Abstract
Substance abuse is one of the most prevalent and costly health problems in the world today. Standard medical therapy is often not curative, and relapse is common. Research over the past several decades on the neural underpinnings of addiction has implicated a network of structures within the brain shown to be altered in patients with substance abuse. The field of neuromodulation aims to utilize this knowledge to treat dysfunctional circuits by targeting and modulating specific brain circuits. While invasive neuromodulation such as DBS and VNS have proven to be effective in treating movement disorders, OCD and epilepsy, there is increasing interest and data with regards to its potential application for the treatment of severe, intractable addiction. Several neuromodulatory techniques and brain targets are currently under investigation in patients with various substance abuse disorders. This review aims to summarize the current state of evidence for neurosurgical neuromodulation as a therapy for substance abuse and addiction, and to provide additional expert opinions as to the obstacles and future directions of this endeavor.
Keywords: Neuromodulation, addiction, substance abuse, deep brain stimulation, nucleus accumbens, vagal nerve stimulation
INTRODUCTION
Substance abuse and addiction are major medical and socioeconomic problems in the United States. In 2009, drug-induced deaths in the U.S. surpassed traffic accidents as the leading external cause of death (Kochanek et al., 2011); in 2015, drug overdoses accounted for 52,404 U.S. deaths. It has been estimated that the total annual cost of drug abuse in the U.S., in terms of healthcare spending, crime and lost productivity, is over $193 billion (US Department of Justice National Drug Intelligence Center, 2011). Furthermore, the burden of addiction is actually growing, due in part to an alarming increase in the rate of opioid abuse (Rudd et al., 2016).
Current therapy for substance abuse is multimodal and includes psychotherapy, behavior modification and pharmacotherapy. The success of these treatments is highly variable, and often dependent on multiple factors, which has remained a point of frustration for patients and physicians alike. Recent efforts to understand the biological basis of addiction have focused on the neural circuitry underlying reward and motivation, which has resulted in the emergence of a of neuromodulation as a treatment modality (Koob and Volkow, 2016). Neuromodulation aims to alter activity in the neuronal circuits underlying reward pathways by influencing neuronal function at an electrophysiologic and/or synaptic level (Burchiel et al., 2015; Lozano and Lipsman, 2013). In this review we present the rationale, indications, and data supporting the use of invasive neuromodulation to treat severe, intractable addictive behavior. We also review some of the major challenges that face the implementation of neuromodulation for treatment-refractory addiction. Finally, we present an overview of future directions for clinical research in this area as we gain deeper insight into the underlying neurobiology and circuitry of addiction.
THE BURDEN OF DISEASE
Alcohol
Alcohol consumption contributed to 5.9% of all global deaths, and to 5.1% of the global burden of disease in 2012 (World Health Organization, 2014). The World Health Organization (WHO) determined that in 2014 alcohol consumption contributed to over 200 disease and injury-related conditions. Alcohol use was found to be the leading risk factor for premature death and disability among people ages 15 to 49 (Lim et al., 2012), and contributed to 25% of total deaths worldwide among people ages 20 to 39 (World Health Organization, 2014). In the U.S., alcohol abuse remains a significant problem; it represents the fourth leading preventable cause of death (Stahre et al., 2014), and in 2016 was responsible for 28% of all driving fatalities (US Department of Transportation National Highway Traffic Safety Administration, 2017). In 2013, the Diagnostic and Statistical Manual (DSM-V) combined the two disorders of “alcohol abuse” and “alcohol dependence” into one overarching disorder: alcohol use disorder (AUD) (American Psychiatric Association, 2013). Using the DSM-V criteria, in 2014 over 16 million American adults and 679,000 adolescents qualified for AUD (SAMHSA, 2014).
Tobacco (nicotine)
Tobacco represents the largest modifiable risk factor for premature death worldwide (World Health Organization, 2015). According to a 2015 report by the WHO, direct tobacco smoking was responsible for the death of approximately 5 million people globally, while indirect (second-hand) tobacco smoking was estimated to kill another 600,000 (World Health Organization, 2015). Globally, an estimated 12% of deaths among adults over 30 years of age were attributable to tobacco in 2012. In the Americas alone, that figure was 16% (World Health Organization, 2015). In 2010, tobacco smoking was estimated to be the cause of 6.3% of disability-adjusted life-years worldwide (Degenhardt et al., 2013).
Other drugs of abuse (cannabis, opioids, cocaine, and other psychostimulants)
In 2012, an estimated 243 million people worldwide (or 5.2% of the world’s population aged 15-64) used an illicit substance – defined as belonging to the cannabis, opioid, cocaine, or amphetamine-type psychostimulant group of drugs (United Nations Office on Drugs and Crime - UNODC, 2014). One study examining the global burden of disease from drug dependence estimated that in 2010, illicit drug dependence was responsible for 20 million disability-adjusted life-years, an increase from 13.1 million years as calculated for 1990 (Degenhardt et al., 2013). This increase was largely attributed to opioid use, which has seen a steep increase largely as a result of increased misuse of prescription opioids. In the United States, deaths from overdoses of prescription opioids more than quadrupled between 1999 and 2010 (Volkow et al., 2014). The United Nations Office on Drugs and Crime (UNODC) estimates that in 2012, there were 183,000 drug-related deaths worldwide (a mortality rate of 40.0 per million persons aged 15-64), the majority of which resulted from drug overdose (United Nations Office on Drugs and Crime - UNODC, 2014).
Taken together, abuse of these substances has led to a predictably (and increasingly) expensive effort to treat addiction in each of its forms. Substance abuse treatment in the United States alone has grown from $9.3 billion annually in 1986 to $20.7 billion annually in 2003 (Mark et al., 2007). In 2006, $24.6 billion in healthcare expenditures could be attributed to alcohol (Bouchery et al., 2011) while in 2010, $170 billion in healthcare expenditures (or 8.7% of annual healthcare spending) could be attributed to cigarette smoking (Xu et al., 2015). In the context of these ballooning expenditures, effective therapies have the potential to dramatically improve resource allocation and (more importantly) patient outcomes.
CURRENT TREATMENT OPTIONS
Addiction treatment is multifaceted, and usually involves some combination of behavioral and medical therapy. Behavioral therapies include cognitive-behavioral therapy (CBT), multidimensional couples and family therapy, and motivational incentives (also known as contingency management therapy) among others. Such therapies are typically administered over the long-term, and aim to alter patient behavior to avoid relapse or other damaging actions on the part of the patient. These therapies continue to evolve and improve, though they still represent just one facet of a multi-factorial approach to addiction treatment (Carroll and Onken, 2005).
Medication-assisted therapies (MAT) for addiction have similarly existed for decades; however, their efficacy in treating substance abuse disorders is limited. Medications for alcohol abuse – including such drugs as naltrexone, acamprosate, and disulfiram – have historically demonstrated modest effect sizes, even in combination with other forms of therapy (Franck and Jayaram-Lindström, 2013; Soyka and Mutschler, 2016). Similarly, modest treatment effects have been observed with medications used to assist in smoking cessation (Wu et al., 2015) and illicit substance abuse (Soyka and Mutschler, 2016).
While a variety of treatment options are currently available to patients with substance abuse disorders, a major issue facing treatment providers is the high rate of relapse in the long term. Without extensive treatment upon discharge from a treatment facility, a majority of patients with substance abuse disorders will relapse regardless of their drug of abuse (Berglund et al., 2003; Dutra et al., 2008). Treatment refractory patients are disproportionately represented in morbidity and mortality data, and represent a cohort of patients for whom new therapies may be especially beneficial.
NEURAL CIRCUITRY OF ADDICTION
From a neuropathophysiological point of view, addiction is viewed as a brain disease wherein dysfunction in neural circuits mediating reward and motivation results in detrimental behavior characterized by intense craving, addiction and relapse (Kuhn et al., 2015). In theory, effective therapies for addiction – those that help avoid relapse in subjects – are virtually all thought to interact with reward and motivation pathways in the brain (Soyka and Mutschler, 2016). Though far from being perfectly understood, these pathways are well studied, and their relevance to substance addiction will be outlined here.
A key structure within the brain reward system is the Nucleus Accumbens (NAc). Located within the ventral striatum, the NAc is thought to play an important role in discriminating and/or integrating signals from multiple limbic areas such as the amygdala, hippocampus, cingulate and prefrontal cortices and motor output areas such as the striatum. Use of almost every known addictive substance has been shown to result in dopamine release in the NAc (Goodman, 2008; Wise, 1996). Activation of these dopamine-modulated circuits produces an initial sense of euphoria; however these particular circuits are also responsible for encoding reward prediction (Volkow et al., 2012). Chronic, repeated drug intake leads to a compensatory downregulation of dopamine-related signaling and alters reward prediction (Ikemoto, 2010). Over time the reward circuitry is altered such that similar doses of the same drug produce diminished reward; this alteration in neural circuitry is in part thought to underlie the development of tolerance and addiction.
Changes in NAc circuitry also lead to conditioning and maladaptive reward prediction, such that previously neutral stimuli can become associated with drug reward. In such circumstances, the NAc may respond to a conditioned stimulus by increasing dopamine signaling in anticipation of substance use. One example of this phenomenon is a “drug-associated cue” – a conditioned stimulus thought to underlie the intense craving associated with substance abuse. When a conditioned stimulus such as a drug-associated cue predicts a reward, its presence provokes a transient increase in NAc dopamine signaling. However, if the reward is not received after a conditioned stimulus, dopamine signaling transiently decreases the moment the reward was expected to arrive (Heinz et al., 2009; Schultz, 2010). Such maladaptive reward prediction circuitry within the NAc is thought to play a substantial role in subject relapse (Jasinska et al., 2014).
