Abstract
Purpose
Potential links may exist between vitamin A intake and myopia via various pathways. In this study, we examined the association between dietary vitamin A intake during adolescence and myopia in early adulthood.
Methods
We performed a prospective analysis utilizing data collected from participants of the Raine Study Gen2. Dietary vitamin A intake, determined via food frequency questionnaires completed at ages 14, 17, and 20 years, was compared with ophthalmic measurements collected at year 20. Low vitamin A levels were defined as <600 µg/day. Regression models were used to adjust for ocular sun exposure level, educational level, and parental myopia as potential confounders.
Results
A total of 642 subjects were analyzed. Although those with adequate vitamin A intakes were less likely to be myopic (P = 0.03), this association became insignificant when adjusted for potential confounding factors in logistic regression modeling (odds ratio, 0.59; 95% confidence interval, 0.98–2.52; P = 0.06).
Conclusions
There were no significant associations between total vitamin A intakes during adolescence and year 20 refractive errors after adjustment for confounders. Replication of this finding and further investigations are essential to rule out the suggestion that sufficient vitamin A intake during adolescence is associated with lower risk of myopia in early adulthood.
Translational Relevance
Our findings are not definitive that ingesting foods high in vitamin A during childhood and adolescence does not have a role for preventing myopia in early adulthood.
Keywords: vitamin A, vitamin A deficiency, myopia, nearsightedness
Introduction
Vitamin A is vital for eye health. It protects against night blindness and corneal thinning, which may lead to corneal perforation and permanent blindness.1 Moreover, vitamin A deficiency is the single most common cause of childhood blindness in developing countries and hence the reason for major programs for vitamin A supplementation in these countries.2–5 In developed countries, including Australia, vitamin A deficiency is rare6 due to comparatively better nutrient intake.7 Nonetheless, subclinical vitamin A deficiency may exist in developed countries and may be associated with different pathologies.
In recent decades, the prevalence of myopia (short-sightedness) has increased, such that many public health researchers are describing it as an epidemic.8,9 There are concerns that rates of sequelae of myopia, such as detached retina and glaucoma, will also increase.10 The increase in myopia involves not only developing countries11–13 but also developed countries such as Australia,14 Singapore,15 and Hong Kong.16 There are various factors leading to such epidemics. Social factors such as the education system may promote extensive near work on computers or paper and reduced time spent outdoors.17 Biological factors include genetics, where pathways triggered by environmental factors control ocular growth, resulting in axial elongation which can lead to myopia.18 Despite evidence that insufficient vitamin A negatively affects eye health, there is currently no research supporting an association between insufficient vitamin A and myopia in a population of adolescents in Australia. Studies to identify ways to prevent or slow the progression of myopia in both developing and developed countries are urgently required, including any that implicate vitamin A deficiency.
We aimed to determine whether variations in dietary intake of vitamin A are associated with measures of myopia in a longitudinal cohort of healthy Australian adolescents.
Methods
This study was granted ethics approval by the Human Research Ethics Committee at the University of Western Australia and was conducted in accordance with the tenets of the Declaration of Helsinki. All participants provided informed consent.
Participants
We analyzed data from participants of the Raine Study, a multigenerational prospective study that has followed parents and their offspring from the second trimester of gestation and is ongoing. Our study utilized data from the offspring (Generation 2, or Gen2).
Raine Study Gen2 participants with dietary vitamin A intake data recorded at 14, 17, and 20 years of age who had refractive errors measured at 20 years of age were included in the analysis for this particular study. Participants with a known ocular history that could affect myopia (e.g., ocular trauma, orthokeratology, refractive surgery) were excluded from the analysis.
Materials and Ophthalmic Data
Post-cycloplegic refraction data were obtained by performing autorefraction with a Nidek ARK-510A (Nidek Co., Ltd., Gamagori, Japan) after tropicamide 1% and phenylephrine 10% eye drops were administered in both eyes. Central corneal thickness (CCT) was measured by an OCULUS Pentacam (OCULUS Optikgeräte GmbH, Wetzlar, Germany). Retinal thickness was measured with optical coherence tomography (Spectralis HRA + OCT; Heidelberg Engineering, Heidelberg, Germany). Axial length was measured using an IOLMaster V.5 (Carl Zeiss Meditec AG, Jena, Germany).
Nutritional Data
Food frequency questionnaires (FFQs) were utilized to estimate nutrition intake. A Commonwealth Scientific and Industrial Research Organisation (CSIRO) FFQ that participants completed at 14 and 17 years of age provided data on the intake of retinol (µg) from animal foods, carotene (µg) from plant foods, and total vitamin A (µg). This FFQ has been demonstrated to adequately rank diet–disease relationships in adolescents and adults.19 The Cancer Council Victoria (CCV) FFQ was administered when the participants were 20 years of age and provided retinol, beta carotene equivalent, alpha carotene, carbohydrate, and zinc data (all measured in µg). These values were referenced from the Australian Food Composition Database, previously known as NUTTAB95, with the exception of data for alpha carotene, which came from the U.S. Department of Agriculture carotenoid database.20
Data Analysis
Spherical equivalent was calculated from the combination of spherical error and half of the cylindrical error. Myopia was then defined as less than –0.5 diopters of spherical equivalence. As there was a high correlation between right and left post-cycloplegic refraction spherical equivalent (Pearson's correlation r = 0.94; P < 0.001), only right spherical equivalent data were used. Similarly, bilateral correlation for CCT (r = 0.96; P < 0.001), global retinal thickness (r = 0.64; P < 0.001), and axial length (r = 0.97; P < 0.001) facilitated using only right eye data.
