Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) causes a highly contagious respiratory disease referred to as COVID‐19. However, emerging evidence indicates that a small but growing number of COVID‐19 patients also manifest neurological symptoms, suggesting that SARS‐CoV‐2 may infect the nervous system under some circumstances. SARS‐CoV‐2 primarily enters the body through the epithelial lining of the respiratory and gastrointestinal tracts, but under certain conditions this pleiotropic virus may also infect peripheral nerves and gain entry into the central nervous system (CNS). The brain is shielded by various anatomical and physiological barriers, most notably the blood–brain barrier (BBB) which functions to prevent harmful substances, including pathogens and pro‐inflammatory mediators, from entering the brain. The BBB is composed of highly specialized endothelial cells, pericytes, mast cells and astrocytes that form the neurovascular unit, which regulates BBB permeability and maintains the integrity of the CNS. In this review, potential routes of viral entry and the possible mechanisms utilized by SARS‐CoV‐2 to penetrate the CNS, either by disrupting the BBB or infecting the peripheral nerves and using the neuronal network to initiate neuroinflammation, are briefly discussed. Furthermore, the long‐term effects of SARS‐CoV‐2 infection on the brain and in the progression of neurodegenerative diseases known to be associated with other human coronaviruses are considered. Although the mechanisms of SARS‐CoV‐2 entry into the CNS and neurovirulence are currently unknown, the potential pathways described here might pave the way for future research in this area and enable the development of better therapeutic strategies.
Keywords: blood–brain barrier, central nervous system, coronavirus, glia, neurogenic inflammation, neuroinflammation, neurons, neurovascular unit
Introduction
On 27 December 2019 the Chinese Center for Disease Control and Prevention announced that it had detected a cluster of patients in Wuhan, China, who had developed severe pneumonia of unknown aetiology, later termed COVID‐19 (coronavirus disease of 2019) [1, 2, 3]. These patients were described as presenting with mainly fever, with a few patients having difficulty in breathing, and on 8 January 2020 the causative agent of COVID‐19 was identified as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) [4]. In the subsequent 7 months, SARS‐CoV‐2 has become a worldwide pandemic, infecting over 18 million people and killing more than 690 000 patients worldwide (https://www.jhu.edu/; https://coronavirus.jhu.edu/), whilst also crippling the world economy. Although COVID‐19 was first described as a respiratory disease, new data show that SARS‐CoV‐2 can infect almost every organ, resulting in an ever‐increasing list of symptoms. In particular, neurological symptoms and disturbances in the central nervous system (CNS) are common in many COVID‐19 patients, and may be a predictor of disease severity. In this review, some of the most recent data on COVID‐19‐associated neurological disease are examined and the possibility that SARS‐CoV‐2 may be infecting the CNS is assessed. In particular, the possible mechanisms of viral entry through the blood–brain barrier (BBB) and the peripheral nerves are examined and the possible long‐term consequences of viral infection in the brain are discussed by surveying data from closely related, neurotropic viruses. Since viral infection of the CNS often has long‐term neurological implications for patients, the possibility that these types of infections could lead to neurodegenerative diseases is discussed.
Neurological symptoms associated with SARS‐CoV‐2 infection: lessons learnt from the past 7 months
Even in the early months of the COVID‐19 pandemic, physicians observed that a significant subset of patients positive for SARS‐CoV‐2 presented with neurological complications, sometimes accompanied by respiratory distress. In February 2020, Li et al. [5] suggested that since SARS‐CoV‐2 shared significant similarities with severe acute respiratory syndrome coronavirus (SARS‐CoV), it was entirely possible that SARS‐CoV‐2 could similarly penetrate the brain and CNS of infected patients through synapses in the medullary cardiorespiratory centre and thereby cause respiratory failure. Quickly thereafter, several studies of severely ill COVID‐19 patients in Wuhan described neurological symptoms including autopsy observations of deceased patients which showed brain tissue oedema and partial neuronal degeneration [6]. In a retrospective study of hospitalized patients with laboratory‐confirmed SARS‐CoV‐2 infection in Wuhan, China, 36.4% of patients exhibited neurological symptoms [7], such as dizziness (16.8%) and headache (13.2%), whilst neurological symptoms were more common in severe versus non‐severe patients (45.5% vs. 30.3%). Several symptoms were more specifically associated with severe disease, such as impaired consciousness (14.8% in severe vs. 2.4% in non‐severe), acute cerebrovascular disease (5.7% vs. 0.8%) and skeletal muscle injury (19.3% vs. 4.8%). Other studies also found incidences of headache, dizziness and confusion in 5%–9% of hospitalized patients [8] and a single study from Wuhan, China, reported similar rates (≈5%) of acute cerebrovascular disease in severely affected COVID‐19 patients [9]. A retrospective analysis of deceased patients in China found a high rate of disorders of consciousness upon admission to the hospital, suggesting neurological complications were an indicator of poor prognosis [10]. In the past 7 months, it has been learnt that the loss of taste and smell is an early sign of SARS‐CoV‐2 infection, affecting approximately 5% of Chinese patients [7] 30%–40% of European patients [11, 12, 13], and is most prevalent in young women [12, 13]. This observation is especially significant since it has been suggested that human coronaviruses, such as SARS‐CoV (in mice) and HCoV‐OC43 (in mice and humans), enter the brain through the olfactory bulb [14]. A recent cross‐sectional study reported that the cumulative incidence of COVID‐19 was higher in patients with active epilepsy compared to control subjects without epilepsy [15]. Another study showed that plasma biomarkers of CNS injury, namely neurofilament light chain protein, a marker for neuronal injury, and glial fibrillary acidic protein, a marker for astrocytic injury, were significantly elevated in patients with moderate and severe COVID‐19 [16]. Histopathological examination of brain specimens from a cohort of 18 COVID‐19 patients showed acute hypoxic injury in the cerebrum and cerebellum and loss of neurons in the cerebral cortex, hippocampus and cerebellar Purkinje cell layer [17]. A retrospective study from China found that COVID‐19 patients over 60 years old and with neurological comorbidities were at a higher risk of developing neurological impairments such as impaired consciousness and cerebrovascular accidents [18].
Gateway to the brain: neurotropic and neuroinvasive potential of SARS‐CoV‐2
Severe acute respiratory syndrome coronavirus 2 belongs to the betacoronavirus genus in the Coronaviridae family [19]. With the exception of HCoV‐229E and HCoV‐NL63 (alphacoronaviruses), human coronaviruses (HCoVs) belong to the betacoronavirus genus. All human coronaviruses have animal origins [20] and cross‐species transmission appears to happen when low affinity binding occurs in receptors closely related between host species. Two strains of SARS‐CoV isolated from palm civets, for example, had high affinity for civet receptor angiotensin converting enzyme 2 (ACE2) and high infectivity in civet cells but these same two strains of SARS‐CoV had low affinity for human ACE2 and therefore low infectivity in human cells [21]. Similar to SARS‐CoV, SARS‐CoV‐2 also binds to human ACE2 with high affinity and is probably the principal entry route into human respiratory cells [22, 23]. Again similar to SARS‐CoV, SARS‐CoV‐2 also requires the transmembrane serine protease 2 (TMPRSS2) for spike protein priming and entry into the host cell [22], although for SARS‐COV‐2 this might vary depending on the cell type [24, 25]. Therefore, cells that express ACE2 and TMPRSS2, such as the glia and neurons, would be plausible targets for SARS‐CoV‐2 infection [26].
