Abstract
Coronavirus disease 2019 (COVID‐19) is a life‐threatening infectious respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). COVID‐19 pandemic causing morbidities and even deaths worldwide revealed that there is urgent need to find pharmacological agents or vaccines. Although there are a lot of agents under investigation, there is no approved agent for the prevention or treatment of the COVID‐19 yet. Treatment of patients remains mainly supportive as well as compassionate use of the agents under investigation. It is well established that excessive inflammatory and immune response and oxidative injury play a critical role in the pathogenesis of COVID‐19. In this review, we aimed to update knowledge about pathogenesis, clinical features, and pharmacological treatment of COVID‐19 and review the potential beneficial effects of ancient antioxidant, anti‐inflammatory, and immunomodulatory molecule melatonin for prevention and treatment of COVID‐19.
Keywords: melatonin, COVID‐19, renin–angiotensin system, antioxidant, anti‐inflammatory, immunomodulatory
Introduction
Coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was first seen as cases of pneumonia of unknown cause in December 2019 in Wuhan, China. Unfortunately, it spread all over the world by human‐to‐human transmission and became a pandemic [1, 2]. According to the situation report published by the World Health Organization (WHO), as of June 28, 2020, 495 760 people died from COVID‐19 and there are 9 843 073 confirmed cases worldwide [3].
COVID‐19 is a respiratory disease caused by SARS‐CoV‐2. Common symptoms of the COVID‐19 are fever, dry cough, and fatigue. The following symptoms may also be seen: shortness of breath, aches and pains, sore throat, diarrhea, nausea, or a runny nose. Although COVID‐19 causes mild to moderate symptoms in most infected individuals, there is a higher risk of developing severe disease and death in people with comorbid disease or over 60 years of age [4]. In cases developing severe disease, it was reported to cause acute lung injury (ALI)/acute respiratory distress syndrome (ARDS), respiratory failure, heart failure, sepsis, and sudden cardiac arrest within a few days [5]. It is well known that ARDS and multiple organ failure if they are not treated enough they may lead to death [6].
Despite all efforts, there is no approved vaccine or pharmacological agent for the prevention or treatment of the COVID‐19 yet. Treatment of patients remains mainly supportive as well as compassionate or off‐label use of the some unapproved agents. It is well known that inappropriate inflammatory and immune response and oxidative damage play an important role in the pathogenesis of COVID‐19 [7, 8].
Melatonin, an indolamine molecule, is mainly produced by pinealocytes in the pineal gland in humans. As a pleiotropic molecule, melatonin exerts substantial anti‐inflammatory, antioxidant, and immunomodulatory properties [9].
In this review, we aimed to update knowledge about pharmacological treatment of COVID‐19 and review the potential beneficial effects of melatonin for COVID‐19 treatment, thus contributing to current scientific literature.
Pathogenesis of COVID‐19
Coronaviruses (CoVs) are belonged to ribonucleic acid (RNA) virus family mainly cause respiratory tract infections in mammals, including humans. Three highly pathogenic human CoVs (HCoVs), including SARS‐CoV, Middle East respiratory syndrome coronavirus (MERS‐CoV), and SARS‐CoV‐2, infecting the lower respiratory tract and causing severe pneumonia even leading to fatal ALI and ARDS, have caused to outbreaks in past two decades [1, 5].
The SARS‐CoV‐2 is known to have four viral structural proteins including spike (S) protein, envelope (E) protein, membrane (M) protein, and nucleocapsid (N) protein. Translation of replicase/transcriptase and viral structural proteins occurs through viral genome acting as messenger RNA (mRNA). The replicase/transcriptase genes consist of open reading frame (ORF) 1a and ORF1b. The viral genome consists of the ORFs encoding replicase/transcriptase and the genes encoding viral structural proteins [6].
The SARS‐CoV‐2 is believed to enter the body via settling on the mainly nasal mucosa through droplets and contacts, then reach the lungs through respiratory system. The SARS‐CoV‐2 may infect the targets expressing angiotensin‐converting enzyme 2 (ACE2) including the lung, heart, renal, and gastrointestinal system when viremia, originating from the lung, occurs [10]. Like the SARS‐CoV, ACE2 was reported to be SARS‐CoV‐2 receptor for host cell entry with its S protein. S1 subunit of S protein is necessary for attachment whereas S2 subunit is necessary for viral fusion and cell entry. Recently, it was shown that transmembrane serine protease 2 (TMPRSS2), cleaves the S protein from S1/S2 and the S2’ site for allowing viral fusion, primes S2 subunit for entry, and a serine protease inhibitor camostat mesylate hinders SARS‐CoV‐2 infection of lung cells [11, 12]. The S protein of SARS‐CoV‐2 binds ACE2 with higher affinity than SARS‐CoV [1].
The results of the clinical and preclinical studies have shown that inflammatory processes including release of cytokines and chemokines play an important role in the pathogenesis of infections caused by HCoVs. In in vitro studies, SARS‐CoV and MERS‐CoV infections were shown to associated with high levels of pro‐inflammatory cytokines (interleukin (IL)‐1β, IL‐6, and tumor necrosis factor (TNF)) and chemokines (C‐C motif chemokine ligand (CCL)‐2, CCL‐3, and CCL‐5); however, low levels of the antiviral factors interferons (IFNs) released by respiratory epithelial cells, dendritic cells (DCs), and macrophages. Elevated levels of cytokines and chemokines cause to neutrophil and monocyte infiltration in the lung, during SARS‐CoV and MERS‐CoV infections [6]. As a result of pro‐inflammatory cytokine/chemokine response during SARS‐CoV and MERS‐CoV infections, a cytokine storm may occurs; lung epithelial and endothelial cells may undergo apoptosis [5, 6]. These changes induce damaging of the alveolar–capillary barrier, alveolar edema, and disruption of gas exchange resulting in ARDS [6].
More recently, it was reported that the nucleotide sequence of SARS‐CoV‐2 is 79.7% identical to the SARS‐CoV and 51.8% identical to the MERS‐CoV [1]. As with SARS‐CoV and MERS‐CoV infections, in the critical patients with COVID‐19 a process called 'cytokine storm' is known to play an important role in the development of ARDS and multiple organ failure [5, 6]. Elevated levels in IL‐1β, IFN‐γ, interferon‐inducible protein 10 (IP‐10), and monocyte chemoattractant protein 1 (MCP‐1), as well as anti‐inflammatory cytokines IL‐4 and IL‐10, have shown in patients with COVID‐19 [13].
Current medications for COVID‐19
Although a considerable amount of experimental researches and clinical trials have been conducted to date, currently there is no FDA‐approved drug for the prevention and treatment of the COVID‐19. As a result, the treatment of patients with COVID‐19 is mostly based on supportive care including oxygen therapy and control of fever, prevention and/or treatment of complications, and mechanical ventilation support in severe cases [7].
A number of repurposed or investigational drugs and adjunctive therapies have been proposed for the treatment of COVID‐19. Some of the most prominent repurposed drugs include chloroquine and hydroxychloroquine, lopinavir/ritonavir, umifenovir, darunavir, darunavir/umifenovir [14], darunavir/cobicistat [15], oseltamivir [16], ribavirin [17], interferon alpha‐1b [18], nitazoxanide [19], ivermectin [20], and camostat mesylate [21]. Adjunctive therapies for patients with COVID‐19 are consisted of corticosteroids, anti‐cytokine, or immunomodulatory agents such as tocilizumab, sarilumab, bevacizumab, fingolimod, eculizumab, and convalescent plasma [22], high‐dose vitamin C [23], dietary supplement of vitamin D [24], and ozone therapy [25]. Also, in recently published preliminary report of the Randomised Evaluation of COVID‐19 therapy (RECOVERY) trial from United Kingdom (UK), easily available and cheap drug dexamethasone (6 mg/day for up to 10 days) treatment was reported to decrease 28‐day mortality rates of COVID‐19 patients under invasive mechanical ventilation or oxygen therapy unlike patients who do not need respiratory support [26]. Some important agents under investigation are summarized in Figure 1 .
Figure 1.
