Abstract
Background
During the COVID-19 pandemic, surgical delays have been common for patients with ductal carcinoma in situ (DCIS) and early-stage estrogen receptor-positive (ER+) breast cancer, often in favor of neoadjuvant endocrine therapy (NET). To understand possible ramifications of these delays, we examined the association between time to operation and pathologic staging and overall survival (OS).
Study Design
Patients with DCIS or ER+ cT1-2N0 breast cancer treated from 2010 through 2016 were identified in the National Cancer Database. Time to operation was recorded. Factors associated with pathologic upstaging were examined using logistic regression analyses. Cox proportional hazard models were used to analyze OS. Analyses were stratified by disease stage and initial treatment strategy.
Results
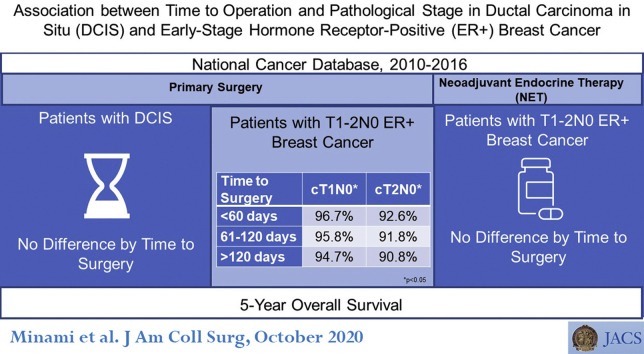
There were 378,839 patients identified. Among those undergoing primary surgical procedure, time to operation was within 120 days in > 98% in all groups. Among cT1-2N0 patients selected for NET, operations were performed within 120 days in 59.6% of cT1N0 and 30.9% of cT2N0 patients. Increased time to operation was associated with increased odds of pathologic upstaging in DCIS patients (ER+: 60 to 120 days: odds ratio 1.15; 95% CI, 1.08 to 1.22; more than 120 days: odds ratio 1.44; 95% CI, 1.24 to 1.68; ER–: 60 to 120 days: NS; more than 120 days: odds ratio 1.36; 95% CI, 1.01 to 1.82; 60 days or less: reference), but not in patients with invasive cancer, irrespective of initial treatment strategy. No difference in OS was seen by time to operation in DCIS or NET patients.
Conclusions
Increased time to operation was associated with a small increase in pathologic upstaging in DCIS patients, but did not impact OS. In patients with cT1-2N0 disease, NET use did not impact stage or OS, supporting the safety of delay strategies in ER+ breast cancer patients during the pandemic.
Abbreviations and Acronyms: BCS, breast-conserving surgery; DCIS, ductal carcinoma in situ; ER, estrogen receptor; NCDB, National Cancer Database; NET, neoadjuvant endocrine therapy; OR, odds ratio; OS, overall survival
Visual Abstract
Continuing Medical Education Credit Information.
Accreditation: The American College of Surgeons is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
AMA PRA Category 1 CreditsTM: The American College of Surgeons designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Of the AMA PRA Category 1 Credits™ listed above, a maximum of 1 credits meet the requirement for Self-Assessment.
To minimize patient exposure and preserve hospital resources during the COVID-19 pandemic, hospitals across the US were asked to sharply decrease the volume of nonurgent surgical cases, including oncologic procedures. The COVID-19 Pandemic Breast Cancer Consortium released recommendations about prioritization of breast cancer operations to aid decision-making, with the recognition that few prospective data existed on the repercussions of the suggested strategies.1 It was recommended that operations for ductal carcinoma in situ (DCIS) be deferred until after resolution of the pandemic, with suggested initiation of neoadjuvant endocrine therapy (NET) in patients with estrogen receptor-positive (ER+) DCIS. NET was similarly recommended as the preferred strategy for patients with early-stage ER+ invasive breast cancer.
The impact of time to operation on breast cancer outcomes in these very-early-stage patients remains unclear. Time to operation has been shown to be associated with increase in upstaging from DCIS to invasive disease on the order of 1% per month, but with excellent survival rates even in upstaged patients.2 In invasive cancer patients, studies using nodal positivity as a surrogate outcomes measure have demonstrated mixed results.3 , 4 In addition, survival analyses have similarly reported varied findings, leaving surgeons without a clear sense of the repercussions that can result from treatment delays.
NET use has primarily been used in patients with stage II and III ER+ disease, demonstrating tumor responses comparable with those seen with neoadjuvant chemotherapy.5 , 6 Survival data in NET patients are more difficult to interpret, not only because of the long disease-free intervals associated with ER+ disease, but also because adjuvant treatment pathways are not standardized in NET patients.5 , 7 There are definitive data in older adults with ER+ disease that demonstrate no overall survival (OS) difference between primary endocrine therapy alone vs operation plus endocrine therapy.8, 9, 10 Use of NET as a delay strategy during the pandemic in patients with early-stage ER+ disease has a basis in high-quality evidence, but given the historical selection bias for NET in postmenopausal patients, it does to a certain extent represent an extrapolation of existing data to a broader population.
The true oncologic ramifications of these delay strategies will be observed over time. Understanding the possible outcomes earlier, however, could be useful in effectively counseling and reassuring patients about surgical delays and the broadened use of NET. Our primary objective was to understand the possible effects of COVID-related surgical delays on breast oncology outcomes by examining the association between time to operation and pathologic staging, with secondary analyses evaluating OS and extent of breast operation.
Methods
Data from 2010 through 2016 were abstracted from the participant user file of the National Cancer Database (NCDB). The NCDB is an oncology dataset that captures approximately 70% of newly diagnosed cancer in the US.1 It is a joint project of the American Cancer Society and the Commission on Cancer of the American College of Surgeons, receiving data from approximately 1,500 Commission on Cancer-accredited cancer programs. This study was deemed exempt from review by the Brigham and Women’s Hospital IRB.
Patients
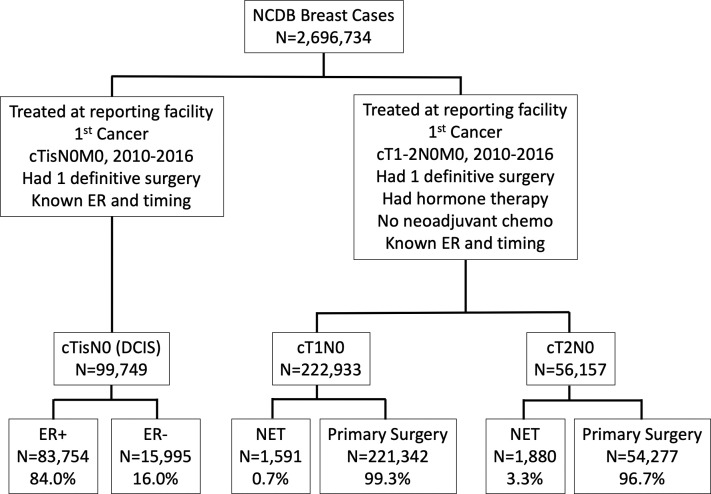
All patients diagnosed January 1, 2010 through December 31, 2016 who underwent breast cancer operations were identified. Given recommendations of the COVID-19 Pandemic Breast Cancer Consortium, the population of interest was patients with DCIS or early-stage (cT1-2N0) ER+ disease.1 Patients who were treated outside the reporting facility, had unknown ER status, unknown surgical timing, unknown surgical pathology data, or who underwent more than 1 operation were excluded. In addition, patients who did not undergo operation within 1 year of diagnosis, who received neoadjuvant chemotherapy, or, in the case of patients with invasive disease, those who did not receive endocrine therapy at all were excluded. An exceedingly small number of patients with DCIS received NET, precluding reliable analysis of this subpopulation, and were excluded. The remaining 99,749 DCIS patients, 222,933 cT1N0, and 56,157 cT2N0 patients were assessed (Fig. 1 ).
Figure 1.
Consolidated Standards of Reporting Trials diagram. ER, estrogen receptor; NCDB, National Cancer Database; NET, neoadjuvant endocrine therapy.
Variables
The exposure of interest was time to operation from time of diagnosis, originally defined as 30 or fewer days, 31 to 60 days, 61 to 90 days, 91 to 120 days, and more than 120 days. As outcomes of interest did not significantly vary from 0 to 30 vs 30 to 60 days or from 60 to 90 vs 90 to 120 days, these categories were collapsed into 60 or fewer days, 61 to 120 days, and more than 120 days. Patient-level variables included age (18 to 39 years, 40 to 49 years, 50 to 59 years, 60 to 69 years, 70 to 79 years, and older than 80 years), race/ethnicity (white, black, Hispanic, Asian, and other/unknown), insurance status (private, Medicare, Medicaid, uninsured, and other/unknown), regional location of the patient’s home ZIP code (metropolitan, urban, or rural). Disease characteristics included tumor grade (classified as 1, 2, or 3) and tumor histology (ductal, lobular, or mixed). Both clinical and pathologic tumor category (Tis, T1, T2, T3, or T4) and nodal category (Nx, N0, N1, N2, or N3) were included. Treatment characteristics included operation type (breast-conserving surgery [BCS] vs mastectomy), radiation therapy (yes/no), and adjuvant chemotherapy (yes/no). Facility type was also incorporated, defined by Commission on Cancer accreditation status as a community cancer program, comprehensive community cancer program, academic/research program, or integrated network cancer program.