At these early stages of our understanding of COVID‐19, SARS‐CoV‐2 infection of the CNS is speculative and, although the mechanisms of SARS‐CoV‐2 entry into the CNS have not yet been studied, extrapolation from closely related viruses points toward some possible routes of infection. SARS‐CoV‐2 may deploy similar mechanisms to other pathogenic neurotropic RNA viruses that bypass protective barriers and access the CNS. Most coronaviruses cause respiratory disease in humans and most immunocompetent hosts display only mild to moderate upper respiratory symptoms [27], suggesting that most coronavirus infections begin in the lungs. However, human coronavirus infections can spread out of the lung and cause neurological complications: SARS‐CoV [28], MERS‐CoV [29], HCoV‐OC43 [19] and HCoV‐229E [30] infections are associated with headache, dizziness, axonopathic polyneuropathy, myopathy, ischaemic stroke, ataxia, febrile seizures, convulsions, loss of consciousness and encephalomyelitis encephalitis [14]. In fact, some of these viruses have been found in the CNS of their hosts [31, 32, 33, 34, 35]. SARS‐CoV has been isolated and cultured from the brain of a severe acute respiratory syndrome (SARS) infected patient, providing evidence that SARS‐CoV is able to infect the human brain [32]. HCoV‐OC43 has been associated with fatal encephalitis in immunocompromised paediatric patients, demonstrating direct evidence for the presence of viral proteins and RNA in neuronal cells in autopsied brain tissue [33, 35]. A first case of SARS‐CoV‐2 meningitis/encephalitis has been reported recently, where RNA has been detected in the cerebrospinal fluid (CSF) [34], suggesting that SARS‐CoV‐2 can invade the CNS. Similar to these neurotropic HCoVs, SARS‐CoV‐2 infection in the lungs of some COVID‐19 patients may also lead to entry into the CNS and this could occur via two main pathways: (i) infection of peripheral nerves and retrograde axonal transport; and/or (ii) haematogenous spread and infection of the cells of the BBB.
Entry through the peripheral nerves
The peripheral organs are highly innervated with neurons that link the peripheral nervous system (PNS) with the CNS and neuroinvasive viruses have evolved several strategies to access the CNS via the transneural route [19, 31, 36]. For example, viruses can manipulate the neuronal cytoskeletal machinery of the microtubules and molecular motors, such as dynein and kinesin, to traffic virions via a retrograde and anterograde transport route, respectively, from the PNS into the CNS [36]. After replication in the neuronal cell body, fully assembled viral particles are released into the synaptic cleft and infection is spread to presynaptic neurons. As will be discussed in more detail below, most RNA viruses including a number of coronaviruses have been shown to enter the CNS by utilizing the transneural pathway [19, 31]. Given the fact that SARS‐CoV‐2 is an RNA virus and has substantial similarity to other coronaviruses (that belong to the same family of viruses), it is hypothesized that it might deploy similar entry routes to access the CNS (Fig. 1).
Figure 1.
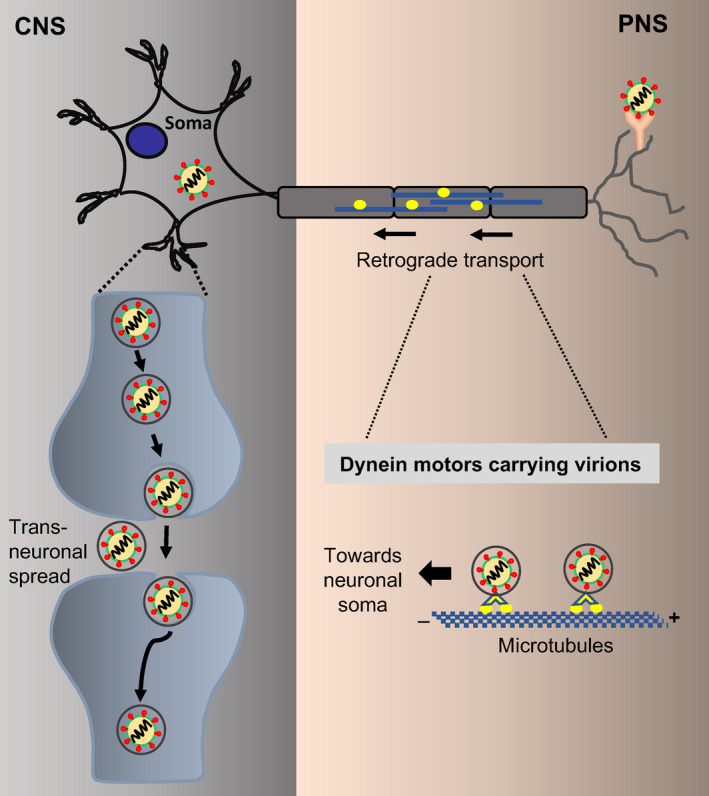
Potential route of SARS‐CoV‐2 entry from the peripheral nervous system (PNS) into the central nervous system (CNS). The virions bind to the receptors on the peripheral nerves and are then transported via the retrograde transport system using microtubule‐associated molecular motor dynein (from + to – end) towards the neuronal cell body (soma). Once inside the soma, the virions undergo transneuronal spread through the synapses to infect the presynaptic neurons. [Colour figure can be viewed at wileyonlinelibrary.com]
Trafficking via olfactory nerves
The olfactory nerve belongs to the PNS and innervates the olfactory epithelium and terminates in the olfactory bulb in the CNS. Many SARS‐CoV‐2 patients have reported anosmia in the early stages of the disease, suggesting the involvement of the olfactory bulb [37]. In mice, ACE2 and TMPRSS2 were expressed in the olfactory epithelium, with higher expression levels in older animals [38]. Another group reported that ACE2 and TMPRSS2 were mainly expressed by non‐neuronal cells of the olfactory epithelium and olfactory bulb in mice, non‐human primates as well as in humans [39]. More recently, neuropilin‐1 (NRP1) has been shown to be abundantly expressed in the respiratory and olfactory epithelium, with highest expression in endothelial cells of small‐ and medium‐sized vessels of the nasal cavity. Furthermore, NRP1‐mediated transport of SARS‐CoV‐2 pseudotyped virus was demonstrated in the CNS of mice [40]. Hence, in conjunction with ACE2, NRP1 could also be used as an additional receptor by SARS‐CoV‐2 to enter into the CNS via olfactory nerves.
Cell culture and animal models of HCoV neuropathogenesis have shown that HCoV‐OC43 enters via the olfactory nerves and spreads within the CNS, where it is mainly confined to the neuroanatomical connections of the trigeminal and olfactory nerves [19]. Porcine haemagglutinating encephalomyelitis coronavirus (PHEV) oro‐nasally infects the nasal mucosa, tonsil, lung and small intestine and then is delivered via the retrograde transport system through peripheral nerves to the medullary neurons [41, 42, 43]. The neurotropic coronavirus, mouse hepatitis virus (MHV), enters the CNS via the olfactory and trigeminal nerves [44]. Viruses can also spread to the spinal cord from the trigeminal nuclei via the reticular formation and the reticulo‐spinal tract [45] and this trans‐synaptic transfer has been reported for avian bronchitis virus (a gammacoronavirus) [46, 47]. Experimental studies using transgenic mice revealed that both SARS‐CoV and MERS‐CoV, when inoculated intranasally, entered the brain, possibly via the olfactory nerves, and thereafter rapidly spread to some specific brain areas including the thalamus and brainstem [29, 48]. Therefore, if viral replication in the nose is sufficiently high, it is possible that these high viral titres could infiltrate the olfactory nerve. Nasal swabs of symptomatic and asymptomatic COVID‐19 patients have higher viral loads than throat swabs [3, 49], suggesting that these cells might be the loci of viral replication and possible reservoirs for dissemination within the nasal cavity to the olfactory nerve.