Total daily dietary vitamin A intake was determined as the sum of retinol, 1/12 of daily beta carotene intake, and 1/24 of daily alpha carotene intake.21 These are equivalent to the addition of the NUTTAB95 retinol and 1/12 of the NUTTAB95 beta carotene equivalent (which includes the correct proportion of alpha carotene) data of the year 20 FFQ.
As there is no accepted definition of low daily vitamin A intake in Australia, we defined low vitamin A intake from the FFQs as <600 µg per day for both sexes. This is based on the Recommended Dietary Intakes of 900 µg for males and 700 µg for females.22
We conducted a prospective study of vitamin A equivalent data (retinol, carotene, total vitamin A) from the 14-, 17-, and 20-year-old FFQs and compared that data to the ophthalmic data taken at 20 years of age. These analyses were compared in both males and females combined and separately. Both sexes were assessed for equal proportions using χ2 goodness-of-fit tests. Two-sided independent sample t-tests were used to compare year 20 refractive errors between males and females. Using logistic regression, year 20 vitamin A data and sex were modeled as predictors and compared with year 20 refractive errors as the dependent variable.
Participants excluded from the analysis were compared with those included on the variables of sex, educational level, parental myopia, dietary vitamin A intake, and myopia.
The association of year 14, 17, and 20 vitamin A and other dietary variables and spherical equivalence were investigated using Pearson's correlation. The difference between year 14 and year 17 vitamin A data were also measured against right spherical equivalence for correlation. Dichotomous myopia categories were compared with dichotomous year 20 vitamin A categories by phi correlation coefficients. In multiple studies, several factors, including educational level,23 sun exposure,23–25 parental myopia,23 carbohydrate intake,26 and zinc intake,27,28 have been associated with myopia. Utilizing each of these independent confounders compared with dichotomous myopic or not-myopic categories, we determined individual odds ratios by binomial logistic regression. Based on results from the univariable associations among the above confounders, vitamin A intake, and right spherical equivalence, we performed a multivariable adjustment for conjunctival ultraviolet autofluorescence (CUVAF), educational level, and parental myopia to determine the association between vitamin A intake and right spherical equivalence. A linear regression model was also generated between year 20 vitamin A data and refractive errors as a continuous measure, adjusting for CUVAF, educational level, and parental myopia.
We utilized SPSS Statistics for Windows 25 (IBM Corp., Armonk, NY) for analysis, and we defined any statistically significant association as P < 0.05.
Results
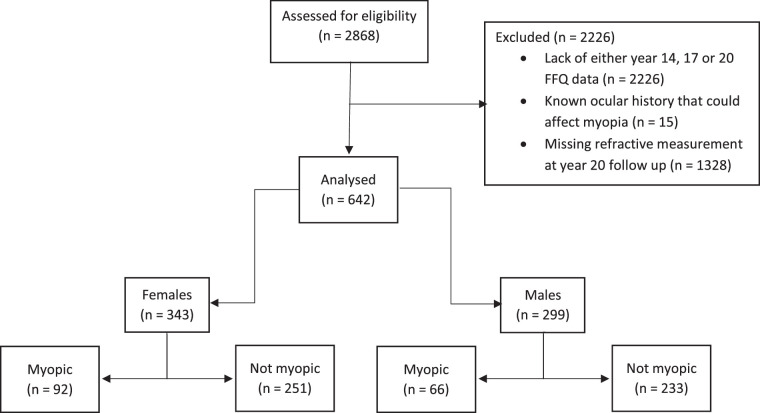
A total of 2868 Gen2 participants were originally included in the Raine Study from the second trimester of gestation. Of these, 642 participants fulfilled the inclusion criteria for our study (343 females, 299 males); 158 of the participants (24.6%) had myopia: 92 females (58.2%) and 66 males (41.8%) (Fig. 1). There was no significant difference between the proportion of all selected females and males: χ2(1) = 3.02, P = 0.08. The mean right spherical equivalent of the sample was –0.09D (SD, 1.35; 95% confidence interval [CI], –0.20 to 0.01). No significant difference in mean right spherical equivalence was demonstrated between females and males (mean right spherical equivalent difference, –0.05D; 95% CI, –0.11 to 0.02; P = 0.16). There was no evidence from the logistic regression model (P = 0.32) to suggest that the strength of the relationship between year 20 vitamin A and year 20 refractive error depended on sex.
Figure 1.
Flow diagram of participants.
