Abstract
Purpose
To quantify the effect of silicone hydrogel crosslink density on the adhesion at corneal epithelial cells/silicone hydrogel contact lens interface.
Methods
A custom-built rheometer, referred to as the live cell monolayer rheometer, was used to measure the adhesive strengths between corneal epithelial cell monolayers and silicone hydrogel lens surfaces. The resulting stress relaxations of senofilcon A–derived silicone hydrogel materials with varying crosslinking densities and delefilcon A were tested. Senofilcon A–like materials labeled L1, L2, L3, L4, and L5 contained crosslinker concentrations of 1.2, 1.35, 1.5, 1.65, and 1.8 wt%, respectively. The residual modulus measured from the live cell monolayer rheometer provided a direct indication of adhesive attachment.
Results
Within the senofilcon-derived series, the adhesive strength shows a surprising minimum with respect to crosslink density. Specifically, L1 (1.20%) has the highest adhesive strength of 39.5 ± 11.2 Pa. The adhesive strength diminishes to a minimum of 11.2 ± 2.1 Pa for L3, whereafter it increases to 14.5 ± 2.5 Pa and 18.1 ± 5.1 Pa for L4 and L5, respectively. The delefilcon A lens exhibits a comparable adhesive strength of 27.8 ± 6.3 Pa to L1.
Conclusions
These results demonstrated that increasing the crosslink density has a nonmonotonic influence on the adherence of lenses to mucin-expressing corneal epithelial cells, which suggests a competition mechanism at the cell/lens interface.
Translational Relevance
Because the adhesiveness of contact lenses to ocular tissues may impact the comfort level for lens wearers and affect ease of removal, this study suggests that lens adhesion can be optimized through the control of crosslink density.
Keywords: contact lenses, corneal epithelium, adhesive strength, crosslinking density
Introduction
The degree of epithelium adhesion against material surfaces influences the material design for many biomedical devices. In the ocular environment, a strong adhesion of the corneal epithelium against contact lenses can induce end-of-day discomfort,1–4 and a diminution of comfort during time of wear of soft contact lenses (SCLs) is the most common reason for contact lens discontinuation.5,6 To develop contact lenses with improved wearer experience, it is both clinically and industrially important to correlate SCL material properties with the wearer comfort. Extensive literature has reported on the coefficient of friction (CoF) measurements of SCLs as a candidate material property that can link to subject comfort.1–4,6 However, CoF measurement results greatly depend on the testing conditions, which can make the replication and interpretation of results difficult.6,7 In addition, kinetic friction force does not reflect the adhesion force between two surfaces, which fundamentally distinguishes the CoF measurement from the adhesive strength measurement of cornea epithelium against SCLs.8 Therefore, we present a customized device to directly measure the adhesive strength at the corneal epithelial cells/SCLs interface through relaxation experiments and suggest a new parameter, the residual modulus, as a candidate material property complementary to CoF for screening and developing contact lens formulations.
A direct adhesion measurement of live cell monolayers against contact lenses is challenging owing to the low adhesive strength of cell monolayers against material surfaces. To resolve this issue, many researchers have used single cell techniques, such as atomic force microscopy and magnetic bead microscopy, to measure the adhesive forces of a single cell against different substrates.9–11 However, single cell techniques overlook the collective behaviors of the corneal epithelium, and substantial variations exist in the adhesive strength measurements owing to variabilities among single cells. Here we modify a customized live cell monolayer rheometer (LCMR) to perform step–strain experiments on cell monolayers adhering onto different substrates.12,13 A rheometer measures the material response to either an imposed stress (force) or strain (deformation). Ideal solids and liquids have simple behaviors under imposed perturbations. However, viscoelastic materials, such as living cells, exhibit more complex behaviors under imposed perturbations. Quantifications of these behaviors contain information about internal material properties. The LCMR captures the epithelium collective behavior by performing step–strain experiments directly on epithelial cell monolayers. In a step–strain experiment, the cell monolayer is subject to a step shearing deformation and the subsequent force response from the cell monolayer is monitored. Modulus of the cell layer, defined as the measured stress over the imposed strain, is obtained as a function of time. For viscoelastic systems such as epithelial cell sheets, the moduli will relax from the peak modulus immediately after the step deformation. The relaxation curve thus obtained elucidates the structural rearrangement of the sample. For viscoelastic solid materials, the modulus will relax to a plateau value at long times. The magnitude of the plateau modulus indicates the resisting strength against the imposed shear movement. The LCMR reduces the biovariability among experiments by measuring the average response from a cell monolayer, and the signal-to-noise ratio in the force measurements is improved by averaging a collective response from the bulk monolayer.