Trafficking via the vagus nerve
It is well established that influenza virus (IV) infects the lungs and spreads to the brain via retrograde axonal transport in the vagus nerve [46, 50]. The vagus nerve travels through the neck and thorax to the stomach and it connects the lung to the gut and brain, also referred to as the lung–gut–brain axis [51]. Since the lungs represent a major reservoir of SARS‐CoV‐2 infection, at least in the early stages of COVID‐19 disease, it is possible that SARS‐CoV‐2 could use the vagus nerve to enter the CNS and travel throughout the lung–gut–brain axis, potentially interfering with all of these systems at different time‐points during infection. This may explain why some patients experience a combination of gastrointestinal, neurological and lung symptoms throughout the course of infection [52].
The gut is a highly innervated organ with a complex gut microbiome and this complex network biochemically interacts with the host CNS, also known as the gut–brain axis. In fact, the gut has often been referred to as a neurological organ since it is innervated by five different classes of neurons: intrinsic enteric neurons, vagal afferents, spinal afferents, parasympathetic efferents and sympathetic efferents [53]. If SARS‐CoV‐2 gains access to this highly complex innervated network, it is possible that it could use the network to penetrate the CNS [54]. Furthermore, enterocytes in the ileum and colon express both ACE2 and TMPRSS2 [52], facilitating viral infection and replication, probably resulting in tissue damage and compromised gut function. In support of this possibility, gastrointestinal symptoms are a feature of SARS‐CoV‐2 infection, especially amongst severe cases. The virus is commonly found in the faeces of mild cases, often detectable even after viral particles are no longer detectable in the sputum [55]. Indeed, MERS‐CoV can infect intestinal epithelial cells and intestinal inoculation of mice with MERS‐CoV leads to viral infection and immune cell invasion into the lungs [56], suggesting that intestinal infection can spread to other organs of the body. It is possible that the enteric nervous system, and specifically the intestinal vagal afferents, may offer another route by which SARS‐CoV‐2 may enter the CNS [54]. Alternatively, the CNS may also be affected indirectly by the release of pro‐inflammatory molecules from gut‐associated leukocytes and glial cells [54]. Intestinal infection by transmissible gastroenteritis virus (TGEV), a porcine alphacoronavirus, results in upregulation of many of the same cytokines observed after SARS‐CoV/SARS‐CoV‐2 infection of the lungs, including interleukin 1β (IL‐1β), IL‐6, tumour necrosis factor (TNF‐α) and IL‐10 [57]. This implies that infection of the gut may have the potential to induce or exacerbate a cytokine storm and contribute to neuroinflammation. The fact that many of these cytokines can promote vascular permeability and leakage resulting in BBB dysfunction suggests that infection in the gut could be another plausible route by which SARS‐CoV‐2 could penetrate the brain.
Trafficking via the neuromuscular junction
Neuromuscular junctions (NMJs) are specialized synapses between the motor neuron and the muscles that control and facilitate muscle movement. Most motor neurons have their cell bodies in the spinal cord, which, in turn, is in synaptic contact with the motor neurons in the CNS. Synapses serve as the portals of entry for the viruses as most of the cell surface receptors to which the viruses bind are concentrated at the synaptic membranes. Rabies virus and poliovirus (both RNA viruses) spread via the NMJ from muscles into the somatic neurons in the spinal cord [36, 58]. Rabies virus particles enter the axons of motor neurons at the NMJ directly by binding to the nicotinic acetylcholine receptors (nAchR) on muscles and neural cell adhesion molecules (NCAM) and p75NTR, two entry receptors present on motor neurons [59]. The nAchR is widely expressed on the post‐synaptic membranes of NMJs in the PNS and therefore is most likely to play a role in virus entry into muscle cells, not neurons. After primary replication within the motor neuron cell bodies, virions travel from one neuron to another neuron along the spinal cord into the CNS. Once inside the CNS, rabies virus utilizes NCAM and p75NTR to exclusively infect neurons and transneuronal spread occurs exclusively between synaptically connected neurons. The infection then moves unidirectionally from post‐synaptic to presynaptic neurons in a retrograde manner and spreads to distant brain regions. Poliovirus, on the other hand, binds to CD155 receptors found on the axonal membranes of motor neurons [60]. The virus replicates in motor neurons within the spinal cord followed by subsequent dissemination of progeny virions into the brain via retrograde transport [61]. PHEV binds to NCAM expressed on the surface of medullary neurons to enter into the CNS. Since SARS‐CoV‐2 belongs to the same genus as PHEV (betacoronavirus), it is possible that it might also deploy neuronal NCAM to enter the CNS. Additionally, previous studies have shown that ACE2 is present on skeletal muscles [62]. Hence, SARS‐CoV‐2 might bind to ACE2 receptors present on skeletal muscles and enter the CNS via retrograde transport. In support of this argument, it has been shown that 19.3% of patients with severe COVID‐19‐related neurological manifestations had skeletal muscle injury [7]. The prevalence of myalgia between 11% and 50% and muscle weakness related to COVID‐19 has been reported in several studies [63, 64, 65]. Acute myositis has also been recognized as a manifestation of COVID‐19 on magnetic resonance imaging scans [66] and, in at least one specific case, an afebrile COVID‐19 patient was hospitalized and did not present any upper and lower airway symptoms but had elevated creatine kinase and C‐reactive protein levels, suggestive of muscle inflammation [66]. During the 2015 MERS‐CoV outbreak in the Republic of Korea, four patients in a cohort of 23 (17.4%) experienced neuropathies and limb weakness, neurological complications that lasted months after initial infection [67]. It is possible that COVID‐19 patients who have experienced SARS‐CoV‐2 infection of the CNS via the NMJ could experience similar complications, and long‐term follow‐up of these patients should be prioritized.
Entry through the blood–brain barrier (BBB)
Normally, viral entry from the blood into the CNS is restricted by the BBB, which forms a structural and functional barrier between the peripheral circulation and the CNS. The BBB is composed of highly specialized cerebrovascular endothelial cells, pericytes, mast cells and astrocytes that function together as a neurovascular unit to maintain homeostasis [68, 69]. The total length of brain capillaries in humans is approximately 600 km with a surface of 15–25 m2, providing a large area for viral invasion [70]. The neurovascular unit serves as the gate‐keeper of the CNS that protects the brain by regulating the cerebral blood flow and limiting the access of pathogens, leukocytes and toxic substances [71, 72]. Human neurotropic RNA viruses have evolved as opportunistic pathogens that can bypass the BBB and gain entry into the CNS by several mechanisms: (i) paracellular transport via a leaky BBB, (ii) transcellular transport by direct infection of the cerebrovascular endothelial cells, (iii) transport via extracellular vesicles, a form of ‘Trojan horse’ trafficking, (iv) transport via receptor‐mediated endocytosis or (v) transport via infected peripheral immune cells, another form of ‘Trojan horse’ trafficking. SARS‐CoV‐2 may utilize similar pathway(s) to overcome the barriers separating the brain from the peripheral blood and gain access into the CNS (Fig. 2).
