ABSTRACT
Transcription factors (TFs) are often used repeatedly during development and homeostasis to control distinct processes in the same and/or different cellular contexts. Considering the limited number of TFs in the genome and the tremendous number of events that need to be regulated, re-use of TFs is necessary. We analyzed how the expression of the homeobox TF, orthodenticle homeobox 2 (Otx2), is regulated in a cell type- and stage-specific manner during development in the mouse retina. We identified seven Otx2 cis-regulatory modules (CRMs), among which the O5, O7 and O9 CRMs mark three distinct cellular contexts of Otx2 expression. We discovered that Otx2, Crx and Sox2, which are well-known TFs regulating retinal development, bind to and activate the O5, O7 or O9 CRMs, respectively. The chromatin status of these three CRMs was found to be distinct in vivo in different retinal cell types and at different stages. We conclude that retinal cells use a cohort of TFs with different expression patterns and multiple CRMs with different chromatin configurations to regulate the expression of Otx2 precisely.
KEY WORDS: Cis-regulatory module, Transcription factor, Otx2, Crx, Sox2
Summary: Retinal cells use transcription factors with different expression patterns and cis-regulatory modules with different chromatin configurations to regulate the cell type- and stage-specific expression of Otx2 precisely.
INTRODUCTION
In the mammalian genome, there are ∼1500 transcription factors (TFs) (Zhou et al., 2017; Vaquerizas et al., 2009), which bind to specific DNA sequences, within cis-regulatory modules (CRMs), to control gene expression and regulate almost every aspect of life. Many are used repeatedly and in a variety of combinations to regulate distinct developmental and homeostatic events. For instance, a handful of TFs, including Pax6, Ptf1a, Sox9, Hnf6 (Onecut1) and Neurod1, regulate pancreatic development in addition to retinal development (Bastidas-Ponce et al., 2017; Emerson et al., 2013; Poché et al., 2008; Ohsawa and Kageyama, 2008). Moreover, within the same organ, TFs can be expressed in mitotic cells, newly postmitotic cells and mature cells to regulate development and function. The re-use of a limited pool of TFs in different cellular contexts probably evolved to maximize their utility in driving the evolution of an amazingly diverse set of cell types and functions. A full definition of how specificity and function are created through the redeployment of TFs is an area of interest in many systems.
The vertebrate neural retina has served as a model system for studying the development of a complex mammalian tissue (Cepko et al., 1996). Access to the developing retina using in vivo electroporation has provided a strong platform for studies of gene regulation and cell fate determination (Matsuda and Cepko, 2007, 2004). The developmental role and regulation of Otx2, a homeobox TF that is important in multiple aspects of retinal development, has been the subject of several such studies. Otx2 is expressed in retinal progenitor cells (RPCs), the mitotic cells of the retina. RPCs that express Otx2 are a subset of those that are about to produce postmitotic daughter cells (Muranishi et al., 2011; Trimarchi et al., 2008). In addition, Otx2 is expressed in mature photoreceptor cells, the rods and cones, and mature bipolar interneurons (Koike et al., 2007; Fossat et al., 2007). Otx2 has been shown to be required for the genesis of photoreceptor cells, bipolar cells and a type of interneuron, horizontal cells, which do not express Otx2 (Koike et al., 2007; Nishida et al., 2003; Sato et al., 2007). The dosage of Otx2 is important for the determination of rods versus bipolar interneurons (Wang et al., 2014) and for the survival and activity of mature photoreceptors, bipolar cells and horizontal cells (Housset et al., 2013; Béby et al., 2010; Bernard et al., 2014). Overexpression of Otx2 in neonatal mouse retinas in vivo, when rods and bipolar cells are being generated, was found to increase the production of bipolar cells at the expense of rod photoreceptors. Knocking down Otx2 levels via short hairpin RNA (shRNA) led to the formation of more rods and fewer bipolar cells (Wang et al., 2014). These and other studies (Martinez-Morales et al., 2001) establish Otx2 as a key gene in the development of several ocular tissues and retinal cell types, posing interesting questions about how it is regulated to achieve this variety of outcomes.
Several studies of the regulation of Otx2 during retinal development have been carried out. One CRM, ‘EELPOT’, can recapitulate Otx2 expression in embryonic mouse retinas (Muranishi et al., 2011). It is positively regulated by Rax and negatively regulated by the Notch signaling pathway. Another CRM, designated ECR2, recapitulates Otx2 expression in a subset of neonatal RPCs and their newly postmitotic daughters (Emerson and Cepko, 2011). ECR2 has not been characterized regarding its TF binding sites (TFBSs) or cognate TFs. In addition, three DNaseI hypersensitive genomic fragments (DHS-2, DHS-4 and DHS-15) flanking the Otx2 gene have been shown to be active in the neonatal mouse retina (Wilken et al., 2015), although their upstream TFs and expression patterns were not characterized. Despite these previous studies, the CRMs that regulate Otx2 expression in mature photoreceptor and bipolar cells are currently unknown. In addition to regulation in these various cell types, the regulation of expression levels of Otx2 is of interest. As rod and cone photoreceptors differentiate, the Otx2 level decreases, whereas it increases in bipolar cells and disappears in differentiating horizontal cells (Trimarchi et al., 2008; Koike et al., 2007; Fossat et al., 2007; Baas et al., 2000). Dose is also important for the survival and function of mature retinal cells, because alleles that reduce the level and activity of Otx2 lead to retinal degeneration (Bernard et al., 2014).
To elucidate the regulation of Otx2 in different cellular contexts, we searched systematically for candidate CRMs for Otx2 using the DNaseI hypersensitivity of genomic regions within 350 kb of the Otx2 gene. Candidates were tested for CRM activity, and seven new Otx2 CRMs were identified. Three of these CRMs, designated O5, O7 and O9, were found to drive strong expression in the postnatal retina in vivo. O5 drives expression primarily in mature bipolar cells. O7 drives expression in mature rods, and O9 drives expression in developing neonatal cells. These three CRMs thus recapitulate Otx2 expression in distinct cell types and at different stages. We also explored the TFBSs and TFs that regulate these CRMs and discovered that Otx2, Crx and Sox2 can bind to and activate O5, O7 and O9, respectively. We also examined the endogenous chromatin status of O5, O7 and O9 CRMs in different retinal cell types and found that these three CRMs show different chromatin states in different cell types. We conclude that the expression of Otx2 in different retinal cellular contexts is not regulated by common regulators. The chromatin accessibility and presence of particular TFs together ensure the expression of Otx2 in a cell type- and stage-specific manner in the retina.
RESULTS
Identification of active CRMs for the Otx2 gene based on chromatin status
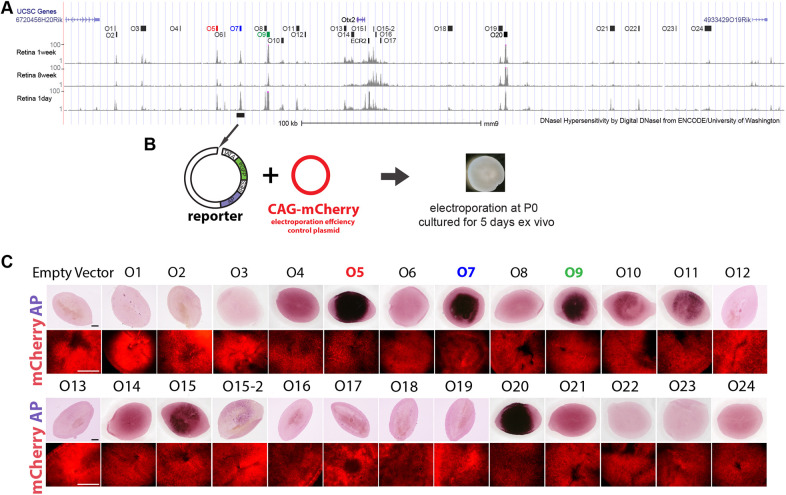
CRMs have been nominated using a variety of methods and features. Chromatin accessibility and histone modifications are two such features (Klemm et al., 2019). The UW ENCODE (University of Washington Encyclopedia of DNA elements) project created high-quality, genome-wide mapping of DNaseI hypersensitive sites (DNaseI HS) for major tissues and cell types in mice and humans, including the mouse neural retina (The ENCODE Project Consortium, 2011; Vierstra et al., 2014). We systematically evaluated 25 DNaseI HS fragments, spanning a region of ∼350 kb surrounding the Otx2 locus, for CRM activity in the retina. Each DNaseI HS fragment was amplified and cloned into the Stagia3 reporter vector, which expresses GFP and alkaline phosphatase (AP) when an active CRM is inserted upstream of a minimal TATA promoter (Billings et al., 2010). When tested in newly explanted retinas ex vivo, seven of the DNaseI HS regions (O5, O7, O9, O10, O11, O15 and O20) drove significant expression of the reporter gene (Fig. 1). Although the precise sequences of the three CRMs reported by Wilken et al. (2015) are not available, based on the approximate coordinates, the O5 and O9 CRMs might overlap with their DHS-2 and DHS-4.
Fig. 1.
Assay of DNaseI hypersensitive regions near the Otx2 gene for CRM activity in the retina. (A) Twenty-five DNaseI hypersensitive regions (The ENCODE Project Consortium, 2011; Vierstra et al., 2014), O1-O24, flanking the Otx2 gene are highlighted. (B) Individual DNaseI hypersensitive regions were cloned into the Stagia3 reporter plasmid and electroporated into P0 mouse retinas. The retinas were cultured as organ cultures for 5 days ex vivo. The CAG-mCherry plasmid served as the electroporation efficiency control. (C) Seven out of the 25 DNaseI hypersensitive regions drove AP expression. The O5, O7 and O9 CRMs (highlighted) became the focus of this study. Scale bars: 0.5 mm.