The adhesive strength against SCLs varies depending on the nature of the surface in contact. Owing to the technological challenges of measuring live cell monolayer properties on conventional rheometers, previous literature has used mica, glass, and extracted mucin layers as approximations of the ocular surface.6,14–16 However, the corneal epithelium presents a soft, viscoelastic contact surface for SCLs, which deviates from the stiff, elastic surfaces that have been tested. In addition, corneal epithelial cells actively respond to the environmental perturbations, such as the mechanical perturbation introduced by SCL wear, which potentially modifies the contact mechanics at the cornea/lens interface. A characterization of the adhesive strength between SCLs and a viscoelastic, active surface was missing in the past studies. We addressed this issue by culturing a monolayer of mucin-producing corneal epithelial cells to mimic the ocular environment.
Various material properties of SCLs can contribute to their adhesive performance, including the water content, the ionic strength, and the surface roughness. Owing to the great variability in commercial SCL formulations, it is challenging to compare the characterizations of SCLs across brands and establish a systematic correlation between a single material property and the observed trend in the adhesion measurements. In this study, we systematically varied the crosslinker contents of senofilcon A–derived materials, which eliminates the contributions from other material properties to the adhesive strength. We chose to vary the crosslinker content because the crosslinking density is known to modulate the mechanical and chemical properties of polymer networks. Park and Robinson14 have observed that the self-adhesion of hydrogel surfaces decreases with crosslinker contents. In addition, the elastic modulus of hydrogels increases with the crosslinking density.17–20 Past studies have established that crosslinking density–induced stiffness change in the substrate can actively modulate cell behaviors.21,22 However, the influence of crosslinking density and stiffness of the hydrogel network on muco-adhesion strength remains unclear. Here, we examined how the crosslinking density of the hydrogel materials modifies the muco-adhesive strength of corneal epithelial cells.
The origin of adhesion at biological interfaces is complex. At cornea/contact lens interfaces, the glycocalyx surrounding the epithelial cell membrane can mediate the adhesion against contact lens surfaces through either hydrophilic or hydrophobic interactions. The ubiquity of charged macromolecules on cell membranes at a physiologic pH provides an additional adhesion mechanism through electrostatic interactions.23–25 The observed adhesion responses of epithelial cell sheets usually result from multiple molecular origins. We proposed a competitive mechanism between a passive viscoelastic contact mechanism and an active modulation of adhesion response from corneal epithelial cells, which results in a nonmonotonic correlation between the crosslinking density of senofilcon A–derived materials and the adhesive strength of corneal epithelial cells. The results suggest that the crosslinker content can be exploited as a simple method to tune the cell adhesion response, which can guide clinicians and engineers alike toward a rational design of SCLs and other biomaterials.
Methods
Silicone Hydrogel Lenses
A series of senofilcon A–derived materials with varying volume contents of triethylene glycol dimethacrylate was used in this study. The crosslinker contents for L1 to L5 are listed in the Table. The crosslinker contents of 1.5% is commercially available and the chosen range of crosslinker contents can lead to viable contact lenses. Delefilcon A lenses were also tested as a comparison. The delefilcon A lens is composed of a silicone hydrogel material in the core with an equilibrium water content of 33% and an interpenetrating crosslinked zone on the surface with a water content of more than 80%.26,27 Atomic force microscopy indentation tests have shown that the elastic modulus of the delefilcon A surface is 25 kPa while hydrated, lower than the reported values for senofilcon A (see the Discussion).2
Table.
Summary of Residual Moduli for the Silicone Hydrogel Materials Used in the Study
| Lenses | Crosslinker Contents (%) | Number of Trials | Mean Residual Moduli ± SE [Pa] |
|---|---|---|---|
| L1 | 1.20 | 6 | 39.5 ± 11.1 |
| L2 | 1.35 | 6 | 16.0 ± 4.0 |
| L3 | 1.50 | 5 | 11.2 ± 2.1 |
| L4 | 1.65 | 10 | 14.5 ± 2.5 |
| L5 | 1.80 | 6 | 18.1 ± 5.1 |
| Delefilcon A | Unknown | 10 | 27.9 ± 6.7 |
SE, standard error.
Lens Cleaning Protocol
The silicone hydrogel lenses with varying crosslinker contents were obtained in sterile glass bottles containing surfactant solution. The delefilcon A contact lenses were obtained in commercial blister packages. Before the experiments, the lenses were cut into a fixed circular area of 73 mm2 with a metal ring. The lenses were cleaned following a previously published protocol to remove the effect of packaging solutions.28 The washed lenses were stored in fresh phosphate-buffered saline for up to 1 week and were transferred with Teflon-coated tweezers.
