Abstract
Objective
This study aimed to assess the association between adipose tissue distribution and severity of clinical course in patients with severe acute respiratory syndrome coronavirus 2.
Methods
For this retrospective study, 143 hospitalized patients with confirmed coronavirus disease 2019 (COVID‐19) who underwent an unenhanced abdominal computed tomography (CT) scan between January 1, 2020, and March 30, 2020, were included. Univariate and multivariate logistic regression analyses were performed to identify the risk factors associated with the severity of COVID‐19 infection.
Results
There were 45 patients who were identified as critically ill. High visceral to subcutaneous adipose tissue area ratio (called visceral adiposity) (odds ratio: 2.47; 95% CI: 1.05‐5.98, P = 0.040) and low mean attenuation of skeletal muscle (called high intramuscular fat [IMF] deposition) (odds ratio: 11.90; 95% CI: 4.50‐36.14; P < 0.001) were independent risk factors for critical illness. Furthermore, visceral adiposity or high IMF deposition increased the risk of mechanical ventilation (P = 0.013, P < 0.001, respectively). High IMF deposition increased the risk of death (P = 0.012).
Conclusions
COVID‐19 patients with visceral adiposity or high IMF deposition have higher risk for critical illness. Therefore, patients with abdominal obesity should be monitored more carefully when hospitalized.
Study Importance.
What is already known?
-
►
Adipose tissue distribution has been considered as an important factor in the body’s response to infection.
What does this study add?
-
►
Visceral adiposity and high intramuscular fat deposition were independent risk factors for critical illness of patients with severe acute respiratory syndrome coronavirus 2, and those factors increased the risks of mechanical ventilation and death.
-
►
In the subgroup under 60 years old, patients with visceral adiposity or high intramuscular fat deposition had higher risk for critical illness.
How might these results change the direction of research or the focus of clinical practice?
-
►
Patients with abdominal obesity should be monitored more carefully when hospitalized.
-
►
Adipose tissue distribution assessed by computed tomography scan could be superior to BMI in risk evaluation of coronavirus disease 2019, as BMI does not provide information on fat distribution.
-
►
Further studies should focus on mechanisms that explain why visceral adiposity and high intramuscular fat deposition are associated with the severity of coronavirus disease 2019.
Introduction
An outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which emerged in Wuhan, has rapidly spread throughout China and become a global health threat (1, 2, 3, 4). As of May 28, SARS‐CoV‐2 had affected over 5,593,631 individuals and resulted in more than 353,334 deaths worldwide, and the cases continue to rise (4). The mortality rate of coronavirus disease 2019 (COVID‐19) so far is about 2.3% to 7.2%, lower than that of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus diseases (1, 2, 3).
In prior studies, approximately 5% to 10% of COVID‐19 patients rapidly developed critical illness in the form of acute respiratory distress syndrome (ARDS) or sepsis with acute organ dysfunction (1, 5, 6). Among those, 71% to 75% of patients required mechanical ventilation, and roughly half ended in death (7, 8). Early identification of patients with high risk for critical illness may help prevent worsening at early stage of the disease and may reduce mortality (9). Advanced age and comorbidity are the most recognized risk factors for adverse outcome of COVID‐19 (1, 2).
The rising prevalence of obesity has been described as a global pandemic and has become a major public health concern (10). Overnutrition, as well as undernutrition, has been considered an important factor in the body’s response to infection for centuries (11). For patients infected with influenza A (H1N1) virus, obesity was identified as a risk factor for hospitalization and mechanical ventilation in several different populations (12, 13). The distribution of adipose tissue has a major influence on the body’s immune system (14). The elevated inflammatory cytokine levels observed in patients with high visceral fat may be associated with increased obesity‐associated morbidity in H1N1 infections (15). Furthermore, lipids can be stored within skeletal muscle when the capacity for fat storage by subcutaneous and visceral tissue are exceeded, known as intramuscular fat (IMF) tissue (14). IMF deposition not only reflects the quality of muscle and poor physical function, it is also an independent risk factor for prognosis after cardiac and oncologic surgery (16, 17, 18, 19).
In the present study, we explored the relationship between abdominal adipose tissue distribution, skeletal muscle area (SMA), IMF deposition, and severity of COVID‐19 in a retrospective cohort of 143 patients who had an unenhanced abdominal computed tomography (CT).
Methods
The study was approved by the Ethics Commission of our institution, and the requirements for informed consent were waived because of its retrospective nature.
Study population
We consecutively identified 170 hospitalized patients with confirmed COVID‐19 who underwent at least one abdominal CT scan (including thoracoabdominal CT) through a review of clinical and imaging databases in Tongji Hospital in Wuhan, China, between January 1, 2020 and March 30, 2020 (Figure 1). The end date for follow‐up was April 17, 2020. Exclusion criteria were as follows: (a) patients with abdominal CT scan 2 weeks prior to the onset of symptoms (n = 8), (b) patients with contrast‐enhanced CT of the abdomen (n = 7), (c) patients with suboptimal image quality for analysis due to artifacts or ascites (n = 5), (d) patients with insufficient scanning coverage for imaging evaluation for subcutaneous adipose tissue (SAT) (n = 3), and (e) patients who died of diseases other than COVID‐19 (n = 4). Indication for CT of the abdomen included abdominal symptoms such as abdominal pain, diarrhea, vomiting, and/or abnormal laboratory tests. Finally, a total of 143 patients with unenhanced abdominal CT were included in this study. For patients with multiple abdominal CT examinations, CT scan with the shortest time interval between imaging and the onset of symptom was used for this analysis.
Figure 1.
