Abstract
The gene encoding glucose oxidase from Aspergillus niger ZM-8 was cloned and transferred to Pichia pastoris GS115, a transgenic strain P. pastoris GS115-His-GOD constructed. The growth curve of P. pastoris GS115-His-GOD was consistent with that of Pichia pastoris GS115-pPIC9K under non-induced culture conditions. Under methanol induction conditions, the growth of the GOD-transgenic strain was significantly lowered than P. pastoris GS115-pPIC9K with the induced-culture time increase, and the optical densities of GOD-transgenic strain reached one-third of that of the P. pastoris GS115-pPIC9K at 51 h. The activity of glucose oxidase in the cell-free supernatant, the supernatant of cell lysate, and the precipitation of cell lysate was 14.3 U/mL, 18.2 U/mL and 0.48 U/mL, respectively. The specific activity of glucose oxidase was 8.3 U/mg, 6.52 U/mg and 0.73 U/mg, respectively. The concentration of hydrogen peroxide formed by glucose oxidase from supernatant of the fermentation medium, the supernatant of the cell lysate, and the precipitation of cell lysate catalyzing 0.2 M glucose was 14.3 μg/mL, 18.2 μg/mL, 0.48 μg/mL, respectively. The combination of different concentrations of glucose oxidase and glucose could significantly inhibit the growth of Agrobacterium and Escherichia coli in logarithmic phase. The filter article containing supernatant of the fermentation medium, supernatant of the cell lysate, and precipitation of cell lysate had no inhibitory effect on Agrobacterium and E. coli. The minimum inhibitory concentration of hydrogen peroxide on the plate culture of Agrobacterium and E. coli was 5.6 × 103 μg/mL and 6.0 × 103 μg/mL, respectively.
Keywords: Aspergillus niger, Pichia pastoris, Glucose oxidase, Transgenic, Antimicrobial activity
Introduction
Since 1929, Fleming’s discovery on bactericides prompted a search for antimicrobial substance in molds of the same genus. Coulthard et al. (1942) first described an antibacterial glucose aerohydrogenes (Notatin, firstly named as penicillin A) from Penicillium notatum Westling. Almost at the same time, other substances including penicillin B and penatin also were isolated from Penicillium and exhibited good antimicrobial activity (Bruggen et al., 1943; Kocholaty, 1943; Roberts et al., 1943). Until 1963, Muller found that penicillin B and penatin were identical to notatin, and all of them belong to Glucose oxidase (GOD) (Bentley, 1963), which played the important roles in the inhibition of microbial growth. Thereafter, Glucose oxidase, referred to as an an ideal enzyme, has attracted the attention of researchers (Bentley, 1963; Park et al., 2000; Crognale et al., 2006; Belyad, Karkhanei & Raheb, 2018; Li et al., 2019; Tu et al., 2019).
Glucose oxidase (β-D-glucose: oxygen 1-oxidoreductase, GOD, EC 1.1.3.4) catalyzes the oxidation of glucose to gluconic acid and hydrogen peroxide in the presence of molecular oxygen according to the following reactions (Hodgkins et al., 1993; Yamaguchi et al., 2007; Meng et al., 2014):
GODs are produced by molds such as Aspergillus niger and Penicillium (Frederick et al., 1990; Meng et al., 2014; Qiu et al., 2016; Farshad, Amin & Catherine, 2018). Many literatures reported that GOD could inhibit the growth of microbes in foods or food preparation media (Tiina & Sandholm, 1989; Yoo & Rand, 1995; Li et al., 2019). And it has been proven that this type of antibacterial compound is hydrogen peroxide (H2O2), which is active against G+ and G− bacteria (Malherbe et al., 2003). This bacteriostatic effect of hydrogen peroxide is mainly attributed to the peroxidation of membrane lipids (Roberts et al., 1943; Piard & Desmazeaud, 1991). In laboratory-scale testing, refrigerated shelf life of GOD-treated fish was improved by 67% over untreated fish (Field et al., 1986). Moreover, GOD was able to inhibit growth of Pseudomonas spp. which are the main psychrotrophic spoilage microorganisms of chilled poultry (Barnes & Impey, 1968; Cox et al., 1975). GODs are also used in many medical applications. Sandholm and his co-workers suggested that all mastitis pathogens were sensitive to the glucose oxidase-lactoperoxidase system (Sandholm et al., 1988). GOD was also used as an antimicrobial agent in oral care (Szynol et al., 2004). The effect of honey on clearing infections in a wide range of wounds, which often did not respond to conventional therapy, was result of the antibacterial activity of hydrogen peroxide that is produced by GOD in honey (Molan, 2001; Bang, Buntting & Molan, 2003; Khadivi et al., 2017).
As above-mentioned descriptions, GODs are prepared mainly from the fermentation of Aspergillus (Tu et al., 2019), Penicillium (Bodade, Khobragade & Arfeen, 2010; Khan et al., 2016), Bacillus sp. (Xu et al., 2018), Cladosporium neopsychrotolerans (Ge et al., 2020), transgenic Trichoderma reesei (Wu et al., 2017), transgenic P. pastoris (Park et al., 2000; Crognale et al., 2006; Yamaguchi et al., 2007; Rocha et al., 2010; Fang et al., 2015; Belyad, Karkhanei & Raheb, 2018), and directly used in industry (Wong, Wong & Chen, 2008) as a control agent against pathogenic microorganism (Hopkinsa et al., 2019; Lee et al., 2019; Li et al., 2019) or a key catalyst for bioelectrochemical applications (Visvanathan et al., 2018; Mano, 2019). However, very little information is available whether a glucose oxidase-secreting microbe could inhibit growth of its surrounding living things and become an ecological bacteriostatic agent. As we all know, chemical control is still the main method used to control the incidence of gray mold, chemical disinfectant could leave unsafe residues on plant materials and can drive resistance in pathogens, as well as contribute to environmental pollution. In this study, we here reported a new biological control strategy (Dal et al., 2008) for controlling the growth of pathogenic microorganism. Specifically, the gene coding for glucose oxidase from A. niger ZM-8 was expressed under the control of inducible alcohol oxidase 1 (AOX-1) promoter in yeast P. pastoris. The antimicrobial property of the glucose oxidase enzyme was evaluated.
Materials and Methods
Strains and plasmids
A 1,749.0 bp GOD gene fragment was amplified from the genomic DNA of Aspergillus niger ZM-8 by the CTAB method (Porebski, Bailey & Baum, 1997). Primers for PCR were designed as Table S1 based on conserved sequences of glucose oxidase gene (No. JO5242) from GenBank Database, and then cloned into plasmid pUC19, The linearized vector pUC19-His-GOD by SmaI was inserted into the S. cerevisiae α-factor secretion signal molecule pPIC9k (Invitrogn, Carlsbad, CA, USA) to generate the expression vector pPIC9k-His-GOD under the action of the promoter AOX-1. The identified recombinant plasmid pPIC9K-His-GOD was linearized by Bgl-П and transformed into P. pastoris GS115 cells by electroporation. The electro-competent P. pastoris GS115 cells were prepared using standard methods (Manivasakam & Schiestl, 1993). The electroporation was performed using a Gene Pulser (Bio-Rad, Hercules, CA, USA) at 1.5 kV, 40.0 μF, and 150.0 Ω according to manufacturer’s instruction.