NEUROMODULATION DEFINED
The term “neuromodulation” refers to a number of different treatment modalities. It encompasses therapies such deep brain stimulation (DBS), vagal nerve stimulation (VNS), transcranial magnetic stimulation (TMS), and transcranial direct current stimulation (tDCS). A summary of targets studied to date in the context of neuromodulation for addiction therapy (as well as other disorders) is illustrated in Figure 1.
Figure 1:
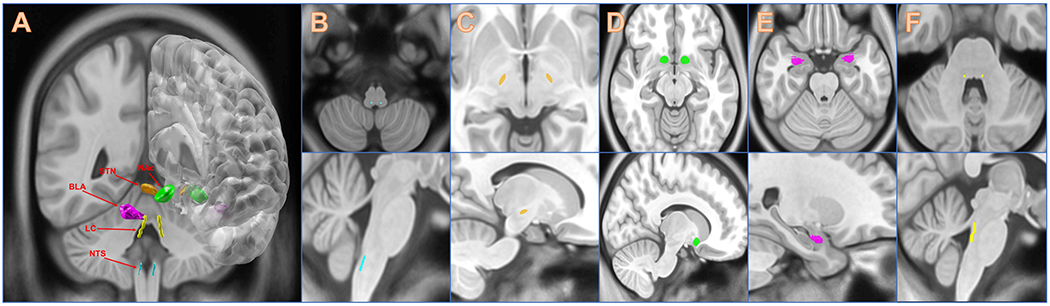
Illustration of cranial targets for invasive neuromodulation. A) All targets, B) Nucleus of the Solitary Tract, C) Subthalamic Nucleus, D) Nucleus Accumbens, E) Basolateral Amygdala, F) Locus Coeruleus.
NEUROMODULATORY INTERVENTIONS FOR ADDICTION
Early efforts
Ablative techniques
Modern-day neuromodulation therapies – while not ablative – were conceived largely as reversible forms of earlier permanent, ablative procedures whereby a specific region of the brain was targeted stereotactically and intentionally destroyed. While generally reserved for patients that are not candidates for neuromodulation, studies on ablative procedures for addiction deserve mention for contextual and historical clarity. A number of studies have been published on ablation for various addictive behaviors. The bilateral anterior cingulotomy – relying on the presumption that the anterior cingulum plays a role in obsessive and compulsive behaviors associated with particular addictive disorders has been studied in over 400 patients across multiple studies. The largest of these studies included 348 heroin-addicted patients, and reported achieving an abstinence rate of 45% after 2 years (Medvedev et al., 2003); however, due to concerns regarding the study’s methods and reported results it failed to garner much interest (Gao and Wang, 2015).
Stereotactic ablation of the NAc for addiction has been studied in similar populations as well. One study conducted by Gao et al. (Gao et al., 2003) reported abstinence in 11 of 28 heroin-addicted subjects over 6 months, while another by Wu et al. (Wu et al., 2010) reported abstinence in 9 of 12 alcoholics at 6 months. On a larger scale, one single-institution study performed NAc ablation on 272 heroin-addicted subjects from 2000-2004 and reported a nearly 70% abstinence rate at one year; a separate long-term cross-sectional analysis of a 100-person subset of these 272 patients found a 5-year abstinence rate of 58% (Li et al., 2013).
Early studies using ablative procedures have been criticized in terms of their study design and execution, especially given the irreversible nature of ablation. Today, given concerns regarding the permanence of ablative therapies, as well as the development of adjustable, and reversible neuromodulatory alternatives, the role of ablative therapies in treating addiction remains unclear.
Modern techniques
Today’s neuromodulatory therapies differ from ablative techniques in two important aspects: they are adjustable and reversible. As a result, while they rely on lessons learned from ablative techniques, especially with regards to anatomy and target selection, they provide a relatively safer and potentially more effective therapy for addiction. For example, stimulation parameters can potentially be adjusted to accommodate clinical changes such as tolerance and relapse.
Deep Brain Stimulation (DBS)
Since its development in the late 1980’s DBS has been shown to be a safe and effective treatment modality for a number of movement disorders (Deuschl et al., 2006; Kupsch et al., 2006; Schuurman et al., 2000). Over the years, efforts have been underway to expand the application of DBS to include certain treatment-refractory psychiatric disorders including depression, obsessive-compulsive disorder (OCD), and Tourette’s syndrome. To date several studies have demonstrated beneficial clinical effects for these indications, which has led to DBS becoming an approved treatment for refractory OCD under the FDA’s humanitarian device exemption (HDE) (Kuhn et al., 2015). While its long-term efficacy for psychiatric diseases is still to be determined, recently there has been substantial effort to investigate its utility for various addictive behaviors as well. These efforts can be largely organized according to the anatomical target of interest and will be the focus of the following discussion. Table 1 summarizes those studies to date which have investigated the utility of DBS for treating addiction.
Table 1:
Literature examining ablative procedures for addiction
| Authors & Year | Study Type | Number, type of subjects | Target | Follow-up time | Results |
|---|---|---|---|---|---|
| Medvedev et al., 2003 | Prospective case series | 187 heroin-dependent subjects | Bilateral cingulate gyrus | 2 years | At minimum 2 years follow-up: -62% achieved long-term abstinence -13% partial improvement in drug use -25% no change / no data |
| Gao et al., 2003 | Prospective case series | 28 opiate-drug dependent subjects | Bilateral NAc | 15 months (mean) | At mean 15 months follow-up: -39% achieved & maintained abstinence |
| Wu et al., 2010 | Prospective case series | 12 alcohol-dependent subjects | Bilateral NAc | 16.6 months (mean) | At 12 months follow-up: -75% achieved & maintained abstinence |
| Li et al., 2013 | Prospective case series | 272 heroin-dependent subjects | Bilateral NAc | 1 year | After 1 year follow-up: -69.5% achieved & maintained abstinence |
NAc = nucleus accumbens
Nucleus Accumbens (NAc)
The NAc was first introduced as a target for modern DBS systems in 2003, as an alternative treatment for OCD and anxiety disorders (Sturm et al., 2003). During the course of an investigation of DBS of the NAc for anxiety, one subject was noted to experience a significant reduction in alcohol consumption (Kuhn et al., 2007). The subject had developed alcohol dependence as a result of his anxiety disorder, which had resulted in alcohol abuse for more than 10 years. While DBS of the NAc did little to alleviate the subject’s anxiety symptoms or severe comorbid depression, he reported losing the urge to consume alcohol shortly after the onset of stimulation. The subject subsequently was able to successfully discontinue his excessive alcohol consumption. These results were observed over the duration of stimulation of the NAc (12 months) (Kuhn et al., 2007).
Nicotine addiction
This finding led Kuhn and colleagues to retrospectively review their series of subjects treated with DBS of the NAc to look for pre-existing nicotine addiction (Kuhn et al., 2009). They identified a total of ten nicotine-addicted subjects with treatment-refractory anxiety and OCD, as well as Tourette’s syndrome. Of these ten patients, three were found to have experienced a long-term remission of their nicotine addiction during stimulation. Comparatively, the voluntary abstinence rate within the general population is estimated to be approximately 9% (Kuhn et al., 2009; Meyer et al., 2003).
A similar finding was reported by Mantione et al. (Mantione et al., 2010) in 2010, wherein a subject who had undergone DBS of the NAc for severe OCD also managed to stop using tobacco. Notably, this subject had a long history of tobacco use, and had tried and failed several times prior to undergoing DBS treatment for her OCD. After her DBS procedure, she remained abstinent at her 2 year follow-up, and denied any craving or withdrawal symptoms upon cessation.
Alcohol addiction
While the first observations of DBS favorably affecting substance abuse behavior in test subjects were noted either incidentally or retrospectively, a small number of patients have since been treated with DBS for the primary indication of substance abuse. In 2009, Müller et al. (Muller et al., 2009) reported the results of their study examining DBS of the NAc in three severely alcohol-addicted subjects. Two of the three subjects (subjects #1 and #2) ceased alcohol consumption completely. For these subjects, alcohol reduction began within the first month of stimulation, and complete cessation was achieved by three and five months, respectively. Both subjects remained abstinent at their fifteen-month follow-up, and both subjects were able to return to the workforce and maintain employment after study completion. The third subject (#3) experienced a significant reduction in alcohol consumption overall. His drinking reduction also began within the first month of stimulation, but he did relapse four times during the fifteen-month follow-up period.
In 2013, that same group (Juergen Voges et al. 2013) performed bilateral NAc DBS for two more subjects with similarly severe alcohol addiction (Voges et al., 2013). Both subjects reported an immediate absence of alcohol craving after DBS initiation. The first subject (subject #4) achieved complete abstinence for 20 months, but subsequently relapsed briefly (1-3 days) on four separate occasions over the following 12 months. The second (#5) achieved complete abstinence for 16 months, but also experienced a series of short (1-3 days) relapses over the following 12 months. Notably, approximately two and a half years after DBS placement surgery, the subject experienced a prolonged relapse lasting over one year, culminating in a hospitalization for a generalized seizure. The subject reported that over the prior year, the effects of stimulation never reached the level he had experienced at the beginning of DBS therapy. Head imaging at that time revealed 10-mm caudoventral dislocation of both brain electrodes. The subject subsequently underwent lead replacement and reported an improvement in symptoms, comparable to what he had experienced when he had achieved abstinence following his initial DBS surgery.
Long term follow-up of these five subjects with chronic alcoholism was published in 2016 (Müller et al., 2016). One of the most salient findings from that report was that the two instances of major (i.e. long-term) relapses that were observed arose in subjects whose DBS system stopped functioning properly. In one instance, a subject was noted to have migration of his DBS leads during a period of prolonged relapse (#5); while another suffered a prolonged relapse corresponding to depletion of his DBS generator battery (#3) (Müller et al., 2016). In effect, both cases of major relapse may serve as examples of unintended within-subject negative control periods. The observed correlation in severity of relapse for subjects with malfunctioning DBS systems supports the working theory that alcohol craving was effectively targeted and treated with appropriate NAc DBS.
