Abstract
Unexpected biomagnifications and bioaccumulation of heavy metals (HMs) in the surrounding environment has become a predicament for all living organisms together with plants. Excessive release of HMs from industrial discharge and other anthropogenic activities has threatened sustainable agricultural practices and limited the overall profitable yield of different plants species. Heavy metals at toxic levels interact with cellular molecules, leading towards the unnecessary generation of reactive oxygen species (ROS), restricting productivity and growth of the plants. The application of various osmoprotectants is a renowned approach to mitigate the harmful effects of HMs on plants. In this review, the effective role of glycine betaine (GB) in alleviation of HM stress is summarized. Glycine betaine is very important osmoregulator, and its level varies considerably among different plants. Application of GB on plants under HMs stress successfully improves growth, photosynthesis, antioxidant enzymes activities, nutrients uptake, and minimizes excessive heavy metal uptake and oxidative stress. Moreover, GB activates the adjustment of glutathione reductase (GR), ascorbic acid (AsA) and glutathione (GSH) contents in plants under HM stress. Excessive accumulation of GB through the utilization of a genetic engineering approach can successfully enhance tolerance against stress, which is considered an important feature that needs to be investigated in depth.
Keywords: heavy metal stress, glycine betaine (GB) accumulation, exogenous application, plants, antioxidant enzymes, genetic engineering
1. Introduction
Over the years metalloids and heavy metals have received substantial consideration in multidisciplinary areas of environmental and geosciences due to their biomagnification, bioaccumulation along with negative ecological impacts [1,2,3,4,5,6,7,8,9,10]. Anthropogenic activities are the main source of HM pollution for both water bodies and soil, as a result causing severe problems for human being as well [11,12,13,14]. Smelting, electroplating, mining, fertilizers, open waste dumping, tanneries, automobile emission, electronic and paper industries have been reported as discharging huge amounts of HMs in the environment as an anthropogenic source [15,16,17]. Certainly, high exposure to heavy metal pollution has harmful effects on human health, including cancer, reproductive and neurological disorders and damage to central nervous system [18]. Human health could be exposed to different HMs in different ways, such as generation of HMs from various sources can produce pollution for the plants consumed by humans [19]. Heavy metals are hazardous pollutants characterized by their toxicity, persistence and non-biodegradability in neighboring environments [20]. Depending upon the concentration of HMs in the surrounding environment and the characteristics of plant species, metals can gather to various degrees in plants.
Plants have the capability to uptake and translocate toxic metals in different parts of their body as reported in several studies [21,22,23,24,25,26]. Uptake of HMs in plants not only inhibits the growth attributes of plants, but also induces toxic effects on consumer health [27,28,29,30,31,32]. Various studies have revealed the harmful impacts of HMs on human health and plant growth and development [33,34,35,36,37,38,39]. High concentrations of harmful metals particularly in soil significantly affects physiological and morphological traits of the plants [40,41]. Under HM stress, plants showed evident symptoms of structural transformation and inhibition of the photosynthesis process [42]. Different HMs such as zinc (Zn), cadmium (Cd), aluminum (Al), chromium (Cr), nickel (Ni) and metalloids like arsenic (As) diminish plant development and growth by producing a number of metabolic alterations in plants [43,44]. Most likely, heavy metal ions stay in the cytoplasm and cause oxidative stress through the excessive formation of reactive oxygen species (ROS), which ultimately restrict cell metabolism [45].
2. Signaling Response in Plants against Heavy Metal Stress
Environmental stresses in plants are caused by intense growth conditions that restrain the normal development and growth of the plants, which may be fatal in severe cases [46]. Different types of environmental stresses like HMs, temperature, drought and salinity are main cause of morphological and physiological changes in plants. All environmental stresses (biotic and a biotic) generate oxidative stress which can easily harm cell components and cause their dysfunction pursued by the uptake and over production of ROS at high rates. Enzyme complexes of nicotinamide adenine dinucleotide phosphaten (NADPH) oxidases generally involved in the generation of ROS, which then usually accumulate in different organelles of cell especially cytoplasm, mitochondria and nucleus [47,48]. Uncontrolled production of ROS results in protein denaturation, carbohydrates oxidation, oxidation of RNA and DNA, lipid peroxidation in cellular compartments, and it severely affects enzymatic activity in plants [49].
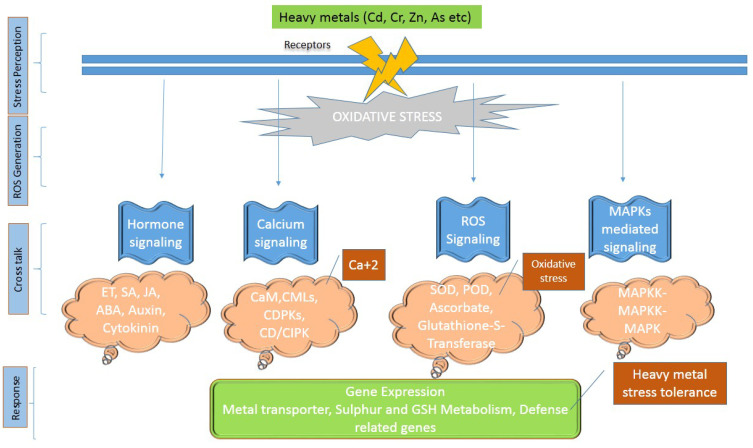
Heavy metals produce oxidative stress by disturbing the ROS stability in cells, and then induce the antioxidant mechanism of plants. For examples, excessive accumulation of Cr leads towards the generation of ROS and without doubt oxidative stress as well [50,51]. Heavy metals at toxic levels obstructs normal metabolic processes in different ways including displacement and disturbance of protein building blocks [52], adversely altering and affecting the authenticity of the cytoplasmic membrane, repressing critical events in plants such as respiration, photosynthesis and enzymatic activities [53]. In plants, heavy metal stress starts diverse signaling paths including signaling of mitogen activated protein kinase mitogen activated protein kinases (MAPKs) are group of protein kinases that perform a vital role during isignal transduction through modulating gene transcription in the nucleus as an appropriate response to changes occurs in the cellular environment, calcium dependent signaling, hormone signaling and signaling of ROS [54]. These signaling pathways increase the expression of responsive stress genes in plants [55]. Transduction of heavy metal stress signaling is started by ion channels/receptors through awareness of stress signals and also through non-protein messengers like hydrogen ions, calcium and cyclic nucleotides as given in (Figure 1). Several studies reported the importance of these signaling molecules and responsive genes in plants against the stress induced by HMs [56,57,58,59,60].
Figure 1.
Heavy metals stress signaling in plants.
Heavy metals stress provoke oxidative stress, osmotic stress and denaturation of proteins in plants, which eventually lead adaptive cellular responses such as acceleration of antioxidant system, speeding up the ROS scavenging, initiation of stress protein and accumulation of well-matched solutes [61]. Commonly, plants produce various types of solutes in response of stress. Most common compatible solutes are polyols, proline, sucrose, trehalose, different compounds of quaternary ammonium like choline O-sulfate, pipecolatebetaine, hydroxyprolinebetaine, alanine betaine and glycine betaine (GB). They are also known as osmoprotectants because they protect plant from dehydration injuries. They have the ability to safeguard plants from harmful stress through various ways such as the stabilization of protein and enzymes as well as safeguarding of membrane integrality and detoxification of ROS [62,63,64]. Application of these compatible solutes can significantly improve crop growth and yield in stressed environment.
3. Accumulation of Glycine Betaine (GB) as an Emerging Signal Molecule in Plants
Many plants accumulate higher concentration of GB against abiotic stress [65]. GB mostly is accumulated in chloroplast, where it is actively involved in safeguarding the Photosystem II (PSII) effectiveness under stressful conditions [64,66]. Accumulation and uptake of GB is more prominent in chloroplasts as compared to other cellular compartments of the plants in shielding the plants facing salinity and oxidative stress [67]. According to He et al. [68], high accumulation of GB enhances the rate of seed germination along with exceptional growth of grains., Accumulation of GB in plants is beneficial because it provides the nitrogen source as a result of better roots growth and germination of seeds. The better growth and earlier sprouting of roots results in greater biomass of wheat seedlings [68]. This suggests that more accumulation of GB results in more enhancements of both the seedlings and seedlings growth in wheat crop [68]. Not all the plants have the mechanism to accumulate GB by natural means, even at lower levels some of the transgenic plants have ability to accumulate GB [69].
Glycine betaine triggers the antioxidant system of the plant by activating the activities of diverse antioxidant enzymes like peroxidase (POX), catalase (CAT) and superoxide dismutase (SOD), which ultimately protects plant from oxidative harm [70]. Stimulation of antioxidant machinery in different plants species under numerous stressful condition by GB is extensively studied and reported in different studies [71,72,73]. Moreover, GB not only improves the development and growth of the plant, but also increases yield through employing much better photosynthesis system in plant [74]. Fundamental mechanisms involve in stress tolerance and degree of yield development by GB is yet not fully exposed. Taking into account the importance of GB, present review highlights the effectiveness and efficiency of GB in remediating the stress induced by HMs along with improvement in plant growth and development.
4. Biosynthesis of Glycine Betaine in Plants
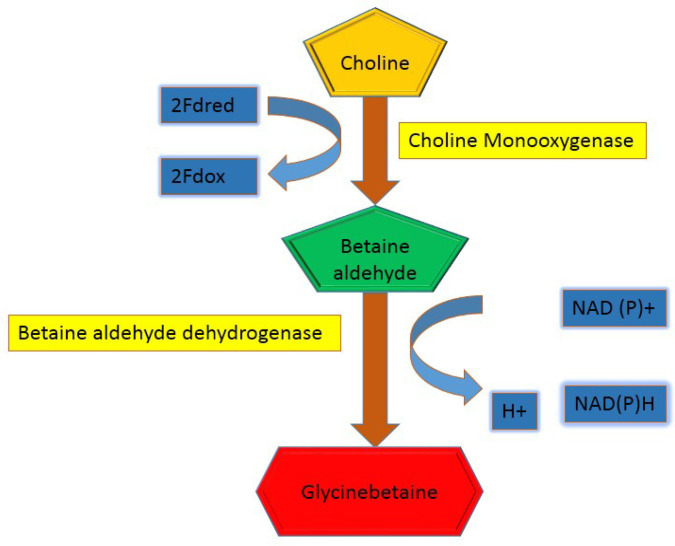
Various kinds of procedures and techniques have been utilized to cut down the heavy metal stress in plants. Use of different osmoprotectants to decrease the stress in plants induced by metal toxicity is one such technique. Glycine betaine (N,N,N-trimethylglycine) is a very vital osmoregulation material, and its concentration differs significantly amongst different species of plants. Glycine betaine in higher plants synthesized in chloroplast from serine through betaine aldehyde, choline and ethanolamine [75]. Choline is altered into betaine aldehyde with the help of choline mono oxygenase, which then eventually converts into glycinebetaine (GB) via betaine aldehyde hydrogenase (Figure 2). Under stressful conditions various plants have the potential to generate the GB as vital compound. Glycine betaine is environmentally safe, non-toxic and water soluble compound [66]. Glycine betaine is extensively engaged in the fortification of plants alongside various stress full conditions including salinity [76,77], drought [78,79,80], and HMs [81,82,83,84,85]. Application of GB reduced the HMs stress on plants by stabilizing the proteins, hunting ROS and protecting photosynthesis process [86].
Figure 2.
Biosynthesis pathway of glycine betaine in higher plants.
5. Role of Glycine Betaine in Plant Growth
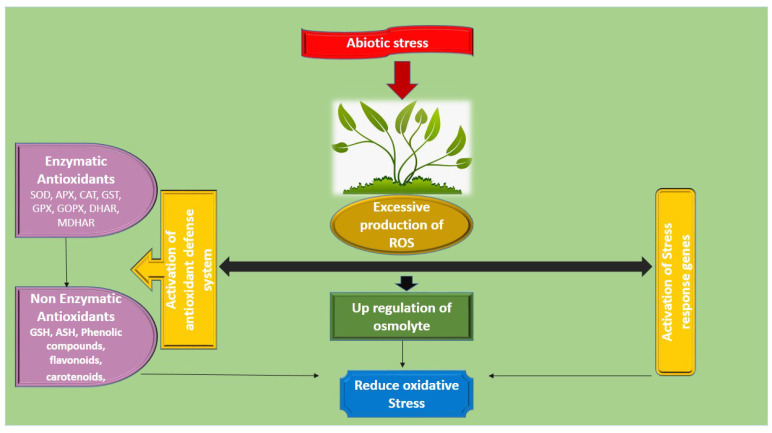
Different metabolites are extensively present in plants, they regulate the growth of plants and support them to survive under environmental stress conditions [87]. These compatible osmolytes maintain plant growth and cell enlargement in stressful environments. Glycine betaine is one such organic osmolyte. Glycine betaine has significant importance in biological and morphological processes in plants. Accumulation of GB in plants increases the yields in terms of number of seeds, fruits and flower size [88]. A number of studies has shown that GB enhances growth of the plant by growing photosynthesis rate and chlorophyll levels in various plants like tomato [67], wheat [76], cotton [82] sweet potato [89] and soybean [90]. Utilization of GB considerably promote the plant growth via boosting up osmolyte accumulation and antioxidant potential [91]. Plants normally have active defense system (antioxidant system) which controls the oxidative stress [35], it includes both enzymatic and non-enzymatic system, and they actively take part in scavenging of ROS (Figure 3). The antioxidant system takes active part in promoting the growth of different plants species through controlling the excessive over-generation of ROS during both non-stressed and stressed environment [35,92,93,94].
