Abstract
New Zealand kauri is an ancient, iconic, gymnosperm tree species that is under threat from a lethal dieback disease caused by the oomycete Phytophthora agathidicida. To gain insight into this pathogen, we determined whether proteinaceous effectors of P. agathidicida interact with the immune system of a model angiosperm, Nicotiana, as previously shown for Phytophthora pathogens of angiosperms. From the P. agathidicida genome, we defined and analysed a set of RXLR effectors, a class of proteins that typically have important roles in suppressing or activating the plant immune system. RXLRs were screened for their ability to activate or suppress the Nicotiana plant immune system using Agrobacterium tumefaciens transient transformation assays. Nine P. agathidicida RXLRs triggered cell death or suppressed plant immunity in Nicotiana, of which three were expressed in kauri. For the most highly expressed, P. agathidicida (Pa) RXLR24, candidate cognate immune receptors associated with cell death were identified in Nicotiana benthamiana using RNA silencing‐based approaches. Our results show that RXLRs of a pathogen of gymnosperms can interact with the immune system of an angiosperm species. This study provides an important foundation for studying the molecular basis of plant–pathogen interactions in gymnosperm forest trees, including kauri.
Keywords: effectors, forest pathogen, kauri dieback, NBS‐LRR immune receptors, Phytophthora agathidicida, RXLR
RXLR effectors of the kauri dieback pathogen Phytophthora agathidicida triggered or suppressed immunity in Nicotiana, showing that RXLRs from a gymnosperm pathogen can interact with an angiosperm immune system.
1. INTRODUCTION
New Zealand kauri (Agathis australis) is an ancient species in the Araucariaceae conifer family and is under threat from kauri dieback disease (Waipara et al., 2013; Bradshaw et al., 2020). New Zealand kauri forests were decimated by European settlers in the 19th century (Beever et al., 2009), and the few forests that remain have protected status, in keeping with their cultural and ecological importance (Wardle, 1991; Ogden, 1995; Lambert et al., 2018). Kauri dieback disease was first noticed in 2006 (Beever et al., 2009), and now occurs throughout the geographic range of New Zealand kauri forests (Waipara et al., 2013; Bradshaw et al., 2020).
Protection of forests from pests and diseases is of paramount importance for many reasons, not least of which is their potential for mitigating climate change (Bastin et al., 2019). Over the last few decades, forest trees in both natural and planted forests have come under increasing threat from invasive pest and disease epidemics. A growing number of these are caused by oomycetes in the genus Phytophthora, such as diebacks of jarrah and alder (Hansen, 2015) and of bunya and hoop pines in the same Araucariaceae family as kauri (Shuey et al., 2019). Recent epidemics have been influenced by factors such as changes in climate (Woods et al., 2016), human‐mediated movement of pathogens (Goss et al., 2011; Wingfield et al., 2015), and rapid evolution or hybridization of the pathogen (Brasier, 2000; Callaghan & Guest, 2015).
Kauri dieback is caused by a highly destructive soilborne species, Phytophthora agathidicida (Weir et al., 2015), which kills fine roots, causes collar rot, and blocks vascular tissues, ultimately killing the tree (Beever et al., 2009). Due to the recent emergence of kauri dieback disease, comparatively little is known about the P. agathidicida–kauri pathosystem and the origin of P. agathidicida. So far, disease management has focused on attempts to prevent its spread, chemical control with phosphite injections, and screening for resistance in the kauri population (Bradshaw et al., 2020). Identification of disease resistance will play an important role in the mitigation of kauri dieback disease in the long term.
Plants can resist pathogens through the recognition of pathogen virulence factors termed effectors (Cook et al., 2015; van der Burgh & Joosten, 2019). By understanding how effectors and their host targets interact at the molecular level, questions about key drivers of pathogen success and failure can be addressed (Ntoukakis & Gifford, 2019). Often, one of the main outputs of pathogen resistance is a localized cell death response, the hypersensitive response (HR), which occurs on recognition of specific effectors by corresponding plant immune receptors. This visual output can be used to identify effector–immune receptor interactions, as well as plant material resistant to pathogens (Rietman et al., 2012; Dangl et al., 2013; Vleeshouwers & Oliver, 2014; Van de Wouw & Idnurm, 2019).
Like other pathogens, Phytophthora species produce effector proteins. The main class of intracellular effectors is the RXLRs (Judelson, 2012), which target a variety of host molecules to manipulate host immunity (Wang & Jiao, 2019). Because plants have evolved to recognize pathogen effector molecules as triggers for defence, pathogens are under strong selection pressure to evade recognition and can achieve this by loss, mutation, or silencing of effectors (Qutob et al., 2013; Anderson et al., 2015; Pais et al., 2018; Wang et al., 2019). In forest health situations such as the kauri–P. agathidicida system, the long lifespan of the host means the pathogen has a considerable time advantage in terms of adaptability in this “arms race” with its host, although phenotypic plasticity due to processes such as epigenetic variation and somatic mutation (Bräutigam et al., 2013, Simberloff & Leppanen, 2019) might enable adaptability in long‐lived trees and these processes deserve further investigation.
Compared to studies of plant–pathogen interactions with angiosperm crop pathogens, little is known about how pathogens of gymnosperms interact with their hosts at a molecular level (Bradshaw et al., 2016; Stewart et al., 2018). The genetic basis of disease resistance has been established for some pine diseases (Sniezko et al., 2014) and some species show major gene resistance (Kinloch et al., 2008; Sniezko et al., 2014). Effector candidates have been identified in fungal pathogens of gymnosperm trees that are similar in structure and function to those of angiosperm pathogens (de Wit et al., 2012; Raffaello & Asiegbu, 2017; Ma et al., 2019) and deserve further exploration in the context of forest health. Studies of effectors in forest pathogens will help to predict their adaptive potential and the dynamics of pathogen–tree coevolution in forests, and serve as tools for detection of immune receptors that could accelerate tree improvement (Keriö et al., 2019).
We tested the hypothesis that RXLR effectors from an oomycete that is pathogenic to a gymnosperm interact with the immune system of model angiosperm plants in a similar way to that of angiosperm pathogens. We defined a set of RXLR effectors in P. agathidicida and performed functional analyses to assess their roles in planta. A model‐plant system was chosen due to the cultural significance and technical limitations associated with using kauri. For one of the RXLR genes that was highly up‐regulated in kauri, the model‐plant system was screened for candidate cognate immune receptors. To the best of our knowledge, this work is the first of its kind for any forest gymnosperm–oomycete pathosystem and provides a foundation for studies of the molecular basis of plant–pathogen interactions in forest trees, including kauri.
2. RESULTS
2.1. Prediction of a set of RXLR effector gene candidates in P. agathidicida
RXLR effectors from Phytophthora species can have important roles in suppressing or activating the plant immune system (Anderson et al., 2015). With this in mind, we used three prediction methods to identify a well‐supported set of 78 RXLR effector candidates (Figure S1) from the genome of P. agathidicida NZFS3770, an isolate collected from Great Barrier Island, New Zealand in 2006 (Studholme et al., 2016). BLAST searches suggested that, of the 78 PaRXLRs, only four (PaRXLR21, PaRXLR35, PaRXLR57, and PaRXLR59) are unique to P. agathidicida.
The amino acid sequences of the 78 predicted RXLR effector candidates were analysed for conserved sequence motifs. Using the motif alignment search tool (MAST), 16 significantly over‐represented motifs were found. In addition to the signal peptide and RXLR motif that were used, in part, for the selection of the 78 candidates, these included W, Y, and L motifs often found in RXLR effectors (Jiang et al., 2008), as well as sequences conserved between related RXLR effector candidates of P. agathidicida (Figures 1 and S2). These results suggest there are common features in the RXLRs of P. agathidicida compared to those of other Phytophthora species.
Figure 1.
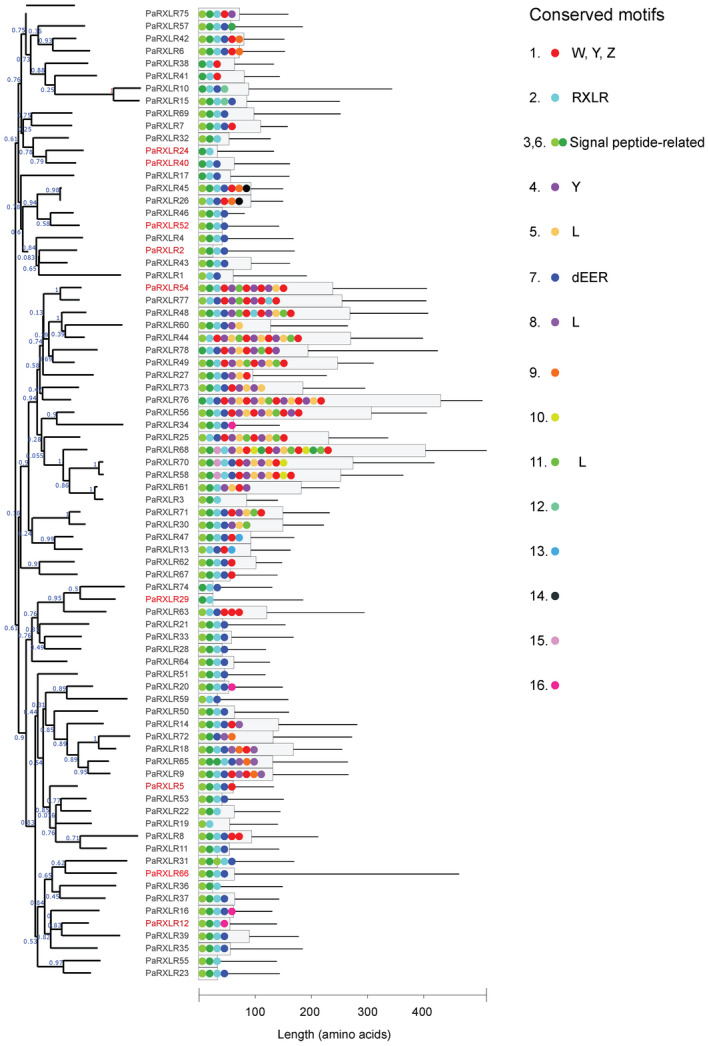
Phylogeny and domain structure of the Phytophthora agathidicida RXLR effector candidates. The dendrogram represents a maximum‐likelihood phylogenetic tree. Numbers on the branches are approximate likelihood ratio test (aLRT) values as reported by PhyML. PaRXLRs with names in red are those that either elicited or suppressed cell death in functional assays. The histogram shows lengths of predicted proteins (thin black lines), according to the scale below, and the proportion of proteins involved in amino acid motifs that were significantly over‐represented among the 78 RXLRs as predicted by MEME (grey boxes). Coloured dots represent the motifs, with the order respected but not drawn to scale. The key indicates conserved motifs, with putative functions or similarities to common RXLR motifs indicated where appropriate; the numbers correspond to those detailed in Figure S2
2.2. P. agathidicida isolates and RXLR effector gene candidates show low genetic diversity
We tested the hypothesis that selection for diversification of P. agathidicida RXLR sequences has occurred in kauri forests by examining sequence variation in isolates from throughout the kauri dieback region in the northern part of New Zealand (Table 1 and Figure 2). The numbers of single nucleotide polymorphisms (SNPs) per genome amongst 12 P. agathidicida isolates, relative to the 37.2 Mb genome reference strain NZFS3770 (Studholme et al., 2016), ranged from 29,701 to 43,737 (Table 1); this equated to sequence differences of only 0.08%–0.12% between the isolates, suggesting low genetic diversity in the population.
Table 1.
