Abstract
Hematopoietic stem cells (HSCs) maintain lifelong production of mature blood cells and regenerate the hematopoietic system after cytotoxic injury. Use of expanding cell surface marker panels and advanced functional analyses have revealed the presence of several immune-phenotypically different HSC subsets with distinct self-renewal and repopulating capacity and bias toward selective lineage differentiation. This chapter summarizes current understanding of the phenotypic and functional heterogeneity within the HSC pool, with emphasis on the immune-phenotypes and functional features of several known HSC subsets, and their roles in steady-state and emergency hematopoiesis, and in aging. The chapter also highlights some of the future research directions to elucidate further the biology and function of different HSC subsets in health and disease states.
Keywords: Hematopoietic stem cells, Hematopoiesis, HSC subsets, Functional heterogeneity, Differentiation bias
Introduction
Hematopoietic stem cells (HSCs) are defined by their ability to sustain life long production of mature blood cells of all lineages, and to repopulate all hematopoietic lineages after radiation or chemically induced cytotoxic insult. Healthy HSCs are capable of (a) long-term multilineage reconstitution and in situ recovery of the hematopoietic system (e.g., after massive cytotoxic injury induced by radiation or chemotherapy) and (b) long-term engraftment and multilineage repopulation after adoptive transplant into preconditioned recipients.
Identification, enrichment, and functional characterization of mouse adult HSCs in 1988 [1] paved the way for more precise isolation and characterization of HSCs. Subsequent studies have catalyzed continuous advancements in phenotypic profiling and enrichment of murine HSCs, as well as functional characterization of HSCs [2–9].
Important progress in identifying human HSCs was accomplished in 1992 through isolation of a candidate human HSC population from the fetal bone marrow [10]. Succeeding studies reported phenotypic and functional characterization of human HSCs residing in the cord blood, adult bone marrow (BM), the peripheral blood of patients after mobilization with granulocyte/macrophage colony-stimulating factor (GM-CSF) or granulocyte colony-stimulating factor (G-CSF), and in the peripheral blood of cancer patients undergoing chemotherapy [11–13]. These studies fueled identification of new human HSC markers and more advanced phenotypic and functional characterization of human HSCs residing in the bone marrow, fetal liver, cord blood, and peripheral blood [14–17].
Remarkably, early on it was apparent that the population of HSCs is heterogeneous [18]. Numerous studies since then have defined phenotypic and functional heterogeneity within HSC pool and have revealed the coexistence of several HSC subsets with distinct proliferation, self-renewal, and differentiation potentials [19–29]. Cumulatively, these findings transformed our perception of HSCs as a functionally uniform pool to that of a heterogeneous pool consisting of different HSC subsets.
Recent review brought up important questions about the origins of HSC heterogeneity [30]. The origins of HSC heterogeneity and intrinsic and extrinsic factors that may shape functional diversification of HSCs are not well understood. These factors may include: (a) differential genetic and epigenetic reprogramming during early development and cell maturation, (b) differential localization in BM niches, and (c) genetic and epigenetic reprogramming brought on by responses to different molecular and cellular stimuli [14, 30].
Phenotypic and Functional Distinction of Short-Term and Long-Term Repopulating Hematopoietic Stem Cells
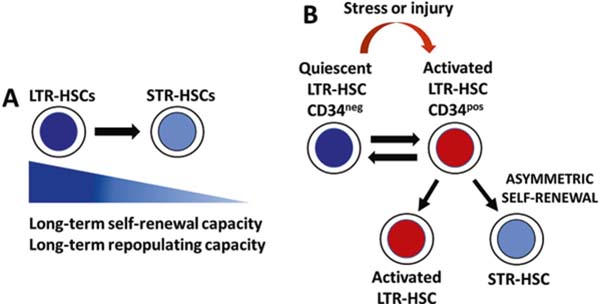
Transplantation of different BM cell populations phenotypically defined by differential expression of cell surface markers revealed that the HSC compartment is heterogeneous in regard to prototypical HSC functional features: self-renewal capacity and lifespan of individual HSC clones, multilineage repopulation of hematopoiesis in lethally irradiated mice, and the duration of repopulation after the transplant. Multiple studies used different sets of cell surface markers, retroviral cell marking, single cell transplant approaches, cellular barcoding, and serial transplantation to establish the existence and define functional properties of short-term repopulating HSCs (STR-HSC) and long-term repopulating HSCs (LTR-HSC) in mice and humans. In mice, STR-HSCs have a limited self-renewal capacity and upon transplantation are able to support hematopoiesis in lethally irradiated recipients for up to several months. In contrast, LTR-HSCs display an extensive self-renewal capacity and can support hematopoiesis for ≥6 months and longer. Serial transplantation remains a standard for in vivo assessment of long-term self-renewal and multilineage repopulating potential of HSCs. In that regard, murine LTR-HSCs can be distinguished from STR-HSC by their capacity to reconstitute hematopoietic systems of lethally irradiated recipients in a successive manner through serial transplantation [27, 31–45] (Fig. 10.1a).
Fig. 10.1.
Functional features of short-term repopulating HSCs (STR-HSC) and long-term repopulating HSCs (LTR-HSC). (a) LTR-HSCs display long-term self-renewal and repopulating capacity, whereas STR-HSCs exhibit short-term self-renewal and repopulating capacity. (b) During homeostasis, the majority of LTR-HSCs are CD34neg, and are quiescent and dividing infrequently. In response to stress and injury, dormant HSCs are activated and start to express CD34, and cycle more frequently. Activated HSCs are maintaining themselves and are generating STR-HSCs through asymmetric self-renewal divisions. After restoring homeostasis of the hematopoietic system, activated HSCs return to a quiescent state
Human LTR-HSCs can maintain human hematopoiesis in serially transplanted recipient immunodeficient mice. Matsuoka et al. have reported that human cord blood CD34+/− cells that do not express thrombopoietin (TPO) receptor myeloproliferative leukemia protein (MPL) maintain long-term human hematopoiesis in primary, secondary, and tertiary recipient immunodeficient mice and represent LTR-HSCs. The population of CD34+ MPL+ cells represents intermediate-term HSCs, whereas CD34- MPL+ cells are short-term repopulating HSCs [46].
Further studies revealed differential CD34 expression on murine LTR-HSCs during developmental stages. During fetal and neonatal period, LTR-HSCs express CD34, whereas LTR-HSCs from adult mice are CD34neg. Notably, the expression of CD34 marker on adult mouse LTR-HSCs is reversible. During steady-state hematopoiesis in adult mice, the majority of LTR-HSCs are CD34neg. However, after cytotoxic injury, LTR-HSCs exist in two “states”: CD34neg and CD34pos states. More importantly, when hematopoiesis achieves steady state after the injury, the CD34pos HSCs revert to CD34neg state [47–49] (Fig. 10.1b).