Figure 2.
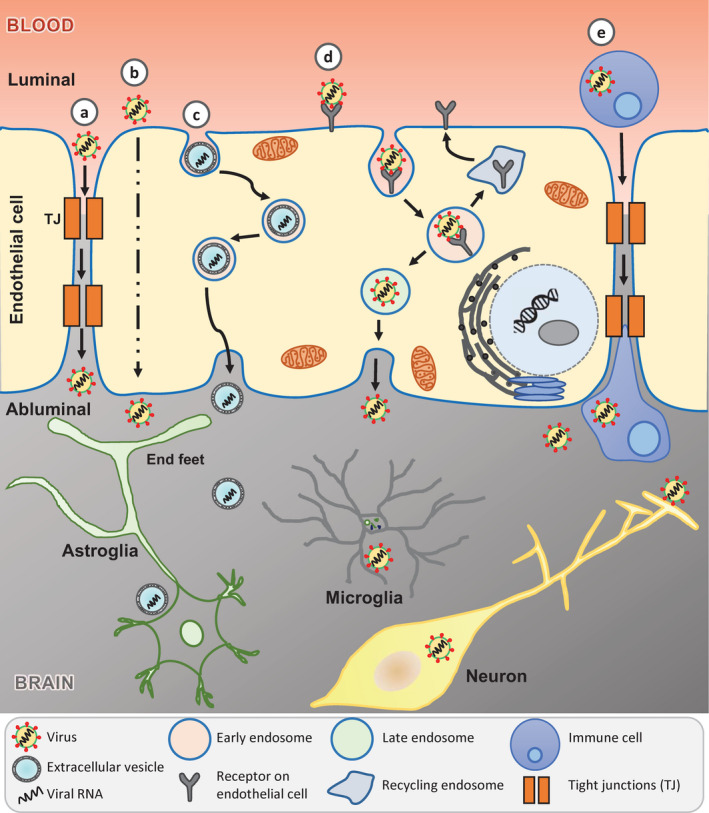
Potential mechanisms of SARS‐CoV‐2 entry into the central nervous system (CNS). The schematic depicts five routes by which SARS‐CoV‐2 could traverse across the blood–brain barrier (BBB) to access the CNS: (a) paracellular transport through leaky BBB due to disrupted tight junctions (TJs); (b) transcellular transport by direct infection of cerebrovascular endothelial cells; (c) transport via extracellular vesicles, a form of ‘Trojan horse’ trafficking; (d) transport via receptor‐mediated endocytosis; (e) transport via infected peripheral immune cells, another form of ‘Trojan horse’ trafficking. [Colour figure can be viewed at wileyonlinelibrary.com]
Paracellular transport through a leaky BBB
The BBB is mainly formed by specialized tight junctions (TJs) between adjacent cerebrovascular endothelial cells [72]. These TJs form a high‐resistance paracellular barrier with a transendothelial electrical resistance of up to 1800 Ω cm2 that maintains BBB integrity by controlling integrity and permeability of nutrients, molecules and cells in and out of the CNS [58, 73]. The TJs consist of transmembrane proteins such as claudin‐3, ‐5 and ‐12 that are essential for the formation of the paracellular barrier and zona occludens‐1 to ‐3 for structural stability. Other integral proteins include occludins and junctional adhesion proteins, namely vascular endothelial cadherin (CD144), for maintenance of the BBB. It has been shown that claudin‐1, zona occludens‐1, junctional adhesion molecule A or 1 and occludins are expressed at a low‐level post‐West Nile virus (WNV) infection [74], which may suggest their degradation. These changes have been associated with disruption of the BBB permeability, especially in the presence of pro‐inflammatory cytokines such as TNF‐α and interleukins [75]. The cytokine storm associated with SARS‐CoV‐2 infection results in increased secretion of pro‐inflammatory cytokines and chemokines such as IL‐6, TNF‐α, macrophage inflammatory protein 1‐alpha, IP‐10 and granulocyte‐colony stimulating factor as well as C‐reactive protein and ferritin [64]. These cytokines and chemokines can bind to specific receptors on the cerebral microvascular endothelium leading to BBB breakdown, neuroinflammation and encephalitis. The loss of BBB integrity could loosen the TJs between the endothelial cells paving the way for paracellular traversal of SARS‐CoV‐2 into the CNS (Fig. 2a). A recent study on Japanese encephalitis virus (JEV) (an RNA virus) suggests that the paracellular mode of trafficking could be one of the potential routes of entry into the CNS [76]. JEV infected mast cells release chymase, a vasoactive protease, which cleaves TJ proteins, including zona occludens‐1 and ‐2, claudin‐5 and occludin, breaking down the BBB and facilitating entry of JEV into the CNS.
Transcellular transport by direct infection of cerebrovascular endothelial cells
Some neurotrophic viruses, such as JEV and WNV, can enter into the CNS via the bloodstream in a process known as viraemia [77]. Following primary replication, the virus is released into the bloodstream which can then directly infect cerebrovascular endothelial cells and traverse transcellularly (Fig. 2b) to release virus into the brain parenchyma. Infection of brain endothelial cells could, in turn, lead to vascular leakage and loss of BBB integrity, further exacerbating viral neuroinvasion and neuroinflammation.
Trafficking via receptor‐mediated endocytosis
The BBB possesses a specialized transcellular transport system to facilitate the supply of nutrients into the brain [78], which is often hijacked by neurotropic viruses to traffic into the CNS. Zika virus (an RNA virus) known to cause microcephaly and other congenital defects in the foetus as well as neurological conditions such as Guillain–Barré syndrome in adults have been shown to cross the intact BBB [79]. This potentially occurs via a receptor‐mediated transcellular pathway followed by infection of neural progenitor cells and inhibition of neuronal differentiation. SARS‐CoV‐2 on the other hand could bind to ACE2 receptors on cerebrovascular endothelial cells [80] directed to clathrin‐coated vesicles to undergo receptor‐mediated endocytosis to gain access to the CNS. This pathway bypasses the need to establish a productive infection of the virus in these cells (Fig. 2d).
Trafficking via infection of peripheral immune cells – a ‘Trojan horse’ mechanism
In the ‘Trojan horse’ strategy of neuroinvasion, the virus hides inside innate immune cells, which traffic across the permeabilized BBB utilizing specific chemokine receptors and can further infect neurons and glial cells (Fig. 2e). Cell adhesion molecules are upregulated during WNV infection and adhesion molecules, particularly intercellular adhesion molecule 1 (ICAM‐1), on endothelial cells and leukocytes play important roles in leukocyte trafficking into the brain. Studies on WNV suggest that cell adhesion molecules may play a role in facilitating migration of peripherally infected leukocytes into the CNS [81, 82]. The virus may migrate within infected leukocytes that enter the CNS [83]. Indeed, human immunodeficiency virus (HIV) infected leukocytes that migrate through the BBB are one of the routes of spread to the CNS [84]. HIV infects CD4‐positive T‐cells and utilizes chemokine CCR5 as a co‐receptor to enter the CNS [85, 86]. Additionally, HIV also infects CD16‐positive monocytes to travel across the BBB and infect brain microglia leading to chronic inflammation and eventually neuronal damage and dementia [36]. Human cytomegalovirus [87, 88], enteroviruses including poliovirus [89] and flaviviruses [90] have also been shown to infect different types of leukocytes and to use them as a reservoir for haematogenous dissemination toward the CNS. It has been shown that ACE2 receptor is expressed on haematopoietic cells, including monocytes and lymphocytes. SARS‐CoV infects monocytes and dendritic cells, whereas MERS‐CoV infects monocytes and T‐cells via dipeptidyl peptidase 4 [91, 92]. It is possible that SARS‐CoV‐2 could infect monocytes and lymphocytes and traffic across the BBB and further infect neural cells. More recently CD147, also known as basigin and EMMPRIN (extracellular matrix metalloproteinase inducer), has been implicated as an alternative entry receptor for SARS‐CoV‐2, which is expressed on activated lymphoid, myeloid, epithelial and neuronal cells in the CNS [93, 94].