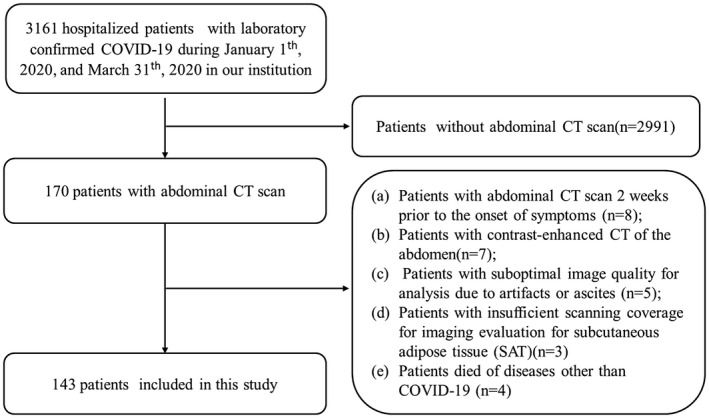
Flowchart for inclusion and exclusion criteria of our cohort.
Data collection
Demographic and clinical parameters were obtained from clinical electronic medical records as follows: sex, age, height, weight, underlying comorbidities, history of surgery, treatment measures, and clinical outcome. Results of laboratory examinations that were closest to the abdominal CT scan were also collected. The durations from onset of symptom to requiring mechanical ventilation, death, or discharge were recorded. Critically ill patients are defined as patients with ARDS or sepsis with acute organ dysfunction (6). BMI was calculated as weight in kilograms divided by height in meters squared.
CT examination
All abdominal CT examinations were performed on designated CT scanners for COVID‐19 patients (uCT 780 [United Imaging, China] or Somatom Force/Somatom Definition AS+ [Siemens Healthineers, Germany]). After patients were placed in the supine and feetfirst position on the couch, images were acquired at breath‐holding following inspiration, using tube voltage 120 kVp, automatic tube current modulation, and slice thickness 10 mm. All images were then reconstructed with a slice thickness of 5 mm.
CT analyses of adipose tissue and skeletal muscle variables
Detailed spatial maps of attenuation coefficients determined by CT provide a reliable and convenient way to quantify tissue areas and mean attenuation within tissue‐specific thresholds of attenuation values. The attenuation values were defined on a Hounsfield unit (HU) scale using calibration points of air (−1,000 HU) and water (0 HU) (20, 21). Abdominal adipose tissue and skeletal muscle variables were measured on a single 5‐mm cross‐sectional CT image at the level of the third lumbar vertebra (L3) using Advance workstation (ADW, GE Healthcare, Milwaukee, Wisconsin), software ADW server 4.6 (22). Two radiologists (LD and XLZ, with 7 and 3 years of experience, respectively) independently analyzed adipose tissue and skeletal muscle variables and were blinded to the patient’s medical record other than the images belonging to patients with COVID‐19 (Figure 2). First, the outer and inner perimeters of SAT were delineated, and only the pixels between the inner and outer perimeters were retained. Then, tissue threshold was set to a minimum value of −190 HU and a maximum value of −30 HU. The applied threshold isolated the area of SAT. The skeletal muscle was obtained using a similar method with tissue‐specific thresholds ranging from −29 to 150 HU. SMA and mean attenuation of the entire skeletal muscle (SMD) were measured. Visceral adipose tissue area (VAT) was obtained after hand tracing the outer perimeter of VAT and removing all pixels outside the contoured area as well as the pixels of intestines, kidney, and other organs in the abdominal cavity. Tissue‐specific thresholds of −150 to −50 HU were applied and resulted in total VAT area. VAT to SAT ratio (VSR) was calculated to indicate abdominal adipose tissue distributions, and high VSR was termed visceral adiposity (23). Low‐density muscle reflects increased muscle lipid content, termed high IMF deposition (22, 23).
Figure 2.
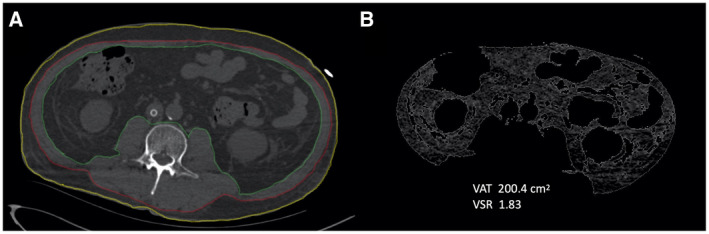
Cross‐sectional computed tomography (CT) images of the third lumbar vertebra used to quantify adipose tissue distribution variables. (A) Subcutaneous adipose tissue (SAT) area is between the yellow and red lines, and skeletal muscle area is between the red and green lines. (B) Visceral adipose tissue (VAT) area is inside the green lines, excluding"/> non‐VAT fat within abdominal organs. VSR, VAT to SAT ratio.
The cutoff value of 100 cm2 was used for VAT and SAT, irrespective of sex and age. This value is widely used as a cutoff point to evaluate visceral fat obesity in Asian populations (24). There are no previously recognized threshold values defining abnormal ranges with regard to VSR, SMA, and SMD for healthy individuals or individuals with a viral infection. Therefore, the sex‐specific median values were used as cutoff points.
Statistical analysis
The interobserver agreements of SMA, the corresponding muscle mean CT attenuation, and SAT and VAT area of the 143 patients measured by the two radiologists independently were analyzed with intraclass correlation coefficient (ICC). In assessing interobserver reproducibility, ICC < 0.2 is considered poor agreement, 0.21‐0.40 fair, 0.41‐0.60 moderate, 0.61‐0.80 good, and 0.81‐1 excellent (25). The mean value of the aforementioned parameters measured by the two radiologists was calculated for further statistical analysis.
