Abstract
With the trend of minimally invasive spine surgery, full-endoscopic lumbar discectomy (FELD) has evolved with the advancement of the optics and instruments. Regarding the techniques, the transforaminal and interlaminar approach remain the major accesses in FELD. Transforaminal endoscopic lumbar discectomy (TELD) is an effective and safe treatment for herniation of the lumbar disc. More and more evidence supports the TELD in enhancing recovery and decreasing surgical complications. However, the learning curve of TELD remains steep, especially at the L5–S1 level. The iliac crest height is an essential factor in the operability of TELD at the L5–S1 level. In the situation of the high iliac crest, TELD is technically challenging even for an experienced surgeon. Therefore, the authors report their techniques of TELD with foraminoplasty step-by-step and the preliminary results in this report.
Keywords: Endoscopic discectomy, Percutaneous lumbosacral region, Iliac crest
INTRODUCTION
Nowadays, full-endoscopic lumbar spine surgery has gained more attention. Since, 3 decades ago, some pioneers have developed the techniques of transforaminal endoscopic lumbar discectomy (TELD) [1-5]. TELD can achieve decompression with minimal injury to collateral soft tissue, which benefits postoperative pain, shorter hospital stay, and early return to daily life or work. Because the surgery is done under local anesthesia, surgeons can avoid neural injury by communicating with the patient directly during the procedure. Though there are advantages in enhancing postoperative recovery, there are several limitations causing failure or complications of TELD. From the anatomical study, the transforaminal approach to L5–S1 level may be challenging due to high iliac crest and narrowed foraminal area that results from large L5 transverse process or hypertrophic facet joint [6-8]. There are various techniques proposed to overcome these anatomical limitations. Some authors have described the endoscopic foraminoplasty technique by using a drill or reamer in performing TELD [9-11]. Nevertheless, there are few reports about the TELD with foraminoplasty focusing on the L5–S1 level with high iliac crest. Therefore, the authors described their techniques step-by-step and reported the preliminary results.
MATERIALS AND METHODS
The Institutional Review Board (IRB) of Changhua Christian Hospital approved the study (IRB No.190905), and all patients had informed consent. The retrospective study enrolled patients with lumbar disc herniation at the level of L5–S1 and high iliac crest undergoing TELD with foraminoplasty from July 2018 to December 2019. All patients had unilateral radiculopathy resistant to conservative treatment. The high iliac crest is defined as the outline of the iliac crest above the mid-L5 pedicle in lateral radiography [12]. The demographics and clinical outcomes of the patients were recorded prospectively. The surgical techniques were described step-by-step. The clinical outcomes were assessed with the visual analogue scale (VAS), Oswestry Disability Index (ODI), and modified MacNab criteria. Statistical calculations were carried out with the IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). The p-value of less than 0.05 was considered statistically significant.
SURGICAL TECHNIQUES
1. Patient Position and Skin Marking
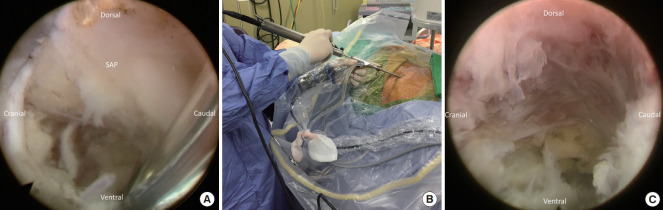
The patient is in the prone position with hips and knees flexion on the Jackson table. Antero-posterior and lateral fluoroscopy are used to depict the appropriate level and outline of the iliac crest. After prepping and draping, the outline of the iliac crest is identified in the Ferguson view of the fluoroscopy. The entry point is marked at the intersection of the tangent line to the iliac crest toward the base of the S1 superior articular process (SAP) in anteroposterior fluoroscopic view, and the trajectory line toward the junction of the S1 SAP and pedicle in lateral fluoroscopic view (Fig. 1).
Fig. 1.