Figure 3.
Abiotic stress response in plants.
In plants, GB plays a vibrant role in stimulation of the antioxidant system [95]. In plants, GB has the ability to extensively boost up the activities and exercise of various antioxidant enzymes such as SOD, CAT and POD [70]. Furthermore, it was perceived that GB assists in improving the chlorophyll content and photosynthesis rate in plants [82,96]. Different studies have reported constructive role of GB in plant growth and biomass under heavy metal stress [95,97,98,99]. Islam et al. [100] investigated that glycine betaine significantly improves cell growth of tobacco plant under Cd stress. Exogenous application of GB extensively improve plant growth, biomass and rate of survival [101,102]. Glycine betaine shields photosynthetic pigments and chlorophyll contents which ultimately results in improved plant growth [103]. Different studies have reported that GB demonstrated encouraging effects on improvement and growth of several plants in stressed conditions [70,83,85,98].
6. Promotive Role of Glycine Betaine in Mitigating Heavy Metal Stress in Plants
Heavy metals are considered very important pollutants in the environment and most of them are very toxic even at very low concentration [104]. A high concentration of HMs uptake in plants causes the excessive generation of ROS which may results in oxidative damage to cellular compartments of the plants [105,106]. Therefore the understanding antioxidant mechanism, oxidative stress along with its mechanism of alleviating the oxidative stress are crucial for plants under heavy metal stress. Glycine betaine also act as osmoprotectants inhibiting the production of ROS and free radicals [107]. Alteration of gene expression has been observed in different studies under HMs stress [55,108]. Glycine betaine regulated all the required gene expressions that create additional antioxidants enzymes SOD, POD and CAT and successfully scavenge the unwarranted ROS in perennial ryegrass under Cd stress [97]. Earlier studies have studied the positive impacts of GB on the mitigation of metals stress in various plants species [81,82,97,109]. Exogenous application of GB reduces the harmful effect of Cd toxicity on vital growth of the Lemna gibba L. plant [110]. Glycine betaine successfully enhances HMs stress tolerance in pants and keeps the cells of plants free from the heavy metal toxicity. There are different studies demonstrating the constructive role of GB application in mitigation of heavy metal stress in various plants (Table 1).
Table 1.
Summary of the application of glycine betaine on various plant species under heavy metal stress.
| Plant Species | Heavy Metal Stress | Effect of Exogenous Glycine Betaine (GB) | References |
|---|---|---|---|
| Wheat | Cr Stress | Glycine Betaine (GB) improved chlorophyll contents, biomass, growth characteristics and protein content. | [81] |
| Cotton | Cd Stress | Glycine Betaine (GB) boosted the plant growth, improved activities of antioxidant enzyme and rate of photosynthesis | [82] |
| Mung Bean | Cr Stress | Glycine Betaine (GB) improved plant growth. | [83] |
| Amaranth | Cd Stress | Glycine Betaine (GB) significantly encouraged the rate of photosynthesis in edible amaranth and considerably improved the chlorophyll content of leaves. | [89] |
| Perennial Ryegrass | Cd Stress | Glycine Betaine (GB) improved stability of cell membrane via decreasing lipid membrane oxidation. | [97] |
| Cotton | Pb Stress | Effectively improved the gas attributes and plant growth under Pb stress. | [99] |
| Wheat | Cd Stress | Glycine Betaine (GB) improved fresh biomass of roots and shoots. | [111] |
| Cucumber | Al Stress | Glycine Betaine (GB) showed significant protective effect on chlorophyll content. | [112] |
| Asian Rice | As Stress | Glycine Betaine (GB) increased the GST and GRX gene expression alongside As stress. | [113] |
| Sorghum | Cr Stress | Glycine Betaine (GB) improved the quality and total yield of sorghum. | [114] |
| Tobacco | Cd Stress | Glycine Betaine (GB) reduces the stomatal closure, accumulation of malondialdehyde (MDA) and damage to leaf. | [115] |
6.1. Improvement in Plant Growth and Biomass
When open to heavy metal toxicity, plants face growth retardation and inhibition. HMs stress results in the considerable reduction of plant growth, biomass, number of leaves, plant height, weight and length of root and stem [111,112]. Moreover, HMs inhibit the process of transpiration, replication and thus altering the growth and cell division of plants [27]. However, application of GB not only acts as an osmoprotection but also enhances plant growth when exposed to HM stress. Glycine betaine improves the plants growth under heavy metal stress by escalating chlorophyll contents and reducing the oxidative damage [47]. Glycine betaine safeguard the photosynthetic pigments and machinery that in results in improvement in growth of the plants [116,117,118]. Glycine betaine application improves the adverse effects of Cd stress in cotton seedlings, efficiently alleviating the Cd induced decline in plant biomass and its growth. Foliar application of GB helps plants to maintain the healthy growth and cell division [82].
Foliar application of GB effectively alleviates the chromium’s noxious effects on wheat and improved the plant growth, roots and shoots weights [81]. Foliar application of GB boosts the growth potency through enhancing root/shoot length, along with dry biomass of the plant [119]. Higher concentration of Cd reduces the both dry and fresh weight of the tobacco, whereas application of GB alleviates the Cd toxicity and improved plant biomass and growth under Cd stress [120]. Treatment of GB significantly mitigate the inhibitory adverse impacts of Cd on important crop wheat (Triticum aestivum L.) growth attributes, and plants showed better dry biomass of shoots under Cd stress [111]. Lead induce growth inhibition were reported in several plants such as Perennial ryegrass (Lolium perenne L.) [100] and harmal (Peganum harmala L.) [121]. Zouari et al. [109] described that supplementation of GB proved to be useful in preventing the Pb content in tissues of olive trees and thus decreasing its intimidating impacts on plant growth. Similarly application of GB restricted the Pb content in juvenile tissues of cotton plants and mitigated its inhibitory adverse effects on growth characteristics [98].
6.2. Enhancement in Rate of Photosynthesis
General heavy metal stress is known to change the plant water relation, which may possibly affect the rate of photosynthesis, ascent of sap, mineral nutrition, water uptake, functioning of stomata and slowdown of chlorophyll biosynthesis and eventually results in restriction of successful photosynthesis [122,123]. In plants, photosynthesis is highly susceptible to HMs stress [124]. Heavy metals toxicity increases the generation of ROS. Heavy metals interaction with different electron transport activities in the mitochondrial membrane and in cellular compartments of chloroplast results in production of ROS [125]. Different studies has reported that toxicity of HMs to various physiological process of plants occurs through (ROS)-prompted lipid peroxidation and also by its by products such as linolenic acid-13-ketotriene, 12-oxo-phytodienoic acid and acrolein which may vigorously distress rate of photosynthesis and PS II [103,126].
Glycine betaine is plentiful mostly in chloroplast where it protects the thylakoid membrane and sustains the photosynthetic activities [127]. Glycine betaine contributes to control of the cytoplasmic dehydration and uphold turgor pressure in leaves of plant subjugated to water deficient state, thus conserving the eminent photosynthetic activities [128]. Exogenous application of GB improves the growth attributes of sorghum by stimulating the leaf expansion, enhancing turgidity and increasing the creation of photosynthetic pigments [129]. The exogenous GB supplement mitigated the decline in photosynthetic activity under Pb stress in Cotton plant. GB boosts the Pb tolerance by enhancing the photosynthetic activities and synthesis of chlorophyll [99]. It is recognized that GB protects the plants photosynthetic activities by raising stomatal conductance, preserving the RuBisCo enzyme activity and conserving the ultrastructure of chloroplast against environmental stress [130].
6.3. Up-Regulation of Antioxidant Defense System
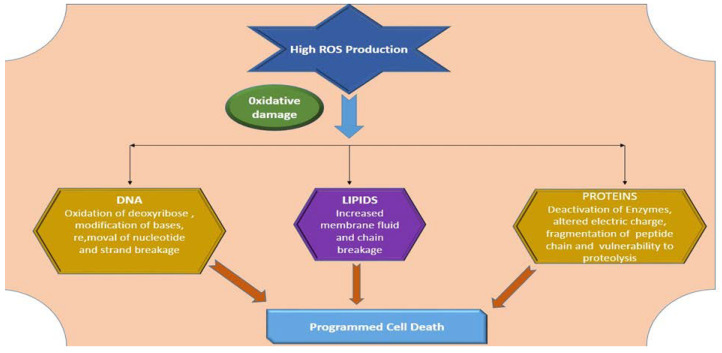
Plants consecutively produce excessive and superfluous ROS due to HMs stress [131]. ROS are key constituents of the signaling pathways, which operate as main regulators of cell physiology and cellular response of plants as a results of several harsh environmental stresses [132]. In several plants, HMs ions attach to protein groups and are competent enough to replace defined cations in binding spots, resulting in the generation of ROS and inactivation of enzymes, that ultimately cause oxidative stresses such as oxidation of amino acids, degradation of proteins, inhibition of vital enzymes, damage to DNA and RNA and membrane lipids peroxidation [133] as shown in Figure 4. An abrupt rise of ROS at an intracellular level is caused owing to the unevenness between scavenging and production of ROS [134,135]. Therefore, generation of ROS should remain in compatible limits within plants.
Figure 4.
Reactive oxygen species (ROS)-induced oxidative damage to DNA, lipids and proteins.
Plants do have a definite scheme of antioxidants like CAT, POX, GSH and SOD functions as reducing agents which cease oxidation reaction by eliminating free radicals. Generation of such molecules inhibits the incidence of oxidative burst [136]. Different reports stated that GB has a task like an osmoslyte, and may increase the antioxidant system to minimize the adversative impacts of HMs toxicity in plants [83,109]. Exogenous application of GB in cotton enhanced the antioxidant system by reducing the oxidative stress, as proved by the declined generation of H2O2 contents, electrolyte leakage (EL) and MDA level in leaf and root of the plant grown under Cd stress conditions [82]. Lou et al. [96] stated that the activities of different operative antioxidant enzymes like SOD, CAT, POX were significantly improved when GB was applied exogenously to Lolium perenne L. under Cd stress. Similarly supplementation of GB promoted the antioxidant enzymes effective activities and restrained Cr accumulation and oxidative damage in wheat [81].
Plants also possess another shield mechanism to inhibit the destructive effects of ROS (i.e., generated as a result of HMs toxicity) is the prevalence of the ascorbate-glutathione (ASC-GSH) cycle [49]. Successful treatment of GB upregulate enzymes activities in the ascorbate-glutathione cycle. Activities of DHAR (dihydro ascorbate reductase), APX (ascorbate peroxidase), MDHAR (monohydro ascorbate reductase), glutathione S-transferase (GST), glyoxalase (Gly I,II) and glutathione peroxidase (GPX) were considerably improved by GB application in mung beans under Cd stress [96]. Increased in APX (ascorbate peroxidase) with the application of GB has been studied in cultured tobacco cells under Cd stress [115] and also reported in cotton under Pb stress [97].
6.4. Upgrading of Metal and Mineral Uptake
Glycine betaine mitigates the lethal impacts of HMs by improving the resistance mechanisms of plants under stressful conditions. Plants have several mechanisms and ways to regulate and sustain homeostasis of metal cellular uptake and much higher accumulation of liberated metal ions [81,137]. Supplementation of GB significantly reduces the concentration of Cr and its total uptake by mung beans in its parts as compared to the pertinent Cr treatment alone [83]. Decrease in Cr uptake in several parts of the plant body occurred due to the defensive function of GB for the plant cell membrane for that reasons, a smaller amount of Cr penetrates the cytoplasm [138]. Application of GB extensively reduces the concentration of Cd in the roots, stem and leaf of the cotton plant [82]. Moreover, the reduction in total Cr accumulation in plant along with the application of GB occurs due to the reasonable competition between Cr and other critical nutrients [139]. Similarly, a decline in several metals including Cd, Pb and Cr has previously been testified in various plant like wheat [81], mung bean [96], cotton [97] and rice [140] with applications of GB.