Categorical variables were presented as number (percentage), and continuous variables were presented as median (interquartile range [IQR]). To compare variables between different groups, the χ2 test or Fisher exact test was used for categorical variables, and the t test or Mann‐Whitney U test was used for continuous variables, when appropriate. Correlations between the continuous adipose tissue and skeletal muscle variables were assessed using Spearman rank correlation analysis. Univariate and multivariate logistic regression analyses were performed to identify the risk factors associated with the severity of COVID‐19. Sex, age, and variables with P < 0.05 at univariate logistic regression analysis were applied to multivariate analysis. The linear relationship between continuous predictor variables and the logit of the outcome was checked. The variance inflation factor was used to check the collinearity of variables included in the multivariate logistic regression equation. No significant interaction was found between variables included in multivariate analyses. Based on the likelihood ratio test, we were allowed to keep sex, age, hypertension, cerebrovascular disease, visceral adiposity, and high IMF deposition in the final model. Cumulative rates of mechanical ventilation and mortality were calculated using Kaplan‐Meier methods, and differences between curves were evaluated using the log‐rank test. Statistical analysis was done with R software version 3.6.1 (R Project for Statistical Computing; www.r‐project.org). A two‐tailed P < 0.05 was considered statistically significant.
Results
Patient characteristics
Critically ill patients were defined as patients with ARDS or sepsis with acute organ dysfunction based on the clinical management of COVID‐19 published by the World Health Organization (6). There were 45 patients who met the criteria for critical illness, while the remaining 98 patients did not (Figure 1). The clinical and physical characteristics of the two groups are summarized in Table 1. No significant difference in age or sex was found between the two groups (P = 0.105; P = 0.107; respectively). The critical illness group had significantly higher height and body weight (P = 0.034; P = 0.022; respectively), but no significant difference was found between the two groups in BMI values (P = 0.148). Hypertension, diabetes, cardiovascular disease, cerebrovascular disease, and malignancy were the most common comorbidities in patients with COVID‐19, and among these comorbidities, hypertension and cerebrovascular disease were significantly more common in the critical illness group (P = 0.03; P = 0.025; respectively).
Table 1.
Baseline characteristics of hospitalized patients with COVID‐19 in Tongji Hospital, Wuhan, China
| Total | Critical illness | Noncritical illness | P value | |
|---|---|---|---|---|
| Age | 66 (56‐73.5) | 67 (60‐75) | 65 (54‐73) | 0.105 |
| Sex (male) | 70 (49.0%) | 27 (60%) | 43 (43.9%) | 0.107 |
| Height (cm) | 165 (160‐170) | 167.5 (161.8‐174) | 163 (158‐169) | 0.034 |
| Weight (kg) | 64 (55‐70) | 68.5 (60‐72) | 60 (55‐66) | 0.022 |
| BMI (kg/m2) | 23.4 (21.9‐25.3) | 24.8 (22.5‐26.1) | 23.0 (21.4‐24.8) | 0.148 |
| Comorbidity (yes or no) | 84 (58.7%) | 31 (68.9%) | 53 (54.1%) | 0.184 |
| Hypertension | 53 (37.1%) | 23 (51.1%) | 30 (30.6%) | 0.03 |
| Diabetes | 28 (19.6%) | 10 (22.2%) | 18 (18.4%) | 0.755 |
| Cardiovascular disease | 18 (12.6%) | 7 (15.6%) | 11 (11.2%) | 0.65 |
| Other heart diseases | 12 (8.4%) | 5 (11.1%) | 7 (7.1%) | 0.518 |
| Cerebrovascular disease | 13 (9.0%) | 8 (17.8%) | 5 (5.1%) | 0.025 |
| COPD | 10 (7.0%) | 3 (6.7%) | 7 (7.1%) | 1 |
| Chronic liver disease | 5 (3.5%) | 0 | 5 (5.1%) | 0.326 |
| Chronic kidney disease | 5(3.5%) | 1 (2.2%) | 4 (4.1%) | 1 |
| Malignancy | 13 (9.1%) | 4 (8.9%) | 9 (9.2%) | 1 |
| History of surgery | 23 (16.1%) | 8 (17.8%) | 15 (15.3%) | 0.898 |
| WBC (109/L) | 6.18 (4.89‐9.60) | 7.39 (4.78‐11.25) | 5.92 (5.00‐8.23) | < 0.001 |
| NBC (109/L) | 4.43 (2.88‐7.67) | 5.87 (3.13‐9.76) | 4.13 (2.79‐6.76) | 0.021 |
| LBC (109/L) | 1.12 (0.74‐1.61) | 0.96 (0.65‐1.24) | 1.19 (0.79‐1.70) | 0.031 |
| Hemoglobin (g/L) | 111 (92.5‐125.5) | 103 (88‐119) | 114 (95.5‐128) | 0.037 |
| PLT (10⁹/L) | 185 (151‐246.5) | 159 (112‐203) | 200.5 (169.5‐259) | 0.007 |
| Albumin (g/L) | 35.9 (31.9‐39.9) | 32.6 (29.4‐37.1) | 37.7 (33.7‐41.6) | < 0.001 |
| Cholesterol (mmol/L) | 3.65 (2.96‐4.51) | 3.03 (2.34‐3.67) | 3.93 (3.35‐4.71) | < 0.001 |
| Glucose (mmol/L) | 6.18 (5.18‐8.05) | 7.07 (6.00‐9.23) | 5.74 (5.02‐7.38) | 0.003 |
| Prognosis | < 0.001 | |||
| Death | 15 (10.5%) | 15 (33.3%) | 0 | |
| Discharged | 115 (80.4%) | 18 (40%) | 97 (99.0%) | |
| Hospitalized | 13 (9.1%) | 12 (26.7%) | 1 (1.0%) | |
| SAT (cm2) | 108.2 (77.0‐156.7) | 108.2 (66.0‐138.5) | 108.8 (83.2‐175.2) | 0.342 |
| VAT (cm2) | 103.4 (60.3‐166.6) | 131.9 (79.2‐185.7) | 90.5 (51.3‐156.1) | 0.042 |
| VSR | 0.94 (0.62‐1.40) | 1.31 (0.79‐1.76) | 0.87 (0.57‐1.31) | 0.003 |
| SMA (cm2) | 96.2 (79.0‐118.2) | 93.3 (77.0‐118.4) | 98.5 (81.7‐117.2) | 0.327 |
| SMD (HU) | 32.3 (23.7‐39.3) | 25.4 (16.3‐30.6) | 35.7 (28.1‐41.3) | < 0.001 |
Categorical variables presented as number (percentage), and continuous variables presented as median (interquartile range). To compare variables between different groups, the χ2 test or Fisher exact test was used for categorical variables, and the t test or Mann‐Whitney U test was used for continuous variables, when appropriate. A two‐tailed P < 0.05 was considered statistically significant.
COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2019; HU, Hounsfield units; LBC, lymphocyte count; NBC, neutrophil count; PLT, platelet count; SAT, subcutaneous adipose tissue; SMA, skeletal muscle area; SMD, the mean attenuation of skeletal muscle; VAT, visceral adipose tissue; VSR, VAT to SAT ratio; WBC, white blood cell.
Among the critical illness group, 88.9% (40 out of 45) of the patients required mechanical ventilation, including 34 patients who required invasive ventilation. Median time from onset of symptom to mechanical ventilation was 16 days (IQR, 10‐24).
At the end of follow‐up, 15 patients died of COVID‐19; median time from onset of symptoms to death was 43 days (IQR, 34.5‐55.5). A total of 115 patients were discharged from the hospital. Median time from illness onset (i.e., before admission) to discharge was 53 days (IQR, 42‐61). Mortality rate among patients with a definite outcome in our cohort was 11.5%. A total of 13 patients were still hospitalized, and most of them were critically ill (92.3%); median time from onset of illness to the end of follow‐up was 80 days (IQR, 74‐87).
Critically ill patients showed higher VAT and VSR and lower SMD
Interobserver agreement between the two radiologists’ measurements of adipose tissue and skeletal muscle variables was excellent (ICC = 0.995 for SAT; ICC = 0.999 for VAT; ICC = 0.909 for SMA; and ICC = 0.993 for SMD).
Critically ill patients had significantly higher VAT and VSR (P = 0.003; P = 0.042; respectively). Median SMD was 25.4 HU and 35.7 HU in critically ill and noncritically ill groups, respectively, and the difference was statistically significant (P < 0.001) (Table 1).
Clinical characteristics among different adipose tissue distribution features
An area of 100 cm2 was used as a cutoff point to distinguish high and normal fat areas for VAT and SAT, irrespective of sex. Median VSR (1.33 in male and 0.71 in female) was used to distinguish high from normal VSR. Similarly, the cutoff value for SMA was 115.5 cm2 in males and 84.7 cm2 in females. The cutoff value for SMD was 32.7 HU in males and 28.9 HU in females.
The clinical characteristics of patients with and without visceral adiposity or high IMF deposition are shown in Table 2. Patients with visceral adiposity or high IMF deposition were significantly older (P = 0.045; P < 0.001; respectively). A relatively larger area of VAT and smaller area of SMA were found in patients with high IMF deposition (P = 0.016; P = 0.006; respectively). There was no difference in sex and complication rate between patients with and without visceral adiposity or high IMF deposition.
Table 2.
Clinical characteristics of patients with and without visceral adiposity or high intramuscular fat deposition
| Visceral adiposity 1 | High IMF deposition 2 | |||||
|---|---|---|---|---|---|---|
| Yes (n = 72) | No (n = 71) | P value | Yes (n = 71) | No (n = 72) | P value | |
| Age (y) | 66 (60‐75) | 65 (51.5‐72.5) | 0.045 | 70 (60.5‐78.5) | 62 (50.5‐67) | < 0.001 |
| Sex | 35 (48.6%) | 35 (49.3%) | 1 | 35 (49.3%) | 35 (48.6%) | 1 |
| Comorbidity (yes or no) | 44 (61.1%) | 40 (56.3%) | 0.682 | 44 (62.0%) | 40 (55.6%) | 0.542 |
| Hypertension | 28 (38.9%) | 25 (35.2%) | 0.778 | 32 (45.1%) | 21 (29.2%) | 0.073 |
| Diabetes | 18 (25%) | 10 (14.1%) | 0.152 | 12 (16.9%) | 16 (22.2%) | 0.555 |
| Cardiovascular disease | 9 (12.5%) | 9 (12.7%) | 1 | 13 (18.3%) | 5 (6.9%) | 0.072 |
| Other heart diseases | 5 (6.9%) | 7 (9.9%) | 0.744 | 7 (9.9%) | 5 (6.9%) | 0.744 |
| Cerebrovascular disease | 9 (12.5%) | 4 (5.6%) | 0.256 | 7 (9.9%) | 6 (8.3%) | 0.979 |
| COPD | 8 (11.1%) | 2 (2.8%) | 0.097 | 7 (9.9%) | 3 (4.2%) | 0.208 |
| Chronic liver disease | 2 (2.8%) | 3 (4.2%) | 0.681 | 1 (1.4%) | 4 (5.6%) | 0.366 |
| Chronic kidney disease | 1 (1.4%) | 4 (5.6%) | 0.209 | 3 (4.2%) | 2 (2.8%) | 0.681 |
| Malignancy | 6 (8.3%) | 7 (9.9%) | 0.979 | 7 (9.9%) | 6 (8.3%) | 0.979 |
| History of surgery | 11 (15.3%) | 12 (16.9%) | 0.971 | 11 (15.5%) | 12 (16.7%) | 1 |
| Prognosis | < 0.001 | < 0.001 | ||||