Outcomes measures
The main measure of interest was the proportion of DCIS, cT1N0, and cT2N0 patients who were upstaged on final surgical pathology. Inclusion criteria were based on T and N categories, and the outcomes measure was based on the 8th edition of the American Joint Committee on Cancer’s AJCC Cancer Staging Manual.11 Patients included in the cT1N0 and cT2N0 groups were recoded by the 8th edition clinical prognostic staging criteria and were deemed “upstaged” if their pathologic prognostic stage was higher than their clinical prognostic stage. Secondary outcomes measures were 5-year OS and type of breast procedure (BCS vs mastectomy).
Statistical analysis
Time to operation was treated as a categorical variable, defined by the date of diagnosis to the date of operation. All tests were 2-sided with a p value < 0.05 considered statistically significant. Analyses were stratified by clinical staging on presentation (DCIS, cT1N0, cT2N0); all DCIS patients (ER+ and ER–) underwent upfront operation, and the cT1N0 and cT2N0 groups were also stratified by initial treatment strategy (NET vs primary operation). Chi-square tests of proportion were performed to test the significance of baseline differences in the study population. Univariable logistic regression models were used to examine factors associated with pathologic upstaging (eTable 1). To test the significance of time to operation controlled for all other patient and hospital covariates, a multivariable analysis was performed using a random intercept, fixed slope, logistic regression model with the hospital as a random effect. Variables for this model were chosen a priori, and included age, race, Charlson Comorbidity Index score, insurance status, facility type, tumor histology, operation type, and time to operation. Tumor grade was not included in this model, given that it is a significant part of the 8th edition prognostic pathologic staging system (ie the outcomes variable). A multivariable logistic regression model was also performed to determine significant factors associated with receipt of NET. Cox proportional hazards models—adjusted for age, race, comorbidity index, tumor grade, histology, operation type, pathologic tumor and nodal category, and adjuvant therapy (chemotherapy and radiation therapy)—were used to compare OS by time from diagnosis to operation by clinical disease stage (DCIS, cT1N0, cT2N0), and initial treatment strategy (NET vs primary operation). All analyses were performed using SPSS, version 19.0 (IBM Corp).
Results
Of the 99,749 DCIS patients, 83,754 (84%) had ER+ disease and 15,995 (16%) had ER– disease. Of the patients with cT1N0 disease, 1,591 (0.7%) underwent NET and the remaining underwent primary operation. A greater percentage of patients (3.3%) with cT2N0 disease underwent NET (n = 1,880). For patients with cT1-2N0 disease, multivariable analysis showed that older age, higher comorbidity index (Charlson Comorbidity Index score ≥ 3: odds ratio [OR] 1.83; 95% CI, 1.41 to 2.39 [reference: Charlson Comorbidity Index of 0]), lobular disease (OR 1.17; 95% CI, 1.06 to 1.29), and cT2 vs cT1 tumor (OR 5.40; 95% CI, 5.01 to 5.81) were among the factors significantly associated with NET receipt (eTable 2).
Clinicopathologic characteristics of the patients in each group are shown in Table 1 . In the DCIS group, 98.2% of patients underwent operations in the first 120 days. Similarly, 99.4% of cT1N0 patients and 99.1% of cT2N0 patients in the primary operation group underwent operation within 120 days. In contrast, 59.6% of the cT1N0 and 30.9% of the cT2N0 NET patients underwent operations within 120 days.
Table 1.
Patient Characteristics
| Characteristic | DCIS patient |
cT1N0 patient |
cT2N0 patient |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ER+ (n = 83,754) |
ER– (n = 15,995) |
NET (n = 1,591) |
Primary operation (n = 221,342) |
NET (n = 1,880) |
Primary operation (n = 54,277) |
|||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Age∗ | ||||||||||||
| 18–39 y | 2,451 | 2.9 | 507 | 3.2 | 29 | 1.8 | 4,818 | 2.2 | 21 | 1.1 | 2,671 | 4.9 |
| 40–49 y | 17,150 | 20.5 | 2,331 | 14.6 | 170 | 10.7 | 30,432 | 13.7 | 157 | 8.4 | 9,827 | 18.1 |
| 50–59 y | 23,593 | 28.2 | 4,661 | 29.1 | 336 | 21.1 | 55,485 | 25.1 | 362 | 19.3 | 13,327 | 24.6 |
| 60–69 y | 23,972 | 28.6 | 48,890 | 30.6 | 492 | 30.9 | 72,239 | 32.6 | 581 | 30.9 | 14,621 | 26.9 |
| 70–79 y | 13,045 | 15.6 | 2,657 | 16.6 | 357 | 22.4 | 45,174 | 20.4 | 449 | 23.9 | 9,223 | 17.0 |
| 80 y or older | 3,543 | 4.2 | 949 | 5.9 | 207 | 13.0 | 13,194 | 6.0 | 310 | 16.5 | 4,608 | 8.5 |
| Race∗ | ||||||||||||
| White | 63,659 | 76.0 | 12,551 | 78.5 | 1,322 | 83.1 | 186,587 | 84.3 | 1,491 | 79.3 | 43,628 | 80.4 |
| Black | 10,665 | 12.7 | 1,643 | 10.3 | 119 | 7.5 | 16,254 | 7.3 | 171 | 9.1 | 4,829 | 8.9 |
| Hispanic | 5,073 | 6.1 | 947 | 5.9 | 93 | 5.8 | 10,278 | 4.6 | 137 | 7.3 | 3,232 | 6.0 |
| Asian | 3,859 | 4.6 | 767 | 4.8 | 47 | 3.0 | 6,961 | 3.1 | 70 | 3.7 | 2,240 | 4.1 |
| Other | 498 | 0.6 | 87 | 0.5 | 10 | 0.6 | 1,262 | 0.6 | 11 | 0.6 | 348 | 0.6 |
| Comorbidity index | ||||||||||||
| 0 | 70,824 | 84.6 | 13,514 | 84.5 | 1,234 | 77.6 | 184,373 | 83.3 | 1,539 | 81.9 | 44,446 | 81.9 |
| 1 | 10,519 | 12.6 | 1,982 | 12.4 | 239 | 15.0 | 29,636 | 13.4 | 241 | 12.8 | 7,757 | 14.3 |
| 2 | 1,853 | 2.2 | 391 | 2.4 | 78 | 4.9 | 5,549 | 2.5 | 68 | 3.6 | 1,524 | 2.8 |
| ≥ 3 | 558 | 0.7 | 108 | 0.7 | 40 | 2.5 | 1,784 | 0.8 | 32 | 1.7 | 550 | 1.0 |
| Insurance∗ | ||||||||||||
| Private | 50,754 | 60.6 | 9,193 | 57.5 | 663 | 41.7 | 117,865 | 53.3 | 713 | 37.9 | 29,021 | 53.5 |
| Medicare | 25,778 | 30.8 | 5,426 | 33.9 | 756 | 47.5 | 86,475 | 39.1 | 953 | 50.7 | 19,431 | 35.8 |
| Medicaid | 4,228 | 5.0 | 789 | 4.9 | 102 | 6.4 | 10,099 | 4.6 | 120 | 6.4 | 3,679 | 6.8 |
| Other government | 931 | 1.1 | 167 | 1.0 | 12 | 0.8 | 2,233 | 1.0 | 20 | 1.1 | 577 | 1.1 |
| Uninsured | 1,225 | 1.5 | 260 | 1.6 | 27 | 1.7 | 2,603 | 1.2 | 43 | 2.3 | 1,016 | 1.9 |
