Abstract
Alzheimer's disease (AD) is a chronic neurodegenerative, and abnormal aggregation of the neurotoxic β amyloid (Aβ) peptide is an early event in AD. The present study aimed to determine the correlation between the nicotinic acetylcholine receptor α7 subunit (α7 nAChR) and Aβ in the brains of patients with AD, and to investigate whether the increased expression levels of the α7 nAChR could alter the neurotoxicity of Aβ. The expression levels of α7 nAChR and Aβ in the brains of patients with AD and healthy brains were analyzed using immunofluorescence. Moreover, SH-SY5Y cells were used to stably overexpress or silence α7 nAChR expression levels, prior to the treatment with or without 1 µmol/l Aβ1-42 oligomer (AβO). The mRNA and protein expression levels of α7 nAChR, synaptophysin (SYP), postsynaptic density of 95 kDa (PSD-95) and synaptosomal-associated protein of 25 kDa (SNAP-25) were subsequently analyzed using reverse transcription-quantitative PCR and western blotting. In addition, the concentration of acetylcholine (ACh) and the activity of acetylcholinesterase (AChE) were analyzed using spectrophotometry, while the cell apoptotic rate was determined using flow cytometry. The expression of Aβ in the brains of patients with AD was found to be significantly increased, whereas the expression of α7 nAChR was significantly decreased compared with the healthy control group. In vitro, the expression levels of α7 nAChR were significantly increased or decreased following the overexpression or silencing of the gene, respectively. Consistent with these observations, the mRNA and protein expression levels of SYP, PSD-95 and SNAP-25 were also significantly increased following the overexpression of α7 nAChR and decreased following the genetic silencing of the receptor. In untransfected or negative control cells, the expression levels of these factors and the apoptotic rate were significantly reduced following the exposure to AβO, which was found to be attenuated by α7 nAChR overexpression, but potentiated by α7 nAChR RNA silencing. However, no significant differences were observed in either the ACh concentration or AChE activity following transfection. Collectively, these findings suggested that α7 nAChR may protect the brains of patients with AD against Aβ, as α7 nAChR overexpression increased the expression levels of SYP, SNAP-25 and PSD-95, and attenuated the inhibitory effect of Aβ on the expression of these synaptic proteins and cell apoptosis. Overall, this indicated that α7 nAChR may serve an important neuroprotective role in AD.
Keywords: SH-SY5Y cells, α7 nicotinic acetylcholine receptor, β-amyloid peptide, synaptic protein, synaptophysin, apoptotic rate, synaptosomal-associated protein of 25 kDa, postsynaptic density of 95 kDa, Alzheimer's disease
Introduction
Alzheimer's disease (AD) is the most common type of neurodegenerative disorder that affects the older population; it is pathologically characterized by the formation of extracellular senile plaques (SP) and intraneuronal neurofibrillary tangles, which result from the deposition of β-amyloid peptide (Aβ) and the aggregation of hyperphosphorylated tau protein, respectively (1). Aβ, a peptide of 38–43 amino acids, serves an important role in the pathogenesis of AD as it aggregates to form soluble oligomers, fibrils and SPs (2). Previous studies have reported that the soluble Aβ oligomer, amyloid peptide oligomers (AβOs), is more toxic than insoluble fibrils (3,4) and is more closely associated with the progression of AD (4,5). The deposition of Aβ plaques is also associated with cross-sectional synaptic network dysfunction, progressive brain atrophy, neuronal calcium homeostasis (6) and long term cognitive decline (7).
Studies conducted on both postmortem brains of individuals with AD and AD model animals have reported that that the synapses are affected during the early stages of neurodegenerative processes (8,9). The primary function of the synapse is to mediate intracellular communication by releasing neurotransmitters from the presynaptic terminal, as well as to modulate neural plasticity (10,11). Synaptic proteins, including vesicle-associated and pre- and post-synaptic membrane proteins, are closely related to cognitive function, and previous studies have shown that the loss of synapses in the brain tissues of patients with AD was associated with cognitive impairment (12–14).
Synaptophysin (SYP), a specific marker of vesicles that reflects the density and distribution of synapses, serves a crucial role in neural plasticity by influencing the synaptic structure and mediating neurotransmitter release via phosphorylation (15). Synaptosomal-associated protein of 25 kDa (SNAP-25), a component of the complex of soluble N-ethylmaleimides sensitive factor attachment protein receptor proteins, is central to synaptic vesicle exocytosis, a process that regulates intracellular calcium dynamics by negatively modulating neuronal voltage-gated calcium channels (16). Postsynaptic density of 95 kDa (PSD-95), a neuronal PSD-95/Dlg/ZO-1 protein, associates with receptors and cytoskeletal elements at synapses, orchestrates synaptic development and regulates synapse stabilization and plasticity (17). Notably, numerous studies have demonstrated that certain chemicals, such as AβOs, can alter the levels of SYP in SH-SY5Y cells, which may be related to the pathogenesis of AD (18–20).
Previous studies have revealed that cholinergic deficits, such as the modification of acetylcholine (ACh) and cholinesterase (ChE), as well as the downregulated expression of nicotinic ACh receptors (nAChRs), are all significant contributors to cognitive impairment in AD (21,22). ACh is stored in the synaptic vesicles and is exocytosed and released into the synaptic cleft to activate nAChRs; activated nAChRs are highly permeable to Ca2+, which is an important signaling molecule involved in the long-lasting modulatory effects of the receptors (21). There are two forms of ChE, acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). It was previously demonstrated that AChE C-terminal peptides modify the regulation of nAChRs (23), which is consistent with another study that reported treatment with an AChE inhibitor increased nAChR expression levels (24). Neuronal nAChRs, members of the super-family of ligand-gated ion channels, are involved in a number of important physiological functions, such as memory and cognition (25). The loss of these receptors from several regions of brains affected by AD is observed in receptor-ligand binding studies, and this loss is positively correlated with the presence of SPs in the temporal lobe (26). The α (α2-α10) and β (β2-β4) subunits of these receptors combine to form both hetero- and homo-pentameric subtypes, of which α4β2 and α7 nAChRs are the predominant subtypes in human brains (27). Moreover, neuronal nAChRs are expressed pre-, post- and extra-synaptically in the brains and mediate postsynaptic responses (28). Notably, electrophysiological studies have shown that the formation of glutamatergic synapses in the hippocampus is promoted by α7 nAChR (29). It has been suggested that α3 and α7 nAChR may change the neuroinflammatory status by inhibiting NF-κB activation and reducing the production of inflammatory cytokines (30–32).
An early sign of AD is the loss of synapses, which is most strongly correlated with cognitive decline (33). Another characteristic of AD, as demonstrated by positron emission tomography, is a deficit of nAChRs in the brain, which is also closely related to cognitive dysfunction (34). In a previous study conducted in α7 nAChR knockout mice, the number of glutamatergic synapses in the hippocampus was severely reduced, whereas the activation of endogenous α7 nAChR was found to increase this number (29); however, the effects of altered α7 nAChR expression on the synaptic proteins are relatively unknown.
The present study aimed to determine whether the altered expression levels of α7 nAChR in SH-SY5Y cells may regulate the expression levels of SYP, PSD-95 and SNAP-25, in addition to their influence on the neurotoxic effect of Aβ on these synaptic proteins, cell apoptosis, and the levels and activity of ACh and AChE.