Table 1 displays the participant characteristics. The mean age at the 20-year follow-up was 20.0 years (range, 18.3–22.1 years; SD, 0.4). Of the 642 subjects included in this analysis, 37 (5.8%) were classified as having low vitamin A intake at year 14. A further 107 (16.7%) and 445 (69.3%) were classified as having low vitamin A intake from the year 17 and year 20 FFQs, respectively. Of the 37 participants with low vitamin A intake at 14 years, 11 had myopia (29.7%). Similarly, of the 107 participants with low vitamin A intake at 17 years and 445 participants with low vitamin A intake at 20 years, 25 (23.4%) and 122 (27.4%), respectively, had myopia.
Table 1.
Characteristics of Participants Who Have and Have Not Been Categorized as Having Myopia
| Participants Included | Participants Excluded | |||||
|---|---|---|---|---|---|---|
| Characteristic | Myopic (n = 158) | Not Myopic (n = 484) | Total | Myopic (n = 158) | Not Myopic (n = 528) | Totala |
| Age (y), mean (SD) | 20.1 (0.39) | 20.0 (0.44) | 20.0 (0.43) | 20.1 (0.54) | 20.1 (0.44) | 20.1 (0.46) |
| Female, n (%) | 92 (58.2) | 251 (51.9) | 343 (53.4) | 68 (43.0) | 232 (43.9) | 1071 (48.1) |
| Vitamin A intake (µg), median (IQR) | ||||||
| Year 14 | 1139 (863–1438) | 1124 (843–1443) | 1129 (708–1317) | 1142 (789–1540) | 1120 (854–1486) | 1102 (832–1486) |
| Year 17 | 966 (686–1236) | 986 (716–1330) | 978 (709–1317) | 923 (752–1269) | 979 (684–1329) | 920 (680–1262) |
| Year 20 | 492 (362–643) | 522 (389–730) | 511 (380–715) | 576 (387–780) | 558 (394–730) | 558 (390–740) |
| Year 20 carbohydrate intake (µg), median (IQR) | 172 (131–224) | 177 (135–230) | 176 (135–228) | 181 (129–250) | 172 (135–230) | 175 (134–235) |
| Year 20 zinc intake (µg), median (IQR) | 11 (8–14) | 12 (8–16) | 12 (8–16) | 12 (9–17) | 11.7 (8–16) | 12 (8–16) |
| Year 20 CUVAF (mm2), median (IQR) | 30.2 (10.1–51.8) | 44.9 (19.3–67.9) | 41.1 (17.2–65.5) | 36.6 (10.9–57.5) | 51.6 (25.8–74.7) | 47.9 (23.0–72.6) |
| Vitamin A status, female, n (%)/male, n (%) | ||||||
| Year 14 low (<600 µg) | 8 (8.7)/3 (4.5) | 19 (7.6)/7 (3.0) | 27 (7.9)/10 (3.3) | 3 (8.6)/5 (8.1) | 14 (10.1)/10 (5.2) | 47 (10.6)/37 (7.0) |
| Year 14 adequate (≥600 µg) | 84 (91.3)/63 (95.5) | 232 (92.4)/226 (97.0) | 316 (92.1)/289 (96.7) | 32 (91.4)/57 (91.9) | 125 (89.9)/182 (94.8) | 397 (89.4)/490 (93.0) |
| Year 17 low (<600 µg) | 16 (17.4)/9 (13.6) | 54 (21.5)/28 (12.0) | 70 (20.4)/37 (12.4) | 3 (20.0)/2 (22.2) | 10 (21.3)/8 (16.7) | 43 (22.1)/22 (14.1) |
| Year 17 adequate (≥600 µg) | 76 (82.6)/57 (8s6.4) | 197 (78.5)/205 (88.0) | 273 (79.6)/262 (87.6) | 12 (80.0)/7 (77.8) | 37 (78.7)/40 (83.3) | 152 (77.9)/134 (85.9) |
| Year 20 low (<600 µg) | 77 (83.7)/45 (68.2) | 205 (81.7)/118 (50.6) | 282 (82.2)/163 (54.5) | 55 (83.3)/44 (55.7) | 184 (89.3)/143 (55.4) | 259 (88.1)/194 (55.4) |
| Year 20 adequate (≥600 µg) | 15 (16.3)/21 (31.8) | 46 (18.3)/115 (49.4) | 61 (17.8)/136 (45.5) | 11 (16.7)/35 (44.3) | 22 (10.7)/115 (44.6) | 35 (11.9)/156 (44/6) |
| Highest education completed, n (%) | ||||||
| Primary school | 1 (0.7) | 3 (0.7) | 4 (0.7) | 2 (1.5) | 14 (3.5) | 18 (2.7) |
| Secondary school (high school) | 110 (76.9) | 304 (70.9) | 414 (72.4) | 92 (69.7) | 275 (69.3) | 456 (68.4) |
| Otherb | 20 (14.0) | 92 (21.4) | 112 (19.6) | 28 (21.2) | 88 (22.2) | 148 (22.2) |
| University | 12 (8.4) | 30 (7.0) | 42 (7.3) | 10 (7.6) | 20 (5.0) | 45 (6.7) |
| Parental myopia, n (%) | ||||||
| No | 100 (69.9) | 357 (82.6) | 457 (79.5) | 95 (72.0) | 327 (82.4) | 530 (79.5) |
| Yes | 43 (30.1) | 75 (17.3) | 118 (20.5) | 37 (28.0) | 70 (17.6) | 137 (20.5) |
IQR, interquartile range.