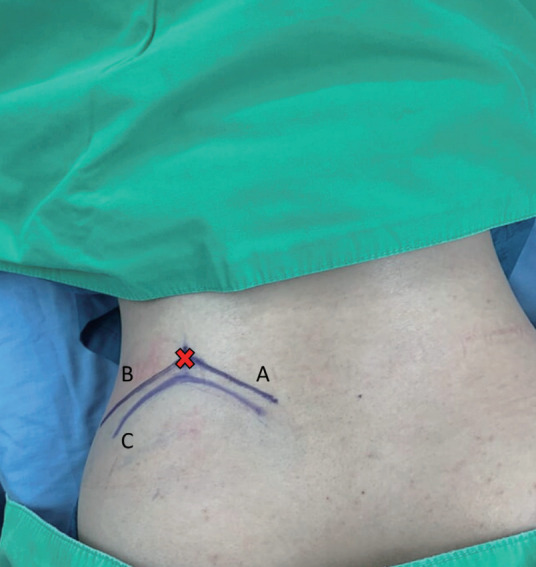
Skin markings are illustrated. The entry point (red cross) is at the intersection of the tangent line (A) to the iliac crest line (C) toward the superior articular process in anteroposterior view and the trajectory line (B) in the lateral view.
2. Anesthesia and Docking the Working Cannula
After planning the entry point and trajectory, the patient is given local infiltration anesthesia with 1% lidocaine preoperatively. Then, a stab incision by 8 mm is made through the skin and fascia with a blade. The cannulated needle is placed from the entry point and along the trajectory to the base of SAP. The target of the needle placement is at the junction of the SAP and pedicle, which are bony structures and easy to identify by fluoroscopic guidance and tactile feedback. Thus, epidurogram and discogram are omitted. After confirming the needle position, diluted lidocaine (0.2%) is given to infiltrate and block the ventral facet. Although the diluted lidocaine may fail to infiltrate the epidural space adequately, the pain from irritation to the annulus is minimal with the current technique. Then, a guidewire is inserted through the cannulated needle and followed by sequential dilators to create the track for working cannula. The position for the working channel is inserted through the dilator under the visualization of the fluoroscopy (Fig. 2).
Fig. 2.
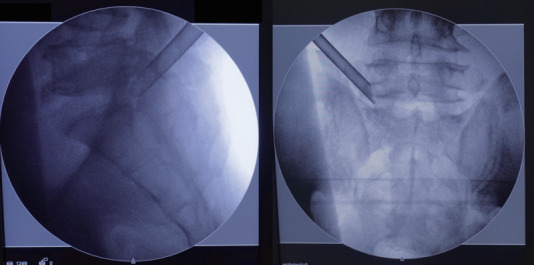
The position for the working cannula is illustrated by left L5–S1 transforaminal endoscopic lumbar discectomy. In the lateral view, the cannula is docked at the junction of superior articular process and pedicle. In the anteroposterior view, the cannula is landed at the lateral border of the foramen.
3. Endoscopic Foraminoplasty
The dilator is then removed, and the endoscope of the working channel by 4.3 mm (SPINENDOS GmbH, Munich, Germany) is inserted through the working channel with continuous sterile saline irrigation. The radiofrequency coagulator (SPINENDOS GmbH) and grasping forceps are utilized to dissect soft tissue away and then define the SAP under direct endoscopic visualization.
A high-speed diamond burr (SPINENDOS GmbH) is used to drill the ventral portion of the SAP and trim the posterior margin of the S1 vertebra for widening the working space in the foramen (Fig. 3). The foraminal ligament is then removed with forceps. Sometimes, a bulging disc in the foraminal area can be found and removed partially with forceps or shrank with a radiofrequency coagulator to maintain the endoscopic vision. After foraminoplasty, the working cannula can be inserted into the lateral recess of the spinal canal under direct endoscopic visualization (Fig. 4).
Fig. 3.
(A) Initial endoscopic view showed the base of the superior articular process (SAP). (B) The use of the endoscopic burr during foraminoplasty. (C) Endoscopic view of the drilled SAP and widened foramen.
Fig. 4.
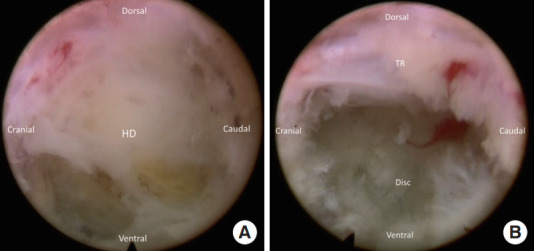
(A) The herniated disc (HD) is exposed after foraminoplasty. (B) The traversing root (TR) is exposed after discectomy.