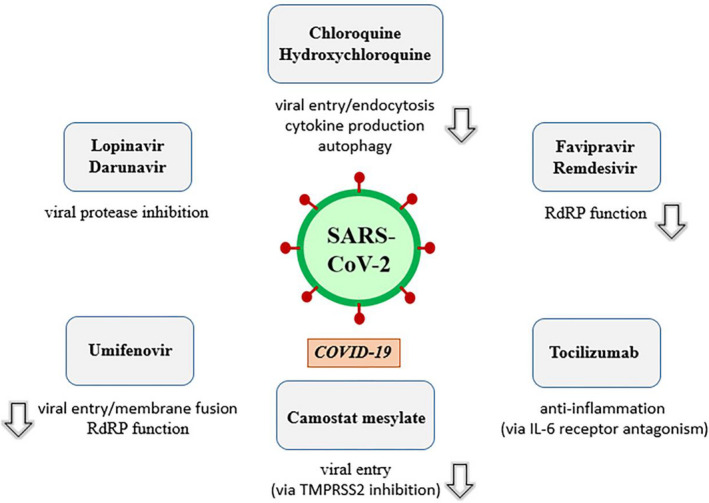
Important pharmacological agents under investigation against COVID‐19 and their mechanism of action. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane serine protease 2; COVID‐19, coronavirus disease 2019; RdRP, RNA‐dependent RNA polymerase; IL‐6, interleukin 6.
Chloroquine and hydroxychloroquine are FDA‐approved drugs with approval dates 1949 and 1955, respectively, for the prevention and treatment of malaria and the treatment of systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) [22, 27, 28]. In addition to inhibition of viral entry and endocytosis, they attenuate cytokine production and inhibit autophagy and lysosomal activity in host cells. Chloroquine and hydroxychloroquine were reported to have in vitro activity against SARS‐CoV‐2 [22]. There are a lot of ongoing clinical trials related to the use of chloroquine and hydroxychloroquine for COVID‐19 [29, 30].
Chloroquine is recommended to use 500 mg twice daily for 10 days for mild, moderate, and severe cases of COVID‐19 [31]. Although the optimal dose for hydroxychloroquine for COVID‐19 remains unclear, according to the result of a study implementing physiologically based pharmacokinetic models, it is recommended to use a loading dose of 400 mg twice daily given orally for the first day, followed by a maintenance dose of 200 mg given twice daily for 4 days [32]. Although hydroxychloroquine has not been approved by the FDA for this indication, FDA published a fact sheet regarding as emergency use authorization of hydroxychloroquine for treatment of COVID‐19 [33].
It should be kept in mind that chloroquine and hydroxychloroquine can cause QT prolongation, retinopathy, hypoglycemia, and neuropsychiatric clinical manifestations. While these drugs are compassionately used, it is should not be forgotten that QT prolongation may result in death by increasing the risk of cardiac arrhythmia. Concomitant use with other drugs (azithromycin, etc.) which prolong QT requires attention [34, 35, 36, 37].
Lopinavir/ritonavir is an available drug combination for the treatment of human immunodeficiency virus (HIV) infection. It has been proposed against SARS‐CoV‐2. It is believed that lopinavir inhibits viral protease, thereby virus remains immature and loses the ability to infect cells. Ritonavir is used to prolong the duration of the effect of lopinavir by inhibiting its hepatic metabolism [38]. Lopinavir/ritonavir is proposed to use at a dose of 400 mg/100 mg twice a day for up to 14 days for COVID‐19. Lopinavir/ritonavir has gastrointestinal adverse effects including nausea, diarrhea, and hepatotoxicity [22].
Darunavir, a protease inhibitor, has been used to treat HIV infection [39]. A study of molecular dynamics simulations and virtual screening proposed darunavir as a repurposed drug for COVID‐19 [40]. Like other antiviral agents, there is off‐label use of darunavir/cobicistat for COVID‐19 [41]. Cobicistat, a CYP3A inhibitor, improves oral bioavailability and reduces systemic clearance of darunavir, resulting in increased plasma concentration [42]. A phase 3 clinical trial of darunavir/cobicistat for COVID‐19 is ongoing [15].
Umifenovir, also called as arbidol, is used for influenza. It has been proposed for COVID‐19 based on its inhibitory effect on RNA‐dependent RNA polymerase (RdRP) [43]. In addition, it targets the S protein/ACE2 interaction and inhibits membrane fusion of the viral envelope. It is under a clinical trial with oral dose of 200 mg every 8 h for COVID‐19 treatment [22, 44].
Camostat mesylate is used for the treatment of pancreatitis. It inhibits TMPRSS2, thereby hinders host cell entry of the virus [22].
Tocilizumab which is used for RA has off‐label use for COVID‐19 treatment, based on anti‐inflammatory effects. It is a recombinant humanized anti‐human IL‐6 receptor monoclonal antibody. In a retrospective study, tocilizumab was found to be associated with improve in the symptoms, oxygenation of blood, and pulmonary opacities in patients with severe COVID‐19 [45]. Tocilizumab was reported to cause abnormal liver function tests, neutropenia and anaphylaxis [46].
Remdesivir is a broad‐spectrum antiviral nucleotide prodrug with potent in vitro antiviral activity against Ebola virus (EBOV), MERS‐CoV, SARS‐CoV, respiratory syncytial virus (RSV), and SARS‐CoV‐2 [47, 48]. Remdesivir, an investigational nucleotide analogue, inhibits viral RdRP [49]. Its proposed dose is 200 mg loading dose, followed by 100 mg daily infusion for COVID‐19 treatment in a clinical trial [22]. In a randomized controlled trial of Ebola virus disease (EVD) therapeutics, remdesivir was reported to may be associated with hypotension followed by cardiac arrest in a patient [50].
Favipiravir is used for influenza in Japan. It acts through its active metabolite favipiravir ribofuranosyl‐5′‐triphosphate, a purine nucleotide which inhibits viral RdRP. It has in vitro activity against SARS‐CoV‐2 [22]. In an open‐label control study, favipiravir was given at an oral dose of 1 600 mg twice a day on first day and 600 mg twice a day on days 2–14 to patients with COVID‐19. It has been reported following side effects due to favipiravir; diarrhea, liver injury, and poor diet [51].
Knowledge about proposed drugs for the treatment of COVID‐19 depends on in vitro and animal studies as well as clinical data with low level of evidence such as case reports, case series, and clinical trials with insufficient sample size and risk of bias in the literature [22]. There is an urgent need for the development of effective prevention and treatment strategies for COVID‐19. In addition, it should not be forgotten that there is also need to continuous public drug information service regarding COVID‐19 treatment [52].
An overview of melatonin
Melatonin, also called as N‐acetyl‐5‐methoxytryptamine, is an indolamine molecule (Figure 2 ). In humans, this hormone is mainly produced by pinealocytes in the pineal gland, then released in blood. Melatonin both regulates circadian rhythm [53], and its biosynthesis in the pineal gland is correlated with the circadian rhythm which is provided by a neural system. When light stimulus reaches the retina, retinohypothalamic tract (RHT), which originates from retinal ganglion cells, sends photic signals to hypothalamic suprachiasmatic nuclei (SCN). GABAergic input originates in SCN reaches the hypothalamic paraventricular nuclei (PVN) which projects directly and indirectly to the preganglionic sympathetic neurons of the first thoracic segments of the spinal cord. Then, nerve fibers forming the conary nerves reach the pineal gland by a projection of the postganglionic sympathetic neurons which release norepinephrine, of the superior cervical ganglia (SCG). As a result, light stimulus inhibits melatonin synthesis through the suppression provided by SCN on the PVN. On the contrary, this suppression on the PVN is being removed in the dark [54, 55].
Figure 2.
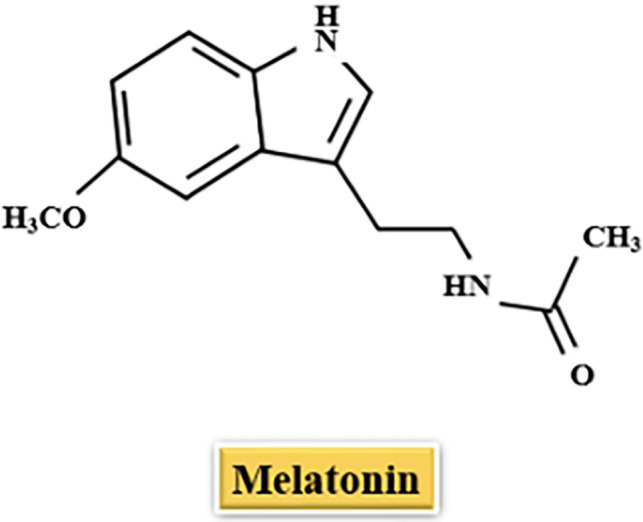
Chemical structure of the melatonin.
Norepinephrine released by the end of the sympathetic neurons bind to α1 and β1 adrenergic receptors in the pinealocytes’ membrane. Melatonin synthesis is induced by activation of cyclic adenosine monophosphate (cAMP)‐protein kinase A (PKA)‐cAMP response element binding protein (CREB) and phospholipase C (PLC)‐Ca2+‐protein kinase C (PKC) cascades due to norepinephrine–receptor binding. Activation of these cascades leads to increase in arylalkylamine N‐acetyltransferase (AANAT) which transforms serotonin to N‐acetylserotonin (NAS) [54, 56].
