64 (1.19–2.26) |
| Asian or Asian British | 1.62 (1.43–1.82) |
| Black | 1.71 (1.44–2.02) |
| Other | 1.33 (1.03–1.73) |
| Obesity | |
| Not obese | 1.0 |
| Obese class 1 (BMI 30–34.9 kg.m‐2) | 1.27 (1.18–1.36) |
| Obese class 2 (BMI 35–39.9 kg.m‐2) | 1.56 (1.41–1.73) |
| Obese class 3 (BMI ≥ 40 kg.m‐2) | 2.27 (1.99–2.58) |
| Blood pressure | |
| Normal | 1.0 |
| Diagnosed hypertension | 1.07 (1.00–1.15) |
| Respiratory disease | |
| Non‐asthma respiratory disease | 1.78 (1.67–1.90) |
| Asthma: no recent oral steroid use | 1.11 (1.02–1.20) |
| Asthma: recent oral steroid use | 1.25 (1.08–1.44) |
| Chronic heart disease | 1.27 (1.20–1.35) |
| Diabetes | |
| Controlled (HbA1c < 58 mmol.mol‐1) | 1.50 (1.40–1.60) |
| Uncontrolled (HbA1c ≥ 58 mmol.mol‐1) | 2.36 (2.18–2.56) |
| No recent HbA1c measure | 1.87 (1.63–2.16) |
| Non‐haematological cancer | |
| Diagnosed < 1 y ago | 1.56 (1.29–1.89) |
| Diagnosed 1–4.9 y ago | 1.19 (1.04–1.35) |
| Diagnosed ≥ 5 y ago | 0.97 (0.88–1.06) |
| Haematological cancer | |
| Diagnosed < 1 y ago | 3.52 (2.41–5.14) |
| Diagnosed 1–4.9 y ago | 3.12 (2.50–3.89) |
| Diagnosed ≥ 5 y ago | 1.88 (1.55–2.29) |
| Liver disease | 1.61 (1.33–1.95) |
| Stroke or dementia | 1.79 (1.67–1.93) |
| Other neurological disorder | 2.46 (2.19–2.76) |
| Kidney disease | 1.72 (1.62–1.83) |
| Organ transplant | 4.27 (3.20–5.70) |
| Spleen diseases | 1.41 (0.93–2.12) |
| Rheumatoid or lupus or psoriatic disease | 1.23 (1.12–1.35) |
| Other immunosuppressive condition | 1.69 (1.21–2.34) |
| Social deprivation | |
| 1 (least deprived) | 1.0 |
| 2 | 1.13 (1.04–1.24) |
| 3 | 1.23 (1.13–1.35) |
| 4 | 1.49 (1.37–1.63) |
| 5 (most deprived) | 1.75 (1.65–1.91) |
| Smoking status | |
| Never | 1.00 |
| Ex‐smoker | 1.25 (1.18–1.33) |
| Current | 0.89 (0.79–0.99) |
Age
Increasing age is the single most important risk factor, and risk of death from COVID‐19 increases logarithmically from the age of approximately 10 years [42], with risk reported to rise 9.5% [23] or 12% [42] for each additional year of age. A 12% rise in risk for each year of age means doubling mortality risk for every additional 5‐6 years of age and a 10‐fold increase for an individual who is 20 years older. The causes are likely multifactorial. As an isolated risk‐factor, risk of death from COVID‐19 increases three‐fold from age 40–49 to age 50–59, and six‐fold to age 60‐69y [23].
Sex
More men are admitted to hospital with COVID‐19 than women (Table 2). Increased mortality for men is less marked in the hospitalised population [15] than in the general population [23] (Table 3 and 4), and for men and women with equivalent disease severity there is little difference in intensive care mortality (Fig. 2) [16].
Ethnicity
There is clear evidence of an excess mortality in the non‐White UK population as highlighted in reports from the Institute for Fiscal Studies, the Centre for Evidence Based Medicine and Public Health England [12, 43, 44]. The causes of this are likely multifactorial. Social geography factors may include: increases in urban dwelling; social deprivation; overcrowded and multi‐generational housing; occupation in key worker and public‐ and patient‐facing roles; and reliance on public transport. Increased prevalence of diabetes, cardiac disease, hypertension, chronic kidney disease and obesity likely contributes. Genetic predisposition and relative insufficiency of vitamin D are also proposed, but unconfirmed, causes.
