Abstract
Molecular signaling pathways involved in cancer have been intensively studied due to their crucial role in cancer cell growth and dissemination. Among them, zinc finger E-box binding homeobox-1 (ZEB1) and -2 (ZEB2) are molecules that play vital roles in signaling pathways to ensure the survival of tumor cells, particularly through enhancing cell proliferation, promoting cell migration and invasion, and triggering drug resistance. Importantly, ZEB proteins are regulated by microRNAs (miRs). In this review, we demonstrate the impact that miRs have on cancer therapy, through their targeting of ZEB proteins. MiRs are able to act as onco-suppressor factors and inhibit the malignancy of tumor cells through ZEB1/2 down-regulation. This can lead to an inhibition of epithelial-mesenchymal transition (EMT) mechanism, therefore reducing metastasis. Additionally, miRs are able to inhibit ZEB1/2-mediated drug resistance and immunosuppression. Additionally, we explore the upstream modulators of miRs such as long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs), as these regulators can influence the inhibitory effect of miRs on ZEB proteins and cancer progression.
Keywords: microRNA, ZEB family, cancer therapy, EMT, drug resistance, immunotherapy, non-coding RNA
1. Introduction
Epithelial-mesenchymal transition (EMT) process was first introduced by Greenburg and his colleagues in 1982 [1]. To date, three major types of EMT have been identified: type I EMT, which occurs during embryogenesis, type II EMT, which is activated during wound healing, tissue regeneration and organ fibrosis, and type III EMT, which occurs during metastasis of cancer cells [2]. EMT is the process of cellular transition wherein epithelial cells are bio-transformed into mesenchymal cells with fibroblast-like properties [3,4,5,6]. In the EMT mechanism, cadherins play a significant role. Cadherins promote cell-cell adhesion and are located at the adherens’ junctions. There are different kinds of cadherins including E, N, P, VE, proto, desmosomal, and FAT cadherins, but N-cadherin and E-cadherin are the most important ones in EMT mechanism. A decrease in E-cadherin levels, and an increase in N-cadherin levels lead to stimulation of EMT, and enhanced migratory ability of cancer cells [7,8]. Additionally, upon EMT stimulation, morphology changes and alterations in cytoskeleton occur in cells and affect their migratory ability and adhesion to neighboring cells. These molecular and structural changes promote the dissemination of cells into other sites [9]. Essentially, this increased cell migration is beneficial in normal cells to accelerate physiological processes such as wound healing and embryogenesis. It has been reported that EMT occurs to provide the required flexibility for mesoderm and neural crest formations [10,11]. However, cancer cells can exploit the EMT mechanism for metastasis to distant sites [12,13,14]. There is increased attention towards the EMT mechanism in cancer therapy not only because of its contribution toward metastasis, but also due to the fact that the EMT mechanism can trigger chemoresistance of cancer cells, and decrease sensitivity to apoptosis [15,16]. Therefore, understanding the molecular pathways regulating EMT is a crucial in the field of cancer studies.
EMT is regulated by a variety EMT-promoting transcription factors (EMT-TFs) such as Snail, Slug, Twist, TBX-2, SIX, transforming growth factor--β (TGF-β), and Zinc finger E-box-binding homeobox protein (ZEB) [17]. These upstream EMT-TFs can induce EMT and promote the biotransformation of cells from epithelial phenotype into mesenchymal phenotype by affecting levels of cadherins. Different studies have shown the involvement of ZEB proteins in modulating EMT during normal development and in pathological conditions [18,19,20,21]. Our aim in the present review is to 1) show that ZEB proteins are able to regulate metastasis of cancer cells via affecting EMT, 2) understand how different microRNAs (miRs) can regulate the ZEB/EMT axis, and 3) demonstrate how other upstream mediators can regulate the miR/ZEB/EMT axis.
2. ZEB Family
The ZEB family, which was first discovered in Drosophila melanogaster, consists of two key members ZEB1 and ZEB2 [22]. Both ZEB1 and ZEB2 possess the amino-terminal (NZF) and carboxy-terminal zinc finger cluster (CZF), thereby allowing them to bind to regulatory DNA sequences in their target promoters [23,24,25]. This has led to their involvement in different biological events, such as embryogenesis, hematopoiesis, and more importantly, EMT. In fact, ZEB proteins are well-known due to their ability in stimulation of EMT [20]. In this section, we provide an overview of ZEB1 and ZEB2 proteins to shed some light on their role in cancer cells.
2.1. ZEB1
ZEB1 gene is located on chromosome 10p11.2, and its protein is made up of two zinc-finger clusters at N- and C-terminal ends, while the middle portion of the ZEB1 protein contains three distinct parts including a homeodomain, a Smad interaction domain and a C-terminal binding protein (CtBP). The CtBP is involved in the regulation of ZEB1 function [26,27]. Primarily, the zinc-finger clusters allow ZEB1 to bind to E-boxes. ZEB1 regulates its downstream effectors through binding to E-promoter DNA sequence (5′-CANNTG-3′) [28]. Various publications have also highlighted ZEB1′s association with enhanced viability and invasiveness of cancer cells. In colorectal cancer (CRC) cells, it was found that tumor suppressor death domain-associated protein (DAXX) is able to prevent ZEB1 modulation on E-cadherin to inhibit the invasion and proliferation of tumor cells. Down-regulation of DAXX enhanced ZEB-1 suppression of E-cadherin, leading to the enhanced proliferation and malignancy of cancer cells [29]. It has also been highlighted that EMT may contribute to chemoresistance of cancer cells [30,31]. In pancreatic cancer, Rho associated coiled coil containing protein kinase 2 (ROCK2) enhances the expression of ZEB1. This in turn leads to ZEB1-mediated EMT induction, which contributes to gemcitabine resistance in pancreatic cancer cells [32]. In CRC cells, TCF4 enhances expression of ZEB1 to promote stemness and migration of cancer cells, thereby promoting chemotherapy resistance [33]. In prostate cancer cells, ZEB1 stimulates up-regulation of ATP-binding cassette subfamily C member 10 (MRP4) to export docetaxel out of cancer cells, resulting in their decreased sensitivity to chemotherapy [34]. These studies support the modulation of ZEB1, and highlights that it may be beneficial in enhancing the efficacy of chemotherapy and in reducing the migratory ability of cancer cells. Overall, ZEB1 is an important mediator to enhance the invasion and proliferation of tumor cells. More importantly, ZEB1 may significantly reduce the efficiency of chemotherapy.
2.2. ZEB2
ZEB2 is another member of ZEB family and is located on chromosome 2q22.3 [25]. Structurally, the N-terminal end of ZEB2 consists of four zinc fingers, while the C-terminal end has three zinc fingers [25]. Similar to ZEB1, ZEB2 appears to play a crucial role in migration and invasion. In non-small cell lung cancer (NSCLC), MDM2 binding protein (MTBP) behaves as an oncogene to increase EMT through ZEB2 up-regulation [35]. This in turn enhanced the migration and metastasis of NSCLC tumor cells. In bladder cancer, it was found that indoleamine-2,3-dioxygenase-1 (IDO1) induces ZEB2 overexpression, which in turns increases the viability and proliferation of cancer cells [36]. ZEB2 has also been found to increase the expression of ETS proto-oncogene 1 (ETS1) to up-regulate other EMT proteins such as matrix metalloproteinase 9 (MMP-9) and Twist [37]. Importantly, ZEB2 is also capable of inducing chemoresistance via EMT activation. Phosphatidylinositol 3-kinase (PI3K)/protein kinase-B (Akt) pathway is a down-stream pathway of ZEB2 that induces EMT by reducing the level of E-cadherin protein, leading to the generation of cisplatin resistance in NSCLC cells [38] In all, ZEB2 appears to mediate EMT, and may be a potential therapeutic target in cancer treatment.
3. MicroRNAs
Non-coding RNAs (ncRNAs) comprise a huge part of human genome and are involved in various molecular pathways and processes [39,40,41,42,43,44,45,46,47,48]. They are divided into two characteristic groups: house-keeping and regulatory molecules [49]. They play a remarkable role in vital biological processes such as apoptosis, autophagy, differentiation, cell cycle, proliferation, and migration by targeting various down-stream molecular pathways [50,51]. Additionally, they contribute to the transcription, post-transcriptional modifications, and signal transduction networking. MiRs are house-keeping molecules belonging to the small nucleolar RNAs (SnoRNAs) family [52,53,54]. In this section, we first provide an introduction about miRs and their biosynthesis, followed by a highlight of their potential roles in cancer.
MiRs are single-stranded RNA molecules with a length of 19–24 nucleotides and may possess regulatory functions [55,56]. In total, 60% of all human genome has a binding site for miRs. This highlights the influence of miRs as they control many cellular processes and their dysregulation is related to the development of diseases [57,58,59,60,61]. MiRs are capable of post-transcriptional regulation of their target through RNA interference. These small RNA molecules bind to their target via 3′-untranslated region (3′-UTR). It has been demonstrated that the level of miRs has a negative relationship with the expression of their down-stream targets [62,63,64]. Moreover, one miR is able to target more than one messenger RNA (mRNA), again highlighting their widespread influence in many cellular processes [65]. In the synthesis of miR, a primary miR (pri-miR) is first produced by the action of RNA polymerase. The pri-miR is long with more than 500 nucleotides. It is then processed by Drosha/Pasha and DICER1 proteins, which cleave the pri-miR to generate a mature miR. Next, the mature miR is incorporated in a complex to form miR-RNA-induced silencing complex assembly [66,67,68,69,70].
MicroRNAs in Cancer Metastasis
When focusing on the cancer context, miRs can have oncogenic, or tumor suppressing properties. Onco-suppressor miRs that inhibit invasion of cancer cells undergo down-regulation during cancer development. Enhancing the expression of such miRs can aid in the down-regulation of factors involved in migration of cancer cells such as PRMT5 [71]. Additionally, MiR-506-3p up-regulation considerably reduces the viability and proliferation of ovarian cancer cells and stimulates apoptotic cell death. Investigation of underlying molecular pathways shows that miR-506-3p inhibits Akt/Forkhead box O3 (FOXO3a) by inhibition of sirtuin 1 (SIRT1) [72]. Elevating the expression of miR-506-3p is a potential strategy in ovarian cancer treatment. In the gastric cancer model, it was also observed that a reverse relationship between miR-612 and nin one binding protein (NOB1) helped reduce the migration and invasion of cervical cancer cells [73]. Similarly, in pancreatic cancer, it was also found that overexpression miRs could lead to better prognosis. Enhancing the expression of miR-519 appears to sensitize pancreatic cancer cells to apoptosis and inhibits their proliferation and migration. This miR prevents the activation of programmed death ligand 1 (PD-L1), under hypoxic conditions to suppress tumorigenesis [74]. In all, various studies have shown that miRs are efficient upstream mediators that target various molecular pathways. Enhancing the expression of tumor suppressor miRs may prove to be an advantageous strategy and extensive research is currently being performed to exploit this strategy [74,75,76,77,78]. Conversely, oncogenic miRs are able to elevate the malignancy and proliferation of cancer cells and are associated with poor prognosis. Their downregulation is of interest in cancer therapy [79,80]. For instance, miR-424-5p is able to induce anoikis resistance to promote migratory ability of cancer cells [81]. The targeting of miRs may therefore be considered a promising candidate in cancer therapy. Interestingly, EMT-TFs are considered as potential down-stream targets of miRs in cancer metastasis. MiR-582-3p and miR-582-5p suppress migration of cancer cells via down-regulation of TGF-β in cancer cells [82]. This concurs that miRs can play a significant role in the regulation of metastasis via targeting different pathways and mechanisms. In the following sections, we focus on the regulation of ZEB proteins by miRs and their association with cancer metastasis and chemoresistance.
4. MicroRNA, ncRNA, and ZEB: Role in EMT and Cancer Metastasis
This section specifically demonstrates the impact that miRs have on cell migration and invasion, through their targeting of ZEB proteins. Upstream modulators of miRs such as lncRNAs and circRNAs are also extensively discussed. As mentioned, miRs are able to act as both onco-suppressor as well as promoter of cancer dissemination. Particularly, they are able to exert these effects through their modulation of ZEB proteins, to result in changes in the EMT mechanism. For instance, it appears that miR-200c plays a dual role in cancer therapy. Some studies have demonstrated that miR-200c elevates the viability and proliferation of tumor cells, while another study showed that miR-200c sensitizes cancer cells into chemotherapy by targeting neurophilin 1 and reducing cancer malignancy [83,84,85,86]. It is believed that miR-200c exerts an inhibitory impact on TGF-β-mediated EMT through down-regulation of both ZEB1 and ZEB2 proteins [87].
4.1. ZEB1
4.1.1. MiRs as Modulators of ZEB1
Breast cancer is one of the leading causes of death among females [88]. Importantly, metastasis is a prevalent concern in breast cancer development [89]. Notably, ZEB1 has been identified as a key player in migration of breast cancer cells. MiR-200a was found to down-regulate ZEB1 in suppressing cancer cell migration. It appears that by down-regulating ZEB1, miR-200a can enhance E-cadherin levels and inhibit EMT [90]. This study demonstrates that the relationship between miRs and ZEB proteins is crucial in the regulation of metastasis. Additionally, other onco-suppressor miRs have also been identified to regulate the migration of cancer cells. MiR-1271 have been found to significantly decrease the viability and proliferation of tumor cells [91,92,93]. In ovarian cancer cells, miR-1271 inhibits EMT via ZEB1 down-regulation (binding into 3′-UTR), leading to the decreased viability, proliferation, invasion, and migration of tumor cells. As a consequence of ZEB1 down-regulation by miR-1271, levels of E-cadherin undergo up-regulation, accompanied by a decrease in the levels of N-cadherin [94]. In gastric cancer cells, expression of miR-203 undergoes down-regulation, resulting in an up-regulation of ZEB1 and resistance of cancer cells to radiotherapy. It has been suggested that enhancing the expression of miR-203 is a potential strategy in sensitizing cancer cells to radiotherapy, since miR-203 binds to the 3′-UTR of ZEB1 to repress its expression. This then results in a decrease in the malignancy of cancer cells and an increased sensitivity to radiotherapy [95]. Additionally, inhibition of ZEB1 by miRs such as miR-101-3p, miR-525-5p and miR-186-5p is also corelated with a diminution in metastasis of cancer cells due to EMT inhibition by E-cadherin up-regulation [96,97,98]. Taken together, the miR/ZEB1 axis is an important factor in cancer dissemination and may be an important and relevant target in cancer therapeutics. A newly published study has investigated efficacy of ursolic acid in affecting miR-220c/ZEB1 axis. Ursolic acid enhances expression of miR-200c, as an onco-suppressor factor that, in turn, reduces expression of TGF-β1, providing the condition for down-regulation of ZEB1 and inhibiting metastasis of CRC cells [97].
As aforementioned, EMT-TFs such as TGF-β can stimulate EMT. ZEB1 engages in a feedback loop with TGF-β and miR, thereby promoting metastasis of cancer cells. Normally, miR-33a-5p suppresses TGF-β to inhibit ZEB1 activation, leading to suppression of metastasis. However, in cancer conditions, TGF-β and ZEB1 cooperate with each other to promote migration of cancer cells. TGF-β can enhance copy numbers of ZEB1, while ZEB1 suppresses miR-33a-5p, an inhibitor of TGF-β signaling. This cooperation between ZEB1 and TGF-β leads to inhibition of miR-33a-5p, and stimulation of EMT [99]. This once again highlights that onco-suppressor miRs may suppress ZEB1 via affecting other EMT-TFs such as TGF-β, and that ZEB1 can form a negative feedback loop with onco-suppressor miRs in promoting metastasis of cancer cells.
The Akt/mammalian target of rapamycin (mTOR) signaling pathway is another pathway that is commonly deregulated in cancer [100,101,102]. Phosphorylated Akt can induce mTOR to promote the motility and invasion of tumor cells [103,104,105,106,107,108]. It appears that miR-205 is able to target the Akt/mTOR signaling pathway to regulate malignancy and progression of cancer cells [109]. By suppressing Akt/mTOR signaling pathway, miR-708 acts as an anti-tumor agent to inhibit ZEB1, leading to the suppressing EMT mechanism [110]. Finally, miR-126 was also found to inhibit ZEB1 to suppress MMP-2, MMP-9, and oncogenic JAK2/STAT3 signaling pathway, leading to the reduced migration and metastasis of cervical cancer cells [111].
Additionally, Wnt signaling pathway contributes to cancer cell growth and dissemination. Abnormal expression of Wnt signaling pathway can be observed in cancers [112,113,114,115,116,117]. Wnt/β-catenin signaling pathway can promote EMT through ZEB1 up-regulation to elevate the invasion and malignancy of tumor cells. Enhancing the expression of miR-33b effectively inhibits Wnt/β-catenin/ZEB1 axis to suppress cancer malignancy through EMT inhibition [118]. Similarly, miR-200a is capable of decreasing gastric adenocarcinoma invasion via down-regulation of Wnt/β-catenin and subsequent suppressing of ZEB1 and ZEB2 [119]. In malignant meningioma, miR-4652-3p down-regulates the expression of ZEB1 by suppressing Wnt and nuclear translocation of β-catenin. Conversely, lncRNA LINC00702 can activate Wnt/β-catenin signaling pathway by sponging miR-4652-3p to induce ZEB1 and promote the metastasis and invasion of malignant meningioma [120]. These studies highlight the fact that firstly, Wnt can promote metastasis of cancer cells via ZEB1 up-regulation; secondly, the Wnt/ZEB1 axis can be inhibited by onco-suppressor miRs; thirdly, miRs affect both expression of Wnt and nuclear translocation of β-catenin; and finally, lncRNAs can regulate miR/Wnt/ZEB1 axis. The mediation of the miR/ZEB1 axis by lncRNAs will be more extensively discussed in the next section.
4.1.2. LncRNAs as Modulators of miR/ZEB1 Axis
Long non-coding RNAs (lncRNAs) belong to a category of ncRNAs with regulatory effect on biological events [121,122]. They consist of at least 200 nucleotides and they are able to function as upstream mediators of miRs [111]. LncRNAs suppress the expression of miRs via acting as competitive endogenous RNA (ceRNA) [123]. The effect of lncRNAs on miR/ZEB1 axis has been investigated in cancer cells. For instance, miR-429 was found to inhibit EMT through ZEB1 inhibition and its expression is typically down regulated in pancreatic cancer cells. MiR-429 can be regulated by lncRNA XIST, which is up-regulated in pancreatic cancer cells to reduce miR-429 levels. This in turn increases ZEB1 expression and promotes EMT. Additionally, through targeting the miR-429/ZEB1 axis, XIST also affects morphology of cancer cells, such that silencing XIST results in a change in cell morphology, from the original spindle shape to a rounded one [124]. In another instance, LncRNA IUR is a onco-suppressor factor that has shown a great capability in suppressing tumorigenesis [125]. LncRNA IUR can inhibit the migration and metastasis of prostate cancer cells via enhancing the expression of miR-200, which in turn inhibits ZEB1 [126].
Conversely, lncRNA TDRG1 is an oncogenic factor that is able to regulate miRs in cancer cells [127,128]. In lung cancer cells, TDRG1 enhances the migration, metastasis, and malignancy of cancer cells by promoting ZEB1 expression through miR-873-5p down-regulation [129]. LncRNA TTN-AS1 is also considered an oncogenic factor that induces ZEB1 through miR-4677-3p down-regulation, leading to the enhanced migration and metastasis of NSCLC cells [130]. LncRNA (Nuclear Enriched Abundant Transcript 1) NEAT1 contributes to enhancing the malignancy of cancer cells [131]. It has been demonstrated that NEAT1 can target miRs to regulate cancer proliferation and migration [132]. In breast cancer cells, NEAT1 reduces the expression of miR-448 to elevate the metastasis and invasion of cancer cells through ZEB1 up-regulation [133]. LncRNA TP73-AS1 reduces the expression of miR-200a to up-regulate ZEB1, leading to the enhanced progression and malignancy of tumor cells. There appears to be a feedback loop, wherein TP73-AS1-activated ZEB1 has a stimulatory effect on the expression of TP73-AS1 to enhance its inhibitory activity on miR-200a, leading to increased induction of ZEB1 [90].
In renal cell carcinoma (RCC), miR-429 typically reduces the expression of ZEB1 to suppress RCC progression. However, miR-429 can be inhibited by SCAMP1, a lncRNA that is activated by oxidative stress [134]. This highlights that stimulation of oxidative stress negatively impacts cancer therapy. Generally, it is believed that enhancing level of oxidative stress can lead to a reduction in the viability of cancer cells by predisposing them into apoptosis [135,136]. However, as mentioned, increasing levels of oxidative stress may also activate lncRNAs involved in cancer metastasis. Therefore, careful considerations are warranted before using oxidative stress in cancer therapy, keeping in mind the possible adverse effects of this treatment method.
MiR-139-5 was also found to suppress ZEB1 levels. However, lncRNA human leukocyte antigen (HLA) complex 5 (HCP5) is able to induce ZEB1 and EMT by suppressing miR-139-5 [137]. LncRNA MAGI2-AS3 has also been explored in cancer and it appears that MAGI2-AS3 is able to modulate molecular pathways such as Fas/FasL to suppress breast cancer, bladder cancer, and hepatocellular carcinoma [138,139]. Particularly, in gastric cancer cells, miR-141/200a diminishes the invasion and migration of tumor cells via suppressing ZEB1. LncRNA MAGI2-AS3 down-regulates the expression of miR-141/200a to induce ZEB1, leading to the stimulation of EMT and enhanced invasion of tumor cells [125].
LncRNA LINC00511 is located on chromosome 17q24.3 and has been associated with increased malignancy in cancer [140]. In glioblastoma (GBM) cells, miR-524-5p inhibits ZEB1 to suppress GBM invasion and migration. LINC00511 has been found to decrease the expression of miR-524-5p to up-regulate YB1 [141]. YB1 is a transcription factor that can enhance the expression of ZEB1 in cancer [128]. The inhibition of miR-524-5p by LINC00511 promotes ZEB1 expression through YB1 up-regulation, leading to enhanced EMT and malignancy of GBM cells [141]. These studies again demonstrate that lncRNAs can disrupt inhibitory effects of miRs on ZEB1 to promote metastasis of cancer cells. In glioma cells, miR-205-3p inhibits TGF-β, while lncRNA linc00645 functions as an upstream mediator and activates TGF-β via suppressing miR-205-3p, leading to an increase in ZEB1 levels and subsequent EMT activation [137].
LncRNA MALAT1 located on the chromosome 11q13, is also suggested to be involved in elevating the malignancy of cancer cells. A variety of factors act as down-stream mediators for lncRNA MALAT1 and it appears that MALAT1 is capable of targeting miRs in cancer cells [105,142,143]. MALAT1 was found to enhance the expression of ZEB1 through miR-143-3p down-regulation, resulting in elevated migration and metastasis of tumor cells [144]. Another downstream target of MALAT1 is miR-429, which is considered as a potential biomarker for diagnosis of different cancers [145]. MALAT1 was found to inhibit miR-429 to accelerate the malignancy and invasion of cervical cancer cells [146]. Notably, miR-429 can inhibit the metastasis of cancer cells and stimulate apoptotic cell death through ZEB1 down-regulation [110]. Interestingly, it has been demonstrated that fine particulate matter (PM2.5, aerodynamic diameter, 2.5 μm) is able to induce oxidative stress, inflammation, genetic mutations, and DNA damage [147,148]. It has been found that miR-204 can reduce the expression of ZEB1 to suppress EMT. PM2.5 activates MALAT1 via stimulation of NF-κB, as an inflammatory pathway. MALAT1 in turn induces ZEB1 through miR-204 down-regulation to enhance the malignancy and invasion of tumor cells via EMT induction [149]. These two studies demonstrate that lncRNAs can affect more than one downstream miR to mediate ZEB1 levels, and that other molecular pathways such as NF-κB can act as upstream mediator of lncRNA/miR/ZEB1 axis.
HOXA distal transcript antisense RNA (HOTTIP) is located at the distal end of HOXA gene cluster [150]. This lncRNA undergoes abnormal expressions in different cancers and it has been shown that HOTTIP is related to the increased proliferation and progression of cancer cells [120]. It is held that lncRNA HOTTIP down-regulates the expression of miR-101 to elevate ZEB1 levels, leading to an increase in EMT [151]. A study has also shown that miR-205 down-regulates the expression of ZEB proteins and HOXD9 to suppress the malignancy and invasion of cancer cells through EMT inhibition [152]. Finally, it has been demonstrated that lncRNA HOXC-AS2 induces ZEB1 by sponging miR-876-5p, leading to the stimulation of EMT and enhanced migration and invasion of tumor cells [153]. Taken together, the relationship between lncRNAs and miRs in the regulation of ZEB1 in cancer cells are dynamic and complicated, and understanding these pathways is an essential part of effective cancer therapy.
4.1.3. CircRNAs as Modulators of miR/ZEB1 Axis
Circular RNAs (circRNAs) are endogenous, conserved ncRNAs that are sometimes employed as biomarkers for cancer diagnosis [154,155]. Similar to lncRNA, circRNAs are able to modulate the expression of their targets [156]. In lung cancer cells, hsa-circ-0023404 decreases the expression of miR-217 to enhance the expression of its target, ZEB1, leading to the increased migration and invasion of cancer cells [157]. The laryngeal carcinoma is considered as one of the common cancers among head and neck tumors and is mainly diagnosed in elder people [158]. In spite of the low incidence rate, this cancer results in high mortality worldwide [159]. It was discovered that miR-200c is capable of inhibiting ZEB1 to prevent the metastasis and invasion of laryngeal cancer cells. Hsa-circ-005748 up-regulates ZEB1 by sponging miR-200c, leading to the metastasis of these cancer cells [160]. Therefore, inhibition of hsa-circ-005748 may in turn increase miR-200c expression to suppress ZEB1 and cancer metastasis. Similarly, in lung adenocarcinoma (LUAD) cells, miR-665 is able to inhibit cancer metastasis via ZEB1 down-regulation. The circ-TSPAN4 enhances the expression of ZEB1 by miR-665 down-regulation to promote the metastasis of LUAD cells [161]. These studies concur that down-regulation of onco-suppressor miRs in cancer cells may also be mediated by upstream circRNAs. This in turn promotes up-regulation of ZEB1 and enhanced metastasis of cancer cells.