Finally, additional studies have supported the notion that NAc stimulation modulates activity in its cortical targets. In 2011 Kuhn et al. (Kuhn et al., 2011) treated a single patient with severe treatment-resistant alcohol addiction with DBS of the NAc. The authors showed that error-related negativity (ERN) potentials – an EEG measure which corresponds to recognizing a performance error, and which is attenuated in alcohol abuse (Heinze, 2009; Ridderinkhof, 2002) – could be modulated by the status of the stimulator (“on” vs. “off”). Higher ERN amplitudes from the anterior mid-cingulate cortex were observed with the stimulator in the “on” position than in the “off” position. The subject achieved complete cessation of alcohol intake by one year of follow-up. The results of the author’s findings would suggest that DBS activation may also target alcohol addiction by improving a subject’s error and decision processing. Indeed this theory has been substantiated by another study, which observed more risk averse and cautious decision-making in one subject during NAc DBS activation versus inactivation (Kuhn et al., 2015; Münte, 2008).
Heroin/opioid addiction
DBS targeting of the NAc has also been studied in the context of heroin addiction, which is of particular interest given heroin’s high addictive potential. The first case was reported in 2011 by Zhou et al. (Zhou et al., 2011), and described DBS targeting of the NAc in a 24-year old male subject with refractory heroin dependence. Following chronic NAc stimulation, the subject remained relapse-free despite having his generator voluntarily switched off 2.5 years after implantation. This finding is particularly interesting, as it suggests that NAc stimulation may permanently modify reward circuitry and restore previously healthy behaviors.
Another heroin-related study was conducted in 2012, wherein a 47-year old male with 22 years of heroin dependence and multiple failed detoxifications underwent bilateral NAc DBS (Valencia-Alfonso et al., 2012). In this study, the authors performed intraoperative electrophysiological recordings from implanted DBS electrodes while subjects viewed drug-related images. The authors reported that of the four electrode contacts, only the dorsal-most contacts located at the border of the internal capsule and the NAc showed cue-induced activity changes. In addition, post-operative stimulation of the dorsal-most contacts led to the greatest improvement in drug abstinence and craving. This study was one of the first to show a direct correspondence between intraoperative stimulation mapping and subsequent clinical response for addiction.
More recently, Kuhn et al. (Kuhn et al., 2014) treated two more chronic opioid-addicted, therapy-resistant subjects with bilateral NAc DBS. Both subjects were successfully weaned off methadone after initiating DBS. Although each subject had a single relapse event a few weeks after implantation, both subsequently achieved complete abstinence from heroin and methadone, which was maintained for more than 1 and 2 years, respectively. Of note, both patients had a history of polysubstance use, including amphetamines, alcohol and benzodiazepines. However, these were consumed recreationally and was not classified as an addiction. Such continued recreational use suggests that NAc stimulation may selectively influence only maladaptive addictive behavior mediated by this circuitry.
Cocaine addiction
To date, a number of studies have examined the utility of DBS in treating cocaine addiction in rat animal models. However, with respect to human subjects, DBS for cocaine addiction is relatively understudied. The first report from human subjects treated with DBS for cocaine addiction was published in 2016 by Gonçalves-Ferreira et al. (Gonçalves-Ferreira et al., 2016). In their study, the authors followed one subject with treatment-refractory cocaine dependence for a total of thirty months after DBS of both the NAc and bed nucleus of the stria terminalis (BNST). Six months after surgery, the subject achieved a significant reduction in cocaine consumption as well as drug craving. This improvement was maintained during a subsequent blinded period of sham stimulation (i.e. stimulator “off”), which the authors speculated may be due to a placebo effect or secondary to synaptic plasticity. The extent to which these results may be generalized to other cocaine-addicted subjects remains to be seen, and will depend on data from future human studies.
Subthalamic Nucleus
The subthalamic nucleus (STN) is an established DBS target for the treatment of Parkinson’s Disease (PD) and OCD (Mallet et al., 2008; Mirza et al., 2017). As a key basal ganglia structure involved in movement, the role of the STN is well-studied. Due in part to some of the non-motor effects observed in patients treated with DBS of the STN, our understanding of the the STN has expanded to include its role within reward and emotion circuitry (Baunez, 2016).
Dopamine Dysregulation Syndrome/ Pathologic Gambling
PD patients taking dopaminergic medication may be subject to dopamine dysregulation syndrome (DDS), characterized by a compulsive, maladaptive pattern of dopaminergic medication use resembling drug abuse. PD patients may also develop impulse control disorders (ICDs) such as pathologic gambling (PG) (Tanwani et al., 2015), hypersexuality and compulsive shopping (Evans et al., 2004). For such patients, in addition to treating symptoms of Parkinson’s disease, DBS of the STN has been shown to reduce the severity of DDS. A number of studies have demonstrated a reduction in DDS and ICD in patients treated with DBS of the STN for PD (Ardouin et al., 2006; Bandini et al., 2007; Knobel et al., 2008; Lim et al., 2009; Witjas et al., 2005). These findings were observed in the context of lowering levodopa equivalents which is often characteristic of STN DBS, and therefore, only modestly suggestive of the applicability of STN for DBS in subjects with addiction. Given its recent rediscovery within reward and addiction circuitry (McGinty et al., 2011; Volkow et al., 2013), however, it is likely to be the subject of future studies for neuromodulation for addiction.
Other targets
As evidence of the increasing level of research interest in DBS (see Figure 2), a number of other DBS targets have been identified in recent years. They include the anterior cingulate cortex (ACC) (Weston, 2012), the basolateral amygdala (BLA) (Langevin, 2012; Volkow et al., 2013), the lateral habenula (LHb) (Friedman et al., 2011, 2010), and the medial forebrain bundle (MFB) (Döbrössy et al., 2015). Each of these structures has been implicated in some form of reward processing or neural circuitry, and as such each serves as a logical target for DBS with respect to treating addiction. None has yet been studied in human trials. Therefore, further data is needed to determine the precise role that each may play as a target for DBS for addiction. For example, data (unpublished) from our own laboratory implicates a role of the amygdala, orbitofrontal cortex and hippocampus in mediating impulsivity and nicotine addiction.
Figure 2:
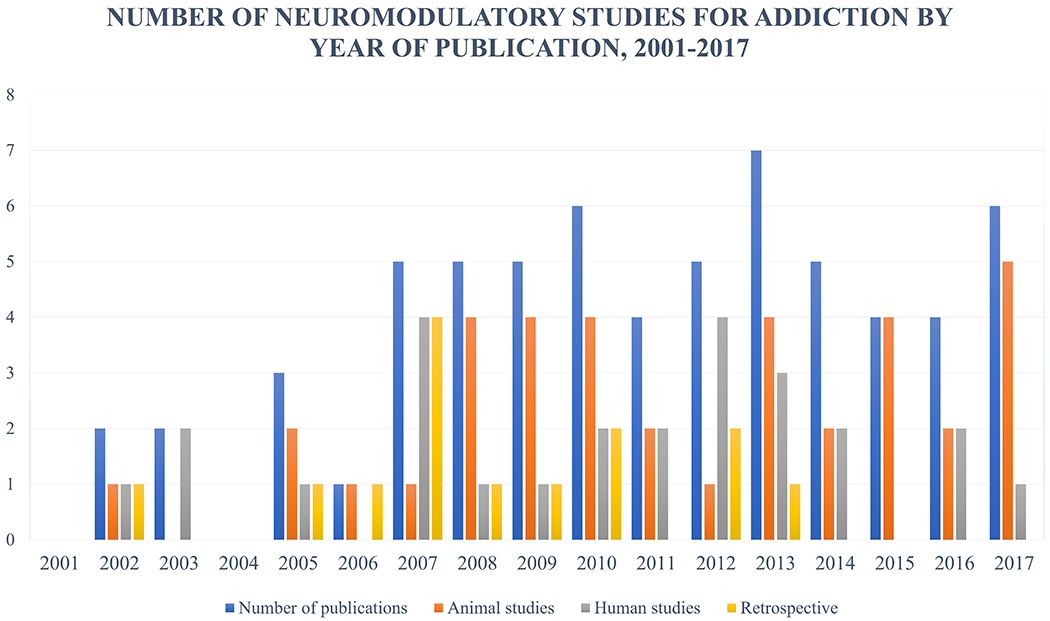
Graphic illustration of number of publications describing pre-clinical or clinical trials investigating brain stimulation techniques for treatment of addictive behaviors. In recent years the proportion of retrospective studies has decreased in favor of prospective studies using addictive behavior as primary endpoints being studied.
Vagal Nerve Stimulation (VNS)
While DBS for addiction directly modulates brain targets within the limbic reward networks, vagal nerve stimulation (VNS) has been shown to modulate limbic networks indirectly via the action of vagal parasympathetic afferent fibers activation of brainstem nuclei which subsequently synapse on limbic brain reward areas. VNS is an approved treatment for epilepsy, and has been has also been well studied as an intervention for patients with major depression and chronic pain (Chakravarthy et al., 2015; Krishna et al., 2016; Moreines et al., 2011). Originally, the vagus nerve was considered to contain exclusively efferent fibers of the parasympathetic nervous system (Groves and Brown, 2005); however, it is now known to be a mixed nerve comprised of 80% afferent and 20% efferent fibers (George et al., 2000). The cell bodies of the afferent nerve fibers exist within the nodose and jugular ganglia, and project to the nucleus of the solitary tract (NTS) (Maier et al., 1998). The NTS in turn has several direct and indirect projections to various brain structures. Thus, afferent components of the vagus nerve have direct and indirect influence over higher-order neural circuits within the brain (Barnes et al., 2003). It is in this context that vagal nerve stimulation has benefitted from considerable attention from researchers as a means to modulate central brain networks involved in addictive behaviors.