Cell Culture
Human telomerase reverse transcriptase-immortalized corneal epithelial cells were generously donated by Professor Suzanne Fleiszig (University of California, Berkeley), and were used between passages 50 and 70. The cells were cultured in a basal medium (EpiLife, LifeTechnologies, Carlsbad, CA) supplemented with human corneal growth supplements (LifeTechnologies), under 37°C and 5% CO2 and were kept between 30% and 80% confluence. Before the experiment, the cells were plated onto a collagen-coated coverslip attached onto an experimental aluminum plate until it reached more than 90% confluence. The medium was switched to CO2 independent medium (Gibco, Gaithersburg, MD) during the experiments.
Experimental Setup: LCMR
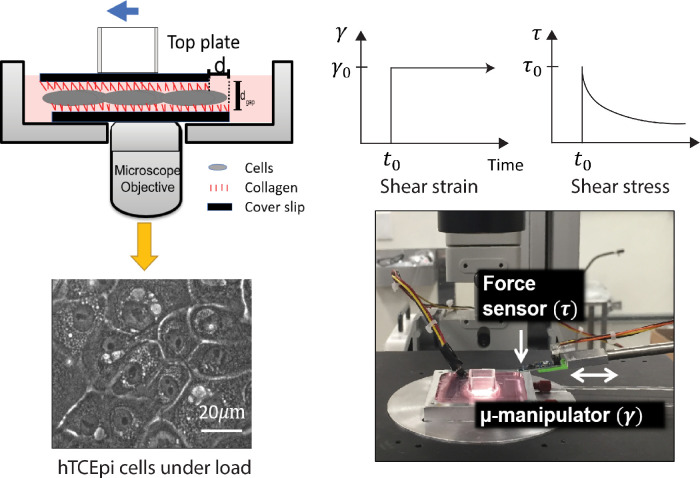
The adhesive strength was measured using a modified version of the LCMR developed in the Fuller laboratory at Stanford University.13,29 A schematic of the instrument is shown in Figure 1. The instrument is composed of a customized aluminum bottom plate, a top plate, and a force sensor (Femto Tools, Buchs, Switzerland) mounted onto a micromanipulator (Sutter Instruments, Navato, CA). A confluent monolayer of human telomerase reverse transcriptase-immortalized corneal epithelial was cultured on a collagen-coated plate glued in the center of the aluminum well. The cell behavior was monitored through an inverted microscope (Nikon Eclipse TE300, 40× air Phase2).
Figure 1.
A schematic illustration of the LCMR. (Top left) A schematic view of the experimental plate in which the human telomerase reverse transcriptase-immortalized corneal epithelial (hTCEpi) monolayer is in contact with collagen coated surfaces. In contact lens adhesion experiments, contact lenses were attached onto the top surface. (Bottom left) hTCEpi cell monolayer visualized through the microscope (Nikon TE300, phase contrast, 40×). (Top right) a schematic of a step strain experiment for a viscoelastic solid in which the shear stress relaxes to a stable plateau value. Bottom right: a photograph of the LCMR.
On the day of experiment, a hydrogel lens was attached onto the collagen-coated or silanized surface on the top plate, which was then gently placed on the cell monolayer. The attachment of the lens against the top plate was confirmed after each experiment. The adhesion between the cell monolayer and the hydrogel material was allowed to develop over a controlled time period. During the experiment, the media in the bottom plate was kept at 37°C through an external heated water bath, and the temperature was monitored by a thermocouple placed in the bottom well. For all the experiments in the present study, the normal stress on the cell monolayer imposed by the weight of the top plates stayed around 500 Pa, as determined by the weight of the top plate over the contact area. As a comparison, the normal pressure of the eyelids against the ocular surfaces was clinically determined to be on the order of 1000 Pa for healthy subjects.30,31 We chose 500 Pa as the normal pressure to minimize damage on corneal epithelial cells during the experiment.
The experiment was controlled by a customized MATLAB code. After the force sensor was brought in contact with the top plate, a user-defined step motion was applied through the micromanipulator. A DAQ board (National Instruments, Austin, TX) collected the voltage readings as a function of time from the force sensor which were converted to force levels, F(t), using a known conversion factor given by the manufacturer. The contact area, A, was determined by previous literature as 18.8 mm2 for a confluent cell monolayer.12,13 The confluence of the cell monolayer was estimated with the microscope, and the monolayer behavior was verified with confocal immunofluorescent images. The calibration of the LCMR was done with silicone oils of known viscosities (see the Supplementary Materials and Supplementary Figures S2 and S3).