| Unknown | 838 | 1.0 | 160 | 1.0 | 31 | 1.9 | 2,067 | 0.9 | 31 | 1.6 | 553 | 1.0 |
| Region | ||||||||||||
| Metro | 70,858 | 84.6 | 13,422 | 83.9 | 1,296 | 81.5 | 182,504 | 82.5 | 1,571 | 83.6 | 44,633 | 82.2 |
| Urban | 9,558 | 11.4 | 1,902 | 11.9 | 218 | 13.7 | 29,281 | 13.2 | 226 | 12.0 | 7,372 | 13.6 |
| Rural | 1,214 | 1.4 | 257 | 1.6 | 33 | 2.1 | 3,917 | 1.8 | 29 | 1.5 | 962 | 1.8 |
| Unknown | 2,124 | 2.5 | 414 | 2.6 | 44 | 2.8 | 5,640 | 2.5 | 54 | 2.9 | 1,310 | 2.4 |
| Facility type∗ | ||||||||||||
| Community | 6,465 | 8.0 | 1,183 | 7.6 | 112 | 7.2 | 19,278 | 8.9 | 132 | 7.1 | 4,757 | 9.2 |
| Comprehensive | 36,644 | 45.1 | 7,176 | 46.3 | 623 | 39.9 | 98,290 | 45.4 | 717 | 38.6 | 23,671 | 45.9 |
| Academic | 25,206 | 31.0 | 4,674 | 30.2 | 524 | 33.5 | 66,491 | 30.7 | 704 | 37.9 | 15,662 | 30.3 |
| Integrated network | 12,988 | 16.0 | 2,455 | 15.9 | 303 | 19.4 | 32,465 | 15.0 | 306 | 16.5 | 7,516 | 14.6 |
| Histology∗ | ||||||||||||
| Ductal | NA | NA | NA | NA | 1,219 | 76.6 | 176,972 | 80.0 | 1,284 | 68.3 | 39,905 | 73.5 |
| Lobular | NA | NA | NA | NA | 199 | 12.5 | 22,192 | 10.0 | 349 | 18.6 | 8,322 | 15.3 |
| Mixed | NA | NA | NA | NA | 173 | 10.9 | 22,178 | 10.0 | 247 | 13.1 | 6,050 | 11.1 |
| Grade∗ | ||||||||||||
| 1 | 11,314 | 13.5 | 263 | 1.6 | 591 | 27.1 | 77,878 | 35.2 | 498 | 26.5 | 8,971 | 16.5 |
| 2 | 33,311 | 39.8 | 2,149 | 13.4 | 805 | 50.6 | 109,108 | 49.3 | 1,040 | 55.3 | 28,762 | 53.0 |
| 3 | 26,257 | 31.4 | 10,903 | 68.2 | 124 | 7.8 | 25,837 | 11.7 | 267 | 14.2 | 14,529 | 26.8 |
| Unknown | 12,872 | 15.4 | 2,680 | 16.8 | 71 | 4.5 | 8,519 | 3.8 | 75 | 4.0 | 2,015 | 3.7 |
| Operative time from diagnosis | ||||||||||||
| < 60 d | 69,539 | 83.0 | 13,197 | 82.5 | 352 | 22.1 | 199,778 | 90.3 | 137 | 7.3 | 47,598 | 87.7 |
| 61–120 d | 12,689 | 15.2 | 2,504 | 15.7 | 596 | 37.5 | 20,208 | 9.1 | 444 | 23.6 | 6,187 | 11.4 |
| > 120 d | 1,526 | 1.8 | 294 | 1.8 | 643 | 40.4 | 1,356 | 0.6 | 1,299 | 69.1 | 492 | 0.9 |
| Operation type∗ | ||||||||||||
| BCS | 60,120 | 71.8 | 9,402 | 58.8 | 1,085 | 68.2 | 170,933 | 77.2 | 1,173 | 62.4 | 26,803 | 49.4 |
| Mastectomy | 23,634 | 28.2 | 6,593 | 41.2 | 506 | 31.8 | 50,409 | 22.8 | 707 | 37.6 | 27,474 | 50.6 |
| Axillary operation type∗ | ||||||||||||
| None | 36,691 | 43.8 | 4,704 | 29.4 | 168 | 10.6 | 5,382 | 59.3 | 186 | 9.9 | 1,174 | 2.2 |
| SLNB | 22,360 | 26.7 | 5,766 | 36.0 | 916 | 57.6 | 131,306 | 11.5 | 952 | 50.6 | 25,783 | 47.5 |
| SLNB/ALND | 3,353 | 4.0 | 676 | 4.2 | 184 | 11.6 | 25,431 | 11.5 | 292 | 15.5 | 10,514 | 19.4 |
| ALND | 2,649 | 3.2 | 958 | 6.0 | 103 | 6.5 | 12,486 | 5.6 | 130 | 6.9 | 4,519 | 8.3 |
| Unknown | 18,701 | 22.3 | 3,891 | 24.3 | 220 | 13.8 | 46,737 | 21.1 | 320 | 17.0 | 12,287 | 22.6 |
| pT∗ | ||||||||||||
| pT0 | 75,075 | 89.6 | 13,192 | 82.5 | 37 | 2.3 | 660 | 0.3 | 17 | 0.9 | 55 | 0.1 |
| pT1 | 8,246 | 9.8 | 2,619 | 16.4 | 1,308 | 82.2 | 197,252 | 89.1 | 684 | 36.4 | 10,641 | 19.6 |
| pT2 | 351 | 0.4 | 151 | 0.9 | 224 | 14.1 | 2,216 | 10.0 | 1,064 | 56.6 | 40,939 | 75.4 |
| pT3 | 61 | 0.1 | 26 | 0.2 | 20 | 1.3 | 1,080 | 0.5 | 95 | 5.1 | 2,429 | 4.5 |
| pT4 | 11 | 0.0 | 7 | 0.0 | 2 | 0.1 | 134 | 0.1 | 20 | 1.1 | 213 | 0.4 |
| pN∗ | ||||||||||||
| pN0 | 58,328 | 69.6 | 12,539 | 78.4 | 1,182 | 74.3 | 184,293 | 83.3 | 1,138 | 60.5 | 34,653 | 63.8 |
| pN1 | 840 | 1.0 | 285 | 1.8 | 247 | 15.5 | 30,129 | 13.6 | 501 | 26.6 | 15,301 | 28.2 |
| pN2 | 64 | 0.1 | 39 | 0.2 | 21 | 1.3 | 2,422 | 1.1 | 60 | 3.2 | 2,617 | 4.8 |
| pN3 | 37 | 0.0 | 12 | 0.1 | 9 | 0.6 | 695 | 0.3 | 30 | 1.6 | 886 | 1.6 |
| pNx | 24,485 | 29.2 | 3,120 | 19.5 | 132 | 8.3 | 3,803 | 1.7 | 151 | 8.0 | 820 | 1.5 |
| Adjuvant treatment | ||||||||||||
| Endocrine therapy∗ | 43,873 | 52.4 | 979 | 6.1 | 1,591 | 100 | 221,342 | 100 | 1,880 | 100 | 54,277 | 100 |
| Chemotherapy∗ | 1,767 | 2.1 | 1,177 | 7.4 | 177 | 11.1 | 39,749 | 18.0 | 284 | 15.6 | 23,139 | 42.6 |
| Radiation∗ | 45,565 | 54.4 | 8,157 | 51.0 | 849 | 53.4 | 161,328 | 72.9 | 1,052 | 56.0 | 326,680 | 60.2 |
ALND, axillary lymph node dissection; BCS, breast-conserving surgery; DCIS, ductal carcinoma in situ; ER, estrogen receptor; NA, not applicable; NET, neoadjuvant endocrine therapy; pT, pathologic tumor category; pN, pathologic nodal category.
p < 0.05.
Among DCIS patients, mastectomy was more common in ER– than ER+ disease (41.2% vs 28.2%; p < 0.01). Among cT1N0 patients, mastectomy was more common in the NET group (31.8%) than in the primary operation group (22.8%) (p < 0.01), and in cT2N0 patients, BCS rates were higher in the NET group compared with the primary operation group (62.4% vs 49.4% respectively; p < 0.01).
Association between time to operation and pathologic upstaging
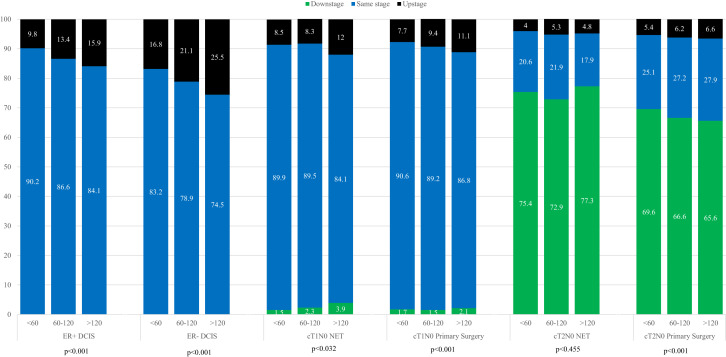
In total, a greater proportion of patients with ER– DCIS (17.6%) were upstaged to invasive disease compared with patients with ER+ DCIS (10.4%) (Table 2 ). The proportion of patients who were upstaged on final pathology increased by time to operation among both the ER+ and ER– DCIS patients (Fig. 2 ; p < 0.001 for both). A small group of patients (< 5%) had invasive disease on final pathology but were missing elements (eg grade or nodal status) needed to accurately stage them per the 8th edition prognostic pathologic staging criteria, and were coded as being upstaged, yet “unknown.” Among the statistically significant factors associated with pathologic upstaging among ER+ DCIS patients on adjusted analysis was time to operation, as patients undergoing operations more than 60 days after diagnosis had an OR of 1.15 (95% CI, 1.08 to 1.22) compared with those who underwent operations within the first 60 days (Table 3 ). Patients with ER– DCIS also had higher odds of being upstaged, but only if they underwent operations more than 120 days after diagnosis (OR 1.36; 95% CI, 1.01 to 1.82). Patients with both ER+ and ER– DCIS undergoing mastectomy were more likely to be upstaged than those undergoing BCS (ER+: OR 2.76; 95% CI, 2.63 to 2.90; ER–: OR 2.14; 95% CI, 1.96 to 2.35).