| Death | 11 (15.3%) | 4 (5.6%) | 13 (18.3%) | 2 (2.8%) | ||
| Discharged | 52 (72.2%) | 63 (88.7%) | 47 (66.2%) | 68 (94.4%) | ||
| Hospitalized | 9 (12.5%) | 4 (5.6%) | 11 (15.5%) | 2 (2.8%) | ||
| SAT (cm2) | 107.5 (69.4‐130.9) | 111.0 (89.0‐171.2) | 0.184 | 126.3 (87.0‐171.6) | 97.6 (68.8‐148.7) | 0.087 |
| VAT (cm2) | 143.6 (90.6‐197.7) | 79.1 (45.7‐116.8) | < 0.001 | 126.3 (74.0‐185.5) | 89.6 (49.9‐145.3) | 0.016 |
| VSR | 1.40 (0.93‐1.82) | 0.61 (0.46‐0.92) | < 0.001 | 1.00 (0.65‐1.52) | 0.89 (0.56‐1.36) | 0.266 |
| SMA (cm2) | 96.4 (78.3‐118.5) | 95.6 (81.0‐115.5) | 0.857 | 91.7 (77.1‐108.8) | 100.1 (87.3‐126.5) | 0.006 |
| SMD (HU) | 31.8 (20.3‐36.8) | 33.0 (24.4‐41.5) | 0.091 | 23.6 (17.0‐28.1) | 39.3 (35.9‐43.0) | < 0.001 |
Categorical variables presented as number (percentage), and continuous variables presented as median (interquartile range). To compare variables between different groups, the χ2 test or Fisher exact test was used for categorical variables, and the t test or Mann‐Whitney U test was used for continuous variables, when appropriate. A two‐tailed P < 0.05 was considered statistically significant.
High VSR was termed visceral adiposity with the cutoff values of 1.33 in male and 0.71 in female.
Low SMD was termed as high IMF deposition with the cutoff values of 32.7 HU in males and 28.9 HU in females.
COPD, chronic obstructive pulmonary disease; CT, computed tomography; HU, Hounsfield units; IMF, intramuscular fat; LBC, lymphocyte count; NBC, neutrophil count; PLT, platelet count; SAT, subcutaneous adipose tissue; SMA, skeletal muscle area; SMD, the mean attenuation of skeletal muscle; VAT, visceral adipose tissue; VSR, VAT to SAT ratio; WBC, white blood cell.
Visceral adiposity and high IMF deposition were independent risk factors for critical illness
The overall rate of critical illness in our study was 31.5%. The rates of critical illness in patients with visceral adiposity were almost twice as much as patients with no visceral adiposity (42.3% vs. 22.5%, P = 0.035; Table 3). Patients with high IMF deposition showed significantly higher rates of critical illness compared with those without high IMF deposition (52.1% vs. 11.1%, P < 0.001; Table 3).
Table 3.
Relationship between severity of COVID‐19 infection and adipose tissue distribution variables
| Group | Critical illness (n = 45) | Noncritical illness (n = 98) | P value |
|---|---|---|---|
| SAT | 1 | ||
| High SAT (n = 78) | 25 (32.1%) | 53 (67.9%) | |
| Normal SAT (n = 65) | 20 (30.8%) | 45 (69.2%) | |
| VAT | 0.103 | ||
| High VAT (n = 73) | 28 (38.3%) | 45 (61.6%) | |
| Normal VAT (n = 70) | 17 (24.3%) | 53 (75.7%) | |
| Visceral adiposity | 0.035 | ||
| Visceral adiposity (n = 72) | 29 (42.3%) | 43 (59.7%) | |
| No visceral adiposity (n = 71) | 16 (22.5%) | 55 (77.5%) | |
| SMA | 0.134 | ||
| High SMA (n = 72) | 18 (25%) | 54 (75%) | |
| Low SMA (n = 71) | 27 (38.0%) | 44 (62.0%) | |
| IMF deposition | < 0.001 | ||
| High IMF deposition (n = 71) | 37 (52.1%) | 34 (47.9%) | |
| No high IMF deposition (n = 72) | 8 (11.1%) | 64 (88.9%) |
Variables presented as number (percentage); the χ2 test or Fisher exact test was used to compare variables between different groups. A two‐tailed P < 0.05 was considered statistically significant.
COVID‐19, coronavirus disease 2019; IMF, intramuscular fat; SAT, subcutaneous adipose tissue; SMA, skeletal muscle area; VAT, visceral adipose tissue.
Both univariate and multivariate logistic regression analyses were used to examine the association of adipose tissue and skeletal muscle variables with the risk of critical illness (Table 4). Univariate logistic regression demonstrated that hypertension, cerebrovascular disease, visceral adiposity, and high IMF deposition was significantly associated with the severity of COVID‐19 infection. Multivariate logistic regression analysis demonstrated that visceral adiposity (odds ratio [OR]: 2.47, 95% CI: 1.05‐5.98, P = 0.040) and high IMF deposition (OR: 11.90, 95% CI: 4.50‐36.14, P < 0.001) were independent risk factors for critical illness.
Table 4.