Residual Modulus Calculation and Data Analysis
The apparent modulus as a function of time was defined as shear stress over shear strain and calculated through the following equation,
| (1) |
where τ(t) is the shear stress as a function of time, γ is the shear strain, and d is the step movement applied by the micromanipulator. The shear stress exerted on the corneal epithelial cell monolayers, τ(t), was defined as shear force over area, or F(t)/A. The shear strain exerted on the corneal epithelial cell monolayer, γ, was defined as the shear distance over the gap height in the normal direction, or d/dgap. The gap, dgap, was measured as the height difference between two focal planes, which varied between 5 and 8 µm. Figure 1 shows a graphical representation of shear stress and shear strain.
The peak modulus was defined as the first modulus value after the step strain was applied. The residual modulus was calculated as the mean modulus during the 20 seconds of the experiment before the retraction of the micromanipulator. Statistical analysis was performed using Welch's t-test owing to the unequal trial numbers among the samples and the small sample size compared with the time points collected for residual moduli.
Results
The human telomerase reverse transcriptase-immortalized corneal epithelial monolayers exhibited a cobblestone morphology. The presence of mucin 1, a major cell membrane-associated glycoprotein, was verified with immunofluorescent staining in the Supplementary Materials and Supplementary Figure S1. A representative graph from the LCMR is shown in Figure 2, where the cell monolayer was in contact with a collagen-coated surface for 2 hours. The peak modulus was relaxed to a stable residual modulus over 10 seconds. The inset shows the same plot on a log–log scale to emphasize the relaxation behavior.
Figure 2.
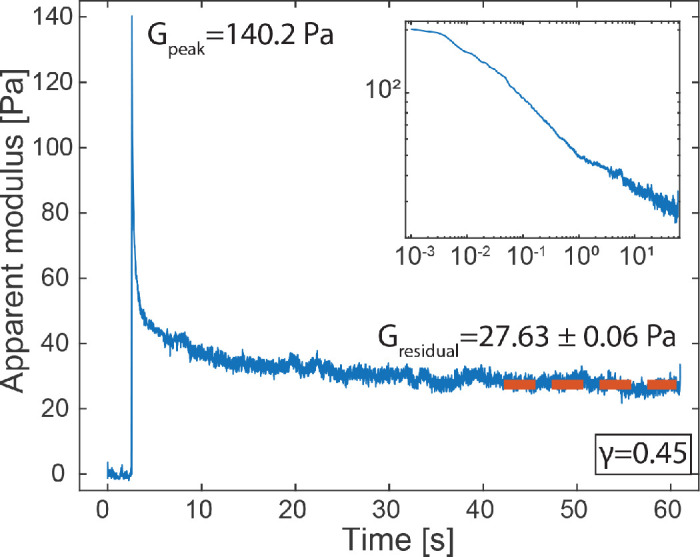
A representative graph obtained from LCMR in which the peak modulus was relaxed to a plateau value at long time scales. Inset: relaxation curve on a log–log scale.
Effect of Adhesion Time
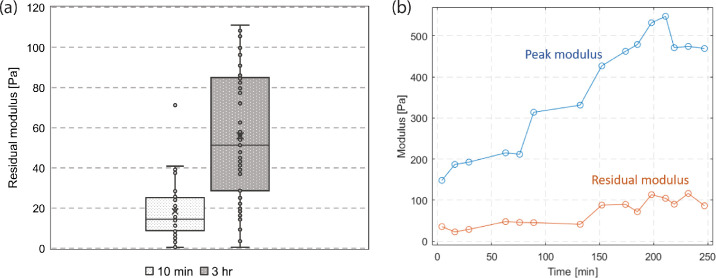
To test the effect of adhesion time, we collected the relaxation curves from multiple cell monolayers that were in contact with delefilcon A lenses for 10 minutes and for 3 to 4 hours. Figure 3a shows that the residual moduli for 10-minute adhesion (18.73 ± 2.1 Pa) were statistically lower than those for long time adhesion (56.0 ± 4.6 Pa). To further illustrate the effect, Figure 3b shows the peak and residual moduli as a function of adhesion time using a cell monolayer against a collagen-coated top plate. The peak modulus increased more than three-fold during the first 3 hours of adhesion. The residual modulus consistently increased during a period of four hours. Data beyond four hours was not collected owing to the cell viability concerns.