Foliar application of GB increases the concentration of important nutrients such as potassium and sodium ions in both the shoot and root of wheat (Triticum aestivum L.) [118]. Mahmood et al. [141] also reported an enhanced content of important ions like Na+ and K+ with higher application of GB in wheat plant. Decrease and increases in the concentrations of micronutrients accumulation by the plants relies upon the difference of GB application approaches and disparity among plant species [81]. The presence of these micronutrients ensures effective plant growth, plant metabolism, synthesis of chlorophyll, seed and fruit development and production of carbohydrates while their shortage encourages irregular growth in plants [142].
6.5. Alleviation of Electrolyte Leakage (EL), Malondialdehyde (MDA) and Hydrogen Peroxide (H2O2)
Malondialdehyde (MDA) is believed to be an indicator of the generation of free radical and cytotoxic outcome of lipid peroxidation in stressed cells [143,144]. MDA is renowned biomarker of the complex oxidative stress [145]. Heavy metal toxicity leads to increases in MDA contents in plants [146]. Antioxidant machinery performs a vigorous part in the decline of MDA contents under heavy metal stress [147,148,149]. Increases in MDA content, H2O2 and EL will breakdown the homeostatic ROS balance which will eventually decrease the actions of vibrant antioxidant enzymes under heavy metal stress. Similarly, previous studies also reported the same effects on antioxidant enzymes and production of ROS in various plants [150,151,152]. Nevertheless, application of GB improves the antioxidant enzymes activities and headed to diminish the oxidative damage as confirmed through declined generation of hydrogen peroxide, EL and robust MDA level in both roots and shoots of the plants under metal stress [81,151].
Glycine betaine encourage the decline of H2O2, EL and MDA levels which specifies that GB effectively mitigates the detrimental impacts of Cd toxicity in cotton [82]. Supplementation of GB (10 mM) significantly reduces the MDA content induced by the Cd toxicity in cultured tobacco cells [118]. Oxidative stress induced by Pb on olive trees was considerably mitigated via exogenous application of GB through considerable reduction in EL, lipid peroxidation (TBARS) and hydrogen peroxide (H2O2) in all tissues analyzed [109]. Similarly, exogenously applied GB effectively detoxifies hydrogen peroxide (H2O2) by increasing antioxidant enzymes activities in Phanerochaete chrysosporium L. under Cd stress [153]. Application of GB reduces the malondialdehyde, hydrogen peroxide and EL conferred resistance in opposition to heavy metal stress by promoting preemptive activities of all important antioxidant enzymes.
7. Involvement of Glycine Betaine against Combinations of Abiotic Stresses in Plants
Plant species naturally accumulate GB as their main osmolyte once they are subjected to various type of abiotic stresses. Glycine betaine accumulate in many species of plants such as wheat (Triticum aestivum L.) [81], cherry tomato (Solanum lycopersicum L. var. cerasiforme) [154], sugar beet (Beta vulgaris L.) [155], maize (Zea mays L.) [156] and tomato (Solanum lycopersicum L.) [157]. Exogenously application of GB has been extensively examined to improve its capacity to alleviate the abiotic stress condition including drought, salt, and temperature. Induction of chilling stress tolerance has also been previously reported in sugarcane, loquat fruit, sweet pepper with the application of GB [158,159,160]. Application of GB reduces the chilling stress in potato plants by improving ROS scavenging, reducing chlorophyll bleaching and stabilization of photosynthetic activities [161]. Application of GB improves the performance of photosynthetic characteristics’ of bread wheat under drought stress [78]. Different studies have reported that GB improved tolerance against drought stress due to its active participation in osmotic adjustment in different plants [162]. Under severe drought stress, GB helps to uphold osmotic adjustment, sustain the sustainability of membranes and boost defense system in bentgrass [80].
Salt stress is also a big challenge for prosperous agriculture. Crops yields frequently decreased because crops fall short to survive salinity [163]. Glycine betaine also enhances salt tolerance in different plants. Its application successfully reduces salt stress in plants like tomato [164], onion [165], rapeseed mustard [166]. The number of nodules, activity of nodules and biological process of nitrogen fixation considerably reduce under salt stress. Under salt stress, GB application considerably enhances the particular nodule activity and nitrogen fixation [167]. Utilization of GB increases plant height, biomass, root length and total chlorophyll in rice plant under harsh salt stress [168]. The application of GB further enhances proline content, soluble sugar along with successful improvement in vital antioxidant systems which offer better conditions for the growth of plants exposed to salinity stress [169]. Plants treated with GB also retain greater antioxidant enzymes movements that restrict oxidative stress under salinity [165,170].
Compatible solutes including polyamines, proline, GB, and soluble sugar accumulation in plants are positively correlated with low temperature stress tolerance [171,172] and extreme temperature [64,173] in plants. Glycine betaine enhanced the growth of potato [174], maize [175], chickpea (Cicer arietinum L.) [176], and cotton [177] under chilling stress conditions. Cooling (<10 ℃) is particularly harmful to the development of chickpea, especially during the reproductive stage. Chilling causes a restriction in pollen tube growth, abnormal functions in male and female gametes and flower abortion. Chilling stress damage to membranes, a decrease in cellular respiration, increase in ROS, the elevation of abscisic acid and cryoprotectants such as GB safeguard the proactive activities of several enzymes and vital proteins, helping in stabilization of membranes structure and maintaining the photosynthetic apparatus under freezing temperatures during chilling stress. As reported previously, accumulation and uptake of GB is linked with the cold tolerance in several species of plants [178].
Foliar application of GB during the bud stage of chickpea showed much better improvement in flowering in the form of successful pollen germination, active pollen viability, tremendous growth in pollen tube, greater receptivity in stigma and extensive viability of ovule in chilling stress conditions. On the other hand, during pod filling, GB treatment resulted in an increase in the number of seeds and its yield, seed weight and pods were significantly improved after treatment. Glycine betaine greatly induced cold stress tolerance in plants through effective improvement in overall leaf water, sucrose and chlorophyll content, meanwhile, it also reduced the ROS and contents of abscisic acid as well [177]. Some plant species such as barley (Hordeum vulgare L.) [179] and spinach [180] synthesize more GB in different compartments of chloroplasts than plants like rockcress (Arabidopsis) [181] and tobacco [182]. Exogenously GB application to chickpea plant, increasing the flower behavior, pod formation and many yield parameters, provides a partially cold tolerance in the plants [176].
Cotton crops (Gossypium hirsutum L.) are very prone to chilling stress [183]. Thus, chilling is considered a major stress that may intimidate the overall production of cotton. It is important to increase the chilling tolerance of cotton production during these periods. Seed germination was significantly improved by glycine betaine application under chilling stress. Seed priming with GB enhanced stress tolerance in plants [184,185]. Seed priming with GB make the cotton seedlings more resilient to chilling stress. Seedlings obtained from GB-treated seeds show higher SOD, CAT and APX activities, thus lowering the H2O2 content of the leaves and reducing cell damage. Treatment with GB modulates the manifestation of some important genes that produce ROS-scavenging enzymes. While plants are in stress conditions, faster buildup of GB occurs in several plants and the content of GB is positively associated with increased tolerance. Application of GB to cotton seeds provides tolerance during the germination stage and vital four-leaf stage of seedlings [177].
8. Application Methods of Glycine Betaine
Different methodological approaches are used to apply the GB at a range of different concentrations, and it performs a substantial role in fortification and safeguarding of physio-chemical attributes of the plants under HM stress. Different types of GB application methods increase the yield, fertility and growth of the plants under stress and non-stress conditions [64,95]. There are different methods of GB application which can be directly used as plant growth supporters.
8.1. Foliar Application of GB
Foliar application of GB is suggested as a valuable mean of inducing tolerance in plants under stress conditions with destitute solute accumulation [118]. Different factors define the usefulness of foliar treatment of GB such as concentration of GB applied, application period and types of plant species on which GB is applied [64]. Foliar supplementation of GB substantially alleviate the harmful results of Cr toxicity on mung bean and enhanced biomass, growth along with total chlorophyll contents [83]. A similar improvement in biomass, chlorophyll content and uptake of essential nutrients were also examined under foliar exogenously applied GB under Cd stress in amaranth [86]. Foliar application of GB also improve the physiochemical attributes under Cd stress by reducing the H2O2, level of MDA and superoxide radicals (O2−) in two cultivar of wheat [110]. Foliar treatment of GB improve the antioxidant enzymes’ activities and reduce the oxidative stress induce by the Cr stress in wheat plant [81].
8.2. Pre-Sowing Seed Treatments
Different studies support the fact that the application of GB is equally effective at very early growing phases of seed germination and seedling establishment [186,187]. Survival of seedlings and germination of seeds under various kinds of environmental stress are vital for crop yield and its establishment [188], which can be efficiently improved by treating the seeds with the application of GB [189,190]. Seed treatment with the supplementation of GB enhanced the activity of the PSII center. Glycine betaine application mitigates the prohibitory impacts of environmental stress during the reparation of PSII center by speeding up the establishment of D1 protein [191].
Glycine betaine seed treatment protects the chloroplast membrane, increases the osmotic adjustment, preserves the photosynthetic pigments and also sustains the turgor pressure by accumulation of both inorganic osmolytes (Mg, Ca and K ions) and organic osmolytes (total amino nitrogen, proline, total soluble proteins and total soluble sugars) [192]. The water potential of plant tissues and cells is harmfully affected under different kind of osmotic stresses. Korkmaz et al. [193] stated that pre-sowing seed treatment with 5mM of GB considerably enhanced the relative water content (RWC) and leaf water potential of pepper seedlings under salt stress. Arafa et al. [194] also described that pre-soaking of sorghum grains with GB improved the germination rate in contrast with the control treatment. Seed pre-sowing treatment along with GB significantly motivated the germination of seeds and improvements in antioxidant systems of the plants. Application of GB at seeds level sustained a higher level of antioxidant enzymes including SOD, APX and CAT activities and it retain H2O2 contents at lower levels in leaves, thus preventing the level of injury occurred to cell membrane in cotton seedling [177].
9. Role of Glycine Betaine in Crop Improvement
The methods of orthodox plant breeding have altered the use of physiological assortment parameters and rely on genetic variability that is already present [195]. The significant variation in the GB contents of wheat [196], barley [197] and maize [198] verified that the behavior of GB in plants was a quantitative character. It was emphasized that there was considerable genetic diversity for GB in several heterotic groups of maize and a recessive allele of a single nuclear gene was responsible. Betaine aldehyde dehydrogenase (BADH) and choline oxidase (COD) are key enzymes which have been used to converse glycinebetaine synthesis in plants, which normally does not synthesis glycinebetaine. Genetically modified tomato plants with choline oxidase and BADH enzymes showed much better development in size and flowering [199]. Non-significant maternal or cytoplasmic effects were determined in the quantitative genetic studies for GB. The higher additive gene effect than non-additives was estimated by incomplete diallele analysis with eight low- and five high-betaine parents in barley. Similarly, the results of scaling tests (generation mean analysis of the parental, F1, F2, and backcross generations) showed a predominantly additive trait and moderately narrow sense heritability degree [195,197]. It is advisable to select individual plants for GB in early generations (F2–F3).
10. Genetic Engineering
Genetic manipulation is one of the most important techniques used now days for the creation of crop resistance to different environmental stresses. Different crops have been established for the upgrading against stress confrontation via increasing generation of different hormone, antioxidant enzymes and organic osmolytes [200,201]. Glycine betaine is an important osmolyte that defends the plant against the detrimental effects of environmental stresses. Genetic engineering creates transgenic plants which contains various genes for the GB biosynthesis pathway, which ultimately develops increased resistance to a widespread environmental strains through various phases of plant development [202]. Choline oxidase (COD) and betaine aldehyde dehydrogenase (BADH) are two main enzymes whichh are chiefly used to create GB synthesis in that plant which does not normally synthesise GB. Zhang et al. [203] described that genetically transformed tomato plant with codA, BADH transgenes showed much better rate of photosynthesis, better chlorophyll content along with higher assimilates content as compared to non-transformed wild plant.