4.2. ZEB2
4.2.1. MiRs as Modulators of ZEB2
MiR-124 is suggested to be an onco-suppressor miR. Recently, an effort has been made to suppress the prostate cancer invasion. It is held that cationic polymer nanoparticles are able to deliver miR-124 in prostate cancer cells to inhibit their proliferation, motility, and colony formation [162]. In TNBC cells, miR-124 effectively decreases the malignancy and invasion of tumor cells by EMT inhibition through ZEB2 down-regulation [163]. Similarly, miR-145 has been widely established as a tumor suppressor. It negatively affects the invasion and migration of thyroid carcinoma cells by down-regulation of NF-κB signaling pathway [164]. Furthermore, lncRNA-ROR down-regulates the expression of miR-145 to remove its inhibitory impact and induce EMT in tumor cells [165]. Importantly, it was found that miR-145 decreases the expression of ZEB2 to inhibit EMT, and consequently, suppress the proliferation, progression, and migration of NSCLC cells [166]. These studies demonstrate that the downregulation of ZEB2 by onco-suppressor miRs can lead to a decrease in the metastasis of cancer cells.
Another onco-suppressor miR is miR-30a. In breast cancer cells, miR-30a suppresses the nuclear translocation of β-catenin to attenuate cancer proliferation and progression, and is associated with favorable prognosis of patients with breast cancer [167]. Furthermore, miR-30a appears to be beneficial in sensitizing cancer cells to chemotherapy via affecting Akt signaling pathway [168]. MiR-30a was found to inhibit ZEB2 to result in a reduction of triple negative breast cancer (TNBC) cells malignancy [169]. Finally, miR-3653 is an onco-suppressor that is down-regulated in hepatocellular carcinoma (HCC) cells [170]. MiR-3653 was found to bind to the 3′-UTR of ZEB2 to diminish its expression, leading to the reduced invasion and malignancy of colon cancer cells [171]. MiR-138-5p uses a same strategy in inhibition of lung adenocarcinoma cell malignancy, by suppressing EMT through ZEB2 inhibition to attenuate metastasis of tumor cells [172].
Osteosarcoma typically has a high recurrence rate and low survival rate [173,174]. Therefore, understanding the pathways involved in malignancy and cancer progression may pave the road for improved treatment of this type of cancer. Investigation of molecular pathways has shown that miR-101 up-regulation inhibits ZEB2 and affects proliferation and metastasis of osteosarcoma cells [175]. Unfortunately, miR-101 is down-regulated in osteosarcoma cells compared to the normal cells. Enhancing the expression of miR-101 may reduce malignancy and progression of osteosarcoma cells.
4.2.2. LncRNAs as Modulators of miR/ZEB2 Axis
In the previous section, we demonstrated that lncRNAs are able to function as ceRNA in affecting miR expression. Notably, increasing evidence has also demonstrated that lncRNAs can effectively target ZEB2 via affecting miRs. For instance, LncRNA HOTAIRM1, which has dual properties as it interacts with both onco-suppressor and oncogenic miRs. HOTAIRM1 is located on human HOXA gene cluster and suggested to be involved in myeloid cell development [176]. A newly published article has shown the anti-tumor activity of lncRNA HOTAIRM1 by up-regulation of ARHGAP24 through miR-106a-5p inhibition [177]. However, it has been found that HOTAIRM1 is related to the elevated migration and invasion of tumor cells [178]. It is held that lncRNA HOTAIRM1 diminishes the expression of miR-873-5p to induce ZEB2, resulting in an increase in cancer cell proliferation and suppressing apoptotic cell death [179].
MiR-505 is also considered an onco-suppressor miR that interacts with IGF-1 and HMGB1 to suppress the growth and malignancy of tumor cells [180,181,182]. Various studies have demonstrated that lncRNAs such as lncRNA CRAL, LEF-AS1, and DLX6-AS1 are able to target miR-505 in different cancers such as gastric cancer, CRC, and breast cancer [183,184,185]. In cervical cancer, lncRNA CTS was found to target miR-505. MiR-505 down-regulates ZEB2 levels to inhibit EMT and invasion of cervical cancer cells. LncRNA CTS, therefore, stimulates ZEB2-mediated EMT through miR-505 sponging, leading to the enhanced viability, proliferation, and malignancy of cervical cancer cells [186]. Taken together, stimulation of ZEB2 by lncRNAs not only enhances metastasis of cancer cells via EMT induction, but also promotes cell proliferation. This decrease in apoptosis by ZEB2 induction is of importance in chemotherapy, since cancer cells can attain chemoresistance via reducing their sensitivity into chemotherapy-mediated apoptosis. As such, targeting miR/ZEB2 axis may be a promising strategy in cancer therapy, as it increases the sensitivity of cancer cells toward chemotherapy.
In gastric cancer cells, miR-203 diminishes cancer metastasis through ZEB2 down-regulation. LncRNA UCA1 enhances the progression and metastasis of tumor cells through disrupting the miR-203/ZEB2 axis [187]. Particularly, lncRNAs can affect upstream transcription factors of ZEB2 in cancer metastasis. In NSCLC cells, slug was found to behave as an upstream mediator to induce EMT through increasing ZEB2 levels. MiR-218 was able to disrupt the Slug/ZEB2 axis to suppress NSCLC migration. Conversely, miR-218 undergoes down-regulation by lncRNA SNHG12 to stimulate Slug/ZEB2 and promote metastasis of NSCLC cells [188].
Glioma is an intracranial tumor that emanates from neuroglial stem or progenitor cells [189]. Again, this is an alarming cancer with high mortality and morbidity rate [190,191]. The migration and invasion of cancer cells into neighboring cells and tissues reduces the survival time of patients [192,193]. It has been demonstrated that lncRNA SNHG5 can inhibit miR-205-5p expression. Reduced miR-205-5p expression triggers the induction of ZEB2, which in turn enhances the migration ability of tumor cells [194]. Up-regulation of miR-205-5p may therefore be beneficial in reducing glioma malignancy.
4.2.3. CircRNAs as Modulators of miR/ZEB2 Axis
Increasing evidence highlights the role of miR-377 as an onco-suppressor in cancer cells. MiR-377 can target Akt signaling to suppress the proliferation and invasion of tumor cells, and induce cell cycle arrest [195]. Normally, miR-377 reduces the expression of ZEB2. In bladder cancer cells, the expression of miR-377 undergoes down-regulation by circZFR to promote cancer metastasis through ZEB2 stimulation [196]. MiR-653 also appears to be an onco-suppressor miR in bladder cancer cells. CircRNA ciRs-6 reduces miR-653 expression to induce March1, leading to the increased proliferation of tumor cells [110]. MiR-653 is similarly suppressed in breast cancer cells, by another circRNA hsa-circ-0004771. Knockdown of hsa-circ-0004771 sensitizes cancer cells to apoptosis and inhibits their progression through miR-653 up-regulation and subsequent inhibition of ZEB2 [197]. Evidently, ZEB2 induction dually enhances proliferation and metastasis of cancer cells. Hence, targeting the circRNA/miR/ZEB2 axis can pave the way into effective inhibition of proliferation and migration of cancer cells.
In renal cancer, patients typically have poorer survival rates and treatment strategies can be improved [198,199,200]. MiR-153 was found to exert inhibitory impact on ZEB2 expression to suppress renal cancer, while circPCNXL2 stimulates ZEB2 expression via miR-153 sponging to elevate the invasion and proliferation of renal cancer cells [201]. Therefore, decreasing the expression of circPCNXL2 may yield an up-regulation of miR-153 and suppresses ZEB2 expression to eliminate renal cancer.
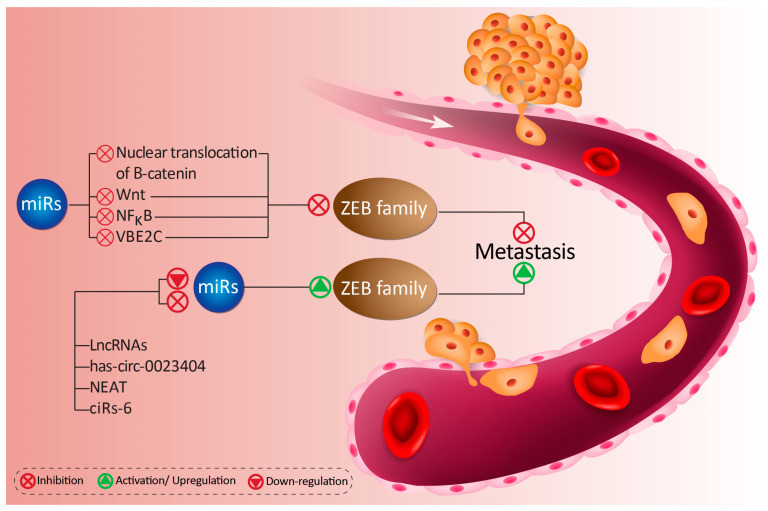
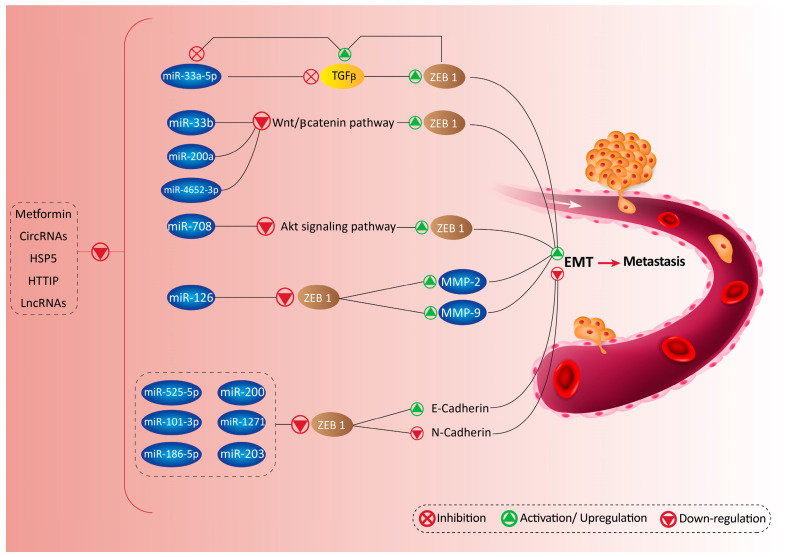
In all, these studies highlight the extensive influence that miRs have on ZEB proteins (Figure 1 and Figure 2). Across a wide range of cancers, different miRs work to inhibit ZEB and halt cancer progression. Additionally, lncRNAs and circRNAs are able to act as upstream mediators of miRs to affect ZEB2 expression. Through the revealing of these molecular pathways, we may better understand these promising candidates in cancer therapy.
Figure 1.
The oncogenic upstream mediators of miRs activating ZEB proteins, which enhances metastasis and migration of cancer cells.
Figure 2.
Metformin, lncRNAs, circRNAs, and other molecular pathways are able to function as upstream mediators of miRs in targeting ZEB proteins, and promote cancer progression.
5. MicroRNAs, ZEB, and Their Role in Tumor Resistance
Multidrug resistance (MDR) is a complicated and challenging phenomenon accounting for cross-resistance towards structurally unrelated drugs [202,203]. It is estimated that approximately 70% of solid and hematological tumors demonstrate MDR. This percentage elevates after chemotherapy, since cancer cells are able to switch among molecular pathways to obtain chemoresistance, and frequent application of chemotherapeutic agents speeds up MDR [204,205]. Therefore, when trying to understand the role of miR and ZEB proteins in cancer, it also important to explore how miR’s modulation on ZEB can affect tumor resistance. Particularly, miR is implicated in tumor resistance. For instance, cisplatin is a potential chemotherapeutic agent with the ability of inhibiting the proliferation and viability of various cancers [206]. In ovarian cancer, it has been reported that miR-137 reduces the expression of MCL1 to sensitize tumor cells into cisplatin-induced apoptosis [207]. In this section, we seek to understand how miR modulation on ZEB can contribute to tumor resistance.
5.1. ZEB1
5.1.1. Paclitaxel Resistance
Paclitaxel (PTX) is a chemotherapeutic agent that is frequently employed in cancer therapy to prevent cell proliferation due to its anti-mitotic capabilities [208]. Unfortunately, PTX resistance is an important obstacle, which has reduced the feasibility of this agent [209,210,211,212]. Notably, ZEB1 can promote cancer cells resistance towards PTX, and down-regulation of ZEB1 may be a key toward re-sensitizing cancer cells to PTX chemotherapy [213]. MiR-124-3p suppresses ZEB1 to sensitize gastric cancer cells into PTX therapy. Circular RNA Circ-PVT1 reverses this axis by sponging miR-124-3p and elevating the expression of ZEB1 to induce PTX resistance in gastric cancer cells [214]. LncRNA NEAT1 was also found to mediate PTX resistance in ovarian cancer cells. Normally, miR-194 undergoes up-regulation to inhibit ZEB1 and subsequently, reduce the malignancy and invasion of cancer cells. LncRNA NEAT1 suppresses the inhibitory effect of miR-194 on ZEB1 to induce the resistance of ovarian cancer cells into PTX chemotherapy [215].
5.1.2. Gemcitabine Resistance
Gemcitabine is a chemotherapeutic agent isolated from deoxycytidine, which is frequently applied in the treatment of breast cancer [216]. Gemcitabine triggers cell cycle arrest by binding into DNA or suppressing ribonucleotide reductase [217,218]. It appears that ZEB1 contributes to the gemcitabine resistance in TNBC cells. This study found that ZEB1 associates with Yes associated protein (YAP) to enhance cancer progression and proliferation and induces chemoresistance. Importantly, ZEB1 was found to be a target of miR-873, and that increasing miR-873 expression down-regulates the expression of YAP and ZEB1, and sensitizes tumor cells into gemcitabine therapy [219].
5.1.3. Cisplatin Resistance
Another important factor to consider when exploring acquired tumor resistance is lncRNA, which can regulate miR, to in turn affect ZEB levels. Prostate cancer-associated transcription 1 (PCAT1) undergoes up-regulation in cancer cells to suppress cell death [220]. In gastric cancer cells, PCAT-1 induces the resistance of cancer cells into cisplatin therapy by stimulation of ZEB1 through miR-128 inhibition, leading to the enhanced progression and malignancy of gastric cancer cells [221]. Therefore, targeting the miR/ZEB1 axis may alleviate cisplatin resistance.
5.1.4. 5-Fluorouracil
The most common chemotherapeutic agent in treatment of cancer is 5-FU [222]. Different molecular pathways are involved in resistance into 5-FU, and miRs are key players [223,224]. LncRNA NEAT1 have been found to possess oncogenic activity and enhance the progression and malignancy of cancer cells via targeting miRs such as miR-144-3p and miR-410 [225,226]. In CRC cells, NEAT1 is involved in 5-FU resistance through miR-34a regulation [227] (Figure 3).
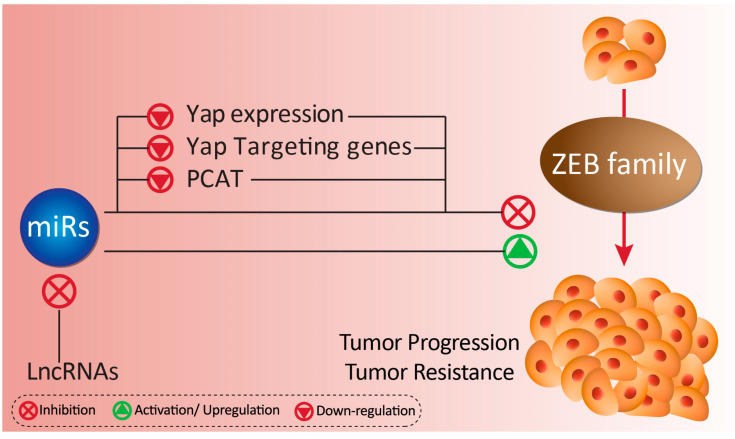
Figure 3.
The miRs as key player in regulation of tumor malignancy via targeting ZEB proteins.
5.2. ZEB2
In osteosarcoma, miR-200b diminishes the progression and motility of tumor cells by inhibition of PI3K/Akt and AMPK signaling pathways, leading to the downregulation of vascular endothelial growth factor (VEGF). LncRNA CCAT2 reverses this axis by induction of VEGF through miR-200b inhibition [228]. It is worth mentioning that enhancing the expression of miR-200b is beneficial in sensitizing cancer cells into chemotherapy, so that arrestin domain containing 3 (ARRDC3) elevates the efficacy of chemotherapy in TNBC cells via miR-200b up-regulation [229]. It appears that up-regulation of miR-200b enhances apoptosis in lung cancer cells and remarkably increases the efficacy of chemotherapy [230]. Another member of miR-200 family, known as miR-200c, sensitizes gastric cancer cells to cisplatin and enhances chemotherapeutic efficacy by suppressing ZEB2 expression [215].
6. MicroRNAs Target ZEB Family in Immune Cells
Other than ZEB’s prevalent role in EMT, ZEB’s involvement with the tumor microenvironment and immune system is also crucial in its mediation of cancer dissemination and development. Tumor cells use immunosuppressive cells such as CD4+ T cells to escape from the anti-cancer activity of CD8+ T cells [231,232,233]. Notably, it has been demonstrated that cytotoxic CD8+ tumor infiltrating lymphocytes (CD8+ TILs) are able to eliminate cancer cells [234], while sustained exposure of tumor cells into CD8+ TILs reduces their anti-tumor activity [235]. It is worth mentioning that PD-1/PD-L1 axis may be involved in driving CD8+ T cell exhaustion and therapies targeting PD-L1 have been explored [236,237,238,239,240]. PD-L1 binds to PD-1 to induce apoptotic cell death in CD8+ T cells and ensure the survival of cancer cells [241,242,243,244].
In diffuse large B cell lymphoma (DLBCL) cells, miR-8890-3p is capable of suppressing ZEB1, while lncRNA SNHG14 conversely reduces the expression of miR-8890-3p to activate ZEB1. Consequently, ZEB1 stimulates PD-L1 to protect cancer cells against the cytotoxic effects of immune cells, resulting in promoting the survival and migration of DLBCL cells [245].
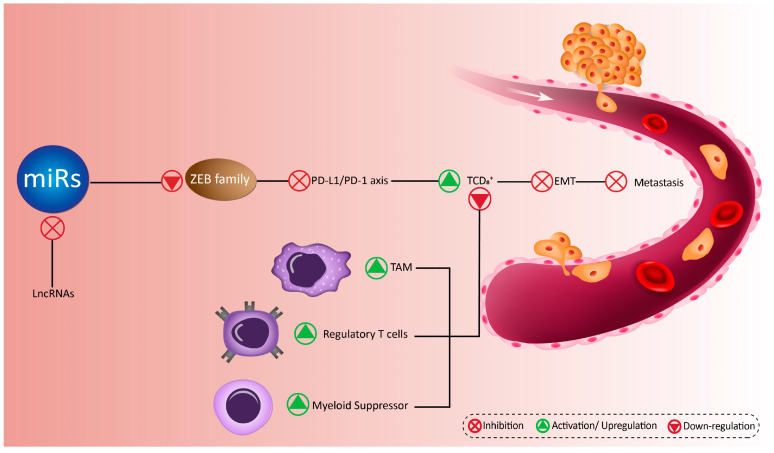
In another instance, it has been reported that ZEB1 is an efficient factor in elevating the malignancy of tumor cells, through the induction of PD-L1 expression to enhance the levels of CD8+ T-cell immunosuppression and cancer metastasis. Enhancing the expression of miR-200 disrupts ZEB1 expression to suppress PD-L1 and immunosuppression, resulting in decreased metastasis and invasion of cancer cells [246]. ZEB1 can induce EMT in breast cancer cells via activation of PD-L1. It has been reported that miR-200 overexpression reduces the levels of ZEB1 to inhibit EMT through interfering with PD-L1 activation, as an immunosuppressive factor [247]. Unfortunately, there are currently no reports about the relationship between miRs and ZEB2 in cancer immunotherapy, and further studies can focus on revealing relationship between miR/ZEB2 axis and cancer immunotherapy. Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6 demonstrate the regulation of ZEB1 and ZEB2 by various miRs proteins in mediating cancer metastasis. Upstream mediators of miR such as lncRNAs and circRNAs are also highlighted in Table 1 through Table 6. Figure 4 further summarizes the effect of miR/ZEB axis on immune system.
Table 1.
ZEB1 regulation by miRs in different cancers.