4. Endoscopic Discectomy
After adjusting the position of the working cannula, the intracanalicular disc herniation can be identified. After partial discectomy, the herniated disc stuck at the annular defect is released. The migrated segment is usually free and able to be pulled out from the canal. The surgeon can use a ball-tip probe to explore the fragment in the epidural space. After discectomy, annuloplasty and epidural hemostasis are conducted with a radiofrequency probe. Sometimes, epidural bleeding may happen after decompression. Radiofrequency bipolar probe or hemostatic agents can usually control and epidural venous bleeding.
5. Final Check Point
After discectomy, the surgeon should check the bleeding, possible residual pathology, and gross appearance of nerve roots. Adequate decompression is defined as the grossly free margin of the nerve roots with good pulsation. Then, the endoscope can be retracted to check the extraforaminal area and the exiting root (Fig. 5).
Fig. 5.
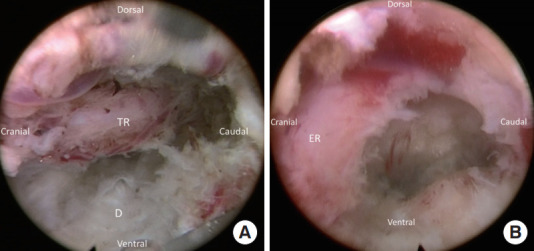
Final view after decompression. (A) The decompressed lateral recess and traversing root (TR). (B) Withdraw the endoscope and rotate the vision toward cranial direction can show the exiting root (ER) to confirm adequate decompression.
Further, extraforaminal discectomy can be performed if there is gross compression of the exiting root by a herniated disc. The drainage tube placement is not necessary after meticulous hemostasis. The wound is closed with one subcutaneous stitch.
RESULTS
Sixteen cases underwent TELD with foraminoplasty at the L5–S1 level in the current series. The demographics are presented in Table 1. The mean age of the patients was 41.7 years old (range, 14–79 years). The mean follow-up time was 8.4 ± 3.8 months (range, 3–12 months). One patient had a history of previous instrumentation surgery at the index level before undergoing TELD. The preoperative mean VAS scores for leg pain significantly decreased from 7.4 ± 1.3 to 1.3 ± 0.8 at the last follow-up (p<0.05) (Table 2). There was no significant difference in VAS scores for back pain (2.3 ± 2.8 preoperatively vs. 1.8 ± 1.1 at the last follow-up). The mean preoperative ODI score was 47.0 ± 12.3. The postoperative ODI scores were 3.1 ± 4.1, 4.2 ± 8.8, 3.2 ± 7.1, and 1.5 ± 2.3, at 1, 3, 6, and 12 months respectively (p<0.05) (Fig. 6). The global results of the MacNab criteria were excellent in 14 patients and good in 2 patients. There were no immediate postoperative complications, such as epidural hematoma or dysesthesia. One patient had early recurrence 2 weeks after the TELD and improved after the repeated TELD. There was no iatrogenic instability and revision for fusion surgery in the current series.
Table 1.
Patients’ demographics
| Variable | Value |
|---|---|
| Age (yr) | 41.7 ± 19.9 |
| Preoperative VAS | |
| Back | 2.3 ± 2.8 |
| Leg | 7.4 ± 1.3 |
| Preoperative ODI | 47.0 ± 12.3 |
| Follow-up time (mo) | 8.4 ± 3.8 |
| Sex | |
| Male | 11 (68.8) |
| Female | 5 (31.2) |
| Body mass index (kg/m2) | |
| < 18.5 | 1 (6.2) |
| 18.5–25 | 9 (56.4) |
| 25–30 | 4 (25.0) |
| > 30 | 2 (12.4) |
| Previous operation history | 1 (6.2) |
| Cigarette smoking | 2 (11.8) |
| Disc degeneration | |
| Grade 2 | 5 (31.3) |
| Grade 3 | 10 (62.5) |
| Grade 4 | 1 (6.2) |
| Herniation site | |
| Central | 4 (25.0) |
| Subarticular | 7 (43.7) |
| Foraminal | 5 (31.3) |
Values are presented as mean±standard deviation or number (%).