Screening of clones and determination of biomass
The recombinant yeast clones were screened on yeast extract peptone dextrose (YPD) (1% (w/v) yeast extract, 2% (w/v) tryptone, 2% (w/v) dextrose, 2% (w/v) agar) plus 1 M sorbitol (YPDS) plates containing 100.0 μg/mL G418 (Invitrogen, Carlsbad, CA, USA) for 2.0–4.0 days. Potential high-level secretion transformants were obtained from the YPDS agar plates containing a higher G418 concentration (300.0 μg/mL). All these potential high-level secretion clones were confirmed by PCR using genomic DNA as the templates.
One colony was picked among several high copy clones obtained from the plate containing P. pastoris GS115-pPIC9k-His-GOD. P. pastoris GS115-pPIC9k was used as a negative control for the experiment. Clones were inoculated in Buffered Glycerol-complex medium (BMGY) (1% (w/v) yeast extract, 2% (w/v) tryptone, 100 mM Potassium Phosphate (pH 6.0), 1.34% (w/v) YNB, 4 × 10−5 D-Biotin, 1% (w/v) glycerol), and cultured at 30 °C until OD600 = 0.60. The culture was then transferred to Buffered methanol-complex medium (BMMY) and cultured at 30 °C. The absorbance of growing culture was measured every 3 h.
Expression of GOD in transgenic P. pastoris GS115
The P. Pastoris strains were cultivated in BMGY medium at 30 °C for 24 h. Biomass was generated after initial growth phase with glycerol as a carbon source. Finally, to induce AOX-1 dependent protein expression, the methanol fed-batch phase was started with methanol feed rate of 0.5 mL/12.0 h. Cell-free supernatant, the supernatant of cell lysate, and the precipitate of cell lysate was collected, and crushed by ultrasonic. The ultrasonication conditions used was 15 s, 25 s, 380 w, 99 times, and then stored at 4 °C (Cereghino & Cregg, 2000).
Analysis of glucose oxidase activity
Pichia pastoris GS115-His-GOD-01 and P. pastoris GS115-pPIC9k were cultured at 30 °C with supplement of 0.5% methanol per 12 h. Activities of glucose oxidase from cell-free supernatant, cell lysate supernatant and precipitation were carried out according to Gemba & Hara (1971) with a slight modification. In detail, the reaction system consisting of 4.0 mL of reaction mixture (0.20 M Acetic acid-Sodium acetate, pH 5.2; 0.2 M glucose and 1.0 mM Indigo Carmine) and 2.0 mL of the appropriately diluted enzyme solution was added into and incubated for 10 min at 37 °C. Then, the reaction was stopped by boiling water bath for 13 min. The absorbance was measured at a wavelength of 615.0 nm. One unit (U) of Glucose oxidase activity was defined as the amount of enzyme, that can oxidize 1.0 μM of β-D-glucose to D-gluconic acid and H2O2 per 3 min at pH 6.0 and 30 °C. All reactions were performed in triplicate. The formula of enzyme activity as follows:
X0: Enzyme activity; A: Absorbance value of trichloroacetic acid instead of glucose as the control; A0: Absorbance value of the sample solution; K: Slope of the standard curve; C0: Intercept of the standard curve. V: the volume of sample solution; F: the dilution factor.
The specific activity of GOD
Protein concentrations of cell-free liquid, cell lysate supernatant and precipitation from P. pastoris GS115-His-GOD-01 and P. pastoris GS115-pPIC9K were determined by the method of Bradford (Hammond & Kruger, 1988). Absorbance was measured at 615 nm wavelength and specific activity of GOD was the value of activity divided by the value of protein concentrations.
Antibacterial effects of glucose and glucose oxidase system on growth of Agrobacterium and E. coli in liquid medium
Glucose oxidase and glucose were used in three dilution-set combinations. The concentration of glucose used were 1.0, 2.5 and 5.0 mg/mL respectively. The GOD was from fermentation supernatant of transgenic P. pastoris GS115-His-GOD-01 that was induced by methanol and fermentation supernatant from P. pastoris GS115-pPIC9K as control. Concentrations of GOD used was 1.0, 5.0 and 10.0 U/mL. The GOD and glucose solutions were added in the medium of YEP or LB and arranged in a Latin-square design to study the effects of substrates and enzyme on growth of Agrobacterium LBA4404 and E. coli DH5α by measuring optical density in 600 nm.
GOD antibacterial activity to Agrobacterium and E. coli on agar plates
The antibacterial activity of GOD produced by P. pastoris GS115-His-GOD-01 against A. tumefaciens LBA4404 and E. coli DH5α (stored in Dr. Ma Jianzhong’s laboratory of Lanzhou University of Technology) was determined. Cultivate to bacterial liquid OD600 = 1.0, spread on YPE (1% (w/v) yeast extract, 1% (w/v) tryptone, 0.5% (w/v) NaCl and 1.5% (w/v ) Agar) plates or LB (1% (w/v) yeast extract, 2% (w/v) tryptone, 2% (w/v) NaCl and 1.5% (w/v) agar) plates, on 51 h after adding methanol, add cell-free liquid, sonicate the supernatant by precipitating, collect the pellet and resuspend the pellet in an ice bath, immerse it in sterile filter paper, and inoculate with 0.20 M A. tumefaciens LBA4404 or E. coli DH5α on the surface of the glucose plate, observe its antibacterial effect.
Antibacterial activity of hydrogen peroxide solution to Agrobacterium and E. coli on agar plates
The inhibitory effects of A. tumefaciens LBA4404 and E. coli DH5α and the minimum hydrogen peroxide concentration to inhibit bacterial growth were detected. A. tumefaciens LBA4404 was cultured with shaking at 28 °C to OD600 = 1.0, and 200 μL was coated on YPE medium, and then put into filter paper with different concentrations of hydrogen peroxide solution, and cultured at 28 °C for 14 h, and observed antibacterial effect. E. coli DH5α was shake-cultured at 37 °C to OD600 = 1.5, 200 μL was applied to LB medium, and then filter paper of different concentrations of hydrogen peroxide solution was placed. The culture was allowed to stand at 37 °C for 14 h to observe the inhibitory effect.
Results
Vector construction and screening of transgenic P. pastoris clones
Pichia pastoris strain GS115 was transformed using linearized pPIC9K-His-GOD as described in materials and methods to yield P. pastoris GS115-His-GOD (Fig. S1). Twelve clones were obtained and confirmed by PCR-testing for gene integration. These clones were then screened on YPDS plates with different concentrations of Geneticin (G418), that is, 100 mM, 200 mM, and 300 mM, respectively. A positive transgenic clone, designated as P. pastoris GS115-His-GOD 01 was grown on the YPDS plate with a high Geneticin concentration and was considered for subsequent experiments.