Trafficking via exosomes – a ‘Trojan horse’ mechanism
Viruses can also hijack the extracellular vesicle (EV) biogenesis pathway to package viral RNA in EVs which can cross the BBB to enter into the CNS and infect neurons and glial cells (Fig. 2c). EVs have emerged as highly sophisticated intercellular communication systems that can transmit information in the form of protein, RNA and lipids to neighbouring and distant cells and change the function of recipient cells [95]. Recent studies have shown that small EVs or exosomes released from human hepatocytes infected with hepatitis C virus can carry full‐length viral RNA, viral proteins and nucleic acids. Transfer of these exosomes to naïve human hepatoma cells resulted in productive infection even in the presence of neutralizing antibodies, supporting the notion that viruses could exploit exosomes for viral transmission and immune evasion [96]. Moreover, infectious exosomes containing viral RNA associated with argonaute 2, heat shock protein 90 and miR‐122 were isolated from the sera of chronic hepatitis C virus infected patients [97]. These studies demonstrate that exosomes can transmit infectious viral genomes to nearby host cells. Other notable examples of viruses using EVs to enter the CNS include human John Cunningham polyomavirus, influenza A virus and HIV [98, 99]. Thus far, there is no experimental evidence to suggest that SARS‐CoV‐2 infected cells can employ exosomes to shuttle infectious RNA, but recent evidence from other pathogenic neurotropic RNA viruses raises the possibility that SARS‐CoV‐2 might deploy similar mechanisms to package viral RNA, cross the BBB and infect neural cells in the CNS.
Neurological consequences of SARS‐CoV‐2 infection
Thus far, some of the mechanisms of SARS‐CoV‐2 infection of the CNS and possible routes of entry have been examined but, much like SARS‐CoV, MERS‐CoV and other neurotropic viruses, it is possible that these infections could have both short‐ and long‐term neurological sequelae in COVID‐19 patients. Once inside the CNS, pathogenic neurotropic viruses can infect neurons and glial cells, leading to the disruption of the complex architecture of neural networks and damage to the nervous system [100]. Microglia and astroglia are the innate immune cells in the CNS that respond to viral infection by expressing interferon‐stimulated genes, including alpha/beta interferon (IFN‐α/β), known as type I interferons, a first line of immune defence in limiting viral dissemination [101]. The upregulation of these genes can induce acute inflammation and possibly encephalitis, meningitis or acute flaccid paralysis and ultimately viral elimination with favourable prognosis outcome. However, in some cases viral antigens can persist even after viral clearance which might lead to chronic inflammation and post‐infection neurological syndromes in surviving hosts.
Recent studies support the possibility that SARS‐CoV‐2 could cause CNS damage either by direct virus‐induced neuronal damage or indirectly by host immune‐mediated damage through exposure to pathogenic levels of inflammatory mediators. A case of acute haemorrhagic necrotizing encephalitis has been reported in a single COVID‐19 patient, suggestive of a cytokine storm [102]. Systemic exposure to pathogenic levels of inflammatory mediators may have a negative impact on brain function and cognition of COVID‐19 patients. SARS‐CoV‐2 infection can directly result in CNS injury. This is consistent with the evidence from other viruses which have direct viral impacts on the CNS, such as SARS‐CoV, MERS‐CoV, HCoV‐OC43, HCoV‐229E and HIV [103, 104].
Impact on the burden of neurological diseases
What does this mean in terms of long‐term neurological effects of COVID‐19? It is still too early to reliably predict the possible long‐term health effects of SARS‐CoV‐2 infection but some general predictions are possible. Since there is substantial population variability in symptoms associated with acute SARS‐CoV‐2 infection, the long‐term consequences of COVID‐19 will also be variable, probably depending on sex and age (at the time of infection) of the patients. However, since other human coronavirus infections cause autoimmune and neurodegenerative diseases in their hosts, it is possible that these may also occur after SARS‐CoV‐2 infection. For instance, HCoV‐OC43 and HCoV‐229E antigens and RNA have been detected in the CSF and brain tissues of multiple sclerosis patients, where viral RNA persists in the absence of infectious virus [31, 105]. Another study showed that antibodies against HCoV‐OC43 and HCoV‐229E were found in the CSF of Parkinson’s disease (PD) patients [106]. Patients infected with other neurotropic viruses, including WNV, JEV, HIV, H5N1 and IV develop parkinsonian symptoms, including tremor, rigidity and bradykinesia, and infection with these viruses may increase the risk of developing PD [107]. Therefore, there is a possibility that the long‐term presence of viral components in the brain of COVID‐19 patients may induce chronic innate and adaptive immune responses that eventually lead to autoimmune and neurodegenerative diseases such as Alzheimer’s disease, PD and multiple sclerosis in susceptible individuals.
The pathological hallmarks of many chronic neurodegenerative diseases are selective, such as progressive loss of neurons, accumulation of misfolded/aggregated proteins like amyloid‐beta and alpha‐synuclein as well as neuroinflammation [108]. As discussed above, COVID‐19 patients present with olfactory and gastrointestinal issues and it is noteworthy that idiopathic olfactory dysfunction and vagal dysfunction are both early and common symptoms of preclinical PD and precede several years before the onset of classical motor dysfunction [109]. With regard to PD aetiology, Braak et al. [110] hypothesized that PD progresses through the nervous system in different stages, which was later revised as the ‘dual‐hit’ hypothesis by Hawkes et al. [111]. According to the ‘dual‐hit’ hypothesis, a neurotropic viral pathogen probably enters into the brain through the olfactory pathway or the enteric nervous system and leads to misfolding and aggregation of alpha‐synuclein protein and its translocation to the midbrain by ‘prion‐like’ propagation throughout the brain as the disease progresses. In an earlier study, Barnett and Perlman had shown that MHV entered the brain through the olfactory system and infected dopaminergic neurons in the substantia nigra and ventral tegmental area, respectively [112]. Interestingly, MHV produced a widespread infection of the A10 group of neurons. It also infected neurons of the A8 and A9 groups, suggesting that MHV can damage the nigrostriatal and mesolimbic dopamine pathway. A growing body of literature has now revealed that the majority of the proteins involved in neurodegenerative diseases are transported in exosomes [113]. Therefore, it is possible that exosomes play an important role in the transport of misfolded alpha‐synuclein across the BBB into the brain parenchyma where it acts as ‘seeds’ in the recipient neural cells, spreading throughout the brain by a ‘prion‐like’ mechanism. Chronic neurodegenerative diseases develop over decades with distinct preclinical, prodromal and clinical phases and the long‐term impacts of SARS‐CoV‐2 on younger patients is not known but could be a serious concern. If SARS‐CoV‐2 alters the long‐term impacts on the CNS as described, they may manifest over years or decades. As recovered COVID‐19 patients age, there might be an upsurge in the number of neurodegenerative diseases. Therefore, it is proposed that patients who have recovered from the acute phase of the SARS‐CoV‐2 infection should be continuously monitored throughout their lives for the development of neurological symptoms associated with neurodegenerative disease.