Figure 3.
The effect of adhesion time on the relaxation behaviors. (a) Residual moduli after contact times of 10 minutes and 3 hours. (b) The peak and residual moduli of a single cell monolayer over adhesion time.
Effect of Crosslinker Content
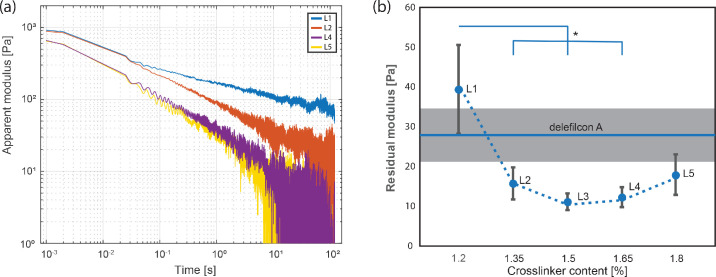
Figure 4 shows the effect of crosslinker contents on the residual modulus of corneal epithelial cells against the silicone hydrogel materials. Each experiment was conducted on a fresh cell monolayer and a fresh silicone hydrogel contact lens. Each mounted contact lens was kept in contact with the cell monolayer surface for approximately 2.5 hours, before a step–strain experiment was performed. To avoid strain hardening or plastic deformation of the cell monolayers, the results from the first step–strain experiment on each sample were used in analysis. The resulting residual moduli and their respective experimental trial numbers are tabulated in the Table. Among senofilcon A–derived materials, L1 has the highest adhesive strength of 39.5 ± 11.2 Pa and the lowest amount of crosslinker incorporated, that is, 1.2 wt %. As the crosslink density of the senofilcon A–derived series increases, the adhesive strength diminishes to a minimum of 11.2 ± 2.1 Pa for L3 (1.50 wt % crosslinker), whereafter it increases to 14.5 ± 2.5 and 18.1 ± 5.1 Pa for L4 (1.65 wt % crosslinker) and L5 (1.80 wt % crosslinker), respectively. The delefilcon A lens exhibits a comparable adhesive strength of 27.8 ± 6.3 Pa to L1, suggesting that the surface of delefilcon A has a low crosslink density and is different compared with L3 to L5. The statistical analysis results are shown in Figure 4. The residual modulus of delefilcon A is statistically higher than L3 (1.50%) and L4 (1.65%).
Figure 4.
The effect of crosslinker content on the relaxation behaviors. (a) Representative relaxation curves from senofilcon A–derived materials. (b) The residual moduli as a function of the crosslinking density in silicone hydrogel. The differences between L3 and delefilcon A, L4 and delefilcon A were confirmed by a one-tailed t-test (P < 0.05). Error bar: standard error. Significance with respect to L1: *P < 0.05.
Discussion
In this study, we investigated the influence of crosslinker content on the adhesive strength of mucin-expressing corneal epithelial cells against silicone hydrogel materials. We quantified the adhesive strength using a live cell monolayer rheometer and observed that the adhesive strength generally increased over contact time. Interestingly, the crosslinker content had a nonmonotonic effect on the adhesive strength. To explain this relationship, we proposed a competition mechanism between a morphology-dominated adhesion mechanics and a stiffness-induced cell response.
The cell monolayer subject to a step–strain deformation shows a relaxation behavior with an initial peak modulus that relaxed to a stable plateau value at a long time scale (Fig. 2), reminiscent of the relaxation behavior from a viscoelastic solid. This behavior agrees with the viscoelastic solid model that has been reported for reconstituted cytoskeletons.32–34 The high initial modulus—more than 100 Pa—reflects the short time behavior of the cell monolayer in response to a mechanical perturbation, during which the molecular structure of the cell cytoskeleton is effectively frozen and the adhesion between the cell surface and the substrate remains intact. After the initial perturbation, the cytoskeletal proteins rearrange toward a minimal energy conformation and weak intermolecular bonds present at the cell/substrate surface break, which both contribute to the relaxation of the initial modulus. At long time scales (60 seconds after the perturbation initiation), the low residual modulus (on the order of 10 Pa) reflects the stable adhesion developed between the cell monolayer and the hydrogel surface. Therefore, we use the residual modulus as an indicator of the adhesive strength of the cell monolayer against the hydrogel material surfaces.