Table 2.
Patient Population by TNM Stage/Primary Treatment, American Joint Committee on Cancer 8th Edition Clinical Prognostic Staging and 8th Edition Pathologic Prognostic Staging
| Cancer type, AJCC 8th edition clinical prognostic stage, pathologic prognostic stage | n | % | Pathologic upstaging |
|---|---|---|---|
| ER+ DCIS (n = 83,754) | |||
| 0 | |||
| 0 | 75,024 | 89.6 | Same stage |
| IA | 5,561 | 6.6 | Upstage |
| IB | 252 | 0.3 | Upstage |
| IIA-B | 162 | 0.4 | Upstage |
| IIIA-C | 46 | 0.0 | Upstage |
| Unknown | 2,709 | 3.2 | Upstage |
| ER– DCIS (n = 15,995) | |||
| 0 | |||
| 0 | 13,175 | 82.4 | Same stage |
| IA | 922 | 5.8 | Upstage |
| IB | 590 | 3.6 | Upstage |
| IIA-B | 298 | 1.9 | Upstage |
| IIIA-C | 81 | 0.5 | Upstage |
| Unknown | 1,926 | 5.8 | Upstage |
| cT1N0 NET (n = 1,395) | |||
| IA | 1,375 | 98.6 | — |
| 0 | 30 | 2.2 | Downstage |
| IA | 1,222 | 88.9 | Same stage |
| IB | 79 | 5.7 | Upstage |
| IIA-B | 33 | 2.4 | Upstage |
| IIIA-C | 11 | 0.8 | Upstage |
| IB | 20 | 1.4 | — |
| 0 | 0 | 0 | Downstage |
| IA | 8 | 40.0 | Downstage |
| IB | 0 | 0 | Same stage |
| IIA-B | 11 | 55.0 | Upstage |
| IIIA-C | 1 | 5.0 | Upstage |
| cT1N0 upfront operation (n = 209,102) | |||
| IA | 204,944 | 98.0 | — |
| 0 | 587 | 0.3 | Downstage |
| IA | 189,159 | 92.3 | Same stage |
| IB | 10,740 | 5.2 | Upstage |
| IIA-B | 2,776 | 1.7 | Upstage |
| IIIA-C | 888 | 0.4 | Upstage |
| IB | 4,158 | 2.0 | — |
| 0 | 11 | 0.3 | Downstage |
| IA | 2,917 | 70.2 | Downstage |
| IB | 0 | 0 | Same stage |
| IIA-B | 1,125 | 27.1 | Upstage |
| IIIA-C | 106 | 2.5 | Upstage |
| cT2N0 NET (n = 1,649) | |||
| IB | 1,288 | 78.1 | — |
| 0 | 11 | 0.9 | Downstage |
| IA | 1,019 | 79.1 | Downstage |
| IB | 229 | 17.8 | Same stage |
| IIA-B | 0 | 0 | Upstage |
| IIIA-C | 29 | 2.3 | Upstage |
| IIA | 332 | 20.1 | — |
| 0 | 2 | 0.6 | Downstage |
| IA | 88 | 26.5 | Downstage |
| IB | 116 | 34.9 | Downstage |
| IIA | 79 | 23.8 | Same stage |
| IIB | 30 | 9.0 | Upstage |
| IIIA-C | 17 | 5.1 | Upstage |
| IIB | 29 | 1.8 | — |
| 0 | 0 | 0 | Downstage |
| IA | 8 | 27.6 | Downstage |
| IB | 0 | 0 | Downstage |
| IIA | 11 | 37.9 | Downstage |
| IIB | 7 | 24.1 | Same stage |
| IIIA-C | 3 | 10.3 | Upstage |
| cT2N0 upfront operation (n = 51,208) | |||
| IB | 33,648 | 65.7 | — |
| 0 | 39 | 0.1 | Downstage |
| IA | 24,929 | 74.1 | Downstage |
| IB | 8,061 | 24.0 | Same stage |
| IIA-B | 0 | 0 | Upstage |
| IIIA-C | 619 | 1.8 | Upstage |
| IIA | 14,831 | 29.0 | — |
| 0 | 6 | 0.0 | Downstage |
| IA | 1,997 | 13.5 | Downstage |
| IB | 6,454 | 43.5 | Downstage |
| IIA | 4,396 | 29.6 | Same stage |
| IIB | 1,412 | 9.5 | Upstage |
| IIIA-C | 566 | 3.9 | Upstage |
| IIB | 2,729 | 5.3 | — |
| 0 | 4 | 0.1 | Downstage |
| IA | 285 | 1.0 | Downstage |
| IB | 0 | 0 | Downstage |
| IIA | 1,746 | 64.0 | Downstage |
| IIB | 520 | 19.1 | Same stage |
| IIIA-C | 174 | 6.4 | Upstage |
AJCC, American Joint Committee on Cancer; DCIS, ductal carcinoma in situ; ER, estrogen receptor; NET, neoadjuvant endocrine therapy.
Figure 2.
Change in American Joint Committee on Cancer 8th edition pathologic prognostic stage by time to operation, stratified by disease subtype and initial treatment strategy; time to operation in days. DCIS, ductal carcinoma in situ; ER, estrogen receptor; NET, neoadjuvant endocrine therapy.
Table 3.