Expression of the GOD affecting the growth of the GOD-transgenic strain
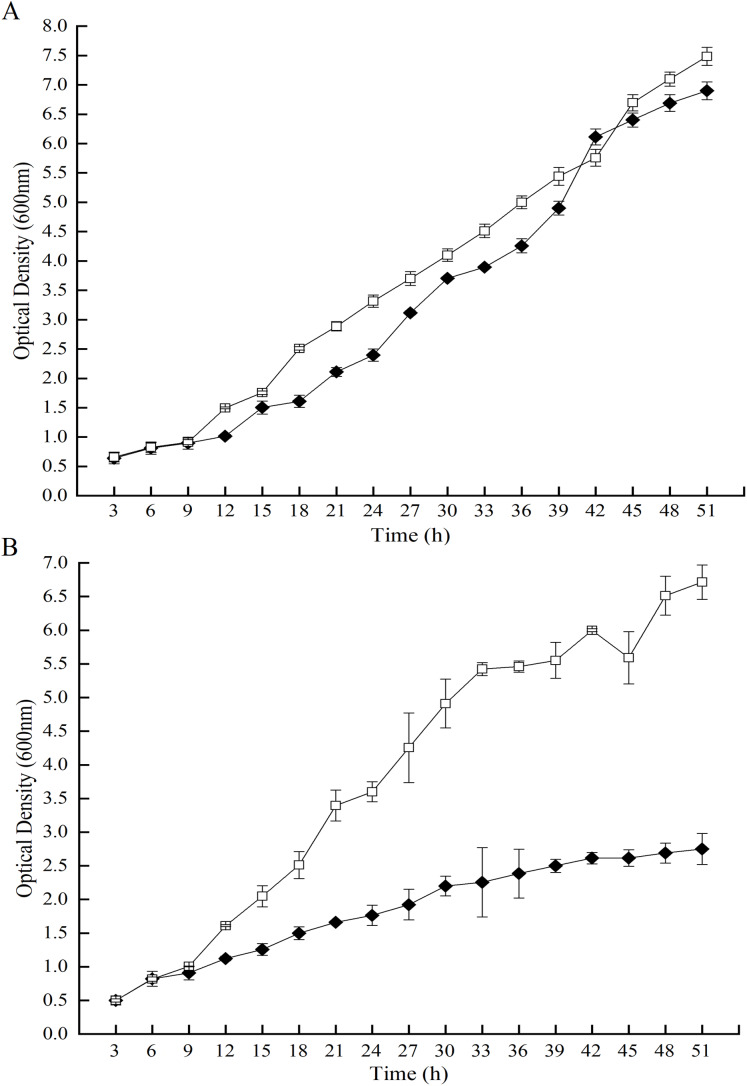
Hydrogen peroxide, one of the products by GOD, injures living cells. In line with this statement, growth of the GOD-transgenic strain, P. pastoris GS115-His-GOD 01 was analyzed. Compared to P. pastoris GS115-pPIC9K, the growth of P. pastoris GS115-His-GOD 01 was slightly decreased during 51st h of incubation under GOD uninduced condition (Fig. 1A). Its optical density at 600 nm was 0.95-fold of that of P. pastoris GS115-pPIC9K at the time point of 51.0 h. However, the growth of P. pastoris GS115-His-GOD 01 was significantly lowered if the GOD was induced by methanol (Fig. 1B). During the growth of 51.0 h, the optical densities of P. pastoris GS115-His-GOD 01 were 0.54-fold of that of P. pastoris GS115-pPIC9K at 18.0h, 0.43-fold at 36 h, and 0.37-fold at 51.0 h, respectively. The inhibited growth of the GOD-transgenic P. pastoris could be attributed to the expression of the foreign GOD and, hereafter, accumulation of H2O2.
Figure 1. Effects of GOD induction on the growth of the P. pastoris GS115-His-GOD 01.
(A) P. pastorisGS115-His-GOD 01 (filled diamonds) and P. pastoris GS115-pPIC9K (hollow squares) were incubated in YPD without methanol for 51 h at 30 °C; (B) P. pastoris GS115-His-GOD 01 (filled diamonds) and P. pastoris GS115-pPIC9K (hollow squares) were incubated in YPD with 0.5% methanol added every 12 h for 51 h at 30 °C.
Activities of the glucose oxidase
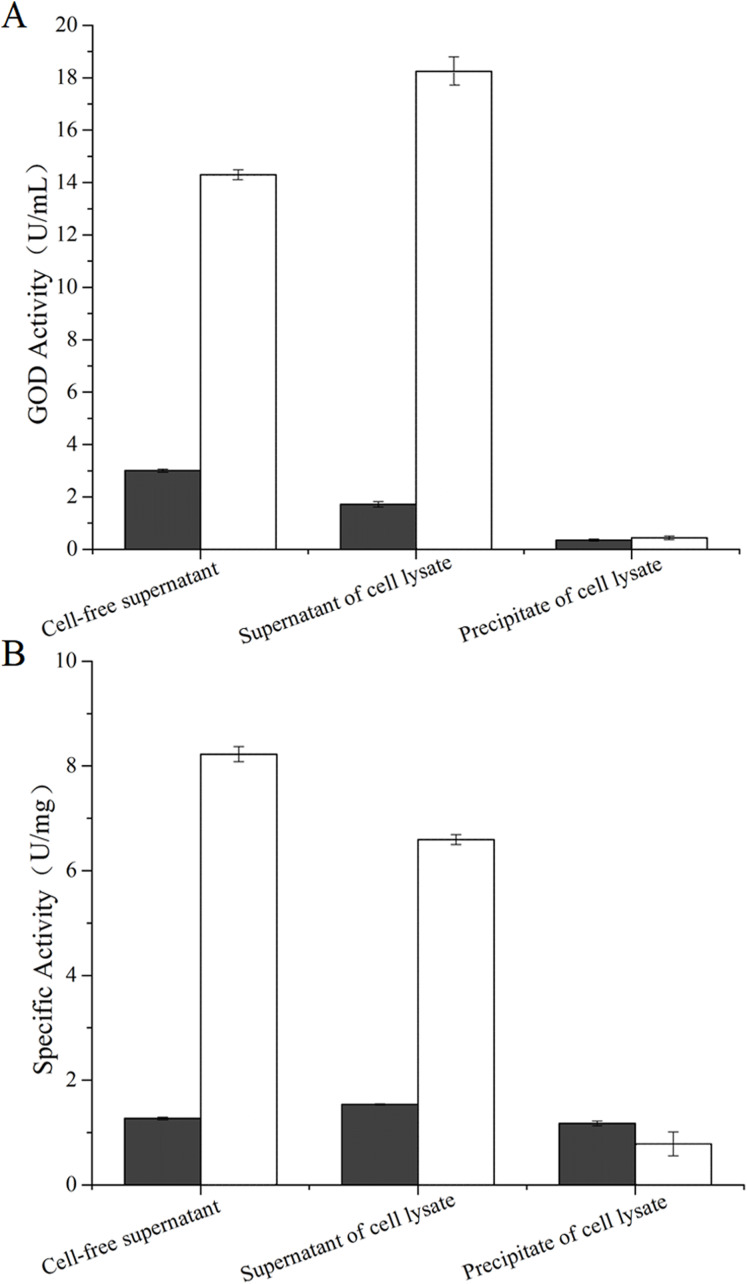
After 51 h-induced incubation, the cultures were processed into three parts of which were the cell-free supernatant, the supernatant and the precipitation of the cell lysates. The activities of the GOD preparations from P. pastoris GS115-His-GOD 01 were 14.27 U/mL in the cell-free supernatant, 18.2 U/mL in the supernatant of the cell lysate, and 0.48 U/mL in the precipitation (Fig. 2A). As a control, the activities of the three GOD preparations from P. pastoris GS115-pPIC9K were 3.22 U/mL, 1.76 U/mL and 0.41 U/mL, respectively (Fig. 2A). The specific activities of the three GOD preparations from P. pastoris GS115-His-GOD 01 were 8.30 U/mg in the cell-free supernatant, 6.52 U/mg in the supernatant of the cell lysate, and 0.73 U/mg in the precipitation, respectively (Fig. 2B). The specific activities of the three preparations from P. pastoris GS115-pPIC9K were 0.859 U/mg, 1.483 U/mg, and 0.529 U/mg, respectively (Fig. 2B). According to the specific activities, the cell-free supernatant of P. pastoris GS115-His-GOD 01 had the highest value, but the supernatant of the cell lysate of P. pastoris GS115-pPIC9K gave the highest specific activity. These results suggested that the native GOD of P. pastoris GS115 was mainly an intracellular enzyme. In the GOD-transgenic P. pastoris GS115, the enzyme was mainly secreted. This is in accordance with that the recombinant GOD was directed to an extra-cellular fraction by a signal peptide, α-mating factor.