Includes missing refractive values.
Includes, for example, technical and further education (vocational tertiary education in Australia).
The mean age of participants excluded from our analysis at the 20-year follow-up was 20.1 years (range, 19.4–22.1 years; SD, 0.5), and there was no significant difference compared to the selected participants (t = 1.2; P = 0.2). Between the included and excluded participants, there was a significant difference found among sex, with χ2(1) = 5.63 and P = 0.018; educational level, with χ2(3) = 10.412 and P = 0.015; and parental myopia, with χ2(1) = 11.498 and P = 0.001. For the included and excluded participants, there was no significant difference between the low and adequate vitamin A intake categories, with χ2(1) = 0.16 and P = 0.69. Regression analysis of dietary vitamin A intake (µg/day) was also not significant (R2 = 0.076; year 20 vitamin A β = 0.015; P = 0.58). There was also no significant difference between these two groups for myopia, with χ2(1) = 0.46 and P = 0.50.
Intake of Vitamin A at Years 14 and 17
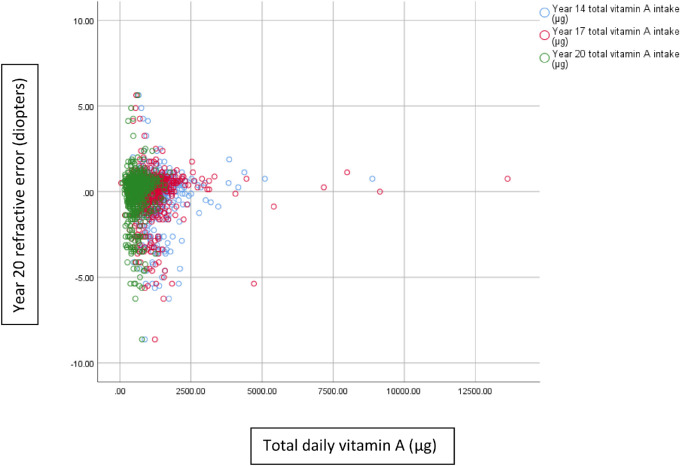
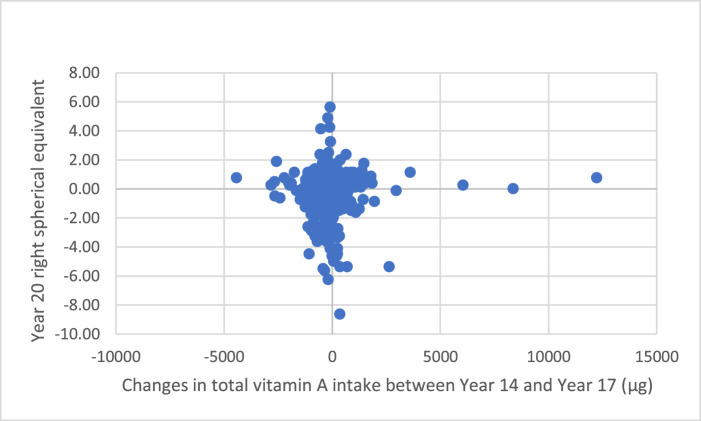
There were no significant linear correlations between refractive error (right spherical equivalent) and year 14 (Pearson correlation r = 0.01; P = 0.84) or year 17 (r = 0.02; P = 0.60) total vitamin A intake in the sample (Fig. 2). We compared vitamin A intake over the 3 years with year 20 refractive errors and found no significant correlation (r = 0.02; P = 0.70) (Fig. 3). There were also no significant correlations between refractive errors and year 14 vitamin A intake of males (r = 0.05; P = 0.44) and females (r = –0.02; P = 0.66). Similarly, year 17 vitamin A intakes of males (r = 0.06; P = 0.29) and females (r = –0.03; P = 0.60) were not significantly correlated with year 20 refractive errors.
Figure 2.
Year 14 (r = 0.008; P = 0.837), year 17 (r = 0.021; P = 0.593), and year 20 (r = 0.058; P = 0.142) total vitamin A intake and year 20 refractive data.
Figure 3.
Changes in total vitamin A intake from year 14 to year 17 and year 20 refractive data (r = 0.015; P = 0.702).
Intake of Vitamin A at Year 20
There was no significant correlation between year 20 vitamin A and refractive error (Pearson correlation r = 0.06; P = 0.14). However, we identified a significant negative association between the myopic group and low vitamin A intake category (Φ = –0.10; P = 0.01). Correlations between ocular axial length and year 20 vitamin A levels (r = 0.01; P = 0.73) and year 20 vitamin A categories (r = –0.03; P = 0.45) were insignificant.
A univariable logistic regression analysis (Table 2) showed that significant predictors of subjects who were myopic included vitamin A intake category, such that those with adequate intakes were less likely to be myopic (odds ratio [OR], 0.61; 95% CI, 0.39–0.96; P = 0.03). Other significant associations observed with myopia were CUVAF (OR, 0.99; 95% CI, 0.98–0.99; P < 0.001) and parental history of myopia (OR, 0.49; 95% CI, 0.32–0.76; P = 0.001). There were no significant associations between eye outcomes and age, sex, intake of vitamin A as a continuous measure, carbohydrate, zinc intakes, or highest education level completed.