We tested the activity of these seven CRMs in vivo by electroporating individual reporter plasmids into mouse retinas at postnatal day (P) 0 (Fig. 2). Electroporation tends to result in expression in RPCs and their postmitotic progeny, which in the neonatal period become primarily rods and bipolar cells, with a small number of amacrine interneurons and Müller glial cells (Matsuda and Cepko, 2007; Young, 1985). Plasmids can be retained in these mature cell types, allowing for a read out of activity in multiple cell types and their RPCs. Retinas were thus harvested at P3 or P14 to examine the activities of these CRMs in developing and mature retinal cells, respectively. The O10, O11, O15 and O20 CRMs were found to drive GFP expression in vivo but were relatively weak (Fig. S1) and were not investigated further.
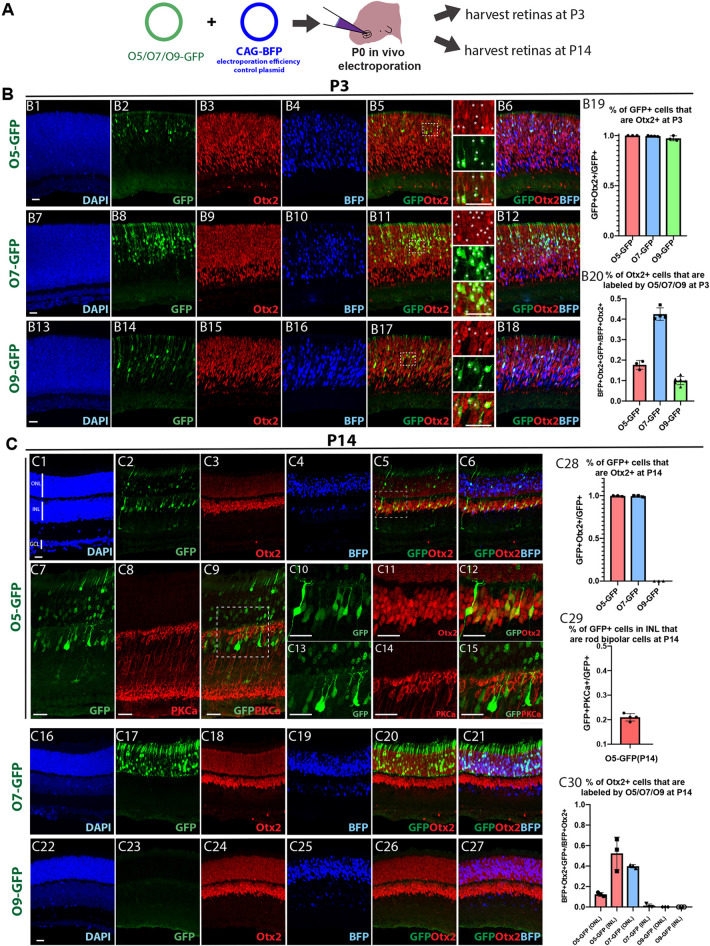
Fig. 2.
O5, O7 and O9 CRMs drove cell type- and stage-specific expression in the retina in vivo at postnatal stages. (A) The O5, O7 or O9 reporter plasmids (O5-GFP, O7-GFP or O9-GFP) were electroporated into P0 mouse retinas in vivo, together with the CAG-BFP plasmid, which served as the electroporation efficiency control. The retinas were harvested at P3 or P14. (B) The activity of the O5 (B1-B6), O7 (B7-B12) and O9 (B13-B18) CRMs in the retina in vivo at P3. The retinal sections were stained with anti-Otx2 antibody and DAPI. The high-magnification view of the highlighted region in B5, B11 and B17 is shown on the right of the image. White stars indicate GFP+ cells. The percentage of GFP+ cells that were Otx2+ and the percentage of Otx2+ cells that were labeled by individual CRMs are quantified in B19 and B20, respectively (mean±s.d.). (C) The activity of the O5 (C1-C15), O7 (C16-C21) and O9 (C22-C27) CRMs in the retina in vivo at P14. The retinal sections were stained with anti-Otx2 (C1-C6, C10-C12 and C16-C27) or anti-PKCa antibody (C7-C9 and C13-C15) and DAPI. C10-C12: high-magnification views of the highlighted region in C5. C13-C15: high-magnification views of the highlighted region in C9. (C28) The percentage of GFP+ cells that were Otx2+ at P14 was quantified for individual CRMs (mean±s.d.). (C29) The percentage of O5-GFP+ cells that were rod bipolar neurons was quantified (mean±s.d.). (C30) The percentage of Otx2+ cells that were labeled by individual CRMs at P14 was quantified (mean±s.d.). Scale bars: 20 μm.
At P3, the O5, O7 and O9 CRMs were found to direct strong GFP expression, mimicking the endogenous expression of Otx2 (Fig. 2B). More than 97% of the GFP+ cells were positive for Otx2 protein expression, as detected using immunohistochemistry (IHC) (Fig. 2B19). O5, O7 and O9 CRMs were active in ∼16, 42 and 10% of the electroporated cells that were Otx2+ (BFP+Otx2+ cells) at P3, respectively (Fig. 2B20). Co-electroporation of multiple CRMs driving different fluorescent proteins revealed that there was a significant number of cells simultaneously expressing the reporters driven by two or all three of these CRMs (Fig. S2). We estimated that O5, O7 and/or O9 CRMs were collectively expressed in ∼50% of the Otx2+ cells at P3 (Fig. S2J).
Given that Otx2 has been shown to be expressed in the G2-M phase of the last cell cycle in a subset of RPCs (Trimarchi et al., 2008), the expression driven by the CRMs was examined in mitotic cells. First, to confirm that a subset of RPCs expressed Otx2, we examined the expression of Otx2 protein in cells labeled by EdU, an S-phase marker (Buck et al., 2008), or Ki67, a pan-cell cycle marker (Scholzen and Gerdes, 2000). A small subset of Otx2+ cells were found to be EdU+ after a 30 min pulse, with 0.1, 0.9 or 5% of O5, O7 or O9 active cells showing EdU staining, respectively (Fig. S3). Approximately 6, 7 or 29% of O5, O7 or O9 active cells, respectively, were Ki67+ (Fig. S3). Thus, the O5 and O7 CRMs were less active in EdU+ cells, whereas the O9 CRM preferentially marked the Otx2+ cells that were cycling at P3 (Fig. S3). These data indicate that the O5, O7 or O9 CRMs mark overlapping, but distinct, populations of cells that express Otx2 in the neonatal mouse retina, with O9 being the most active in mitotic cells.
At P14, when the retina is almost fully developed, Otx2 is expressed at higher levels in bipolar cells and relatively lower levels in photoreceptor cells (Fig. 2C3). The O5 CRM was observed to mimic this pattern (Fig. 2C1-C6). In the inner nuclear layer, the majority of O5-GFP+ cells were found to be cone bipolar cells, as recognized by the characteristic axonal projection pattern and PMCA1 immunostaining (Behrens et al., 2016; Star et al., 2012) (Fig. 2; Fig. S4). In addition, ∼20% of O5-GFP+ cells in the inner nuclear layer were positive for protein kinase C alpha (PKCa; also known as Prkca) (Fig. 2C7-C15,C29), a well-established marker of rod bipolar cells (Negishi et al., 1988; Shekhar et al., 2016). Given that the estimated cone bipolar to rod bipolar ratio is ∼2.6:1 in mouse retina (Strettoi et al., 2010), the O5 CRM is biased towards labeling cone bipolar cells. By contrast, the activity of the O7 CRM was restricted to rod photoreceptors at P14 (Fig. 2C16-C21), and the O9 CRM was negative at P14 (Fig. 2C22-C30).
Taken together, the O5, O7 and O9 CRMs direct expression recapitulating the endogenous Otx2 expression in different retinal cell populations and at different developmental stages. The O9 CRM preferentially labels Otx2+ RPCs and their newly postmitotic daughters, and its activity is silenced in differentiated cell types. In mature retinas (P14 or later), the O5 CRM drives expression predominantly in bipolar cells and in a relatively small subset of rods, and the O7 CRM directs expression only in rod photoreceptors.
The O5 CRM is required for expression of Otx2 in bipolar cells
In order to determine whether the Otx2 CRMs identified above are necessary for endogenous Otx2 expression, we deleted them from the genome in vivo. We electroporated a Cas9 plasmid and appropriate single guide RNAs (sgRNAs) into the mouse retina in vivo for this purpose. Plasmids encoding sgRNAs targeting the 5′ or 3′ region of the O5 CRM, in addition to O5-Cas9 (O5 CRM drove Cas9 expression) and CAG-mCherry plasmids, were co-electroporated into mouse retinas at P0 in vivo (Fig. 3A,B). Previous studies have shown that there is a high efficiency of co-electroporating multiple plasmids into the same retinal cells in vivo (Matsuda and Cepko, 2004, 2007). We first examined the efficiency of CRISPR-mediated O5 deletion by amplifying genomic DNA from fluorescence-activated cell sorting (FACS)-sorted individual retinal cells that had received the plasmid mix. There were ∼30% cells with either heterozygous or homozygous deletion of the O5 CRM at P14 (Fig. S5). To quantify the effects of the O5 CRM deletion on Otx2 levels, endogenous Otx2 transcripts were detected and quantified by single molecule fluorescent RNA in situ hybridization (smFISH) (Raj et al., 2008; Wang et al., 2012). The number of smFISH puncta had been found to be well correlated with mRNA levels. We dissociated the retinas into single-cell preparations to increase the accuracy of quantification. The electroporated cells that received the plasmid mix were labeled by mCherry (Fig. 3C-F). Bipolar cells were labeled by IHC for Chx10 (Vsx2). The number of Otx2 smFISH puncta in mCherry+Chx10+ bipolar cells with or without the O5 CRM deletion was quantified (Fig. 3G). The number of bipolar cells with low Otx2 mRNA levels was significantly increased when the O5 CRM was deleted, demonstrating that the O5 CRM is necessary for the wild-type level of Otx2 mRNA in bipolar cells.