During genetic manipulation gene products primarily target the chloroplast in codA transgenic plants. Transgenic plants predominately accumulate GB in chloroplast, and display tolerance to different types of abiotic stresses [67]. Transgenic reports over the years have demonstrated that those plants which generate additional GB have improved the resistance to various environmental stresses [204,205]. The important gene (BADH) betaine aldehyde dehydrogenase plays a very crucial function in plants under stressful conditions. Qin et al. [206] stated that BADH transgenic soybeans have shown a 6–17% increase in germination index, POX activity enhanced by 1–7% and decreased in contents of MDA by 1.5–13% in comparison to the control treatment. Increase in the accumulation of GB through genetic engineering could be effectively exploited as an essential implement to mitigate the heavy metal stress confrontation in plants. Cloning of (BADH) genes will further upon up the new horizons to create stress-tolerant crop cultivars [207].
11. Conclusions
Glycine betaine is a key organic osmolyte, which usually accumulates in a diverse range of plants against different environmental stresses. Also, it assists the plants to recover from severe stress quickly. In this review, we aimed to explore the impacts of GB on growth traits along with the mechanism engaged in plants to mitigate the metals stress. Exogenous application of GB boosts and improves yield and growth well as physiological attributes of plants. Glycine betaine application reduces heavy metals toxicity by increasing the robust activities of imperative antioxidant enzymes. Exogenously applied GB lessens the ROS formed in various plants under heavy metal stress. Thinking about the ability of exogenous GB as scavenger of ROS, it providentially transforms into expressive means to oppose the unfavorable impacts of environmental stress, thus reducing annual loss of agriculture. Furthermore, GB application maintains the water relation and turgor pressure in plant cells and also raises the rate of photosynthesis. The foliar method of GB application was found to be more efficient in alleviating the heavy metal stress and improving the growth attributes of the plants. Application of GB showed ameliorative impacts on growth characteristics of plants. Further investigation is required to fill the gap concerning the physiological response of GB and its application during signal transduction paths in several plants exposed to heavy metal stress. A wide range of experimental studies are required to examine how GB improves the nutrient uptake at molecular level and how GB collaborate with physio-chemical processes of the plants. Genetic manipulation and further understanding of inducible GB genes and their products could easily improve our knowledge of GB improvement to stress tolerance and resistance in plants.
Acknowledgments
The authors are grateful to Higher Education Commission (HEC) Islamabad, Pakistan for its support.
Funding
The authors are grateful to Higher Education Commission (HEC) Islamabad, Pakistan for its support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dixit R., Wasiullah E.Y., Malaviya D., Pandiyan K., Singh U., Sahu A., Shukla R., Singh B., Rai J., Sharma P., et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability. 2015;7:2189–2212. doi: 10.3390/su7022189. [DOI] [Google Scholar]
- 2.Peng W., Li X., Xiao S., Fan W. Review of remediation technologies for sediments contaminated by heavy metals. J. Soils Sediments. 2018;18:1701–1719. doi: 10.1007/s11368-018-1921-7. [DOI] [Google Scholar]
- 3.Seleiman M.F., Santanen A., Jaakkola S., Ekholm P., Hartikainen H., Stoddard F.L., Mäkelä P.S.A. Biomass yield and quality of bioenergy crops grown with synthetic and organic fertilizers. Biomass Bioenerg. 2013;59:477–485. doi: 10.1016/j.biombioe.2013.07.021. [DOI] [Google Scholar]
- 4.Seleiman M.F., Santanen A., Kleemola J., Stoddard F.L., Mäkelä P.S.A. Improved sustainability of feedstock production with sludge and interacting mychorriza. Chemosphere. 2013;91:1236–1242. doi: 10.1016/j.chemosphere.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Seleiman M.F., Santanen A., Stoddard F.L., Mäkelä P.S.A. Feedstock quality and growth of bioenergy crops fertilized with sewage sludge. Chemosphere. 2012;89:1211–1217. doi: 10.1016/j.chemosphere.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Shahid M.J., Ali S., Shabir G., Siddique M., Rizwan M., Seleiman M.F., Afzal M. Comparing the performance of four macrophytes in bacterial assisted floating treatment wetlands for the removal of trace metals (Fe, Mn, Ni, Pb, and Cr) from polluted river water. Chemosphere. 2020;243:125353. doi: 10.1016/j.chemosphere.2019.125353. [DOI] [PubMed] [Google Scholar]
- 7.Seleiman M.F., Kheir A.S. Maize productivity, heavy metals uptake and their availability in contaminated clay and sandy alkaline soils as affected by inorganic and organic amendments. Chemosphere. 2018;204:514–522. doi: 10.1016/j.chemosphere.2018.04.073. [DOI] [PubMed] [Google Scholar]
- 8.Salem H.S., Abdel-Salam A., Abdel-Salam M.A., Seleiman M.F. Phytoremediation of metal and metalloids from contaminated soil. In: Hasanuzzaman M., Nahar K., Fujita M., editors. Plants under Metal and Metalloid Stress- Responses, Tolerance and Remediation. Springer Nature Pte Ltd.; Singapore: 2018. pp. 249–262. Series Soil Biology. Chapter 9. [Google Scholar]
- 9.Seleiman M.F., Selim S., Jaakkola S., Mäkelä P. Chemical composition and in vitro digestibility of whole-crop maize fertilized with synthetic fertilizer or digestate and harvested at two maturity stages in boreal growing conditions. Agric. Food Sci. 2017;26:47–55. doi: 10.23986/afsci.60068. [DOI] [Google Scholar]
- 10.Abdel-Salam A., Salem H.M., Abdel-Salam M.A., Seleiman M.F. Phyto and chemical removal of heavy metal-contaminated soils. In: Sherameti I., Varma A., editors. Heavy Metal Contamination of Soils: Monitoring and Remediation. Volume 44. Springer; Cham, Switzerland: 2015. pp. 299–308. Series Soil Biology. [Google Scholar]
- 11.Seleimana M.F., Santanen A., Mäkelä P.S.A. Recycling sludge on cropland as fertilizer—Advantages and risks. Resour. Conserv. Recycl. 2020;155:104647. doi: 10.1016/j.resconrec.2019.104647. [DOI] [Google Scholar]
- 12.Qayyum M.F., Rehman M.Z., Ali S., Rizwan M., Naeem A., Maqsood M.A., Khalid H., Rinklebe J., Ok Y.S. Residual effcts of monoammonium phosphate, gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere. 2017;174:515–523. doi: 10.1016/j.chemosphere.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Ali S., Rizwan M., Zaid A., Arif M.S., Yasmeen T., Hussain A., Shahid M.R., Bukhari S.A.H., Hussain S., Abbasi G.H. 5-Aminolevulinic Acid-Induced Heavy Metal Stress Tolerance and Underlying Mechanisms in Plants. J. Plant Growth Regul. 2018;37:1423–1433. doi: 10.1007/s00344-018-9875-y. [DOI] [Google Scholar]
- 14.Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Heavy metals toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taiwo A.M., Gbadebo A.M., Oyedepo J.A., Ojekunle Z.O., Alo O.M., Oyeniran A.A., Onalaja O.J., Ogunjimi D., Taiwo O.T. Bioremediation of industrially contaminated soil using compost and plant technology. J. Hazard Mater. 2016;304:166–172. doi: 10.1016/j.jhazmat.2015.10.061. [DOI] [PubMed] [Google Scholar]
- 16.Rizwan M., Ali S., Adrees M., Ibrahim M., Tsang D.C., Rehman M.Z., Zahir Z.A., Rinklebe J., Tack F.M., Ok Y.S. A critical review on effcts, tolerance mechanisms and management of cadmium in vegetables. Chemosphere. 2017;182:90–105. doi: 10.1016/j.chemosphere.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Jacob J.M., Karthik C., Saratale R.G., Smita S., Kumar D., Prabakar D., Kadirvelu K., Pugazhendhi A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018;217:56–70. doi: 10.1016/j.jenvman.2018.03.077. [DOI] [PubMed] [Google Scholar]
- 18.Huang T.L., Huang L.Y., Fu S.F., Trinh N.N., Huang H.J. Genomic profiling of rice roots with short-and long-term chromium stress. Plant Mol. Biol. 2014;86:157–170. doi: 10.1007/s11103-014-0219-4. [DOI] [PubMed] [Google Scholar]
- 19.Kohzadi S., Shahmoradi B., Ghaderi E., Loqmani H., Maleki A. Concentration, Source, and Potential Human Health Risk of Heavy Metals in the Commonly Consumed Medicinal Plants. Biol. Trace Elem. Res. 2019;187:41–50. doi: 10.1007/s12011-018-1357-3. [DOI] [PubMed] [Google Scholar]
- 20.Ali H., Khan E., Ilahi I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019;2019:6730305. doi: 10.1155/2019/6730305. [DOI] [Google Scholar]
- 21.Sofy M., Seleiman M.F., Alhammad B.A., Alharbi B.A., Mohamed H.I. Minimizing adverse effects of Pb stress on maize yield, macro elements, and physiological and biochemical traits by combined treatment with jasmonic acid, salicylic acid, and proline. Agronomy. 2020;10:699. doi: 10.3390/agronomy10050699. [DOI] [Google Scholar]
- 22.Saleem M.H., Ali S., Seleiman M.F., Rizwan M., Rehman M., Akram N.A., Liu L., Alotaibi M., Al-Ashkar I., Mubushar M. Assessing the Correlations between Different Traits in Copper-Sensitive and Copper-Resistant Varieties of Jute (Corchorus capsularis L.) Plants. 2019;8:545. doi: 10.3390/plants8120545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seleiman M.F., Alotaibi M., Alhammad B., Alharbi B., Refay Y., Badawy S. Effects of ZnO nanoparticles and biochar of rice straw and cow manure on characteristics of contaminated soil and sunflower productivity, oil quality, and heavy metals uptake. Agronomy. 2020;10:790. doi: 10.3390/agronomy10060790. [DOI] [Google Scholar]
- 24.Chandra R., Yadav S., Yadav S. Phytoextraction potential of heavy metals by native wetland plants growing on chlorolignin containing sludge of pulp and paper industry. Ecol. Eng. 2017;98:134–145. doi: 10.1016/j.ecoleng.2016.10.017. [DOI] [Google Scholar]
- 25.Willscher S., Jablonski L., Fona Z., Rahmi R., Wittig J. Phytoremediation experiments with Helianthus tuberosus under different pH and heavy metal soil concentrations. Hydrometallurgy. 2017;168:153–158. doi: 10.1016/j.hydromet.2016.10.016. [DOI] [Google Scholar]
- 26.Nayak A.K., Panda S.S., Basu A., Dhal N.K. Enhancement of toxic Cr (VI), Fe, and other heavy metals phytoremediation by the synergistic combination of native Bacillus cereus strain and Vetiveria zizanioides L. Int. J. Phytoremediat. 2018;20:682–691. doi: 10.1080/15226514.2017.1413332. [DOI] [PubMed] [Google Scholar]
- 27.Malar S., Vikram S.S., JC Favas P., Peruma V. Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart)] Bot. Stud. 2014;55:1–11. doi: 10.1186/s40529-014-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tauqeer H.M., Ali S., Rizwan M., Ali Q., Saeed R., Iftikhar U., Ahmad R., Farid M., Abbasi G.H. Phytoremediation of heavy metals by Alternanthera bettzickiana: Growth and physiological response. Ecotoxicol. Environ. Saf. 2016;126:138–146. doi: 10.1016/j.ecoenv.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 29.Abbas T., Rizwan M., Ali S., Rehman M.Z., Qayyum M.F., Abbas F., Hannan F., Rinklebe J., Ok Y.S. Effct of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L,) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017;140:37–47. doi: 10.1016/j.ecoenv.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 30.Khan A., Fatima H., Ghania A., Nadeem M., Aziz A., Hussain M., Ikram M. Improving Salinity Tolerance in Brassica (Brassica napus var, Bsa and Brassica campestris var, Toria) by Exogenous Application of Proline and Glycine Betaine. Pak. J. Sci. Ind. Res. Ser. B Boil. Sci. 2018;1:1–8. [Google Scholar]