Table 2.
Univariable and Multivariable Associations with Myopia in a Cohort of Young Adultsa
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Odds Ratio | 95% Confidence Interval | P | Odds Ratio | 95% Confidence Interval | P |
| Age | 1.199 | 0.792–1.817 | 0.39 | 1.403 | 0.880–2.235 | 0.16 |
| Sex (female) | 0.773 | 0.538–1.111 | 0.16 | 0.907 | 0.594–1.383 | 0.65 |
| Vitamin A intake, total daily | ||||||
| Year 14 (low/adequate categories) | 0.759 | 0.366–1.573 | 0.46 | — | — | — |
| Year 17 (low/adequate categories) | 1.085 | 0.666–1.769 | 0.74 | — | — | — |
| Year 20 (low/adequate categories) | 0.610 | 0.389–0.955 | 0.03 | 1.569 | 0.975–2.524 | 0.06 |
| Year 14 (absolute amount/mg) | 0.962 | 0.711–1.303 | 0.80 | — | — | — |
| Year 17 (absolute amount/mg) | 0.819 | 0.612–1.097 | 0.18 | — | — | — |
| Year 20 (absolute amount/mg) | 0.489 | 0.238–1.004 | 0.05 | — | — | — |
| Year 20 daily carbohydrate intake/mg | 0.435 | 0.051–3.740 | 0.45 | — | — | — |
| Year 20 daily zinc intake/µg | 0.974 | 0.946–1.002 | 0.07 | — | — | — |
| CUVAF | 0.988 | 0.982–0.994 | <0.001 | 0.988 | 0.981–0.994 | <0.001 |
| Highest education completed | ||||||
| Primary school | 1 (ref) | 1 (ref) | ||||
| Secondary school (high school) | 0.833 | 0.079–8.827 | 0.88 | 0.786 | 0.072–8.605 | 0.84 |
| Otherb | 0.905 | 0.447–1.829 | 0.78 | 1.091 | 0.512–2.325 | 0.82 |
| University | 0.543 | 0.238–1.241 | 0.15 | 0.594 | 0.248–1.421 | 0.24 |
| Parental myopia | ||||||
| No parents | 1 (ref) | 1 (ref) | ||||
| One or both parents | 0.489 | 0.316–0.755 | 0.001 | 0.481 | 0.306–0.757 | 0.002 |
The odds of myopia decrease with adequate vitamin A intake and increased sun exposure and increase with parental history of myopia. The multivariable analysis included age, sex, year 20 total daily vitamin A intake categories, CUVAF, highest education completed, and parental myopia.
Includes, for example, technical and further education (vocational tertiary education in Australia).
We performed a multivariable analysis adjusting for age, sex, CUVAF, highest education completed, and parental myopia. Despite finding no association between education level in our univariable analysis we adjusted for it, as a study utilizing the exact cohort of participants demonstrated an association.23 A possible reason for this may be due to our selection criteria, which excluded many participants for not having relevant FFQ data. Age and sex were adjusted, as they were baseline characteristics. Linear regression comparing the continuous variables of refractive error and year 20 vitamin A data as a continuous measure, adjusting for the above confounders, demonstrated no significant association (R2 = 0.24; year 20 vitamin A β = 0.06; P = 0.17).
In an adjusted logistic regression model, the association between adequate vitamin A at year 20 and lower risk of myopia was no longer significant (OR, 0.59; 95% CI, 0.98–2.52; P = 0.06) (Table 2). There were 399 participants (260 females, 139 males) with low year 20 vitamin A intakes. In this subgroup, the refractive error was similar between females and males (difference of means of right spherical equivalence, 0.08D; 95% CI, –0.18 to 0.34; P = 0.53).
Discussion
We did not find any correlation between quantitative values of vitamin A intake and refractive errors. There was a significant association between myopia and low vitamin A category, indicating that, although there does not appear to be a linear relationship with vitamin A and myopia, a threshold may exist where suboptimal intakes increase risk. However, this association disappeared when accounting for confounding factors in the analysis.