Fig. 3.
Deletion of the O5 CRM reduced Otx2 transcript levels in bipolar cells. (A,B) The O5-sgRNA5′-1 or -2 plasmid targets the 5′ end of the O5 CRM in the mouse genome, and the O5-sgRNA3′-1 or -2 plasmid targets the 3′ of the O5 CRM. The O5-Cas9 plasmid expressed Cas9 under the control of the O5 CRM. The CAG-mCherry plasmid served as the electroporation efficiency control. These plasmids were co-electroporated into P0 retinas in vivo. At P14, retinas were harvested and dissociated into single cells. The transcript levels of Otx2 in mCherry+Chx10(Vsx2)+ bipolar cells were detected and quantified by smFISH. (C,D) The level of the Otx2 transcript in control retinal cells. mCherry marked the electroporated cells. Chx10 IHC labeled bipolar cells. The Otx2 transcript was detected by smFISH (green signal). Each green dot in C4 represents one mRNA molecule. D1-D4 are high-magnification views of the highlighted region in C1. White arrows indicate mCherry+Chx10+ bipolar cells. (E,F) The level of Otx2 transcripts in retinal cells that had the CRISPR constructs to delete the O5 CRM. F1-F4 are high-magnification views of the highlighted region in E1. (G) Quantification of the Otx2 transcript levels. The y-axis represents the number of Otx2 transcripts (green dots) in individual bipolar cells based on smFISH (C4, D3, E4 and F3). Filled circles in the plot represent cells, with each filled circle representing one cell. The percentages of cells that expressed different levels of Otx2 are indicated. sgRNA-pair1: O5-sgRNA5′-1 and O5-sgRNA3′-1; sgRNA-pair2: O5-sgRNA5′-2 and O5-sgRNA3′-2. Student's two-tailed t-test. ****P<0.0001. Scale bars: 10 μm.
Assay for the endogenous role of the O9 CRM was not successful. Owing to the extended time needed to accumulate significant levels of Cas9 protein and the fact that the O9 CRM is only active transiently in Otx2+ progenitor/precursor cells, we were not able to delete the O9 CRM quickly enough to assay its endogenous activity. Deletion of the O7 CRM by CRISPR did not result in significant downregulation of Otx2 mRNA levels (data not shown). Its function might be compensated by other Otx2 CRMs, such as O5, which has activity in rods (Fig. 2C2).
Identification of TFBSs and TFs for O5, O7 or O9 activity
To understand how O5, O7 or O9 CRMs are regulated, we searched for TFBSs and TFs that are required for activation of these CRMs. We first determined the minimal sequences within each CRM that are required for CRM activity. The O5, O7 or O9 CRMs were truncated into smaller fragments based on the positions of bioinformatically predicted TFBSs (Shannon and Richards, 2020; Grant et al., 2011). Each fragment was individually cloned into the Stagia3 reporter and tested in mouse retinas ex vivo (Fig. 4A,C,E). The O5-8, O7-1 and O9-12 elements were found to drive strong expression of the AP reporter gene. We then mutated individual predicted TFBSs on O5-8, O7-1 or O9-12 to investigate the identity of their cognate TFs (Fig. 4; Fig. S6). Mutation of the Otx2 binding site (Otx2 BS) completely abolished the activity of the O5-8 CRM (Fig. 4B). Mutation of the Crx binding site (Crx BS) reduced the activity of the O7-1 element (Fig. 4D). Mutation of the Sox2 binding site (Sox2 BS) reduced the activity of the O9-12 element (Fig. 4F).
Fig. 4.
Otx2, Crx or Sox2 activated O5, O7 or O9 CRM, respectively. (A,C,E) The minimal elements within O5 (A), O7 (C) or O9 (E), which were sufficient to drive reporter activity (AP signals), were discovered by creating deletion constructs of individual CRMs. Each CRM was truncated into smaller elements based on the availability of predicted TFBSs. Each truncated element was incorporated into the Stagia3 reporter plasmid and tested for its ability to drive AP expression in retinas ex vivo. CAG-mCherry plasmid was used as the electroporation efficiency control. Strong AP signals often absorbed the fluorescent mCherry signals (e.g. O5-2). Scale bars: 0.5 mm. (B,D,F) The Otx2, Crx or Sox2 binding sites were required for the activity of O5-8 (B), O7-1 (D) or O9-12 (F) elements, respectively. The mutation or deletion of these binding sites abolished reporter activity. Scale bars: 0.5 mm. (G-J) Otx2, Crx or Sox2 was sufficient for reporter activity in HEK293T cells. The CAG-mCherry plasmid was the transfection efficiency control. In the O5-8-mOtx2-GFP plasmid, the Otx2 binding site was mutated. In the O7-1-mCrx-GFP plasmid, the Crx binding site was mutated. In the O9-12-mSox2-GFP plasmid, the Sox2 binding site was mutated. (J) Ratio of the percentage of GFP+ cells to mCherry+ cells. Student's two-tailed t-test. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. Scale bars: 50 μm.
The ability of Otx2, Crx or Sox2 to activate O5-8, O7-1 or O9-12 was investigated further in HEK293T cells in vitro, which do not normally activate the Otx2 CRMs. Otx2 was found to be sufficient to activate the wild-type O5-8, but not the element with the Otx2 binding site mutation (Fig. 4G). Crx and Sox2 behaved in a similar manner on their respective elements (Fig. 4H-J).
To examine whether Otx2, Crx or Sox2 binds directly to O5, O7 or O9 CRMs, we performed electrophoretic mobility shift assays (EMSAs) (Fig. S7A-C). The biotin-labeled CRMs were used as DNA probes. Strong electrophoretic mobility bands were detected when the CRM probes were mixed with TF-enriched nuclear extracts. When unlabeled cold probe or antibody was included, the primary EMSA band disappeared, suggesting that the binding was specific. In addition, the binding of Otx2, Crx or Sox2 to O5, O7 or O9 CRM in vivo was confirmed by analysis of the published Otx2 and Crx ChIP-seq datasets (Samuel et al., 2014; Corbo et al., 2010) from mouse retina or ChIP-qPCR experiments (Fig. S7D,E). These results demonstrate that Otx2, Crx or Sox2 can regulate O5, O7 or O9 CRMs via direct binding and are likely to contribute to the cell type- and stage-specific activation of these CRMs.
To investigate whether the expression of Otx2, Crx and Sox2 match the predicted patterns for their roles in regulating the Otx2 CRMs, we checked their expression by IHC. At neonatal stages, O5-GFP+, O7-GFP+ or O9-GFP+ cells were positive for Otx2, Crx or Sox2, respectively (Fig. 2B; Fig. S8). However, not all Otx2+, Crx+ or Sox2+ electroporated cells turned on O5, O7 or O9 CRM by P3. This could be attributable to the levels of the TFs, which might not be high enough in certain retinal cells. To test this possibility, we overexpressed Otx2, Crx or Sox2 in P0 retinas electroporated by the CRM reporter plasmids. This led to increased numbers of cells that turned on O5, O7 or O9 CRM plasmids within 24 h, respectively (Fig. S9). These data suggest that Otx2, Crx or Sox2 can activate O5, O7 or O9 CRM reporters at neonatal stages and that the levels of these TFs might be limiting for plasmid CRM activity.
In mature retinas, Otx2 is expressed at higher levels in bipolar cells than in rods, which matches the pattern of O5-GFP expression (Fig. 2C). Notably, compared with cone bipolar cells, rod bipolar cells express relatively lower levels of Otx2 (Fig. S10A-C). Consistent with this, the majority of O5-GFP+ bipolar cells had the morphology of cone bipolar cells (Fig. 2C2), suggesting that the level of Otx2 is important for the expression pattern of O5 CRM activity in bipolar cells. Interestingly, Crx is expressed at a higher level in rods than in bipolar cells (Glubrecht et al., 2009; Shekhar et al., 2016) (Fig. S10B), and the O7-GFP, which requires a Crx binding site, was found to be active only in rods. It is possible that the level of Crx is not high enough to activate O7-GFP in bipolar cells. However, overexpression of Crx specifically in bipolar cells, via the Chx164 bipolar CRM (Kim et al., 2008), did not lead to activation of O7-GFP in bipolar cells (data not shown). Likewise, Sox2 is expressed in Müller glial cells in the adult retina (Surzenko et al., 2013), but the O9-GFP CRM, which requires the Sox2 binding site, was not active in these cells at P14. We compared the levels of Sox2 in P3 retinal cells with those in P14 Müller glial cells by quantitative smFISH (Fig. S10F,G). The mRNA level of Sox2 in mature Müller glial cells was found to be only slightly lower than that in RPCs at P3, suggesting that the level of Sox2 is not responsible for the loss of O9 activity in mature Müller glial cells. It is possible that unknown transcriptional repressors of O7 or O9 CRMs exist in mature bipolar or Müller glial cells, a hypothesis that needs to be investigated.