Potential neuropathological effects of current COVID‐19 therapies
At this time, there are no therapeutics or vaccines approved by the US Food and Drug Administration (FDA) to specifically cure, treat or prevent COVID‐19. However, the FDA continues to issue emergency approvals for ‘off‐label’ drugs to treat severe COVID‐19 patients. These drugs include antiviral drugs (remdesivir, chloroquine and hydroxychloroquine) and immunomodulators (tocilizumab, canakinumab and anakinra), and some of them may contribute to neurological dysfunction. For example, chloroquine and hydroxychloroquine, which were commonly used earlier in the pandemic, could potentially increase the likelihood of neurological disorders, especially in the elderly [114]. The use of hydroxychloroquine has been associated with neuropsychiatric manifestations including irritability, nervousness and psychosis, possibly by its ability to cross the BBB [115]. Corticosteroids, another common group of medications used to treat severe cases of COVID‐19, have also been associated with psychiatric symptoms, especially when administered at high doses [116]. The use of immunomodulators, such as tocilizumab (monoclonal antibody to IL‐6 receptor), canakinumab (monoclonal antibody to IL‐1β) and anakinra (IL‐1 receptor antagonist), is in clinical trials to dampen cytokine responses in severely ill COVID‐19 patients; however, they have poor BBB penetration into the CNS [117]. The use of tocilizumab has been associated with multifocal cerebral thrombotic microangiopathy and adverse neurological effects on the CNS [118]. Finally, mechanical ventilation was, and continues to be, the principal medical intervention used to treat severe COVID‐19 patients. However, some recent studies are raising the possibility that the use of mechanical ventilation can contribute to neurological injury, which can fuel further lung damage [119].
Indirect effects of COVID‐19 on patients with neurological disease
The current pandemic has spread globally to every country, infected millions of people and remains a major public health concern. Whilst governments around the world are using various measures, such as lockdowns, quarantine and contact tracing, to control the spread of infection, it is worth noting that these approaches might significantly impact patients with chronic neurological disorders in unintended ways. For example, the COVID‐19 quarantine in Italy created uncertainty and confusion about the availability of clinical services and continuity of care amongst PD patients [120]. Negative effects on mental health have been observed in those whose daily life was disrupted by the various public health measures [121]. Many drugs used to treat people with chronic neurological diseases are being repurposed to fight COVID‐19 [122, 123, 124], potentially leading to shortages and contributing to extra stress and worsening of disease symptoms.
Conclusions
Although SARS‐CoV‐2 and its methods of infection are rapidly being learnt about, there is still much to learn. In this review, information has been extrapolated from other neurotropic viruses to make some predictions and it is clear that SARS‐CoV‐2 has the potential to infect the CNS and cause long‐term neurological damage in COVID‐19 patients. The next few years will be critical if the long‐term effects of this pandemic on the neurological health of this population are to be determined. Governments are urged to create a framework and a national registry of patients who have been infected by SARS‐CoV‐2. Patients, particularly those with neurological symptoms, must be tracked regularly and consistently at ongoing time‐points over their lifetime using advanced neuroimaging and biochemical analysis of biomarkers to map the degenerative process. To this end, the Spanish Neurological Society has implemented a registry of neurological manifestations in patients with confirmed COVID‐19. Román et al. have emphasized the need for COVID‐19 international neurological databanks to report all cases of new‐onset, acute, delayed and any long‐latency neurological disorders associated with SARS‐CoV‐2 infection during the COVID‐19 pandemic [125]. The development of a regional, national or international registry will not only provide a database but will also help develop strategies to better combat COVID‐19‐mediated neurological manifestations. In addition, a systematic research approach in this area will allow better understanding of the neurological impact of SARS‐CoV‐2 infection on different groups of the population, its impact on the CNS and COVID‐19‐associated long‐term neurological consequences.
Disclosure of conflicts of interest
None.
Acknowledgements
The National Research Council Canada is thanked for intramural funding.
Reproduced with the permission of the Minister of National Research Council Canada.
Contributor Information
M. Kulka, Email: marianna.kulka@nrc‐cnrc.gc.ca.
J. K. Sandhu, Email: jagdeep.sandhu@nrc‐cnrc.gc.ca.
Data availability statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579: 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV‐2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol 2020; 92: 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8: 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology 2020; 77: 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asadi‐Pooya AA, Simani L. Central nervous system manifestations of COVID‐19: a systematic review. J Neurol Sci 2020; 413: 116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Y, Wang M, Zhou Y, et al. Acute cerebrovascular disease following COVID‐19: a single center, retrospective, observational study Stroke Vasc Neurol 2020; 5: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130: 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID‐19 in patients presenting with influenza‐like symptoms. Int Forum Allergy Rhinol 2020; 10: 806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giacomelli A, Pezzati L, Conti F, et al. Self‐reported olfactory and taste disorders in SARS‐CoV‐2 patients: a cross‐sectional study. Clin Infect Dis 2020; 71: 889–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol 2020; 277(8): 2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 2019; 12: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cabezudo‐García P, Ciano‐Petersen NL, Mena‐Vázquez N, Pons‐Pons G, Castro‐Sánchez MV, Serrano‐Castro PJ. Incidence and case fatality rate of COVID‐19 in patients with active epilepsy. Neurology 2020; 95: e1417–e1425. [DOI] [PubMed] [Google Scholar]
- 16. Kanberg N, Ashton NJ, Andersson L‐M, et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID‐19. Neurology 2020; 95: e1754–e1759. [DOI] [PubMed] [Google Scholar]
- 17. Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of COVID‐19. N Engl J Med 2020; 383: 989–992992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiong W, Mu J, Guo J, et al. New onset neurologic events in people with COVID‐19 infection in three regions in China. Neurology 2020; 95: e1479–1487. [DOI] [PubMed] [Google Scholar]
- 19. Dube M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ. Axonal transport enables neuron‐to‐neuron propagation of human coronavirus OC43. J Virol 2018; 92: e00404–e00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 2019; 17: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guan Y, Zheng BJ, He YQ, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 2003; 302: 276–278. [DOI] [PubMed] [Google Scholar]
- 22. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS‐CoV‐2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020; 9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoffmann M, Kleine‐Weber H, Pöhlmann S. A multibasic cleavage site in the spike protein of SARS‐CoV‐2 is essential for infection of human lung cells. Mol Cell 2020; 78: 779–784.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS‐CoV‐2 on virus entry and its immune cross‐reactivity with SARS‐CoV. Nat Commun 2020; 11: 1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qi J, Zhou Y, Hua J, et al. The scRNA‐seq expression profiling of the receptor ACE2 and the cellular protease TMPRSS2 reveals human organs susceptible to COVID‐19 infection. bioRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vabret A, Dina J, Brison E, Brouard J, Freymuth F. Human coronaviruses. Pathol Biol (Paris) 2009; 57: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glass WG, Subbarao K, Murphy B, Murphy PM. Mechanisms of host defense following severe acute respiratory syndrome‐coronavirus (SARS‐CoV) pulmonary infection of mice. J Immunol 2004; 173: 4030–4039. [DOI] [PubMed] [Google Scholar]