Notably, studies of adult human HSCs have also documented reversible in vivo expression of CD34, and revealed that both CD34neg and CD34pos HSC subsets exhibit long-term in vivo multilineage engraftment capacity [50, 51].
Expression of CD34 on mouse LTR-HSCs is also a marker of HSC dormancy and activation. During homeostasis, the majority of infrequently dividing and dormant HSCs are in the CD34neg subset, whereas CD34pos subset contains active self-renewing HSCs. In response to stress and injury (e.g., infections, acute blood cell loss, chemotherapy, or irradiation-induced cytotoxicity), dormant CD34neg HSCs switch to actively cell cycling CD34pos state. Importantly, once the blood system is regenerated and the homeostasis is re-established, activated HSCs return to dormancy [52–55]. These observations indicate that HSCs can reversibly switch from dormant to activated state in response to hematopoietic stress and injury. Moreover, these studies have revealed that reversible CD34 expression demarcates activation states of HSCs during homeostatic and pathological conditions.
In response to stress and injury (e.g., infections, acute blood cell loss, chemotherapy, or irradiation-induced cytotoxicity), HSCs exit dormancy to restore the homeostasis of the hematopoiesis, and then return to a quiescent state (Fig. 10.1b). Return of HSCs to a quiescent state minimizes replicative stress and DNA damage accumulation in HSCs and is extremely important for maintaining the fitness of individual HSCs and entire HSC pool, thus preventing HSC exhaustion and possible bone marrow failure [56, 57].
Lineage-Biased Hematopoietic Stem Cell Subsets
Initial prevailing concept was that LTR-HSCs are uniform in their self-renewal, long-term repopulating, and multilineage differentiation capacities. However, transplantation of single HSCs and in vivo tracking of their progeny and lineage differentiation potential in a serial transplant setting have revealed quite a functional diversity and consequential heterogeneity among LTR-HSCs. These studies have defined several HSC subsets that exhibit lineage differentiation bias and differential propensity in vivo to generate myeloid and lymphoid lineages. While these lineage- biased HSCs can generate all hematopoietic lineages, the ratios of lymphoid and myeloid cells they produce differ [19, 23, 26, 58].
Lineage-biased HSC subsets that exhibit distinct lineage output profiles in vivo and are present in young and old mice were isolated and characterized based on differential levels of expression of signaling lymphocytic activation molecule 1 (SLAMf1) or CD150. CD150 is one of the SLAM family markers used to distinguish HSCs from multipotent progenitors (MPPs) and downstream oligopotent progenitors [27, 33].
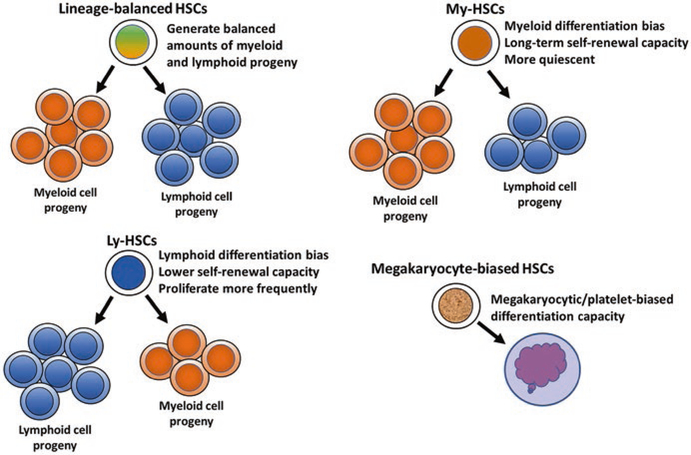
The CD150hi subset of LTR-HSCs, named Myeloid-biased HSCs (My-HSCs), predominantly generated myeloid lineages and has stably maintained the same differentiation pattern and self-renewal potential throughout serial transplantation. In addition, the lymphoid progeny of My-HSCs exhibited reduced response to IL-7, a cytokine with an important role in differentiation and proliferation of lymphoid cells. The CD150low HSC subset, named Lymphoid-biased HSCs (Ly-HSCs), largely generated lymphoid lineages (Fig. 10.2). The third CD150med HSC subset produces balanced amounts of myeloid and lymphoid progeny and represents lineage- balanced HSC subset [19, 23, 26, 58, 59] (Fig. 10.2).
Fig. 10.2.
Lineage-biased HSC subsets exhibit in vivo lineage differentiation bias. Lineage-balanced HSCs produce balanced amounts of myeloid and lymphoid progeny. Myeloid-biased HSCs exhibit long-term self-renewal capacity and myeloid differentiation bias. Lymphoid-biased HSCs display lymphoid differentiation bias and lower self-renewal capacity and proliferate more frequently. Megakaryocyte-biased HSCs display megakaryocytic/platelet-biased differentiation capacity
In addition to differential lineage outputs, lineage-biased HSC subsets exhibit different self-renewal and LTR capacity, and differentiation kinetics. Among lineage- biased HSCs My-HSCs are more quiescent and exhibit the highest long-term self-renewal and LTR capacity. In contrast, Ly-HSCs divide more frequently and exhibit lesser and shorter lasting self-renewal potential, as observed after serial transplantation of clonally derived Ly-HSCs [19, 23, 26, 58, 60]. Serial transplantation experiments have also revealed the stability of lineage bias among lineage-biased HSC subsets, indicating that the biased differentiation predisposition is a stable functional feature of these HSC subsets.
Expression of CD41 (platelet integrin alpha chain 2b) was reported to also distinguish myeloid-biased and lymphoid-biased HSC subsets [61]. CD41pos HSC subset is more quiescent and selectively expresses key transcription factors driving myelo-erythroid and megakaryocyte differentiation. CD41neg HSC subset on the other hand divides more frequently and exhibits lymphoid lineage gene priming. In serial transplantation experiments, CD41pos HSCs exhibited robust long-term repopulation capacity and marked bias toward myeloid differentiation, whereas CD41neg HSCs exhibited attenuated long-term repopulation capacity and marked bias toward generating lymphoid progeny. Analysis of CD41 KO mice revealed significant decreases in platelet, erythrocyte, and all leukocyte lineages. Furthermore, loss of CD41 resulted in perturbed hematopoiesis and decreased survival and quiescence of HSCs [61].
In addition to myeloid and lymphoid-biased HSC subsets, several studies have detected the presence of HSC subsets that display megakaryocytic/platelet-biased differentiation capacity [59, 62–65] (Fig. 10.2).