Unsurprisingly, risk is not equal among different non‐White ethnicities. An approximately 50% excess of non‐White, particularly Black patients in intensive care was an early signal of the greater impact of COVID‐19 on these populations [45]. The Institute for Fiscal studies report indicated that Black and Pakistani populations’ mortality was increased 3.7‐ and 2.9‐fold, respectively, compared with the White population [43]. An ISARIC study (Harrison et al, unpublished data https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3618215) of more than > 30,000 UK patients hospitalised with COVID‐19 up until 8 May 2020 reported that patients from all non‐White ethnicities were younger than White patients and were less likely to have most comorbidities, with the exception of diabetes which had an increased prevalence. Patients from all non‐White ethnicities were more likely to require admission to intensive care than White patients (21–24% vs. 13%, OR 1.3) and to need invasive mechanical ventilation. Increased risk persisted when adjusted for baseline characteristics and comorbidities. Mortality was increased for patients of South Asian ethnicity (OR 1.2), but not among other non‐White ethnicities, with diabetes a contributory but not fully explanatory factor. The OpenSAFELY study [23] reported an approximately 60–70% increase in mortality across the UK population in people of all non‐White ethnicities.
Healthcare workers
Concerns about deaths among front‐line healthcare staff have featured in mainstream and social media. Approximately 200 healthcare staff have died in the UK with COVID‐19. Reports described deaths of first 119 [46] and then 203 UK health and social care workers with COVID‐19, the latter including 166 healthcare workers [11]. A related report indicated an increased risk of death in younger female healthcare workers [47]. Although deaths among healthcare workers are reportable to the NHS, robust data on this has yet to be published. Office for National Statistics data have at times noted no increase in risk [48] and at others, a small increase. In the latest report, published in late June and relating to data from May, male healthcare workers (but not female healthcare workers) in England and Wales had a statistically significant and approximately 30% higher rate of death involving COVID‐19 than an age‐ and sex‐matched comparator population [49]. Nurses and nursing assistants or auxiliaries were notably affected. Of note, mortality in social care workers of both sexes was approximately doubled [49].
The above studies show clear evidence that among the UK healthcare workers who have died, including doctors, a disproportionate number have been of non‐White ethnicities [11, 46]. Among 166 UK healthcare workers who died with COVID‐19, 64% were non‐White, compared with 21% of the NHS workforce and 13% of the wider population [11]. Ninety‐four percent of doctors who died were non‐White [11]. Conversely, these healthcare worker deaths were notable for an absence of anaesthetists and intensivists [11, 46], despite anaesthetists alone accounting for 12% of hospital staff [50]. Non‐medical theatre and intensive care staff were also under‐represented, but the death of a male intensive care nurse is a salutary reminder that no one is exempt from risk [11]. A broader review of deaths among healthcare workers, focusing on anaesthetists and intensivists, also suggested low rates of mortality in other countries [51].
Comorbidity
Almost all COVID‐19 studies report increased risk in patients with comorbidities. In the ISARIC study, 77.5% of hospitalised patients and 89% who died had major comorbidities [15]. While several health conditions have been examined in detail, even for some common conditions, risk analyses are uncertain or conflicting. For uncommon or rare conditions, data are sparse and risk estimates prone to marked uncertainty or fluctuating estimates of risk. The impact of deconditioning or frailty from chronic health conditions has not been widely presented. For some comorbidities, the interrelation between other factors such as age and ethnicity has not been disentangled and increased risks may be due to interaction between these factors.
Diabetes mellitus
Diabetes is an important risk‐factor for harm from COVID‐19 and may be particularly important in some non‐White ethnic populations. Risk may be increased both by direct effects of the disease and by its complications. Jankowski et al. (Jankowski et al., unpublished data https://www.medrxiv.org/content/10.1101/2020.05.05.20091967v3) reported increased risk of hospitalisation in patients with diabetes with or without complications (Table 2) while the OpenSAFELY study [23] identified a higher risk of death in poorly controlled diabetes (HbA1c ≥ 58mmol/L) compared with controlled diabetes (Table 4).
Chronic heart disease
Chronic heart disease is limited by inconsistency of definition. Jankowski et al. (Jankowski et al., unpublished data https://www.medrxiv.org/content/10.1101/2020.05.05.20091967v3) reported that chronic heart disease doubles the risk of hospital admission with COVID‐19. It is consistently reported as a risk‐factor for severe disease and poor outcomes in large observational studies. This is notable because of the significant element of vascular dysfunction, including microvascular and major thrombosis, associated with severe COVID‐19. The ICNARC dataset provides no information on cardiac disease as a risk‐factor, but only captures very severe cardiac disease in past medical history. The ISARIC [15] and OpenSAFELY [23] studies are divergent, reporting a 2.7‐ and 1.3‐fold increase in risk, respectively (Tables 3 and 4).
Chronic respiratory disease
Chronic respiratory disease also suffers from lack of a precise definition. In large observational studies, chronic respiratory disease appears to be less prominently associated with severe illness or poor outcomes than cardiac and cardiovascular diseases. Based on analyses by Jankowski et al. (Jankowski et al., unpublished data https://www.medrxiv.org/content/10.1101/2020.05.05.20091967v3), ISARIC [15] and ICNARC [16], it appears both asthma and chronic respiratory disease increase risk of hospitalisation but further impact on mortality appears less marked (Tables 2, 3, 4). The impact of non‐asthmatic chronic pulmonary disease is greater than asthma. Among those with asthma in the OpenSAFELY study [23], only those with recent use of oral corticosteroids were reported to have significantly increased risk of mortality.