| MiR | Down-Stream Target | Cancer Type | Major Outcomes | Refs |
|---|---|---|---|---|
| MiR-23a | ZEB1 | Intraocular tumor | A negative feedback loop between miR-23a and ZEB1 regulates EMT and overexpression of miR-23a inhibits EMT by ZEB1 down-regulation | [248] |
| MiR-23b | ZEB1 | Bladder cancer | MiR-23b induces apoptosis and cell cycle arrest, and decreases the invasion and EMT through ZEB1 inhibition | [249] |
| MiR-33b | ZEB1 | Melanoma | Cordycepin enhances the expression of miR-33b to inhibit ZEB1 and induces mesenchymal-epithelial transition in cancer cells, resulting in decreased invasion and migration of cancer cells | [250] |
| MiR-126 | ZEB1 | Osteosarcoma | Inhibition of EMT, migration, and metastasis of cancer cells through ZEB1 down-regulation | [251] |
| MiR-128 | ZEB1 | Prostate cancer | MiR-128 sensitizes cancer cells into cisplatin chemotherapy by ZEB1 down-regulation and decreasing the malignancy and invasion of cancer cells | [252] |
| MiR-130b | ZEB1 | Endometrial cancer | The miR-130b down-regulates the expression of ZEB1 to inhibit the malignancy and invasion of cancer cells | [253] |
| MiR-139-5p | ZEB1/2 | Hepatocellular carcinoma | Reduced invasion, migration, metastasis, and EMT by ZEB1/2 down-regulation through miR-139-5p | [254] |
| Glioblastoma multiforme | Suppressing the invasion and migration of cancer cells through ZEB1/2 inhibition | [255] | ||
| MiR-141 and miR-146b-5p | AUF1/ZEB1 | Osteosarcoma | These miRs are able to down-regulate the expression of AUF1 to repress ZEB1, resulting in an increase in epithelial markers (E-cadherin and Epcam) and a decrease in mesenchymal markers (N-cadherin and Vimentin) | [256] |
|
MiR-144
MiR-144 |
ZEB1/2 | Breast cancer | MiR-144 is an onco-suppressor that inhibits EMT and migration invasion through ZEB1/2 down-regulation | [257] |
| Thyroid cancer | MiR-144 down-regulates the expression of ZEB1/2 to prevent cancer progression and proliferation | [258] | ||
| MiR-150 | ZEB1 | Esophageal squamous cell carcinoma | MiR-150 degrades ZEB1 to induce mesenchymal-epithelial transition (MET), resulting in a decrease in tumor depth, lymph node metastasis, and lymphatic invasion | [259] |
| Ovarian cancer | Suppressing the malignancy and invasion of cancer cells through ZEB1 inhibition | [260] | ||
| MiR-199a-3p | ZEB1 | Melanoma | The administration of gambogic acid is associated with up-regulation of miR-199a-3p and subsequent inhibition of ZEB1 to suppress cancer progression both in vitro and in vivo | [261] |
| MiR-199b | ZEB1 | Non-small cell lung cancer | Suppressing the proliferation, migration, and invasion of cancer cells through ZEB1 down-regulation | [262] |
| MiR-200 | ZEB1-FAK/Src | Human lung cancer | The miR-200 up-regulation decreases the invasion and malignancy of cancer cells through enhancing ZEB1 expression and subsequent activation of FAK/Src | [263] |
| ZEB1 | Endometrial carcinoma | The expression of miR-200 undergoes down-regulation in endometrial carcinoma cells to induce ZEB1 and subsequently, EMT mechanism to elevate the invasion and malignancy of cancer cells | [264] | |
| Lymphoma | Generation of a less aggressive behavior by ZEB1 inhibition through miR-200 | [265] | ||
| Insulinoma mouse model | Overexpression of miR-200 is associated with ZEB1 inhibition and decreased migration and proliferation of cancer cells | [266] | ||
| MiR-200b | ZEB1 | Osteosarcoma | Overexpression of miR-200b is associated with down-regulation of ZEB1 and decreased invasion and malignancy of cancer cells | [267] |
| Human hepatocellular carcinoma | By down-regulation of ZEB1, miR-200b reduces the stemness of cancer cells | [268] | ||
| MiR-200b and miR-141 | ZEB1 | Non-small cell lung cancer | The overexpression of miR-200b and miR-141 is related to the inhibition of ZEB1 and sensitizing cancer cells into nintedanib | [269] |
| MiR-200c | ZEB1 | Human colon cancer | Suppressing the invasion and migration of cancer cells through ZEB1 down-regulation | [270] |
| Gastric carcinoma | Overexpression of miR-200c is related to the ZEB1 down-regulation and enhanced levels of E-cadherin protein | [271] | ||
| Human bladder cancer | Administration of sulforaphane is associated with miR-200c induction and subsequently, inhibition of ZEB1 and malignancy of cancer cells | [272] | ||
| Non-small cell lung carcinoma | The cyclamen pseudibericum extract up-regulates miR-200c to induce ZEB1 down-regulation, resulting in suppressing cancer progression and proliferation | [273] | ||
| Non-small cell lung cancer | MiR-200c sensitizes cancer cells to the gefitinib-mediated apoptosis by down-regulation of ZEB1 | [274] | ||
| Lung cancer | MiR-200c sensitizes lung cancer cells into crizotinib chemotherapy by inhibition of ZEB1, and subsequently, EMT inhibition | [275] | ||
| MiR-200c and miR-141 | ZEB1 | Glioma cell | The miR-200c and -141 synergistically inhibit ZEB1 to prevent the malignancy and invasion of cancer cells | [276] |
| ZEB1/2 | Gastric cancer | MiR-200c/141 significantly decreases ZEB1/2 expression to suppress cancer malignancy | [277] | |
| MiR-203 | ZEB1 | Non-small cell lung cancer | The administration of silymarin enhances the expression of miR-203 to inhibit ZEB1 and elevate the levels of E-cadherin, resulting in suppressing cancer | [278] |
| MiR-204 | ZEB1 | Prostate cancer | MiR-204 up-regulation sensitizes cancer cells into docetaxel-mediated apoptosis through ZEB1 down-regulation | [279] |
| MiR-205 | ZEB1 | Ovarian cancer | MiR-205 enhances the invasion and migration of cancer cells via ZEB1 up-regulation. Reducing the expression of miR-205 is of interest in suppressing the malignancy of cancer cells | [280] |
| Prostate cancer | By inhibition of ZEB1, miR-205 sensitizes cancer cells into radiotherapy and induces DNA damage | [281] | ||
| Breast cancer | MiR-205 sensitizes cancer cells into radiotherapy and prevents DNA repair by ZEB1 down-regulation | [282] | ||
| MiR-205-5p | ZEB1 | Prostatic carcinoma | Suppressing the migration and invasion of cancer cells by ZEB1 down-regulation | [283] |
| MiR-340 | ZEB1/TGF-β | Breast cancer | MiR-340 inhibits ZEB1 to suppress TGF-β-mediated cancer progression | [284] |
| ZEB1 | Osteosarcoma | MiR-340 down-regulates the expression of ZEB1 to sensitize cancer cells into cisplatin-mediated apoptotic cell death | [285] | |
| MiR-409-3p | ZEB1 | Breast cancer | MiR-409-3p binds to the 3′-UTR of ZEB1 to inhibit the progression and metastasis of cancer cells | [286] |
| MiR-429 | ZEB1 | Ovarian cancer | Down-regulation of miR-429 is related to the resistance of cancer cells into cisplatin chemotherapy. Up-regulation of miR-429 suppresses ZEB1 to sensitize cancer cells into apoptosis | [287] |
| Oral squamous cell carcinoma | MiR-429 suppresses the viability and progression of cancer cells via ZEB1 down-regulation | [288] | ||
| Human thyroid cancer | MiR-429 binds to the 3′-UTR to inhibit ZEB1, resulting in suppressing invasion of cancer cells | [110] | ||
| MiR-431 | ZEB1 | Hepatocellular carcinoma | MiR-431 suppresses the migration and invasion capabilities of cancer cells through inhibition of ZEB1-mediated EMT | [289] |
| MiR-448 | ZEB1/2 | Breast cancer | The miR-448 significantly reduces the expressions of ZEB1/2 to inhibit the malignancy and invasion of cancer cells via EMT down-regulation | [290] |
| MiR-455 | ZEB1 | Non-small cell lung cancer | The miR-455 reduces the expression of ZEB1 to inhibit the malignancy of cancer cells | [291] |
| MiR-484 | Smad2/ZEB1 | Cervical cancer | Overexpression of miR-484 inhibits Smad2/ZEB1 to suppress cancer malignancy and miR-484 expression can be considered as a biomarker | [292] |
| MiR-508 | ZEB1 | Renal cell carcinoma | Up-regulation of miR-508 significantly reduces the expression of ZEB1 to inhibit EMT, leading to a decrease in cancer migration and metastasis | [293] |
| MiR-508-3p | ZEB1 | Triple negative breast cancer | Suppressing the invasion and EMT of cancer cells by down-regulation of ZEB1 | [294] |
| MiR-574-3p | ZEB1 | Human gastric carcinoma | MiR-574-3p reduces the expression of ZEB1 by binding into 3′-UTR to decrease the malignancy of cancer cells, and simultaneously, sensitize cancer cells into cisplatin therapy | [295] |
| MiR-590-3p | ZEB1/2 | Glioblastoma multiforme | Decreased invasion and migration of cancer cells by ZEB1/2 down-regulation | [296] |
| MiR-641 | ZEB1 | Cervical cancer | Negatively affecting the proliferation, migration, and invasion of cancer cells through ZEB1 down-regulation | [297] |
| MiR-652 | ZEB1 | Pancreatic cancer | Acidic microenvironment of tumor cells induces EMT through ZEB1 up-regulation. Enhancing the expression of miR-652 inhibits acidic-mediated EMT and ZEB1 induction | [298] |
| MiR-655 | TGF-β/ZEB1 | Pancreatic cancer | MiR-655 inhibits TGF-β/ZEB1 axis to suppress EMT in cancer cells | [299] |
| MiR-675-5p | UBQLN1/ZEB1/miR200 | Pancreatic cancer | The miR-675-5p reduces the malignancy of cancer cells and ZEB1 protein by up-regulation of UBQLN1 and down-regulation of miR-200 | [300] |
| MiR-873-5p | ZEB1 | Colorectal cancer | The inhibitory effect of miR-873-5p on the migration, EMT formation, and invasion of cancer cells is mediated through ZEB1 down-regulation | [301] |
| MiR-875-5p | EGFR/ZEB1 | Prostate cancer | By suppressing EGFR/ZEB1 axis, miR-875-5p inhibits EMT mechanism and sensitizes cancer cells to radiotherapy | [302] |
| MiR-1271 | ZEB1 | Pancreatic cancer | Suppressing the invasion, progression, and EMT in cancer cells by ZEB1 down-regulation | [303] |
| MiR-1236-3p | ZEB1 | High-grade serous ovarian carcinoma | There is a negative relationship between miR-1236-3p and ZEB1 to suppress the migration and invasion of cancer cells | [304] |
| MiR-1236-3p | ZEB1 | Breast cancer | ZEB1 inhibition by miR-1236-3p contributes to the inhibitory effect of this miR on the migration and invasion of cancer cells | [305] |
| MiR-3662 | ZEB1 | Melanoma | Amelioration of invasiveness and malignancy of cancer cells by ZEB1 down-regulation | [306] |
Table 2.
miR/ZEB1 regulation by lncRNAs in different cancers.
| LncRNA | MiR | Down-Stream Target | Cancer Type | Major Outcomes | Refs |
|---|---|---|---|---|---|
| LncRNA DANCR | MiR-33a-5p | ZEB1 | Esophageal squamous cell carcinoma | MiR-33a-5p suppresses cancer malignancy via reducing ZEB1 expression. LncRNA DANCR sponges miR-33a-5p to enhances the invasion via ZEB1 induction | [307] |
| LncRNA SNHG6 | MiR-101-3p | ZEB1 | Hepatocellular carcinoma | LncRNA SNHG6 down-regulates the expression of miR-101-3p to induce ZEB1 and enhance the malignancy of cancer cells | [308] |
| LncRNA PTAR | MiR-101-3p | ZEB1 | Serous ovarian cancer | LncRNA PTAR decreases the expression of miR-101-3p to induce ZEB1 and EMT mechanism, leading to the invasion and metastasis of cancer cells | [309] |
| LncRNA NNT-AS1 | MiR-142-3p | ZEB1 | Breast cancer | Enhancing the progression of cancer cells by sponging miR-142-3p and induction of ZEB1 | [310] |
| LncRNA TUG1 | MiR-142-3p | ZEB1 | Hepatocellular carcinoma | By down-regulation of miR-142-3p, lncRNA TUG1 enhances the expression of ZEB1 to ensure the proliferation and malignancy of cancer cells | [311] |
| LncRNA SNHG16 | MiR-140-5p | ZEB1 | Esophageal squamous cell carcinoma | The lncRNA SNHG16 functions as an oncogenic factor and neutralizes the inhibitory effect of miR-140-5p on ZEB1 to induce EMT and enhance the migration and invasion of cancer cells | [312] |
| MiR-205 | ZEB1 | Osteosarcoma | SNHG16 reduces the expression of miR-205 to elevate the expression of ZEB1, resulting in an increase in the viability, proliferation, and migration of cancer cells | [313] | |
| LncRNA HOTAIR | MiR-217 | ZEB1 | Osteosarcoma | By reducing the expression of miR-217, lncRNA HOTAIR enhances the expression of ZEB1 and improves their malignancy | [314] |
| MiR-23b-3p | ZEB1 | Hepatocellular carcinoma | The miR-23b-3p inhibits ZEB1 and lncRNA HOTAIR prevents the inhibitory effect of miR-23b-3p on ZEB1 to induce EMT | [315] | |
| lncRNA UCA1 | Has-miR-145 | ZEB1/2-FSCN1 | Bladder cancer | There is a reverse relationship between lncRNA UCA1 and has-miR-145. Decreased expression of has-miR-145 enhances the expression of ZEB1/2 and FSCN1 to elevate the migration and invasion of cancer cells | [316] |
| MiR-204-5p | ZEB1 | Glioma cells | By sponging miR-204-5p, lncRNA UCA1 stimulates ZEB1 and activates EMT mechanism | [317] | |
| LncRNA ZEB1-AS1 | MiR-200c/141 | ZEB1 | Glioma cancer | LncRNA ZEB1-AS1 down-regulates the expression of miR-200c/141 to induce ZEB1 and enhance the malignancy and invasion of cancer cells | [318] |
| MiR-409-3p | ZEB1 | Non-small cell lung cancer | A feedback loop is involved, so that lncRNA ZEB1-AS1 induces ZEB1 through miR-409-3p down-regulation, leading to the metastasis and survival of cancer cells | [319] | |
| MiR-101 | ZEB1 | Colorectal cancer | Elevating the proliferation and migration of cancer cells via down-regulation of MiR-101 and up-regulation of ZEB1 by lncRNA ZEB1-AS1 | [320] | |
| LncRNA MIAT | MiR-150-5p | ZEB1 | Osteosarcoma | The miR-150-5p is down-regulated by MIAT to induce ZEB1 and enhance the malignancy of cancer cells | [321] |
| LncRNA MAGI1-IT1 | MiR-200a | ZEB1/2 | Ovarian cancer | Via competitively binding into miR-200a, lncRNA MAGI1-IT1 enhances the expression of ZEB1/2 to ensure the invasion and metastasis of cancer cells | [293] |
| LncRNA HULC | MiR-200a-3p | ZEB1 | Hepatocellular carcinoma | By sequestering miR-200a-3p, lncRNA HULC stimulates ZEB1 to enhance the malignancy and progression of tumor cells | [322] |
| LncRNA NEAT1 | MiR-204 | ZEB1 | Nasopharyngeal carcinoma | MiR-204 inhibits EMT through ZEB1 down-regulation, and lncRNA NEAT1 reverse this axis to enhance the proliferation and viability of cancer cells | [323] |
| LncRNA MINCR | MiR-223 | ZEB1-Akt/PI3K | Nasopharyngeal carcinoma | MINCR induces ZEB1 by sponging miR-223, resulting in activation of Akt/PI3K and resistance of cancer cells into radiotherapy | [324] |
| LncRNA CAT104 | MiR-381 | ZEB1 | Gastric carcinoma | LncRNA CAT104 down-regulates the expression of miR-381 to enhances ZEB1 levels, resulting in enhanced invasion of cancer cells. Additionally, there is a negative feedback loop between ZEB1 and miR-381. | [325] |
| LncRNA ZNF469-3 | MiR-574-5p | ZEB1 | Triple negative breast cancer | The reverse relationship between ZNF469-3 and miR-574-5p paves the road for up-regulation of ZEB1 and subsequent activation of EMT, leading to the cancer progression and malignancy | [326] |
Table 3.
miR/ZEB1 regulation by various molecular pathways in different cancers.
| Upstream Mediator | MiR | Down-Stream Target | Cancer Type | Major Outcomes | Refs |
|---|---|---|---|---|---|
| ELF3 | MiR-141-3p | ZEB1 | Hepatocellular carcinoma | Overexpression of miR-141-3p down-regulates ZEB1. The ELF3 reduces the expression of miR-141-3p to induce ZEB1 and EMT mechanism | [327] |
| SPROUTY-2 | MiR-200/miR-150 | ZEB1 | Colon cancer | By reducing the expression of miR-200/miR-150, SPROUTY-2 induces ZEB1 to facilitate the mesenchymal phenotype acquisition of cancer cells | [286] |
| STAT3 | MiR-200 | ZEB1 | Invasive breast carcinoma | ZEB1 stimulation by miR-200 down-regulation via STAT3-dependent manner enhances the EMT acquisition in cancer cells | [328] |
| TGF-β1 | MiR-200 | ZEB1/2 | Non-small cell lung cancer | The administration of decitabine induces miR-200 expression through TGF-β1 inhibition to down-regulate ZEB1/2, leading to the suppressing EMT and migration of cancer cells | [329] |
| GRHL2 | MiR-200b/a | ZEB1 | Ovarian cancer | GRHL2 down-regulates the expression of ZEB1 by miR-200a/b overexpression to preserve the epithelial phenotype | [330] |
| 53BP1 | MiR-200b and miR-429 | ZEB1 | Breast cancer | The 53BP1 enhances the expression of miR-200b and miR-429 to elevate E-cadherin levels and suppress EMT mechanism through ZEB1 down-regulation | [331] |
| Mel-18 | MiR-205 | ZEB1/2 | Breast cancer | Mel-18 enhances the expression of miR-205 to inhibit ZEB1/2, resulting in decreased progression and invasion of cancer cells | [332] |
| ΔNp63α | MiR-205 | ZEB1 | Cervical squamous cell carcinoma | ΔNp63α alleviates cancer progression and malignancy by enhancing the expression of miR-205, subsequently down-regulating of ZEB1, and consequently, inhibition of EMT, and enhancing E-cadherin levels | [333] |
| KCNQ1OT1 | MiR-217 | ZEB1 | Colorectal cancer | KCNQ1OT1 inhibits miR-217 to stimulate ZEB1 and EMT mechanism in cancer cells. There is a feedback loop, so that ZEB1 also enhances the expression of KCNQ1OT1 to elevate its inhibitory effect on miR-217 | [334] |
| Circ008913 | MiR-889 | DAB2IP/ZEB1 | Skin carcinogenesis | Arsenite down-regulates the expression of circ008913 to up-regulate miR-889. Then, a decrease occurs in DAB2IP to induce ZEB1 and carcinogenesis | [335] |
| Pituitary tumor-transforming gene 1 | MiR-3666 | ZEB1 | Cervical cancer | The expression of miR-3666 reduces to neutralize its inhibitory impact of ZEB1, and consequently, elevate the metastasis and progression of cancer cells | [289] |
Table 4.
ZEB2 regulation by miRs in different cancers.
| MiR | Down-Stream Target | Cancer Type | Major Outcomes | Refs |
|---|---|---|---|---|
| MiR-29b | TET1/ZEB2 | Breast cancer | The miR-29b is an oncogene miR that inhibits TET1 to induce ZEB2 expression, leading to the EMT and colony formation of cancer cells | [336] |
| MiR-30a-5p | ZEB2 | Renal cancer | The miR-30a-5p reduces the expression of ZEB2 to be related with desirable prognosis of cancer cells | [337] |
| MiR-101 | ZEB2 | Osteosarcoma | Suppressing the invasion and proliferation of cancer cells through ZEB2 down-regulation | [175] |
| MiR-124 | ZEB2 | Triple negative breast cancer | MiR-124 diminishes the expression of ZEB2 to inhibit the EMT and invasion of cancer cells | [338] |
| MiR-129 | Wnt-β-catenin/ZEB2 | Non-small cell lung cancer | The miR-129 disrupts Wnt/ZEB2 axis to inhibit EMT | [339] |
| MiR-132 | ZEB2 | Colorectal cancer | Reducing the invasion and metastasis of cancer cells through ZEB2 down-regulation | [340] |
| Lung cancer | Diminishing the migration and invasion of cancer cells through ZEB2 inhibition | [341] | ||
| MiR-138 | ZEB2 | Bladder cancer | The miR-138 binds to the 3′-UTR of ZEB2 to inhibit the metastasis and invasion of cancer cells | [289] |
| MiR-141 | ZEB2 | Hepatocellular carcinoma | The miR-141 decreases the expression of ZEB2 to induce apoptosis and diminish viability and proliferation of cancer cells | [342] |
| Renal cancer | The administration of honokiol is associated with miR-141 induction and subsequent downregulation of ZEB2 to inhibit the malignancy of cancer cells | [343] | ||
| MiR-145 | ZEB2 | Non-small cell lung cancer | MiR-145 acts as an onco-suppressor miR that negatively affects the expression of ZEB2 to inhibit the progression and malignancy of cancer cells | [166] |
| Prostate cancer | There is a negative feedback loop between miR-145 and ZEB2, so that overexpression of miR-145 down-regulates the expression of ZEB2 to ensure the reduced viability and proliferation of cancer cells | [344] | ||
| MiR-145-5p | ZEB2 | Gastric cancer | The miR-145-5p decreases the levels of N-cadherin by ZEB2 down-regulation | [345] |
| MiR-153 | ZEB2 | Ovarian cancer | Acting as an onco-suppressor miR and reduces ZEB2 expression to EMT inhibition | [346] |
| MiR-154 | ZEB2 | Non-small cell lung cancer | The miR-154 exerts an anti-tumor impact by ZEB2 down-regulation | [347] |
| Hepatocellular carcinoma | The miR-154 functions as an onco-suppressor miR by inhibition ZEB2 expression and reducing cancer malignancy and proliferation | [348] | ||
| MiR-155 and FOXP3 | ZEB2 | Colorectal cancer | The miR-155 and FOXP3 inhibit ZEB2 expression to suppress EMT via E-cadherin level up-regulation and Vimentin level downregulation | [307] |
| MiR-187 | ZEB2 | Osteosarcoma | The miR-187 decreases the expression of ZEB2 to inhibit the malignancy and migration of tumor cells | [349] |
| MiR-200 | ZEB1/2 | Ovarian cancer | The cancer cells acquire an epithelial phenotype by enhancing the expression of miR-200 and subsequent inhibition of ZEB1 and ZEB2 proteins | [350] |
| ZEB2 | Breast cancer | As an onco-suppressor miR, miR-200 decreases the expression of ZEB2 and its targets gene Snail1 to induce mesenchymal to epithelial transition | [351] | |
| MiR-200a | ZEB2 | Nasopharyngeal carcinoma | Suppressing the growth and invasion of cancer cells through ZEB2 down-regulation | [352] |
| Hepatocellular carcinoma | The miR-200a diminishes the expression of ZEB2 to suppress EMT and invasion of cancer cells | [353] | ||
| Ovarian cancer | The miR-200a increases the levels of E-cadherin by EMT inhibition and ZEB2 down-regulation | [354] | ||
| MiR-200b | ZEB2 | Gastric carcinoma | Inhibition of ZEB2 by miR-200b suppresses invasion, metastasis, and migration of cancer cells | [355] |
| Glioma | Reducing the growth and metastasis of ZEB2 inhibition | [356] | ||
| MiR-200c | ZEB2 | Ovarian cancer | MiR-200c reduces the expression of ZEB2 to inhibit EMT by enhancing E-cadherin levels and reducing Vimentin levels | [357] |
| Non-small cell lung cancer | The miR-200c inhibits EMT mechanism by ZEB2 down-regulation | [358] | ||
| MiR-200c-3p | ZEB2 | Prostate carcinoma | The miR-200c-3p functions as an anti-tumor miR that inhibits the progression and invasion of cancer cells through ZEB2 down-regulation | [359] |
| MiR-203 | ZEB2 | Lung adenocarcinoma and nasopharyngeal carcinoma | MiR-203 enhances the efficacy of cisplatin in chemotherapy and eradication of cancer cells, and also inhibits their invasion by EMT down-regulation through ZEB2 inhibition | [360,361] |
| MiR-205 | ZEB2 | Renal cell carcinoma | The miR-205 is related to the favorable prognosis and reduced invasion of cancer cells through ZEB2 down-regulation | [362] |
| MiR-206 | ZEB2 | Renal cancer | Decreasing the proliferation of tumor cells through ZEB2 down-regulation | [363] |
| MiR-211-5p | ZEB2 | Hepatocellular carcinoma | The miR-211-5p suppresses the metastasis of cancer cells via ZEB2 down-regulation | [215] |
| MiR-215 | ZEB2 | Non-small cell lung cancer | The in vitro and in vivo experiments demonstrate the potential of miR-215 in down-regulation of ZEB2 and suppressing the invasion, progression, and malignancy of cancer cells, and induction of apoptotic cell death | [364] |
| MiR-335 | ZEB2 | Colorectal cancer | The inhibition of metastasis and invasion of cancer cells through ZEB2 down-regulation | [365] |
| Papillary thyroid cancer | Through reducing the expression of ZEB2, miR-335 suppresses the growth and metastasis of cancer cells | [366] | ||
| MiR-338-3p | ZEB2 | Gastric cancer | MiR-338-3p diminishes the expression of ZEB2 to inhibit EMT in cancer cells | [367] |
| MiR-454-3p and miR-374b-5p | ZEB2 | Bladder cancer | Reducing the expression of ZEB2 significantly decreases the migration and invasion of cancer cells | [325] |
| MiR-506 | ZEB2 | Gastric carcinoma | The miR-506 suppresses metastasis through ZEB2 down-regulation | [130] |
| MiR-545 | Wnt-β-catenin/ZEB2 | Non-small cell lung cancer | The miR-545 reduces the expression of Wnt/β−catenin to down-regulate the expression of ZEB2, leading to the decreased migration and invasion of cancer cells | [368] |
| MiR-598 | ZEB2 | Non-small cell lung cancer | The in vitro experiment demonstrated that miR-598 decreases the expression of ZEB2 to inhibit the migration and metastasis of cancer cells | [369] |
| MiR-622 | ZEB2 | Glioma | The increased expression of miR-622 is related to the desirable prognosis via ZEB2 down-regulation | [370] |
| MiR-769-3p | Wnt-β-catenin/ZEB2 | Glioma | The miR-769-3p down-regulates the expression of Wnt and inhibits nuclear translocation of β−catenin to suppress ZEB2, leading to the decreased viability, proliferation and invasion of cancer cells | [371] |
| MiR-940 | ZEB2 | Glioma | Inhibition of cancer progression and EMT through ZEB2 down-regulation | [372] |
| MiR-1179 | ZEB2 | Hepatocellular carcinoma | The miR-1179 reduces the expression of ZEB2 to inhibit cancer progression and malignancy | [373] |
| MiR-3653 | ZEB2 | Colon cancer | Suppressing metastasis and EMT by inhibition of ZEB2 | [171] |
Table 5.
MiR/ZEB2 regulation by lncRNAs in different cancers.
| LncRNA | MiR | Down-Stream Target | Cancer Type | Major Outcomes | Refs |
|---|---|---|---|---|---|
| LncRNA TUG1 | MiR-142 | ZEB2 | Bladder cancer | The lncRNA TUG1 stimulates ZEB2 through miR-142 down-regulation to inhibit apoptosis and enhance the proliferation of cancer cells | [374] |
| LncRNA ROR | MiR-145 | ZEB2 | Hepatocellular carcinoma | The lncRNA ROR elevates the expression of ZEB2 through miR-145 sponging to inhibit the EMT and malignancy of cancer cells | [375] |
| LncRNA MALAT1 | MiR-200s | ZEB2 | Kidney carcinoma | The lncRNA MALAT1 induces ZEB2 via miR-200s sponging, predisposing cancer cells into growth and proliferation | [376] |
| MiR-204 | ZEB2 | Breast cancer | The negative relationship between MALAT1 and miR-204 results in ZEB2 induction to enhance the migration and invasion of cancer cells | [377] | |
| LncRNA UCA1 | MiR-203 | ZEB2 | Gastric cancer | This lncRNA sponges miR-203 to induce ZEB2, leading to the enhanced malignancy, invasion, and proliferation of tumor cells | [187] |
| LncRNA SNHG5 | MiR-205-5p | ZEB2 | Glioma | LncRNA SNHG5 stimulates ZEB2 by sponging miR-205-5p to elevate the proliferation of cancer cells | [194] |
| LncRNA UICLM | MiR-215 | ZEB2 | Colorectal cancer | The in vivo and in vitro experiments demonstrated that lncRNA induces ZEB2 via miR-215 down-regulation to enhance the migration and malignancy of cancer cells | [378] |
| LncRNA SNHG12 | MiR-218 | Slug/ZEB2 | Non-small cell lung cancer | MiR-218 inhibits Slug/ZEB2 axis to suppress EMT in cancer cells. LncRNA SNHG16 activates Slug/ZEB2 axis through miR-218 sponging | [188] |
| LncRNA XIST | MiR-367 and miR-141 | ZEB2 | Non-small cell lung cancer | The lncRNA XIST up-regulates the expression of ZEB2 by inhibition of miR-367 and miR-141, leading to the TGF- β-induced EMT | [379] |
| LncRNA UCA1 | MiR-498 | ZEB2 | Esophageal cancer | The lncRNA UCA1 inhibits ZEB2 via miR-498 down-regulation to suppress the migration, proliferation, invasion, and EMT | [7] |
| LncRNA CTS | MiR-505 | ZEB2 | Cervical cancer | Down-regulation of miR-505 by CTS is associated with increased malignancy of cancer cells through ZEB2 induction | [186] |
| LncRNA HOTAIRM1 | MiR-873-5p | ZEB2 | Glioma | LncRNA HOTAIRM1 decreases the expression of miR-873-5p by sponging to up-regulate the expression of ZEB2, leading to an increase in progression of glioma cells and a decrease in apoptotic cell death | [179] |
Table 6.
miR/ZEB2 regulation by various molecular pathways in different cancers.
| Upstream Mediator | MiR | Down-Stream Target | Cancer Type | Major Outcomes | Refs |
|---|---|---|---|---|---|
| P53 | MiR-30a | ZEB2 | Breast cancer | P53 stimulates the expression of miR-30a to upregulate ZEB2, resulting in reduced viability, proliferation, and invasion of cancer cells | [169] |
| EZH2-DNMT1 | MiR-142-3p | ZEB2 | Nasopharyngeal carcinoma | The EZH2-DNMT1 induces ZEB2 through miR-142-3p sponging, resulting in an increase in cancer progression | [380] |
| CircNUP214 | MiR-145 | ZEB2 | Thyroid cancer | CircNUP214 induces ZEB2 through miR-145 down-regulation to enhance the malignancy and progression of cancer cells | [381] |
| CircPCNXL2 | MiR-153 | ZEB2 | Renal cancer | The circPCNXL2 stimulates the expression of ZEB2 through miR-153 down-regulation to suppress the malignancy and invasion of cancer cells | [201] |
| FOXP3 | MiR-155 | ZEB2 | Human breast cancer | FOXP3 and miR-155 synergistically down-regulate the expression of ZEB2 to diminish the invasiveness of cancer cells | [382] |
| Akt/ERK | MiR-200c | ZEB2 | Gastric cancer | The inhibition of Akt/ERK enhances the expression of miR-200c to suppress IGF-I-mediated ZEB2, leading to the reduced invasion and EMT of cancer cells | [383] |
| β1 integrin | TGF-β/miR-200 | ZEB2 | Triple negative breast cancer | Enhancing the expression of β1 integrin reduces the metastasis of cancer cells into lung. This is followed by disrupting TFG−β/miR-200/ZEB2, elevating the E-cadherin levels, and restoring the cohesion of cells | [384] |
| CircZFR | MiR-377 | ZEB2 | Bladder cancer | Enhanced progression and malignancy of cancer cells result from down-regulation of miR-377 by circZFR and subsequent induction of ZEB2 | [196] |
| Hsa-circ-0004771 | MiR-653 | ZEB2 | Breast cancer | MiR-653 reduces the expression of ZEB2 and is associated with desirable prognosis. Hsa-circ-0004771 diminishes miR-653 expression to induce ZEB2, leading to the inhibition of apoptosis and enhanced migration and invasion of cancer cells | [197] |
Figure 4.