Multivariable Analysis of Factors Associated with Upgrade by the American Joint Committee on Cancer 8th Edition Prognostic Staging
| Patient characteristic | ER+ DCIS |
ER– DCIS |
NET |
Primary operation |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | ||||||||
| 18–39 y | Too few events | — | Too few events | — | Too few events | — | Too few events | — |
| 40–49 y | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| 50–59 y | 1.00 (0.94–1.07) | 0.99 | (0.77 0.68–0.87) | < 0.01 | (0.84 0.50–01.40) | 0.50 | (1.00 0.96–1.05) | 0.90 |
| 60–69 y | 1.05 (0.98–1.13) | 0.19 | (0.67 0.59–0.78) | < 0.01 | (0.61 0.35–1.06) | 0.08 | (0.87 0.83–0.92) | < 0.01 |
| 70–79 y | 1.14 (1.03–1.25) | 0.01 | (0.64 0.53–0.78) | < 0.01 | (0.81 0.44–1.51) | 0.51 | (0.84 0.79–0.89) | < 0.01 |
| 80 y or older | 1.32 (1.15–1.51) | < 0.01 | (0.61 0.47–0.78) | < 0.01 | (1.56 0.82–2.98) | 0.17 | (1.22 1.13–1.32) | < 0.01 |
| Race | ||||||||
| White | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| Black | 0.98 (0.91–1.05) | 0.49 | (1.26 1.10–1.45) | < 0.01 | (1.27 0.78–2.10) | 0.34 | (1.46 1.38–1.53) | < 0.01 |
| Hispanic | 1.12 (1.01–1.24) | 0.03 | (1.08 0.89–1.30) | 0.45 | (1.19 0.65–2.18) | 0.58 | (1.19 1.11–1.27) | < 0.01 |
| Asian | 1.22 (1.09–1.36) | < 0.01 | (1.41 1.15–1.71) | < 0.01 | (0.92 0.39–2.18) | 0.85 | (0.93 0.85–1.01) | 0.10 |
| Comorbidity index | ||||||||
| 0 | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| 1 | 1.13 (1.05–1.21) | < 0.01 | (1.13 0.99–1.29) | 0.06 | (0.94 0.60–1.45) | 0.76 | (1.05 1.01–1.10) | 00.02 |
| 2 | 1.33 (1.14–1.54) | < 0.01 | (1.27 0.97–1.67) | 0.08 | (1.21 0.62–2.38) | 0.58 | (1.13 1.03–1.24) | 0.01 |
| ≥ 3 | 1.36 (1.05–1.76) | 0.02 | (1.26 0.73–2.16) | 0.40 | (1.92 0.85–4.31) | 0.11 | (1.12 0.95–1.31) | 0.18 |
| Insurance | ||||||||
| Private | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| Medicare | 0.93 (0.87–0.99) | 0.04 | 0.93 0.81–1.06) | 0.27 | (1.54 0.99–2.37) | 0.06 | (0.98 0.93–1.02) | 0.31 |
| Medicaid | 1.06 (0.95–1.18) | 0.28 | 1.30 1.08–1.57) | 0.01 | (1.69 0.97–2.95) | 0.06 | (1.27 1.19–1.36) | < 0.01 |
| Uninsured | 0.90 (0.74–1.09) | 0.28 | 0.84 0.60–1.18) | 0.31 | (0.55 0.12–2.44) | 0.43 | (1.28 1.13–1.44) | < 0.01 |
| Facility type | ||||||||
| Community | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| Comprehensive | 1.26 (1.14–1.38) | < 0.01 | (1.26 1.05–1.52) | < 0.01 | (1.60 0.80–3.20) | 0.18 | (0.96 0.91–1.02) | 0.17 |
| Academic | 1.53 (1.38–1.69) | < 0.01 | (1.81 1.49–2.19) | < 0.01 | (1.81 0.91–3.63) | 0.09 | (0.96 0.90–1.02) | 0.15 |
| Integrated network | 1.29 (1.15–1.44) | < 0.01 | (1.54 1.25–1.89) | < 0.01 | (1.64 0.79–3.44) | 0.19 | (0.91 0.86–0.97) | 0.01 |
| Histology | ||||||||
| Ductal | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| Lobular | NA | — | NA | — | (1.75 1.21–2.53) | < 0.01 | (1.50 1.44–1.57) | < 0.01 |
| Mixed | 0.13 (0.12–0.14) | < 0.01 | (0.08 0.06–0.10) | < 0.01 | (1.25 0.80–1.96) | 0.32 | (1.13 1.08–1.19) | < 0.01 |
| cT | ||||||||
| cT1 | NA all cTis | — | — | — | 1 | Ref | 1 | Ref |
| cT2 | — | — | — | — | (0.36 0.26–0.50) | < 0.01 | (0.47 0.45–0.49) | < 0.01 |
| Operation type | ||||||||
| BCS | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| Mastectomy | 2.76 (2.63–2.90) | < 0.01 | (2.14 1.96–2.35) | < 0.01 | (3.07 2.26–4.17) | < 0.01 | (2.82 2.73–2.91) | < 0.01 |
| Operation timing | ||||||||
| <60 d | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| 60–120 d | 1.15 (1.08–1.22) | < 0.01 | (1.09 0.97–1.23) | 0.14 | (1.01 0.64–1.61) | 0.96 | (0.92 0.88–0.97) | < 0.01 |
| > 120 d | 1.44 (1.24–1.68) | < 0.01 | (1.36 1.01–1.82) | 0.04 | (1.46 0.94–2.29) | 0.09 | (1.06 0.90–1.25) | 0.48 |
BCS, breast-conserving surgery; cT, clinical tumor category, DCIS, ductal carcinoma in situ; ER, estrogen receptor; NA, not applicable; NET, neoadjuvant endocrine therapy; OR, odds ratio; Ref, reference.
In total, a larger proportion of T1N0 NET patients (9.7%) were upstaged compared with the T2N0 NET patients (4.8%) (Table 2). The proportion of patients who were upstaged on final pathology increased by time to operation among the T1N0 NET patients (p < 0.03), but not the T2N0 NET patients (p = 0.455) (Fig. 2). Of the cT2N0 NET patients, 80% of those with clinical stage IB disease, 62% of those with stage IIA disease, and 65.5% of those with stage IIB disease were downstaged on final pathology. On adjusted analysis, NET patients undergoing operations more than 60 days after diagnosis were not more likely to be upstaged on final pathology compared with those undergoing operations in 60 or fewer days (Table 3). Patients with lobular disease were more likely to be upstaged (OR 1.75; 95% CI, 1.21 to 2.53) compared with those with ductal or mixed histologies. NET patients undergoing mastectomy also had higher odds of upstaging on final pathology (OR 3.07; 95% CI, 2.26 to 4.17), although those who were initially staged as having cT2 disease compared with those with cT1 disease were less likely to be upstaged (OR 0.36; 95% CI, 0.26 to 1.47).
Among primary operation patients, 7.5% of cT1N0 patients and 5.4% of cT2N0 patients were upstaged on final pathology. The proportion of patients who were upstaged increased by time to operation (cT1N0: 7.7% in the fewer than 60 days group compared with 11.1% in the more than 120 days group; cT2N0: 5.4% in the fewer than 60 days group compared with 6.6% in the more than 120 days group) (p < 0.001 for both). However, on adjusted analysis, time to operation was not significantly associated with pathologic upstaging. Patients with lobular disease and mixed histologies were more likely to be upstaged (lobular: OR 1.50; 95% CI, 1.21 to 2.53; mixed: 1.13, 95% CI, 1.08 to 1.19) compared with those with a ductal histology. Mastectomy patients in this group, as in the DCIS and NET groups, had higher odds of upstaging on final pathology (OR 2.82; 95% CI, 2.73 to 2.91). As in the NET group, those who were initially staged as having cT2 disease compared with those with cT1 disease were less likely to be upstaged (OR 0.47; 95% CI, 0.45 to 0.49).
Association between time to operation and overall survival
Median follow-up time ranged from 33.7 months to 42.4 months by subgroup (Table 4 ). No significant difference in OS was seen by time to operation among the ER+ (p = 0.085) and ER– DCIS patients (p = 0.669), cT1N0 NET patients (p = 0.129), or cT2N0 NET patients (p = 0.538). In the primary operation group, a slight decrease in OS was seen in both the cT1N0 group (OS 96.7% in the fewer than 60 days to operation group vs 94.7% in the more than 120 days to operation group; p < 0.001) and the cT2N0 group (OS was 92.6% in the fewer than 60 days to operation group vs 90.8% in the more than 120 days to operation group; p = 0.046).
Table 4.
Cox Proportional Hazards Model Comparing 5-year Overall Survival by Disease Subtype, 2010 to 2015, According to Clinical Disease Stage and Initial Treatment Strategy
| Variable | DCIS |
NET |
Primary operation |
|||
|---|---|---|---|---|---|---|
| ER+ | ER– | cT1N0 | cT2N0 | cT1N0 | cT2N0 | |
| Median follow-up time, mo | 41.6 | 42.4 | 33.7 | 36.3 | 40.1 | 40.9 |
| Time to operation | ||||||
| < 60 d | 98.1 | 97.0 | 94.6 | 87.8 | 96.7 | 92.6 |
| 61–120 d | 97.9 | 96.8 | 97.6 | 91.8 | 95.8 | 91.8 |
| > 120 d | 97.4 | 96.6 | 96.9 | 91.5 | 94.7 | 90.8 |
| p Value timing | 0.085 | 0.669 | 0.129 | 0.538 | < 0.001 | 0.046 |
Adjusted for age, race, Charlson Comorbidity Index score, insurance, region, grade, histology, operation type, path T/N, adjuvant therapy.
DCIS, ductal carcinoma in situ; ER, estrogen receptor; NET, neoadjuvant endocrine therapy.
Association between time to operations and breast operation
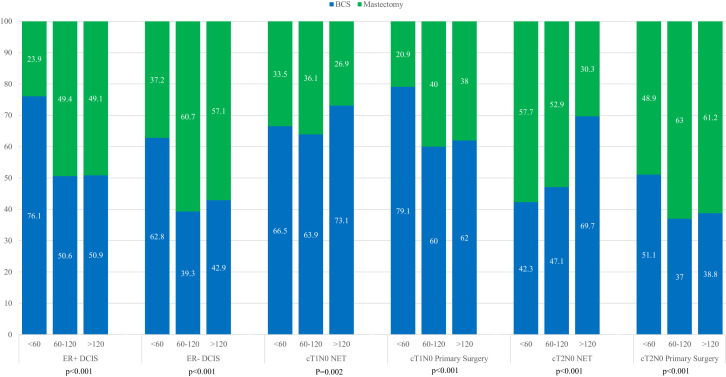
Among patients with DCIS and among those with cT1-2N0 disease undergoing primary operation, mastectomy rates increased with longer time to operation (eg ER+ DCIS: 23.9% in the fewer than 60 days group vs 49.1% in the more than 120 days group; ER– DCIS: 37.2% in the 60 or fewer days group vs 57.1% in the more than 120 days group; p < 0.001 for both). In the NET groups, however, mastectomy rates overall decreased by time, with the clearest trend in the cT2N0 NET group (57.7% in the 60 or fewer days group vs 30.3% in the more than 120 days group; p < 0.001) (Fig. 3 ).