Obesity
Mild obesity appears to have little impact on risk from COVID‐19. Increasing risk as body mass index rises significantly may be difficult to separate from the risk of concurrent conditions (e.g. hypertension, diabetes and asthma) but multivariate analyses suggest severe obesity as an independent risk‐factor. Jankowski et al. (Jankowski et al., unpublished data https://www.medrxiv.org/content/10.1101/2020.05.05.20091967v3) reported a modest increase in risk of hospitalisation in patients with a BMI > 35 kg.m‐2 and ISARIC [15] reported an increase in mortality in hospitalised patients (Table 2 and 3). The ICNARC report indicates intensive care mortality risk rising linearly with increasing obesity, reaching a 50% increase at a BMI of approximately 55 kg.m‐2 (Fig. 2) [16]. The OpenSAFELY study [23] reported progressively increasing mortality risk in all classes of obesity (Table 4).
Immunosuppression and rheumatological conditions
Interpretation of risk here is complex and uncertain. Immunosuppression is likely variously defined. A review from the global rheumatology alliance used multivariate analysis to explore factors associated with hospitalisation due to COVID‐19 in 600 patients with rheumatological conditions [52]. Higher doses of prednisolone (≥ 10 mg.day‐1) were associated with increased likelihood of admission but use of non‐steroidal or disease modifying drugs and biological agents were not. Jankowski et al. (Jankowski et al., unpublished data https://www.medrxiv.org/content/10.1101/2020.05.05.20091967v3) reported a dramatic 11‐fold increase in risk in hospitalisation of patients with rheumatological conditions, but the population prevalence used for this estimate appears low (Table 2). Additionally, ICNARC reports a 50% increased mortality in severely immunocompromised patients in intensive care (Fig. 2) [16]. The OpenSAFELY study [23] reported only a small increase in mortality risk in patients with rheumatological conditions and a moderate increase in other immunosuppressive conditions (Table 4).
Malignancy
Current and recent malignancy (diagnosed up to a year ago) is a risk‐factor for death from COVID‐19, particularly haematological malignancy, and this risk persists beyond 5 years after diagnosis (Tables 3 and 4).
Renal disease
Chronic renal disease, if severe, impacts outcome from severe COVID‐19 because it increases the risk of requiring renal replacement therapy, which is needed in up to a quarter of patients on intensive care, and is associated with increased mortality [16]. The OpenSAFELY study [23] reported a 70% increase in mortality associated with kidney disease, defined as eGFR < 60 mL.min‐1.m‐2 (Grades 3 and above).
Hypertension
Numerous observational studies of hospitalised patients with COVID‐19 report notable associations between hypertension and poor outcome [53]. The LOW‐HARM risk prediction tool (Soto‐Mota et al, unpublished data https://www.medrxiv.org/content/10.1101/2020.05.26.20111120v2) reported hypertension was associated with a doubling of mortality in hospitalised patients. The OpenSAFELY study [23] reported only a modest impact of diagnosed hypertension on overall mortality. The role of pre‐existing hypertension on progression from moderate to severe disease likely requires more exploration.
Mental health disorders
There is a notable lack of information on mental health conditions as a risk‐factor for COVID‐19 severity. Pre‐existing mental health conditions and the impact of managing patients with COVID‐19 on the mental health of those with or without pre‐exiting mental health conditions are important, but are not considered here further.
Other diseases
Other health conditions that impact outcomes from COVID‐19 are listed in Tables 3 and 4. However, there is no doubt that this list is not exhaustive. Particularly for uncommon and atypical conditions, clinical judgement will be required to estimate risk.
Pregnancy
There is no clear, robust evidence of an increased risk attributable to pregnancy. A Royal College of Obstetricians and Gynaecologists review states there is no increased risk of infection and no increased risk of severe illness [54]. A study from the UK obstetric surveillance system (UKOSS) reported on 427 pregnant women admitted to UK hospitals with confirmed SARS‐CoV‐2 infection between 1 March and 14 April 2020 [55]. Admission rate was 4.9 per 1000 maternities (95%CI 4.5–5.4). Eighty‐one percent of admissions were in the third trimester and half after 34 weeks gestation. Risk‐factors were: non‐White ethnicity (OR 4.5); maternal age > 35 years (OR 1.35); BMI > 30 kg.m‐2 (OR 2.2); and comorbidity (OR 1.52), of which chronic hypertension was most prominent. Nine percent of hospitalised women required intensive care admission and four (approximately 1%) required extracorporeal membrane oxygenation. Five (1.2%) died, though causation was not proven. The authors estimated a mortality of 5.6 (95%CI 1.8–13.1) per 100,000 maternities (though at the time of reporting, nine women remained in intensive care), compared with the UK all‐cause maternal mortality rate in 2015–2017 of 9.2 per 100,000. The UK guidance includes pregnancy with significant cardiac disease as an indication for shielding [56], and many take the pragmatic view that women in the third trimester of pregnancy should be actively protected from SARS‐CoV‐2 exposure.