When β1‐adrenergic receptor on the pinealocyte’s membrane is being stimulated, cAMP level and PKA activity increases, respectively, and CREB is being stimulated followed by the increase in production of N‐acetyltransferase (NAT). Alpha1‐adrenergic receptors strengthen β1‐adrenergic activity by increasing PLC activity which leads to subsequently increase in the level of cytosolic Ca2+ and the activity of protein kinase C (PKC) and prostaglandins [55].
Melatonin is synthesized from tryptophan through four consecutive enzymatic reactions. First step is the conversion of tryptophan to 5‐hydroxytryptophan by tryptophan hydroxylase. In the second step, 5‐hydroxytryptophan is decarboxylated to serotonin. Then, serotonin is converted to N‐acetylserotonin (NAS) by AANAT followed by conversion of NAS to melatonin by acetylserotonin O‐methyltransferase (ASMT) [54, 57].
Melatonin exerts both lipophilic and hydrophilic properties. Melatonin is not stored in the pinealocytes and can easily across the blood brain barrier depend on its amphiphilic feature. It immediately enters the cerebrospinal fluid (CSF) and blood, once synthesized in the pineal gland [53]. Melatonin binds to albumin about 70% in the blood. It has been reported to have a half‐life of about 3–45 min based on its biphasic elimination when orally administered. Also, it has a half‐life of about 30 min following intravenous infusion [55].
Melatonin is transformed to 6‐hydroxymelatonin (6‐HM) by CYP1A2 and conjugated to 6‐sulfatoxymelatonin (6‐SM) in the liver and also kidney. These metabolites eliminated through urine [54]. The main urinary metabolite of melatonin is 6‐SM in humans [55].
In the central nervous system (CNS), melatonin is enzymatically, pseudoenzymatically or nonenzymatically metabolized to N1‐acetyl‐N2‐formyl‐5‐methoxykynuramine (AFMK) which is converted to N‐acetyl‐5‐methoxykynuramine (AMK) by undergoing deformylation [56]. Melatonin, AFMK, and AMK have free radical scavenging activity and protective effects against oxidative damage. AFMK is the poorest scavenger among them. Generally, protective effects against oxidative damage may be ordered as follows: AMK > melatonin > AFMK [58].
Melatonin, is known to have substantial anti‐inflammatory properties and activates a number of antioxidative enzymes such as glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT), regulates expression of several defensive enzymes and diminishes lipid peroxidation and apoptosis. It is clear that oxidative stress markedly triggers apoptosis [9]. Also, melatonin has several pleiotropic effects by distinct mechanisms. In this regard, melatonin, one of the most powerful natural antioxidants, directly interacts with reactive oxygen and nitrogen species, providing antioxidant effects independently of its cellular receptors and mobilizing the intracellular antioxidant enzymatic system. Moreover, melatonin has specific cellular membrane receptors including MT1 (MTNR1A in humans) and MT2 (MTNR1B in humans). These receptors are heterotrimeric Gi/Go and Gq/11 protein‐coupled receptors which interact with adenylyl cyclase (AC), phospholipase A2 (PLA2), and PLC, resulting in reduced cAMP and cGMP production and/or increment in diacylglycerol (DAG) and inositol 1,4,5‐trisphosphate (IP3) formation. MT1 and MT2 receptors are found in nearly all peripheral tissues in addition to the CNS. Melatonin has been reported to bind to retinoid orphan receptors/retinoid Z receptors (ROR/RZR nuclear receptors) [54]. Also, melatonin has been shown to bind to a cytosolic enzyme, quinone reductase 2 (QR2), previously called as MT3 receptor [59].
Experimental use of the melatonin against viral infections
Melatonin has been reported to be effective against several viral infections [60]. Melatonin exerts indirect antiviral properties by reducing inflammation and oxidative stress, and also by modulating immune responses [5].
Ebola virus disease is a rapidly progressive and highly lethal disease caused by EBOV. The most important cause of death due to EVD is hemorrhagic shock syndrome (HSS) [61]. There is currently no FDA‐approved antiviral agent for the treatment of patients with EVD. Although there are investigational antiviral drugs such as regeneron (REGN‐EB3) and mAb114 for EVD, treatment mainly remains supportive [62]. EBOV causes severe vascular leakage also called as Ebola hemorrhagic shock syndrome (EHSS) by activating the Rho/Rho‐associated protein kinase (Rho/ROCK) pathway which leads to actin bundle formation and a tensile force relaxing the junctions between the vascular endothelial cells (VECs). Recently, melatonin was found to effective against Ebola hemorrhagic shock syndrome in an engineered microvessel‐on‐a‐chip model. In this study, melatonin was observed to reduce vascular permeability by inhibiting Rho/ROCK signaling in VECs [61].
Respiratory syncytial virus may lead to severe lung disease because of excessive inflammatory immune response in childhood. In this regard, oral pre‐administration of melatonin at dose of 5 mg/kg twice daily for 3 days was shown to result in a significant decrement of oxidative lung damage and inflammation in mice infected with RSV. It was reported that melatonin, markedly inhibited the increment of malondialdehyde (MDA), nitric oxide (NO), and hydroxyl radical (·OH) levels and restored the reduced glutathione (GSH) and SOD levels in the lung due to its antioxidant and free radical scavenger effects. Also, melatonin significantly inhibited inflammation by reducing elevated level of serum TNF‐α [63]. The protective effect of melatonin against RSV infection has also been shown in vitro study. According to the result of this study, melatonin pre‐treatment was observed to inhibit the elevation of toll‐like receptor 3 (TLR3)‐mediated inflammatory gene expression including nuclear nuclear factor kappa‐light‐chain enhancer of activated B cells (NF‐κB), TNF‐α and inducible nitric oxide synthase (iNOS) without affecting TLR3 protein, in RSV‐infected macrophages. It was suggested that melatonin suppresses the elevation of TNF‐α and iNOS expression by inhibiting NF‐κB nuclear translocation [64].
Venezuelan equine encephalitis (VEE) is a viral infection caused by VEE virus (VEEV) which especially affects human and equines. The inflammation induced by VEEV is associated with a high mortality rate in mice. According to data obtained from an experimental study, while melatonin application for pre‐treatment (three days before the infection and continuing until the end of the experiment) and treatment (during the infection) increased the survival rate, melatonin application for post‐treatment (24 h after the infection) was found to be ineffective on survival in mice infected with VEEV, suggesting melatonin has a preventive effect on VEE infection. Melatonin administrated at a dose of 500 mcg/kg by subcutaneous (sc) injection in all in vivo experiments of mentioned study. Melatonin reduced the apoptosis in the brain of infected mice and in the VEEV‐infected neuroblastoma cells. On the contrary, melatonin was reported to cause apoptosis of uninfected neuroblastoma cells [65]. Melatonin was also shown to protect mice infected with the VEEV by decreasing mortality rate, postponing the onset of the disease, and reducing viral load in the brain as well as in the blood. Also, it has been reported that melatonin diminishes the cell destruction in the chicken embryo fibroblasts infected with the VEEV [66].
Melatonin also was shown to have protective effect against acute liver failure caused by rabbit hemorrhagic disease virus (RHDV) in an experimental study. It was reported that melatonin exerts anti‐inflammatory effect including decrement in TNF‐α and IL‐6, and decreases viral replication by inhibiting hepatic sphingosine kinase 1 (SphK1)/sphingosine‐1‐phosphate (S1P) signaling pathway in rabbits infected with RHDV [67].
Melatonin also was reported to protect minks from Aleutian disease (AD) is a viral disease which leads to lesions in the kidney, liver, lungs, and arteries because of hypergammaglobulinemia and immune complexes caused by AD parvovirus. Melatonin was observed to markedly reduced mortality rate in minks infected with AD virus. It was suggested that protective effect of melatonin may be due to its free radical scavenger, immunomodulator, and antioxidant enzyme‐inducing properties [68].