Materials and methods
Chemicals and reagents
The anti-α7 nAChR rabbit polyclonal antibody (cat. no. GTX10096), anti-α7 nAChR rabbit polyclonal antibody (cat. no. GTX64511), fluorescent FITC-conjugated anti-rabbit IgG antibody (cat. no. BA1105), fluorescence Cy3-conjugated anti-mouse IgG antibody (cat. no. BA1031), anti-SNAP-25 rabbit monoclonal antibody (cat. no. GTX62566) and the anti-SD-95 rabbit monoclonal antibody (cat. no. GTX61948) were all purchased from GeneTex, Inc. The anti-Aβ mouse polyclonal antibody (cat. no. 803001) was purchased from BioLegend, Inc., and the anti-SYP rabbit monoclonal antibody (cat. no. ab32127) was obtained from Abcam. The horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody (cat. no. 7074) was purchased from Cell Signaling Technology, Inc., while the HRP-conjugated anti-mouse IgG antibody (cat. no. SC-2005) and anti-β-actin mouse monoclonal antibody (cat. no. SC-81178) were obtained from Santa Cruz Biotechnology, Inc.
Control short hairpin RNA (shRNA) plasmid-B (cat. no. SC-108065), α7 nAChR shRNA plasmid (h; cat. no. SC-42532-sh) and GFP shRNA plasmid (A.vic; cat. no. SC-45924-sh) were purchased from Santa Cruz Biotechnology, Inc. E.coli DH5α competent cells purchased from Beijing Solarbio Science and Technology Co., Ltd. The Alexa 488-conjugated goat anti-rabbit IgG antibody, DMEM and FBS were obtained from Thermo Fisher Scientific, Inc. The pcDNA3.1 plasmid, G418 solution, X-treme GENE HP DNA Transfection reagent, First Start Universal SYBR Green Master mix (Rox) and First Strand cDNA Synthesis kit were obtained from Roche Diagnostics GmbH. The Annexin V-APC/7-AAD apoptosis kit was purchased from Multi Sciences, while the Hyper Performance Chemiluminescence film and ECL Plus reagent were obtained from Cytiva. AChE assay kits (cat. no. A024-1-1) and ACh assay kits (cat. no. A105-3) were obtained from Nanjing Jiancheng Bioengineering Institute and the quantitative (q)PCR primer pairs for α7 nAChR, SYP, PSD-95, SNAP-25 and β-actin were designed based on the corresponding gene sequences and synthesized by Shanghai Genecore Bio Technologies Co., Ltd. DMEM and FBS purchased from Thermo Fisher Scientific, Inc. The pcDNA3.1-GFP plasmid was kindly donated by Professor Jian-Jiang Zhou of Guizhou Medical University (Guizhou, China), while Aβ1-42, puromycin, 1,1,1,3,3,3- hexafluoro-2-propanol (HFIP), goat serum, DMSO and the other general chemicals used were purchased from Sigma-Aldrich; Merck KGaA.
Patient samples
The study was approved by the Ethics Committee of the Affiliated Hospital of Guizhou Medical University. All the brain tissue blocks had been donated voluntarily by the patient or with the consent of the family. Post-mortem brain samples from the Netherlands Brain Bank were obtained and characterized according to the specific clinical and neuropathological criteria (35). According to their medical history, clinical manifestations and laboratory tests, the donor was diagnosed as ‘probable AD’ by excluding other possible causes of dementia, such as stroke. The clinical diagnosis was performed according to the National Institute of Neurological and Communication Disorders and Stroke and the Association of Alzheimer's Disease and Related Diseases criteria (36), and the severity of dementia was assessed according to the Global Deterioration Scale (37). The control brain samples had no known history or symptoms of any neurological or psychiatric disorder.
The temporal cortex, frontal cortex and hippocampus of nine patients with AD (men, 3; women, 6; age range, 70–100 years) and nine controls (men, 4; women, 5; age range, 65–100 years) were studied; the mean age at mortality for the patients with AD was 81.5±7.1 years, while the mean age at mortality of the controls was 79.4±9.2 years. The interval between mortality and autopsy in the patients with AD and the controls was 5.1±1.0 and 8.0±3.4 h, respectively.
AβO preparation
Synthetic human Aβ1-42 was suspended in pre-cooled HFIP at a final concentration of 1 mM; the solution was incubated at room temperature for 60 min and subsequently on ice for 10 min (38). Aliquots were transferred into non-siliconized microcentrifuge tubes and the HFIP was allowed to evaporate overnight in a hood at room temperature, which was then stored at −80°C (38). Prior to the treatment of cells, Aβ1-42 was dissolved in DMSO to obtain a final concentration of 5 mmol/l. For the preparation of oligomers, the solution was subsequently diluted with modified DMEM and incubated for 24 h at 4°C.
Double-labelling immunofluorescence
The co-localization of α7 nAChR and Aβ was analyzed using double staining. Human brain tissue wax blocks were provided by the Netherlands Brain Bank, and were cut into 6-µm sections. The sections were heated at 58°C for 1 h, placed in xylene I, xylene II, xylene III (15 min each) and 100% ethanol (10 min), 90% ethanol (10 min), 70% ethanol (10 min), deparaffinized and hydrated. After three washes in PBS and microwaving in 0.01 M citric acid buffer (pH 6.0) at 70°C for 20 min for the antigen repair, sections were subsequently blocked with goat serum for 1 h at 37°C prior to incubation with primary antibodies against α7 nAChR (1:100) and Aβ (1:100) overnight at 4°C. Following the primary antibody incubation, the sections were washed three times in PBS and incubated with either a FITC-conjugated goat anti-mouse IgG antibody (1:100) or a Alexa 488-conjugated goat anti-rabbit IgG antibody (1:100) for 1 h at room temperature. The sections were subsequently rinsed three times in PBS and mounted with VECTASHIELD (Vector Laboratories, Inc.).
The immunofluorescence procedure was performed by an independent neuropathologist and the analysis of the results were performed by another researcher. The brain regions analyzed included the hippocampus, temporal lobe and frontal lobe. The integrated optical density (IOD) of immunofluorescence staining for α7 nAChR and Aβ was determined by counting randomly selected nine fields of vision using a fluorescent microscope (magnification, ×200 or ×400).
Cell culture and treatments
SH-SY5Y cells, derived from human neuroblastoma cells, were purchased from the German Collection of Microorganisms and Cell Cultures GmbH. Cells were cultured in DMEM, supplemented with 10% FBS, 100 mg/ml streptomycin and 100 U/ml penicillin, and maintained in a humidified atmosphere containing 5% CO2 at 37°C. To maintain their neuronal characteristics, the cells were treated following ≤3 passages with 1 mM AβO at 37°C for 24 h, in combination with the knockdown or overexpression of α7 nAChR.
Construction of the pcDNA3.1-α7 nAChR expression plasmid
Total RNA was extracted from the SH-SY5Y cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. A total of 1 µg RNA was reverse transcribed into cDNA using a First Strand cDNA synthesis kit. The following primers pairs were used for reverse transcription (RT-)qPCR, which were designed according to the GenBank sequence no. BC-037571: α7 nAChR forward, 5′-CCCAAGCTTATGCAGGAGGCAGATATCAGTGGC-3′ and reverse, 5′-CGCGGATCCTTACGCAAAGTCTTTGGACACG-3′. The final PCR product obtained was 966-bp long. The final PCR products were subsequently separated by 1% agarose gel electrophoresis and the 966-bp fragment was purified using an agarose gel extraction kit. This fragment was then ligated into the pcDNA3.1 plasmid to create the recombinant pcDNA3.1-α7 nAChR plasmid, which was amplified in competent E. coli DH5a cells. The pcDNA3.1-α7 nAChR plasmid was subsequently purified, digested with HindIII (1 µl) and BamHI (1 µl) at 37°C for 1 h in a water bath, visualized with ethidium bromide and detected using agarose gel electrophoresis. The plasmid was sequenced by Sangon Biotech Co., Ltd. to validate its identity.
Transfection of the pcDNA3.1-α7 nAChR or α7 nAChR shRNA plasmid (h) into SH-SY5Y cells and selection of stable clones
SH-SY5Y cells, maintained and cultured aforementioned, were stably transfected with the pcDNA3.1-α7 nAChR, empty pcDNA 3.1, pcDNA 3.1-GFP, control shRNA, α7 nAChR shRNA plasmid (h) or GFP shRNA plasmid using X-treme GENE HP DNA Transfection reagent, according to the manufacturer's protocol. The pcDNA 3.1 and control shRNA plasmid-B, which do not target any endogenous transcript, were used as negative controls, and pcDNA 3.1-GFP and GFP shRNA plasmids served as the controls for the efficiency of transfection.