VAS, visual analogue scale; ODI, Oswestry Disability Index.
Table 2.
Clinical outcomes
| Variable | Score | p-value |
|---|---|---|
| VAS for back pain | ||
| Preoperative | 2.3 ± 2.8 | |
| Postoperative | 1.8 ± 1.1 | NS |
| VAS for leg pain | ||
| Preoperative | 7.4 ± 1.3 | |
| Postoperative | 1.3 ± 0.8 | < 0.05 |
| ODI | ||
| Preoperative | 47.0 ± 12.3 | |
| Postoperative 1 month | 3.1 ± 4.1 | < 0.05 |
| Postoperative 3 months | 4.2 ± 8.8 | < 0.05 |
| Postoperative 6 months | 3.2 ± 7.1 | < 0.05 |
| Postoperative 1 year | 1.5 ± 2.3 | < 0.05 |
Values are presented as mean±standard deviation or number (%).
VAS, visual analogue scale; ODI, Oswestry Disability Index; NS, not significant.
Fig. 6.
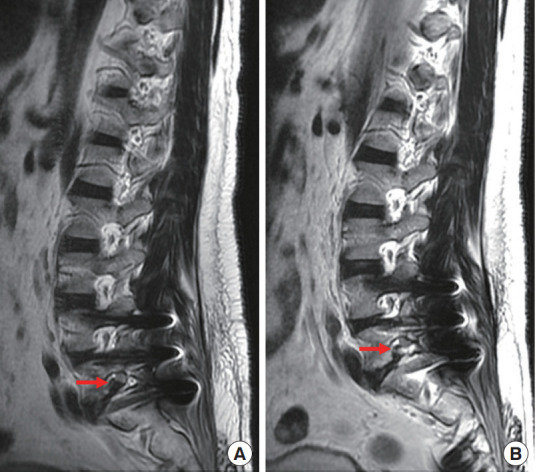
The preoperative (A) and postoperative (B) magnetic resonance imaging showed decompressed root after removal of foraminal disc herniation (red arrow).
1. Case Illustration (Supplementary video clip 1)
A 40-year-old man with underlying hypertension has sustained left lower limb pain for 14 days. He just underwent an operation of the posterior lumbar interbody fusion with cortical bone trajectory screws fixation for subluxation at L4–5 and L5–S1 level at another hospital 3 weeks before. The VAS of leg pain was 8 and aggravated while walking. The findings of the physical examination showed no significant loss of bilateral muscle power, but the straight leg raise test was positive at 60° over the left side. The ODI assessment scored a 23; thus, 46% disability was noted. Magnetic resonance imaging revealed foraminal herniated intervertebral disc and retained Gelfoam sponge at the L5–S1 level causing compression (Fig. 7). Thus, he underwent TELD with foraminoplasty. He was pain-free after TELD. The 1-month ODI score was 0. There had been no recurrence during the 3-month follow-up period.
Fig. 7.
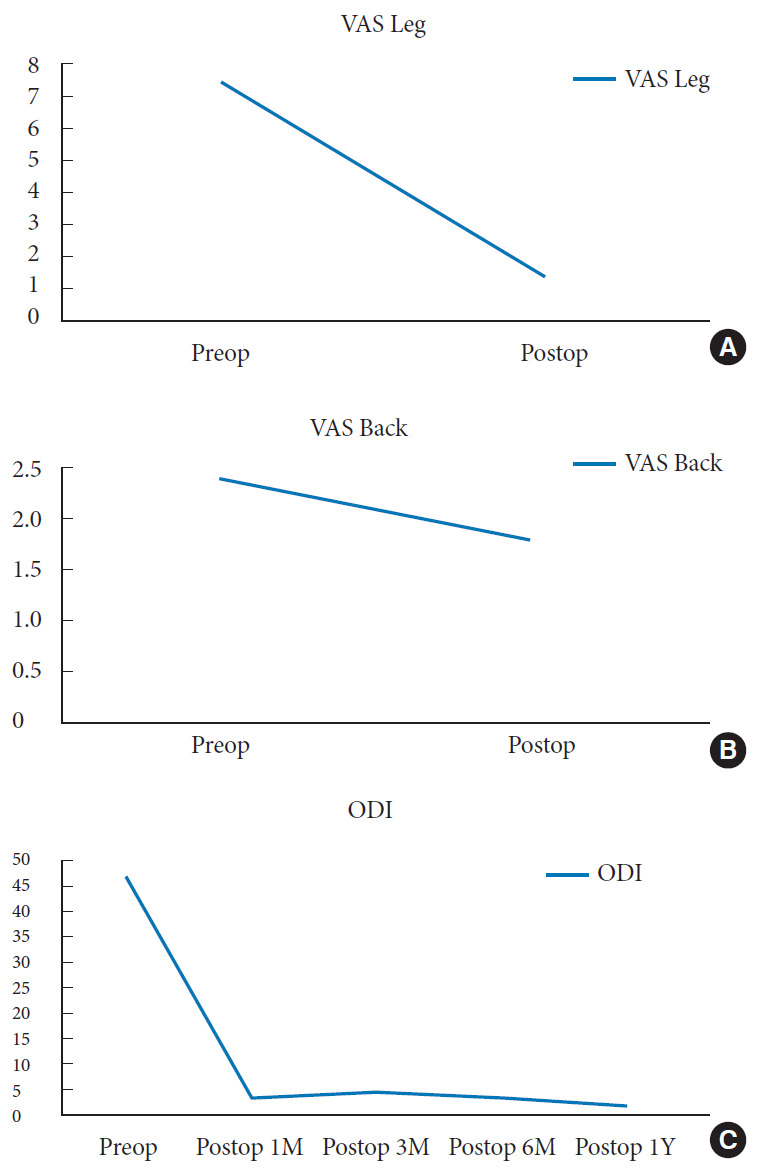
The clinical outcomes: (A) visual analogue scale (VAS) for leg pain, (B) VAS for back pain, and (C) preoperative (preop) and postoperative (postop) Oswestry Disability Index (ODI) scores. 1M, 1 month; 3M, 3 months; 6M, 6 months; 1Y, 1 year.
DISCUSSION