Platelets are generated in the bone marrow through megakaryopoiesis, and together with red blood cells comprise >90% of the cells produced daily. In addition to their role in thrombosis and wound healing, activated platelets modulate innate and adaptive immune responses to infections [66, 67]. Rapid consumption of platelets due to excessive bleeding, injury, and infection can lead to transient thrombocytopenia, which must be resolved by rapid platelet replenishment.
Analysis of Vwf-eGFP BAC transgenic mice with eGFP reporter driven by the megakaryocyte-associated von Willebrand factor (VWF) identified vWF− and vWF+ HSC subsets. Functional analysis of lineage differentiation bias of vWF+ and vWF− HSCs at the clonal level revealed that vWF+ HSCs displayed long-term repopulating capacity with platelet/myeloid-biased repopulation of recipients, while vWF− HSCs showed long-term repopulating capacity with clear lymphoid-biased repopulation of recipients [59]. Single-cell transplantation and single-cell RNASeq expression analysis detected a subset of HSCs expressing high levels of von Willebrand factor (VWF), which could represent a population of megakaryocyte- biased HSCs [64].
During steady-state hematopoiesis, there is a log-fold range in the level of cell surface expression of stem cell factor (SCF) receptor c-kit on HSCs. Since c-kit expression is the obligatory marker for isolation of HSCs, and SCF and c-kit have an important role in HSC function and maintenance, Shin et al. investigated the functional and genetic properties of HSCs expressing low and high levels of c-kit. Functional and transcriptome analyses in that study have revealed that HSCs expressing the same level of CD150 marker, but different level of c-kit, are functionally diverse subsets [63]. HSCs with low level of surface c-kit expression exhibited enhanced self-renewal and long-term repopulating potential. In addition, c-kitlow HSCs are more quiescent and display delayed multilineage differentiation. In contrast, HSCs with high level of surface c-kit expression displayed increased cell cycling and rapid multilineage differentiation kinetics, but impaired long-term repopulation capacity and self-renewal after serial transplant. In vitro and in vivo studies of the differentiation potential of c-kitlow and c-kithi HSCs have also shown that c-kithi HSC subset possess much higher megakaryocyte differentiation capacity and gives rise more rapidly to megakaryocytes than c-kitlow HSCs, and thus exhibits a megakaryocytic lineage bias [63].
In human hematopoiesis, study by Matsuoka et al. demonstrated that human cord blood CD34− and CD34+ HSCs represent myeloid-biased and lymphoid-biased HSC subsets with long-term repopulating capacities [68]. A recent study has confirmed that the CD34− subset of cord blood HSCs represents the most primitive human LTR-HSCs, which also display megakaryocyte/erythrocyte lineage priming and much higher megakaryocyte/erythrocyte differentiation potential in vivo than the CD34+ HSC subset [69].
It is evident that the LTR-HSC pool is functionally and immunophenotypically diverse, encompassing HSC subsets with different self-renewal and long-term repopulating capacity, proliferation and differentiation kinetics, and lineage differentiation bias.
Expanding cell surface marker panels and advancing technologies for purification and genetic and functional analysis of a small population of cells will undoubtedly lead to more definitive immunophenotypic and functional classification of known lineage-biased HSC subsets in homeostasis, and perhaps the discovery of new types of lineage-biased HSCs.
It will be interesting and important to determine whether lineage-biased HSC subsets are developmentally independent, or if there is a developmental hierarchy involved, such as the one described for murine CD41pos and CD41neg HSC subsets [61], and how are they maintained and regulated [70–72].
There is still much to be learned about: (a) the origins of HSC heterogeneity and developmental emergence of different HSC subsets [30] and (b) functional properties (self-renewal and LTR capacity, differentiation kinetics, and profile) and cell surface immunophenotypes of different subsets of HSCs under pathophysiological conditions (e.g., inflammatory and oxidative stress, infections) and during and after cytotoxic injury (radiation, chemotherapy).
Hematopoietic Stem Cells and Emergency Hematopoiesis
In the last decade, it became apparent that hematopoiesis is a very adaptable and responsive process that can quickly react to external stimuli such as infections and change to meet the need for the specific type of blood cells. Viral and bacterial infections and associated inflammation mobilize present innate immune effector cells (granulocytes, monocytes) to contain or eradicate pathogens. During infection, innate immune effector cells are rapidly used and need to be continuously replenished. Continuous need for newly generated innate immune effector cells causes a shift from steady-state hematopoiesis to emergency hematopoiesis (EH), which ensures increased output of innate immune effector cells and fast innate immune responses. Both EH and subsequent adaptive immune response are necessary to control and clear viral and bacterial infections.
A number of studies have established that EH involves and starts with a response of HSCs to infections. HSCs express pattern-recognition receptors (PRRs) such as toll-like receptors (TLRs) that recognize pathogen-associated molecular patterns (PAMPs). TLR engagement by bacterial or viral PAMPs activates the NF-κB and interferon regulatory factor (IRF) pathways, which mediate inflammation and antibacterial and antiviral defense. HSCs are also responsive to inflammatory cytokines and chemokines that are being produced in response to pathogens. Thus, HSCs can “detect” infection via PAMPs and inflammatory cytokines and chemokines and initiate rapid generation of innate immune cells. During EH, inflammatory cytokines (e.g., Type I and Type II IFNs and IL-6) and chemokines activate HSCs, which results in increased proliferation and temporary expansion of HSCs pool, myeloid lineage-biased differentiation and mobilization of HSCs into peripheral blood, and migration to spleen (extramedullary hematopoiesis) [73–83].
Several studies have reported that exposure to recurring infections and chronic inflammation are eroding self-renewal and repopulating capacity of LTR-HSCs, leading to reduction of HSC pool and sometimes even to their depletion [74, 76, 77, 84–87].
Notably, in an animal model of Lymphocytic choriomeningitis virus (LCMV) infection, IFNγ was found to promote differentiation of myeloid-biased HSCs but not lymphoid-biased HSCs during an innate immune response to infection [88]. Other studies reported that Ehrlichia muris infection causes activation of LTR-HSCs and myeloid differentiation bias, and that TLR-mediated stimulation of HSCs promotes myeloid differentiation and activates myeloid-biased HSCs [71, 77, 79]. Thus, it appears that pathogens are preferentially inducing differentiation of My-HSC subset.
As mentioned earlier, the platelets play an important role in innate and adaptive immune responses to infections [66, 67]. Infections can increase platelet turnover, which can lead to transient thrombocytopenia. In that situation, the hematopoietic system must quickly ramp up the production of platelets.