Figure 2. Activities and specific activities of the GOD in the different preparations.
(A) The activities of the glucose oxidase in the cell-free supernatant, the supernatant of cell lysate, and the precipitate of cell lysate. (B) The specific activities of the glucose oxidase in the cell-free supernatant, the supernatant of cell lysate, and the precipitate of cell lysate. Data from P. pastoris GS115-pPIC9K are exhibited in black columns and P. pastoris GS115-His-GOD 01 in colorless columns.
The concentration of hydrogen peroxide from GOD catalyzed glucose
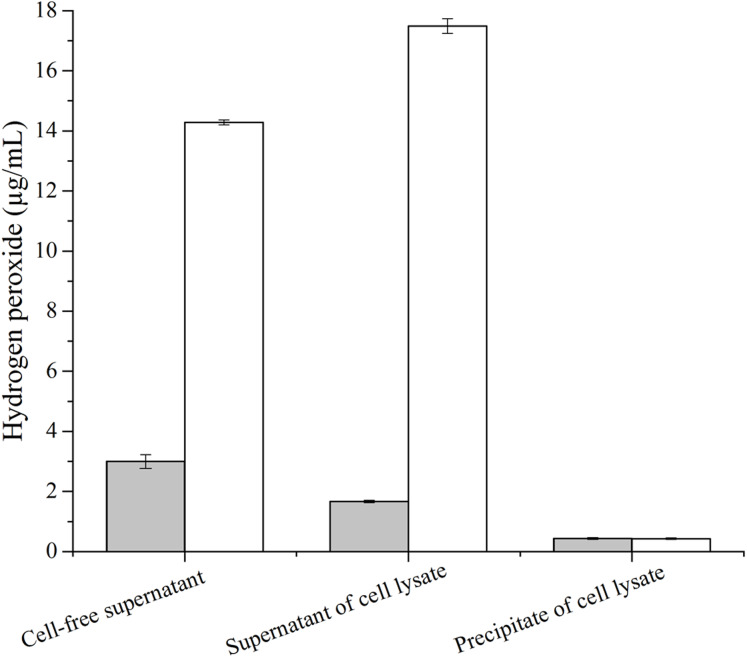
The concentration of hydrogen peroxide produced by GOD from P. pastoris GS115-His-GOD-01 and P. pastoris GS115-pPIC9K catalytic glucose was showed in Fig. 3. According to the results in Fig. 3, the concentration of hydrogen peroxide was 14.3 μg/mL and 3.05 μg/mL in cell-free supernatant. The concentration of H2O2 from the supernatant of cell lysate was 18.2 μg/mL and 1.86 μg/mL, however, that of the precipitate of cell lysate was 0.48 μg/mL and 0.46 μg/mL. These results indicated that the GOD could be secreted out of the cells with the form of soluble protein.
Figure 3. Production of hydrogen peroxide by the GOD preparations.
Hydrogen peroxides produced by GOD from P. pastoris GS115-His-GOD 01 are in colorless columns, and in black columns from P. pastoris GS115-pPIC9K. Note: Cell-free supernatant, the GOD from the supernatant after fermentation; supernatant of cell lysate, the GOD from the supernatant of the cell lysate by ultrasonication; precipitate of cell lysate, the GOD from the precipitation of cell lysate by ultrasonication.
Inhibition of the GOD preparations on the growth of A. tumefaciens LBA4404 and E. coli in liquid medium
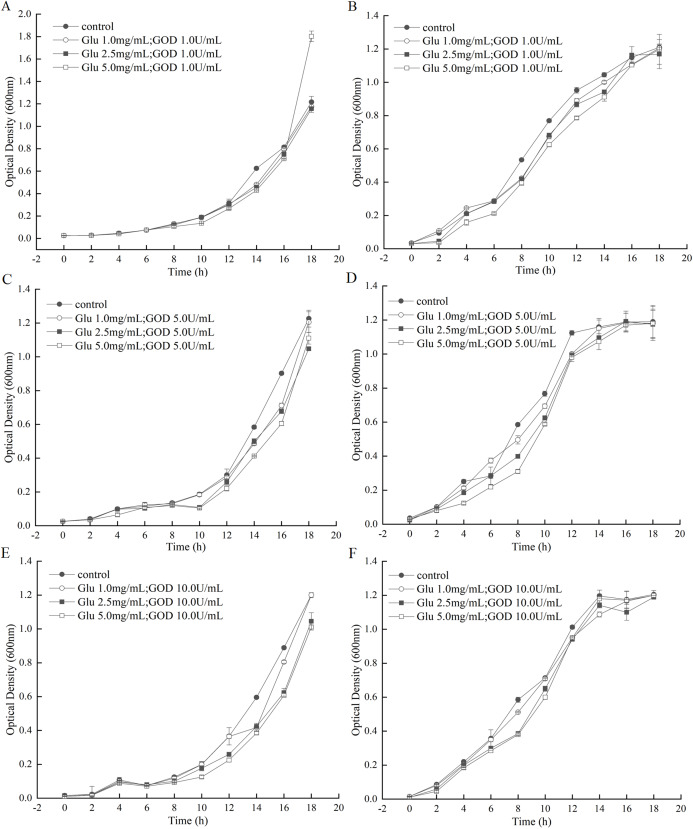
The GOD was prepared in fermentation supernatant of P. pastoris GS115-His-GOD 01. When the concentration of GOD was set as 1.0 U/mL, the growth curve of A. tumefaciens LBA4404 and E. coli DH5α under the gradually increasing of Glu were shown in Figs. 4A and 4B. From these two figures, almost no inhibition to the growth of A. tumefaciens LBA4404 compared with the control that was added with equal volume of P. pastoris GS115-pPIC9K fermentation supernatant. However, the marked inhibition to the growth of E. coli DH5α were observed from 4th h to 14th h with substrate concentration increasing (Fig. 4B). When the concentration of GOD was set as 5.0 U/mL, a slight inhibition on the growth of A. tumefaciens LBA4404 after 14th h with substrate concentration increasing (Fig. 4C), and the same change trends with Fig. 4B can be observed for the inhibition on E. coli DH5α from Fig. 4D. With the increasing of Glu concentration, the delay of growth of A. tumefaciens LBA4404 also be observed by the given GOD concentration of 10 U/mL (Fig. 4E), and the minor effect on E. coli DH5α also could be found in Fig. 4F. Conclusions were drawn from Fig. 4, it showed these combinations did not completely inhibit growth of A. tumefaciens and LBA4404 E. coli DH5α, but influenced the time at which growth was initiated. Delay of growth initiation was greatest with the enzyme concentration, 5.0 U/mL, and the impact increased also with substrate concentration.