At the L5–S1 level, TELD is challenging due to some anatomical obstacles. The surgeon should assess the iliac crest height and the inclination of the disc at the L5–S1 level. Both these factors cause a steeper trajectory angle and possible failure to reach the herniated disc. The critical factor in succeeding TELD is the location and inclination of the working cannula toward the pathology. The entry point and the target can usually determine the trajectory of the cannula. As for the entry point, there are 2 choices reported in the literature, included supra-iliac and transiliac entry. The transiliac approach is to gain a direct route toward the lesion by drilling a hole through the ilium [13]. The diameter of the hole on the ilium determines the mobility of the working cannula. Though TELD at the L5–S1 level can be done in a lateral approach, the transiliac access is technically demanding and only demonstrated on few case reports. While using the supra-iliac entry at L5–S1 TELD, high iliac crest might be a significant concern in the procedure. The situation of the high iliac crest leads to the increased inclination of the trajectory, which makes the cannula toward a more ventral aspect and away from the centrally herniated disc [14]. Choi and Park [12] reported a series of the L5–S1 TELD in 100 patients and simplified the relation of iliac crest height and L5–S1 disc level with 2-dimensional radiography. By supra-iliac entry, TELD at L5–S1 can achieve a good outcome in 92% of the patients. In high iliac crest patients whose iliac crest is above the mid-L5 pedicle in lateral radiography, foraminoplasty may be considered for transforaminal access of L5–S1 disc herniation.
The techniques of foraminoplasty were initially proposed to reach the migrated disc by widening the foramen during TELD [10,11]. The SAP is the main obstacle in transforaminal endoscopic access to the epidural space in the spinal canal. After the undercutting of the ventral part of the SAP, the working zone can be widened, and the endoscopic trajectory can directly target the herniated disc at the ventral epidural space [15]. With the application of foraminoplasty, TELD might be feasible in not only migrated disc but also L5–S1 herniated disc with a high iliac crest [16]. The foraminoplasty can be performed by using the bone trephines, an endoscopic burr, or side-firing Ho: YAG laser. The foraminoplasty by bone trephines is usually conducted under the guidance of the fluoroscopy. It is an efficient procedure, but it may theoretically cause a higher risk of nerve injury and uncontrolled bleeding.