In an effort to understand how the hematopoietic system counteracts infection-induced thrombocytopenia, Haas et al. discovered a new stem-like megakaryocyte-committed progenitor cell population (SL-MkPs) in the HSC compartment. Although SL-MkPs share some of the features with multipotent HSCs, this cell subset is unipotent and produces only megakaryocyte lineage [89]. In the steady-state hematopoiesis, SL-MkPs are in a quiescent state, and do not participate much in a steady-state megakaryopoiesis. However, infection-related inflammation induces emergency megakaryopoiesis, activates this cell subset, and promotes rapid differentiation into megakaryocytes and generation of platelets [89]. These findings further illustrate how the entire HSC compartment and different HSC subsets are equipped to respond to and rapidly deal with diverse emergency conditions. It will be interesting to study further the features and function of SL-MkPs subset in real infection scenarios.
Overall, further studies are necessary to determine the effects of acute and chronic bacterial and viral infections on the function of different lineage-biased HSCs in young, middle-aged, and old hosts.
Aging of Hematopoietic Stem Cell Subsets
Aging brings on wide-ranging adverse changes in the function of the hematopoietic system and causes profound and distinct functional and quantitative changes among HSC subsets which are at the core of altered and dysregulated hematopoiesis and immunity. The hallmarks of the aged hematopoietic system are enhanced myelopoiesis and increased production of myeloid cells, impaired lymphopoiesis and decreased output of lymphoid cells, declining function of the immune system, and anemia. Functional impairments of the adaptive immune system and dysregulation of innate immunity are contributing to (a) increased susceptibility to and occurrence of serious infections, (b) impaired would healing, and (c) increased incidence of inflammatory and autoimmune diseases, and hematological and other malignancies. All these adverse effects of the hematopoietic system aging are significantly increasing morbidity and mortality among the elderly [90–94].
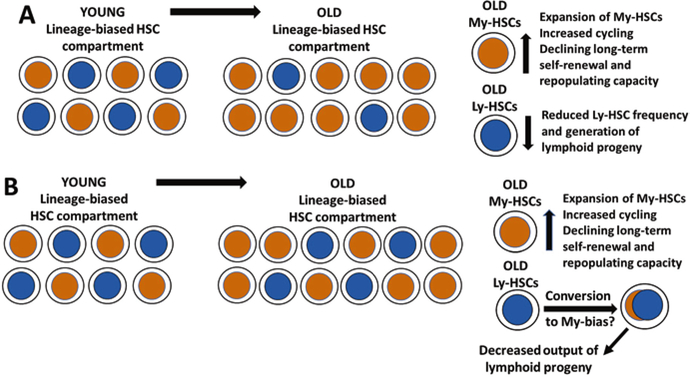
In young and middle-aged mice, the stable balance is maintained between myeloid-biased, lymphoid-biased, and balanced HSC subsets [23, 58–60] (Fig. 10.3a). In older mice, the pool of myeloid-biased HSCs is expanding, and that subset becomes a predominant type of HSCs in very old mice. More importantly, old myeloid-biased HSCs display increased cycling, declining long-term self-renewal and repopulating capacity, and reduced generation of mature blood cells in comparison to young myeloid-biased HSCs [19, 91, 92, 95–97] (Fig. 10.3a).
Fig. 10.3.
Age-induced changes in lineage-biased HSC subsets. (a) In a current model of HSC aging, the subset of My-HSCs expands, but their long-term self-renewal and repopulating capacity are declining. In contrast, the pool of Ly-HSCs is reduced, contributing to reduced lymphopoiesis and decreased output of lymphoid progeny. (b) In an alternative newly proposed model, the numbers of old Ly-HSCs do not change, but their lineage differentiation bias could be switching to myelopoiesis, resulting in decreased output of lymphoid progeny
Functional and transcriptome analysis of murine young and old HSCs at the single-cell level identified upregulated platelet-lineage gene expression and functional platelet bias at the single-cell level among old HSCs. Moreover, the pool of old HSCs contains a high proportion of previously unknown platelet-restricted HSCs that almost exclusively produce platelets [98]. Observation that inactivation of FOG-1 transcription factor (involved in normal development of megakaryocytes and erythrocytes in mice and humans) increases lymphoid lineage output, suggested that platelet-biased priming of HSCs may contribute to reduced production of lymphoid cell lineages [98].
Significantly reduced and altered lymphopoiesis and decreased output of T and B cell lineages in old age are thought to be caused in part by reduction of the pool of lymphoid-biased HSCs and their age-related functional decline [91, 99, 100] (Fig. 10.3a). The most recent paper reported that phenotypic and functional changes in the aged immune system are largely due to functional and epigenetic changes in old HSCs, which are defining the transcriptional profile and impaired function of their T and B cell progeny [101]. Regrettably, the LTR-HSCs analyzed in that study were not separated into lymphoid-biased and myeloid-biased HSC subsets.
Previous studies have reported reduced frequency of lymphoid-biased HSCs in old mice, leading to conclusion that age-related impaired lymphopoiesis is due to loss of lymphoid-biased HSCs.
Dorshkind group [102] and our group as well (unpublished data) have observed that when the total numbers of HSCs and lymphoid-biased HSCs are determined instead of their frequency, the number of lymphoid-biased HSCs is not statistically different between young and old mice [102] (Fig. 10.3b). More importantly, the transcriptome profiles of old My-HSCs and Ly-HSCs were found to be similar, suggesting that old Ly-HSCs acquire myeloid-biased characteristics. Based on these findings, Kong et al. are suggesting a revised model of Ly-HSC aging, wherein the switch to myeloid-biased transcriptional profile rather than reduction of the pool of old Ly-HSCs contributes to diminished lymphopoiesis in old age [102] (Fig. 10.3b). Further studies of old Ly-HSCs are needed to elucidate further genetic and functional changes undergoing in that HSC subset and to understand better how these age-associated changes impact the lymphopoiesis and function of immune system in old age.
Notably, the age-induced functional and quantitative changes of HSCs subsets in old mice and elderly humans are quite similar. The number of HSCs in elderly humans is expanded as well, and old HSCs are predominantly myeloid biased and are less quiescent, but with reduced self-renewal and repopulating capacity. More importantly, aged human HSCs display transcriptional upregulation of genes associated with cell cycle, myeloid lineage specification, and myeloid malignancies. Moreover, the pool of aged myeloid-biased HSCs contains HSCs that carry genetic and epigenetic changes, which are increasing the risk of developing age-associated hematopoietic diseases such as myelodysplastic syndromes (MDS), myeloproliferative disorders (MPDs), bone marrow failure disorders, and myeloid leukemias [90–94, 103].
A plethora of cell-intrinsic factors and changes (increased myeloid priming and differentiation signaling pathways, downregulated lymphoid priming and lymphoid differentiation signaling pathways, impaired response to genotoxic stress, epigenetic changes, etc.) and cell-extrinsic factors (aged microenvironment, aged HSC niches, inflammation, oxidative stress) contribute to aging-related functional changes of lineage-biased HSC subsets [104–108].