How tumor microenvironment components are affected by the relationship between miRs and ZEB proteins, and their regulation by lncRNAs.
7. Conclusions
In this article, we provided a comprehensive review about the relationship between miRs and ZEB family in cancer cells and how this relationship affects the progression and metastasis of tumor cells. After miRs discovery, an exponential amount of research has been performed to understand their role in different biological processes such as cell differentiation, apoptosis, and migration. We typically observe aberrant miR expression in cancer cells and restoring the normal expression of miRs may be crucial in cancer therapy. It is also vital to explore the relevance of ZEB1 and ZEB2 proteins in cancer therapy. It has been reported that ZEB proteins are able to enhance the proliferation and malignancy of tumor cells. One of the most important pathways affected by ZEB proteins is the EMT mechanism. It appears that induction of EMT by ZEB proteins not only enhances the progression and metastasis of cancer cells, but also stimulates drug resistance. Therefore, revealing the underlying molecular pathways involved in ZEB regulation can be beneficial for further studies in the field of cancer therapy and elevating the efficacy of chemotherapy. In this review, we also detailed how and which miRs affect ZEB proteins in various cancers. We consolidated the factors that may function as upstream modulators to negatively affect miRs, leading to the induction of ZEB expression. As it is shown in Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6, lncRNAs and circRNAs can act as oncogenic factors. These upstream mediators induce and enhance the expression of ZEB1 and -2 through sponging their target miRs, resulting in an increase in malignancy and invasion of tumor cells. Identification of these factors and further targeting of them can significantly diminish the malignancy of tumor cells and pave the road for the effective cancer therapy. Finally, we highlighted ZEB1’s role in immunosuppression. Through it, we identified a crucial knowledge gap wherein the relationship between miRs, ZEB2, and immune cells in the cancer context is still a mystery. In all, we dissected the different effects of miR on ZEB proteins, which may in turn help us develop better treatment strategies in attenuating metastasis of cancer cells.
Abbreviations
EMT, epithelial-to-mesenchymal transition; PCD, programmed cell death; MET, mesenchymal-to-epithelial transition; TGF-β, transforming growth factor-beta; EMT-TFs, EMT-promoting transcription factors; ZEB, zinc finger E-box-binding homeobox; CZF, carboxy-terminal zinc finger cluster; CtBP, C-terminal binding protein; CRC, colorectal cancer; DAXX, death domain-associated protein; ROCK2, Rho associated coiled-coil containing protein kinase 2; NSCLC, non-small cell lung cancer; MTBP, MDM2 binding protein; IDO1, indoleamine-2,3-dioxygenase-1; MMP, matrix metalloproteinase; PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase B; ncRNAs, non-coding RNAs; SnoRNAs, small nucleolar RNAs; 3′-UTR, 3′-untranslated region; mRNA, messenger RNA; pri-miR, primary miR; SIRT1, sirtuin 1; FOXO3a, Forkhead box O3; NOB1, nin one binding protein; PD-L1, programmed death ligand 1; OSCC, oral squamous cell carcinoma; mTOR, mammalian target of rapamycin; lncRNAs, long non-coding RNAs; ceRNA, competitive endogenous RNA; NEAT1, Nuclear Enriched Abundant Transcript 1; RCC, renal cell carcinoma; HLA, human leukocyte antigen; HCP5, HLA complex 5; HOTTIP, HOXA distal transcript antisense RNA; α-SMA, α-smooth muscle actin; circRNAs, circular RNAs; LUAD, lung adenocarcinoma; TNBC, triple negative breast cancer; HCC, hepatocellular carcinoma; MDR, multidrug resistance; P-gp, P-glycoprotein; PTX, paclitaxel; YAP, Yes-associated protein; PCAT1, prostate cancer-associated transcription 1; VEGF, vascular endothelial growth factor; ARRDC3, arrestin domain containing 3; CD8+ TILs, CD8+ tumor infiltrating lymphocytes; DLBCL, diffuse large B cell lymphoma.
Author Contributions
M.A., H.L.A., E.R.M., S.M., V.Z., K.H., S.S., A.Z., and M.N. did the literature review presented in this study and wrote the manuscript. A.Z. and M.N. did the figures. E.R.M., S.M., V.Z., K.H., and S.S. did the tables. R.M. and A.P.K. conceived the idea for this review and directed the entire study. R.M., H.L.A., and A.P.K. finalized this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
A.P.K. is supported by the National Medical Research Council of Singapore. A.P.K. is also supported by the National Medical Research Council of Singapore and the Singapore Ministry of Education under its Research Centres of Excellence initiative to Cancer Science Institute of Singapore, National University of Singapore.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wu X., Xin Z., Zou Z., Lu C., Yu Z., Feng S., Pan P., Hao G., Dong Y., Yang Y. Seminars in Cancer Biology. Elsevier; Amsterdam, The Netherlands: 2019. SRY-related high-mobility-group box 4: Crucial regulators of the EMT in cancer. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y., Zhou B.P. Epithelial-mesenchymal transition—a hallmark of breast cancer metastasis. Cancer Hallm. 2013;1:38–49. doi: 10.1166/ch.2013.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz V.M., de Herreros A.G. Seminars in Cancer Biology. Elsevier; Amsterdam, The Netherlands: 2016. F-box proteins: Keeping the epithelial-to-mesenchymal transition (EMT) in check; pp. 71–79. [DOI] [PubMed] [Google Scholar]
- 4.Kar R., Jha N.K., Jha S.K., Sharma A., Dholpuria S., Asthana N., Chaurasiya K., Singh V.K., Burgee S., Nand P. A “NOTCH” Deeper into the Epithelial-To-Mesenchymal Transition (EMT) Program in Breast Cancer. Genes. 2019;10:961. doi: 10.3390/genes10120961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seccia T., Caroccia B., Piazza M., Rossi G.P. The Key Role of Epithelial to Mesenchymal Transition (EMT) in Hypertensive Kidney Disease. Int. J. Mol. Sci. 2019;20:3567. doi: 10.3390/ijms20143567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty S., Mir K.B., Seligson N.D., Nayak D., Kumar R., Goswami A. Integration of EMT and cellular survival instincts in reprogramming of programmed cell death to anastasis. Cancer Metastasis Rev. 2020;39:553–566. doi: 10.1007/s10555-020-09866-x. [DOI] [PubMed] [Google Scholar]
- 7.Wang P., Liu X., Han G., Dai S., Ni Q., Xiao S., Huang J. Downregulated lncRNA UCA1 acts as ceRNA to adsorb microRNA-498 to repress proliferation, invasion and epithelial mesenchymal transition of esophageal cancer cells by decreasing ZEB2 expression. Cell Cycle. 2019;18:2359–2376. doi: 10.1080/15384101.2019.1648959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seton-Rogers S. Epithelial–mesenchymal transition: Untangling EMT’s functions. Nat. Rev. Cancer. 2015;16:1. doi: 10.1038/nrc.2015.6. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz M., Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Martinez R., Ramkumar N., Anderson K.V. p120-catenin regulates WNT signaling and EMT in the mouse embryo. Proc. Natl. Acad. Sci. USA. 2019;116:16872–16881. doi: 10.1073/pnas.1902843116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moly P.K., Cooley J.R., Zeltzer S.L., Yatskievych T.A., Antin P.B. Gastrulation EMT is independent of P-cadherin downregulation. PLoS ONE. 2016;11:e0153591. doi: 10.1371/journal.pone.0153591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J., Zheng Y., Zhang H., Liu Y., Sun H., Zhang P. Galectin-1 induces metastasis and epithelial-mesenchymal transition (EMT) in human ovarian cancer cells via activation of the MAPK JNK/p38 signalling pathway. Am. J. Transl. Res. 2019;11:3862–3878. [PMC free article] [PubMed] [Google Scholar]
- 13.Chi Y., Wang F., Zhang T., Xu H., Zhang Y., Shan Z., Wu S., Fan Q., Sun Y. miR-516a-3p inhibits breast cancer cell growth and EMT by blocking the Pygo2/Wnt signalling pathway. J. Cell. Mol. Med. 2019;23:6295–6307. doi: 10.1111/jcmm.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou P., Li Y., Li B., Zhang M., Liu Y., Yao Y., Li D. NMIIA promotes tumor growth and metastasis by activating the Wnt/β-catenin signaling pathway and EMT in pancreatic cancer. Oncogene. 2019;38:5500–5515. doi: 10.1038/s41388-019-0806-6. [DOI] [PubMed] [Google Scholar]
- 15.Liu W., Yang Y.J., An Q. LINC00963 Promotes Ovarian Cancer Proliferation, Migration and EMT via the miR-378g/CHI3L1 Axis. Cancer Manag. Res. 2020;12:463–473. doi: 10.2147/CMAR.S229083. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Schulz A., Gorodetska I., Behrendt R., Fuessel S., Erdmann K., Foerster S., Datta K., Mayr T., Dubrovska A., Muders M.H. Linking NRP2 With EMT and Chemoradioresistance in Bladder Cancer. Front. Oncol. 2019;9:1461. doi: 10.3389/fonc.2019.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afzal Ashaie M., Hoque Chowdhury E. Cadherins: The superfamily critically involved in breast cancer. Curr. Pharm. Des. 2016;22:616–638. doi: 10.2174/138161282205160127095338. [DOI] [PubMed] [Google Scholar]
- 18.Carpinelli M.R., de Vries M.E., Auden A., Butt T., Deng Z., Partridge D.D., Miles L.B., Georgy S.R., Haigh J.J., Darido C., et al. Inactivation of Zeb1 in GRHL2-deficient mouse embryos rescues mid-gestation viability and secondary palate closure. Dis. Models Mech. 2020 doi: 10.1242/dmm.042218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho H.J., Oh N., Park J.H., Kim K.S., Kim H.K., Lee E., Hwang S., Kim S.J., Park K.S. ZEB1 Collaborates with ELK3 to Repress E-Cadherin Expression in Triple-Negative Breast Cancer Cells. Mol. Cancer Res. MCR. 2019;17:2257–2266. doi: 10.1158/1541-7786.MCR-19-0380. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimoto S., Tanaka F., Morita H., Hiraki A., Hashimoto S. Hypoxia-induced HIF-1alpha and ZEB1 are critical for the malignant transformation of ameloblastoma via TGF-beta-dependent EMT. Cancer Med. 2019;8:7822–7832. doi: 10.1002/cam4.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang W., Li Z., Dong X., Zhao N., Liu Y., Wang C., Chen J. Schisandrin B inhibits TGF-beta1-induced epithelial-mesenchymal transition in human A549 cells through epigenetic silencing of ZEB1. Exp. Lung Res. 2019;45:157–166. doi: 10.1080/01902148.2019.1631906. [DOI] [PubMed] [Google Scholar]
- 22.Soen B., Vandamme N., Berx G., Schwaller J., Van Vlierberghe P., Goossens S. ZEB Proteins in Leukemia: Friends, Foes, or Friendly Foes? HemaSphere. 2018;2:e43. doi: 10.1097/HS9.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comijn J., Berx G., Vermassen P., Verschueren K., van Grunsven L., Bruyneel E., Mareel M., Huylebroeck D., van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol. Cell. 2001;7:1267–1278. doi: 10.1016/S1097-2765(01)00260-X. [DOI] [PubMed] [Google Scholar]
- 24.Verschueren K., Remacle J.E., Collart C., Kraft H., Baker B.S., Tylzanowski P., Nelles L., Wuytens G., Su M.T., Bodmer R., et al. SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5’-CACCT sequences in candidate target genes. J. Biol. Chem. 1999;274:20489–20498. doi: 10.1074/jbc.274.29.20489. [DOI] [PubMed] [Google Scholar]
- 25.Remacle J.E., Kraft H., Lerchner W., Wuytens G., Collart C., Verschueren K., Smith J.C., Huylebroeck D. New mode of DNA binding of multi-zinc finger transcription factors: deltaEF1 family members bind with two hands to two target sites. EMBO J. 1999;18:5073–5084. doi: 10.1093/emboj/18.18.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Y., Sawada J.-i., Sui G., Affar E.B., Whetstine J.R., Lan F., Ogawa H., Luke M.P.-S., Nakatani Y., Shi Y. Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature. 2003;422:735–738. doi: 10.1038/nature01550. [DOI] [PubMed] [Google Scholar]
- 27.Zhang P., Sun Y., Ma L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14:481–487. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shargh S.A., Sakizli M., Khalaj V., Movafagh A., Yazdi H., Hagigatjou E., Sayad A., Mansouri N., Mortazavi-Tabatabaei S.A., Khorshid H.R.K. Downregulation of E-cadherin expression in breast cancer by promoter hypermethylation and its relation with progression and prognosis of tumor. Med. Oncol. 2014;31:250. doi: 10.1007/s12032-014-0250-y. [DOI] [PubMed] [Google Scholar]
- 29.Peiffer D.S., Wyatt D., Zlobin A., Piracha A., Ng J., Dingwall A.K., Albain K.S., Osipo C. DAXX Suppresses Tumor-Initiating Cells in Estrogen Receptor–Positive Breast Cancer Following Endocrine Therapy. Cancer Res. 2019;79:4965–4977. doi: 10.1158/0008-5472.CAN-19-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang F., Ren C., Wang J., Wang S., Yang L., Han X., Chen Y., Tong G., Yang G. The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncogenesis. 2019;8:1–15. doi: 10.1038/s41389-019-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y., Zhou Y., He J., Sun H., Jin Z. Long non-coding RNA H19 mediates ovarian cancer cell cisplatin-resistance and migration during EMT. Int. J. Clin. Exp. Pathol. 2019;12:2506. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y., Zhou Y., Wang K., Li T., Zhang M., Yang Y., Wang R., Hu R. ROCK2 Confers Acquired Gemcitabine Resistance in Pancreatic Cancer Cells by Upregulating Transcription Factor ZEB1. Cancers. 2019;11:1881. doi: 10.3390/cancers11121881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun S., Yang X., Qin X., Zhao Y. TCF4 promotes colorectal cancer drug resistance and stemness via regulating ZEB1/ZEB2 expression. Protoplasma. 2020 doi: 10.1007/s00709-020-01480-6. [DOI] [PubMed] [Google Scholar]
- 34.Orellana-Serradell O., Herrera D., Castellon E.A., Contreras H.R. The transcription factor ZEB1 promotes chemoresistance in prostate cancer cell lines. Asian J. Androl. 2019;21:460–467. doi: 10.4103/aja.aja_1_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan B., Han H., Wu L., Xiong Y., Zhang J., Dong B., Yang Y., Chen J. MTBP promotes migration and invasion by regulation of ZeB2-mediated epithelial–mesenchymal transition in lung cancer cells. OncoTargets Ther. 2018;11:6741. doi: 10.2147/OTT.S167963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai Y.-S., Jou Y.-C., Tsai H.-T., Cheong I.-S., Tzai T.-S. Urologic Oncology: Seminars and Original Investigations. Elsevier; Amsterdam, The Netherlands: 2019. Indoleamine-2, 3-dioxygenase-1 expression predicts poorer survival and up-regulates ZEB2 expression in human early stage bladder cancer. [DOI] [PubMed] [Google Scholar]
- 37.Yalim-Camci I., Balcik-Ercin P., Cetin M., Odabas G., Tokay N., Sayan A.E., Yagci T. ETS1 is coexpressed with ZEB2 and mediates ZEB2-induced epithelial-mesenchymal transition in human tumors. Mol. Carcinog. 2019;58:1068–1081. doi: 10.1002/mc.22994. [DOI] [PubMed] [Google Scholar]
- 38.Wu D.M., Zhang T., Liu Y.B., Deng S.H., Han R., Liu T., Li J., Xu Y. The PAX6-ZEB2 axis promotes metastasis and cisplatin resistance in non-small cell lung cancer through PI3K/AKT signaling. Cell Death Dis. 2019;10:349. doi: 10.1038/s41419-019-1591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ElKhouly A.M., Youness R., Gad M. MicroRNA-486-5p and MicroRNA-486-3p: Multifaceted Pleiotropic Mediators in Oncological and Non-Oncological Conditions. Non-coding RNA Res. 2020;5:11–21. doi: 10.1016/j.ncrna.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delihas N. Discovery and characterization of the first non-coding RNA that regulates gene expression, micF RNA: A historical perspective. World J. Biol. Chem. 2015;6:272. doi: 10.4331/wjbc.v6.i4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anastasiadou E., Jacob L.S., Slack F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer. 2018;18:5. doi: 10.1038/nrc.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X., Tang F.R., Arfuso F., Cai W.Q., Ma Z., Yang J., Sethi G. The Emerging Role of Long Non-Coding RNAs in the Metastasis of Hepatocellular Carcinoma. Biomolecules. 2019;10:66. doi: 10.3390/biom10010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Z., Wang Y.Y., Xin H.W., Wang L., Arfuso F., Dharmarajan A., Kumar A.P., Wang H., Tang F.R., Warrier S., et al. The expanding roles of long non-coding RNAs in the regulation of cancer stem cells. Int. J. Biochem. Cell Biol. 2019;108:17–20. doi: 10.1016/j.biocel.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Cheng J.T., Wang L., Wang H., Tang F.R., Cai W.Q., Sethi G., Xin H.W., Ma Z. Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells. 2019;8:1178. doi: 10.3390/cells8101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pourhanifeh M.H., Mahjoubin-Tehran M., Karimzadeh M.R., Mirzaei H.R., Razavi Z.S., Sahebkar A., Hosseini N., Mirzaei H., Hamblin M.R. Autophagy in cancers including brain tumors: Role of MicroRNAs. Cell Commun. Signal. 2020;18:88. doi: 10.1186/s12964-020-00587-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yousefi F., Shabaninejad Z., Vakili S., Derakhshan M., Movahedpour A., Dabiri H., Ghasemi Y., Mahjoubin-Tehran M., Nikoozadeh A., Savardashtaki A., et al. TGF-β and WNT signaling pathways in cardiac fibrosis: Non-coding RNAs come into focus. Cell Commun. Signal. 2020;18:87. doi: 10.1186/s12964-020-00555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jamali Z., Taheri-Anganeh M., Shabaninejad Z., Keshavarzi A., Taghizadeh H., Razavi Z.S., Mottaghi R., Abolhassan M., Movahedpour A., Mirzaei H. Autophagy regulation by microRNAs: Novel insights into osteosarcoma therapy. IUBMB Life. 2020;72:1306–1321. doi: 10.1002/iub.2277. [DOI] [PubMed] [Google Scholar]
- 48.Naeli P., Yousefi F., Ghasemi Y., Savardashtaki A., Mirzaei H. The Role of MicroRNAs in Lung Cancer: Implications for Diagnosis and Therapy. Curr. Mol. Med. 2020;20:90–101. doi: 10.2174/1566524019666191001113511. [DOI] [PubMed] [Google Scholar]
- 49.Losko M., Kotlinowski J., Jura J. Long noncoding RNAs in metabolic syndrome related disorders. Mediat. Inflamm. 2016;2016:5365209. doi: 10.1155/2016/5365209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goh J.N., Loo S.Y., Datta A., Siveen K.S., Yap W.N., Cai W., Shin E.M., Wang C., Kim J.E., Chan M., et al. microRNAs in breast cancer: Regulatory roles governing the hallmarks of cancer. Biol. Rev. Camb. Philos. Soc. 2016;91:409–428. doi: 10.1111/brv.12176. [DOI] [PubMed] [Google Scholar]
- 51.Varghese E., Liskova A., Kubatka P., Samuel S.M., Büsselberg D. Anti-Angiogenic Effects of Phytochemicals on miRNA Regulating Breast Cancer Progression. Biomolecules. 2020;10:191. doi: 10.3390/biom10020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magalhaes M., Jorge J., Goncalves A.C., Sarmento-Ribeiro A.B., Carvalho R., Figueiras A., Santos A.C., Veiga F. miR-29b and retinoic acid co-delivery: A promising tool to induce a synergistic antitumoral effect in non-small cell lung cancer cells. Drug Deliv. Transl. Res. 2020 doi: 10.1007/s13346-020-00768-7. [DOI] [PubMed] [Google Scholar]
- 53.Ragheb M.A., Soliman M.H., Elzayat E.M., Mohamed M.S., El-Ekiaby N., Abdelaziz A.I., Abdelwahab A.A. MiR-520c-3p Modulates Doxorubicin-chemosensitivity in HepG2 cells. Anti-Cancer Agents Med. Chem. 2020 doi: 10.2174/1871520620666200502004817. [DOI] [PubMed] [Google Scholar]
- 54.Taherdangkoo K., Kazemi Nezhad S.R., Hajjari M.R., Tahmasebi Birgani M. miR-485-3p suppresses colorectal cancer via targeting TPX2. Bratisl. Lek. Listy. 2020;121:302–307. doi: 10.4149/bll_2020_048. [DOI] [PubMed] [Google Scholar]
- 55.Park J.H., Jeong G.H., Lee K.S., Lee K.H., Suh J.S., Eisenhut M., van der Vliet H.J., Kronbichler A., Stubbs B., Solmi M., et al. Genetic variations in MicroRNA genes and cancer risk: A field synopsis and meta-analysis. Eur. J. Clin. Investig. 2020 doi: 10.1111/eci.13203. [DOI] [PubMed] [Google Scholar]
- 56.Salomão K.B., Pezuk J.A., de Souza G.R., Chagas P., Pereira T.C., Valera E.T., Brassesco M.S. MicroRNA dysregulation interplay with childhood abdominal tumors. Cancer Metastasis Rev. 2019;38:783–811. doi: 10.1007/s10555-019-09829-x. [DOI] [PubMed] [Google Scholar]
- 57.Seo H.A., Moeng S., Sim S., Kuh H.J., Choi S.Y., Park J.K. MicroRNA-Based Combinatorial Cancer Therapy: Effects of MicroRNAs on the Efficacy of Anti-Cancer Therapies. Cells. 2020;9:29. doi: 10.3390/cells9010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiao X., Qian X., Wu L., Li B., Wang Y., Kong X., Xiong L. microRNA: The Impact on Cancer Stemness and Therapeutic Resistance. Cells. 2020;9:8. doi: 10.3390/cells9010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu J., Bao J., Kim M., Yuan S., Tang C., Zheng H., Mastick G.S., Xu C., Yan W. Two miRNA clusters, miR-34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc. Natl. Acad. Sci. USA. 2014;111:E2851–E2857. doi: 10.1073/pnas.1407777111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen C.Z., Schaffert S., Fragoso R., Loh C. Regulation of immune responses and tolerance: The micro RNA perspective. Immunol. Rev. 2013;253:112–128. doi: 10.1111/imr.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Follert P., Cremer H., Béclin C. MicroRNAs in brain development and function: A matter of flexibility and stability. Front. Mol. Neurosci. 2014;7:5. doi: 10.3389/fnmol.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takahashi R.-u., Miyazaki H., Ochiya T. The role of microRNAs in the regulation of cancer stem cells. Front. Genet. 2014;4:295. doi: 10.3389/fgene.2013.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raza U., Zhang J.D., Şahin Ö. MicroRNAs: Master regulators of drug resistance, stemness, and metastasis. J. Mol. Med. 2014;92:321–336. doi: 10.1007/s00109-014-1129-2. [DOI] [PubMed] [Google Scholar]
- 64.Filipowicz W., Bhattacharyya S.N., Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 65.Eulalio A., Huntzinger E., Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132:9–14. doi: 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 66.Sartorius K., Makarova J., Sartorius B., An P., Winkler C., Chuturgoon A., Kramvis A. The Regulatory Role of MicroRNA in Hepatitis-B Virus-Associated Hepatocellular Carcinoma (HBV-HCC) Pathogenesis. Cells. 2019;8:1504. doi: 10.3390/cells8121504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Syed S.N., Frank A.C., Raue R., Brune B. MicroRNA-A Tumor Trojan Horse for Tumor-Associated Macrophages. Cells. 2019;8:1482. doi: 10.3390/cells8121482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Handa H., Murakami Y., Ishihara R., Kimura-Masuda K., Masuda Y. The Role and Function of microRNA in the Pathogenesis of Multiple Myeloma. Cancers. 2019;11:1738. doi: 10.3390/cancers11111738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cosentino G., Plantamura I., Cataldo A., Iorio M.V. MicroRNA and Oxidative Stress Interplay in the Context of Breast Cancer Pathogenesis. Int. J. Mol. Sci. 2019;20:5143. doi: 10.3390/ijms20205143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo Y., Jia Y., Wang S., Liu N., Gao D., Zhang L., Lin Z., Wang S., Kong F., Peng C. Downregulation of MUTYH contributes to cisplatin-resistance of esophageal squamous cell carcinoma cells by promoting Twist-mediated EMT. Oncol. Rep. 2019;42:2716–2727. doi: 10.3892/or.2019.7347. [DOI] [PubMed] [Google Scholar]