Influenza virus causes respiratory tract infections likely by apoptosis and production of reactive oxygen species (ROS) in the airway epithelial cells. Melatonin exerts protective and therapeutic effects against influenza virus. In a recent study, efficacy of melatonin was investigated on a murine model of influenza A infection. Melatonin was administered as prophylactically (20 mg/kg or 200 mg/kg sc) or therapeutically (200 mg/kg sc). Prophylaxis or treatment with 200 mg/kg melatonin increased survival rate. Treatment with 200 mg/kg melatonin was reported to result in reduced levels of TNF‐α, IL‐6, and IFN‐γ, and elevated levels of IL‐10 and TGF‐β in bronchoalveolar lavage fluid (BALF) reflecting production in lung. In addition, melatonin treatment was reported to decrease pulmonary leukocyte infiltrates and the phosphorylation of NF‐κB p65 in the lung homogenate of mice, suggesting reduced levels of pro‐inflammatory cytokines associated with reduced activation of NF‐κB. According to this study, supplementation of melatonin to ribavirin treatment resulted in higher survival rate than ribavirin alone [69]. On the contrary, long‐term dietary supplementation of melatonin was reported not to have significant effect on lung IL‐1β, IL‐6, and TNF‐α levels, plasma IL‐6 level, H2O2 production by lung cells, liver 4‐hydroxynonenal (4‐HNE), and MDA levels, pulmonary viral titers, preventing the weight loss and decreased food intake in mice infected with influenza [70]. The lack of studies makes it difficult to comment on whether melatonin is effective in the prophylaxis or treatment of influenza. More studies are needed to elucidate the beneficial effects of melatonin on influenza infection.
According to aforementioned data obtained from experimental studies in the literature, melatonin may be a potential therapeutic agent for the prevention of disease development and reducing disease severity as well as mortality rates in numerous viral infections.
Melatonin and COVID‐19
Oxidized products are released during viral infections and replication [5]. Oxidative damage and inflammation have an important role in the pathogenesis of viral infections. Damage of many organs may occur as a result of these pathological processes [1]. Melatonin, a powerful endogen antioxidant, free radical scavenger, and anti‐inflammatory molecule, has been reported to exert beneficial effects on viral diseases [60]. In addition, the effectiveness of melatonin has been demonstrated in many different conditions associated with inflammation ischemia–reperfusion (I/R) injury and oxidative stress. Our results showed that physiological melatonin concentration has an important role in the protection from I/R injury in the body [71]. In this context, we previously observed that melatonin is a protective agent against liver damage, induced by pinealectomy, renal I/R or myocardial I/R [72, 73, 74], I/R injury of brain [75], heart [76] and flap injury [77], testicular injury [78], radiation damage [79], cerebral vasospasm after subarachnoidal hemorrhage [80] and colitis [9] in rats. In this context, there are several possible conditions increasing free radicals in COVID‐19 patients as follows: excessive inflammation, cytokine storm, hypoxemia, and respiratory support by mechanical ventilation. It is well known that mechanical ventilation can cause ventilator‐induced lung injury (VILI) which includes alveolar damage, lung edema, accumulation of immune cells, pro‐inflammatory cytokine discharge, and the exaggerated ROS generation. Unfortunately, VILI is a condition which can result in sequelae and even death. Recently, ramelteon, a melatonin receptor agonist, has been reported to protect lung from VILI by increasing IL‐10 production in a rat model [81]. Decreased level of intracellular heme oxygenase 1 (HO‐1), an antiviral, anti‐inflammatory, antioxidant, and cytoprotective stress protein, may exert an important role in the pathogenesis of COVID‐19 [82]. Melatonin is known to increase a group of antioxidant proteins including HO‐1 by activating NF‐E2‐related factor 2 (Nrf2) [83, 84]. In this regard, melatonin was proposed to use for COVID‐19 treatment because of its HO‐1 enhancing property [82]. According to our experiences and available literature data, it seems to us that melatonin may also be beneficial in COVID‐19 treatment [5], and anti‐inflammatory properties COVID‐19 blockade of heme synthesis could limit the HO‐1 stress tolerance function, contributing to host fragility.
As a good news, recently, melatonin was proposed to be a potential candidate drug as an adjuvant treatment for patients with COVID‐19 [5]. Also, melatonin was found to be candidate drug for COVID‐19 in a network‐based drug repurposing study. Melatonin was predicted to indirectly interact with the human CoVs‐associated cellular proteins, including ACE2, B‐cell lymphoma 2 (Bcl‐2)‐like protein 1 (BCL2L1), JUN, and inhibitor of NF‐κB kinase subunit beta (IKBKB). Also, according to this study, combination of mercaptopurine and melatonin may be a potential treatment for COVID‐19 by synergistically targeting papain‐like protease, ACE2, c‐Jun signaling, and anti‐inflammatory cascades [1].
The possible anti‐inflammatory effects of melatonin that may be beneficial in the ALI/ARDS induced by COVID‐19 involves upregulation of sirtuin‐1 (SIRT1), suppression of NF‐κB activation, and stimulation of NF‐E2‐related factor 2 (Nrf2), thus a decrement in the pro‐inflammatory cytokines (TNF‐α, IL‐1β, IL‐6, and IL‐8) and increment in the level of anti‐inflammatory cytokine IL‐10 [5]. In addition, melatonin exerts cardioprotective effects by modulating apoptosis and autophagy via elevating expression of SIRT1 in septic mice [85]. Preclinical and clinical studies have reported that melatonin reduces pro‐inflammatory cytokines in many diverse conditions. In this regard, melatonin has been reported to dose‐dependently reduce TNF‐α and IL‐1β production but not IL‐6 in human RA synovial fibroblasts [86]. Melatonin administration (orally 25 mg daily for 6 months) has been reported to reduce serum levels of TNF‐α, IL‐1β, IL‐6, lipoperoxides, and NO catabolites in patients with relapsing–remitting multiple sclerosis (RRMS) [87]. In a double‐blind, placebo‐controlled clinical trial, melatonin (orally 6 mg daily for 8 weeks) administration significantly decreased serum level of IL‐6 and high‐sensitivity C‐reactive protein (hs‐CRP) but not TNF‐α in type 2 diabetes mellitus (DM) patients with chronic periodontitis [88]. Melatonin has been reported to reduce TNF and IL‐6 levels, and increase IL‐10 level in human placental trophoblasts which have been undergone hypoxia/reoxygenation [89]. Melatonin administration (10 mg at 09:00 h and 60 mg at 21:00 h for 3 months) has been reported to reduce plasma levels of IL‐1β, IL‐2, IL‐6, and TNF‐α in patients with Charcot–Marie–Tooth Neuropathy [90]. In a double‐blind, placebo‐controlled clinical trial, melatonin administration (6 mg daily for 40 days) has been reported to reduce plasma levels of TNF‐α and IL‐6 in obese women [91]. Melatonin administration (25 mg/kg ip 30 min before each caerulein injection) has been reported to result in decrease in IL‐1β and TNF‐α, and increase in IL‐4 serum levels in rats with caerulein‐induced acute pancreatitis [92]. Melatonin administration (10 mg/kg ip) has been reported to reduce TNF‐α serum level in rats with Escherichia coli‐induced pyelonephritis [93]. These data of in vivo, in vitro, and clinical studies indicate that melatonin may be potential supportive agent in counteracting with cytokine storm in patients with COVID‐19.
Melatonin improves proliferation and maturation of natural killer (NK) cells, T and B lymphocytes, granulocytes, and monocytes, thus supports immune response. Melatonin augments antigen presentation in macrophages; thus, complement receptor 3, MHC class I and class II, and CD4 antigens are upregulated. Given the level of neutrophils, lymphocytes and CD8+ T cells may decrease in peripheral blood in COVID‐19 patients, melatonin may also be useful as an immunoregulator [5]. Also, melatonin has been reported to blunt the NF‐kB induction and decrease the NLRP3 expression in heart of mice with polymicrobial sepsis induced by the cecal ligation and puncture (CLP) model. It is well established that the nucleotide oligomerization domain (NOD)‐like receptor 3 (NLRP3) inflammasome plays an important role in the innate immune response during inflammatory states [94]. In addition, melatonin has been reported to reduce the macrophage and neutrophil infiltration in the lung by inhibiting NLRP3 inflammasome in experimental models. This effect may be another reason for using melatonin in the treatment of COVID‐19 [5].
Currently, in Spain, there is an ongoing multicenter randomized placebo‐controlled phase 2/3 clinical trial 'MeCOVID' investigating whether melatonin has an efficacy in the prophylaxis of COVID‐19 among healthcare workers. Status of this trial is not yet recruiting. Four hundred fifty participants between the ages of 18–65 are estimated to be included in the study. It is known that that the peak blood level of melatonin is higher in younger children and SARS‐CoV‐2 appears to less affect them when compared to other groups of age. In this regard, the researchers supposed that approximately reaching the melatonin levels in children may protect from infection or hinder progression to severe disease even if the infection occurs. For this purpose, melatonin with prolonged release will be administered orally at a dose of 2 mg daily before bedtime for 12 weeks. Confirmed symptomatic infection rate will be considered as primary outcome measure of the study [95].