Continued functional and molecular profiling of murine and human HSC subsets during aging are necessary for development of therapeutic approaches that would: (a) slow down or attenuate HSC aging and (b) modify bias of old HSC subsets toward selective lineage differentiation with the goal to improve the function of old HSCs and hematopoietic and immune systems in elderly.
Concluding Remarks and Future Directions
Ongoing progress in HSC research revealed increasing cellular and functional heterogeneity and complexity of the HSC compartment. Current understanding indicates that the HSC pool is composed of functionally diverse and yet synergistic array of HSC subsets. Combined, these HSC subsets can cover most if not all “bases” to maintain optimal functioning of the hematopoietic system in steady state and during emergency conditions (e.g., inflammatory and oxidative stress), and to rapidly regenerate the hematopoietic system after cytotoxic injury and restore its homeostasis.
It is very important and clinically highly relevant to amplify our understanding of the function of all HSC subsets in a steady state and especially under pathophysiological and stress conditions. Below are some of the future research directions with a clinical relevance, whose outcomes could pave the way for targeted modification of genetic and epigenetic makeup and functional properties (e.g., lineage differentiation bias) of different HSC subsets to improve function of the hematopoietic and immune systems in aging and disease:
Analysis of clonal succession and dominance among different HSC subsets from young to old age, and in homeostatic versus pathophysiological conditions
Analysis of the function of different HSC subsets during acute and chronic infections (acute and chronic emergency hematopoiesis) in young, middle-aged, and old hosts
Analysis of the function of different HSC subsets during acute and chronic inflammation
Characterization of the effects of pre-therapy cancer progression on the function of all HSC subsets (Cancer Hematopoiesis)
Characterization of the acute effects of cancer and therapy (radiation and chemotherapy) on the function of all HSC subsets in tumor-bearing mouse models and in cancer patients
Characterization of the long-lasting effects of cancer and therapy (radiation and chemotherapy) on the function of all HSC subsets in murine cancer survivor models and in cancer survivors.
References
- 1.Spangrude GJ, Heimfeld S, Weissman IL (1988) Purification and characterization of mouse hematopoietic stem cells. Science 241(4861):58–62 [DOI] [PubMed] [Google Scholar]
- 2.Jurecic R, Van NT, Belmont JW (1993) Enrichment and functional characterization of Sca-1+WGA+, Lin-WGA+, Lin-Sca-1+, and Lin-Sca-1+WGA+ bone marrow cells from mice with an Ly-6a haplotype. Blood 82(9):2673–2683 [PubMed] [Google Scholar]
- 3.Li CL, Johnson GR (1992) Long-term hemopoietic repopulation by Thy-1lo, Lin-, Ly6A/E+ cells. Exp Hematol 20(11):1309–1315 [PubMed] [Google Scholar]
- 4.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Nishikawa S, Miura Y, Suda T (1991) Enrichment and characterization of murine hematopoietic stem cells that express c-kit molecule. Blood 78(7):1706–1712 [PubMed] [Google Scholar]
- 5.Spangrude GJ (1989) Enrichment of murine haemopoietic stem cells: diverging roads. Immunol Today 10(10):344–350 [DOI] [PubMed] [Google Scholar]
- 6.Spangrude GJ, Scollay R (1990) A simplified method for enrichment of mouse hematopoietic stem cells. Exp Hematol 18(8):920–926 [PubMed] [Google Scholar]
- 7.Spangrude GJ, Smith L, Uchida N, Ikuta K, Heimfeld S, Friedman J, Weissman IL (1991) Mouse hematopoietic stem cells. Blood 78(6):1395–1402 [PubMed] [Google Scholar]
- 8.Weissman IL, Heimfeld S, Spangrude G (1989) Haemopoietic stem cell purification. Immunol Today 10(6):184–185 [DOI] [PubMed] [Google Scholar]
- 9.Weissman IL, Shizuru JA (2008) The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood 112(9):3543–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B (1992) Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci U S A 89(7):2804–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE (1997) Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A 94(10):5320–5325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray L, DiGiusto D, Chen B, Chen S, Combs J, Conti A, Galy A, Negrin R, Tricot G, Tsukamoto A (1994) Analysis of human hematopoietic stem cell populations. Blood Cells 20(2–3):364–369 [PubMed] [Google Scholar]
- 13.Murray L, Chen B, Galy A, Chen S, Tushinski R, Uchida N, Negrin R, Tricot G, Jagannath S, Vesole D et al. (1995) Enrichment of human hematopoietic stem cell activity in the CD34+Thy-1+Lin- subpopulation from mobilized peripheral blood. Blood 85(2):368–378 [PubMed] [Google Scholar]
- 14.Guezguez B, Campbell CJ, Boyd AL, Karanu F, Casado FL, Di Cresce C, Collins TJ, Shapovalova Z, Xenocostas A, Bhatia M (2013) Regional localization within the bone marrow influences the functional capacity of human HSCs. Cell Stem Cell 13(2):175–189 [DOI] [PubMed] [Google Scholar]
- 15.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE (2011) Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 333(6039):218–221 [DOI] [PubMed] [Google Scholar]
- 16.Reckzeh K, Kizilkaya H, Helbo AS, Alrich ME, Deslauriers AG, Grover A, Rapin N, Asmar F, Grønbæk K, Porse B, Borregaard N, Vestweber D, Nerlov C, Theilgaard-Mönch K (2018) Human adult HSCs can be discriminated from lineage-committed HPCs by the expression of endomucin. Blood Adv 2(13):1628–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sitnicka E, Buza-Vidas N, Larsson S, Nygren JM, Liuba K, Jacobsen SE (2003) Human CD34+ hematopoietic stem cells capable of multilineage engrafting NOD/SCID mice express flt3: distinct flt3 and c-kit expression and response patterns on mouse and candidate human hematopoietic stem cells. Blood 102(3):881–886 [DOI] [PubMed] [Google Scholar]
- 18.Uchida N, Fleming WH, Alpern EJ, Weissman IL (1993) Heterogeneity of hematopoietic stem cells. Curr Opin Immunol 5(2):177–184 [DOI] [PubMed] [Google Scholar]
- 19.Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, Rossi DJ (2010) Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A 107(12):5465–5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benveniste P, Frelin C, Janmohamed S, Barbara M, Herrington R, Hyam D, Iscove NN (2010) Intermediate-term hematopoietic stem cells with extended but time-limited reconstitution potential. Cell Stem Cell 6(1):48–58 [DOI] [PubMed] [Google Scholar]