Figure 4. Inhibition effects of the GOD preparations on growth of A. tumefaciens LBA4404 and E. coli DH5α in liquid media.
(A and B) Shows the inhibition effects of the GOD (1.0 U/mL) on growth of A. tumefaciens LBA4404 and E. coli DH5α with the final concentrations of glucose were 1.0 mg/mL, 2.5 mg/mL, 5.0 mg/mL, respectively. (C and D) Displayed the inhibition effects of the GOD (5.0 U/mL) on growth of A. tumefaciens LBA4404 and E. coli DH5α with the final concentrations of glucose were 1.0 mg/mL, 2.5 mg/mL, 5.0 mg/mL, respectively. (E and F) Displayed the inhibition effects of the GOD (10.0 U/mL) on growth of A. tumefaciens LBA4404 and E. coli DH5α with the final concentrations of glucose were 1.0 mg/mL, 2.5 mg/mL, 5.0 mg/mL, respectively.
Antibacterial effects of glucose and glucose oxidase on growth of A. tumefaciens and E. coli on agar plates
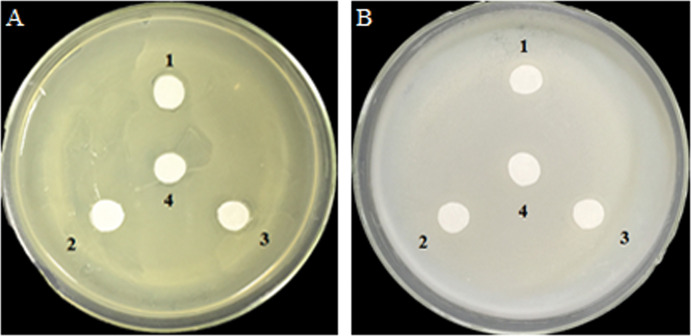
Analysis of the antibacterial activity of hydrogen peroxide (H2O2) produced by GOD catalyzed substrates glucose. A. tumefaciens LBA4404 (Fig. 5A) and E. coli DH5α (Fig. 5B) were plated on YPE or LB which were contained 0.2 M glucose. Filter papers were soaked by cell-free supernatant, the supernatant of cell lysate, and the precipitate of cell lysate from P. pastoris GS115-His-GOD 01, cell-free supernatant of P. pastoris GS115-pPIC9K as the negative control. The results showed that H2O2 derived from glucose which catalyzed by GOD had no effect on the growth of A. tumefaciens LBA4404 and E. coli DH5α.
Figure 5. Inhibition effects of the GOD preparations on growth of A. tumefaciens LBA4404 (A) and E. coli DH5α (B) in solid media.
(1) Denotes the GOD preparations from the cell-free supernatant of P. pastoris GS115-His-GOD 01; (2) is the supernatant of cell-lysate of P. pastoris GS115-His-GOD 01 by ultrasonication; (3) is the precipitate of cell-lysate of P. pastoris GS115-His-GOD 01 by ultrasonication; (4) represents the negative control with the sterile water.
Antibacterial activity of hydrogen peroxide solution to A. tumefaciens LBA4404 and E. coli DH5α
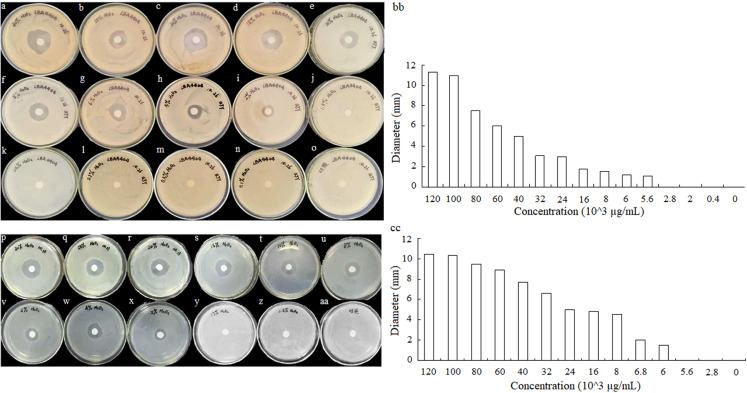
To detect the minimum concentration of hydrogen peroxide solution inhibit the growth of A. tumefaciens LBA4404 and E. coli DH5α, the sterile filter articles were soaked with a volume of 10 μL hydrogen peroxide that was diluted to different concentrations. Different concentrations of hydrogen peroxide solution effect on A. tumefaciens LBA4404 and E. coli DH5α were determined. As shown in Figs. 6A–O and 6P–AA, the inhibition effect of H2O2 on the two strains gradually decreased with the concentration reducing. The diameters of inhibition zone for these strains also were displayed in Figs. 6BB and 6CC, implying that the minimum concentration of hydrogen peroxide solution inhibits the growth of A. tumefaciens LBA4404 and E. coli DH5α was 5.6 × 103 μg/mL and 6.0 × 103 μg/mL.
Figure 6. Different concentrations of hydrogen peroxide solution effect on A. tumefaciens LBA4404 (A–O) and E. coli DH5α (P–AA) on agar plates.
The inhibit effects of different concentrations of hydrogen peroxide solutions on the growth of A. tumefaciens LBA4404 (BB), and E. coli DH5α (CC).