We would like to point out that the adhesive strength measured with LCMR is fundamentally different from the CoF measured on a conventional tribometer. CoF measurements indicate the kinetic friction force at the interface, which correlates with the energy loss during an adhesion loading/unloading cycle, but CoF does not imply the degree of adhesive strength.8 During blinking and contact lens wear, adhesion forces contribute to the mechanical resistance at the ocular surface against contact lens materials and, therefore, we propose that adhesive strength measurements could be used alongside with CoF measurements to reflect the degree of wearer discomfort under the physiologic conditions.
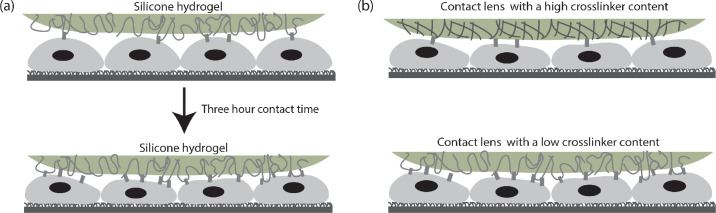
The three-fold increase in the residual modulus after a 3-hour contact time can be explained by the dynamics of adhesion process schematically shown in Figure 5a. The adhesive force at the cell/lens interface originates from intermolecular interactions between the glycocalyx of the corneal epithelial cells and the polymer chains present at the surface of SCL hydrogels. Over time, polymer chains in the SCLs diffuse toward the cell/lens interface and form noncovalent intermolecular associations (including physical, ionic, Van der Waals, and hydrogen bonding) with glycoproteins present at the cell surface, which increases the adhesive strength at the cell/lens interface. A similar trend is observed in artificial viscoelastic interfaces where the adhesive strength increases over contact time between two polymeric surfaces.31
Figure 5.
A schematic of the proposed viscoelastic contact model that represents the contact between the ocular surface and the contact lens material. (a) A longer contact time results in stronger intermolecular interactions. (b) A contact lens with a higher crosslinker content exposes less potential sites for intermolecular interactions.
The decrease in the adhesive strengths at low crosslinker contents (1.20%, 1.35%, and 1.50%) suggests the importance of chain flexibility in the adhesion process. Polymer chains in low crosslinker content hydrogels are more flexible, which can create an interfacial region with more depth for contact and subsequent entanglement with the glycoproteins present at the cell surface. The mesh size of the polymer network is larger in low crosslinker content hydrogels, which provides more space for interpenetration of glycoproteins and a higher chance of intermolecular entanglement/association. The sharp decrease in the residual modulus from L1 (1.20%) to L2 (1.35%) and L3 (1.50%) reflects the ability of crosslinker content to modulate the adhesive strength, which agrees with the observation made by Park and Robinson14 on hydrogel muco-adhesion. Although we have not characterized the chemical modifications introduced by the crosslinker contents, the results show that a morphology-dominated adhesion model correlates well with the observed trend in the adhesive strength. We note that the high standard error on the residual moduli for hydrogels with lower crosslinker contents might result from the introduction of surface heterogeneity through the uneven spreading of the crosslinkers inside the L1 and DT1 silicone hydrogel materials, although this error scales with the magnitude of the means for both L1 and DT1, relative to those of L2 to L5.
The interesting increase in the residual modulus for L4 (Fig. 4) suggests that competitive mechanical mechanisms may contribute to the relaxation processes (Fig. 5). It is known that the elastic modulus of polymer networks increases with crosslinker contents. Several studies have suggested that cells can respond to a stiffer substrate by generating more focal adhesion complexes, resulting in a flatter morphology of the cells, which might indicate a stronger adhesion against the surface.21,22 The relaxation curves obtained from LCMR are a mixed response from the cell mechanics and the adhesive strength at the interface. At low crosslinker contents, the nonspecific interactions between the glycocalyx and the polymer networks in hydrogels dominates the adhesive strength of the cell monolayer. At high crosslinker contents, however, a more elastic cell response in the presence of a high-modulus substrate compensates the loss in adhesive strength owing to less intermolecular interactions, which results in a net increase of adhesive strength. The increased adhesive strength at 1.8% crosslinker content, therefore, might reflect the increase in cell monolayer modulus in response to a stiffer surface. Because lower adhesion at the lens/cornea interface is desired for easy removal, the nonmonotonic correlation between adhesive strength and crosslinker contents of the lens material indicates that an optimal crosslinker contents should be determined for each lens material to reduce adhesion, facilitate removal, and improve patient comfort. In addition, contact lenses encounter multiple material surfaces during manufacturing before contacting patients’ ocular surfaces. Therefore, the adhesive strengths of the lens materials against various surfaces during the manufacturing process and storage also contribute to the optimal level of crosslinker contents in developing contact lenses.