- 31.Peters D.E., Eebu C., Nkpaa K.N. Potential Human Health Risk Assessment of Heavy Metals via Consumption of Root Tubers from Ogoniland, Rivers State, Nigeria. Biol. Trace Elem. Res. 2018;186:568–578. doi: 10.1007/s12011-018-1330-1. [DOI] [PubMed] [Google Scholar]
- 32.Intawongse M., Kongchouy N., Dean J.R. Bioaccessibility of heavy metals in the seaweed Caulerparacemosa var, corynephora: Human health risk from consumption. Instrum. Sci. Technol. 2018;46:628–644. doi: 10.1080/10739149.2018.1427105. [DOI] [Google Scholar]
- 33.Chibuike G.U., Obiora S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014;2014:12. doi: 10.1155/2014/752708. [DOI] [Google Scholar]
- 34.Ashfaque F., Inam A., Sahay S., Iqbal S. Influence of Heavy Metal Toxicity on Plant Growth, Metabolism and Its Alleviation by Phytoremediation—A Promising Technology. J. Agric. Ecol. Res. Int. 2016;6:1–19. doi: 10.9734/JAERI/2016/23543. [DOI] [Google Scholar]
- 35.Khan M., Iqbal R., Khan N.A. Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress. Springer; Cham, Switzerland: 2017. [Google Scholar]
- 36.Seneviratne M., Rajakaruna N., Rizwan M., Madawala H.M.S.P., Ok Y.S., Vithanage M. Heavy metal-induced oxidative stress on seed germination and seedling development: A critical review. Environ. Geochem. Health. 2017;41:1813–1831. doi: 10.1007/s10653-017-0005-8. [DOI] [PubMed] [Google Scholar]
- 37.Farid M., Ali S., Rizwan M., Ali Q., Saeed R., Nasir T., Abbasi G.H., Rehmani M.I., Ata-Ul-Karim S.T., Bukhari S.A., et al. Phytomanagement of chromium contaminated soils through sunflower under exogenously applied 5-aminolevulinic acid. Ecotoxicol. Environ. Saf. 2018;151:255–265. doi: 10.1016/j.ecoenv.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Genthe B., Kapwata T., Le Roux W., Chamier J., Wright C.Y. The reach of human health risks associated with metals/metalloids in water and vegetables along a contaminated river catchment: South Africa and Mozambique. Chemosphere. 2018;199:1–9. doi: 10.1016/j.chemosphere.2018.01.160. [DOI] [PubMed] [Google Scholar]
- 39.Rizwan M., Ali S., Rehman M.Z., Rinklebe J., Tsang D.C.W., Bashir A., Maqbool A., Tack F.M.G., Ok Y.S. Cadmium phytoremediation potential of Brassica crop species: A review. Sci. Total Environ. 2018;631–632:1175–1191. doi: 10.1016/j.scitotenv.2018.03.104. [DOI] [PubMed] [Google Scholar]
- 40.Babst-Kosteckaa A.A., Waldmannc P., Frérotd H., Vollenweider P. Plant adaptation to metal polluted environments—Physiological, morphological, and evolutionary insights from Biscutella laevigata. Environ. Exp. Bot. 2016;127:1–13. doi: 10.1016/j.envexpbot.2016.03.001. [DOI] [Google Scholar]
- 41.Shanying H.E., Xiaoe Y., Zhenli H.E., Baligar V.C. Morphological and Physiological Responses of Plants to Cadmium Toxicity: A Review. Pedosphere. 2017;27:421–438. [Google Scholar]
- 42.Khan A., Khan S., Khan M.A., Qamar Z., Waqas M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015;22:13772–13799. doi: 10.1007/s11356-015-4881-0. [DOI] [PubMed] [Google Scholar]
- 43.Anjum S.A., Ashraf U., Khan I., Tanveer M., Ali M., Hussain I., Wang L.C. Chromium and aluminum phyto-toxicity in maize; morphophysiological responses and metal uptake. Clean-Soil Air Water. 2016;44:915–1084. doi: 10.1002/clen.201500532. [DOI] [Google Scholar]
- 44.Anjum S.A., Tanveer M., Hussain S., Ashraf U., Khan I., Wang L.C. Alteration in growth, leaf gas exchange, and photosynthetic pigments of maize plants under combined cadmium and arsenic stress. Water Air Soil Pollut. 2017;228:13. doi: 10.1007/s11270-016-3187-2. [DOI] [Google Scholar]
- 45.Pongrac P., Zhao F.J., Razinger J., Zrimec A., Regvar M. Physiological responses to Cd and Zn in two Cd/Zn hyperaccumulating Thlaspi species. Environ. Exp. Bot. 2009;66:479–486. doi: 10.1016/j.envexpbot.2009.03.010. [DOI] [Google Scholar]
- 46.Lämke J., Bäurle I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017;18:124. doi: 10.1186/s13059-017-1263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demidchik V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015;109:212–228. doi: 10.1016/j.envexpbot.2014.06.021. [DOI] [Google Scholar]
- 48.Zaid A., Wani S.H. Bioactive Molecules in Plant Defense. Springer Science and Business Media LLC; Berlin, Germany: 2019. Reactive Oxygen Species Generation, Scavenging and Signaling in Plant Defense Responses; pp. 111–132. [Google Scholar]
- 49.Noctor G., Foyer C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 50.Steffens B. The role of ethylene and ROS in salinity, heavy metal, and flooding responses in rice. Front. Plant Sci. 2014;5:1–5. doi: 10.3389/fpls.2014.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J., Zhao M., Pei L., Zhang R., Liu X., Wei L., Yang M., Xu Q. Oxidative stress and DNA damage in a long-term hexavalent chromiumexposed population in North China: A cross-sectional study. BMJ Open. 2018;8:021470. doi: 10.1136/bmjopen-2017-021470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002;53:1–11. doi: 10.1093/jexbot/53.366.1. [DOI] [PubMed] [Google Scholar]
- 53.Hossain M.A., Piyatida P., da Silva J.A.T., Fujita M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxifiation of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012;2012:37. doi: 10.1155/2012/872875. [DOI] [Google Scholar]
- 54.Kumar S., Trivedi P.K. Heavy Metal Stress Signalling in Plants. Plant Metal Interaction (Emerging Remediation Techniques) Elsevier; Amsterdam, The Netherlands: 2016. pp. 585–603. [Google Scholar]
- 55.Tiwari S., Lata C. Heavy Metal Stress, Signaling, and Tolerance Due to Plant-Associated Microbes: An Overview. Front. Plant Sci. 2018;9:452. doi: 10.3389/fpls.2018.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J.H., Cao L., Dou S.Z. Bioaccumulation of heavy metals and health risk assessment in three benthic bivalves along the coast of Laizhou Bay, China. Mar. Pollut. Bull. 2017;117:98–110. doi: 10.1016/j.marpolbul.2017.01.062. [DOI] [PubMed] [Google Scholar]
- 57.Dubey S., Shri M., Misra P., Lakhwani D., Bag S.K., Asif M.H. Heavy metals induce oxidative stress and genome-wide modulation in transcriptome of rice root. Funct. Integr. Genom. 2014;14:401–417. doi: 10.1007/s10142-014-0361-8. [DOI] [PubMed] [Google Scholar]
- 58.Singh I., Shah K. Exogenous application of methyl jasmonate lowers the effect of cadmium-induced oxidative injury in rice seedlings. Phytochemistry. 2014;108:57–66. doi: 10.1016/j.phytochem.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Dutta S., Mitra M., Agarwal P., Mahapatra K., De S., Sett U., Roy S. Oxidative and genotoxic damages in plants in response to heavy metal stress and maintenance of genome stability. Plant Signal. Behav. 2018;13:e1460048. doi: 10.1080/15592324.2018.1460048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiwari S., Lata C., Chauhan S., Prasad P., Prasad M.A. Functional genomic perspective on drought signalling and its crosstalk with phytohormone-mediated signalling pathways in plants. Curr. Genom. 2017;18:469–482. doi: 10.2174/1389202918666170605083319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rhodes D., Hanson A.D. Quaternary ammonium and tertiary sulfonium compounds in higher-plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993;44:357–384. doi: 10.1146/annurev.pp.44.060193.002041. [DOI] [Google Scholar]
- 63.Bohnert H.J., Jensen R.G. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996;14:89–97. doi: 10.1016/0167-7799(96)80929-2. [DOI] [Google Scholar]
- 64.Ashraf M., Foolad M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007;59:206–216. doi: 10.1016/j.envexpbot.2005.12.006. [DOI] [Google Scholar]
- 65.Umar J., Aliyu A., Shehu K., Abubakar L. Influence of Salt Stress on Proline and Glycine Betaine Accumulation in Tomato (Solanum lycopersicum L.) J. Hort. Plant. 2018;1:19–25. doi: 10.18052/www.scipress.com/JHPR.1.19. [DOI] [Google Scholar]
- 66.Yildirima E., Ekincia M., Turanb M., Dursuna A., Kula R., Parlakova F. Roles of glycine betaine in mitigating deleterious effect of salt stress on lettuce (Lactuca sativa L.) Arch. Agron. Soil Sci. 2015;61:1673–1689. doi: 10.1080/03650340.2015.1030611. [DOI] [Google Scholar]
- 67.Park E.J., Jeknic Z., Pino M.T., Murata N., Chen T.H. Glycinebetaine accumulation is more effective in chloroplasts than in the cytosol for protecting transgenic tomato plants against abiotic stress. Plant Cell Environ. 2007;30:994–1005. doi: 10.1111/j.1365-3040.2007.01694.x. [DOI] [PubMed] [Google Scholar]
- 68.He C., Zhang W., Gao Q., Yang A., Hu X., Zhang J. Enhancement of drought resistance and biomass by increasing the amount of glycine betaine in wheat seedlings. Euphytica. 2011;177:151–167. doi: 10.1007/s10681-010-0263-3. [DOI] [Google Scholar]
- 69.Sakamoto A., Murata N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002;25:163–171. doi: 10.1046/j.0016-8025.2001.00790.x. [DOI] [PubMed] [Google Scholar]
- 70.Rasheed R., Iqbal M., Ashraf M.A., Hussain I., Shafiq F., Yousaf A., Zaheer A. Glycine betaine counteracts the inhibitory effects of waterlogging on growth, photosynthetic pigments, oxidative defence system, nutrient composition, and fruit quality in tomato. J. Hortic. Sci. Biotechnol. 2017;93:385–391. doi: 10.1080/14620316.2017.1373037. [DOI] [Google Scholar]
- 71.Hisyam B., Alam M.A., Naimah N., Jahan M.S. Roles of Glycinebetaine on Antioxidants and Gene Function in Rice Plants Under Water Stress. Asian J. Plant Sci. 2017;16:132–140. [Google Scholar]
- 72.Yadu S., Dewangan T.L., Chandrakar V., Keshavkant S. Imperative roles of salicylic acid and nitric oxide in improving salinity tolerance in Pisum sativum L. Physiol. Mol. Biol. Plants. 2017;23:43–58. doi: 10.1007/s12298-016-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao W.Q., Lei Y.K., Yang P., Li Q.S., Wang L.L., He B.Y., Xu Z.M., Zhou C., Ye H.J. Exogenous Glycinebetaine Promotes Soil Cadmium Uptake by Edible Amaranth Grown during Subtropical Hot Season. Int. J. Environ. Res. Public Health. 2018;15:1794. doi: 10.3390/ijerph15091794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen T.H., Murata N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008;13:499–505. doi: 10.1016/j.tplants.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 75.Hanson A.D., Scott N.A. Betaine synthesis from radioactive precursors in attached, water-stressed barley leaves. Plant Physiol. 1980;66:342–348. doi: 10.1104/pp.66.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian F., Wang W., Liang C., Wang X., Wang G., Wang W. Over accumulation of glycine betaine makes the function of the thylakoid membrane better in wheat under salt stress. Crop J. 2017;5:73–82. doi: 10.1016/j.cj.2016.05.008. [DOI] [Google Scholar]
- 77.Khan A., Khan S., Khan M.A., Aamir M., Ullah H.J., Nawab J., Rehman I.U., Shah J. Heavy metals effects on plant growth and dietary intake of trace metals in vegetables cultivated in contaminated soil. Int. J. Environ. Sci. Technol. 2018;16:2295–2304. doi: 10.1007/s13762-018-1849-x. [DOI] [Google Scholar]