Taken together, O5, O7 or O9 CRMs are active in cells expressing Otx2, Crx or Sox2, but not all Otx2+, Crx+ or Sox2+ cells turn on the corresponding CRMs. The levels of TFs appear to be important for CRM activity at neonatal stages. In the differentiated retina, the activity of repressors, particularly in mature bipolar and Müller glia with respect to the O7 and O9 CRMs, might contribute to the regulation of the CRM activity.
Otx2, Crx and Sox2 are required for the activation of O5, O7 and O9 CRM in the retina
To investigate whether Otx2, Crx and Sox2 are required for O5, O7 and O9 activities in different retinal cell types at different stages in vivo, we used shRNA constructs to knock down these TFs. To test the necessity of Otx2 in bipolar cells for O5 activation, Otx2 was knocked down using microRNA-based shRNA cassettes (Otx2-shRNA1 or Otx2-shRNA2), which were inserted into the 3′ UTR of the mCherry gene (Wang et al., 2014). To target differentiated bipolar cells specifically, we used the bipolar specific CRM, 164 bp Chx10 CRM (Chx164) (Kim et al., 2008). Although the Chx164 CRM is biased towards labeling rod bipolar cells, i.e. less active in cone bipolar cells, it is the most effective driver for specific targeting of differentiated bipolar cells, and not RPCs, where Chx10 is also expressed. The Chx164-mCherry-Otx2-shRNA1/2 plasmid or control Chx164-mCherry-LacZ-shRNA plasmid was co-electroporated with the O5-GFP plasmid into P0 retinas in vivo. The retinas were harvested at P14 and the activity of the O5 CRM was assayed in cells marked by mCherry expression. The percentage of mCherry+GFP+ cells among mCherry+ cells was significantly reduced in the presence of Otx2-shRNA compared with the level in LacZ-shRNA controls (Fig. 5A), suggesting that Otx2 is necessary for the activation of the O5 CRM in mature bipolar cells. Likewise, knocking down Crx in differentiated rods using the rod-specific rhodopsin promoter (Matsuda and Cepko, 2007) significantly decreased the activity of the O7 CRM in vivo (Fig. 5B). Knocking down Sox2 in P0 retinas ex vivo greatly suppressed the activity of the O9 CRM (Fig. 5C). These data demonstrate that Otx2, Crx and Sox2 are required for the activation of the O5, O7 and O9 CRMs, respectively.
Fig. 5.
Otx2, Crx or Sox2 was required for the activation of O5, O7 or O9 CRM in the retina in vivo. (A) Knocking down Otx2 using shRNA diminished O5 activity in bipolar cells. Control LacZ-shRNA (A1), Otx2-shRNA1 (A2) or Otx2-shRNA2 was placed in the 3′ UTR of mCherry and driven by the Chx164 CRM (Kim et al., 2008), which directs expression in mature bipolar cells. The shRNA-containing plasmid and O5-GFP plasmid were co-electroporated into retinas in vivo at P0. Retinas were harvested and examined at P14. White arrows indicate mCherry+GFP+ cells. (A3) Quantification of the percentage of mCherry+ cells that turned on the O5 CRM. Student's two-tailed t-test. *P<0.05; **P<0.01. Scale bars: 20 μm. (B) Knocking down Crx in rods diminished O7 CRM activity. Control LacZ-shRNA (B1), Crx-shRNA1 (B2) or Crx-shRNA2 (data not shown) was placed in the 3′ UTR of mCherry and driven by the Rho promoter (Matsuda and Cepko, 2007, 2004), which directs expression in mature rods. The shRNA-containing plasmid and O7-GFP plasmid were co-electroporated into retinas in vivo at P0. Retinas were harvested and examined at P14. Scale bars: 20 μm. (C). Knocking down Sox2 diminished O9 activity in retinal cells at P1. Control LacZ-shRNA (C2,C3), Sox2-shRNA1 (C4) or Sox2-shRNA2 was placed in the 3′ UTR of mCherry and driven by the ubiquitous CAG promoter. The shRNA-containing plasmids were electroporated into P0 retinas with the O9-GFP plasmid. (C1) The retinas were cultured ex vivo for 24 h. (C5) Quantification of the percentage of mCherry+ cells that turned on the O9 CRM. Student's two-tailed t-test. **P<0.01; ***P<0.001. Scale bars: 20 μm.
The chromatin status of Otx2 CRMs in different retinal cell types
To gain a better understanding of how multiple Otx2 CRMs collectively control Otx2 transcription in the retina, we examined the chromatin status of endogenous O5, O7 or O9 CRMs in the genome in different retinal cell types and at different stages.
Heterochromatic regions of the genome, which are marked by histone3 lysine9 trimethylation (H3K9me3) or histone3 lysine 27 trimethylation (H3K27me3) (Becker et al., 2017), are typically resistant to DNaseI, whereas active or poised CRMs are hypersensitive to DNaseI. DNase-seq and ATAC-seq allow for genome-wide profiling of the ‘open’ regions in the genome (Klemm et al., 2019). We analyzed the published DNase-seq and ATAC-seq data (Vierstra et al., 2014; Hughes et al., 2017; Jorstad et al., 2017; Zibetti et al., 2019; Murphy et al., 2019) to investigate whether the Otx2 CRMs were open or closed in retinal bipolar cells, rods, Müller glial cells, P2 RPCs and P1 retinal cells (Fig. 6). Genomic sequences corresponding to O5, O7 and O9 CRMs were found to be open in P2 RPCs and P1 retinal cells, which are composed of retinal progenitors/precursors and differentiated cells. In mature bipolar cells, the O7 CRM was less accessible, compared with the O5 and O9 CRMs. In rods, all three CRMs were found to be open. In Müller glial cells, the O5 and O7 CRMs were closed, and the O9 CRM was less accessible than in other cell types. Therefore, the endogenous O5, O7 and O9 CRMs have different chromatin accessibilities in different retinal cell types and at different stages during development. Notably, although it is unknown whether the chromatin status of O5, O7 and O9 CRMs is regulated in a similar fashion in human retina, these three CRMs were open in human retinal cells, as revealed by human ATAC-seq datasets (Wang et al., 2018; Xie et al., 2020) (Fig. S12).
Fig. 6.
The chromatin status of O5, O7 and O9 CRMs in different retinal cell types and at different stages. The published ATAC-seq (P2 RPC, bipolar, rod and Müller glial cells) (Hughes et al., 2017; Jorstad et al., 2017; Zibetti et al., 2019; Murphy et al., 2019) and DNase-seq (P0 retinal cells) (Vierstra et al., 2014) results were re-analyzed to reveal the chromatin status of O5, O7 and O9 CRMs. O5-8, O7-1 and O9-12: minimal sequences that are required for CRM activity.
DISCUSSION
We analyzed the regulation of Otx2, a gene required for the formation of several retinal cell types at different times in development. We wished to understand not only how its temporal and cell type-specific transcription are orchestrated, but also how its level is controlled. Its level can contribute to the choice of a rod versus a bipolar fate (Wang et al., 2014) and can influence the survival and function of particular mature retinal cells (Bernard et al., 2014). We first used plasmid electroporation of genomic regions defined by their chromatin accessibility to identify several CRMs, O5, O7 and O9. These elements showed differential activity regarding cell types and the timing of their expression. The binding sites that are required for CRM activity were defined and were used successfully to direct a search for the cognate TFs. The identified TFs were found to be necessary, but not sufficient, for the expression patterns of these CRMs. This led us to investigate the accessibility of the CRMs in the chromatin of particular cell types in vivo. The O5, O7 and O9 CRMs were found to display differential chromatin accessibility in different cell types. Based on these data, we propose a model to explain how the stage- and cell type-specific expression of Otx2 is achieved in the retina (Fig. 7A).
Fig. 7.
TFs and the chromatin status of CRMs collectively control the cell type- and stage-specific expression of Otx2 in the retina. (A) The model of the cell type- and stage-specific regulation of Otx2 expression in the retina. In retinal progenitor/precursor cells, O5, O7 and O9 CRMs are all active. The O9 CRM, which can be activated by Sox2, preferentially drives Otx2 expression in retinal progenitor cells and their newly postmitotic progeny. Differentiated rods express high levels of Crx and low levels of Otx2. The O7 CRM, which can be activated by Crx, is the main CRM that is used to activate Otx2 transcription in rods. Differentiated bipolar cells express relatively higher levels of Otx2 and lower levels of Crx. Apart from the reduced expression of Crx, the O7 CRM is less accessible in bipolar cells. The O5 CRM is thus the main CRM that drives Otx2 transcription in bipolar cells. Although Müller glia express Sox2, which can activate the O9 CRM, Sox2 is not able to direct Otx2 transcription in these cells, perhaps owing to the presence of a repressor. Taken together, different TFs, their levels and the chromatin status of individual CRMs regulate the cell type- and stage-specific expression of the Otx2 gene. (B) The CRMs regulating Otx2 expression in different tissues. The O5, O7, O9, O10, O11, O15 and O20 CRMs collectively regulated Otx2 expression in embryonic and postnatal retina. The EELPOT (Muranishi et al., 2011), O6 and O8 CRMs (Perez-Cervantes et al., 2020) were active in embryonic retina, but silenced in postnatal retina. The AN1 and AN2 CRMs were active in anterior neuroectoderm (Kurokawa et al., 2014). The FM1, FM2 and FM3 CRMs drove expression in forebrain/midbrain (Kurokawa et al., 2014, 2004a). The CM or VE CRM directed expression in cephalic mesenchyme (CM) or visceral endoderm (VE), respectively (Kimura et al., 2000, 1997). (C) High-magnification view of the promoter region of the Otx2 gene.