In comparison, the endoscopic burr can help with removal and hemostasis of bone under endoscopic visualization. However, it may take more time to do the foraminoplasty with endoscopic burr than with trephines. Recently, there is a newly designed trephine (TESSYS Isee) used under endoscopic visualization [17]. There is still a lack of comparative study to prove one of the tools superior to the other. Thus, it depends on the surgeon's preference and experience and the availability of instruments to perform foraminoplasty.
In recent years, there has been a trend to treat the herniated disc at the L5–S1 level with interlaminar endoscopic lumbar discectomy (IELD). IELD was proved to be safe and effective in treating the disc herniation at the L5–S1 level [18,19]. From anatomical consideration, the interlaminar window is wider at the L5–S1 level throughout the lumbosacral spine [20]. The recent systemic review revealed that TELD has comparable clinical efficacy and safety compared with IELD. Besides, IELD is superior to TELD in less operative time and radiation exposure [21]. However, the surgeons should manipulate the neural structures before discectomy during ILED. The procedure may cause obvious irritation to root during operation. Thus, IELD is usually performed under general or epidural anesthesia.
In comparison, neural structures are usually seen after decompression in TELD, so local anesthesia is feasible. Patients may take less anesthetic risk when undergoing TELD, especially for elderly or patients with multiple comorbidities. Furthermore, the risk of postoperative dysesthesia is less in TELD. The location of the herniation and the degree of migration may be one of the factors in deciding the surgical route. From a retrospective study in Korea, TELD is preferred for shoulder type, centrally located, and recurrent disc herniation, while IELD is preferred for axillary type and migrated discs, especially those of a high-grade migration [22]. In the current series, the authors proposed the technique of foraminoplasty by drilling the SAP base and posterior vertebral body along the superior vertebral notch of the sacrum. By this way, even highly migrated disc can be removed.
There are limitations in the current report. First, it is a retrospective study and the sample size is small. The follow-up time is short term. There is a lack of 3-dimensional images to evaluate the surgical techniques from our case series. The learning curve for TELD with foraminoplasty is steep, and it cannot be studied in the short report.
CONCLUSION
TELD is an effective and safe treatment of the herniated disc at the L5–S1 level. In the situation of the high iliac crest, surgeons can use supra-iliac access and foraminoplasty to reach the herniated disc at the L5–S1 level.
Footnotes
The authors have nothing to disclose.
SUPPLEMENTARY MATERIALS
Supplementary video clip 1 can be found via https://doi.org/10.14245/ns.2040166.060.v.1.
REFERENCES
- 1.Kambin P. Arthroscopic microdiskectomy. Mt Sinai J Med. 1991;58:159–64. [PubMed] [Google Scholar]
- 2.Kambin P, O'Brien E, Zhou L, et al. Arthroscopic microdiscectomy and selective fragmentectomy. Clin Orthop Relat Res. 1998;(347):150–67. [PubMed] [Google Scholar]
- 3.Mathews HH. Transforaminal endoscopic microdiscectomy. Neurosurg Clin N Am. 1996;7:59–63. [PubMed] [Google Scholar]
- 4.Savitz MH. Same-day microsurgical arthroscopic lateral-approach laser-assisted (SMALL) fluoroscopic discectomy. J Neurosurg. 1994;80:1039–45. doi: 10.3171/jns.1994.80.6.1039. [DOI] [PubMed] [Google Scholar]
- 5.Mayer HM, Brock M. Percutaneous endoscopic discectomy: surgical technique and preliminary results compared to microsurgical discectomy. J Neurosurg. 1993;78:216–25. doi: 10.3171/jns.1993.78.2.0216. [DOI] [PubMed] [Google Scholar]
- 6.Ebraheim NA, Xu R, Huntoon M, et al. Location of the extraforaminal lumbar nerve roots. An anatomic study. Clin Orthop Relat Res. 1997;(240):230–5. doi: 10.1097/00003086-199707000-00030. [DOI] [PubMed] [Google Scholar]