- 71.Sun C.M., Zhang G.M., Qian H.N., Cheng S.J., Wang M., Liu M., Li D. MiR-1266 suppresses the growth and metastasis of prostate cancer via targeting PRMT5. Eur. Rev. Med. Pharmacol. Sci. 2019;23:6436–6444. doi: 10.26355/eurrev_201908_18525. [DOI] [PubMed] [Google Scholar]
- 72.Xia X.Y., Yu Y.J., Ye F., Peng G.Y., Li Y.J., Zhou X.M. MicroRNA-506-3p inhibits proliferation and promotes apoptosis in ovarian cancer cell via targeting SIRT1/AKT/FOXO3a signaling pathway. Neoplasma. 2020;67:344–353. doi: 10.4149/neo_2020_190517N441. [DOI] [PubMed] [Google Scholar]
- 73.Jin Y., Zhou X., Yao X., Zhang Z., Cui M., Lin Y. MicroRNA-612 inhibits cervical cancer progression by targeting NOB1. J. Cell. Mol. Med. 2020;24:3149–3156. doi: 10.1111/jcmm.14985. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Nong K., Zhang D., Chen C., Yang Y., Yang Y., Liu S., Cai H. MicroRNA-519 inhibits hypoxia-induced tumorigenesis of pancreatic cancer by regulating immune checkpoint PD-L1. Oncol. Lett. 2020;19:1427–1433. doi: 10.3892/ol.2019.11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.ZHAO L., YU A., ZHANG Y., WANG X., HAN B., WANG X. MicroRNA-149 suppresses the malignant phenotypes of ovarian cancer via downregulation of MSI2 and inhibition of PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24:55–64. doi: 10.26355/eurrev_202001_19895. [DOI] [PubMed] [Google Scholar]
- 76.Allahverdi A., Arefian E., Soleimani M., Ai J., Nahanmoghaddam N., Yousefi-Ahmadipour A., Ebrahimi-Barough S. MicroRNA-4731-5p delivered by AD-mesenchymal stem cells induces cell cycle arrest and apoptosis in glioblastoma. J. Cell. Physiol. 2020 doi: 10.1002/jcp.29472. [DOI] [PubMed] [Google Scholar]
- 77.Guo X., Piao H., Zhang Y., Sun P., Yao B. Overexpression of microRNA-129-5p in glioblastoma inhibits cell proliferation, migration, and colony-forming ability by targeting ZFP36L1. Bosn. J. Basic Med. Sci. 2019 doi: 10.17305/bjbms.2019.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang W., He B. MiR-760 inhibits the progression of non-small cell lung cancer through blocking ROS1/Ras/Raf/MEK/ERK pathway. Biosci. Rep. 2020:BSR20182483. doi: 10.1042/BSR20182483. [DOI] [PubMed] [Google Scholar]
- 79.Huang C., Liu J., Pan X., Peng C., Xiong B., Feng M., Yang X. miR-454 promotes survival and induces oxaliplatin resistance in gastric carcinoma cells by targeting CYLD. Exp. Ther. Med. 2020;19:3604–3610. doi: 10.3892/etm.2020.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qin X., Wang X.Y., Fei J.W., Li F.H., Han J., Wang H.X. MiR-20a promotes lung tumorigenesis by targeting RUNX3 via TGF-beta signaling pathway. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/20-12a. [DOI] [PubMed] [Google Scholar]
- 81.Liu X., Fu Y., Zhang G., Zhang D., Liang N., Li F., Li C., Sui C., Jiang J., Lu H., et al. miR-424-5p Promotes Anoikis Resistance and Lung Metastasis by Inactivating Hippo Signaling in Thyroid Cancer. Mol. Ther. Oncolytics. 2019;15:248–260. doi: 10.1016/j.omto.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang S., Zou C., Tang Y., Wa Q., Peng X., Chen X., Yang C., Ren D., Huang Y., Liao Z., et al. miR-582-3p and miR-582-5p Suppress Prostate Cancer Metastasis to Bone by Repressing TGF-β Signaling. Mol. Ther. Nucleic Acids. 2019;16:91–104. doi: 10.1016/j.omtn.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vescarelli E., Gerini G., Megiorni F., Anastasiadou E., Pontecorvi P., Solito L., De Vitis C., Camero S., Marchetti C., Mancini R., et al. MiR-200c sensitizes Olaparib-resistant ovarian cancer cells by targeting Neuropilin 1. J. Exp. Clin. Cancer Res. CR. 2020;39:3. doi: 10.1186/s13046-019-1490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meng Z., Zhang R., Wang Y., Zhu G., Jin T., Li C., Zhang S. miR-200c/PAI-2 promotes the progression of triple negative breast cancer via M1/M2 polarization induction of macrophage. Int. Immunopharmacol. 2019:106028. doi: 10.1016/j.intimp.2019.106028. [DOI] [PubMed] [Google Scholar]
- 85.Basu S., Chaudhary A., Chowdhury P., Karmakar D., Basu K., Karmakar D., Chatterjee J., Sengupta S. Evaluating the role of hsa-miR-200c in reversing the epithelial to mesenchymal transition in prostate cancer. Gene. 2020;730:144264. doi: 10.1016/j.gene.2019.144264. [DOI] [PubMed] [Google Scholar]
- 86.Tang H., Song C., Ye F., Gao G., Ou X., Zhang L., Xie X., Xie X. miR-200c suppresses stemness and increases cellular sensitivity to trastuzumab in HER2+ breast cancer. J. Cell. Mol. Med. 2019;23:8114–8127. doi: 10.1111/jcmm.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin H., Yang L., Tian F., Nie S., Zhou H., Liu J., Chen W. Up-regulated LncRNA-ATB regulates the growth and metastasis of cholangiocarcinoma via miR-200c signals. OncoTargets Ther. 2019;12:7561–7571. doi: 10.2147/OTT.S217676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DeSantis C.E., Ma J., Gaudet M.M., Newman L.A., Miller K.D., Goding Sauer A., Jemal A., Siegel R.L. Breast cancer statistics, 2019. CA A Cancer J. Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 89.Abolghasemi M., Tehrani S.S., Yousefi T., Karimian A., Mahmoodpoor A., Ghamari A., Jadidi-Niaragh F., Yousefi M., Kafil H.S., Bastami M., et al. MicroRNAs in breast cancer: Roles, functions, and mechanism of actions. J. Cell. Physiol. 2020;235:5008–5029. doi: 10.1002/jcp.29396. [DOI] [PubMed] [Google Scholar]
- 90.Zou Q., Zhou E., Xu F., Zhang D., Yi W., Yao J. A TP73-AS1/miR-200a/ZEB1 regulating loop promotes breast cancer cell invasion and migration. J. Cell. Biochem. 2018;119:2189–2199. doi: 10.1002/jcb.26380. [DOI] [PubMed] [Google Scholar]
- 91.Zhu Y., Yang Z., Luo X.H., Xu P. Long noncoding RNA TTN-AS1 promotes the proliferation and migration of prostate cancer by inhibiting miR-1271 level. Eur. Rev. Med. Pharmacol. Sci. 2019;23:10678–10684. doi: 10.26355/eurrev_201912_19766. [DOI] [PubMed] [Google Scholar]
- 92.Tian Y., Chen Y.Y., Han A.L. MiR-1271 inhibits cell proliferation and metastasis by targeting LDHA in endometrial cancer. Eur. Rev. Med. Pharmacol. Sci. 2019;23:5648–5656. doi: 10.26355/eurrev_201907_18300. [DOI] [PubMed] [Google Scholar]
- 93.Yao H., Sun Q., Zhu J. miR-1271 enhances the sensitivity of colorectal cancer cells to cisplatin. Exp. Ther. Med. 2019;17:4363–4370. doi: 10.3892/etm.2019.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiao Y., Zhu G., Yu J., Li Y., Wu M., Zhao J., Tian X. miR-1271 inhibits growth, invasion and epithelial–mesenchymal transition by targeting ZEB1 in ovarian cancer cells. OncoTargets Ther. 2019;12:6973. doi: 10.2147/OTT.S219018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang Y., Jin S., Tan S., Shen Q., Xue Y. MiR-203 acts as a radiosensitizer of gastric cancer cells by directly targeting ZEB1. OncoTargets Ther. 2019;12:6093–6104. doi: 10.2147/OTT.S197539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li J., Xia L., Zhou Z., Zuo Z., Xu C., Song H., Cai J. MiR-186-5p upregulation inhibits proliferation, metastasis and epithelial-to-mesenchymal transition of colorectal cancer cell by targeting ZEB1. Arch. Biochem. Biophys. 2018;640:53–60. doi: 10.1016/j.abb.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 97.Zhang L., Cai Q.Y., Liu J., Peng J., Chen Y.Q., Sferra T.J., Lin J.M. Ursolic acid suppresses the invasive potential of colorectal cancer cells by regulating the TGF-beta1/ZEB1/miR-200c signaling pathway. Oncol. Lett. 2019;18:3274–3282. doi: 10.3892/ol.2019.10604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fan M.J., Zou Y.H., He P.J., Zhang S., Sun X.M., Li C.Z. Long non-coding RNA SPRY4-IT1 promotes epithelial-mesenchymal transition of cervical cancer by regulating the miR-101-3p/ZEB1 axis. Biosci. Rep. 2019;39 doi: 10.1042/BSR20181339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dai Y., Wu Z., Lang C., Zhang X., He S., Yang Q., Guo W., Lai Y., Du H., Peng X., et al. Copy number gain of ZEB1 mediates a double-negative feedback loop with miR-33a-5p that regulates EMT and bone metastasis of prostate cancer dependent on TGF-β signaling. Theranostics. 2019;9:6063–6079. doi: 10.7150/thno.36735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharma N., Nanta R., Sharma J., Gunewardena S., Singh K.P., Shankar S., Srivastava R.K. PI3K/AKT/mTOR and sonic hedgehog pathways cooperate together to inhibit human pancreatic cancer stem cell characteristics and tumor growth. Oncotarget. 2015;6:32039. doi: 10.18632/oncotarget.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ong P.S., Wang L.Z., Dai X., Tseng S.H., Loo S.J., Sethi G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharmacol. 2016;7:395. doi: 10.3389/fphar.2016.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singh S.S., Yap W.N., Arfuso F., Kar S., Wang C., Cai W., Dharmarajan A.M., Sethi G., Kumar A.P. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015;21:12261–12273. doi: 10.3748/wjg.v21.i43.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang J., Wang X., Wen G., Ren Y. miRNA2055p functions as a tumor suppressor by negatively regulating VEGFA and PI3K/Akt/mTOR signaling in renal carcinoma cells. Oncol. Rep. 2019;42:1677–1688. doi: 10.3892/or.2019.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Z., Luo S., Wu M., Huang C., Shi H., Song X. LncRNA GHET1 promotes cervical cancer progression through regulating AKT/mTOR and Wnt/beta-catenin signaling pathways. Biosci. Rep. 2020;40 doi: 10.1042/bsr20191265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yin C., Lin X., Wang Y., Liu X., Xiao Y., Liu J., Snijders A.M., Wei G., Mao J.H., Zhang P. FAM83D promotes epithelial-mesenchymal transition, invasion and cisplatin resistance through regulating the AKT/mTOR pathway in non-small-cell lung cancer. Cell. Oncol. (Dordrecht) 2020 doi: 10.1007/s13402-020-00494-9. [DOI] [PubMed] [Google Scholar]
- 106.Cai C., Dang W., Shilei L., Huang L., Li Y., Li G., Yan S., Jiang C., Song X., Hu Y., et al. ANTXR1/TEM8 promotes gastric cancer progression through activation of the PI3K/AKT/mTOR signaling pathway. Cancer Sci. 2020 doi: 10.1111/cas.14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cui Z., Han B., Wang X., Li Z., Wang J., Lv Y. Long Non-Coding RNA TTN-AS1 Promotes the Proliferation and Invasion of Colorectal Cancer Cells by Activating miR-497-Mediated PI3K/Akt/mTOR Signaling. OncoTargets Ther. 2019;12:11531–11539. doi: 10.2147/OTT.S229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ren S., Liu J., Feng Y., Li Z., He L., Li L., Cao X., Wang Z., Zhang Y. Knockdown of circDENND4C inhibits glycolysis, migration and invasion by up-regulating miR-200b/c in breast cancer under hypoxia. J. Exp. Clin. Cancer Res. CR. 2019;38:388. doi: 10.1186/s13046-019-1398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen W., Kong K.-K., Xu X.-K., Chen C., Li H., Wang F.-Y., Peng X.-F., Zhang Z., Li P., Li J.-L. Downregulation of miR-205 is associated with glioblastoma cell migration, invasion, and the epithelial-mesenchymal transition, by targeting ZEB1 via the Akt/mTOR signaling pathway. Int. J. Oncol. 2018;52:485–495. doi: 10.3892/ijo.2017.4217. [DOI] [PubMed] [Google Scholar]
- 110.Sun S., Hang T., Zhang B., Zhu L., Wu Y., Lv X., Huang Q., Yao H. miRNA-708 functions as a tumor suppressor in colorectal cancer by targeting ZEB1 through Akt/mTOR signaling pathway. Am. J. Transl. Res. 2019;11:5338–5356. [PMC free article] [PubMed] [Google Scholar]
- 111.Xie R., Wang M., Zhou W., Wang D., Yuan Y., Shi H., Wu L. Long Non-Coding RNA (LncRNA) UFC1/miR-34a Contributes to Proliferation and Migration in Breast Cancer. Med. Sci. Monit. 2019;25:7149–7157. doi: 10.12659/MSM.917562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Du H., Wang X., Dong R., Hu D., Xiong Y. miR-601 inhibits proliferation, migration and invasion of prostate cancer stem cells by targeting KRT5 to inactivate the Wnt signaling pathway. Int. J. Clin. Exp. Pathol. 2019;12:4361–4379. [PMC free article] [PubMed] [Google Scholar]
- 113.Kong B., Lv Z.D., Xia J., Jin L.Y., Yang Z.C. DLC-3 suppresses cellular proliferation, migration, and invasion in triple-negative breast cancer by the Wnt/beta-catenin pathway. Int. J. Clin. Exp. Pathol. 2019;12:1224–1232. [PMC free article] [PubMed] [Google Scholar]
- 114.Guo Y., Li B., Yan X., Shen X., Ma J., Liu S., Zhang D. Bisphenol A and polychlorinated biphenyls enhance the cancer stem cell properties of human ovarian cancer cells by activating the WNT signaling pathway. Chemosphere. 2019;246:125775. doi: 10.1016/j.chemosphere.2019.125775. [DOI] [PubMed] [Google Scholar]
- 115.Gao X.H., Zhang Y.L., Zhang Z.Y., Guo S.S., Chen X.B., Guo Y.Z. MicroRNA-96-5p represses breast cancer proliferation and invasion through Wnt/beta-catenin signaling via targeting CTNND1. Sci. Rep. 2020;10:44. doi: 10.1038/s41598-019-56571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pohl S.G., Brook N., Agostino M., Arfuso F., Kumar A.P., Dharmarajan A. Wnt signaling in triple-negative breast cancer. Oncogenesis. 2017;6:e310. doi: 10.1038/oncsis.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Surana R., Sikka S., Cai W., Shin E.M., Warrier S.R., Tan H.J., Arfuso F., Fox S.A., Dharmarajan A.M., Kumar A.P. Secreted frizzled related proteins: Implications in cancers. Biochim. Biophys. Acta. 2014;1845:53–65. doi: 10.1016/j.bbcan.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 118.Qu J., Li M., An J., Zhao B., Zhong W., Gu Q., Cao L., Yang H., Hu C. MicroRNA-33b inhibits lung adenocarcinoma cell growth, invasion, and epithelial-mesenchymal transition by suppressing Wnt/beta-catenin/ZEB1 signaling. Int. J. Oncol. 2015;47:2141–2152. doi: 10.3892/ijo.2015.3187. [DOI] [PubMed] [Google Scholar]
- 119.Cong N., Du P., Zhang A., Shen F., Su J., Pu P., Wang T., Zjang J., Kang C., Zhang Q. Downregulated microRNA-200a promotes EMT and tumor growth through the wnt/beta-catenin pathway by targeting the E-cadherin repressors ZEB1/ZEB2 in gastric adenocarcinoma. Oncol. Rep. 2013;29:1579–1587. doi: 10.3892/or.2013.2267. [DOI] [PubMed] [Google Scholar]
- 120.Shang A., Wang W., Gu C., Chen C., Zeng B., Yang Y., Ji P., Sun J., Wu J., Lu W., et al. Long non-coding RNA HOTTIP enhances IL-6 expression to potentiate immune escape of ovarian cancer cells by upregulating the expression of PD-L1 in neutrophils. J. Exp. Clin. Cancer Res. 2019;38:411. doi: 10.1186/s13046-019-1394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abolghasemi M., Tehrani S.S., Yousefi T., Karimian A., Mahmoodpoor A., Ghamari A., Jadidi-Niaragh F., Yousefi M., Kafil H.S., Bastami M., et al. Critical roles of long noncoding RNAs in breast cancer. J. Cell. Physiol. 2020;235:5059–5071. doi: 10.1002/jcp.29442. [DOI] [PubMed] [Google Scholar]
- 122.Vafadar A., Shabaninejad Z., Movahedpour A., Mohammadi S., Fathullahzadeh S., Mirzaei H.R., Namdar A., Savardashtaki A., Mirzaei H. Long Non-Coding RNAs As Epigenetic Regulators in Cancer. Curr. Pharm. Des. 2019;25:3563–3577. doi: 10.2174/1381612825666190830161528. [DOI] [PubMed] [Google Scholar]
- 123.Yang C., Di Wu L.G., Liu X., Jin Y., Wang D., Wang T., Li X. Competing endogenous RNA networks in human cancer: Hypothesis, validation, and perspectives. Oncotarget. 2016;7:13479. doi: 10.18632/oncotarget.7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shen J., Hong L., Yu D., Cao T., Zhou Z., He S. LncRNA XIST promotes pancreatic cancer migration, invasion and EMT by sponging miR-429 to modulate ZEB1 expression. Int. J. Biochem. Cell Biol. 2019;113:17–26. doi: 10.1016/j.biocel.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 125.Li D., Wang J., Zhang M., Hu X., She J., Qiu X., Zhang X., Xu L., Liu Y., Qin S. LncRNA MAGI2-AS3 Is Regulated by BRD4 and Promotes Gastric Cancer Progression via Maintaining ZEB1 Overexpression by Sponging miR-141/200a. Mol. Ther. Nucleic Acids. 2020;19:109–123. doi: 10.1016/j.omtn.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun L., Chen T., Li T., Yu J. LncRNA IUR downregulates ZEB1 by upregulating miR-200 to inhibit prostate carcinoma. Physiol. Genom. 2019;51:607–611. doi: 10.1152/physiolgenomics.00062.2019. [DOI] [PubMed] [Google Scholar]
- 127.Jiang H., Jiang Y., Zhang T., Mo K., Su S., Wang A., Zhu Y., Huang G., Zhou R. The lncRNA TDRG1 promotes cell proliferation, migration and invasion by targeting miR-326 to regulate MAPK1 expression in cervical cancer. Cancer Cell Int. 2019;19:152. doi: 10.1186/s12935-019-0872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Deng S.-j., Chen H.-y., Ye Z., Deng S.-c., Zhu S., Zeng Z., He C., Liu M.-l., Huang K., Zhong J.-x. Hypoxia-induced LncRNA-BX111 promotes metastasis and progression of pancreatic cancer through regulating ZEB1 transcription. Oncogene. 2018;37:5811–5828. doi: 10.1038/s41388-018-0382-1. [DOI] [PubMed] [Google Scholar]
- 129.Hu X., Mu Y., Wang J., Zhao Y. LncRNA TDRG1 promotes the metastasis of NSCLC cell through regulating miR-873-5p/ZEB1 axis. J. Cell. Biochem. 2019 doi: 10.1002/jcb.29559. [DOI] [PubMed] [Google Scholar]
- 130.Zhong Y., Wang J., Lv W., Xu J., Mei S., Shan A. LncRNA TTN-AS1 drives invasion and migration of lung adenocarcinoma cells via modulation of miR-4677-3p/ZEB1 axis. J. Cell. Biochem. 2019;120:17131–17141. doi: 10.1002/jcb.28973. [DOI] [PubMed] [Google Scholar]
- 131.Zhang M., Wu W., Wang Z., Wang X. lncRNA NEAT1 is closely related with progression of breast cancer via promoting proliferation and EMT. Eur. Rev. Med. Pharmacol. Sci. 2017;21:1020–1026. [PubMed] [Google Scholar]
- 132.Tan X., Wang P., Lou J., Zhao J. Knockdown of lncRNA NEAT1 suppresses hypoxia-induced migration, invasion and glycolysis in anaplastic thyroid carcinoma cells through regulation of miR-206 and miR-599. Cancer Cell Int. 2020;20:132. doi: 10.1186/s12935-020-01222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jiang X., Zhou Y., Sun A.J., Xue J.L. NEAT1 contributes to breast cancer progression through modulating miR-448 and ZEB1. J. Cell. Physiol. 2018;233:8558–8566. doi: 10.1002/jcp.26470. [DOI] [PubMed] [Google Scholar]
- 134.Shao Q., Wang Q., Wang J. LncRNA SCAMP1 regulates ZEB1/JUN and autophagy to promote pediatric renal cell carcinoma under oxidative stress via miR-429. Biomed. Pharmacother. 2019;120:109460. doi: 10.1016/j.biopha.2019.109460. [DOI] [PubMed] [Google Scholar]
- 135.Kirtonia A., Sethi G., Garg M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell. Mol. Life Sci. 2020 doi: 10.1007/s00018-020-03536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Aggarwal V., Tuli H.S., Varol A., Thakral F., Yerer M.B., Sak K., Varol M., Jain A., Khan M.A., Sethi G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules. 2019;9:735. doi: 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang C., Sun J., Liu W., Yang Y., Chu Z., Yang T., Gui Y., Wang D. Long noncoding RNA HCP5 contributes to epithelial-mesenchymal transition in colorectal cancer through ZEB1 activation and interacting with miR-139-5p. Am. J. Transl. Res. 2019;11:953–963. [PMC free article] [PubMed] [Google Scholar]
- 138.Pu J., Wang J., Wei H., Lu T., Wu X., Wu Y., Shao Z., Luo C., Lu Y. lncRNA MAGI2-AS3 Prevents the Development of HCC via Recruiting KDM1A and Promoting H3K4me2 Demethylation of the RACGAP1 Promoter. Mol. Ther. Nucleic Acids. 2019;18:351–362. doi: 10.1016/j.omtn.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang Y., Yang H., Xu M., Zhang H., Sun M., Mu P., Dong T., Du S., Liu K. Long non-coding RNA (lncRNA) MAGI2-AS3 inhibits breast cancer cell growth by targeting the Fas/FasL signalling pathway. Human Cell. 2018;31:232–241. doi: 10.1007/s13577-018-0206-1. [DOI] [PubMed] [Google Scholar]
- 140.Zhao X., Liu Y., Li Z., Zheng S., Wang Z., Li W., Bi Z., Li L., Jiang Y., Luo Y. Linc00511 acts as a competing endogenous RNA to regulate VEGFA expression through sponging hsa-miR-29b-3p in pancreatic ductal adenocarcinoma. J. Cell. Mol. Med. 2018;22:655–667. doi: 10.1111/jcmm.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Du X., Tu Y., Liu S., Zhao P., Bao Z., Li C., Li J., Pan M., Ji J. LINC00511 contributes to glioblastoma tumorigenesis and epithelial-mesenchymal transition via LINC00511/miR-524-5p/YB1/ZEB1 positive feedback loop. J. Cell. Mol. Med. 2019;24:1474–1487. doi: 10.1111/jcmm.14829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hao T., Wang Z., Yang J., Zhang Y., Shang Y., Sun J. MALAT1 knockdown inhibits prostate cancer progression by regulating miR-140/BIRC6 axis. Biomed. Pharmacother. 2020;123:109666. doi: 10.1016/j.biopha.2019.109666. [DOI] [PubMed] [Google Scholar]
- 143.Wei S., Liu Q. Long noncoding RNA MALAT1 modulates sepsis-induced cardiac inflammation through the miR-150-5p/NF-kappaB axis. Int. J. Clin. Exp. Pathol. 2019;12:3311–3319. [PMC free article] [PubMed] [Google Scholar]
- 144.Chen L., Yao H., Wang K., Liu X. Long non-coding RNA MALAT1 regulates ZEB1 expression by sponging miR-143-3p and promotes hepatocellular carcinoma progression. J. Cell. Biochem. 2017;118:4836–4843. doi: 10.1002/jcb.26158. [DOI] [PubMed] [Google Scholar]
- 145.Wang Y., Yu X.J., Zhou W., Chu Y.X. MicroRNA-429 inhibits the proliferation and migration of esophageal squamous cell carcinoma cells by targeting RAB23 through the NF-kappaB pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24:1202–1210. doi: 10.26355/eurrev_202002_20172. [DOI] [PubMed] [Google Scholar]
- 146.Shen F., Zheng H., Zhou L., Li W., Xu X. Overexpression of MALAT1 contributes to cervical cancer progression by acting as a sponge of miR-429. J. Cell Physiol. 2019;234:11219–11226. doi: 10.1002/jcp.27772. [DOI] [PubMed] [Google Scholar]