Possible Mechanisms Linking Vitamin A Insufficiency with Myopia
Despite our analysis demonstrating no association between myopia and vitamin A intake with data from the selected cohort, we believe that there is a theoretical association between myopia and vitamin A. There are plausible mechanisms that support this hypothesis. Elongation of the vitreous chamber of the eye is the most common cause of myopia.29 There are significant amounts of evidence, including from animal studies, suggesting that dopamine affects eye elongation.30–32 One animal study indicated dopamine was a key neurotransmitter released by bipolar cells to modulate the layers of the retina by synaptic or volume transmission.33 It is also known that genetic variation of dopamine receptor gene transcription is associated with myopia.34 In situations of dysregulated (low) dopamine signaling or levels, myopic eye growth ensues.35
Various genes associated with myopia, particularly high myopia, have been identified.34,36–38 Some identified genes are linked to ocular refraction and pathological myopia.37 Various genetic pathways—for neurotransmission, photoreceptor morphogenesis and retinoic acid metabolism, ion channel activity, development of ocular and central nervous systems, and extracellular matrix development and remodeling—are implicated in refractive errors.37
Furthermore, various animal studies have demonstrated that retinoic acid acts a chemical signal for regulating eye growth.39–41 In these studies, retinoic acid concentrations were elevated during form deprivation, where some spatial frequencies were removed from the retinal image. This is perhaps a compensatory mechanism, as during the development of form-deprivation myopia, where axial growth results in hyperopic defocus, retinoid acid levels declined in the choroid and sclera.39 One possible pathway that we propose is that insufficiency of vitamin A, or transcription issues of related genes for retinoic acid metabolism, may result in inadequate production of retinoic acid. This, in turn, may upset the regulation of eye growth and result in abnormal axial elongation, causing myopia. Genetic factors also play a part in vitamin A absorption in the gut, thus impacting serum vitamin A concentrations.42
Another possible pathway supporting the association between vitamin A and myopia is the relationship between retinitis pigmentosa (RP) and myopia. In clinical trials, supplementation with vitamin A appeared to slow progression of RP, a genetic cause of night blindness (nyctalopia).43–45 RP is the most common genetically inherited ocular disease and is associated with over 50 different genes.46 We have identified three genes common to RP and vitamin A. RDH5 and RGR are associated with RP, retinoic acid metabolism, and myopia. RPE65 is common to RP and vitamin A deficiency. This finding may indicate a significant link between myopia and vitamin A deficiency. The various genetic pathways mentioned above are involved in possible mechanisms whereby insufficient vitamin A may modify the activation or inhibition of the pathways, resulting in myopia. This evidence supports further research into the association of vitamin A and myopia.
Strengths
There was no selection bias, as the analyzed participants were recruited from the Raine Study. This study has been demonstrated to have internal and external validity, because at year 20 the characteristics of the cohort members were similar to those of the general population of Western Australian young adults.47 Furthermore, by utilizing longitudinal data from the same cohort of participants, we were able to determine associations over a 3-year period, providing more comprehensive information than a cross-sectional analysis. We compared the dietary vitamin A intake in year 14 and year 17 with refractive errors attained at year 20. The availability of various factors associated with myopia—CUVAF, highest education completed, and parental myopia—from the same cohort allowed us to correct for them as confounders to provide a more accurate outcome.
Limitations
No serum measurements of vitamin A levels were available from our data. Our vitamin A intakes were solely attained from the FFQs, which provided a retrospective estimate of an individual's dietary intake of vitamin A over a year. Thus, data on vitamin A intake are likely to be limited by recall bias.48 FFQ responses may also be subjected to confounding factors such as mood, attention span, and frequency of exposure to the stimulus.49 Moreover, vitamin A insufficiency is very unlikely in our cohort of participants based in Australia. Thus, the spectrum of vitamin A intake may not have been sufficiently varied to compare against refractive data due to this selection bias. We acknowledge a difference between the proportions of participants categorized as having low vitamin A intake by the CSIRO (year 14 and 17) and by the CCV (year 20) FFQs. A study comparing the FFQs concluded that limits of agreement with diet records were poor for vitamin A data and that a significant drawback is an overestimation of the low proportion.50 This may be another reason why we were not able to draw any linear associations between quantitative values of vitamin A intake and myopia. However, comparing categories rather than continuous values yielded an association following a binomial regression analysis, reflecting a non-dose–response relationship between vitamin A intake and risk of myopia.
Axial elongation does not progress linearly with age, as axial elongation ceases by teenage years,51 allowing transient changes as young adults.52 Our participants had only a single measurement of ophthalmic data at 20 years old. If signals affecting long-term eye growth have ceased at that point, adequate vitamin A intakes may not affect myopia, with the exception of preventing transient changes.
Studies involving more precise vitamin A measurements from a larger cohort of participants across a wider range of ages may delineate these associations more clearly.
Conclusions
We found no evidence that young adults with low vitamin A intakes are likely to have myopia at 20 years of age. Although the link between myopia and vitamin A does not appear to be linear, a threshold may exist for optimal vitamin A intake and axial elongation. Further studies incorporating more precise measurements of dietary vitamin A and adopting a broader spectrum of participants of younger ages and countries of residence would delineate associations more robustly.
Acknowledgments
The authors thank the Raine Study participants and their families, and they thank the Raine Study and Lions Eye Institute research staff for cohort coordination and data collection.
Funding for core management of the Raine Study was provided by the University of Western Australia, Curtin University, Telethon Kids Institute, Women and Infants Research Foundation, Edith Cowan University, Murdoch University, University of Notre Dame Australia, and Raine Medical Research Foundation. The eye data collection of the Raine Study Gen2 20-year follow-up was funded by a National Health and Medical Research Council (NHMRC) grant (1021105) and by the Ophthalmic Research Institute of Australia, Alcon Research Institute, Lions Eye Institute, Canadian Institutes of Health Research, and the Australian Foundation for the Prevention of Blindness. The Raine Study Gen2 17-year follow-up was funded by NHMRC grant 353514, and the Raine Study Gen2 14-year follow-up was funded by NHMRC Grant 211912.