At the neonatal stage (P0-P3), the chromatin regions of O5, O7 and O9 CRMs are open. Their regulators, Otx2, Crx and Sox2, are present and collectively regulate the expression of Otx2 via these three CRMs in overlapping retinal cell populations. The levels of these TFs are important for CRM activity at this stage. In differentiated retinas (P14 or older), each CRM and its TF regulate Otx2 expression in a distinct manner. In bipolar cells, the O5 CRM is more accessible than O7 CRM and the O9-12 region within the O9 CRM, where Sox2 binds (Fig. 6). Given that Sox2 is not expressed in bipolar cells, the O9 CRM is not active in these cells. The expression of Otx2 in bipolar cells is self-regulated in a feed-forward fashion via the O5 CRM. In rod photoreceptors, all three CRMs are open. The O9 CRM is not active in rods, probably owing to the absence of Sox2. The O5 CRM is weakly active in rods, probably owing to the low expression of Otx2 in rods. Crx primarily regulates the transcription of Otx2 via the O7 CRM in rod photoreceptors. In addition, Otx2 is not expressed by mature Müller glial cells. Although Sox2 is present, the O9-12 region within the O9 CRM is not accessible in Müller glial cells. The O9 CRM is not active in Müller glial cells. Overall, retinal cells use the availability of TFs, the levels of TFs and the chromatin accessibility of multiple CRMs to regulate Otx2 expression precisely in a stage- and cell type-specific manner in the postnatal retina in vivo.
Otx2 also is expressed in the retina at embryonic stages (Muranishi et al., 2011). We examined the activities of the candidate Otx2 CRMs (Fig. 1) in embryonic day (E) 14.5 retinas ex vivo. Interestingly, all the CRMs that are active in the postnatal retina, including O5, O7 and O9, were found to be active at E14.5. We recently used noncoding RNA (ncRNA) profiling to identify photoreceptor CRMs and found that the O5, O7 and O9 CRMs overlapped with regions that showed transcription of ncRNA in the embryonic retina (Perez-Cervantes et al., 2020). Furthermore, regions of the chick genome with sequence homology to the mouse O5 and O7 CRMs showed CRM activity in the developing embryonic chick retina. Interestingly, as in the mouse, Otx2 regulated the chick O5 CRM (N.L., unpublished observations). However, despite the activity of some CRMs at both embryonic and postnatal stages, a few Otx2 CRMs active at embryonic stages were no longer active in the postnatal retina. The O6, O8 and EELPOT CRMs (Fig. 7B) (Muranishi et al., 2011) are active in the embryonic retina, but are silent postnatally. Different mechanisms might control the developmental silencing of these CRMs. The O6 and O8 CRMs are DNaseI hypersensitive in the neonatal retina; therefore, it is likely that it is the absence of their TFs, which are currently unknown, that leads to their lack of activity. The EELPOT CRM is not DNaseI hypersensitive in the postnatal retina (Fig. 7B); therefore, it might not be accessible by its TFs, such as Rax, which is present at postnatal stages. These data suggest that additional CRMs contribute to the regulation of Otx2 expression at embryonic stages and that the silencing of embryonic CRMs at late stages is caused by chromatin remodeling and/or dynamic expression of TFs.
Apart from the retina, Otx2 is essential for early embryonic development and brain development (Beby and Lamonerie, 2013; Acampora, 1999). Several CRMs have been identified to control Otx2 expression in visceral endoderm (VE), anterior neuroectoderm (AN), cephalic mesenchyme (CM) and developing forebrain/midbrain (FM) (Kurokawa et al., 2004a,b; Kimura et al., 2000, 1997). The published VE and CM CRMs are located in the promoter region of the Otx2 gene and partly overlap with the O15 CRM, which drives weak expression in the neonatal retina (Fig. 1). Interestingly, none of the other distal Otx2 CRMs is shared among tissues (Fig. 7B). We examined the chromatin accessibility of the nonretinal Otx2 CRMs in the retina to investigate whether this is attributable to differential chromatin states. The published anterior neuroectoderm CRMs (AN1 and AN2) (Kurokawa et al., 2004b, 2014) are not significantly open in the postnatal retina, whereas two out of three mouse forebrain/midbrain CRMs (FM1 and FM3, corresponding to O19 and O22) (Kurokawa et al., 2004a, 2014) are open but inactive in the retina. This further suggests that the activities of the tissue-specific Otx2 CRMs are regulated not only by differential chromatin states, but also by the availability of their TFs. Taken together, the expression of Otx2 is regulated by many distinct CRMs and TFs in different developmental and cellular contexts (Table S2).
Although the present study improves our understanding of the complex regulation of Otx2, it provokes additional questions and suggests future directions. First, how is the dynamic, differential accessibility of Otx2 CRMs established in different tissues and cell types? TFs and chromatin remodelers are key regulators of the nucleosome occupancy and positioning, which are primary determinants of chromatin accessibility (Klemm et al., 2019). Given that chromatin remodelers lack sequence specificity, sequence-specific TFs are proposed to play central roles in establishing the differential accessibility of Otx2 CRMs. It is not known whether the TFs that initiate remodeling of chromatin accessibility are the same TFs that activate CRMs. Otx2 and Sox2, which can activate the O5 and O9 CRMs in the retina, can function as pioneer factors and regulate chromatin remodeling in other systems (Iwafuchi-Doi and Zaret, 2014; Boulay et al., 2017). Crx can regulate the chromatin remodeling of genes associated with photoreceptor differentiation (Ruzycki et al., 2018). It is highly possible that these tissue- or cell type-specific TFs help to shape the chromatin accessibility, together with other components of the chromatin remodeling machinery. Additional studies are needed to determine whether and how Otx2, Crx and Sox2 regulate the differential remodeling of chromatin accessibility in the retina. Second, how are the differential expression levels of Otx2 established in different cellular contexts? Our data suggest that the levels of TFs could help to establish and maintain the differential expression levels of Otx2 in rod and bipolar cells. The O5 CRM is strongly active in bipolar cells, which express high levels of the O5 regulator, Otx2, creating a feed-forward loop, which is likely to maintain high Otx2 levels in bipolar cells. By contrast, the level of Otx2 in rod photoreceptors is low, probably not high enough to stimulate the self-reinforcement mechanism. Instead, Crx, which is highly expressed in rods, regulates Otx2 expression via the O7 CRM. Additional factors, which titrate down Otx2 levels in rod photoreceptors to prevent the feed-forward loop operating in bipolar cells, need to be identified to complete our understanding. Additional mechanisms, involving the Notch signaling pathway and TFs, including Blimp1 (Prdm1), Vsx2 and Sox2, contribute to the regulation of Otx2 expression in RPCs and newly postmitotic cells (Muranishi et al., 2011; Wang et al., 2014; Kim et al., 2008). All these factors will need to be integrated for the dynamic and specific regulation of Otx2 in different cellular contexts. Third, what are the advantages of using multiple TFs and CRMs to regulate Otx2 expression in different cellular contexts? This strategy can significantly increase the robustness of the system. When one CRM element is mutated or disrupted, other CRMs could compensate for its function. For instance, when the O7 CRM was deleted by CRISPR/Cas9, we did not observe significant downregulation of Otx2 transcription (data not shown). In addition, different cellular contexts probably maintain distinct regulatory environments. This strategy can ensure the activation of Otx2 in response to different signals, to maximize the utility of TFs in the genome.
In addition to the data regarding Otx2 regulation provided by these studies, the CRMs identified here provide reagents for labeling and manipulating distinct cell types in the retina via in vivo plasmid DNA electroporation. The O5 CRM is primarily active in cone bipolar cells, the O9 CRM in neonatal retinal progenitor cells and newly postmitotic cells, and the O7 CRM marks rod photoreceptors.
In summary, we studied how the expression of a key regulator of retinal development, Otx2, is regulated, in order to understand the specifics of its expression. In addition, we wished to gain some insight into the mechanisms that might account more generally for the dynamic and cell type-specific regulation of a gene. We showed that multiple CRMs and a cohort of TFs collectively control the stage- and tissue/cell type-specific expression of Otx2. The chromatin accessibility of CRMs and the presence of sequence-specific upstream TFs are both required for activation of Otx2 expression in different cellular contexts.
MATERIALS AND METHODS
Animals
Wild-type mouse neonates were obtained from timed pregnant CD1 mice (Charles River Laboratories, #022). All animal studies were approved by the Administrative Panel on Laboratory Animal Care (APLAC) at Stanford University.
Plasmid construction
The CAG-mCherry plasmid was from Matsuda and Cepko (2004). The Stagia3 vector was from Billings et al. (2010). The CAG-BFP plasmid was from Tang et al. (2015). All other plasmids were constructed by restrictive enzyme-based cloning or Gibson assembly methods (Gibson et al., 2009).
Otx2 CRM reporter plasmids (Fig. 1)
The 25 candidate Otx2 CRMs were amplified from the mouse genome by primers shown in Table S1 and cloned into the Stagia3 vector. The sequences of these CRMs are shown in Table S3.
CRISPR plasmids (Fig. 3; Fig. S5)
To generate the O5-Cas9 plasmid, the Cas9 fragment was amplified by PCR from Px330 plasmid (Addgene 42230) (Cong et al., 2013) and replaced the GFP fragment in the O5-GFP plasmid. To generate the O5-sgRNA5′ and O5-sgRNA3′ plasmids, complementary oligonucleotides (Table S1) were annealed and cloned into the modified Px330 plasmid, which does not express Cas9.