Risk calculators for healthcare workers
Smith and Spiegelhalter have argued that there is a need to “communicate realistic levels of risk as they apply to different groups, not to reassure or frighten but to allow informed personal decisions in a setting of necessary uncertainty” [57]. This is particularly important for healthcare workers as shielding is paused. Several authors have created risk calculators directed at ‘workers’ or ‘healthcare workers’ that calculate risk of harm dependent on pre‐existing personal characteristics. The ISARIC study links to an interactive risk tool based on its findings (at https://isaric4c.net/risk), enabling estimation of mortality after hospital admission. Jankowski et al. (Jankowski et al., unpublished data https://www.medrxiv.org/content/10.1101/2020.05.05.20091967v3) used population disease prevalence data and risk ratios from ISARIC (Docherty et al., unpublished data https://www.medrxiv.org/content/10.1101/2020.04.23.20076042v1) to estimate risk of death compared with a healthy woman aged 50 and validated this using the OpenSAFELY methodology [23]. Measures of relative risk were then adjusted and combined into low, middle and high risk categories. Coggon et al. (Coggon et al., unpublished data https://www.medrxiv.org/content/10.1101/2020.05.21.20108969v1) used data from the OpenSAFELY study and, as previously suggested [58], converted increased relative risk into the equivalent of increased age to create the ‘COVID‐age’ tool. This enables the user to understand risk in terms of how their personal status ‘ages’ them in terms of risk of death from COVID‐19. In the version of this tool used by the Association of Local Authority Medical Advisors [59], COVID‐age is categorised into low, moderate, high and very high vulnerability, and workplace considerations are stated on this basis. National Health Service Employers [60] use a tool developed by the Faculty of Occupational Heath (Kunti et al., unpublished data http://www.fom.ac.uk/wp‐content/uploads/Risk‐Reduction‐Framework‐for‐NHS‐staff‐at‐risk‐of‐COVID‐19‐infection‐12‐05‐20.pdf) which describes the need for a wider view of workplace and workforce risk, but only refers to personal risk in descriptive terms and does not use the available information regarding quantitative increases in risk. The Welsh COVID‐19 workforce risk assessment tool uses broad categorisation of personal risk to create a score, which is allocated as low, high or very high risk and allocates graded actions based on risk categories [61].
There are limitations to all these tools. First, most only use personal (mostly biological) factors to assess risk. Although several state they are directed at healthcare professionals, they make little or no explicit assessment of the interaction between personal and work‐related environmental risk or mitigation of the latter. Without considering environmental risk, the tools are not work‐focused, but simply population risk tools. In their effort to simplify use, several tools group risk scores together to create categories of risk. This creates several problems. Risk is a continuous variable and categorisation is artificial, creating problems for those who are just below or just above a cut‐off. Categorisation also lends itself to definitive allocation of specific actions to specific categories, as seen in several tools. Failure to consider workplace and role‐related risks and categorisation mean that the tools are rigid and do not adapt to the dynamic nature of risk, which occurs with variations in prevalence and mitigation. This is particularly important because, as described above, increasing environmental risk disproportionately increases risk for individuals with the highest relative risks. A risk tool should arguably therefore avoid arbitrary categorisation and should include personal, environmental and mitigating factors to enable dynamic ‘point‐of‐time’ risk assessments (Fig. 3). Because of the limitations of the underlying data on which COVID‐19 risk tools are based, it is likely that they are best used as a guide to risk and to inform discussions, rather than determine outcomes. In Appendix 1, a risk aid is presented for this purpose which aims to acknowledge the continuous nature of personal risk and the dynamic impact of environmental risk.
Figure 3.