After 24 h of transfection incubation at 37°C, 1×103 cells/ml transfected with pcDNA 3.1-α7 nAChR (1.5 mg/ml) and pcDNA 3.1 (1.5 mg/ml) were selected for 800 µg/ml G418 administration for 14 days at 37°C, while cells transfected with control shRNA plasmid-B or α7 nAChR shRNA plasmid (h) were selected for 4 µg/ml puromycin administration 14 days at 37°C. When resistant cell clones appeared, individual clones were collected and re-cultured to 60–80% confluence in successive passages until cells demonstrated the overexpression or genetic silencing of α7 nAChR. Subsequent experimentations were performed after 24 h.
Analysis of mRNA encoding the α7 nAChR subunit, SYP, PSD-95 and SNAP-25 using RT-qPCR
The mRNA expression levels of the α7 nAChR subunit, SYP, PSD-95, SNAP-25 and β-actin were determined using RT-qPCR, according to the manufacturer's instructions of each kit or reagent. Cells were collected for RNA extraction when the cell density reached 80%. In brief, 1 µg total RNA was extracted from the SH-SY5Y cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and the reverse transcription of RNA to cDNA was performed using a First Strand cDNA synthesis kit at 42°C for 60 min, 70°C for 5 min and 4°C for 10 min. qPCR was subsequently performed using an ABI Step One Plus system and First Start Universal SYBR Green Master (ROX), with the following thermocycling conditions: Initial denaturation at 95°C 10 min, followed by 40 cycles at 95°C for 15 sec, annealing temperature for 60 sec and 72°C for 60 sec. The primer pairs for α7 nAChR, SYP, PSD-95, SNAP-25 or β-actin are presented in Table I. Expression levels were determined using the 2−ΔΔCq method (39). The experiment was repeated nine times independently and were quantified using SDS v1.4 software (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Table I.
Primers used for determining the level of mRNA encoding α7nAChR, SYP, PSD-95, SNAP-25 or β-actin by reverse transcription-quantitative PCR.
| Gene | Primer sequence | Amplicon size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| α7nAChR | 5′ACCACTCACCGTCTACTTCTCC 3′ | 167 | 58 |
| 5′CATCTGGGAAACGAACAGTCTT 3′ | |||
| SYP | 5′GCTGTCAGATGTGAAGATGGC 3′ | 192 | 52 |
| 5′CCTGTCTCCTTAAACACGAACC 3′ | |||
| PSD-95 | 5′ CTCAGGGTCAACGACAGCAT 3′ | 114 | 58 |
| 5′ GACATAGAGGCGAACGATGG 3′ | |||
| SNAP-25 | 5′ GGATATGGGCAATGAGATCG 3′ | 115 | 58 |
| 5′ CAGCATCTTTGTTGCACGTT 3′ | |||
| β-actin | 5′TGGCACCACACCTTCTACAATG3′ | 103 | 58 |
| 5′TCATCTTCTCGCGGTTGGC 3′ |
Analysis of the α7 nAChR subunit, SYP, PSD-95 and SNAP-25 expression levels using western blotting
The protein expression levels of α7 nAChR, SYP, PSD-95 and SNAP-25 in cell lysates were quantified using western blotting. Cells were washed three times with pre-chilled PBS in a 6-well plate. Then, cells were incubated on ice for 3 h with 60 µl/well RIPA buffer containing protease and phosphatase inhibitors (cat. no. P0013B; Beyotime Institute of Biotechnology). The supernatant was collected after centrifugation at 4°C for 15 min at 4,024.8 × g, and the concentration of the supernatant protein was assessed with a Bradford Protein Assay kit (cat. no. P0006; Beyotime Institute of Biotechnology), according to the manufacturer's instructions. Proteins were loaded (12 µg/lane)and proteins were separated by 10% SDS-PAGE. The separated proteins were transferred to PVDF membranes and blocked with 5% fat-free dry milk at room temperature for 2 h. The membranes were incubated with primary antibodies against α7 nAChR (1:200), SYP(1:500), PSD-95 (1:500) and SNAP-25 (1:200) at 4°C overnight. Following the primary antibody incubation, Membranes were washed three times with TBS buffer with 0.1% Tween-20 and subsequently incubated with a HRP-conjugated secondary antibody [1:5,000 (anti-mouse) or 1:2,000 (anti-rabbit)] at room temperature for 1 h. Protein bands were detected with an ECL kit, according to the manufacturer's instructions. After stripping these antibodies with buffer containing detergent (cat. no. P0025; Beyotime Institute of Biotechnology), the membranes were probed again with antibodies against β-actin (1:6,000) at 37°C for 2 h and then incubated with a HRP-conjugated anti-mouse IgG secondary antibody 37°C for 60 min. Expression levels of the target proteins were normalized to β-actin and the resulting ratio was presented as a percentage of the corresponding control value. Semi-quantification was performed using ImageJ software (v1.46; National Institutes of Health).
Flow cytometric analysis of apoptosis
Cell apoptosis was determined using an Annexin V-APC/7-AAD apoptosis kit, according to the manufacturer's protocol. Cells were harvested and washed three times with pre-cooled PBS. Following centrifugation at 400.8 × g at room temperature for 5 min, cells were resuspended in 500 µl binding buffer containing 5 µl Annexin V-APC and 10 µl 7-ADD and incubated in the dark at room temperature for 5 min. Apoptotic cells were analyzed using a BD FACSVerse flow cytometer (BD Biosciences) and FlowJo software (FlowJo LLC; v.10.6.2). Results are presented as the percentage of early + late apoptotic cells.
Analysis of the concentration of ACh and the activity of AChE in cell supernatant using spectrophotometry
The concentration of ACh and activity of AChE were measured according to manufacturer's protocol of the AChE assay kits and ACh assay kits. The substrate solution was reacted with ACh at room temperature for 15 min to produce a brown compound. ACh was synthesized by choline and acetyl-CoA under the catalytic action of choline acetyl translocase (choline acetylase). The absorbance OD value was measured at 550 nm, with the depth of color being proportional to the concentration of ACh.
AChE is mainly located in the synaptic space of cholinergic nerve terminals and is released under biological stimulation (40). AChE hydrolyzed ACh to cholic acid and acetic acid, and subsequently choline was reacted with mercapto color reagent to produce a 5-thio-2-nitro-benzoic acid anion, which produced a yellow color. The catalytic activity of AChE was measured at a wavelength of 412 nm.
Statistical analysis
Statistical analysis was performed using SPSS v22.0 software (IBM Corp.) and data are presented as the mean ± standard deviation. Pearson correlation analysis was used to determine the correlation, statistical differences between two groups were determined using a two-tail Student's t-test and statistical differences between multiple groups were determined using a one-way ANOVA followed by a Student-Newman-Keuls or Tukey's post hoc test (n=9). P<0.05 was considered to indicate a statistically significant difference.