Disclosure: F.J. Ng, None; D.A. Mackey, None; T.A. O'Sullivan, None; W.H. Oddy, None; S. Yazar, None
References
- 1. Imdad A, Mayo-Wilson E, Herzer K, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst Rev. 2017; 3: CD008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilbert C, Foster A. Childhood blindness in the context of VISION 2020–the right to sight. Bull World Health Organ. 2001; 79: 227–232. [PMC free article] [PubMed] [Google Scholar]
- 3. Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ. 2011; 343: d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Guideline: vitamin A supplementation for infants and children 6–59 months of age. Available at: https://apps.who.int/iris/bitstream/handle/10665/44664/9789241501767_eng.pdf?sequence=1. Accessed February 1, 2019.
- 5. United Nations Children's Fund. Vitamin A supplementation: a decade of progress. Available at: https://www.unicef.org/publications/files/Vitamin_A_Supplementation.pdf. Accessed February 1, 2019.
- 6. World Health Organization. WHO global database on vitamin A deficiency 2006. Available at: https://www.who.int/vmnis/vitamina/data/database/countries/aus_vita.pdf?ua=1. Accessed February 1, 2019.
- 7. Koay CL, Patel DK, Tajunisah I, Subrayan V, Lansingh VC. A comparative analysis of avoidable causes of childhood blindness in Malaysia with low income, middle income and high income countries. Int Ophthalmol. 2015; 35: 201–207. [DOI] [PubMed] [Google Scholar]
- 8. Wu PC, Huang HM, Yu HJ, Fang PC, Chen CT. Epidemiology of myopia. Asia Pac J Ophthalmol (Phila). 2016; 5: 386–393. [DOI] [PubMed] [Google Scholar]
- 9. Ansah JP, Koh V, de Korne DF, et al.. Projection of eye disease burden in Singapore. Ann Acad Med Singapore. 2018; 47: 13–28. [PubMed] [Google Scholar]
- 10. Cooper J, Tkatchenko AV. A review of current concepts of the etiology and treatment of myopia. Eye Contact Lens. 2018; 44: 231–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh NK, James RM, Yadav A, et al.. Prevalence of myopia and associated risk factors in schoolchildren in North India. Optom Vis Sci. 2019; 96: 200–205. [DOI] [PubMed] [Google Scholar]
- 12. Dong YH, Liu HB, Wang ZH, et al.. Prevalence of myopia and increase trend in children and adolescents aged 7-18 years in Han ethnic group in China, 2005-2014 [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2017; 38: 583–587. [DOI] [PubMed] [Google Scholar]
- 13. Gao Z, Meng N, Muecke J, et al.. Refractive error in school children in an urban and rural setting in Cambodia. Ophthalmic Epidemiol. 2012; 19: 16–22. [DOI] [PubMed] [Google Scholar]
- 14. French AN, Morgan IG, Burlutsky G, Mitchell P, Rose KA. Prevalence and 5- to 6-year incidence and progression of myopia and hyperopia in Australian schoolchildren. Ophthalmology. 2013; 120: 1482–1491. [DOI] [PubMed] [Google Scholar]
- 15. Saw SM, Matsumura S, Hoang QV. Prevention and management of myopia and myopic pathology. Invest Ophthalmol Vis Sci. 2019; 60: 488–499. [DOI] [PubMed] [Google Scholar]
- 16. Fan DS, Lam DS, Lam RF, et al.. Prevalence, incidence, and progression of myopia of school children in Hong Kong. Invest Ophthalmol Vis Sci. 2004; 45: 1071–1075. [DOI] [PubMed] [Google Scholar]
- 17. Morgan IG, French AN, Ashby RS, et al.. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018; 62: 134–149. [DOI] [PubMed] [Google Scholar]
- 18. Riddell N, Crewther SG. Integrated comparison of GWAS, transcriptome, and proteomics studies highlights similarities in the biological basis of animal and human myopia. Invest Ophthalmol Vis Sci. 2017; 58: 660–669. [DOI] [PubMed] [Google Scholar]
- 19. Ambrosini GL, de Klerk NH, O'Sullivan TA, Beilin LJ, Oddy WH. The reliability of a food frequency questionnaire for use among adolescents. Eur J Clin Nutr. 2009; 63: 1251–1259. [DOI] [PubMed] [Google Scholar]
- 20. Cancer Council Victoria. Dietary Questionnaire for Epidemiological Studies Version 3.2 (DQES v3.2) user guide. Available at: https://www.cancervic.org.au/downloads/cec/DQES%20guide%20Sept%202015_2.pdf. Accessed May 12, 2020.
- 21. Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 22. Office of Dietary Supplements, National Institutes of Health. Vitamin A: fact sheet for health professionals. Available at: https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/. Accessed March 1, 2019.