Figure 3.
Breast operation type by time to operation, stratified by disease subtype and initial treatment strategy; time to operation in days. BCS, breast-conserving surgery; DCIS, ductal carcinoma in situ; ER, estrogen receptor; NET, neoadjuvant endocrine therapy.
Discussion
Oncologic surgery triage recommendations during the COVID-19 pandemic were based on best available evidence, but admittedly, as unprecedented circumstances forced unconventional practices, some extrapolations from existing data were necessary. Questions remain about the possible effect of the proposed delay strategies on oncologic outcomes. Our study found that on adjusted analyses, surgical delays of more than 120 days were associated with pathologic upstaging in patients with DCIS but not in those with invasive disease. In DCIS patients and cT1-2N0 patients treated with NET, no survival differences were noted by time to operation.
The anxiety of nonoperative management of DCIS stems from known sampling error on core biopsy with pathologic upstaging rates ranging from 10% to 30% at the time of operation.2 , 12 Although nonoperative treatment of non-high-grade DCIS is being explored in randomized clinical trials in Europe and the US (eg LORD [Low Risk DCIS],13 LORIS [Low Risk DCIS],14 and COMET [Comparison of Operative to Monitoring and Endocrine Therapy for Low-Risk DCIS] trials),15 these trials are enrolling highly selected patients and have yet to report their results. In addition, retrospective data have shown that in patients meeting LORIS criteria, upstage rates at the time of operation might be 7% to 20%.16 , 17 The prospect of leaving patients with undiagnosed and untreated invasive disease can engender discomfort in surgeons and patients alike.
The association between pathologic upstaging and time to operation in DCIS patients has previously been studied in the NCDB, with results consistent with ours, although patients were not stratified by ER status.2 The possible mitigating effect of NET on disease progression in ER+ DCIS has been demonstrated in a small series,18 and was part of the recommended delay strategies during the pandemic. Although there were too few DCIS patients on NET to render an adequate analysis in the current study, taken together, these data suggest that initiation of NET in patients with ER+ DCIS is a reasonable delay strategy. For patients with ER– DCIS in whom NET is not a viable option, the consequences of leaving disease in place for longer periods of time remain unclear. Our findings are similar to a previous single-institution study that demonstrated a significantly higher upgrade rate among patients with ER– DCIS,17 although it is unclear to what extent this phenomenon is driven by grade vs ER status alone.
A previous study has suggested that longer time to operation in DCIS patients can have a small but statistically significant impact on OS.2 This is likely mediated by the increased rates of invasive disease found on excision, although a previous analysis of Surveillance, Epidemiology, and End Results program data suggests that breast cancer-specific mortality can be observed even in women who never had an invasive cancer diagnosis.19 The lack of survival difference by time to operation in our analysis might be due to short follow-up time and to the fact that we did not separate the upstaged patients from the patients with pure DCIS on final pathology. Given that the clinical question during the pandemic applied to patients in limbo between their DCIS diagnosis and surgical intervention, our finding that OS does not differ by time to operation in both ER+ and ER– disease answers a different question than addressed by previous studies.
For patients with early-stage ER+ breast cancer, NET is not currently widely used in the US.20 , 21 Similar in-breast tumor response rates have been noted in patients undergoing NET compared with those undergoing neoadjuvant chemotherapy,5 with 50% to 80% of women seeing partial or complete in-breast responses.6 , 22 Although patients with cT1N0 disease were included in studies of primary endocrine therapy alone, which examined the difference in survival between older women undergoing primary endocrine therapy vs operation and endocrine therapy,8, 9, 10 NET has primarily been explored in patients with stage II to III disease in whom the intent was to downstage the primary tumor.5 As such, the NET population in the NCDB, especially those with clinical T1 disease included in this study, represents a highly selected group. As our multivariable analysis shows, there are specific patient characteristics (ie older age, higher Charlson Comorbidity Index score, and not having private insurance) and disease characteristics (lower grade and having a cT2 tumor compared with a cT1 tumor) that were associated with a higher likelihood of receiving NET. The bias toward older patients with more comorbidities is not surprising, as NET studies have demonstrated the possibility of favorable tumor response with a more favorable toxicity profile than chemotherapy,5 but does limit the applicability of our findings to the wider population affected by the COVID-19 surgical delays.
The lack of association between time to operation and pathologic upstaging in the NET group in our study is to be expected, given the tumor response rates reported in previous NET studies, and supports the recommendations made by the COVID-19 Pandemic Breast Cancer Consortium. The absence of a survival difference by time to operation is also consistent with the lack of difference in OS found in the primary endocrine therapy trials.8, 9, 10 In addition, most patients with cT2N0 disease were downstaged on final pathology. Although the absolute number of patients is small, there might be some positive, albeit unintended, effects of this particular COVID strategy.
The relationship between time to operation and pathologic upstaging in patients with invasive breast cancer has been mixed. One modeling study in pregnant patients found an association between time to operation and increased risk of positive nodes, with a 3-month delay in operation carrying an associated 2.6% increase in risk of positive lymph nodes. This has not been validated in an actual patient population.3 In a population of clinically node-negative patients with early-stage breast cancer treated at the MD Anderson Cancer Center, time to operation was not associated with pathologically positive lymph nodes on adjusted analyses.4
Survival analyses have similarly reported mixed results, with some reporting a statistically significant association between time to operation and decreased survival,23, 24, 25 and others finding no such association.26, 27, 28 Our analysis found a slight decrease in OS (approximately 2%) at a median follow-up of 40 months in patients who underwent primary operation more than 120 days after diagnosis compared with those who underwent operation within the first 60 days. Although a survival difference might be surprising in early-stage disease, this is consistent with a Surveillance, Epidemiology, and End Results program/NCDB analysis from Bleicher and colleagues,24 which found that time to operation was associated with lower OS in patients with stage I/II disease, but not with stage III disease. Although we were able to adjust for certain patient, disease, and treatment factors, there are likely unmeasured factors underlying delays in treatment in this population, which might be responsible for the slight decrement in OS. For example, previous studies have noted that cohorts who undergo operation farther out from the time of diagnosis have higher proportions of Medicaid, uninsured, black, Hispanic, and lower-income patients.25 In addition, among a cohort of Medicaid patients in North Carolina, differences in survival by time to treatment were noted not among early-stage patients, but among late-stage patients.23 The relationship between survival and time to treatment might be significantly modified by socioeconomic and disease factors. Yet as increased time to operation during the COVID-19 pandemic was driven by different factors, the small OS difference might not hold true when outcomes of these patients are analyzed in the future.
Unmeasured factors likely also underlie the association between time to operation and the extent of breast operation. The increase in mastectomy rates by time to operation in the DCIS and primary operation groups might not be linked to extent of disease; rather it might be a factor as simple as surgical scheduling that accounts for the greater proportions of mastectomies among patients undergoing operations more than 60 days after diagnosis. Reconstruction, although not explored in our current analysis, has previously been associated with longer time to operation.4 , 24 Extent of breast operations in early-stage disease is also subject to patient preference, with a variety of non-disease-related factors driving a patient’s decision to undergo mastectomy.29, 30, 31
Our study has several limitations. First, as mentioned previously, the applicability of our findings is limited, given that that populations experiencing delays in surgical therapy and who were selected for NET in this retrospective analysis are more select then the patients who have experienced surgical delays during the pandemic. We would assert, however, that these are the best data currently available to study the possible outcomes of oncologic surgical delays. Second, to take advantage of the most current variables in the NCDB (eg HER2 status), and given that NET use in the US has only recently begun to be more widely adopted, our analysis was limited to 2010 through 2016, rendering our follow-up time relatively short. Third, using the American Joint Committee on Cancer 8th edition prognostic staging allowed us to more accurately judge rates of meaningful (ie prognostic) differences in upstaging, but the pathologic staging system does require more known data points (ie tumor grade, nodal status, HR-status, and HER-2 status). As such, not all of the patients with invasive cancer could be definitively staged pathologically. Finally, adherence to endocrine therapy cannot be assessed in the NCDB; this is a significant issue in breast cancer patients on endocrine therapy, which can influence OS.32 Due to the retrospective nature of the NCDB, however, our reported survival data likely capture a “real-world” population of adherers and nonadherers alike.
Conclusions
In this analysis, surgical delays of more than 60 days were associated with pathologic upstaging in patients with DCIS but not in those with invasive disease. No survival differences in patients with DCIS or early-stage ER+ breast cancer on NET were noted by time to operation. Although the applicability of these data to the patients experiencing surgical delays during the COVID-19 pandemic is limited, surgeons and patients might find some reassurance in these findings, as these 2 groups represent patients significantly affected by the surgical triage recommendations of the COVID-19 Pandemic Breast Cancer Consortium. Future study of outcomes of patients treated during this time will be required to determine the actual impact of COVID-related surgical delays and delay strategies.