- 78.Gupta N., Thind S.K., Bains N.S. Glycine betaine application modifies biochemical attributes of osmotic adjustment in drought-stressed wheat. Plant Growth Regul. 2013;72:221–228. doi: 10.1007/s10725-013-9853-0. [DOI] [Google Scholar]
- 79.Yang N., Wang C.L., He W.P., Qu Y.Z., Li Y.S. Photosynthetic characteristics and effects of exogenous glycine of Chorispora bungeana under drought stress. Photosynthetica. 2016;54:459–467. doi: 10.1007/s11099-016-0187-9. [DOI] [Google Scholar]
- 80.Liu N., Lin S., Huang B. Differential Effects of Glycine Betaine and Spermidine on Osmotic Adjustment and Antioxidant Defense Contributing to Improved Drought Tolerance in Creeping Bentgrass. J. Am. Soc. Hortic. Sci. 2017;142:20–26. doi: 10.21273/JASHS03962-16. [DOI] [Google Scholar]
- 81.Ali S., Chaudhary A., Rizwan M., Anwar H.T., Adrees M., Farid M., Irshad M.K., Hayat T., Anjum S.A. Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.) Environ. Sci. Pollut. Res. 2015;22:10669–10678. doi: 10.1007/s11356-015-4193-4. [DOI] [PubMed] [Google Scholar]
- 82.Farooq M.A., Ali S., Hameed A., Bharwana S.A., Rizwan M., Ishaque W., Farid M., Mahmood K., Iqbal Z. Cadmium stress in cotton seedlings: Physiological, photosynthesis andoxidative damages alleviated by glycinebetaine. S. Afr. J. Bot. 2016;104:61–68. doi: 10.1016/j.sajb.2015.11.006. [DOI] [Google Scholar]
- 83.Jabeen N., Abbas Z., Iqbal M., Rizwan M., Jabbar A., Farid M., Ali S., Ibrahim M., Abbas F. Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch. Agron. Soil Sci. 2016;62:648–662. doi: 10.1080/03650340.2015.1082032. [DOI] [Google Scholar]
- 84.Xalxo R., Yadu B., Chakraborty P., Chandrakar V., Keshavkant S. Modulation of nickel toxicity by glycinebetaine and aspirin in Pennisetum typhoideum. Acta Biol. Szeged. 2017;61:163–171. [Google Scholar]
- 85.Yao W., Xu T., Farooq S.U., Jin P., Zheng Y. Glycine betaine treatment alleviates chilling injury in zucchini fruit (Cucurbita pepo L.) by modulating antioxidant enzymes and membrane fatty acid metabolism. Postharvest Biol. Technol. 2018;144:20–28. doi: 10.1016/j.postharvbio.2018.05.007. [DOI] [Google Scholar]
- 86.Nusrat N., Shahbaz M., Perveen S. Modulation in growth, photosynthetic efficiency, activity of antioxidants and mineral ions by foliar application of glycine betaine on pea (Pisum sativum L.) under salt stress. Acta Physiol. Plant. 2014;36:2985–2998. doi: 10.1007/s11738-014-1670-1. [DOI] [Google Scholar]
- 87.Ahanger M.A., Tyagi S.R., Wani M.R., Ahmad P. Drought tolerance: Roles of organic osmolytes, growth regulators and mineral nutrients. In: Ahmad P., Wani M.R., editors. Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment. Volume 1. Springer; New York, NY, USA: 2014. pp. 25–56. [Google Scholar]
- 88.Ahanger M.A., Gul F., Ahmad P., Akram N.A. Plant Metabolites and Regulation Under Environmental Stress. Elsevier Inc.; Amsterdam, The Netherlands: 2018. Environmental Stresses and Metabolomics—Deciphering the Role of Stress Responsive Metabolites; pp. 53–67. [Google Scholar]
- 89.Fan W., Zhang M., Zhang H., Zhang P. Improved Tolerance to Various Abiotic Stresses in Transgenic Sweet Potato (Ipomoea batatas) Expressing Spinach Betaine Aldehyde Dehydrogenase. PLoS ONE. 2012;7:e37344. doi: 10.1371/journal.pone.0037344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen P., Yan K., Shao H., Zhao S. Physiological Mechanisms for High Salt Tolerance in Wild Soybean (Glycine soja) from Yellow River Delta, China: Photosynthesis, Osmotic Regulation, Ion Flux and antioxidant Capacity. PLoS ONE. 2013;8:e83227. doi: 10.1371/journal.pone.0083227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malekzadeh P. Inflence of exogenous application of glycine betaine on antioxidative system and growth of salt-stressed soybean seedlings (Glycine max L.) Physiol. Mol. Biol. Plants. 2015;21:225–232. doi: 10.1007/s12298-015-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akram N.A., Ashraf M., Al-Qurainy F. Aminolevulinic acidinduced changes in some key physiological attributes and activities of antioxidant enzymes in sunflwer (Helianthus annuus L.) plants under saline regimes. Sci. Hortic. 2012;142:143–148. doi: 10.1016/j.scienta.2012.05.007. [DOI] [Google Scholar]
- 93.Noctor G., Mhamdi A., Foyer C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014;164:1636–1648. doi: 10.1104/pp.113.233478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.You J., Chan Z.L. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015;6:1092. doi: 10.3389/fpls.2015.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khan M.I.R., Iqbal N., Masood A., Mobin M., Anjum N.A., Khan N.A. Modulation and significance of nitrogen and sulfur metabolism in cadmium challenged plants. Plant Growth Regul. 2016;78:1–11. doi: 10.1007/s10725-015-0071-9. [DOI] [Google Scholar]
- 96.Lou Y., Yang Y., Hu L., Liu H., Xu Q. Exogenous glycinebetaine alleviates the detrimental effect of Cd stress on perennial ryegrass. Ecotoxicology. 2015;24:1330–1340. doi: 10.1007/s10646-015-1508-7. [DOI] [PubMed] [Google Scholar]
- 97.Ali Q., Anwar F., Ashraf M., Saari N., Perveen R. Ameliorating effects of exogenously applied proline on seed composition, seed oil quality and oil antioxidant activity of maize (Zea mays L.) under drought stress. Int. J. Mol. Sci. 2013;14:818–835. doi: 10.3390/ijms14010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hossain M.A., Hasanuzzaman M., Fujita M. Up-regulation of antioxidant and glyoxalase systems by exogenous glycine betaine and proline in mung bean confer tolerance to cadmium stress. Physiol. Mol. Biol. Plants. 2010;16:259–272. doi: 10.1007/s12298-010-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bharwana S.A., Ali S., Farooq M.A., Iqbal N., Hameed A., Abbas F., Ahmad M.S.A. Glycine betaine-induced lead toxicity tolerance related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. Turk. J. Bot. 2014;38:281–292. doi: 10.3906/bot-1304-65. [DOI] [Google Scholar]
- 100.Islam M.M., Anamul-Hoque M., Okuma E., Banu N.A., Shimoishi Y., Nakamura Y., Murata Y. Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J. Plant Physiol. 2009;166:1587–1597. doi: 10.1016/j.jplph.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 101.Molla M.R., Ali M.R., Hasanuzzaman M., Al-Mamun M.H., Ahmed A., Nazim-ud-Dowla M.A.N., Rohman M.M. Exogenous proline and betaine-induced upregulation of glutathione transferase and glyoxalase I in lentil (Lens culinaris) under drought stress. Not. Bot. Horti. Agrobot. Cluj Napoca. 2014;42:73–80. doi: 10.15835/nbha4219324. [DOI] [Google Scholar]
- 102.Raza M.A.S., Saleem M.F., Moazzam J.M., Khan I. Impact of foliar applied glycinebetaine on growth and Physiology of wheat (Triticum Aestivum L.) Under drought. Pak. J. Agric. Sci. 2014;51:327–334. [Google Scholar]
- 103.Cha-um S., Samphumphuang T., Kirdmanee C. Glycinebetaine alleviates water deficit stress in indica rice using proline accumulation, photosynthetic efficiencies, growth performances and yield attributes. Aust. J. Crop Sci. 2013;7:213–218. [Google Scholar]
- 104.Masindi V., Muedi K. Environmental contamination by heavy Metals. In: Hosam El-Din M., Saleh Refaat Aglan F., editors. Heavy Metals. IntechOpen; Rijeka, Croatia: 2018. [DOI] [Google Scholar]
- 105.Ruciniska-Sobkowiak R. Oxidative stress in plants exposed to heavy metals. Postepy Biochemii. 2010;56:191–200. [PubMed] [Google Scholar]
- 106.Shahid M., Pourrut B., Dumat C., Nadeem M., Aslam M., Pinelli E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contamin. Toxicol. 2014;232:1–44. doi: 10.1007/978-3-319-06746-9_1. [DOI] [PubMed] [Google Scholar]
- 107.Fariduddin Q., Varshney P., Yousuf M., Ali A., Ahmad A. Dissecting the role of Glycine Betaine in Plants under Abiotic Stress. Plant Stress. 2013;7:8–18. [Google Scholar]
- 108.Hasan M.K., Cheng Y., Kanwar M.K., Chu X.Y., Ahammed G.J., Qi Z.Y. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017;8:1492. doi: 10.3389/fpls.2017.01492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zouari M., Elloumi N., Labrousse P., Ben Rouina B., Ben Abdallah F., Ben Ahmed C. Olive trees response to lead stress: Exogenous proline provided better tolerance than glycine betaine. S. Afr. J. Bot. 2018;118:158–165. doi: 10.1016/j.sajb.2018.07.008. [DOI] [Google Scholar]
- 110.Duman F., Aksoy A., Aydin Z., Temizgul R. Effects of Exogenous Glycinebetaine and Trehalose on Cadmium Accumulation and Biological Responses of an Aquatic Plant (Lemna gibba L.) Water Air Soil Pollut. 2011;217:545–556. doi: 10.1007/s11270-010-0608-5. [DOI] [Google Scholar]
- 111.Rasheed R., Ashraf M.A., Hussain I., Haider M.Z., Kanwal U., Iqbal M. Exogenous proline and glycinebetaine mitigate cadmium stress in two genetically different spring wheat (Triticum aestivum L.) cultivars. Braz. J. Bot. 2014;37:399–406. doi: 10.1007/s40415-014-0089-7. [DOI] [Google Scholar]
- 112.Stepien P., Gediga K., Piszcz U., Karmowska K. Effects of the exogenous glycine betaine on photosynthetic apparatus in cucumber leaves challenging Al stress; Proceedings of the 18th International Conference on Heavy Metals in the Environment; Ghent, Belgium. 12–15 October 2016. [Google Scholar]
- 113.Dubeya A.K., Kumara N., Ranjana R., Gautam A., Pande V., Sanyal I., Mallick S. Application of glycine reduces arsenic accumulation and toxicity in Oryza sativa L. by reducing the expression of silicon transporter genes. Ecotoxicol. Environ. Saf. 2018;148:410–417. doi: 10.1016/j.ecoenv.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 114.Kumar P., Tokas J., Singal H.R. Amelioration of Chromium VI Toxicity in Sorghum (Sorghum bicolor L.) using Glycine Betaine. Sci. Rep. 2019;9:16020. doi: 10.1038/s41598-019-52479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.He X., Richmond M.E., Williams D.V., Zheng W., Wu F. Exogenous Glycine betaine reduces cadmium uptake and mitigates cadmium toxicity in two tobacco genotypes differing in cadmium tolerance. Int. J. Mol. Sci. 2019;20:1612. doi: 10.3390/ijms20071612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ahmad R., Ali S., Abid M., Rizwan M., Ali B., Tanveer A., Ghani M.A. Glycinebetaine alleviates the chromium toxicity in Brassica oleracea L. by suppressing oxidative stress and modulating the plant morphology and photosynthetic attributes. Environ. Sci. Pollut. Res. 2020;27:1101–1111. doi: 10.1007/s11356-019-06761-z. [DOI] [PubMed] [Google Scholar]
- 117.Kurepin L.V., Ivanov A.G., Zaman M., Pharis R.P., Hurry V., Hüner N.P. Photosynthesis: Structures, Mechanisms, and Applications. Springer; Cham, Switzerland: 2017. Interaction of glycine betaine and plant hormones: Protection of the photosynthetic apparatus during abiotic stress; pp. 185–202. [Google Scholar]
- 118.Anjum S.A., Farooq M., Wang L.C., Xue L.L., Wang S.G., Wang L., Chen M. Gas exchange and chlorophyll synthesis of maize cultivars are enhanced by exogenously-applied glycinebetaine under drought conditions. Plant Soil Environ. 2011;57:326–331. doi: 10.17221/41/2011-PSE. [DOI] [Google Scholar]