Phytophthora agathidicida isolates with sequenced genomes
| ID | NZFS a | Year b | Location b | Sequence accession numbers c | Mbases reads | SNPs d per genome | Genes with SNPs | RXLRs with SNPs e |
|---|---|---|---|---|---|---|---|---|
| A | 3770 | 2006 | Great Barrier Island | SRX1116283 | 4,619 | |||
| B | 3772 | 2013 | Waitakeres, Huia | SRX1116282 | 4,057 | |||
| 1 | 3118 | 2009 | Waitakeres, Huia | SRX4575879 | 4,765 | 41,720 | 1,433 | 8 |
| 2 | 3126 | 2006 | Maungaroa beach | SRX4575884 | 4,797 | 41,709 | 1,418 | 6 |
| 3 | 3128 | 2009 | Waitakeres, Huia | SRX4575881 | 7,030 | 43,737 | 1,436 | 7 |
| 4 | 3616 | 2001 | Great Barrier Island | SRX4575880 | 4,614 | 29,701 | 869 | 2 |
| 5 | 3687 | 2011 | Waipoua Forest | SRX4575875 | 4,768 | 43,493 | 1,476 | 6 |
| 6 | 3815 | 2014 | Coromandel | SRX4575874 | 4,558 | 42,358 | 1,688 | 7 |
| 7 | 3869 | 2014 | Arapahoe | SRX4575877 | 4,667 | 42,634 | 1,662 | 7 |
| 8 | 3885 | 2014 | Whenuanui, Ruawai | SRX4575876 | 4,799 | 43,146 | 1,673 | 6 |
| 9 | 4288 | 1972 | Great Barrier Island | SRX4575883 | 3,828 | 41,131 | 950 | 3 |
| 10 | 4289 | 2010 | Raetea | SRX4575882 | 4,798 | 42,749 | 1,487 | 6 |
| 11 | 4290 | 2010 | Waipoua Forest | SRX4575885 | 5,122 | 42,789 | 1,483 | 7 |
| 12 | 4291 | 2014 | Coromandel | SRX4575878 | 4,879 | 42,976 | 1,691 | 7 |
NZFS (New Zealand Forest Service collection) number of the P. agathidicida isolate.
Year and location (in New Zealand) of isolate collection.
Accession numbers for GenBank sequence read archive Bioprojects. 3770 and 3772 are those from Studholme et al (2016).
Total number of single nucleotide polymorphism (SNP) sites in the resequenced genomes compared to that of NZFS3770.
All isolates had all 78 RXLR genes; the numbers indicate how many of those 78 had SNPs. Chi‐square analysis to compare the proportions of these RXLR genes and other genes with SNPs showed no significant difference for any of the resequenced strains (p > .6).
Figure 2.
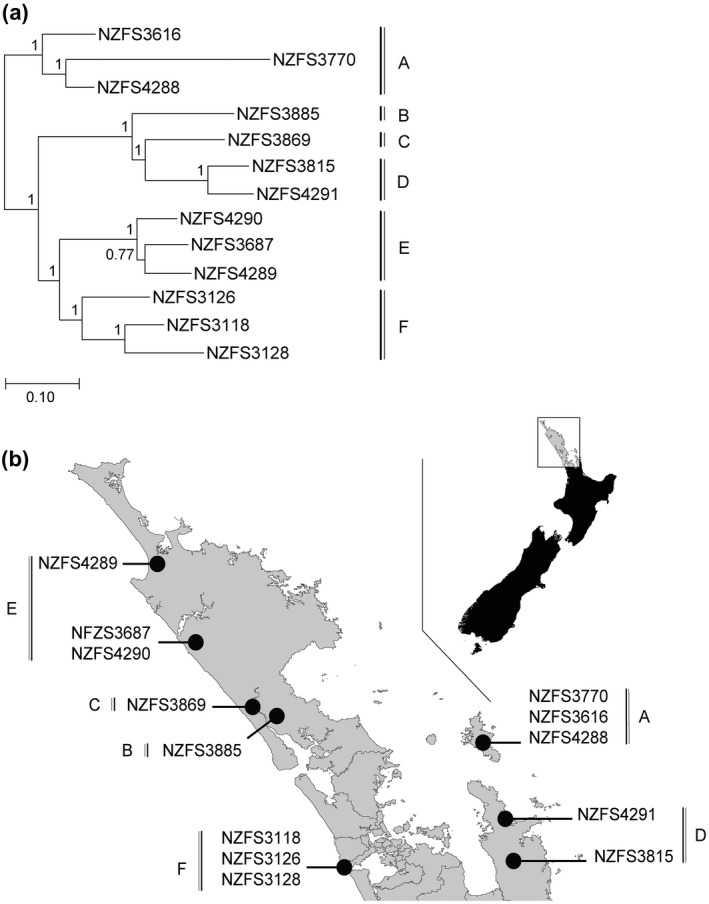
Phylogeny and location of all Phytophthora agathidicida samples. The phylogeny was computed using maximum likelihood on concatenated variable single nucleotide polymorphism (SNP) loci from all 12 resequenced P. agathidicida genomes, determined by comparison to the reference NZFS3770 genome. Numbers on the branches are approximate likelihood ratio test (aLRT). Main groupings in the phylogeny, indicated by letters, are reported on the map, which represents the top of the North Island of New Zealand.
Amongst the 78 RXLR effector gene candidates studied in this project, only 10 had SNPs in their corresponding coding sequence, showing they are mostly identical amongst the isolates studied (Table 1). This low SNP rate was similar to that of the rest of genome; there was no significant enrichment in the proportion of RXLR effector gene candidates with SNPs compared to that of all other genes for any of the isolates studied (Tables 1, S3, and S5). Thus, we found no evidence for selection for RXLR sequence diversification among these samples.
2.3. P. agathidicida RXLR effector candidates induce cell death in Nicotiana spp.
To gain insight into how P. agathidicida RXLRs interact with the plant immune system, the RXLRs were screened for the ability to trigger cell death in model angiosperm Nicotiana spp. Cell death is often indicative of immune system activation on recognition of an effector by a corresponding plant immune receptor, and is termed the HR (Wang et al., 2011). Using an Agrobacterium tumefaciens‐mediated transient transformation assay (ATTA), eight of the P. agathidicida (Pa) RXLR effector candidates consistently triggered cell death in Nicotiana tabacum, and two of these also triggered cell death in N. benthamiana when tested in at least three independent experiments (Figures 3 and S3). All eight had BLASTP hits to other Phytophthora RXLRs, with some orthologous to functionally characterized RXLR effectors in other species based on reciprocal best BLASTP hits. Most notable among these was PaRXLR24, which is orthologous to P. sojae Avh238 and P. parasitica pPE4 (Table 2), highlighting the potential importance of these RXLRs for pathogens of both gymnosperms and angiosperms.
Figure 3.
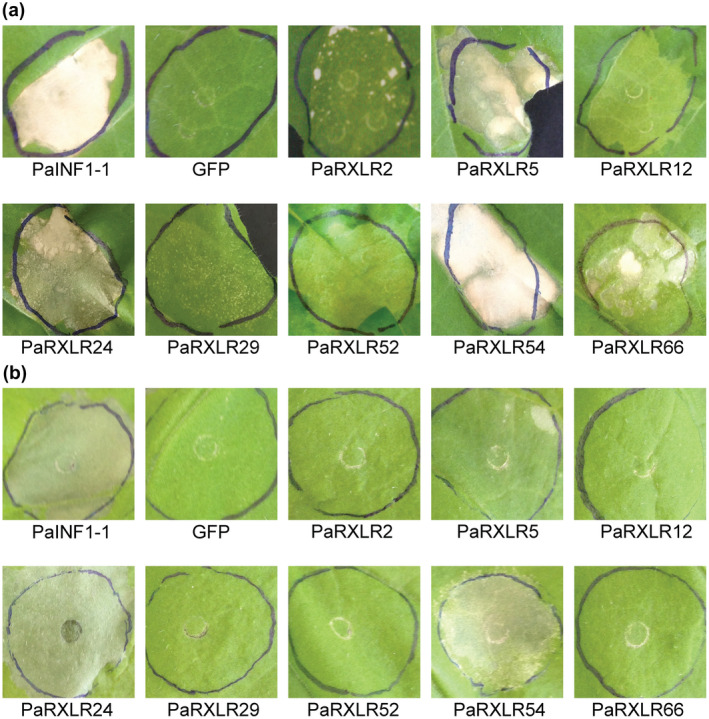
Eight RXLR effector candidates of Phytophthora agathidicida trigger cell death in Nicotiana spp. Agrobacterium tumefaciens GV3101 strains carrying P. agathidicida INF1‐1 (PaINF1‐1; positive control), green fluorescent protein (GFP; negative control) or P. agathidicida (Pa) RXLR candidates were infiltrated into 5‐week‐old leaves of Nicotiana tabacum (a) or Nicotiana benthamiana (b). Leaves were photographed 7 days post infiltration. Six P. agathidicida RXLR effector candidates triggered cell death in N. tabacum only, whilst two others (PaRXLR24 and PaRXLR54) triggered cell death in both N. tabacum and N. benthamiana. The experiment was repeated three times with consistent results (Figure S3).
Table 2.
Features of the nine RXLR effector candidates from Phytophthora agathidicida that induced or suppressed cell death in Nicotiana spp.
| Name | Best BLAST hit | E‐value | GenBank ID | % amino acid identity | Cell death‐triggering activity f | Suppressed RXLR‐triggered immunity g | |
|---|---|---|---|---|---|---|---|
| Nt | Nb | ||||||
| PaRXLR2 | Phytophthora cactorum hypothetical protein a | 2.0E−38 | RAW43538.1 | 53 | Weak | No | No |
| PaRXLR5 b | Phytophthora parasitica P1569 hypothetical protein a | 6.0E−22 | ETI36999.1 | 45 | Strong | No | No |
| PaRXLR12 | Phytophthora megakarya hypothetical protein | 4.0E−21 | OWZ07307.1 | 41 | Weak | No | No |
| PaRXLR24 c | P. parasitica pPE4 a | 3.0E−48 | XP_008889734.1 | 65 | Strong | Strong | No |
| PaRXLR29 | P. parasitica hypothetical protein | 9.0E−19 | XP_008894466.1 | 38 | Weak | No | No |
| PaRXLR52 d | Phytophthora sojae hypothetical protein a | 7.0E−56 | XP_009533161.1 | 62 | Weak | No | No |
| PaRXLR54 | Phytophthora palmivora avirulence protein | 5.0E−100 | POM79043.1 | 45 | Strong | Strong | No |
| PaRXLR66 e | P. megakarya RXLR protein | 2.0E−27 | OWZ17774.1 | 37 | Weak | No | No |
| PaRXLR40 | P. palmivora RXLR protein a | 1.0E−15 | POM65748.1 | 38 | No | No | Yes |
Reciprocal top BLAST hit.
Ortholog of P. nicotianae Avh8 (KUG01203.1). E value 2e−21, 43.4% amino acid identity.
Ortholog of P. sojae Avh238 (AEK81002.1). E value 8e−28, 46.4% amino acid identity.
Ortholog of P. palmivora Avr1b‐1 (POM62647.1). E value 4e−39, 60.7% amino acid identity.
Only PaRXLR66 showed single nucleotide polymorphism variation among 14 P. agathidicida genomes.
RXLR triggered cell death on N. tabacum (Nt) or N. benthamiana (Nb).
RXLR suppressed Avr3a‐R3a and PaRXLR24‐triggered cell death on N. benthamiana.