Univariate and multivariate analysis of adipose tissue distribution for predicting severity of COVID‐19
| Characteristic | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age | 1.03 | 1‐1.05 | 0.066 | 0.98 | 0.95‐1.02 | 0.270 |
| Sex (male) | 1.92 | 0.94‐3.98 | 0.075 | 2.11 | 0.91‐5.04 | 0.086 |
| Comorbidity (yes or no) | 1.88 | 0.9‐4.05 | 0.097 | |||
| Hypertension | 2.37 | 1.15‐4.93 | 0.020 | 1.83 | 0.75‐4.52 | 0.185 |
| Diabetes | 1.27 | 0.52‐2.99 | 0.59 | |||
| Cardiovascular disease | 1.46 | 0.5‐3.99 | 0.47 | |||
| Other heart diseases | 1.62 | 0.46‐5.4 | 0.43 | |||
| Cerebrovascular disease | 4.02 | 1.26‐14.07 | 0.021 | 4.4 | 0.99‐21.84 | 0.057 |
| COPD | 0.93 | 0.19‐3.52 | 0.917 | |||
| Chronic liver disease | 0 | NA | 0.988 | |||
| Chronic kidney disease | 0.53 | 0.03‐3.74 | 0.58 | |||
| Malignancy | 0.96 | 0.25‐3.15 | 0.955 | |||
| History of surgery | 1.2 | 0.45‐3.01 | 0.709 | |||
| High SAT | 1.06 | 0.52‐2.17 | 0.869 | |||
| High VAT | 1.94 | 0.95‐4.05 | 0.072 | |||
| Visceral adiposity | 2.32 | 1.13‐4.89 | 0.024 | 2.47 | 1.05‐5.98 | 0.040 |
| High SMA | 1.84 | 0.9‐3.82 | 0.095 | |||
| High IMF deposition | 8.71 | 3.81‐22.07 | < 0.001 | 11.90 | 4.50‐36.14 | < 0.001 |
Sex, age, and variables with P < 0.05 at univariate logistic regression analysis were applied to multivariate analysis. A two‐tailed P < 0.05 was considered statistically significant.
COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2019; IMF, intramuscular fat; NA, not available ; OR, odds ratio; SAT, subcutaneous adipose tissue; SMA, skeletal muscle area; VAT, visceral adipose tissue.
Furthermore, patients with visceral adiposity or high IMF deposition had significantly higher risk of mechanical ventilation compared with patients without those adipose tissue distribution features (P = 0.013; P < 0.001; respectively; Figure 3A‐3B). No statistical significance was found for visceral adiposity and increased risk of death in patients with COVID‐19 (P = 0.056; Figure 3C). High IMF deposition increased the risk of death (P = 0.012; Figure 3D).
Figure 3.
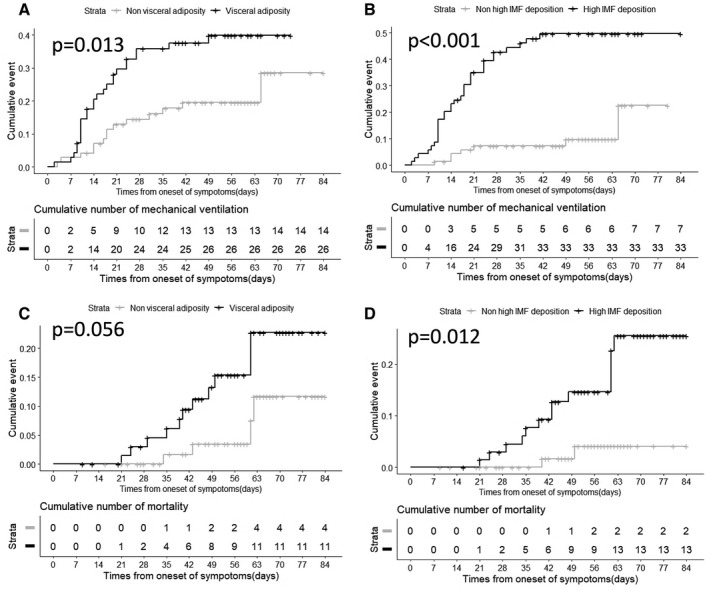
Cumulative rates of mechanical ventilation and mortality were calculated using Kaplan‐Meier methods, and differences between curves were evaluated using the log‐rank test. (A) Time‐dependent risk of mechanical ventilation from the time of onset between patients with or without visceral adiposity. (B) Time‐dependent risk of mechanical ventilation between patients with or without high intramuscular fat (IMF) deposition. (C) Time‐dependent risk of death from the time of onset between patients with or without visceral adiposity. (D) Time‐dependent risk of death between patients with or without high IMF deposition. (Two of the enrolled individuals were excluded from analysis because record of time of onset was unavailable.)
Patients aged < 60 years with visceral adiposity and high IMF deposition had higher risk for critical illness
As adipose tissue distribution is usually associated with age, we studied the relationship between them. Whereas SAT, VAT, and VSR were not correlated with age (R = −0.162, P = 0.054; R = −0.063, P = 0.454; and R = 0.127, P = 0.130; respectively), SMA and SMD were moderately correlated with age (R = −0.333, P < 0.001 and R = −0.459, P < 0.001; respectively). Otherwise, SMD was not correlated with VSR (R = −0.025, P = 0.760).
We divided the patients into two subgroups based on age. In the group younger than 60 years old (n = 44), patients with visceral adiposity (OR: 7.58, 95% CI: 1.7‐42.21, P = 0.011) and high IMF deposition (OR: 18.67, 95% CI: 3.64‐147.42, P = 0.001) had higher risks for critical illness. In the subgroup older than 60 years old (n = 99), only high IMF deposition (OR: 6.21, 95% CI: 2.39‐18.47, P < 0.001) was significantly associated with the severity of COVID‐19 infection.
Discussion
In this study, we analyzed the impact of adipose tissue distribution on the severity of COVID‐19 infection in a hospitalized cohort. Visceral adiposity and high IMF deposition were independent risk factors associated with critical illness, and those factors increased the risks of mechanical ventilation and death. Moreover, in the subgroup younger than 60 years old, patients with visceral adiposity or high IMF deposition had higher risk for critical illness, even though sample size of the subgroup was small.