Results
Expression levels of α7 nAChR and Aβ in the brains of patients with AD and healthy brains
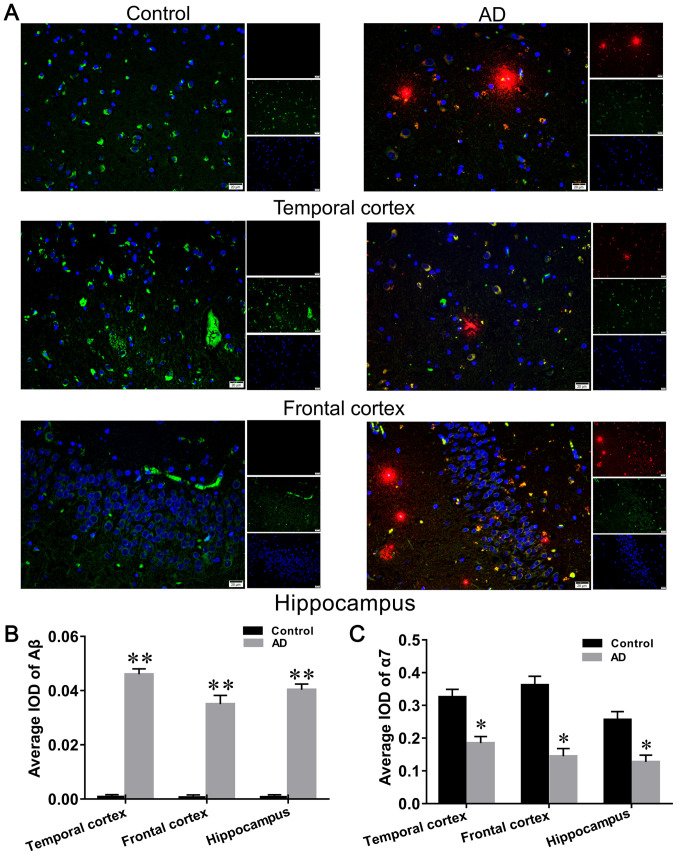
Aβ was observed to be expressed mainly in the cytoplasmic and extracellular forms of both hippocampal and cortical neurons (Fig. 1A), and was found to be expressed at high levels in the brain of patients with AD compared with the controls (Fig. 1B). α7nAChR was observed to be mainly expressed in the cytoplasm of hippocampal and cortical nerves (Fig. 1A), and its distribution in the brain of patients with AD was significantly decreased compared with the healthy control group (Fig. 1C).
Figure 1.
Expression levels of α7 nAChR and Aβ in the brains of patients with AD and normal brains. (A) Images of the immunofluorescence double staining in the control and AD groups. (B) Aβ is expressed in the cytoplasm and extracellular matrix of cells. It was also found highly expressed in the brain slices of patients with AD, whereas the expression levels were minimal in healthy control brain sections following immunofluorescence double staining. (C) α7 nAChR was found expressed in the cytoplasm and reduced expression levels were observed in brain sections of patients with AD compared with healthy human brain sections, following immunofluorescence double staining. The cell nuclei are stained in blue using DAPI. Aβ-positive neurons are indicated in red and α3 nAChR-positive neurons are green. Scale bar, 20 µm. Data are presented as the mean ± standard deviation (n=9). *P<0.05 and **P<0.01 vs. control group. α7 nAChR, nicotinic acetylcholine receptor α7 subunit; Aβ, β-amyloid; AD, Alzheimer's disease; IOD, integrated optical density.
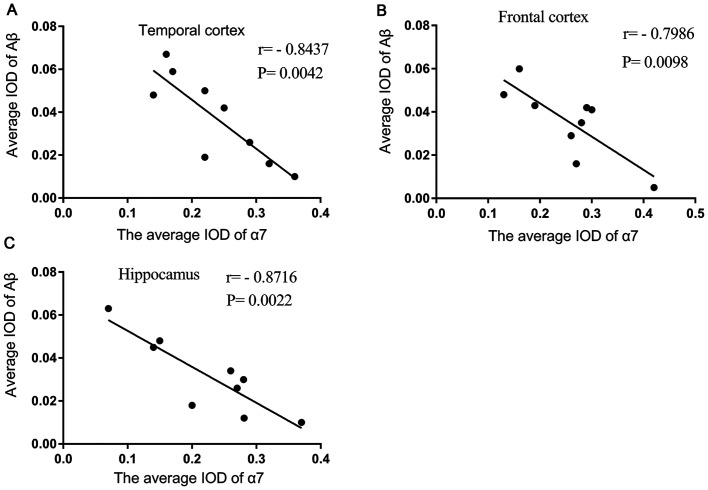
Mean IOD of α7nAChR is negatively correlated with the mean IOD of Aβ in the brains of patients with AD
The mean IOD of α7 nAChR and Aβ observed by immunofluorescence double staining were demonstrated to be strongly negatively correlated with each other in the temporal cortex (Fig. 2A), frontal cortex (Fig. 2B) and hippocampus (Fig. 2C) of brain sections of patients with AD.
Figure 2.
Mean IOD of α7 nAChR is negatively correlated with the mean IOD of Aβ in the brain of patients with AD. Mean IOD of α7 nAChR and Aβ were found to be negatively correlated with each other in the (A) temporal cortex, (B) frontal cortex and (C) hippocampus following immunofluorescence double staining of brain slices of patients with AD. Data was obtained using correlation analysis (n=9). P<0.05 α7 nAChR vs. Aβ. IOD, integrated optical density; α7 nAChR, nicotinic acetylcholine receptor α7 subunit; Aβ, β-amyloid; AD, Alzheimer's disease.
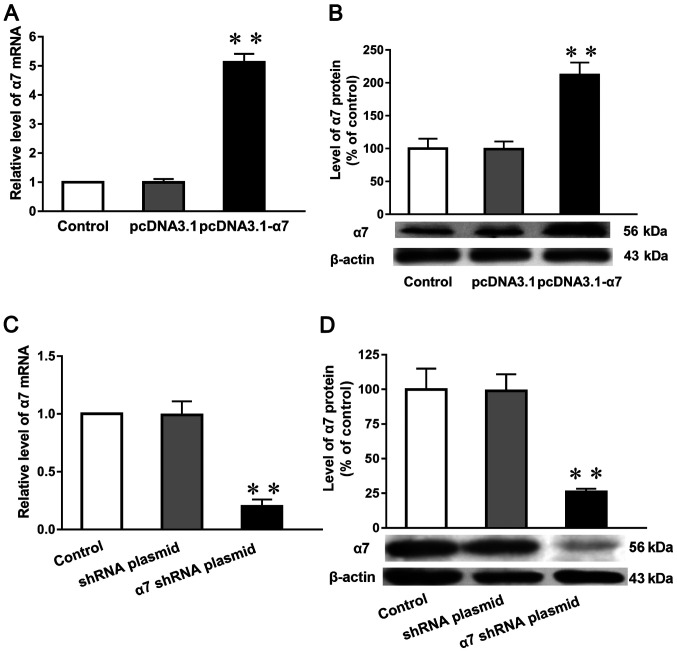
Expression levels of α7 nAChR mRNA and protein in SH-SY5Y cells in which α7 nAChR is overexpressed or silenced
Compared with both untransfected cells (normal control) and those transfected with the empty plasmid (negative control), the mRNA and protein expression levels of the α7 nAChR subunit (Fig. 3A and B) in cells overexpressing the receptor were increased by 433 and 110%, respectively, in SH-SY5Y cells. Moreover the mRNA and protein expression levels were reduced by 95% (Fig. 3C) and 80% (Fig. 3D), respectively, following the genetic silencing of α7 nAChR.
Figure 3.
Expression levels of α7 nAChR mRNA and protein in SH-SY5Y cells in which α7 nAChR is overexpressed or silenced. (A) mRNA and (B) protein expression levels of α7 nAChR in SH-SY5Y cells overexpressing α7 nAChR. (C) mRNA and (D) protein expression levels of α7 nAChR in SH-SY5Y cells with silenced α7 nAChR expression. β-actin was used as an internal standard. Expression levels were determined using reverse transcription-quantitative PCR and western blotting. Data are presented as the mean ± standard deviation (n=9).**P<0.01 vs. control. α7 nAChR, nicotinic acetylcholine receptor α7 subunit; shRNA, short hairpin RNA.