Discussion
As evident from the abovementioned reviews, Glucose oxidase acts as a bacteriostatic agent by catalyzing hydrogen peroxide production via glucose oxidation (Wong, Wong & Chen, 2008; Bankar et al., 2009). At present, large-scale production of the enzyme was completed by good stability strains and fermentation technology (Ge et al., 2020). Although, many strains (Bodade, Khobragade & Arfeen, 2010; Khan et al., 2016; Wu et al., 2017) have been screened for producing this enzyme efficiently, only a few strains could be applied to commercial production. Therefore, more strategies need to be developed by constructing different bioreactor, modified enzyme, or enzyme engineering technology. Compared with a mass of glucose oxidase as an antibacterial agent applied in food preservation (Lee et al., 2019; Li et al., 2019), uses the GOD-transgenic strains or their fermented supernatants directly are easy, inexpensive, and widely available (Dal et al., 2008). However, little information is available whether a glucose oxidase-secreting microbe could inhibit the growth of its surrounding living things. As Dal et al. (2008) description, yeast could inhibit the gray mold growth, the novel viewpoint could be used to the development of bacteriostatic agent by genetic manipulations. Studies have found that most P. pastoris expression systems use methanol-induced ethanol oxidase promoters to express GOD (Crognale et al., 2006; Yamaguchi et al., 2007; Belyad, Karkhanei & Raheb, 2018), and the concentration of methanol directly affects cell growth and protein expression (Cereghino et al., 2002; Xiao, Wang & Chen, 2004; Daly & Hearn, 2005). Crognale et al. (2006) described a genetically modified P. pastoris X 33 with the gene encoding the GOX from P. variabile P16, and the activity only reached at 50 U/mL. Kovačević et al. (2014) cloned several mutated glucose oxidase genes from A. niger M12 and expressed them in P. pastoris KM71H. The highest activity of the GOD came up to 17.5 U/mL of fermentation media. Gu et al. (2015) reported recently that a yield of GOD reached 21.81 g/L, with an activity of 1972.9 U/mL, in P. pastoris S17 of which is a genetically modified strain by manipulating genes involved in protein folding machinery and abnormal folding stress responses. Belyad, Karkhanei & Raheb (2018) also cloned the GOD gene from A. niger ATCC 9029 and inserted into the pPIC9 vector for protein expression in P. pastoris GS115 by the alcohol oxidase promoter, but no the expression ability and enzyme activity were introduced. Although the above literatures has been reported the produce of GOD, there are no detailed determination on the activity of glucose oxidase in supernatant of the fermentation medium, the supernatant of the cell lysate, and the precipitation of cell lysate. In particular, there is no evidence of hydrogen peroxide production and bacteriostatic properties analysis. In this article, the GOD-encoding gene from A. niger ZM-8 was cloned and transferred into P. pastoris GS115 to yield a transgenic strain, which can excrete GOD to medium by the way of methanol induction. The activity of glucose oxidase in supernatant of the fermentation medium, the supernatant of the cell lysate, and the precipitation of cell lysate was 14.3 U/mL, 18.2 U/mL and 0.48 U/mL, respectively. Corresponding these determined samples, The concentration of hydrogen peroxide formed by glucose oxidase can reached at 14.3 μg/ml, 18.2 μg/ml, 0.48 μg/ml, respectively. According to our results, the GOD-transgenic P. pastoris has to produce more enzyme molecules or higher active enzymes in order to inhibit microbes. Although the growth of P. pastoris GS115-His-GOD was found to be seriously inhibited during the period of methanol induction, its fermented supernatants containing the GOD activity can really reduce the growth of E. coli and A. tumefaciens in liquid culture (Fig. 4). In contrast, the GOD-soaked filter papers didn’t exhibit any inhibition to the growth of A. tumefaciens and E. coli on the solid medium (Fig. 5). At present, it was not sure that it resulted from no enough oxygen or no enough GOD. As shown in Fig. 6, hydrogen peroxide can inhibit growth of A. tumefaciens and E. coli on solid medium, and the concentrations at least are 5.6 × 103 μg/mL and 6.0 × 103 μg/mL, respectively. To reach the concentration of hydrogen peroxide, the activity of the GOD produced from the transgenic strain should be at least increased 300-fold. To achieve antibacterial applications by GOD-transgenic P. pastoris directly, there will be more studies to be done in enzyme activity improvement and oxygen-offering system. These results could provide a new insight on bacteriostatic agent.
Conclusion
This study cloned a gene encoding Aspergillus niger ZM-8 glucose oxidase and transferred it to P. pastoris to form a transgenic strain GS115-His-GOD. Compared with GS115-pPIC9K, the transgenic P. pastoris GS115-His-GOD could express glucose oxidase by methanol induction, The hydrogen peroxide could be produced with Glucose as the substrate during the fermentation process, and exhibited the inhibition activities on the growth of A. tumefaciens and E. coli.
Supplemental Information
Letters in italics designate restriction endonuclease sites. GOD, the gene for Aspergillus niger glucose oxidase; alpha, ɑ-mating factor; AOX1, the promoter of alcohol oxidase 1; HISorf, the open reading frame of the histidine deaminase gene 4. The vector was linearized with Bgl II before transformation.
Funding Statement
This study was financially supported by Chinese National Natural Science Foundation (Nos. 31760028 and 31460032), the Fundamental Research Funds for Key Laboratory of Drug Screening and Deep Processing for Traditional Chinese and Tibetan Medicine of Gansu Province (No. KZZY20180605), and the Youth Talent Support Program of Lanzhou University of Technology (No. 2018). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Yonggang Wang, Email: wangyg@lut.cn.
Jianzhong Ma, Email: majz@lut.cn.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Yonggang Wang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Jiangqin Wang performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Feifan Leng performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Jianzhong Ma conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Alnoor Bagadi performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The data is available in the figures and a Supplemental File.
References
- Bang, Buntting & Molan (2003).Bang LM, Buntting C, Molan P. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. Journal of Alternative and Complementary Medicine. 2003;9(2):267–273. doi: 10.1089/10755530360623383. [DOI] [PubMed] [Google Scholar]
- Bankar et al. (2009).Bankar SB, Bule MV, Singhal RS, Ananthanarayan L. Glucose oxidase-an overview. Biotechnology Advances. 2009;27(4):489–501. doi: 10.1016/j.biotechadv.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Barnes & Impey (1968).Barnes EM, Impey CS. Psychrophilic spoilage bacteria of poultry. Journal of Applied Bacteriology. 1968;31(1):97–107. doi: 10.1111/j.1365-2672.1968.tb00345.x. [DOI] [PubMed] [Google Scholar]