Body composition not only reflects nutritional status but has significant influence on metabolic activity and inflammatory response (14). Several methods are available for the evaluation of those components, and BMI is the most commonly used one. High BMI was significantly associated with critical illness for patients with COVID‐19 in a large prospective cohort study (26). However, no significant difference in BMI value was found between critical illness and noncritical illness groups in our cohort. The inconsistent results may be due to the small sample size of our cohort. As BMI is an indirect measurement of adipose tissue, it cannot provide information on fat distribution. More recent data have proposed that visceral adiposity and ectopic fat accumulation contribute to various features of metabolic syndrome, over and above the BMI (14). A cross‐sectional image of CT scans at the level of the L3 could quantify adipose tissue and skeletal muscle, and currently this is a widely used method to evaluate adipose tissue distribution (23). In this study, visceral adiposity and high IMF deposition were significantly associated with critical illness for patients with COVID‐19. This suggested that the fat distribution rather than the total fat may be a better indicator of risk for critical illness of patients with COVID‐19.
In this study, we found that VAT rather than SAT deposition was associated with the severity of COVID‐19 infection. Previous studies have shown that VAT was different from SAT in pro‐inflammatory cytokine production (27). Visceral fat was supposed to be an endocrine organ and had pro‐inflammatory characteristics (14). Different studies have demonstrated that people with visceral adiposity have higher concentrations of circulating inflammatory cytokines compared with lean individuals (14, 28). Tumor necrosis factor‐α, interleukin (IL)‐6, and IL‐1b are the main inflammatory cytokines secreted by VAT from adipocytes and resident macrophages, while anti‐inflammatory cytokines such as adiponectin are secreted from subcutaneous adipocytes (29). In SARS‐CoV‐2 infected individuals, studies have reported that IL‐6, IL‐10, and tumor necrosis factor‐α surged during illness and declined during recovery (30, 31). And a portion of severe patients with COVID‐19 had an elevated cytokine profile resembling cytokine storm in severe acute respiratory syndrome and Middle East respiratory syndrome (32). The dysregulated and exuberant immune responses could exacerbate lung damage as well as lead to other fatal complications (33). Moreover, excessive adipose tissue, especially VAT, can also alter cell‐mediated immune responses through the increase in leptin and the decrease in adiponectin (34). The immune dysfunction caused by visceral fat aggravating COVID‐19‐induced immune damage may be the reason why patients with visceral adiposity were more susceptible to developing a serious illness. In addition, abdominal obesity can profoundly alter pulmonary function by diminishing exercise capacity and augmenting airway resistance, resulting in increased work of breathing (35). For supine patients, ventilation may be more difficult with the decreased diaphragmatic excursion.
The present study showed that high IMF deposition was significantly associated with risk of critical illness and requiring mechanical ventilation in patients with COVID‐19. There was a moderate correlation between SMD and age, and high IMF deposition was more common in older patients in our study, but high IMF deposition was still a significant risk factor for critical illness when age was adjusted for statistically. In the subgroup under 60 years old, patients with high IMF deposition had higher risk for critical illness; however, the 95% confidence intervals of the odds ratios were large. This may be due partly to the small sample size of the subgroup. Previous studies have shown that high IMF deposition contributes to muscle weakness independent of the age‐associated loss in muscle mass, which is consistent with our findings (19). A previous study reported that IMF deposition delayed and blunted immune responses, especially natural killer lymphocytes involved in innate immunity (14). Montano Loza et al. demonstrated that the presence of skeletal muscle loss and high IMF depositionincreased the risk of sepsis‐related death in patients with cirrhosis, probably due to impaired immunity (36). Therefore, high IMF deposition would impair immune function of COVID‐19 patients and increase the risk of complications and mortality.
To our knowledge, this is the first study that has investigated the association between adipose tissue distribution and severity of COVID‐19 infection. There are several limitations of our study. First, it is a retrospective, single‐center study with a small sample of hospitalized patients, therefore larger, multicenter studies are needed to confirm the results of this study. Second, the possibility of selection bias in patient inclusion in the study group must be considered, as abdominal CT scan is not a routine examination for COVID‐19 patients. Besides, physical activity levels were not analyzed in our study, although they have been widely associated with adipose tissue distribution in previous studies (16, 28). The association between smoking and severity of COVID‐19 is controversial; it was not analyzed in our study because electronic medical records for smoking were not complete in our hospital during the outbreak period (37, 38). Nevertheless, our study offered the new insight that visceral adiposity and high IMF deposition are significantly associated with the severity of COVID‐19 infection. The assessment of adipose distribution and IMF deposition may help with risk stratification of patients with COVID‐19 upon hospital admission.
Conclusion
Our findings show that patients with visceral adiposity or high IMF deposition were more likely to develop critical illness when infected with COVID‐19. Therefore, patients with abdominal obesity should be monitored more carefully when hospitalized.
Funding agencies
This work is supported by grants from the National Natural Science Foundation of China (NSFC) (grant numbers 81701657, 81801695, and 81771801).
Disclosure
The authors declared no conflict of interest.
Author contributions
ZL, XH, YS, and DH designed the study; YY, XH, LD, and XZ conducted the research; YY analyzed the data; YY, ZL, and IRK wrote the manuscript; ZL and XH have primary responsibility for final content. All authors gave final approval of the submitted and published versions.
Acknowledgments
Data described in the manuscript, code book, and analytic code will be made available upon request.
Contributor Information
Xuemei Hu, Email: mayjuly3720@163.com.
Zhen Li, Email: zhenli@hust.edu.cn.
References
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA 2020;323:1775‐1776. [DOI] [PubMed] [Google Scholar]
- 3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 721314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Coronavirus disease (COVID‐19) Situation Report – 129. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200528‐covid‐19‐sitrep‐129.pdf?sfvrsn=5b154880_2. Updated May 28, 2020, Accessed May 28, 2020.