2.4. PaRXLR40 can suppress RXLR‐triggered immunity
Having identified RXLRs that induce cell death, we then screened for those with potential virulence functions. Some Phytophthora RXLR effectors suppress plant immunity to facilitate pathogen infection (Deb et al., 2018; Dalio et al., 2018). Thus, we investigated whether P. agathidicida RXLR effector candidates can suppress immunity triggered by an elicitin protein, INF1‐1, or by effector proteins. The P. infestans elicitin protein INF1 triggers an HR in N. benthamiana (Kamoun et al., 1998), and this response can be suppressed by the P. infestans RXLR effector Avr3a (Bos et al., 2006). We identified three paralogs of INF1 in the P. agathidicida genome (PaINF1‐1, PaINF1‐2, and PaINF1‐3; Figure S4a). As with P. infestans INF1, infiltration of P. agathidicida PaINF1‐1 into N. benthamiana also induced cell death that could be suppressed by P. infestans Avr3a (Figure S4b), so PaINF1‐1 and Avr3A were used as elicitor and suppressor controls, respectively. In the suppression assays, PaRXLR effectors were infiltrated into N. benthamiana leaves 24 hr before infiltration of the PaINF1‐1 elicitor, but none of the PaRXLR candidate effectors tested could suppress PaINF1‐1‐triggered cell death (Figure S4b).
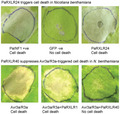
We next investigated whether any of the PaRXLRs could suppress effector‐triggered cell death immunity elicited by the P. infestans RXLR Avr3a (Engelhardt et al., 2012) in the presence of its cognate potato immune receptor protein R3a (Armstrong et al., 2005). Out of the PaRXLR effector candidates tested, only PaRXLR40 consistently suppressed Avr3a/R3a‐triggered cell death in three independent experiments (Figure 4a).
Figure 4.
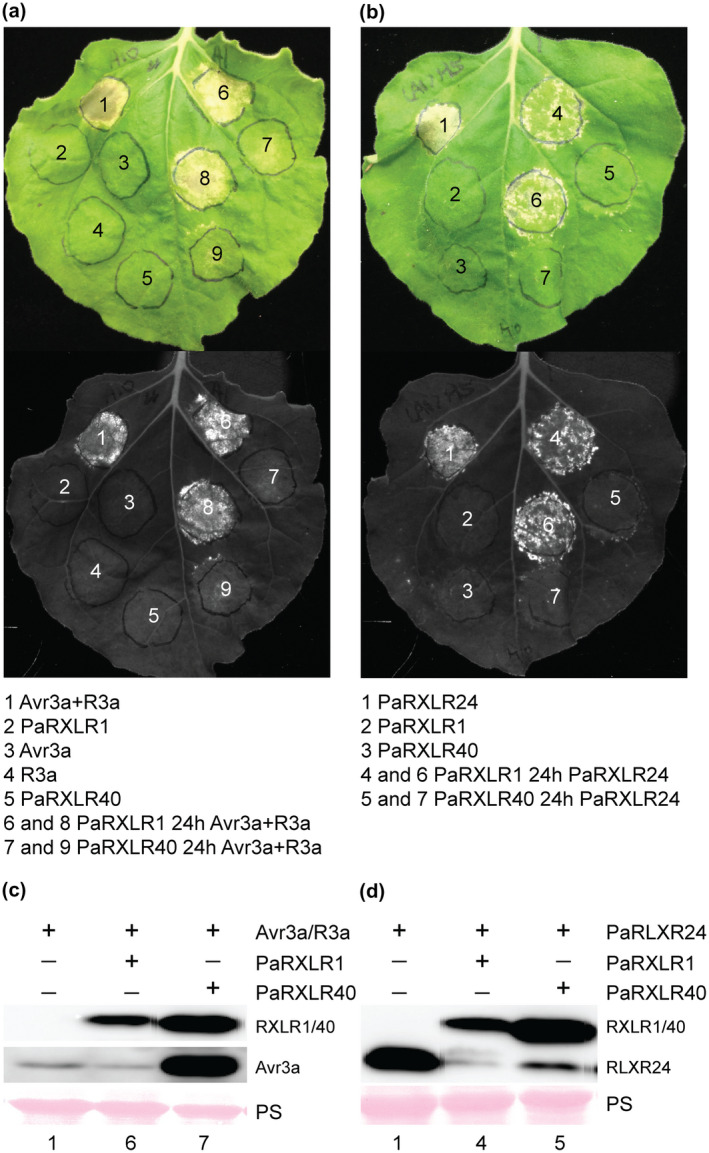
Suppression of RXLR‐triggered immunity by PaRXLR40. Suppression of (a) Avr3a and R3a‐triggered cell death and (b) PaRXLR24‐triggered cell death by PaRXLR40 on 5‐week‐old Nicotiana benthamiana. Avr3a, R3a, and PaRXLR were labelled with GFP, HA, and FLAG tags, respectively. Agrobacterium tumefaciens carrying cell death elicitors were infiltrated 24 hr after infiltration of Phytophthora agathidicida RXLR effector candidate PaRXLR40, or the negative suppression control, PaRXLR1. Photographs with visible light (top) and UV (bottom) were taken 7 days post‐infiltration of cell death elicitors. Suppression is shown by lack of cell death at infiltration spots (a) 7 and 9 and (b) 5 and 7. The experiment was repeated three times with consistent results. (c) and (d) Protein immunoblots of total proteins extracted from N. benthamiana leaves collected 3 days post‐infiltration confirmed the presence of elicitors and PaRXLR effector candidates. Representative protein loading is shown by Ponceau staining (PS).
Given that PaRXLR40 suppressed cell death triggered by Avr3a/R3a, we tested whether it could also suppress cell death triggered by an effector from P. agathidicida, PaRXLR24. Coinfiltration of PaRXLR40 24 hr after PaRXLR24 suppressed cell death in N. benthamiana leaves, suggesting that PaRXLR40 can suppress PaRXLR24‐induced immunity (Figure 4b).
To confirm that suppression of cell death by PaRXLR40 was not due to nonspecific inhibition of elicitor gene expression, protein immunoblots were performed to verify the presence of the elicitors and effector proteins after coinfiltration into N. benthamiana. As expected, the cell death elicitors Avr3a and PaRXLR24, and the PaRXLR40 suppressor, were detected in all relevant samples (Figure 4c,d). However, R3a (hemagglutinin [HA]‐tagged) was unable to be detected by protein immunoblotting. Because C‐terminal‐tagged R3a was previously shown to be nonfunctional (Engelhardt et al., 2012), in this study we used R3a with a centrally located HA tag (replacing amino acids 1,167 to 1,175), which may have affected detection of the HA tag or protein stability. However, the expression of R3a‐HA in the suppression assay samples was verified by reverse transcription (RT)‐PCR, with Avr3a as a positive control (Figure S5). Thus, in this study PaRXLR40 was shown to be a specific suppressor of immunity triggered by effectors, including cell death triggered by PaRXLR24, an effector with which it shares 55.1% amino acid identity (Figure S6c).
2.5. Both PaRXLR24 and PaRXLR40 are expressed in planta
To indicate whether any of the nine P. agathidicida RXLR effector candidates that triggered or suppressed cell death in Nicotiana spp. have the potential to be functional in kauri, we determined their expression in kauri (Table S6). Roots and leaves of kauri inoculated with P. agathidicida were collected at intervals up to 72 hr post‐inoculation and relative quantitative RT‐PCR was performed on RNA from these and from in‐culture samples. None of the nine P. agathidicida RXLR effector candidates were expressed in culture. Whilst most of the RXLRs tested showed no or low expression in planta, PaRXLR24, PaRXLR40, and PaRXLR12 were expressed at all four time points in both leaf and root samples, although with expression remaining low until 24 hr, and with higher levels of expression in roots than leaves (Figure 5 and Table S6). Thus, PaRXLR24 and PaRXLR40, which respectively showed cell death elicitor and suppression activity in the model angiosperm N. benthamiana, were expressed in the kauri host.
Figure 5.
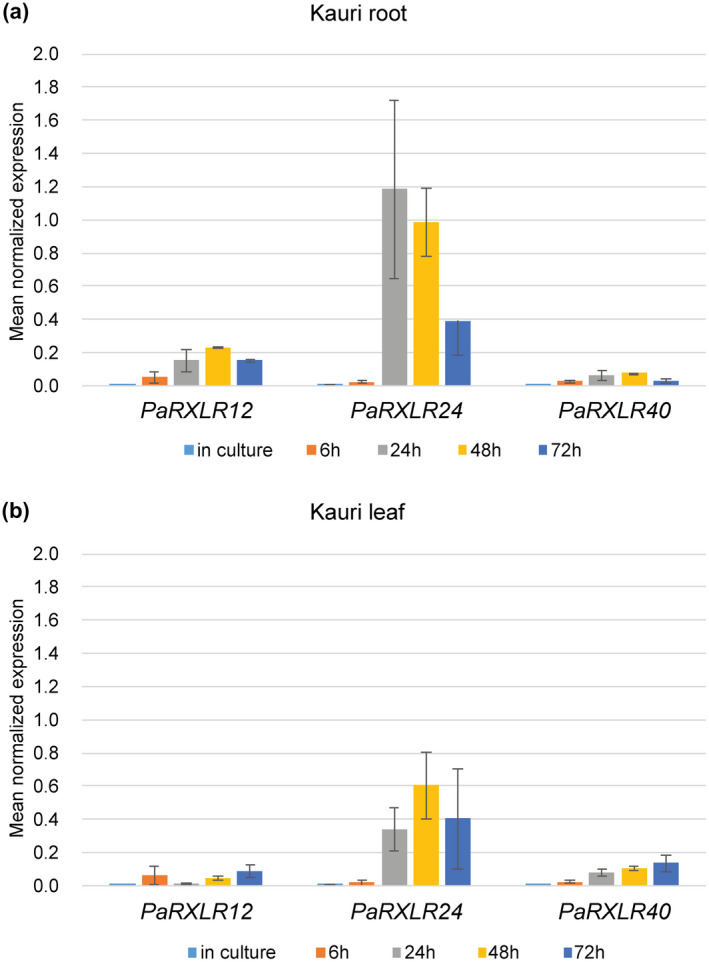
PaRXLR24 and PaRXLR40 are expressed in kauri tissue. Gene expression was analysed in (a) roots and (b) leaves of kauri inoculated with Phytophthora agathidicida mycelium for the nine RXLRs that either triggered cell death or suppressed effector triggered defence in Nicotiana spp. Expression of PaRXLR genes in vitro (mycelium) and in planta (6, 24, 48, and 72 hr post‐inoculation) was normalized to the geometric mean of three P. agathidicida housekeeping genes, β‐tubulin, actin, and elongation factor 2. The normalized means are shown with standard error bars. Only three (PaRXLR12, PaRXLR24, and PaRXLR40) were expressed in kauri (full results in Table S6).
2.6. Identification of an amino acid required for cell death induction by PaRXLR24
PaRXLR24, the candidate effector that caused strong cell death in both Nicotiana species, is an ortholog of virulence factors pPE4 of P. parasitica (Huang et al., 2019) and Avh238 of P. sojae (Wang et al., 2011) (Table 2 and Figure 6). The amino acids critical for cell death induction by P. sojae Avh238 have been identified (Yang et al., 2017) and we tested the hypothesis that equivalent amino acids show a similar function in PaRXLR24. The 79th amino acid of Avh238 (histidine) was shown to be critical for its cell death‐inducing activity, whilst the 51st and 76th amino acids had minor roles (Yang et al., 2017). Alignment of the predicted amino acid sequence of PaRXLR24 with those of isoforms of P. sojae Avh238 identified the equivalent positions of these three amino acids in PaRXLR24 (Figure 6a), and site‐directed mutagenesis was used to mutate them. Mutated isoforms of PaRXLR24, in which proline 59 or alanine 78 had been replaced with serine (as found in the nonfunctional Avh238 P7076), did not affect its ability to trigger cell death. However, mutation of PaRXLR24 isoleucine 81, either alone or in combination with the other mutations, showed reduced cell death compared to the wild type (WT) (Figure 6b). PaRXLR24 WT and mutant proteins were detected in extracts from infiltrated N. benthamiana leaves by immunoblotting, suggesting that the loss of cell death‐inducing ability by PaRXLR24I81N mutants was not due to protein instability (Figure 6c). Thus, the ability of both PaRXLR24 and P. sojae Avh238 to induce cell death in N. benthamiana is dependent on an amino acid that occurs in the equivalent position in the two proteins.