- 21.Benz C, Copley MR, Kent DG, Wohrer S, Cortes A, Aghaeepour N, Ma E, Mader H, Rowe K, Day C, Treloar D, Brinkman RR, Eaves CJ (2012) Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell 10(3):273–283 [DOI] [PubMed] [Google Scholar]
- 22.Copley MR, Beer PA, Eaves CJ (2012) Hematopoietic stem cell heterogeneity takes center stage. Cell Stem Cell 10(6):690–697 [DOI] [PubMed] [Google Scholar]
- 23.Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee SJ, Brinkman R, Eaves C (2007) Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell 1(2):218–229 [DOI] [PubMed] [Google Scholar]
- 24.Hock H (2010) Some hematopoietic stem cells are more equal than others. J Exp Med 207:1127–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Clevers H (2010) Coexistence of quiescent and active adult stem cells in mammals. Science 327:542–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morita Y, Ema H, Nakauchi H (2010) Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med 207:1173–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oguro H, Ding L, Morrison SJ (2013) SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell 13(1):102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raaijmakers MH, Scadden DT (2008) Divided within: heterogeneity within adult stem cell pools. Cell 135:1006–1008 [DOI] [PubMed] [Google Scholar]
- 29.Schroeder T (2010) Hematopoietic stem cell heterogeneity: subtypes, not unpredictable behavior. Cell Stem Cell 6:203–207 [DOI] [PubMed] [Google Scholar]
- 30.Crisan M, Dzierzak E (2016) The many faces of hematopoietic stem cell heterogeneity. Development 143(24):4571–4581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adolfsson J et al. (2001) Upregulation of Flt3 expression within the bone marrow Lin(−) Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity 15:659–669 [DOI] [PubMed] [Google Scholar]
- 32.Christensen JL, Weissman IL (2001) Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A 98(25):14541–14546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121(7):1109–1121 [DOI] [PubMed] [Google Scholar]
- 34.Lemischka IR, Raulet DH, Mulligan RC (1986) Developmental potential and dynamic behavior of hematopoietic stem cells. Cell 45(6):917–927 [DOI] [PubMed] [Google Scholar]
- 35.Lemischka IR (1992) What we have learned from retroviral marking of hematopoietic stem cells. Curr Top Microbiol Immunol 177:59–71 [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Papa EF, Dooner MS, Machan JT, Johnson KW, Goldberg LR, Quesenberry PJ, Colvin GA (2012) Homing and long-term engraftment of long- and short-term renewal hematopoietic stem cells. PLoS One 7(2):e31300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu R, Neff NF, Quake SR, Weissman IL (2011) Tracking single hematopoietic stem cells in vivo using high-throughput sequencing in conjunction with viral genetic barcoding. Nat Biotechnol 29:928–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKenzie JL, Gan OI, Doedens M, Dick JE (2005) Human short-term repopulating stem cells are efficiently detected following intrafemoral transplantation into NOD/SCID recipients depleted of CD122+ cells. Blood 106(4):1259–1261 [DOI] [PubMed] [Google Scholar]
- 39.Morrison SJ, Weissman IL (1994) The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity 1(8):661–673 [DOI] [PubMed] [Google Scholar]
- 40.Nakauchi H, Sudo K, Ema H (2001) Quantitative assessment of the stem cell self-renewal capacity. Ann N Y Acad Sci 938:18–24 [DOI] [PubMed] [Google Scholar]
- 41.Osawa M, Hanada K, Hamada H, Nakauchi H (1996) Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273:242–245 [DOI] [PubMed] [Google Scholar]
- 42.Randall TD, Lund FE, Howard MC, Weissman IL (1996) Expression of murine CD38 defines a population of long-term reconstituting hematopoietic stem cells. Blood 87(10):4057–4067 [PubMed] [Google Scholar]
- 43.Szilvassy SJ, Humphries RK, Lansdorp PM, Eaves AC, Eaves CJ (1990) Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc Natl Acad Sci U S A 87(22):8736–8740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagers AJ, Weissman IL (2006) Differential expression of alpha2 integrin separates long-term and short-term reconstituting Lin-/loThy1.1(lo)c-kit+ Sca-1+ hematopoietic stem cells. Stem Cells 24(4):1087–1094 [DOI] [PubMed] [Google Scholar]
- 45.Yang L et al. (2005) Identification of Lin(−)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood 105:2717–2723 [DOI] [PubMed] [Google Scholar]
- 46.Matsuoka Y, Takahashi M, Sumide K, Kawamura H, Nakatsuka R, Fujioka T, Sonoda Y (2017) CD34 antigen and the MPL receptor expression defines a novel class of human cord blood-derived primitive hematopoietic stem cells. Cell Transplant 26(6):1043–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuoka S, Ebihara Y, Xu M, Ishii T, Sugiyama D, Yoshino H, Ueda T, Manabe A, Tanaka R, Ikeda Y, Nakahata T, Tsuji K (2001) CD34 expression on long-term repopulating hematopoietic stem cells changes during developmental stages. Blood 97(2):419–425 [DOI] [PubMed] [Google Scholar]
- 48.Ogawa M, Tajima F, Ito T, Sato T, Laver JH, Deguchi T (2001) CD34 expression by murine hematopoietic stem cells. Developmental changes and kinetic alterations. Ann N Y Acad Sci 938:139–145 [DOI] [PubMed] [Google Scholar]
- 49.Sato T, Laver JH, Ogawa M (1999) Reversible expression of CD34 by murine hematopoietic stem cells. Blood 94:2548–2554 [PubMed] [Google Scholar]
- 50.Dao MA, Arevalo J, Nolta JA (2003) Reversibility of CD34 expression on human hematopoietic stem cells that retain the capacity for secondary reconstitution. Blood 101(1):112–118 [DOI] [PubMed] [Google Scholar]
- 51.Zanjani ED, Almeida-Porada G, Livingston AG, Zeng H, Ogawa M (2003) Reversible expression of CD34 by adult human bone marrow long-term engrafting hematopoietic stem cells. Exp Hematol 31(5):406–412 [DOI] [PubMed] [Google Scholar]
- 52.Baumgartner C, Toifl S, Farlik M, Halbritter F, Scheicher R, Fischer I, Sexl V, Bock C, Baccarini M (2018) An ERK-dependent feedback mechanism prevents hematopoietic stem cell exhaustion. Cell Stem Cell 22(6):879–892.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cabezas-Wallscheid N, Buettner F, Sommerkamp P, Klimmeck D, Ladel L, Thalheimer FB, Pastor-Flores D, Roma LP, Renders S, Zeisberger P, Przybylla A, Schönberger K, Scognamiglio R, Altamura S, Florian CM, Fawaz M, Vonficht D, Tesio M, Collier P, Pavlinic D, Geiger H, Schroeder T, Benes V, Dick TP, Rieger MA, Stegle O, Trumpp A (2017) Vitamin A-retinoic acid signaling regulates hematopoietic stem cell dormancy. Cell 169(5):807–823 [DOI] [PubMed] [Google Scholar]