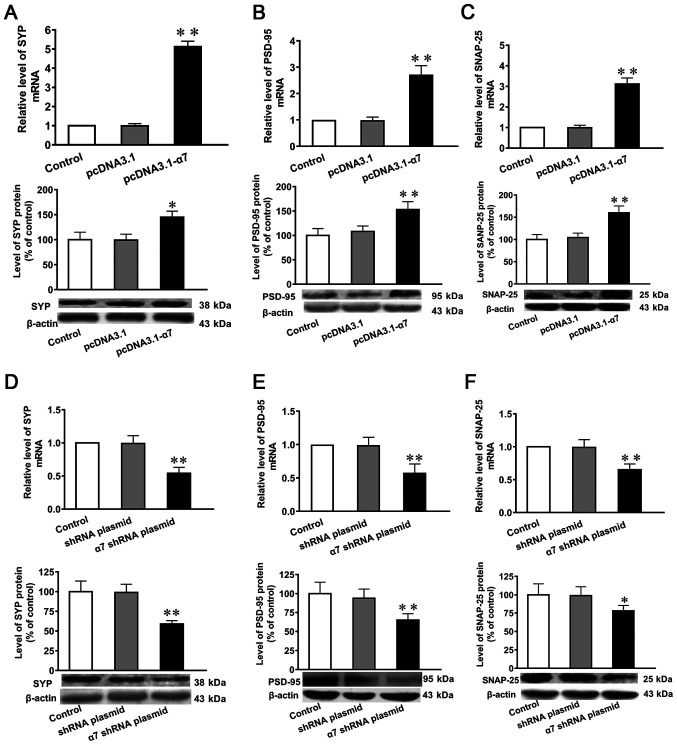
mRNA and protein expression levels of SYP, PSD-95 and SNAP-25 in SH-SY5Y cells in which α7 nAChR is overexpressed or silenced
Compared with untransfected controls and cells transfected with the empty plasmid, the mRNA and protein expression levels of SYP, PSD-95 and SNAP-25 were significantly increased in SH-SY5Y cells overexpressing α7 nAChR (Fig. 4A-C). Furthermore, the mRNA and protein expression levels of SYP, PSD-95 and SNAP-25 were significantly decreased in SH-SY5Y cells in which α7 nAChR was silenced (Fig. 4D-F).
Figure 4.
Expression levels of SYP, PSD-95 and SNAP-25 in SH-SY5Y cells overexpressing α7 nAChR or with silenced α7 nAChR expression. Expression levels of mRNA and protein were determined using reverse transcription-quantitative PCR and western blotting, respectively. mRNA and protein expression levels of (A) SYP, (B) PSD-95 and (C) SNAP-25 after α7 nAChR overexpression. mRNA and protein expression levels of (D) SYP, (E) PSD-95 and (F) SNAP-25 after α7 nAChR silencing. Data are presented as the mean ± standard deviation (n=9). *P<0.05 and **P<0.01 vs. control. SYP, synaptophysin; PSD-95, postsynaptic density of 95 kDa; SNAP-25, synaptosomal-associated protein of 25 kDa; α7 nAChR, nicotinic acetylcholine receptor α7 subunit; shRNA, short hairpin RNA.
Concentration of ACh and the activity of AChE in SH-SY5Y cells in which the expression of α7 nAChR is overexpressed or silenced
No significant differences were observed in the concentration of ACh or the activity of AChE in SH-SY5Y cells with overexpressed or silenced α7 nAChR expression compared with the control cells (Table II).
Table II.
Concentration of ACh and the activity of AChE in SH-SY5Y cells in which expression of α7 nAChR was overexpressed or silenced.
| Group | ACh concentration (µg/ml) | AChE activity (U/ml) |
|---|---|---|
| Control | 25.32±1.82 | 0.79±0.18 |
| pcDNA 3.1 | 25.75±2.07 | 0.85±0.23 |
| α7 nAChR-pcDNA 3.1 | 27.72±4.02 | 0.71±0.20 |
| Control shRNA plasmid B | 26.59±3.82 | 0.74±0.16 |
| α7 nAChR shRNA plasmid B | 29.97±2.78 | 0.82±0.22 |
ACh, acetylcholine; AChE, acetylcholinesterase; α7 nAChR, nicotinic acetylcholine receptor α7 subunit; shRNA, short hairpin RNA.
mRNA and protein expression levels of SYP, PSD-95 and SNAP-25 in SH-SY5Y cells in which α7 nAChR is overexpressed or silenced and exposed to AβO
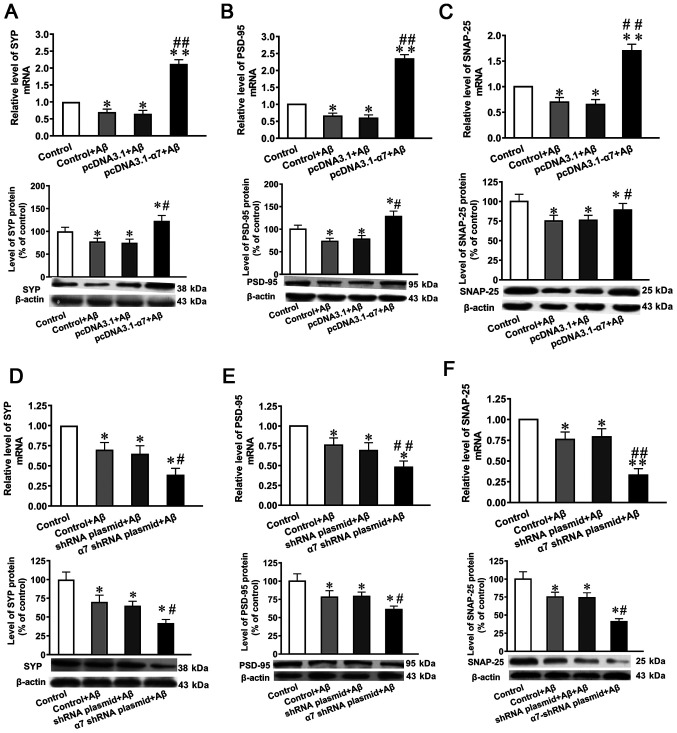
Compared with the untransfected control or negative control, the mRNA and protein expression levels of SYP, PSD-95 and SNAP-25 were significantly decreased following the exposure to AβO compared with the control group (Fig. 5A-F). In addition, the overexpression of α7 nAChR attenuated the toxicity of AβO (Fig. 5A-C), while α7 nAChR silencing potentiated the toxic effects induced by AβO (Fig. 5D-F).
Figure 5.
Expression levels of SYP, PSD-95 and SNAP-25 in SH-SY5Y cells following the overexpression of α7 nAChR or silencing of α7 nAChR, and exposure to AβO. mRNA and protein expression levels of (A) SYP, (B) PSD-95 and (C) SNAP-25 after α7 nAChR overexpression. mRNA and protein expression levels of (D) SYP, (E) PSD-95 and (F) SNAP-25 after α7 nAChR silencing. The expression levels of mRNA and protein were determined using reverse transcription-quantitative PCR and western blotting, respectively. Data are presented as the mean ± standard deviation (n=9). *P<0.05 and **P<0.01 vs. control; ##P<0.01, #P<0.05 vs. empty plasmid. SYP, synaptophysin; PSD-95, postsynaptic density of 95 kDa; SNAP-25, synaptosomal-associated protein of 25 kDa; α7 nAChR, nicotinic acetylcholine receptor α7 subunit; AβO, Aβ1-42 oligomer; shRNA, short hairpin RNA.