Figure 6.
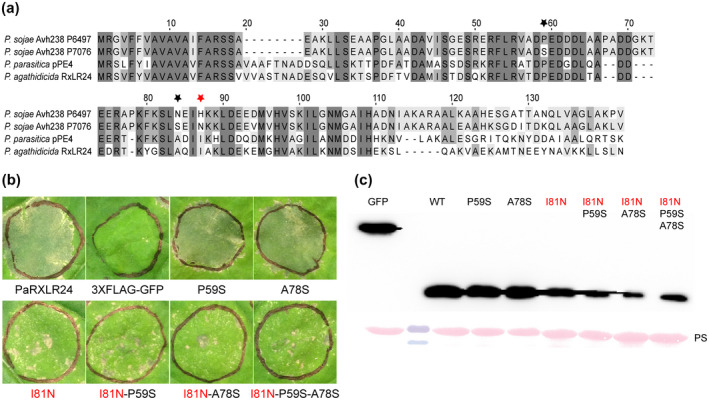
Identification of amino acids required for PaRXLR24 cell death‐triggering activity. (a) Amino acid alignment of Phytophthora sojae Avh238 (two alleles), Phytophthora parasitica pPE4, and PaRXLR24. The red star indicates the position of the 79th amino acid (histidine) in the active P6497 allele of Avh238 required for cell death‐inducing activity (Yang et al., 2017), and the corresponding 81st amino acid (isoleucine) in PaRXLR24. Black stars indicate positions of amino acids shown to affect cell death‐inducing activity in Avh238 and corresponding amino acids in PaRXLR24. (b) Agroinfiltration of PaRXLR24 wild‐type and mutants on 5‐week‐old Nicotiana benthamiana leaves. The experiment was repeated three times with consistent results. 3 × FLAG‐GFP was used as negative control. The PaRXLR24P59S and PaRXLR24A78S single (black star) mutants showed similar levels of cell death as the wild type. PaRXLRI81N single (red star), double and triple mutants showed reduced cell death compared to the wild type. Photographs were taken 7 days post‐infiltration. (c) Western blotting confirmed the stability of wild‐type (WT) and mutant PaRXLR24 proteins in total leaf extracts.
One of the Avh238 isoforms of P. sojae, Avh238 P7076, which is unable to able to elicit cell death, was shown to suppress INF1‐triggered defence (Wang et al., 2011). To determine if PaRXLR24 has the ability to suppress cell death, a function that could normally be masked because of its own cell death induction activity, the single (I81N) and triple (I81N‐P59S‐A78S) cell death‐deficient mutants of PaRXLR24 were tested. Neither of these mutants were able to suppress either PaINF1‐1‐ or Avr3a/R3a‐triggered cell death (Figure S6). Thus, while PaRXLR24 and P. sojae Avh238 are similar in requiring a specific amino acid at an equivalent position for cell‐death activity, they differ in respect of their ability to suppress INF1‐triggered defence.
2.7. Potential NBS‐LRR receptors for PaRXLR24 identified in N. benthamiana
Next, we sought to identify potential plant targets for PaRXLR24. Plant nucleotide‐binding site and leucine‐rich repeat (NBS‐LRR) immune receptors can recognize RXLR effectors from Phytophthora pathogens (Lee & Yeom, 2015), thus a hairpin library for silencing of NBS‐LRR‐encoding genes (Brendolise et al., 2017) was used to identify potential N. benthamiana NBS‐LRR receptors that recognize PaRXLR24. Two pooled hairpin constructs (HP7 and HP14) that each target six or eight NBS‐LRRs, out of a total of 48 pools targeting 345 NBS‐LRRs, were able to suppress PaRXLR24‐triggered cell death (Figure S7). Then, hairpin constructs targeting individual NBS‐LRRs from the HP7 and HP14 pools showed that HP7‐1, HP7‐2, HP14‐6, and HP14‐8 gave the most effective suppression of PaRXLR24‐triggered cell death (Figure 7). Whilst silencing efficiencies of these hairpins showed none fully suppressed their NBS‐LRR target (Table S7), hairpin HP7‐1 suppressed PaRXLR24‐triggered cell death in 75% of the trials (Figure 7) and was predicted to target an NBS‐LRR with similarity to an RPM1‐like immune receptor (El Kasmi et al., 2017) (Tables 3 and S7). Hairpin HP7‐2 showed the most consistent and strong suppression of PaRXLR24‐triggered cell death (in 81% of infiltration spots) and appears to target an R1‐like NBS‐LRR (Ballvora et al., 2002) (Figure 7, and Tables 3 and S7). From the HP14 hairpin pool, HP14‐6 and HP14‐8 both suppressed PaRXLR24‐triggered cell death in about 60% of infiltration spots and their predicted N. benthamiana NBS‐LRR targets were similar to putative late blight resistance protein R1‐like proteins of Nicotiana species (Figure 7, and Tables 3 and S7).
Figure 7.
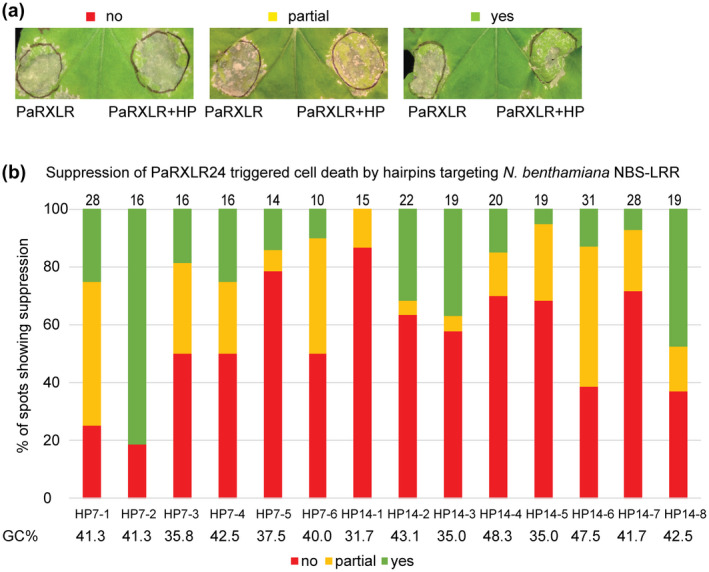
Identification of NBS‐LRRs required for PaRXLR24‐triggered cell death. Agrobacterium‐mediated screening was used to identify NBS‐LRRs required for PaRXLR24‐triggered cell death in 5‐week‐old Nicotiana benthamiana. Hairpin constructs (HP) that targeted individual NBS‐LRRs were infiltrated 48 hr before PaRXLR24. Photographs were taken 7 days after PaRXLR24 infiltration. (a) Leaves showing examples of suppression scoring, with no suppression (red), partial suppression (yellow), and strong suppression (green) of PaRXLR24‐triggered cell death by hairpin constructs. (b) Percentages of infiltration spots showing different levels of suppression by individual hairpin constructs that silenced NBS‐LRRs from hairpin pools 7 and 14. Numbers of infiltration sites counted are shown above each bar. NBS‐LRRs targeted by hairpin constructs HP7‐1, HP7‐2, HP14‐6, and HP14‐8 suppressed PaRXLR24‐triggered cell death more than 50% of the time
Table 3.
Identification of Nicotiana benthamiana NBS‐LRR candidates involved in recognition of PaRLXR24
| NBS‐LRR Genome v. 0.4.4 (v. 1.0.1) a | Hairpin screen | VIGS screen | NBS‐LRR type | |||||
|---|---|---|---|---|---|---|---|---|
| Hairpin b | Suppression (%) c | Silencing efficiency (% ± SD) d | TRV construct g | Suppression (%) f | Ion leakage (%) g | Silencing efficiency (% ± SD) d | ||
| 8754g0022.1 (05566g02009.1; 05566g02008.1) | HP7‐1 | 75.0 | 33.7 ± 10.7 | TRV‐NLR3 | Yes | 51.3* | 82.5 ± 5.0 | RPM1‐like h |
| TRV‐NLR5 | Yes | 60.1* | 79.6 ± 3.0 | |||||
| 1428g0007.1 (05653g00005.1) | HP7‐2 | 81.3 | 11.9 ± 19.1 | TRV‐NLR6 | Yes | 40.8* | 58.1 ± 7.0 | R1‐A‐like i |
| 4955g0024.1 (03461g05022.1) | HP14‐6 | 61.3 | 38.1 ± 9.9 | TRV‐NLR1 | No | 82.4 | 65.0 ± 6.0 | R1‐B‐like i |
| 5032g0004.1 (03924g01008.1; 15476g01014.1) | HP14‐8 | 63.2 | 38.1 ± 15.1 | TRV‐NLR2 | No | 83.9 | 77.1 ± 4.0 | R1‐B‐like |
| TRV‐NLR4 | No | 71.7 | 88.7 ± 3.0 | |||||
Top NBS‐LRR candidates predicted from the N. benthamiana genome v. 0.4.4 (NbS0000 numbers) and equivalent gene models from the genome v. 1.0.1 in parentheses (Niben101Scf numbers). Two of the v. 0.4.4 candidates each had two matches in the N. benthamiana v. 1.0.1 genome.
Hairpin construct used to silence N. benthamiana NBS‐LRR, designed using N. benthamiana genome v. 0.4.4 NBS‐LRR gene models.
Percentage of infiltration spots showing full or partial suppression of PaRXLR24‐triggered cell death.
Silencing efficiency of NBS‐LRR candidates determined by reverse transciptionPCR. 100% is complete loss of NBS‐LRR expression.
Virus‐induced gene silencing (VIGS) construct used to silence N. benthamiana NBS‐LRR, designed using N. benthamiana genome v. 1.0.1.
Infiltration spots showing suppression of PaRXLR24‐triggered cell death on VIGS‐silenced N. benthamiana plants (see Figure S8).
Ion leakage of PaRXLR24‐infiltrated spots in TRV‐NLR1‐6 silenced plants, shown as % conductivity compared to boiled leaf samples. Asterisks (*) indicate PaRXLR24 values significantly different in TRV‐NLR‐silenced plant versus GFP‐silenced plant while values for GFP‐infiltrated control sites on the same plants were not significant.
El Kasmi et al. (2017).
Ballvora et al. (2002).
To further assess whether the candidate NBS‐LRRs are required for recognition of PaRXLR24, those targeted by hairpins HP7‐1, HP7‐2, HP14‐6, and HP14‐8 were silenced by virus‐induced gene silencing (VIGS) in N. benthamiana (Velásquez et al., 2009). Because the hairpin construct library was designed using NBS‐LRRs predicted from an older annotated genome of N. benthamiana, Niben.genome.v. 0.4.4 (Bombarely et al., 2012; Brendolise et al., 2017), the VIGS constructs were targeted to equivalent updated gene models in the Niben v. 1.0.1 genome (Grosse‐Holz et al., 2018) (Tables 3 and S7). The NBS‐LRR targeted by hairpin HP7‐1 had two hits in the Niben v. 1.0.1 genome (Table S7); these shared 98.8% nucleotide identity and are adjacent to each other on the same contig. Similarly, the NBS‐LRR targeted by hairpin HP14‐8 also had two hits in the Niben v. 1.0.1 genome (Table S7), with those hits sharing 82.9% nucleotide identity and being located on different contigs in the genome assembly. In total six VIGS constructs (TRV‐NLR1 to ‐NLR6) were designed to silence the four candidates (Tables 3 and S7).