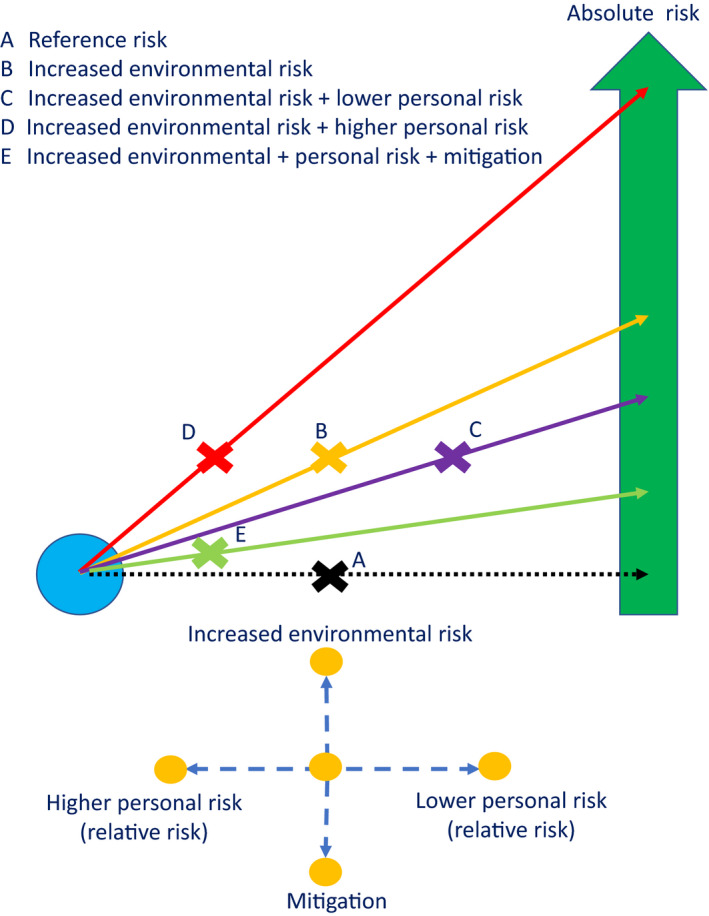
The dynamic interaction of environmental and personal risk. Baseline risk in represented by the yellow cross and the black dotted line. As environmental risk is increased, the cross moves upward and mitigation moves it down. Increased or decreased personal risk moves the cross left or right, respectively. Overall risk is shown on the green arrow. [Colour figure can be viewed at wileyonlinelibrary.com]
Conclusions
Healthcare workers are at increased risk of infection, harm and death from COVID‐19. Close and prolonged exposure, to individuals infectious with SARS‐CoV‐2, promotes infection. A person’s individual characteristics influence the subsequent risk of clinically important COVID‐19, the necessity for hospital or intensive care admission and death. While relative risk is often calculated, absolute risk is more dynamic and more important. Individual risk interacts with environmental risk and mitigation factors to determine overall risk. Hospitals create a unique environment of increased risk for staff. Anaesthetists and intensivists are likely to be at particularly high risk of exposure but available evidence suggests that mitigation has been effective for these individuals. A tool to guide individual risk assessment and to inform discussions consequent on that assessment should include personal factors, environmental factors and mitigation measures to enable a dynamic ‘point‐of‐time’ risk assessment.
Acknowledgements
No external funding or competing interests declared.
Appendix 1. Risk assessment tool
Most risk toolkits consider only personal risk. Overall risk is an interaction between personal risk, environmental risk and mitigation measures and this aid attempts to capture all three factors.
Data from several studies enables estimation of relative risk of death from COVID‐19 in a population with certain characteristics, compared to a reference population. The calculated relative risk applies to a population of people with the same characteristics but does not predict individual outcome.
Relative risk remains unchanged as the risk in the environment increases (i.e. in settings when community or in‐hospital risk of infection increases) but the absolute risk increases. The absolute risk increases for those at increased relative risk than for those without it (see Box 1). Mitigation reduces risk.
It should be emphasised that this tool is not validated to correlate with increasing risk. It is designed to provide a structure for evaluating estimated risk which enables discussion between the individual and relevant colleagues. No arbitrary cut off is suggested for any actions as such decisions are nuanced.
Step 1. Individual personal relative risk ratio
Complete the table using risk scores from Table 4. If necessary, Figure 2a–e or Table 3 may also be used.
| Relative risk/Hazard ratio | |
|---|---|
| Score for your age | |
| Score for your sex | |
| Score for your ethnicity | |
| Score for medical conditions | |
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| Other factors (e.g. smoking, social deprivation) | |
| Overall personal risk ratio (multiply all numbers in column 2) |
Step 2. Calculate work‐related environmental factors
Environmental factors do not impact on the relative risk an individual has of harms from SARS‐CoV‐2 and COVID‐19 but will amplify changes in absolute risk. As such they can be considered as (unquantified) multipliers of risk for such individuals. Score one point for each environmental risk factor or mitigation failure that is present. Based on current evidence and practices anaesthesia and intensive care are not judged high risk.
| Present | Not present | |
|---|---|---|
| Environment risk | ||
| High prevalence of SARS‐CoV‐2 in local community (as informed by local or national information) | ||
| Rising prevalence of SARS‐CoV‐2 in local community (Ro > 1) | ||
| High prevalence of SARS‐CoV‐2 in hospital (as informed by local or national information) | ||
| Travel to or from work on public transport | ||
| Working in a patient‐facing role caring for patients with COVID‐19 | ||
| Working in high risk specialty or setting* | ||
| Mitigation failures | ||
| Failure of effective segregation of patients by risk of COVID‐19 | ||
| Lack of adherence to social distancing within working environment | ||
| Failure of standard infection control precautions | ||
| Failure of transmission‐based precautions | ||
| Inadequate provision or use of PPE | ||
Anaesthesia and ICU are not judged high risk at present.
Step 3. Final risk assessment
Final relative risk scores.
| Individual personal relative risk ratio | Environmental risk factors and mitigation | |
|---|---|---|
| Scores |
The tool enables consideration of all relevant factors. Interpretation of the final outcome and any actions to be undertaken relies on individual judgement of the extent/severity of each factor. However, raised individual personal risk (e.g. relative risk > 3) combined with multiple environmental risk factors (e.g. ≥ 3) should raise particular concern.