Obesity
To date, few animal models have been used to investigate the potential utility of VNS as therapy for addiction (Childs et al., 2017; Liu et al., 2011), albeit with promising results. With respect to human trials, existing data supporting the potential for VNS as a therapy for addiction are purely observational. In 2007 Pardo et al. (Pardo et al., 2007) reported an incidental finding from a study of VNS for obese subjects with refractory depression. The authors noted that – independent of changes in mood symptoms – subjects were found to have experienced significant, gradual weight loss during stimulation. Similar findings of weight loss have been reported from a retrospective analysis of VNS for epilepsy patients (Burneo et al., 2002), in which 8 of 32 (25%) subjects experienced significant weight loss during stimulation. Moreover, their weight loss was associated with increasing stimulator current intensities. While obesity in these subjects may not necessarily imply an “addiction” to food, the observations suggest that VNS may modulate appetetive behavior. Further study of VNS within the context of addiction is warranted.
Transcranial Stimulation Techniques
In addition to surgical techniques (i.e. DBS and VNS), neuromodulation can also be performed through a nonsurgical transcutaneous transcranial approach. Transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (TCDS) are two such strategies with potential therapeutic utility. While their precise mechanism is not fully understood (Kuhn 2015), both recent studies have described the application of both techniques for the treatment of addiction. Relying on recent neuroimaging data which suggests that certain cortical areas of the brain may play an important role in addictive behavior, a number of studies have explored TCDS and TMS of the dorsolateral prefrontal cortex (DLPFC) (Boggio et al., 2008). To date, studies have been published describing TCDS or TMS for patients with alcohol dependence (Boggio et al., 2008; da Silva et al., 2013; Gorelick et al., 2014; Klauss et al., 2014), nicotine addiction (Boggio et al., 2009; Fecteau et al., 2014; Fregni et al., 2008; Gorelick et al., 2014), cocaine addiction (Batista et al., 2015; Camprodon et al., 2007; Conti and Nakamura-Palacios, 2014; Politi et al., 2008; Terraneo et al., 2016), and food addiction (Bou Khalil and El Hachem, 2014; Sauvaget et al., 2015).
The potential for transcranial techniques in various addictive behaviors is promising, but it must also be considered with the important disclaimer that both tDCS and TMS may produce temporary effects with a washout period in between administrations, and as such do not portend the same long-term benefits of more directly-targeted therapies like DBS or VNS. For transcranial modalities, long-term benefits would likely require chronic therapy administered on a regular basis which poses unique challenges in subjects with addiction.
Degree of invasiveness / safety profiles
An important consideration when comparing these forms of neuromodulation is the relative safety of each therapy. Excluding ablative procedures, DBS is considered to be the most invasive of those therapeutic modalities discussed. Given that this technique involves implantation of electrodes intracranially, it carries risks which are absent for VNS and transcranial techniques. The most serious complication for DBS surgery is intracranial hemorrhage which is reported to occur in 1.9% to 4.1% of cases, while permanent neurologic complications have been reported to occur in approximately 2% (Knotkova, 2015). One report of 319 consecutive patients treated with DBS for various movement disorders from 1995-2005 recorded and categorized adverse events (AEs) in three stages: 1) intraoperative AEs, which took place during surgery or anesthesia, including isolated seizure (1.2%), intracerebral hemorrhage (0.6%), intraventricular hemorrhage (0.6%) and a large subdural hematoma (0.3%); 2) perioperative AEs, which occurred within 2 weeks of surgery, such as headache (15.0%), confusion (5.0%), and hallucinations (2.8%); and 3) long-term AEs, defined as occurring more than two weeks after surgery, including dysarthria (4.0%), worsening gait (3.8%), cognitive dysfunction (4.0%), and infection (4.4%). Revisions was required for 7.8% of patients, for indications including infection, lead fracture (3.1%), and lead migration (2.6%) (Kenney et al., 2007).
Comparatively, VNS is considerably less invasive since it does not involve an intracranial electrode placement. That said, VNS is still consists of a surgically implanted device, and a number of complications have been reported for this technique related to both the surgery and to stimulation of the vagus nerve itself. One study which aggregated data from 454 patients treated with VNS for epilepsy reported complications as follows: 1) during and immediately after surgery, AEs included infection (3% to 6%), reversible vocal cord paralysis (0.7%), lower facial weakness (0.7%), and bradycardia with asystole (0.1%) which was only reported during implantation and interrogation of vagal nerve leads; 2) within three months of surgery, AEs included hoarseness (37.2%), voice alternation (37.2%), throat pain (11% - 28.1%), cough (42% - 45%), paresthesia (25%), headache (20%), dyspnea (16%), general pain (17%) and neck pain (17%), all of which were rated as “mild” or “moderate”, and did not require adjustment of stimulation parameters; and finally 3) after long-term follow-up (5 years), AEs included hoarseness (18.7%), dyspnea (3.2%), paresthesia (1.5%), throat pain (4.7%) and cough (1.5%), nearly all of which were associated with the stimulation-on state (Ben-Menachem, 2001).
Nonsurgical transcranial techniques are at a considerable advantage in this regard, as they do not require any surgical intervention and therefore manage to avoid many of the intraoperative and postoperative complications associated with DBS and VNS. One systematic review of TMS included 1,001 patients, with AEs in only 5% of patients. Of these, the most common AEs included headache or other facial pain (46%), lightheadedness (22%), non-specific discomfort (10%), or muscle contraction (10%). Seizure was reported in one subject during stimulation (Oberman et al., 2011). Another systematic review of tDCS including 3,836 subjects reported AEs to include itching, tingling, headache, burning sensation and general discomfort, although each of these was found to occur with similar frequency in sham stimulation, suggesting that AEs for TMS could be largely attributable to contact with the transcranial hardware, as opposed to stimulation per se (Brunoni et al., 2011).
Ethical considerations
As these techniques become more prevalent and their efficacy improves, time should be taken to consider the ethical implications of treating patients with substance use disorders in this manner. Addicted patients are especially vulnerable as study participants, largely as a result of the various social, economic, and psychological stressors which they experience to a disproportionate degree (Luigjes et al., 2015). Previous studies have also reported that patients with addiction often seek financial compensation from clinical trial participation as a source of income (Ali et al., 2016; Luigjes et al., 2015). Therefore, this factor should be considered when planning future trial of neuromodulation for addiction, both to avoid subjects whose primary motive is financial, and to mitigate concerns regarding subject coercion or undue influence (Ali et al., 2016).
Appropriate, comprehensive rehabilitation from substance abuse and addiction is not limited to treating craving and withdrawal symptoms, but ought to include the appropriately tailored social, emotional and financial rehabilitation to ensure that abstinence is maximally achieved and maintained. As a corollary, while patient selection is a vital component of any major medical treatment, attention must be paid to ensure patients requesting neuromodulatory therapy are not denied purely as a consequence of their social or financial circumstances. Requiring a multidisciplinary team focused on each of the various components of a patient’s rehabilitation will be vital in ensuring that neuromodulation therapies are afforded the highest probability of success.
FIVE-YEAR VIEW
Although the deliberate use of DBS in the treatment of addiction is relatively nascent, this review is a testament to the progress which has been made towards understanding the neuronal circuitry underlying reward and its subsequent dysregulation resulting in addiction. The next five years will no doubt see this advancement continue, but progress will require overcoming a number of critical challenges. A few issues in particular must be addressed given the therapy of interest and the indication at hand.
Obstacles to trial design and completion
First and foremost, trials which target addiction are at an immediate disadvantage with respect to recruitment and retention of study subjects. Addicted subjects are by virtue of their disease particularly challenging to study from scientific, logistical and ethical perspectives. Given that DBS requires both a surgical intervention and long-term follow-up, appropriate and ethical subject recruitment is critical. Substance abuse subjects tend to have more serious social and medical problems than typical study participants, which can increase the barriers to receiving DBS and exacerbate issues with compliance (Luigjes et al., 2015). In this respect, clinical trial design and execution is disproportionately influenced by the substance of abuse being studied. Moreover, the resources required to recruit and monitor subjects addicted to nicotine would differ considerably from those required to study subjects addicted to methamphetamines and opiates. Given the incidence of relapse with substance abuse, participants must be willing and able to commit to prolonged follow-up at regular intervals. The presence of an adequate support system is paramount in ensuring adherence to monitoring visits and testing, as well as in notifying investigators of adverse events as they arise. Finally, participation in a study on addictive behavior requires that subjects openly admit to behaviors which can carry considerable social stigmata or sense of embarrassment. Compared to other DBS indications, addiction faces an uphill battle with respect to enrolling subjects willing to undergo the emotional and psychological stress of confronting their addiction.
As a therapeutic intervention, DBS falls under the umbrella of “brain surgery”, and consequently may be stigmatized by subjects, their families, the media, and even other physicians. The disparity in perceived seriousness between DBS and other forms of therapy for addiction has no doubt contributed to difficulty in recruiting study subjects. In 2015 Luigjes et al. (Luigjes et al., 2015) questioned the feasibility of DBS for addiction after experiencing considerable difficulty recruiting just eight subjects for a pilot study aimed at determining the feasibility of DBS for treatment-refractory cocaine and/or heroin addiction in the Netherlands. Similar issues were not observed for a DBS trial for patients with OCD at the same institution. Fear of having a “surgical procedure”, lack of insight into the severity of addiction as a disease (Luigjes et al., 2015), and a reluctance by clinicians to consider addiction as a true brain disorder (Heyman, 2013; Levy, 2013) likely contributed to the observed discrepancy. Though not explicitly reported in most DBS trials, it is likely that this phenomenon beguiles the procedure as an investigational therapy, and may not improve without addressing the underlying apprehension that participants and clinicians alike may have towards DBS for addictive indications.