The VIGS‐silenced plants showed no difference in phenotype compared to TRV‐GFP‐silenced control plants (Figure S8), as expected. N. benthamiana plants with TRV‐NLR3, ‐NLR5, and ‐NLR6, corresponding to NBS‐LRRs silenced by hairpin HP7‐1 and HP7‐2, showed suppression of PaRXLR24‐induced cell death (Figure S8). This was supported by the observation that PaRXLR24‐infiltrated spots showed significant reduction in ion leakage in VIGS‐silenced plants compared to GFP‐silenced plants (Tables 3 and S7). Although NBS‐LRRs silenced by HP14‐6 and HP14‐8 showed suppression of PaRXLR24‐triggered cell death (Figure 7), those results were not confirmed in VIGS assays, where PaRXLR24‐infiltrated spots showed the same levels of cell death and ion leakage in VIGS‐silenced plants as in GFP‐silenced plants (Figure S8, Tables 3 and S7). Together the VIGS and hairpin silencing results suggest that NBS‐LRRs silenced by hairpins HP7‐1 and HP7‐2 may be involved in PaRXLR24 recognition in N. benthamiana. Thus, an RXLR from a Phytophthora species that is pathogenic to a gymnosperm can be recognized by immune receptors from a model angiosperm plant.
3. DISCUSSION
3.1. Identification and functional analysis of P. agathidicida RXLR effector candidates
There are currently few studies of molecular plant–microbe interactions involving gymnosperm tree pathogens, despite their immense importance for forest health. Phytophthora species are particularly notorious pathogens of forest gymnosperms (Hansen, 2015; Shuey et al., 2019; Bradshaw et al., 2020) and there is an urgent need to understand how they interact with plants in order to develop new methods of disease control. To help address this knowledge gap, we identified 78 RXLR effector candidates from the kauri dieback pathogen P. agathidicida. Eight of the PaRXLRs tested elicited cell death in Nicotiana spp.; this proportion of cell‐death eliciting RXLRs is similar to those found in studies with P. sojae (11/169) (Wang et al., 2011) and Plasmopara viticola (10/83) (Liu et al., 2018). None of the PaRXLRs tested were able to suppress immunity elicited by P. agathidicida INF1‐1. This is in contrast to other studies in which 23 of 49 P. sojae RXLRs and 52 of 78 P. viticola RXLRs could suppress INF‐triggered cell death (Wang et al., 2011; Liu et al., 2018).
3.2. P. agathidicida RXLR genes showed low genetic diversity and only some were expressed
In our study, genome analysis of 13 isolates of P. agathidicida from across the geographic range of kauri in New Zealand showed a lower level of nucleotide diversity (99.9% identical) based on pairwise SNP analysis. Among 78 PaRXLRs, only 10 showed polymorphism; of those only one was shown to elicit cell death in N. benthamiana but was not expressed in kauri. This low level of RXLR diversity was similar to the overall genome diversity, suggesting lack of enrichment for RXLR polymorphisms. This finding is concordant with an asexually reproducing population that is not endemic to New Zealand. Asexual reproduction is common among Phytophthora pathogens, and asexual lineages have been shown to cause epidemics (Pais et al., 2018).
Of the nine PaRXLRs that either elicited or suppressed cell death in N. benthamiana, only three were expressed in kauri. Whilst expression levels were generally lower in leaves than roots, consistent with P. agathidicida being a root pathogen, there were similar patterns of expression in the two tissue types, with PaRXLR24 most highly expressed in both. P. agathidicida has been shown to cause lesions on kauri leaves (Herewini et al., 2018) and our expression results indicate that some aspects of plant–pathogen interactions may be consistent across tissues. The observation that most of the PaRXLRs tested were not expressed in kauri was not surprising. Not all RXLR genes are expressed in planta, with lack of expression being one mechanism to evade recognition by cognate immune receptors (Gilroy et al., 2011; Pais et al., 2018).
Studies with other Phytophthora species have shown that the timing of in planta RXLR gene expression is important during infection (Wang et al., 2011; Cooke et al., 2012; Yin et al., 2017). In our study, the expression of PaRXLR40 peaked later than PaRXLR24 in both kauri root and leaf. These results, combined with our finding that PaRXLR40 is also able to suppress PaRXLR24 or Avr3a/R3a‐triggered cell death, suggest that PaRXLR40 may suppress downstream defence responses triggered by PaRXLR24.
3.3. PaRXLR24 as an ortholog of P. sojae Avh238
Because of its high expression in kauri and its strong cell‐death eliciting function in N. benthamiana, PaRXLR24 was compared to the orthologous P. sojae Avh238 in more detail. Site‐directed mutagenesis of PaRXLR24 identified that isoleucine 81 is important for PaRXLR24‐triggered cell death. An equivalent mutant version of its ortholog P. sojae Avh238 (H79th) also lost the ability to trigger cell death but revealed a cryptic virulence function as it was able to suppress INF1‐triggered defence. Suppression assays with cell‐death negative mutants of PaRXLR24 suggested that PaRXLR24 cannot suppress INF1‐triggered defence, and therefore does not appear to show the same virulence function as Avh238. P. sojae Avh238 interacts with, and destabilizes, type 2 1‐aminocyclopropane‐1‐carboxylic acid synthase (ACS), which interrupts ethylene biosynthesis that is required for resistance against P. sojae in soybean (Yang et al., 2017). Ethylene is an important hormone in plant defence against pathogens (Broekgaarden et al., 2015). However, it is not known if ethylene is involved in defence against P. agathidicida in kauri and whether PaRXLR24 shares the same host target as Avh238.
3.4. Potential immune receptor targets were found in N. benthamiana
In this study, NBS‐LRRs were identified from the angiosperm model‐plant N. benthamiana that specifically recognized RXLR effectors from P. agathidicida, which is pathogenic to a gymnosperm. It was previously shown that NBS‐LRR receptors from distantly related species can confer disease resistance, such as a maize NBS‐LRR enhancing resistance to a bacterial pathogen in Arabidopsis and rice plant hosts (Xu et al., 2018), indicating highly conserved mechanisms of plant defence. Our work supports the premise that these mechanisms may be very broadly conserved at the molecular level between gymnosperm and angiosperm systems.
Both of the top candidate NBS‐LRRs that recognized PaRXLR24 showed similarity to characterized immune receptors. The NBS‐LRR silenced by HP7‐2 showed similarity to late‐blight resistance protein R1 (Ballvora et al., 2002), which is encoded by the major R1 resistance gene cluster in potato (Kuang et al., 2005). R1 is involved in defence against P. infestans Avr1, an RXLR effector that directly interacts with host exocyst component Sec5, potentially disrupting the host vesicle trafficking system required for defence (Du et al., 2015). The other candidate NBS‐LRR, silenced by HP7‐1, showed similarity to Arabidopsis immune receptor RPM1 (El Kasmi et al., 2017). RPM1 guards RIN4, a conserved plant immunity signalling hub and a strong activator of plant defence (Toruño et al., 2019). Phosphorylation of RIN4 in the presence of pathogen effectors such as Pseudomonas syringae type III effectors AvrRpm1 and AvrB leads to activation of RPM1‐mediated downstream signal transduction and plant defence response (Toruño et al., 2019).
The hairpin‐based RNA silencing method used to identify NBS‐LRRs had some limitations. Inaccurate annotation of the N. benthamiana gene models may have led to over‐ or underestimation of functional NBS‐LRRs. Indeed, different numbers of targets were identified in the two versions of the N. benthamiana genome. Furthermore, due to similarities between N. benthamiana NBS‐LRR gene family sequences, off‐target silencing could occur (Guo et al., 2016; Brendolise et al., 2017). It is possible that the initial positive screening results with the R1‐like NBS‐LRRs targeted by HP14‐6 and HP14‐8 may have been off‐targets related to the stronger R1‐like HP7‐2 NBS‐LRR candidate. Confirmation of the NBS‐LRRs candidates in independent experiments using VIGS lent strong support for potential roles of NBS‐LRRs targeted by HP7‐1 and/or HP7‐2 in recognition of PaRXLR24 in N. benthamiana, but no support for those targeted by HP14‐6 and HP14‐8.
3.5. Implications for kauri dieback
There are few studies of molecular plant–microbe interactions involving forest trees, particularly gymnosperms. Indications from this work are that P. agathidicida may use similar molecular tools as other Phytophthora species that are principally angiosperm pathogens and it is feasible that immune receptors identified in model‐plants may enable the development of genetic markers for resistance in kauri. At the time of writing, the kauri genome sequence was not available. Meanwhile there is more that needs to be learned about the responses of kauri tissue to the effector proteins themselves and their effects on the ability of P. agathidicida to cause disease. There are also early indications that P. agathidicida may be able to colonize other gymnosperm hosts as well as some angiosperms, including Myrtaceae (Bradshaw et al., 2020).
In the event that immune receptors that recognize specific PaRXLRs can be identified in kauri, the implications for the continued health of such a long‐lived forest tree need consideration. Studies of short‐rotation crop pathogens and an increasing number of tree species have warned of the breakdown of major gene resistance due to rapidly evolving pathogens (Kinloch et al., 2008; Stam & McDonald, 2018). In agricultural crops, durability of resistance can be increased by pyramiding immune receptors and by selecting those that recognize effectors with important virulence functions that may incur a fitness cost if lost or mutated (Vleeshouwers & Oliver, 2014; Moscou & Van Esse, 2017). In forest trees the basis of resistance is very broad, including qualitative as well as quantitative genetic resistance (Ennos, 2015; Fraser et al., 2016), along with a complexity of biotic and abiotic environmental factors that can influence plant health in forests (Sniezko, 2006; Feau & Hamelin, 2017; Sniezko & Koch, 2017; Bradshaw et al., 2020). The long‐lived nature of trees also means that understanding the evolutionary ecology of the forest is critical to ensure durable resistance (Ennos, 2015). Thus, a wholistic approach to tree health involving all aspects from genetic resistance to population diversity to the dynamic microbiome is needed (Desprez‐Loustau et al., 2016; Feau & Hamelin, 2017; Moscou & Van Esse, 2017; Sniezko & Koch, 2017; Bradshaw et al., 2020). A deeper knowledge of the biology underlying plant–pathogen interactions that influence resistance and susceptibility will help illuminate the path forward.
3.6. Conclusions
Our work reveals how a Phytophthora pathogen of a gymnosperm tree species interacts with plants at the molecular level in ways consistent with those of angiosperm pathosystems and provides a foundation for studying the molecular basis of plant–pathogen interactions in gymnosperm trees. Notably, candidate immune receptors identified using this approach might ultimately provide molecular markers for resistance breeding in forest trees.
4. EXPERIMENTAL PROCEDURES
4.1. RXLR gene identification, motif and orthology predictions
RXLR effector gene candidates were predicted from the genome sequence of P. agathidicida strain NZFS3770 (Studholme et al., 2016) (GenBank: GCA_001314445.1). Gene models were computed using Augustus v. 2.5.5 (Stanke & Morgenstern, 2005), from which 78 RXLR effector gene candidates were identified by combining three prediction methods (Bhattacharjee et al., 2006; Whisson et al., 2007; Win et al., 2007) (Figure S1), using HMMER v. 3.0 (Finn et al., 2011) and SignalP v. 3.0 for signal peptide prediction (Bendtsen et al., 2004). Predicted nucleotide and amino acid sequences for these RXLRs are in Table S1 and on GenBank (accession numbers MT503101–MT503178). Maximum‐likelihood phylogenetic analysis was done as previously described (Ozturk et al., 2019). Over‐represented amino acid motifs in the 78 RXLR effector candidates were identified using default parameter values in the MEME suite motif alignment search tool (MAST) (Bailey et al., 2009).