CRM deletion analysis (Fig. 4)
The truncated CRM O5-1 to O5-8 were amplified by PCR and cloned into the Stagia3 vector plasmid individually. Mutation variants of O5-8, O7-1 or O9-12 were generated by annealing complementary oligonucleotides of individual mutated fragments and ligating them into the EcoRI and XhoI digested Stagia3 plasmid. The sequences of the truncated and mutated CRMs are shown in Table S3.
Cell type-specific knockdown and overexpression of Otx2, Crx or Sox2 (Fig. 5)
The Chx164-mCherry-lacZ-shRNA or Chx164-mCherry-Otx2-shRNA plasmid was generated by replacing the CAG promoter of the CAG-mCherry-lacZ-shRNA (Addgene 73978; Wang et al., 2014) or CAG-mCherry-Otx2-shRNA1/2 (Wang et al., 2014) plasmid with the 164 bp Chx10 CRM and basic TATA box promoter (Kim et al., 2008). The Rho-mCherry-lacZ-shRNA or Rho-mCherry-Crx-shRNA plasmid was generated by replacing the CAG promoter of CAG-mCherry-lacZ-shRNA (Addgene 73978; Wang et al., 2014) or CAG-mCherry-Crx-shRNA (Wang et al., 2014) plasmid with the 2.2 kb bovine rhodopsin promoter (Matsuda and Cepko, 2007). The RNAi cassette against Sox2 gene was obtained via the BLOCK-iT PolII miR RNAi Express (Life Technologies) and inserted into the RNAi expression vector CAG-mCherry-miR155 (a gift from Dr Jianming Jiang, National University of Singapore). The sequences of the Sox2 shRNA cassettes are shown in Table S1. The efficiency of different Sox2-shRNA cassettes was tested by reporter assay using HEK293T cells (ATCC-CRL-3216, recently authenticated and tested for contamination; Fig. S11) and antibody staining (Fig. 5). The CAG-Sox2 plasmid was generated by cloning the ORF of mouse Sox2 gene into the CAG-GFP backbone (Addgene 11150; Matsuda and Cepko, 2004). The GFP fragment was replaced by the Sox2 ORF. The CAG-filler-DNA plasmid was generated by deleting the GFP fragment from the CAG-GFP plasmid.
In vivo and ex vivo plasmid electroporation into the retina
Ex vivo and in vivo retina electroporations were carried out as previously described (Matsuda and Cepko, 2004, 2007; Wang et al., 2014). For ex vivo electroporation, five pulses of 25 V, 50 ms each at intervals of 950 ms were applied to dissected retinas. For in vivo electroporation, five pulses of 80 V, 50 ms each at intervals of 950 ms were applied to neonatal mouse pups. All ex vivo and in vivo electroporation experiments were repeated with at least three biological replicates. Plasmids were electroporated with a concentration of 500 ng/μl to 1 μg/μl per plasmid.
Histology and immunohistochemistry
Dissected mouse eyeballs were fixed in 4% paraformaldehyde (PFA) in 1× PBS (pH 7.4) for 2 h at room temperature. Retinas were then dissected and equilibrated at room temperature in a series of sucrose solutions (5% sucrose in 1× PBS, 5 min; 15% sucrose in 1× PBS, 15 min; 30% sucrose in 1× PBS, 1 h; 1:1 mixed solution of OCT and 30% sucrose in PBS, 4°C, overnight), frozen and stored at −80°C. A Leica CM3050S cryostat (Leica Microsystems) was used to prepare 20 μm cryosections. Retinal cryosections were washed in 1× PBS briefly, incubated in 0.2% Triton, 1× PBS for 20 min, and blocked for 30 min in blocking solution, which consisted of 0.1% Triton, 1% bovine serum albumin and 10% donkey serum (Jackson ImmunoResearch Laboratories) in 1× PBS. Slides were incubated with primary antibodies diluted in blocking solution in a humidified chamber at room temperature for 2 h or at 4°C overnight. After washing in 0.1% Triton 1× PBS three times, slides were incubated with secondary antibodies and DAPI (Sigma-Aldrich; D9542) for 30 min to 2 h, washed three times with 0.1% Triton, 1× PBS and mounted in Fluoromount-G (Southern Biotechnology Associates).
The following primary antibodies were used: chicken anti-GFP (Abcam, AB13970, 1:1000), goat anti-RFP (Fisher Scientific, NC1578084, 1:500), rabbit anti-Otx2 (VWR, 10091-640, 1:1000), rabbit anti-Crx (Fisher Scientific, NBP215964, 1:500), mouse anti-Ki67 (BD Biosciences, 550609, 1:200), sheep anti-Chx10 (Exalpha Biologicals, X1179P, 1:500), rabbit anti-PMCA1 (Abcam, AB3528, 1:100) and mouse anti-Sox2 (Santa Cruz Biotechnology, sc-365823) antibodies. EdU detection was performed with a Click-iT EdU Alexa Fluor 647 imaging kit (C10340, Invitrogen). AP activity was detected by an AP detection kit (Sigma, SCR004).
Dissociation of retinal tissues and quantitative single molecule FISH
Retinal tissues were dissociated as described previously (Trimarchi et al., 2008). After dissociation, retinal cells were left on slides treated with poly-d-lysine (0.1 mg/ml; Millipore) for 45 min at 37°C. Retinal cells were then fixed on the slides in 4% PFA in 1× PBS (pH 7.4) for 15 min at room temperature. The cells were stained immediately or dehydrated by going through a 50, 70 and 100% ethanol gradient, and stored in 100% ethanol at −20°C. FISH probes were purchased from RNAscope. The commercial protocol provided by RNAscope was followed. The slides were imaged by Zeiss confocal LSM 880. Images were analyzed by Zen software (Zeiss) and quantified with Fiji software.
EMSA and ChIP-qPCR
EMSAs were performed as described previously (Wang et al., 2014). Roughly 1×106 293T cells were transfected with 1 μg of CAG-LacZ, CAG-Otx2, CAG-Crx or CAG-Sox2 plasmid using polyethylenimine (1 mg/ml, 4 μl per 1 μg DNA; 9002-98-6; Polysciences). Nuclear extracts were prepared from these cells or P3 mouse retinas using NE-PER nuclear and cytoplasmic extraction reagent kit (Pierce). Complementary oligonucleotides were ordered, annealed and labeled with the DNA 3′ End Biotinylation kit (Pierce). Chemiluminescent Nucleic Acid Detection Module (Pierce) was used to detect biotin-labeled probes after EMSA. ChIP-qPCRs were performed by using the EZ-ChIP kit (Millipore Sigma, 17-371) and Quantstudio 3 qPCR machine.
Imaging and analysis
All images of retinal sections were acquired with a Zeiss LSM880 inverted confocal microscope. Retinal explants were imaged with a Leica M165 FC microscope. HEK293T cells were imaged with a Zeiss Axio Observer 3 microscope. Images in Figs 2, 3 and 5 were maximum projections of 5 μm tissues and were quantified with Fiji software.
Prediction of TFBSs
To predict TFBSs located in CRMs, the Position Weight Matrix (PWM) annotated by MotifDB was used (Shannon and Richards, 2020). Based on these PWMs, FIMO (Grant et al., 2011) was used to search for potential TFBSs with the default cut-off exponent value of <10−4.
DNase-seq and ATAC-seq and data analysis
We downloaded the BigWig file for DNase-seq of mouse newborn retina tissue from the ENCODE portal (https://doi.org/10.1093/nar/gkx1081; https://www.encodeproject.org/) with the following identifier: ENCSR000CNV (Vierstra et al., 2014). ATAC-seq data for rod photoreceptors were from Hughes et al. (2017) and downloaded from GEO (Sample GSM2199333). Raw ATAC-seq data for bipolar cells were from Murphy et al. (2019) (GEO GSM3791362), and Müller glia were from Jorstad et al. (2017) and downloaded from NCBI Sequence Read Archive (accession SRX2881310). ATAC-seq data for P2 RPCs were from Zibetti et al. (2019) (SRX2895840). Briefly, reads were aligned to mouse mm10 genome using Bowtie2 (-X 2000) (Langmead and Salzberg, 2012) and processed with SAMtools to remove duplicates and MT reads (Li et al., 2009). We generated BigWig coverage tracks using deepTools bamCoverage (Ramírez et al., 2016) with the following parameters: --binSize 1 -p=max --normalizeUsing CPM --region “chr14” --ignoreForNormalization chrX. BigWig for all datasets were visualized in IGV Genome browser. Footprinting analyses for O5, O7 and O9 CRMs were performed on the corresponding bam files using HINT-ATAC (Li et al., 2019) with the command rgt-hint footprinting --organism=mm10 --atac-seq. The RGT suite from http://www.regulatory-genomics.org/motif-analysis was used to identify the motifs overlapping with predicted footprints with the command rgt-motifanalysis matching --organism=mm10.
Statistics
The sample size (n) was determined by using G*power software (two groups, the effect size of 0.5, α error at 0.05 and power set at 0.95). GraphPad Prism v.8 (GraphPad) was used to perform statistical analysis. Student's t-tests (two tailed) were used with the appropriate parameters. Statistical significance was defined as a P value <0.05.