- 23. McKnight CM, Sherwin JC, Yazar S, et al.. Myopia in young adults is inversely related to an objective marker of ocular sun exposure: the Western Australian Raine cohort study. Am J Ophthalmol. 2014; 158: 1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramamurthy D, Lin Chua SY, Saw SM. A review of environmental risk factors for myopia during early life, childhood and adolescence. Clin Exp Optom. 2015; 98: 497–506. [DOI] [PubMed] [Google Scholar]
- 25. Rose KA, Morgan IG, Ip J, et al.. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008; 115: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 26. Galvis V, Tello A, Camacho PA, Parra MM, Merayo-Lloves J. Bio-environmental factors associated with myopia: an updated review [in English, Spanish]. Arch Soc Esp Oftalmol. 2017; 92: 307–325. [DOI] [PubMed] [Google Scholar]
- 27. Fedor M, Socha K, Urban B, et al.. Serum concentration of zinc, copper, selenium, manganese, and Cu/Zn Ratio in children and adolescents with myopia. Biol Trace Elem Res. 2017; 176: 1–9. [DOI] [PubMed] [Google Scholar]
- 28. Huibi X, Kaixun H, Qiuhua G, Yushan Z, Xiuxian H. Prevention of axial elongation in myopia by the trace element zinc. Biol Trace Elem Res. 2001; 79: 39–47. [DOI] [PubMed] [Google Scholar]
- 29. Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res. 2006; 82: 185–200. [DOI] [PubMed] [Google Scholar]
- 30. Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004; 108: 17–40. [DOI] [PubMed] [Google Scholar]
- 31. Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013; 114: 106–119. [DOI] [PubMed] [Google Scholar]
- 32. Huang F, Zhang L, Wang Q, et al.. Dopamine D1 receptors contribute critically to the apomorphine-induced inhibition of form-deprivation myopia in mice. Invest Ophthalmol Vis Sci. 2018; 59: 2623–2634. [DOI] [PubMed] [Google Scholar]
- 33. Farshi P, Fyk-Kolodziej B, Krolewski DM, Walker PD, Ichinose T. Dopamine D1 receptor expression is bipolar cell type-specific in the mouse retina. J Comp Neurol. 2016; 524: 2059–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohngemach S, Feldkaemper M, Schaeffel F. Pineal control of the dopamine D2-receptor gene and dopamine release in the retina of the chicken and their possible relation to growth rhythms of the eye. J Pineal Res. 2001; 31: 145–154. [DOI] [PubMed] [Google Scholar]
- 35. Zhou X, Pardue MT, Iuvone PM, Qu J. Dopamine signaling and myopia development: what are the key challenges. Prog Retin Eye Res. 2017; 61: 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jacobi FK, Zrenner E, Broghammer M, Pusch CM. A genetic perspective on myopia. Cell Mol Life Sci. 2005; 62: 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wojciechowski R, Cheng CY. Involvement of multiple molecular pathways in the genetics of ocular refraction and myopia. Retina. 2018; 38: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tedja MS, Wojciechowski R, Hysi PG, et al.. Genome-wide association meta-analysis highlights light-induced signaling as a driver for refractive error. Nat Genet. 2018; 50: 834–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bitzer M, Feldkaemper M, Schaeffel F. Visually induced changes in components of the retinoic acid system in fundal layers of the chick. Exp Eye Res. 2000; 70: 97–106. [DOI] [PubMed] [Google Scholar]
- 40. McFadden SA, Howlett MH, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004; 44: 643–653. [DOI] [PubMed] [Google Scholar]
- 41. Troilo D, Nickla DL, Mertz JR, Summers Rada JA. Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Invest Ophthalmol Vis Sci. 2006; 47: 1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Borel P, Desmarchelier C. Genetic variations associated with vitamin a status and vitamin A bioavailability. Nutrients. 2017; 9: E246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berson EL, Weigel-DiFranco C, Rosner B, Gaudio AR, Sandberg MA. Association of vitamin A supplementation with disease course in children with retinitis pigmentosa. JAMA Ophthalmol. 2018; 136: 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berson EL, Rosner B, Sandberg MA, et al.. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993; 111: 761–772. [DOI] [PubMed] [Google Scholar]
- 45. Berson EL, Rosner B, Sandberg MA, et al.. Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin A. Arch Ophthalmol. 2010; 128: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsin A, Betts-Obregon B, Grigsby JG. Visual cycle proteins: structure, function, and roles in human retinal disease. J Biol Chem. 2018; 293: 13016–13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Straker L, Mountain J, Jacques A, et al.. Cohort profile: the Western Australian Pregnancy Cohort (Raine) Study–Generation 2. Int J Epidemiol. 2017; 46: 1384–1385j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shim JS, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health. 2014; 36: e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krall EA, Dwyer JT, Ann Coleman K. Factors influencing accuracy of dietary recall. Nutr Res. 1988; 8: 829–841. [Google Scholar]
- 50. Ambrosini GL, Mackerras D, de Klerk NH, Musk AW. Comparison of an Australian food-frequency questionnaire with diet records: implications for nutrition surveillance. Public Health Nutr. 2003; 6: 415–422. [DOI] [PubMed] [Google Scholar]
- 51. Goss DA, Cox VD, Herrin-Lawson GA, Nielsen ED, Dolton WA. Refractive error, axial length, and height as a function of age in young myopes. Optom Vis Sci. 1990; 67: 332–338. [DOI] [PubMed] [Google Scholar]
- 52. Mallen EA, Kashyap P, Hampson KM. Transient axial length change during the accommodation response in young adults. Invest Ophthalmol Vis Sci. 2006; 47: 1251–1254. [DOI] [PubMed] [Google Scholar]