4.2. A. tumefaciens‐mediated transient transformation assays
RXLR effector gene candidates and PaINF1‐1 were PCR‐amplified from genomic (g)DNA of P. agathidicida isolate NZFS3616 (the primers used are listed in Table S2). Single, double, and triple mutant versions of PaRXLR24 were made with a QuickChange II site‐directed mutagenesis kit (Agilent) using wild‐type (WT) PaRXLR24 template cloned into SmaI‐digested pICH41021 (Yanisch‐Perron et al., 1985). The RXLR PCR products, PaRXLR24 WT and mutant plasmids, along with either signal peptide PR1α (apoplastic) or N‐3 × FLAG tag (cytoplasmic) (Integrated DNA Technologies), were used as entry modules for Golden Gate assembly (Engler et al., 2008) into the Agrobacterium expression vector pICH86988 (Weber et al., 2011).
Verified plasmid constructs were transformed into A. tumefaciens GV3101 (Holsters et al., 1980). Three of the PaRXLRs could not be cloned so only 75 were screened for their ability to induce cell death. For these cell death screening assays, overnight cultures of transformed A. tumefaciens GV3101 were resuspended in buffer (10 mM MgCl2, 10 mM MES‐KOH pH 5.6, 100 μM acetosyringone) and infiltrated into N. benthamiana or N. tabacum leaves at a final OD600 of 1.0 (Ma et al., 2012). The 73 PaRXLRs that did not trigger cell death on N. benthamiana were tested for suppression of cell death. For suppression assays, A. tumefaciens carrying cell‐death elicitor genes were infiltrated 24 hr after P. agathidicida RXLR effectors at OD600 of 0.4 for all constructs (Wang et al., 2011). Symptoms were scored 7 days post‐infiltration.
4.3. Protein immunoblotting and RT‐PCR
To verify protein production in suppression assays, N. benthamiana leaves were infiltrated as described for suppression screening, harvested after 3 days, then snap‐frozen in liquid nitrogen. Total proteins were extracted using GTEN protein extraction buffer (Choi et al., 2018). Twenty microlitres of total protein extract was separated by SDS‐PAGE (10%–12% polyacrylamide). Gel electrophoresis was performed at 110 V for 2–3 hr in running buffer (25 mM Tris‐HCl, 192 mM glycine, 0.1% SDS) (Laemmli, 1970). Proteins were transferred to PVDF membrane (Sigma‐Aldrich) in transfer buffer (25 mM Tris‐HCl, 192 mM glycine, 20% (vol/vol) methanol) overnight at 30 V. Proteins were probed with mouse anti‐GFP (1/200), ‐HA (1/1000) (Santa Cruz Biotechnology) or ‐FLAG (1/5000) (Sigma‐Aldrich) primary antibody and chicken anti‐mouse IgG‐HRP (1/20,000) (Santa Cruz Biotechnology) secondary antibody. Membranes were treated with chemiluminescent substrate (SuperSignal West Dura Extended Duration; Thermo Fisher Scientific) and protein bands detected using a C600 Gel Imaging System (Azure Biosystem).
Because R3a‐HA2 could not be detected on a western blot, gene expression was verified by RT‐PCR. RNA was extracted using a Spectrum Plant Total RNA Kit (Sigma‐Aldrich) and cDNA synthesised using random primers (QuantiTect Reverse Transcription Kit, Qiagen). Gene‐specific primers were used to amplify R3a‐HA2 and GFP‐Avr3a from cDNA samples using Phusion Flash High‐Fidelity PCR Master Mix (Thermo Fisher Scientific).
4.4. Expression of P. agathidicida RXLR genes in planta
Quantitative RT‐PCR was used to determine expression of PaRXLRs in kauri tissue. Using P. agathidicida isolate 3813 (Herewini et al., 2018), grown in carrot broth and V8 juice (Horner & Hough, 2014; Herewini et al., 2018), small pieces of mycelium were placed onto fine root tips of 8‐month‐old susceptible kauri seedlings (HTHF‐2017‐MW8‐G). For leaves, a small surface wound was made 0.5 cm from the base of each leaf prior to inoculation. The roots were then sealed between wet paper towels in a plastic cassette and incubated at 17°C with 14.5 hr light:9.5 hr dark. Leaf and root samples were collected from three infected seedlings at 6, 24, 48, and 72 hr post‐inoculation, with one leaf or two root tips from each seedling (up to 2 cm from infection point). Samples were snap‐frozen in liquid nitrogen and stored at –80°C. All kauri plant material was respectfully destroyed at the completion of the experimental work.
RNA was extracted from the samples and cDNA synthesised as above. One microlitre of 2‐fold diluted cDNA was mixed with 5 µl of 2 × SensiFAST SYBR No‐ROX (Bioline) and 0.5 mM forward and reverse primer in a total volume of 10 µl. Two technical replicates for each of three biological replicates were subject to RT‐PCR, using 40 cycles of 95°C for 5 s, 60°C for 10 s, and 72°C for 20 s. Relative expression of P. agathidicida RXLR gene candidates was calculated with the Q‐Gene method (Muller et al., 2002), using the geometric mean of three P. agathidicida housekeeping genes, β‐tubulin, actin, and translation elongation factor 2, as reference (Table S1).
4.5. Genome sequencing and SNP analysis of 12 P. agathidicida isolates
Twelve isolates of P. agathidicida (Table 1) were grown in clarified carrot broth (Herewini et al., 2018) for 7 days at 17°C, with gDNA extracted from freeze‐dried mycelium (Moller et al., 1992). P. agathidicida gDNA was sequenced on an Illumina HiSeq 2500 at the Australian Genome Research Facility, using Illumina gDNA shotgun library preparation and HiSeq HT chemistry with 125 bp paired‐end reads (Illumina).
Raw DNA sequence data were processed with fastq‐mcf to remove primer and sequencing adapter sequences (Aronesty, 2011), then quality trimmed to a Phred score of >20 using SolexaQA v. 3.1.4 (Cox et al., 2010). Data quality was analysed using FastQC v. 0.11.5 (Bioinformatics, 2015). Sequence data are available from the Sequence Read Archive (SRA): BioProject PRJNA486676.
Paired‐end reads were mapped to the NZFS3770 reference genome (Studholme et al., 2016) with Bowtie 2 v. 2.2.6 (Langmead & Salzberg, 2012). SNPs in the 12 genomes were compared to the reference genome using FreeBayes v. 1.1.0‐46 (Garrison & Marth, 2012), with ploidy set to 2 (diploid). The FreeBayes VCF files were annotated based on P. agathidicida NZFS3770 gene models using SnpEff v. 4.3t, with default parameters and quality filtering at Q > 30 (Cingolani et al., 2012). Numbers of SNPs in coding sequences were determined using bedtools (Quinlan & Hall, 2010) (Table S3). Homozygous SNPs were extracted from VCF files and an alignment built by concatenating all 5,851 SNPs. A phylogeny was built using the poppr R package (Kamvar et al., 2014).
4.6. Screening for N. benthamiana receptors
Screening for N. benthamiana NBS‐LRR receptors involved in PaRXLR24 recognition was performed by RNA silencing (Brendolise et al., 2017). A. tumefaciens GV3101 cells carrying the constructs were infiltrated into 5‐week‐old N. benthamiana leaves with final OD600 of 0.2 for hairpin constructs and 0.4 for PaRXLR24. The 48 sets of pooled hairpins (Brendolise et al., 2017) were used in the first round of screening. Hairpins targeting individual NBS‐LRR genes from two positive pools (Table S4) were then used in the final screening. Symptoms were assessed 7 days post‐infiltration. Top individual NBS‐LRR candidates were assessed further using VIGS (Table S4), carried out as described previously (Wang et al., 2018), with silencing constructs developed using PCR primers (Table S2). Cell death was quantified by ion leakage as described previously (Jing et al., 2016). Silencing efficiencies of individual hairpins were determined using quantitative RT‐PCR, with RNA and cDNA prepared as above. NBS‐LRR gene expression in hairpin‐infiltrated N. benthamiana leaves is shown as a percentage of that in noninfiltrated leaves (n = 3) with expression of elongation factor 1‐α (EF1a) used for normalization.
Supporting information
ACKNOWLEDGMENTS
The work was supported by the Tertiary Education Commission of New Zealand via Bio‐Protection Research Centre grants, along with in‐kind support through the Scion Healthy Trees, Healthy Future programme (MBIE, C04X1305). Kauri germplasm was provided with permission from Taoho Patuawa on behalf of the Te Roroa Iwi Trust. J.W. was supported by the Gatsby Charitable foundation. Thanks to Dr Cyril Brendolise (Plant & Food Research, New Zaland) for provision of hairpin constructs, Professor Paul Birch and Dr Piers Hemsley (University of Dundee, UK) for GFP‐Avr3a and R3a‐HA2 constructs, Professor Jonathan Jones and Mark Youles (The Sainsbury Laboratory, UK) for plasmids pICH41021 and pICH86988, and Wenyue Zheng and Yuyin Wang (Nanjing Agricultural University, China) for assistance with VIGS screening.
Guo Y, Dupont P‐Y, Mesarich CH, et al. Functional analysis of RXLR effectors from the New Zealand kauri dieback pathogen Phytophthora agathidicida . Molecular Plant Pathology. 2020;21:1131–1148. 10.1111/mpp.12967
DATA AVAILABILITY STATEMENT
Sequence data of RXLR effector gene candidates from P. agathidicida strain NZFS3770 are available from GenBank at https://www.ncbi.nlm.nih.gov/ as genome assembly GCA_001314445.1 and accession numbers MT503101–MT503178. Sequence data of 12 isolates of P. agathidicida are available from the Sequence Read Archive (SRA) https://www.ncbi.nlm.nih.gov/bioproject/ as PRJNA486676.