- 54.Wilson A, Oser GM, Jaworski M, Blanco-Bose WE, Laurenti E, Adolphe C, Essers MA, Macdonald HR, Trumpp A (2007) Dormant and self-renewing hematopoietic stem cells and their niches. Ann N Y Acad Sci 1106:64–75 [DOI] [PubMed] [Google Scholar]
- 55.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lió P, Macdonald HR, Trumpp A (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135(6):1118–1129 [DOI] [PubMed] [Google Scholar]
- 56.Nakamura-Ishizu A, Takizawa H, Suda T (2014) The analysis, roles and regulation of quiescence in hematopoietic stem cells. Development 141(24):4656–4666 [DOI] [PubMed] [Google Scholar]
- 57.Walter D, Lier A, Geiselhart A, Thalheimer FB, Huntscha S, Sobotta MC, Moehrle B, Brocks D, Bayindir I, Kaschutnig P, Muedder K, Klein C, Jauch A, Schroeder T, Geiger H, Dick TP, Holland-Letz T, Schmezer P, Lane SW, Rieger MA, Essers MA, Williams DA, Trumpp A, Milsom MD (2015) Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature 520(7548):549–552 [DOI] [PubMed] [Google Scholar]
- 58.Muller-Sieburg CE, Cho RH, Karlsson L, Huang JF, Sieburg HB (2004) Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood 103(11):4111–4118 [DOI] [PubMed] [Google Scholar]
- 59.Sanjuan-Pla A, Macaulay IC, Jensen CT, Woll PS, Luis TC, Mead A, Moore S, Carella C, Matsuoka S, Bouriez Jones T, Chowdhury O, Stenson L, Lutteropp M, Green JC, Facchini R, Boukarabila H, Grover A, Gambardella A, Thongjuea S, Carrelha J, Tarrant P, Atkinson D, Clark SA, Nerlov C, Jacobsen SE (2013) Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature 502(7470):232–236 [DOI] [PubMed] [Google Scholar]
- 60.Sieburg HB, Cho RH, Dykstra B, Uchida N, Eaves CJ, Muller-Sieburg CE (2006) The hematopoietic stem compartment consists of a limited number of discrete stem cell subsets. Blood 107(6):2311–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gekas C, Graf T (2013) CD41 expression marks myeloid-biased adult hematopoietic stem cells and increases with age. Blood 121(22):4463–4472 [DOI] [PubMed] [Google Scholar]
- 62.Carrelha J, Meng Y, Kettyle LM, Luis TC, Norfo R, Alcolea V, Boukarabila H, Grasso F, Gambardella A, Grover A, Högstrand K, Lord AM, Sanjuan-Pla A, Woll PS, Nerlov C, Jacobsen SEW (2018) Hierarchically related lineage-restricted fates of multipotent haematopoietic stem cells. Nature 554(7690):106–111 [DOI] [PubMed] [Google Scholar]
- 63.Shin JY, Hu W, Naramura M, Park CY (2014) High c-Kit expression identifies hematopoietic stem cells with impaired self-renewal and megakaryocytic bias. J Exp Med 211(2):217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson NK, Kent DG, Buettner F, Shehata M, Macaulay IC, Calero-Nieto FJ, Sánchez Castillo M, Oedekoven CA, Diamanti E, Schulte R, Ponting CP, Voet T, Caldas C, Stingl J, Green AR, Theis FJ, Göttgens B (2015) Combined single-cell functional and gene expression analysis resolves heterogeneity within stem cell populations. Cell Stem Cell 16(6):712–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woolthuis CM, Park CY (2016) Hematopoietic stem/progenitor cell commitment to the megakaryocyte lineage. Blood 127(10):1242–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Assinger A (2014) Platelets and infection – an emerging role of platelets in viral infection. Front Immunol 5:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yeaman MR (2014) Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol 12(6):426–437 [DOI] [PubMed] [Google Scholar]
- 68.Matsuoka Y, Sumide K, Kawamura H, Nakatsuka R, Fujioka T, Sasaki Y, Sonoda Y (2015) Human cord blood-derived primitive CD34-negative hematopoietic stem cells (HSCs) are myeloid-biased long-term repopulating HSCs. Blood Cancer J 5:e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sumide K, Matsuoka Y, Kawamura H, Nakatsuka R, Fujioka T, Asano H, Takihara Y, Sonoda Y (2018) A revised road map for the commitment of human cord blood CD34-negative hematopoietic stem cells. Nat Commun 9(1):2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borot F, Lin CS, Snoeck HW, Mukherjee S, Wang TC (2017) Bone marrow myeloid cells regulate myeloid-biased hematopoietic stem cells via a histamine-dependent feedback loop. Cell Stem Cell 21(6):747–760.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen X, Deng H, Churchill MJ, Luchsinger LL, Du X, Chu TH, Friedman RA, Middelhoff M, Ding H, Tailor YH, Wang ALE, Liu H, Niu Z, Wang H, Jiang Z, Renders S, Ho SH, Shah SV, Tishchenko P, Chang W, Swayne TC, Munteanu L, Califano A, Takahashi R, Nagar KK, Renz BW, Worthley DL, Westphalen CB, Hayakawa Y, Asfaha S, Borot F, Lin CS, Snoeck HW, Mukherjee S, Wang TC (2017) Bone marrow myeloid cells regulate m yeloid- biased hematopoietic stem cells via a histamine-dependent feedback loop. Cell Stem Cell 21(6):747–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pinho S, Marchand T, Yang E, Wei Q, Nerlov C, Frenette PS (2018) Lineage-biased hematopoietic stem cells are regulated by distinct niches. Dev Cell 44(5):634–641.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boiko JR, Borghesi L (2012) Hematopoiesis sculpted by pathogens: toll-like receptors and inflammatory mediators directly activate stem cells. Cytokine 57(1):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burberry A, Zeng MY, Ding L, Wicks I, Inohara N, Morrison SJ, Núñez G (2014) Infection mobilizes hematopoietic stem cells through cooperative NOD-like receptor and toll-like receptor signaling. Cell Host Microbe 15(6):779–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Glatman Zaretsky A, Engiles JB, Hunter CA (2014) Infection-induced changes in hematopoiesis. J Immunol 192(1):27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.King KY, Goodell MA (2011) Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol 11(10):685–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.MacNamara KC, Jones M, Martin O, Winslow GM (2011) Transient activation of hematopoietic stem and progenitor cells by IFNγ during acute bacterial infection. PLoS One 6(12):e28669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manz MG, Boettcher S (2014) Emergency granulopoiesis. Nat Rev Immunol 14(5):302–314 [DOI] [PubMed] [Google Scholar]