Tackling these issues will be essential to achieving progress with respect to neuromodulation for addiction. In some respects, the disadvantages related to studying substance-abusing participants may be mitigated by focusing on specific subsets of substance abusers. In this regard, nicotine addiction may be the ideal candidate substance for further study. Tobacco smoking is relatively common with a reported prevalence of smoking amongst adults in the United States estimated to be 19% (Centers for Disease Control and Prevention, 2012). While the burden of disease related to smoking is tremendous, daily smokers usually do not face the same scale of social and legal stigma as abusers of substances such as alcohol, cocaine or opiates (Ahern et al., 2007; Room, 2005). As such, their participation in and adherence to clinical trial protocols may be more likely.
Despite the large numbers of potential study subjects with nicotine addiction, recruitment for an intervention such as DBS, however, may fall victim to the same issues reported by Luigjes et al. (Luigjes et al., 2015). Given that smoking tobacco produces its negative health effects over the long term, subjects with an active addiction may not feel compelled to undergo an invasive treatment, especially if they are younger and feel healthy. In this regard, it is possible that less invasive modalities are more likely to succeed in these subjects. In this respect, VNS and transcranial techniques offer some advantages, as they are far less invasive than DBS and do not come with the stigma of “psychosurgery”. Thus, potential participants and their primary care provides may be more amenable to consider these options as potential experimental therapies. Furthermore, studies using these less invasive techniques combined with neuroimaging may provide additional data regarding the circuitry underlying addiction and pave the way for more informed trials of DBS for addiction.
Testing multiple targets
While current evidence is strongly suggestive of a central role of the ventral tegmental area and NAc in reward processing and addictive behavior, fewer studies have searched for common drug-induced molecular changes in other areas of the brain associated with addiction (Nestler, 2005). Such studies are essential to the progress of neuromodulatory therapy, as their findings will contribute to a more comprehensive understanding of the addiction process and may lead to the development of new targets for neuromodulation therapy. It is likely that in the next five years our understanding of a successful neuromodulatory treatment will involve stimulating multiple targets simultaneously, perhaps even through multiple modalities, such that each target can be adjusted independently to achieve an optimal treatment result. Such an approach would be consistent with our evolving understanding of the reward networks within the brain much the same way that the study of Parkinson’s disease arrived at a working hypothesis of the underlying basal ganglia circuitry(DeLong and Wichmann, 2007).
Improving measures of efficacy
An important limitation in testing any intervention for addiction lies in the ambiguity in measurable outcomes for addicted subjects. While substance use and disuse are measurable, addictive behavior consists of many physiologic, psychiatric and emotional components which are difficult to measure objectively. Quantifying efficacy can therefore be difficult, given the variety of imprecise symptoms and behaviors implicated by a given intervention. In recent years, several studies have reported discrepancies in brain activation between addicted and non-addicted subjects using functional MRI (fMRI) (Asensio et al., 2010; Bickel et al., 2007; Goudriaan et al., 2010). Though not yet broadly applicable, imaging biomarkers such as those being discovered with fMRI, DTI and PET imaging modalities have tremendous potential within addiction research, as they may more definitively classify and track subjects during a given intervention.
A multidisciplinary approach
While the field of invasive neuromodulation will continue to expand and improve in the coming years, successful treatment for refractory substance abuse and addiction will require a multidisciplinary approach, wherein each patient’s treatment plan is overseen by neurosurgeons, psychiatrists, primary care providers, ethicists and social workers. As mentioned previously, a singular focus on neuromodulation therapy may jeopardize a patient’s chances at recovery. We argue that effectively reducing craving and withdrawal symptoms is necessary but not sufficient for achieving and maintaining abstinence from an addictive substance or behavior. Particularly with respect to maintenance, a multidisciplinary approach can help mitigate those social, personal, and financial factors which so often lead to relapse in addicted patients (White and Kelly, 2011). Just as substance addiction is not the result of any one single neurologic or anatomical defect, its treatment will require the input of multiple specialists working in concert to maximize the efficacy of a given neuromodulatory therapy.
SUMMARY/CONCLUSION
Neuromodulation therapy – a therapy which exerts its effects by targeting dysfunctional neural circuitry – stands poised to dramatically influence the field of addiction. As our understanding of addictive behavior and its associated neural underpinnings has evolved, new neuronal targets for therapy have emerged. The current state of neuromodulation therapy for addiction is in testing these targets for their clinical role in various addictive behaviors. As described in this review, certain therapeutic modalities and brain targets have generated greater interest with more experimental human data than others. While further study is warranted to establish a more concrete understanding of the mechanisms underlying the success of neuromodulation in treating addiction, we are confident that the future of addiction therapy will include a component of neuromodulation as part of a multidisciplinary team-based approach to each individual patient. As clinicians and patients begin to embrace the concept of addiction as a brain disorder, therapies aiming to treat dysfunctional neural networks will follow as intuitive, logical solutions to a serious brain disease. The next five years will likely see a great deal of progress towards that end, and as such we encourage the continued efforts of all those tasked with advancing the field of neuromodulation for the betterment of the human condition.
Table 2:
Literature examining DBS surgery for addiction
| Authors & Year | Study Type | Number, type of subjects | Target | Follow-up time | Results |
|---|---|---|---|---|---|
| Alcohol Dependence | |||||
| Kuhn et al., 2007 | Prospective case report | 1 alcohol-dependent subject | Bilateral NAc | 12 months | At 12 months follow-up: -Subject no longer drank to excess -Subject endorsed a reduction in urge to drink |
| Heinz et al., 2009 | Prospective case series | 3 alcohol-dependent subjects | Bilateral NAc | 14 months | At 14 months follow-up: -2/3 (67%) achived & maintained abstinence -3/3 (100%) experienced significant reduction in urge to consume alcohol |
| Muller et al., 2009 | Prospective case series | 3 alcohol-dependent subjects | Bilateral NAc | 1 year | At 1 year follow-up: -2/3 subjects achieved and maintained abstinence -1/3 subjects markedly reduced alcohol consumption |
| Voges et al., 2013 | Prospective case series | 5 alcohol-dependent subjects | Bilateral NAc | 38 months (mean) | At mean 38 months follow-up: -2/5 subjects (40%) achieved and maintained abstinence -3/5 (60%) achieved reduction in relapse frequency and severity |
| Muller et al., 2016 | Prospective case series | 5 alcohol-dependent subjects | Bilateral NAc | 8 years (maximum) | After 3 years of follow-up: -5/5 subjects (100%) experienced a disappearance in urge to consume alcohol -2/5 (40%) achieved and maintained abstinence -2/5 (40%) achieved reduction in relapse frequency and severity |
| Nicotine | |||||
| Kuhn et al., 2009 | Prospective case series | 10 nicotine-dependent subjects | Unilateral NAc (n=5) Bilateral NAc (n=5) |
30 months | At 30 months follow-up: -Mean score on FTND decreased from 4.44 → 3.33 |
| Mantione et al., 2010 | Prospective case report | 1 nicotine-dependent subject | Bilateral NAc | 2 years | At 2 years follow-up: -Subject achieved and maintained abstinence from smoking. |
| Cocaine | |||||
| Gonçalves-Ferreira et al., 2016 | Case report, double-blind, cross-over study | 1 cocaine-dependent subject | Bilateral NAc, bilateral BNST | 24 months | At 24 months follow-up: -Subject experienced significant reduction in subjective craving for cocaine use -Weeks free of cocaine consumption was significantly reduced (40.9% pre-DBS, 68% at 24 months post-DBS) |
| Heroin | |||||
| Zhou et al., 2011 | Case report | 1 heroin-dependent subject | Bilateral NAc | 6 years (stimulation for 2.5 years, stimulator removed for 3.5 years) | At 6 years follow-up: -Subject achieved and maintained abstinence from heroin drug abuse. |
| Valencia-Alfonso et al., 2012 | Case report | 1 heroin-dependent subject | Bilateral NAc, bilateral ALIC | 6 months | At 6 months follow-up: -Subject achieved and maintained abstinence (with exception of one 14-day relapse) |
| Kuhn et al., 2014 | Case series (piloting phase to a clinical trial) | 2 heroin-dependent subjects | Bilateral NAc | 24 months | At 24 months follow-up: -2/2 (100%) of subjects achieved and maintained abstinence from chronic heroin abuse |
NAc = nucleus accumbens; ALIC = anterior limb of internal capsule; BNST = bed nucleus of stria terminalis; FTND = Fagerstrom test for nicotine dependence
HIGHLIGHTS:
Substance abuse and addiction are serious and costly socioeconomic problems
Neuromodulation therapies have shown significant promise for treating addiction
Recently, DBS and transcranial techniques enjoy substantial research interest
The NAc is the most well-studied target with respect to DBS for addiction
Apprehension regarding the invasiveness of such procedures must be addressed
Acknowledgments
The authors have no acknowledgements to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Competing interests
The authors have no competing interests to declare.