- 29. Li K, Wohlford‐Lenane C, Perlman S, et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis 2016; 213: 712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Talbot PJ, Ekande S, Cashman NR, Mounir S, Stewart JN. Neurotropism of human coronavirus 229E. Adv Exp Med Biol 1993; 342: 339–346. [DOI] [PubMed] [Google Scholar]
- 31. Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol 2000; 74: 8913–8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu J, Zhong S, Liu J, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine MIG in pathogenesis. Clin Infect Dis 2005; 41: 1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nilsson A, Edner N, Albert J, Ternhag A. Fatal encephalitis associated with coronavirus OC43 in an immunocompromised child. Infect Dis (Lond) 2020; 52: 419–422. [DOI] [PubMed] [Google Scholar]
- 34. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. Int J Infect Dis 2020; 94: 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morfopoulou S, Brown JR, Davies EG, et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med 2016; 375: 497–498. [DOI] [PubMed] [Google Scholar]
- 36. Miller KD, Schnell MJ, Rall GF. Keeping it in check: chronic viral infection and antiviral immunity in the brain. Nat Rev Neurosci 2016; 17: 766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID‐19 pandemic. Rhinology 2020; 58: 295–298. [DOI] [PubMed] [Google Scholar]
- 38. Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R. Expression of the SARS‐CoV‐2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem Neurosci 2020; 11: 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brann DH, Tsukahara T, Weinreb C, et al. Non‐neuronal expression of SARS‐CoV‐2 entry genes in the olfactory system suggests mechanisms underlying COVID‐19‐associated anosmia. Sci Adv 2020; 6: eabc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cantuti‐Castelvetri L, Ojha R, Pedro L, et al. Neuropilin‐1 facilitates SARS‐CoV‐2 cell entry and provides a possible pathway into the central nervous system. bioRxiv 2020. [Google Scholar]
- 41. Mengeling WL, Boothe AD, Ritchie AE. Characteristics of a coronavirus (strain 67N) of pigs. Am J Vet Res 1972; 33: 297–308. [PubMed] [Google Scholar]
- 42. Andries K, Pensaert MB. Immunofluorescence studies on the pathogenesis of hemagglutinating encephalomyelitis virus infection in pigs after oronasal inoculation. Am J Vet Res 1980; 41: 1372–1378. [PubMed] [Google Scholar]
- 43. Li YC, Bai WZ, Hirano N, et al. Neurotropic virus tracing suggests a membranous‐coating‐mediated mechanism for transsynaptic communication. J Comp Neurol 2013; 521: 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perlman S, Jacobsen G, Afifi A. Spread of a neurotropic murine coronavirus into the CNS via the trigeminal and olfactory nerves. Virology 1989; 170: 556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Perlman S, Evans G, Afifi A. Effect of olfactory bulb ablation on spread of a neurotropic coronavirus into the mouse brain. J Exp Med 1990; 172: 1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Matsuda K, Park CH, Sunden Y, et al. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza A virus in mice. Vet Pathol 2004; 41: 101–107. [DOI] [PubMed] [Google Scholar]
- 47. Chasey D, Alexander DJ. Morphogenesis of avian infectious bronchitis virus in primary chick kidney cells. Arch Virol 1976; 52: 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008; 82: 7264–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sungnak W, Huang N, Becavin C, et al. SARS‐CoV‐2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020; 26: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matsuda K, Shibata T, Sakoda Y, et al. In vitro demonstration of neural transmission of avian influenza A virus. J Gen Virol 2005; 86: 1131–1139. [DOI] [PubMed] [Google Scholar]
- 51. Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Front Psychiatry 2018; 9: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang H, Kang Z, Gong H, et al. Digestive system is a potential route of COVID‐19: an analysis of single‐cell coexpression pattern of key proteins in viral entry process. Gut 2020; 69: 1010–1018. [Google Scholar]
- 53. Holzer P, Schicho R, Holzer‐Petsche U, Lippe IT. The gut as a neurological organ. Wien Klin Wochenschr 2001; 113: 647–660. [PubMed] [Google Scholar]
- 54. Esposito G, Pesce M, Seguella L, Sanseverino W, Lu J, Sarnelli G. Can the enteric nervous system be an alternative entrance door in SARS‐CoV‐2 neuroinvasion? Brain Behav Immun 2020; 87: 93–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tian Y, Rong L, Nian W, He Y. Review article: Gastrointestinal features in COVID‐19 and the possibility of faecal transmission. Aliment Pharmacol Ther 2020; 51: 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhou J, Li C, Zhao G, et al. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci Adv 2017; 3: eaao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xia L, Yang Y, Wang J, Jing Y, Yang Q. Impact of TGEV infection on the pig small intestine. Virol J 2018; 15: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe 2013; 13: 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lafon M. Modulation of the immune response in the nervous system by rabies virus. Curr Top Microbiol Immunol 2005; 289: 239–258. [DOI] [PubMed] [Google Scholar]
- 60. Ren R, Racaniello VR. Human poliovirus receptor gene expression and poliovirus tissue tropism in transgenic mice. J Virol 1992; 66: 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ohka S, Nihei C, Yamazaki M, Nomoto A. Poliovirus trafficking toward central nervous system via human poliovirus receptor‐dependent and ‐independent pathway. Front Microbiol 2012; 3: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cabello‐Verrugio C, Morales MG, Rivera JC, Cabrera D, Simon F. Renin‐angiotensin system: an old player with novel functions in skeletal muscle. Med Res Rev 2015; 35: 437–463. [DOI] [PubMed] [Google Scholar]
- 63. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Beydon M, Chevalier K, Al Tabaa O, et al. Myositis as a manifestation of SARS‐CoV‐2 [published online ahead of print]. Ann Rheum Dis 2020. [DOI] [PubMed] [Google Scholar]
- 67. Kim JE, Heo JH, Kim HO, et al. Neurological complications during treatment of Middle East respiratory syndrome. J Clin Neurol 2017; 13: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Langen UH, Ayloo S, Gu C. Development and cell biology of the blood–brain barrier. Annu Rev Cell Dev Biol 2019; 35: 591–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Correale J, Villa A. Cellular elements of the blood–brain barrier. Neurochem Res 2009; 34: 2067–2077. [DOI] [PubMed] [Google Scholar]
- 70. Begley DJ, Brightman MW. Structural and functional aspects of the blood–brain barrier. Prog Drug Res 2003; 61: 39–78. [DOI] [PubMed] [Google Scholar]
- 71. McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat Rev Immunol 2011; 11: 318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Daneman R, Prat A. The blood–brain barrier. Cold Spring Harb Perspect Biol 2015; 7: a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Miner JJ, Diamond MS. Mechanisms of restriction of viral neuroinvasion at the blood–brain barrier. Curr Opin Immunol 2016; 38: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Suen WW, Prow NA, Hall RA, Bielefeldt‐Ohmann H. Mechanism of West Nile virus neuroinvasion: a critical appraisal. Viruses 2014; 6: 2796–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yarlagadda A, Alfson E, Clayton AH. The blood–brain barrier and the role of cytokines in neuropsychiatry. Psychiatry (Edgmont) 2009; 6: 18–22. [PMC free article] [PubMed] [Google Scholar]
- 76. Hsieh JT, St John AL. Japanese encephalitis virus and its mechanisms of neuroinvasion. PLoS Pathog 2020; 16: e1008260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gonzalez‐Scarano F, Tyler KL. Molecular pathogenesis of neurotropic viral infections. Ann Neurol 1987; 22: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis 2010; 37: 13–25. [DOI] [PubMed] [Google Scholar]