- 7.Reulen HJ, Müller A, Ebeling U. Microsurgical anatomy of the lateral approach to extraforaminal lumbar disc herniations. Neurosurgery. 1996;39:345–50. doi: 10.1097/00006123-199608000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Mirkovic SR, Schwartz DG, Glazier KD. Anatomic considerations in lumbar posterolateral percutaneous procedures. Spine (Phila Pa 1976) 1995;20:1965–71. doi: 10.1097/00007632-199509150-00001. [DOI] [PubMed] [Google Scholar]
- 9.Yeung AT, Yeung CA. Advances in endoscopic disc and spine surgery: foraminal approach. Surg Technol Int. 2003;11:255–63. [PubMed] [Google Scholar]
- 10.Schubert M, Hoogland T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Oper Orthop Traumatol. 2005;17:641–61. doi: 10.1007/s00064-005-1156-9. [DOI] [PubMed] [Google Scholar]
- 11.Choi G, Lee SH, Lokhande P, et al. Percutaneous endoscopic approach for highly migrated intracanal disc herniations by foraminoplastic technique using rigid working channel endoscope. Spine (Phila Pa 1976) 2008;33:E508–15. doi: 10.1097/BRS.0b013e31817bfa1a. [DOI] [PubMed] [Google Scholar]
- 12.Choi KC, Park CK. Percutaneous endoscopic lumbar discectomy for L5-S1 disc herniation: consideration of the relation between the iliac crest and L5-S1 disc. Pain Physician. 2016;19:E301–8. [PubMed] [Google Scholar]
- 13.Choi G, Kim JS, Lokhande P, et al. Percutaneous endoscopic lumbar discectomy by transiliac approach: a case report. Spine (Phila Pa 1976) 2009;34:E443–6. doi: 10.1097/BRS.0b013e31817c4f39. [DOI] [PubMed] [Google Scholar]
- 14.Tezuka F, Sakai T, Abe M, et al. Anatomical considerations of the iliac crest on percutaneous endoscopic discectomy using a transforaminal approach. Spine J. 2017;17:1875–80. doi: 10.1016/j.spinee.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Sairyo K, Higashino K, Yamashita K, et al. A new concept of transforaminal ventral facetectomy including simultaneous decompression of foraminal and lateral recess stenosis: technical considerations in a fresh cadaver model and a literature review. J Med Invest. 2017;64:1–6. doi: 10.2152/jmi.64.1. [DOI] [PubMed] [Google Scholar]
- 16.Choi KC, Shim HK, Park CJ, et al. Usefulness of percutaneous endoscopic lumbar foraminoplasty for lumbar disc herniation. World Neurosurg. 2017;106:484–92. doi: 10.1016/j.wneu.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Xiong C, Li T, Kang H, et al. Early outcomes of 270-degree spinal canal decompression by using TESSYS-ISEE technique in patients with lumbar spinal stenosis combined with disk herniation. Eur Spine J. 2019;28:78–86. doi: 10.1007/s00586-018-5655-4. [DOI] [PubMed] [Google Scholar]
- 18.Ruetten S, Komp M, Godolias G. A New full-endoscopic technique for the interlaminar operation of lumbar disc herniations using 6-mm endoscopes: prospective 2-year results of 331 patients. Minim Invasive Neurosurg. 2006;49:80–7. doi: 10.1055/s-2006-932172. [DOI] [PubMed] [Google Scholar]
- 19.Choi G, Lee SH, Raiturker PP, et al. Percutaneous endoscopic interlaminar discectomy for intracanalicular disc herniations at L5-S1 using a rigid working channel endoscope. Neurosurgery. 2006;58(1 Suppl):ONS59–68. doi: 10.1227/01.neu.0000192713.95921.4a. [DOI] [PubMed] [Google Scholar]
- 20.Sakçı Z, Önen MR, Fidan E, et al. Radiologic anatomy of the lumbar interlaminar window and surgical considerations for lumbar interlaminar endoscopic and microsurgical disc surgery. World Neurosurg. 2018;115:e22–6. doi: 10.1016/j.wneu.2018.03.049. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Jing X, Li C, et al. Percutaneous endoscopic lumbar discectomy for L5S1 lumbar disc herniation using a transforaminal approach versus an interlaminar approach: a systematic review and meta-analysis. World Neurosurg. 2018;116:412–20. doi: 10.1016/j.wneu.2018.05.075. e2. [DOI] [PubMed] [Google Scholar]
- 22.Choi KC, Kim JS, Ryu KS, et al. Percutaneous endoscopic lumbar discectomy for L5-S1 disc herniation: transforaminal versus interlaminar approach. Pain Physician. 2013;16:547–56. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.