Inhibition of melatonin by most viruses suggests that modulation of melatonin may be useful in the management of viral infections [96]. Although melatonin has not direct effects on viral replication or transcription, based on its antioxidant and anti‐inflammatory properties, it may be a potential drug to reduce the severity of clinical symptoms. Melatonin also may decrease mortality rate among patients with viral disease and save them time to recover [1]. Given the antioxidant and anti‐inflammatory effects, melatonin seems to be a potential agent for attenuation of COVID‐19 infection. As an adjuvant therapeutic agent, melatonin may be useful in COVID‐19 and related complications including ALI and ARDS likely by immune regulation, anti‐inflammation, and antioxidation [5]. There is urgent necessity to a lot of well‐designed preclinical and clinical studies investigating efficacy of melatonin for COVID‐19 treatment.
Melatonin‐RAS relationship: benefits for COVID‐19
ACE2, a homologue of ACE, is found as two types: membrane‐bound and soluble. Membrane‐bound ACE2 is a type I transmembrane metallopeptidase which comprises an extracellular catalytic domain, receptor for SARS‐CoV‐2, and a transmembrane anchor. Soluble ACE2 circulates in the blood and has no anchor [97]. ACE2 operates as a monocarboxypeptidase with its extracellular catalytic domain which degrades angiotensin II (Ang II) and angiotensin I (Ang I) into angiotensin 1–7 (Ang 1‐7) and angiotensin 1‐9 (Ang 1‐9), respectively [98]. In addition, ACE2 possibly exerts enzymatic effect on other substrates including apelin, des‐arginine bradykinin, and neurotensin. Ang 1‐7–mitochondrial assembly (MAS) receptor binding leads to vasodilation, anti‐inflammatory, and anti‐fibrotic effects. Thus, Ang 1‐7 establishes the balance by eliminating the harmful effects caused by Ang II [97].
ACE2 is located primarily in the lung (airways and type II alveolar cells), heart, kidney, and intestine. It is also found in oral and nasal mucosa, skin, lymph nodes, thymus, bone marrow, spleen, liver, testis, and brain [97].
Endocytosis of viral particles–ACE2 complex leads to decrease in membrane‐bound ACE2 expression. In addition, upregulation in protease activity of a disintegrin and metalloproteinase 17 (ADAM17) which separating 2 domains of ACE2 from each other results in sheds extracellular catalytic domain of ACE2 into the circulation. Thus, while activity of the protective ACE2/Ang 1‐7/MAS receptor axis is decreasing, increased activity of harmful ACE/Ang II/ Angiotensin II receptor type 1 (AT1 receptor) axis is occurred. Increased level of Ang II leads to further upregulation of ADAM17 activity through the AT1 receptors and downstream extracellular signal‐regulated kinase (ERK)/ p38 mitogen‐activated protein kinase (MAPK) signaling pathways. ADAM17 also associated with the liberation of membrane‐bound precursors of TNF‐α, IFN‐γ, and IL‐4 into the circulation. It is well established that IL‐4 and IFN‐γ reduce ACE2 expression [99. Therefore, reduction in tissue ACE2 levels may result in increased lung damage and tissue fibrosis seen in COVID‐19 cases [97]. COVID‐19 not only affects the lungs but also causes acute cardiac and renal damage, myocarditis, arrhythmias, and gut and liver pathologies. These events are considered to be related to the loss of tissue ACE2 as a result of COVID‐19 [99]. In this context, SARS‐CoV infection is also known to cause cardiac dysfunction, arrhythmias, and even cardiac death. SARS‐CoV infection has been reported to cause myocardial dysfunction by reducing myocardial ACE2 expression in mice [96]. Remarkably high Ang II plasma levels which correlated with viral load and pulmonary damage have been reported in patients with COVID‐19 [100]. This increase in Ang II levels in patients with COVID‐19 seems to be result of a decrease in tissue ACE2 levels. Also, reduced plasma Ang II/Ang 1‐7 ratios have been reported in recombinant human ACE2 (rhACE2)‐treated patients with pulmonary arterial hypertension and ALI. Furthermore, ACE inhibitors and ARBs have protective effects against dramatic results of cardiovascular disease partially by elevating ACE2 levels [99]. Based on this relationship, it has been recommended that patients under ACEI and ARB treatment should not discontinue their medication during COVID‐19 pandemic if there is no clinical indication [101]. Soluble form of ACE2 has been reported to block SARS‐CoV replication in the monkey kidney cell line. In addition, SARS‐CoV‐2 has been reported to be neutralized by ACE2 fused to the Fc portion of immunoglobulin. In this regard, soluble rhACE2 has been proposed to be useful in COVID‐19 treatment by preventing SARS‐CoV‐2–membrane‐bound ACE2 interaction [102]. In this regard, an open‐label, randomized, and controlled clinical trial was started in China. In this study, it was planned to intravenously administrate 0.4 mg/kg rhACE2 twice a day for 7 days. However, this study was withdrawn due to lack of Center for Drug Evaluation (CDE) approval before participants were enrolled [103].
There is an ongoing open‐label, randomized, and controlled phase 1 clinical trial in Egypt. In this study, it is aimed to investigate the potential beneficial effects of recombinant bacterial ACE2 receptors‐like enzyme of B38‐CAP (rbACE2) on an estimated 24 adult patients with COVID‐19. The study has not started to be recruited yet. 0.4 mg/kg rbACE2 will be intravenously administrated to the intervention group twice a day for 7 days [104].
According to the aforementioned preclinical and clinical data, reduced tissue expression of ACE2 and thus shift of RAS balance toward ACE/Ang II/AT1 pathway seem to be an important factor in the pathogenesis of multiple organ damage including lung, heart, kidney, and liver in patients with COVID‐19. These data suggest that restoring tissue ACE2 levels and also preventing shift of RAS balance toward ACE/Ang II/AT1 pathway may be an effective approach in the treatment of COVID‐19. RAS modulating effects of melatonin has been reported in several experimental studies ( Table I ).
Table I.
Effects of melatonin on RAS components in in vivo experiments.
| Investigators | Disease model | Animals | Dosing | Effects |
|---|---|---|---|---|
| Tain, Y. L. et al. [105] | Programmed hypertension (PH) induced by prenatal dexamethasone (DEX) administration | Female 12‐16 weeks old Sprague–Dawley (SD) rats | 0.01% melatonin in drinking water during pregnancy and lactation | Increase in ACE2 and AT2R expression as well as MAS receptor protein levels in the kidney of male offspring |
| Wu, T. H. et al. [106] | PH induced by neonatal DEX administration | Male neonate offspring of female 12‐16 weeks old SD rats | 0.01% melatonin in drinking water during the lactation period | Increase in ACE2 expression in the kidney and heart of male offspring |
| Tain, Y. L. et al. [107] | PH induced by maternal caloric restriction | Female 12‐16 weeks old SD rats | 0.01% melatonin in drinking water during pregnancy | Increase in ACE2 expression and protein levels in the kidney of the male offspring |
| Tain, Y. L. et al. [108] | PH induced by maternal exposure to continuous light | Female 12‐16 weeks old SD rats |
50 mg/day ip agomelatine during pregnancy and lactation 0.01% melatonin in drinking water during pregnancy and lactation |
Agomelalatine Decrease in expression of ACE and ACE2 Increase in expression of AT2 receptor and MAS receptor in kidney. Melatonin Decrease in renal ACE expression. |
| Tain, Y. L. et al. [109] | PH induced by prenatal DEX and postnatal high‐fat diet | Female 12‐16 weeks old SD rats | 0.01% melatonin in drinking water during pregnancy and lactation | Increase in renal expression of AT2 receptor and MAS receptor in male offspring |
Maternal melatonin application (0.01% melatonin in drinking water during pregnancy and lactation) has been reported to increase ACE2 and AT2R expression as well as MAS receptor protein levels in the kidney of male offspring rats with prenatal dexamethasone (DEX)‐induced programmed hypertension. In this study, investigators have suggested that elevated renal MAS protein levels play an important role in the prevention of DEX‐induced programmed hypertension by melatonin [105].
Melatonin administration to pups (0.01% melatonin in drinking water during the lactation period) has been reported to increase ACE2 expression in the kidney and heart of male offspring rats with neonatal DEX‐induced programmed hypertension [106]. Maternal melatonin treatment (0.01% melatonin in drinking water during pregnancy) increased ACE2 expression and protein levels in the kidney of the adult offspring exposed to maternal caloric restriction [107].