Supplementary Material
Acknowledgements
The members of the Cepko and Wang laboratories provided valuable discussion and support for this project.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: C.S.Y.C., C.L.C., S.W.; Methodology: C.S.Y.C., N.L., A.E.D., Z.J., S.W.; Software: C.S.Y.C., N.L., Z.J.; Validation: C.S.Y.C., N.L., A.E.D., Z.J., S.W.; Formal analysis: C.S.Y.C., N.L., Z.J., S.W.; Investigation: C.S.Y.C., A.E.D., S.W.; Resources: R.Z., L.L., M.-R.W., C.L., Z.J., S.W.; Data curation: C.S.Y.C., N.L., A.E.D., L.L., M.-R.W., S.W.; Writing - original draft: C.S.Y.C., S.W.; Writing - review & editing: N.L., C.L.C., S.W.; Visualization: N.L., C.L.C., S.W.; Supervision: C.L.C., S.W.; Project administration: S.W.; Funding acquisition: S.W.
Funding
Support was provided by the American Diabetes Association (1-16-INI-16 to S.W.) and P30 to Stanford Ophthalmology (to S.W.), and by the Howard Hughes Medical Institute (to C.L.C). N.L. was supported by postdoctoral fellowships from the Schweizerischer Nationalfonds zur Förderung der wissenschaftlichen Forschung and the Human Frontiers Science Program, and by the National Institutes of Health (R01EY029771). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at https://dev.biologists.org/lookup/doi/10.1242/dev.187922.supplemental
Peer review history
The peer review history is available online at https://dev.biologists.org/lookup/doi/10.1242/dev.187922.reviewer-comments.pdf
References
- Acampora D. (1999). Otx genes in corticogenesis and brain development. Cereb. Cortex 9, 533-542. 10.1093/cercor/9.6.533 [DOI] [PubMed] [Google Scholar]
- Baas D., Bumsted K. M., Martinez J. A., Vaccarino F. M., Wikler K. C. and Barnstable C. J. (2000). The subcellular localization of OTX2 is cell-type specific and developmentally regulated in the mouse retina. Mol. Brain Res. 78, 26-37. 10.1016/S0169-328X(00)00060-7 [DOI] [PubMed] [Google Scholar]
- Bastidas-Ponce A., Scheibner K., Lickert H. and Bakhti M. (2017). Cellular and molecular mechanisms coordinating pancreas development. Development 144, 2873-2888. 10.1242/dev.140756 [DOI] [PubMed] [Google Scholar]
- Beby F. and Lamonerie T. (2013). The homeobox gene Otx2 in development and disease. Exp. Eye Res. 111, 9-16. 10.1016/j.exer.2013.03.007 [DOI] [PubMed] [Google Scholar]
- Béby F., Housset M., Fossat N., Le Greneur C., Flamant F., Godement P. and Lamonerie T. (2010). Otx2 gene deletion in adult mouse retina induces rapid RPE dystrophy and slow photoreceptor degeneration. PLoS ONE 5, e11673 10.1371/journal.pone.0011673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J. S., McCarthy R. L., Sidoli S., Donahue G., Kaeding K. E., He Z., Lin S., Garcia B. A. and Zaret K. S. (2017). Genomic and proteomic resolution of heterochromatin and its restriction of alternate fate genes. Mol. Cell 68, 1023-1037.e15. 10.1016/j.molcel.2017.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens C., Schubert T., Haverkamp S., Euler T. and Berens P. (2016). Connectivity map of bipolar cells and photoreceptors in the mouse retina. eLife 5, e20041 10.7554/eLife.20041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C., Kim H.-T., Torero Ibad R., Lee E. J., Simonutti M., Picaud S., Acampora D., Simeone A., Di Nardo A. A., Prochiantz A. et al. (2014). Graded Otx2 activities demonstrate dose-sensitive eye and retina phenotypes. Hum. Mol. Genet. 23, 1742-1753. 10.1093/hmg/ddt562 [DOI] [PubMed] [Google Scholar]
- Billings N. A., Emerson M. M. and Cepko C. L. (2010). Analysis of Thyroid Response Element Activity during Retinal Development. PLoS ONE 5, e13739 10.1371/journal.pone.0013739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay G., Awad M. E., Riggi N., Archer T. C., Iyer S., Boonseng W. E., Rossetti N. E., Naigles B., Rengarajan S., Volorio A. et al. (2017). OTX2 activity at distal regulatory elements shapes the chromatin landscape of Group 3 medulloblastoma. Cancer Discov. 7, 288-301. 10.1158/2159-8290.CD-16-0844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck S. B., Bradford J., Gee K. R., Agnew B. J., Clarke S. T. and Salic A. (2008). Detection of S-phase cell cycle progression using 5-ethynyl-2′-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2′-deoxyuridine antibodies. BioTechniques 44, 927-929. 10.2144/000112812 [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Austin C. P., Yang X., Alexiades M. and Ezzeddine D. (1996). Cell fate deternination in the vertebrate retina. Proc. Natl. Acad. Sci. USA 93, 589-595. 10.1073/pnas.93.2.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L., Ran F. A., Cox D., Lin S., Barretto R., Habib N., Hsu P. D., Wu X., Jiang W., Marraffini L. A. et al. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819-823. 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo J. C., Lawrence K. A., Karlstetter M., Myers C. A., Abdelaziz M., Dirkes W., Weigelt K., Seifert M., Benes V., Fritsche L. G. et al. (2010). CRX ChIP-seq reveals the cis-regulatory architecture of mouse photoreceptors. Genome Res. 20, 1512-1525. 10.1101/gr.109405.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson M. M. and Cepko C. L. (2011). Identification of a retina-specific Otx2 enhancer element active in immature developing photoreceptors. Dev. Biol. 360, 241-255. 10.1016/j.ydbio.2011.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson M. M., Surzenko N., Goetz J. J., Trimarchi J. and Cepko C. L. (2013). Otx2 and Onecut1 promote the fates of cone photoreceptors and horizontal cells and repress rod photoreceptors. Dev. Cell 26, 59-72. 10.1016/j.devcel.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossat N., Le Greneur C., Béby F., Vincent S., Godement P., Chatelain G. and Lamonerie T. (2007). A new GFP-tagged line reveals unexpected Otx2 protein localization in retinal photoreceptors. BMC Dev. Biol. 7, 122 10.1186/1471-213X-7-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. G., Young L., Chuang R.-Y., Venter J. C., Hutchison C. A. and Smith H. O. (2009). Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343-345. 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- Glubrecht D. D., Kim J.-H., Russell L., Bamforth J. S. and Godbout R. (2009). Differential CRX and OTX2 expression in human retina and retinoblastoma. J. Neurochem. 111, 250-263. 10.1111/j.1471-4159.2009.06322.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C. E., Bailey T. L. and Noble W. S. (2011). FIMO: scanning for occurrences of a given motif. Bioinformatics 27, 1017-1018. 10.1093/bioinformatics/btr064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housset M., Samuel A., Ettaiche M., Bemelmans A., Beby F., Billon N. and Lamonerie T. (2013). Loss of Otx2 in the adult retina disrupts retinal pigment epithelium function, causing photoreceptor degeneration. J. Neurosci. 33, 9890-9904. 10.1523/JNEUROSCI.1099-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. EO., Enright J. M., Myers C. A., Shen S. Q. and Corbo J. C. (2017). Cell Type-specific epigenomic analysis reveals a uniquely closed chromatin architecture in mouse rod photoreceptors. Sci. Rep. 7, 43184 10.1038/srep43184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwafuchi-Doi M. and Zaret K. S. (2014). Pioneer transcription factors in cell reprogramming. Genes Dev. 28, 2679-2692. 10.1101/gad.253443.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorstad N. L., Wilken M. S., Grimes W. N., Wohl S. G., VandenBosch L. S., Yoshimatsu T., Wong R. O., Rieke F. and Reh T. A. (2017). Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature 548, 103-107. 10.1038/nature23283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. S., Matsuda T. and Cepko C. L. (2008). A core paired-type and POU homeodomain-containing transcription factor program drives retinal bipolar cell gene expression. J. Neurosci. 28, 7748-7764. 10.1523/JNEUROSCI.0397-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura C., Takeda N., Suzuki M., Oshimura M., Aizawa S. and Matsuo I. (1997). Cis-acting elements conserved between mouse and pufferfish Otx2 genes govern the expression in mesencephalic neural crest cells. Development124, 3929-3941. [DOI] [PubMed]
- Kimura C., Yoshinaga K., Tian E., Suzuki M., Aizawa S. and Matsuo I. (2000). Visceral endoderm mediates forebrain development by suppressing posteriorizing signals. Dev. Biol. 225, 304-321. 10.1006/dbio.2000.9835 [DOI] [PubMed] [Google Scholar]
- Klemm S. L., Shipony Z. and Greenleaf W. J. (2019). Chromatin accessibility and the regulatory epigenome. Nat. Rev. Genet. 20, 207-220. 10.1038/s41576-018-0089-8 [DOI] [PubMed] [Google Scholar]
- Koike C., Nishida A., Ueno S., Saito H., Sanuki R., Sato S., Furukawa A., Aizawa S., Matsuo I., Suzuki N. et al. (2007). Functional roles of Otx2 transcription factor in postnatal mouse retinal development. Mol. Cell. Biol. 27, 8318-8329. 10.1128/MCB.01209-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa D., Kiyonari H., Nakayama R., Kimura-Yoshida C., Matsuo I. and Aizawa S. (2004a). Regulation of Otx2 expression and its functions in mouse forebrain and midbrain. Development 131, 3319-3331. 10.1242/dev.01220 [DOI] [PubMed] [Google Scholar]
- Kurokawa D., Takasaki N., Kiyonari H., Nakayama R., Kimura-Yoshida C., Matsuo I. and Aizawa S. (2004b). Regulation of Otx2 expression and its functions in mouseepiblast and anterior neuroectoderm. Development 131, 3307-3317. 10.1242/dev.01219 [DOI] [PubMed] [Google Scholar]