References
- 1. Public Health England . Transmission characteristics and principles of infection prevention and control. https://www.gov.uk/government/publications/wuhan‐novel‐coronavirus‐infection‐prevention‐and‐control/transmission‐characteristics‐and‐principles‐of‐infection‐prevention‐and‐control (accessed 29/06/2020).
- 2. Cook TM, Harrop‐Griffiths W. Kicking on while it’s still kicking off – getting surgery and anaesthesia restarted after COVID‐19. Anaesthesia 2020; 75: 1273–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature 2020; 581: 465–9. [DOI] [PubMed] [Google Scholar]
- 4. Leung NHL, Ch DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nature Medicine 2020; 26: 676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cook TM. Personal protective equipment during the coronavirus disease (COVID) 2019 pandemic ‐ a narrative review. Anaesthesia 2020; 75: 920–7. [DOI] [PubMed] [Google Scholar]
- 6. Public Health England . New government recommendations for England NHS hospital trusts and private hospital providers. https://www.gov.uk/government/publications/wuhan‐novel‐coronavirus‐infection‐prevention‐and‐control/new‐government‐recommendations‐for‐england‐nhs‐hospital‐trusts‐and‐private‐hospital‐providers (accessed 29/06/2020).
- 7. Public Health England . Introduction and organisational preparedness. https://www.gov.uk/government/publications/wuhan‐novel‐coronavirus‐infection‐prevention‐and‐control/introduction‐and‐organisational‐preparedness (accessed 29/06/2020).
- 8. Liu Y, Yan L‐M, Wan L, et al. Viral dynamics in mild and severe cases of COVID‐19. Lancet Infectious diseases 2020; 20: 656–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Public Health England . COVID‐19 personal protective equipment (PPE). https://www.gov.uk/government/publications/wuhan‐novel‐coronavirus‐infection‐prevention‐and‐control/covid‐19‐personal‐protective‐equipment‐ppe#ppe‐guidance‐by‐healthcare‐context (accessed 29/06/2020).
- 10. Tran K, Cimon K, Severn M, Pessoa‐Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012; 7: e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kearney L, Lennane S, Woodman E, Kursumovic E, Cook TM. At least 23 nationalities among NHS staff killed by covid. Health Service Journal. https://www.hsj.co.uk/workforce/at‐least‐23‐nationalities‐among‐nhs‐staff‐killed‐by‐covid/7027666.article (accessed 29/06/2020). [Google Scholar]
- 12. Public Health England . Beyond the data: Understanding the impact of COVID‐19 on BAME groups. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/892376/COVID_stakeholder_engagement_synthesis_beyond_the_data.pdf (accessed 29/06/2020).
- 13. Horby P, Lim WS, Emberson JR et al. Dexamethasone in hospitalized patients with Covid‐19 — preliminary report. New England Journal of Medicine 2020. Epub 17 Jul. 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 14. Office for National Statistics . Coronavirus (COVID‐19) Infection Survey pilot: 12 June 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/12june2020#antibody‐tests‐for‐covid‐19 (accessed 29/06/2020).
- 15. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. British Medical Journal 2020; 369: m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Intensive Care National audit and Research Centre . ICNARC report on COVID‐19 in critical care 26 June 2020. https://www.icnarc.org/Our‐Audit/Audits/Cmp/Reports (accessed 29/06/2020)
- 17. Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID‐19: a systematic review and meta‐analysis of observational studies. Anaesthesia 2020; 75: 1340–49. [DOI] [PubMed] [Google Scholar]
- 18. Office for National Statistics . Deaths registered weekly in England and Wales, provisional: week ending 5 June 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregisteredweeklyinenglandandwalesprovisional/weekending5june2020 (accessed 29/06/2020).
- 19. Office for National Statistics . Deaths registered by place of occurrence: week ending 5 June 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregisteredweeklyinenglandandwalesprovisional/weekending5june2020#deaths‐registered‐by‐place‐of‐occurrence (accessed 29/06/2020).
- 20. Intensive Care National audit and Research Centre . ICNARC report on COVID‐19 in critical care 5 June 2020. https://www.icnarc.org/Our‐Audit/Audits/Cmp/Reports (accessed 5/06/2020).
- 21. Office for National Statistics . Coronavirus (COVID‐19) roundup. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19roundup/2020‐03‐26#:~:text=The%20majority%20of%20deaths%20involving,aged%2085%20years%20and%20over (accessed 29/06/2020).