REFERENCES
- Ahern J, Stuber J, Galea S, 2007. Stigma, discrimination and the health of illicit drug users. Drug Alcohol Depend. 88, 188–196. 10.1016/j.drugalcdep.2006.10.014 [DOI] [PubMed] [Google Scholar]
- Ali R, Difrancesco MF, Ho AL, Kampman KM, Caplan AL, Halpern CH, 2016. Attitudes Toward Treating Addiction with Deep Brain Stimulation. Brain Stimul. 10.1016/j.brs.2016.03.009 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. DSM-V, American Journal of Psychiatry. 10.1176/appi.books.9780890425596.744053 [DOI] [Google Scholar]
- Ardouin C, Voon V, Worbe Y, Abouazar N, Czernecki V, Hosseini H, Pelissolo A, Moro E, Lhommée E, Lang AE, Agid Y, Benabid AL, Pollak P, Mallet L, Krack P, 2006. Pathological gambling in Parkinson’s disease improves on chronic subthalamic nucleus stimulation. Mov. Disord 21, 1941–1946. 10.1002/mds.21098 [DOI] [PubMed] [Google Scholar]
- Asensio S, Romero MJ, Palau C, Sanchez A, Senabre I, Morales JL, Carcelen R, Romero FJ, 2010. Altered neural response of the appetitive emotional system in cocaine addiction: An fMRI Study. Addict. Biol 15, 504–516. 10.1111/j.1369-1600.2010.00230.x [DOI] [PubMed] [Google Scholar]
- Bandini F, Primavera A, Pizzorno M, Cocito L, 2007. Using STN DBS and medication reduction as a strategy to treat pathological gambling in Parkinson’s disease. Park. Relat. Disord 13, 369–371. 10.1016/j.parkreldis.2006.07.011 [DOI] [PubMed] [Google Scholar]
- Barnes A, Duncan R, Chisholm JA, Lindsay K, Patterson J, Wyper D, 2003. Investigation into the mechanisms of vagus nerve stimulation for the treatment of intractable epilepsy, using 99mTc-HMPAO SPET brain images. Eur. J. Nucl. Med. Mol. Imaging 30, 301–305. 10.1007/s00259-002-1026-8 [DOI] [PubMed] [Google Scholar]
- Batista EK, Klauss J, Fregni F, Nitsche MA, Nakamura-Palacios EM, 2015. A randomized placebo-controlled trial of targeted prefrontal cortex modulation with bilateral tDCS in patients with crack-cocaine dependence. Int. J. Neuropsychopharmacol 18, 1–11. 10.1093/ijnp/pyv066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunez C, 2016. The Subthalamic Nucleus and Reward-Related Processes, in: Soghomonian J-J. (Ed.), The Basal Ganglia. pp. 319–337. [Google Scholar]
- Ben-Menachem E, 2001. Vagus nerve stimulation, side effects, and long-term safety. J. Clin. Neurophysiol 18, 415–418. [DOI] [PubMed] [Google Scholar]
- Berglund M, Thelander S, Salaspuro M, Franck J, Andreasson S, Ojehagen A, 2003. Treatment of Alcohol Abuse: An Evidence-Based Review. Alcohol. Clin. Exp. Res 27, 1645–1656. 10.1097/01.ALC.0000090144.99832.19 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA, 2007. Behavioral and neuroeconomics of drug addiction: Competing neural systems and temporal discounting processes. Drug Alcohol Depend. 10.1016/j.drugalcdep.2006.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Liguori P, Sultani N, Rezende L, Fecteau S, Fregni F, 2009. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci. Lett 463, 82–86. 10.1016/j.neulet.2009.07.041 [DOI] [PubMed] [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, Basaglia A, Fregni F, 2008. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: A double-blind, sham-controlled study. Drug Alcohol Depend. 92, 55–60. 10.1016/j.drugalcdep.2007.06.011 [DOI] [PubMed] [Google Scholar]
- Bou Khalil R, El Hachem C, 2014. Potential role of repetitive transcranial magnetic stimulation in obesity. Eat. Weight Disord. - Stud. Anorexia, Bulim. Obes 19, 403–407. 10.1007/s40519-013-0088-x [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD, 2011. Economic costs of excessive alcohol consumption in the U.S., 2006. Am. J. Prev. Med 41, 516–524. 10.1016/j.amepre.2011.06.045 [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F, 2011. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int. J. Neuropsychopharmacol 10.1017/S1461145710001690 [DOI] [PubMed] [Google Scholar]
- Burchiel K, Liker MA, Lozano AM, 2015. Deep brain stimulation: current assessment, new applications, and future innovations. Neurosurg. Focus 38, 1–2. 10.3171/2015.4.FOCUS15179.Disclosure [DOI] [PubMed] [Google Scholar]
- Burneo J, Faught E, Knowlton R, Morawetz R, Kuzniecky R, 2002. Weight loss associated with vagus nerve stimulation. Neurology 59, 463–464. [DOI] [PubMed] [Google Scholar]
- Camprodon JA, Martínez-Raga J, Alonso-Alonso M, Shih MC, Pascual-Leone A, 2007. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 86, 91–94. 10.1016/j.drugalcdep.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Carroll KM, Onken LS, 2005. Behavioral therapies for drug abuse. Am. J. Psychiatry 162, 1452–1460. 10.1176/appi.ajp.162.8.1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2012. Current cigarette smoking among adults - United States, 2011. MMWR Morb. Mortal. Wkly. Rep 61, 889–894. 10.1136/tobaccocontrol-2012-050529 [DOI] [PubMed] [Google Scholar]
- Chakravarthy K, Chaudhry H, Williams K, Christo PJ, 2015. Review of the Uses of Vagal Nerve Stimulation in Chronic Pain Management. Curr. Pain Headache Rep 19 10.1007/s11916-015-0528-6 [DOI] [PubMed] [Google Scholar]
- Childs JE, DeLeon J, Nickel E, Kroener S, 2017. Vagus nerve stimulation reduces cocaine seeking and alters plasticity in the extinction network. Learn. Mem 24, 35–42. 10.1101/lm.043539.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti C, Nakamura-Palacios E, 2014. Bilateral Transcranial Direct Current Stimulation Over Dorsolateral Prefrontal Cortex Changes the Drug-cued Reactivity in the Anterior Cingulate Cortex of Crack-. Brain Stimul. 7, 2013–2015. [DOI] [PubMed] [Google Scholar]
- da Silva MC, Conti CL, Klauss J, Alves LG, do Nascimento Cavalcante HM, Fregni F, Nitsche MA, Nakamura-Palacios EM, 2013. Behavioral effects of transcranial Direct Current Stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J. Physiol. Paris 107, 493–502. 10.1016/j.jphysparis.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, Freedman G, Burstein R, Johns N, Engell RE, Flaxman A, Murray CJL, Vos T, 2013. Global burden of disease attributable to illicit drug use and dependence: Findings from the Global Burden of Disease Study 2010. Lancet 382, 1564–1574. 10.1016/S0140-6736(13)61530-5 [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T, 2007. Circuits and circuit disorders of the basal ganglia. Arch. Neurol 10.1001/archneur.64.1.20 [DOI] [PubMed] [Google Scholar]
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, Daniels C, Deutschländer A, Gruber D, Hamel W, Herzog J, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reu A, 2006. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med 355, 896–908. 10.1056/NEJMoa060281 [DOI] [PubMed] [Google Scholar]
- Döbrössy MD, Furlanetti LL, Coenen VA, 2015. Electrical stimulation of the medial forebrain bundle in pre-clinical studies of psychiatric disorders. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2014.11.018 [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW, 2008. A meta-analytic review of psychosocial interventions for substance use disorders. Am. J. Psychiatry 165, 179–87. 10.1176/appi.ajp.2007.06111851 [DOI] [PubMed] [Google Scholar]
- Evans AH, Katzenschlager R, Paviour D, O’Sullivan JD, Appel S, Lawrence AD, Lees AJ, 2004. Punding in Parkinson’s disease: Its relation to the dopamine dysregulation syndrome. Mov. Disord 19, 397–405. 10.1002/mds.20045 [DOI] [PubMed] [Google Scholar]
- Fecteau S, Agosta S, Hone-Blanchet A, Fregni F, Boggio P, Ciraulo D, Pascual-Leone A, 2014. Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: A preliminary study. Drug Alcohol Depend. 140, 78–84. 10.1016/j.drugalcdep.2014.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck J, Jayaram-Lindström N, 2013. Pharmacotherapy for alcohol dependence: Status of current treatments. Curr. Opin. Neurobiol 10.1016/j.conb.2013.05.005 [DOI] [PubMed] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS, 2008. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: A randomized, sham-controlled study. J. Clin. Psychiatry 69, 32–40. 10.4088/JCP.v69n0105 [DOI] [PubMed] [Google Scholar]
- Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E, Ben-Tzion M, Ami-Ad L, Yaka R, Yadid G, 2010. Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology 59, 452–459. 10.1016/j.neuropharm.2010.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E, Ben-Tzion M, Yadid G, 2011. Electrical stimulation of the lateral habenula produces an inhibitory effect on sucrose self-administration. Neuropharmacology 60, 381–387. 10.1016/j.neuropharm.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Wang X, 2015. Stereotactic Neurosurgery for Drug Addiction, in: Neurosurgical Treatments for Psychiatric Disorders. pp. 161–173. [Google Scholar]
- Gao G, Wang X, He S, Li W, Wang Q, Liang Q, Zhao Y, Hou F, Chen L, Li A, 2003. Clinical Study for Alleviating Opiate Drug Psychological Dependence by a Method of Ablating the Nucleus accumbens with Stereotactic Surgery. Stereotact. Funct. Neurosurg 81, 96–104. 10.1159/000075111 [DOI] [PubMed] [Google Scholar]
- George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, Lisanby S, Burt T, Goldman J, Ballenger JC, 2000. Vagus nerve stimulation: a new tool for brain research and therapy. Biol. Psychiatry 47, 287–95. 10.1016/S0006-3223(99)00308-X [DOI] [PubMed] [Google Scholar]