- 119.Bhatti K.H., Anwar S., Nawaz K., Hussain K., Siddiqi E.H., Sharif R.U., Talat A., Khalid A. Effect of Exogenous Application of Glycinebetaine on Wheat (Triticum aestivum L.) Under Heavy Metal Stress. Middle-East J. Sci. Res. 2013;14:130–137. [Google Scholar]
- 120.Bai X.Y., Dong Y.J., Wang Q.H., Xu L.L., Kong J., Liu S. Effects of lead and nitric oxide on photosynthesis, antioxidative ability, and mineral element content of perennial ryegrass. Biol. Plantarum. 2015;59:163–170. doi: 10.1007/s10535-014-0476-8. [DOI] [Google Scholar]
- 121.Mahdavian K., Ghaderian S.M., Schat H. Pb accumulation, Pb tolerance, antioxidants, thiols, and organic acids in metallicolous and non-metallicolous Peganum harmala L. under Pb exposure. Environ. Exp. Bot. 2016;126:21–31. doi: 10.1016/j.envexpbot.2016.01.010. [DOI] [Google Scholar]
- 122.Cuypers A., Smeets K., Vangronsveld J. Heavy Metal Stress in Plants. In: Hirt H., editor. Plant Stress Biology: From Genomics to Systems Biology. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2009. [Google Scholar]
- 123.Pourrut B., Pohu A.L., Pruvot C., Garçon G., Verdin A., Waterlot C., Bidar G., Shirali P., Douay F. Assessment of flash-aided phytostabilisation of highly contaminated soils after an 8-year field trial Part 2. Influence on plants. Sci. Total Environ. 2011;409:4504–4510. doi: 10.1016/j.scitotenv.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 124.Sheetal K.R., Singh S.D., Anand A., Prasad S. Heavy metal accumulation and effects on growth, biomass and physiological processes in mustard. Ind. J. Plant Physiol. 2016;21:219–223. doi: 10.1007/s40502-016-0221-8. [DOI] [Google Scholar]
- 125.Chen F., Gao J., Zhou Q. Toxicity assessment of simulated urban runoff containing polycyclic musks and cadmium in Carassius auratus using oxidative stress biomarkers. Environ. Pollut. 2012;162:91–99. doi: 10.1016/j.envpol.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 126.Farmer E.E., Mueller M.J. ROS-Mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013;64:429–450. doi: 10.1146/annurev-arplant-050312-120132. [DOI] [PubMed] [Google Scholar]
- 127.Genard H., LeSaos J., Hillard J., Tremolieres A., Boucaud J. Effect of salinity on lipid composition, glycine betaine content and photosynthetic activity in chloroplasts of Suaeda maritime. Plant Physiol. Biochem. 1991;29:421–427. [Google Scholar]
- 128.Iqbal N., Ashraf M., Ashraf M.Y. Glycinebetaine, an osmolyte of interest to improve water stress tolerance in sunflower (Helianthus annuus L.): Water relations and yield. S. Afr. J. Bot. 2008;74:274–281. doi: 10.1016/j.sajb.2007.11.016. [DOI] [Google Scholar]
- 129.Ibrahim A.H., Aldesuquy H.S. Glycine Betaine and Shikimic Acid—Induced Modification in Growth Criteria, Water Relation and Productivity of Droughted Sorghum bicolor Plants. Phyton Horn. 2003;43:351–361. [Google Scholar]
- 130.Nomura M., Hibino T., Takabe T., Sugiyama T., Yokota A., Miyake H., Takabe T. Transgenically produced glycinebetaine protects ribulose-1, 5-biophosphate carboxylase/oxygenase from inactivation of Synechococcus sp. PCC7942 under salt stress. Plant Cell Physiol. 1998;32:425–432. doi: 10.1093/oxfordjournals.pcp.a029386. [DOI] [Google Scholar]
- 131.Feigl G., Lehotai N., Molnár Á., Ördög A., Rodríguez-Ruiz M., Palma J.M., Corpas F.J., Erdei L., Kolbert Z. Zinc induces distinct changes in the metabolism of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Ann. Bot. 2015;116:613–625. doi: 10.1093/aob/mcu246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Das K., Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014;2:53. doi: 10.3389/fenvs.2014.00053. [DOI] [Google Scholar]
- 133.Maleki M., Ghorbanpour M., Kariman K. Physiological and antioxidative responses of medicinal plants exposed to heavy metals stress. Plant Gene. 2017;11:247–254. doi: 10.1016/j.plgene.2017.04.006. [DOI] [Google Scholar]
- 134.Miller G., Suzuki N., Ciftci-Yilmaz S., Ron M. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 135.Srivastava S., Dubey R.S. Manganese-excess induces oxidative stress, lowers the pool of antioxidants and elevates activities of key antioxidative enzymes in rice seedlings. Plant Growth Regul. 2011;64:1–16. doi: 10.1007/s10725-010-9526-1. [DOI] [Google Scholar]
- 136.Luis A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015;66:2827–2837. doi: 10.1093/jxb/erv099. [DOI] [PubMed] [Google Scholar]
- 137.Rehman M.Z., Rizwan M., Ghafoor A., Naeem A., Ali S., Sabir M., Qayyum M.F. Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soil and its phyto-availability to wheat and rice under rotation. Environ. Sci. Pollut. Res. 2015;22:16897–16906. doi: 10.1007/s11356-015-4883-y. [DOI] [PubMed] [Google Scholar]
- 138.Giri J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal. Behav. 2011;6:1746–1751. doi: 10.4161/psb.6.11.17801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shahbaz M., Zia B. Does exogenous application of glycinebetaine through rooting medium alter rice (Oryza sativa L.) mineral nutrient status under saline conditions. J. Appl. Bot. Food Qual. 2012;84:54–60. [Google Scholar]
- 140.Cao F., Liu L., Ibrahim W., Cai Y., Wu F. Alleviating effects of exogenous glutathione, glycinebetaine, brassinosteroids and salicylic acid on cadmium toxicity in rice seedlings (Oryza Sativa) Agrotechnology. 2013;2:107–112. doi: 10.4172/2168-9881.1000107. [DOI] [Google Scholar]
- 141.Mahmood T., Ashraf M., Shahbaz M. Does exogenous application of Glycine betaine as a Pre sowing seed treatment improves growth and regulate some key physiological attributes in wheat plants grown under water deficit conditions. Pak. J. Bot. 2009;41:1291–1302. [Google Scholar]
- 142.Tripathi D.K., Singh S., Singh S., Mishra S., Chauhan D.K., Dubey N.K. Micronutrients and their diverse role in agricultural crops: Advances and future prospective. Acta Physiol. Plant. 2015;37:1–14. doi: 10.1007/s11738-015-1870-3. [DOI] [Google Scholar]
- 143.Hu R., Sunc K., Suc X., Pana Y., Zhang Y. Physiological responses and tolerance mechanisms to Pb in two xerophils: Salsola passerina Bunge and Chenopodium album L. J. Hazard. Mater. 2012;205–206:131–138. doi: 10.1016/j.jhazmat.2011.12.051. [DOI] [PubMed] [Google Scholar]
- 144.Li B., Shi J.B., Wang X., Meng M., Huang L., Qi XL., He B., Ye Z.H. Variations and constancy of mercury and methylmercury accumulation in rice grown at contaminated paddy field sites in three Provinces of China. Environ. Pollut. 2013;181:91–97. doi: 10.1016/j.envpol.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 145.Yonny M.E., Rodríguez Torressi A., Nazareno M.A., Cerutti S. Development of a novel, sensitive, selective, and fast methodology to determine malondialdehyde in leaves of melon plants by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Anal. Methods Chem. 2017;9 doi: 10.1155/2017/4327954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Patel A., Pandey V., Patra D.D. Metal absorption properties of Mentha spicata grown under tannery sludge amended soil—Its effect on antioxidant system and quality. Chemosphere. 2016;147:67–73. doi: 10.1016/j.chemosphere.2015.12.097. [DOI] [PubMed] [Google Scholar]
- 147.Farooq M.A., Ali S., Hameed A., Ishaque W., Mahmood K., Iqbal Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 2013;96:242–249. doi: 10.1016/j.ecoenv.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 148.Ali B., Qian P., Jin R., Ali S., Khan M., Aziz R., Tian T., Zhou W. Physiological and ultra-structural changes in Brassica napus seedlings induced by cadmium stress. Biol. Plant. 2014;58:131–138. doi: 10.1007/s10535-013-0358-5. [DOI] [Google Scholar]
- 149.Zaheer I.E., Ali S., Rizwan M., Bareen F.E., Abbas Z., Bukhari S.A.H., Wijaya L., Alyemeni M.N., Ahmad P. Zinc-lysine prevents chromium-induced morphological, photosynthetic, and oxidative alterations in spinach irrigated with tannery wastewater. Environ. Sci. Pollut. Res. 2019;26:28951–28961. doi: 10.1007/s11356-019-06084-z. [DOI] [PubMed] [Google Scholar]
- 150.Najeeb U., Jilani G., Ali S., Sarwar M., Xu L., Zhou W. Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L, and its remediation through exogenous citric acid. J. Hazard. Mater. 2011;186:565–574. doi: 10.1016/j.jhazmat.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 151.Saidi I., Ayouni M., Dhieb A., Chtourou Y., Chaïbi W., Djebali W. Oxidative damages induced by short-term exposure to cadmium in bean plants: Protective role of salicylic acid. S. Afr. J. Bot. 2013;85:32–38. doi: 10.1016/j.sajb.2012.12.002. [DOI] [Google Scholar]
- 152.Ehsan S., Ali S., Noureen S., Mahmood K., Farid M., Ishaque W., Shakoor M.B., Rizwan M. Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol. Environ. Saf. 2014;106:164–172. doi: 10.1016/j.ecoenv.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 153.Xu P., Zeng G., Huang D., Dong H., Lai C., Chen M., Tang W., Li F., Leng Y., Cheng M., et al. Cadmium induced hydrogen peroxide accumulation and responses of enzymatic antioxidants in Phanerochaete chrysosporium. Ecol. Eng. 2015;75:110–115. doi: 10.1016/j.ecoleng.2014.11.060. [DOI] [Google Scholar]
- 154.Al Hassan M., Fuertes M.M., Ramos Sánchez F.J., Vicente O., Boscaiu M. Effects of Salt and Water Stress on Plant Growth and on Accumulation of Osmolytes and Antioxidant Compounds in Cherry Tomato. Not BotHortiAgrobo. 2015;43:1–11. [Google Scholar]
- 155.Kito K., Yamamori T., Theerawitaya C., Cha-um S., Fukaya M., Rai V., Takabe T. Differential Effects of Excess Potassium and Sodium on Plant Growth and Betaine Accumulation in Sugar Beet. J. Agron. Agric. Asp. 2017;3:1–7. [Google Scholar]
- 156.Sozharajan R., Natarajan S. NaCl stress causes changes in photosynthetic pigments and accumulation of compatible solutes in Zea mays L. J Appl Advan Res. 2016;1:3–9. doi: 10.21839/jaar.2016.v1i1.6. [DOI] [Google Scholar]
- 157.De la Torre-González A., Montesinos-pereira D., Blasco B., Ruiz J.M. Influence of the proline metabolism and glycine betaine on tolerance to salt stress in tomato (Solanum lycopersicum L.) commercial genotypes. J. Plant Physiol. 2018;231:329–336. doi: 10.1016/j.jplph.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 158.Rasheed R., Wahid A., Ashraf M., Basra S.M.A. Role of proline and glycinebetaine in improving chilling stress tolerance in sugarcane buds at sprouting. Int. J. Agric. Biol. 2010;12:1–8. [Google Scholar]
- 159.Jin P., Zhang Y., Shan T., Huang Y., Xu J., Zheng Y. Low-Temperature Conditioning Alleviates Chilling Injury in Loquat Fruit and Regulates Glycine Betaine Content and Energy Status. J. Agric. Food Chem. 2015;63:3654–3659. doi: 10.1021/acs.jafc.5b00605. [DOI] [PubMed] [Google Scholar]
- 160.Wang Q., Ding T., Zuo J., Gao L., Fan L. Amelioration of postharvest chilling injury in sweet pepper by glycine betaine. Postharvest Biol. Technol. 2016;112:114–120. doi: 10.1016/j.postharvbio.2015.07.008. [DOI] [Google Scholar]
- 161.Ahmad R., Hussain J., Jamil M., Kim M.D., Kwak S.S., Shah M.M., El-Hendawy S.E., Al-Suhaibani N.A., Rehman S. Glycinebetaine Synthesizing Transgenic Potato Plants Exhibit Enhanced Tolerance To Salt And Cold Stresses. Pak. J. Bot. 2014;46:1987–1993. [Google Scholar]
- 162.Burgess P., Huang B. Effects of sequential application of plant growth regulators and osmoregulants on drought tolerance of creeping bentgrass. Crop Sci. 2013;54:837–844. doi: 10.2135/cropsci2013.03.0200. [DOI] [Google Scholar]