- 79.Megías J, Yáñez A, Moriano S, O’Connor JE, Gozalbo D, Gil ML (2012) Direct Toll-like receptor-mediated stimulation of hematopoietic stem and progenitor cells occurs in vivo and promotes differentiation toward macrophages. Stem Cells 30(7):1486–1495 [DOI] [PubMed] [Google Scholar]
- 80.Schuettpelz LG, Link DC (2013) Regulation of hematopoietic stem cell activity by inflammation. Front Immunol 4:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yáñez A, Goodridge HS, Gozalbo D, Gil ML (2013) TLRs control hematopoiesis during infection. Eur J Immunol 43(10):2526–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boettcher S, Manz MG (2017) Regulation of inflammation- and infection-driven hematopoiesis. Trends Immunol 38(5):345–357 [DOI] [PubMed] [Google Scholar]
- 83.Welner RS, Kincade PW (2014) 9–1-1: HSCs respond to emergency calls. Cell Stem Cell 14(4):415–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Esplin BL, Shimazu T, Welner RS, Garrett KP, Nie L, Zhang Q, Humphrey MB, Yang Q, Borghesi LA, Kincade PW (2011) Chronic exposure to a TLR ligand injures hematopoietic stem cells. J Immunol 186(9):5367–5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hirche C, Frenz T, Haas SF, Döring M, Borst K, Tegtmeyer PK, Brizic I, Jordan S, Keyser K, Chhatbar C, Pronk E, Lin S, Messerle M, Jonjic S, Falk CS, Trumpp A, Essers MAG, Kalinke U (2017) Systemic virus infections differentially modulate cell cycle state and functionality of long-term hematopoietic stem cells in vivo. Cell Rep 19(11):2345–2356 [DOI] [PubMed] [Google Scholar]
- 86.Kobayashi H, Kobayashi CI, Nakamura-Ishizu A, Karigane D, Haeno H, Yamamoto KN, Sato T, Ohteki T, Hayakawa Y, Barber GN, Kurokawa M, Suda T, Takubo K (2015) Bacterial c-di-GMP affects hematopoietic stem/progenitors and their niches through STING. Cell Rep 11(1):71–84 [DOI] [PubMed] [Google Scholar]
- 87.Takizawa H, Fritsch K, Kovtonyuk LV, Saito Y, Yakkala C, Jacobs K, Ahuja AK, Lopes M, Hausmann A, Hardt WD, Gomariz Á, Nombela-Arrieta C, Manz MG (2017) Pathogen- induced TLR4-TRIF innate immune signaling in hematopoietic stem cells promotes proliferation but reduces competitive fitness. Cell Stem Cell 21(2):225–240.e5 [DOI] [PubMed] [Google Scholar]
- 88.Matatall KA, Shen CC, Challen GA, King KY (2014) Type II interferon promotes differentiation of myeloid-biased hematopoietic stem cells. Stem Cells 32(11):3023–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haas S, Hansson J, Klimmeck D, Loeffler D, Velten L, Uckelmann H, Wurzer S, Prendergast ÁM, Schnell A, Hexel K, Santarella-Mellwig R, Blaszkiewicz S, Kuck A, Geiger H, Milsom MD, Steinmetz LM, Schroeder T, Trumpp A, Krijgsveld J, Essers MA (2015) Inflammation- induced emergency megakaryopoiesis driven by hematopoietic stem cell-like megakaryocyte progenitors. Cell Stem Cell 17(4):422–434 [DOI] [PubMed] [Google Scholar]
- 90.Bowman RL, Busque L, Levine RL (2018) Clonal hematopoiesis and evolution to hematopoietic malignancies. Cell Stem Cell 22(2):157–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cho RH, Sieburg HB, Muller-Sieburg CE (2008) A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood 111:5553–5561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gazit R, Weissman IL, Rossi DJ (2008) Hematopoietic stem cells and the aging hematopoietic system. Semin Hematol 45:218–224 [DOI] [PubMed] [Google Scholar]
- 93.Pang WW, Schrier SL, Weissman IL (2017) Age-associated changes in human hematopoietic stem cells. Semin Hematol 54(1):39–42 [DOI] [PubMed] [Google Scholar]
- 94.Shlush LI (2018) Age-related clonal hematopoiesis. Blood 131(5):496–504 [DOI] [PubMed] [Google Scholar]
- 95.Dykstra B, Olthof S, Schreuder J, Ritsema M, de Haan G (2011) Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J Exp Med 208(13):2691–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL (1996) The aging of hematopoietic stem cells. Nat Med 2:1011–1016 [DOI] [PubMed] [Google Scholar]
- 97.Sudo K, Ema H, Morita Y, Nakauchi H (2000) Age-associated characteristics of murine hematopoietic stem cells. J Exp Med 192:1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grover A, Sanjuan-Pla A, Thongjuea S, Carrelha J, Giustacchini A, Gambardella A, Macaulay I, Mancini E, Luis TC, Mead A, Jacobsen SE, Nerlov C (2016) Single-cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nat Commun 7:11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Linton PJ, Dorshkind K (2004) Age-related changes in lymphocyte development and function. Nat Immunol 5:133–139 [DOI] [PubMed] [Google Scholar]
- 100.Wang JW, Geiger H, Rudolph KL (2011) Immunoaging induced by hematopoietic stem cell aging. Curr Opin Immunol 23:532–536 [DOI] [PubMed] [Google Scholar]
- 101.Leins H, Mulaw M, Eiwen K, Sakk V, Liang Y, Denkinger M, Geiger H, Schirmbeck R (2018) Aged murine hematopoietic stem cells drive aging-associated immune remodeling. Blood 132(6):565–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kong Y, Pioli PD, Montecino-Rodriguez E, Casero D, Dorshkind K (2018) Lymphoid biased hematopoietic stem cells acquire a myeloid pattern of gene expression with age. J Immunol 200(1 Supplement):103.6 [Google Scholar]
- 103.Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL (2011) Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A 108(50):20012–20017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beerman I (2017) Accumulation of DNA damage in the aged hematopoietic stem cell compartment. Semin Hematol 54(1):12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Busque L, Buscarlet M, Mollica L, Levine RL (2018) Concise review: age-related clonal hematopoiesis: stem cells tempting the devil. Stem Cells 36(9):1287–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elias HK, Bryder D, Park CY (2017) Molecular mechanisms underlying lineage bias in aging hematopoiesis. Semin Hematol 54(1):4–11 [DOI] [PubMed] [Google Scholar]
- 107.Kramer A, Challen GA (2017) The epigenetic basis of hematopoietic stem cell aging. Semin Hematol 54(1):19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Latchney SE, Calvi LM (2017) The aging hematopoietic stem cell niche: phenotypic and functional changes and mechanisms that contribute to hematopoietic aging. Semin Hematol 54(1):25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]