- Belyad, Karkhanei & Raheb (2018).Belyad F, Karkhanei AA, Raheb J. Expression, characterization and one step purification of heterologous glucose oxidase gene from Aspergillus niger ATCC, 9029 in Pichia pastoris. EuPA Open Proteomics. 2018;19:1–5. doi: 10.1016/j.euprot.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley (1963).Bentley R. Glucose oxidase. In: Boyer PD, Lardy H, Myrbäck K, editors. The Enzymes. Vol. 7. New York: Academic Press; 1963. pp. 567–586. [Google Scholar]
- Bodade, Khobragade & Arfeen (2010).Bodade RG, Khobragade CN, Arfeen S. Optimization of culture conditions for glucose oxidase production by a Penicillium chrysogenum SRT 19 strain. Engineering in Life Science. 2010;10(1):35–39. doi: 10.1002/elsc.200900030. [DOI] [Google Scholar]
- Bruggen et al. (1943).Bruggen JTV, Reithel FJ, Cain CK, Katzman PA, Doisy EA, Muir RD, Roberta EC, Gaby WL, Homan DM, Jones LR. Penicillin B: purification and mode of action. Journal of Biological Chemistry. 1943;148(2):365–378. doi: 10.1086/394673. [DOI] [Google Scholar]
- Cereghino et al. (2002).Cereghino GPL, Cereghino JL, Ilgen C, Cregg JM. Production of recombinant proteins in fermenter cultures of the yeast Pichia pastoris. Current Opinion in Biotechnology. 2002;13(4):329–332. doi: 10.1016/S0958-1669(02)00330-0. [DOI] [PubMed] [Google Scholar]
- Cereghino & Cregg (2000).Cereghino JL, Cregg JM. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiology Reviews. 2000;24(1):45–66. doi: 10.1111/j.1574-6976.2000.tb00532.x. [DOI] [PubMed] [Google Scholar]
- Coulthard et al. (1942).Coulthard CE, Short WF, Michaelis R, Sykes G, Skrimshire GEH, Standfast AFB, Birkinshaw JH, Raistrick H. Notatin: an antibacterial glucose aerohydrogenes from Penicillium notatum Westling. Nature. 1942;150:634–635. doi: 10.1038/150634a0. [DOI] [Google Scholar]
- Cox et al. (1975).Cox NA, Juven BJ, Thomson JE, Mercuri AJ, Chew V. Spoilage odors in poultry meat produced by pigmented and nonpigmented Pseudomonas. Poultry Science. 1975;54(6):2001–2006. doi: 10.3382/ps.0542001. [DOI] [Google Scholar]
- Crognale et al. (2006).Crognale S, Pulci V, Brozzoli V, Petruccioli M, Federici F. Expression of Penicillium variabile P16 glucose oxidase gene in Pichia pastoris and characterization of the recombinant enzyme. Enzyme and Microbial Technology. 2006;39(6):1230–1235. doi: 10.1016/j.enzmictec.2006.03.005. [DOI] [Google Scholar]
- Dal et al. (2008).Dal BG, Monaco C, Rollan MC, Lampugnani G, Arteta N, Abramoff C, Ronco L, Stocco M. Biocontrol of postharvest grey mould on tomato by yeasts. Journal of Phytopathology. 2008;156(5):257–263. doi: 10.1111/j.1439-0434.2007.01351.x. [DOI] [Google Scholar]
- Daly & Hearn (2005).Daly R, Hearn MT. Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. Journal of Molecular Recognition. 2005;18(2):119–138. doi: 10.1109/4.953490. [DOI] [PubMed] [Google Scholar]
- Fang et al. (2015).Fang J, You R, Song C, Guo RF. Production of glucose oxidase and its application in feed industry. Feed Review. 2015;10:1–7. doi: 10.3969/j.issn.1001-0084.2015.10.001. [DOI] [Google Scholar]
- Farshad, Amin & Catherine (2018).Farshad D, Amin Z, Catherine M. In silico and in vivo analysis of signal peptides effect on recombinant glucose oxidase production in nonconventional yeast yarrowia lipolytica. World Journal of Microbiology and Biotechnology. 2018;34(9):128. doi: 10.1007/s11274-018-2512-x. [DOI] [PubMed] [Google Scholar]
- Field et al. (1986).Field CE, Pivarnik LF, Barnett SM, Rand AG., Jr Utilization of glucose oxidase for extending the shelf-life of fish. Journal of Food Science. 1986;51(1):66–70. doi: 10.1111/j.1365-2621.1986.tb10837.x. [DOI] [Google Scholar]
- Frederick et al. (1990).Frederick KR, Tung JT, Emerick RS, Masiarz FR, Schopter LM. Glucose oxidase from Aspergillus niger: cloning, gene sequence, secretion from Saccharomyces cerevisiae and kinetic analysis of a yeast-derived enzyme. Journal of Biological Chemistry. 1990;265(7):3793–3802. doi: 10.1021/bi00461a029. [DOI] [PubMed] [Google Scholar]
- Ge et al. (2020).Ge JZ, Jiang X, Liu WN, Wang Y, Huang HQ, Bai YG, Su XY, Yao B, Luo HY. Characterization, stability improvement, and bread baking applications of a novel cold-adapted glucose oxidase from Cladosporium neopsychrotolerans SL16. Food Chemistry. 2020;310:1–8. doi: 10.1016/j.foodchem.2019.125970. [DOI] [PubMed] [Google Scholar]
- Gemba & Hara (1971).Gemba T, Hara F. A new automated enzymatic method for determination of blood glucose by coupling of cupric histamine system. Ikagaku Shinpojumu. 1971;1(1):106–108. [Google Scholar]
- Gu et al. (2015).Gu L, Zhang J, Du G, Chen J. Multivariate modular engineering of the protein secretory pathway for production of heterologous glucose oxidase in Pichia pastoris. Enzyme and Microbial Technology. 2015;68:33–42. doi: 10.1016/j.enzmictec.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Hammond & Kruger (1988).Hammond JBW, Kruger NJ. The Bradford method for protein quantization. Methods in Molecular Biology. 1988;32:9–15. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- Hodgkins et al. (1993).Hodgkins M, Mead D, Ballance DJ, Goodey A, Sudbery P. Expression of the glucose oxidase gene from Aspergillus niger in Hansenula polymorpha and its use as reporter gene to isolate regulatory mutants. Yeast. 1993;9(6):625–635. doi: 10.1002/yea.320090609. [DOI] [PubMed] [Google Scholar]
- Hopkinsa et al. (2019).Hopkinsa EJ, Huclb P, Scanlonc MG, Nickersona MT. Effects of glucose oxidase and organic acids on the properties of a model low sodium dough prepared from Harvest and Pembina CWRS wheat. Journal of Cereal Science. 2019;89:1–8. doi: 10.1016/j.jcs.2019.102802. [DOI] [Google Scholar]
- Khadivi et al. (2017).Khadivi DF, Darvishi F, Dezfulian M, Madzak C. Expression and characterization of glucose oxidase from Aspergillus niger in Yarrowia lipolytica. Molecular Biotechnology. 2017;59(8):307–314. doi: 10.1007/s12033-017-0017-8. [DOI] [PubMed] [Google Scholar]
- Khan et al. (2016).Khan I, Qayyum S, Ahmed S, Niaz Z, Fatima N, Chi ZM. Molecular cloning and sequence analysis of a pvgox gene encoding glucose oxidase in Penicillium viticola F1 strain and it’s expression quantitation. Gene. 2016;592(2):291–302. doi: 10.1016/j.gene.2016.07.032. [DOI] [PubMed] [Google Scholar]
- Kocholaty (1943).Kocholaty W. Purification and properties of penatin: the second antibacterial substance produced by Penicillium notatum Westling. Science. 1943;2:73–86. doi: 10.1126/science.97.2512.186. [DOI] [PubMed] [Google Scholar]
- Kovačević et al. (2014).Kovačević G, Blažić M, Draganić B, Ostafe R, Jankulović MG, Fischer R. Cloning, heterologous expression, purification and characterization of M12 mutant of Aspergillus niger glucose oxidase in yeast Pichia pastoris KM71H. Molecular Biotechnology. 2014;56(4):305–311. doi: 10.1007/s12033-013-9709-x. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2019).Lee I, Cheon HJ, Adhikari MD, Tran TD, Yeon KM, Kim MI, Kim J. Glucose oxidase-copper hybrid nanoflowers embedded with magnetic nanoparticles as an effective antibacterial agent. International Journal of Biological Macromolecules. 2019;155:1520–1531. doi: 10.1016/j.ijbiomac.2019.11.129. [DOI] [PubMed] [Google Scholar]