- 163.Liu X.M., Kim K.E., Kim K.C., Nguyen X.C., Han H.J., Jung M.S., Kim H.S., Kim S.H., Park H.C., Yun D.J., et al. Cadmium activates Arabidopsis MPK3 and MPK6 via accumulation of reactive oxygen species. Phytochemistry. 2010;71:614–618. doi: 10.1016/j.phytochem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 164.Wei D., Zhang W., Wang C., Meng Q., Li G., Chen T.H.H., Yang X. Genetic engineering of the biosynthesis of glycinebetaine leads to alleviate salt-induced potassium efflux and enhances salt tolerance in tomato plants. Plant Sci. 2017;257:74–83. doi: 10.1016/j.plantsci.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 165.Rady M.O.A., Semida W.M., Abd El-Mageed T.A., Hemida K.A., Rady M.M. Up-regulation of antioxidative defense systems by glycine betaine foliar application in onion plants confer tolerance to salinity stress. Sci. Hortic. 2018;240:614–622. doi: 10.1016/j.scienta.2018.06.069. [DOI] [Google Scholar]
- 166.Kumar A., Dharamvir Kumar M., Jangra M., Kumar N. Growth and Development of Rapeseed Mustard and Other Field Crops under Different Sowing Dates. Int. J. Pure Appl. Biosci. 2018;6:144–156. doi: 10.18782/2320-7051.7101. [DOI] [Google Scholar]
- 167.Sabagh A.E.L., Sorour S., Ragab A., Saneoka H., Islam M.S. The Effect of Exogenous Application of Proline and Glycine Betaineon the Nodule Activity of Soybean under Saline Condition. J. Agric. Biotechnol. 2017;2:1–5. [Google Scholar]
- 168.Yao T.C., Shaharuddin N.A., Ling H.C., Mahmood M. Exogenous Application of Glycine Betaine Alleviates Salt Induced Damages More Efficiently Than Ascorbic Acid In In Vitro Rice Shoots. Aust. J. Basic Appl. Sci. 2016;10:58–65. [Google Scholar]
- 169.Alasvandyari F., Mahdavi B. Effect of Glycinebetaine On Growth and Antioxidant Enzymes of Safflower Under Salinity Stress Condition. Agric. For. 2017;63:85–95. doi: 10.17707/AgricultForest.63.3.09. [DOI] [Google Scholar]
- 170.Shams M., Yildirim E., Ekinci M., Turan M., Dursun A., Parlakova F., Kul R. Exogenously applied glycine betaine regulates some chemical characteristics and antioxidative defence systems in lettuce under salt stress. Hortic. Environ. Biotechnol. 2016;57:225–231. doi: 10.1007/s13580-016-0021-0. [DOI] [Google Scholar]
- 171.Xing W., Rajashekar C.B. Glycinebetaine involvement in freezing tolerance and water stress is Arabidopsis thaliana. Environ. Exp. Bot. 2001;46:21–28. doi: 10.1016/S0098-8472(01)00078-8. [DOI] [PubMed] [Google Scholar]
- 172.Hussain H.A., Men S., Hussain S., Chen Y., Ali S., Zhang S., Zhang K., Li Y., Xu Q., Liao C., et al. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019;9:3890. doi: 10.1038/s41598-019-40362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ashraf M., Harris J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004;166:3–16. doi: 10.1016/j.plantsci.2003.10.024. [DOI] [Google Scholar]
- 174.Somersalo S., Kyei-Boahen S., Pehu E. Exogenous glycine betaine application as a possibility to increase low temperature tolerance of crop plants. Nordisk Jordbruksforsk. 1996;78:10. [Google Scholar]
- 175.Chen W.P., Li P.H., Chen T.H.H. Glycine betaine increases chilling tolerance and reduces chilling-induced lipid peroxidation in Zea mays L. Plant Cell Environ. 2000;23:609–618. doi: 10.1046/j.1365-3040.2000.00570.x. [DOI] [Google Scholar]
- 176.Nayyar H., Chander K., Kumar S., Bains T. Glycine betaine mitigates cold stress damage in Chickpea. Agron. Sustain. Dev. 2005;25:381–388. doi: 10.1051/agro:2005033. [DOI] [Google Scholar]
- 177.Cheng C., Pei L.M., Yin T.T., Zhang K.W. Seed treatment with glycine betaine enhances tolerance of cotton to chilling stress. J. Agric. Sci. 2018;156:323–332. doi: 10.1017/S0021859618000278. [DOI] [Google Scholar]
- 178.Wang L., Shan T., Xie B., Ling C., Shao S., Jin P., Zheng Y. Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem. 2019;272:530–538. doi: 10.1016/j.foodchem.2018.08.085. [DOI] [PubMed] [Google Scholar]
- 179.Jagendorf T.A., Takabe T. Inducers of glycine betaine synthesis in barley. Plant Physiol. 2001;127:1827–1835. doi: 10.1104/pp.010392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Di Martino C., Delfine S., Pizzuto R., Loreto F., Fuggi A. Free amino acids and glycine betaine in leaf osmoregulation of spinach responding to increasing salt stress. New Phytol. 2003;158:455–463. doi: 10.1046/j.1469-8137.2003.00770.x. [DOI] [PubMed] [Google Scholar]
- 181.Sulpice R., Tsukaya H., Nonaka H., Mustardy L., Chen T.H., Murata N. Enhanced formation of flowers in salt-stressed Arabidopsis after genetic engineering of the synthesis of glycine betaine. Plant J. 2003;36:165–176. doi: 10.1046/j.1365-313X.2003.01873.x. [DOI] [PubMed] [Google Scholar]
- 182.Nuccio M.L., McNeil S.D., Ziemak M.J., Hanson A.D., Jain R.K., Selvaraj G. Choline Import into Chloroplasts Limits Glycine Betaine Synthesis in Tobacco: Analysis of Plants Engineered with a Chloroplastic or a Cytosolic Pathway. Metab. Eng. 2000;2:300–311. doi: 10.1006/mben.2000.0158. [DOI] [PubMed] [Google Scholar]
- 183.Zhao J., Li S., Jiang T., Liu Z., Zhang W., Jian G., Qi F. Chilling stress—The key predisposing factor for causing Alternaria alternata infection and leading to cotton (Gossypium hirsutum L.) leaf senescence. PLoS ONE. 2012;7:e36126. doi: 10.1371/journal.pone.0036126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang Q., Rue K. The effect of glycine betaine priming on seed germination of six turfgrass species under drought, salinity, or temperature stress. HortScience. 2014;49:1454–1460. doi: 10.21273/HORTSCI.49.11.1454. [DOI] [Google Scholar]
- 185.Ahmed N., Zhang Y., Yu H., Gabar A., Zhou Y., Li Z., Zhang M. Seed priming with Glycine betaine improve seed germination characteristics and antioxidant capacity of wheat (Triticum aestivum L.) seedlings under water-stress conditions. Appl. Ecol. Environ. Res. 2019;17:8333–8350. doi: 10.15666/aeer/1704_83338350. [DOI] [Google Scholar]
- 186.Grumet R., Isleib G.T., Hanson A.D. Genetic Control of Glycinebetaine Level in Barley. Crop Sci. 1985;25:618–622. doi: 10.2135/cropsci1985.0011183X002500040010x. [DOI] [Google Scholar]
- 187.Iqbal N., Ashraf M.Y. Does Seed Treatment with Glycinebetaine Improve Germination Rate and Seedling Growth of Sunflower (Helianthus Annuus L.) Under Osmotic Stress. Pak. J. Bot. 2006;38:1641–1648. [Google Scholar]
- 188.Naidu B.P. Production of betaine from Australian Melaleuca spp. for use in agriculture to reduce plant stress. Aust. J. Exp. Agric. 2003;43:1163–1170. doi: 10.1071/EA02223. [DOI] [Google Scholar]
- 189.Demir I., Oztokat C. Effect of salt priming on germination and seedling growth at low temperatures in watermelon seeds during development. Seed Sci. Technol. 2003;31:765–770. doi: 10.15258/sst.2003.31.3.26. [DOI] [Google Scholar]
- 190.Farooq M., Basra S.M.A., Ahmad N. Improving the performance of transplanted rice by seed priming. Plant Growth Regul. 2007;51:129–137. doi: 10.1007/s10725-006-9155-x. [DOI] [Google Scholar]
- 191.Allakhverdiev S.I., Los D.A., Mohanty P., Nishiyama Y., Murata N. Glycinebetaine alleviates the inhibitory effectof moderate heat stress on the repair of photosystem II during photoinhibition. Biochim. Biophys. Acta. 2007;1767:1363–1371. doi: 10.1016/j.bbabio.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 192.Hassanein R.A., Hassanein A.A., Haider A.S., Hashem H.A. Improving salt tolerance of Zea mays L. plants by presoaking their grains in glycine betaine. Aust. J. Basic Appl. Sci. 2009;3:928–942. [Google Scholar]
- 193.Korkmaz A., Sirikci R., Kocacınar F., Özlem Deger O., Demirkırıan A.R. Alleviation of salt-induced adverse effects in pepper seedlings by seed application of glycinebetaine. Sci. Hortic. 2012;148:197–205. doi: 10.1016/j.scienta.2012.09.029. [DOI] [Google Scholar]
- 194.Arafa A.A., Khafagy M.A., El-Banna M.F. The effect of glycinebetaine or ascorbic acid on grain germination and leaf structure of sorghum plants grown under salinity stress. Aust. J. Crop Sci. 2009;3:294–304. [Google Scholar]
- 195.Heuer B. Influence of exogenous application of proline and glycinebetaine on growth of salt-stressed tomato plants. Plant Sci. 2003;165:693–699. doi: 10.1016/S0168-9452(03)00222-X. [DOI] [Google Scholar]
- 196.Corol D., Ravel C., Rakszegi M., Bedo Z., Charmet G., Beale M.E. Effects of genotype and environment on the contents of betaine, choline, and trigonelline in cereal grains. J. Agric. Food Chem. 2012;60:5471–5481. doi: 10.1021/jf3008794. [DOI] [PubMed] [Google Scholar]
- 197.Moharramnejad S., Sofalian O., Valizadeh M., Asgari A., Shiri M. Proline, glycine betaine, total phenolics and pigment con-tents in response to osmotic stress in maize seedlings. J. Biosci. Biotechnol. 2015;4:313–319. [Google Scholar]
- 198.Lerma C., Roch P.J., Ju G.C., Yang W.J., Hanson A.D., Rhodes D. Betaine deficiency in maize: Complementation tests and metabolic basis. Plant Physiol. 1991;95:1113–1119. doi: 10.1104/pp.95.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang W.J., Nadolska-Orczyk A., Wood K.V., Hahn D.T., Rich P.J., Wood A.J., Saneoka H., Premachandra G.S., Bonham C.C., Rhodes J.C. Near-Isogenic Lines of Maize Differing for Glycinebetaine. Plant Physiol. 1995;107:621–630. doi: 10.1104/pp.107.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang T., Liang J., Wang M., Li D., Liu Y., Chen T.H., Yang X. Genetic engineering of the biosynthesis of glycine betaine enhances the fruit development and size of tomato. Plant Sci. 2019;280:355–366. doi: 10.1016/j.plantsci.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 201.Munns R., Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 202.Ashraf M., Akram N.A., Arteca R.N., Foolad M.R. The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Crit. Rev. Plant Sci. 2010;29:162–190. doi: 10.1080/07352689.2010.483580. [DOI] [Google Scholar]
- 203.Zhang H., Dong H., Li W., Sun Y., Chen S., Kong X. Increased glycine betaine synthesis and salinity tolerance in ahcmo transgenic cotton lines. Mol. Breed. 2009;23:289–298. doi: 10.1007/s11032-008-9233-z. [DOI] [Google Scholar]
- 204.Kishitani S., Takanami T., Suzuki M., Oikawa M., Yokoi S., Ishitani M. Compatibility of glycinebetaine in rice plants: Evaluation using transgenic rice plants with a gene for peroxisomal betaine aldehyde dehydrogenase from barley. Plant Cell Environ. 2000;23:107–114. doi: 10.1046/j.1365-3040.2000.00527.x. [DOI] [Google Scholar]
- 205.Khan M.S., Yu X., Kikuchi A., Asahina M., Watanabe K.N. Genetic engineering of glycine betaine biosynthesis to enhance abiotic stress tolerance in plants. Plant Biotechnol. 2009;26:125–134. doi: 10.5511/plantbiotechnology.26.125. [DOI] [Google Scholar]
- 206.Qin D., Zhao C.L., Liu X.Y., Wang P.W. Transgenic soybeans expressing betaine aldehyde dehydrogenase from Atriplex canescens show increased drought tolerance. Plant Breed. 2017;136:699–709. doi: 10.1111/pbr.12518. [DOI] [Google Scholar]
- 207.Hashemi F.S.G., Ismail M.R., Rafii M.Y., Aslani F., Miah G., Muharam F.M. Critical multifunctional role of the betaine aldehyde dehydrogenase gene in plants. Biotechnol. Equip. 2018;32:818–829. [Google Scholar]