In an experimental study investigating effects of maternal agomelatine (melatonin receptor agonist) and melatonin treatment during pregnancy and lactation on programmed hypertension in male offspring rats of mother exposed to continuous light, several changes have been reported on RAS. In this study, it has been reported that maternal agomelatine (50 mg/day ip) administration decreased expression of ACE and ACE2 but increased expression of ang II type 2 receptor (AT2 receptor) (mediating beneficial effects of Ang II) and MAS receptor in kidney. Additionally, maternal melatonin application (0.01% in drinking water) reduced renal ACE expression (108).
Maternal melatonin therapy (0.01% melatonin in drinking water during pregnancy and lactation) has been reported to increase renal expression of AT2 receptor and MAS receptor in prenatal DEX and postnatal high‐fat diet‐induced programmed hypertension in male offspring rats [109].
Conclusion
Considering the limited number of studies mentioned above, melatonin may be a potential agent to prevent multiple organ injuries and subsequent disease progression as well as sequelae in patients with COVID‐19 due to its modulating effects on RAS, and antioxidant, anti‐inflammatory, free radical scavenger, and antiviral and immunomodulatory effects. Ultimately, the results of the ongoing trial of melatonin in the prophylaxis of COVID‐19 on healthcare workers and additional clinical and preclinical studies will offer more strong evidences to science world.
Conflict of interest
The authors declare no conflict of interest.
Funding
The study is not supported by any source of funding.
References
- 1. Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network‐based drug repurposing for novel coronavirus 2019‐nCoV/SARS‐CoV‐2. Cell Discov. (2020) 6 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pneumonia of unknown cause – China: World Health Organization; 2020 [Available from: https://www.who.int/csr/don/05‐january‐2020‐pneumonia‐of‐unkown‐cause‐china/en/].
- 3. Coronavirus disease (COVID‐19) Situation Report– 160: World Health Organization; 2020. [Available from: https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200628‐covid‐19‐sitrep‐160.pdf?sfvrsn=2fe1c658_2.
- 4. Coronavirus: World Health Organization; 2020. [Available from: https://www.who.int/health‐topics/coronavirus#tab=tab_3.
- 5. Zhang R., Wang X., Ni L. et al. COVID‐19: Melatonin as a potential adjuvant treatment. Life Sci. (2020) 250 117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ye Q., Wang B., Mao J. Cytokine storm in COVID‐19 and treatment. J. Infect. (2020) 80 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clinical management of severe acute respiratory infection (SARI) when COVID‐19 disease is suspected: interim guidance, 13 March 2020. World Health Organization, 2020. 1–22. [Google Scholar]
- 8. Martín Giménez V.M., Inserra F., Tajer C.D. et al. Lungs as target of COVID‐19 infection: Protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci. (2020) 254 117808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tasdemir S., Parlakpinar H., Vardi N., Kaya E., Acet A. Effect of endogen‐exogenous melatonin and erythropoietin on dinitrobenzene sulfonic acid‐induced colitis. Fundam. Clin. Pharmacol. (2013) 27 299–307. [DOI] [PubMed] [Google Scholar]
- 10. Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS‐CoV‐2 infection‐a review of immune changes in patients with viral pneumonia. Emerg. Microb. Infect. (2020) 9 727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffmann M., Kleine‐Weber H., Schroeder S. et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181 271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID‐19: Their roles in pathogenesis. J. Microbiol. Immunol. Infect. (2020) S1684‐1182(20) 30082–30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C., Wang Y., Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). (2020) 395 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Costanzo M., De Giglio M.A.R., Roviello G.N. SARS CoV‐2: Recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr. Med. Chem. (2020) 27 4536–41. [DOI] [PubMed] [Google Scholar]
- 15. Efficacy and Safety of Darunavir and Cobicistat for Treatment of COVID‐19 (DC‐COVID‐19) (2020) [Available from: https://clinicaltrials.gov/ct2/show/NCT04252274?term=NCT04252274&draw=2&rank=1.
- 16. Hydroxychloroquine, Oseltamivir and Azithromycin for the Treatment of COVID‐19 Infection: An RCT (PROTECT) 2020 [Available from: https://clinicaltrials.gov/ct2/show/NCT04338698?term=oseltamivir&cond=COVID&draw=2&rank=1.
- 17. New Antiviral Drugs for Treatment of COVID‐19 2020 [Available from: https://clinicaltrials.gov/ct2/show/NCT04392427?term=ribavirin&cond=COVID&draw=2&rank=2.
- 18. Experimental Trial of rhIFNα Nasal Drops to Prevent 2019‐nCOV in Medical Staff. (2020) [Available from: https://clinicaltrials.gov/ct2/show/NCT04320238?term=interferon+alpha&cond=COVID&draw=2&rank=1.
- 19. The Efficacy of Ivermectin and Nitazoxanide in COVID‐19 Treatment 2020 [Available from: https://clinicaltrials.gov/ct2/show/NCT04351347?term=NCT04351347&cond=COVID&draw=2&rank=1.
- 20. Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA‐approved drug ivermectin inhibits the replication of SARS‐CoV‐2 in vitro. Antivir. Res. (2020) 178 104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Combination Therapy With Camostat Mesilate + Hydroxychloroquine for COVID‐19 (CLOCC) 2020 [Available from: https://clinicaltrials.gov/ct2/show/NCT04338906?term=NCT04338906&draw=2&rank=1.
- 22. Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for Coronavirus disease 2019 (COVID‐19). JAMA (2020) 323 1824–36. [DOI] [PubMed] [Google Scholar]
- 23. Vitamin C Infusion for the Treatment of Severe 2019‐nCoV Infected Pneumonia 2020 [Available from: https://clinicaltrials.gov/ct2/show/NCT04264533?term=NCT04264533&draw=2&rank=1.
- 24. Vitamin D on Prevention and Treatment of COVID‐19 (COVITD‐19) 2020 [Available from: https://clinicaltrials.gov/ct2/show/NCT04334005?term=NCT04334005&draw=2&rank=1.
- 25. Hernández A., Papadakos P.J., Torres A. et al. Two known therapies could be useful as adjuvant therapy in critical patients infected by COVID‐19. Rev. Esp. Anestesiol. Reanim. (2020) 67 245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L. et al. Effect of dexamethasone in hospitalized patients with COVID‐ 19: preliminary report. (2020).
- 27. Drugs@FDA: FDA‐Approved Drugs, Hydroxychloroquine sulfate: US Food and Drug Administration; [Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=009768.
- 28. Drugs@FDA: FDA‐Approved Drugs, Chloroquine phosphate: US Food and Drug Administration; [Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=006002.
- 29. Studies found for: hydroxychloroquine | COVID [Available from: https://clinicaltrials.gov/ct2/results?cond=COVID&term=hydroxychloroquine&cntry=&state=&city=&dist=&Search=Search.
- 30. Studies found for: chloroquine|COVID [Available from: https://clinicaltrials.gov/ct2/results?cond=COVID&term=chloroquine&cntry=&state=&city=&dist=.
- 31. [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases. (2020) 43 185‐8. [DOI] [PubMed] [Google Scholar]
- 32. Yao X., Ye F., Zhang M. et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome Coronavirus 2 (SARS‐CoV‐2). Clin. Infect. Dis. (2020) 71 732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fact sheet for health care providers emergency use authorization (EUA) of hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of COVID‐19 in certain hospitalized patients: US Food and Drug Administration; (2020) [Available from: https://www.fda.gov/media/136537/download.
- 34. Kalil A.C. Treating COVID‐19‐off‐label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA. (2020) 323 1897–8. [DOI] [PubMed] [Google Scholar]
- 35. Marmor M.F., Kellner U., Lai T.Y., Melles R.B., Mieler W.F. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. (2016) 123 1386–94. [DOI] [PubMed] [Google Scholar]
- 36. Unübol M., Ayhan M., Guney E. Hypoglycemia induced by hydroxychloroquine in a patient treated for rheumatoid arthritis. J. Clin. Rheumatol. (2011) 17 46–7. [DOI] [PubMed] [Google Scholar]
- 37. Mascolo A., Berrino P.M., Gareri P. et al. Neuropsychiatric clinical manifestations in elderly patients treated with hydroxychloroquine: a review article. Inflammopharmacology. (2018) 26 1141–9. [DOI] [PubMed] [Google Scholar]
- 38. Zhu S., Guo X., Geary K., Zhang D. Emerging therapeutic strategies for COVID‐19 patients. Discoveries (Craiova, Romania). (2020) 8 e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spagnuolo V., Castagna A., Lazzarin A. Darunavir for the treatment of HIV infection. Expert Opin. Pharmacother. (2018) 19 1149–63. [DOI] [PubMed] [Google Scholar]
- 40. Pant S., Singh M., Ravichandiran V., Murty U.S.N., Srivastava H.K. Peptide‐like and small‐molecule inhibitors against Covid‐19. J. Biomol. Struct. Dynam. (2020) 38 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zanon D., Manca A., De Nicolò A. et al. Data on the stability of darunavir/cobicistat suspension after tablet manipulation. Data Brief. (2020) 30 105552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Burger D.M., Calmy A., Marzolini C. Cobicistat: A case of mislabelled drug‐drug interaction risk? Br. J. Clin. Pharmacol. (2020) 86 834–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen C., Huang J., Cheng Z., Wu J., Chen S., Zhang Y. et al.Favipiravir versus arbidol for COVID‐19: a randomized clinical trial. (2020).