REFERENCES
- Anderson, R.G. , Deb, D. , Fedkenheuer, K. and McDowell, J.M. (2015) Recent progress in RXLR effector research. Molecular Plant‐Microbe Interactions, 28, 1063–1072. [DOI] [PubMed] [Google Scholar]
- Armstrong, M.R. , Whisson, S.C. , Pritchard, L. , Bos, J.I.B. , Venter, E. , Avrova, A.O. et al (2005) An ancestral oomycete locus contains late blight avirulence gene Avr3a, encoding a protein that is recognized in the host cytoplasm. Proceedings of the National Academy of Sciences of the United States of America, 102, 7766–7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronesty, E. (2011) Ea‐Utils: command‐line tools for processing biological sequencing data. Durham, NC: Expression Analysis. [Google Scholar]
- Bailey, T.L. , Boden, M. , Buske, F.A. , Frith, M. , Grant, C.E. , Clementi, L. et al (2009) MEME SUITE: tools for motif discovery and searching. Nucleic Acids Research, 37, W202–W208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballvora, A. , Ercolano, M.R. , Weiß, J. , Meksem, K. , Bormann, C.A. , Oberhagemann, P. et al (2002) The R1 gene for potato resistance to late blight (Phytophthora infestans) belongs to the leucine zipper/NBS/LRR class of plant resistance genes. The Plant Journal, 30, 361–371. [DOI] [PubMed] [Google Scholar]
- Bastin, J.‐F. , Finegold, Y. , Garcia, C. , Mollicone, D. , Rezende, M. , Routh, D. et al (2019) The global tree restoration potential. Science, 365, 76–79. [DOI] [PubMed] [Google Scholar]
- Beever, R.E. , Waipara, N.W. , Ramsfield, T.D. , Dick, M.A. and Horner, I.J. (2009) Kauri (Agathis australis) under threat from Phytophthora . Phytophthoras in Forests and Natural Ecosystems, 74, 74–85. [Google Scholar]
- Bendtsen, J.D. , Nielsen, H. , von Heijne, G. and Brunak, S. (2004) Improved prediction of signal peptides: SignalP 3.0. Journal of Molecular Biology, 340, 783–795. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee, S. , Hiller, N.L. , Liolios, K. , Win, J. , Kanneganti, T.‐D. , Young, C. et al (2006) The malarial host‐targeting signal is conserved in the Irish potato famine pathogen. PLoS Pathogens, 2, e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bioinformatics, B. (2015) FastQC: a quality control tool for high throughput sequence data. Cambridge: Babraham Institute. [Google Scholar]
- Bombarely, A. , Rosli, H.G. , Vrebalov, J. , Moffett, P. , Mueller, L.A. and Martin, G.B. (2012) A draft genome sequence of Nicotiana benthamiana to enhance molecular plant–microbe biology research. Molecular Plant‐Microbe Interactions, 25, 1523–1530. [DOI] [PubMed] [Google Scholar]
- Bos, J.I. , Kanneganti, T.D. , Young, C. , Cakir, C. , Huitema, E. , Win, J. et al (2006) The C‐terminal half of Phytophthora infestans RXLR effector AVR3a is sufficient to trigger R3a‐mediated hypersensitivity and suppress INF1‐induced cell death in Nicotiana benthamiana . The Plant Journal, 48, 165–176. [DOI] [PubMed] [Google Scholar]
- Bradshaw, R.E. , Bellgard, S.E. , Black, A. , Burns, B.R. , Gerth, M.L. , McDougal, R.L. et al (2020) Phytophthora agathidicida: research progress, cultural perspectives and knowledge gaps in the control and management of kauri dieback in New Zealand. Plant Pathology, 69, 3–16. [Google Scholar]
- Bradshaw, R.E. , Guo, Y. , Sim, A.D. , Kabir, M.S. , Chettri, P. , Ozturk, I.K. et al (2016) Genome‐wide gene expression dynamics of the fungal pathogen Dothistroma septosporum throughout its infection cycle of the gymnosperm host Pinus radiata . Molecular Plant Pathology, 17, 210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier, C.M. (2000) The rise of hybrid fungi. Nature, 405, 134–135. [DOI] [PubMed] [Google Scholar]
- Bräutigam, K. , Vining, K.J. , Lafon‐Placette, C. , Fossdal, C.G. , Mirouze, M. , Marcos, J.G. et al (2013) Epigenetic regulation of adaptive responses of forest tree species to the environment. Ecology and Evolution, 3, 399–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendolise, C. , Montefiori, M. , Dinis, R. , Peeters, N. , Storey, R.D. and Rikkerink, E.H. (2017) A novel hairpin library‐based approach to identify NBS–LRR genes required for effector‐triggered hypersensitive response in Nicotiana benthamiana . Plant Methods, 13, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekgaarden, C. , Caarls, L. , Vos, I.A. , Pieterse, C.M. and Van Wees, S.C. (2015) Ethylene: traffic controller on hormonal crossroads to defense. Plant Physiology, 169, 2371–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burgh, A.M. and Joosten, M.H. (2019) Plant immunity: thinking outside and inside the box. Trends in Plant Science, 24, 587–601. [DOI] [PubMed] [Google Scholar]
- Callaghan, S. and Guest, D. (2015) Globalisation, the founder effect, hybrid Phytophthora species and rapid evolution: new headaches for biosecurity. Australasian Plant Pathology, 44, 255–262. [Google Scholar]
- Choi, S. , Jayaraman, J. and Sohn, K.H. (2018) Arabidopsis thaliana SOBER 1 (SUPPRESSOR OF AVRBST‐ELICITED RESISTANCE 1) suppresses plant immunity triggered by multiple bacterial acetyltransferase effectors. New Phytologist, 219, 324–335. [DOI] [PubMed] [Google Scholar]
- Cingolani, P. , Platts, A. , Wang, L.L. , Coon, M. , Nguyen, T. , Wang, L. et al (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso‐2; iso‐3. Fly, 6, 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, D.E. , Mesarich, C.H. and Thomma, B.P.H.J. (2015) Understanding plant immunity as a surveillance system to detect invasion. Annual Review of Phytopathology, 53, 541–563. [DOI] [PubMed] [Google Scholar]
- Cooke, D.E. , Cano, L.M. , Raffaele, S. , Bain, R.A. , Cooke, L.R. , Etherington, G.J. et al (2012) Genome analyses of an aggressive and invasive lineage of the Irish potato famine pathogen. PLoS Pathogens, 8, e1002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, M.P. , Peterson, D.A. and Biggs, P.J. (2010) SolexaQA: at‐a‐glance quality assessment of Illumina second‐generation sequencing data. BMC Bioinformatics, 11, 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalio, R.J.D. , Maximo, H.J. , Oliveira, T.S. , Dias, R.O. , Breton, M.C. , Felizatti, H. et al (2018) Phytophthora parasitica effector PpRxLR2 suppresses Nicotiana benthamiana immunity. Molecular Plant‐Microbe Interactions, 31, 481–493. [DOI] [PubMed] [Google Scholar]
- Dangl, J.L. , Horvath, D.M. and Staskawicz, B.J. (2013) Pivoting the plant immune system from dissection to deployment. Science, 341, 746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb, D. , Anderson, R.G. , How‐Yew‐Kin, T. , Tyler, B.M. and McDowell, J.M. (2018) Conserved RxLR effectors from oomycetes Hyaloperonospora arabidopsidis and Phytophthora sojae suppress PAMP‐ and effector‐triggered immunity in diverse plants. Molecular Plant‐Microbe Interactions, 31, 374–385. [DOI] [PubMed] [Google Scholar]
- Desprez‐Loustau, M.‐L. , Aguayo, J. , Dutech, C. , Hayden, K.J. , Husson, C. , Jakushkin, B. et al (2016) An evolutionary ecology perspective to address forest pathology challenges of today and tomorrow. Annals of Forest Science, 73, 45–67. [Google Scholar]
- Du, Y. , Mpina, M.H. , Birch, P.R.J. , Bouwmeester, K. and Govers, F. (2015) Phytophthora infestans RXLR effector AVR1 interacts with exocyst component Sec5 to manipulate plant immunity. Plant Physiology, 169, 1975–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kasmi, F. , Chung, E.‐H. , Anderson, R.G. , Li, J. , Wan, L. , Eitas, T.K. et al (2017) Signaling from the plasma‐membrane localized plant immune receptor RPM1 requires self‐association of the full‐length protein. Proceedings of the National Academy of Sciences of the United States of America, 114, E7385–E7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt, S. , Boevink, P.C. , Armstrong, M.R. , Ramos, M.B. , Hein, I. and Birch, P.R. (2012) Relocalization of late blight resistance protein R3a to endosomal compartments is associated with effector recognition and required for the immune response. The Plant Cell, 24, 5142–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler, C. , Kandzia, R. and Marillonnet, S. (2008) A one pot, one step, precision cloning method with high throughput capability. PLoS ONE, 3, e3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennos, R.A. (2015) Resilience of forests to pathogens: an evolutionary ecology perspective. Forestry, 88, 41–52. [Google Scholar]
- Feau, N. and Hamelin, R.C. (2017) Say hello to my little friends: how microbiota can modulate tree health. New Phytologist, 215, 508–510. [DOI] [PubMed] [Google Scholar]
- Finn, R.D. , Clements, J. and Eddy, S.R. (2011) HMMER web server: interactive sequence similarity searching. Nucleic Acids Research, 39, W29–W37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, S. , Martín‐García, J. , Perry, A. , Kabir, M.S. , Owen, T. , Solla, A. et al (2016) A review of Pinaceae resistance mechanisms against needle and shoot pathogens with a focus on the Dothistroma–Pinus interaction. Forest Pathology, 46, 453–471. [Google Scholar]
- Garrison, E. and Marth, G. (2012) Haplotype‐based variant detection from short‐read sequencing. arXiv, 1207.3907 [q‐bio.GN]. https://arxiv.org/abs/1207.3907v2. [Google Scholar]
- Gilroy, E.M. , Breen, S. , Whisson, S.C. , Squires, J. , Hein, I. , Kaczmarek, M. et al (2011) Presence/absence, differential expression and sequence polymorphisms between PiAVR2 and PiAVR2‐like in Phytophthora infestans determine virulence on R2 plants. New Phytologist, 191, 763–776. [DOI] [PubMed] [Google Scholar]
- Goss, E.M. , Larsen, M. , Vercauteren, A. , Werres, S. , Heungens, K. and Grünwald, N.J. (2011) Phytophthora ramorum in Canada: evidence for migration within North America and from Europe. Phytopathology, 101, 166–171. [DOI] [PubMed] [Google Scholar]
- Grosse‐Holz, F. , Kelly, S. , Blaskowski, S. , Kaschani, F. , Kaiser, M. and van der Hoorn, R.A. (2018) The transcriptome, extracellular proteome and active secretome of agroinfiltrated Nicotiana benthamiana uncover a large, diverse protease repertoire. Plant Biotechnology Journal, 16, 1068–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Q. , Liu, Q. , A. Smith, N. , Liang, G. and Wang, M.‐B. (2016) RNA silencing in plants: mechanisms, technologies and applications in horticultural crops. Current Genomics, 17, 476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, E.M. (2015) Phytophthora species emerging as pathogens of forest trees. Current Forestry Reports, 1, 16–24. [Google Scholar]
- Herewini, E.M. , Scott, P.M. , Williams, N.M. and Bradshaw, R.E. (2018) In vitro assays of Phytophthora agathidicida on kauri leaves suggest variability in pathogen virulence and host response. New Zealand Plant Protection, 71, 285–288. [Google Scholar]
- Holsters, M. , Silva, B. , Van Vliet, F. , Genetello, C. , De Block, M. , Dhaese, P. et al (1980) The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid, 3, 212–230. [DOI] [PubMed] [Google Scholar]
- Horner, I.J. and Hough, E.G. (2014) Pathogenicity of four Phytophthora species on kauri: in vitro and glasshouse trials. New Zealand Plant Protection, 67, 54–59. [Google Scholar]
- Huang, G. , Liu, Z. , Gu, B. , Zhao, H. , Jia, J. , Fan, G. et al (2019) An RXLR effector secreted by Phytophthora parasitica is a virulence factor and triggers cell death in various plants. Molecular Plant Pathology, 20, 356–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, R.H. , Tripathy, S. , Govers, F. and Tyler, B.M. (2008) RXLR effector reservoir in two Phytophthora species is dominated by a single rapidly evolving superfamily with more than 700 members. Proceedings of the National Academy of Sciences of the United States of America, 105, 4874–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing, M. , Guo, B. , Li, H. , Yang, B. , Wang, H. , Kong, G. et al (2016) A Phytophthora sojae effector suppresses endoplasmic reticulum stress‐mediated immunity by stabilizing plant binding immunoglobulin proteins. Nature Communications, 7, 11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judelson, H.S. (2012) Dynamics and innovations within oomycete genomes: insights into biology, pathology, and evolution. Eukaryotic Cell, 11, 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun, S. , van West, P. , Vleeshouwers, V.G. , de Groot, K.E. and Govers, F. (1998) Resistance of Nicotiana benthamiana to Phytophthora infestans is mediated by the recognition of the elicitor protein INF1. The Plant Cell, 10, 1413–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamvar, Z.N. , Tabima, J.F. and Grünwald, N.J. (2014) Poppr: an R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ, 2, e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keriö, S. , Daniels, H.A. , Gomez‐Gallego, M. , Tabima, J.F. , Lenz, R.R. , Sondreli, K.L. et al (2019) From genomes to forest management—Tackling invasive Phytophthora species in the era of genomics. Canadian Journal of Plant Pathology, 42, 1–29. [Google Scholar]