- 22. Kontopantelis E, Stevens RJ, et al. Spatial distribution of clinical computer systems in primary care in England in 2016 and implications for primary care electronic medical record databases: a cross‐sectional population study. British Medical Journal Open 2018; 8: e020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williamson EJ, Walker AJ, Bhaskaran K et al. OpenSAFELY: factors associated with COVID‐19 death in 17 million patients. Nature 2020; 584: 430–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. NHS England . Patient Notification System (CPNS). Coronavirus (COVID‐19) cases in the UK About the data. https://coronavirus.data.gov.uk/about#england‐covid‐19‐associated‐deaths (accessed 29/06/2020).
- 25. Nuffield Department of Primary Care Health Services . Development of a COVID‐19 risk prediction model. https://www.phc.ox.ac.uk/research/primary‐care‐epidemiology/covid‐19‐risk‐tool (accessed 29/06/2020).
- 26. NHS Digital . NHS Workforce Statistics – March 2020. https://digital.nhs.uk/data‐and‐information/publications/statistical/nhs‐workforce‐statistics/march‐2020 (accessed 29/06/2020).
- 27. Katikireddi SV, Leyland AH, McKee M, Ralston K, Stuckler D. Patterns of mortality by occupation in the UK, 1991–2011: a comparative analysis of linked census and mortality records. Lancet Public Health 2017; 2: e501–e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steensels D, Oris E, Coninx L, et al. Hospital‐Wide SARS‐CoV‐2 Antibody Screening in 3056 Staff in a Tertiary Center in Belgium. JAMA 2020; 324: 195–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Journal of the American Medical Association 2020; 323: 1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) — China, 2020. China CDC Weekly 2020; 2: 113–22. [PMC free article] [PubMed] [Google Scholar]
- 31. Livingston E, Bucher K. Coronavirus Disease 2019 (COVID‐19) in Italy. Journal of the American Medical Association 2020; 323: 1335. [DOI] [PubMed] [Google Scholar]
- 32. Instituto de Salud Carlos III . Informe sobre la situación de COVID‐19 en personal sanitaria en España. Fecha del informe: 07–05‐020 (Situation in healthcare professionals as of May 7, 2020). https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes%20COVID‐19/COVID‐19%20en%20Espa%C3%B1a.%20Situaci%C3%B3n%20en%20Sanitarios%20a%2007%20de%20mayo%20de%202020.pdf (accessed 29/06/2020).
- 33. Office for National Statistics . Coronavirus (COVID‐19) Infection Survey pilot: 5 June 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/5june2020#characteristics‐of‐people‐testing‐positive‐for‐covid‐19 (accessed 29/06/2020).
- 34. Public Health England . Weekly Coronavirus Disease 2019 (COVID‐19) Surveillance Report. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/891721/Weekly_COVID19_Surveillance_Report_‐_week_24.pdf (accessed 29/06/2020).
- 35. Houlihan CF, Vora N, Byrne T et al. Pandemic peak SARS‐CoV‐2 infection and seroconversion rates in London frontline health‐care workers. Lancet 2020; 396: e6–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Treibel TA, Manisty C, Burton M, et al. COVID‐19: PCR screening of asymptomatic healthcare workers at London hospital. Lancet 2020; 395: 1608–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rivett L, Sridhar S, Sparkes D, et al. Screening of healthcare workers for SARS‐CoV‐2 highlights the role of asymptomatic carriage in COVID‐19 transmission. Elife. 2020; 9: e58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yao W, Wang JB, et al. Emergency tracheal Intubation in 202 patients with COVID‐19 in Wuhan, China: lessons learned and expert recommendations. British Journal of Anaesthesia 2020; 125: e28–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. El‐Boghdadly K, Wong DJN, Owen R, et al. Risks to healthcare workers following tracheal intubation of patients with COVID‐19: a prospective international multicentre cohort study. Anaesthesia 2020; 75: 1437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cook TM, Harrop GW. Updated guidance on Personal Protective Equipment (PPE) for clinicians. The Faculty of Intensive Care Medicine, The Intensive Care Society, The Association of Anaesthestists, The Royal College of Anaesthetists. 2020. https://icmanaesthesiacovid‐19.org/personal‐protective‐equipment‐ppe‐for‐clinicians (accessed 29/06/2020).
- 41. De Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS‐CoV‐2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross‐sectional study. Lancet Infectious Diseases 2020; 20: 1034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spiegelhalter D.What have been the fatal risks of Covid, particularly to children and younger adults? Medium. June 2020. https://medium.com/wintoncentre/what‐have‐been‐the‐fatal‐risks‐of‐covid‐particularly‐to‐children‐and‐younger‐adults‐a5cbf7060c49 (accessed 29/06/2020).
- 43. Platt L, Warwick R.Are some ethnic groups more vulnerable to COVID‐19 than others? The Institute for Fiscal Studies. May 2020. ISBN 978‐1‐912805‐75‐4 https://www.ifs.org.uk/inequality/chapter/are‐some‐ethnic‐groups‐more‐vulnerable‐to‐covid‐19‐than‐others/ (accessed 29/06/2020).