- 44. Clinical Study of Arbidol Hydrochloride Tablets in the Treatment of Pneumonia Caused by Novel Coronavirus 2020 [Available from: https://clinicaltrials.gov/ct2/show/NCT04260594?term=NCT04260594&cond=COVID&draw=2&rank=1.
- 45. Xu X., Han M., Li T. et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc. Natl Acad. Sci. USA. (2020) 117 10970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jones G., Ding C. Tocilizumab: a review of its safety and efficacy in rheumatoid arthritis. Clin. Med. Insights Arthritis Musculoskelet. Disord. (2010) 3 81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sheahan T.P., Sims A.C., Leist S.R. et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nat. Commun. (2020) 11 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang M., Cao R., Zhang L. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. (2020) 30 269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gordon C.J., Tchesnokov E.P., Woolner E. et al. Remdesivir is a direct‐acting antiviral that inhibits RNA‐dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. (2020) 295 6785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mulangu S., Dodd L.E., Davey R.T. Jr et al. A randomized, controlled trial of ebola virus disease therapeutics. N. Engl. J. Med. (2019) 381 2293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cai Q., Yang M., Liu D. et al. Experimental treatment with favipiravir for COVID‐ 19: an open‐label control study. Engineering (Beijing, China). (2020). 10.1016/j.eng.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Larrouquere L., Gabin M., Poingt E. et al. Genesis of an emergency public drug information website by the French Society of Pharmacology and Therapeutics during the COVID‐19 pandemic. Fundam. Clin. Pharmacol. (2020) 34 389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hemati K., Pourhanifeh M.H., Dehdashtian E. et al. Melatonin and morphine: potential beneficial effects of co‐use. Fundam. Clin. Pharmacol. (2020). 10.1111/fcp.12566 [DOI] [PubMed] [Google Scholar]
- 54. Amaral F.G.D., Cipolla‐Neto J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. (2018) 62 472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Macchi M.M., Bruce J.N. Human pineal physiology and functional significance of melatonin. Front. Neuroendocrinol. (2004) 25 177–95. [DOI] [PubMed] [Google Scholar]
- 56. Cipolla‐Neto J., Amaral F.G.D. Melatonin as a hormone: new physiological and clinical insights. Endocr. Rev. (2018) 39 990–1028. [DOI] [PubMed] [Google Scholar]
- 57. Brzezinski A. Melatonin in humans. N. Engl. J. Med. (1997) 336 186–195. [DOI] [PubMed] [Google Scholar]
- 58. Galano A., Tan D.X., Reiter R.J. On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J. Pineal Res. (2013) 54 245–57. [DOI] [PubMed] [Google Scholar]
- 59. Slominski R.M., Reiter R.J., Schlabritz‐Loutsevitch N., Ostrom R.S., Slominski A.T. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol. Cell. Endocrinol. (2012) 351 152–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Srinivasan V., Mohamed M., Kato H. Melatonin in bacterial and viral infections with focus on sepsis: a review. Recent Patents Endocr. Metab. Immune Drug Discov. (2012) 6 30–9. [DOI] [PubMed] [Google Scholar]
- 61. Junaid A., Tang H., van Reeuwijk A. et al. Ebola Hemorrhagic Shock Syndrome‐on‐a‐Chip. iScience. (2020) 23(1) 100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ebola (Ebola Virus Disease), Treatment: Centers for Disease Control and Prevention; [Available from: https://www.cdc.gov/vhf/ebola/treatment/index.html.
- 63. Huang S.H., Cao X.J., Liu W., Shi X.Y., Wei W. Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J. Pineal Res. (2010) 48 109–16. [DOI] [PubMed] [Google Scholar]
- 64. Huang S.H., Cao X.J., Wei W. Melatonin decreases TLR3‐mediated inflammatory factor expression via inhibition of NF‐kappa B activation in respiratory syncytial virus‐infected RAW264.7 macrophages. J Pineal Res. (2008) 45 93–100. [DOI] [PubMed] [Google Scholar]
- 65. Montiel M., Bonilla E., Valero N. et al. Melatonin decreases brain apoptosis, oxidative stress, and CD200 expression and increased survival rate in mice infected by Venezuelan equine encephalitis virus. Antivir. Chem. Chemother. (2015) 24 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bonilla E., Valero‐Fuenmayor N., Pons H., Chacín‐Bonilla L. Melatonin protects mice infected with Venezuelan equine encephalomyelitis virus. Cell. Mol. Life Sci. (1997) 53 430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Crespo I., San‐Miguel B., Sánchez D.I. et al. Melatonin inhibits the sphingosine kinase 1/sphingosine‐1‐phosphate signaling pathway in rabbits with fulminant hepatitis of viral origin. J. Pineal Res. (2016) 61 168–76. [DOI] [PubMed] [Google Scholar]
- 68. Ellis L.C. Melatonin reduces mortality from Aleutian disease in mink (Mustela vison). J. Pineal Res. (1996) 21 214–7. [DOI] [PubMed] [Google Scholar]
- 69. Huang S.‐H., Liao C.‐L., Chen S.‐J. et al. Melatonin possesses an anti‐influenza potential through its immune modulatory effect. J. Funct. Foods. (2019) 58 189–98. [Google Scholar]
- 70. Han S.N., Meydani M., Wu D. et al. Effect of long‐term dietary antioxidant supplementation on influenza virus infection. J. Gerontol. Ser. A Biol. Sci. Med. Sci. (2000) 55 B496–503. [DOI] [PubMed] [Google Scholar]
- 71. Sahna E., Parlakpinar H., Ozturk F., Cigremis Y., Acet A. The protective effects of physiological and pharmacological concentrations of melatonin on renal ischemia‐reperfusion injury in rats. Urol. Res. (2003) 31 188–93. [DOI] [PubMed] [Google Scholar]
- 72. Sahna E., Parlakpinar H., Vardi N., Ciğremis Y., Acet A. Efficacy of melatonin as protectant against oxidative stress and structural changes in liver tissue in pinealectomized rats. Acta Histochem. (2004) 106 331–6. [DOI] [PubMed] [Google Scholar]
- 73. Fadillioglu E., Kurcer Z., Parlakpinar H., Iraz M., Gursul C. Melatonin treatment against remote organ injury induced by renal ischemia reperfusion injury in diabetes mellitus. Arch. Pharm. Res. (2008) 31 705–12. [DOI] [PubMed] [Google Scholar]
- 74. Colak C., Parlakpinar H., Ozer M.K., Sahna E., Cigremis Y., Acet A. Investigating the protective effect of melatonin on liver injury related to myocardial ischemia‐reperfusion. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. (2007) 13: Br251‐4. [PubMed] [Google Scholar]
- 75. Kavakli A., Sahna E., Parlakpinar H., Yahsi S., Ogeturk M., Acet A. The effects of melatonin on focal cerebral ischemia‐reperfusion model. Saudi Med. J. (2004) 25 1751–2. [PubMed] [Google Scholar]
- 76. Sahna E., Parlakpinar H., Turkoz Y., Acet A. Protective effects of melatonin on myocardial ischemia/reperfusion induced infarct size and oxidative changes. Physiol. Res. (2005) 54 491–5. [PubMed] [Google Scholar]
- 77. Gurlek A., Celik M., Parlakpinar H., Aydogan H., Bay‐Karabulut A. The protective effect of melatonin on ischemia‐reperfusion injury in the groin (inferior epigastric) flap model in rats. J. Pineal Res. (2006) 40 312–7. [DOI] [PubMed] [Google Scholar]
- 78. Eşrefoğlu M., Gül M., Parlakpinar H., Acet A. Effects of melatonin and caffeic acid phenethyl ester on testicular injury induced by myocardial ischemia/reperfusion in rats. Fundam. Clin. Pharmacol. (2005) 19 365–72. [DOI] [PubMed] [Google Scholar]
- 79. Karaer I., Simsek G., Gul M. et al. Melatonin protects inner ear against radiation damage in rats. Laryngoscope. (2015) 125 E345–9. [DOI] [PubMed] [Google Scholar]