- 147.Szigeti T., Dunster C., Cattaneo A., Cavallo D., Spinazze A., Saraga D.E., Sakellaris I.A., de Kluizenaar Y., Cornelissen E.J., Hanninen O., et al. Oxidative potential and chemical composition of PM2.5 in office buildings across Europe—The OFFICAIR study. Environ. Int. 2016;92–93:324–333. doi: 10.1016/j.envint.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 148.Valavanidis A., Vlachogianni T., Fiotakis K., Loridas S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health. 2013;10:3886–3907. doi: 10.3390/ijerph10093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Luo F., Wei H., Guo H., Li Y., Feng Y., Bian Q., Wang Y. LncRNA MALAT1, an lncRNA acting via the miR-204/ZEB1 pathway, mediates the EMT induced by organic extract of PM2.5 in lung bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;317:L87–Ll98. doi: 10.1152/ajplung.00073.2019. [DOI] [PubMed] [Google Scholar]
- 150.Wang K.C., Yang Y.W., Liu B., Sanyal A., Corces-Zimmerman R., Chen Y., Lajoie B.R., Protacio A., Flynn R.A., Gupta R.A. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang S., Wang W., Liu G., Xie S., Li Q., Li Y., Lin Z. Long non-coding RNA HOTTIP promotes hypoxia-induced epithelial-mesenchymal transition of malignant glioma by regulating the miR-101/ZEB1 axis. Biomed. Pharmacother. 2017;95:711–720. doi: 10.1016/j.biopha.2017.08.133. [DOI] [PubMed] [Google Scholar]
- 152.Dai B., Zhou G., Hu Z., Zhu G., Mao B., Su H., Jia Q. MiR-205 suppresses epithelial-mesenchymal transition and inhibits tumor growth of human glioma through down-regulation of HOXD9. Biosci. Rep. 2019;39 doi: 10.1042/BSR20181989. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 153.Dong N., Guo J., Han S., Bao L., Diao Y., Lin Z. Positive feedback loop of lncRNA HOXC-AS2/miR-876-5p/ZEB1 to regulate EMT in glioma. OncoTargets Ther. 2019;12:7601–7609. doi: 10.2147/OTT.S216134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kulcheski F.R., Christoff A.P., Margis R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J. Biotechnol. 2016;238:42–51. doi: 10.1016/j.jbiotec.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 155.Meng S., Zhou H., Feng Z., Xu Z., Tang Y., Li P., Wu M. CircRNA: Functions and properties of a novel potential biomarker for cancer. Mol. Cancer. 2017;16:94. doi: 10.1186/s12943-017-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Conn S.J., Pillman K.A., Toubia J., Conn V.M., Salmanidis M., Phillips C.A., Roslan S., Schreiber A.W., Gregory P.A., Goodall G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 157.Liu C., Zhang Z., Qi D. Circular RNA hsa_circ_0023404 promotes proliferation, migration and invasion in non-small cell lung cancer by regulating miR-217/ZEB1 axis. OncoTargets Ther. 2019;12:6181–6189. doi: 10.2147/OTT.S201834. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 158.Re M., Magliulo G., Gioacchini F.M., Bajraktari A., Bertini A., Çeka A., Rubini C., Ferrante L., Procopio A.D., Olivieri F. Expression levels and clinical significance of miR-21-5p, miR-let-7a, and miR-34c-5p in laryngeal squamous cell carcinoma. BioMed. Res. Int. 2017;2017:3921258. doi: 10.1155/2017/3921258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wei Q., Yu D., Liu M., Wang M., Zhao M., Liu M., Jia W., Ma H., Fang J., Xu W. Genome-wide association study identifies three susceptibility loci for laryngeal squamous cell carcinoma in the Chinese population. Nat. Genet. 2014;46:1110. doi: 10.1038/ng.3090. [DOI] [PubMed] [Google Scholar]
- 160.Fu D., Huang Y., Gao M. Hsa_circ_0057481 promotes laryngeal cancer proliferation and migration by modulating the miR-200c/ZEB1 axis. Int. J. Clin. Exp. Pathol. 2019;12:4066. [PMC free article] [PubMed] [Google Scholar]
- 161.Ying X., Zhu J., Zhang Y. Circular RNA circ-TSPAN4 promotes lung adenocarcinoma metastasis by upregulating ZEB1 via sponging miR-665. Mol. Genet. Genom. Med. 2019;7:e991. doi: 10.1002/mgg3.991. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 162.Conte R., Valentino A., Di Cristo F., Peluso G., Cerruti P., Di Salle A., Calarco A. Cationic Polymer Nanoparticles-Mediated Delivery of miR-124 Impairs Tumorigenicity of Prostate Cancer Cells. Int. J. Mol. Sci. 2020;21:869. doi: 10.3390/ijms21030869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen J., Zhong Y., Li L. miR-124 and miR-203 synergistically inactivate EMT pathway via coregulation of ZEB2 in clear cell renal cell carcinoma (ccRCC) J. Transl. Med. 2020;18:69. doi: 10.1186/s12967-020-02242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen L., Hu N., Wang C., Zhao H. HOTAIRM1 knockdown enhances cytarabine-induced cytotoxicity by suppression of glycolysis through the Wnt/beta-catenin/PFKP pathway in acute myeloid leukemia cells. Arch. Biochem. Biophys. 2020;680:108244. doi: 10.1016/j.abb.2019.108244. [DOI] [PubMed] [Google Scholar]
- 165.Li J., Zhang S., Wu L., Pei M. Interaction between LncRNA-ROR and miR-145 contributes to epithelial-mesenchymal transition of ovarian cancer cells. Gen. Physiol. Biophys. 2019;38:461–471. doi: 10.4149/gpb_2019028. [DOI] [PubMed] [Google Scholar]
- 166.Liu Q., Chen J., Wang B., Zheng Y., Wan Y., Wang Y., Zhou L., Liu S., Li G., Yan Y. miR-145 modulates epithelial-mesenchymal transition and invasion by targeting ZEB2 in non-small cell lung cancer cell lines. J. Cell. Biochem. 2018 doi: 10.1002/jcb.28126. [DOI] [PubMed] [Google Scholar]
- 167.Miao Y., Wang L., Zhang X., Xing R.-G., Zhou W.-W., Liu C.-R., Zhang X.-L., Tian L. miR-30a inhibits breast cancer progression through the Wnt/β-catenin pathway. Int. J. Clin. Exp. Pathol. 2019;12:241. [PMC free article] [PubMed] [Google Scholar]
- 168.Wang T., Chen G., Ma X., Yang Y., Chen Y., Peng Y., Bai Z., Zhang Z., Pei H., Guo W. MiR-30a regulates cancer cell response to chemotherapy through SNAI1/IRS1/AKT pathway. Cell Death Dis. 2019;10:153. doi: 10.1038/s41419-019-1326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Di Gennaro A., Damiano V., Brisotto G., Armellin M., Perin T., Zucchetto A., Guardascione M., Spaink H.P., Doglioni C., Snaar-Jagalska B.E. A p53/miR-30a/ZEB2 axis controls triple negative breast cancer aggressiveness. Cell Death Differ. 2018;25:2165–2180. doi: 10.1038/s41418-018-0103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang L., Zhang T., Deng Z., Sun L. MicroRNA3653 inhibits the growth and metastasis of hepatocellular carcinoma by inhibiting ITGB1. Oncol. Rep. 2019;41:1669–1677. doi: 10.3892/or.2019.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhu W., Luo X., Fu H., Liu L., Sun P., Wang Z. miR-3653 inhibits the metastasis and epithelial-mesenchymal transition of colon cancer by targeting Zeb2. Pathol. Res. Pract. 2019;215:152577. doi: 10.1016/j.prp.2019.152577. [DOI] [PubMed] [Google Scholar]
- 172.Zhu D., Gu L., Li Z., Jin W., Lu Q., Ren T. MiR-138-5p suppresses lung adenocarcinoma cell epithelial-mesenchymal transition, proliferation and metastasis by targeting ZEB2. Pathol. Res. Pract. 2019;215:861–872. doi: 10.1016/j.prp.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 173.Eleutério S.J.P., Senerchia A.A., Almeida M.T., Costa C.M.D., Lustosa D., Calheiros L.M., Barreto J.H.S., Brunetto A.L., Macedo C.R.P.D., Petrilli A.S. Osteosarcoma in patients younger than 12 years old without metastases have similar prognosis as adolescent and young adults. Pediatric Blood Cancer. 2015;62:1209–1213. doi: 10.1002/pbc.25459. [DOI] [PubMed] [Google Scholar]
- 174.Wang S.-N., Luo S., Liu C., Piao Z., Gou W., Wang Y., Guan W., Li Q., Zou H., Yang Z.-Z. miR-491 inhibits osteosarcoma lung metastasis and chemoresistance by targeting αB-crystallin. Mol. Ther. 2017;25:2140–2149. doi: 10.1016/j.ymthe.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lin H., Zheng X., Lu T., Gu Y., Zheng C., Yan H. The proliferation and invasion of osteosarcoma are inhibited by miR-101 via targetting ZEB2. Biosci. Rep. 2019;39 doi: 10.1042/BSR20181283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu N., Feng S., Li H., Chen X., Bai S., Liu Y. Long non-coding RNA MALAT1 facilitates the tumorigenesis, invasion and glycolysis of multiple myeloma via miR-1271-5p/SOX13 axis. J. Cancer Res. Clin. Oncol. 2020;146:367–379. doi: 10.1007/s00432-020-03127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chao H., Zhang M., Hou H., Zhang Z., Li N. HOTAIRM1 suppresses cell proliferation and invasion in ovarian cancer through facilitating ARHGAP24 expression by sponging miR-106a-5p. Life Sci. 2020:117296. doi: 10.1016/j.lfs.2020.117296. [DOI] [PubMed] [Google Scholar]
- 178.Shi T., Guo D., Xu H., Su G., Chen J., Zhao Z., Shi J., Wedemeyer M., Attenello F., Zhang L., et al. HOTAIRM1, an enhancer lncRNA, promotes glioma proliferation by regulating long-range chromatin interactions within HOXA cluster genes. Mol. Biol. Rep. 2020;47:2723–2733. doi: 10.1007/s11033-020-05371-0. [DOI] [PubMed] [Google Scholar]
- 179.Lin Y.H., Guo L., Yan F., Dou Z.Q., Yu Q., Chen G. Long non-coding RNA HOTAIRM1 promotes proliferation and inhibits apoptosis of glioma cells by regulating the miR-873-5p/ZEB2 axis. Chin. Med. J. (Engl.) 2020;133:174–182. doi: 10.1097/CM9.0000000000000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ren L., Yao Y., Wang Y., Wang S. MiR-505 suppressed the growth of hepatocellular carcinoma cells via targeting IGF-1R. Biosci. Rep. 2019;39:BSR20182442. doi: 10.1042/BSR20182442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tian L., Wang Z.Y., Hao J., Zhang X.Y. miR-505 acts as a tumor suppressor in gastric cancer progression through targeting HMGB1. J. Cell. Biochem. 2019;120:8044–8052. doi: 10.1002/jcb.28082. [DOI] [PubMed] [Google Scholar]
- 182.Shi H., Yang H., Xu S., Zhao Y., Liu J. miR-505 functions as a tumor suppressor in glioma by targeting insulin like growth factor 1 receptor expression. Int. J. Clin. Exp. Pathol. 2018;11:4405. [PMC free article] [PubMed] [Google Scholar]
- 183.Gong X., Huang A. LEF-AS1 participates in occurrence of colorectal cancer through adsorbing miR-505 and promoting KIF3B expression. Eur. Rev. Med. Pharmacol. Sci. 2019;23:9362–9370. doi: 10.26355/eurrev_201911_19429. [DOI] [PubMed] [Google Scholar]
- 184.Wang Z., Wang Q., Xu G., Meng N., Huang X., Jiang Z., Chen C., Zhang Y., Chen J., Li A., et al. The long noncoding RNA CRAL reverses cisplatin resistance via the miR-505/CYLD/AKT axis in human gastric cancer cells. RNA Biol. 2020:1–14. doi: 10.1080/15476286.2019.1709296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhao P., Guan H., Dai Z., Ma Y., Zhao Y., Liu D. Long noncoding RNA DLX6-AS1 promotes breast cancer progression via miR-505-3p/RUNX2 axis. Eur. J. Pharmacol. 2019;865:172778. doi: 10.1016/j.ejphar.2019.172778. [DOI] [PubMed] [Google Scholar]
- 186.Feng S., Liu W., Bai X., Pan W., Jia Z., Zhang S., Zhu Y., Tan W. LncRNA-CTS promotes metastasis and epithelial-to-mesenchymal transition through regulating miR-505/ZEB2 axis in cervical cancer. Cancer Lett. 2019;465:105–117. doi: 10.1016/j.canlet.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 187.Gong P., Qiao F., Wu H., Cui H., Li Y., Zheng Y., Zhou M., Fan H. LncRNA UCA1 promotes tumor metastasis by inducing miR-203/ZEB2 axis in gastric cancer. Cell Death Dis. 2018;9:1–14. doi: 10.1038/s41419-018-1170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang Y., Liang S., Yu Y., Shi Y., Zheng H. Knockdown of SNHG12 suppresses tumor metastasis and epithelial-mesenchymal transition via the Slug/ZEB2 signaling pathway by targeting miR-218 in NSCLC. Oncol. Lett. 2019;17:2356–2364. doi: 10.3892/ol.2018.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Weller M., Wick W., Aldape K., Brada M., Berger M., Pfister S.M., Nishikawa R., Rosenthal M., Wen P.Y., Stupp R. Glioma. Nat. Rev. Dis. Primers. 2015;1:1–18. doi: 10.1038/nrdp.2015.17. [DOI] [PubMed] [Google Scholar]
- 190.Ostrom Q.T., Bauchet L., Davis F.G., Deltour I., Fisher J.L., Langer C.E., Pekmezci M., Schwartzbaum J.A., Turner M.C., Walsh K.M. Response to “the epidemiology of glioma in adults: A ‘state of the science’review”. Neuro-Oncology. 2015;17:624–626. doi: 10.1093/neuonc/nov022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hanif F., Muzaffar K., Perveen K., Malhi S.M., Simjee S.U. Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. APJCP. 2017;18:3. doi: 10.22034/APJCP.2017.18.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rodriguez A., Tatter S.B. Laser ablation of recurrent malignant gliomas: Current status and future perspective. Neurosurgery. 2016;79:S35–S39. doi: 10.1227/NEU.0000000000001442. [DOI] [PubMed] [Google Scholar]
- 193.Hu B., Wang Q., Wang Y.A., Hua S., Sauvé C.-E.G., Ong D., Lan Z.D., Chang Q., Ho Y.W., Monasterio M.M. Epigenetic activation of WNT5A drives glioblastoma stem cell differentiation and invasive growth. Cell. 2016;167:1281–1295.e18. doi: 10.1016/j.cell.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Meng X., Deng Y., Lv Z., Liu C., Guo Z., Li Y., Liu H., Xie B., Jin Z., Lin F. LncRNA SNHG5 Promotes Proliferation of Glioma by Regulating miR-205-5p/ZEB2 Axis. OncoTargets Ther. 2019;12:11487. doi: 10.2147/OTT.S228439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wu H., Liu H.Y., Liu W.J., Shi Y.L., Bao D. miR-377-5p inhibits lung cancer cell proliferation, invasion, and cell cycle progression by targeting AKT1 signaling. J. Cell. Biochem. 2019;120:8120–8128. doi: 10.1002/jcb.28091. [DOI] [PubMed] [Google Scholar]
- 196.Zhang W.Y., Liu Q.H., Wang T.J., Zhao J., Cheng X.H., Wang J.S. CircZFR serves as a prognostic marker to promote bladder cancer progression by regulating miR-377/ZEB2 signaling. Biosci. Rep. 2019;39 doi: 10.1042/BSR20192779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xie R., Tang J., Zhu X., Jiang H. Silencing of hsa_circ_0004771 inhibits proliferation and induces apoptosis in breast cancer through activation of miR-653 by targeting ZEB2 signaling pathway. Biosci. Rep. 2019;39:BSR20181919. doi: 10.1042/BSR20181919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA Cancer J. Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 199.Ljungberg B., Campbell S.C., Cho H.Y., Jacqmin D., Lee J.E., Weikert S., Kiemeney L.A. The epidemiology of renal cell carcinoma. Eur. Urol. 2011;60:615–621. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 200.Znaor A., Lortet-Tieulent J., Laversanne M., Jemal A., Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015;67:519–530. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 201.Zhou B., Zheng P., Li Z., Li H., Wang X., Shi Z., Han Q. CircPCNXL2 sponges miR-153 to promote the proliferation and invasion of renal cancer cells through upregulating ZEB2. Cell Cycle. 2018;17:2644–2654. doi: 10.1080/15384101.2018.1553354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Riganti C., Contino M. New Strategies to Overcome Resistance to Chemotherapy and Immune System in Cancer. Int. J. Mol. Sci. 2019;20:4783. doi: 10.3390/ijms20194783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nedeljković M., Damjanović A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells. 2019;8:957. doi: 10.3390/cells8090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sajid A., Raju N., Lusvarghi S., Vahedi S., Swenson R.E., Ambudkar S.V. Synthesis and Characterization of Bodipy-FL-Cyclosporine A as a Substrate for Multidrug Resistance-Linked P-Glycoprotein (ABCB1) Drug Metab. Dispos. Biol. Fate Chem. 2019;47:1013–1023. doi: 10.1124/dmd.119.087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.van Hoppe S., Jamalpoor A., Rood J.J.M., Wagenaar E., Sparidans R.W., Beijnen J.H., Schinkel A.H. Brain accumulation of osimertinib and its active metabolite AZ5104 is restricted by ABCB1 (P-glycoprotein) and ABCG2 (breast cancer resistance protein) Pharmacol. Res. 2019;146:104297. doi: 10.1016/j.phrs.2019.104297. [DOI] [PubMed] [Google Scholar]
- 206.Shen X., Wang Y., Xi L., Su F., Li S. Biocompatibility and paclitaxel/cisplatin dual-loading of nanotubes prepared from poly(ethylene glycol)-polylactide-poly(ethylene glycol) triblock copolymers for combination cancer therapy. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2019;27:1025–1035. doi: 10.1016/j.jsps.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen W., Du J., Li X., Zhi Z., Jiang S. microRNA-137 downregulates MCL1 in ovarian cancer cells and mediates cisplatin-induced apoptosis. Pharmacogenomics. 2020;21:195–207. doi: 10.2217/pgs-2019-0122. [DOI] [PubMed] [Google Scholar]
- 208.Abu Samaan T.M., Samec M., Liskova A., Kubatka P., Büsselberg D. Paclitaxel’s Mechanistic and Clinical Effects on Breast Cancer. Biomolecules. 2019;9:789. doi: 10.3390/biom9120789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Khodaverdi S., Jafari A., Movahedzadeh F., Madani F., Yousefi Avarvand A., Falahatkar S. Evaluating Inhibitory Effects of Paclitaxel and Vitamin D3 Loaded Poly Lactic Glycolic Acid Co-Delivery Nanoparticles on the Breast Cancer Cell Line. Adv. Pharm. Bull. 2020;10:30–38. doi: 10.15171/apb.2020.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Miyagawa Y., Yanai A., Yanagawa T., Inatome J., Egawa C., Nishimukai A., Takamoto K., Morimoto T., Kikawa Y., Suwa H., et al. Baseline neutrophil-to-lymphocyte ratio and c-reactive protein predict efficacy of treatment with bevacizumab plus paclitaxel for locally advanced or metastatic breast cancer. Oncotarget. 2020;11:86–98. doi: 10.18632/oncotarget.27423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gao L., Zhao P., Li Y., Yang D., Hu P., Li L., Cheng Y., Yao H. Reversal of PGlycoprotein-Mediated Multidrug Resistance by Novel Curcumin Analogues in Paclitaxel-resistant Human Breast Cancer Cells. Biochem. Cell Biol. 2020 doi: 10.1139/bcb-2019-0377. [DOI] [PubMed] [Google Scholar]
- 212.Bashmail H.A., Alamoudi A.A., Noorwali A., Hegazy G.A., Ajabnoor G.M., Al-Abd A.M. Thymoquinone Enhances Paclitaxel Anti-Breast Cancer Activity via Inhibiting Tumor-Associated Stem Cells Despite Apparent Mathematical Antagonism. Molecules. 2020;25:426. doi: 10.3390/molecules25020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sakata J., Utsumi F., Suzuki S., Niimi K., Yamamoto E., Shibata K., Senga T., Kikkawa F., Kajiyama H. Inhibition of ZEB1 leads to inversion of metastatic characteristics and restoration of paclitaxel sensitivity of chronic chemoresistant ovarian carcinoma cells. Oncotarget. 2017;8:99482–99494. doi: 10.18632/oncotarget.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Liu Y.Y., Zhang L.Y., Du W.Z. Circular RNA circ-PVT1 contributes to paclitaxel resistance of gastric cancer cells through the regulation of ZEB1 expression by sponging miR-124-3p. Biosci. Rep. 2019;39 doi: 10.1042/bsr20193045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jiang G., Wen L., Deng W., Jian Z., Zheng H. Regulatory role of miR-211-5p in hepatocellular carcinoma metastasis by targeting ZEB2. Biomed. Pharmacother. 2017;90:806–812. doi: 10.1016/j.biopha.2017.03.081. [DOI] [PubMed] [Google Scholar]
- 216.Papa A.-L., Sidiqui A., Balasubramanian S.U.A., Sarangi S., Luchette M., Sengupta S., Harfouche R. PEGylated liposomal Gemcitabine: Insights into a potential breast cancer therapeutic. Cell. Oncol. 2013;36:449–457. doi: 10.1007/s13402-013-0146-4. [DOI] [PubMed] [Google Scholar]
- 217.Samanta K., Setua S., Kumari S., Jaggi M., Yallapu M.M., Chauhan S.C. Gemcitabine Combination Nano Therapies for Pancreatic Cancer. Pharmaceutics. 2019;11:574. doi: 10.3390/pharmaceutics11110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Goel S., Sinha R.J., Bhaskar V., Aeron R., Sharma A., Singh V. Role of gemcitabine and cisplatin as neoadjuvant chemotherapy in muscle invasive bladder cancer: Experience over the last decade. Asian J. Urol. 2019;6:222–229. doi: 10.1016/j.ajur.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang H., Liu K., Chi Z., Zhou X., Ren G., Zhou R., Li Y., Tang X., Wang X.J. Interplay of MKP-1 and Nrf2 drives tumor growth and drug resistance in non-small cell lung cancer. Aging. 2019;11:11329–11346. doi: 10.18632/aging.102531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yuan Q., Chu H., Ge Y., Ma G., Du M., Wang M., Zhang Z., Zhang W. LncRNA PCAT1 and its genetic variant rs1902432 are associated with prostate cancer risk. J. Cancer. 2018;9:1414. doi: 10.7150/jca.23685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ji X., Guo H., Yin S., Du H. miR-139-5p functions as a tumor suppressor in cervical cancer by targeting TCF4 and inhibiting Wnt/beta-catenin signaling. OncoTargets Ther. 2019;12:7739–7748. doi: 10.2147/OTT.S215796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Guo S.S., Wang Y., Fan Q.X. Raddeanin A promotes apoptosis and ameliorates 5-fluorouracil resistance in cholangiocarcinoma cells. World J. Gastroenterol. 2019;25:3380–3391. doi: 10.3748/wjg.v25.i26.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nakagawa Y., Kuranaga Y., Tahara T., Yamashita H., Shibata T., Nagasaka M., Funasaka K., Ohmiya N., Akao Y. Induced miR-31 by 5-fluorouracil exposure contributes to the resistance in colorectal tumors. Cancer Sci. 2019;110:2540–2548. doi: 10.1111/cas.14090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhao P., Ma Y.G., Zhao Y., Liu D., Dai Z.J., Yan C.Y., Guan H.T. MicroRNA-552 deficiency mediates 5-fluorouracil resistance by targeting SMAD2 signaling in DNA-mismatch-repair-deficient colorectal cancer. Cancer Chemother. Pharmacol. 2019;84:427–439. doi: 10.1007/s00280-019-03866-7. [DOI] [PubMed] [Google Scholar]
- 225.Shan G., Tang T., Xia Y., Qian H.-J. Long non-coding RNA NEAT1 promotes bladder progression through regulating miR-410 mediated HMGB1. Biomed. Pharmacother. 2020;121:109248. doi: 10.1016/j.biopha.2019.109248. [DOI] [PubMed] [Google Scholar]
- 226.Wang W., Ge L., Xu X.J., Yang T., Yuan Y., Ma X.L., Zhang X.H. LncRNA NEAT1 promotes endometrial cancer cell proliferation, migration and invasion by regulating the miR-144-3p/EZH2 axis. Radiol. Oncol. 2019;53:434–442. doi: 10.2478/raon-2019-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Liu F., Ai F.Y., Zhang D.C., Tian L., Yang Z.Y., Liu S.J. LncRNA NEAT1 knockdown attenuates autophagy to elevate 5-FU sensitivity in colorectal cancer via targeting miR-34a. Cancer Med. 2020;9:1079–1091. doi: 10.1002/cam4.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Liu J., Kong D., Sun D., Li J. Long non-coding RNA CCAT2 acts as an oncogene in osteosarcoma through regulation of miR-200b/VEGF. Artif. Cells Nanomed. Biotechnol. 2019;47:2994–3003. doi: 10.1080/21691401.2019.1640229. [DOI] [PubMed] [Google Scholar]