- Kurokawa D., Ohmura T., Sakurai Y., Inoue K., Suda Y. and Aizawa S. (2014). Otx2 expression in anterior neuroectoderm and forebrain/midbrain is directed by more than six enhancers. Dev. Biol. 387, 203-213. 10.1016/j.ydbio.2014.01.011 [DOI] [PubMed] [Google Scholar]
- Langmead B. and Salzberg S. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods9, 357-359. 10.1038/nmeth.1923 [DOI]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G. and Durbin R.. 1000 Genome Project Data Processing Subgroup (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078-2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Schulz M. H., Look T., Begemann M., Zenke M. and Costa I. G. (2019). Identification of transcription factor binding sites using ATAC-seq. Genome Biol. 20, 45 10.1186/s13059-019-1642-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Morales J. R., Signore M., Acampora D., Simeone A. and Bovolenta P. (2001). Otx genes are required for tissue specification in the developing eye. Development 128, 2019-2030. [DOI] [PubMed] [Google Scholar]
- Matsuda T. and Cepko C. L. (2004). Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. USA 101, 16-22. 10.1073/pnas.2235688100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T. and Cepko C. L. (2007). Controlled expression of transgenes introduced by in vivo electroporation. Proc. Natl. Acad. Sci. USA 104, 1027-1032. 10.1073/pnas.0610155104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranishi Y., Terada K., Inoue T., Katoh K., Tsujii T., Sanuki R., Kurokawa D., Aizawa S., Tamaki Y. and Furukawa T. (2011). An essential role for RAX homeoprotein and NOTCH-HES signaling in Otx2 expression in embryonic retinal photoreceptor cell fate determination. J. Neurosci. 31, 16792-16807. 10.1523/JNEUROSCI.3109-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. P., Hughes A. E. O., Lawrence K. A., Myers C. A. and Corbo J. C. (2019). Cis-regulatory basis of sister cell type divergence in the vertebrate retina. eLife 8, e48216 10.7554/eLife.48216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi K., Kato S. and Teranishi T. (1988). Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci. Lett. 94, 247-252. 10.1016/0304-3940(88)90025-0 [DOI] [PubMed] [Google Scholar]
- Nishida A., Furukawa A., Koike C., Tano Y., Aizawa S., Matsuo I. and Furukawa T. (2003). Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat. Neurosci. 6, 1255-1263. 10.1038/nn1155 [DOI] [PubMed] [Google Scholar]
- Ohsawa R. and Kageyama R. (2008). Regulation of retinal cell fate specification by multiple transcription factors. Brain Res. 1192, 90-98. 10.1016/j.brainres.2007.04.014 [DOI] [PubMed] [Google Scholar]
- Perez-Cervantes C., Smith L. A., Nadadur R. D., Hughes A. EO., Wang S., Corbo J. C., Cepko C., Lonfat N. and Moskowitz I. P. (2020). Enhancer transcription identifies cis-regulatory elements for photoreceptor cell types. Development 147, dev184432 10.1242/dev.184432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poché R. A., Furuta Y., Chaboissier M.-C., Schedl A. and Behringer R. R. (2008). Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Müller glial cell development. J. Comp. Neurol. 510, 237-250. 10.1002/cne.21746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A., van den Bogaard P., Rifkin S. A., van Oudenaarden A. and Tyagi S. (2008). Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods 5, 877-879. 10.1038/nmeth.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F., Ryan D. P., Grüning B., Bhardwaj V., Kilpert F., Richter A. S., Heyne S., Dündar F. and Manke T. (2016). deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160-W165. 10.1093/nar/gkw257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzycki P. A., Zhang X. and Chen S. (2018). CRX directs photoreceptor differentiation by accelerating chromatin remodeling at specific target sites. Epigenet. Chromatin 11, 42 10.1186/s13072-018-0212-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel A., Housset M., Fant B. and Lamonerie T. (2014). Otx2 ChIP-seq reveals unique and redundant functions in the mature mouse retina. PLoS ONE 9, e89110 10.1371/journal.pone.0089110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Inoue T., Terada K., Matsuo I., Aizawa S., Tano Y., Fujikado T. and Furukawa T. (2007). Dkk3-Cre BAC transgenic mouse line: a tool for highly efficient gene deletion in retinal progenitor cells. Genesis 45, 502-507. 10.1002/dvg.20318 [DOI] [PubMed] [Google Scholar]
- Scholzen T. and Gerdes J. (2000). The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 182, 311-322. [DOI] [PubMed] [Google Scholar]
- Shannon P. and Richards M. (2020). MotifDb: an annotated collection of protein-DNA binding sequence motifs. R package version 1.30.0. 10.18129/B9.bioc.MotifDb
- Shekhar K., Lapan S. W., Whitney I. E., Tran N. M., Macosko E. Z., Kowalczyk M., Adiconis X., Levin J. Z., Nemesh J., Goldman M. et al. (2016). Comprehensive classification of retinal bipolar neurons by single-cell transcriptomics. Cell 166, 1308-1323.e30. 10.1016/j.cell.2016.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Star E. N., Zhu M., Shi Z., Liu H., Pashmforoush M., Sauve Y., Bruneau B. G. and Chow R. L. (2012). Regulation of retinal interneuron subtype identity by the Iroquois homeobox gene Irx6. Development 139, 4644-4655. 10.1242/dev.081729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E., Novelli E., Mazzoni F., Barone I. and Damiani D. (2010). Complexity of retinal cone bipolar cells. Prog. Retin. Eye Res. 29, 272-283. 10.1016/j.preteyeres.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surzenko N., Crowl T., Bachleda A., Langer L. and Pevny L. (2013). SOX2 maintains the quiescent progenitor cell state of postnatal retinal Muller glia. Development 140, 1445-1456. 10.1242/dev.071878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. CY., Rudolph S., Dhande O. S., Abraira V. E., Choi S., Lapan S. W., Drew I. R., Drokhlyansky E., Huberman A. D., Regehr W. G. et al. (2015). Cell type-specific manipulation with GFP-dependent Cre recombinase. Nat. Neurosci. 18, 1334-1341. 10.1038/nn.4081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ENCODE Project Consortium. (2011). A user's guide to the encyclopedia of DNA elements (ENCODE). PLoS Biol. 9, e1001046 10.1371/journal.pbio.1001046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi J. M., Stadler M. B. and Cepko C. L. (2008). Individual retinal progenitor cells display extensive heterogeneity of gene expression. PLoS ONE 3, e1588 10.1371/journal.pone.0001588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquerizas J. M., Kummerfeld S. K., Teichmann S. A. and Luscombe N. M. (2009). A census of human transcription factors: function, expression and evolution. Nat. Rev. Genet. 10, 252-263. 10.1038/nrg2538 [DOI] [PubMed] [Google Scholar]
- Vierstra J., Rynes E., Sandstrom R., Zhang M., Canfield T., Hansen R. S., Stehling-Sun S., Sabo P. J., Byron R., Humbert R. et al. (2014). Mouse regulatory DNA landscapes reveal global principles of cis-regulatory evolution. Science 346, 1007-1012. 10.1126/science.1246426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Flanagan J., Su N., Wang L.-C., Bui S., Nielson A., Wu X., Vo H.-T., Ma X.-J. and Luo Y. (2012). RNAscope. J. Mol. Diagn. 14, 22-29. 10.1016/j.jmoldx.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Sengel C., Emerson M. M. and Cepko C. L. (2014). A gene regulatory network controls the binary fate decision of rod and bipolar cells in the vertebrate retina. Dev. Cell 30, 513-527. 10.1016/j.devcel.2014.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zibetti C., Shang P., Sripathi S. R., Zhang P., Cano M., Hoang T., Xia S., Ji H., Merbs S. L. et al. (2018). ATAC-Seq analysis reveals a widespread decrease of chromatin accessibility in age-related macular degeneration. Nat. Commun. 9, 1364 10.1038/s41467-018-03856-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken M. S., Brzezinski J. A., La Torre A., Siebenthall K., Thurman R., Sabo P., Sandstrom R. S., Vierstra J., Canfield T. K., Hansen R. et al. (2015). DNase I hypersensitivity analysis of the mouse brain and retina identifies region-specific regulatory elements. Epigenet. Chromatin 8, 8 10.1186/1756-8935-8-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Zhang W., Zhang M., Akhtar T., Li Y., Yi W., Sun X., Zuo Z., Wei M., Fang X. et al. (2020). Chromatin accessibility analysis reveals regulatory dynamics of developing human retina and hiPSC-derived retinal organoids. Sci. Adv. 6, eaay5247 10.1126/sciadv.aay5247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W. (1985). Cell differentiation in the retina of the mouse. Anat. Rec. 212, 199-205. 10.1002/ar.1092120215 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Liu M., Xia X., Gong T., Feng J., Liu W., Liu Y., Zhen B., Wang Y., Ding C. et al. (2017). A mouse tissue transcription factor atlas. Nat. Commun. 8, 15089 10.1038/ncomms15089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibetti C., Liu S., Wan J., Qian J. and Blackshaw S. (2019). Epigenomic profiling of retinal progenitors reveals LHX2 is required for developmental regulation of open chromatin. Commun. Biol. 2, 142 10.1038/s42003-019-0375-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.