- Kinloch, B.B. , Davis, D.A. and Burton, D. (2008) Resistance and virulence interactions between two white pine species and blister rust in a 30‐year field trial. Tree Genetics & Genomes, 4, 65–74. [Google Scholar]
- Kuang, H. , Wei, F. , Marano, M.R. , Wirtz, U. , Wang, X. , Liu, J. et al (2005) The R1 resistance gene cluster contains three groups of independently evolving, type I R1 homologues and shows substantial structural variation among haplotypes of Solanum demissum . The Plant Journal, 44, 37–51. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the dead of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lambert, S. , Waipara, N. , Black, A. , Mark‐Shadbolt, M. and Wood, W. (2018) Indigenous biosecurity: māori responses to kauri dieback and myrtle rust in Aotearoa New Zealand In: Urquhart J., Marzano M. and Potter C.E. (Eds.) The Human Dimensions of Forest and Tree Health. New York: Springer, pp. 109–137. [Google Scholar]
- Langmead, B. and Salzberg, S.L. (2012) Fast gapped‐read alignment with Bowtie 2. Nature Methods, 9, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H.‐A. and Yeom, S.‐I. (2015) Plant NB‐LRR proteins: tightly regulated sensors in a complex manner. Briefings in Functional Genomics, 14, 233–242. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Lan, X. , Song, S. , Yin, L. , Dry, I.B. , Qu, J. et al (2018) In planta functional analysis and subcellular localization of the oomycete pathogen Plasmopara viticola candidate RXLR effector repertoire. Frontiers in Plant Science, 9, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L. , Lukasik, E. , Gawehns, F. and Takken, F.L. (2012) The use of agroinfiltration for transient expression of plant resistance and fungal effector proteins in Nicotiana benthamiana leaves In: Joseph H. and Howlett B.J. (Eds.) Plant Fungal Pathogens. New York: Springer, pp. 61–74. [DOI] [PubMed] [Google Scholar]
- Ma, Z.G. , Liu, J.J. and Zamany, A. (2019) Identification and functional characterization of an effector secreted by Cronartium ribicola . Phytopathology, 109, 942–951. [DOI] [PubMed] [Google Scholar]
- Moller, E.M. , Bahnweg, G. , Sandermann, H. and Geiger, H.H. (1992) A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies and infected plant tissues. Nucleic Acids Research, 20, 6115–6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscou, M.J. and Van Esse, H.P. (2017) The quest for durable resistance. Science, 358, 1541–1542. [DOI] [PubMed] [Google Scholar]
- Muller, P. , Janovjak, H. , Miserez, A. and Dobbie, Z. (2002) Processing of gene expression data generated by quantitative real‐time RT‐PCR. BioTechniques, 33, 514. [PubMed] [Google Scholar]
- Ntoukakis, V. and Gifford, M.L. (2019) Plant–microbe interactions: tipping the balance. Journal of Experimental Botany, 70, 4583–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden, J. (1995) The long‐term conservation of forest diversity in New Zealand. Pacific Conservation Biology, 2, 77–90. [Google Scholar]
- Ozturk, I.K. , Dupont, P.‐Y. , Chettri, P. , McDougal, R. , Böhl, O.J. , Cox, R.J. et al (2019) Evolutionary relics dominate the small number of secondary metabolism genes in the hemibiotrophic fungus Dothistroma septosporum . Fungal Biology, 123, 397–407. [DOI] [PubMed] [Google Scholar]
- Pais, M. , Yoshida, K. , Giannakopoulou, A. , Pel, M.A. , Cano, L.M. , Oliva, R.F. et al (2018) Gene expression polymorphism underpins evasion of host immunity in an asexual lineage of the Irish potato famine pathogen. BMC Evolutionary Biology, 18, 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan, A.R. and Hall, I.M. (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics, 26, 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qutob, D. , Chapman, B.P. and Gijzen, M. (2013) Transgenerational gene silencing causes gain of virulence in a plant pathogen. Nature Communications, 4, 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello, T. and Asiegbu, F.O. (2017) Small secreted proteins from the necrotrophic conifer pathogen Heterobasidion annosum sl. (HaSSPs) induce cell death in Nicotiana benthamiana . Scientific Reports, 7, 8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietman, H. , Bijsterbosch, G. , Cano, L.M. , Lee, H.‐R. , Vossen, J.H. , Jacobsen, E. et al (2012) Qualitative and quantitative late blight resistance in the potato cultivar Sarpo Mira is determined by the perception of five distinct RXLR effectors. Molecular Plant‐Microbe Interactions, 25, 910–919. [DOI] [PubMed] [Google Scholar]
- Shuey, L.S. , Pegg, K. , Dodd, S. , Manners, A.G. , White, D. , Burgess, T.I. et al (2019) Araucaria dieback – a threat to native and plantation forests In: Edwards J. (Eds.), Australasian Plant Pathology Society Conference APPS 2019 Strong Foundations, Future Innovations. Melbourne, Australia: Australasian Plant Pathology Society. [Google Scholar]
- Simberloff, D. and Leppanen, C. (2019) Plant somatic mutations in nature conferring insect and herbicide resistance. Pest Management Science, 75, 14–17. [DOI] [PubMed] [Google Scholar]
- Sniezko, R.A. (2006) Resistance breeding against nonnative pathogens in forest trees—Current successes in North America. Canadian Journal of Plant Pathology, 28, S270–S279. [Google Scholar]
- Sniezko, R.A. and Koch, J. (2017) Breeding trees resistant to insects and diseases: putting theory into application. Biological Invasions, 19, 3377–3400. [Google Scholar]
- Sniezko, R.A. , Smith, J. , Liu, J.‐J. and Hamelin, R.C. (2014) Genetic resistance to fusiform rust in southern pines and white pine blister rust in white pines—A contrasting tale of two rust pathosystems—Current status and future prospects. Forests, 5, 2050–2083. [Google Scholar]
- Stam, R. and McDonald, B.A. (2018) When resistance gene pyramids are not durable‐the role of pathogen diversity. Molecular Plant Pathology, 19, 521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke, M. and Morgenstern, B. (2005) AUGUSTUS: a web server for gene prediction in eukaryotes that allows user‐defined constraints. Nucleic Acids Research, 33, W465–W467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, J.E. , Kim, M.‐S. and Klopfenstein, N.B. (2018) Molecular genetic approaches toward understanding forest‐associated fungi and their interactive roles within forest ecosystems. Current Forestry Reports, 4, 72–84. [Google Scholar]
- Studholme, D.J. , McDougal, R.L. , Sambles, C. , Hansen, E. , Hardy, G. , Grant, M. et al (2016) Genome sequences of six Phytophthora species associated with forests in New Zealand. Genomics Data, 7, 54–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toruño, T.Y. , Shen, M. , Coaker, G. and Mackey, D. (2019) Regulated disorder: posttranslational modifications control the RIN4 plant immune signaling hub. Molecular Plant‐Microbe Interactions, 32, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Wouw, A.P. and Idnurm, A. (2019) Biotechnological potential of engineering pathogen effector proteins for use in plant disease management. Biotechnology Advances, 37, 107387–107396. [DOI] [PubMed] [Google Scholar]
- Velásquez, A.C. , Chakravarthy, S. and Martin, G.B. (2009) Virus‐induced gene silencing (VIGS) in Nicotiana benthamiana and tomato. Journal of Visualized Experiments, 28, e1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleeshouwers, V.G.A.A. and Oliver, R.P. (2014) Effectors as tools in disease resistance breeding against biotrophic, hemibiotrophic, and necrotrophic plant pathogens. Molecular Plant‐Microbe Interactions, 27, 196–206. [DOI] [PubMed] [Google Scholar]
- Waipara, N. , Hill, S. , Hill, L. , Hough, E. and Horner, I. (2013) Surveillance methods to determine tree health, distribution of kauri dieback disease and associated pathogens. New Zealand Plant Protection, 66, 235–241. [Google Scholar]
- Wang, Q. , Han, C. , Ferreira, A.O. , Yu, X. , Ye, W. , Tripathy, S. et al (2011) Transcriptional programming and functional interactions within the Phytophthora sojae RXLR effector repertoire. The Plant Cell, 23, 2064–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. and Jiao, F. (2019) Effectors of Phytophthora pathogens are powerful weapons for manipulating host immunity. Planta, 250, 413–425. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Tyler, B.M. and Wang, Y. (2019) Defense and counterdefense during plant‐pathogenic oomycete infection. Annual Review of Microbiology, 73, 667–696. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Xu, Y. , Sun, Y. , Wang, H. , Qi, J. , Wan, B. et al (2018) Leucine‐rich repeat receptor‐like gene screen reveals that Nicotiana RXEG1 regulates glycoside hydrolase 12 MAMP detection. Nature Communications, 9, 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle, P. (1991) Vegetation of New Zealand. Cambridge University Press. [Google Scholar]
- Weber, E. , Engler, C. , Gruetzner, R. , Werner, S. and Marillonnet, S. (2011) A modular cloning system for standardized assembly of multigene constructs. PLoS ONE, 6, e16765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir, B.S. , Paderes, E.P. , Anand, N. , Uchida, J.Y. , Pennycook, S.R. , Bellgard, S.E. et al (2015) A taxonomic revision of Phytophthora clade 5 including two new species, Phytophthora agathidicida and P. cocois . Phytotaxa, 205, 21–38. [Google Scholar]
- Whisson, S.C. , Boevink, P.C. , Moleleki, L. , Avrova, A.O. , Morales, J.G. , Gilroy, E.M. et al (2007) A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature, 450, 115–118. [DOI] [PubMed] [Google Scholar]
- Win, J. , Morgan, W. , Bos, J. , Krasileva, K.V. , Cano, L.M. , Chaparro‐Garcia, A. et al (2007) Adaptive evolution has targeted the C‐terminal domain of the RXLR effectors of plant pathogenic oomycetes. The Plant Cell, 19, 2349–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield, M.J. , Brockerhoff, E.G. , Wingfield, B.D. and Slippers, B. (2015) Planted forest health: the need for a global strategy. Science, 349, 832–836. [DOI] [PubMed] [Google Scholar]
- de Wit, P.J.G.M. , van der Burgt, A. , Okmen, B. , Stergiopoulos, I. , Abd‐Elsalam, K.A. , Aerts, A.L. et al (2012) The genomes of the fungal plant pathogens Cladosporium fulvum and Dothistroma septosporum reveal adaptation to different hosts and lifestyles but also signatures of common ancestry. PLoS Genetics, 8, e1003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, A.J. , Martín‐García, J. , Bulman, L. , Vasconcelos, M.W. , Boberg, J. , La Porta, N. et al (2016) Dothistroma needle blight, weather and possible climatic triggers for the disease's recent emergence. Forest Pathology, 46, 443–452. [Google Scholar]
- Xu, Y. , Liu, F. , Zhu, S. and Li, X. (2018) The maize NBS‐LRR gene ZmNBS25 enhances disease resistance in rice and Arabidopsis . Frontiers in Plant Science, 9, 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B. , Wang, Q.Q. , Jing, M.F. , Guo, B.D. , Wu, J.W. , Wang, H.N. et al (2017) Distinct regions of the Phytophthora essential effector Avh238 determine its function in cell death activation and plant immunity suppression. New Phytologist, 214, 361–375. [DOI] [PubMed] [Google Scholar]
- Yanisch‐Perron, C. , Vieira, J. and Messing, J. (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mpl8 and pUC19 vectors. Gene, 33, 103–119. [DOI] [PubMed] [Google Scholar]
- Yin, J.L. , Gu, B. , Huang, G.Y. , Tian, Y. , Quan, J.L. , Lindqvist‐Kreuze, H. et al (2017) Conserved RXLR effector genes of Phytophthora infestans expressed at the early stage of potato infection are suppressive to host defense. Frontiers in Plant Science, 8, 2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data of RXLR effector gene candidates from P. agathidicida strain NZFS3770 are available from GenBank at https://www.ncbi.nlm.nih.gov/ as genome assembly GCA_001314445.1 and accession numbers MT503101–MT503178. Sequence data of 12 isolates of P. agathidicida are available from the Sequence Read Archive (SRA) https://www.ncbi.nlm.nih.gov/bioproject/ as PRJNA486676.