We also noticed the high adhesive strength of cell monolayers against delefilcon A lens (Fig. 4). Dunn et al.2 have reported that delefilcon A has a hydrophilic surface with an exceedingly low elastic modulus compared with other silicone hydrogel materials of similar formulations. As mentioned, flexible chains at low crosslinker contents can favor intermolecular bonding with cell surface proteins. A sharp increase in adhesive strength at ultralow crosslinker contents is also observed by Park and Robinson.14 Additionally, the hydrophilic nature of the delefilcon A hydrogel surface could provide potential hydrogen bonding sites with the glycosylated membrane-associated proteins on the corneal epithelial cells. Both factors can contribute to the observed high residual modulus for delefilcon A.
Overall, we characterized the adhesive strength of corneal epithelial cells against a series of systematically varied silicone hydrogel materials. For comparative purposes, an additional silicone hydrogel material (i.e., DT1) with a surface having very different structure and mechanical properties was tested. The results illustrate that the crosslinking density can be used to tune the adhesive strength of corneal epithelial cells against silicone hydrogel materials. A simple viscoelastic contact model can readily explain the observed changes in adhesive strengths with time and with crosslinker contents. This correlation can be exploited by the contact lens industry, drug delivery, and tissue engineering alike as a simple method of modulating epithelial cell muco-adhesive strength.
Supplementary Material
Acknowledgments
Supported by a grant from Johnson & Johnson Vision Care, Inc.
Disclosure: C. Liu, None; C.W. Scales, Johnson & Johnson Vision Care, Inc. (E); None; G.G. Fuller, Johnson & Johnson Vision Care, Inc. (F) None
References
- 1. Stapleton F, Tan J.. Impact of contact lens material, design, and fitting on discomfort. Eye Contact Lens. 2017; 43: 32–39. [DOI] [PubMed] [Google Scholar]
- 2. Dunn AC, Urueña JM, Huo Y, Perry SS, Angelini TE, Sawyer WG. Lubricity of surface hydrogel layers. Tribol Lett. 2013; 49: 371–378. [Google Scholar]
- 3. Jacob JT. Biocompatibility in the development of silicone-hydrogel lenses. Eye Contact Lens. 2013; 39: 13–19. [DOI] [PubMed] [Google Scholar]
- 4. Tighe BJ. A decade of silicone hydrogel development: surface properties, mechanical properties, and ocular compatibility. Eye Contact Lens. January 2013:1. [DOI] [PubMed] [Google Scholar]
- 5. Young G, Veys J, Pritchard N, Coleman S. A multi-centre study of lapsed contact lens wearers. Ophthalmic Physiol Opt. 2002; 22: 516–527. [DOI] [PubMed] [Google Scholar]
- 6. Sterner O, Aeschlimann R, Zürcher S, et al.. Friction measurements on contact lenses in a physiologically relevant environment: effect of testing conditions on friction measurements on contact lenses. Invest Ophthalmol Vis Sci. 2016; 57: 5383–5392. [DOI] [PubMed] [Google Scholar]
- 7. Shoaib T, Espinosa-Marzal RM.. Insight into the Viscous and Adhesive Contributions to Hydrogel Friction. Tribology Letters. 2018; 66: 96. [Google Scholar]
- 8. Israelachvili JN, Chen Y-L, Yoshizawa H. Relationship between adhesion and friction forces. J Adhes Sci Technol. 1994; 8: 1231–1249. [Google Scholar]
- 9. Rico F, Roca-Cusachs P, Gavara N, Farré R, Rotger M, Navajas D. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys Rev E. 2005; 72: 021914. [DOI] [PubMed] [Google Scholar]
- 10. Bausch AR, Ziemann F, Boulbitch AA, Jacobson K, Sackmann E. Local measurements of viscoelastic parameters of adherent cell surfaces by magnetic bead microrheometry. Biophys J. 1998; 75: 2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kollmannsberger P, Tanja Mierke C, Fabry B. Nonlinear viscoelasticity of adherent cells is controlled by cytoskeletal tension. Soft Matter. 2011; 7: 3127–3132. [Google Scholar]