- Li et al. (2019).Li XJ, Xie XF, Xing FG, Xu L, Zhang J, Wang ZD. Glucose oxidase as a control agent against the fungal pathogen Botrytis cinerea in postharvest strawberry. Food Control. 2019;105:277–284. doi: 10.1016/j.foodcont.2019.05.037. [DOI] [Google Scholar]
- Malherbe et al. (2003).Malherbe DF, Toit MD, Otero RRC, Rensburg PV, Pretorius IS. Expression of the Aspergillus niger glucose oxidase gene in Saccharomyces cerevisiae and its potential applications in wine production. Applied Microbiology & Biotechnology. 2003;61(5–6):502–511. doi: 10.1007/s00253-002-1208-0. [DOI] [PubMed] [Google Scholar]
- Manivasakam & Schiestl (1993).Manivasakam P, Schiestl RH. High efficiency transformation of Saccharomyces cerevisiae by electroporation. Nucleic Acids Research. 1993;21(18):4414–4415. doi: 10.1093/nar/21.18.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano (2019).Mano N. Engineering glucose oxidase for bioelectrochemical applications. Bioelectrochemistry. 2019;128:218–240. doi: 10.1016/j.bioelechem.2019.04.015. [DOI] [PubMed] [Google Scholar]
- Meng et al. (2014).Meng Y, Zhao M, Yang M, Zhang Q, Meng Y. Production and characterization of recombinant glucose oxidase from Aspergillus niger expressed in Pichia pastoris. Letters in Applied Microbiology. 2014;58(4):393–400. doi: 10.1111/lam.12202. [DOI] [PubMed] [Google Scholar]
- Molan (2001).Molan PC. Potential of honey in the treatment of wounds and burns. American Journal of Clinical Dermatology. 2001;2(1):13–19. doi: 10.2165/00128071-200102010-00003. [DOI] [PubMed] [Google Scholar]
- Park et al. (2000).Park EH, Shin YM, Lim YY, Kwon TH, Kim DH, Yang MS. Expression of glucose oxidase by using recombinant yeast. Journal of Biotechnology. 2000;81(1):35–44. doi: 10.1016/S0168-1656(00)00266-2. [DOI] [PubMed] [Google Scholar]
- Piard & Desmazeaud (1991).Piard JC, Desmazeaud MJ. Inhibiting factors produced by lactic acid bacteria. 1. oxygen metabolites and catabolism end-products. Dairy Science and Technology. 1991;71(5):525–541. doi: 10.1051/lait:1991541. [DOI] [Google Scholar]
- Porebski, Bailey & Baum (1997).Porebski S, Bailey LG, Baum BR. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Molecular Biology Reporter. 1997;15(1):8–15. doi: 10.1007/BF02772108. [DOI] [Google Scholar]
- Qiu et al. (2016).Qiu ZJ, Guo YF, Bao XM, Hao JR, Sun GY, Peng BY, Bi WX. Expression of Aspergillus niger glucose oxidase in yeast Pichia pastoris SMD1168. Biotechnology & Biotechnological Equipment. 2016;30(5):998–1005. doi: 10.1080/13102818.2016.1193442. [DOI] [Google Scholar]
- Roberts et al. (1943).Roberts EC, Cain CK, Muir RD, Reithel FJ, Doisy EA. Penicillin b, an antibacterial substance from Penicillium notatum. Journal of Biological Chemistry. 1943;147(1):47–58. doi: 10.1515/bchm2.1943.277.4-6.284. [DOI] [Google Scholar]
- Rocha et al. (2010).Rocha SN, José AN, María EC, María IGS, Gombert AK. Heterologous expression of glucose oxidase in the yeast Kluyveromyces marxianus. Microbial Cell Factories. 2010;9(1):4. doi: 10.1186/1475-2859-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandholm et al. (1988).Sandholm M, Ali-Vehmas T, Kaartinen L, Junnila M. Glucose oxidase (GOD) as a source of hydrogen peroxide for the lactoperoxidase (LPO) system in milk: antibacterial effect of the GOD-LPO system against mastitis pathogens. Journal of Veterinary Medicine. 1988;35(1–10):346–352. doi: 10.1111/j.1439-0450.1988.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Szynol et al. (2004).Szynol A, De Soet JJ, Sieben-Van TE, Bos JW, Frenken LG. Bactericidal effects of a fusion protein of llama heavy-chain antibodies coupled to glucose oxidase on oral bacteria. Antimicrobial Agents and Chemotherapy. 2004;48(9):3390–3395. doi: 10.1128/AAC.48.9.3390-3395.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiina & Sandholm (1989).Tiina M, Sandholm M. Antibacterial effect of the glucose oxidase-glucose system on food-poisoning organisms. International Journal of Food Microbiology. 1989;8(2):165–174. doi: 10.1016/0168-1605(89)90071-8. [DOI] [PubMed] [Google Scholar]
- Tu et al. (2019).Tu T, Wang Y, Huang H, Wang Y, Jiang X, Wang Z, Yao B, Luo H. Improving the thermostability and catalytic efficiency of glucose oxidase from Aspergillus niger by molecular evolution. Food Chemistry. 2019;281:163–170. doi: 10.1016/j.foodchem.2018.12.099. [DOI] [PubMed] [Google Scholar]
- Visvanathan et al. (2018).Visvanathan R, Jayathilake C, Liyanage R, Sivakanesan R. Applicability and reliability of the glucose oxidase method in assessing α-amylase activity. Food Chemistry. 2018;1–28:265–272. doi: 10.1016/j.foodchem.2018.09.114. [DOI] [PubMed] [Google Scholar]
- Wong, Wong & Chen (2008).Wong CM, Wong KH, Chen XD. Glucose oxidase: natural occurrence, function, properties and industrial applications. Applied Microbiology and Biotechnology. 2008;78(6):927–938. doi: 10.1007/s00253-008-1407-4. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2017).Wu Y, Sun X, Xue X, Luo H, Su X. Overexpressing key component genes of the secretion pathway for enhanced secretion of an Aspergillus niger glucose oxidase in Trichoderma reesei. Enzyme and Microbial Technology. 2017;106:83–87. doi: 10.1016/j.enzmictec.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Xiao, Wang & Chen (2004).Xiao SK, Wang L, Chen YQ. The study on improving expression levels of heterologous gene in Pichia pastoris. Biotechnology Bulletin. 2004;30(2):23–26. doi: 10.3969/j.issn.1002-5464.2004.02.006. [DOI] [Google Scholar]
- Xu et al. (2018).Xu D, Sun L, Li C, Wang Y, Ye R. Inhibitory effect of glucose oxidase from, Bacillus, sp. CAMT22370 on the quality deterioration of pacific white shrimp during cold storage. LWT. 2018;92:339–346. doi: 10.1016/j.lwt.2018.02.025. [DOI] [Google Scholar]
- Yamaguchi et al. (2007).Yamaguchi M, Tahara Y, Nakano A, Taniyama T. Secretory and continuous expression of Aspergillus niger glucose oxidase gene in Pichia pastoris. Protein Expression and Purification. 2007;55(2):273–278. doi: 10.1016/j.pep.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Yoo & Rand (1995).Yoo W, Rand AG. Antibacterial effect of glucose oxidase on growth of Pseudomonas fragi, as related to pH. Journal of Food Science. 1995;60(4):868–871. doi: 10.1111/j.1365-2621.1995.tb06249.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Letters in italics designate restriction endonuclease sites. GOD, the gene for Aspergillus niger glucose oxidase; alpha, ɑ-mating factor; AOX1, the promoter of alcohol oxidase 1; HISorf, the open reading frame of the histidine deaminase gene 4. The vector was linearized with Bgl II before transformation.
Data Availability Statement
The following information was supplied regarding data availability:
The data is available in the figures and a Supplemental File.