- 79. Alimonti JB, Ribecco‐Lutkiewicz M, Sodja C, et al. Zika virus crosses an in vitro human blood–brain barrier model. Fluids Barriers CNS 2018; 15: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Verma S, Lo Y, Chapagain M, et al. West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: transmigration across the in vitro blood–brain barrier. Virology 2009; 385: 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dai J, Wang P, Bai F, Town T, Fikrig E. ICAM‐1 participates in the entry of West Nile virus into the central nervous system. J Virol 2008; 82: 4164–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Diamond MS, Klein RS. West Nile virus: crossing the blood–brain barrier. Nat Med 2004; 10: 1294–1295. [DOI] [PubMed] [Google Scholar]
- 84. Kim WK, Corey S, Alvarez X, Williams K. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol 2003; 74: 650–656. [DOI] [PubMed] [Google Scholar]
- 85. Joseph SB, Arrildt KT, Sturdevant CB, Swanstrom R. HIV‐1 target cells in the CNS. J Neurovirol 2015; 21: 276–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Deng H, Liu R, Ellmeier W, et al. Identification of a major co‐receptor for primary isolates of HIV‐1. Nature 1996; 381: 661–666. [DOI] [PubMed] [Google Scholar]
- 87. Bentz GL, Jarquin‐Pardo M, Chan G, Smith MS, Sinzger C, Yurochko AD. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J Virol 2006; 80: 11539–11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chan G, Nogalski MT, Stevenson EV, Yurochko AD. Human cytomegalovirus induction of a unique signalsome during viral entry into monocytes mediates distinct functional changes: a strategy for viral dissemination. J Leukoc Biol 2012; 92: 743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rhoades RE, Tabor‐Godwin JM, Tsueng G, Feuer R. Enterovirus infections of the central nervous system. Virology 2011; 411: 288–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Neal JW. Flaviviruses are neurotropic, but how do they invade the CNS? J Infect 2014; 69: 203–215. [DOI] [PubMed] [Google Scholar]
- 91. Law HK, Cheung CY, Ng HY, et al. Chemokine up‐regulation in SARS‐coronavirus‐infected, monocyte‐derived human dendritic cells. Blood 2005; 106: 2366–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chu H, Zhou J, Wong BH, et al. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J Infect Dis 2016; 213: 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang K, Chen W, Zhou Y‐S, et al. SARS‐CoV‐2 invades host cells via a novel route: CD147‐spike protein. bioRxiv 2020. [Google Scholar]
- 94. Iacono KT, Brown AL, Greene MI, Saouaf SJ. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp Mol Pathol 2007; 83: 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020; 367: eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ramakrishnaiah V, Thumann C, Fofana I, et al. Exosome‐mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci USA 2013; 110: 13109–13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bukong TN, Momen‐Heravi F, Kodys K, Bala S, Szabo G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2‐miR122‐HSP90. PLoS Pathog 2014; 10: e1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. van Dongen HM, Masoumi N, Witwer KW, Pegtel DM. Extracellular vesicles exploit viral entry routes for cargo delivery. Microbiol Mol Biol Rev 2016; 80: 369–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Morris‐Love J, Gee GV, O'Hara BA, et al. JC polyomavirus uses extracellular vesicles to infect target cells. mBio 2019; 10: e00379‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ludlow M, Kortekaas J, Herden C, et al. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol 2016; 131: 159–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. MacMicking JD. Interferon‐inducible effector mechanisms in cell‐autonomous immunity. Nat Rev Immunol 2012; 12: 367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID‐19‐associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 2020; 296: E119–E120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Meessen‐Pinard M, Le Coupanec A, Desforges M, Talbot PJ. Pivotal role of receptor‐interacting protein kinase 1 and mixed lineage kinase domain‐like in neuronal cell death induced by the human neuroinvasive coronavirus OC43. J Virol 2017; 91: e01513–e01516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Garden GA. Microglia in human immunodeficiency virus‐associated neurodegeneration. Glia 2002; 40: 240–251. [DOI] [PubMed] [Google Scholar]
- 105. Murray RS, Brown B, Brian D, Cabirac GF. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann Neurol 1992; 31: 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fazzini E, Fleming J, Fahn S. Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson's disease. Mov Disord 1992; 7: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jang H, Boltz DA, Webster RG, Smeyne RJ. Viral parkinsonism. Biochim Biophys Acta 2009; 1792: 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dugger BN, Dickson DW. Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol 2017; 9: a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wu Y, Le W, Jankovic J. Preclinical biomarkers of Parkinson disease. Arch Neurol 2011; 68: 22–30. [DOI] [PubMed] [Google Scholar]
- 110. Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003; 24: 197–211. [DOI] [PubMed] [Google Scholar]
- 111. Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual‐hit hypothesis. Neuropathol Appl Neurobiol 2007; 33: 599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Barnett EM, Perlman S. The olfactory nerve and not the trigeminal nerve is the major site of CNS entry for mouse hepatitis virus, strain JHM. Virology 1993; 194: 185–191. [DOI] [PubMed] [Google Scholar]
- 113. Howitt J, Hill AF. Exosomes in the pathology of neurodegenerative diseases. J Biol Chem 2016; 291: 26589–26597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pahan P, Pahan K. Smooth or risky revisit of an old malaria drug for COVID‐19? J Neuroimmune Pharmacol 2020; 15: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Manzo C, Gareri P, Castagna A. Psychomotor agitation following treatment with hydroxychloroquine. Drug Saf Case Rep 2017; 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kenna HA, Poon AW, de los Angeles CP, Koran LM. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci 2011; 65: 549–560. [DOI] [PubMed] [Google Scholar]
- 117. Nellan A, McCully CML, Cruz Garcia R, et al. Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood 2018; 132: 662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jewell P, Ansorge O, Kuker W, Irani SR, Zamboni G. Tocilizumab‐associated multifocal cerebral thrombotic microangiopathy. Neurol Clin Pract 2016; 6: e24–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pelosi P, Rocco PR. The lung and the brain: a dangerous cross‐talk. Crit Care 2011; 15: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Schirinzi T, Cerroni R, Di Lazzaro G, et al. Self‐reported needs of patients with Parkinson's disease during COVID‐19 emergency in Italy. Neurol Sci 2020; 41: 1373–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhu S, Wu Y, Zhu CY, et al. The immediate mental health impacts of the COVID‐19 pandemic among people with or without quarantine managements. Brain Behav Immun 2020; 87: 56–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta‐1b, lopinavir‐ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID‐19: an open‐label, randomised, phase 2 trial. Lancet 2020; 395: 1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Novi G, Mikulska M, Briano F, et al. COVID‐19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult Scler Relat Disord 2020; 42: 102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Rejdak K, Grieb P. Adamantanes might be protective from COVID‐19 in patients with neurological diseases: multiple sclerosis, parkinsonism and cognitive impairment. Mult Scler Relat Disord 2020; 42: 102163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Román GC, Reis J, Spencer PS, et al. COVID‐19 international neurological registries. Lancet Neurol 2020; 19: 484–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.