- 5. Poston JT, Patel BK, Davis AM. Management of critically ill adults with COVID‐19. JAMA 2020;323:1839‐1841. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization . Clinical management of COVID‐19. https://www.who.int/publications/i/item/clinical-management-of-covid-19. Updated May 27, 2020, Accessed May 28, 2020.
- 7. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid‐19 in critically ill patients in the Seattle Region—case series. N Engl J Med 2020;382:2012‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med 2020;8:475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID‐19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care 2020;10:33. doi: 10.1186/s13613-020-00650-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis 2006;6:438‐446. [DOI] [PubMed] [Google Scholar]
- 12. Fezeu L, Julia C, Henegar A, et al. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta‐analysis. Obes Rev 2011;12:653‐659. [DOI] [PubMed] [Google Scholar]
- 13. Louie JKAM, Winter K, Jean C, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A (H1N1) infection in California. JAMA 2009;302:1896‐1902. [DOI] [PubMed] [Google Scholar]
- 14. Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med 2020;7:22. doi: 10.3389/fcvm.2020.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Honce R, Schultz‐Cherry S. Impact of obesity on influenza a virus pathogenesis, immune response, and evolution. Front Immunol 2019;10:1071. doi: 10.3389/fimmu.2019.01071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. West MA, Dijk DPJ, Gleadowe F, et al. Myosteatosis is associated with poor physical fitness in patients undergoing hepatopancreatobiliary surgery. J Cachexia Sarcopenia Muscle 2019;10:860‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ataseven B, Luengo TG, du Bois A, et al. Skeletal muscle attenuation (sarcopenia) predicts reduced overall survival in patients with advanced epithelial ovarian cancer undergoing primary debulking surgery. Ann Surg Oncol 2018;25:3372‐3379. [DOI] [PubMed] [Google Scholar]
- 18. Yamashita M, Kamiya K, Matsunaga A, et al. Prognostic value of psoas muscle area and density in patients who undergo cardiovascular surgery. Can J Cardiol 2017;33:1652‐1659. [DOI] [PubMed] [Google Scholar]
- 19. Martin L, Birdsell L, MacDonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539‐1547. [DOI] [PubMed] [Google Scholar]
- 20. Gomez‐Perez SL, Haus JM, Sheean P, et al. Measuring abdominal circumference and skeletal muscle from a single cross‐sectional computed tomography image: a step‐by‐step guide for clinicians using National Institutes of Health ImageJ. JPEN J Parenter Enteral Nutr 2016;40:308‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldman LW. Principles of CT and CT technology. J Nucl Med Technol 2007;35:115‐128. [DOI] [PubMed] [Google Scholar]
- 22. Tanaka M, Okada H, Hashimoto Y, et al. Relationship between nonalcoholic fatty liver disease and muscle quality as well as quantity evaluated by computed tomography. Liver Int 2020;40:120‐130. [DOI] [PubMed] [Google Scholar]
- 23. Fujiwara N, Nakagawa H, Kudo Y, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 2015;63:131‐140. [DOI] [PubMed] [Google Scholar]
- 24. Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity . New criteria for 'obesity disease' in Japan. Circ J 2002;66:987‐992. [DOI] [PubMed] [Google Scholar]
- 25. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979;86:420‐428. [DOI] [PubMed] [Google Scholar]
- 26. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020:m1966. doi: 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghazarian M, Luck H, Revelo XS, Winer S, Winer DA. Immunopathology of adipose tissue during metabolic syndrome. Turk Patoloji Derg 2015;31:172‐180. [DOI] [PubMed] [Google Scholar]
- 28. Stefan N, Häring H‐U, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol 2013;1:152‐162. [DOI] [PubMed] [Google Scholar]
- 29. Barchetta I, Cimini FA, Ciccarelli G, Baroni MG, Cavallo MG. Sick fat: the good and the bad of old and new circulating markers of adipose tissue inflammation. J Endocrinol Invest 2019;42:1257‐1272. [DOI] [PubMed] [Google Scholar]
- 30. Sarzi‐Puttini P, Giorgi V, Sirotti S, et al. COVID‐19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol 2020;38:337‐342. [PubMed] [Google Scholar]
- 31. Conti P, Ronconi G, Caraffa A, et al. Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by coronavirus‐19 (COVI‐19 or SARS‐CoV‐2): anti‐inflammatory strategies. J Biol Regul Homeost Agents 2020;34:327‐331. [DOI] [PubMed] [Google Scholar]
- 32. Pedersen SF, Ho YC. SARS‐CoV‐2: a storm is raging. J Clin Invest 2020;130:2202‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS‐CoV‐2 infection‐a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect 2020;9:727‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Venken K, Seeuws S, Zabeau L, et al. A bidirectional crosstalk between iNKT cells and adipocytes mediated by leptin modulates susceptibility for T cell mediated hepatitis. J Hepatol 2014;60:175‐182. [DOI] [PubMed] [Google Scholar]
- 35. Dietz W, Santos‐Burgoa C. Obesity and its implications for COVID‐19 mortality. Obesity (Silver Spring) 2020;28:1005. [DOI] [PubMed] [Google Scholar]
- 36. Montano‐Loza AJ. New concepts in liver cirrhosis: clinical significance of sarcopenia in cirrhotic patients. Minerva Gastroenterol Dietol 2013;59:173‐186. [PubMed] [Google Scholar]
- 37. Patanavanich R, Glantz SA. Smoking is associated with COVID‐19 progression: a meta‐analysis. Nicotine Tob Res 2020;22:1653‐1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID‐19). Eur J Intern Med 2020;75:107‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]