- 229.Soung Y.H., Chung H., Yan C., Ju J., Chung J. Arrestin Domain Containing 3 Reverses Epithelial to Mesenchymal Transition and Chemo-Resistance of TNBC Cells by Up-Regulating Expression of miR-200b. Cells. 2019;8:692. doi: 10.3390/cells8070692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fang S., Zeng X., Zhu W., Tang R., Chao Y., Guo L. Zinc finger E-box-binding homeobox 2 (ZEB2) regulated by miR-200b contributes to multi-drug resistance of small cell lung cancer. Exp. Mol. Pathol. 2014;96:438–444. doi: 10.1016/j.yexmp.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 231.Vinay D.S., Ryan E.P., Pawelec G., Talib W.H., Stagg J., Elkord E., Lichtor T., Decker W.K., Whelan R.L., Kumara H.S. Seminars in Cancer Biology. Elsevier; Amsterdam, The Netherlands: 2015. Immune evasion in cancer: Mechanistic basis and therapeutic strategies; pp. S185–S198. [DOI] [PubMed] [Google Scholar]
- 232.Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int. Immunol. 2016;28:383–391. doi: 10.1093/intimm/dxw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Davis R.J., Van Waes C., Allen C.T. Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and Tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol. 2016;58:59–70. doi: 10.1016/j.oraloncology.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Restifo N.P., Dudley M.E., Rosenberg S.A. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat. Rev. Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wherry E.J. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 236.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.-J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chen L., Flies D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hamid O., Robert C., Daud A., Hodi F.S., Hwu W.-J., Kefford R., Wolchok J.D., Hersey P., Joseph R.W., Weber J.S. Safety and tumor responses with lambrolizumab (anti–PD-1) in melanoma. N. Engl. J. Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Youngblood B., Wherry E.J., Ahmed R. Acquired transcriptional programming in functional and exhausted virus-specific CD8 T cells. Curr. Opin. HIV AIDS. 2012;7:50. doi: 10.1097/COH.0b013e32834ddcf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 242.Freeman G.J., Sharpe A.H., Kuchroo V.K. Protect the killer: CTLs need defenses against the tumor. Nat. Med. 2002;8:787–789. doi: 10.1038/nm0802-787. [DOI] [PubMed] [Google Scholar]
- 243.Hobo W., Maas F., Adisty N., de Witte T., Schaap N., van der Voort R., Dolstra H. siRNA silencing of PD-L1 and PD-L2 on dendritic cells augments expansion and function of minor histocompatibility antigen–specific CD8+ T cells. Blood J. Am. Soc. Hematol. 2010;116:4501–4511. doi: 10.1182/blood-2010-04-278739. [DOI] [PubMed] [Google Scholar]
- 244.Zha H., Han X., Zhu Y., Yang F., Li Y., Li Q., Guo B., Zhu B. Blocking C5aR signaling promotes the anti-tumor efficacy of PD-1/PD-L1 blockade. Oncoimmunology. 2017;6:e1349587. doi: 10.1080/2162402X.2017.1349587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhao L., Liu Y., Zhang J., Liu Y., Qi Q. LncRNA SNHG14/miR-5590-3p/ZEB1 positive feedback loop promoted diffuse large B cell lymphoma progression and immune evasion through regulating PD-1/PD-L1 checkpoint. Cell Death Dis. 2019;10:1–15. doi: 10.1038/s41419-019-1886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chen L., Gibbons D.L., Goswami S., Cortez M.A., Ahn Y.H., Byers L.A., Zhang X., Yi X., Dwyer D., Lin W., et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 2014;5:5241. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Noman M.Z., Janji B., Abdou A., Hasmim M., Terry S., Tan T.Z., Mami-Chouaib F., Thiery J.P., Chouaib S. The immune checkpoint ligand PD-L1 is upregulated in EMT-activated human breast cancer cells by a mechanism involving ZEB-1 and miR-200. Oncoimmunology. 2017;6:e1263412. doi: 10.1080/2162402X.2016.1263412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wang Y., Luo Y., Guan W., Zhao H. Role of miR-23a/Zeb1 negative feedback loop in regulating epithelial-mesenchymal transition and tumorigenicity of intraocular tumors. Oncol. Lett. 2018;16:2462–2470. doi: 10.3892/ol.2018.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Majid S., Dar A.A., Saini S., Deng G., Chang I., Greene K., Tanaka Y., Dahiya R., Yamamura S. MicroRNA-23b functions as a tumor suppressor by regulating Zeb1 in bladder cancer. PLoS ONE. 2013;8:e67686. doi: 10.1371/journal.pone.0067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhang P., Huang C., Fu C., Tian Y., Hu Y., Wang B., Strasner A., Song Y., Song E. Cordycepin (3’-deoxyadenosine) suppressed HMGA2, Twist1 and ZEB1-dependent melanoma invasion and metastasis by targeting miR-33b. Oncotarget. 2015;6:9834–9853. doi: 10.18632/oncotarget.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jiang R., Zhang C., Liu G., Gu R., Wu H. MicroRNA-126 Inhibits Proliferation, Migration, Invasion, and EMT in Osteosarcoma by Targeting ZEB1. J. Cell. Biochem. 2017;118:3765–3774. doi: 10.1002/jcb.26024. [DOI] [PubMed] [Google Scholar]
- 252.Sun X., Li Y., Yu J., Pei H., Luo P., Zhang J. miR-128 modulates chemosensitivity and invasion of prostate cancer cells through targeting ZEB1. Jpn. J. Clin. Oncol. 2015;45:474–482. doi: 10.1093/jjco/hyv027. [DOI] [PubMed] [Google Scholar]
- 253.Dong P., Karaayvaz M., Jia N., Kaneuchi M., Hamada J., Watari H., Sudo S., Ju J., Sakuragi N. Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis. Oncogene. 2013;32:3286–3295. doi: 10.1038/onc.2012.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Qiu G., Lin Y., Zhang H., Wu D. miR-139-5p inhibits epithelial-mesenchymal transition, migration and invasion of hepatocellular carcinoma cells by targeting ZEB1 and ZEB2. Biochem. Biophys. Res. Commun. 2015;463:315–321. doi: 10.1016/j.bbrc.2015.05.062. [DOI] [PubMed] [Google Scholar]
- 255.Yue S., Wang L., Zhang H., Min Y., Lou Y., Sun H., Jiang Y., Zhang W., Liang A., Guo Y., et al. miR-139-5p suppresses cancer cell migration and invasion through targeting ZEB1 and ZEB2 in GBM. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015;36:6741–6749. doi: 10.1007/s13277-015-3372-8. [DOI] [PubMed] [Google Scholar]
- 256.Al-Khalaf H.H., Aboussekhra A. MicroRNA-141 and microRNA-146b-5p inhibit the prometastatic mesenchymal characteristics through the RNA-binding protein AUF1 targeting the transcription factor ZEB1 and the protein kinase AKT. J. Biol. Chem. 2014;289:31433–31447. doi: 10.1074/jbc.M114.593004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pan Y., Zhang J., Fu H., Shen L. miR-144 functions as a tumor suppressor in breast cancer through inhibiting ZEB1/2-mediated epithelial mesenchymal transition process. OncoTargets Ther. 2016;9:6247–6255. doi: 10.2147/OTT.S103650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Guan H., Liang W., Xie Z., Li H., Liu J., Liu L., Xiu L., Li Y. Down-regulation of miR-144 promotes thyroid cancer cell invasion by targeting ZEB1 and ZEB2. Endocrine. 2015;48:566–574. doi: 10.1007/s12020-014-0326-7. [DOI] [PubMed] [Google Scholar]
- 259.Yokobori T., Suzuki S., Tanaka N., Inose T., Sohda M., Sano A., Sakai M., Nakajima M., Miyazaki T., Kato H., et al. MiR-150 is associated with poor prognosis in esophageal squamous cell carcinoma via targeting the EMT inducer ZEB1. Cancer Sci. 2013;104:48–54. doi: 10.1111/cas.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Jin M., Yang Z., Ye W., Xu H., Hua X. MicroRNA-150 predicts a favorable prognosis in patients with epithelial ovarian cancer, and inhibits cell invasion and metastasis by suppressing transcriptional repressor ZEB1. PLoS ONE. 2014;9:e103965. doi: 10.1371/journal.pone.0103965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Liang L., Zhang Z., Qin X., Gao Y., Zhao P., Liu J., Zeng W. Gambogic Acid Inhibits Melanoma through Regulation of miR-199a-3p/ZEB1 Signalling. Basic Clin. Pharmacol. Toxicol. 2018;123:692–703. doi: 10.1111/bcpt.13090. [DOI] [PubMed] [Google Scholar]
- 262.Wang J., Zhou F., Yin L., Zhao L., Zhang Y., Wang J. MicroRNA-199b targets the regulation of ZEB1 expression to inhibit cell proliferation, migration and invasion in nonsmall cell lung cancer. Mol. Med. Rep. 2017;16:5007–5014. doi: 10.3892/mmr.2017.7195. [DOI] [PubMed] [Google Scholar]
- 263.Ungewiss C., Rizvi Z.H., Roybal J.D., Peng D.H., Gold K.A., Shin D.H., Creighton C.J., Gibbons D.L. The microRNA-200/Zeb1 axis regulates ECM-dependent beta1-integrin/FAK signaling, cancer cell invasion and metastasis through CRKL. Sci. Rep. 2016;6:18652. doi: 10.1038/srep18652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Asakura T., Yamaguchi N., Ohkawa K., Yoshida K. Proteasome inhibitor-resistant cells cause EMT-induction via suppression of E-cadherin by miR-200 and ZEB1. Int. J. Oncol. 2015;46:2251–2260. doi: 10.3892/ijo.2015.2916. [DOI] [PubMed] [Google Scholar]
- 265.Huang W.T., Kuo S.H., Cheng A.L., Lin C.W. Inhibition of ZEB1 by miR-200 characterizes Helicobacter pylori-positive gastric diffuse large B-cell lymphoma with a less aggressive behavior. Mod. Pathol. Off. J. USA Can. Acad. Pathol. Inc. 2014;27:1116–1125. doi: 10.1038/modpathol.2013.229. [DOI] [PubMed] [Google Scholar]
- 266.Title A.C., Hong S.J., Pires N.D., Hasenohrl L., Godbersen S., Stokar-Regenscheit N., Bartel D.P., Stoffel M. Genetic dissection of the miR-200-Zeb1 axis reveals its importance in tumor differentiation and invasion. Nat. Commun. 2018;9:4671. doi: 10.1038/s41467-018-07130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Li Y., Zeng C., Tu M., Jiang W., Dai Z., Hu Y., Deng Z., Xiao W. MicroRNA-200b acts as a tumor suppressor in osteosarcoma via targeting ZEB1. OncoTargets Ther. 2016;9:3101–3111. doi: 10.2147/ott.s96561. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 268.Tsai S.C., Lin C.C., Shih T.C., Tseng R.J., Yu M.C., Lin Y.J., Hsieh S.Y. The miR-200b-ZEB1 circuit regulates diverse stemness of human hepatocellular carcinoma. Mol. Carcinog. 2017;56:2035–2047. doi: 10.1002/mc.22657. [DOI] [PubMed] [Google Scholar]
- 269.Nishijima N., Seike M., Soeno C., Chiba M., Miyanaga A., Noro R., Sugano T., Matsumoto M., Kubota K., Gemma A. miR-200/ZEB axis regulates sensitivity to nintedanib in non-small cell lung cancer cells. Int. J. Oncol. 2016;48:937–944. doi: 10.3892/ijo.2016.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chen M.L., Liang L.S., Wang X.K. miR-200c inhibits invasion and migration in human colon cancer cells SW480/620 by targeting ZEB1. Clin. Exp. Metastasis. 2012;29:457–469. doi: 10.1007/s10585-012-9463-7. [DOI] [PubMed] [Google Scholar]
- 271.Kurata A., Yamada M., Ohno S.I., Inoue S., Hashimoto H., Fujita K., Takanashi M., Kuroda M. Expression level of microRNA-200c is associated with cell morphology in vitro and histological differentiation through regulation of ZEB1/2 and E-cadherin in gastric carcinoma. Oncol. Rep. 2018;39:91–100. doi: 10.3892/or.2017.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Shan Y., Zhang L., Bao Y., Li B., He C., Gao M., Feng X., Xu W., Zhang X., Wang S. Epithelial-mesenchymal transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail, ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J. Nutr. Biochem. 2013;24:1062–1069. doi: 10.1016/j.jnutbio.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 273.Karagur E.R., Ozay C., Mammadov R., Akca H. Anti-invasive effect of Cyclamen pseudibericum extract on A549 non-small cell lung carcinoma cells via inhibition of ZEB1 mediated by miR-200c. J. Nat. Med. 2018;72:686–693. doi: 10.1007/s11418-018-1204-z. [DOI] [PubMed] [Google Scholar]
- 274.Zhou G., Zhang F., Guo Y., Huang J., Xie Y., Yue S., Chen M., Jiang H., Li M. miR-200c enhances sensitivity of drug-resistant non-small cell lung cancer to gefitinib by suppression of PI3K/Akt signaling pathway and inhibites cell migration via targeting ZEB1. Biomed. Pharmacother. 2017;85:113–119. doi: 10.1016/j.biopha.2016.11.100. [DOI] [PubMed] [Google Scholar]
- 275.Gao H.X., Yan L., Li C., Zhao L.M., Liu W. miR-200c regulates crizotinib-resistant ALK-positive lung cancer cells by reversing epithelial-mesenchymal transition via targeting ZEB1. Mol. Med. Rep. 2016;14:4135–4143. doi: 10.3892/mmr.2016.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Guo E., Wang Z., Wang S. MiR-200c and miR-141 inhibit ZEB1 synergistically and suppress glioma cell growth and migration. Eur. Rev. Med. Pharmacol. Sci. 2016;20:3385–3391. [PubMed] [Google Scholar]
- 277.Zhou X., Wang Y., Shan B., Han J., Zhu H., Lv Y., Fan X., Sang M., Liu X.D., Liu W. The downregulation of miR-200c/141 promotes ZEB1/2 expression and gastric cancer progression. Med. Oncol. (Northwood Lond. Engl.) 2015;32:428. doi: 10.1007/s12032-014-0428-3. [DOI] [PubMed] [Google Scholar]
- 278.Singh T., Prasad R., Katiyar S.K. Therapeutic intervention of silymarin on the migration of non-small cell lung cancer cells is associated with the axis of multiple molecular targets including class 1 HDACs, ZEB1 expression, and restoration of miR-203 and E-cadherin expression. Am. J. Cancer Res. 2016;6:1287–1301. [PMC free article] [PubMed] [Google Scholar]
- 279.Wu G., Wang J., Chen G., Zhao X. microRNA-204 modulates chemosensitivity and apoptosis of prostate cancer cells by targeting zinc-finger E-box-binding homeobox 1 (ZEB1) Am. J. Transl. Res. 2017;9:3599–3610. [PMC free article] [PubMed] [Google Scholar]
- 280.Niu K., Shen W., Zhang Y., Zhao Y., Lu Y. MiR-205 promotes motility of ovarian cancer cells via targeting ZEB1. Gene. 2015;574:330–336. doi: 10.1016/j.gene.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 281.El Bezawy R., Tinelli S., Tortoreto M., Doldi V., Zuco V., Folini M., Stucchi C., Rancati T., Valdagni R., Gandellini P., et al. miR-205 enhances radiation sensitivity of prostate cancer cells by impairing DNA damage repair through PKCepsilon and ZEB1 inhibition. J. Exp. Clin. Cancer Res. CR. 2019;38:51. doi: 10.1186/s13046-019-1060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhang P., Wang L., Rodriguez-Aguayo C., Yuan Y., Debeb B.G., Chen D., Sun Y., You M.J., Liu Y., Dean D.C., et al. miR-205 acts as a tumour radiosensitizer by targeting ZEB1 and Ubc13. Nat. Commun. 2014;5:5671. doi: 10.1038/ncomms6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Li L., Li S. miR-205-5p inhibits cell migration and invasion in prostatic carcinoma by targeting ZEB1. Oncol. Lett. 2018;16:1715–1721. doi: 10.3892/ol.2018.8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Hou L.K., Yu Y., Xie Y.G., Wang J., Mao J.F., Zhang B., Wang X., Cao X.C. miR-340 and ZEB1 negative feedback loop regulates TGF-beta- mediated breast cancer progression. Oncotarget. 2016;7:26016–26026. doi: 10.18632/oncotarget.8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Yan H., Zhang B., Fang C., Chen L. miR-340 alleviates chemoresistance of osteosarcoma cells by targeting ZEB1. Anti-Cancer Drugs. 2018;29:440–448. doi: 10.1097/CAD.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 286.Barbachano A., Fernandez-Barral A., Pereira F., Segura M.F., Ordonez-Moran P., Carrillo-de Santa Pau E., Gonzalez-Sancho J.M., Hanniford D., Martinez N., Costales-Carrera A., et al. SPROUTY-2 represses the epithelial phenotype of colon carcinoma cells via upregulation of ZEB1 mediated by ETS1 and miR-200/miR-150. Oncogene. 2016;35:2991–3003. doi: 10.1038/onc.2015.366. [DOI] [PubMed] [Google Scholar]
- 287.Zou J., Liu L., Wang Q., Yin F., Yang Z., Zhang W., Li L. Downregulation of miR-429 contributes to the development of drug resistance in epithelial ovarian cancer by targeting ZEB1. Am. J. Transl. Res. 2017;9:1357–1368. [PMC free article] [PubMed] [Google Scholar]
- 288.Lei W., Liu Y.E., Zheng Y., Qu L. MiR-429 inhibits oral squamous cell carcinoma growth by targeting ZEB1. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015;21:383–389. doi: 10.12659/msm.893412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Sun K., Zeng T., Huang D., Liu Z., Huang S., Liu J., Qu Z. MicroRNA-431 inhibits migration and invasion of hepatocellular carcinoma cells by targeting the ZEB1-mediated epithelial-mensenchymal transition. FEBS Open Bio. 2015;5:900–907. doi: 10.1016/j.fob.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 290.Ma P., Ni K., Ke J., Zhang W., Feng Y., Mao Q. miR-448 inhibits the epithelial-mesenchymal transition in breast cancer cells by directly targeting the E-cadherin repressor ZEB1/2. Exp. Biol. Med. (Maywood, NJ, USA) 2018;243:473–480. doi: 10.1177/1535370218754848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Li Y.J., Ping C., Tang J., Zhang W. MicroRNA-455 suppresses non-small cell lung cancer through targeting ZEB1. Cell Biol. Int. 2016;40:621–628. doi: 10.1002/cbin.10584. [DOI] [PubMed] [Google Scholar]
- 292.Hu Y., Xie H., Liu Y., Liu W., Liu M., Tang H. miR-484 suppresses proliferation and epithelial-mesenchymal transition by targeting ZEB1 and SMAD2 in cervical cancer cells. Cancer Cell Int. 2017;17:36. doi: 10.1186/s12935-017-0407-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 293.Gao H., Li X., Zhan G., Zhu Y., Yu J., Wang J., Li L., Wu W., Liu N., Guo X. Long noncoding RNA MAGI1-IT1 promoted invasion and metastasis of epithelial ovarian cancer via the miR-200a/ZEB axis. Cell Cycle. 2019;18:1393–1406. doi: 10.1080/15384101.2019.1618121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 294.Guo S.J., Zeng H.X., Huang P., Wang S., Xie C.H., Li S.J. MiR-508-3p inhibits cell invasion and epithelial-mesenchymal transition by targeting ZEB1 in triple-negative breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2018;22:6379–6385. doi: 10.26355/eurrev_201810_16050. [DOI] [PubMed] [Google Scholar]
- 295.Wang M., Zhang R., Zhang S., Xu R., Yang Q. MicroRNA-574-3p regulates epithelial mesenchymal transition and cisplatin resistance via targeting ZEB1 in human gastric carcinoma cells. Gene. 2019;700:110–119. doi: 10.1016/j.gene.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 296.Pang H., Zheng Y., Zhao Y., Xiu X., Wang J. miR-590-3p suppresses cancer cell migration, invasion and epithelial-mesenchymal transition in glioblastoma multiforme by targeting ZEB1 and ZEB2. Biochem. Biophys. Res. Commun. 2015;468:739–745. doi: 10.1016/j.bbrc.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 297.Yao R., Zheng H., Wu L., Cai P. miRNA-641 inhibits the proliferation, migration, and invasion and induces apoptosis of cervical cancer cells by directly targeting ZEB1. OncoTargets Ther. 2018;11:8965–8976. doi: 10.2147/OTT.S190303. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 298.Deng S., Li X., Niu Y., Zhu S., Jin Y., Deng S., Chen J., Liu Y., He C., Yin T., et al. MiR-652 inhibits acidic microenvironment-induced epithelial-mesenchymal transition of pancreatic cancer cells by targeting ZEB1. Oncotarget. 2015;6:39661–39675. doi: 10.18632/oncotarget.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Harazono Y., Muramatsu T., Endo H., Uzawa N., Kawano T., Harada K., Inazawa J., Kozaki K. miR-655 Is an EMT-suppressive microRNA targeting ZEB1 and TGFBR2. PLoS ONE. 2013;8:e62757. doi: 10.1371/journal.pone.0062757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wang J., Zhang Y., Wei H., Zhang X., Wu Y., Gong A., Xia Y., Wang W., Xu M. The mir-675-5p regulates the progression and development of pancreatic cancer via the UBQLN1-ZEB1-mir200 axis. Oncotarget. 2017;8:24978–24987. doi: 10.18632/oncotarget.15330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Li G., Xu Y., Wang S., Yan W., Zhao Q., Guo J. MiR-873-5p inhibits cell migration, invasion and epithelial-mesenchymal transition in colorectal cancer via targeting ZEB1. Pathol. Res. Pract. 2019;215:34–39. doi: 10.1016/j.prp.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 302.El Bezawy R., Cominetti D., Fenderico N., Zuco V., Beretta G.L., Dugo M., Arrighetti N., Stucchi C., Rancati T., Valdagni R., et al. miR-875-5p counteracts epithelial-to-mesenchymal transition and enhances radiation response in prostate cancer through repression of the EGFR-ZEB1 axis. Cancer Lett. 2017;395:53–62. doi: 10.1016/j.canlet.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 303.Liu H., Wang H., Liu X., Yu T. miR-1271 inhibits migration, invasion and epithelial-mesenchymal transition by targeting ZEB1 and TWIST1 in pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2016;472:346–352. doi: 10.1016/j.bbrc.2016.02.096. [DOI] [PubMed] [Google Scholar]
- 304.Wang Y., Yan S., Liu X., Zhang W., Li Y., Dong R., Zhang Q., Yang Q., Yuan C., Shen K., et al. miR-1236-3p represses the cell migration and invasion abilities by targeting ZEB1 in high-grade serous ovarian carcinoma. Oncol. Rep. 2014;31:1905–1910. doi: 10.3892/or.2014.3046. [DOI] [PubMed] [Google Scholar]
- 305.Liang T.C., Fu W.G., Zhong Y.S. MicroRNA-1236-3p inhibits proliferation and invasion of breast cancer cells by targeting ZEB1. Eur. Rev. Med. Pharmacol. Sci. 2019;23:9988–9995. doi: 10.26355/eurrev_201911_19565. [DOI] [PubMed] [Google Scholar]
- 306.Zhu L., Liu Z., Dong R., Wang X., Zhang M., Guo X., Yu N., Zeng A. MicroRNA-3662 targets ZEB1 and attenuates the invasion of the highly aggressive melanoma cell line A375. Cancer Manag. Res. 2019;11:5845–5856. doi: 10.2147/CMAR.S200540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zhang C., Wang L., Yang J., Fu Y., Li H., Xie L., Cui Y. MicroRNA-33a-5p suppresses esophageal squamous cell carcinoma progression via regulation of lncRNA DANCR and ZEB1. Eur. J. Pharmacol. 2019;861:172590. doi: 10.1016/j.ejphar.2019.172590. [DOI] [PubMed] [Google Scholar]
- 308.Chang L., Yuan Y., Li C., Guo T., Qi H., Xiao Y., Dong X., Liu Z., Liu Q. Upregulation of SNHG6 regulates ZEB1 expression by competitively binding miR-101-3p and interacting with UPF1 in hepatocellular carcinoma. Cancer Lett. 2016;383:183–194. doi: 10.1016/j.canlet.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 309.Liang H., Yu T., Han Y., Jiang H., Wang C., You T., Zhao X., Shan H., Yang R., Yang L., et al. LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol. Cancer. 2018;17:119. doi: 10.1186/s12943-018-0870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Li Y., Lv M., Song Z., Lou Z., Wang R., Zhuang M. Long non-coding RNA NNT-AS1 affects progression of breast cancer through miR-142-3p/ZEB1 axis. Biomed. Pharmacother. 2018;103:939–946. doi: 10.1016/j.biopha.2018.04.087. [DOI] [PubMed] [Google Scholar]