- 44. Razaq A, Harrison D, Karunanithi S, et al. BAME COVID‐19 DEATHS – what do we know? Rapid data and evidence review. Centre for Evidence Based Medicine. May 2020. https://www.cebm.net/covid‐19/bame‐covid‐19‐deaths‐what‐do‐we‐know‐rapid‐data‐evidence‐review/ (accessed 29/06/2020).
- 45. Intensive Care National Audit and Research Centre . ICNARC report on COVID‐19 in critical care 17 April 2020. Published 17 April June 2020. https://www.icnarc.org/Our‐Audit/Audits/Cmp/Reports (accessed 17/06/2020)
- 46. Cook TM, Kursumovic E, Lennane S. Deaths of NHS staff from covid‐19 analysed. Health Service Journal. https://www.hsj.co.uk/exclusive‐deaths‐of‐nhs‐staff‐from‐covid‐19‐analysed/7027471.article (accessed 29/06/2020). [Google Scholar]
- 47. Cook TM, Kursumovic E, Lennane S, Kearney L, Woodman E. Younger female NHS workers may face greater risk of death from coronavirus. Health Service Journal. https://www.hsj.co.uk/coronavirus/younger‐female‐nhs‐workers‐may‐face‐greater‐risk‐of‐death‐from‐coronavirus/7027560.article (accessed 29/06/2020). [Google Scholar]
- 48. Coronavirus (COVID‐19) related deaths by occupation, England and Wales: deaths registered up to and including 20 April 2020, Office for National Statistics. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/causesofdeath/bulletins/coronaviruscovid19relateddeathsbyoccupationenglandandwales/deathsregistereduptoandincluding20april2020#men‐and‐coronavirus‐related‐deaths‐by‐occupation (accessed 05/07/2020).
- 49. Office for National Statistics . Coronavirus (COVID‐19) related deaths by occupation, England and Wales: deaths registered between 9 March and 25 May 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/causesofdeath/bulletins/coronaviruscovid19relateddeathsbyoccupationenglandandwales/deathsregisteredbetween9marchand25may2020#deaths‐involving‐covid‐19‐among‐men‐and‐women‐health‐and‐social‐care‐workers (accessed 29/06/2020).
- 50. NHS digital . HCHS staff by HEE region, staff group and nationality, March 2019 AH2747. https://digital.nhs.uk/data‐and‐information/find‐data‐and‐publications/supplementary‐information/2019‐supplementary‐information‐files/hchs‐staff‐by‐hee‐region‐staff‐group‐and‐nationality‐march‐2019‐ah2747 (accessed 29/06/2020).
- 51. Kursumovic E, Lennane S, Cook TM. Deaths in healthcare workers due to COVID‐19: the need for robust data and analysis. Anaesthesia 2020; 75: 989–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gianfrancesco M, Hyrich KL, Al‐Adely S, et al. Characteristics associated with hospitalisation for COVID‐19 in people with rheumatic disease: data from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Annals of Rheumatic Disease 2020; 79: 859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cook TM. The importance of hypertension as a risk factor for severe illness and mortality in COVID‐19. Anaesthesia 2020; 75: 976–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Royal College of Midwives and Royal College of Obstetricians and Gynaecologists . Coronavirus (COVID‐19) Infection in Pregnancy. Version 10.12. Royal College of Obstetricians and Gynaecologists 19 June 2020. https://www.rcog.org.uk/globalassets/documents/guidelines/2020‐06‐18‐coronavirus‐covid‐19‐infection‐in‐pregnancy.pdf (accessed 29/06/2020)
- 55. Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS‐CoV‐2 infection in UK: national population based cohort study. British Medical Journal 2020; 369: m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Public Health England . Guidance on shielding and protecting people who are clinically extremely vulnerable from COVID‐19. https://www.gov.uk/government/publications/guidance‐on‐shielding‐and‐protecting‐extremely‐vulnerable‐persons‐from‐covid‐19/guidance‐on‐shielding‐and‐protecting‐extremely‐vulnerable‐persons‐from‐covid‐19#clinically‐extremely‐vulnerable‐groups (accessed 29/06/2020)
- 57. Smith GD, Spiegelhalter D. Shielding from covid‐19 should be stratified by risk. British Medical Journal 2020; 369: m2063. [DOI] [PubMed] [Google Scholar]
- 58. Spiegelhalter D. How old are you really? Communicating chronic risk through “effective age” of your body and organs. BMC Medical Informatics and Decision Making 2016; 16: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Association of Local Authority Medical Advisors . Covid‐19 Medical Risk Assessment. https://alama.org.uk/covid‐19‐medical‐risk‐assessment/ (accessed 29/06/2020).
- 60. Risk assessments for staff. NHS Employers. 29 May 2020 https://www.nhsemployers.org/covid19/health‐safety‐and‐wellbeing/risk‐assessments‐for‐staff (accessed 29/06/2020).
- 61. Welsh Government . Welsh COVID‐19 workforce risk assessment tool. May 2020. https://gov.wales/covid‐19‐workforce‐risk‐assessment‐tool (accessed 29/06/2020).