Apoptotic rate of SH-SY5Y cells in which the expression of α7 nAChR is overexpressed or silenced and simultaneously exposed to AβO
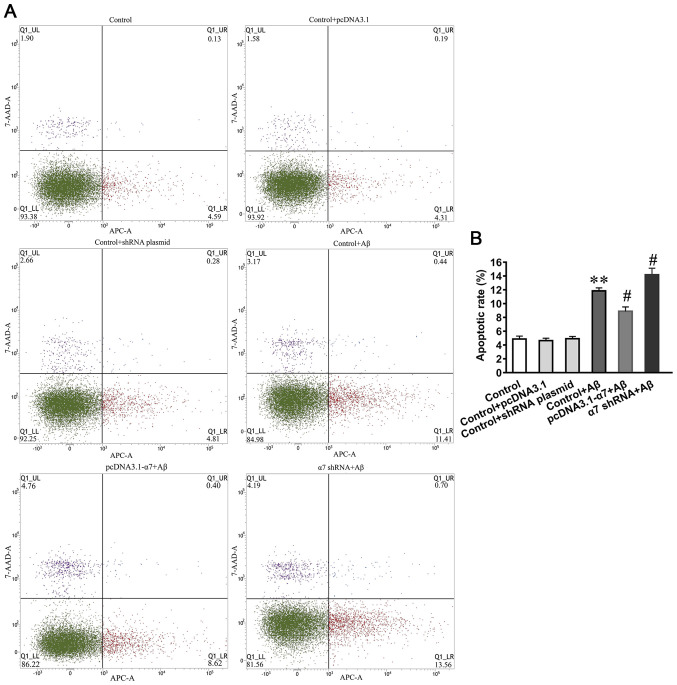
To investigate the neurotoxic effects of AβO on cell apoptosis and the neuroprotective function of α7 nAChR, the apoptotic rate was determined using an Annexin V-APC/7AAD apoptosis kit and flow cytometry (Fig. 6A). The results demonstrated that the apoptotic rates were significantly increased in SH-SY5Y cells treated with AβO, and that the overexpression of α7 nAChR attenuated the AβO-induced apoptotic rate, whereas α7 nAChR silencing increased the AβO-induced apoptotic rate (Fig. 6B).
Figure 6.
Apoptotic rate of SH-SY5Y cells, which are simultaneously exposed to α7 nAChR overexpression or gene silencing and AβO. (A) Apoptotic rate was determined using flow cytometry and (B) data are presented as the mean ± standard deviation (n=9). **P<0.01 vs. control; #P<0.05 vs. control + AβO. α7 nAChR, nicotinic acetylcholine receptor α7 subunit; AβO, Aβ1-42 oligomer; shRNA, short hairpin RNA.
Discussion
The present study successfully established cell lines stably transfected with either the pcDNA3.1-α7 nAChR or α7 nAChR shRNA plasmid, and subsequently demonstrated that the mRNA and protein expression levels of α7 nAChR were significantly increased and decreased, respectively. In the present study, the levels of upregulation and downregulation of α7 nAChR were not consistent. Since not all post-transcriptional products are translated into proteins, the increase of mRNA is not necessarily synchronized with the expression of proteins. Under different conditions of up- and downregulation, the efficiency of transcription and translation differs.
Previous studies have shown that alterations in nicotinic cholinergic mechanisms contribute to brain dysfunction (41). ACh is degraded by AChE to choline at cholinergic synapses to modulate synaptic transmission, and choline is suggested to be a full agonist of α7 nAChR (42). A previous study revealed that an α7 nAChR agonist increased the release of ACh transiently (43). In the present study, the effects of the altered expression levels of α7 nAChR on the concentration of ACh and the activity of AChE was investigated in SH-SY5Y cells. The findings indicated that there were no significant differences in either ACh concentration or AChE activity in the treated cells. The possible reason for the emergence of this result is that α-7nAChR can increase the release of ACh and affect the activity of AChE under short-term conditions; however, under the conditions of the present study, this change was not observed. The present results suggested that changes in the expression levels of α7 nAChR in SH-SY5Y cells may not affect ACh concentrations and AChE activity, which requires further investigation.
Numerous studies have reported that α7 nAChR served an important role in cognitive processes in brains of patients with AD, which may be related to the inhibition of Aβ toxicity and modulation of synaptic transmission and plasticity (44–46).
The findings of the present study suggested that the expression levels of Aβ in the brains of patients with AD were increased compared with the healthy control group, and the expression levels of α7 nAChR in brain sections of patients with AD were decreased. Immunofluorescence double staining identified that the expression levels of Aβ were decreased in the brain regions exhibiting higher expression levels of α7 nAChR; a negative correlation was found between α7 nAChR and Aβ in the temporal lobe, frontal lobe and hippocampus of patients with AD, which suggested that α7 nAChR may have a neuroprotective effect in the brains of patients with AD. Subsequently, the neuroprotective effect of α7 nAChR in SH-SY5Y was investigated. It was speculated that the correlation may be due to the neuronal loss induced by Aβ by Aβ itself. However, due to the lack of human brain tissue specimens, the present study did not perform hematoxylin-eosin staining.
In the present study, mRNA and protein expression levels of SYP, SNAP-25 and PSD-95 were significantly increased in the neuronal cells overexpressing α7 nAChR, while decreased expression levels were observed following α7 nAChR RNA silencing. These results suggested that α7 nAChR may be involved in regulating the expression of these proteins, in addition to other synaptic proteins, which would establish a link to synaptic stabilization and plasticity. It has been revealed that SYP levels in the superior temporal and inferior parietal cortex are ~5% lower in patients with severe AD compared with individuals with no cognitive impairment (47). Our previous study reported significantly decreased expression levels of SYP protein and mRNA in the brains of patients with AD, mice carrying the amyloid protein precursor (APP)SWE mutation and rats injected with Aβ1-42 (48). Furthermore, the expression levels of PSD-95 and SNAP-25 are decreased in the cerebral cortex of 3×Tg-AD mice (49). The findings of the present study also indicated that α7 nAChR may provide a link to synaptic stabilization, plasticity and transmission in the nervous system, which may be related to the protective function of this receptor against cognitive decline during the pathogenesis of AD. However, α7 nAChR may regulate synaptic plasticity by regulating the level of calcium ions, which will be the subject of future studies.
Aβ exerts its synaptotoxicity by suppressing long-term potentiation, impairing synaptic plasticity and inducing neuronal dysfunction (50). The synaptotoxic effect of Aβ42 peptides was suggested to be one of the most important aspects of the pathogenic process resulting in AD (51), and significantly reduced expression levels of SYP, PSD-95 and SNAP-25 have been revealed in the brains of mice influenced by Aβ (52–54). Moreover, the present study detected significant reductions in the expression levels of SYP, PSD-95 and SNAP-25 in SH-SY5Y cells exposed to AβO, which may reflect the synaptotoxic effects induced by Aβ.
In the present study, the inhibitory effects of Aβ on the expression levels of SYP, PSD-95 and SNAP-25 were significantly attenuated following the increased expression levels of α7 nAChR and potentiated following α7 nAChR RNA silencing. These observations indicated that α7 nAChR may also prevent the loss of synaptic proteins caused by Aβ in vivo. It has been previously reported that the inhibition of the expression levels of α7 nAChR in SH-SY5Y cells by RNA interference increases the toxicity and expression levels of Aβ, which subsequently elevates the expression levels of β-site APP-cleaving enzyme 1 (BACE1) and decreases the expression levels of BACE2 (38,55). In the present study, the expression levels of synaptic proteins were reduced to a greater extent by Aβ in the presence of α7 nAChR silencing, which may be related to the reduced production of Aβ caused by α7 nAChR. In addition, it was found that the treatment of SH-SY5Y cells with AβO significantly increased the apoptotic rate, while the proliferation of the cells was poor (data not shown), which was attenuated by α7 nAChR overexpression and potentiated by α7 nAChR silencing. These findings suggested that α7 nAChR may effectively decrease AβO-induced neuronal apoptosis, which may be connected to the increased expression of synaptic proteins.