Author Contributions
Study conception and design: Minami, Kantor, King, Mittendorf
Acquisition of data: Kantor, Weiss
Analysis and interpretation of data: Minami, Kantor, Weiss, King, Mittendorf
Drafting of manuscript: Minami, Kantor
Critical revision: Minami, Kantor, Weiss, Nakhlis, King, Mittendorf
Footnotes
CME questions for this article available athttp://jacscme.facs.org
Disclosure Information: Authors have nothing to disclose. Timothy J Eberlein, Editor-in-Chief, has nothing to disclose. Ronald J Weigel, CME Editor, has nothing to disclose.
Disclosures outside the scope of this work: Dr King receives speaker honoraria from Genomic Health. Dr Mittendorf receives research support from GlaxoSmithKline, provides compensated service on scientific advisory boards for Merck, Genomic Health, Sellas Lifesciences and uncompensated service on steering committees for Bristol-Myers Squibb, Eli Lilly, and Roche/Genentech. Dr Mittendorf’s institution receives clinical trial funding fromAstraZeneca, EMD Serono, Roche/Genentech (MD Anderson Cancer Center) and Roche/Genentech (Dana Farber Cancer Institute). All other authors have nothing to disclose.
Support: Dr Minami’s institution receives research support from Conquer Cancer Foundation (Young Investigator Award, 2020-2021) and the American College of Surgeons (Faculty Research Fellowship, 2020-2022).
Disclaimer: Dr Mittendorf serves on the board of directors for the American Society of Clinical Oncology and as a scientific advisor for the Susan G Komen for the Cure Foundation.
Appendix
eTable 1.
Univariable Analysis of Factors Associated with Upgrade by the American Joint Committee on Cancer 8th Edition Prognostic Staging
| Patient characteristic | ER+ DCIS |
ER– DCIS |
NET |
Primary operation |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | ||||||||
| 18–39 y | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| 40–49 y | 0.44 (0.40–0.49) | < 0.01 | 0.67 (0.55–0.82) | < 0.01 | 0.55 (0.22–1.33) | 0.18 | 0.57 (0.53–0.61) | < 0.01 |
| 50–59 y | 0.38 (0.34–0.42) | < 0.01 | 0.45 (0.37–0.55) | < 0.01 | 0.39 (0.17–0.92) | 0.03 | 0.50 (0.47–0.54) | < 0.01 |
| 60–69 y | 0.36 (0.32–0.40) | < 0.01 | 0.35 (0.28–0.42) | < 0.01 | 0.28 (0.12–0.66) | 0.01 | 0.40 (0.38–0.44) | < 0.01 |
| 70–79 y | 0.37 (0.33–0.41) | < 0.01 | 0.32 (0.26–0.40) | < 0.01 | 0.42 (0.18–0.99) | 0.05 | 0.38 (0.35–0.41) | < 0.01 |
| 80+ y | 0.42 (0.36–0.48) | < 0.01 | 0.30 (0.23–0.39) | < 0.01 | 0.74 (0.21–1.77) | 0.50 | 0.56 (0.51–0.61) | < 0.01 |
| Race | ||||||||
| White | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| Black | 1.05 (0.98–1.12) | 0.16 | 1.31 (1.15–1.49) | < 0.01 | 1.46 (0.93–2.28) | 0.10 | 1.48 (1.41–1.55) | < 0.01 |
| Hispanic | 1.14 (1.05–1.25) | < 0.01 | 1.33 (1.13–1.57) | < 0.01 | 0.97 (0.55–1.71) | 0.93 | 1.28 (1.20–1.36) | < 0.01 |
| Asian | 1.24 (1.12–1.37) | < 0.01 | 1.45 (1.21–1.73) | < 0.01 | 0.95 (0.43–2.07) | 0.90 | 1.01 (0.93–1.09) | 0.87 |
| Charlson Comorbidity Index | ||||||||
| 0 | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| 1 | 1.10 (1.03–1.17) | 0.01 | 1.08 (0.96–1.22) | 0.20 | 0.99 (0.65–1.51) | 0.98 | 1.04 (1.00–1.09) | 0.05 |
| 2 | 1.22 (1.06–1.41) | 0.01 | 1.14 (0.89–1.47) | 0.31 | 1.39 (0.74–2.64) | 0.31 | 1.09 (0.99–1.20) | 0.05 |
| ≥ 3 | 1.32 (1.03–1.69) | 0.03 | 0.95 (0.57–1.57) | 0.83 | 2.19 (1.02–4.70) | 0.04 | 1.09 (0.93–1.27) | 0.30 |
| Insurance | ||||||||
| Private | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| Medicare | 0.89 (0.84–0.93) | < 0.01 | 0.69 (0.63–0.76) | < 0.01 | 1.36 (1.00–1.84) | 0.05 | 0.84 (0.82–0.87) | < 0.01 |
| Medicaid | 1.18 (1.07–1.30) | < 0.01 | 1.48 (1.25–1.75) | < 0.01 | 1.77 (1.07–2.94) | 0.03 | 1.32 (1.24–1.40) | < 0.01 |
| Uninsured | 1.13 (0.95–1.35) | 0.17 | 1.06 (0.78–1.44) | 0.72 | 0.53 (0.13–2.21) | 0.39 | 1.32 (1.18–1.48) | < 0.01 |
| Facility type | ||||||||
| Community | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| Comprehensive | 1.26 (1.14–1.39) | < 0.01 | 1.24 (1.04–1.49) | 0.02 | 1.54 (0.78–3.01) | 0.21 | 0.99 (0.93–1.04) | 0.65 |
| Academic | 1.40 (1.27–1.54) | < 0.01 | 1.69 (1.41–2.04) | < 0.01 | 1.68 (0.85–3.29) | 0.13 | 1.02 (0.96–1.08) | 0.56 |
| Integrated network | 1.25 (1.12–1.39) | < 0.01 | 1.48 (1.21–1.80) | < 0.01 | 1.52 (0.74–3.12) | 0.26 | 0.96 (0.90–1.02) | 0.17 |
| Histology | ||||||||
| Ductal | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| Lobular | NA | — | NA | — | 1.66 (1.18–2.35) | < 0.01 | 1.54 (1.48–1.61) | < 0.01 |
| Mixed | 0.14 (0.13–1.15) | < 0.01 | 0.08 (0.07–0.10) | < 0.01 | 1.23 (0.81–1.88) | 0.37 | 1.17 (1.11–1.22) | < 0.01 |
| cT | ||||||||
| cT1 | NA (all cTis) | Ref | NA (all cTis) | — | 1 | Ref | 1 | Ref |
| cT2 | — | — | — | — | 0.47 (0.35–0.63) | < 0.01 | 0.67 (0.64–0.70) | < 0.01 |
| Operation type | ||||||||
| BCS | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| Mastectomy | 2.82 (2.70–2.95) | < 0.01 | 2.36 (2.17–2.56) | < 0.01 | 2.80 (2.11–3.72) | < 0.01 | 2.60 (2.53–2.68) | < 0.01 |
| Operation timing | ||||||||
| < 60 d | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| 60–120 d | 1.43 (1.35–1.52) | < 0.01 | 1.32 (1.19–1.47) | < 0.01 | 0.97 (0.63–1.51) | 0.90 | 1.21 (1.16–1.27) | < 0.01 |
| > 120 d | 1.75 (1.52–2.01) | < 0.01 | 1.70 (1.30–2.21) | < 0.01 | 1.01 (0.67–1.51) | 0.98 | 1.42 (1.21–1.66) | < 0.01 |
BCS, breast-conserving surgery; cT, clinical tumor category, DCIS, ductal carcinoma in situ; ER, estrogen receptor; NET, neoadjuvant endocrine therapy; OR, odds ratio; Ref, reference.
eTable 2.