- Gonçalves-Ferreira A, Do Couto FS, Rainha Campos A, Lucas Neto LP, Gonçalves-Ferreira D, Teixeira J, 2016. Deep Brain Stimulation for Refractory Cocaine Dependence. Biol. Psychiatry 79, e87–e89. 10.1016/j.biopsych.2015.06.023 [DOI] [PubMed] [Google Scholar]
- Goodman A, 2008. Neurobiology of addiction. An integrative review. Biochem. Pharmacol 75, 266–322. 10.1016/j.bcp.2007.07.030 [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Zangen A, George MS, 2014. Transcranial magnetic stimulation in the treatment of substance addiction. Ann. N. Y. Acad. Sci 1327, 79–93. 10.1111/nyas.12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, De Ruiter MB, Van Den Brink W, Oosterlaan J, Veltman DJ, 2010. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: An fMRI study. Addict. Biol 15, 491–503. 10.1111/j.1369-1600.2010.00242.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves DA, Brown VJ, 2005. Vagal nerve stimulation: A review of its applications and potential mechanisms that mediate its clinical effects. Neurosci. Biobehav. Rev 29, 493–500. 10.1016/j.neubiorev.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grüsser SM, Grace AA, Wrase J, 2009. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict. Biol 14, 108–118. 10.1111/j.1369-1600.2008.00136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze H-J, 2009. Counteracting incentive sensitization in severe alcohol dependence using deep brain stimulation of the Nucleus accumbens: clinical and basic science aspects. Front. Hum. Neurosci 3, 1–11. 10.3389/neuro.09.022.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman GM, 2013. Addiction and choice: Theory and new data. Front. Psychiatry 4 10.3389/fpsyt.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, 2010. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci. Biobehav. Rev 35, 129–150. 10.1016/j.neubiorev.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y, 2014. Factors modulating neural reactivity to drug cues in addiction: A survey of human neuroimaging studies. Neurosci. Biobehav. Rev 38, 1–16. 10.1016/j.neubiorev.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney C, Simpson R, Hunter C, Ondo W, Almaguer M, Davidson A, Jankovic J, 2007. Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J. Neurosurg 106, 621–625. 10.3171/jns.2007.106.4.621 [DOI] [PubMed] [Google Scholar]
- Klauss J, Penido Pinheiro LC, Silva Merlo BL, Correia Santos G de A., Fregni F, Nitsche MA, Miyuki Nakamura-Palacios E, 2014. A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int. J. Neuropsychopharmacol 17, 1793–1803. 10.1017/S1461145714000984 [DOI] [PubMed] [Google Scholar]
- Knobel D, Aybek S, Pollo C, Vingerhoets FJG, Berney A, 2008. Rapid resolution of dopamine dysregulation syndrome (DDS) after subthalamic DBS for parkinson disease (PD). Cogn. Behav. Neurol 21, 187–189. 10.1097/WNN.0b013e318185e6e2 [DOI] [PubMed] [Google Scholar]
- Knotkova H, 2015. Textbook of Neuromodulation. 10.1007/978-1-4939-1408-1 [DOI] [Google Scholar]
- Kochanek KD, Xu J, Murphy SL, Minino AM, Kung H, 2011. Deaths : Final Data for 2009. Natl. Vital Stat. Reports 60, 1–166. https://doi.org/May8,2013 [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry 3, 760–773. 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna V, Sammartino F, Kon N, King K, 2016. Neuromodulation for Epilepsy. Neurosurg. Clin. N. Am 27, 123–131. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Bauer R, Pohl S, Lenartz D, Huff W, Kim EH, Klosterkoetter J, Sturm V, 2009. Observations on unaided smoking cessation after deep brain stimulation of the nucleus accumbens. Eur. Addict. Res 15, 196–201. 10.1159/000228930 [DOI] [PubMed] [Google Scholar]
- Kuhn J, Grundler TOJ, Bauer R, Huff W, Fischer AG, Lenartz D, Maarouf M, Bührle C, Klosterkötter J, Ullsperger M, Sturm V, 2011. Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addict. Biol 16, 620–623. 10.1111/j.1369-1600.2011.00337.x [DOI] [PubMed] [Google Scholar]
- Kuhn J, Lenartz D, Huff W, Lee S, Koulousakis A, Klosterkoetter J, Sturm V, 2007. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J. Neurol. Neurosurg. Psychiatry 78, 1152–3. 10.1136/jnnp.2006.113092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J, Möller M, Lenartz D, Christian PB, Veerle V-V, 2015. Neuromodulation for Addiction, in: Knotkova H, Rasche D (Eds.), Textbook of Neuromodulation. Springer, pp. 247–255. [Google Scholar]
- Kuhn J, Moller M, Treppmann JF, Bartsch C, Lenartz D, O J, G T., Maarouf M, Brosig A, Barnikol UB, Klosterkötter J, Sturm V, 2014. Deep brain stimulation of the nucleus accumbens and its usefulness in severe opioid addiction. Mol. Psychiatry 19, 145–146. 10.1038/mp.2012.196 [DOI] [PubMed] [Google Scholar]
- Kupsch A, Benecke R, Müller JJ-U, Trottenberg T, Schneider G-H, Poewe W, Eisner W, Wolters A, Müller JJ-U, Deuschl G, others, Pinsker MO, Skogseid IM, Roeste GK, Vollmer-Haase J, Brentrup A, Krause M, Tronnier V, Schnitzler A, Voges J, Nikkhah G, Vesper J, Naumann M, Volkmann J, 2006. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N. Engl. J. Med 355, 1978–1990. 10.1056/NEJMoa063618 [DOI] [PubMed] [Google Scholar]
- Langevin J-P, 2012. The amygdala as a target for behavior surgery. Surg. Neurol. Int 3, 40 10.4103/2152-7806.91609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N, 2013. Addiction is not a brain disease (and it matters). Front. Psychiatry 4 10.3389/fpsyt.2013.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wang J, Wang XL, Chang CW, Ge SN, Gao L, Wu HM, Zhao HK, Geng N, Gao GD, 2013. Nucleus accumbens surgery for addiction. World Neurosurg. 80, 9–19. 10.1016/j.wneu.2012.10.007 [DOI] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng ATA, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FGR, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Hanafiah KM, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CDH, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJC, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, Van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJL, Ezzati M, 2012. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, O’Sullivan SS, Kotschet K, Gallagher DA, Lacey C, Lawrence AD, Lees AJ, O’Sullivan DJ, Peppard RF, Rodrigues JP, Schrag A, Silberstein P, Tisch S, Evans AH, 2009. Dopamine dysregulation syndrome, impulse control disorders and punding after deep brain stimulation surgery for Parkinson’s disease. J. Clin. Neurosci 16, 1148–1152. 10.1016/j.jocn.2008.12.010 [DOI] [PubMed] [Google Scholar]
- Liu H, Liu Y, Yu J, Lai M, Zhu H, Sun A, Chen W, Zhou W, 2011. Vagus nerve stimulation inhibits heroin-seeking behavior induced by heroin priming or heroin-associated cues in rats. Neurosci. Lett 494, 70–74. 10.1016/j.neulet.2011.02.059 [DOI] [PubMed] [Google Scholar]
- Lozano AM, Lipsman N, 2013. Probing and Regulating Dysfunctional Circuits Using Deep Brain Stimulation. Neuron 77, 406–424. 10.1016/j.neuron.2013.01.020 [DOI] [PubMed] [Google Scholar]
- Luigjes J, van den Brink W, Schuurman PR, Kuhn J, Denys D, 2015. Is deep brain stimulation a treatment option for addiction? Addiction 110, 547–548. 10.1111/add.12773 [DOI] [PubMed] [Google Scholar]
- Maier SF, Goehler LE, Fleshner M, Watkins LR, 1998. The role of the vagus nerve in cytokine-to-brain communication, in: Annals of the New York Academy of Sciences. pp. 289–300. 10.1111/j.1749-6632.1998.tb09569.x [DOI] [PubMed] [Google Scholar]
- Mallet L, Polosan M, Jaafari N, Baup N, Welter M-L, Fontaine D, du Montcel ST, Yelnik J, Chéreau I, Arbus C, Raoul S, Aouizerate B, Damier P, Chabardès S, Czernecki V, Ardouin C, Krebs M-O, Bardinet E, Chaynes P, Burbaud P, Cornu P, Derost P, Bougerol T, Bataille B, Mattei V, Dormont D, Devaux B, Vérin M, Houeto J-L, Pollak P, Benabid A-L, Agid Y, Krack P, Millet B, Pelissolo A, Group SS, 2008. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N. Engl. J. Med 359, 2121–2134. 10.1056/NEJMoa0708514 [DOI] [PubMed] [Google Scholar]
- Mantione M, Van De Brink W, Schuurman PR, Denys D, 2010. Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens: Therapeutic and research implications: Case report. Neurosurgery 66 10.1227/01.NEU.0000360570.40339.64 [DOI] [PubMed] [Google Scholar]
- Mark TL, Levit KR, Buck JA, Coffey RM, Vandivort-Warren R, 2007. Mental health treatment expenditure trends, 1986–2003. Psychiatr. Serv 58, 1041–8. 10.1176/appi.ps.58.8.1041 [DOI] [PubMed] [Google Scholar]
- McGinty VB, Hayden BY, Heilbronner SR, Dumont EC, Graves SM, Mirrione MM, du Hoffmann J, Sartor GC, España RA, Millan EZ, DiFeliceantonio AG, Marchant NJ, Napier TC, Root DH, Borgland SL, Treadway MT, Floresco SB, McGinty JF, Haber S, 2011. Emerging, reemerging, and forgotten brain areas of the reward circuit: Notes from the 2010 Motivational Neural Networks conference. Behav. Brain Res 10.1016/j.bbr.2011.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev SV, Anichkov AD, Poliakov II, 2003. Physiological mechanisms of the effectiveness of bilateral stereotactic cingulotomy in treatment of strong psychological dependence in drug addiction. Hum. Physiol 29, 492–497. [PubMed] [Google Scholar]