In conclusion, the expression levels of Aβ in the brain of patients with AD were significantly increased compared with the healthy control group, whereas the expression levels of α7 nAChR in the brain of patients with AD were significantly decreased compared with the healthy control group. Furthermore, it was demonstrated that the expression levels of α7 nAChR and Aβ were negatively correlated with each other in the brain of patients with AD. The mRNA and protein expression levels of SYP, SNAP-25 and PSD-95 were all significantly increased following the overexpression of α7 nAChR, but were reduced following RNA silencing of this receptor. In un-transfected or negative control cells, the expression levels of these factors and the apoptotic rate were significantly reduced following the exposure to AβO, which was found to be attenuated by α7 nAChR overexpression, but potentiated by α7 nAChR RNA silencing. Overall, these findings suggested that α7 nAChR may serve an important neuroprotective role related to the pathogenesis of AD and it may be useful for future drug developments in AD.
Acknowledgements
The authors would like to thank the Netherlands Brain Bank for providing the brain tissue samples.
Funding
This work was supported by grants from the Chinese National Natural Science Foundation (grant no. 81860207), the Program for Changjiang Scholars and Innovative Research Team in University (grant no. IRT13058), the Guizhou Foundation [grant. nos. (2014)06 and 2014(6008)], The Guizhou Science and Technology Foundation [grant no. (2019)1437] and the Guizhou Province Graduate Education Innovation Program Foundation [grant nos. YJSCXJH(2018)040 and YJSCXJH(2019)070].
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request
Authors' contributions
XLQ and JMR designed the study, JMR wrote and revised the manuscript. JMR and SLZ analyzed the data and revised the manuscript. SLZ, XLW and ZZG performed the experiments. ZZG helped perform the analysis of the data and provided constructive discussions. All authors read and approved the final manuscript
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Affiliated Hospital of Guizhou Medical University. All the brain tissue blocks had been donated voluntarily by the patient or with the consent of the family.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bloom GS. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 2.Amemori T, Jendelova P, Ruzicka J, Urdzikova LM, Sykova E. Alzheimer's disease: Mechanism and approach to cell therapy. Int J Mol Sci. 2015;16:26417–26451. doi: 10.3390/ijms161125961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreira ST, Lourenco MV, Oliveira MM, De Felice FG. Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer's disease. Front Cell Neurosci. 2015;9:191. doi: 10.3389/fncel.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viola KL, Klein WL. Amyloid β oligomers in Alzheimer's disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015;129:183–206. doi: 10.1007/s00401-015-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesné SE, Sherman MA, Grant M, Kuskowski M, Schneider JA, Bennett DA, Ashe KH. Brain amyloid-β oligomers in ageing and Alzheimer's disease. Brain. 2013;136:1383–1398. doi: 10.1093/brain/awt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magi S, Castaldo P, Macrì ML, Maiolino M, Matteucci A, Bastioli G, Gratteri S, Amoroso S, Lariccia V. Intracellular calcium dysregulation: Implications for Alzheimer's disease. BioMed Res Int. 2016;2016:6701324. doi: 10.1155/2016/6701324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jagust W. Is amyloid-β harmful to the brain? Insights from human imaging studies. Brain. 2016;139:23–30. doi: 10.1093/brain/awv326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overk CR, Masliah E. Pathogenesis of synaptic degeneration in Alzheimer's disease and Lewy body disease. Biochem Pharmacol. 2014;88:508–516. doi: 10.1016/j.bcp.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheng M, Sabatini BL, Südhof TC. Synapses and Alzheimer's disease. Csh Perspect Biol. 4 doi: 10.1101/cshperspect.a005777. doi.org/10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrill JA, Chen H, Streifel KM, Yang D, Mundy WR, Lein PJ. Ontogeny of biochemical, morphological and functional parameters of synaptogenesis in primary cultures of rat hippocampal and cortical neurons. Mol Brain. 2015;8:10. doi: 10.1186/s13041-015-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Morales V, Montero F, González-Forero D, Rodríguez-Bey G, Gómez-Pérez L, Medialdea-Wandossell MJ, Domínguez-Vías G, García-Verdugo JM, Moreno-López B. Membrane-derived phospholipids control synaptic neurotransmission and plasticity. PLoS Biol. 2015;13:e1002153. doi: 10.1371/journal.pbio.1002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang DB, Kinoshita Y, Kinoshita C, Uo T, Sopher BL, Cudaback E, Keene CD, Bilousova T, Gylys K, Case A, et al. Loss of endophilin-B1 exacerbates Alzheimer's disease pathology. Brain. 2015;138:2005–2019. doi: 10.1093/brain/awv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcello E, Epis R, Saraceno C, Di Luca M. Synaptic dysfunction in Alzheimer's disease. Adv Exp Med Biol. 2012;970:573–601. doi: 10.1007/978-3-7091-0932-8_25. [DOI] [PubMed] [Google Scholar]
- 14.Wang DB, Kinoshita Y, Kinoshita C, Uo T, Sopher BL, Cudaback E, Keene CD, Bilousova T, Gylys K, Case A, et al. Loss of endophilin-B1 exacerbates Alzheimer's disease pathology. Brain. 2015;138:2005–2019. doi: 10.1093/brain/awv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sivanesan S, Tan A, Rajadas J. Pathogenesis of Abeta oligomers in synaptic failure. Curr Alzheimer Res. 2013;10:316–323. doi: 10.2174/1567205011310030011. [DOI] [PubMed] [Google Scholar]
- 16.Antonucci F, Corradini I, Fossati G, Tomasoni R, Menna E, Matteoli M. SNAP-25, a known presynaptic protein with emerging postsynaptic functions. Front Synaptic Neurosci. 2016;8:7. doi: 10.3389/fnsyn.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Yuan J, Pang J, Ma J, Han B, Geng Y, Shen L, Wang H, Ma Q, Wang Y, Wang M. Effects of chronic stress on cognition in male SAMP8 mice. Cell Physiol Biochem. 2016;39:1078–1086. doi: 10.1159/000447816. [DOI] [PubMed] [Google Scholar]
- 18.Xi YD, Zhang DD, Ding J, Yu HL, Yuan LH, Ma WW, Han J, Xiao R. Genistein inhibits Aβ25-35-induced synaptic toxicity and regulates CaMKII/CREB pathway in SH-SY5Y cells. Cell Mol Neurobiol. 2016;36:1151–1159. doi: 10.1007/s10571-015-0311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito S, Ménard M, Atkinson T, Brown L, Whitfield J, Chakravarthy B. Relative expression of the p75 neurotrophin receptor, tyrosine receptor kinase A, and insulin receptor in SH-SY5Y neuroblastoma cells and hippocampi from Alzheimer's disease patients. Neurochem Int. 2016;101:22–29. doi: 10.1016/j.neuint.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Gray NE, Zweig JA, Kawamoto C, Quinn JF, Copenhaver PF. STX, a novel membrane estrogen receptor ligand, protects against amyloid-β toxicity. J Alzheimers Dis. 2016;51:391–403. doi: 10.3233/JAD-150756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer's disease: Targeting the cholinergic system. Curr Neuropharmacol. 2016;14:101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park D, Choi EK, Cho TH, Joo SS, Kim YB. Human neural stem cells encoding ChAT gene restore cognitive function via acetylcholine synthesis, Aβ elimination, and neuroregeneration in APPswe/PS1dE9 mice. Int J Mol Sci. 2020;21:21. doi: 10.3390/ijms21113958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badin AS, Eraifej J, Greenfield S. High-resolution spatio-temporal bioactivity of a novel peptide revealed by optical imaging in rat orbitofrontal cortex in vitro: Possible implications for neurodegenerative diseases. Neuropharmacology. 2013;73:10–18. doi: 10.1016/j.neuropharm.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Galimberti D, Scarpini E. Old and new acetylcholinesterase inhibitors for Alzheimer's disease. Expert Opin Investig Drugs. 2016;25:1181–1187. doi: 10.1080/13543784.2016.1216972. [DOI] [PubMed] [Google Scholar]