- 12. Elkins CM, Qi QM, Fuller GG. Corneal cell adhesion to contact lens hydrogel materials enhanced via tear film protein deposition. PLoS One. 2014; 9: e105512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hollenbeck EC, Antonoplis A, Chai C, Thongsomboon W, Fuller GG, Cegelski L. Phosphoethanolamine cellulose enhances curli-mediated adhesion of uropathogenic Escherichia coli to bladder epithelial cells. Proc Natl Acad Sci USA. 2018; 115: 10106–10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park H, Robinson JR.. Mechanisms of mucoadhesion of poly(acrylic acid) hydrogels. Pharm Res. 1987; 4: 457–464. [DOI] [PubMed] [Google Scholar]
- 15. Leung S-HS, Robinson JR.. Polyanionic polymers in bio- and mucoadhesive drug delivery. In: Polyelectrolyte Gels. Vol 480. ACS Symposium Series. American Chemical Society; 1992: 269–284. [Google Scholar]
- 16. Zappone B, Ruths M, Greene GW, Jay GD, Israelachvili JN. Adsorption, lubrication, and wear of lubricin on model surfaces: polymer brush-like behavior of a glycoprotein. Biophys J. 2007; 92: 1693–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tobolsky AV, Katz D, Takahashi M, Schaffhauser R. Rubber elasticity in highly crosslinked systems: crosslinked styrene, methyl methacrylate, ethyl acrylate, and octyl acrylate. J Polymer Sci A. 1964; 2: 2749–2758. [Google Scholar]
- 18. Francius G, Hemmerlé J, Ohayon J, et al.. Effect of crosslinking on the elasticity of polyelectrolyte multilayer films measured by colloidal probe AFM. Microsc Res Tech. 69: 84–92. [DOI] [PubMed] [Google Scholar]
- 19. Fiejdasz S, Horak W, Lewandowska-Łańcucka J, Szuwarzyński M, Salwiński J, Nowakowska M. Tuning of elasticity and surface properties of hydrogel cell culture substrates by simple chemical approach. J Colloid Interface Sci. 2018; 524: 102–113. [DOI] [PubMed] [Google Scholar]
- 20. Flory PJ, Rabjohn N, Shaffer MC. Dependence of tensile strength of vulcanized rubber on degree of cross-linking. J Polymer Sci. 1949; 4: 435–455. [Google Scholar]
- 21. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006; 126: 677–689. [DOI] [PubMed] [Google Scholar]
- 22. Swift J, Ivanovska IL, Buxboim A, et al.. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013; 341: 1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hattrup CL, Gendler SJ.. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008; 70: 431–457. [DOI] [PubMed] [Google Scholar]
- 24. Shurer CR, Colville MJ, Gupta VK, et al.. Genetically encoded toolbox for glycocalyx engineering: tunable control of cell adhesion, survival, and cancer cell behaviors. ACS Biomater Sci Eng. 2018; 4: 388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuo JC-H, Gandhi JG, Zia RN, Paszek MJ. Physical biology of the cancer cell glycocalyx. Nat Phys. 2018; 14: 658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qiu Y, Pruitt JD, Thekveli SJ, Tucker RC, Nelson J. Silicone hydrogel lenses with water-rich surfaces. February 2012. US Patent US20120026458A1.
- 27. Qiu Y, Samuel NT, Pruitt JD, et al.. Silicone hydrogel lens with a crosslinked hydrophilic coating. February 2012. US Patent US13193651.
- 28. Bhamla MS, Chai C, Rabiah NI, Frostad JM, Fuller GG. Instability and breakup of model tear films. Invest Ophthalmol Vis Sci. 2016; 57: 949–958. [DOI] [PubMed] [Google Scholar]
- 29. Elkins CM. A Cell Monolayer Rheometer Measuring the Mechanical Properties of Living Cells. Stanford, CA:Stanford University; 2014. Thesis. [Google Scholar]
- 30. Yamaguchi M, Shiraishi A.. Relationship between eyelid pressure and ocular surface disorders in patients with healthy and dry eyes. Invest Ophthalmol Vis Sci. 2018; 59: DES56–DES63. [DOI] [PubMed] [Google Scholar]
- 31. Miller D. Pressure of the lid on the eye. Arch Ophthalmol. 1967; 78: 328–330. [DOI] [PubMed] [Google Scholar]
- 32. Tharmann R, Claessens MMAE, Bausch AR. Viscoelasticity of isotropically cross-linked actin networks. Phys Rev Lett. 2007; 98: 088103. [DOI] [PubMed] [Google Scholar]
- 33. Koenderink GH, Dogic Z, Nakamura F, et al.. An active biopolymer network controlled by molecular motors. Proc Natl Acad Sci USA. 2009; 106: 15192–15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yao NY, Becker DJ, Broedersz CP, et al.. Nonlinear viscoelasticity of actin transiently cross-linked with mutant α-actinin-4. J Mol Biol. 2011; 411: 1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.