- Meyer C, Rumpf HJ, Schumann A, Hapke U, John U, 2003. Intentionally reduced smoking among untreated general population smokers: Prevalence, stability, prediction of smoking behaviour change and differences between subjects choosing either reduction or abstinence. Addiction 98, 1101–1110. 10.1046/j.1360-0443.2003.00475.x [DOI] [PubMed] [Google Scholar]
- Mirza S, Yazdani U, Dewey III R, Patel N, Dewey RB, Miocinovic S, Chitnis S, 2017. Comparison of Globus Pallidus Interna and Subthalamic Nucleus in Deep Brain Stimulation for Parkinson Disease: An Institutional Experience and Review. Parkinsons. Dis. 2017, 1–15. 10.1155/2017/3410820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreines JL, McClintock SM, Holtzheimer PE, 2011. Neuropsychologic effects of neuromodulation techniques for treatment-resistant depression: A review. Brain Stimul. 4, 17–27. 10.1016/j.brs.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U, Sturm V, Voges J, Heinze H-J, Galazky I, Büntjen L, Heldmann M, Frodl T, Steiner J, Bogerts B, 2016. Nucleus Accumbens Deep Brain Stimulation for Alcohol Addiction – Safety and Clinical Long-term Results of a Pilot Trial. Pharmacopsychiatry 3–6. 10.1055/s-0042-104507 [DOI] [PubMed] [Google Scholar]
- Muller UJ, Sturm V, Voges J, Heinze H-JJ, Galazky I, Heldmann M, Scheich H, Bogerts B, Müller UJ, Sturm V, Voges J, Heinze H-JJ, Galazky I, Heldmann M, Scheich H, Bogerts B, Muller UJ, Sturm V, Voges J, Heinze H-JJ, Galazky I, Heldmann M, Scheich H, Bogerts B, 2009. Successful treatment of chronic resistant alcoholism by deep brain stimulation of nucleus accumbens: first experience with three cases. Pharmacopsychiatry 42, 288–291. 10.1055/s-0029-1233489 [DOI] [PubMed] [Google Scholar]
- Münte TF, 2008. Nucleus accumbens is involved in human action monitoring: evidence from invasive electrophysiological recordings. Front. Hum. Neurosci 1 10.3389/neuro.09.011.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, 2005. Is there a common molecular pathway for addiction? Nat. Neurosci 8, 1445–1449. 10.1038/nn1578 [DOI] [PubMed] [Google Scholar]
- Oberman L, Edwards D, Eldaief M, Pascual-Leone A, 2011. Safety of theta burst transcranial magnetic stimulation: a systematic review of the literature. J. Clin. Neurophysiol 28, 67–74. 10.1097/WNP.0b013e318205135f.Safety [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Sheikh SA, Kuskowski MA, Surerus-Johnson C, Hagen MC, Lee JT, Rittberg BR, Adson DE, 2007. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int. J. Obes. (Lond) 31, 1756–9. 10.1038/sj.ijo.0803666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi E, Fauci E, Santoro A, Smeraldi E, 2008. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am. J. Addict 17, 345–6. 10.1080/10550490802139283 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, 2002. Alcohol Consumption Impairs Detection of Performance Errors in Mediofrontal Cortex. Science (80-. ). 298, 2209–2211. 10.1126/science.1076929 [DOI] [PubMed] [Google Scholar]
- Room R, 2005. Stigma, social inequality and alcohol and drug use. Drug Alcohol Rev. 10.1080/09595230500102434 [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L, 2016. Increases in Drug and Opioid-Involved Overdose Deaths — United States, 2010–2015. MMWR. Morb. Mortal. Wkly. Rep 65, 1445–1452. 10.15585/mmwr.mm655051e1 [DOI] [PubMed] [Google Scholar]
- SAMHSA, 2014. Substance Abuse and Mental Health Services Administration. Crisis Services: Effectiveness, CostEffectiveness, and Funding Strategies. Subst. Abus. Ment. Heal. Serv. Adm [Google Scholar]
- Sauvaget A, Trojak B, Bulteau S, Jiménez-Murcia S, Fernández-Aranda F, Wolz I, Menchón JM, Achab S, Vanelle JM, Grall-Bronnec M, 2015. Transcranial direct current stimulation (tDCS) in behavioral and food addiction: A systematic review of efficacy, technical, and methodological issues. Front. Neurosci 9 10.3389/fnins.2015.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, 2010. Dopamine signals for reward value and risk: basic and recent data. Behav. Brain Funct 6, 24 10.1186/1744-9081-6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurman PR, Bosch DA, Bossuyt PMM, Bonsel GJ, van Someren EJW, de Bie RMA, Merkus MP, Speelman JD, 2000. A Comparison of Continuous Thalamic Stimulation and Thalamotomy for Suppression of Severe Tremor. N. Engl. J. Med 342, 461–468. 10.1056/NEJM200002173420703 [DOI] [PubMed] [Google Scholar]
- Soyka M, Mutschler J, 2016. Treatment-refractory substance use disorder: Focus on alcohol, opioids, and cocaine. Prog. Neuro-Psychopharmacology Biol. Psychiatry 70, 148–161. 10.1016/j.pnpbp.2015.11.003 [DOI] [PubMed] [Google Scholar]
- Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X, 2014. Contribution of Excessive Alcohol Consumption to Deaths and Years of Potential Life Lost in the United States. Prev. Chronic Dis 11, 130293 10.5888/pcd11.130293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, Klosterkötter J, 2003. The nucleus accumbens: A target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J. Chem. Neuroanat 26, 293–299. 10.1016/j.jchemneu.2003.09.003 [DOI] [PubMed] [Google Scholar]
- Tanwani P, Fernie BA, Nikčević AV, Spada MM, 2015. A systematic review of treatments for Impulse Control Disorders and related behaviours in Parkinson’s disease. Psychiatry Res. 225, 402–406. 10.1016/j.psychres.2014.12.005 [DOI] [PubMed] [Google Scholar]
- Terraneo A, Leggio L, Saladini M, Ermani M, Bonci A, Gallimberti L, 2016. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. Eur. Neuropsychopharmacol 26, 37–44. 10.1016/j.euroneuro.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime - UNODC, 2014. World Drug Report.
- US Department of Justice National Drug Intelligence Center, 2011. The Economic Impact of Illicit Drug Use.
- US Department of Transportation National Highway Traffic Safety Administration, 2017. Research Note 2016 Fatal Motor Vehicle Crashes : Overview.
- Valencia-Alfonso CE, Luigjes J, Smolders R, Cohen MX, Levar N, Mazaheri A, Van Den Munckhof P, Schuurman PR, Van Den Brink W, Denys D, 2012. Effective deep brain stimulation in heroin addiction: A case report with complementary intracranial electroencephalogram. Biol. Psychiatry 71, e35–e37. 10.1016/j.biopsych.2011.12.013 [DOI] [PubMed] [Google Scholar]
- Voges J, Müller U, Bogerts B, Münte T, Heinze HJ, 2013. Deep brain stimulation surgery for alcohol addiction. World Neurosurg. 80, S28.e21–S28.e31. 10.1016/j.wneu.2012.07.011 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Frieden TR, Hyde PS, Cha SS, 2014. Medication-Assisted Therapies — Tackling the Opioid-Overdose Epidemic. N. Engl. J. Med 370, 2063–2066. 10.1056/NEJMp1402780 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, 2012. Addiction circuitry in the human brain. Annu. Rev. Pharmacol. Toxicol 52, 321–36. 10.1146/annurev-pharmtox-010611-134625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD, 2013. Unbalanced neuronal circuits in addiction. Curr. Opin. Neurobiol 10.1016/j.conb.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston CSE, 2012. Another major function of the anterior cingulate cortex: The representation of requirements. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2011.04.014 [DOI] [PubMed] [Google Scholar]
- White W, Kelly JF, 2011. Recovery management: What if we really believed that addiction was a chronic disorder?, in: Addiction Recovery Management. pp. 67–84. 10.1007/978-1-60327-960-4_5 [DOI] [Google Scholar]
- Wise RA, 1996. Neurobiology of addiction. Curr. Opin. Neurobiol 10.1016/S0959-4388(96)80079-1 [DOI] [PubMed] [Google Scholar]
- Witjas T, Baunez C, Henry JM, Delfini M, Regis J, Cherif AA, Peragut JC, Azulay JP, 2005. Addiction in Parkinson’s disease: Impact of subthalamic nucleus deep brain stimulation. Mov. Disord. 20, 1052–1055. 10.1002/mds.20501 [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2015. WHO global report on trends in prevalence of tobacco smoking 2015. WHO Mag. 359. https://doi.org/9789241564922 [Google Scholar]
- World Health Organization, 2014. Global status report on alcohol and health 2014. World Heal. Organ 1–100. https://doi.org//entity/substance_abuse/publications/global_alcohol_report/en/index.html
- Wu HM, Wang XL, Chang CW, Li N, Gao L, Geng N, Ma JH, Zhao W, Gao GD, 2010. Preliminary findings in ablating the nucleus accumbens using stereotactic surgery for alleviating psychological dependence on alcohol. Neurosci. Lett 473, 77–81. 10.1016/j.neulet.2010.02.019 [DOI] [PubMed] [Google Scholar]
- Wu L, Sun S, He Y, Zeng J, 2015. Effect of smoking reduction therapy on smoking cessation for smokers without an intention to quit: An updated systematic review and meta-analysis of randomized controlled trials. Int. J. Environ. Res. Public Health. 10.3390/ijerph120910235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Bishop EE, Kennedy SM, Simpson SA, Pechacek TF, 2015. Annual healthcare spending attributable to cigarette smoking: An update. Am. J. Prev. Med 48, 326–333. 10.1016/j.amepre.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Xu J, Jiang J, 2011. Deep brain stimulation of nucleus accumbens on heroin-seeking behaviors: A case report. Biol. Psychiatry. 10.1016/j.biopsych.2011.02.012 [DOI] [PubMed] [Google Scholar]