Multivariable Analysis of Factors Associated with Neoadjuvant Endocrine Therapy Receipt
| Patient characteristic | OR (95% CI) | p Value |
|---|---|---|
| Age | ||
| 18–39 y | 1 | Ref |
| 40–49 y | 1.02 (0.73–1.41) | 0.93 |
| 50–59 y | 1.41 (1.02–1.93) | 0.04 |
| 60–69 y | 1.73 (1.26–2.38) | < 0.01 |
| 70–79 y | 1.92 (1.38–2.66) | < 0.01 |
| 80+ y | 2.63 (1.88–3.69) | < 0.01 |
| Race | ||
| White | 1 | Ref |
| Black | 1.06 (0.93–1.21) | 0.38 |
| Hispanic | 1.35 (1.17–1.56) | < 0.01 |
| Asian | 1.07 (0.88–1.30) | 0.50 |
| Charlson Comorbidity Index | ||
| 0 | 1 | Ref |
| 1 | 0.88 (0.79–0.97) | 0.01 |
| 2 | 1.32 (1.10–1.59) | < 0.01 |
| ≥ 3 | 1.83 (1.41–2.39) | < 0.01 |
| Insurance | ||
| Private | 1 | Ref |
| Medicare | 1.16 (1.05–1.29) | < 0.01 |
| Medicaid | 1.47 (1.26–1.70) | < 0.01 |
| Uninsured | 1.53 (1.18–1.99) | < 0.01 |
| Region | ||
| Metro | 1 | Ref |
| Urban | 1.02 (0.91–1.13) | 0.77 |
| Rural | 1.06 (0.81–1.39) | 0.66 |
| Unknown | 1.17 (0.95–1.45) | 0.15 |
| Facility type | ||
| Community | 1 | Ref |
| Comprehensive | 1.13 (0.97–1.30) | 0.11 |
| Academic | 1.62 (1.40–1.87) | < 0.01 |
| Integrated Network | 1.59 (1.36–1.87) | < 0.01 |
| Histology | ||
| Ductal | 1 | Ref |
| Lobular | 1.17 (1.06–1.29) | < 0.01 |
| Mixed | 1.09 (0.98–1.22) | 0.12 |
| Grade | ||
| 1 | 1 | Ref |
| 2 | 0.80 (0.74–0.87) | < 0.01 |
| 3 | 0.45 (0.40–0.51) | < 0.01 |
| cT | ||
| cT1 | 1 | Ref |
| cT2 | 5.40 (5.01–5.81) | <0.01 |
cT, clinical tumor category; OR, odds ratio; Ref, reference.
References
- 1.Dietz J., Moran M., Isakoff S. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. https://www.facs.org/-/media/files/quality-programs/napbc/asbrs_napbc_coc_nccn_acr_bc_covid_consortium_recommendations.ashx Available at: [DOI] [PMC free article] [PubMed]
- 2.Ward W.H., DeMora L., Handorf E. Preoperative delays in the treatment of DCIS and the associated incidence of invasive breast cancer. Ann Surg Oncol. 2020;27:386–396. doi: 10.1245/s10434-019-07844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nettleton J., Long J., Kuban D. Breast cancer during pregnancy: quantifying the risk of treatment delay. Obstet Gynecol. 1996;87:414–418. doi: 10.1016/0029-7844(95)00470-x. [DOI] [PubMed] [Google Scholar]
- 4.Wagner J.L., Warneke C.L., Mittendorf E.A. Delays in primary surgical treatment are not associated with significant tumor size progression in breast cancer patients. Ann Surg. 2011;254:119–124. doi: 10.1097/SLA.0b013e318217e97f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spring L.M., Gupta A., Reynolds K.L. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2:1477–1486. doi: 10.1001/jamaoncol.2016.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis M.J., Suman V.J., Hoog J. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J Clin Oncol. 2011;29:2342–2349. doi: 10.1200/JCO.2010.31.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colleoni M., Sun Z., Price K.N. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group Trials I to V. J Clin Oncol. 2016;34:927–935. doi: 10.1200/JCO.2015.62.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fennessy M., Bates T., MacRae K., Riley D. Late follow-up of a randomized trial of surgery plus tamoxifen versus tamoxifen alone in women aged over 70 years with operable breast cancer. Br J Surg. 2004;91:699–704. doi: 10.1002/bjs.4603. [DOI] [PubMed] [Google Scholar]
- 9.Mustacchi G., Ceccherini R., Milani S. Tamoxifen alone versus adjuvant tamoxifen for operable breast cancer of the elderly: long-term results of the phase III randomized controlled multicenter GRETA trial. Ann Oncol. 2003;14:414–420. doi: 10.1093/annonc/mdg117. [DOI] [PubMed] [Google Scholar]
- 10.Hind D., Wyld L., Reed M.W. Surgery, with or without tamoxifen, vs tamoxifen alone for older women with operable breast cancer: cochrane review. Br J Cancer. 2007;96:1025–1029. doi: 10.1038/sj.bjc.6603600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuliano A.E., Connolly J.L., Edge S.B. Breast cancer—major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:290–303. doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 12.Brennan M.E., Turner R.M., Ciatto S. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;260:119–128. doi: 10.1148/radiol.11102368. [DOI] [PubMed] [Google Scholar]
- 13.Elshof L.E., Tryfonidis K., Slaets L. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ—The LORD study. Eur J Cancer. 2015;51:1497–1510. doi: 10.1016/j.ejca.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Francis A., Thomas J., Fallowfield L. Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur J Cancer. 2015;51:2296–2303. doi: 10.1016/j.ejca.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Hwang E.S., Hyslop T., Lynch T. The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: a phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS) BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilewskie M., Stempel M., Rosenfeld H. Do LORIS trial eligibility criteria identify a ductal carcinoma in situ patient population at low risk of upgrade to invasive carcinoma? Ann Surg Oncol. 2016;23:3487–3493. doi: 10.1245/s10434-016-5268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimm L.J., Ryser M.D., Partridge A.H. Surgical upstaging rates for vacuum assisted biopsy proven DCIS: implications for active surveillance trials. Ann Surg Oncol. 2017;24:3534–3540. doi: 10.1245/s10434-017-6018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang E.S., Hyslop T., Hendrix L.H. Phase II single-arm study of preoperative letrozole for estrogen receptor-positive postmenopausal ductal carcinoma in situ: CALGB 40903 (Alliance) J Clin Oncol. 2020 Mar 3 doi: 10.1200/JCO.19.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narod S.A., Iqbal J., Giannakeas V. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1:888–896. doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
- 20.Chiba A., Hoskin T.L., Heins C.N. Trends in neoadjuvant endocrine therapy use and impact on rates of breast conservation in hormone receptor-positive breast cancer: a National Cancer Data Base study. Ann Surg Oncol. 2017;24:418–424. doi: 10.1245/s10434-016-5585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss A., Wong S., Golshan M. Patterns of axillary management in stages 2 and 3 hormone receptor-positive breast cancer by initial treatment approach. Ann Surg Oncol. 2019;26:4326–4336. doi: 10.1245/s10434-019-07785-y. [DOI] [PubMed] [Google Scholar]
- 22.Bear H.D., Wan W., Robidoux A. Using the 21-gene assay from core needle biopsies to choose neoadjuvant therapy for breast cancer: a multicenter trial. J Surg Oncol. 2017;115:917–923. doi: 10.1002/jso.24610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLaughlin J.M., Anderson R.T., Ferketich A.K. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol. 2012;30:4493–4500. doi: 10.1200/JCO.2012.39.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bleicher R.J., Ruth K., Sigurdson E.R. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2:330–339. doi: 10.1001/jamaoncol.2015.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polverini A.C., Nelson R.A., Marcinkowski E. Time to treatment: measuring quality breast cancer care. Ann Surg Oncol. 2016;23:3392–3402. doi: 10.1245/s10434-016-5486-7. [DOI] [PubMed] [Google Scholar]
- 26.Brazda A., Estroff J., Euhus D. Delays in time to treatment and survival impact in breast cancer. Ann Surg Oncol. 2010;17(Suppl 3):291–296. doi: 10.1245/s10434-010-1250-6. [DOI] [PubMed] [Google Scholar]
- 27.Sainsbury R., Johnston C., Haward B. Effect on survival of delays in referral of patients with breast-cancer symptoms: a retrospective analysis. Lancet. 1999;353:1132–1135. doi: 10.1016/s0140-6736(99)02374-0. [DOI] [PubMed] [Google Scholar]
- 28.Comber H., Cronin D.P., Deady S. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005;98:238–239. [PubMed] [Google Scholar]
- 29.Sivell S., Elwyn G., Edwards A., Manstead A.S. Factors influencing the surgery intentions and choices of women with early breast cancer: the predictive utility of an extended theory of planned behaviour. BMC Med Inform Decis Mak. 2013;13:92. doi: 10.1186/1472-6947-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamelinck V.C., Bastiaannet E., Pieterse A.H. A prospective comparison of younger and older patients' preferences for breast-conserving surgery versus mastectomy in early breast cancer. J Geriatr Oncol. 2018;9:170–173. doi: 10.1016/j.jgo.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Dicks E., Roome R., Chafe J. Factors influencing surgical treatment decisions for breast cancer: a qualitative exploration of surgeon and patient perspectives. Curr Oncol. 2019;26 doi: 10.3747/co.26.4305. e216–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hershman D.L., Shao T., Kushi L.H. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]