- 80. Aladag M.A., Turkoz Y., Parlakpinar H., Ozen H., Egri M., Unal S.C. Melatonin ameliorates cerebral vasospasm after experimental subarachnoidal haemorrhage correcting imbalance of nitric oxide levels in rats. Neurochem. Res. (2009) 34 1935–44. [DOI] [PubMed] [Google Scholar]
- 81. Wu G.C., Peng C.K., Liao W.I., Pao H.P., Huang K.L., Chu S.J. Melatonin receptor agonist protects against acute lung injury induced by ventilator through up‐regulation of IL‐10 production. Respirat. Res. (2020) 21 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hooper P.L. COVID‐19 and heme oxygenase: novel insight into the disease and potential therapies. Cell Stress Chaperones. (2020) 1 711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen Y., Yuan T., Zhang H. et al. Activation of Nrf2 attenuates pulmonary vascular remodeling via inhibiting endothelial‐to‐mesenchymal transition: an insight from a plant polyphenol. Int. J. Biol. Sci. (2017) 13 1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ahmadi Z., Ashrafizadeh M. Melatonin as a potential modulator of Nrf2. Fundam. Clin. Pharmacol. (2020) 34 11–9. [DOI] [PubMed] [Google Scholar]
- 85. Zhang W.X., He B.M., Wu Y., Qiao J.F., Peng Z.Y. Melatonin protects against sepsis‐induced cardiac dysfunction by regulating apoptosis and autophagy via activation of SIRT1 in mice. Life Sci. (2019) 217 8–15. [DOI] [PubMed] [Google Scholar]
- 86. Huang C.C., Chiou C.H., Liu S.C. et al. Melatonin attenuates TNF‐α and IL‐1β expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. J. Pineal Res. (2019) 66 e12560. [DOI] [PubMed] [Google Scholar]
- 87. Sánchez‐López A.L., Ortiz G.G., Pacheco‐Moises F.P. et al. Efficacy of melatonin on serum pro‐inflammatory cytokines and oxidative stress markers in relapsing remitting multiple sclerosis. Arch. Med. Res. (2018) 49 391–8. [DOI] [PubMed] [Google Scholar]
- 88. Bazyar H., Gholinezhad H., Moradi L. et al. The effects of melatonin supplementation in adjunct with non‐surgical periodontal therapy on periodontal status, serum melatonin and inflammatory markers in type 2 diabetes mellitus patients with chronic periodontitis: a double‐blind, placebo‐controlled trial. Inflammopharmacology (2019) 27 67–76. [DOI] [PubMed] [Google Scholar]
- 89. Sagrillo‐Fagundes L., Assunção Salustiano E.M., Ruano R., Markus R.P., Vaillancourt C. Melatonin modulates autophagy and inflammation protecting human placental trophoblast from hypoxia/reoxygenation. J. Pineal Res. (2018) 65 e12520. [DOI] [PubMed] [Google Scholar]
- 90. Chahbouni M., López M.D.S., Molina‐Carballo A. et al. Melatonin treatment reduces oxidative damage and normalizes plasma pro‐inflammatory cytokines in patients suffering from charcot‐marie‐tooth neuropathy: a pilot study in three children. Molecules (Basel, Switzerland) (2017) 22 1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mesri Alamdari N., Mahdavi R., Roshanravan N., Lotfi Yaghin N., Ostadrahimi A.R., Faramarzi E. A double‐blind, placebo‐controlled trial related to the effects of melatonin on oxidative stress and inflammatory parameters of obese women. Horm. Metab. Res. (2015) 47 504–8. [DOI] [PubMed] [Google Scholar]
- 92. Carrasco C., Marchena A.M., Holguín‐Arévalo M.S. et al. Anti‐inflammatory effects of melatonin in a rat model of caerulein‐induced acute pancreatitis. Cell Biochem. Funct. (2013) 31 585–90. [DOI] [PubMed] [Google Scholar]
- 93. Sener G., Tuğtepe H., Velioğlu‐Oğünç A., Cetinel S., Gedik N., Yeğen B.C. Melatonin prevents neutrophil‐mediated oxidative injury in Escherichia coli‐induced pyelonephritis in rats. J. Pineal Res. (2006) 41 220–7. [DOI] [PubMed] [Google Scholar]
- 94. Rahim I., Djerdjouri B., Sayed R.K. et al. Melatonin administration to wild‐type mice and nontreated NLRP3 mutant mice share similar inhibition of the inflammatory response during sepsis. J. Pineal Res. (2017) 63 e12410. [DOI] [PubMed] [Google Scholar]
- 95. Efficacy of melatonin in the prophylaxis of Coronavirus disease 2019 (COVID‐19) among healthcare workers (MeCOVID) 2020 [Available from: https://clinicaltrials.gov/ct2/show/NCT04353128?term=melatonin&cond=COVID&draw=1&rank=1.
- 96. Anderson G., Reiter R.J. Melatonin: Roles in influenza, Covid‐19, and other viral infections. Rev. Med. Virol. (2020) 30 e2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zemlin A.E., Wiese O.J., Coronavirus disease 2019 (COVID‐19) and the renin‐angiotensin system: A closer look at angiotensin‐converting enzyme 2 (ACE2). Annals Clin. Biochem. Int. J. Lab. Med. (2020) 57: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gheblawi M., Wang K., Viveiros A. et al. Angiotensin‐converting enzyme 2: SARS‐CoV‐2 receptor and regulator of the renin‐angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. (2020) 126 1456–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wang K., Gheblawi M., Oudit G.Y. Angiotensin converting enzyme 2: a double‐edged sword. Circulation (2020) 142 426–8. [DOI] [PubMed] [Google Scholar]
- 100. Liu Y., Yang Y., Zhang C. et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. (2020) 63 364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Talreja H., Tan J., Dawes M. et al. A consensus statement on the use of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in relation to COVID‐19 (corona virus disease 2019). N. Zeal. Med. J. (2020) 133 85–7. [PubMed] [Google Scholar]
- 102. Batlle D., Wysocki J., Satchell K. Soluble angiotensin‐converting enzyme 2: a potential approach for coronavirus infection therapy? Clin. Sci. (2020) 134 543–545. [DOI] [PubMed] [Google Scholar]
- 103. Recombinant Human Angiotensin‐converting Enzyme 2 (rhACE2) as a Treatment for Patients With COVID‐19 2020 [Available from: https://clinicaltrials.gov/ct2/show/study/NCT04287686?term=recombinant+human+ACE2&cond=COVID‐19&draw=2.
- 104. Recombinant Bacterial ACE2 Receptors ‐Like Enzyme of B38‐CAP Could be Promising COVID‐19 Infection‐ and Lung Injury Preventing Drug Better Than Recombinant Human ACE2 (Bacterial ACE2) 2020 [Available from: https://clinicaltrials.gov/ct2/show/NCT04375046?term=NCT04375046&draw=2&rank=1.
- 105. Tain Y.L., Chen C.C., Sheen J.M. et al. Melatonin attenuates prenatal dexamethasone‐induced blood pressure increase in a rat model. J. Am. Soc. Hypertens. (2014) 8 216–26. [DOI] [PubMed] [Google Scholar]
- 106. Wu T.‐H., Kuo H.‐C., Lin I.‐C., Chien S.‐J., Huang L.‐T., Tain Y.‐L. Melatonin prevents neonatal dexamethasone induced programmed hypertension: Histone deacetylase inhibition. J. Steroid Biochem. Mol. Biol. (2014) 144 253–259. [DOI] [PubMed] [Google Scholar]
- 107. Tain Y.L., Huang L.T., Hsu C.N., Lee C.T. Melatonin therapy prevents programmed hypertension and nitric oxide deficiency in offspring exposed to maternal caloric restriction. Oxid. Med. Cell. Longev. (2014) 2014 283180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tain Y.L., Lin Y.J., Chan J.Y.H., Lee C.T., Hsu C.N. Maternal melatonin or agomelatine therapy prevents programmed hypertension in male offspring of mother exposed to continuous light. Biol. Reprod. (2017) 97 636–43. [DOI] [PubMed] [Google Scholar]
- 109. Tain Y.L., Sheen J.M., Yu H.R. et al. Maternal melatonin therapy rescues prenatal dexamethasone and postnatal high‐fat diet induced programmed hypertension in male rat offspring. Front. Physiol. (2015) 6 377. [DOI] [PMC free article] [PubMed] [Google Scholar]