- 311.He C., Liu Z., Jin L., Zhang F., Peng X., Xiao Y., Wang X., Lyu Q., Cai X. lncRNA TUG1-Mediated Mir-142-3p Downregulation Contributes to Metastasis and the Epithelial-to-Mesenchymal Transition of Hepatocellular Carcinoma by Targeting ZEB1. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018;48:1928–1941. doi: 10.1159/000492517. [DOI] [PubMed] [Google Scholar]
- 312.Zhang K., Chen J., Song H., Chen L.B. SNHG16/miR-140-5p axis promotes esophagus cancer cell proliferation, migration and EMT formation through regulating ZEB1. Oncotarget. 2018;9:1028–1040. doi: 10.18632/oncotarget.23178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhu C., Cheng D., Qiu X., Zhuang M., Liu Z. Long Noncoding RNA SNHG16 Promotes Cell Proliferation by Sponging MicroRNA-205 and Upregulating ZEB1 Expression in Osteosarcoma. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018;51:429–440. doi: 10.1159/000495239. [DOI] [PubMed] [Google Scholar]
- 314.Wang B., Qu X.L., Liu J., Lu J., Zhou Z.Y. HOTAIR promotes osteosarcoma development by sponging miR-217 and targeting ZEB1. J. Cell. Physiol. 2019;234:6173–6181. doi: 10.1002/jcp.27394. [DOI] [PubMed] [Google Scholar]
- 315.Yang T., He X., Chen A., Tan K., Du X. LncRNA HOTAIR contributes to the malignancy of hepatocellular carcinoma by enhancing epithelial-mesenchymal transition via sponging miR-23b-3p from ZEB1. Gene. 2018;670:114–122. doi: 10.1016/j.gene.2018.05.061. [DOI] [PubMed] [Google Scholar]
- 316.Xue M., Pang H., Li X., Li H., Pan J., Chen W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016;107:18–27. doi: 10.1111/cas.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Liang C., Yang Y., Guan J., Lv T., Qu S., Fu Q., Zhao H. LncRNA UCA1 sponges miR-204-5p to promote migration, invasion and epithelial-mesenchymal transition of glioma cells via upregulation of ZEB1. Pathol. Res. Pract. 2018;214:1474–1481. doi: 10.1016/j.prp.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 318.Meng L., Ma P., Cai R., Guan Q., Wang M., Jin B. Long noncoding RNA ZEB1-AS1 promotes the tumorigenesis of glioma cancer cells by modulating the miR-200c/141-ZEB1 axis. Am. J. Transl. Res. 2018;10:3395–3412. [PMC free article] [PubMed] [Google Scholar]
- 319.Qu R., Chen X., Zhang C. LncRNA ZEB1-AS1/miR-409-3p/ZEB1 feedback loop is involved in the progression of non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2018;507:450–456. doi: 10.1016/j.bbrc.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 320.Xiong W.C., Han N., Wu N., Zhao K.L., Han C., Wang H.X., Ping G.F., Zheng P.F., Feng H., Qin L., et al. Interplay between long noncoding RNA ZEB1-AS1 and miR-101/ZEB1 axis regulates proliferation and migration of colorectal cancer cells. Am. J. Transl. Res. 2018;10:605–617. [PMC free article] [PubMed] [Google Scholar]
- 321.Jin H., Jin X., Chai W., Yin Z., Li Y., Dong F., Wang W. Long non-coding RNA MIAT competitively binds miR-150-5p to regulate ZEB1 expression in osteosarcoma. Oncol. Lett. 2019;17:1229–1236. doi: 10.3892/ol.2018.9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Gao H., Li S.P., Xu H.X., Yu Y., He J.D., Wang Z., Xu Y.J., Wang C.Y., Zhang H.M., Zhang R.X., et al. LncRNA HULC enhances epithelial-mesenchymal transition to promote tumorigenesis and metastasis of hepatocellular carcinoma via the miR-200a-3p/ZEB1 signaling pathway. Oncotarget. 2016;7:42431–42446. doi: 10.18632/oncotarget.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lu Y., Li T., Wei G., Liu L., Chen Q., Xu L., Zhang K., Zeng D., Liao R. The long non-coding RNA NEAT1 regulates epithelial to mesenchymal transition and radioresistance in through miR-204/ZEB1 axis in nasopharyngeal carcinoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016;37:11733–11741. doi: 10.1007/s13277-015-4773-4. [DOI] [PubMed] [Google Scholar]
- 324.Zhong Q., Chen Y., Chen Z. LncRNA MINCR regulates irradiation resistance in nasopharyngeal carcinoma cells via the microRNA-223/ZEB1 axis. Cell Cycle (Georgetown, Tex.) 2020;19:53–66. doi: 10.1080/15384101.2019.1692176. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 325.Yuan G., Quan J., Dong D., Wang Q. Long Noncoding RNA CAT104 Promotes Cell Viability, Migration, and Invasion in Gastric Carcinoma Cells Through Activation of MicroRNA-381-Inhibiting Zinc Finger E-box-Binding Homeobox 1 (ZEB1) Expression. Oncol. Res. 2018;26:1037–1046. doi: 10.3727/096504017X15144748428127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wang P.S., Chou C.H., Lin C.H., Yao Y.C., Cheng H.C., Li H.Y., Chuang Y.C., Yang C.N., Ger L.P., Chen Y.C., et al. A novel long non-coding RNA linc-ZNF469-3 promotes lung metastasis through miR-574-5p-ZEB1 axis in triple negative breast cancer. Oncogene. 2018;37:4662–4678. doi: 10.1038/s41388-018-0293-1. [DOI] [PubMed] [Google Scholar]
- 327.Zheng L., Xu M., Xu J., Wu K., Fang Q., Liang Y., Zhou S., Cen D., Ji L., Han W., et al. ELF3 promotes epithelial-mesenchymal transition by protecting ZEB1 from miR-141-3p-mediated silencing in hepatocellular carcinoma. Cell Death Dis. 2018;9:387. doi: 10.1038/s41419-018-0399-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Guo L., Chen C., Shi M., Wang F., Chen X., Diao D., Hu M., Yu M., Qian L., Guo N. Stat3-coordinated Lin-28-let-7-HMGA2 and miR-200-ZEB1 circuits initiate and maintain oncostatin M-driven epithelial-mesenchymal transition. Oncogene. 2013;32:5272–5282. doi: 10.1038/onc.2012.573. [DOI] [PubMed] [Google Scholar]
- 329.Zhang N., Liu Y., Wang Y., Zhao M., Tu L., Luo F. Decitabine reverses TGF-beta1-induced epithelial-mesenchymal transition in non-small-cell lung cancer by regulating miR-200/ZEB axis. Drug Des. Dev. Ther. 2017;11:969–983. doi: 10.2147/DDDT.S129305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Chung V.Y., Tan T.Z., Tan M., Wong M.K., Kuay K.T., Yang Z., Ye J., Muller J., Koh C.M., Guccione E., et al. GRHL2-miR-200-ZEB1 maintains the epithelial status of ovarian cancer through transcriptional regulation and histone modification. Sci. Rep. 2016;6:19943. doi: 10.1038/srep19943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Kong X., Ding X., Li X., Gao S., Yang Q. 53BP1 suppresses epithelial-mesenchymal transition by downregulating ZEB1 through microRNA-200b/429 in breast cancer. Cancer Sci. 2015;106:982–989. doi: 10.1111/cas.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Lee J.Y., Park M.K., Park J.H., Lee H.J., Shin D.H., Kang Y., Lee C.H., Kong G. Loss of the polycomb protein Mel-18 enhances the epithelial-mesenchymal transition by ZEB1 and ZEB2 expression through the downregulation of miR-205 in breast cancer. Oncogene. 2014;33:1325–1335. doi: 10.1038/onc.2013.53. [DOI] [PubMed] [Google Scholar]
- 333.Zhao W., Wang H., Han X., Ma J., Zhou Y., Chen Z., Zhou H., Xu H., Sun Z., Kong B., et al. DeltaNp63alpha attenuates tumor aggressiveness by suppressing miR-205/ZEB1-mediated epithelial-mesenchymal transition in cervical squamous cell carcinoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016;37:10621–10632. doi: 10.1007/s13277-016-4921-5. [DOI] [PubMed] [Google Scholar]
- 334.Bian Y., Gao G., Zhang Q., Qian H., Yu L., Yao N., Qian J., Liu B., Qian X. KCNQ1OT1/miR-217/ZEB1 feedback loop facilitates cell migration and epithelial-mesenchymal transition in colorectal cancer. Cancer Biol. Ther. 2019;20:886–896. doi: 10.1080/15384047.2019.1579959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Xiao T., Xue J., Shi M., Chen C., Luo F., Xu H., Chen X., Sun B., Sun Q., Yang Q., et al. Circ008913, via miR-889 regulation of DAB2IP/ZEB1, is involved in the arsenite-induced acquisition of CSC-like properties by human keratinocytes in carcinogenesis. Met. Integr. Biometal Sci. 2018;10:1328–1338. doi: 10.1039/C8MT00207J. [DOI] [PubMed] [Google Scholar]
- 336.Wang H., An X., Yu H., Zhang S., Tang B., Zhang X., Li Z. MiR-29b/TET1/ZEB2 signaling axis regulates metastatic properties and epithelial-mesenchymal transition in breast cancer cells. Oncotarget. 2017;8:102119–102133. doi: 10.18632/oncotarget.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Chen Z., Zhang J., Zhang Z., Feng Z., Wei J., Lu J., Fang Y., Liang Y., Cen J., Pan Y., et al. The putative tumor suppressor microRNA-30a-5p modulates clear cell renal cell carcinoma aggressiveness through repression of ZEB2. Cell Death Dis. 2017;8:e2859. doi: 10.1038/cddis.2017.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Ji H., Sang M., Liu F., Ai N., Geng C. miR-124 regulates EMT based on ZEB2 target to inhibit invasion and metastasis in triple-negative breast cancer. Pathol. Res. Pract. 2019;215:697–704. doi: 10.1016/j.prp.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 339.Li X., Li C., Bi H., Bai S., Zhao L., Zhang J., Qi C. Targeting ZEB2 By microRNA-129 In Non-Small Cell Lung Cancer Suppresses Cell Proliferation, Invasion And Migration Via Regulating Wnt/beta-Catenin Signaling Pathway And Epithelial-Mesenchymal Transition. OncoTargets Ther. 2019;12:9165–9175. doi: 10.2147/OTT.S217536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Zheng Y.B., Luo H.P., Shi Q., Hao Z.N., Ding Y., Wang Q.S., Li S.B., Xiao G.C., Tong S.L. miR-132 inhibits colorectal cancer invasion and metastasis via directly targeting ZEB2. World J. Gastroenterol. 2014;20:6515–6522. doi: 10.3748/wjg.v20.i21.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.You J., Li Y., Fang N., Liu B., Zu L., Chang R., Li X., Zhou Q. MiR-132 suppresses the migration and invasion of lung cancer cells via targeting the EMT regulator ZEB2. PLoS ONE. 2014;9:e91827. doi: 10.1371/journal.pone.0091827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wu S.-M., Ai H.-W., Zhang D.-Y., Han X.-Q., Pan Q., Luo F.-L., Zhang X.-L. MiR-141 targets ZEB2 to suppress HCC progression. Tumor Biol. 2014;35:9993–9997. doi: 10.1007/s13277-014-2299-9. [DOI] [PubMed] [Google Scholar]
- 343.Li W., Wang Q., Su Q., Ma D., An C., Ma L., Liang H. Honokiol suppresses renal cancer cells’ metastasis via dual-blocking epithelial-mesenchymal transition and cancer stem cell properties through modulating miR-141/ZEB2 signaling. Mol. Cells. 2014;37:383–388. doi: 10.14348/molcells.2014.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ren D., Wang M., Guo W., Huang S., Wang Z., Zhao X., Du H., Song L., Peng X. Double-negative feedback loop between ZEB2 and miR-145 regulates epithelial-mesenchymal transition and stem cell properties in prostate cancer cells. Cell Tissue Res. 2014;358:763–778. doi: 10.1007/s00441-014-2001-y. [DOI] [PubMed] [Google Scholar]
- 345.Jiang S.B., He X.J., Xia Y.J., Hu W.J., Luo J.G., Zhang J., Tao H.Q. MicroRNA-145-5p inhibits gastric cancer invasiveness through targeting N-cadherin and ZEB2 to suppress epithelial-mesenchymal transition. OncoTargets Ther. 2016;9:2305–2315. doi: 10.2147/ott.s101853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 346.Zhou J., Xie M., Shi Y., Luo B., Gong G., Li J., Wang J., Zhao W., Zi Y., Wu X., et al. MicroRNA-153 functions as a tumor suppressor by targeting SET7 and ZEB2 in ovarian cancer cells. Oncol. Rep. 2015;34:111–120. doi: 10.3892/or.2015.3952. [DOI] [PubMed] [Google Scholar]
- 347.Lin X., Yang Z., Zhang P., Liu Y., Shao G. miR-154 inhibits migration and invasion of human non-small cell lung cancer by targeting ZEB2. Oncol. Lett. 2016;12:301–306. doi: 10.3892/ol.2016.4577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 348.Pang X., Huang K., Zhang Q., Zhang Y., Niu J. miR-154 targeting ZEB2 in hepatocellular carcinoma functions as a potential tumor suppressor. Oncol. Rep. 2015;34:3272–3279. doi: 10.3892/or.2015.4321. [DOI] [PubMed] [Google Scholar]
- 349.Fei D., Zhao K., Yuan H., Xing J., Zhao D. MicroRNA-187 exerts tumor-suppressing functions in osteosarcoma by targeting ZEB2. Am. J. Cancer Res. 2016;6:2859–2868. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 350.Bendoraite A., Knouf E.C., Garg K.S., Parkin R.K., Kroh E.M., O’Briant K.C., Ventura A.P., Godwin A.K., Karlan B.Y., Drescher C.W., et al. Regulation of miR-200 family microRNAs and ZEB transcription factors in ovarian cancer: Evidence supporting a mesothelial-to-epithelial transition. Gynecol. Oncol. 2010;116:117–125. doi: 10.1016/j.ygyno.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Perdigao-Henriques R., Petrocca F., Altschuler G., Thomas M., Le M., Tan S., Hide W., Lieberman J. miR-200 promotes the mesenchymal to epithelial transition by suppressing multiple members of the Zeb2 and Snail1 transcriptional repressor complexes. Oncogene. 2016;35:158–172. doi: 10.1038/onc.2015.69. [DOI] [PubMed] [Google Scholar]
- 352.Xia H., Ng S.S., Jiang S., Cheung W.K., Sze J., Bian X.-W., Kung H.-f., Lin M.C. miR-200a-mediated downregulation of ZEB2 and CTNNB1 differentially inhibits nasopharyngeal carcinoma cell growth, migration and invasion. Biochem. Biophys. Res. Commun. 2010;391:535–541. doi: 10.1016/j.bbrc.2009.11.093. [DOI] [PubMed] [Google Scholar]
- 353.Yang X., Wang J., Qu S., Zhang H., Ruan B., Gao Y., Ma B., Wang X., Wu N., Li X., et al. MicroRNA-200a suppresses metastatic potential of side population cells in human hepatocellular carcinoma by decreasing ZEB2. Oncotarget. 2015;6:7918–7929. doi: 10.18632/oncotarget.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Wu Q., Guo R., Lin M., Zhou B., Wang Y. MicroRNA-200a inhibits CD133/1+ ovarian cancer stem cells migration and invasion by targeting E-cadherin repressor ZEB2. Gynecol. Oncol. 2011;122:149–154. doi: 10.1016/j.ygyno.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 355.Kurashige J., Kamohara H., Watanabe M., Hiyoshi Y., Iwatsuki M., Tanaka Y., Kinoshita K., Saito S., Baba Y., Baba H. MicroRNA-200b regulates cell proliferation, invasion, and migration by directly targeting ZEB2 in gastric carcinoma. Ann. Surg. Oncol. 2012;19(Suppl. 3):S656–S664. doi: 10.1245/s10434-012-2217-6. [DOI] [PubMed] [Google Scholar]
- 356.Li J., Yuan J., Yuan X., Zhao J., Zhang Z., Weng L., Liu J. MicroRNA-200b inhibits the growth and metastasis of glioma cells via targeting ZEB2. Int. J. Oncol. 2016;48:541–550. doi: 10.3892/ijo.2015.3267. [DOI] [PubMed] [Google Scholar]
- 357.Lu Y.-m., Shang C., Ou Y.-l., Yin D., Li Y.-N., Li X., Wang N., Zhang S.-l. miR-200c modulates ovarian cancer cell metastasis potential by targeting zinc finger E-box-binding homeobox 2 (ZEB2) expression. Med. Oncol. 2014;31:134. doi: 10.1007/s12032-014-0134-1. [DOI] [PubMed] [Google Scholar]
- 358.Jiao A., Sui M., Zhang L., Sun P., Geng D., Zhang W., Wang X., Li J. MicroRNA-200c inhibits the metastasis of non-small cell lung cancer cells by targeting ZEB2, an epithelial-mesenchymal transition regulator. Mol. Med. Rep. 2016;13:3349–3355. doi: 10.3892/mmr.2016.4901. [DOI] [PubMed] [Google Scholar]
- 359.Zhang J., Zhang H., Qin Y., Chen C., Yang J., Song N., Gu M. MicroRNA-200c-3p/ZEB2 loop plays a crucial role in the tumor progression of prostate carcinoma. Ann. Transl. Med. 2019;7:141. doi: 10.21037/atm.2019.02.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Duan X., Fu Z., Gao L., Zhou J., Deng X., Luo X., Fang W., Luo R. Direct interaction between miR-203 and ZEB2 suppresses epithelial–mesenchymal transition signaling and reduces lung adenocarcinoma chemoresistance. Acta Biochim. Biophys. Sin. 2016;48:1042–1049. doi: 10.1093/abbs/gmw099. [DOI] [PubMed] [Google Scholar]
- 361.Jiang Q., Zhou Y., Yang H., Li L., Deng X., Cheng C., Xie Y., Luo X., Fang W., Liu Z. A directly negative interaction of miR-203 and ZEB2 modulates tumor stemness and chemotherapy resistance in nasopharyngeal carcinoma. Oncotarget. 2016;7:67288. doi: 10.18632/oncotarget.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Chen Z., Tang Z.Y., He Y., Liu L.F., Li D.J., Chen X. miRNA-205 is a candidate tumor suppressor that targets ZEB2 in renal cell carcinoma. Oncology Res. Treat. 2014;37:658–664. doi: 10.1159/000368792. [DOI] [PubMed] [Google Scholar]
- 363.Chen X.F., Guo J.F., Xu J.F., Yin S.H., Cao W.L. MiRNA-206 inhibits proliferation of renal clear cell carcinoma by targeting ZEB2. Eur. Rev. Med. Pharmacol. Sci. 2019;23:7826–7834. doi: 10.26355/eurrev_201909_18992. [DOI] [PubMed] [Google Scholar]
- 364.Hou Y., Zhen J., Xu X., Zhen K., Zhu B., Pan R., Zhao C. miR-215 functions as a tumor suppressor and directly targets ZEB2 in human non-small cell lung cancer. Oncol. Lett. 2015;10:1985–1992. doi: 10.3892/ol.2015.3587. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 365.Sun Z., Zhang Z., Liu Z., Qiu B., Liu K., Dong G. MicroRNA-335 inhibits invasion and metastasis of colorectal cancer by targeting ZEB2. Med. Oncol. (Northwood Lond. Engl.) 2014;31:982. doi: 10.1007/s12032-014-0982-8. [DOI] [PubMed] [Google Scholar]
- 366.Kan Q., Su Y., Yang H. MicroRNA-335 is downregulated in papillary thyroid cancer and suppresses cancer cell growth, migration and invasion by directly targeting ZEB2. Oncol. Lett. 2017;14:7622–7628. doi: 10.3892/ol.2017.7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Huang N., Wu Z., Lin L., Zhou M., Wang L., Ma H., Xia J., Bin J., Liao Y., Liao W. MiR-338-3p inhibits epithelial-mesenchymal transition in gastric cancer cells by targeting ZEB2 and MACC1/Met/Akt signaling. Oncotarget. 2015;6:15222. doi: 10.18632/oncotarget.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Cui J., Pan G., He Q., Yin L., Guo R., Bi H. MicroRNA-545 targets ZEB2 to inhibit the development of non-small cell lung cancer by inactivating Wnt/beta-catenin pathway. Oncol. Lett. 2019;18:2931–2938. doi: 10.3892/ol.2019.10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Tong X., Su P., Yang H., Chi F., Shen L., Feng X., Jiang H., Zhang X., Wang Z. MicroRNA-598 inhibits the proliferation and invasion of non-small cell lung cancer cells by directly targeting ZEB2. Exp. Ther. Med. 2018;16:5417–5423. doi: 10.3892/etm.2018.6825. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 370.Song Q., Pang H., Qi L., Liang C., Wang T., Wang W., Li R. Low microRNA-622 expression predicts poor prognosis and is associated with ZEB2 in glioma. OncoTargets Ther. 2019;12:7387–7397. doi: 10.2147/OTT.S218161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Wang K., Yang S., Gao Y., Zhang C., Sui Q. MicroRNA-769-3p inhibits tumor progression in glioma by suppressing ZEB2 and inhibiting the Wnt/beta-catenin signaling pathway. Oncol. Lett. 2020;19:992–1000. doi: 10.3892/ol.2019.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Xu R., Zhou F., Yu T., Xu G., Zhang J., Wang Y., Zhao L., Liu N. MicroRNA-940 inhibits epithelial-mesenchymal transition of glioma cells via targeting ZEB2. Am. J. Transl. Res. 2019;11:7351–7363. [PMC free article] [PubMed] [Google Scholar]
- 373.Gao H.B., Gao F.Z., Chen X.F. MiRNA-1179 suppresses the metastasis of hepatocellular carcinoma by interacting with ZEB2. Eur. Rev. Med. Pharmacol. Sci. 2019;23:5149–5157. doi: 10.26355/eurrev_201906_18179. [DOI] [PubMed] [Google Scholar]
- 374.Liu Q., Liu H., Cheng H., Li Y., Li X., Zhu C. Downregulation of long noncoding RNA TUG1 inhibits proliferation and induces apoptosis through the TUG1/miR-142/ZEB2 axis in bladder cancer cells. OncoTargets Ther. 2017;10:2461–2471. doi: 10.2147/OTT.S124595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Li C., Lu L., Feng B., Zhang K., Han S., Hou D., Chen L., Chu X., Wang R. The lincRNA-ROR/miR-145 axis promotes invasion and metastasis in hepatocellular carcinoma via induction of epithelial-mesenchymal transition by targeting ZEB2. Sci. Rep. 2017;7:4637. doi: 10.1038/s41598-017-04113-w. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 376.Xiao H., Tang K., Liu P., Chen K., Hu J., Zeng J., Xiao W., Yu G., Yao W., Zhou H. LncRNA MALAT1 functions as a competing endogenous RNA to regulate ZEB2 expression by sponging miR-200s in clear cell kidney carcinoma. Oncotarget. 2015;6:38005. doi: 10.18632/oncotarget.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Wang Y., Zhou Y., Yang Z., Chen B., Huang W., Liu Y., Zhang Y. MiR-204/ZEB2 axis functions as key mediator for MALAT1-induced epithelial-mesenchymal transition in breast cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2017;39:1010428317690998. doi: 10.1177/1010428317690998. [DOI] [PubMed] [Google Scholar]
- 378.Chen D.L., Lu Y.X., Zhang J.X., Wei X.L., Wang F., Zeng Z.L., Pan Z.Z., Yuan Y.F., Wang F.H., Pelicano H., et al. Long non-coding RNA UICLM promotes colorectal cancer liver metastasis by acting as a ceRNA for microRNA-215 to regulate ZEB2 expression. Theranostics. 2017;7:4836–4849. doi: 10.7150/thno.20942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Li C., Wan L., Liu Z., Xu G., Wang S., Su Z., Zhang Y., Zhang C., Liu X., Lei Z. Long non-coding RNA XIST promotes TGF-β-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Lett. 2018;418:185–195. doi: 10.1016/j.canlet.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 380.Li Y., He Q., Wen X., Hong X., Yang X., Tang X., Zhang P., Lei Y., Sun Y., Zhang J., et al. EZH2-DNMT1-mediated epigenetic silencing of miR-142-3p promotes metastasis through targeting ZEB2 in nasopharyngeal carcinoma. Cell Death Differ. 2019;26:1089–1106. doi: 10.1038/s41418-018-0208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Li X., Tian Y., Hu Y., Yang Z., Zhang L., Luo J. CircNUP214 sponges miR-145 to promote the expression of ZEB2 in thyroid cancer cells. Biochem. Biophys. Res. Commun. 2018;507:168–172. doi: 10.1016/j.bbrc.2018.10.200. [DOI] [PubMed] [Google Scholar]
- 382.Brown C.Y., Dayan S., Wong S.W., Kaczmarek A., Hope C.M., Pederson S.M., Arnet V., Goodall G.J., Russell D., Sadlon T.J. FOXP3 and miR-155 cooperate to control the invasive potential of human breast cancer cells by down regulating ZEB2 independently of ZEB1. Oncotarget. 2018;9:27708. doi: 10.18632/oncotarget.25523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Li H., Xu L., Li C., Zhao L., Ma Y., Zheng H., Li Z., Zhang Y., Wang R., Liu Y., et al. Ubiquitin ligase Cbl-b represses IGF-I-induced epithelial mesenchymal transition via ZEB2 and microRNA-200c regulation in gastric cancer cells. Mol. Cancer. 2014;13:136. doi: 10.1186/1476-4598-13-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Truong H.H., Xiong J., Ghotra V.P., Nirmala E., Haazen L., Le Devedec S.E., Balcioglu H.E., He S., Snaar-Jagalska B.E., Vreugdenhil E., et al. beta1 integrin inhibition elicits a prometastatic switch through the TGFbeta-miR-200-ZEB network in E-cadherin-positive triple-negative breast cancer. Sci. Signal. 2014;7:ra15. doi: 10.1126/scisignal.2004751. [DOI] [PubMed] [Google Scholar]