- 25.Fukunaga K, Yabuki Y. SAK3-induced neuroprotection is mediated by nicotinic acetylcholine receptors. In: Nicotinic Acetylcholine Receptor Signaling in Neuroprotection. In: Akaike A, Shimohama S, Yoshimi Misu Y, editors. Springer; Berlin: 2018. pp. 159–171. [PubMed] [Google Scholar]
- 26.Hernandez CM, Kayed R, Zheng H, Sweatt JD, Dineley KT. Loss of alpha7 nicotinic receptors enhances beta-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer's disease. J Neurosci. 2010;30:2442–2453. doi: 10.1523/JNEUROSCI.5038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gil SM, Metherate R. Enhanced sensory-cognitive processing by activation of nicotinic acetylcholine receptors. Nicotine Tob Res. 2019;21:377–382. doi: 10.1093/ntr/nty134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71:155–165. doi: 10.1016/j.neuron.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozada AF, Wang X, Gounko NV, Massey KA, Duan J, Liu Z, Berg DK. Glutamatergic synapse formation is promoted by α7-containing nicotinic acetylcholine receptors. J Neurosci. 2012;32:7651–7661. doi: 10.1523/JNEUROSCI.6246-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue Y, Liu R, Cheng W, Hu Y, Li J, Pan X, Peng J, Zhang P. GTS-21 attenuates lipopolysaccharide-induced inflammatory cytokine production in vitro by modulating the Akt and NF-κB signaling pathway through the α7 nicotinic acetylcholine receptor. Int Immunopharmacol. 2015;29:504–512. doi: 10.1016/j.intimp.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Tyagi E, Agrawal R, Nath C, Shukla R. Inhibitory role of cholinergic system mediated via alpha7 nicotinic acetylcholine receptor in LPS-induced neuro-inflammation. Innate Immun. 2010;16:3–13. doi: 10.1177/1753425909104680. [DOI] [PubMed] [Google Scholar]
- 32.Liao Y, Qi XL, Cao Y, Yu WF, Ravid R, Winblad B, Pei JJ, Guan ZZ. Elevations in the levels of NF-κB and inflammatory chemotactic factors in the brains with Alzheimer's disease - One mechanism may involve α3 nicotinic acetylcholine receptor. Curr Alzheimer Res. 2016;13:1290–1301. doi: 10.2174/1567205013666160703174254. [DOI] [PubMed] [Google Scholar]
- 33.Domínguez-Álvaro M, Montero-Crespo M, Blazquez-Llorca L, Insausti R, DeFelipe J, Alonso-Nanclares L. Three-dimensional analysis of synapses in the transentorhinal cortex of Alzheimer's disease patients. Acta Neuropathol Commun. 2018;6:20. doi: 10.1186/s40478-018-0520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauwens M, Mottaghy FM, Bucerius J. PET imaging of the human nicotinic cholinergic pathway in atherosclerosis. Curr Cardiol Rep. 2015;17:67. doi: 10.1007/s11886-015-0614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, et al. Research criteria for the diagnosis of Alzheimer's disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 36.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 37.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 38.Wang XL, Deng YX, Gao YM, Dong YT, Wang F, Guan ZZ, Wei H, Qi XL. Activation of α7 nAChR by PNU-282987 improves synaptic and cognitive functions through restoring the expression of synaptic-associated proteins and the CaM-CaMKII-CREB signaling pathway. Aging (Albany NY) 2020;12:543–570. doi: 10.18632/aging.102640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Dunant Y, Gisiger V. Ultrafast and slow cholinergic transmission. Different involvement of acetylcholinesterase molecular forms. Molecules. 2017;22:1300. doi: 10.3390/molecules22081300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 42.Albiñana E, Luengo JG, Baraibar AM, Muñoz MD, Gandía L, Solís JM, Hernández-Guijo JM. Choline induces opposite changes in pyramidal neuron excitability and synaptic transmission through a nicotinic receptor-independent process in hippocampal slices. Pflugers Arch. 2017;469:779–795. doi: 10.1007/s00424-017-1939-5. [DOI] [PubMed] [Google Scholar]
- 43.Huang M, Felix AR, Kwon S, Lowe D, Wallace T, Santarelli L, Meltzer HY. The alpha-7 nicotinic receptor partial agonist/5-HT3 antagonist RG3487 enhances cortical and hippocampal dopamine and acetylcholine release. Psychopharmacology (Berl) 2014;231:2199–2210. doi: 10.1007/s00213-013-3373-5. [DOI] [PubMed] [Google Scholar]
- 44.Stoiljkovic M, Kelley C, Nagy D, Hurst R, Hajós M. Activation of α7 nicotinic acetylcholine receptors facilitates long-term potentiation at the hippocampal-prefrontal cortex synapses in vivo. Eur Neuropsychopharmacol. 2016;26:2018–2023. doi: 10.1016/j.euroneuro.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Lagostena L, Trocme-Thibierge C, Morain P, Cherubini E. The partial alpha7 nicotine acetylcholine receptor agonist S 24795 enhances long-term potentiation at CA3-CA1 synapses in the adult mouse hippocampus. Neuropharmacology. 2008;54:676–685. doi: 10.1016/j.neuropharm.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 46.Inestrosa NC, Godoy JA, Vargas JY, Arrazola MS, Rios JA, Carvajal FJ, Serrano FG, Farias GG. Nicotine prevents synaptic impairment induced by amyloid-β oligomers through α7-nicotinic acetylcholine receptor activation. Neuromolecular Med. 2013;15:549–569. doi: 10.1007/s12017-013-8242-1. [DOI] [PubMed] [Google Scholar]
- 47.Counts SE, Nadeem M, Lad SP, Wuu J, Mufson EJ. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65:592–601. doi: 10.1097/00005072-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Cao Y, Xiao Y, Ravid R, Guan ZZ. Changed clathrin regulatory proteins in the brains of Alzheimer's disease patients and animal models. J Alzheimers Dis. 2010;22:329–342. doi: 10.3233/JAD-2010-100162. [DOI] [PubMed] [Google Scholar]
- 49.Carvalho C, Santos MS, Oliveira CR, Moreira PI. Alzheimer's disease and type 2 diabetes-related alterations in brain mitochondria, autophagy and synaptic markers. Biochim Biophys Acta. 2015;1852:1665–1675. doi: 10.1016/j.bbadis.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Ferreira ST, Klein WL. The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer's disease. Neurobiol Learn Mem. 2011;96:529–543. doi: 10.1016/j.nlm.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tu S, Okamoto S, Lipton SA, Xu H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease. Mol Neurodegener. 2014;9:48. doi: 10.1186/1750-1326-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Yu L, Yang H, Li C, Hui Z, Xu Y, Zhu X. Oridonin attenuates synaptic loss and cognitive deficits in an Aβ1-42-induced mouse model of Alzheimer's disease. PLoS One. 2016;11:e0151397. doi: 10.1371/journal.pone.0151397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu SJ, Yang C, Zhang Y, Su RY, Chen JL, Jiao MM, Chen HF, Zheng N, Luo S, Chen YB, et al. Neuroprotective effect of β-asarone against Alzheimer's disease: Regulation of synaptic plasticity by increased expression of SYP and GluR1. Drug Des Devel Ther. 2016;10:1461–1469. doi: 10.2147/DDDT.S93559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chauhan NB, Lichtor T, Siegel GJ. Aging potentiates Abeta-induced depletion of SNAP-25 in mouse hippocampus. Brain Res. 2003;982:219–227. doi: 10.1016/S0006-8993(03)03011-7. [DOI] [PubMed] [Google Scholar]
- 55.Qi XL, Nordberg A, Xiu J, Guan ZZ. The consequences of reducing expression of the alpha7 nicotinic receptor by RNA interference and of stimulating its activity with an alpha7 agonist in SH-SY5Y cells indicate that this receptor plays a neuroprotective role in connection with the pathogenesis of Alzheimer's disease. Neurochem Int. 2007;51:377–383. doi: 10.1016/j.neuint.2007.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request